Abstract
A mathematical model integrating tumor angiogenesis and tumor-targeted cytotoxicity by immune cells was developed to identify the therapeutic window of two distinct modes to treat cancer: (1) an anti-angiogenesis treatment based on the monoclonal antibody bevacizumab that targets tumor vasculature, and (2) immunotherapy involving the injection of unlicensed dendritic cells to boost the anti-tumor adaptive response. The angiogenic cytokine Vascular Endothelial Growth Factor (VEGF) contributes to the immunosuppressive tumor microenvironment, which is responsible for the short-lived therapeutic effect of cancer-targeted immunotherapy. The effect of immunosuppression on the width of the therapeutic window of each treatment was quantified. Experimental evidence has shown that neutralizing immunosuppressive cytokines results in an enhanced immune response against infections and chronic diseases. The model was used to determine treatment protocols involving the combination of anti-VEGF and unlicensed dendritic cell injections that enhance tumor regression. The model simulations predicted that the most effective method to treat tumors involves administering a series of biweekly anti-VEGF injections to disrupt angiogenic processes and limit tumor growth. The simulations also verified the hypothesis that reducing the concentration of the immunosuppressive factor VEGF prior to an injection of unlicensed dendritic cells enhances the cytotoxicity of CD8+ T cells and results in complete tumor elimination. Feasible treatment protocols for tumors that are diagnosed late and have grown to a relatively large size were identified.
Keywords: Immunotherapy, Monoclonal antibody, VEGF, Mathematical modeling, Immunosuppression, Tumor angiogenesis
1. Introduction
Cancer is one of the most prevalent diseases in the world (Jemal et al., 2010; Mathers et al., 2001; Hayat et al., 2007). In spite of recent advancements in cancer treatment, the battle against this deadly disease still rages on. Traditional treatment modalities include chemotherapy, radiotherapy and surgery. Unfortunately, each of these types of treatment has its own disadvantages, including damage to nearby healthy tissue due to chemical toxicity or ionizing radiation (Schwartz, 1999; Park, 2014; Azinovic et al., 2001; Jellema et al., 2007; Lipshultz et al., 2013; Azim et al., 2011), an increased risk of a second primary cancer due to treatment of the first cancer (Schaapveld et al., 2008; Hodgson et al., 2007; Palumbo et al., 2013; Bokemeyer and Schmoll, 1995), or loss of the treated organ. However, progress in the fight against cancer continues to be made, as novel modes to treat cancer are developed and tested. Mathematical modeling has become an essential tool that is used to simulate tumor–immune cell interactions and to predict the therapeutic effect of various cancer treatments (Bender et al., 2013; Ribba et al., 2014; Enderling and Chaplain, 2014; Louzoun et al., 2014; Joshi et al., 2009; Eftimie et al., 2011; Wilson and Levy, 2012). As new breakthroughs are made in the field of cancer biology and immunology, models are developed that account for these discoveries (Liao et al., 2014; Poleszczuk et al., 2015; Arciero et al., 2004; Stevens and Mackey, 2013).
A promising strategy to combat cancer is to reduce the supply of nutrients that is transported to the tumor via angiogenesis, i.e. the sprouting of new blood vessels from existing vasculature. The key cytokine that drives normal and pathological angiogenesis is the Vascular Endothelial Growth Factor (VEGF) (Verheul and Pinedo, 2000; Hoeben et al., 2004). The release of VEGF by tumor cells experiencing hypoxic conditions causes VEGF to bind to VEGF receptors on the surface of endothelial cells, which serve as the building blocks of existing vasculature. These activated endothelial cells then sprout and migrate into the tumor site, leading to tumor angiogenesis and enhanced tumor growth. The objective of administering an anti-angiogenic injection is to disrupt the process of new vasculature growth into a solid tumor mediated by VEGF. One way to block tumor angiogenesis is to inject the humanized VEGF antibody bevacizumab (Avastin®) to impede the activation of the receptor VEGFR-2 by VEGF (Shih and Lindley, 2006; Ferrara et al., 2005).
Mounting evidence shows that VEGF also plays a major role in the immunosuppression of innate and adaptive immune system cells (Liu et al., 2012; Shi et al., 2014; Voron et al., 2014; Voron et al., 2015; Saito et al., 1998). VEGF suppresses their anti-tumor function due to the capability of these cells of expressing VEGF receptors once they have been activated and have migrated to the tumor site (Kaur et al., 2014; Murdoch et al., 2008; Ziogas et al., 2012). Gabrilovich and colleagues showed that VEGF is capable of disrupting dendritic cell function and can impede their maturation from CD34+ hematopoietic precursors (Gabrilovich et al., 1996). CD8+ T cell function is also affected by VEGF (Voron et al., 2015). Experimental results have shown that administration of bevacizumab can reverse the maturation defect of dendritic cells by neutralizing VEGF (Osada et al., 2008). Thus, another reason for administering anti-VEGF therapy is to eliminate the immunosuppressive tumor microenvironment by reducing the concentration of free VEGF in the tumor. The neutralization of VEGF would allow immune cells to carry out their tumor-targeted cytotoxic activities unimpeded. There are, however, side effects tied to the continuous use of anti-angiogenic treatments based on monoclonal antibodies (Wu and Huang, 2008; Widakowich et al., 2007). Additionally, continued use of an anti-angiogenic therapy may eventually impede the transport of adjuvant drugs due to pruning of tumor vasculature. Nevertheless, treatments involving monoclonal antibodies targeting VEGF have been moderately successful for treating colon, breast, kidney and lung cancer, and have extended the life of many patients up to several months.
Cancer cells are characterized by a set of hallmarks acquired through genetic or epigenetic modifications (Hanahan and Weinberg, 2011). These changes confer them with certain properties that allow them to survive and replicate in a hostile environment. A key hallmark of cancer cells is their ability to limit, or even escape, tumor-targeted immunosurveillance (Kim et al., 2007). Through genetic and epigenetic modifications, cancer cells reduce their antigenicity by turning off genes that lead to expression of certain tumor-associated antigens in the surface of their cell membrane that, if present, would trigger a strong immune reaction. The typically-low antigenicity of tumor cells, combined with their ability to stimulate angiogenesis, is what drives tumor growth and metastasis.
In order to counteract the ability of tumor cells to dampen or evade a tumor-targeted immune response, immunotherapy has been proposed as an alternative treatment to kill tumor cells. This treatment often exploits the presence of certain molecules found mainly on the surface of tumor cells to make the tumor more antigenic, or visible, to immune cells. One type of immunotherapy involves injecting primed or genetically modified live immune cells, such as natural killer cells, dendritic cells, or cytotoxic T lymphocytes, into the patient to enhance the immune response against cancer cells. Another immunotherapy approach involves injecting antigens found on the surface of tumor cells. An advantage of both strategies is that they tend to elicit a stronger and specific immune response that destroys cancer cells but spares healthy tissue. An immunotherapy strategy aimed at killing tumor cells directly involves the injection of cytokines such as TNF-α, IFN-γ or IL-2 to force tumor cells to undergo apoptosis or to keep them from replicating.
Immunotherapy administered as monotherapy has had limited success in a clinical setting. Although it has been observed that multiple types of immune cells tend to migrate to the tumor microenvironment, these activated cells are often anergic and develop functions that are pro-tumor rather than anti-tumor. A reason for this anergy may be the generation of a weak immune response due to persistently low tumor antigenicity in spite of efforts to boost the immune system (Escors, 2014). Experimental evidence has shown that immunosuppressive factors secreted by tumor cells, including VEGF, TGF-β and IL-10 are responsible for creating a tumor microenvironment that is strongly immunosuppressive (Gorelik and Flavell, 2002; Ghiringhelli et al., 2005; Della Porta et al., 2005; Ohm et al., 2003; Akdis and Blaser, 2001; Oyama et al., 1998; Pinzon-Charry et al., 2005; Kusmartsev and Gabrilovich, 2006), and leads to the loss of tumor-targeted cytotoxicity by native, as well as injected, immune cells over time. Even if the tumor-targeted cytotoxicity of immune cells were to remain active for an extended period of time, cancer cells tend to be genetically unstable and eventually develop a resistance to immunotherapy.
The mixed results of immunotherapy have motivated researchers to look for ways to extend its therapeutic window. Based on the fact that VEGF stimulates the development of new vessels from existent vasculature and that, at the same time, it acts as an immunosuppressor, researchers have conducted experiments to elucidate the multiple therapeutic effects of an anti-VEGF treatment, especially when coupled with other modes of treatment. Multiple reports suggest that an anti-angiogenic treatment can reduce tumor vasculature while simultaneously enhancing the anti-tumor immune response (Selvaraj et al., 2014; Oelkrug and Ramage, 2014). Since an anti-VEGF treatment involving a VEGF antibody works by decreasing the concentration of free VEGF from the tumor microenvironment, it is expected that administering an anti-VEGF treatment prior to the administration of a DC treatment will lead to treatment synergy characterized by a decreased tumor vascularization and a stronger anti-tumor immune response.
The ideal cancer treatment would be one that requires only a limited number of injections in order to trigger an anti-tumor immune response that is long-lasting and that minimizes unwanted side effects. The dual role of VEGF as a pro-angiogenic and immunosuppressive factor has motivated researchers to work on developing anti-cancer vaccines based on VEGF or VEGFR antigen (Li et al., 2002; Gavilondo et al., 2014; Morera et al., 2012; Wang et al., 2010; Okaji et al., 2006). Some of these vaccines have been tested in animal models and in clinical trials and have been deemed safe. However, in the cases where there was complete tumor eradication, a regimen of periodic injections of VEGF antigen was required. A long-term therapeutic effect produced by a small number of vaccinations is yet to be observed.
The mathematical model presented in this article was developed to serve as a basic platform for cancer treatment design. The model was used to test the hypothesis that a combination of an anti-VEGF treatment with immunotherapy involving injections of unlicensed DC can lead to synergy and tumor eradication by suppressing the suppressor (VEGF). The model predicts a specific therapeutic window for an anti-VEGF treatment and for immunotherapy when each is administered alone, or in combination, during which the treatment can eliminate the tumor. The model predicts that if the therapeutic window of each treatment has passed, a series of anti-VEGF injections followed by injections of unlicensed DC will lead to treatment synergy, resulting in the eventual elimination of the tumor without the need for a continuous injection regimen or an excessively high dose, both of which can lead to serious side effects.
2. Materials and methods
The present model integrates approaches aimed at simulating tumor angiogenesis, the adaptive tumor-targeted immune response and immunosuppression mediated by tumor-secreted factors. The model by Robertson-Tessi et al. (2012) was used as the basis of the interaction between the tumor and the immune system due in part to its realism and modeling approach that makes it easy to expand to include other types of immune cells, cytokines and anti-cancer treatments. In their paper, the authors provide a detailed account of the biological underpinnings of tumor–immune system interactions and a rationale for the terms used in their model. Their model considered the dynamics of unlicensed and licensed dendritic cells, and of proliferating and active CD4+ helper T cells and CD8+ effector T cells. These authors included the immunosuppressive effect of regulatory T cells (Tregs) and of the tumor-secreted cytokines TGF-β and IL-10. The anti-tumor effect of IL-2 secreted by proliferating CD4+ helper T cells was also included. By generating a set of bifurcation plots, they also illustrated the effect of the level of tumor antigenicity and tumor growth rate on the effectiveness of the immune system in eliminating a tumor in the absence of any treatment. However, their model did not include any angiogenic processes, and tumor growth was governed by a function that did not depend on the extent of tumor vascularization. Moreover, their results indicated that immunotherapy consisting of injections of unlicensed dendritic cells given as monotherapy may not be effective in eliminating a tumor, since their model predicted an eventual increase in tumor growth after the treatment ends.
Continued tumor growth depends on sustained angiogenesis to increase tumor vasculature. Angiogenesis, in turn, depends on the relative concentrations of VEGF, Angiopoietin-1 (Ang-1) and Angiopoietin-2 (Ang-2). Ang-1 leads to vasculature maturation by reinforcing the adhesion of endothelial cells (EC) with each other. Ang-2 destabilizes vasculature and leads to vessel sprouting when VEGF is present and to vascular regression in the absence of VEGF. Cameron and Davis (2009) took the lead in modeling this aspect of tumor angiogenesis and their approach served as the basis for tumor angiogenesis in the present model. Their model assumed that the maturation of EC and the total length of tumor vasculature both depend on the ratio of the concentration of VEGF to the number of EC. A logistic tumor growth was assumed such that the carrying capacity of tumor cells depends on the total length of vasculature inside the tumor. Since VEGF also acts as an immunosuppresor, a term similar to that used in Robertson-Tessi et al. (2012) to model immunosuppression by TGF-β was introduced. In their work, Cameron and Davis demonstrated that only a temporary therapeutic benefit could be achieved with the anti-VEGF treatment they simulated, similar to the predictions of the model by Robertson-Tessi et al. for DC immunotherapy. These predictions of treatment failure motivated the author to test whether it is possible to achieve synergy between these two treatments in a way that leads to permanent elimination of a fastgrowing tumor of very low antigenicity.
The present model consists of 18 ordinary differential equations (ODE) describing the dynamics of tumor cells, endothelial cells, immune cells, IL-2, IL-10, TGF-β, VEGF and changes in tumor vasculature. As the ODEs are highly nonlinear and represent a stiff system, MATLAB’s stiff system solver ode23 (Mathworks, Natick, MA) was used to solve the system numerically. All the parameter values were taken from published in vivo and in vitro experiments involving human subjects and murine models, except the parameter values defining the strength of VEGF immunosuppression, which were assumed to be the same as those defining the immunosuppressive strength of TGF-β. Injections of anti-VEGF and DC were simulated by defining a new initial condition at the time point when an anti-VEGF or DC injection is administered, increasing the current concentration of anti-VEGF or the number of unlicensed DC by the amount of anti-VEGF or unlicensed DC injected, and continuing to solve the system of ODEs from that time point while applying the new initial conditions.
Eqs. (1)–(18) represent the model of tumor growth that was used to test the hypothesis that an anti-VEGF treatment followed by a DC immunotherapy leads to tumor eradication via treatment synergy.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
An injection of anti-VEGF leads to an increase in the current concentration of anti-VEGF, which binds to free VEGF and reduces the concentration of both free VEGF and free VEGF antibody. This type of treatment decreases the rate of tumor angiogenesis. An injection of unlicensed DC boosts the anti-cancer activity of CD8+T cells. Table 1 lists the parameter values that were used in all the simulations.
Table 1.
Model parameters.
Table 2 lists the initial conditions that were used to run the model up to the time when an injection was first administered, if any. These initial conditions were specifically chosen to consider a case where a tumor has the potential to grow to a very large size by initiating processes that can increase tumor vasculature in a short amount of time. A fully developed, but small, vasculature was assumed to be initially present in the periphery of the tumor. To facilitate the process of angiogenesis and vasculature maturation, the concentration of Ang-1 was initially set to be 10 times that of Ang-2. The initial tumor population consisted of a single tumor cell and, therefore, the initial free VEGF concentration was set to zero due to the fact that tumor cells do not experience hypoxia when their density is low. It was assumed that there were 10,000 activated endothelial cells present initially, a single licensed dendritic cell and a single activated effector CD8+ T cell. The initial conditions of all the other model variables were set to zero, including the number of activated CD4+ helper T cells, activated CD8+ T cells and the concentration of pro-and antitumor cytokines.
Table 2.
Initial conditions used in all the simulations.
| Variable | Definition | Initial value | Units |
|---|---|---|---|
| T | Number of cancer cells | 1 | cell |
| U | Number of mature unlicensed dendritic cells | 0 | cell |
| D | Number of mature licensed dendritic cells | 1 | cell |
| AE | Number of activating/proliferating effector memory CD8+ T cells | 0 | cell |
| E | Number of activated effector memory CD8+ T cells | 1 | cell |
| AH | Number of activating/proliferating memory helper CD4+ T cells | 0 | cell |
| H | Number of activated memory helper CD4+ T cells | 0 | cell |
| AR | Number of activating/proliferating regulatory T cells | 0 | cell |
| R | Number of activated regulatory T cells | 0 | cell |
| Y | Number of endothelial cells | 10,000 | cell |
| C | Concentration of IL-2 | 0 | ng mL−1 |
| S | Concentration of TGF-β | 0 | ng mL−1 |
| I | Concentration of IL-10 | 0 | ng mL−1 |
| A1 | Concentration of Angiopoietin-1 | 10 | ng mL−1 |
| A2 | Concentration of Angiopoietin-2 | 1 | ng mL−1 |
| V | Concentration of free VEGF | 0 | ng mL−1 |
| Va | Concentration of anti-VEGF | 0 | ng mL−1 |
| B | Length of tumor vasculature | 1000 | μm |
To explore the possibility of synergy between two distinct antitumor treatments, the model was used to compare the effect of administering a “standard anti-VEGF treatment” consisting of 6 biweekly injections of 7 × 108 ng of anti-VEGF versus a “standard dendritic cell treatment” consisting of 15 biweekly injections of 5 × 106 unlicensed dendritic cells for three distinct cases: (1) immunosuppression by TGF-β and VEGF are both turned off, (2) immunosuppression by TGF-β is turned on but immunosuppression by VEGF is turned off, and (3) the combined immunosuppressive effect of TGF-β and VEGF is turned on.
The dosage and the schedule of the standard anti-VEGF treatment were selected to be consistent with phase II clinical trials involving the use of bevacizumab to treat recurrent malignant glioma (Vredenburgh et al., 2007). In general, the recommended dose varies by cancer type and period between injections. For example, it is recommended to administer 10 mL (25 mL) of bevacizumab per kilogram of body weight to treat glioblastoma multiforme (metastatic colon carcinoma), if it is administered every two weeks. The chosen dosage of unlicensed DC in the model is one that is typically administered (Brossart et al., 2000). A 14-day interval between DC injections is optimal in the sense that it has been observed that a different period between injections leads to a longer time needed for complete tumor removal. The fact that a 2-week period between DC injections is optimal is not surprising, since it is consistent with the timescale of T cell expansion and decay (Robertson-Tessi et al., 2012).
3. Results
Table 3 shows the predicted number of tumor cells, activated endothelial cells, total length of tumor vasculature and VEGF concentration for different levels of tumor antigenicity, when immunosuppression by TGF-β and VEGF are both turned off (indicated by the top number within each table cell), and when no anti-tumor treatment is administered. The maximum simulation time was 4000 days (~11 years). For the lowest tumor antigenicity considered in the model (a = 1 × 10−5), it is predicted that the adaptive immune system will not be able to arrest tumor growth. In this case, the anti-tumor immune response will be very weak and, consequently, effector T cells will not be able to kill tumor cells at a rate fast enough to overcome the replication rate of tumor cells. However, for higher levels of tumor antigenicity, the adaptive immune system will stabilize tumor size as long as immunosuppressive effects are weak or nonexistent. In addition to regulating tumor growth, the immune system will also maintain the number of endothelial cells, tumor vasculature and VEGF concentration at a stable equilibrium. This model prediction suggests that if there is no immunosuppressive effect by TGF-β and VEGF, and as long as the antigenicity of the tumor is not too low, namely, if parameter a is of the order O(10−4) or greater, tumor size will remain small. If the immune system were to effectively kill cancer cells at this constant rate, there would be no tumor growth even if cancer cells continue to replicate. Additionally, the model predicts that if tumor size is already stabilized and remains constant due to the anti-tumor action of the adaptive immune system, either a standard anti-angiogenic treatment or standard DC immunotherapy administered at any point in time will eliminate the remaining tumor cells. This result suggests that an anti-VEGF treatment and a DC treatment both have an “infinite” therapeutic window to begin treatment if tumor growth has already been stabilized by the adaptive immune system prior to the administration of one of these treatments.
Table 3.
Effect of immunosuppression on the number of tumor cells as a function of tumor antigenicity.
| Antigenicity | Tumor cells | Endothelial cells | Vasculature (μm) | VEGF (ng) |
|---|---|---|---|---|
| 10−5 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 10−4 | 1.98 × 107 | 8.81 × 108 | 2.84 × 106 | 1.77 × 104 |
| ∞ | ∞ | ∞ | ∞ | |
| 10−3 | 1.73 × 106 | 6.14 × 107 | 3.64 × 105 | 1240 |
| ∞ | ∞ | ∞ | ∞ | |
| 10−2 | 1.73 × 105 | 0 | 5.62 × 104 | 93.4 |
| ∞ | ∞ | ∞ | ∞ | |
| 10−1 | 8.59 × 105 | 2.22 × 107 | 2.91 × 105 | 449 |
| ∞ | ∞ | ∞ | ∞ | |
| 1 | 9.42 × 105 | 2.59 × 107 | 2.93 × 105 | 524 |
| ∞ | ∞ | ∞ | ∞ | |
| 101 | 1.16 × 106 | 3.55 × 107 | 3.06 × 105 | 717 |
| 2.00 × 105 | 0 | 26670 | 185.4 | |
| 102 | 1.43 × 106 | 4.80 × 107 | 3.31 × 105 | 969 |
| 3.86 × 104 | 0 | 5.19 × 103 | 35.7 | |
| 103 | 1.57 × 104 | 0 | 4.11 × 103 | 9.9 |
| 2.04 × 104 | 0 | 2.79 × 103 | 18.7 | |
| 104 | 9.60 × 103 | 0 | 2.27 × 103 | 6.4 |
| 1.39 × 104 | 0 | 1.93 × 103 | 12.7 | |
| 105 | 8.69 × 103 | 0 | 1.93 × 103 | 6.1 |
| 1.25 × 104 | 0 | 1.74 × 103 | 11.4 |
Table 3 illustrates the non-linear relationship between the concentration of free VEGF (which can be used as a biomarker of tumor size) and the level of tumor antigenicity when immunosuppression by TGF-β and VEGF is turned off (top value of each table cell). In the model, as tumor antigenicity increases a = 1 × 10−4 (and up to a = 1 × 10−2), the adaptive immune system is stimulated to kill cancer cells more effectively, which reduces tumor size and the total amount of VEGF secreted by tumor cells. At a = 2.68 × 10−3 VEGF concentration decreases below a threshold value, leading to the collapse of the activated endothelial cell population (which explains the entries with zero active EC). This has the effect of further arresting the growth of the tumor and of its vasculature. Increasing tumor antigenicity from a = 10−2 to a = 102 leads to an increase in VEGF concentration, the activated EC population and total tumor vasculature. However, beyond a = 102 the tumor antigenicity will be high enough to elicit an even stronger immune reaction. This immune response results in a further decrease in VEGF concentration below its threshold value for EC activation due to the increased tumor cell death, leading again to the collapse of the EC population, and to an even greater reduction of tumor size and tumor vasculature.
Although the above predictions only apply to the case of no immunosuppression by TGF-β and VEGF, they suggest that immunotherapy is a promising mode to treat cancer, since it increases the anti-tumor immune response by increasing the net antigenicity of the tumor. Based on these simulations, an immunotherapy that increases the tumor antigenicity level to be in the order of O(10−4) can lead to the stabilization of tumor growth, as long as the immunosuppressive tumor microenvironment is neutralized prior to the administration of immunotherapy.
Table 3 also shows the predicted number of tumor cells, activated endothelial cells, total length of tumor vasculature and VEGF concentration for different levels of tumor antigenicity, when a combined immunosuppressive effect by TGF-β and VEGF is present (indicated by the bottom number within each table cell), and without any treatment. The maximum simulation time was also 4000 days. For the lowest level of tumor antigenicity used in the model (a = 1 × 10−5) up to an intermediate level (a=1), it is predicted that the adaptive immune system will not be able to kill cancer cells at a rate fast enough to stop tumor growth. For low to intermediate antigenicity values, immunosuppression will be sufficiently strong that it will reduce tumor cytotoxicity significantly, leading to continued tumor vascularization and growth. Consequently, the number of tumor cells, activated endothelial cells, tumor vasculature and VEGF concentration will continue to increase. If the tumor antigenicity level is in the order O(101) or higher, the adaptive immune system alone will be able to arrest tumor growth, but it may not be able to eliminate the tumor completely.
Comparing the results of no immunosuppression versus immunosuppression presented in Table 3 allows the quantification of the negative effect that TGF-β and VEGF have on the functionality of cancer-targeted immune cells. In the presence of immunosuppression, only tumors of a relatively high antigenicity can be stabilized by the immune system without the need for an antitumor treatment. These results demonstrate that it is not sufficient to prime immune cells to destroy tumor cells. The immunosuppressive tumor microenvironment must also be modified to ensure that the immune cells that penetrate the tumor maintain their cytotoxic effect against tumor cells long after the immunotherapy has ended. The model predicts that a “tip of the balance” in favor of strengthening immunosuppression and reducing tumor antigenicity will lead to tumor persistence, whereas high tumor antigenicity alone could lead to tumor clearance even in the presence of moderate immunosuppressive effects.
In nature, the tip of the balance favors low antigenicity and high immunosuppression. Therefore, to ensure that the analysis and predictions of the model are clinically relevant and conform to experimental observations, the results presented hereafter pertain only to the case of very low tumor antigenicity (a = 1 × 10−5).
In the model, the growth coefficient γ1 determines how fast tumor cells replicate. This parameter can be set to a constant value in the range 0.1–1 (day−1) to simulate a slow-growing or a fast-growing tumor. An aspect of tumor growth regulation that was studied with the model is the extent to which the growth rate of a tumor of very low antigenicity determines whether the adaptive immune system alone will be able to eliminate the tumor. Table 4 shows the results when the immunosuppressive effect of TGF-β and VEGF is turned off (indicated by the top number within each table cell), and when it is turned on (indicated by the bottom number within each table cell), for different values of the tumor growth coefficient γ1, when no anti-tumor treatment is administered. The maximum simulation time was again 4000 days. These data indicate that regardless of whether the immunosuppressive effect of TGF-β and VEGF is off or on, the faster a tumor grows, the more destabilizing this growth rate is on tumor–immune system dynamics. In particular, the immune system alone cannot control tumors that have an intermediate to high growth coefficient (0.5≤γ1≤1) because these tumor cells grow at a faster rate than the rate at which they are killed by immune cells (Robertson-Tessi et al., 2012). In this case, the number of tumor cells, activated endothelial cells, tumor vasculature and the concentration of VEGF will continue to increase. However, the model predicts that tumors having a low to intermediate growth coefficient in the range 0.1<γ1<0.4 will be stabilized by the immune system in the absence of any anti-tumor treatment. Interestingly, the model predicts that if a tumor of very low antigenicity (a = 1 × 10−5) were to grow very slowly (γ1=0.1), it is not likely to initially elicit a strong immune response due to the fact that few unlicensed dendritic cells will come in contact with tumor cells to become mature unlicensed DCs (see the first term in the right-hand side of Eq. (2)), and the tumor will continue to grow over time. By the time the tumor has grown to a size large enough to elicit a stronger immune response (due to a faster rate of unlicensed DC maturation via an increase in the frequency of DC contact with tumor cells), it will be able to escape destruction due to the large number of tumor cells that are already present and that are actively dividing, and the fact that the immune response is still relatively weak due to the low antigenicity of the tumor.
Table 4.
Number of tumor cells for different tumor growth coefficients and immunosuppression levels.
| Growth coefficient | Tumor cells | Endothelial cells | Vasculature (μm) | VEGF (ng) |
|---|---|---|---|---|
| 0.1 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 0.2 | 8.63 × 104 | 0 | 1.15 × 104 | 80 |
| 8.63 × 104 | 0 | 1.15 × 104 | 80 | |
| 0.3 | 1.44 × 105 | 0 | 1.92 × 104 | 133.4 |
| 1.44 × 105 | 0 | 1.92 × 104 | 133.4 | |
| 0.4 | 2.23 × 105 | 0 | 2.97 × 104 | 206.6 |
| 2.23 × 105 | 0 | 2.97 × 104 | 206.6 | |
| 0.5 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 0.6 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 0.7 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 0.8 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 0.9 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ | |
| 1 | ∞ | ∞ | ∞ | ∞ |
| ∞ | ∞ | ∞ | ∞ |
Therefore, a factor that is predicted by the model to play a key role in determining whether a tumor will be eliminated by the immune system or whether it will escape immune destruction is not only the magnitude of the elicited immune response, but also how early this immune response is triggered by the tumor (or through immunotherapy). This suggests the existence of a therapeutic window during which immunotherapy should be started to be successful in eliminating the tumor.
The results based on administration of an anti-tumor treatment are presented in Section 3.1 through Section 3.3. All the simulations assumed a tumor of very low antigenicity (a = 1 × 10−5) that grows at a fast rate (γ1=0.69). This combination of parameter values was selected in order to simulate a realistic worst-case scenario to test the effectiveness of different anti-tumor treatment protocols in treating an aggressive tumor that elicits a very weak immune response.
3.1. Anti-VEGF treatment
3.1.1. No immunosuppression by TGF-β or VEGF
When only a standard anti-VEGF treatment is administered, there is a therapeutic window for the time of the first anti-VEGF injection of 157 days<t<586 days that leads to tumor eradication. Administering the standard anti-VEGF treatment too early is ineffective because early on, when the tumor is relatively small, there is not sufficient VEGF in the tumor for anti-VEGF to neutralize it, and the injected VEGF antibodies will be wasted without any benefit to the patient. Starting the standard anti-VEGF treatment on day 586 or after, will also be ineffective in treating the tumor because, by then, there will be too much VEGF, leading to a large amount of tumor vasculature and a high tumor cell carrying capacity. This will result in too many tumor cells for the standard treatment protocol to be effective. The model predicts that in order to eliminate the tumor if the therapeutic window of a standard anti-VEGF treatment has ended and no anti-VEGF treatment has been started, more anti-VEGF injections will be required in order to decrease the tumor size substantially. For example, a modified anti-VEGF treatment started on day 680 and consisting of 20 biweekly anti-VEGF injections will be successful in eradicating the tumor.
An important consideration when designing a treatment protocol and schedule is the minimization of treatment cytotoxicity and cost. These constraints impose a limit on the feasibility of an anti-VEGF treatment protocol. If the tumor has been detected very late and no practical modified anti-VEGF treatment can eradicate the tumor, other forms of treatment should be pursued first (chemotherapy or radiotherapy). Once the tumor size is reduced significantly by these alternative modes of treatment, an anti-VEGF treatment can be started to eliminate the remaining tumor cells.
3.1.2. Immunosuppression by TGF-β only
There is a therapeutic window for the time of the first anti-VEGF injection of 157 days<t<554 days that leads to tumor eradication. The model predicts that in order to eliminate the tumor if the therapeutic window of a standard anti-VEGF treatment has ended and no treatment has been administered, more injections, a greater frequency of them, and/or a greater anti-VEGF dose than prescribed in the standard treatment will be required. For example, a modified anti-VEGF treatment started on day 620 and consisting of 10 biweekly anti-VEGF injections will be successful in eradicating the tumor. In contrast, starting an anti-VEGF treatment of biweekly injections on day 660 would be unsuccessful even if 40 or more injections were administered, suggesting that chemotherapy or radiotherapy would be better initial treatment options at this stage.
3.1.3. Immunosuppression by TGF-β and VEGF
There is a therapeutic window for a standard anti-VEGF treatment of 157 days<t<546 days specifying when the first injection should be given to ensure that the tumor is eliminated. As the immunosuppressive effects of TGF-β and VEGF were incorporated into the model one at a time, the therapeutic window of a standard anti-VEGF treatment became narrower, but not as drastically as the therapeutic window of a standard DC treatment (see Section 3.2). If the therapeutic window of a standard anti-VEGF treatment has passed and no anti-VEGF treatment has been started, the model predicts that an extended anti-VEGF treatment will be successful in eradiating the tumor if started no more than a few months after the therapeutic window of a standard anti-VEGF treatment ends. An example of a successful treatment is to give 25 biweekly anti-VEGF injections starting on day 630.
3.2. Dendritic cell treatment
3.2.1. No immunosuppression by TGF-β or VEGF
When only a standard DC treatment is administered, there is a therapeutic window for the time of the first DC injection of 144 days<t<447 days that leads to tumor eradication. Starting a standard DC treatment after its therapeutic window has passed will be ineffective because, by then, there will be too many tumor cells. Only if the standard DC treatment protocol were to be modified by increasing the number or frequency of DC injections, and this modified DC treatment were to begin soon after the therapeutic window has passed, would there be eradication of the tumor. For example, to eliminate a tumor when a DC treatment is started on day 465 would require at least 30 weekly DC injections. However, the model predicts that no practical or biologically realistic extended DC treatment will be able to eliminate a tumor if it is diagnosed several months after the therapeutic window of a standard DC treatment has ended.
3.2.2. Immunosuppression by TGF-β Only
There is a therapeutic window for the time of the first injection of a standard DC treatment of 147 days<t<326 days that leads to tumor eradication. This window is significantly narrower than the one for the case when no immunosuppressive effect by TGF-β and VEGF was considered. Starting a standard DC treatment after the therapeutic window for a standard DC treatment has passed (on day 326) will be ineffective because, by then, there will be too many tumor cells and the concentration of TGF-β will be high enough to significantly suppress the function of the immune cells, including the injected DC. Only if the standard DC treatment protocol were to be modified by increasing the number or frequency of DC injections, and this modified treatment were to begin soon after the therapeutic window has passed, the tumor would be eradicated. For example, eliminating a tumor when DC treatment is started on day 330 would require at least 31 weekly DC injections.
3.2.3. Immunosuppression by TGF-β and VEGF
When the immunosuppressive effects of TGF-β and VEGF are both considered, a standard DC treatment could not eradicate the tumor no matter when the treatment was started. Hence, there is no therapeutic window for a standard DC treatment when a tumor of low antigenicity is able to suppress the immune system through the secretion of multiple immunosuppressive cytokines. This result suggests that a standard DC-based immunotherapy will not eliminate the tumor, unless ways to reduce the immunosuppressive effect of the tumor microenvironment are first implemented. Moreover, no feasible and safe extended DC treatment was found that could eradicate the tumor.
3.3. Combined anti-VEGF and dendritic cell treatments
3.3.1. No immunosuppression by TGF-β or VEGF
Case 1: A standard anti-VEGF treatment is given first, and it is followed by a standard DC treatment after the anti-VEGF treatment ends. If a standard anti-VEGF treatment is started within its therapeutic window of 157 days<t<586 days, the tumor will be eradicated and a standard DC treatment will not be necessary. If the therapeutic window for a standard anti-VEGF treatment has passed, a viable option is to achieve treatment synergy by administering a standard anti-VEGF treatment on day 645 followed by a standard DC treatment starting on day 740.
Case 2: A standard DC treatment is given first, and it is followed by a standard anti-VEGF treatment after the DC treatment ends. If a standard DC treatment is started within its therapeutic window of 144 days<t<447 days, the tumor will be eradicated and a standard anti-VEGF treatment will not be necessary. If the therapeutic window for a standard DC treatment has passed but the therapeutic window of a standard anti-VEGF treatment has not passed (the time is 446 days<t<586 days), a standard anti-VEGF treatment alone will eliminate the tumor. From day 586 and on, the best option is to only administer an extended anti-VEGF treatment to eradicate the tumor (e.g. 12 biweekly anti-VEGF injections given starting on day 670).
3.3.2. Immunosuppression by TGF-β Only
Case 1: An anti-VEGF treatment is given first, and it is followed by a DC treatment soon after the anti-VEGF treatment ends. If a standard anti-VEGF treatment is started within the anti-VEGF therapeutic window of 157 days<t<554 days, the tumor will be eradicated and a standard DC treatment will not be necessary. However, if the standard anti-VEGF treatment is initiated after its own therapeutic window ends on day 554, the model predicts that following this standard anti-VEGF treatment with a standard DC treatment will lead to treatment synergy and success only if the standard anti-VEGF treatment is started in no more than a few months after its therapeutic window ends. For example, a standard anti-VEGF treatment started on day 626 followed by a standard DC treatment that begins on day 720 will be successful in eliminating the tumor.
Case 2: A standard DC treatment is given first, and it is followed by a standard anti-VEGF treatment after the DC treatment ends. If a standard DC treatment is started within its therapeutic window of 147 days<t<326 days, the tumor will be eradicated and a standard anti-VEGF treatment will not be necessary. If the therapeutic window for a standard DC treatment has passed, but the therapeutic window for a standard anti-VEGF treatment has not passed (i.e. the current time is 325 days<t<554 days), administering a standard anti-VEGF treatment will eliminate the tumor. If treatment is started on day 554 or after, viable options include administering only an extended anti-VEGF treatment or aiming for treatment synergy as discussed in Case 1 above.
3.3.3. Immunosuppression by TGF-β and VEGF
Case 1: An anti-VEGF treatment is given first, and it is followed by a DC treatment after the anti-VEGF treatment ends. In this case, if a standard anti-VEGF treatment is initiated after its therapeutic window of 157 days<t<546 days ends, then an example of a strategy that eliminates the tumor is a standard anti-VEGF treatment started on day 600 and followed by a standard DC treatment starting on day 720.
Case 2: A sequence where a standard DC treatment is administered first and a standard anti-VEGF treatment is given after the DC treatment ends, is not recommended because a standard DC treatment has no therapeutic effect no matter when the first DC injection is given. If the therapeutic window of a standard anti-VEGF treatment has passed and no cancer treatment of any kind has been administered, then an alternative to eradicate the tumor is to give as many anti-VEGF injections as are necessary until the tumor is eradicated.
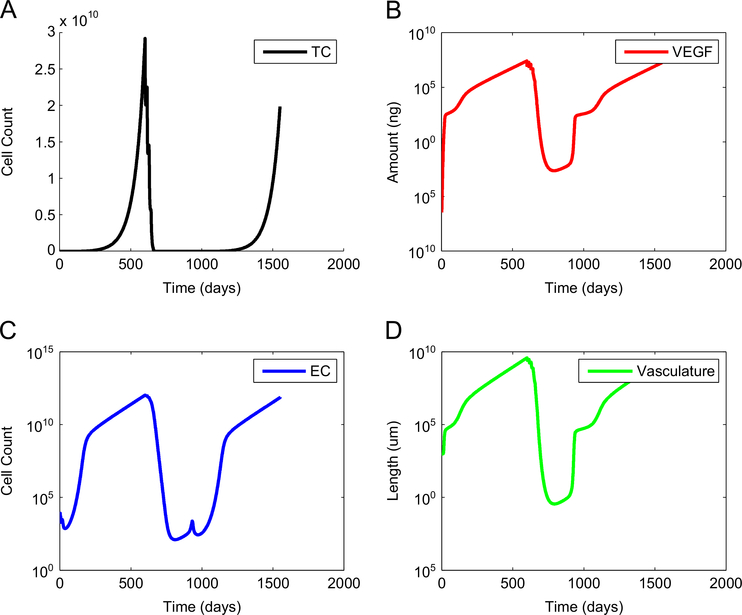
The results presented in Figs. 1–4 are based on a tumor of low antigenicity (a = 1 × 10−5) that grows at a fast rate (γ1=0.69) and that secretes the immunosuppressors TGF-β and VEGF. Fig. 1 illustrates a case where tumor size and the amount of free VEGF are both reduced by a standard anti-VEGF treatment started on day 600, but without extending this anti-angiogenic treatment, and without following it with DC immunotherapy. The outcome is an eventual growth of the tumor over time and failure of the standard anti-VEGF treatment.
Fig. 1.
Tumor escape when a standard anti-angiogenesis treatment is not continued, and is not combined with a treatment of unlicensed dendritic cells. Although tumor size is reduced significantly by a standard anti-VEGF treatment started on day 600, the tumor will eventually grow if the anti-VEGF treatment is not expanded, or if no follow-up DC immunotherapy is administered. (A) number of tumor cells, (B) concentration of free VEGF, (C) number of endothelial cells, and (D) total tumor vasculature.
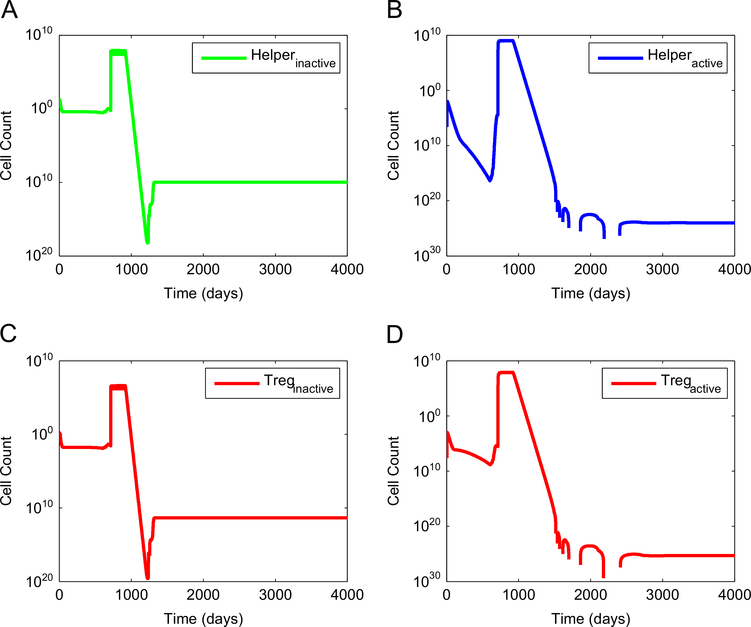
Fig. 4.
Dynamics of anti-tumor CD4+ helper T cells and of pro-tumor regulatory T cells when synergy between an anti-angiogenic treatment and a DC treatment is achieved. A standard anti-VEGF treatment was started on day 600 and was followed by a standard DC treatment started on day 710. Since regulatory T cells are stimulated by licensed DC, the number of Tregs initially increases following unlicensed DC treatment. However, this type of immunotherapy also activates helper T cells and CTL that target the tumor. The activation of anti-tumor cells outweighs the activation of pro-tumor cells. Hence, the end result is complete tumor elimination. (A) number of inactive helper T cells, (B) number of active helper T cells, (C) number of inactive Tregs, and (D) number of active Tregs.
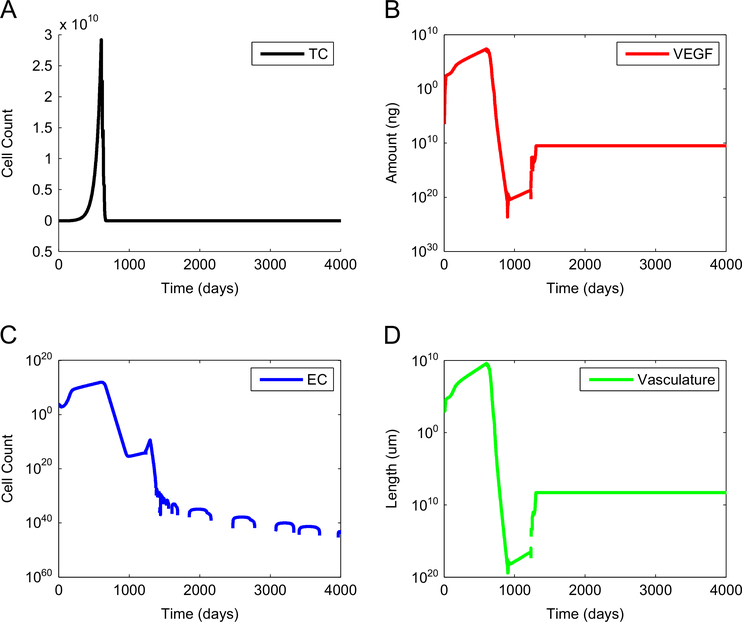
Fig. 2 illustrates complete tumor elimination when a standard anti-VEGF treatment started on day 600 is followed by a standard DC immunotherapy starting on day 710. The standard anti-VEGF treatment decreased the concentration of free VEGF low enough to allow a standard DC treatment to effectively eliminate the tumor without the need for a constant or a high-dose regimen of DC injections. Without this substantial decrease in VEGF, a standard DC immunotherapy started on day 710 (or on any other day) would not have been successful in eliminating the tumor. Thus, Fig. 2 illustrates the fact that the combined regulatory effect of a standard anti-VEGF treatment and a standard DC treatment is stronger than the effect of either treatment given as monotherapy.
Fig. 2.
Tumor elimination via synergy between an anti-angiogenic treatment and a DC treatment. A standard anti-VEGF treatment started on day 600 decreases tumor size, and the concentration of free VEGF, low enough to make a standard DC treatment started on day 710 effective at eliminating the remaining tumor cells. (A) number of tumor cells, (B) concentration of free VEGF, (C) number of endothelial cells, and (D) total tumor vasculature.
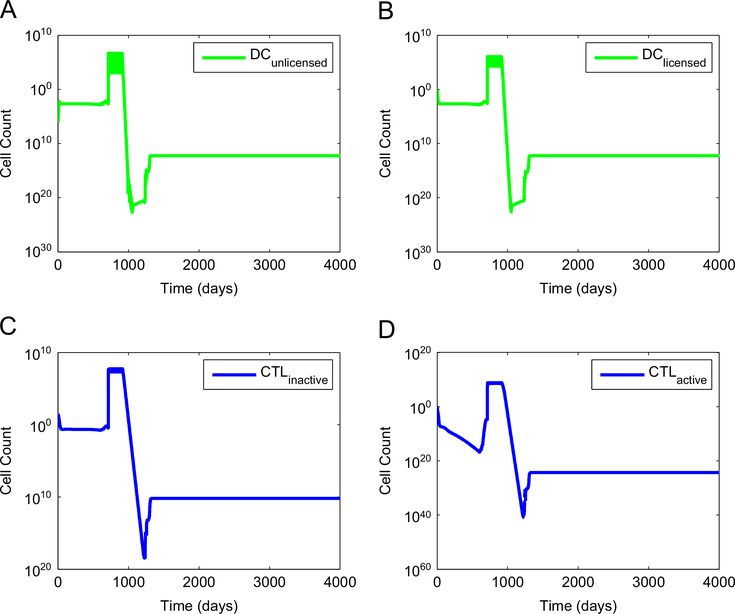
Figs. 3 and 4 show the corresponding dynamics of tumor-targeted immune cells and pro-tumor regulatory T cells for the case when synergy between a standard anti-VEGF treatment and a DC treatment is achieved. The injection of unlicensed DC leads to a sharp increase in the number of active CD8+ T cells and of CD4+ helper T cells that target the tumor. This temporary boost of immune cell activity, coupled with a reduction of the immunosuppressive effect of VEGF effected by the anti-VEGF treatment, is sufficient to eradicate the tumor.
Fig. 3.
Dynamics of tumor-targeted dendritic cells and CTL when synergy between an anti-angiogenic treatment and a DC treatment is achieved. A standard anti-VEGF treatment was started on day 600 and was followed by a standard DC treatment started on day 710. This treatment combination was effective at eliminating the tumor. (A) number of unlicensed dendritic cells, (B) number of licensed dendritic cells, (C) number of inactive CTL, and (D) number of active CTL.
Based on the model simulations, the extent of tumor antigenicity and the level of immunosuppression interact to determine the therapeutic time window of a standard anti-VEGF treatment and of a standard DC treatment. Many more injections with a shorter period between them will be required if these treatments are not started during their respective therapeutic window. Achieving synergy between these two treatments helps to reduce the total number of injections needed of each type, reduces the injection frequency required for treatment success, and the toxicity that may occur when a single type of treatment is given for an extended period of time. Since the time span from the time of tumor development to its diagnosis determines tumor size (here defined as the total number of cancer cells that form a solid tumor), a therapeutic time window actually specifies the lower and upper bounds of tumor size above and below which, respectively, a standard anti-cancer treatment will be effective in eliminating the tumor. Therefore, tumor size at the start of treatment may be used as a marker to determine which of these two treatments will be the most practical and safer to implement, and whether a combination immunotherapy should be administered.
When a tumor exhibits low antigenicity, and the combined immunosuppressive effect of TGF-β and VEGF is strong, an anti-VEGF treatment alone is predicted to be the most effective method to reduce tumor size. An advantage of a standard anti-VEGF treatment over a standard DC treatment, when TGF-β and VEGF both suppress the immune system, is that the former treatment is not affected by these immunosuppressors. This has the effect of making an anti-VEGF treatment effective at eliminating a tumor over a wide therapeutic time window that is robust with respect to the level of tumor immunosuppression. On the other hand, the therapeutic time window of a standard DC treatment becomes narrower as the combined concentration of TGF-β and VEGF increases over time. This means that standard DC immunotherapy will be effective at eliminating only small, slow-growing tumors of a possibly undetectable size, if tumor-induced immunosuppression is strong.
In spite of the predicted success of an anti-VEGF treatment at eliminating a tumor relative to that of a DC treatment, the success of an anti-VEGF monotherapy has been modest in a clinical setting. Fig. 1 suggests that an anti-VEGF therapy alone cannot eliminate a tumor without the assistance of an activated tumor-targeted immune system. Fig. 2 indicates that the successful removal of a tumor by an anti-VEGF treatment is partially due to having an active immune system that takes over in the final stages of anti-VEGF treatment, when the concentrations of immunosuppressive and angiogenic cytokines have been reduced significantly. In effect, the success of an anti-VEGF treatment targeting cancer derives from the fact that it reduces tumor size by decreasing the carrying capacity of the tumor and by reducing immunosuppression, both of which allow the suppressed, anergic immune system to become reactivated and clear the remaining tumor cells.
Table 5 summarizes the effect of incremental immunosuppression by TGF-β and VEGF on the therapeutic time window and tumor size window of the standard anti-cancer treatments that were simulated using the model defined by Eqs. (1)–(18).
Table 5.
Therapeutic windows of standard anti-cancer treatments.
| Level of immunosuppression | Standard treatment | Therapeutic window |
|---|---|---|
| No immunosuppression by TGF-β or VEGF | Anti-VEGF | 157 days < time < 586 days 1.78 × 107 cells < size < 2.38 × 1010 cells |
| Unlicensed DC |
144 days < time < 447 days 1 × 107 cells < size < 3.13 × 109 cells |
|
| Immunosuppression by TGF-β | Anti-VEGF |
157 days < time < 554 days 1.78 × 107 cells < size < 1.50 × 1010 cells |
| Unlicensed DC |
147 days < time < 326 days 1.15 × 107 cells < size < 5.16 × 108 cells |
|
| Immunosuppression by TGF-β and VEGF | Anti-VEGF |
157 days < time < 546 days 1.78 × 107 cells < size < 1.33 × 1010 cells |
| Unlicensed DC | There is no therapeutic window for a standard DC treatment that will lead to tumor elimination when both TGF-β and VEGF act as immunosuppressors. |
A therapeutic window of time or, equivalently, of tumor size, is useful only if this window includes the tumor size threshold for detection. If the model were to predict therapeutic windows only for tumors of undetectable sizes, the results would be useless for developing anti-tumor treatment protocols. A tumor size threshold for 80% probability of detection is estimated to be on the order of 108 cancer cells. This is based on the probability of detecting a tumor of a given diameter calculated in Michaelson et al. (2003), and the formula given in Michaelson et al. (1999) for approximating the number of cancer cells as a function of tumor diameter
| (19) |
where d is the tumor diameter in centimeters and B is the number of cells per cubic centimeter. Given that most cancer cells have a diameter of approximately 20 μm, the typical solid tumor density is estimated to be B=108 cancer cells per cubic centimeter (Michaelson et al., 1999). Solving (19) for d gives
| (20) |
which can be used to estimate the diameter of a tumor consisting of n cells and assuming a tumor density of B cancer cells per cubic centimeter. Based on (20), the ranges of solid tumor diameter that a standard anti-VEGF treatment and a standard DC treatment would be able to eliminate are listed in Table 6. These results indicate that as immunosuppression increases, it sets a limit on the maximum tumor size that can be eliminated by either treatment. These results also suggest that an extended anti-VEGF treatment would be appropriate to administer to reduce the size of tumors that are detected late, are highly vascularized, and have grown to a relatively large size. The potential side effects of an extended anti-angiogenic treatment may be avoided by switching to DC immunotherapy as soon as the size of the tumor has been reduced significantly and the immunosuppressive effect of VEGF and TGF-β has been neutralized.
Table 6.
Therapeutic tumor diameter of standard anti-cancer treatments.
| Level of immunosuppression | Standard treatment | Therapeutic tumor diameter |
|---|---|---|
| No Immunosuppression by TGF-β or VEGF | Anti-VEGF Unlicensed DC |
0.70 cm < diameter < 7.69 cm 0.58 cm < diameter < 3.91 cm |
| Immunosuppression by TGF-β | Anti-VEGF Unlicensed DC |
0.70 cm < diameter < 6.59 cm 0.60 cm < diameter < 2.14 cm |
| Immunosuppression by TGF-β and VEGF | Anti-VEGF Unlicensed DC |
0.70 cm < diameter < 6.33 cm There is no tumor diameter for a standard DC treatment that will lead to tumor elimination when both TGF-β and VEGF act as immunosuppressors. |
The therapeutic windows and tumor diameter ranges presented in Tables 5 and 6 indicate that a standard anti-VEGF treatment can be used to treat a detectable tumor at all levels of immunosuppression. However, this may or may not be the case for DC immunotherapy, depending on how strong the immunosuppression is. When a tumor is diagnosed, it may be of a size large enough that the immunosuppressive tumor microenvironment will make even an extended DC treatment ineffective. Experimental and modeling efforts are underway to develop methods aimed at improving tumor detection at earlier stages (Lutz et al., 2008). These efforts will help to decrease the tumor-detection size threshold to be well within the therapeutic window of DC-based immunotherapy predicted by the model.
Adherence to recommended screening guidelines, especially by at-risk individuals of a certain age, race and sex, will be crucial to the diagnosis of a tumor at an early stage, when the therapeutic window for an anti-VEGF or DC treatment is still active and can lead to tumor elimination. Early detection will help avoid the need for chemotherapy or radiotherapy. In a clinical setting, a cost-benefit analysis, as well as a comparison of the side effects of an anti-angiogenic treatment versus immunotherapy, will also play a role in determining whether anti-VEGF injections, DC injections or a combination of both should be administered.
Since the initial number of tumor cells was set to a single tumor cell, all the model simulations assumed a zero initial VEGF concentration. If free VEGF were already present before an anti-VEGF treatment begins, then, the greater the initial concentration of VEGF, the more this would cause the therapeutic time window for administering anti-VEGF to shift to the left. This shift would allow the possibility of giving anti-VEGF injections much earlier to eliminate the tumor, since there would be a substantial amount of VEGF early on that could be neutralized by anti-VEGF. However, the maximum day limit to begin anti-VEGF treatment and be effective in treating the tumor would be smaller. Similarly, the model predicts that a significant initial concentration of free VEGF in or nearby the tumor would cause the therapeutic time window for administering a standard DC treatment to shift to the left. This prediction is to be expected because the tumor vasculature and carrying capacity would be greater and the tumor would grow at a faster rate compared to the case when there is little or no VEGF initially, thus reducing the maximum day limit when a standard DC treatment should be started in order to effectively eliminate the tumor.
The model simulations highlighted the advantages of an anti-angiogenesis treatment based on injecting a monoclonal VEGF antibody to eliminate tumors up to a size of 1010 cancer cells, regardless of the presence or absence of an immunosuppressive effect by TGF-β and VEGF. Neutralizing tumor-secreted VEGF by administering anti-VEGF is sufficient to decrease the immunosuppressive effect of VEGF, allowing immune cells that are activated by tumor antigens to migrate to the tumor site and perform their cytotoxic activity. However, due to the low antigenicity of tumors, the process of DC licensing and of CD4+ and CD8+ T cell activation may take too long. This delay in activating the immune system to target the tumor may lead to tumor growth, and escape, before the immune system has had a chance to fight it. For this reason, combining an anti-VEGF treatment with immunotherapy to achieve a faster and enhanced immune response is a more appropriate strategy to eliminate a tumor than anti-VEGF monotherapy.
A complex issue that was not addressed in the model assumptions is the fact that activated immune cells use the peripheral and tumor vasculature to reach and penetrate the tumor. Although anti-VEGF can limit the effect of immunosuppression within the tumor and can reduce tumor angiogenesis, it can also have the adverse effect of disrupting useful vasculature, leading to reduced trafficking of activated immune cells to the tumor site. Optimal control theory could be implemented to determine the best anti-VEGF treatment protocol that will maximize tumor size reduction while also minimizing unwanted side effects.
Further expansion and analysis of the model could involve the simulation of adoptive immunotherapy involving ex vivo generated cytotoxic T lymphocytes (CTL). This approach would bypass the immunosuppressive effect that limits the expansion of CTL. Moreover, the model can be expanded to simulate immunotherapy where CTLs are genetically modified to express chimeric antigen receptors (CAR) in order to bypass the need for MHC signaling, which tumor cells often suppress (Maher and Davies, 2004). The CAR approach is likely to become increasingly important in the future development of an anti-cancer vaccine.
Although the integration of tumor angiogenesis, a tumor-targeted immune response and the immunosuppressive effect of tumor secreted cytokines made the present model more realistic, there are important aspects that were not addressed. For example, the time it takes dendritic cells to migrate to lymph nodes and spleen to activate CD8+ T cells was ignored. The model also ignored the time delay that exists due to migration of effector T cells to the tumor site. Time delays are known to destabilize the dynamics of many biological systems, leading to oscillations in the number of tumor cells over time. The predictions based on the analysis of the present model assumed no delays in the immune system response, leading to a possible overestimation of the therapeutic benefit of an anti-VEGF treatment and of immunotherapy.
The model did not consider the pro- and anti-tumor effect of M1 and M2 macrophages, or the therapeutic benefit of IL-12, IFN-γ and TNF-α, all of which are secreted by immune cells at different rates depending on the type and strength of the stimulus. The effect of these cells and cytokines can be incorporated into the model as more information on their dynamics and precise roles becomes available. The difficulty of incorporating these additional components into the model lies in the fact that immune cells can switch from being anti-tumor to being pro-tumor (Sánchez-reyes et al., 2014; Chimal-Ramírez et al., 2013). This phenotypic switch of immune cells needs to be modeled accurately in order to realistically simulate their contribution to hindering (or perpetuating) tumor growth in the context of the tumor microenvironment. Similarly, certain cytokines can be anti- or pro-tumor depending on the spatial and temporal context. The multifaceted role of certain cytokines would need to be elucidated more clearly before including them in the model, in order to avoid introducing a bias that would artificially tip the balance in favor of tumor regression or tumor growth, making a treatment seem more or less effective, respectively, than it has the potential to be. Robertson-Tessi et al. (2012) included in their model the immunosuppressive effect of IL-10 and the anti-tumor effect of IL-2. Hence, IL-2 and IL-10 were incorporated into the present model to keep their modeling approach as intact as possible to quantify the effect of introducing tumor angiogenesis and an anti-VEGF treatment. This is the reason why anti-tumor cytokines such as IFN-γ and IL-4 were not included.
Tumor angiogenesis does not only depend on the amount of VEGF secreted by tumor cells, but also on the density, and type, of VEGF receptors present on endothelial cells. Multiple VEGF receptors, including co-receptors and antagonistic soluble receptors are known to exist. A compartmental, mechanistic modeling approach that can simulate the key aspects of tumor angiogenesis would make the model more realistic. This type of model has previously been developed to simulate the transport of VEGF between the blood, normal tissue and tumor compartments, to simulate the binding dynamics of VEGF with its receptors, and to quantify the therapeutic effect of administering a monoclonal antibody targeting VEGF (Finley et al., 2013). A molecularly-detailed model of angiogenesis could be expanded to include mechanistic information on the immune system and the transport of immune cells from one compartment to another. Expanding such a compartmental model will require accurate information, obtained from published work or by designing new experiments, on the kinetic and geometric parameters of immune cells, their cytokines and their receptors. Once the necessary information becomes available, a multi-compartmental pharmacokinetic model of tumor-immune system interactions could serve as a framework for designing an anti-tumor vaccine.
TGF-β is an angiogenic factor secreted by tumor cells that also contributes to tumor vascularization (Elliott and Blobe, 2005). Thus, the tumor carrying capacity could be defined as a function of both VEGF and TGF-β. This modeling aspect could be important if TGF-β and VEGF can synergize to enhance tumor vascularization. Since VEGF is known to be the major mediator of angiogenesis, the tumor carrying capacity was assumed to depend only on the amount of free VEGF available. However, certain patients become resistant to anti-VEGF therapy or are unresponsive to this type of treatment (Prager et al., 2011). Hence, other means to target angiogenic pathways have been explored, including treatments targeting TGF-β (Connolly et al., 2012; Ganapathy et al., 2010). The model can be used to design an anti-angiogenic treatment that only targets TGF-β, or that targets both TGF-β and VEGF. As with any cancer treatment, the level of toxicity and possible side effects need to be addressed. A pharmacokinetic model that includes a compartment representing normal tissue will serve to design a treatment protocol that maximizes tumor regression, while minimizing the damage to healthy cells.
In the future, the model will be expanded to include a cancer-targeted innate immune response characterized by natural killer cells and a humoral response that produces antibodies against tumor cells or against tumor-secreted factors. An anti-tumor vaccine that is able to elicit antigen presentation and antibody production by CD40-activated B cells is promising, since there is evidence indicating that the immunosuppressive factors IL-10, TGF-β and VEGF do not affect the antigen-presenting function of CD40-activated B cells (Shimabukuro-vornhagen et al., 2012). Also, it is known that the tumor microenvironment contributes to the immunosuppression of natural killer cells (Baginska et al., 2013). An inverse correlation between NK cell activation and Treg activity has been observed, and VEGF and TGF-β play a key role in promoting the activity of Tregs against NK cells (Ghiringhelli et al., 2005; Wada et al., 2009). The expanded model will incorporate the antagonistic functions of Tregs and NK cells and will be used to quantify the role that tumor-secreted cytokines and tumor-associated cells play in suppressing the innate, adaptive and humoral immune responses.
4. Conclusion
A model simulating tumor angiogenesis and tumor–immune system interactions predicted that, in spite of strong immunosuppressive effects, an anti-VEGF treatment has a wide, and robust, therapeutic window of tumor size that leads to the elimination of detectable tumors. In contrast, the therapeutic window of dendritic cell-based immunotherapy becomes considerably narrower as the immunosuppressive effects in the tumor microenvironment become stronger. The model predicted synergy when an anti-VEGF treatment is administered first to reduce immunosuppression, and is immediately followed by immunotherapy to boost and enhance the tumor-targeted immune response. Biologically-feasible treatment protocols were designed that lead to tumor elimination and that do not require a permanent administration of anti-VEGF or dendritic cell injections. These results lay the groundwork for a future pharmacokinetic model that could be used to develop an anti-tumor vaccine.
HIGHLIGHTS.
Distinct therapeutic windows are predicted to exist for an anti-VEGF treatment and for DC immunotherapy.
Conditions leading to synergy between an anti-VEGF treatment and DC immunotherapy were identified.
The model integrates tumor angiogenesis, an adaptive immune response and immunosuppression.
Tumor size at diagnosis can be used as a marker to select the most feasible type of treatment.
Acknowledgments
Funding source
There is no financial support to report.
Footnotes
Conflict of interest statement
The author discloses that there are no potential conflicts of interest.
References
- Azinovic I, Calvo F, Puebla F, et al. , 2001. Long-term normal tissue effects of intraoperative electron radiation therapy (Ioert): late sequelae, tumor recurrence, and second malignancies. Int. J. Radiat. Oncol. Biol. Phys. 49, 597–604. [DOI] [PubMed] [Google Scholar]
- Azim H, de Azambuja E, Colozza M, Bines J, Piccart MJ, 2011. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann. Oncol. 22, 1939–1947. 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- Arciero J, Jackson T, Kirschner D, 2004. A mathematical model of tumor-immune evasion and siRNA treatment. Discret. Contin. Dyn. Syst.-Ser. B 4, 39–58. [Google Scholar]
- Akdis CA, Blaser K, 2001. Mechanisms of interleukin-10-mediated immune suppression. Immunology 103, 131–136. 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokemeyer C, Schmoll H, 1995. Treatment of testicular cancer and the development of secondary malignancies. J. Clin. Oncol. 13, 283–292. [DOI] [PubMed] [Google Scholar]
- Bender BC, Schindler E, Friberg LE, 2013. Population pharmacokinetic pharmacodynamic modelling in oncology: a tool for predicting clinical response. Br. J. Clin. Pharmacol, 1–42. 10.1111/bcp.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, et al. , 2000. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 96, 3102–3108. [PubMed] [Google Scholar]
- Baginska J, Viry E, Paggetti J, Medves S, Berchem G, et al. , 2013. The critical role of the tumor microenvironment in shaping natural killer cell-mediated antitumor immunity. Front. Immunol. 4, 1–13. 10.3389/fimmu.2013.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MA, Davis AL, 2009. A Mathematical Model of Angiogenesis in Glioblastoma Multiforme. Arizona State University. [Google Scholar]
- Chimal-Ramírez GK, Espinoza-Sánchez N. a, Fuentes-Pananá EM, 2013. Protumor activities of the immune response: Insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J. Oncol. 2013, 1–16. 10.1155/2013/835956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EC, Freimuth J, Akhurst RJ, 2012. Complexities of TGF-Beta targeted cancer therapy. Int. J. Biol. Sci. 8, 964–978. 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, et al. , 2005. Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology 68, 276–284. 10.1159/000086784. [DOI] [PubMed] [Google Scholar]
- Enderling H, Chaplain MAJ, 2014. Mathematical modeling of tumor growth and treatment. Curr. Pharm. Des. 20, 1–7. [DOI] [PubMed] [Google Scholar]
- Eftimie R, Bramson JL, Earn DJD, 2011. Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull. Math. Biol. 73, 2–32. 10.1007/s11538-010-9526-3. [DOI] [PubMed] [Google Scholar]
- Escors D, 2014. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J. Sci. 2014, 1–25. 10.1155/2014/734515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RL, Blobe GC, 2005. Role of transforming growth factor beta in human cancer. J. Clin Oncol. 23, 2078–2093. 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Novotny W, 2005. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 333, 328–335. 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- Finley SD, Dhar M, Popel AS, 2013. Compartment model predicts VEGF secretion and investigates the effects of VEGF trap in tumor-bearing mice. Front. Oncol. 3, 196 10.3389/fonc.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D, Chen H, et al. , 1996. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Flavell R, 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2, 46–53. 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, et al. , 2005. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 202, 919–929. 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilondo JV, Hernández-Bernal F, Ayala-Ávila M, de la Torre a, V., de la Torre J, et al. , 2014. Specific active immunotherapy with a VEGF vaccine in patients with advanced solid tumors. results of the CENTAURO antigen dose escalation phase I clinical trial. Vaccine 32, 2241–2250. 10.1016/j.vaccine.2013.11.102. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, et al. , 2010. Targeting the transforming growth factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer 9, 122 10.1186/1476-4598-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, et al. , 2005. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 202, 1075–1085. 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat MJ, Howlader N, Reichman ME, Edwards BK, 2007. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 12, 20–37. 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, et al. , 2007. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J. Clin. Oncol. 25, 1489–1497. 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. , 2004. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 56, 549–580. 10.1124/pr.56.4.3.549. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Jemal A, Center MM, DeSantis C, Ward EM, 2010. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 19, 1893–1907. 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- Jellema AP, Slotman BJ, Doornaert P, Leemans CR, Langendijk J. a, 2007. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 69, 751–760. 10.1016/j.ijrobp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Joshi B, Wang X, Banerjee S, Tian H, Matzavinos A, et al. , 2009. On immunotherapies and cancer vaccination protocols: a mathematical modelling approach. J. Theor. Biol. 259, 820–827. 10.1016/j.jtbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Kaur S, Chang T, Singh SP, Lim L, Mannan P, et al. , 2014. CD47 signaling regulates the immunosuppressive activity of VEGF in T Cells. J. Immunol. 193, 3914–3924. 10.4049/jimmunol.1303116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, 2007. Cancer immunoediting from immune surveillance to immune escape. Immunology 121, 1–14. 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI, 2006. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 25, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, et al. , 2013. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 128, 1927–1955. 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- Louzoun Y, Xue C, Lesinski GB, Friedman A, 2014. A mathematical model for pancreatic cancer growth and treatments. J. Theor. Biol. 351, 74–82. 10.1016/j.jtbi.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K-L, Bai X-F, Friedman A, 2014. Mathematical modeling of interleukin-35 promoting tumor growth and angiogenesis. PLoS One 9, e110126 10.1371/journal.pone.0110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang L, Chang X, Cheng Y, Cheng H, et al. , 2012. Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin. J. Cancer Res. 24, 130–137. 10.1007/s11670-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang M-N, Li H, King KD, Bassi R, et al. , 2002. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J. Exp. Med. 195, 1575–1584. 10.1084/jem.20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AM, Willmann JK, Cochran FV, Ray P, Gambhir SS, 2008. Cancer screening: a mathematical model relating secreted blood biomarker levels to tumor sizes. PLoS Med. 5, 1287–1297. 10.1371/journal.pmed.0050170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Boschi-pinto C, Lopez AD, 2001. Cancer incidence, mortality and survival by site for 14 regions of the world. World Health Organisation, vol. 13, pp. 1–47. [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE, 2008. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 8, 618–631. 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Morera Y, Bequet-Romero M, Ayala M, Pérez PP, Castro J, et al. , 2012. Antigen dose escalation study of a VEGF-based therapeutic cancer vaccine in non human primates. Vaccine 30, 368–377. 10.1016/j.vaccine.2011.10.082. [DOI] [PubMed] [Google Scholar]
- Michaelson J, Satija S, Moore R, Weber G, Halpern E, et al. , 2003. Estimates of the sizes at which breast cancers become detectable on mammographic and clinical grounds. J. Women Imaging 5, 3–10. 10.1097/00130747-200302000-00002. [DOI] [Google Scholar]
- Michaelson JS, Halpern E, Kopans DB, 1999. Breast cancer: computer simulation method for estimating optimal intervals for screening. Radiology 212, 551–560. [DOI] [PubMed] [Google Scholar]
- Maher J, Davies ET, 2004. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br. J. Cancer 91, 817–821. 10.1038/sj.bjc.6602022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Chong G, et al. , 2008. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol. Immunother. 57, 1115–1124. 10.1016/j.jsbmb.2011.07.002.Identification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, et al. , 2003. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101, 4878–4886. 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, et al. , 1998. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa-B activation in hemopoietic progenitor cells. J. Immunol. 160, 1224–1232. [PubMed] [Google Scholar]
- Oelkrug C, Ramage JM, 2014. Enhancement of T cell recruitment and infiltration into tumours. Clin. Exp. Immunol. 178, 1–8. 10.1111/cei.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaji Y, Tsuno NH, Saito S, Yoneyama S, Tanaka M, et al. , 2006. Vaccines targeting tumour angiogenesis-a novel strategy for cancer immunotherapy. Eur. J. Surg Oncol. 32, 363–370. 10.1016/j.ejso.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Park HJ, 2014. Chemotherapy induced peripheral neuropathic pain. Korean J. Anesth. 67, 4–7. 10.4097/kjae.2014.67.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo MO, Kavan P, Miller WH, Panasci L, Assouline S, et al. , 2013. Systemic cancer therapy: achievements and challenges that lie ahead, Front. Pharmacol. 10.3389/fphar.2013.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszczuk J, Hahnfeldt P, Enderling H, 2015. Therapeutic implications from sensitivity analysis of tumor angiogenesis models. PLoS One 101371, e0120007 10.1371/journal.pone.0120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon-Charry A, Maxwell T, López JA, 2005. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol. Cell Biol. 83, 451–461. 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- Prager G, Poettler M, Unseld M, Zielinski C, 2011. Angiogenesis in cancer: anti-VEGF escape mechanisms. Transl. Lung Cancer Res. 1, 14–25. 10.3978/j.issn.2218-6751.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribba B, Holford NH, Magni P, Trocóniz I, Gueorguieva I, et al. , 2014. A review of mixed-effects models of tumor growth and effects of anticancer drug treatment used in population analysis. CPT Pharmacomet. Syst. Pharmacol. 3, e113 10.1038/psp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson-Tessi M, El-Kareh A, Goriely A, 2012. A mathematical model of tumor–immune interactions. J. Theor. Biol. 294, 56–73. 10.1016/j.jtbi.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Schwartz CL, 1999. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist 4, 45–54. [PubMed] [Google Scholar]
- Schaapveld M, Visser O, Louwman MJ, De Vries EGE, Willemse PHB, et al. , 2008. Risk of new primary nonbreast cancers after breast cancer treatment: a dutch population-based study. J. Clin. Oncol. 26, 1239–1246. 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- Stevens A, Mackey MC (Eds.), 2013. Mathematical Methods and Models in Biomedicine. Springer, p. 426. [Google Scholar]
- Shih T, Lindley C, 2006. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin. Ther. 28, 1779–1802. 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yu P, Zeng D, Qian F, Lei X, et al. , 2014. Suppression of vascular endothelial growth factor abrogates the immunosuppressive capability of murine gastric cancer cells and elicits antitumor immunity. FEBS J. 281, 3882–3893. 10.1111/febs.12923. [DOI] [PubMed] [Google Scholar]
- Saito H, Tsujitani S, Ikeguchi M, Maeta M, Kaibara N, 1998. Relationship between the expression of vascular endothelial growth factor and the density of dendritic cells in gastric adenocarcinoma tissue. Br. J. Cancer 78, 1573–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Raundhal M, Patidar A, Saha B, 2014. Anti-VEGF antibody enhances the antitumor effect of CD40. Int. J. Cancer 135, 1983–1988. 10.1002/ijc.28833. [DOI] [PubMed] [Google Scholar]
- Sánchez-reyes K, Bravo-cuellar A, Hernández-flores G, Lerma-díaz JM, Javesuárez LF, et al. , 2014. Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-Like suppressor phenotype with change in toll-like receptor profile. Biomed. Res. Int. 2014, 1–11. 10.1155/2014/683068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro-vornhagen A, Draube A, Liebig TM, Rothe A, Kochanek M, et al. , 2012. The immunosuppressive factors IL-10, TGF-β, and VEGF do not affect the antigen-presenting function of CD40-activated B cells. J. Exp. Clin. Cancer Res. 31, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul HMW, Pinedo HM, 2000. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGFReceptor kinase inhibitors. Clin. Breast Cancer 1, S80–S84. 10.3816/CBC.2000.s.015. [DOI] [PubMed] [Google Scholar]
- Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, et al. , 2014. Control of the immune response by pro-angiogenic factors. Front. Oncol. 4, 70 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voron T, Colussi O, Marcheteau E, Pernot S, Zinzindohoué F, et al. , 2015. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148. 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon D. a, et al. , 2007. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 13, 1253–1259. 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- Wilson S, Levy D, 2012. A mathematical model of the enhancement of tumor vaccine efficacy by immunotherapy. Bull. Math Biol. 74, 1485–1500. 10.1007/s11538-012-9722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Huang CTCD, 2008. Anti-angiogenic therapeutic drugs for treatment of human cancer. Cancer Mol. 4, 37–45. [Google Scholar]
- Widakowich C, de Castro G, de Azambuja E, Dinh P, Awada A, 2007. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist 12, 1443–1455. 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- Wang B, Kaumaya PTP, Cohn DE, 2010. Immunization with synthetic VEGF peptides in ovarian cancer. Gynecol. Oncol. 119, 564–570. 10.1016/j.ygyno.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, et al. , 2009. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 29, 881–888. [PubMed] [Google Scholar]
- Ziogas A, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, et al. , 2012. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 107, 1869–1875. 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]