ABSTRACT
Background
Multisite pain, including low-back and knee pain, is a major health issue that greatly decreases quality of life.
Objectives
This study analyzed the effects of l-serine, which provides necessary components for nerve function, and EPA, which exerts anti-inflammatory properties, on pain scores of adults with pain in at least the low back and knee for ≥3 mo.
Methods
This was a randomized, double-blind, placebo-controlled, parallel-group study. The Japan Low Back Pain Evaluation Questionnaire (JLEQ) and Japanese Knee Osteoarthritis Measure (JKOM) were applied as primary outcomes. The Brief Pain Inventory (BPI) and safety evaluation were secondary outcomes. We enrolled 120 participants aged ≥20 y (36 men and 84 women: mean ± SD age = 40.8 ± 10.9 y). The participants were randomly allocated to either the active group (daily ingestion of 594 mg l-serine and 149 mg EPA) or placebo group. The study period consisted of 8-wk dosing and 4-wk posttreatment observation. ANCOVA between groups for each time point was conducted using the baseline scores as covariates.
Results
The JLEQ scores (active compared with placebo: 14.2 ± 11.2 compared with 19.0 ± 10.2) at week 8 were lower in the active group (P < 0.001). The JKOM scores at week 4 (11.7 ± 9.0 compared with 13.9 ± 7.9), week 8 (10.4 ± 7.9 compared with 13.1 ± 7.1), and week 12 (10.3 ± 7.4 compared with 13.8 ± 7.5) were lower in the active group (P ≤ 0.04). Additionally, the active group had 11–27% better scores compared with the placebo group for BPI1 (worst pain), BPI3 (average pain), and BPI5D (pain during moving) at week 4 (P ≤ 0.028) and week 8 (P ≤ 0.019), respectively, and BPI5D was 23% better in the active group at week 12 (P = 0.007). No adverse events were observed.
Conclusions
l-Serine and EPA were effective for pain relief in adults with low-back and knee pain after multiplicity adjustment.
This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry as UMIN000035056.
Keywords: multiple site pain, neuropathic pain, low-back and knee pain, clinical, Japan Low Back Pain Evaluation Questionnaire (JLEQ), Japanese Knee Osteoarthritis Measure (JKOM), Brief Pain Inventory (BPI)
Introduction
Pain often occurs concurrently at multiple sites, such as the lower back, neck, shoulders, hip, and knee area (1). Low-back pain (LBP) and knee pain constitute major health issues in the adult population (2). Up to 41.4% of the Japanese adult population experiences musculoskeletal pain, with the lower back being the common site of pain for both sexes (3). This study demonstrated that the neck and shoulder area showed the highest prevalence of pain (20.3%), followed by the lower back area (19.1%), and the hip and knee areas (11.1%). A community-based survey targeting >4000 participants in Japan demonstrated that the number of people with these types of pain increases with age (4). Such pain is associated with daily activity impairment as well as loss of work productivity, which significantly impacts quality of life (QOL) (1, 5). In a survey of Japanese adults and the UK Biobank study, greater pain severity and higher number of pain sites were associated with higher presenteeism (6, 7). Another study demonstrated that the total incremental cost of health care due to pain was $635 billion in the United States (8). Current care for LBP, such as nonsteroidal anti-inflammatory drugs, only offers temporary relief with limited effectiveness and poses a high risk of gastrointestinal side effects (5).
Similarly, knee pain is a common complaint experienced by people of all ages. It has been estimated that ≥25% of the elderly population has chronic knee pain, defined as pain occurring on most days of a recent month (9). Knee pain affects daily life and reduces mobility, which can progress to further disabling symptoms. Glucosamine and chondroitin are popular supplements that are commercially available worldwide, but purported benefits have not been consistently supported in the literature (10). Thus, interventions to effectively manage such chronic pain are sought.
l-Serine (l-Ser) is an amino acid that is essential for maintaining normal functions of the nervous system. It is a precursor for the synthesis of phosphoglycerols and complex macromolecules such as sphingolipids and glycolipids, which are important membrane components and myelin constituents (11). When neuronal cells are cultured under serine-deficient conditions, the concentrations of phosphatidylserine and sphingolipids decrease. Demyelination contributes to the development of neuropathic pain by disrupting the molecular and structural features of nerve fibers (12). Patients with hereditary l-Ser deficiency are reported to have polyneuropathy (13). Additionally, there is a report that the l-Ser concentration in blood decreases with age (14). These findings indicate the importance of l-Ser for maintaining normal function of the nervous system, which could be linked to providing beneficial support in chronic pain management.
Furthermore, numerous studies have found that dietary supplementation with ω-3 PUFAs, mainly as combinations of EPA and DHA, is efficacious in reducing joint swelling and pain, morning stiffness, and nonsteroidal anti-inflammatory drug usage in rheumatoid arthritis patients (15, 16). EPA, an ω-3 lipid, exerts anti-inflammatory properties after being metabolized to the anti-inflammatory lipid mediator resolvin E1. Metabolites such as resolvin E1 compete with metabolites from ω-6 PUFAs to promote the resolution of the inflammatory cycle and have been increasingly recognized as important players in the attenuation of inflammation and regulation of autoimmunity (17, 18). Resolvin E1 is reported to alleviate pain and hyperalgesia in response to heat and mechanical stimuli (19–22), and findings indicate that resolvin E1 abolishes TNF-α–evoked N-methyl-d-aspartic acid (NMDA) receptor hyperactivity in spinal dorsal horn neurons, thereby normalizing the spinal synaptic plasticity that has been implicated in generating pain hypersensitivity (21). These reports suggest that EPA supplementation can be beneficial for chronic pain induced by inflammation.
In particular, localized inflammation of the dorsal root ganglion (DRG), which extends its axons to the peripheral nerves, has been proposed to play an important role in neuropathic pain. Inflammatory processes within the DRG per se change excitability of the DRG neurons (23). Early work has demonstrated the importance of the ω-3 PUFAs, EPA and DHA, in attenuating inflammation in the DRG (24). Also, interestingly, a previous study has shown that the l-Ser biosynthesis system in the DRG is affected in a nonclinical model of painful peripheral neuropathy (25). The authors demonstrated that the localized expression of l-Ser biosynthesis enzyme in satellite cells, and not in neuronal cell bodies, plays an important role, and reported decreased l-Ser biosynthesis in the DRG in a paclitaxel-induced hyperalgesia model. Administration of l-Ser improved peripheral hyperalgesia and improved sensory nerve conduction velocity in this model. Based on the above findings, we hypothesized that the combination of l-Ser, which provides necessary components for maintaining nerve function, and EPA, which exerts anti-inflammatory properties, could synergistically alleviate chronic pain, especially in the DRG. In this study, we targeted the generally healthy adult population that experiences pain in multiple body sites. The study aimed to determine the effects of l-Ser and EPA on the pain scores of participants with pain in at least the low back and knee for ≥3 mo. The study was a randomized, double-blind, placebo-controlled, parallel-group design and used multiple validated measures for evaluating LBP and knee pain.
Methods
Trial design
This study was an 8-wk randomized, double-blind, placebo-controlled, parallel-group study followed by a 4-wk posttreatment observation period.
Ethics statements
This study was conducted in accordance with the Declaration of Helsinki. All the participants were informed of the nature of the experimental procedure before written informed consent was obtained. The study was approved by the ethics committee of Kobuna Orthopedic Surgery and Ajinomoto Co, Inc, and was registered at the University Hospital Medical Information Network Clinical Trials Registry as UMIN000035056.
Inclusion and exclusion criteria
We enrolled a generally healthy adult population. Study participants were considered eligible if they met the following criteria: 1) aged ≥20 y; and 2) having pain in at least the low back and knee for ≥3 mo based on a PainDETECT score of 13–38, which includes pain with neuropathic components, ranging from a mixed phenotype of nociceptive and neuropathic pain as well as pain highly indicative as being neuropathic (26). Exclusion criteria included: 1) having nociceptive pain, including wounds, burn injury, and bruising; 2) taking constant medication that affects pain; 3) having clear causes of pain, such as hernia, spinal canal stenosis, or knee osteoarthropathy; 4) having a history of surgery for the same pain in the past; 5) having psychiatric problems as assessed by the Brief Scale for Psychiatric Problems in Orthopaedic Patients; 6) taking functional food or supplements that could influence the outcome of the study; 7) taking amino acid, protein, or EPA supplements; 8) being pregnant or lactating; and 9) having allergies to fish or soy food.
Study design, randomization, and blinding
Sample size was calculated considering type 1 (α) error and type 2 (β) error based on the statistical significance test of the primary end point. Referring to the internal pilot single-arm open-label study, participants who had neuropathic LBP exhibited improvement of the pain score assessed by a 10-point scale as a mean of 2.1 ± 1.6 (n = 31) after ingesting the l-Ser and EPA for 8 wk. In addition, participants who had neuropathic knee pain exhibited similar improvement by a mean of 2.1 ± 1.5 (n = 36). Based on these results, the mean effect sizes on neuropathic LBP and knee pain were both estimated as 2.1, the SDs were conservatively estimated as 1.81, and for the placebo group, the mean effect size was estimated as 1.0 (27), and the SD was equally set to 1.81. Under these settings, the fixed sequence procedure was used, that is, the score for LBP was first tested by the t test, and then the score for knee pain was tested by the t test only when a significant difference was observed for LBP. As a result, when 58 participants were included in each treatment group, the power (1 − β) = 90.1% for LBP and 81.2% for knee pain at the α = 0.05 level for the entire procedure. Considering dropouts in each group, the final sample size was determined to be 60 participants in each group.
There were 360 applicants, and 120 participants were included in the study (Figure 1). We randomly allocated participants to either an l-Ser + EPA supplementation group or a placebo group, and afterwards checked that the following variables were equally distributed in both groups: sex, the Japan Low Back Pain Evaluation Questionnaire (JLEQ) score, and the Japanese Knee Osteoarthritis Measure (JKOM) score. Randomization and allocation were concealed from the researchers, clinicians at the medical institutions, and participants until the final analyses were completed. The allocation table was sealed and stored until key opening by an independent controller.
FIGURE 1.
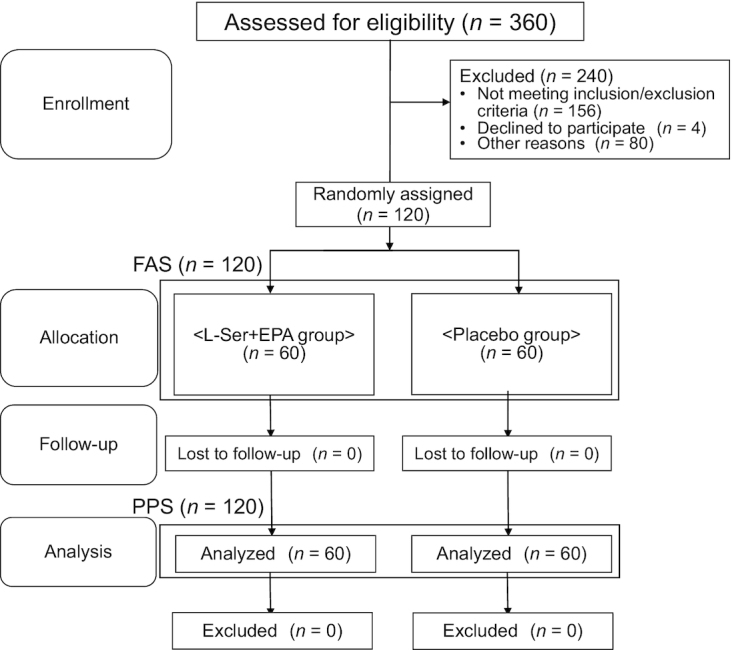
CONSORT diagram for study recruitment. Flow diagram of enrollment and allocation to either the l-serine (l-Ser) + EPA supplementation group or the placebo group of the study. FAS, full analysis set; PPS, per protocol set.
Participants were provided with either l-Ser + EPA supplementation or placebo, 4 capsules/d. To eliminate the effects of eating meals, they were instructed to ingest the capsules ≥2 h after dinner, before going to bed. Both the active sample and placebo were encapsulated in a soft vegetable film capsule. One l-Ser + EPA capsule contained 148.4 mg l-Ser and 148.4 mg purified fish oil, which contained 37.2 mg EPA, 18 mg beeswax, and 9.5 mg soy lecithin on average. One placebo capsule contained 148.4 mg dextrin, 148.4 mg safflower oil, 18 mg beeswax, and 9.5 mg soy lecithin on average. The total daily intake in the active group was 594 mg l-Ser and 149 mg EPA. Twenty-eight capsules were packaged in an aluminum pouch, corresponding to supplementation for 1 wk. These capsules were manufactured by Sunsho Pharmaceutical Co Ltd.
The study period consisted of 8 wk of the dosing period, and 4 wk of the posttreatment observation period (Figure 2). During the posttreatment period, the participants were instructed to continue with the same lifestyle behavior but to stop ingestion of all study capsules, including both active and placebo. The compliance was confirmed by collecting empty aluminum pouches used for packaging active or placebo capsules after the trial. The primary outcomes of the study included JLEQ and Japanese Orthopedic Association (JOA) scores for LBP, and the JKOM score for knee pain. The secondary outcomes included pain assessment by the Brief Pain Inventory (BPI), QOL assessment by EuroQOL 5 Dimensions 5-level (EQ-5D-5L), and a safety evaluation performed by a clinician based on the blood and urine laboratory test results. JLEQ, JKOM, JOA, BPI, EQ-5D-5L, and medical interviews were conducted at baseline and weeks 4 and 8 (dosing period), and week 12 (posttreatment period). The medical interviews included inquiry about the following items: age, gender, medical history, and allergy history (only at baseline), the condition of participants, and general health issues (at each visit).
FIGURE 2.
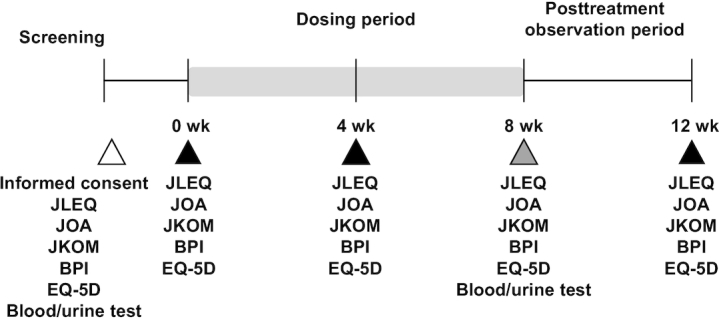
Study flow and outcome measurements during 8 wk of the dosing period and 4 wk of the posttreatment observation period. Scoring on the Japan Low Back Pain Evaluation Questionnaire (JLEQ), Japanese Knee Osteoarthritis Measure (JKOM), Japanese Orthopedic Association (JOA), and Brief Pain Inventory (BPI), quality-of-life (QOL) assessment by EuroQOL 5 Dimensions 5-level (EQ-5D-5L), and medical interviews were conducted at baseline and weeks 4 and 8 (dosing period), and week 12 (posttreatment period).
Outcome measurement
LBP evaluation
To evaluate the LBP, JLEQ and JOA were used. JLEQ is a self-administered, disease-specific measure for assessing the extent of LBP (28, 29). It consists of the visual analog scale (VAS) for the degree of LBP, the JLEQ-I, and a total of 30 questions. These questions include 7 questions regarding LBP related to activities of daily living over the last several days (JLEQ-II), 17 questions regarding problems due to LBP over the last several days (JLEQ-III), and 6 questions regarding health and psychological condition in the last month (JLEQ-IV). These 30 questions in the latter 3 domains are each ranked on a 5-point scale from no impairment (0 points) to serious impairment (4 points) and then added to produce a total score (maximum 120 points). The total score and scores for each domain (JLEQ-I through -IV) were compared between the active and placebo groups.
The JOA score is used to evaluate the therapeutic outcome of LBP. It is a disease-specific measure for assessing the intensity of LBP from the clinician's point of view (30). It consists of 14 questions with a 3–4-point scale, including 3 questions regarding subjective symptoms, 3 questions regarding objective responses, 7 questions regarding activities of daily living, and 1 question regarding bladder function. These 14 questions in the 4 domains are ranked on the 3–4-point scale and then added according to the designated method to produce a total score (maximum: 29 points; minimum: 6 points) (30).
Knee pain evaluation
To evaluate knee pain, JKOM was used. It is a self-administered, disease-specific measure for assessing the extent of knee pain and discomfort (31). It consists of a VAS for the degree of knee pain, JKOM-I, and a total of 25 questions. These questions include 8 questions regarding pain and stiffness in the knee over the last several days (JKOM-II), 10 questions regarding problems in daily life due to knee pain over the last several days (JKOM-III), 5 questions regarding usual activities in the last month (JKOM-IV), and 2 questions regarding general health status in the last month (JKOM-V). These 25 questions in the latter 4 domains are ranked on a 5-point scale from no impairment (0 points) to serious impairment (4 points) and then added to produce a total score (maximum 100 points). The total score and scores for each domain (JKOM-I through -V) were compared between the active and placebo groups.
Overall pain assessment and QOL assessment
The BPI was used to evaluate the intensity of the overall pain and the impact of the pain on daily life; it consists of 8 questions (32–34). These 8 questions are ranked on an 11-point scale from no impairment (0 points) to serious impairment (10 points).
The EQ-5D-5L was used to evaluate health-related QOL, and consists of 5 questions. These 5 questions are ranked on a 5-point scale from no impairment (1 point) to serious impairment (5 points) and then analyzed according to the reported method (35).
Safety evaluation
Systolic and diastolic blood pressure and pulse rate measurements were conducted at baseline and weeks 4, 8, and 12. At baseline and week 8, blood was collected under fasting conditions in the morning. The following blood parameters were measured: white blood cell (WBC) count, RBC count, hemoglobin (Hb), hematocrit (Ht), platelet count, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, total bilirubin, alkaline phosphatase, γ-glutamyl transpeptidase, urea nitrogen, creatinine, uric acid, sodium, chlorine, potassium, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, fasting plasma glucose concentration (FPG), and glycated hemoglobin (HbA1c). The urine samples were obtained at baseline and week 8, and the following urine parameters were measured: urine protein, urine glucose, and urine occult blood. All plasma biochemical variables were measured by LSI Medience Corporation using automatic analyzer LST-008α (Hitachi High-Tech Corp) and JCA-BM8060 (JEOL Ltd) biochemistry analyzers with Sysmex, Nittobo Medical, and LSI Medience reagents using standardized procedures and fresh samples. FPG was determined using a glucose glucokinase assay (LSI Medience Corp) and HbA1c concentrations were measured by LSI Medience using an automated biochemical analyzer JCA-BM9130 and JCA-BM9030 (JEOL Ltd). WBC counts were determined by flow cytometry as part of a full blood count. RBC and platelet counts were determined by electrical resistance detection. Hb concentration was determined by the SLS-Hb method, and Ht was measured by erythrocyte pulse peak detection. A clinician evaluated the safety of the supplementation based on these results and adverse events.
Statistical analysis
Data are summarized as the means and SDs. Statistical significance of differences between the l-Ser + EPA group and the placebo group was assessed by ANCOVA using the initial scores at baseline as covariates at each time point, at a significance level of 5%. All statistical analyses were performed using IBM SPSS Statistics Ver. 24 or R version 3.5.0 (R Foundation) (36).
The statistical analysis was predetermined before key opening. The primary decision was planned in advance to be conducted based on the total scores of JLEQ and JKOM at week 8, and these 2 outcomes were tested by a fixed sequence procedure; the statistical significance test for JKOM score was conducted only when a significant improvement in the JLEQ score was observed. Thus, the type I error was controlled under the α = 5% level regarding the primary outcome. For other outcome measures, such as JLEQ and JKOM scores at other time points, subdomains of JLEQ and JKOM, JOA score, BPI, and EQ-5D-5L, statistical multiplicity was not considered because these do not affect the primary decision.
Results
Participants and compliance
We enrolled 60 participants in both groups (Figure 1). There were no dropouts, and all participants completed the study protocol; thus, the full analysis set included all study participants. The compliance rate in this study was high, such that >95% of capsules were consumed throughout the study in both groups. Mean age, height, weight, BMI, JLEQ, JKOM, and JOA scores at baseline are listed in Table 1. Fifty-one participants (active compared with placebo: 22 compared with 29) had pain in multiple body sites, such as the neck and shoulders, in addition to LBP and knee pain.
TABLE 1.
General characteristics of the study participants in the l-serine (l-Ser) + EPA supplementation group and the placebo group at week 01
| l-Ser + EPA (n = 60) | Placebo (n = 60) | |
|---|---|---|
| Participants men/women (menopause) | 18/42 (5) | 18/42 (6) |
| Age, y | 40.3 ± 11.0 | 41.4 ± 10.9 |
| Height, cm | 163 ± 9 | 163 ± 9 |
| Weight, kg | 59.1 ± 9.3 | 60.6 ± 13.0 |
| BMI, kg/m2 | 22.3 ± 3.1 | 22.7 ± 3.2 |
| JLEQ-I2 | 42.9 ± 15.7 | 41.6 ± 16.0 |
| JLEQ score3 | 28.4 ± 13.4 | 27.3 ± 12.1 |
| JKOM-I4 | 34.8 ± 17.4 | 31.3 ± 15.6 |
| JKOM score5 | 18.2 ± 9.3 | 18.4 ± 7.9 |
| JOA | 21.4 ± 2.6 | 21.8 ± 2.2 |
1Mean age, height, weight, BMI, and JLEQ, JKOM, and JOA scores at baseline are demonstrated. Data are expressed as means ± SDs. JKOM, Japanese Knee Osteoarthritis Measure; JLEQ, Japan Low Back Pain Evaluation Questionnaire; JOA, Japanese Orthopedic Association; VAS, visual analogue scale.
2JLEQ-I: VAS for the degree of low-back pain.
3The JLEQ score is the sum of the JLEQ-II, -III, and -IV scores.
4JKOM-I: VAS for the degree of knee pain.
5The JKOM score is the sum of the JKOM-II, -III, -IV, and -V scores.
LBP relief
Figure 3A and Supplemental Table 1 show the JLEQ scores, evaluating the issues regarding LBP. Supplemental Table 2 presents the prevalence of participants with each issue in the JLEQ questionnaire. JLEQ scores decreased in both groups at weeks 4, 8, and 12 compared with baseline. As a primary outcome, at week 8, ANCOVA using the baseline scores as a covariate demonstrated significantly lower scores in the l-Ser + EPA group compared with the placebo group. Additionally, at week 8, a significant decrease in all 4 domains (I: VAS for the degree of LBP; II: LBP related to activities of daily living over the last several days; III: problems due to LBP over the last several days; and IV: health and psychological condition in the last month) was detected in the l-Ser + EPA group compared with the placebo group. The JOA score was not significantly different between the 2 groups.
FIGURE 3.
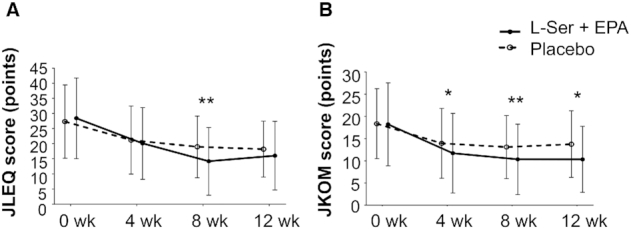
JLEQ (A) and JKOM (B) scores in the l-serine (l-Ser) + EPA supplementation group and the placebo group at weeks 0, 4, 8, and 12. Changes in JLEQ score (A) and JKOM score (B) from all study participants at weeks 0, 4, 8, and 12. The JLEQ score is the sum of the JLEQ-II, -III, and -IV scores, and the JKOM score is the sum of the JKOM-II, -III, -IV, and -V scores. Data are expressed as the means ± SDs, n = 60 in each group. ANCOVA using the baseline score as a covariate was performed to detect differences between the 2 groups by each time point. *, **Significantly different from placebo group: *P < 0.05, **P < 0.01. JKOM, Japanese Knee Osteoarthritis Measure; JLEQ, Japan Low Back Pain Evaluation Questionnaire.
Knee pain relief
Figure 3B and Supplemental Table 1 show the JKOM scores, evaluating issues regarding knee pain. Supplemental Table 3 demonstrates the prevalence of participants with each issue in the JKOM questionnaire. Although JKOM scores decreased in both groups at weeks 4, 8, and 12 compared with baseline, ANCOVA using the baseline scores as a covariate demonstrated significantly lower scores in the l-Ser + EPA group compared with the placebo group at week 8 as the primary outcome. In addition, significantly lower scores in the l-Ser + EPA group compared with the placebo group were observed at weeks 4 and 12.
Additionally, a significant decrease in the following 2 domains (I: VAS for the degree of knee pain; and II: pain and stiffness in the knee over the last several days) was detected in the l-Ser + EPA group compared with the placebo group at week 8. Interestingly, significant differences between these 2 groups were also detected in the posttreatment observation period at week 12 in the following domains: I: VAS for the degree of knee pain; II: pain and stiffness in the knee over the last several days; III: problems in daily life due to knee pain over the last several days; and IV and V: usual activities in the last month and general health status in the last month.
QOL and overall pain relief
On the BPI, which evaluates the intensity of overall pain and the impact of pain on daily life, both groups showed significant improvement in all parameters from baseline to weeks 4, 8, and 12 (Table 2). ANCOVA demonstrated significant improvement in the l-Ser + EPA group compared with the placebo group at week 4 on BPI1: pain at its worst in the last 24 h; BPI3: pain on average; and BPI5D: intensity of the pain on moving. Similarly, at week 8, the BPI1, BPI3, and BPI5D scores were significantly lower in the l-Ser + EPA group than in the placebo group. In the posttreatment observation period at week 12, significant differences in BPI1 and BPI3 were not detected, whereas a significant decrease in BPI5D remained. Supplemental Table 4 shows the improvement in the health-related QOL based on EQ-5D-5L due to l-Ser + EPA supplementation, at week 8.
TABLE 2.
Brief Pain Inventory (BPI) results of the all study participants in the l-serine (l-Ser) + EPA supplementation group and the placebo group at weeks 0, 4, 8, and 12
| Baseline | Week 4 | Week 8 | Posttreatment observation period: week 12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| l-Ser + EPA | Placebo | l-Ser + EPA | Placebo | P value2 | l-Ser + EPA | Placebo | P value2 | l-Ser + EPA | Placebo | P value2 | |
| BPI1: pain at its worst in the last 24 h | 4.13 ± 1.63 | 4.12 ± 1.33 | 2.92 ± 1.51 | 3.35 ± 1.27 | 0.028* | 2.35 ± 1.19 | 2.90 ± 1.19 | 0.004** | 2.9 ± 1.5 | 3.2 ± 1.3 | 0.169 |
| BPI2: pain at its least in the last 24 h | 1.38 ± 1.04 | 1.27 ± 0.92 | 0.83 ± 0.83 | 0.88 ± 1.01 | 0.415 | 0.60 ± 0.74 | 0.65 ± 0.88 | 0.467 | 0.6 ± 1.0 | 0.6 ± 0.9 | 0.793 |
| BPI3: pain on average | 3.03 ± 1.39 | 2.77 ± 1.16 | 1.98 ± 1.16 | 2.22 ± 1.09 | 0.020* | 1.58 ± 0.98 | 1.87 ± 0.97 | 0.019* | 2.0 ± 1.1 | 2.0 ± 1.0 | 0.493 |
| BPI4: pain right now | 2.80 ± 1.55 | 2.62 ± 1.57 | 1.57 ± 1.35 | 1.70 ± 1.27 | 0.281 | 1.10 ± 1.12 | 1.33 ± 0.97 | 0.104 | 1.1 ± 1.3 | 1.4 ± 1.3 | 0.251 |
| BPI5A: intensity of pain: lying | 2.08 ± 1.63 | 2.05 ± 1.42 | 1.30 ± 1.28 | 1.30 ± 1.31 | 0.931 | 1.00 ± 1.19 | 1.08 ± 1.25 | 0.608 | 0.8 ± 1.4 | 0.9 ± 1.3 | 0.788 |
| BPI5B: intensity of pain: sitting | 2.75 ± 1.71 | 2.53 ± 1.59 | 1.83 ± 1.36 | 1.78 ± 1.33 | 0.797 | 1.33 ± 1.27 | 1.52 ± 1.31 | 0.197 | 1.7 ± 1.7 | 1.4 ± 1.3 | 0.413 |
| BPI5C: intensity of pain: standing | 3.28 ± 1.79 | 3.03 ± 1.57 | 1.93 ± 1.40 | 1.93 ± 1.30 | 0.512 | 1.53 ± 1.28 | 1.82 ± 1.41 | 0.069 | 2.0 ± 1.6 | 2.0 ± 1.5 | 0.664 |
| BPI5D: intensity of pain: moving | 3.60 ± 1.68 | 3.33 ± 1.66 | 2.22 ± 1.39 | 2.72 ± 1.33 | 0.001** | 1.77 ± 1.25 | 2.43 ± 1.31 | 0.001** | 2.1 ± 1.6 | 2.8 ± 1.6 | 0.007** |
1BPI scores from all study participants at weeks 0, 4, 8 (dosing period), and 12 (posttreatment observation period). The BPI scores based on 8 questions are shown. Data are expressed as the means ± SDs, n = 60 in each group.
P values were obtained from ANCOVA between groups by each time point, using the scores at baseline as covariates. *,**Significant difference between supplementation and placebo groups: *P < 0.05, **P < 0.01.
To evaluate the effects of l-Ser and EPA on multiple pain sites, an additional subgroup analysis was conducted on a study subset of 51 participants (active compared with placebo: 22 compared with 29) who had pain in multiple body sites, such as the neck and shoulders, in addition to LBP and knee pain. The results of the subgroup analysis of the 51 participants are shown in Supplemental Table 5. ANCOVA demonstrated a significant improvement in the l-Ser + EPA group compared with the placebo group at week 4 on BPI1: pain at its worst in the last 24 h; BPI3: pain at its worst in the last 24 h; BPI4: pain right now; BPI5B: intensity of pain when sitting; and BPI5D: intensity of pain when moving. Similarly, at week 8, the BPI1, BPI3, BPI4, BPI5B, and BPI5D scores were significantly lower in the l-Ser + EPA group than in the placebo group.
Safety evaluations
No adverse events, including blood and urine laboratory test results, were observed. The assessment of safety was made through medical interviews with each participant, and no issues were reported. Supplemental Table 6 shows the results of blood and urine laboratory tests.
Discussion
This randomized, double-blind, placebo-controlled, parallel-group study examined the effect of oral l-Ser + EPA (594 mg and 149 mg daily, respectively) supplementation for 8 wk on back and knee pain intensity in healthy adults with LBP and knee pain. As the primary outcomes of the study, LBP, as assessed by JLEQ, and knee pain, as assessed by JKOM, were both significantly improved by l-Ser and EPA supplementation at week 8. The ANCOVA of JLEQ demonstrated significantly lower scores at week 8, assessed by both total scoring and scoring for each domain. The ANCOVA of JKOM demonstrated significantly lower scores at all time points: weeks 4, 8, and 12. The QOL related to pain was also significantly improved at all time points, as demonstrated by the BPI scores at weeks 4, 8, and 12. As demonstrated in a subgroup analysis of the 51 participants who had pain in multiple body sites, the l-Ser + EPA supplementation also relieved the symptoms of participants who had multiple-site pain. We also observed a very high compliance rate as well as study completion rate, and adverse events were not observed in either of the groups. These results suggest that l-Ser and EPA supplementation is both safe and effective in improving pain and QOL in adults with chronic and nonspecific pain in multiple body sites, including LBP and knee pain.
In this study, JOA was also used for the evaluation of LBP, but the scores were not significantly different between the 2 groups. Compared with JLEQ, which is a patient-reported outcome that focuses more on QOL aspects of pain, JOA is a physician-reported measure that has been widely used to evaluate the clinical results of various surgical and nonsurgical interventions performed on patients with LBP. Therefore, the characteristics of the healthy adult study population could have been related to the limitations in JOA results.
LBP is considered chronic if it has been present for >3 mo. The etiology of chronic LBP is, in most cases (≤85%), unknown or nonspecific, whereas specific causes (specific spinal pathology and neuropathic/radicular disorders) are uncommon (2). Often, the condition or injury that triggered the pain can be healed and undetectable, but the pain can continue. Recent studies have investigated the potential relation between central sensitization and chronic pain disorders. Enhanced excitability in the central nervous system is an important phenomenon observed in people with chronic LBP (37, 38) and also occurs in various other pain conditions (39, 40). Given the increasing evidence supporting the clinical significance of central sensitization in chronic pain, effective methods designed to normalize pain physiology are required.
There have been several clinical studies regarding the oral administration of l-Ser. Oral l-Ser administration has shown effectiveness in patients with hereditary sensory autonomic neuropathy type I, who suffer a debilitating, progressive disorder of peripheral nerves that results in sensory loss and neuropathic pain (41–43). Additionally, an exploratory study suggested that l-Ser can slow disease progression in patients with amyotrophic lateral sclerosis (44, 45). Kiya et al. (25) reported that the administration of l-Ser improved peripheral hyperalgesia in a paclitaxel-induced hyperalgesia model, especially focusing on the function of l-Ser in the DRG.
In vitro studies have also demonstrated that l-Ser is essential for maintaining normal functions of the nervous system. Savoca et al. (46) demonstrated that l-Ser is an important factor for the morphological differentiation of neurons in vitro through the observation of marked effects on dendritogenesis and axon length when l-Ser was added to developing neurons. Additionally, a study conducted in an animal model of brain injury revealed that l-Ser plays a role in inducing the proliferation and differentiation of neural stem cells and promoting the repair of nerve injury (47). Additionally, whereas demyelination contributes to the development of neuropathic pain by disrupting the molecular and structural features of nerve fibers (12), l-Ser is an important component for neural cell membrane and myelin formation (11). These findings indicate that l-Ser is an essential factor for neural cells to function properly.
EPA competes with arachidonic acid, thereby suppressing the production of eicosanoids and inflammation. It has also been reported that the anti-inflammatory lipid mediator resolvin E1 is produced from EPA via an intracellular biosynthetic pathway when activated neutrophils adhere to vascular endothelial cells in local inflammation. In addition to its anti-inflammatory properties, resolvin E1 suppresses NMDA hyperfunction caused by transient activity of receptor potential cation channel subfamily V member 1 and TNF-α by inhibiting extracellular signal-regulated kinase signaling in spinal dorsal horn neurons. Through this mechanism, pain and hyperalgesia in response to heat and mechanical stimuli are alleviated (20–22). These reports suggest that EPA is useful for the treatment of neuropathic pain triggered by inflammation.
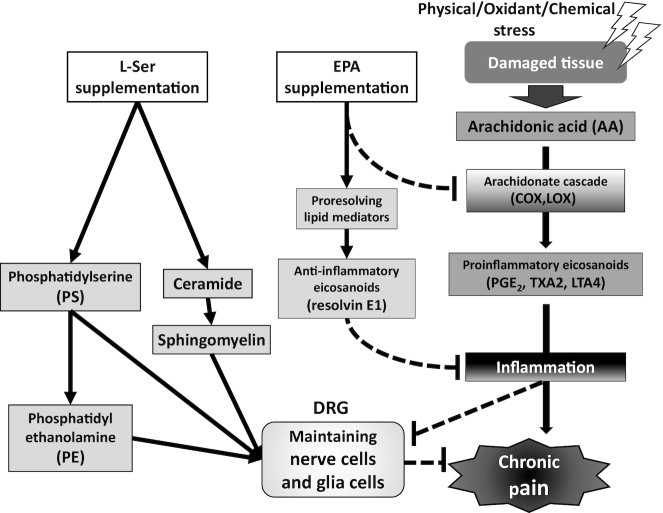
l-Ser could support neuronal function in damaged nerve fibers by providing essential components required for normal neuronal function. EPA could exert anti-inflammatory properties and reduce local inflammation, thereby suppressing pain signals from neuronal fibers (Figure 4). Table 2 demonstrates that l-Ser and EPA supplementation alleviated overall pain, and Supplemental Table 5 demonstrates that the l-Ser + EPA supplementation also relieved the symptoms of participants who had multiple-site pain such as the neck and shoulders, in addition to LBP and knee pain, suggesting that l-Ser and EPA might act on similar pathways in these multiple sites and improve neuropathic pain. l-Ser and EPA might synergistically work in the DRG, by providing necessary components for nerve function and mitigating local inflammation. Additional studies in the future are necessary to clarify the detailed mechanism. The results from the posttreatment observation period in the current study demonstrated a significant difference in the degree of knee pain between the l-Ser + EPA group and the placebo group, indicating that l-Ser and EPA provide continued effects even after supplementation has ceased. Compared with other pain management methods that are often used for temporary pain relief, l-Ser and EPA could have the potential to provide more lasting effects.
FIGURE 4.
Possible mechanism of pain relief from combinatory administration of l-serine (l-Ser) and EPA in relieving chronic low-back pain and knee pain. l-Ser provides essential components in support of damaged nerve cells and glial cells, and thus plays an important role in maintaining normal functions of the nervous system. EPA competes with arachidonic acid, thereby suppressing the production of eicosanoids and inflammation. EPA also produces the anti-inflammatory lipid mediator resolvin E1. We hypothesize that l-Ser and EPA synergistically work in the dorsal root ganglion (DRG), by simultaneously providing necessary components to maintain nerve function and mitigating local inflammation in the DRG. COX, cyclooxygenase; LOX, lipoxygenase; LTA4, leukotriene A4; TXA2, thromboxane A2.
There were several limitations in this study. Firstly, evaluation during the l-Ser and EPA ingestion period was performed only at weeks 4 and 8. We do not have data regarding effects on the pain scores with shorter-term and longer-term ingestion. Secondly, only a limited subgroup analysis was conducted in this study to examine the relation between background information and improvement in outcome scores. In particular, because the l-Ser concentration decreases with aging (14), stratification analysis using biomarkers for efficacy prediction is also important. Thirdly, we did not collect dietary records in this study. In the future, dietary intake should be assessed for potential deficiencies or excess intake of nutrients such as l-Ser and EPA, which could provide additional insight to the mechanistic rationale.
In conclusion, this randomized, double-blind, placebo-controlled, parallel-group study demonstrated that supplementation with l-Ser and EPA improved the pain scores of a generally healthy adult population with pain in at least the low back and knee for ≥3 mo. The compliance rate was high throughout the study, and no adverse events (including abnormal blood and urine laboratory test results) were observed. To our knowledge, this is the first study to report beneficial effects of the combinatory administration of l-Ser and EPA in improving such pain. Although further research is needed to clarify the underlying mechanism related to this effect, l-Ser and EPA supplementation could provide effective pain management and improve QOL in people with chronic LBP and knee pain.
Supplementary Material
Acknowledgments
We thank the study team at Kobuna Orthopedic Clinic. We also thank Dr Tokuhashi at Nihon University for scientific advice.
The authors’ responsibilities were as follows—IS, AY, M Takeshita, YS, KS, M Takada, MH, TM: designed the study; IS, AY, M Takeshita, YK: conducted the study and analyzed the data; IS, AY, M Takeshita, NN, KN: wrote the paper; KN: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Ajinomoto Co, Inc, funded the study and participated in the study design, implementation, analysis, and interpretation of the data.
Author disclosures: IS, AY, M Takeshita, YS, KS, NN, M Takada, MH, TM, and KN are employees of Ajinomoto Co, Inc. YK conducted research funded by Ajinomoto Co, Inc.
Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviation used: BPI, Brief Pain Inventory; DRG, dorsal root ganglion; EQ-5D-5L, QOL assessment by EuroQOL 5 Dimensions 5-level; FPG, fasting plasma glucose level; Hb, hemoglobin; HbA1c, glycated hemoglobin; Ht, hematocrit; JKOM, Japanese Knee Osteoarthritis Measure; JLEQ, Japan Low Back Pain Evaluation Questionnaire; JOA, Japanese Orthopedic Association; LBP, low-back pain; l-Ser, l-serine; NMDA, N-methyl-d-aspartic acid; QOL, quality of life; WBC, white blood cell.
Contributor Information
Ikuko Sasahara, Institute of Food Sciences and Technologies, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Akiko Yamamoto, Institute of Food Sciences and Technologies, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Masamichi Takeshita, Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Yasuyo Suga, Institute of Food Sciences and Technologies, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Katsuya Suzuki, Institute of Food Sciences and Technologies, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Natsumi Nishikata, Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Michihiro Takada, Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Masaki Hashimoto, Direct Marketing Department, Ajinomoto Co, Inc, Tokyo, Japan.
Tomoyuki Mine, Institute of Food Sciences and Technologies, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
Yasuo Kobuna, Kobuna Orthopedic Clinic, Maebashi, Gunma, Japan.
Kenji Nagao, Research Institute for Bioscience Products & Fine Chemicals, Ajinomoto Co, Inc, Kawasaki, Kanagawa, Japan.
References
- 1. Miranda H, Kaila-Kangas L, Heliovaara M, Leino-Arjas P, Haukka E, Liira J, Viikari-Juntura E. Musculoskeletal pain at multiple sites and its effects on work ability in a general working population. Occup Environ Med. 2010;67:449–55. [DOI] [PubMed] [Google Scholar]
- 2. Sanzarello I, Merlini L, Rosa MA, Perrone M, Frugiuele J, Borghi R, Faldini C. Central sensitization in chronic low back pain: a narrative review. J Back Musculoskelet Rehabil. 2016;29:625–33. [DOI] [PubMed] [Google Scholar]
- 3. Suka M, Yoshida K. Musculoskeletal pain in Japan: prevalence and interference with daily activities. Mod Rheumatol. 2005;15:41–7. [DOI] [PubMed] [Google Scholar]
- 4. Kamada M, Kitayuguchi J, Lee IM, Hamano T, Imamura F, Inoue S, Miyachi M, Shiwaku K. Relationship between physical activity and chronic musculoskeletal pain among community-dwelling Japanese adults. J Epidemiol. 2014;24:474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yiengprugsawan V, Steptoe A. Impacts of persistent general and site-specific pain on activities of daily living and physical performance: a prospective analysis of the English Longitudinal Study of Ageing. Geriatr Gerontol Int. 2018;18:1051–7. [DOI] [PubMed] [Google Scholar]
- 6. Tsuji T, Matsudaira K, Sato H, Vietri J, Jaffe DH. Association between presenteeism and health-related quality of life among Japanese adults with chronic lower back pain: a retrospective observational study. BMJ Open. 2018;8:e021160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan F, Byrne KS, Ramakrishnan R, Ferreira M, Dwyer T, Jones G. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: results from UK Biobank study. J Sci Med Sport. 2019;22:444–9. [DOI] [PubMed] [Google Scholar]
- 8. Gaskin DJ, Richard P. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 9. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dadabo J, Fram J, Jayabalan P. Noninterventional therapies for the management of knee osteoarthritis. J Knee Surg. 2019;32:46–54. [DOI] [PubMed] [Google Scholar]
- 11. Perry DK, Hannun YA. The role of ceramide in cell signaling. Biochim Biophys Acta. 1998;1436:233–43. [DOI] [PubMed] [Google Scholar]
- 12. Wei Z, Fei Y, Su W, Chen G. Emerging role of Schwann cells in neuropathic pain: receptors, glial mediators and myelination. Front Cell Neurosci. 2019;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Crabben SN, Verhoeven-Duif NM, Brilstra EH, Van Maldergem L, Coskun T, Rubio-Gozalbo E, Berger R, de Koning TJ. An update on serine deficiency disorders. J Inherit Metab Dis. 2013;36:613–9. [DOI] [PubMed] [Google Scholar]
- 14. Kouchiwa T, Wada K, Uchiyama M, Kasezawa N, Niisato M, Murakami H, Fukuyama K, Yokogoshi H. Age-related changes in serum amino acids concentrations in healthy individuals. Clin Chem Lab Med. 2012;50:861–70. [DOI] [PubMed] [Google Scholar]
- 15. Calder PC. Session 3: joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘Nutrition and autoimmune disease’ PUFA, inflammatory processes and rheumatoid arthritis. Proc Nutr Soc. 2008;67:409–18. [DOI] [PubMed] [Google Scholar]
- 16. Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107(Suppl 2):S171–84. [DOI] [PubMed] [Google Scholar]
- 17. Watson JE, Kim JS, Das A. Emerging class of omega-3 fatty acid endocannabinoids and their derivatives. Prostaglandins Other Lipid Mediat. 2019;143:106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee HN, Surh YJ. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochem Pharmacol. 2012;84:1340–50. [DOI] [PubMed] [Google Scholar]
- 19. Seki H, Tani Y, Arita M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009;89:126–30. [DOI] [PubMed] [Google Scholar]
- 20. Goldberg RJ, Katz J.. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–23. [DOI] [PubMed] [Google Scholar]
- 21. Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7., 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang JG, Strong JA, Xie W, Zhang JM. Local inflammation in rat dorsal root ganglion alters excitability and ion currents in small-diameter sensory neurons. Anesthesiology. 2007;107:322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paterniti I, Impellizzeri D, Di Paola R, Esposito E, Gladman S, Yip P, Priestley JV, Michael-Titus AT, Cuzzocrea S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies. J Neuroinflammation. 2014;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiya T, Kawamata T, Namiki A, Yamakage M. Role of satellite cell-derived L-serine in the dorsal root ganglion in paclitaxel-induced painful peripheral neuropathy. Neuroscience. 2011;174:190–9. [DOI] [PubMed] [Google Scholar]
- 26. Matsubayashi Y, Takeshita K, Sumitani M, Oshima Y, Tonosu J, Kato S, Ohya J, Oichi T, Okamoto N, Tanaka S. Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PLoS One. 2013;8:e68013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarumizu C, Ohno T, Handa S, Matsuoka S, Orimo H. Effectiveness of a compound supplement containing piperines, theanine, creatine, α-lipoic acid, and proteoglycan for low back pain: a double-blind placebo-controlled parallel comparison study. J Pain Relief. 2015;4:190. [Google Scholar]
- 28. Shirado O, Doi T, Akai M, Fujino K, Hoshino Y, Iwaya T. Development of new disease-specific QOL measure for patients with chronic low back pain: Japan Low back pain Evaluation Questionnaire (JLEQ). J Lumbar Spine Disord. 2007;13:225–35. [Google Scholar]
- 29. Shirado O, Doi T, Akai M, Fujino K, Hoshino Y, Iwaya T. An outcome measure for Japanese people with chronic low back pain: an introduction and validation study of Japan Low Back Pain Evaluation Questionnaire. Spine. 2007;32:3052–9. [DOI] [PubMed] [Google Scholar]
- 30. Kim K, Isu T. An overview of clinical scoring systems applicable for lumbar spine surgery. Spinal Surgery. 2015;29:18–25. [Google Scholar]
- 31. Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H, Nasu T. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol. 2005;32:1524–32. [PubMed] [Google Scholar]
- 32. Uki J, Mendoza T, Cleeland CS, Nakamura Y, Takeda F. A brief cancer pain assessment tool in Japanese: the utility of the Japanese Brief Pain Inventory—BPI-J. J Pain Symptom Manage. 1998;16:364–73. [DOI] [PubMed] [Google Scholar]
- 33. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. [DOI] [PubMed] [Google Scholar]
- 34. Takahashi N, Kasahara S, Yabuki S. The objective evaluation for pain, and QOL. Jpn J Rehabil Med. 2016;53:596–603. [Google Scholar]
- 35. Ikeda S, Shiroiwa T, Igarashi A, Noto S, Fukuda T, Saito S, Shimozuma K. Developing a Japanese version of the EQ-5D-5L value set. J Natl Inst Public Health. 2015;64:47–55. [Google Scholar]
- 36. Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.; [Google Scholar]
- 37. O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007;11:415–20. [DOI] [PubMed] [Google Scholar]
- 38. Imamura M, Chen J, Matsubayashi SR, Targino RA, Alfieri FM, Bueno DK, Hsing WT. Changes in pressure pain threshold in patients with chronic nonspecific low back pain. Spine. 2013;38:2098–107. [DOI] [PubMed] [Google Scholar]
- 39. Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043–56. [DOI] [PubMed] [Google Scholar]
- 40. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129:343–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fridman V, Suriyanarayanan S, Novak P, David W, Macklin EA, McKenna-Yasek D, Walsh K, Aziz-Bose R, Oaklander AL, Brown R et al. . Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology. 2019;92:e359–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Auranen M, Toppila J, Suriyanarayanan S, Lone MA, Paetau A, Tyynismaa H, Hornemann T, Ylikallio E. Clinical and metabolic consequences of L-serine supplementation in hereditary sensory and autonomic neuropathy type 1C. Cold Spring Harb Mol Case Stud. 2017;3(6): pii: a002212 doi:10.1101/mcs.a002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garofalo K, Penno A, Schmidt BP, Lee HJ, Frosch MP, von Eckardstein A, Brown RH, Hornemann T, Eichler FS. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J Clin Invest. 2011;121:4735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bradley WG, Miller RX, Levine TD, Stommel EW, Cox PA. Studies of environmental risk factors in amyotrophic lateral sclerosis (ALS) and a phase I clinical trial of L-serine. Neurotox Res. 2018;33:192–8. [DOI] [PubMed] [Google Scholar]
- 45. Levine TD, Miller RG, Bradley WG, Moore DH, Saperstein DS, Flynn LE, Katz JS, Forshew DA, Metcalf JS, Banack SA et al. . Phase I clinical trial of safety of L-serine for ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:107–11. [DOI] [PubMed] [Google Scholar]
- 46. Savoca R, Ziegler U, Sonderegger P. Effects of L-serine on neurons in vitro. J Neurosci Methods. 1995;61:159–67. [DOI] [PubMed] [Google Scholar]
- 47. Sun L, Qiang R, Yang Y, Jiang ZL, Wang GH, Zhao GW, Ren TJ, Jiang R, Xu LH. L-serine treatment may improve neurorestoration of rats after permanent focal cerebral ischemia potentially through improvement of neurorepair. PLoS One. 2014;9:e93405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.