Abstract
It is demanded to supply foods with good quality for all the humans. With the advent of aging society, palatable and healthy foods are required to improve the quality of life and reduce the burden of finance for medical expenditure. Food hydrocolloids can contribute to this demand by versatile functions such as thickening, gelling, stabilising, and emulsifying, controlling texture and flavour release in food processing. Molar mass effects on viscosity and diffusion in liquid foods, and on mechanical and other physical properties of solid and semi-solid foods and films are overviewed. In these functions, the molar mass is one of the key factors, and therefore, the effects of molar mass on various health problems related to noncommunicable diseases or symptoms such as cancer, hyperlipidemia, hyperglycemia, constipation, high blood pressure, knee pain, osteoporosis, cystic fibrosis and dysphagia are described. Understanding these problems only from the viewpoint of molar mass is limited since other structural characteristics, conformation, branching, blockiness in copolymers such as pectin and alginate, degree of substitution as well as the position of the substituents are sometimes the determining factor rather than the molar mass. Nevertheless, comparison of different behaviours and functions in different polymers from the viewpoint of molar mass is expected to be useful to find a common characteristics, which may be helpful to understand the mechanism in other problems.
Keywords: Structure, Rheology, Function, Processing, Nutrition
Graphical abstract
Highlights
-
•
Examples of rheology control by molar mass are shown.
-
•
High and low molar mass compounds often show opposite effects.
-
•
Effects of lower mass compounds on the properties of food polymers are discussed.
-
•
Molar mass effects in physiological function of foods are discussed.
-
•
Molar mass effects in flavour release are discussed.
1. Introduction
To contribute to the universal demand for secure and sustainable food supply, food colloids science and technology can do many things. It can create palatable foods from underutilized resources, and can reduce the food loss. It is reported that there are many people who die from the insufficient supply of foods and malnutrition while food was wasted by improper distribution in some regions as pointed out by the World Health Organization (WHO). International Union of Food Science and Technology (IUFoST) aims to supply sufficient foods helping secure the world's food supply and eliminate world hunger. While the life expectancy in Japan is the longest in the world, it is demanded to extend the healthy life expectancy, which is defined as the period in which the person can enjoy life without any limitation in day-to-day activities resulting from health problems, because the increase of the life-style related diseases make some people ill, which lowers quality of life and causes a significant financial burden.
Palatable, healthy, and sustainable foods are demanded. From the nutritional standpoint it is possible to have a completely adequate diet in the form of fluid foods that require no mastication. However, few people are content to live on such a diet. Modern food and pharmaceutical companies sell manna-like foods, which proclaim to be palatable and healthy. But, people don't eat them every day. Bourne (2002) raises the great number of dentists in the developed countries which supports his argument. Even if ready-made convenient foods are palatable and good for health, most people like to eat something different every time when the situation allows except in a serious calamity such as the earthquake and typhoon/hurricane. Palatable and healthy foods are even more demanded in such a situation as in the age of covid-19 because disadvantaged persons, such as refugees and patients, are under the harsh stresses.
Recent studies clarified that the palatability is closely related with human health through the postprandial thermogenesis (LeBlanc, 2000; LeBlanc & Labrie, 1997) which was found to be induced by brown adipose tissue (BAT) (Okamatsu-Ogura et al., 2019). Small molecules such as resveratrol, bile acid, genipin, short fatty acids like butyric acid, have been reported to increase thermogenic activities in animal or cellular models (Gao et al., 2009). Nguo et al. (2018) examined the effect of chain length of fatty acids on mean-induced thermogenesis (MIT) since monounsaturated fatty acids induced greater MIT than saturated fatty acids. They found that MIT was independent of the chain length. Therefore, the palatability plays a role not only in mental and spiritual domain, but also it is correlated with physiological function. Texture and flavour are key factors governing the palatability and molar mass effects on these factors are discussed in the present review.
Most foods except beverages are gels, and gels can realize a broad range of textures from fluid-like to solid-like (Djabourov, Nishinari, & Ross-Murphy, 2013). Although traditional techniques such as extrusion cooking (Isobe & Noguchi, 1988) and structuring by transglutaminase (Motoki & Seguro, 1998) have been effective to modify food texture, a new gel technology using e.g. 3D printing will be able to produce palatable and healthy foods from plant proteins. People demand the diversity. A flexible manufacturing system which can manage to produce small quantity but various kinds is required in production of foods. This is a characteristic feature demanded for food industry different from other industries where most products can be exactly the same and produced massively at a lower price as much as possible although artificial intelligence (AI) technology may be able to make custom-made products for each individuals in the future.
Since foods generally consist of multiple components, and are changing comparatively rapidly in comparison with other industrial products made of hard materials such as metals, ceramics, and synthetic plastics, therefore the shelf-life is comparatively shorter. While super strong and unchangeable products are required in the other industry, food should be fragile in a sense that it should be chewable and digested by enzymes to be processed in oral and gastrointestinal organs.
It is well known that not only the molar mass but also the structural characteristics such as linkage mode of glucose in polysaccharides make the performance diversity even for similar molar mass compounds. Functional proteins have their specific molar mass because their role in the life phenomenon is designed naturally. Therefore, it seems to be absurd to dare to write an overview on the effects of molar mass in food quality and health related properties. The authors tried to find some common characteristics or rules governing the mechanism which could be applied to other materials or phenomena within food and health problems.
Molecular weight (MW) is a term most widely used although IUPAC recommends to replace it with molar mass. As an intrinsic characteristic quantity of a matter, it should not depend on the gravity and should be the same on the earth and on the other planet. However, many papers have been using the former term. In some communities, a concept of the molecular weight is used to represent the mass of the particle even if this particle is composed of different molecules. Starch is known to consist of different molecules, a linear molecule amylose and a branched molecule amylopectin, but many starch chemists use a term starch molecule or a term molecular weight of starch. Strictly speaking, a term starch molecule should be “molecules constituting a starch”. Although the detailed discussion of the definition of molar mass or molecular weight is not a main topic of this paper, it is important to clarify the definition of molar mass especially when the structure and properties of complex heterogeneous molecules extracted from natural resources are discussed.
1.1. Developing new palatable and healthy foods
Omission test can identify necessary taste ingredients to make an imitation food such as scallop, crab, sea urchin. However, the combination of these tastants in liquid does not give the pleasure or satisfaction for a man to actually chew a real food. Successful examples are kanikama (crab stick) and ikura (salmon roe) where texture is reproduced. Omission tests were done by Watanabe, Lan, Yamaguchi, and Konosu (1990). They found eight taste-active components, glycine, alanine, glutamic acid, arginine, adenosine monophosphate, sodium, potassium, and chloride ions and stated that glycogen enhanced continuity, complexity, fullness, mildness and thickness. Since glycogen is a highly branched polysaccharide, the molar mass is a determining factor of its thickening function. The necessity of inorganic ions to make imitation foods realistic is understood by the cross modal interaction of different sensations and psychophysical effects, which will be discussed in section 9 Molar mass effect on flavour intensity.
The concept of koku was recently re-visited by Nishimura and Egusa (2014) and koku was defined as a phenomenon perceived when humans feel complexity, mouthfulness, long-lastingness with a good balance between taste, odor and tactile sensation. A simple taste such as pure sweetness or bitterness was excluded from koku. The calcium sensing receptor (CaSR) was found as the receptor for koku by Ajinomoto group (Ohsu et al., 2010) who found that various extracellular CaSR agonists enhance sweet, salty, and umami tastes, although they have no taste themselves. Nishimura et al. (2016) reported that the enhancing effect of retronasal aroma by umami compounds. Although this term koku was originally a Japanese word like umami, it was used now internationally, for example, Dunkel, Koster, and Hofmann (2007) found that a nearly tasteless aqueous extract consisting of γ-glutamyl-cysteinyl-β-alanine and some other compounds extracted from beans (Phaseolus vulgaris L.) enhanced its mouthfulness and complexity and induced a much more long-lasting savory taste sensation on the tongue when added to a model chicken broth. They stated that it was exactly the same koku what was proposed by Japanese research groups. Recently, the addition of a kokumi peptide, γ-Glu-Val-Gly was found to improve the reduced-fat peanut butter (Miyamura, Jo, Kuroda, & Kouda, 2015) and chicken consommé (Miyaki, Kawasaki, Kuroda, Miyamura, & Kouda, 2015). A research group of Kyoto University and Fuji Oil Ltd recently reported that the combined use of γ-glutamyl peptides and oligosaccharides, raffinose and stachyose, present in soybeans can increase the kokumi intensity, which suggests that soybean extracts or soymilk can be used to enhance the kokumi taste sensation in food products (Shibata et al., 2017). These papers described successfully the substances which are useful to design palatable foods, but it is necessary to add the viewpoint of the texture which contribute to especially the important factor of koku, the continuity and fullness. This was already pointed out by a pioneering work of Watanabe et al. (1990) which stated that the glycogen, a polymer not a small molecule, played an important role to make a scallop taste. Molar mass problem is involved in physical and chemical aspects in designing koku or palatability. A systematic understanding of the kind of amino acids, their arrangement order, and their length as well as their effects on the viscosity is required for the design. To make the taste research more practical, the texture should be taken into account, and also the role of salt should be understood from the viewpoint of taste-odor, or taste-taste interaction. This will be discussed later in section 9 Molar mass effect on flavour intensity.
Granular protein can prevent the shrinking and dripping of liquid, during frying, of hamburger steak consisting of minced meat with granular protein. Size and shape of this granular soy protein can be controlled by changing the extruder outlet, composition of raw materials, running condition of extrusion (Isobe & Noguchi, 1988). Not only the fibrous texture but also juiciness is an important texture characteristics to produce an authentic meat mouthfeel (Puolanne, 2017; Warner, 2017).
Low molar mass and high molar mass emulsifiers act differently on the surface of oil droplets. While low molar mass emulsifiers may adsorb faster on the surface of oil droplets because their diffusion coefficient is larger than that of high molar mass ones, high molar mass emulsifiers may show a steric stabilisation effect (Dickinson, 2009). The steric repulsion is dependent not only on molar mass of the polymer forming layers surrounding droplets but also on the conformation and branching (Dickinson, 2018).
1.2. Why molar mass?
All the hydrocolloid scientists know the importance of molar mass, for example, to control the viscosity of fluid foods, high molar mass polysaccharides can increase the viscosity at a very low dose (concentration) if the conformation is not completely random coil. Random coil polysaccharides such as pullulan and dextran are not effective to increase the viscosity. The solution viscosity of low molar mass saccharide is much lower than that of higher molar mass saccharide, i.e. polysaccharide. Though there have been many papers studying food processing or health related problems by changing the dose (concentration) of different polysaccharides without controlling the molar mass, it is evident that it is not possible to compare the concentration dependence or the different effects of these polysaccharides when the molar mass of each polysaccharide is different. In the industrial application of these thickening and gelling polysaccharides, the solubility and the hydration rate and extent are important, which are related to the molar mass and other structural characteristics. In electrolytic copolymers such as pectins and alginates or chitosans, the degree of blockiness, type of cations/anions, the position of cations/anions, degree of substitution (methoxylation/acetylation) are also determining factors of the function. In cellulose derivatives, the degree of substitution and its position and length are determining factor of the function. Although the conformation or the stiffness of polysaccharide chain plays important roles in their performance, it is worthwhile to pay attention to the molar mass effect. In the present paper, the terms molar mass, molecular mass, and molecular weight represented in Da (Dalton) or g/mol are used interchangeably. Since it is generally time/energy consuming to obtain food polymers with narrow molar mass distribution, most published papers used polydisperse polymers. Nevertheless, high molar mass and low molar mass have been shown to affect the function/property differently as will be discussed in the present review. The molar mass of some polysaccharides have not been determined with high precision because their structures are heterogeneous and difficult to purify to be subjected to molar mass determination.
Since fibrils of globular protein appeared, the concept of globular protein that forms a gel only at high concentrations changed drastically. It was reported that fibrils of β-lactoglobulin can form a gel at a very low concentration (Veerman, Sagis, & van der Linden, 2003) and the transglutaminase was shown effective to enhance the gelling ability at low protein concentration (Wu, Nishinari, Gao, Zhao, Zhang, Fang, et al., 2016). It is necessary to check the safety of fibrils of β-lactoglobulin as food materials, and Bateman, Ye, and Singh (2010) showed that BLG fibrils, prepared by heating at pH 2 and 80 °C for 20 h, could be digested in simulated gastric fluid. They found that the fibrils were digested completely by pepsin within 2 min and that the peptides in the fibrils with molar mass of 2000–8000 Da could be digested to smaller peptides (mostly < 2000 Da) by pepsin.
Since the publication on the harmful effect of lower mass carrageenan (Tobacman, 2001), it became an active research area (McKim, Willoughby, Blakemore, & Weiner, 2019). McKim et al. (2019) criticizes the assertion of Tobacman (2001) stating that the induced tumour promoting effect of carrageenan reported by Tobacman and her supporters is based on an unrealistic experiment using a very low molar mass carrageenan prepared in a non-physiological condition at a higher temperature > 80 °C and at a very low pH 0.7–1.3, and such a harsh degradation never occurs in normal digestion process in human gastrointestinal organs. This will be discussed later in 6.5.4. Here, the molar mass seems to be a key word to understand the safety issues.
In pharmaceutical/medical sciences, structure/function of heparin has been studied extensively since its discovery hundred years ago mainly because of its anti-thrombin action. Heparin is a naturally occurring sulphated polysaccharide with MW ranging from 5 to 40 kDa belonging to the family of glycosaminoglycan. Since forty years ago, low molecular weight heparin (LMWH) prepared by chemical or enzymatic hydrolysis or ultrasonic degradation has attracted more attention because of its stronger activity. Since the structure of heparin extracted from different sources is heterogeneous, and difficult to clarify the structure/function relation, chemoenzymatic synthesis has been performed to make a better product (Hemker, 2016; Oduah, Linhardt, & Sharfstein, 2016; Wang, Liu, & Voglmeir, 2020). This research area is vibrant and a plethora of papers have been published, 3895 papers in 2019, 2171 papers in 2020 and 15 papers in 2021 (heparin × molecular weight) according to Science Direct. It was reported recently that LMWH reduced the mortality in severe COVID-19 patients (Tan et al., 2020) although the optimum molar mass was not yet determined.
In the nutrition science, it was believed that proteins are hydrolysed into amino acids to be absorbed at intestinal walls. Ejima, Nakamura, Suzuki, and Sato (2018) and Ejima, Yamada, and Sato (2019) reported, however, that di- or tri-peptides were detected in human plasma after ingestion of maize and wheat gluten hydrolysates, and aspartic dipeptides in rat plasma after administration of liver protein hydrolysates. This detection became possible by adopting a new high-sensitive method liquid chromatography-electrospray ionization-tandem mass spectrometer (LC-MS/MS) via multiple reaction monitoring. The same research group led by Sato (2017) detected di-peptide in human plasma after ingestion of collagen or elastin hydrolysates. They emphasize the importance of detecting bioactive compounds such as dipeptides in the body because it can be related with the biological response upon ingestion of the protein hydrolysate.
A similar situation is developing in the other part of the body. Synovial fluids play a key role for the smooth movement of joints of knee or any other part conferring the lubrication as well as shock absorption. Hyaluronan (also called hyaluronic acid, HA) is a main ingredient, also occurring in connective, epithelial, and neural tissues e.g., in eyes, umbilical cord, skin, blood vessels, heart valves (Laurent & Fraser, 1992). Many elderly persons suffer from knee joint pain from osteoarthritis (OA), and the traditional therapy was an injection of hyaluronic acid (Balazs & Denlinger, 1985). Since hyaluronan is hydrolysed by endogenous hyaluronidase and cannot function for a long time, cross-linked hyaluronan was introduced, which can keep longer (Balazs & Denlinger, 1985). HA a copolymer consisting of glucuronic acid and N-acetyl-glucosamine, and occurring naturally, but is also produced by microbial fermentation. Its molar mass is reported to be increased with increasing UDP-N-acetylglucosamine (UDP = uridine diphosphate) concentration (Chen, Marcellin, Hung, & Nielsen, 2009). Physiological functions of HA have been found strongly influenced by molecular weight (MW) and some examples are discussed in section 6.4.
Since human skin plays a role of barrier to prevent the penetration of harmful molecules, higher molar mass compounds above 500 Da are believed to be not able to penetrate (Bos & Meinardi, 2000). Recently, the topical cream on the knee was proposed, and oral therapy, i.e. hyaluronan as a healthy food was commercialized. In a traditional nutrition science, it is believed that all the nutrients are digested into monomers and absorbed in the intestine. Oe et al. (2016) summarised a recent study on the efficacy of ingested HA in treating knee pain based on data from randomized, double-blind, placebo-controlled trials as well as the mechanism of action and safety of dietary HA.
In all therapies, injection, topical, oral ingestion, the molar mass is recognized as an important factor. The increase in molar mass of the permeant (Fluorescein isothiocyanate-dextran) was found to decrease the transport (Medi, Layek, & Singh, 2017).
Electroporation method as a transdermal drug delivery which allows the penetration of higher molar mass drugs has been attracting attention (Kawai et al., 2008; Kawai, 2012; Lombry, Dujardin, & Preat, 2000) (Fig. 1 ).
Fig. 1.
Molar mass of a hydrocolloid molecule increases when it is reconstructed or synthesized while it decreases when it is degrade or digested.
Since food is a complex material consisting of many ingredients, and the human body also consists of various parts and is a complex system in which each part interact each other. In each specific problem, the degradation and reconstruction of molecules are governed by specific enzymes and thus different problems cannot be compared directly each other, but methods of study are sometimes similar thus a study on one problem can learn from another study, and finally the total aspects should be overviewed to solve each specific problem. It is the authors’ hope that each specialists share their own area with other areas to collaborate to find a better solution.
The viscosity of the liquid foods is in most cases determined by food macromolecules and the molar mass is the determining factor together with the structural characteristics, conformation, molecular shape, stiffness, and degree of branching. Some problems of the mixture of high molar mass and low molar mass polymers, which have an important practical significance in medical problems such as hyaluronate related with knee pain (in 6.4), and alginate related with cystic fibrosis (in 6.5), and also the interaction between polysaccharides and proteins in food industry are also discussed.
There have not been many studies on the effect of molar mass distribution by mixing monodisperse polymers because it is time/energy consuming to get well fractionated polymers with wide molar mass range. The elasticity of solid or semi-solid, gel-like foods is also dependent on molar mass and also the structure of junction zones, the network density and the elastically active network chains, which are also dependent on molar mass. Electric charges, ionic strength and pH are also important factors, and therefore the effect of molar mass is not isolated from these factors. Nevertheless, it is expected that extraction of common features in various phenomena which are strongly influenced by the molar mass of polymers which play an important role is useful. From this overview, unresolved problem may find a clue from the other problems. Although it is difficult to find a general rule to understand the role of molar mass in food characteristics and disease therapy, effects of molar mass on structural formation and breakdown in food and health are overviewed in the present paper.
1.3. How to control the molar mass?
There are many methods to obtain or analyse polysaccharides and proteins with different molar masses (Table 1 ). Each methods have advantages and disadvantages. For example, oligomers of sulphated polysaccharides have been attracted much attention because of their biological activities, and depolymerised by ultrasonication, gamma-irradiation, acid or enzymatic hydrolysis. Sulphate groups tend to be removed by acid hydrolysis, while the structure of the repeating units was retained by gamma-irradiation in fucoidan (Choi & Kim, 2013). In another report, the sulphate content of fucoidan slightly increased by ultrasonic degradation (Guo et al., 2014). In ultrasonic degradation of xanthan, removal of pyruvate groups from native xanthan increased the thermal stability keeping the helical conformation, and lowered the sensitivity of molecular conformation to the salt concentration, and thus led to lower degradation efficiency (Li & Feke, 2015).
Table 1.
Methods of molar mass control.
Some methods of molar mass change, decrease or increase, applied to polysaccharides and proteins in food and health industries are shown in Table 1.
2. Molar mass effect on the viscosity and diffusion
2.1. Molar mass effect on the viscosity
The intrinsic viscosity (also called limiting viscosity number) is often determined by an Ubbelohde type capillary viscometer. The relative viscosity is defined as the ratio of the viscosity of the solution to that of the solvent η rel = η/η s , and the specific viscosity (relative viscosity increment) η sp = (η -η s)/η s = η rel -1 per unit concentration is extrapolated to zero concentration to obtain the intrinsic viscosity [η] = Lim (C→0) η sp/C. The intrinsic viscosity has a unit of the inverse of the concentration, and represents the volume the polymer occupies in the solution. Therefore stiff chains or an expanded chain molecules show a high intrinsic viscosity value while compact or less expanded molecules show a lower value.
The relation between the intrinsic viscosity and molar mass of polymers, called Mark-Houwink-Sakurada (MHS) equation, is written as
| [η] = K Ma |
Where K and a depend on solvent quality, a < 1/2 for poor solvent, a = 1/2 for θ solvent, a > 1/2 for good solvent (Doi, 2013; Tanaka, 2011). The MHS exponent a is reported as 0.5 for amylose (in 0.33 M KCl), dextran (water), and 0.8 for stiff chains such as locust bean gum, 0.8–1.1 for anionic polysaccharides such as carboxymethyl cellulose, alginate, κ-carrageenan, xanthan, pectin (Walstra, 2003). Solution properties of pullulan was extensively studied (Kawahara et al., 1984; Buliga & Brant, 1987; Nishinari et al., 1991) and the reported MHS exponent 0.65 was coincident in all the references. The MHS exponent of guar gum was reported as 0.51 (Funami et al., 2005a,b) while that of fenugreek was 0.67 (Funami et al., 2008a,b). Polymers with larger values of [η] are used for thickening agents because they can increase the viscosity of the solution by the addition of a small quantity. The concentration (C) dependence of the viscosity of polymer solutions is usually represented by double logarithmic plot of the zero shear specific viscosity η sp vs coil overlap parameter, C[η], since this master curve was observed for many polysaccharides. The intrinsic viscosity has units of reciprocal concentration, so the product with concentration is dimensionless. Since the viscosity of polymer solutions is generally dependent on shear rate, the viscosity obtained by the extrapolation to zero shear rate is used to understand the concentration dependence systematically.
The critical coil overlap concentration C* is approximately given by 4/[η], and at this concentration the zero shear viscosity was found η sp ~ 10 (see Fig. 2 ). The slope above the critical concentration was found about 3.3 for most random coil polymers including the data for polystyrene in toluene, but this slope was approximately 4.4 for guar gum and locust bean gum for which the critical concentration was found a little bit lower than for the other random coil polymers. The deviation of the behaviour for galactomannans (guar gum and locust bean gum) may be due to specific attractive interactions between side groups on the polymer chains or the stiffness of polymer chains. It should be reminded that a typical behaviour shown in Fig. 2 is limited to the viscosity at very low shear rates.
Fig. 2.
Zero shear specific viscosity as a function of the coil overlap parameter, C[η], for a range of random coil polysaccharides. At C[η] ~ 4 the transition from dilute to concentrated solution behaviour is found for a number of random coil polysaccharides (Morris, Cutler, Ross-Murphy, Rees, & Price, 1981).
As typical shear rate dependence of the viscosity for the solutions of water soluble polymers, data for cellulose derivatives and cereal β-glucans are shown in Fig. 3 A and B. While all the glucose residues are linked by β-(1 → 4) in cellulose which is insoluble in water, in cereal β-glucans about one third of the linkages between the glucose residues are β-(1 → 3) linkages in addition to β-(1 → 4) linkages, and more soluble in water. Cellulose derivatives are soluble and have been studied extensively. For cellulose derivative compounds with different molar masses but approximately the same degree of substitution, the steady shear viscosity of 2% solutions as a function of shear rate is shown in Fig. 3A. A similar shear rate dependence of the steady shear viscosity for beta glucans from oat, barley, wheat flour and wheat bran is shown in Fig. 3B. Another example is shown for galactomannan solutions in Fig. 3C.
Fig. 3.
A. Shear rate dependence of the viscosity for 2% sulfoethylcarboxy-methylcellulose with different MW in 0.1MNaNO3 solution. MW (g/mol)/106: ■, 1.49; □, 0.86; ▲, 0.76; △, 0.68; ●, 0.44; ○, 0.22 (Clasen & Kulicke, 2001). B. Shear rate dependence of the viscosity for 2% cereal β-D-glucans with different MW in water, at 25 °C. MW (g/mol)/106: Oat □, 1.2; barley ■, 0.8; wheat flour ○, 0.4; wheat bran ●, 0.3 (Cui & Wood, 2000). C. Shear rate dependence of the viscosity for 1% solutions of galactomannans from Delonix regia with different MW 4.86 × 105 Da (♦), 4.15 × 105 Da ( ), 2.85 × 105 Da (●), and 1.95 × 105 Da (
), 2.85 × 105 Da (●), and 1.95 × 105 Da ( ). (Rodriguez-Canto et al., 2019).
). (Rodriguez-Canto et al., 2019).
The following features are noted: 1) the viscosity at lower shear rates increases with increasing molar mass; 2) the viscosity decreases with increasing shear rate, which is called shear thinning; 3) The viscosity of the solution of the lowest molar mass does not depend so much on the shear rate, and shows approximately a constant value at lower shear rates. This is called a Newtonian plateau. Because of the limited sensitivity of the rheometer, the viscosity at very low shear rates cannot often be measured. In this case, the viscosity of the solution of the lowest molar mass was not measured below the shear rate of 3 s−1 (Fig. 3A) or 0.1 s−1 (Fig. 3B) or 2 s−1 (Fig. 3C). It should be noted that some published papers erroneously showed a steep rise of the viscosity at lower shear rate with decreasing shear rate, which were probably caused by neglecting the low sensitivity limit of the sensor.
2.2. Molar mass effect on the diffusion
Diffusion is a ubiquitous phenomenon of motion of molecules or particles in any phase (gas, liquid, or solid) caused by the concentration gradient. This is caused not by an external force but by spontaneous collision in Brownian movement (Doi, 2013; Tanaka, 2011). The diffusion is described by a Fick's equation
| ∂C/∂t = D ∂2C/∂x2, |
where C is the concentration (mol/m3), t the time (s), x the position (m) in one dimension. This can be generalized to three dimension.
Rate of diffusion of aroma compounds in a gas phase, carbon dioxide diffusion into egg white through the pore of shell during storage, that of seasonings such as salts, acid, and of sugars in pickling and salting as well as release of tastants in oral cavity, and release of nutrients and drugs in gastrointestinal tract, plasticizers motion in packaging films, and many others, …all play important roles in food and health.
Diffusion coefficient is given by Stokes-Einstein equation
| D = kT / (6πRHη), |
where k is the Boltzmann constant (1.38 × 10−23 J K−1), T is the temperature (K), R H is the hydrodynamic radius of a spherical particle (m), η the viscosity of the surrounding medium (Pa.s). This equation is valid only for an infinitely dilute solution, and for a particle which is larger than the solvent molecule.
The hydrodynamic radius R H is related with the radius of gyration R g = KM ν (Flory Equation, ν is called Flory exponent) by ρ = R g/R H.. For linear, flexible chains in the theta solvent ρ = 1.505, while in the limit of large excluded-volume effects (ν = 0.587) a value of ρ = 1.78 is obtained. Polydispersity increases this value by 14% (i.e. ρ = 2.05) for a most probable (Schulz-Flory) distribution with a polydispersity index PI, the ratio of weight average molecular weight M w and the number average molecular weight M n, PI = M w/M n = 2 (Burchard, 1994).
The Flory exponent ν is related with the mass fractal dimension d f by ν = 1/d f. Some typical values of ν and d f are known: ν = 0.6 (or 0.588), d f = 1.67 (or 1.70) for linear random coil polymer in good solvent), ν = 0.5, d f = 2.0 for linear random coil polymer behaving like a Gaussian chain in theta solvent, ν = 1.0, d f = 1.0 for rigid rod, ν = 0.5, d f = 2.0 for thin disc, ν = 0.33, d f = 3.0 for homogeneous sphere. The fractal dimension represents to which extent the space is filled, and therefore, completely packed sphere with no empty space shows d f = 3.0. When a rigid rod molecule rotates, it can sweep a large volume with low density and thus its d f is 1 much smaller than d f of a sphere (Burchard, 1994).
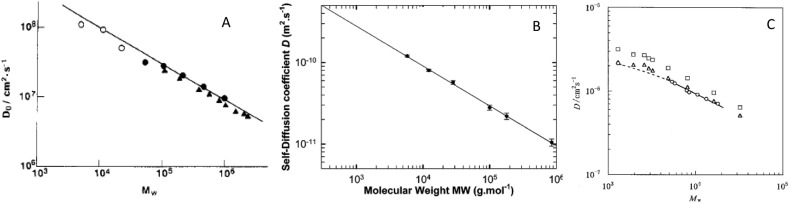
Diffusion coefficient of biomolecules has been measured by ultracentrifugation, dynamic light scattering (DLS), nuclear magnetic resonance (NMR), and fluorescence correlation spectroscopy (FCS). Globular protein molecules have been approximated as spheres, and thus the diffusion coefficient is expected to be related with molar mass as follows: D ~ M -ν, where the exponent ν is theoretically estimated as 0.33 for a sphere.
Summarizing many experimental data for globular proteins, ν = 0.39 and d f = 2.56 was found (Evans, 2020; Augé et al., 2009; Enright et al., 2005). Globular proteins are an example of a class of chemical species where values of ν tend towards 0.33 while the ν value measured in strongly denaturing solutions increased tended to approach 0.6, similar to the exponent expected for a random coil polymer in a good solvent as mentioned above. This difference in the ν value can be used to distinguish between folded, disordered and denatured proteins (Evans, 2020). Dudás and Bodor (2019) acquired diffusion coefficients of 12 globular proteins and 10 intrinsically disordered proteins (IDPs). They reported ν = 0.382 for native folded globular proteins, and ν = 0.492 for intrinsically disordered proteins (Fig. 4 ). The value close to 0.5 indicated that the elongated and loose structures similar to a random coil polymer shown below for pullulan (0.51 by Nishinari et al. (1991); 0.49 by Viel, Capitani, Mannina, and Segre (2003) and poly(ethylene oxide) (0.52–0.55 by Hakansson, Nyden, and Soderman (2000)).
Fig. 4.
Logarithmic representation of diffusion coefficients as a function of molecular weight for folded proteins (red circles), IDPs (blue squares), viscosity corrected denatured folded proteins (open green circles), and IDPs (open green squares) (Dudás et al., 2019).
Pullulan has been studied extensively as a water soluble, model random coil polysaccharide. The diffusion coefficient of pullulan as a function of molar mass is shown in Fig. 5 A and B. The exponent ν was found as 0.51 (Nishinari et al., 2016). The exponent 0.38–0.41 was reported for hydroxyethyl starch (Kuz’mina, Moiseev, Krylov, Yashkir, & Merkulov, 2015) indicating the deviation of these molecules from random coil conformation.
Fig. 5.
A. Double-logarithmic plot of DO against MW for pullulan samples at 25 °C: (●) from PCS, (○) from the classical boundary formation, Nishinari et al. (1991), (▲) (Kato et al., 1984). B. Double-logarithmic plot of D against MW for six pullulan fractions as determined by DOSY experiments recorded at 300 K in dilute D2O solution (Viel et al., 2003). C. Double-logarithmic plot of D for cycloamylose in 0.5 N aqueous NaOH at 25 °C (○). □, Monte Carlo data; △, Monte Carlo data recalculated with two glucose units taken as one bead and corrected for the effects of bead diameter and fluctuating hydrodynamic interaction (Nakata et al., 2003).
Cycloamylose, a cyclic (1 → 4)-α-D-glucan is a large ring cyclodextrin (CD) (cyclic (1 → 4)-α-D-glucans with DP = 6, 7 and 8 have been known as α-CD, β-CD, and γ- CD described later in 9.3.2 Effect of molar mass of carriers on the retention of aroma compounds) has been attracting much attention (Fenyvesi & Szente, 2016). Nakata, Amitani, Norisuye, and Kitamura (2003) studied the conformation of seven cycloamylose samples with weight-average molecular weight from 5 × 103 to 1.8 × 104. They determined the MW and diffusion coefficient D for cycloamylose samples from sedimentation equilibrium (in dimethylsulphoxide) and by dynamic light scattering (in 0.5 N aqueous NaOH). Dependence of D on MW is shown in Fig. 5C. The exponent α was found as 0.53. They noticed, taking into account that correction is made for the effect of fluctuating hydrodynamic interaction, that measured translational diffusion coefficients in the aqueous NaOH agreed fairly well with Monte Carlo data and also with the prediction from the Yamakawa–Fujii theory for the associated Kratky-Porod ring combined with relevant theoretical expressions for the expansion factors (Nakata et al., 2003).
The Stokes-Einstein equation is valid only for infinitely dilute solution. For finite concentration, a corresponding relation
| Dc = kT / (6πξHη) |
Where ξ H is hydrodynamic correlation length, should be used (De Gennes, 1979). Diffusion coefficient of pullulan was found to decrease with increasing concentration for lower MW (5.5 kDa −23.8 kDa) observed by ultracentrifuge and for higher Mw (53.9 kDa–478 kDa) observed by PCS (Nishinari et al., 1991). Diffusion coefficient of αS- casein as a function of concentration is shown in Fig. 6 A (Kusova, Sitnitsky, Idiyatullin, Bakirova, & Zuev, 2018). This dependence can be generalized by scaling as shown in Fig. 6B (Nesmelova, Melnikova, Ranjan, & Skirda, 2019).
Fig. 6.
A. Concentration dependences of the protein self-diffusion coefficient (D) as a function of protein volume fraction for αS-casein at 30 °C (squares), 5 °C (circles) (Kusova et al., 2018). B. Concentration dependence of the diffusion coefficient of αS -casein (red squares), the master curve representing the concentration dependence of the diffusion coefficient for globular proteins (black symbols) and for flexible polymers (light blue line), and the theoretical concentration dependence for Brownian rigid spheres (blue line). Concentration is normalized by a critical volume fraction φ0. The asymptote with zero slope is shown by black dashed line. All curves are normalized by the procedure that merely leads to the shift of the concentration dependence along the horizontal and vertical axes (Nesmelova et al., 2019). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
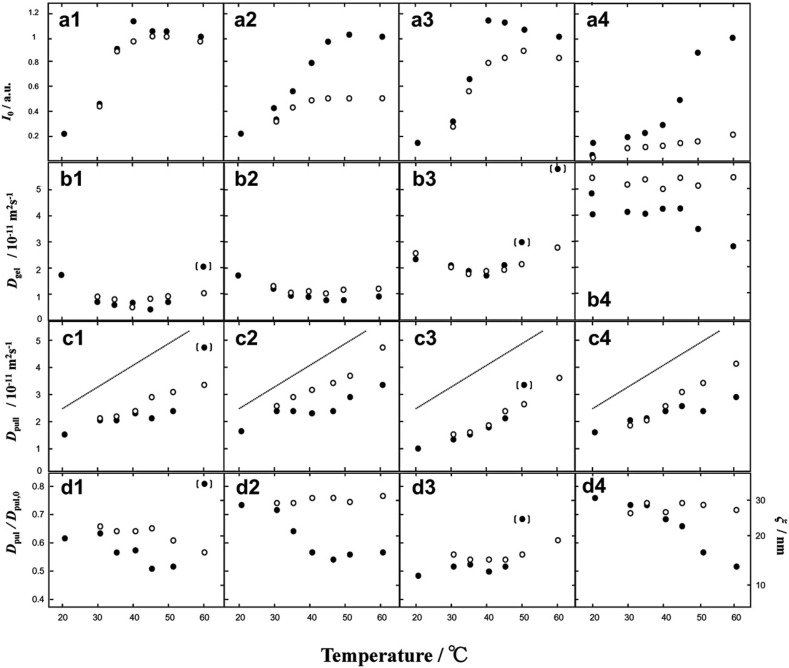
Diffusion coefficient of probe molecules introduced in gelling polymers was used to estimate the mesh size of gel networks. Shimizu, Brenner, Liao, and Matsukawa (2012) using pullulan (MW112 kDa) studied the gelation of potassium type deacylated gellan by a pulse-gradient-stimulated-echo (PGSTE) pulse sequence. The echo signal intensity I 0 = I(0) of a methyl proton in rhamnose residue in gellan in the absence of field gradient decreased at T I(0), 36, 43, 38 and 45–50 °C for 2 mM CaCl2, 5 mM CaCl2, 40 mM KCl and 80 mM KCl, respectively (Fig. 7 ), that reflects the decrease in total random coil content and the helix formation and the aggregation, in agreement with results obtained by circular dichroism (CD) measurements (Ogawa et al., 2006; Morris, Nishinari, & Rinaudo, 2012). The diffusion coefficient of gellan chains, D gel, for solutions with 2 mM CaCl2, 5 mM CaCl2 and 40 mM KCl remained nearly constant during cooling down to T I(0) and increased with further cooling, as shown in Fig. 7b. The decrease of solute gellan concentration at T I(0) might cause a decrease in the local viscosity because solubilized random coil gellan molecules were incorporated into helices and their aggregates. The increase of D gel at T I(0) was more pronounced than expected from the decrease of local viscosity. Therefore, it is considered that the MW distribution of the gellan remaining as a random coil as a solute among the network of aggregates was shifted to lower MW values because higher molar mass gellan chains were preferentially incorporated to junction zones than lower MW chains. This is consistent with the observation that shorter chains preferentially eluded out from gellan gels when gels were immersed in solvents (Hossain & Nishinari, 2009). On the other hand, D gel for the solution with 80 mM KCl showed a different behaviour, that is, D gel became higher with decreasing temperature down to T I(0), remained almost constant with further cooling, increased during holding time at 20 °C (12 h) and remained almost constant during reheating. This behaviour probably reflects the collapse of the solute gellan chain, which is caused by effective shielding (high salt concentration) of repulsion between negative charges on the carboxylate ions (Ogawa et al., 2006).
Fig. 7.
Temperature dependence of the NMR echo signal intensity at 1.3 ppm, I(0), the echo signal intensity without the gradient pulse, for gellan (a), diffusion coefficient of gellan Dgel (b), diffusion coefficient of pullulan Dpull (c) and Dpull/Dpull,0 (d) in 1% gellan solution containing 0.1% pullulan, and the following ionic compositions: 2 mM CaCl2 (1), 5 mM CaCl2 (2), 40 mM KCl (3) and 80 mM KCl (4). Closed circles, cooling; open circles, heating. Data points inside brackets indicate D values that are overestimated by convection. The solid straight lines indicate Dpul in dilute solution. The value ξ at the right vertical axis in (d) is the hydrodynamic mesh size (Shimizu et al., 2012).
The diffusion coefficient of pullulan decreased with decreasing temperature, and it was concluded that pullulan chains were not involved directly in the aggregation of gellan, and remained dissolved during the gelling process, probably due to the high solubility of pullulan in water. The diffusion of pullulan is thought to be restricted by the hydrodynamic interaction with the solute gellan as well as the network of aggregates. The restriction became smaller with decreasing solute gellan concentration and increasing network pore size, both of which result from the thickening of the aggregates.
Since probe molecules (pullulan) in the solution have no intermolecular interaction with the host polymer (gellan) except the hydrodynamic interaction, the diffusion is expressed as follows (Cukier, 1984; De Gennes, 1979; Matsukawa & Ando, 1996):
| DinHost/DinPure = exp (-Rh/ξ) | (4) |
Where D inHost and D inPure are D of the probe molecule in the host polymer solution and that in pure dilute solution, respectively, and ξ is the hydrodynamic mesh size which represents the hydrodynamic mesh size made of the solute gellan and the network of aggregates. Values of ξ are shown in the right vertical axis in Fig. 7d. The change in the microscopic surroundings of pullulan during the cooling process is shown schematically in Fig. 8 .
Fig. 8.
A schematic representation of the microscopic environment of pullulan above (a), at (b) and below (c) TI(0) (Shimizu et al., 2012).
When a physical polymer gel is immersed in a solvent, molecular chains which are not connected to the junction zones were found to release out from the gel to the solvent (Djabourov et al., 2013). It has been observed that the immersion of potassium type deacylated gellan gels in water or electrolyte solution induces chain release, and this release is more noticeable for shorter chains. Ultimately the gel becomes eroded and then disintegrates, and the rate of collapse depends on polymer concentration, original molecular mass and the initial salt content of the gels and the solvent. Salt diffusion from the gels into the solution is faster than chain release; chains which lose condensed or bound ions cannot retain a helical conformation, and so they diffuse out into the solution. The storage Young's modulus E′0 of gellan gels immersed in various solvents was observed as a function of time. For the first 3 h, E′0 increased both in water and in Me4N Cl solution, a solution which inhibits the helix aggregation of gellan. Subsequently, E′0 for a gel immersed in water decreased because of the release of chains contributing to the network (Hossain & Nishinari, 2009). Funami et al., 2008a, Funami et al., 2008b reported that a gellan gel collapsed after 8 h immersion in pure water. They estimated the diffusion coefficient (1.6–2.8) × 10 −11 m2 s−1, which was slightly larger than diffusion coefficient ranging from 0.6 × 10 −11 m2 s−1 to 1.0 × 10 −11 m2 s−1 at 25 °C in 25 mM NaCl for molar mass range from 2.2 × 10 5 to 1.3 × 10 5 reported by Takahashi et al. (2004). This is reasonable since molecular chains released out from the gel network were shorter than un-released chains.
De Silva, Poole-Warren, Martens, & in het Panhuis (2013) studied the chain release of also deacylated but Ca2+ cross-linked gellan gels at 37 °C in a phosphate buffer saline (PBS). These authors also found the chain release as mass loss up to 168 days. Since the CD ellipticity was proportional to the gellan concentration, they estimated the diffusion coefficient of gellan from the CD data D = 1.1 × 10 −13 m2 s−1, which was two orders of magnitude smaller than the reported value of deacylated gellan at 40 °C (Takahashi et al., 2004). They ascribed this difference to the retardation of the mobility of gellan molecules in gel network.
3. Molar mass effect on mechanical properties of gels and solids
Molar mass is a key for the gel formation. When the material changes from sol state to gel state, the molecules are connected each other spanning the whole space in the vessel, and this is called the percolation at which the molar mass is thought to diverge to infinity (De Gennes, 1979; Nishinari, 2009; Tanaka, 2011; Tokita, 1989). Gelling polysaccharides and proteins have been used widely for controlling the food texture. Mechanical strength of gels can be indexed by the elastic modulus and/or fracture stress/strain. Although the oral processing is a dynamic process, that is time dependent, only the fracture stress has been used to characterize the mechanical properties quite often neglecting the intermediate stress/strain of the compression/shearing in the mastication. For example, in fishery industry in Japan a so-called “gel rigidity”, which was defined as the ratio of the force to deformation at break using a spherical probe of 5 mm diameter, has been used widely. Though it may have some merit, it does not distinguish two concave and convex curves connecting the origin and the break point in the force-deformation plot. We hope that these distinction will be taken into account in the near future.
3.1. Minimum chain length for helix formation
Since the average number of residues per helical turn to form α-helix has been established as 3.6, and 13 atoms are involved in the ring formation by hydrogen bond, α-helix is also called 3.613-helix. The average number of residues per helical turn for other protein helices is also determined as 3.0 for 310-helix, and 4.4 for π-helix (https://biomedapps.curtin.edu.au/biochem/tutorials/prottute/index.htm , Interactive Protein Structure Tutorial, Retrieved Feb 2, 2020).
While the helix structure is studied extensively in proteins, that is not so much studied in polysaccharides. The helix formation is the prerequisite for gel formation for agarose, gellan, carrageenan (double helices) , schizophyllan (triple helices), however the minimum molar mass required to form a double helix or triple helix has not been so much studied because of the difficulty to get samples. For gellan, Ogawa et al. (2006) using 6 sodium type samples of different molar masses almost free from cations did not observe the step-wise increase in the intrinsic viscosity for the lowest molar mass sample with decreasing temperature (Fig. 9 A). It was thought that the chain length was too short to form double helices. This assertion is corroborated by DSC thermograms which ceased to show exothermic peaks (Fig. 9B) or endothermic peaks on cooling or heating below a certain molar mass and by circular dichroism experiments. The DSC exothermic peak accompanying the coil to helix transition shifted slightly to lower temperatures with decreasing molar mass which can be fitted with a zipper model (Nishinari, Koide, Williams, & Phillips, 1990).
Fig. 9.
A. Temperature dependence of intrinsic viscosity for Na-gellan aqueous solutions with 25 mmol NaCl. Molar mass G1 > G2 > G3 > G4 > G5> G6 (Ogawa et al., 2006). B. DSC cooling curves for 1% Na-gellan aqueous solutions with 25 mmol NaCl. Molar mass G1 > G2 > G3 > G4 > G5. No peak was observed for G6. (Ogawa et al., 2006). C. Temperature dependence of specific optical rotation [α]300 of κ-carrageenan oligomers with different DPs and polymer at 300 nm in the presence of 2.5 M KCI (Rochas, Rinaudo, & Vincedon, 1983).
A similar tendency was reported for κ-carrageenan. Rochas, Rinaudo, & Vincendon (1983) found that the optical specific rotation [α] at 300 nm did not increase with lowering temperature for oligomers with DP < 4 (Fig. 9C) which was corroborated by 13C NMR observation.
Stokke, Smidsrød, Zanetti, Strand, and Skjåk-Bræk (1993) estimated eight contiguous guluronic residues were necessary to form an egg box junction in the presence of calcium ions, while Powell, Morris, Gidley, and Rees (1982) estimated at least 14 contiguous non-esterified galacturonic acid residues were required to form a stable dimeric junction zone in the gelation of pectin. Bowman et al. (2016) confirmed the previous result of Stokke et al. (1993) by AFM observation.
3.2. Gelation of amylose and oat β-glucan with different molar masses
Amylose gelation is known to be a triggering in the early stage of retrogradation of starch, but only a few studies have been published on the gelation kinetics using amylose samples with different chain lengths (Clark, Gidley, Richardson, & Ross-Murphy, 1989). Fig. 10 A shows the gelation process of 2% solutions of amylose with different chain lengths. Storage shear modulus G′ increased fast and then reached a plateau value earlier in lower molar mass samples. The plateau value increased with increasing molar mass within the DP range from 250 to 1100 however the highest molar mass DP 2550 still continued to increase even after 10,000 min (Clark et al., 1989).
Fig. 10.
A. Storage shear modulus G' (10 rad/s) vs time for 2% aqueous amyloses having chain lengths (DP) as shown beside each curve. Hot solutions were introduced in rheometer which had been kept at 25 °C beforehand (Clark et al., 1989). B. Time dependence of G' (thick line) and G″ (thin line) for oat β-glucan (OGL) solutions (10% w/v) with different molar masses (35, 65, 110, 140 kDa) (frequency 1 Hz, 25 °C) (Lazaridou et al., 2003).
Cereal (Oat, barley, wheat) β-glucans are linear homopolysaccharides composed of d-glucopyranosyl residues (Glcp) linked via a mixture of β- (1 → 3) and β-(1 → 4) linkages (Lazaridou et al., 2003). The chain consists mainly of β-(1 → 4) –linked β-d-glucose in blocks that are separated by single (1 → 3)-linkage. The resultant structure is a polysaccharide built mainly from β-(1 → 3)-linked cellotriosyl (58–72%) and cellotetraosyl (20–34%) units, but a minor amount of sequences with consecutive (1 → 4) linkages longer than the tetraose type and up to 14 glucosyl units (Cui & Wood, 2000). Lazaridou et al. (2003) extracted and purified oat β-glucans (OGL) and obtained five samples with different molar masses 35, 65, 110, 140 and 250 kDa. Gelation process of these OGL solutions is shown in Fig. 10B. Storage modulus G′ of lower molar mass samples showed faster increase and reached a plateau earlier than higher molar mass samples. The highest molar masse sample (MW 250 kDa) did not show any tendency to gel, i.e., G' < G″, under the same experimental conditions even after 200 h of storage (Fig. 10B, inset) (Lazaridou et al., 2003).
The concentration dependence of plateau modulus of amylose gels and β-glucan gels is shown in Fig. 11 . While the plateau storage modulus of amylose gels in the concentration range from 1 to 3%, the modulus is higher for higher molar mass than for lower molar mass (Fig. 11A), the modulus of β-glucan gels in the concentration range from 4 to 12%, the modulus is higher for lower molar mass than for lower molar mass (Fig. 11B).
Fig. 11.
A. Experimental G' (10 rad/s, 25 °C) values vs concentration (% w/v) for gels of amylose with different molar masses: (●) DP = 300, [□] DP = 660, (▲) DP = 1100. Theoretical fits obtained by using the Clark-Ross-Murphy approach (f = 3, 6, and 10) are indicated by broken lines (Clark et al., 1989). B. Concentration dependence of plateau value of G' (1 Hz, 25 °C) for gels of oat β-glucan with MW = 35 kDa (◇) and 110 kDa (△) (Lazaridou et al., 2003).
In amylose gelation, the plateau value increased with increasing molar mass as shown in Fig. 10A, while in β-glucan gelation, the plateau value seemed to decrease with increasing molar mass as shown in Fig. 10B, though for slow gelling samples with higher molar mass did not reach the plateau within 100 h. The apparent inconsistency between Fig. 11A and B, that the apparent plateau modulus increases with increasing MW in the former (amylose) while it is smaller for higher MW in the latter (β-glucan), originates from the non-equilibrium nature of these apparent plateau moduli. As shown in Fig. 11B, the gelation proceeds much slower in β-glucan than in amylose, it is impossible to compare directly with Fig. 11A and B. This reminds us the slow gelation rate of gelatin solutions. The storage modulus of 1.95% (w/w) gelatin/water increased faster at lower temperatures but it has not reached an equilibrium plateau but continued to increase even after 100 h (Nijenhuis, 1981). It is also necessary to take into account the molar mass range and in addition molecular conformation, flexible or stiff, when the modulus of different gelling polymers are compared as a function of molar mass. The effect of minor component of intermediate DP higher than 4 and a small amount of protein in β-glucan seems to be difficult to be quantified accurately, and is a future problem.
It should be noted that there is a critical molar mass and a critical concentration below which no gelation occurs. For amylose this critical molar mass was in between DP = 110 and DP = 250, and below DP = 110 only precipitates were observed. The critical concentration for gelation of amylose was found to depend on the molar mass (Gidley & Bulpin, 1989).
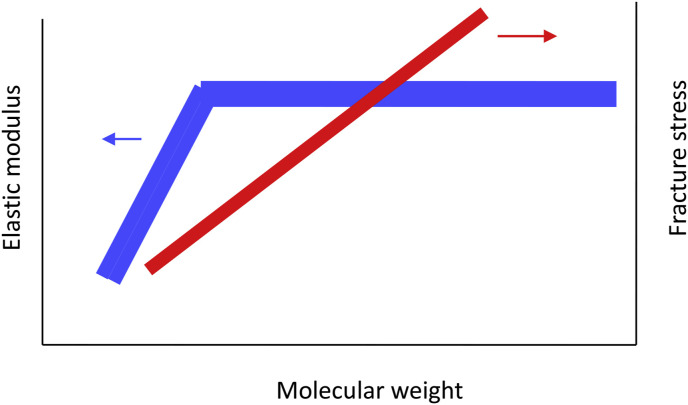
3.3. Elastic modulus and fracture stress of gels as a function of molar mass
Since it is difficult to prepare samples with different molecular weight and with a narrow molecular weight distribution, there have not been so many studies on the molecular weight dependence of elastic modulus of gels. Saunders and Ward (1955) studied rheological properties of gelatin gels with different molecular weights indexed by intrinsic viscosity, and showed that the elastic modulus increased with increasing molecular weight up to a certain value and then levelled off whilst the breaking stress continued to increase with increasing molecular weight (Saunders & Ward, 1955). More recent study (Eysturskarð, Haug, Elharfaoui, Djabourov, & Draget, 2009) on fish gelatin showed a similar tendency. In addition, Eysturskarð (2010) showed that the addition of lower mass gelatin decreased the elastic modulus of gelatin gels.
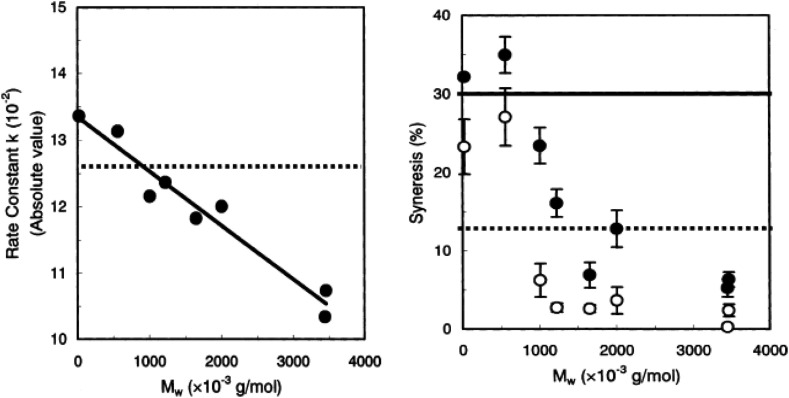
Shear modulus of alginate gels as a function of weight average degree of polymerisation was reported to show two regions; the initial steep ascending region and then levelled off and show a long horizontal region. Smidsrød (1974) showed this tendency for both alginate gels and κ-carrageenan gels. He suggested that the difference of the plateau values, independent of the DP, is due to the difference in the number or the strength of the junctions in alginate and κ-carrageenan. Twenty years later, the same group Draget, Skjåk Braæk, and Smidsrød (1994) reported the molar mass dependence of apparent Young's modulus E app and storage shear modulus G′ of gels of alginate. In the range of molar mass from 300 kDa to 700 kDa, they observed that both E app and G′ increased with increasing molar mass and did not show the molar mass independency as shown in Fig. 12 . It should be mentioned that they noticed that elastic modulus increased with increasing guluronic acid residues as reported previously from the same group (Smidsrød & Haug, 1972). This comparison is difficult because it is difficult to obtain the series of samples with the same molar mass and only different in the guluronic acid residue content. Quite a similar behaviour was reported for κ-carrageenan gels with different molecular weights (Rochas et al., 1990). These tendencies can be summarised as shown in Fig. 12. The elastic modulus increases steeply with increasing molar mass, and then it levels off above a certain molar mass.
Fig. 12.
Schematic representation of the relation between the elastic modulus of gels and molecular weight of gelatin, κ-carrageenan, and alginate based on Saunders and Ward (1955), Rochas et al. (1990), Smidsrød (1974), Mitchell (1980).
The temperature dependence of the elastic modulus has been attracting much attention. Whether the elasticity of agarose is entropic or energetic has been debated for a decade just after the 2nd world war in Japan (Nishinari, 2000a). Nishinari, Watase, and Ogino (1984) tried to separate the entropic term and energetic term from the observed temperature dependence of Young's modulus E′ for gels of agarose with different molar masses (indexed by the intrinsic viscosity) and with the same sulphate content and the 3,6 anhydro-l-galactose content, both of which are important factors influencing the helix and gel formation (Nishinari & Fang, 2017). The Young's modulus E′ increased with increasing molar mass and temperature for higher molar mass fractions (Fig. 13 A). The tendency observed for the increase of E′ with increasing molar mass is consistent with the above Fig. 12 for gels of gelatin, κ-carrageenan, and alginate. The elastic modulus was determined as dynamic Young's modulus E′ by observing longitudinal vibrations of a cylindrically moulded gel, which is free from slippage. While E′ decreased monotonically for a gel of agarose with a low molar mass (F1), E′ of other gels with higher molar masses (F2, F3, F4, F5) increased gradually from 5 °C up to a certain temperature T max, and then decreased (Nishinari et al., 1984). This temperature T max shifted to higher temperatures with increasing molar mass and concentration (Fig. 13A). It was found that the entropic part decreased while the energetic part increased with increasing temperature. The increase of E′ with increasing temperature was explained by a reel-chain model (Nishinari, Koide, & Ogino, 1985).
Fig. 13.
A. Storage Young's modulus E′ of gels of agarose with different molar masses MW(F1) < MW(F2) < MW(F3) < MW(F4) < MW(F5) as a function of temperature (Nishinari et al., 2016). B. Strain-Stress curves of 2.4% gels of agarose with different molar masses MW(f1) < MW(f2) < MW(f3) < MW(f4). Stress values were taken after 2 min strain (Watase; Nishinari, 1983)..
Stress relaxation of agarose gels with different molar masses were observed, and the relaxation spectra were obtained using time-temperature superposition (Watase & Nishinari, 1983). It was shown that box type spectra extended to longer relaxation times with increasing molar mass at a constant concentration, and that both breaking stress and breaking strain increased with increasing molar mass (Fig. 13B).
More recently, Moritaka. Yamanaka, Kobayashi, Ishihara, & Nishinari (2019) examined the size distribution of the masticated fragments of agarose gels, and found that the size distribution depended on molar mass as well as a mouthful size. As expected, the size of masticated fragments decreased with decreasing molar mass of agarose, which forms more brittle gels broken at lower fracture strains.
3.4. Molar mass effect of alginate, pectin, methylcellulose and konjac glucomannan in rheology control
Alginate is unbranched binary copolymer of (1 → 4)-β-linked-d-mannuronic acid (M) and α-l-guluronic acid (G), which occur in a blockwise distribution pattern containing M-, G- and MG-blocks (Draget & Taylor, 2011). Although an egg-box model proposed by a research group of Unilever Research has been widely accepted, the detailed mechanism of the binding of Ca2+ to alginate at a molecular level was not understood. Fang et al. (2007) examined the binding process by stepwise addition of small amounts of CaCl2 into dilute alginate solutions, of different molecular weights and mannuronate/guluronate ratios, and by the investigations using high-sensitivity isothermal titration calorimetry (ITC), Ca2+ -selective potentiometry, and relative viscometry. The results revealed three distinct and successive steps in the binding of calcium to alginate with increased concentration of Ca ions. They were assigned to (i) interaction of Ca2+ with a single guluronate unit forming monocomplexes; (ii) propagation and formation of egg-box dimers via pairing of these monocomplexes; and (iii) lateral association of the egg-box dimers, generating multimers. The boundaries between these steps were found critical, and they were closely correlated with the Ca/guluronate stoichiometry expected for egg-box dimers and multimers with 2/1 helical chains. In this multi-step binding of Ca to alginate, the dimerization process was found to be highly critical, only occurring when the stoichiometry of egg-box dimers is met, that is, Ca/G = 0.25, one calcium ion per four guluronate residues. The formation of egg-box dimers and their subsequent association are thermodynamically equivalent processes and can be fitted by a model of independent binding sites. In addition, the step (iii) showed different association modes depending on the molar mass of alginate. While the relative viscosity η r continues to increase in (iii) indicating that lateral association of egg-box dimers for lower molar mass alginate occurs between different dimers, ηr decreases in (iii) for higher molar mass alginate indicating the association makes the volume of aggregates smaller.
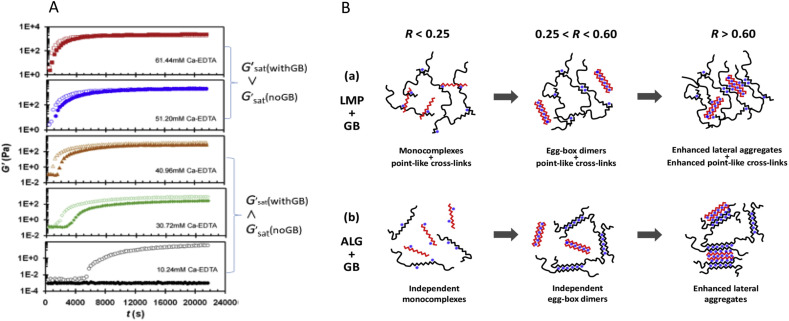
Fig. 14A shows the gelation profiles of 5 mg/mL ALG with and without addition of 5 mg/mL oligoguluronate, guluronate block (GB) at various concentrations of Ca-EDTA. It is well known that alginate forms an inhomogeneous gel by a rapid ionic reaction, and the slow release of calcium from calcium carbonate or Ca-EDTA in the presence of slow acidifier like glucono-delta-lactone is used to make a homogeneous alginate gel (Draget, Ǿstgaard, & Smidsrød, 1991; Thu, Smidsrød, & SkjåkBraæk, 1996). Gelation kinetics and equilibrium gel properties of alginate aqueous solutions induced by in-situ release of Ca ions from Ca-EDTA during D-glucono-delta-lactone (GDL) hydrolysis were observed and the modulatory effects of GB were analysed quantitatively. It is apparent that when Ca-EDTA < 51.20 mM the systems without 5 mg/mL GB gelled faster than those with the addition of 5 mg/mL GB and also attained a higher value of saturated equilibrium storage modulus. It manifests an inhibitory effect exerted by the addition of GB. On the contrary, when Ca-EDTA > 51.20 mM, the systems with 5 mg/mL GB tended to end up with a higher saturated equilibrium storage modulus than those without 5 mg/mL GB. Moreover, the gelation kinetics seems not to be altered significantly by the addition of GB.
Fig. 14.
A. Gelation profiles of storage modulus G′ versus time at fixed ALG and GB concentrations and various Ca-EDTA concentrations. ALG, 5 mg/mL; GB, 0 mg/mL (open symbol) and 5 mg/mL (closed symbol) (Liao et al., 2015). B. Proposed mechanisms of GB modulating the gelation of LMP in different Ca concentration regimes (a), and comparison to the case of ALG (b). The black lines represent macromolecular LMP or ALG. The red zigzag lines represent oligomer GB. The blue dots represent Ca2+ ions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) (Zhang et al., 2019).
The effect of guluronate block GB depends on the ratio of calcium ions to guluronate unit, R = Ca/G. The addition of GB shows an inhibitory effect in the range of 0.25 < R (Ca/G) < 0.60, and promotive effect in the range of R > 0.60 based on static and dynamic viscoelastic measurements. Mixed egg-box dimers between ALG and GB were ruled out because of cooperativity requirement of dimerisation. The promotive effect in the higher Ca concentration regime was assigned to the role of GB dimers participating in and enhancing the lateral aggregation of ALG dimers. In conclusion, short chain guluronate block is found to inhibit the gelation of alginate at low concentration of Ca2+ ions while it enhances the gelation at higher Ca2+ concentration. Inhibitory effect is attributed to the binding of calcium ions to shorter guluronate chains. Those findings may be useful in food processing and also have some therapeutical significance in the rheology of sputum as discussed in the following section.
More recently, Zhang, Hu, et al. (2019) studied the effect of lower molar mass (10 kDa) alginate, GB, on gelling behaviour of high molar mass (118 kDa) low methoxyl pectin (LMP) and high molar mass (240 kDa) alginate. GB was found to promote the gelation of LMP in the range of R ([Ca]/[guluronate + galacturonate]) < 0.25 and could make non-gelling systems gellable. This is significantly different from the case of alginate where no gelation could be induced at all. In the range of 0.25 < R < 0.60, the addition of GB was found to inhibit the gelation of LMP, whereas it had a negligible effect on the gelation of alginate as long as a fixed R was considered. In the range of R > 0.60, GB was found to promote the gelation of LMP again, which is similar to the case of alginate as described above (Fig. 14 B).
Polysaccharides are frequently used to reduce the fat content, and to improve the texture and water holding capacity (WHC) of protein gels. Yao, Zhou, Chen, Ma, Li, & Chen (2018) recently examined the effect of the addition of sodium alginate (SA) with three different molar masses on the WHC of chicken breast myosin gels. They found that WHC increased simultaneously with turbidity and thermal stability, accompanied with the decrease in surface hydrophobicity with increasing molar mass of added SA. They noticed that the addition of sodium alginate shifted the thermal transition temperature to higher temperatures detected by DSC with the higher contribution by a higher molar mass SA, which is consistent with the same stabilisation by the addition of flaxseed gum to salt-soluble meat protein, but is contradictory with the reported thermal destabilisation of myofibrillar, sarcoplasmic and connective tissue protein by the addition of SA. They observed that inhomogeneity of cavities formed in SA-protein gel network was enhanced by the higher molar mass SA, which resulted in the increase in WHC because they thought that large cavities could store more water.
3.5. Molar mass effect in cryogels and heat set gels
Some polysaccharides such as locust bean gum and cereal β-glucan as well as poly(vinyl alcohol) (PVA) form cryogels after freezing and thawing cycles while most polysaccharides such as agarose, carrageenans donot but only framework structure remains after freezing because water exudes out and not retained (Djabourov et al., 2013). Lazaridou and Biliaderis (2004) examined the effect of molar mass of oat, barley and wheat β-glucan on the small and large deformation rheology of cryogels. They found that the elastic modulus, compression modulus and true stress at 40% deformation all increased with increasing number of freezing cycles, concentration and molar mass as had been found previously for PVA cryogels (Djabourov et al., 2013). Fig. 15 A shows the compression stress σ -Hencky strain εH curves of oat β-glucan cryogels. Compressive stress increased with increasing molar mass which is in good agreement with Fig. 15B which shows the force-extension curves for cryogels of PVA with different DPs 500 (curve (1)), 1000 (curve (2)), 1700 (curve (3)), and 2400 (curve (4)). However, the storage shear modulus G′ of oat β - glucan cryogels showed opposite tendency as illustrated in Fig. 15C. The finding that G′ increased with increasing concentration of β-glucan is a common phenomenon.
Fig. 15.
A. Compression stress (σ)-Hencky strain εH curves of cryogels of oat β-glucans with different molar masses (110 kDa, 140 kDa and 200 kDa). Initial solution concentration 3% w/v, 12 freeze–thaw cycles (Lazaridou & Biliaderis, 2004). B. Force-extension curves for 15 wt % PVA gels (DS = 98.5 ± 0.5 mol %, number of freeze-thaw cycles = 3) of various DP (1): 500; (2), 1000; (3), 1700; (4), 2400. PVA gels of square shape (110 mm × 110 mm) with 3 mm thick was clamped between metal plates having 18 mm diameter hole in the center, and were subjected to compression using 5 mm diameter spherical metal probe at 50 mm/min (Watase & Nishinari, 1985). C. Storage modulus G′ recorded at 5 °C, 1.06 Hz, and 0.1% strain, cryogels of oat β-glucan with different molar masses 65 kDa, 110 kDa, 140 kDa and 200 kDa, and with different concentrations 1, 2, and 3% obtained after 12 freeze–thaw cycles (Lazaridou & Biliaderis, 2004).
Holding periods of samples at − 18 °C for 24 h, and at 5 °C for 24 h is defined as one freezing–thawing cycle in oat glucan (A & C) while one cycle of PVA cryogel formation (B) was as follows: holding PVA solutions at −20 °C for 24 h, then it was raised to 15 °C at a constant rate of 0.194 K/min, and hold there for 7 h. Then, the gel was cooled to −20 °C at the same rate and held at −20 °C for 11 h (one cycle).
However, the G′ values of cryogels decreased with increasing molecular size. Lazaridou and Biliaderis (2004) had found a similar trend in their earlier study for cereal β-glucan gels formed at room temperature and it had been attributed to the higher mobility of shorter chains (Lazaridou et al., 2003, 2004). Their interpretation was as follows: the molecular size has a significant impact on the viscosity of polymer solutions, thus influencing the diffusion rates of the interacting polysaccharide chains. However, it is difficult to understand this explanation because the stress-strain curves shown in Fig. 15A, the Young's modulus estimated from the initial slope of the curve is the largest for the gel from the higher molar mass oat glucan 200 kDa and the smallest for the gel from the lowest molar mass oat glucan 110 kDa. Unfortunately, gels from 65 kDa were so weak to keep the shape to do the compression measurements. The inconsistency between the Young's modulus from the slope of stress-strain curves in Fig. 15A and the storage shear modulus shown in Fig. 15C should be studied further.
Heat set gel formation also depends on molar mass. The gelation process of solutions of methyl cellulose (MC) with different molar masses and with approximately the same methoxy content (29 wt%) was observed by measuring G′ and G″ at a constant temperature 55 °C. The gelation time at which G′ began to increase above the base line was shorter in a higher molar mass MC solution. This rheological tendency is in accordance with DSC observation in which an endothermic peak appeared on heating accompanying the gelation. The longer chains have a greater tendency to form junction zones which are induced mainly by hydrophobic interaction (Nishinari, Hoffmann, Moritaka, Kohyama, & Nishinari, 1997). Whether the gel formation of MC is caused by spinodal decomposition or by the nucleation and growth is still a matter of hot debate (McAllister, Schmidt, Dorfman, Lodge, & Bates, 2015). More detailed studies on the gelation of MC changing molar masses at a constant degree of substitution, preferably using regioselective substitution, are expected in the future.
The gelation of KGM occurs through the alkali-induced removal of acetyl groups which confer the solubility for this polysaccharide (Nishinari, 2000b). KGM forms a thermally stable gel by deacetylation upon addition of alkaline coagulant and the gelation of KGM is promoted by heating, in contrast to many other cold-set thermo-reversible gels. The increase in the concentration or molecular weight of KGM shortened the gelation time and increased the rate constant of gelation, and the resulted gels show higher modulus (Yoshimura & Nishinari, 1999; Zhang et al., 2001). This is consistent with the general tendency observed in gelatin, carrageenan, alginate and agarose gels as described above. The effect of the degree of acetylation (DA) on the gelation of KGM was studied (Huang, Takahashi, Kobayashi, Kawase, & Nishinari, 2002; Gao & Nishinari, 2004). To understand the effect of molar mass on the gelation, it is necessary to obtain KGM sample with a constant degree of acetylation (DA) with different molar masses, but this has not yet been done because of the difficulty of sample preparation. The same difficulty is encountered in the study of the interaction of KGM with other polysaccharides such as κ-carrageenan, agarose, starch or proteins such as gelatin and myofibrillar proteins although these mixtures have been used in food products without understanding the detailed mechanism. The more detailed knowledge will improve further the products.
3.6. Molar mass effects in oleogels
Crystallinity of solid fat is generally much higher than high molar mass polysaccharides and proteins, and the linear elastic range is much narrower than in semi-solid foods mainly composed of polysaccharides and proteins. Spreadability of semi-crystalline solid fat such as butter and margarine is an important functionality during consumption, and this characteristics is different from high molar mass polysaccharide and protein food gels.
Since the intake of trans fatty acid was reported to pose a health risk (Mozaffarian, Katan, Ascherio, Stampfer, & Willett, 2006), an alternative technique to solidify the oil without using partially hydrogenated oils which contain trans fatty acids has been sought by food industry. Oleogels can produce three-dimensional crystalline networks and structuring oils (Marangoni & Garti, 2018). Ethyl cellulose (EC) has been used as oil structuring materials. Gravelle, Barbut, Quinton, and Marangoni (2014) examined the oleogel composed of canola oil or soybean oil in the presence of EC with or without surfactants. Fig. 16 shows the effects of molar mass of EC on the mechanical properties of oleogels.
Fig. 16.
Back Extrusion force for oleogels (soybean oil) as a function of mass fraction of EC (ΦEC) with different molar masses, 28.6 ± 6.2 kDa (●), 51.9 ± 10 kDa (■), and 72.8 ± 15 kDa (▲) (Gravelle et al., 2014).
As is shown in Fig. 16, the gel strength was found to increase with mass fraction of EC in a power law fashion, and also to increase with increasing molar mass of EC. From the cryo-SEM observation of partially de-oiled gels, it was found that the internal structure consisted of oil droplets entrapped within a network of interconnected strands or bundles of EC, which formed a scaffolding to support the gel (Zetzl et al., 2014). Therefore, Gravelle et al. (2014) interpreted that reducing the MW of the gelator EC would produce a less entangled network with shorter strands/bundles, having fewer interconnections per structural unit, hence the gel strength was reduced. The finding that the gel strength increased with increasing molar mass of EC was also found for EC oleogels of other vegetable oils, canola, soybean, and flaxseed oil (Zetzl et al., 2014).
Replacing trans and saturated fatty acids in margarines or shortenings has been pursued by bakery industry to produce pastries, croissants, and danishes. Blake and Marangoni (2015) examined the effect of composition of a fat substitute for pastries on rheological properties. This fat substitute is called rolled-in shortenings which are folded into dough to form thin laminated layers between dough layers. Therefore, this fat substitute should have an appropriate rheological property, plastic enough to be spread easily, yet enough hard and with a certain level of yield stress so that it can fit into dough layers, and with high melting temperature. Chain length of fatty acid is one of most important factor controlling the rheology of this fat substitute. In the optimisation of the variable parameter of Coasun (developed in Marngoni's lab) they found that monoglyceride of chains of 18 carbons (Coasun C18) is better than Coasun16 or Coasun 22. Thus, they found the optimum chain length of monoglyceride in the production of pastries, croissants, and danishes.
3.7. Molar mass effect in protein gelation
Rennet-induced gelation of casein micelles has been studied extensively because it is the basis of cheese production. Niki et al. (1994) prepared the casein micelles with different sizes by differential centrifugation. Since the κ-casein, the substrate of rennet enzyme chymosin, exists mainly on the surface of casein micelles, the smaller micelles are richer in κ-casein content. Therefore, glycomacropeptide is liberated faster in smaller casein micelles as shown in Fig. 17 A.
Fig. 17.
A. Time course of glycomacropeptide (GMP) liberated from different casein micelles after renneting. Casein micelle solution (3%) in artificial milk serum. The enzymatic reaction was performed at 30 °C (Niki et al., 1994). B. The relationship between the micellar diameter and G′sat (open circles), G″sat (closed circles) and loss tan δ (open squares). The experiments were carried out at 30 °C. Casein micelle solution: 3% (Niki et al., 1994).
The saturated value of the storage shear modulus was largest in the gel formed by the smallest casein micelles (Fig. 17B). Then, the smallest casein micelles can form a gel faster and can form a stronger gel. In this case, the gelation is dominated not only the size of the casein micelles but the difference of the distribution of κ-casein in the casein micelles should also be taken into consideration.
Recent trend to exploit the possibility to use biowaste or to add more values to plant proteins is becoming more and more active (Petrusan, Rawel, & Huschek, 2016). Felix et al. (2017) examined enzymatic hydrolysates of pea protein isolate expecting the improvement of functional properties. They found that although the solubility increased by hydrolysis, the gelling ability was reduced. It should be noticed that the lipid content and the surface hydrophobicity decreased by enzymatic hydrolysis, and therefore, the decrease in gelling ability was the result of all these interactions. Gelling ability of plant proteins was reported to decrease by enzymatic hydrolysis, but a limited enzymatic hydrolysis makes the buried peptide groups exposed on the surface, and the ionizable groups liberated thus increasing the protein-protein interaction and leading to strengthen the gelling ability (Wouters, Rombouts, Fierens, Brijs, & Delcour, 2016). Since transglutaminase (TGase) can make new covalent bonds, ε-(γ-Glu)-Lys crosslinks, it has been used widely to increase the gelling ability of many proteins such as soy proteins, sunflower protein, canola proteins (Wouters et al., 2016). Here, the molar mass decrease by hydrolysis and then its increase by TGase are the main governing principle of the decrease and increase in the gelling ability.
3.8. Molar mass effect on properties of solid foods
To control the mechanical properties of solid foods, it may be useful to remind the fundamentals of glass transition (Bhandari & Roos, 2017). Glass means the amorphous solid not only the glass used for windows or cups. On lowering the temperature, the solid behaviour changes from rubber-like to solid-like at the glass transition temperature T g. This transition is different from a simple liquid to a simple solid (crystal) transition, and was studied extensively for amorphous polymers. The solid-like amorphous glass is in a non-equilibrium super cooled liquid state, and different from an equilibrium crystalline state. Large scale main chain motion is allowed and the amorphous solid is ductile/plastic above T g, while the large scale motion is frozen and the solid becomes brittle below T g. This can be detected as a drastic decrease of elastic modulus from ca 109 Pa to 106 Pa on raising the temperature. In the first-order transitions such as melting and boiling, there is a discontinuity in the volume–temperature (V-T) or enthalpy-temperature (H-T) plot, while in the glass transition, these changes are gradual. Therefore, the first order temperature derivative of volume or enthalpy, the volumetric coefficient of expansion α = (∂V/∂T)p/V or the heat capacity at constant pressure C p = (∂H/∂T)p, are often used to detect the glass transition, by dilatometry or DSC/DTA (Cowie & Arrighi, 2007; Sperling, 2005). The glass transition strongly depends on the cooling/heating rate which is not so much the case for the first order transition as in the crystallization/melting of low molar mass compounds.
Above the glass transition temperature T g, the amorphous polymer behaves rubber-like and shows a larger strain at break, but below T g, the polymer showed a brittle fracture. The glass transition temperature of synthetic polymers has been studied extensively, and a Fox-Flory equation T g = T g0 – K/M, where T g0 is the T g of the polymer when the molar mass M approaches infinity, and K is a constant (Nunes, Martin, & Johnson, 1982). Unfortunately, this equation was not found to fit well to experiments for polysaccharides and proteins, though not so many papers have been published. Since fractural behaviour of solid state is crucial in the application of plastics, effects of molar mass was studied using polystyrene with different molar masses from 24.5 kDa to 1500 kDa in the early days (Onogi, Matsumoto, & Kamei, 1972) as shown in Fig. 18 . The elongation test of film (3 cm length and 1 cm width) was performed at the elongation rate from 0.028 to 0.56 cm/s and at a temperature range from 115 to 180 °C. They found that stress σ f and strain γ f at fracture as a function of elongation rate dγ f/dt could be superposed by shifting horizontally as had been done for linear viscoelastic studies (Ferry, 1980). The shift factor was found to be similar to that used in WLF equation. The resulted master curves of σ f or strain γ f as a function of dγ f/dt was divided into four regions, I. Brittle to ductile transition, II. Ductile, IV. Viscous fluid, and III. Between II and III. The molar mass dependence was more clearly observed at regions III and IV. In other words, mechanical properties are less sensitive to molar mass in the region I and II. At regions III and IV in master curves log σ f or γ f vs log s T dγ f/dt were obtained by superposition with a suitable shift factor s T (Fig. 18). Higher MW film showed a higher fracture stress or fracture strain at the same elongation rate where the elongation rate is slow.
Fig. 18.
Master curves of σf (left) and γf (right) for narrow molar mass -distribution polystyrenes L54 (600 kDa), L51 (388 kDa), and L52 (131 kDa) at 130 °C (Onogi et al., 1972).
Further, from the observation of master curves for polydisperse samples with a similar MW, they found that lower MW components lower the fracture stress and fracture strain at longer time scales. To show this more clearly, they performed the elongation tests comparing 4 samples, L54 (narrow distribution MW 600 kDa) and blend samples of L54 with low MW polystyrene 24.5 kDa at blending ratios 80, 70 and 60%. As the content of the high-MW component decreases, σ f at a given rate of strain decreased remarkably, and the master curves of γ f plotted against the ultimate rate of strain shifted to the high rate of strain or short time-scale side. This was summarised as follows: “Onogi et al. (1972) showed that a 60% mixture of 600 kDa with a 25 kDa at component reduces the fracture strain of the high MW component by some 50% and the fracture stress by an order of magnitude for low strain rates above Tg. From these experiments it was concluded that the low MW components have a very strong effect on the long relaxation times and act simply as a plasticiser for the high MW component” (Wellinghoff & Baer, 1975). Recently, molar mass effect on mechanical properties transition was examined for polycarbonate (Ohara & Kodama, 2019).
In food science/technology, it should be reminded that water plays an important role of plasticiser (Bhandari & Roos, 2017). Amorphous solid polymers can be made more pliable by lowering its T g, and this can be achieved by incorporating quantities of high-boiling point, low-molar-mass compounds in the material. These are called plasticizers and must be compatible with the polymer (Cowie & Arrighi, 2007). In foods, water is a most important plasticiser, and starch hydrolysates, maltodextrins of different molar masses have also been widely used. The extent of hydrolysis is usually represented by DE (dextrose equivalent). Starch hydrolysates with DE < 20 are called maltodextrins, while those with DE > 20 are called glucose syrups or glucose solids. DE of glucose is 100, and that of starch is zero (White, Hudson, & Adamson, 2003). The T g for maltodextrins with different DEs were reported as T g = 180 °C for DE5 (MW3600), T g = 160 °C for DE10 (MW1800), T g = 155 °C for DE18, T g = 141 °C for DE20 (MW900), T g = 121 °C for DE25 (MW 720), and T g = 100 °C for DE 36 (MW 500), T g = 92 °C for DE52 (maltose). Langrish, Chan, and Kota (2007) proposed T g = 176.3–1.4DE. This agrees qualitatively with a Fox-Flory equation mentioned above, predicting that T g approaches to 176.3 °C at infinite molar mass of maltodextrin which is similar to non-degraded starch. However, the reported values donot agree exactly with ca 188 °C (Roos & Karel, 1991). The effectiveness of the concept of the glass transition in low temperature preservation of food was shown in many foods, e.g. in frozen starch products. Below the glass transition temperature amylose and amylopectin chains mobility is reduced, preventing the molecular association leading to the restructuring (retrogradation) (Zaritzky, 2010). Maltodextrins are used widely as drying aids in spray drying of, for example, skim milk. It is desirable to prevent powders sticking and it is effective to raise the T g so that the surface of powders might become less sticky; below the glass transition temperature the powders are in glassy state and less sticky. Bakery products formulations taking into account the glass transition were recently reviewed (Wang & Zhou, 2017).
As will be discussed later in section 9.3 Effects of molar mass on aroma compounds and carriers on the retention of aroma compounds, the diffusion of aroma in the carrier (mainly polysaccharide, protein and lipid) is strongly influenced by its state. The diffusion coefficient of aroma compounds is known to decrease when the carrier materials changes from rubbery state to glass state (Moran, Yin, Cadwallader, & Padua, 2014).
Since polysaccharides and proteins have hydrophilic residues such as hydroxyl groups or amide groups, the glass transition is influenced by these intermolecular interactions. Main-chain motion is restricted owing to these intermolecular interactions and the glass transition temperature is higher than that of the hydrophilic polymer in the completely dry state (Hatakeyama & Quinn, 1999). In most dried proteins and polysaccharides no glass transition or melting is observed until decomposition of the main chain occurs because intramolecular and intermolecular hydrogen bonds stabilise the high order structure of these polymers. Glycerol, sorbitol, or dimethylsulphoxide were used to plasticise the amylose/starch films to study the glass transition at temperatures lower than the temperature at which main chain scission occurs (Nakamura & Tobolsky, 1967; Sahari, Sapuan, Zainudin, & Maleque, 2012).
On the other hand, introducing a small amount of water to a hydrophilic polymer may disrupt the intermolecular bonds, thereby enhancing the main-chain motion. In this case T g shifts to lower temperatures in the presence of water. Hydrophilic polymers stored under ambient conditions contain a certain amount of bound water. In most practical applications the observed thermal and mechanical properties of the polymer reflect the presence of a nominal amount of water (Hatakeyama & Quinn, 1999).
3.9. Molar mass effect of sugar and polyols on the glass transition of gelatin
Since gelatin has been widely used in food industry although its usage in photographic film has been reduced, understanding the interaction of water and gelatin remains important. DSC heating curves for gelatin gels of different concentrations from 23.2 to 57.7 wt%, quenched from room temperature to −150 °C using liquid nitrogen, are shown in Fig. 19 A. A step-like change in baseline observed at around −100 °C was attributed to glass transition. The glass transition temperature T g is shown by an arrow in Fig. 19A. T g shifted to higher temperatures with decreasing gelatin concentration beyond 55%. It is reported that various kinds of hydrogels form glassy state by quenching from a sol state to −150 °C, and a part of water molecules associated closely with matrix polymers solidify into an amorphous state (Hatakeyama & Hatakeyama, 1998). By heating, amorphous ice associated with matrix become mobile. The free molecular motion commences in gelatin gels with amorphous ice, which is detected as T g in heating DSC curves. A broad exothermic deviation is observed in samples containing a small amount of water (Fig. 18A, curves a-c), which was attributed to the cold crystallization. Water molecules solidified in an amorphous state are mobilized at T g on heating, and molecular motion is enhanced by successive heating and reorganised as unstable ice just before melting. This is confirmed by the fact that the exotherm disappeared if the sample is annealed at a temperature lower than melting. This suggests that cold crystallization can be observed for thermally unstable samples prepared by quenching (Hatakeyama & Hatakeyama, 1998).
Fig. 19.
A. DSC heating curves for gelatin gels of different concentrations: (a) 57.7%; (b) 55.0%; (c) 51.2%; (d) 46.9%; (e) 35.2% (f) 23.2%; Gels were quenched from the room temperature to −150 °C by liquid nitrogen, and then heated at 5 °C/min (Nishinari, Watase, & Hatakeyama, 1997). B. Relationship between phase-transition temperatures and water content Wc (g/g) for gelatin water system. Tg: glass-transition temperature, Tpm: pre-melt crystallization, Tcc: cold crystallization temperature, Tm: melting temperature (Nishinari et al., 1997). (●) Tg. from Reutner et al. (1985). C. Relation between the lowest Tg value (Tg,min) and the Wc where the lowest Tg was observed for various kinds of water-polysaccharide systems. Gellan (closed circle), xanthan gum (1), cellulose sulphate (2), Alginic acid (3), carboxymethylcellulose (4), hyaluronic acid (5) (Hatakeyama, et al. (1999). D. Glass-transition temperature Tg and melting temperature Tm of 40% gelatin gels containing sugars or polyols of various concentrations as a functions of molecular weight of sugars or polyols added. (○), 0.2 M; (●) 0.5 M (Nishinari, Watase et al., 1997).
The phase transition temperatures of gel are shown as a function of water content W c in Fig. 19B. The water content W c is defined as W c (g/g) = W w /W s (g/g), where W w is the weight of water in the system, and W s is that of completely dried sample. At water content range higher than W c = 0.78 (a water content between curves a and b in Fig. 19A), movement of gelatin molecules is restricted by the presence of ice, and on this account, T g shifted to higher temperatures with increasing water content. It was difficult to obtain a complete dissolution for gelatin solutions above 60% (Molar mass of gelatin was 11,000 Da and the isoelectric point was 6.7). However, T g shifted to higher temperatures with decreasing water content W c around W c = 0.78. Reutner et al. (1985) studied the glass transition in gelatin-water systems in the broad concentration range by DSC. T g observed for gelatin water systems by Reutner et al. are also plotted in Fig. 19B. (closed circles). T g observed at lower water content by Reutner et al. (closed circles) locate at the extension of our data (open circles) as shown in Fig. 19B, however, a noticeable discrepancy appeared around W c = 0.7–1.5. These results could not be compared directly because of the difference in samples (MW = 50,000 and isoelectric point 7.5) and the condition of measurement (scan rate 10 °C/min) in the experiments of Reutner et al. (1985). The remarkable difference of T g at W c = 0.7–1.5 should be attributed to this difference. Reutner et al. could achieve a complete quenching to the homogeneous glass, whilst the sample quantity of Nishinari et al. experiment was much larger than Reutner et al. and the system might be separated into two phases, a phase of concentrated gelatin solution and a phase of almost pure water which exist as a homogeneous glass and ice, respectively, at liquid nitrogen temperature. However, if Reutner et al. added much water, they also might have found the shift of T g to higher temperatures with increasing water content above a certain W c. It is well known that T g of a polymer shifts to lower temperatures by the addition of a plasticiser (Cowie & Arrighi, 2007). T g shifted to lower temperatures with decreasing gelatin concentration, i.e. with increasing water content below W c = 0.78, since water molecules play a role of plasticiser. It is also well known that T g shifts to higher temperatures in the presence of a large amount of water, because in such a case the whole system cannot be converted to glassy state, but some ice crystals are formed, and they inhibit the molecular motion of polymer chains (Hatakeyama & Hatakeyama, 1998). The glass-transition temperature T g of gelatin gels as a function of gelatin concentration, therefore, showed a minimum around gelatin concentration 56% (W c = 0.78) (Fig. 19B).
This phenomenon which shows the minimum of T g as a function of water content is widely observed in many polysaccharides-water systems. The relation between W c and the temperature T g,min at which T g becomes minimum is shown in Fig. 19C for gellan (closed circle), xanthan gum, cellulose sulphate, alginic acid, carboxymethylcellulose, hyaluronic acid (Hatakeyama, Nakamura, Takahashi, & Hatakeyama, 1998).
The glass-transition temperature T g and the melting temperature T m of 40% gelatin gels containing sugars (deoxyribose, ribose, glucose, sucrose, raffinose, maltotriose, maltotetraose, α-cyclodextrin, maltohexaose) or polyols (ethylene glycol, propylene glycol, glycerin) with different concentrations as a function of molecular weight of sugars or polyols is shown in Fig. 19D. T g shifted to higher temperatures with increasing molecular weight of sugars or polyols added, and it ranged from 165 to 190 K. T m also shifted to higher temperatures with increasing molecular weight of sugars or polyols added, but it ranged from 268 to 273 K, and the shift was not so pronounced in comparison to that for T g. It is reasonable to see that T g was lowered by adding higher sugar or polyol. However, the efficiency as a plasticiser of each sugar or polyol to lower the glass transition temperature is reduced with increasing molar mass of sugar because their glass transition temperature is higher than that of water (−135 °C = 138 K), and their unfrozen water content per unit mass is also increasing with molar mass (Levine & Slade, 1987). In addition, the structural change induced by the addition of sugars and polyols should be taken into account. It was hypothesized that the number of junction zone of gels, which are formed by the aggregated triple helices in gelatin gels, increased but the size of each junction zone decreased with increasing concentration of sugars (Nishinari et al., 2016). The most part of unfrozen water is surmised to exist in junction zoned from a low temperature DSC study (Watase, Nishinari, & Hatakeyama, 1985). These hypotheses should be further examined using sugars and polyols systematically.
3.10. Molar mass effects on physical properties of films
Edible films made from polysaccharides, proteins, lipids, or the combination, are used to protect foods from contamination of harmful microorganism and oxidation, and also used as carriers of antimicrobial additives to improve their stability and conrol the release (Zhong, Zhuang, Gu, & Zhao, 2019). Most frequently referenced mechanical properties of films in food industry are tensile strength (TS) and elongation at break (EB). Other important parameters in food packaging are water vapor permeability (WVP) and oxygen permeability (OP). These parameters are known to be influenced by molar mass, plasticiser, film production methods including casting, extruding, temperature control, and drying rate. There have been not so many papers reporting the effect of molar mass. In contrast, many papers report the effects of plasticizers, methods of production of films without controlling the molar mass.
Chitosan films are widely used in food and pharmaceutical industry. Chitosan (CH) is a cationic polysaccharide composed of randomly distributed (1,4)-linked 2-amino-2-deoxy-β-d-glucose units, and is obtained by deacetylation of chitin (Rinaudo, 2006). As expected, TS of chitosan films prepared from chitosan solutions with different solvents, acetic acid, citric acid, lactic acid, and malic acid, all increased with increasing molar mass, while EB also showed the same tendency except the film prepared using citric acid. The EB of films prepared from citric acid was found to decrease with increasing molar mass (Park, Marsh, & Rhim, 2002). Park et al. (2002) found that WVP was not influenced significantly by the molar mass of chitosan. They also found that OP of films prepared with malic acid was the lowest, followed by acetic, lactic, and citric acid.
More recently, films of CH with three different molar masses (110 kDa, 50 kDa and 7 kDa) and with approximately the same degree of deacetylation 85.5%) were prepared with and without glycerol as a plasticiser to see the effect of molar mass (Liu et al., 2020). As expected TS and EB increased with increasing MW in all the films with different glycerol contents. WVP and OP decreased with increasing MW. They further applied these CH films for packaging fresh strawberries. They found the weight loss was reduced and the colour was maintained by CH films, and in addition by virtue of antibacterial properties of CH, thus it was useful to extend the shelf life. They found that the film with high MW (110 kDa), high CH content and 50% glycerol/chitosan (w/w) showed the best performance among 27 types of films with different CH and glycerol contents.
The effect of molar mass of chitosan on the film properties of chitosan (CH) -corn starch (CS) composite (50:50 mixing ratio) was studied using low, medium and high molecular weight (LMW, MMW and HMW) chitosan (Bof, Bordagaray, Locaso, & García, 2015). WVP of films of chitosan (CH) alone decreased with increasing molar mass (Fig. 20 A), which was contradictory with the previous report by Park et al. (2002). Bof et al. (2015) ascribed the molar mass dependence of WVP to the development of a more compact structure by higher polymerisation degree chitosan as was observed earlier (Leceta, Guerrero, & de la Caba, 2013). While WVP of the CS/CH blend film with HMM CH is approximately the same to that of HMW CS alone (Fig. 19A), values of WVP for the CS/CH blend film with LMW CH and MMW CH were significantly lower than those for LMW CH and MMW CH alone. This was interpreted as follows: The developed interactions between CH and CS, mainly hydrogen bonding type, reduced the hydrophilic groups availability of chitosan matrices, leading to a reduction in water vapor transmission rate.
Fig. 20.
A. Water vapor permeability of films based on corn starch (CS), chitosan (CH) and a 50:50 blend of them. LMW, MMW and HMW correspond to low, medium and high molecular weight chitosan (Bof et al., 2015). B. Water vapor permeability (WVP) for films of κ-carrageenan, galactomannan with different molar masses, Native (non-degraded), high (HMWH), medium (MMWH), and low (LMWH) MW hydrolysates, and their mixtures. a-f; Bars with different superscripts indicate significant difference (p<0.05) between them (Rodriguez et al., 2020).
More recently, effects of molar mass on blend films of galactomannan and κ-carrageenan were studied using partially hydrolysed galactomannans (Rodriguez-Canto, Cerqueira, Chel-Guerrero, Pastrana, & Aguilar-Vega, 2020). From a widely accepted view that the film strength may decrease with decreasing molar mass, it seems to be of no use to lower the molar mass, however, the molar mass reduction leads to lower the viscosity of the solution, which is advantageous for fluid transportation by pumping to lower the energy cost and also for spray drying coating (Garcia, Gómez-Guillén, López-Caballero, & Barbosa-Canovas, 2016). Rodriguez-Canto et al. (2019) examined the solubility and rheological properties of hydrolysed galactomannan extracted from Delonix regia, and found that the solubility increased with decreasing molar mass (i.e., with increasing the dosage of enzyme for degradation and the reaction time). Rodriguez-Canto et al. (2020) using three hydrolysed galactomannans, the high MW hydrolysate (HMWH) with 4.15 × 105 Da, the medium MW hydrolysate (MMWH) with 2.85 × 105 Da and the low MW (LMWH) hydrolysates with 1.95 × 105 Da, in addition to the native Delonix regia galactomannan (DRGM) with 4.86 × 105 Da, examined the properties TS, EB, and WVP of films of galactomannan alone and composite films of galactomannan and κ-carrageenan (60:40 wt % mixing ratio). While TS was increased by mixing DRGM hydrolysate with κ-carrageenan and the composite with LWWH shows the lowest TS, this composite showed the lowest WVP (Fig. 20B), which was attributed to a more compact molecular arrangement with physical bonding interactions, such as higher hydrogen bonding, in the structure of the films as a consequence of galactomannan shorter chain length (Rodriguez-Canto et al., 2020). This is the opposite tendency as described above for CC/CH film where the longer chain CH was believed to make the structure more compact. The finding that TS of the composite of κ-carrageenan with HMWH was lower than that of κ-carrageenan with MMWH was interpreted that the molar mass above MWWH was higher than the limiting value of the molar mass above which no increase of mechanical strength was observed. As was widely known, the mechanical strength of solid polymers is not increased with increasing molar mass above a certain limiting value of molar mass (Nunes et al., 1982).
Electrostatic spraying (ES) has attracted much attention recently as a method for encapsulation in food and pharmaceutical industry (Barringer & Sumonsiri, 2015). Zhong et al. (2019) applied ES for chitosan with different molar masses LM = 6.55 kDa, MM = 12.93 kDa, and HM = 47.70 kDa. They found that WVP (g∙mm/(m2∙d∙kPa) ranged from 73 (0.5% chitosan) to 80 (1% chitosan) for LM, from 49 (0.5% chitosan) to 58 (1% chitosan) for MM, from 26 (0.5% chitosan) to 47 (1% chitosan) 26–47 for HM, indicating that WVP decreased with increasing molar mass and with decreasing concentration of chitosan. They also found that TS increased with increasing molar mass and decreased with increasing concentration. From measurement of the electric conductivity of the solution and microstructure of the film by SEM, they found that as the conductivity of CH solution became higher with MW increased, more effective coverage and complete film formation without pinholes was accomplished. Their results are consistent with those of Bof et al. (2015), but not with Park et al. (2002), both of whom prepared the film by casting method.
Water plays a role of plasticiser of edible films, and in addition to water, polyols and sugars are also used (Harnkarnsujarit, 2017). Effects of the addition of polyethylenglycol (PEG) with lower molecular weight (MW 300, 400, 600, 800, 1500, 4000, 10,000, and 20,000 Da) to gelatin film were examined (Cao, Yang, & Fu, 2009). It was found that TS and elastic modulus increased while EB decreased with increasing molar mass of PEG. It was also found that the higher MW PEG (>800) showed higher WVP, and the authors concluded that lower MW PEG were more effective to decrease the moisture transfer between the food and the surrounding atmosphere.
As described above, some contradictions among previous papers about the molar mass effects on the film properties, TS, EB, and WVP, have been found, and these should be clarified to develop further application of films. More systematic study should be done for polysaccharides and proteins to get more basic knowledge to develop further the utilization of polysaccharide and protein films. Film thickness, plasticizers, deformation rate, production method, all these affect the film characteristics. Not only trial and error approach but also more systematic study to make films are expected in future.
4. Effect of the coexistence of lower mass compounds with polymers
4.1. Effect of molar mass of β-lactoglobulin hydrolysates on the gelation of κ-carrageenan
Interaction of polysaccharide and protein has been studied by many research groups because of many reasons. Conformation and conformational transitions are essential for the biological and technological functions of natural polyelectrolytes, e.g., DNA. Cao et al. (2016) tried to clarify how the conformational transition of natural polyelectrolyte is affected and tuned by electrostatic complexation with protein as encountered in many biological processes. A model protein/polyelectrolyte system, β-lactoglobulin (β-lg) and κ-carrageenan (κ-car), was studied to see how β-lg affects the conformational change and gelation of κ-car (Cao et al., 2016). DSC curves of κ-car during cooling at different mixing ratio r = β-lg/κ-car were not influenced by the addition of β-lg at higher pH than the isoelectric point (IEP) of β-lg (pH = 5.2) where no electrostatic complexation occurs. On the other hand, at lower pH = 4.7, the DSC exothermic peak on cooling accompanying the conformational and gelling transition of κ-car was suppressed with increasing mixing ratio r = β-lg/κ-car. It was suggested that β-lg/κ-car forms soluble complexes on cooling at low r, but insoluble complexes are formed at high r. Relative extent of conformational transition of κ-car φ (r) was defined as a ratio of DSC enthalpy change ΔH (r) for the mixture with mixing ratio r to that of κ-car without addition of β-lg ΔH(0): φ (r) = ΔH(r)/ΔH(0). The extent φ (r) as a function of β-lg/κ-car mixing ratio r at different pHs decreased more steeply at lower pH below φ = 0.93 (Fig. 21 B). This decrease of φ (r) as a function of r was analysed more quantitatively by using McGhee-von and Hippel (1974) theory which was used to understand the binding of protein to DNA. The number of binding sites consecutively occupied by one protein molecule to κ-car and the binding constant were found to increase with decreasing pH. In addition to DSC results, the number of binding sites consecutively occupied by one protein molecule to κ-car and the binding constant determined from ITC measurement also showed a good agreement with the results from DSC. This inhibitive effects of β-lg on the helix formation and gelation of κ-car were further studied by using β-lg hydrolysates with different molar masses. It was found that larger hydrolysates (>2000 Da) had an inhibitory effect on the conformational transition of κ-car, while shorter hydrolysates (<1000 Da) tended to promote it (Fig. 21A).
Fig. 21.
A. Onset temperature (To) and B. relative extent (φ) of the conformational transition of κ-car as influenced by β-lg and its hydrolysates as a function of mixing ratio r = β-lg/κ-car at pH4.7. Molar masses of β-lg and its hydrolysates; β-lg (19,100 Da) > S1 (2053 Da) > S2 (921 Da)> S3 (127 Da) (Cao et al., 2016).
4.2. Effect of molar mass of added KGM on the properties of gellan
On cooling gellan solutions, coil-to-helix transition occurs as shown by a step-like change of the intrinsic viscosity (Section 3.1, Fig. 9A), and this transition can be also monitored as a step-like change of loss modulus G″ as shown in Fig. 22 A (Nishinari, Miyoshi, Takaya, & Williams, 1996). The addition of konjac glucomannan (KGM) inhibit this transition, and the degree of inhibition increased with increasing molar mass of the added KGM as shown in Fig. 22B. Longer KGM chains inhibited the gelation of gellan more than shorter KGM chains (Nishinari et al., 1996). However, the constituent monosaccharide glucose and mannose of KGM both enhanced the helix and gel formation (Miyoshi, Takaya, & Nishinari, 1998). Both coil-to-helix transition temperature and gelation temperature shifted to higher temperatures on cooling by the addition of glucose or mannose. On heating the gellan gum gels containing glucose or mannose after cooling, the storage modulus began to decrease accompanying the gel to sol transition at a certain temperature, and this temperature shifted to higher temperatures with increasing concentration of added glucose or mannose.
Fig. 22.
A. Temperature dependence of storage modulus G′ and loss modulus G″ during cooling or heating process for a 1.6% solution of gellan gum alone. (○) G′, (△) G″, cooling; (●) G′, (▲) G″, heating; cooling and heating rate: 0.5 °C/min; angular frequency ω = 0.1 rad/s; ΔG represents the relaxational strength, and TM stands for the midpoint temperature of the transition (Nishinari et al., 1996). B. Relaxation strength ΔG for gellan/KGM mixtures (total polysaccharide concentration 1.6%) as a function of mixing ratio. (♦) Gellan gum alone, (□) gellan/ND mixtures, (○) gellan/LM-1 mixtures, (△) gellan/LM-2 mixtures. Molar mass, (1.17 × 106 (ND), 9.5 × 105 (LM-1) and 2.51 × 105 (LM-2). ND, non-degraded native konjac glucomannan (Miyoshi et al., 1998).
As shown in the previous section 4.1. Effect of molar mass of β-lactoglobulin hydrolysates on the gelation of κ-carrageenan, lysine and S3 (lower molar mass (<1000 Da) hydrolysate of β-lg) enhanced the gelation of κ-carrageenan but β-lg and its higher molar mass (>2000 Da) hydrolysate inhibited the gelation of κ-carrageenan. Since these two gel-promotion or gel-inhibition phenomena show a common effect, there may be a boundary molar mass for KGM and its hydrolysate above which the inhibition by adding KGM and below which the promotion by adding KGM hydrolysate occurs. Although the promotion of gelation and the increase in the elastic modulus of resulted gels by the addition of a monosaccharide and disaccharide for agarose, κ-carrageenan, or gellan is well documented (Nishinari & Fang, 2016), the mechanism whether it is due to the increase in the effective concentration of polymers by the hydration of a monosaccharide and disaccharide or it is due to the direct binding of residue in the polymer with the hydroxyl group in a monosaccharide and disaccharide is still a matter of debate (see Stenner, Matubayasi, & Shimizu, 2016).
4.3. Effects of molar mass of non-starch polysaccharides and amylose/amylopectin in the gelatinization and retrogradation of starch
Since starch is an important ingredient of many cereals, tubers, pulses and other crops and an important energy source, and used to control texture of many processed foods, its gelatinization and retrogradation have been studied extensively (Sjöö & Nilsson, 2918; BeMiller, 2018). Effects of the addition of non-starch polysaccharide (NSP) on the gelatinization and retrogradation of starch have been reviewed (BeMiller, 2011; Funami, 2009). Thermorheological measurements using RVA (Rapid Viscoanalyzer) were widely employed because of the insolubility of starch, and it is easy to use RVA in spite of ambiguous characteristics obtained (Chantaro, Pongsawatmanit, & Nishinari, 2013). RVA parameters, pasting temperature, peak viscosity, breakdown and setback are often used. The study of the interaction between starch and non-starch polysaccharides should take into account that it depends not only on molar mass of NSP but also on the degree of branching, chain stiffness, electric charges of NSP, amylose/amylopectin ratio, the lipid or protein content in starch.
Effects of the addition of 0.1–1.0% galactomannans (guar gum (GG), tara gum (TG), locustbean gum (LBG)) and KGM on the gelatinization and retrogradation of 5% wheat starch based on rheological and DSC measurements were studied (Funami et al., 2005a), and it was reported that the RVA peak viscosity (PV) was found to increase with increasing concentration of galactomannans, and the PV was in the following order PV(LBG) > PV(TG) > PV(GG) while the radius of gyration R g was in the opposite order R g (LBG) < R g (TG) < R g(GG), the order of which is also in accord with the molar mass MW(LBG) < MW (TG) < MW (GG). Galactomannans were found to inhibit the swelling of starch granules and the average diameter of starch granules in the presence of these NSP was as follows: KGM (26.8 ± 1.3 mm) < GG (32.2 ± 1.5 mm) < TG (33.0 ± 0.9 mm) < LBG (35.6 ± 3.6 mm), which did not necessarily agree with the order of the RVA peak viscosity: KGM > LBG > TG > GG. This suggested indirectly interactions between amylose-like component of amylopectin and galactomannan chains are also important in addition to the viscosity enhancing of galactomannans. Leached amylose was found reduced by galactomannans: the leached amylose for the control (5% wheat starch) 51.6 ± 1.0% was reduced by 0.5% polysaccharides; KGM (16.2 ± 1.0%), LBG (17.5 ± 0.7%), TG(19.1 ± 0.1%), GG (19.1 ± 1.1%), i.e. the order of the reduction agreed with the order of the RVA peak viscosity. This suggested that increase in viscosity of the continuous phase should prevent the diffusion of amylose from the starch granules, leading to decrease in the amount of leached amylose. These NSPs increased the effective concentration of starch and RVA peak viscosity, but never led to gel formation judging from small deformation oscillatory measurements, G' < G″ and tan δ was increased. These NSPs accelerated the short term retrogradation but retarded the long term retrogradation.
Since the interaction between NSP and starch depends on the molar mass of NSP, amylose/amylopectin ratio and the concentration of starch, it is necessary to compare the effects of NSP with different molar masses using different starches with different amylose/amylopectin ratios to understand better the mechanism. Funami et al. (2005b), using guar gums with eight different molar masses (g/mol) (G1 = 34.6 × 105, G2 = 34.5 × 105, G3 = 20.1 × 105, G4 = 16.5 × 105, G5 = 12.2 × 105, G6 = 10.1 × 105, G7 = 4.7 × 105, G8 = 0.02 × 105), and three maize starch samples with different amylose contents 50%, 26% and 14% prepared by mixing high amylose maize (61.9% amylose), normal maize (26.2% amylose) and waxy maize (1.4% amylose), studied the effect of molar mass employing the same methods as mentioned above. For the 5% and 15 normal maize, the addition of 0.5% guar gum increased the RVA peak viscosity and setback values (with some exceptions), and this increase was found more pronounced with increasing molar mass of guar gum. The onset of the viscosity increase on heating was shifted to lower temperatures with increasing molar mass of guar gum which was thought to interact with amylose, and the increasing of the RVA peak viscosity was attributed to the interaction between guar and amylopectin.
It was shown that the short term retrogradation was retarded by the addition of 0.5% guar gum to 5% maize starch, which was suggested by the increase of the loss tangent as a function of molar mass, and this retarding effect was shown to be correlated with the decreasing leached amylose. This leached amylose in starch-guar system was found to decrease with RVA peak viscosity values (Funami, Kataoka, Omoto, Goto, Asai, & Nishinari (2005c). For the longer term retrogradation, higher molar mass guar G3 and G6 were effective to retard the retrogradation in higher amylose 5% starch (50% amylose) than in low amylose starch (26% amylose), which indicated that high molar mass guar suppress effectively the gelation of amylose until 5 days. For a higher starch concentration (15% maize starch), the rate constant k of retrogradation kinetic equation (J = J 0exp (-kt), where t is the storage time, J 0 is the creep compliance J at t = 0, and k is the rate constant) was found to decrease with increasing molar mass of the added guar gum (0.5%) for higher molar mass than 10.1 × 105 (G6), however, the lower molar mass guar gum, G7 and G8 were found to promote the retrogradation (Fig. 23 A).
Fig. 23.
A. Rate constant k for 15% normal maize starch/0.5% guar system in a kinetic eq. J(t) = J0exp(-kt) describing the relationship between creep compliance and storage time at 4 °C for 14 days as a function of molecular weight of guar gum. Dotted line in the figure represents the value for the control (without guar); 12.6 × 10 −2 (Funami et al., 2005c). B. Syneresis for 5% normal maize starch/0.5% guar system after storage at 4 °C for either 7 or 14 days as a function of molecular weight of guar gum. Dotted line in the figure represents the syneresis for the control (5% normal maize starch without added guar) after storage for 7 days; 13.4%, whereas solid line stands for the syneresis for the control after storage for 14 days; 30.6%. ○, after storage for 7 days; ●, after storage for 14 days (Funami et al., 2005c).
Syneresis for 5% normal starch was quantified with and without guar (0.5%) (Fig. 23B). For the control, syneresis was estimated to be 13.4 ± 1.5 and 30.6 ± 0.5% after storage at 4 °C for 7 and 14 days, respectively. The addition of guar samples with MW values higher than 10.1 × 105 g/mol (i.e. G6) decreased syneresis of the starch system in comparison with the control at each storage period. This effect of guar might include not only the hydration or the water-holding of guar itself but also the retardation of starch retrogradation, especially the inhibition of amylose-amylose associations (Lee, Baek, Cha, Park, & Lim, 2002). The effect of guar was lowered markedly, however, at a longer storage period for starch/G5 or G6 system. Guar samples G7 and G8, on the contrary, increased syneresis of the system in comparison with the control at each storage period, suggesting that the addition of guars with relatively low MW values (i.e. 5.0 × 105 g/mol) should rather promote long-term retrogradation of starch (Funami et al., 2005c). Syneresis of 8 % maize starch gels with and without 1% high MW or low MW pectin showed a similar tendency (Zhang et al., 2018); the addition of pectin decreased the syneresis, however, the lower MW pectin reduced syneresis more than higher MW pectin, which was contradictory with the data for wheat-guar system reported by Funami et al. (2005c). Zhang et al. (2018) examined the syneresis of maize starch with and without a high and low MW pectins after freeze-thawing, and reported that syneresis was increased with increasing cycle of freeze-thawing and that pectin reduced the syneresis. However, in this experiment the lower MW pectin was more effective to reduce the syneresis, which is opposite in the result for maize-guar system shown in Fig. 23B. Whether this contradiction is due to the difference in the syneresis observed in the starch stored at 4 °C and that subjected to freeze-thawing is not clear, and should be studied further.
Corn fibre gum (CFG) has been attracting much attention because of its adhesive, thickening, stabilising, emulsifying and film forming functions, and Qiu, Yadav, Liu, Chen, Tatsumi, & Yin et al. (2016) examined its effect on gelatinization and retrogradation of maize starch and wheat starch using three CFG with different molar masses (CFG-1 = 186 kDa, CFG-2 = 290 kDa, and CFG-3 = 338 kDa). CFG is an arabinoxylan (hemicellulose B) isolated from deoiled and destarched corn fibre by an alkaline hydrogen peroxide extraction process (Yadav, Fishman, Chau, Johnston, & Hicks, 2007). CFG is a highly branched polysaccharide and contains some proteinaceous portion which confers emulsifying activity as in gum arabic. Although the degree of branching and monosaccharide (arabinose, galactose, glucose, xylose and glucuronic acid) contents are not exactly the same in these three CFG samples, effects of CFG on the gelatinization and retrogradation of starch could be interpreted mainly from the viewpoint of MW, and Qiu et al. (2016) found similar tendencies as for the effects of guar gum reported by Funami et al. (2005b); RVA peak viscosity decreased by adding CFG and this decreasing was more pronounced in lower molar mass CFG, and the RVA peak viscosity increased with increasing molar mass of CFG for both maize starch and wheat starch. Also, the leached amylose was found to decrease with increasing molecular weight of CFG for both wheat and maize starch as shown in Fig. 24 together with the results for guar gum taken from Funami et al. (2005c).
Fig.24.

Leached amylose (%) as a function of the molecular weight of the added non-starch polysaccharide (NSP) guar (Funami et al., 2005b), corn fiber gum (Qiu et al., 2016), KGM (Ma, Zhu, Wang, Wang, & Wang, 2019).
A similar phenomenon was found: the addition of pectin to maize starch decreased the RVA peak viscosity and the decrease was more pronounced for a lower MW pectin (Zhang et al., 2018). More recently, a similar phenomenon was reported for the effect of barley β-glucan on the peak viscosity of RVA (Dangi, Yadav, & Yadav, 2020). Dangi, Yadav, & Yadav, 2020 measured rheological properties of rice flour mixed with native β-glucan and with partially hydrolysed β-glucan, and found that the RVA peak viscosity decreased by adding β-glucan, and the extent of the decrease was more pronounced by the addition of hydrolysed β-glucan. Dangi, Yadav, & Yadav, 2019 reported that the RVA peak viscosity for barley starch decreased by adding lower MW pectin (pectin hydrolysate) but the peak viscosity was higher when native pectin (high MW) was added. Dangi, Yadav, and Yadav (2019a) reported that the RVA peak viscosity for pearl millet starch increased by adding even lower MW guar (guar hydrolysate), and the peak viscosity was higher when high MW guar was added. This was also reported for wheat starch-arabinoxylan system (Hou et al., 2020). The RVA peak viscosity in the gelatinization of wheat starch during heating was found to be suppressed by the presence of arabinoxylan (AX), and this effect was more pronounced for lower molar mass AX. It was interpreted by the insertion of lower molar mass AX in the molecular chain of amylose which limit the formation of double helices. This interpretation was based on the lowering of the enthalpy of amylose -lipid complex formation and melting on cooling and heating DSC, and this decrease was more pronounced in the presence of lower molar mass AX. Although the amylose –lipid complex formation has been studied extensively by X-ray diffraction, NMR, and other methods, the insertion of short polysaccharide chains have not been studied, and should be studied further. As for the RVA peak viscosity, non-starch polysaccharides inhibit the gelatinization and decreased the value, but opposite tendency was also found. Whether the increase in the peak viscosity by the addition of non-starch polysaccharide is due to its own high viscosity or not should be clarified systematically.
The so-called setback values of RVA were not consistent among literatures probably because of its structure break down nature in the measurement as pointed out in Chantaro et al. (2013).
Effects of chain length on the gelatinization and retrogradation of starch have been extensively studied (Crofts et al., 2019; Zhu & Liu, 2020). Super-long (extra-long) chains (SLC) in amylopectin were found in starches in rice, maize, barley, wheat, millet and sorghum. A positive linear relation of SLC with granule-bound starch synthase I was found (Inouchi, 2010), and it was reported that SLC promoted the retrogradation (Lin et al., 2019).
Effects of annealing at 20 and 50 °C on gelatinization DSC thermograms of maize, wheat and potato starches were analysed by a zipper model approach (Kohyama & Sasaki, 2006; Nishinari et al., 1990). Ungelatinised starch sample was assumed to consist of M molecular zippers which are aggregated double helices. In other words, ordered region in starch is assumed to consist of zippers, and each zipper consists of N parallel links that can be opened from both ends when starch is subjected to heating. They found no change in amylose and amylopectin chain length distribution by annealing temperature. It was suggested that more links N were generated in each ordered amylopectin region, and the number of ordered regions M was reduced by annealing at a higher temperature, and the total number of links in amylopectin, represented by the product (N × M), was found unchanged for cereal starches and only slightly increased for potato starch.
In addition to molar mas effect, the structural complexity of starch, the chain length distribution of amylose and amylopectin, degree of branching, and the mixing ratio and concentration effect also influence the gelatinization and retrogradation. It is not easy to examine all these aspects in one laboratory, and the collaborative research is required.
4.4. Effect of molar mass of added arabinoxylan on steamed bread quality
Since both gluten and starch play important roles to determine the bread quality, effects of non-starch polysaccharides on both gluten and starch should be studied to understand the effects on bread quality (Lagrain, Wilderjans, Glorieux, & Delcour, 2012; Wang et al., 2019). Wang et al. (2019) studied the effects of water-extractable arabinoxylan (WEAX) with different molar masses extracted from wheat bran on the heat-induced polymerisation of gluten and steamed bread quality. Since there have been contradictory reports on the effects of WEAX on the gluten network, one reported the strengthening while the other reported the weakening of the network, Wang et al. (2019) extracted four arabinoxylan fractions with different molar masses using enzymes and ethanol from wheat bran, and used two fractions F40 (MW = 491 kDa) and F60 (MW = 454 kDa). They found that the added WEAX reduced the heat-induced polymerisation of gluten, and this reduction rate was more pronounced for the lower molar mass fraction F60. Since the loaf volume was found to decrease with increasing heat-induced polymerisation of gluten, it was suggested that the lower molar mass fraction F60 was more suitable for the production of steamed bread. However, they also stated that excessive inhibition of gluten polymerisation resulted in the weakened gas retention capacity and thus the loaf volume could not be increased. In addition, the two fractions are different not only in molar mass but also in ferulic acid content, and monosaccharide (especially arabinose and mannose), more detailed study is required to understand the mechanism.
5. Phase separation induced fractionation
When two polymer solutions are mixed, phase separations due to thermodynamic incompatibility or synergistic interaction due to weak secondary molecular forces occur. Phase separation is classified into aggregative phase separation and segregative phase separation (Fang, Li, Inoue, Lundin, & Appelqvist, 2006). Tolstoguzov (2003) overviewed the application of phase diagram of many combinations of protein - polysaccharide, protein - protein, polysaccharide - polysaccharide. Zasypkin, Braudo, and Tolstoguzov (1997) reported the segregative phase separation of gelatin (pH4.9, MW = 300 kDa) – dextran - water systems using dextran with different MWs: 20 kDa, 65 kDa and 500 kDa. Below the concentration of gelatin or dextran 1.2 × 10 −2 g/g, the mixture of these two biopolymer solutions forms a single phase solution, but above this concentration the mixture is separated into two phases, gelatin rich solution and a dextran rich solution. The addition of small quantity (<0.5%) of dextran accelerated the gel formation of gelatin, and this accelerating action of dextran was found more remarkable with increasing molar mass of dextran as shown by the decreasing of the compliance of gels; a higher mass dextran expedited gelation of gelatin even at a lower concentration.
Edelman et al. (2003) reported the phase separation of gelatin – dextran - water systems. After mixing equal volume 5% gelatin and 5% dextran solutions and equilibrated at 60 °C for 20 h, the composition of upper and lower phases are determined (Fig. 25 ).
Fig. 25.
Effect of dextran molar mass on the molar mass distributions in coexisting phases at 60 °C. Α gelatin, Β dextran. Lines upper set, rich phase; lines lower set, poor phase; symbols (▲,●,■), components before phase separation. Mixtures: -, sample A2 (Gelatin 170 kDa + dextran 282 kDa); —, sample D (Gelatin 170 kDa + dextran 148 kDa). Native material before mixing: ▲, gelatin 170 kDa; ●, dextran 148 kDa; ■, dextran 282 kDa. In 0.1 M NaCl. Overall concentration of both gelatin and dextran is 5 wt% (Edelman et al., 2003).
While the molar mass distribution in the gelatin rich phase was not influenced by the molar mass of dextran in both gelatin rich and gelatin poor phases (Fig. 25A), the dextran content increased with decreasing molar mass of dextran in the initial mixture in dextran poor phase (lines lower set in Fig. 25B). The gelation of gelatin collected from gelatin enriched phase and gelatin depleted phase were compared with that of the initial native gelatin sample, and it was found that G′ (initial native) > G′ (gelatin enriched) > G′ (gelatin depleted). It was ascribed to the higher molar mass of gelatin in gelatin enriched phase than that of gelatin in gelatin depleted phase. This experimental finding was interpreted as the physical molar mass fractionation induced by phase separation. However, this fractionation was interpreted not only by molar mass difference but also by chemical composition of gelatin such as amino acid sequence thus leading to the finding G′ (initial native) > G′ (gelatin enriched) in spite of MW (gelatin enriched) > MW (initial native). Edelman et al. (2003) defined the concept of degree of fractionation = c x,poor,m/c x,rich,m, in which c x,poor,m and c x,rich,m are the concentrations of component x gelatin or dextran with a degree of polymerisation m in the depleted ("poor") and the enriched ("rich") phase. The value of m = 1 corresponds to a monomer. This degree of fractionation decreased with increasing m, and could fit the decrease of gelatin or dextran content with increasing molar mass in dextran poor phase shown in Fig. 25 (A or B) (lines lower set). Similar fractionation induced by phase separation was reported for maltodextrin-agarose (Loret, Schumm, Pudney, Frith, & Fryer, 2005).
Mao et al. (2013) found the phase separation induced fractionation in the mixtures of gum arabic (GA) and sugar beet pectin (SBP). GA has been used widely as an emusifier and is known to consist of three components namely arabinogalactan (fraction 1, AG); arabinogalactan protein (fraction 2, AGP) and glycoprotein (fraction 3, GP). Each fraction contains a range average of different molecular weight components which makes GA polydisperse (Al-Assaf, Phillips, Aoki, & Sasaki, 2007). SBP is used widely as an emulsifier because of its amphiphilic nature originating from the protein moiety, acetyl groups and the carbohydrate fraction. When mixtures of GA and SBP solutions stand quiescently, they are separated into GA rich lower phases and SBP rich upper phases (Fig. 26 A).
Fig. 26.
A. Phase diagram of GA/SBP mixtures at 25 °C: cloud points (■) determined by visual observation and binodal points determined by phase composition analysis for mixed systems with two different SBP starting concentrations: (▲,△) 0.8% SBP and (●,○) 1.0% SBP. The solid and open symbols represent the upper and lower phase, respectively. The broken lines are tie-lines. Closed circle ● on the straight line for 1.0% SBP represents the initial composition of GA and SBP before mixing, and after the phase separation this point moves to ● (in the upper phase) and ○ (in the lower phase) at the end of the tie-line, respectively (Mao et al., 2013). B. Change in the droplet size D4,3 (volume weighted mean diameter) of the emulsions containing 15% MCT and 5% GA, 5% FGA, 1% SBP or 1% FSBP during storage time for day 0, 3 and 7 at 60 °C (Mao et al., 2013).
After the phase separation, molar mass of GA and AGP in the lower phase increased remarkably. This fractionated GA (FGA) was shown to have higher emulsification ability: Emulsion droplets of 15% MCT (medium chain triglyceride) stabilised by FGA were smaller and remained small after storage at 60 °C for 7 days as shown in Fig. 26B (Mao et al., 2013).
Hu et al. (2018) found a stronger extent of molecular fractionation for higher molar mass GA (EM10) with MW of 4.07 × 106 g/mol and a polydispersity index (M w/M n) of 8.0 than for lower molar mass STD (standard gum arabic) with 0.55 × 106 g/mol and 2.5. In the phase separation induced fractionation of gum arabic/hyaluronan mixed systems at a fixed HA concentration of 0.25%, the AGP content was found to increase from 34% to 55% when the EM10 concentration was decreased from 5% to 2.5%. The AGP content of standard gum arabic also increased from 11% to 18% when the concentration of STD was decreased from 10% to 5%. The extent of increase in AGP content was much more significant for EM10/HA system than STD/HA system. This is in line with the previous findings that the fraction of higher molecular weight tends to segregate while the lower molecular weight fraction has relatively higher tolerance to coexistence (Edelman et al., 2003; Loret et al., 2005). Hu et al. (2018) further examined the effects of salt on the phase separation-induced fractionation of GA/HA mixtures. Sodium chloride reduced the electrostatic repulsion and thus phase separation of GA/HA which led to the decrease in AGP content obtained from phase separation-induced fractionation. They also examined the effect of temperature and found that AGP content obtained from phase separation-induced fractionation increased and then decreased, and showed a maximum at around 40 °C. The increase in AGP with increasing temperature was attributed to the decrease in the compatibility between GA and HA due to the increasing interaction of these polymers with the solvent, which led to the acceleration of phase separation. On the other hand, the decrease in AGP was ascribed to the decrease in molar mass of GA and HA caused by hydrolysis. However, this effect of temperature on the AGP content cannot be generalized because, for example, Edelman et al. (2003) have recognized no such effect of temperature on the phase separation behaviour of GA and dextran.
6. Molar mass effects in the stabilisation of suspensions and emulsions
Biopolymer are also used for stabilising dispersed particles or emulsion droplets by forming a steric stabilising layer, and higher molar mass biopolymers are more effective to make a thicker layer (Dickinson, 2003).
SBP molecules can be crosslinked by oxidative enzymes such as laccase and horse radish peroxide (HRP) resulting in the increase of molar mass of SBP. Molar mass of SBP was increased by this treatment with HRP; MW = 1.28 × 106 g/mol modified with 0.0025 U/mL HRP, MW = 1.55 × 106 g/mol modified with 0.0033 U/mL HRP, MW = 1.86 × 106 g/mol modified with 0.0050 U/mL HRP (Zhang, Shi, et al., 2015). Fig. 27 A shows the mean droplet size of the emulsion of medium chain triglyceride (MCT) prepared using the crosslinked SBP by HRP as a function of the time just after the preparation. As is clearly shown, the crosslinked SBP by HRP was more effective to stabilise the emulsion by virtue of the increased molar mass.
Fig. 27.
A. Volume weighted mean diameter D4, 3 of control and modified SBP stabilised-emulsions as a function of storage time at 60 °C. Molar mass of SBP was highest when treated with 0.0050U/mL. d-HRP stands for deactivated HRP in boiling water for 600 s (Zhang, Zhai, et al., 2015). B. Photographs of SeNPs stabilised by GA or AHGA during 60 days storage. Day “0” = immediately after sample synthesis, day “15”, “30”, “45”, “60” = standing time after synthesis (Kong et al., 2014). C. Photographs of the emulsions stabilised with BLG-modified ASY. Photo 0 h (the control, lower photos) and that with BLG-modified ASY-24 h (reacted for 24 h, upper photos) (Bi et al., 2017).
Selenium is an essential micronutrient although it is toxic in large doses. Its deficiency is believed to cause some diseases such as Kashin-Beck disease, and thus selenium nanoparticles (SeNPs) were prepared by the stabilisation of gum arabic (Kong et al., 2014). SeNPs are not stable without any dispersant and show aggregation. Chitosan was found effective to stabilise the SeNPs (Funami et al., 2008a, Funami et al., 2008b), which will be discussed later. GA was found effective to stabilise SeNPs, and SeNPs (particle size of 34.9 nm) were found to be stabilised in gum arabic aqueous solutions for ca. 30 days (Fig. 27B). The alkali-hydrolysed GA (AHGA) was also prepared and its efficiency in stabilising SeNPs was found to be less effective than GA. It was concluded that the branched structure of GA as well as molar mass were important for the stabilisation. It was also found that hydroxyl radical scavenging ability and 2, 2-diphenyl-1- picrylhydrazyl (DPPH) scavenging ability of GA-SeNPs were found higher than those of AHGA-SeNPs. Physiological function of Se will be discussed in 6.7.2.
Al-Assaf et al. (2007) obtained a series of thermally treated gum arabic samples among which they found an optimized physical treatment condition. The sample with increasing AGP content was found most suitable for emulsifying agent. Gum Acacia Seyal (ASY) is less valued than is gum Acacia Senegal, due to its poor emulsifying ability. Bi et al. (2017) modified this ASY via Maillard reaction with β-lactoglobulin resulting in the increase in AGP thus the emulsification ability of ASY was enhanced. ASY modified in this way contained a two-fold AGP content and 3.5 times higher average molar mass, and ASY-stabilised emulsions were found resistant against severe conditions, such as high saline or low pH conditions (Fig. 27C). More recently, in the study of the stability of emulsions stabilised by conjugates prepared by Maillard reaction between whey protein hydrolysates and linear dextrin with three DPs (24, 48, 65), the highest stability was found for the emulsion prepared with the linear dextrin with the highest DP, which conferred the highest steric hindrance (Pan et al., 2020).
Pickering emulsion stabilised by solid particles have been studied in food area (Murray, 2019) because of its long term stability. Fang et al. (2020) prepared amphiphilic sodium alginate colloid particles by Ugi reaction grafting hydrophobic octylamine chain to the hydrophilic sodium alginate chain, and prepared Ugi-Alg with three different molar masses (685 kDa, 307 kDa and 48 kDa). It was found that this amphiphilic Ugi-Alg formed self-assembled micelles, and the critical aggregation concentration was lowest for the highest molar mass Ugi-Alg, and this micelle with higher molar mass Ugi-Alg formed a more compact and stable micelle. It was also found that emulsions of soybean oil/water and styrene/water were stabilised better by higher molar mass than lower molar mass Ugi-Alg, which were proposed to be used as a drug delivery based on a model experiment of curcumin release. It is expected that the study on the safety of this kind of hydrophobically modified polysaccharides in food use will be clarified in the near future.
Maltodextrin is often used with a small molecule emulsifier to stabilise the emulsion. Klinkesorn, Sophanodora, Chinachoti, and McClements (2004) examined the critical flocculation concentration (CFC) of maltodextrin in the corn-oil-water emulsion stabilised by Tween 80, and found that CFC decreased with increasing molar mass of maltodextrin (Fig. 28 ). This was interpreted by the enhancement of the depletion flocculation with increasing the molar mass of maltodextrin. They explained this by calculating the depletion attraction potential assuming the proposal of Sperry (1982).
Fig. 28.
Critical flocculation concentration (CFC) of maltodextrins with different molecular weights in 5 wt% corn oil emulsions stabilised by Tween 80 after 24 h storage at room temperature (Klinkesorn et al., 2004).
6.1. Effect of molar mass of polysaccharides on physiological function
When a word β - glucan is used, it usually means (1 → 3) - β - glucans contained in cell walls of fungi, bacteria and cereals. Structure, properties and functions depend on the source and the extraction, preparation methods (Bacic, Fincher, & Stone, 2009). The simplest (1 → 3)- β-glucans are linear, unbranched chains as found in callose, curdlan, paramylon and pachyman. Some (1,3) - β - glucans have side-chain-branch such as chromistan and fungal laminarins and the fungal mucilage glucans, the (1,3)- β - glucosyl chain residues are substituted to varying degrees at C(O)6 by single β - Glc residues or in some instances by short (1,3) - β - oligoglucosyl chains. The (1,3; 1,4) - β - glucans from cereals and grasses, other embryophytes, lichens and some other taxa are unsubstituted, linear molecules with sequences mostly of two or three (1,4) - linked β - Glc residues, but with longer sequences of up to 15 β -Glc residues, joined by single (1,3) - linkages (Stone, 2009).
As can be seen in the book (Bacic et al., 2009) and in the subsequent recent papers, intensive studies have been performed because of the potential of (1 → 3) – β - glucans for its beneficial function such as anti-tumour, anti-oxidant, anti-inflammatory activities.
6.2. Effect of molar mass of (1 → 3) – β - glucans on anti-tumour activity
Anti-tumour activity of plant origin, especially of polysaccharides extracted from mushrooms, has been attracting much attention because anticancer drugs used in chemotherapy were found cytotoxic or the irradiation therapy damaged not only cancer cells but also the normal cells (Chakraborty et al., 2019; Chihara, Hamuro, Maeda, Arai, & Fukuoka, 1970; Meng, Liang, & Luo, 2016; Mizuno, 1996; Wasser, 2002; Zhang, Cui, Cheung, & Wang, 2007). Action mechanisms of anti-tumour activity are classified into direct and indirect. Indirect action refers to the immuno-enhancing activity, that is, activating T and B lymphocytes, macrophages and natural killer (NK) cells, and stimulating production of interferons (IFNs), interleukins (ILs), and other cytokines. Through this mechanism, the formation and growth of tumour cells are inhibited. Direct action of mushroom polysaccharides has been reported such as inhibiting tumour cell proliferation and/or inducing their death by apoptosis (Lemieszek & Rzeski, 2012; Mizuno, 1996; Wasser, 2002; Zhang et al., 2007).
Anti-tumour activity of β-(1 → 3)- glucans such as schizophyllan, lentinan, grifolan, and others have been studied extensively. It is well known that schizophyllan (SPG) takes a triple helix conformation in water, and random coil in DMSO or alkaline solvent (Gidley & Nishinari, 2009; Norisuye, 1985), and it has been used as anti-tumour agent. Tumour inhibition ratio was evaluated by the inhibiting effect of the anti-tumour active agent to reduce the weight of the tumour after treating the tumour, and thus defined as IR = (W 0 – W)/W 0, where W 0 is the average tumour weight of the control group, and W is that of the treated group. Some β-(1 → 3)- glucans such as schizophyllan, scleroglucan, curdlan, lentinan show a high inhibition ratio close to 1, indicating that the tumour degenerate with the lapse of time after the treatment, while pachyman and laminaran did not show such an activity (Chihara, 1977; Kojima, Tabata, Itoh, & Yanaki, 1986).
Kojima et al. (1986) examined the molar mass effect on the anti-tumour activity of SPG. They found that with decreasing molar mass SPG triple helix fraction decreased steeply at M = 5 × 104, and below that molar mass SPG was found random coil, and examined the molecular weight dependence of the tumour inhibition ratio using Sarcoma 180 ascites for the random-coil and the triple helix species of SPG. They concluded that schizophyllan has anti-tumour activity against Sarcoma 180 only when it has a molecular weight higher than 5 × 104 and a triple helical structure in aqueous solution (Fig. 29 ). Zhang, Li, Xu, and Zeng (2005) also reported that the triple helix conformation is necessary for lentinan, also a β-(1 → 3) glucan having β-(1 → 6) branching structure as SPG, to show anti-tumour activity, and thus lower molar mass lentinan is not effective.
Fig. 29.
A, Tumour inhibition ratio as a function of molar mass of schizophyllan (SPG) in water; B, Molar mass ratios for extensively sonicated samples. MW of SPG in water is 3 times of that in DMSO because high MW SPG takes a tripe helical conformation in water while it is dissolved in a random coil conformation in DMSO (Kojima et al., 1986; Norisuye, 1985).
A plethora of papers concerning the anti-tumour activities of some other polysaccharides have been reported. Some polysaccharides with lower molar mass was also reported to be effective in anti-tumour and immunomodulatory activities. A polysaccharide extracted from Artemisia argyi (MW = 5169Da) was reported to show immune-stimulatory effects (Bao, Yuan, Wang, Liu, & Lan, 2013). Zhong et al. (2015) examined the molar mass dependence of the anti-tumour activity using sonicated SPG samples. They got three fractions MW/Da: 5.78 × 106 (USPG40), 6.99 × 105 (USPG60), 4.89 × 104, (USPG80) by ultrasonic treatment followed by fractional precipitation using ethanol from original SPG which has the molar mass of 7.38 × 106. They found that the highest anti-tumour activity for USPG60 which has a lower MW than non-degraded SPG and USPG40 (Fig. 30 ), which seems to be contradictory to the results shown in Fig. 29. According to Fig. 29 (Kojima et al., 1986; Norisuye, 1985), the anti-tumour activity is decreased below the molar mass of ca 106, and the lower anti-tumour activity found for USPG80 than for USPG60 (Fig. 30) is in accordance with the previous finding shown in Fig. 29.
Fig. 30.
Release effects of serum samples containing SPG and USPG (ultrasonic treated SPG) on IL-2 and TNF-α level. Control (without SPG), SPG (7380 kDa), USPG40 (5780 kDa), USPG60 (699 kDa), USPG80 (48.9 kDa). USPG60 exhibited higher immune-regulatory and anti-tumour activity than other USPG fractions. A–D: Bars without the same superscripts differ significantly (p < 0.05) (Zhong et al., 2015).
More recently, effect of molar mass of SPG on the anti-inflammatory activity was reported by using SPG fractions degraded by ultrasonication (Du et al., 2016). Du et al. (2016) examined the anti-inflammatory activity by measuring the inhibition of nitric oxide (NO) which is known to be a Janus-faced biological agent and is involved in a series of both physiological and pathophysiological processes (Garzon-Porras et al., 2020). While playing an important anti-inflammatory role, NO molecules diffuse rapidly because of its lower mass, and thus macromolecules making NO release slower have been pursued (Garzon-Porras et al., 2020). Du et al. (2016) found that both native and degraded SPG inhibited the production of NO, but the lower mass SPG was more effective.
However, the criteria used to estimate the anti-tumour activity are different in research groups unfortunately, and thus the direct comparison is not possible. This should be further clarified.
Mo et al. (2017) reported that (1 → 3)- β –glucan from Saccharomyces cerevisiae had anti-tumour effects in S180 tumour cells, and the effect was found dose dependent but the molar mass of β –glucan was not changed in their experiments.
Anti-tumour activity of oat β –glucan was studied on three cell lines: HaCaT - normal, immortal cell line of the transformed phenotype in vitro, the first stable line of adult human keratinocytes presenting normal differentiation keratinocytes, and A549 - human epithelial lung cancer cell line, H69AR – human multidrug resistant small cell lung cancer cell line and normal human (Choromanska et al., 2018). Choromanska et al. (2018) found that low MW oat β –glucan did not affect so much the cell viability while high MW one effectively lower the cancer cells but not normal cells with increasing dose (Fig. 31 A).
Fig. 31.
A. The effect of high MW β - glucan on the cells viability. Error bars shown are means ± SD for n ≥ 3.*P ≤ 0.05 (Choromanska et al., 2018). B. Inhibition of hypotonic sodium chloride solution-induced hemolysis of human erythrocytes by β - glucan (400 μg/ml). On the graph marked course of hemolysis with 300 μg/mL LMW β -glucan (dash line ---) and 300 μg/mL HMW β glucan (small dots line ····) after 24 h of incubation. Course of hemolysis without β - glucan (control –) marked on the graph solid line (Choromanska et al., 2018).
While Choromanska et al. (2018) found some straight cytotoxic effects of β –glucan in A549 and H69AR cell line, no decrease of tumour cells proliferation was initiated in the other research group (Chan, Chan, & Sze, 2009). Choromanska et al. (2018) stated it necessary to evaluate the effect of β –glucan not only on cancer cells but also on normal human red blood cells. Thus, they examined the protective effects of β –glucan against hemolysis and found the increased effect in a dose dependent manner as shown in Fig. 31B. The protective effect to inhibit the hemolysis was found more pronounced for high MW β - glucan (dotted line) than for low MW one (broken line).
All these evidences suggest that the immune response is in part non-specific, determined by size rather than by chemical structure (Zhang, Kong, Fang, Nishinari, & Phillips, 2013), but more studies are required to determine the relation between the anti-tumour activity and the structure of the polysaccharide including molar mass, conformation, degree of branching. Anti-tumour activity is discussed again in Section 6.5 on Effect of molar mass of anionic polysaccharides on physiological function.
6.3. Effect of molar mass of β-(1 → 3)- glucans on anti-oxidant activity
Anti-oxidant active functions of β-(1 → 3)- glucans have been reported to depend on the molar mass. A Polish group (Błaszczyk et al., 2015) studied the effects of (1,3; 1,4) - β -glucans extracted and modified from oat with different molar masses, high MW (2,179,700 g/mol) and low MW (69,700 g/mol) on the alleviation of inflammatory condition and lipid peroxidation. Low MW β-(1 → 3) - glucan was manufactured by multistep freeze-milling (Harasym et al., 2015). The enteritis was induced in rats by intravenous injection of Escherichia coli lipopolysaccharides (LPSs). Both high and low MW oat β-glucans were found beneficial, but high MW oat β - glucan was proved to be more effective in decreasing stress oxidation in animals with LPS induced enteritis. Low MW oat β –glucan was found more effective in alleviating parameters in animals without administered LPS.
A similar difference between high and low MW barley β - glucans in the antioxidant activity was reported (Kofuji et al., 2012). Kofuji et al. (2012) found that the radical scavenge = (amount of hydroxy radical scavenged)/(amount of hydroxy radical generated) decreased about 50% by lowering the molar mass from 40,000 to 100,000 Da to 2000 Da.
Zhao et al. (2014) studied the effects of β - glucans extracted from oat, wheat and barley on the levels of lipid, superoxide dismutase (SOD), malondialdehyde in the liver of type 2 diabetic mice. The molar mass was found in the order of oat (172 kDa), wheat (635 kDa) and barley (743 kDa). The reduction of total cholesterol, triglyceride and malondialdehyde as well as the increase of SOD in the liver by the administration of β - glucans were found more conspicuous with increasing MW. Although the polydispersity in the MW distribution was different in three cereals, the difference in the effectiveness was believed due to mainly the difference in MW.
Since it has been demonstrated that molar mass, conformation, chemical modification, and solubility of β - glucans significantly affect their anti - cancer, immune - modulating, and anti - inflammatory properties, further studies are necessary to understand the mechanism using better defined samples (Du, Lin, Bian, & Xu, 2015).
6.4. Effect of chain lengths of hyaluronan on physiological function
Hyaluronan (HA) is found ubiquitously in the extracellular matrix (ECM) in a human body, 4100 μg/g in umbilical cord, 1400–3600 μg/g in synovial fluid, 8.5–18 μg/g in the thoracic lymph, 140–338 μg/g in the vitreous body and 200 μg/g in dermis (Fraser, Laurent, & Laurent, 1997). HA is a copolymer consisting of N-acetyl-d-glucosamine (GlcNAc) and sodium d-glucuronate (GlcANa). The HA chain is known to be stiff (Nishinari, Mo, Takahashi, Kubota, & Okamoto, 2000; Takahashi et al., 2003), and the viscoelasticity is the basis of its shock absorbing role in vocal fold (Dicker et al., 2014), synovial fluids (Balazs & Denlinger, 1985). Physiological effect of HA depends on molar mass, and the molar mass effects on the HA function in synovial fluids, wound healing and cancer are overviewed here.
6.4.1. HA in synovial fluids
Balazs (1968) reported mechanical spectra for synovial fluids taken for a healthy young adult, and a healthy elderly person and an osteoarthritis (OA) patient. A cross-over frequency at which the storage modulus and loss modulus coincide was found to shift to higher frequencies with increasing age, and no such crossover was found for a synovial fluid from an old person. Similar but more complete data were collected from normal 61 samples and OA 72samples by Kawata, Okamoto, Endo, and Tsukamoto (2000). Kawata et al. (2000) showed that the mechanical spectra of an OA patient becomes closer to that of a normal healthy person by adding high molar mass HA (Fig. 32 C).
Fig. 32.
Competitive inhibition in A (a) and B (a) and Enhancement of entanglement or association in A(b) and B(b). Solid curves in A represent G′ and G″ of HA while broken lines stand for G′ and G″ of HA to which 60 disaccharide (A(a)) or 50 disaccharide (A(b)) units were added (Fujii et al., 1996). HA short chains inhibit the entanglement or association of longer HA molecular chain in B (a) (competitive inhibition) while they can rather contribute to the entanglement or association in B (b). C. Effect of the addition of low and high MW HA to the synovial fluid of OA patients (Kawata et al., 2000). This is consistent with A (b) and B (b) but not with A (a) and B (a).
Since it was reported that molar mass of HA decreases or the concentration of HA decreases with aging, it is an important therapeutic problem how the rheology changes when short chains (sHA) coexist with long chains HA. If G′ and G″ of HA are decreased by adding sHA, is it better to remove sHA? Welsh et al. (1980) reported that the addition of 60 disaccharide units drastically changed the mechanical spectra of HA from an entanglement solution behaviour in which G′ and G″ show a crossover at ca 3–6 rad/s to a dilute solution behaviour in which G′ was smaller than G″ at all the angular frequency range from 0.1 rad/s to 30 rad/s and both moduli are strongly frequency dependent (Fig. 32A(a)). However, Fujii et al. (1996) found a slight increase of both moduli on adding 50 disaccharide units (Fig. 32A(b)). They prepared HA short chains with various chain lengths not only by enzymatic degradation which was employed by Welsh et al. (1980) but also by ultrasonic degradation and pyrolysis. One possible explanation for the discrepancy suggested by Morris (a senior author of Welsh et al., 1980) is the possibility of surviving enzyme used for obtaining short chain HA samples (Present authors are grateful to Prof. Morris for his suggestion).
The addition of small amount (0.01–0.1 M) of salts, sodium chloride, potassium chloride or calcium chloride was found to decrease both G′ and G″ while the addition of glucose increased both moduli (Kobayashi, Okamoto, & Nishinari, 1994). Since sHA chains consist of N-acetyl-d-glucosamine (GlcNAc) and sodium d-glucuronate (GlcANa), the addition of sHA includes a chain-length effect, a sugar effect, and a salt effect. Therefore, effects of the addition of sHA were explained in terms of the superposition of these three distinct effects. A shift factor “A” which depends on these three effects was developed to explain these additive effects quantitatively. Fujii et al. (1996) proposed schematic representation shown in Fig. 32B, where sHA inhibit the entanglement or association of longer HA molecular chains (Fig. 32B (a)), which was called competitive inhibition by Welsh et al. (1980), while sHA enhance the entanglement or association of longer HA molecular chains (Fig. 32B (b)). Based on the experimental finding that the addition of sHA of 50 disaccharide units to HA solutions increased both moduli (Fig. 32A (b)), the competitive inhibition explanation was not employed by Fujii et al. (1996).
The addition of HA moved G′ and G″ curves of OA synovial fluid to the region of higher modulus and the crossover point appeared (Fig. 32C). The addition of higher MW HA shifts the crossover point of a synovial fluid of an OA patient to lower frequency and higher modulus region approaching to the crossover point of normal synovial fluid. While the addition of lower MW HA shifted the crossover point of OA synovial fluid to higher modulus, the crossover frequency appeared at a higher frequency region far from the normal synovial fluid.
Therefore, in contrast to the proposed competitive inhibition mechanism by the addition of sHA for the drastic decrease of both moduli (Welsh et al., 1980), another possible mechanism was proposed by Fujii et al. (1996) to explain the observed increase of both moduli.
Bursitis is known to be a common knee pain symptom which is induced by excessive synovial fluids. Effects of injection of high or low molar mass HA to the joint synovial fluid of OA patients were studied, and it was found that higher MW HA decreased some specific protein such as apolipoprotein A-1 and interleukin 1 beta which are thought to be related to inflammatory processes lipid transport, and subsequent pain in synovial fluid, and increased some proteins such as transthyretin which are believed to be anti-aging, more efficiently than lower MW HA (Chen et al., 2014).
More detailed description about the molar mass effect on rheology of HA synovial fluids are found in Lapčik, Lapčik, de Smedt, Demeester, and Chabreček (1998), Fam, Bryant, and Kontopoulou (2007), Maytin (2016), and Gupta, Lall, Srivastava, and Sinha (2019).
6.4.2. HA in morphogenesis, cancer and would healing
HA is enriched in tumour cells, and the effect of HA on tumour progression and metastasis depends on molar mass (Dicker et al., 2014). HA accumulation in tumour is determined by the balance between the HA synthase and the degradation by hyaluronidase. Hyaluronidases produce HA short chains to stimulate endothelial cell proliferation and budding of new capillaries that promote angiogenesis to allow tumour expansion. It was found that hyaluronidase-fragmented HA induced an angiogenesis, formation of new blood vessels. This angiogenesis was found to be induced by oligosaccharides ranging from 4 to 25 disaccharide units of HA but not by native non-degraded HA (West, Hampson, Arnold, & Kumar, 1985).
Tolg et al. (2017) reported that HA synthesis was required for mammary gland branching and 10 kDa HA fragments strongly stimulate branching, but the activity of HA decreases with increasing molecular weight and 500 kDa HA strongly inhibited this morphogenetic process. HA was used as wound healing therapy. Here again, the physiological function is found to depend strongly on molar mass, and high molar mass HA and low molar mass HA were reported to function in opposite directions: while high molar mass HA has already been used in would healing based on its anti-inflammatory and immunosuppressive properties, low molar mass HA was reported to be a potent proinflammatory molecule.
Oligo HA, 2–10 disaccharide units, were shown to stimulate endothelial proliferation at a lower concentration than native higher molar mass HA. In addition, the lesion area was found completely recovered when the endothelial layer was exposed to oligo HA for 40 h after injury, while the wound remained half recovered pretreated with native HA at the same condition (Gao, Yang, Mo, Liu, & He, 2008).
More examples of the different effects of molar mass of HA on physiology/symptoms have been discussed in detail in previous reviews (Cyphert, Trempus, & Grantziotis, 2015; Dicker et al., 2014; Litwiniuk, Krejner, & Grzela, 2016; Turley, Noble, & Bourguignon, 2002).
6.5. Effect of molar mass of anionic polysaccharides on physiological function
Prebiotics from marine algae have been reviewed (Fedorov, Ermakova, Zvyagintseva, & Stonik, 2013; Gurpilhares, Cinelli, Sima, Pessoa, & Sette, 2019). Treatment of cancer has been studied extensively, and many methods have been proposed. Since cancer cell life is controlled in cell cycle as other cells, modulation of cell cycle or cell cycle arrest has been studied, inducing the cancer cell death called apoptosis, prevention of angiogenesis which nourished cancer cells, inhibiting cellular migration to prevent the metastasis, the diffusion of cancer cells to the other sites, enhancing the activity of natural killer NK cells (Wu, Sun, Su, Yu, Yu, & Zhang, 2016; Tomasetti & Vogelstein, 2017). For the last few decades, depolymerised polysaccharides have been applied for their anti-tumour activities mainly because they have better solubility than native polysaccharides, and can penetrate into cells because of its smaller size (Wu et al., 2016). However, some contradictory results have been reported, and it is necessary to compare carefully data from different laboratories.
6.5.1. Alginate
Pseudomonas aeruginosa produces extracellular alginate which interacts with tracheobronchial mucin. Thick and sticky mucus is accumulated. Lung disease results from clogging of the airways due to mucus build-up, decreased mucociliary clearance, and resulting inflammation. This is one of main signs and symptoms of cystic fibrosis. In the pulmonary clearance mechanism, tracheobronchial mucus traps inhaled particles and bacteria, which should be carried away by mucociliary transport. However, Pseudomonas aeruginosa, an opportunistic human pathogen cannot be easily removed by this mechanism.
Mucin is known to consist of heavily glycosylated regions and sparingly glycosylated region, and these units are linked by intra- and inter-molecular disulfide bonds (Fuongfuchat, Jamieson, Blackwell, & Gerken, 1996). Funami et al., 2008a, Funami et al., 2008b reported that the storage modulus of an ex vivo sputum sample from a cystic fibrosis patient after pre-shear (60 s at 20 s−1) decreased by the addition of sodium chloride, but much more decreased by guluronate block, GB (degree of polymerisation, Dp20) and by the addition of GB (Dp10) decreased to 1/6 of the control sputum without addition of NaCl or GB (Fig. 33 ).
Fig. 33.
The initial mechanical response of an ex vivo sputum sample from a cystic fibrosis patient after pre-shear (60 s at 20 s−1) in the presence of guluronate blocks with different degree of polymerisation, and in the presence of NaCl corresponding to the increase in ionic strength imposed by the oligoguluronates (Draget & Taylor, 2011).
Immunogenic response was also reported for high molar mass alginate, which was lost by depolymerisation (Draget & Taylor, 2011). Polymannuronate (PM) block was found more effective for tuber necrosis factor α (TNF-α) production than polyguluronate (PG) (Berntzen et al. (1998)). They found that the molar mass reduction from PM (MW = 35,000) to (MW = 5500) reduced the TNF-α-inducing potency by a factor of 10–100. As for the stronger activity of PM than PG, Draget and Taylor (2011) suggested that mannuronate groups not incorporated in gel network can more directly trigger an immune response. On the other hand, Ueno et al. (2012) reported that PG showed a stronger macrophage-stimulating activity than PM. The same research group of Oda (Iwamoto et al., 2005; Xu et al., 2014) recognized that oligomannuronate (DP7) and oligoguluronate (DP8) having unsaturated terminal structure with a double bond showed the strongest TNF-α-inducing potency, and that saturated oligomers of PG and PM showed no or less potency. They concluded that the unsaturated terminal structure, molecular size, and M/G ratio, are important in macrophage activation. It is necessary to study further the mechanism of immunomodulative action of alginate using well defined alginate with different molar mass, M/G ratio and blockiness.
6.5.2. Pectin/galactan
Pectin, used widely in food industry for its gelling, thickening (apple, citrus), emulsifying (sugar beet) abilities, has been studied for its beneficial physiological function, dietary fibre and anti-tumour activities (Leclere, Van Cutsem, & Michiels, 2013). Since it was found that pH-modified pectin binds to galectin-3 which plays important roles in suppressing cancer cell apoptosis, decreasing sensitivity of cancer cells to chemotherapeutic drugs, thus leading to cancer progression and metastasis, investigations to inhibit galectin-3 activity have been done extensively (Leclere et al., 2013). Zhang, Shi, et al. (2016) prepared sub-fractions of type-I rhamnogalacturonan-rich pectins and homogalacturonan-rich pectins by acid hydrolysis of citrus pectin, and studied the anti-galectin-3 activity of these pH-modified citrus pectin (MCP), MCP-1a, MCP-2a, MCP-2b, MCP-3Sa, MCP-3Sb, MCP-3P, MCP-4a and MCP-4b, with MW 162, 130, 8, 170, 11, 20, 180, and 39 kDa, respectively. The inhibition of galectin-3 activity by MCP was evaluated by the hemagglutination assay, and MCP-2 (MW = 130 kDa) was found to show the lowest minimum inhibition concentration, indicating the best inhibitory performance. Since citrus pectin fractions are heterogeneous, it is difficult to understand the mechanism of the inhibition.The same research group (Zheng et al., 2018) purified a series of galacto-oligosaccharides (GOs) from potato galactan, with DP up to 15. They evaluated prebiotic effects of GOs on Lactobacillus strains including L. rhamnosus JAAS8 and L. plantarum strains K25 and S52, and found that JAAS8 grew on all GOs up to DP15, K25 grew only on GOs up to DP5, and S52 grew only on GO1. Since they found the different growth rates for each strains, they expect that further study on the molar mass effect will be interesting.
6.5.3. Ulvan
Ulvan is a sulphated polysaccharide, extracted from green algae Ulva and Entermorpha, mainly composed of rhamnose, xylose, glucose, glucuronic acid, iduronic acid, and sulphate, with smaller amounts of mannose, arabinose, and galactose (Lahaye & Robic, 2007). By controlling the pressure and temperature, different molar mass compounds U1 (MW = 64.5 kDa) and U2 (MW = 28.2 kDa) were prepared from ulvan (MW = 151.6 kDa), and their effects on lipid metabolism in male Wistar rats were studied (Pengzhan et al., 2003). Diet of ulvan, U1 and U2 group contains 0.5 g/100 g each ulvan, U1 and U2 in addition to the control diet consisting of maize starch (35 g), cholesterol (1 g), wheat flour (45 g), cholic acid (0.2 g), lard (4 g), yolk (7 g), methylthiouracil (0.2 g), wheat bran (6.6 g), sodium chloride (1 g). These authors reported that diet supplemented with ulvan significantly reduced serum total cholesterol (TC) (−45.2%) and low density lipoprotein cholesterol (LDLC) (−54.1%) concentrations in comparison with diet for control rats (P < 0.05) although no marked effects on serum total glyceride (TG) and high density lipoprotein cholesterol (HDLC) concentrations was shown. As for the diet supplemented with lower mass fractions U1 and U2, TG was decreased and HDLC was increased, which is more beneficial, in comparison with diet for control rats. However, the TC and LDL were rather increased in comparison with control and non-degraded ulvan although the difference was not significant. Since the main difference between non-degraded ulvan and degraded fractions U1, and U2 was the molar mass because the sulphate and uronic content were not so different, the above mentioned physiological difference found in rats fed with the non-degraded ulvan and degraded U1 and U2 samples might be attributed to the molar mass difference. However, it is difficult to find a conclusive mechanism from these data because lower molar mass U2 increased HDL but increased also TC, TG and LDL than in U1 and non-degraded ulvan fed rats.
6.5.4. Carrageenans
Antioxidant activity of carrageenans was found to increase by gamma-irradiated degradation into oligosaccharides (Abad, Relleve, Racadio, Aranilla, & de la Rosa, 2013). Among κ-, ι-, λ-carrageenan samples, κ-carrageenan showed the highest DPPH radical scavenging activity, and this activity was increased with increasing concentration of carrageenan, and with decreasing molar mass. DPPH scavenging activity of carrageenans was found lower than that of ascorbic acid. Increase in irradiation dose led to the decrease in molar mass of carrageenans and increase in reducing sugar (Abad et al., 2013). The authors expect the possible application of these carrageenan oligosaccharides in healthy food production retarding the lipid peroxidation in shelf stable meat products although they also think it necessary to confirm the safety issue. Sun et al. (2015) examined the antioxidant activity of κ-carrageenan oligosaccharides prepared by four methods, using free radical depolymerisation, mild acid hydrolysis, κ-carrageenase digestion and partial reductive hydrolysis. As in a previous report (Abad et al., 2013), they also recognized the importance of the reducing sugar content which increased simultaneously with decreasing molar mass in addition to the sulphate content.
Low molecular weight carrageenans were reported to exert anti-tumour effects by promoting the immune system in mice inoculated with S180 tumour cell suspension (Fedorov et al., 2013). However, as will be discussed below, some negative function such as inducing colitis has been also reported.
Anti-colon-cancer effects of kappa-carrageenan from red sea weed Kappaphycus alvarezii were studied using two fractions with different molar masses F1 (MW = 67 kDa) and F2 (MW = 27 kDa) (Raman & Doble, 2015). Unfortunately, not only MW but also other chemical characteristics were also different; galactose (54.1% (F1) and 80.6% (F2)), uronic acid (45.6% (F1), 19.4% (F2)), sulphur (6.1% (F1), 5.4% (F2)). High molar mass (>200 kDa) carrageenan was not used because of its poor solubility, processability and the difficulty in uptake. HEK293 (human embryonic kidney cells), L6 (rat myoblast cells) and HCT116 (human colon cancer cells) lines were used to examine the anticancer activity of F1 and F2. Both of F1 and F2 were found to reduce the number of viable HCT116 cells in a dose-dependent manner (reduction of viable cells from 95.9 to 79.6% and from 90.5 to 63.5%, with increase in the concentration of F1 and F2 from 0 to 1000 μg/ml, although no cytotoxicity was found in HEK293 and L6. The reduction in HCT116 cells was more pronounced in F2 than in F1. This was corroborated by morphological changes of cells in HEK293, L6 and HCT116 observed using inverted fluorescent microscope. The cell number was reduced with treating by F1 and F2, and apoptosis (60–80%) with fragmentation and nuclei condensation were observed. The intracellular reactive oxygen species (ROS) determined using 2′,7′-dichlorofluorescin diacetate (DCFH-DA) increased in F1 and F2 treated cells in HCT116 cell lines while no variation with respect to the control was observed in HEK293 and L6 cell lines. Based on further analysis of cell cycle (G1, S and G2), Raman et al. (2015) found that G1 phase arrest for F2 treated cells was higher than for F1 treated cells, and they ascribed this to the difference in molar mass and the sulphur content as reported by others (Fedorov, Ermakova, Zvyagintseva, & Stonik, 2013).
On the other hand, some research group (McKim et al., 2019) criticized the assertion of the groups (Tobacman, 2001) which showed the harmful effect of carrageenans, stating that this assertion originated from the unrealistically extreme sample preparation as mentioned in 1.2 Why molar mass? The debate continues. Shang et al. (2017) using commercially sold κ-, ι-, λ-carrageenan samples examined the effects of intake of carrageenans on the induction of colitis in C57BL/6 J mice. They found some differences in these three carrageenans, for example, κ-carrageenan seemed to be a promoter for the growth of Helicobacteraceae, while ι- and λ-carrageenan appeared to be inhibitors for the growth of this bacterium. They also found that non-fermentable κ- and ι-carrageenans inhibited the growth of this bacterium Desulfovibrio, probably due to the antibacterial activity of κ- and ι-carrageenans, while λ-carrageenan promoted the growth of this bacterium. Most importantly, the authors found all κ-, ι-, λ-carrageenans significantly decreased the populations of Akkermansia muciniphila which is a potent anti-inflammatory commensal bacterium in the gut. Thus, they concluded that this decrease of anti-inflammatory bacterium caused by the intake of carrageenans led to induce the colitis. This is surely an interesting point as the authors stated. Although the pathological analyses were done in detail, unfortunately the detailed information on the molar mass and the sulphate content, which might have some influences on the pathology, were not described.
More recently, another group (Mi et al., 2020) reported that the inflammation promoting action of carrageenan (MW was determined as 365 kDa) was only found in the high-fat diet group which consumed carrageenan in drinking water. These authors estimated the inflammation promoting action in terms of disease activity index, myeloperoxidase activity, tumour necrosis factor-alpha (TNF-α), Toll-like receptor 4 (TLR4), and they found that mRNA expression of TLR4 and TNF-α (pro-inflammatory cytokines) did not significantly differ in low-fat diet model, which was in contrast with the data of Shang et al. (2017). This discrepancy was attributed to the molar mass difference between the purchased carrageenan from Sigma in Shang et al. (2017) and extracted (native) carrageenan used in Mi et al. (2020).
Some groups assert the harmfulness of carrageenans especially lower mass ones while the other groups report the beneficial functions of carrageenans especially degraded ones. However, there have been no reports unfortunately using carrageenans with different molar masses with controlling sulphate and cation content. This should be important to improve the understanding the mechanism of carrageenan-induced colitis or its safety.
Ai et al. (2018) examined the anti-inflammatory effects of native carrageenans and the depolymerised oligosaccharides on lipopolysaccharide (LPS) treated murine microglia BV-2 cell line. By stimulation with LPS via TLR-4, microglia cells are expected to secrete TNF-α and interleukin 6 (IL-6), two representative pro-inflammatory cytokines, to induce acute, inflammatory responses in the host. Therefore, microglia BV-2 cell line treated with LPS was believed to be one of representative models for studying the anti-inflammatory potentials of target samples (Jeng, Hou, Wang, & Ping, 2005). Native κ-carrageenan (96.2 kDa), ι-carrageenan (113.5 kDa), λ-carrageenan (MW not determined) samples were heated at acidic pH and oligo κ-carrageenan (0.5 kDa), oligo ι-carrageenan (4.1 kDa) were obtained (Ai et al., 2018). Pretreatments of most of polymeric carrageenans at 250–500 μg/mL were found significantly to increase the TNF-α level, implying the co-inflammatory effects with LPS. The co-inflammatory effectiveness of pure carrageenans at 125 μg/mL was notable for λ-carrageenan, followed by ι-carrageenan, and insignificantly for κ-carrageenan. Sulphate content is κ-car < ι-car < λ-car. This suggested a non-negligible role of sulphate content. Oligo ι-carrageenan and κ-carrageenan were found to decrease TNF-α significantly, and were expected to be anti-inflammatory agents. The authors found that anti-inflammatory effects of carrageenan oligosaccharides in their study were comparable to those found for chitin oligosaccharides at 100–500 μg/mL. Ai et al. (2018) further speculated that their polymeric carrageenans may have no permeability into BV-2 cells, as in the case of human colon cancer cells Caco-2, and suggested that polymeric carrageenans may bind toward TLR-4 moieties of BV-2 cells and inducing pro-inflammatory cytokines, like LPS interaction or like the mechanism of carrageenans binding to human colonic epithelium cells. They recognized the necessity of further study to clarify the effects of oligo carrageenans on the signaling pathways.
While many papers reported the beneficial physiological effects of low molar mass and the sulphate content of carrageenans, Sokolova et al. (2020) studied the effect of carrageenans on the lipid metabolism, and concluded that a low degree of sulphate and high molar mass were a prerequisite for the ability of carrageenans to modulate prostaglandin E2 synthesis. Prostaglandin E2 is known to be a lipid mediator that contributes substantial value to immune regulation and the maintenance of gastrointestinal homeostasis, including mucus secretion, gastric cytoprotection and mucosal blood flow (Sokolova et al., 2020).
6.5.5. Fucoidans
Fucoidans extracted from brown seaweeds are composed of mainly sulphated l-fucose residues attached to each other by α-1,3- or interchangeable α-1,3- and α-1,4-bond, and their anticancer and cancer preventive properties have been extensively studied (Fedorov et al., 2013; Wu, Sun, Su, Yu, Yu, & Zhang, 2016). The anticancer activity of fucoidans from sporophyll of Undaria pinnatifida was found to increase by lowering their molecular weight by mild hydrolysis (Yang et al., 2008). Yang et al. (2008) prepared 5 fucoidan samples by heating fucoidan solution in 0.01 N HCl with MW ranging from 260 kDa to 5100 kDa. Anticancer activity of the native and partially hydrolysed fucoidans was determined using sulphorhodamine B assay, which was based on the measurement of cellular protein content. The human lung cancer cell line, A549 was used in their study. Anticancer activity was found to increase with increasing MW and was found highest at MW = 490 kDa. These authors suggested that lower MW fucoidan hydrolysates had greater molecular mobility and interact more effectively with cancer cells, but further lower MW hydrolysates could not be effective probably because of the partial removal of sulphate groups from fucoidan polymers during hydrolysis. Although the authors did not report the decrease in sulphate groups quantitatively, it seems to be consistent with some previous papers reporting the increase in the anticancer activity with increasing sulphation (Cho, Lee, & You, 2011).
You, Yang, Lee, and Lee (2010) examined the molar mass dependence of anticancer activity of hydrolysed fucoidan F >30k (MW > 30 kDa), F5-30k (MW = 5–30 kDa), F<5k (MW < 5 kDa). They found the highest activity in F5-30k, and the reason for the lower activity of the lowest MW F<5k was attributed to the removal of sulphate groups during hydrolysis because they also believed that sulphate played an important role as in previous reports. They also examined the conformation of fucoidan hydrolysates based on the relation between the radius of gyration and the weight average molecular weight obtained from the HPSEC-MALLS measurement. They reported that the highest MW fraction F >30k was compact spherical conformation and the anionic sulphate groups available to bind proteins on the cell surface may be hidden inside the chains while F5-30k fraction took more loose and entangled conformation allowing sulphate groups in the chain are available to bind the proteins. It was unfortunate that the sulphate content of F5-30k fraction was higher (ca 35.5%) than the other two fractions F >30k and F<5k, which makes the speculation difficult; which of the two, sulphate content or the conformation, was more influential to the activity. In addition, the monosaccharide composition was reported to be slightly different. It is indeed not so easy to obtain clear-cut sample fractions.
On the other hand, Clement et al. (2010) examined the structural effects of fucoidan hydrolysates F1 (MW = 3090 Da, sulphate = 37%), F2 (MW = 3200 Da, sulphate = 34%), and a synthetic pentasaccharide (MW = 1420 Da, sulphate = 45.8%) on the complementary activity. The complement system, an essential part of the innate immune system, is involved in various autoimmune diseases, e.g., rheumatoid arthritis, systemic lupus erythematosus. Activation of the complement system by autoantibodies results in immune activation and tissue damage (Biewenga et al., 2020). Complement is activated through three major ways: the classical pathway depending on antigen-bound antibody recognition and including the proteins C1q, C1r, C1s, C2, C4 and C1Inh, the lectin pathway involving the mannose-binding lectin MBL—this pathway merges with the classical pathway at the C4 activation step—and the alternative pathway triggered by contact on microbial and foreign surface (Clement et al., 2010; Lambris, Reid, & Volanakis, 1999). Clement et al. (2010) found a remarkable difference in the complement inhibition IC50 (μg) by hemolysis of sensitized erythrocytes for the three oligosaccharides F1 (32 μg), F2 (3 μg) and pentasaccharide (>50 μg), where IC50 is the amount (μg) of fucoidan inhibiting 50% of the complement-mediated hemolysis. The result indicated that F2 was the strongest inhibitor of complement although the MW was similar with F1 and sulphate content was even lower than F1. Clement et al. (2010) analysed the structure of the oligosaccharides by NMR and molecular modelling, and concluded that the branching in F2 made an effective interaction with complement protein and also create more additional binding sites than linear oligosaccharides. In the anti-tumour activity of SPG, the triple helical conformation was believed to be a key factor, and the critical minimum MW, below which no triple helices were formed, was reported. For fucoidan, no such criterion was proposed probably because of the structural heterogeneity.
Choi and Kim (2013) prepared depolymerised fucoidan by gamma-irradiation with MW from 217 kDa (non-irradiated fucoidan) to 7 kDa. It was confirmed that sulphate content (820 μg/mL in 0.1% fucoidan solution) was only slightly decreased but not significantly changed by the irradiation. They estimated the cytotoxicity of fucoidan fractions by human breast cancer cells (MCF-7), and found that cytotoxicity increase with increasing concentration of fucoidan fractions and with decreasing MW below 16 kDa. Cytotoxicity was also analysed using human stomach cancer cells (AGS) and human hepatocellular carcinoma cells (HepG2), and similar molar mass dependence was observed. Moreover, inhibitory effect of lower MW fucoidan on cell transformation was examined using TPA (12-O-tetradecanoylphorbol-13-acetate) –induced neoplastic cell transformation in JBG mouse epidermis cells. TPA is known to be a highly potent tumour promoter and is widely used for cell transformation. JB6 mouse epidermal cells were treated with TPA with or without native and irradiated fucoidan samples. It was shown that fucoidan inhibits the formation of TPA-promoted neoplastic cell transformation of JB6 cells in a concentration dependent manner; cell transformation was decreased by the treatment of irradiated fucoidan (7 kDa) even at 1 μg/mL with statistical significance (p < 0.05). They attributed the inhibitory effect of lower MW fucoidan to the easier access of small fucoidan into the target protein molecules.
Guo et al. (2014) using ultrasonic depolymerised fucoidan fractions from sea cucumber, examined the antioxidant activity by DPPH radical scavenging activity assay and oxygen radical absorbance capacity (ORAC) analysis. Sulphate groups, which were believed to play an important role in antioxidant activity, were found slightly increased. They found that antioxidant activity increased first and then decreased with increasing molar mass. The higher antioxidant activity in the depolymerised fucoidan was attributed to more free hydroxyl and amino groups and higher contents of reducing sugars at the same mass concentration. However, excessive depolymerisation was thought to lead to the higher production of free radicals thus reducing the antioxidant activity.
Lahrsen, Schoenfeld, et al. (2018) prepared low MW fucoidan by hydrothermal treatment and by hydrogen peroxide treatment. Starting from the native fucoidan (38 kDa) they obtained depolymerised fucoidan (MW ranging from 10.3 to 34.4 kDa) by hydrothermal treatment, and those (MW ranging from 4.9 to 25.0 kDa) by H2O2 treatment. They found that the antioxidant activity represented by radical scavenging potential using DPPH assay was not changed significantly for hydrothermally treated depolymerised fucoidan (MW = 24.0, 15.1. and 10.3 kDa) but decreased for H2O2 treated ones (MW = 12.9, 25.0 and 9.1 kDa). They also examined the anti-proliferative and cytotoxic effects of fucoidan and its depolymerised fraction on cancer cells using Raji cells, a Burkitt lymphoma cell line, and found that hydrothermal treatment did not reduce the antiproliferative effect but H2O2 treatment eliminated this effect. They attributed this elimination of antioxidant and anti-proliferative effects to the removal of phenolic compounds (e.g. phlorotannins) or terpenoids (e.g. fucoxanthin) during the H2O2 treatment. Lahrsen, Liewert, et al. (2018) further studied the molar mass effects of fucoidans on pharmacological activities using differently depolymerised fucoidans as described above. Polymorphonuclear (PMN) elastase, a kind of proteinases, is known to digest invading pathogens and tissue debris. During this phagocytosis, PMN elastase is excreted to extracellular surrounding, and its activity is controlled by α1-proteinase inhibitor. The concentration of the complex of PMN elastase with α1-proteinase inhibitor gives the measure of the activity of the PMN granulocytes. Lahrsen, Schoenfeld, et al. (2018) found that inhibitory effect of PMN elastase decreased with decreasing molar mass of fucoidan fractions. They further examined the activities relevant for treating inflammation diseases, inhibition of complement mediated hemolysis, inhibition of C5a generation, potentiation of C1 esterase inhibitor, and also undesired effects, inhibition of coagulation and activation of factor XII. They found all these activity of depolymerised fraction in relation to native fucoidan decreased with decreasing MW. They compared the anticoagulant activity of depolymerised fractions of fucoidan with that of unfractionated heparin, which is used as anticoagulant. They found that all the activities of depolymerised fractions of fucoidan were much lower than those of heparin which were attributed to the very low DS (degree of sulphation) for depolymerised fractions of fucoidan ca. 0.5–0.6 vs. 1.0 for heparin. The anticoagulant activity of depolymerised fractions of fucoidan was much lower than that of unfractionated heparin. Since heparin is known to have an adverse effect of bleeding, Lahrsen, Schoenfeld, et al. (2018) expected that even high dosages of depolymerised fractions of fucoidan might be applicable without increasing the bleeding risk. They also pointed out that the affinity of heparin to anti-thrombin is so high that in vivo, i.e. in the presence of anti-thrombin, all other activities are considerably weakened due to its binding to anti-thrombin. Then they concluded that their in vitro data were in line with animal experiments showing that the anti-inflammatory activity of fucoidan is superior to that of heparin (Pomin, 2015).
6.5.6. Ascophyllans
Bioactive characteristics of a polysaccharide ascophyllan extracted from a brown seaweed Ascophyllum nodosum have been studied extensively. It has similar but distinct structure characteristics from fucoidans by the presence of a backbone of β-(1, 4)-d-mannuronic acid with fucose-containing branches (3-O-d-xylosyl-l-fucose-4-sulphate) (Yu et al., 2020). Yu et al. (2020) reported that lower mass (MW = 6.96 kDa) ascophyllan hydrolysed by alginate lyase showed stronger wound healing and antimicrobial activity than native ascophyllan (MW = 390 kDa). Wound healing assay was performed by evaluating effects of ascophyllan and its hydrolysate (LMWAs-L) on the migration of human skin fibroblast (HSF) cells. The effect was evaluated by the percent of open area (POA) of the scratch on the HSF cell monolayer. No inhibitory effects of both ascophyllan and LMWAs-L on the viabilities of HSF cells in the range of experimental concentration (0–200 μg/mL) were found, and rather both these saccharides were shown to promote significantly HSF cell proliferation. Moreover, the promotion of LMWAs-L was more significant than that of native ascophyllan. POA was found to decrease with the lapse of time after 12, 24 and 36 h after treatment by ascophyllan and LMWAs-L, and the decrease was more pronounced when treated by LMWAs-L than by native ascophyllan (Fig. 34 ). Although the detailed mechanism for the difference of effectiveness between a high and low MW polysaccharides was not elucidated, the authors interpreted as follows: Since the molecular size of LMWAs-L is much lower than that of native ascophyllan, the molar concentration of LMWAs-L should be much higher than those of native ascophyllan under the same mass concentration conditions. Therefore, the higher molar concentration of LMWAs-L than ascophyllan can effectively and sufficiently bind with some receptors on the cell-surface of HSF cells, which promotes the migration of HSF cells.
Fig. 34.
Wound healing activity represented by open area of the scratch on the human skin fibroblast monolayer treated by ascophyllan and LMWAs-L (lower MW ascophyllan). LMWAs-L showed a faster decrease of open area indicating a faster healing. Different letters suggest significant differences, P < 0.05 (Yu et al., 2020).
The authors further examined the antibacterial activity using a gram-positive bacteria Staphyrococcus aureus and gram-negative bacteria Escherichia coli. Both ascophyllan and LMWAs-L were found to inhibit the growth of these bacteria, and the effect was found more pronounced for LMWAs-L. The authors explained the growth inhibition by binding of the polysaccharides with some compounds in the cell walls, cytoplasmic membranes, and DNA of bacteria, which led to the disruption of these cell components, causing the dissolution of the protein and leakage of essential molecules, and ultimately results in the death of bacteria. As for the different effectiveness of molar mass, they attributed to more polyanionic property of a lower mass LMWAs-L that combined with the membrane proteins causing a membrane-disrupting effect.
6.5.7. Molar mass effect of polysaccharides on the activity of bone morphogenic protein (BMP)
With the aging of society, the number of osteoporosis-related fractures is increasing (Nishizawa et al., 2019). Bone morphogenic protein (BMP) have been studied because of its stimulating function of bone regeneration (Peschel, Zhang, Fischer, & Groth, 2012; Song et al., 2018). Among them, BMP-2 has been attracting more attention, however it has some problems, fast proteolytic degradation, and the high dose treatment may lead to adverse side effects. Therefore, some polysaccharides heparin (HP), dextran sulphate, sulphated cellulose and chitosan have been used as adjuvant to stabilise BMP-2 and enhance its osteogenic activity (Peschel et al., 2012; Song et al., 2018). Song et al. (2018) investigated the enhancing effect of sulphated polysaccharide extracted from the gonad of pacific abalone (Haliotis discus hannai Ino) named AGSP and compared with those of chondroitin sulphate (CS) and heparin. MW and sulphate content of three polysaccharides were; AGSP (6.6 kDa 12.4%), CS (130 kDa, 18.5%) and heparin (35 kDa, 28.4%). The main structure residue of AGSP was determined as →3)-GlcA(1 → 3)-Gal(1→ with sulphated branches comprised of prevalent Gal and minor Glc, and →4)-β-GlcA(1 → 2)-α-Man(1→ residue was also found. Osteogenic activity of these derivatives was studied in cooperation with BMP-2 using C2C12 myoblast cells as a model system measuring alkaline phosphatase (ALP) activity (Peschel et al., 2012; Song et al., 2018). All HP, CS and AGSP enhanced the BMP-2-induced osteogenic activity with increasing polysaccharide concentration up to 50 μg/mL but HP with highest sulphate content decreased the activity above that concentrations, which was attributed to negative impact of HP on cell viability at higher concentrations (Song et al., 2018). This negative effect of HP was in good agreement with a previous study (Peschel et al., 2012). It was believed that higher sulphate contents as well as higher MWs of polysaccharides were harmful for cell viability because of their higher charge density (Chae, Jang, & Nah, 2005; Peschel et al., 2012). Thus, Song et al. (2018) believed that AGSP with a lower sulphate content and a lower MW seems more promising in safety than HP and CS. Further, they expected that AGSP might be absorbed better in human body by virtue of its lower MW (Chae et al., 2005), indicating AGSP may be more promising in the absorption profile.
6.6. Effect of molar mass of neutral polysaccharides on physiological function
6.6.1. Konjac glucomannan
Konjac glucomannan has been traditionally used in Asian countries, and many papers on its texture modifying function, physiologically beneficial function such as prebiotics, lowering cholesterol and blood sugar, preventing constipation have been published (Jiang, Li, Shi, & Xu, 2018; Nishinari, 2000b; Nishinari & Gao, 2007; Nishinari et al., 2016; Nishinari, Takemasa, Zhang, & Takahashi, 2007; Tester & Al-Ghazzewi, 2016, 2017). Most of these beneficial functions were recognized both in unhydrolysed and hydrolysed KGM but some authors found stronger effects in hydrolysed KGM than in unhydrolysed KGM (native KGM) (Chen, Fan, Chen, & Chan, 2005; Yang et al., 2017; Wang et al., 2018). It was reported that konjac glucomannan hydrolysates could stimulate the growth of probiotic microorganisms such as lactic acid bacteria (LAB) but inhibit the growth of undesirable ones such as Escherichia coli, Clostridium perfringens, Listeria monocytogens, and Propionibacterium acnes (Bateni et al., 2013). Based on the hypothesis that prebiotic roles of KGM ingested are not limited to gastrointestinal tract but also in other part of the body, hydrolysed KGM was applied to wound healing, treatment of vaginal infection, and reduction of acune vulgaris (Tester & Al-Ghazzewi, 2016, 2017).
Employing a one ear punch test for Wister mice, the intake of hydrolysed KGM was found to accelerate the wound healing (Al-Ghazzewi, Elamir, Tester, & Elzagoze, 2015). In the vaginal disease, the healthy ecosystem maintained by lactobacilli was decreased by the growth of Candida albicans, an opportunistic pathogenic yeast. Introduction of KGM hydrolysates pessaries together with nystatin (antifungal drug) in the vagina was found effective to recover the healthy ecosystem (Tester et al., 2012). Treatment of antifungal alone led to the decrease of beneficial microorganism while the addition of KGM hydrolysate was found to mitigate it.
6.6.2. Mucilages
Chinese yam (nagaimo) has been used in China, Japan and Asian countries because of its special mucilaginous texture, and believed to have some health beneficial properties. Mucilage was reported to consist of mannose, glucose, arabinose, galactose, rhamnose, xylose and some other saccharides and amino acids (Ma et al., 2020). Zhang, Wang, Liu, and Li (2016) degraded mucilage of Dioscorea opposita (DisP) by oxidative degradation to obtain lower mass compounds because they thought higher molar mass than 100 Da impeded further exploitation. They examined antioxidant and anti-mutagenic activities of four different DisP mucilage, DisP (MW = 132 kDa), LP1 (MW = 94 kDa), LP2 (MW = 36 kDa), LP3 (MW = 9 kDa). Both hydroxyl radical assay and superoxide radical assay showed that the scavenging activity of these mucilages were increased with increasing concentration and with decreasing molar mass. Anti-mutagenic activities showed the same order as in antioxidant activities although authors were not sure for the mechanism of these beneficial functions.
6.7. Effect of molar mass of chitosans on physiological function
6.7.1. Effect of molar mass of chitosans containing selenium on anti-tumour activities
Selenium (Se) is an essential micronutrient, and is contained in some plant and microbial polysaccharides. Se compounds can be classified into two groups based on Se speciation, namely organic Se compounds (selenoamino acid, selenoprotein, Se-containing polysaccharide) and inorganic Se compounds (selenite, selenate). Compared with inorganic Se compounds, organic Se compounds have the advantages of higher bioavailability and lower toxicity (Cheng, Wang, He, & Wei, 2018). Cheng et al. (2018) reviewed the preparation, structure and physiological functions of Se-containing polysaccharide such as antioxidant, antitumor, immune-enhancement, hepatoprotective, antidiabetic, anti-inflammatory, and neuroprotective activities. They concluded that natural Se-containing polysaccharide is Se-conjugated macromolecule, with Se content and molecular weight generally ranged from 0 to 50 μg/g and 103–106 Da, respectively. They expect further development and utilization of Se-containing polysaccharide, and pointed out the importance of comparison of toxicity and bioavailability of these different polysaccharides.
Recently, Se containing nanoparticles have been actively studied to improve the bioavailability by stabilising Se by polysaccharides, chitosan, curdlan, and mushroom polysaccharides (Song et al., 2020). Chitosan (CH or CS), a cationic polysaccharide widely found in the exoskeleton of crustaceans, insects and fungi, was proved to be effective to stabilise selenium nano particles (SeNPs) (Song et al., 2020; Zhang, Zhai, et al., 2015). Zhang, Zhai, et al. (2015) prepared CS (<3 kDa)-Se0NPs and CS(200 kDa)-Se0NPs (Se0 stands for the element Se with zero oxidation state), and examined the release of Se from the nanoparticles in different environments, pH 1.2 with pepsin, pH 7.4 with pancreatin, simulated sweat, and in free radical systems. They found that selenium release rate from CS (<3 kDa)-Se0NPs was lower than that from CS (200 kDa)-Se0NPs under all tested conditions. They attributed this to the loose and incompact structure in the CS-SeNPs prepared with higher MW CS, which were observed by TEM.
Song et al. (2020) stabilised Se0NPs using CS with three different molar masses, 3 k, 65 k, and 600 k Da. They examined the anti-tumour activities of CS stabilised Se0NPs, CS (3 k)-Se0NPs, CS (65 k)-Se0NPs, and CS (600 k)-Se0NPs both in vitro and in vivo. The effect of these CS stabilised Se0NPs on the viability of human liver cancer cell line HepG2 was examined, and the cell viability was reduced in a concentration/molar mass – dependent manner, i.e., the cell viability was reduced more pronouncedly with increasing concentration of CS stabilised Se0NPs and molar mass of CS used to stabilise the nanoparticles. The experimental finding that nanoparticles stabilised by high MWCH showed a higher zeta potential (Song et al., 2020; Zhang, Zhai, et al., 2015) may explain preferential uptake and also released more selenium leading to a higher toxicity to HepG2 cells. These authors used BALB/c nude mice model with HepG2 xenografts to evaluate the in vivo antitumor efficacy of CS-Se0NPs with different CS molar mass and selenium concentrations. Although body weights of mice did not decrease significantly by the administration of NPs, the tumour volume and weight were reduced significantly (Fig. 35 ). Therefore, the authors concluded that CS-Se0NPs have promising anti-tumour activity with less side effects, and the higher molar mass CS (600 kDa) was more effective.
Fig. 35.
Evolution of the tumour volume in BALB/c nude mice with HepG2 xenografts administered with CS-Se0NPs over 18 days treatment (Song et al., 2020). CS(3 k)-Se0NPs and CS(600 k)-Se0NPs represent zero valent Se nanoparticles stabilised with chitosan MW 3 kDa and 600 kDa, respectively.
Since it was clarified that the highest MW chitosan among several MW chitosans (<3 kDa, 3 kDa, 65 kDa, 200 kDa, and 600 kDa) showed the highest performance, it is expected in the future to be clarified whether higher molar mass chitosan (>600 kDa) or a chitosan with MW in between 200 kDa and 600 kDa shows a stronger bioactivity or not.
6.7.2. Effect of molar mass of chitosans on anti-fungal activity
Sporotrichosis is a zoonotic mycosis, caused by species belonging to the S. schenckii complex, including S. schenckii, S. luriei, S. brasiliensis, and so on. This disease occurs in several parts of the world, including Latin America, South Africa, India, and Japan (Garcia et al., 2020). Garcia et al. (2020) studied the effect of molar mass of chitosan on the anti-microbial activity on the S. brasiliensis. They used three chitosan (CH) samples with different MW and DD (degree of deacetylation) CHLMW (MW = 206 kg/mol, DD = 79%), CHMMW (MW = 467 kg/mol, DD = 84%), CHHMW (MW = 984 kg/mol, DD = 82%). They found that the lowest MW chitosan CHLMW was most effective to inhibit both the biomass and metabolic activity of the adhesion and mature phase of S. brasiliensis. Unfortunately, not only the molar mass but also DD was different in three samples, it was difficult to see whether the different antifungal activity was due to only the difference in MW or not.
7. Digestion – from high to lower molar mass
7.1. In vitro digestion studies
Digestion is the breakdown process of food substances to small molecules which can be absorbed. Digestion begins from the oral processing, and then digestive organs which secrete digestive enzymes. Carbohydrates are decomposed into monosaccharides, proteins are into amino acids and fats are into fatty acids and glycerol, monoacylglycerol. Food digestion process consists of physical process and chemical process. Physical process is the breakdown foods from the larger size food to smaller size, increasing the surface area thus more susceptible to digestive enzymes. Chemical process is the catalytic reaction which breakdowns food fragments into molecular length scales and further into structural elements, monosaccharides, amino acids and fatty acids and glycerol, which are ready to be absorbed at intestines. Food processing and cooking improve the palatability and facilitate the enzymatic reaction. Gelatinized starch, denatured proteins, and emulsified lipids are more susceptible for enzymatic reaction.
While in vivo test is required finally to understand the digestion and absorption of foods, in vitro test has many advantages, rapid, less expensive, reproducible, easy sampling, free from the individual differences, and therefore is suitable for screening better candidates from many samples, and in addition it is possible to compare the results obtained in different laboratories. Thus, the international protocol for the digestion study was proposed (Minekus et al., 2014; Brodkorb et al., 2019). The digestion experiment consists of three phases: oral phase using simulated salivary fluid (SSF), gastric phase using simulated gastric fluid (SGF), and intestinal phase using simulated intestinal fluid (SIF). To compare experimental results obtained in different laboratories, it was found necessary to assure the harmonisation of key parameters, transit times, pH, the activity of enzymes (Egger et al., 2016, 2017). An example of SDS-PAGE of the digested skim milk powder as a model protein obtained in 12 different laboratories of the collaborative research group is shown in Fig. 36 .
Fig. 36.
SDS-PAGE of hydrolysed skim milk powder (SMP) after gastric (Upper figure) and intestinal (Lower) in vitro digestion. a, b, Comparison of results from in-house protocols performed in individual laboratories 1–12 (a), with the harmonised protocol, performed in seven different laboratories (b). Undigested SMP is shown as a control; specific protein bands are highlighted with arrows: Casein fragments, partly hydrolysed casein; pancreatin, bands originating from pancreatin. M, molecular weight marker (Brodkorb et al., 2019; Egger et al., 2016).
7.2. Effect of chain length of dietary fibre on hyperglycemia
The definition of the dietary fibre has been a problem among researchers, and Kiriyama et al. (2006) proposed a wider concept luminacoids as follows: “dietary components which are not digested and/or absorbed in the human small intestine and which exert physiological effect that are useful in maintaining good health via the gastrointestinal tract”. This comprehensive terminology should be classified into starch and non-starch components. Dietary fibre is a major component of non-starch substances and can be sub-classified into polysaccharides and lignin. Since it is different from the definition in the other countries, the summary of the discussion is cited below: “1) The term “dietary fibre” should be retained because the tentative dietary goal in Japan has been set for dietary fibre which is a major component of indigestible ingredients and people are encouraged to take it daily. 2) Greater confusion will be caused if low molecular weight compounds such as sugar alcohols and indigestible oligosaccharides (degree of polymerisation: 2 to 9) and substances such as resistant starches and proteins are classified into dietary fibre even if these substances have physiological functions similar to those of dietary fibre. Sugar alcohols should be classified into poorly digested and/or absorbed groups. 3) It should not be defined based on a single analytical method. An analytical method that enables precise measurement should be applied to each substance according to the classification system; there is no single analytical method that can be applied to all components of dietary fibre. 4) The terms of “water-soluble”, “water-insoluble,” “fermentable” and “non-fermentable,” which describe the attributes of individual indigestible components, are inappropriate for use in general classification.”
As for the problem mentioned in item 4), the present authors have been also feeling confusing. In some textbooks or papers, konjac glucomannan (KGM) is classified into “water –soluble dietary fibre” while cellulose is classified into “water –insoluble dietary fibre”. KGM has been eaten in Japan and in some other East Asian countries. Since KGM is a neutral polysaccharide that consists of β-1, 4-linked d-mannose and d-glucose with about one in 19 units being acetylated. The solubility of KGM depends on the acetyl content, and because of the variation of this, some KGM samples would not dissolve molecularly and only a dispersion is obtained. However, since the dietary fibre is structurally and functionally diverse, the categorisation based on the solubility is not sufficient (Gidley & Yakubov, 2019).
Dietary fibre has been studied more than 50 years for its beneficial effects on health, but its definition has been a matter of debate. After Codex Alimentarius issued the definition of dietary fibre in 2009: Dietary fibre is carbohydrate polymers that are not digested in the small intestine of humans and categorised into the following three materials 1) edible carbohydrate polymers naturally occurring in the food; 2) carbohydrate polymers, which have been obtained from food raw material by physical, enzymatic, or chemical means; and 3) synthetic carbohydrate polymers (Jones, 2013). In the early stage of the discussion, oligosaccharides with degree of polymerisation DP < 10 were excluded, but now these are also included if these are not hydrolysed in the small intestine and fermented in the large intestine (Jones, 2013).
The importance of glycemic index (GI) as a valid and reproducible method of classifying carbohydrate foods was confirmed and there was consensus that diets low in GI and glycemic load (GL) were relevant to the prevention and management of diabetes and coronary heart disease, and probably obesity (Augustin et al., 2015). Glycemic lowering function is one of the most important beneficial function of dietary fibres. Jenkins et al. (2008) compared the effect of a low glycemic index or a high cereal fibre diet on type 2 diabetes. A low glycemic index or a high cereal fibre diet was taken by 210 participants with type 2 diabetes treated with anti-hyperglycemic medications for 6 months, and glycated hemoglobin A1c decreased in the group which took a low glycemic index diet compared with the group which took a high cereal fibre diet. However, the mechanism of lowering postprandial glucose level is not well understood.
Guar gum and its hydrolysates have been attracting much attention because they can be used to treat the constipation (Yamatoya et al., 1995) and lowering the blood glucose (Tsuda et al., 1998), and it is still being studied recently (Mudgil et al., 2018). In this application, the degradation to reduce the molar mass is effective to increase the intake because the viscosity of native guar solution is too high when the concentration is raised. It is based on the hypothesis that the beneficial dietary fibre effect is retained after lowering the molar mass.
Although the residence time of foods in the oral cavity is not so long, starchy foods like custards are partially digested during oral processing. The effect of α-amylase on the perceived texture during oral processing was shown in the experiment that changed the concentration of added α-amylase or acarbose, an amylase inhibitor (de Wijk, Prinz, Engelen, & Weenen, 2004). In this experiment, de Wijk et al. (2004) used starch-based vanilla custards and non-starch-based vanilla carboxymethyl cellulose (CMC) custards. It should be also mentioned that saliva incorporated in bolus acts later and also stimulates secretion of digestive liquids in the gastrointestinal organs, which likely contribute to digest the food (Nishinari et al., 2016).
Digestion of starch has been known to be influenced by granular size of starch. It is well known that granules of potato starch are much larger than those of maize starch. Fig. 37 a shows hydrolysis kinetics of potato starch suspension by α-amylase while the corresponding kinetics of maize starch with the same concentration is shown in Fig. 37b (Dhital, Warren, Butterworth, Ellis, & Gidley, 2017). Larger granules (>55 μm) of potato starch were hydrolysed more slowly than smaller granules (<20 μm). This difference in hydrolysis rate was interpreted as resulting from differences in available surface area; smaller granules having more available surface area compared with that of larger granules, on the same weight basis.
Fig. 37.
Hydrolysis kinetics of potato starch (A) and maize starch (B) with varying granule sizes (Dhital et al., 2017).
In contrast, the rate of amylolytic hydrolysis of maize starch were not so much influenced by granular sizes of maize starch; larger granules (>20 μm) compared with smaller granules (<10 μm) were hydrolysed almost at the same rate. This similarity in hydrolysis extent was explained by the structural difference in large and small granules: there are surface pores and channels in maize starch granules that can dramatically increase the available surface area for amylase catalysed hydrolysis while there are no such pores, channels and cavity in potato starch (Dhital et al., 2017). The same research group compared the starch hydrolysis of barley and sorghum, and found that the hydrolysis of barley was faster than sorghum for the similar grain fragment sizes. It was interpreted that more coexisting protein bodies/matrices in sorghum hinder the amylase accessibility toward the starch granules. The hydrolysis extent was found negatively correlated with particle size, as milling to smaller fragments can break down the cell wall and protein matrices increasing the accessibility of amylases towards the starch granules (Dhital et al., 2017). As was described for the rennet gelation of casein micelles with different micelle sizes (3.7 Molar mass effect in protein gelation), the gelation was governed by not simply the micelles sizes but was influenced by the microstructural difference.
It is now widely recognized that postprandial increase of GI is lower in the slowly digestible starch (SDS) than in the rapidly digestible starch (RDS), and the rate of the digestion has been studied extensively (Englyst et al., 2018). Though the digestion process of starch is complex involving the diffusion of enzyme to the substrate, adsorption of enzyme to starch granules, and the subsequent catalytic reaction, also interfered by the coexisting protein, lipid, and other compounds, an improved analytical method to study the digestion is developed recently (Yu et al., 2018). Effects of chain length of amylose and amylopectin on the digestion rate of starch have recently been studied (Gong, Cheng, Gilbert, & Li, 2019; Martinez et al., 2018). Gong et al. (2019) analysed the chain length distribution by size –exclusion chromatograph (SEC) and fluorophore-assisted carbohydrate electrophoresis (FACE) for freeze-dried retrograded starch (16 different varieties rice) samples after subjecting to in vitro enzyme digestion. The starch digestion was monitored by concentration of glucose, and the digestion curves are fitted to the first order kinetics. In combination with structural observation by SEM and DSC results, they concluded that starch samples with higher amounts of amylose short-medium chains and relatively shorter amylose medium chains showed slower digestion, which they attributed to the denser small cells forming the gel matrix, limiting its susceptibility to digestion enzymes (Fig. 38 ). Since amylopectin is digested faster, it was not found to decrease in the digestion rate.
Fig. 38.
Schematic representation showing the effect of chain length distribution of starch on the digestion process. Starches with different weight distribution of hydrodynamic volume, Vh, as a function of degree of polymerisation (DP) with different chain length distribution show different digestion processes. Higher amounts of amylose short-medium chains and relatively shorter amylose medium chains form the compact retrograded amylose networks into which digestive enzymes cannot penetrate (Gong et al., 2019).
The function of preventing the absorption of saccharides has been ascribed to the high viscosity of dietary fibres. Glucose absorption was slowed by the simultaneous ingestion of viscous dietary fibre such as guar and pectin, and this effect was shown to increase with the viscosity of the ingested gum. The destruction of the viscous character of guar by hydrolysis prevented this action (Jenkins et al., 1978). It was shown that water-soluble dietary fibre with high viscosity slows down the gastric emptying (Ebihara, Masuhara, & Kiriyama, 1981). Wood et al. (1994) examined the effect of viscosity using oat gum (OG) mainly consisting of (1 → 3)(1 → 4)-β-glucan on plasma glucose and insulin levels of healthy humans consuming 50 g glucose. They found that increasing the dose of OG successively reduced the plasma glucose and insulin responses relative to a control without gum. They further found that the reduction of the viscosity of OG by acid hydrolysis reduced or eliminated the capacity to decrease postprandial glucose and insulin levels.
The glycemic index lowering effect of polysaccharides was ascribed to the high viscosity (Jenkins et al., 1978) as mentioned above, and this was recently reconfirmed by McRorie (2015) and McRorie and McKeown (2017), who proposed the mechanism of glycemic control as slowing the interaction of digestive enzymes and nutrients by the high viscosity of high molar mass β-glucans, guar gum, and psyllium. They also stated that soluble non-viscous fibres such as wheat bran, inulin were not effective for glycemic control at physiological doses although some papers reported a beneficial function at extremely high doses.
A significant increase in the slowly digestible starch (SDS) and resistant starch (RS) was reported for maize starch-pectin systems using different MW pectins (Zhang et al., 2018). Both SDS and RS increased after storage at 4 °C for 14 days both in maize starch with and without pectin. This increase was found more pronounced in the presence of pectin and its effect was more conspicuous for higher molar mass pectin, which was consistent with the relative crystallinity estimated by X-ray diffraction; starch in the presence of higher MW pectin showed a higher relative crystallinity. However, the relative crystallinity was found lower in the starch with pectins than in starch alone. Although it is believed that the higher crystalline starch was believed to be slowly or hardly digested than less crystalline starch, this was found not to be the case in this study, and should be studied further.
Molar mass effect of lowering blood glucose by oat β-glucan was examined using glucose drinks and gels with different MW β-glucan (Fig. 39 A) (Kwong, Wolever, Brummer, & Tosh, 2013). Drinks were solutions containing either no β-glucan (ND); 4 g low MW β-glucan (LD); or 4 g high MW β-glucan (HD). Viscoelastic gels contained either 4 g low MW β-glucan (4LG); 2 g low plus 2 g high MW β-glucan (2H2LG); or 3 g high plus 1 g low MW β-glucan (3H1LG). ND was used as the control glucose solution. Glycemic response was found largest in the glucose drink ND (●) and the smallest in the glucose drink containing the high MW β-glucan HD (○). The β-glucan viscoelastic gels containing low MW β-glucan 4LG (▲) was not found effective to reduce the blood glucose rise. It is clear that the higher MW is effective to lower the blood glucose, and it is lost when it is in a gel state.
Fig. 39.
A. Postprandial glycemic response curves elicited by the test foods, drinks and gels (mean ± SEM, n = 15). ND, control glucose drink; β-glucan drinks (LD, with low MW β-glucan; HD, with high MW β-glucan) and β-glucan viscoelastic gels (4LG, 2H2LG and 3H1LG). Values of the same time point with different letters are significantly different (P < 0.05). Error bars are omitted if they overlap (Kwong, Wolever, Brummer, & Tosh, 2013). B. Average value of blood glucose as a function of time after the intake of glucose solution (25% solution), Xyloglucan A (Glucose solution containing high molar mass xyloglucan), Xyloglucan B (Glucose solution containing low molar mass xyloglucan) (Nishinari et al., 2020).
To see what is the key factor of the dietary fibre which lowers glycemic index, two solutions of xyloglucan with high and low molar masses but with approximately the same steady shear viscosity at a lower shear rate 0.5 s−1 were ingested by nine healthy male subjects. The blood was taken every 30 min up to 120 min after the ingestion of the mixed solution of glucose and xyloglucan. The rise of blood glucose was blunted by ingesting both solutions of xyloglucan with high and low molar masses, and the effect of the solution of higher molar mass xyloglucan was slightly higher (Fig. 39B), which was in line with the previous report that the lowering effect was mainly governed by the product of the peak value of molar mass and the concentration of the ingested dietary fibre oat gum (Wood et al., 2000). Molar mass was more important than the concentration within the range in Nishinari et al. (2020) since the mass of the polysaccharide is almost nine times higher in the solution of lower mass xyloglucan than in the solution of higher molar mass xyloglucan. This is not contradictory with another previous theory (Jenkins et al., 1978) asserting that the high viscosity is the determining factor because the polymer solution viscosity is higher when the molar mass is high at the fixed concentration as shown in Fig. 3 in Section 2.
Although more detailed studies are required to get a clearer insight on the mechanism of the blood-glucose-lowering-effect of xyloglucan, the results exclude an explanation that attributes this lowering effect to the binding of glucose with dietary fibre xyloglucan which are expelled out from the body without being absorbed. If this explanation is valid, the Xyloglucan B sample would have shown a greater effect, which was not the case.
In the intervention study on health benefit of dietary fibres, baseline diet, mode of feeding (e.g., whether fed alone or with meals, as a single bolus in a liquid or added to foods), the total dose, characteristics of the subjects, and other important study conditions should be taken into account (Jones, 2013). Although an intervention study such as glucose intake is useful to assess the acute glycemic effects, long-term (multimonth) data from well-controlled intervention clinical studies are necessary to establish a clinically meaningful health benefit for improved glycemic control (McRorie, 2015).
Recently, it was reported that α-glucosidase inhibitory effect of polysaccharides from blackcurrant (Xu et al., 2018a, b, c) or from blackberry (Dou et al., 2019) was enhanced by ultrasound degradation of the polysaccharide.
Xu et al. (2018a) prepared two degraded polysaccharides (U-400, MW = 1.89 × 104 kDa, and U-600, MW = 1.32 × 104 kDa) from blackcurrant (Ribes nigrum L.) polysaccharide (BCP, MW = 3.26 × 104 kDa) by different ultrasound powers of 400 W and 600 W, respectively. Monosaccharide composition, consisting of arabinose, galacturonic acid, rhamnose, galactose, mannose, and glucose, was not so much changed by ultrasonication except the doubling of uronic acid content. It was also found that the reducing sugar content and thermal stability increased with the increase of ultrasound intensity. Xu et al. (2018a) found that all the activities of the antioxidant (hydroxyl and superoxide radicals scavenging, lipid peroxidation inhibition, and DNA damage protection activities), α-amylase and α-glucosidase inhibition activities of BCP were enhanced by ultrasonic degradation: these activities increased in the order of U-600 > U-400 > BCP. Xu et al. (2018b) prepared two degraded BCP (DBCP-1, MW = 1.30 × 104 kDa, and DBCP-2, MW = 9.62 × 103 kDa) from blackcurrant polysaccharide (BCP, MW = 3.26 × 104 kDa) by using Fe2+ with different concentrations of H2O2 solution. Monosaccharide composition and the glycosidic linkage patterns were fount not changed as in a previous report (Xu et al., 2018a). Inhibition activities of α-amylase and α-glucosidase were found increased with decreasing MW; DBCP-2 > DBCP-1 >BCP as in the previous report (Xu et al., 2018a). According to the authors’ deduction that the activities increase with decreasing molar mass, DBCP-2 (MW = 9.62 × 103 kDa obtained by using Fe2+ with H2O2 solution) must show higher inhibition activities than U-600 (MW = 1.32 × 104 kDa obtained by ultrasonic degradation), but unfortunately the authors have not described the comparison. Although the molar mass seems to be the key factor to determine the activities, slight difference in monosaccharide composition or linkage patterns may inhibit the comparison or not is expected to be clarified. The authors submitted another paper before these two reports concerning the effect of sulphation on the activities of BCP (Xu et al., 2018c). They performed the sulphation of BCP and found that the inhibition of α-amylase activity increased with increasing degree of sulphation (DS) and the dose. Since MW slightly increased and monosaccharide composition also changed with increasing DS, they could not identify the determining factor for bioactivities among spatial structural, MW, monosaccharide composition, the configuration of glycosidic bonds, and the sulphate content of the polysaccharides.
Dou et al. (2019) prepared blackberry polysaccharide BBP (MW = 591 kDa) consisting of arabinose, galactose, glucose, galacturonic acid, and glucuronic acid. By ultrasound degradation for 8, 16 and 24 h, they obtained three fractions BBP-8 (MW = 364), BBP-16 (MW = 250) and BBP-24 (MW = 177 KDa), and noticed that primary structure was retained. A slight increase of reducing sugar with decreasing MW may have some effect, which also was noticed in the degradation performed to improve the bioactivity in other polysaccharides. Fig. 40 shows the IC50 (the concentration of the polysaccharide required to inhibit 50% of the α-glucosidase activity) of BBP, BBP-8, BBP-16, BBP-24, together with acarbose, an α-glucosidase inhibitor, widely used for diabetes treatment.
Fig. 40.
IC50 of BBP, BPP-8, BPP-16, BPP-24, together with acarbose (Dou et al., 2019).
As shown in Fig. 40, the lowest MW BBP-24 showed the lowest IC50 indicating the highest inhibitory effect of α-glucosidase activity. Dou et al. (2019) analysed the decrease in fluorescent intensity (fluorescent quenching) of α-glucosidase with increasing concentration of BBP and its degraded compounds based on fluorescence resonance energy transfer (FRET) method (Kandagal et al., 2006; Wu et al., 2020). Dou et al. (2019) found that the binding affinity and binding site number of inhibitors (BBP) to α-glucosidase were affected by the molar mass of BBP which was in line with Fig. 40.
7.3. Molar mass effect in lipolysis-hyperlipidemia
Digestion of lipids dispersed as oil droplets in foods has been studied actively and as expected the lipolysis proceeds faster for small droplets because of the larger interfacial area which accelerate the access of the lipase, and in addition the coalescence and flocculation are generally slower than for larger droplets (Dickinson, 2018). To understand the molar mass effect of the lipolysis, it is desirable to change molar mass keeping the droplet size constant. Probably because of this difficulty, there have been not so much study on the molar mass effect of lipolysis.
It was, however, reported that depolymerised guar gum also reduces the plasma cholesterol (Grundy et al., 2016). Using this lower viscosity polysaccharide, these authors showed that total and LDL cholesterol were reduced significantly at the end of guar bread period relative to baseline and control concentrations. This decrease was equivalent to those observed in the previous studies where non-depolymerised guar was used (Ellis, Dawoud, & Morris, 1991; Jenkins et al., 1980). It was thought that, in addition to the rheological effects, the galactomannan acts also as a physical barrier to enzyme-substrate interactions and the release of nutrients from the food matrix (Grundy et al., 2016). Guar galactomannan also inhibits the action of pancreatic amylase by binding noncompetitively with the enzyme. These binding mechanisms explain the reduction of postprandial hyperglycemia and plasma insulin concentration in diabetic and non-diabetic human subjects.
Wood believed that the high viscosity of β-glucan in the gut is the governing factor to lower the cholesterol (Tosh, 2013). His group obtained β-glucan from various cereals, oat, barley, wheat flour and wheat bran. LDL cholesterol was significantly lowered in the subjects who were given high molar mass β-glucan cereals, and low molar mass β-glucan was not effective. They concluded that intake of 3 g of oat β-glucan/d with a high molar mass (2.21 × 106 g/mol) or a medium molar mass (0.53 × 106 g/mol) lowered LDL cholesterol, but a lower molar mass (0.21 × 106 g/mol) β-glucan was less effective (Wolever, Tosh, Gibbs, & Brand-Miller, 2010). They recognized that the viscosity is a very important criterion for lowering the LDL cholesterol as was asserted (P = 0.001), and concluded that log(MW × C) was a significant determinant of LDL cholesterol (P = 0.003) by analysis of covariance (Fig. 41 A). They also noticed that treatment effects were not significantly influenced by age, sex, study center, or baseline LDL cholesterol. The ineffectiveness of low molar mass β-glucan to lower LDL cholesterol was confirmed later (Hu et al., 2015).
Fig. 41.
A. Serum LDL cholesterol as a function of log(MW × C), B. Serum LDL cholesterol as a function of log(viscosity). Values are means ± SEMs for the 5 treatments: 1, wheat-bran cereal (n = 87); 2, 4 g low-MW β-glucan (n = 63); 3, 3 g medium-MW β-glucan (n = 64); 4, 4 g medium-MW β-glucan (n = 67); 5, 3 g high-MW β-glucan (n = 86). Solid lines are regression equations (Wolever et al., 2010).
Using enzymatic degradation of oat beta glucan, Gamel, Abdel-Aal, Ames, Duss, and Tosh (2014) showed that LDL cholesterol as well as blood glucose decreased with increasing viscosity of oat slurry observed by RVA. Wang and Ellis (2014) surveyed the literature on the blood-cholesterol and -glucose lowering effect of β-glucan, and reconfirmed that this MW dependent effect was found both in the experimental study in healthy subjects who consumed glucose drinks containing extracted and purified β-glucan samples of different doses and MW values, and in the study in which β-glucan was incorporated into a food matrix such as baked goods and breakfast cereals. However, not all the studies showed such beneficial effects of β-glucan when it was incorporated into some foods. In the study of Kerckhoffs, Hornstra, and Mensink (2003), it was found that LDL cholesterol was lowered in subjects taking bread and cookies rich in β-glucan while the lowering function was not recognized when β-glucan was taken in the form of orange juice containing β-glucan. Wang and Ellis (2014) pointed out that depolymerisation of β-glucan and/or reduced solubility in food may account for the attenuation in efficacy reported for the latter products.
Effects of molar mass of β-glucan added to canola oil emulsion on the lipolysis were recently studied (Zhai, Gunness, & Gidley, 2020). As has been reported, the lipolysis of emulsion foods is influenced by mixing methods and emulsifier types. Non-food emulsifier Triton X-100 was used because the initial droplet shape using this emulsifier was more uniform than that using WPI or lecithin. Oil droplets were initially mixed with the emulsifier solution by stirring at 800 rpm for 2 h at room temperature. Then barley β-glucan (BG) solution was added at a mass ratio of 1:1 with stirring at 800 rpm for another 2 h at 20 °C to form the final emulsion. Ten milliliter of emulsion was incubated with 10 mL of the simulated intestinal juice and the mixtures were stirred at 100 rpm for 2 h at 37 °C. Free fatty acid (FFA) release was measured after 2 h by a titrimetric method (Fig. 42 A).
Fig. 42.
A. Time course of free fatty acids released by lipolysis in emulsions containing different molar masses of barley beta-glucan (BG). BGL: Low molar mass barley BG; BGM: Medium molar mass barley BG; BGH: High molar mass barley BG. Different letters show statistical difference at p < 0.05 (Zhai et al., 2020). B. Free fatty acids (FFAs) release during the intestinal digestion process in the presence of pectins (HMP. MMP and LMP). Control corresponds to the emulsions without addition of pectin (Espinal-Ruiz et al., 2016).
As is shown in Fig. 42A, FFA release was retarded by the presence of BG and the retardation degree depended on the molar mass. Higher molar mass BG retarded the release of FFA than lower molar mass BG. The molar mass dependence was explained by depletion mechanism which was used to explain the molar mass effect of maltodextrin on the critical flocculation concentration (Klinkesorn et al., 2004), discussed in section 6. Molar mass effects in the in the stabilisation of suspensions and emulsions.
Wilcox, Brownlee, Richardson, Dettmar, and Pearson (2014) examined the inhibitory effects of the addition of alginates on the pancreatic lipase. Lipase inhibition was found to increase with increasing the guluronate content, fraction of guluronate dimers, guluronate trimers and the number of guluronate blocks greater than one in the alginate polymer, but the significant role of molar mass was not found in the range tested in their study. Mackie, Bajka, and Rigby (2016) also found the effect of alginate on delaying the absorption rate of lipid in the small intestine, and they ascribed this effect to the high viscosity. Yao, Nie, et al. (2018) and Yao, Zhou, et al. (2018) also studied the effects of a polysaccharide gum arabic (GA) and low molecular surfactant Tween 80 (T80) on the lipid digestion. As has been widely reported, with increasing addition of T80, the protecting GA layer of the GA-stabilised emulsion droplets was replaced by T80. About 15% of GA still remained on the interface when T80 concentration increased to ~4%. Addition of T80 stiffened the network formed at the droplets interface. The kinetics of lipid digestion exhibited a biphasic rate, an induction period followed by a speeding up process. It was caused by the adsorption behaviour of lipase on the emulsion interface during the lipolysis process.
Espinal-Ruiz, Restrepo-Sánchez, Narváez-Cuenca, and McClements (2016) studied the effect of the addition of pectin with different molar masses and different degrees of methoxylation (high methoxylated pectin (HMP), medium methoxylated pectin (MMP) and low methoxylated pectin (LMP)) on the lipid digestion. Molar mass of the three pectins were 181 kDa (HMP), 148 kDa (MMP), and 130 kDa (LMP). Values of zeta potential at pH 7 were −28.2 mV (HMP), −34.8 mV (MMP), and −47.5 mV (LMP), respectively. The finding that higher molar mass pectin HMP retarded the lipid digestion than lower molar mass pectin LMP (Fig. 42B) was explained by a closed structure around the lipid droplets induced by a stronger depletion flocculation in the emulsion with a higher molar mass HMP, while the emulsion with a lower mass LMP having more anionic charges formed an open structure due to its electrostatic repulsion with negatively charged lipid droplets, and therefore allow the access of lipase to the surface of oil droplets and promoting the digestion. Since HMP has higher molar mass which contributes more to the depletion, but lower anionic charges which repel less droplets, it is difficult to estimate separately the effects of molar mass and electric charge which retarded the digestion.
Guo, Bellissimo, and Rousseau (2017) demonstrated the free fatty acid release by the action of lipase using a standardised pH-stat digestion procedure (Minekus et al., 2014) was delayed in gels with the same ingredients as emulsion; while the lipolysis proceeded faster in the WPI-stabilised emulsion, it was delayed in the WPI-emulsion gelled by heating in the presence of NaCl/CaCl2 although they found only a slight difference in the final amount of released fatty acid after the digestion experiment of 120 min. Taking also into account the structural observation using CLSM and diffusion behaviour of FITC-dextran in emulsion gels, they suggested that denser protein network formed by the addition of CaCl2 in addition to NaCl protected the dispersed oil droplets and thus delayed the lipid digestion. Mat, Souchon, Michon, and Le Feunteun (2020) pointed out the importance to study the interplay between proteolysis and lipolysis because in real food emulsions the digestion of proteins which stabilise oil droplets influences strongly the digestion of lipids. They prepared 6 different model foods consisting of 15 wt% whey protein with or without 10 wt% rapeseed oil, four of which were heated at 80 °C to gel. They found that the proteolysis in gastric phase (pH 3) was faster in a liquid food at the initial stage but at later (120min), more proteins were digested, and in addition, they found the similar tendency also in the intestinal phase. This was explained by the higher susceptibility of heat-denatured whey protein than native whey protein. They found it was consistent with in vivo study in which a higher protein content was found in the caecum for the liquid-food-fed-rats than for the solid-food-fed-rats. The degree of lipid hydrolysis in the intestinal phase (pH7) is shown for four different food models, Liquid Fine Emulsion (LFE), Gelled Coarse Emulsion (GCE), Gelled Fine Emulsion (GFE), and Gelled Coarse Emulsion with a modified o/w interface (GCEi) in Fig. 43 .
Fig. 43.
Evolution of the degree of hydrolysis (DH) of lipids for LFE (Liquid Fine Emulsion), GCE (Gelled Coarse Emulsion), GFE (Gelled Fine Emulsion), and GCEi (Gelled Coarse Emulsion with a modified o/w interface) measured by pH-stat in the in vitro intestinal digestion (pH = 7.0) (Mat et al., 2020).
As in the proteolysis at gastric phase, the lipolysis in the intestinal phase was found also influenced by the physical state of the continuous phase. Since oil droplets were entrapped in the protein gel in GCE, GCEi, GFE, they were stabilised throughout the intestinal reactions, whereas droplets in LFE could more easily enter into contact, resulting in coalescence and creaming. The authors concluded that the physical state of food model, gelled or non-gelled, fine or coarse oil droplets in the emulsion are determining factor of the digestion kinetics.
8. Effect of molar mass of thickening agent used for safe swallowing
With the advent of aged society, the number of persons having difficulties in mastication and deglutition is increasing, and pneumonia caused by aspiration - the transport of a bolus into the trachea instead of the esophagus - of food through the airway (Brito de la Fuente, Turcanu, Ekberg, & Gallegos, 2017; Kohyama, 2015; Steele et al., 2015; Turcanu et al., 2015, 2018), and is now the third most important cause of death in Japan after malignant neoplasm and heart disease in elderly. It has been empirically known that the risk of the aspiration can be reduced by thickening liquids such as water, tea, fruit juice with thickeners (Cichero, 2013; Funami, 2017; Funami, Ishihara, Nakauma, Kohyama, & Nishinari, 2012; Newman, Vilarde, Clavé, & Speyer, 2016; Nishinari et al., 2016). Efficacy of the viscosity on the prevention of the aspiration has been studied by many research groups. It has long been known that fast flow of bolus through the pharynx makes it difficult for airway protection. The thickening of liquids makes the flow more slow, allowing the coordination of safe swallowing, and thus it is effective to reduce the risk of aspiration. Bolus transit velocity has been reported from 8.3 to 50 cm/s at pharyngeal phase, and from 2.9 to 14.2 cm/s at esophageal phase (Newman et al., 2016). Swallowing shear rate has also been studied by many groups, however, because of the complex structure of organs for swallowing the estimated values are not always agree with each other (Nishinari et al., 2019). Even though there is not a common international standard for dysphagia fluids yet available, the most accepted guideline (National Dysphagia Diet Task Force) classifies Thickened fluids (TFs) only according to their shear viscosity, at a shear rate of 50 s−1 at 25 °C, into four different stages: thin (1–50 mPa s), nectar (50–350 mPa s), honey (350–1750 mPa s) and spoon-thick or pudding-like (>1750 mPa s) (Cichero, 2013; Turcanu et al., 2015, 2018).
Fig. 44 shows the prevalence of the aspiration for various boli with different viscosities. Patients, served foods, and serving conditions are different thus it is difficult to compare the absolute values of different research groups, however, it can be seen that the prevalence of aspiration seem to decrease with increasing viscosity of boli and was lowest for the highest viscosity of spoon-thick viscosity.
Fig. 44.
Prevalence of patients with aspiration (measured by VFS (videofluoroscopy) or FEES (fiberoptic endoscopic evaluation of swallowing) according to the level of viscosity. Different symbols are taken from published reports by different research groups. A broken line represents the mean values (Newman, Vilarde, Clavé, & Speyer, 2016).
Three polysaccharide solutions which show the same steady shear viscosity at 50 s−1 but different shear thinning behaviors (Fig. 45 ) were used to see the effects of rheology of bolus on the risk of aspiration under the guidance of medical doctors (Nishinari et al., 2011). Sample 1 (G1), 2.0 wt% guar gum solution stirred at room temperature for 10 h, then heated at 75 °C for 1 h. Sample 2 (G2), 2.6 wt% guar gum solution stirred at room temperature for 10 h, then heated at 75 °C for 1 h and sterilized at 121 °C for 20 min. Sample 3 (X2), 4.1 wt% xanthan solution stirred at room temperature for 10 h, then heated at 75 °C for 1 h and sterilized at 121 °C for 20 min. All these solutions contain X-ray contrast medium which was affirmed to be non-toxic for lung. The order of the magnitude of the viscosity at higher shear rates and that at lower shear rates is opposite: η (X2) > η (G1) > η (G2) at lower shear rate but η (X2) < η (G1) < η (G2) at higher shear rates. Thirty-two observations were analysed, and it was found that the incidence of aspiration was 4 for X2, 5 for G1 and 7 for G2. The experimental observation that a higher molar mass guar G1 showed more shear thinning than a lower molar mass G2 is consistent with widely observed behaviour for water-soluble polymer solutions (See Fig. 3).
Fig. 45.
Shear rate dependence of the viscosity of xanthan and guar solutions showing the crossover at 50 s−1 (Nishinari et al., 2011).
Thus, there is a general tendency that the viscosity at lower shear rate seems to be more important rather than that at 50 s−1 or higher shear rate. The probability of the aspiration seems to decrease with increasing the viscosity at lower shear rate (Table 2 ). Since the number of observation is not sufficiently high, this supposition should be confirmed with larger number of data in the future. It should be mentioned here that when the concentration of guar gum was higher to increase the viscosity at lower shear rates, panellists found the difficulty to swallow. Therefore, solutions of the guar gum with lower molar mass which is less shear thinning than xanthan solutions seem to be not suitable as a thickening agent to lower the risk of aspiration. On the other hand, shear thinning solutions were generally evaluated easy to swallow.
Table 2.
The relation between the viscosity at lower shear rates and the rate of aspiration (Nishinari et al., 2011).

Extensional rheology has been studied in dysphagia because the bolus is believed to be subjected to extensional flow when bolus is squeezed between the tongue and the palate and also in the subsequent pharyngeal phase in swallowing process. In addition, both bolus elasticity and the cohesiveness of bolus were found to be important for dysphagia treatment, and the extensional viscosity measurements have been performed (Brito de la Fuente et al., 2017; Nishinari et al., 2019; Nyström, Qazi, Bülow, Ekberg, & Stading, 2015; Turcanu et al., 2018). Cohesiveness of bolus was suggested to correlate well with the breakup time of the fluid extensional test (Nishinari et al., 2019).
Turcanu et al. (2018) examined the effect of saliva on the extensional behaviour of thickened fluids for dysphagia, and found that the breakup time of starch based fluids became shorter even during a short time in comparison with non-starch based gum solutions.
Since excessive thickening could cause additional side effects, such as lowering the palatability and the subsequent reduction of liquid intake, leading to dehydration and malnutrition, the optimum thickening level should be identified. At the same time, apart from its viscosity, the bolus cohesiveness is another key factor which influences the aspiration. The cohesiveness is shown to be highly correlated with the extensibility which can be characterized by the break-up time in the fluid extension test, and it is suggested that high cohesiveness could play an important role in preventing aspiration when the rheological properties of test solutions were compared (Nishinari et al., 2019). It is also known that the breakup time of polymer solutions increases with increasing molar mass (Tirtaatmadja, McKinley, & Cooper-White, 2006).
To develop further the application of gelling biopolymers for dysphagia, it is necessary to study the rheological and related properties when gels are masticated. Rheology and structure of gels depend on intrinsic factors such as chemical structure, molar mass and its distribution, and extrinsic factors such as concentration, gelation rate. Fracture stress and strain, deformability (brittleness), miscibility with saliva should be studied, and related with the taste and aroma, which affect the salivation and bolus formation. Rheological and structural propertiess of masticated gels (bolus) should be studied.
9. Molar mass effect on flavour intensity
9.1. Taste receptors and molar mass
Receptors of some basic taste such as sweetness, bitterness, and sourness have been identified by Zuker's group (Zhang, Jin, Zhang, Ding, O'Keefe, Ye, & Zuker, 2019).
It is generally known that oligosaccharides with higher degree of polymerisation (DP) and polysaccharides donot give sweet taste, but it is also empirically known that when starch is masticated for a long time in the mouth, it gives some sweetness sensation as mentioned earlier. To understand this problem more quantitatively, it is necessary to notice that carbohydrates have three characteristics, monomer unit, the number of monomer units (DP), and the type of linkages connecting these units (Lim & Pullicin, 2019). Maltodextrin was fractionated by using ethanol-water solvents based on the different solubility of saccharides of different DPs: S1 (average DP 7), S2 (average DP 14) and S3 (average DP 44) (Balto et al., 2016). Using a triangle test in which subjects are asked to discriminate 3 samples including blank stimuli containing tasteless methylcellulose to balance the viscosity, they found that subjects discriminated S1 and S2 but not S3. They noticed that the detectability of the average DP 7 was higher than that of the average DP 14 at both 6% and 8% concentrations. They concluded that humans can perceive the taste of α-glucan with DP up to 14 but below 44, and the upper limit is still not known (Lapis, Penner, & Lim, 2014; 2016). They further examined the discriminability of 3 maltooligosaccharides, DP 3–4, DP 5–6, DP 6–7 together with glucose (DP1), maltose (DP2) and maltotriose (DP3) in the presence and absence of sweetness inhibitor lactisole (Fig. 46 ).
Fig. 46.
Discriminability of glucose (DP 1), maltose (DP 2), maltotriose (DP 3), and additional maltooligosaccharide preparations (DP 3–4, 5–6, and 6–7) at 75 mM in the absence (light bars) and presence (dark bars) of the sweetness inhibitor lactisole. Subjects performed discrimination tests. The proportion of correct responses (right y-axis) was used to obtain the discriminability (d′). The dashed horizontal line indicates significant discriminability based on d′ analysis at p < 0.05 (Lim et al., 2019).
All six stimuli were perceived, and the discriminability (d′) among all six stimuli was not significantly different in the absence of lactisole, but in the presence of lactisole it was found that glucose (DP 1), maltose (DP 2) and maltotriose (DP3) were not discriminated because their sweetness was suppressed, while 3 maltooligosaccharides, DP 3–4, DP 5–6, DP 6–7 were detected indicating that these maltooligosaccharides were discriminated. These results led these authors to think that there are unidentified receptor which is different from those already discovered recently by Zuker's group.
World Health Organization (2015) recommends to reduce intake of monosaccharides and disaccharides added to foods and beverages, and sugars naturally present in honey, syrups and fruit juices (Di Monaco, Miele, Cabisidan, & Cavella, 2018). Since oligosaccharides have been attracting much attention by virtue of their prebiotic function, their sweetness intensity has been studied (Ruiz-Aceituno, Hernandez-Hernandez, Kolida, Moreno, & Methven, 2018) using ten commercial and four novel prebiotics (4-galacosyl-kojibiose, lacturosucrose, lactosyl-oligofructosides and raffinosyl-oligofructosides). Based on the sensory evaluation and principal component analysis, Ruiz-Aceituno et al. (2018) concluded that chain length was the most important determining factor for sweetness intensity of these carbohydrates rather than the types of linkage or the presence of ketose groups. Disaccharides (lactose, lactulose, kojibiose, leucrose, maltulose, turanose and others) showed higher sweetness (relative sweetness 49.8–68.3 to sucrose sweetness 100), than trisaccharides (4-galactosyl-kojibiose and lacturosucrose) (41.4–46.5), which in turn exhibited higher sweetness than mixtures of oligosaccharides having DP above 3 (11.2–37.1).
9.2. Effect of molar mass of thickening and gelling agents on taste intensity
It is generally believed that the perceived sweetness intensity is decreased with increasing concentration of coexisting thickening agents (Morris, 1993). Experimental results of Morris showed that this decrease of sweetness intensity did not depend on the kind of thickening agent such as guar gum, alginate, carboxymethyl cellulose but only on the viscosity (Fig. 47 ). Nottingham group using guar and dextran with different molar masses examined the saltiness intensity as a function of the zero shear viscosity and polymer concentration (Fig. 48 ) (Koliandris et al., 2010).
Fig.47.
Variation in perceived thickness(●), sweetness (▲), and flavour intensity (■), and in objective zero-shear viscosity (η0, ○, right-hand axis) for samples incorporating the same fixed concentrations of sucrose and strawberry flavouring, but different concentrations (C) of a random coil polysaccharide (guar gum). C* represents the coil-overlap concentration (see Fig. 2 in section 2) (Morris, 1993).
Fig. 48.
Salty taste intensity is determined by zero shear viscosity and the concentration of a thickener. The dashed lines correspond to iso-saltiness. Saltiness intensity increases with increasing line number (Koliandris et al., 2010).
They found that saltiness intensity decreased with increasing viscosity at lower shear rate, which was consistent with results on sweetness intensity by Morris (1993) shown in Fig. 47. The difference in saltiness intensity was recognized only when the zero shear viscosities are very different. They also found that the saltiness intensity increased with increasing polymer concentration at a constant zero shear viscosity. This observation became possible because they used dextran which is not commonly used as a thickener in food industry because dextran is not so effective as a thickener in comparison with guar, locustbean gum, and xyloglucan. The reason why dextran is not effective as a thickener may be attributed to its chain flexibility caused by alpha 1,6 linkage than other polysaccharides. Therefore, it was necessary to add a large amount of dextran with lower molar mass to make a solution with the same zero shear viscosity of solutions of guar or high molar mass dextran. The saltiness increasing effect of the addition of a low molar mass dextran may be attributed to one of the following reasons: the effective concentration of salt increases because water is hydrated with dextran, or the direct interaction of salt with some part of glucose residues of dextran. It is difficult to conclude which one or the other mechanism is responsible for this effect at present.
As for solid foods, Morris (1993) found a good correlation between the intensity of the flavour release and the fracture strain; he found that the sweetness intensity and strawberry flavour intensity decreased with increasing fracture strain. It was understood as the decrease in the surface area of the fragments from which taste and aroma compounds were released. Clark (2002) showed that the overall flavour intensity decreased linearly with increasing hardness for dessert gels. However, a deformable/cohesive gel consisting of 0 4% low acyl gellan, xanthan, and locust bean gum did not show a good flavour release than other gels having the same hardness because the flavour remained trapped in the gel. Although gelatin gel is deformable/cohesive, it showed a better flavour release in the mouth than other gels having the same hardness because it melted at body temperature. Therefore, both interpretations of Morris (1993) and Clark (2002) are not contradictory but complementary. As described in Section 3.3 Elastic modulus and fracture stress of gels as a function of molar mass, lower molar mass gelling agents form less deformable and brittle gels, these will form gels with higher flavour release. In addition to the fracture strain, the flavour release is known to be enhanced by syneresis (Nishinari & Fang, 2016). The relation between water and aroma holding capacity and the molar mass of the food matrix should be further studied.
9.3. Effect of molar mass of aroma compounds and carrier on the retention of aroma compounds
Since flavour (odor and taste) is an important factor together with texture governing the acceptability of foods, many papers on the flavour release have been published. There have been many reports on the effects of viscosity of the liquids on the flavour release (Roberts, Elmore, Langley, & Bakker, 1990). The effects of concentration or elastic modulus of gels on the aroma release have also been studied (Baek, Linforth, Blake, & Taylor, 1999; Boland, Delahunty, & van Ruth, 2006).
It is generally believed that the aroma compound release was suppressed with increasing viscosity or elasticity of food matrix. Aroma compounds are mostly volatile and many of them are bioactive, and have been encapsulated by coating, or encapsulating for protecting from environmental stress, oxygen, moisture, light, heat, and pH. The coating/encapsulating materials are called walls or carriers, and they are selected from polysaccharides, proteins and lipids and some small molecules. (Shishir, Xie, Sun, Zheng, & Chen, 2018; Gupta et al., 2016).
To develop further a better method for aroma retention or release, it is necessary to understand the flavour characteristics and carrier (polysaccharides, proteins and lipids) characteristics, sample preparation before encapsulation (e.g. initial emulsion formation before spray drying), method of encapsulation (e.g spray drying emulsification, extrusion), and operating condition of encapsulation (operating conditions of spray drying) (Saifullah, Shishir, Ferdowsi, Rahman, & Vuong, 2019).
Since molar mass plays an important role in flavour characteristic and carrier, these two factors are discussed in this section.
9.3.1. Effect of molar mass of aroma compounds on the flavour release
Aroma molecules are generally volatile and odorous organic molecules, mostly in gaseous or liquid forms. These molecules are generally lipophilic and lower molecular weight between 100 and 250, belonging to hydrocarbons, alcohols, aldehydes, ketones, esters, acids and sulfides group of compounds (Gupta et al., 2016).
The general tendency of the retention has been found is in the order of acids < aldehydes < esters ≤ ketones ≤ alcohols, indicating that acids tend to most easily to release. Therefore, it can be concluded that retention of volatiles depends on their molecular weight, relative volatility, polarity, and type of compound. Generally, small, very volatile, and water-soluble flavours are lost to a greater extent than the larger, less volatile, and water-insoluble flavourings (Gupta et al., 2016; Saifullah et al., 2019).
To understand systematically the aroma retention or release, it is logical to examine the influence of molar mass, chemical groups, polarity and volatility of aroma compounds retained in various carriers, glucose, maltose, maltodextrin, starch, thickening or gelling polysaccharides, proteins, and lipids. A general tendency that higher molar mass aroma compounds are better retained was recognized (Moran, Yin, Cadwallader, & Padua, 2014). The relative retention was in the following order, ethyl propionate (MW = 102 g/mol) < ethyl butyrate (MW = 116) < isoamyl butyrate (MW = 158) < n-butyl pentanoate (MW = 158) in glucose, maltose (DE 50), maltodextrin (DE 41.4) and maltodextrin (DE 28.5) as a carrier of aroma compounds (Goubet, Le Quere, & Voilley, 1998). However, the retention of ethyl butyrate was higher than that of isoamyl butyrate in maltose and maltodextrin (DE 28.5), which was an exception to the above mentioned general rule.
A similar trend was found for the relative retention of 8 compounds, 1, acetaldehyde (MW = 44); 2, dimethyl sulphide (MW = 64); 3, diacetyl (MW = 86); 4, allyl isocyanate (MW = 99); 5, ethyl butyrate (MW = 116); 6, citral (MW = 152); 7, octanol (MW = 130); 8, butyric acid (MW = 88) in different starches from maize, potato and pea (Jørgensen et al., 2012) (Fig. 49 ). The order of the boiling point (Bp) of these compounds is in the same order of MW; higher the MW the higher the boiling point, but some exceptions. The Bp = 162 °C of the 8th compound butyric acid (MW88) was higher than Bp = 120 °C of 5th compound ethyl butyrate (MW116).
Fig. 49.
Retention (% w/w) of 8 aroma compounds by starch granules from maize, potato, pea and control (evaporation without starch). From light to dark gray from left to right: control (evaporation without starch), maize, potato, and pea. 1, acetaldehyde (MW = 44 Da); 2, dimethyl sulphide (MW = 64 Da); 3, diacetyl (MW = 86 Da); 4, allyl isocyanate (MW = 99 Da); 5, ethyl butyrate (MW = 116 Da); 6, citral (MW = 152 Da); 7, octanol (MW = 130 Da); 8, butyric acid (MW = 88 Da). Error bars indicate ±SD. Different letters denote significant difference (p < 0.05) between starches (Jørgensen et al., 2012).
Some exceptions for the order of retention were also found. Although MW of the 8th butyric acid (88 Da) is lower than those of the 4th allyl isocyanate (99 Da) and 5th ethyl butyrate (116 Da), it showed a higher retention than those compounds.
Low MW aroma compounds (i.e. acetaldehyde, dimethyl sulphide, diacetyl, allyl isothiocyanate and ethyl butyrate) binding to native starch granules exhibited positive retention of flavour as compared to pure aroma as shown in Fig. 49 (Jørgensen et al., 2012). The left light gray bar showing the retention without starch is smaller than those with darker gray bars which represent the retention in the starch indicating that starch binds low MW aroma compounds. In contrast, the high MW aroma compounds (i.e. citral, octanol and butyric acid) showed a negative retention, i.e. starch-induced aroma evaporation. This has been attributed to the starch-mediated exposure from a large surface area from which aroma compounds can evaporate. In addition, these high MW aroma compounds diffuse less rapidly through the starch granule matrix. However, the extreme high retention of the 2nd compound dimethyl sulphide in comparison to the 1st compound acetaldehyde and the 3rd compound diacetyl suggested the specific interaction with starch as suggested by Goubet et al. (1998).
Recently, more detailed study on the retention/release of 51 odorant molecules consisting of four chemical families (alcohols, aldehydes, ketones and esters) was performed. Odorant molecules were also classified by the structure of their carbon chain (linear or branched, with or without double bonds). Water, pectin, dairy and dairy-pectin gels were used as matrix of odorants (Ayed et al., 2014). As a general tendency, alcohols exhibited the higher retention for all the matrices, and unsaturated odorants having double bonds showed also the higher retention in all the 4 chemical families. Thus, the unsaturated alcohols exhibited the highest retention. However, it was not possible to find any rule governing the retention from the viewpoint of molar mass, and also from the hydrophobicity or polarity, and any other general rules could not be found.
Aldehydes, ketones and alcohols with different chain lengths in proteins of fish, meat and plant have been studied (Cao, Zhou, Wang, Sun, & Pan, 2018; Wang & Arntfield, 2016; Zhou, Zhao, Su, & Sun, 2014). It seems that more release (less retention) was found in the following order; alcohols (1-pentanol, 1-hexanol, 1-octen-3-ol, 1-octenol) > aldehydes (pentanal, hexanal, heptanal, octanal) > ketones (2-pentnon, 2-hexanone, 2-heptanon, 2-octanone), and longer chain (higher molar mass) compounds seem to be more retained probably because of stronger hydrophobic interactions. The hydrophobicity is represented by partition coefficient. The water-air partition coefficient (logPwa) representing the volatility (from water) of aroma compounds and an octanol-air partition coefficient (logPoa) predicting the aroma release from O/W emulsions have been used to predict the aroma release (Tamaru, Igura, & Shimoda, 2018). Values of log P for hexanal, heptanal, and octanal are 1.85, 2.29 and 2.78, hexan-1-ol, octan-1-ol, and nonan-1-ol are 1.82, 2.8 and 3.3 (Ayed et al., 2014), and this value increases with increasing chain length and hydrophobicity.
However, some exceptions were noted, aldehydes were found more retained than ketones in vicilins (Heng et al., 2004). Nonanal was found more released than pentanal, hexanal and octanal although the latter three compounds release followed a general rule that shortest chain pentanal was released most and the longest chain octanal was least released in G-actin (Cao et al., 2018). In addition to molar mass, with or without double bonds, relative volatility, polarity, type of flavour, size of flavour core and concentration of flavour should be taken into account to understand the retention of flavour (Ayed et al., 2014; Saifullah et al., 2019).
9.3.2. Effect of molar mass of carriers on the retention of aroma compounds
Maltodextrins, cyclodextrins, pectin, sodium alginate, gums, whey protein concentrate, or zein, and their mixtures are commonly used for the encapsulation of flavour and aroma (Saifullah et al., 2019). As described in section 3.8 Molar mass effects on properties of solid foods and 3.10 Molar mass effects on physical properties of films, the glass transition temperature of these polymers decreases with decreasing molar mass and moisture content. The mobility of aroma compounds encapsulated by these polymers is higher in rubbery state than in glassy state. Therefore, to prevent the loss of aroma compounds during storage, wall/carrier materials should be kept in glassy state. It is generally found that the concentration of oxidation products of encapsulated flavours during storage is lower for low molecular weight matrices, in particular when produced by spray drying. This is related to the matrix free volume, which decreases with the decreasing molecular weight of the matrix constituents (Ubbink & Schoonman, 2013).
Cyclodextrins (CDs) composed of 6, 7 and 8 glucopiranose units α-CD, β-CD, and γ- CD are produced commercially. They form cone-shaped structures with a hydrophobic cavity and an outer hydrophilic area, enabling the reversible binding of hydrophobic molecules in their cavity to form supramolecular host-guest complexes (Sonnendecker et al., 2019). They are used to encapsulate food flavours and aromas, orange aroma by α-cyclodextrin, d-limonene, menthol, and shiitake flavour by β-CD, and encapsulated aromas are stable during storage (Fenyvesi & Szente, 2016). Storage stability of complexes of essential oil (anise oil, lemon, majoram oil, and garlic oil) with β-CD were shown to be good even after 14 years of storage (Fenyvesi & Szente, 2016). Since this complex formation is conditioned by the cavity of the host CD and the hydrophobic guest aroma, it can happen that two of the smaller guests are included into one larger cavity (Fenyvesi & Szente, 2016). Large ring CD up to DP 37 has been prepared, and proved non-toxic, but is not produced in a great quantity. Sonnendecker et al. (2019) reported that CDs with DP = 10 and 11 were effective for the enantiomeric separation of several enantiomeric pharmaceuticals (Sonnendecker et al., 2019). Although the larger CDs were thought to be unsuitable for enatiomeric discrimination because of the increasing flexibility of larger CD rings, CD10 (CD with DP = 10) to CD12 (CD with DP = 12) were believed to be different from CD6 (α-CD) to CD8 (γ-CD) while still providing sufficient rigidity to form stable complexes. Thus, CD10 to CD12 were thought to act as chiral selectors (Sonnendecker et al., 2019). Therefore, the flexibility is thought important for the performance of larger ring CDs. Recently, Takemasa, Yuguchi, and Kitamura (2020) studied the conformation of CDs based on SAXS, and confirmed the flexibility of large ring cyclodexrins (Nakata et al., 2003). Since the structure and size of cavity of large ring CDs are different from conventional CDs (α-CD, β-CD, and γ- CD), further development in aroma modification is expected in the future.
Although the molar mass is an important determining factor of rheological properties of matrix/wall/carrier, the perceived flavour intensity is influenced by many other factors. While some research groups found that the perceived flavour intensity decreased with increasing gel rigidity, other groups found the opposite tendency. The former tendency might be understood by assuming the decrease of released aroma compounds by instrumental observation, but it was also possible to explain by the increase of mastication time and strength for stronger gels for the latter tendency (Boland et al., 2006). Protocol of the sensory evaluation has not been unified, which makes difficult to compare the results reported by different research groups.
9.4. Cross modal interaction-interaction among texture-taste-aroma
Other factors such as binding of aroma compounds with carriers, taste-odor interaction should also be taken into account to understand whole aspects of aroma release (Gupta et al., 2016).
As has been widely reported, texture, taste and aroma interact with each other before and during eating. For example, when isoamylacetate (IAA) which is a key compound of banana and sucrose solution are injected to the mouth, most subjects perceived banana flavour, but when the sucrose solution is replaced by water, most subjects donot perceive the banana flavour. This is understood by the fact that most humans have never eaten banana from which sugars are removed, and they always perceive a banana flavour key compound in the presence of sugars (Hort & Hollowood, 2004).
10. Conclusion
It is well known that gel formation of food biopolymers is strongly influenced by the molar mass, ionic strength, pH, temperature and other environmental conditions. Some typical examples were discussed. However, needless to say, it is important to study the effect of detailed chemical structure, the degree of substitution and the position of the substituent in cellulose derivatives, KGM, pectin, alginate, galactomannans, native gellans, and other gelling polysaccharides and proteins. Although it is possible to prepare the rigorously controlled cellulose derivatives by regio-selective substitution, it is difficult to get a large amount of sample at present, which makes the collaborative works very difficult. As for the polyelectrolytes such as ionic polysaccharides, gellans, carrageenans, pectins, alginates, chitins, and others, effects of the type of cations or anions present are sometimes more important than a small difference in molar mass. The large scale preparation methods to study polysaccharides with narrow molar mass distribution are not well established in addition to the above-mentioned requirement of chemical purity.
Collaborative research using a common sample is expected to be useful. Molecular level study such as rearrangements of biopolymers during structure formation has been reported and this may be distinguished from syneresis (this is also a kind of rearrangement) causing slippage in rheological measurements. Although Pickering emulsion is widely studied, the mechanism is not yet clarified. It is necessary to study the emulsion activity and stability using the solid particles with different lengths and flexibilities and lipophilicities. Again, a common sample distributed in the collaborative work will be useful. It is necessary to combine the fundamentals and applications in different disciplines. Collaborative research work done in IFOGEST using a common protocol of in vitro digestion study and in the Japanese research group using a common gellan sample proved to be fruitful, and such collaborative research is expected to flourish in many food hydrocolloids related areas.
Declaration of competing interest
There is no conflict of interest.
Acknowledgements
The research was supported by the grants from the National Natural Science Foundation of China (31671811), the State Key Research and Development Plan “Modern food processing and food storage and transportation technology and equipment” (No. 2017YFD0400200), and the Science and Technology Commission of Shanghai Municipality (No.18JC1410801).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.foodhyd.2020.106110.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abad L.V., Relleve L.S., Racadio C.D.T., Aranilla C.T., de la Rosa A.M. Antioxidant activity potential of gamma irradiated carrageenan. Applied Radiation and Isotopes. 2013;79:73–79. doi: 10.1016/j.apradiso.2013.04.035. [DOI] [PubMed] [Google Scholar]
- Ai L., Chung Y., Lin S., Jeng K.G., Lai P.F., Xiong Z. Carrageenan polysaccharides and oligosaccharides with distinct immunomodulatory activities in murine microglia BV-2 cells. International Journal of Biological Macromolecules. 2018;120:633–640. doi: 10.1016/j.ijbiomac.2018.08.151. [DOI] [PubMed] [Google Scholar]
- Al-Assaf S., Phillips G.O., Aoki H., Sasaki Y. Characterization and properties of Acacia senegal (L.) willd. Var. Senegal with enhanced properties (Acacia (sen) SUPER GUMTM): Part 1—controlled maturation of Acacia senegal var. Senegal to increase viscoelasticity, produce a hydrogel form and convert a poor into a good emulsifier. Food Hydrocolloids. 2007;21:319–328. [Google Scholar]
- Al-Ghazzewi F., Elamir A., Tester R., Elzagoze A. Effect of depolymerized konjac glucomannan on wound healing. Bioactive Carbohydrate & Dietary Fibre. 2015;5:125–128. [Google Scholar]
- Augé S., Schmit P.O., Crutchfield C.A., Islam M.T., Harris D.J., Durand E. NMR measure of translational diffusion and fractal dimension. Application to molecular mass measurement. The Journal of Physical Chemistry B. 2009;113:1914–1918. doi: 10.1021/jp8094424. [DOI] [PubMed] [Google Scholar]
- Augustin L.S.A., Kendall C.W.C., Jenkins D.J.A., Willett W.C., Astrup A., Barclay A.W. Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the international carbohydrate quality consortium (ICQC) Nutrition, Metabolism, and Cardiovascular Diseases. 2015;25:795–815. doi: 10.1016/j.numecd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Ayed C., Lubbers S., Andriot I., Merabtine Y., Guichard E., Tromelin A. Impact of structural features of odorant molecules on their retention/release behaviours in dairy and pectin gels. Food Research International. 2014;62:846–859. [Google Scholar]
- Bacic A., Fincher G.B., Stone B.A., editors. Chemistry, biochemistry, and biology of (1 → 3)- β –glucans and related polysaccharides. 2nd ed. Elsevier Amsterdam; 2009. [Google Scholar]
- Baek I., Linforth R.S.T., Blake A., Taylor A.J. Sensory perception is related to the rate of change of volatile concentration in-nose during eating of model gels. Chemical Senses. 1999;24:155–160. doi: 10.1093/chemse/24.2.155. [DOI] [PubMed] [Google Scholar]
- Balazs E.A. Vol. 34. University of Michigan Medical Center; 1968. pp. 255–259. (Viscoelastic properties of hyaluronic acid and biological lubrication). Journal, Special Issue. [PubMed] [Google Scholar]
- Balazs E.A., Denlinger J.L. Sodium hyaluronate and joint function. Journal of Equine Veterinary Science. 1985;5:217–228. [Google Scholar]
- Balto A.S., Lapis T.J., Silver R.K., Ferreira A.J., Beaudry C.M., Lim J. On the use of differential solubility in aqueous ethanol solutions to narrow the DP range of food-grade starch hydrolysis products. Food Chemistry. 2016;197:872–880. doi: 10.1016/j.foodchem.2015.10.120. [DOI] [PubMed] [Google Scholar]
- Bao X., Yuan H., Wang C., Liu J., Lan M. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydrate Polymers. 2013;98:1236–1243. doi: 10.1016/j.carbpol.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Barringer S.A., Sumonsiri N. Electrostatic coating technologies for food processing. Annual Review of Food Science and Technology. 2015;6:157–169. doi: 10.1146/annurev-food-022814-015526. [DOI] [PubMed] [Google Scholar]
- Bateman L., Ye A., Singh H. In vitro digestion of β-lactoglobulin fibrils formed by heat treatment at low pH. Journal of Agricultural and Food Chemistry. 2010;58:9800–9808. doi: 10.1021/jf101722t. [DOI] [PubMed] [Google Scholar]
- Bateni E., Tester R., Al-Ghazzewi F., Bateni S., Alvani K., Piggott J. The use of konjac glucomannan hydrolysates (GMH) to improve the health of the skin and reduce acne vulgaris. The American Journal of Dermatology and Venereology. 2013;2:10–14. [Google Scholar]
- Beer M.U., Wood P.J., Weisz J. Simple and rapid method for evaluation of Mark- Houwink- Sakurada constants of linear random coil polysaccharides using molecular weight and intrinsic viscosity determined by high performance size exclusion chromatography :Application to guar galactomannan. Carbohydrate Polymers. 1999;39:377–380. [Google Scholar]
- Beer M.U., Wood P.J., Weisz J., Fillion N. Effect of cooking and storage on the amount and molecular weight of (1-3)(1-4)-β-D-glucan extracted from oat products by an in vitro digestion system. Cereal Chemistry. 1997;74:705–709. [Google Scholar]
- BeMiller J.N. Pasting, paste, and gel properties of starch-hydrocolloid combinations. Carbohydrate Polymers. 2011;86:386–423. [Google Scholar]
- BeMiller J.N. Carbohydrate chemistry for food scientists. 3rd ed. AACC International Press; WP: 2018. 6 starches: Molecular and granular structures and properties. [Google Scholar]
- Berntzen G., Flo T.H., Medvedev A., Kilaas L., Skjak-Brak G., Sundan A. The tumor necrosis factor-inducing potency of lipopolysaccharide and uronic acid polymers is increased when they are covalently linked to particles. Clinical and Diagnostic Laboratory Immunology. 1998;5:355–361. doi: 10.1128/cdli.5.3.355-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari B., Roos Y.H., editors. Non-equilibrium states and glass transitions in foods processing effects and product-specific implications. Woodhead Publ; 2017. [Google Scholar]
- Biewenga M., Sarasqueta A.F., Tushuizen M.E., de Jonge-Muller E.S.M., van Hoek B., Trouw L.A. The role of complement activation in autoimmune liver disease. Autoimmunity Reviews. 2020;19:102534. doi: 10.1016/j.autrev.2020.102534. [DOI] [PubMed] [Google Scholar]
- Bi B., Yang H., Fang Y., Nishinari K., Phillips G.O. Characterization and emulsifying properties of β-lactoglobulin-gum Acacia Seyal conjugates prepared via the Maillard reaction. Food Chemistry. 2017;214:614–621. doi: 10.1016/j.foodchem.2016.07.112. [DOI] [PubMed] [Google Scholar]
- Blake A.I., Marangoni A.G. Factors affecting the rheological properties of a structured cellular solid used as a fat mimetic. Food Research International. 2015;74:284–293. doi: 10.1016/j.foodres.2015.04.045. [DOI] [PubMed] [Google Scholar]
- Bof M.J., Bordagaray V.C., Locaso D.E., García M.A. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocolloids. 2015;51:281–294. [Google Scholar]
- Boland A.B., Delahunty C.M., van Ruth S.M. Influence of the texture of gelatin gels and pectin gels on strawberry flavour release and perception. Food Chemistry. 2006;96:452–460. [Google Scholar]
- Bos J.D., Meinardi M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Experimental Dermatology. 2000;9:165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- Bourne M.C. 2nd ed. Academic Press; New York: 2002. Food texture and viscosity. [Google Scholar]
- Bowman K.A., Aarstad O.A., Nakamura M., Stokke B.T., Skjåk-Bræk G., Round A.N. Single molecule investigation of the onset and minimum size of the calcium-mediated junction zone in alginate. Carbohydrate Polymers. 2016;148:52–60. doi: 10.1016/j.carbpol.2016.04.043. [DOI] [PubMed] [Google Scholar]
- Brito de la Fuente E., Turcanu M., Ekberg O., Gallegos C. Springer; 2017. Rheological aspects of swallowing and dysphagia: Shear and elongational flows. Dysphagia 687–716, part of the medical radiology. [Google Scholar]
- Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols. 2019;14:991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- Buliga G.S., Brant D.A. Temperature and molecular weight dependence of the unperturbed dimensions of aqueous pullulan International. Journal of Biological Macromolecules. 1987;9:71–76. [Google Scholar]
- Burchard W. Light scattering. In: Ross-Murphy S.B., editor. Physical techniques for the study of food biopolymers. Springer Science+Business Media; Dordrecht: 1994. pp. 151–213. [Google Scholar]
- Byun E.H., Kim J.H., Sung N.Y., Choi J., Lim S.T., Kim K.H. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiation Physics and Chemistry. 2008;77:781–786. [Google Scholar]
- Błaszczyk K., Wilczak J., Harasym J., Gudej S., Suchecka D., Królikowski Impact of low and high molecular weight oat beta-glucan on oxidative stress and antioxidant defense in spleen of rats with LPS induced enteritis. Food Hydrocolloids. 2015;51:272–280. doi: 10.1039/c4fo00638k. [DOI] [PubMed] [Google Scholar]
- Cao Y., Li S., Fang Y., Nishinari K., Phillips G.O. Conformational transition of polyelectrolyte as influenced by electrostatic complexation with protein. Biomacromolecules. 2016;17:3949–3956. doi: 10.1021/acs.biomac.6b01335. [DOI] [PubMed] [Google Scholar]
- Cao N., Yang X., Fu Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocolloids. 2009;23:729–735. [Google Scholar]
- Cao J.X., Zhou C.Y., Wang Y., Sun Y.Y., Pan D.D. The effect of oxidation on the structure of G-actin and its binding ability with aroma compounds in carp grass skeletal muscle. Food Chemistry. 2018;240:346–353. doi: 10.1016/j.foodchem.2017.07.068. [DOI] [PubMed] [Google Scholar]
- Chae S.Y., Jang M.K., Nah J.W. Influence of molecular weight on oral absorption of water soluble chitosans. Journal of Controlled Release. 2005;102:383–394. doi: 10.1016/j.jconrel.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Chakraborty I., Sen I.K., Mondal S., Rout D., Bhanja S.K., Maity G.N. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatalysis and Agricultural Biotechnology. 2019;22:101425. [Google Scholar]
- Chan G.C., Chan W.K., Sze D.M. The effects of beta-glucan on human immune and cancer cells. Journal of Hematology & Oncology. 2009;2(25):11. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantaro P., Pongsawatmanit R., Nishinari K. Effect of heating-cooling on rheological properties of tapioca starch paste with and without xanthan gum. Food Hydrocolloids. 2013;13:183–194. [Google Scholar]
- Chanyongvorakul Y., Matsumura Y., Nonaka M., Motoki M., Mori T. Physical properties of soy bean and broad bean 11S globulin gels formed by transglutaminase reaction. Journal of Food Science. 1995;60:483–488. & 493. [Google Scholar]
- Chen H.L., Fan Y.H., Chen M.E., Chan Y. Unhydrolysed and hydrolysed konjac glucomannans modulated caecal and faecal microflora in Balb/c mice. Nutrition. 2005;21:1059–1064. doi: 10.1016/j.nut.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Cheng L., Wang Y., He X., Wei X. Preparation, structural characterization and bioactivities of Se-containing polysaccharide: A review. International Journal of Biological Macromolecules. 2018;120:82–92. doi: 10.1016/j.ijbiomac.2018.07.106. [DOI] [PubMed] [Google Scholar]
- Chen C.P., Hsu C.C., Pei Y.C., Chen R.L., Zhou S., Shen H.C. Changes of synovial fluid protein concentrations in supra-patellar bursitis patients after the injection of different molecular weights of hyaluronic acid. Experimental Gerontology. 2014;52:30–35. doi: 10.1016/j.exger.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Chen W.Y., Marcellin E., Hung J., Nielsen L.K. Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. Journal of Biological Chemistry. 2009;284:18007–18014. doi: 10.1074/jbc.M109.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara G. Pharmacological activity of the polysaccharides. Kobunshi (High. Polymers, Japan) 1977;26:117–123. [Google Scholar]
- Chihara G., Hamuro J., Maeda Y.Y., Arai Y., Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom) Cancer Research. 1970;30:2776–2781. [PubMed] [Google Scholar]
- Choi J.I., Kim H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydrate Polymers. 2013;97:358–362. doi: 10.1016/j.carbpol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Choi J., Kim H.J., Lee J.W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chemistry. 2011;129:520–523. doi: 10.1016/j.foodchem.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Cho M.L., Lee B.Y., You S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules. 2011;16:291–297. doi: 10.3390/molecules16010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choromanska A., Kulbacka J., Harasym J., Oledzki R., Szewczyk A., Saczko J. High- and low-molecular weight oat beta-glucan reveals antitumor activity in human epithelial lung cancer. Pathology and Oncology Research. 2018;24:583–592. doi: 10.1007/s12253-017-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichero J.A.Y. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutrition Journal. 2013;12(54):8. doi: 10.1186/1475-2891-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. Influence of hydrocolloids on flavour release and sensory― instrumental correlations. In: Phillips G.O., Williams P.A., editors. Gums and Stabilisers for the food industry 11. Royal Society of Chemistry; Cambridge: 2002. pp. 217–225. [Google Scholar]
- Clark A.H., Gidley M.J., Richardson R.K., Ross-Murphy S.B. Rheological studies of aqueous amylose gels: The effect of chain length and concentration on gel modulus. Macromolecules. 1989;22:346–351. [Google Scholar]
- Clasen C., Kulicke W.-M. Determination of viscoelastic and rheo-optical of material functions of water-soluble cellulose derivatives. Progress in Polymer Science. 2001;26:1839–1919. [Google Scholar]
- Clement M.J., Tissot B., Chevolot L., Adjadj E., Du Y., Curmi P.A. NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology. 2010;20:883–894. doi: 10.1093/glycob/cwq046. [DOI] [PubMed] [Google Scholar]
- Cowie J.M.G., Arrighi V. 3rd ed. CRC Press; 2007. Polymers: Chemistry and physics of modern materials. [Google Scholar]
- Crofts N., Itoh A., Abe M., Miura S., Oitome N.F., Bao J. Three major nucleotide polymorphisms in the waxy gene correlated with the amounts of extra-long chains of amylopectin in rice cultivars with S or L-type Amylopectin. Journal of Applied Glycoscience. 2019;66:37–46. doi: 10.5458/jag.jag.JAG-2018_005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Wood P.J. Relationships between structural features, molecular weight and rheological properties of cereal β-D-glucans. In: Nishinari K., editor. Hydrocolloids: PART 1 physical chemistry and industrial applications of gels, polysaccharides and proteins. Elsevier Science Publishers; London: 2000. pp. 159–168. [Google Scholar]
- Cukier R. Diffusion of Brownian spheres in semidilute polymer solutions. Macromolecules. 1984;17:252–255. [Google Scholar]
- Cyphert J.M., Trempus C.S., Garantziotis S. Size matters: Molecular weight specificity of hyaluronan effects in cell biology. International Journal of Cell Biology. 2015;8 doi: 10.1155/2015/563818. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi N., Yadav B.S., Yadav R.B. Pasting, rheological, thermal and gel textural properties of pearl millet starch as modified by guar gum and its acid hydrolysate. International Journal of Biological Macromolecules. 2019;139:387–396. doi: 10.1016/j.ijbiomac.2019.08.012. [DOI] [PubMed] [Google Scholar]
- Dangi N., Yadav B.S., Yadav R.B. Pectin and its acid hydrolysate for the modification of hydration, pasting, thermal and rheological properties of barley starch. International Journal of Biological Macromolecules. 2019;152:969–980. doi: 10.1016/j.ijbiomac.2019.10.183. [DOI] [PubMed] [Google Scholar]
- Dangi N., Yadav B.S., Yadav R.B. Pasting, rheological, and dough mixing behavior of rice flour as affected by the addition of native and partially hydrolyzed β-glucan concentrate. Journal of Texture Studies. 2020;51:650–662. doi: 10.1111/jtxs.12520. [DOI] [PubMed] [Google Scholar]
- De Gennes P.-G. Cornell University Press; USA: 1979. Scaling concepts in polymer physics. [Google Scholar]
- De Silva D.A., Poole-Warren L.A., Martens P.J., in het Panhuis M. Mechanical characteristics of swollen gellan gum hydrogels. Journal of Applied Polymer Science. 2013;130:3374–3383. [Google Scholar]
- Dhital S., Warren F.J., Butterworth P.J., Ellis P.R., Gidley M.J. Mechanisms of starch digestion by α-amylase—structural basis for kinetic properties. Critical Reviews in Food Science and Nutrition. 2017;57:875–892. doi: 10.1080/10408398.2014.922043. [DOI] [PubMed] [Google Scholar]
- Di Monaco R., Miele N.A., Cabisidan E.K., Cavella S. Strategies to reduce sugars in food. Current Opinion in Food Science. 2018;19:92–97. [Google Scholar]
- Dicker K.T., Gurski L.A., Pradhan-Bhatt S., Witt R.L., Farach-Carson M.C., Jia X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomaterialia. 2014;10:1558–1570. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocolloids. 2009;23:1473–1482. [Google Scholar]
- Dickinson E. Hydrocolloids acting as emulsifying agents - how do they do it? Food Hydrocolloids. 2018;78:2–14. [Google Scholar]
- Djabourov M., Nishinari K., Ross-Murphy S.B. Physical gels from biological and synthetic polymers. Cambridge University Press; 2013. [Google Scholar]
- Doi M. Oxford University Press; 2013. Soft matter physics. [Google Scholar]
- Dou Z., Chen C., Fu X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocolloids. 2019;96:568–576. [Google Scholar]
- Draget K.I., Skjåk Braæk G., Smidsrød O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydrate Polymers. 1994;25:31–38. [Google Scholar]
- Draget K.I., Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloids. 2011;25:251–256. [Google Scholar]
- Draget K.I., Ǿstgaard K., Smidsrød O. Homogeneous alginate gels: A technical approach. Carbohydrate Polymers. 1991;14:159–178. [Google Scholar]
- Dudás E.F., Bodor A. Quantitative, diffusion NMR based analytical tool to distinguish folded, disordered, and denatured biomolecules. Analytical Chemistry. 2019;91:4929–4933. doi: 10.1021/acs.analchem.8b05617. [DOI] [PubMed] [Google Scholar]
- Du B., Lin C., Bian Z., Xu B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends in Food Science & Technology. 2015;41:49–59. [Google Scholar]
- Du B., Zeng H., Yang Y., Bian Z., Xu B. Anti-inflammatory activity of polysaccharide from Schizophyllum commune as affected by ultrasonication. International Journal of Biological Macromolecules. 2016;91:100–105. doi: 10.1016/j.ijbiomac.2016.05.052. [DOI] [PubMed] [Google Scholar]
- Dunkel A., Koster J., Hofmann T. Molecular and sensory characterization of γ-glutamyl peptides as key contributors to the kokumi taste of edible beans (Phaseolus vulgaris L.) Journal of Agricultural and Food Chemistry. 2007;55:6712–6719. doi: 10.1021/jf071276u. [DOI] [PubMed] [Google Scholar]
- Ebihara K., Masuhara R., Kiriyama S. Major determinants of plasma glucose-flattening activity of a water-soluble dietary fiber: Effects of konjac mannan on gastric emptying and intraluminal glucose-diffusion. Nutrition Reports International. 1981;23:1145–1156. [Google Scholar]
- Edelman M.W., Tromp R.H., van der Linden E. Phase separation- induced fractionation in molar mass in aqueous mixtures of gelatin and dextran. Physical Review E. 2003;67(2) doi: 10.1103/PhysRevE.67.021404. 021404. [DOI] [PubMed] [Google Scholar]
- Egger L., Ménard O., Delgado-Andrade C., Alvito P., Assunção R., Balance S. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Research International. 2016;88:217–225. [Google Scholar]
- Egger L., Schlegel P., Baumann C., Stoffers H., Guggisberg D., Brügger C. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Research International. 2017;102:567–574. doi: 10.1016/j.foodres.2017.09.047. [DOI] [PubMed] [Google Scholar]
- Ejima A., Nakamura M., Suzuki Y.A., Sato K. Identification of food-derived peptides in human blood after ingestion of corn and wheat gluten hydrolysates. Journal of Food Bioactives. 2018;2:104–111. [Google Scholar]
- Ejima A., Yamada K., Sato K. Presence of isomerized aspartic dipeptides in a porcine liver protein hydrolysate and their bioavailability upon ingestion. Bioactive Compounds in Health and Disease. 2019;2:155–169. [Google Scholar]
- Ellis P.J., Dawoud F.M., Morris E.R. Blood glucose, plasma insulin and sensory responses to guar containing wheat breads: Effects of molecular weight and particle size of guar gum. British Journal of Nutrition. 1991;66:363–379. doi: 10.1079/bjn19910041. [DOI] [PubMed] [Google Scholar]
- Englyst K., Goux A., Meynier A., Quigley M., Englyst H., Brack O. Inter- laboratory validation of the starch digestibility method for determination of rapidly digestible and slowly digestible starch. Food Chemistry. 2018;245:1183–1189. doi: 10.1016/j.foodchem.2017.11.037. [DOI] [PubMed] [Google Scholar]
- Enright M.B., Leitner D.M. Mass fractal dimension and the compactness of proteins. Physical Review E. 2005;71 doi: 10.1103/PhysRevE.71.011912. [DOI] [PubMed] [Google Scholar]
- Espinal-Ruiz M., Restrepo-Sánchez L.-P., Narváez-Cuenca C.-E., McClements D.J. Impact of pectin properties on lipid digestion under simulated gastrointestinal conditions: Comparison of citrus and banana passion fruit (Passiflora tripartita var. mollissima) pectins. Food Hydrocolloids. 2016;52:329–342. [Google Scholar]
- Evans R. The interpretation of small molecule diffusion coefficients: Quantitative use of diffusion-ordered NMR spectroscopy. Progress in Nuclear Magnetic Resonance Spectroscopy. 2020;117:33–69. doi: 10.1016/j.pnmrs.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Eysturskarð J. 2010. Mechanical properties of gelatin gels; effect of molecular weight and molecular weight distribution. PhD Thesis, NTNU, Norway) [Google Scholar]
- Eysturskarð J., Haug I.J., Elharfaoui N., Djabourov M., Draget K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocolloids. 2009;23:1702–1711. [Google Scholar]
- Fam H., Bryant J.T., Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007;44:59–74. [PubMed] [Google Scholar]
- Fang Y., Al-Assaf S., Phillips G.O., Nishinari K., Funami T., Williams P.A. Multiple steps and critical behaviors of the binding of calcium to alginate. Journal of Physical Chemistry. 2007;B111:2456–2462. doi: 10.1021/jp0689870. [DOI] [PubMed] [Google Scholar]
- Fang Y.P., Li L.B., Inoue C., Lundin L., Appelqvist I. Associative and segregative phase separations of gelatin/kappa-carrageenan aqueous mixtures. Langmuir. 2006;22:9532–9537. doi: 10.1021/la061865e. [DOI] [PubMed] [Google Scholar]
- Fang X., Zhao X., Yu G., Zhang L., Feng Y., Zhou Y. Effect of molecular weight and pH on the self-assembly microstructural and emulsification of amphiphilic sodium alginate colloid particles. Food Hydrocolloids. 2020;103:105593. [Google Scholar]
- Fedorov S.N., Ermakova S.P., Zvyagintseva T.N., Stonik V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Marine Drugs. 2013;11:4876–4901. doi: 10.3390/md11124876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M., Perez-Puyana V., Romero A., Guerrero A. Development of thermally processed bioactive pea protein gels: Evaluation of mechanical and antioxidant properties. Food and Bioproducts Processing. 2017;101:74–83. [Google Scholar]
- Fenyvesi E., Szente L. Nanoencapsulation of flavors and aromas by cyclodextrins. Encapsulations. 2016;2:769–782. [Google Scholar]
- Fernando A., Herman A., Sue B., Laurence C., Riccardo C., Wolfgang D. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to an application on the use of partially depolymerised guar gum as a food additive. EFSA Journal. 2007;5:514. [Google Scholar]
- Ferry J.D. 3rd ed. Wiley; 1980. Viscoelastic properties of polymers. [Google Scholar]
- Frandsen K.E.H., Simmons T.J., Dupree P., Poulsen J.-C.N., Hemsworth G.R., Ciano L. The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nature Chemical Biology. 2016;12:298–303. doi: 10.1038/nchembio.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R.E.J., Laurent C.T., Laurent B.G.U. Hyaluronan: Its nature, distribution, functions and turnover. Journal of Internal Medicine. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- Fujii K., Kawata M., Kobayashi Y., Okamoto A., Nishinari K. Effects of the addition of hyaluronate segments with different chain lengths on the viscoelasticity of hyaluronic acid solutions. Biopolymers. 1996;38:583–591. doi: 10.1002/(SICI)1097-0282(199605)38:5%3C583::AID-BIP4%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Funami T. Functions of food polysaccharides to control the gelatinization and retrogradation behaviors of starch in an aqueous system in relation to the macromolecular characteristics of food polysaccharides. Food Science and Technology Research. 2009;15:557–568. [Google Scholar]
- Funami T. In vivo and rheological approaches for characterizing food oral processing and usefulness of polysaccharides as texture modifiers- A review. Food Hydrocolloids. 2017;68:2–14. [Google Scholar]
- Funami T., Ishihara S., Nakauma M., Kohyama K., Nishinari K. Texture design for products using food hydrocolloids. Food Hydrocolloids. 2012;26:412–420. [Google Scholar]
- Funami T., Kataoka Y., Noda S., Hiroe M., Ishihara S., Asai I. Functions of fenugreek gum with various molecular weights on the gelatinization and retrogradation behaviors of corn starch-1: Characterizations of starch and investigations of corn starch/fenugreek gum composite system at a relatively high starch concentration; 15 w/v% Food Hydrocolloids. 2008;22:763–776. [Google Scholar]
- Funami T., Kataoka Y., Noda S., Hiroe M., Ishihara S., Asai I. Functions of fenugreek gum with various molecular weights on the gelatinization and retrogradation behaviors of corn starch-2: Characterizations of starch and investigations of corn starch/fenugreek gum composite system at a relatively low starch concentration; 5 w/v% Food Hydrocolloids. 2008;22:777–787. [Google Scholar]
- Funami T., Kataoka Y., Omoto T., Goto Y., Asai I., Nishinari K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch. Food Hydrocolloids. 2005;19:1–13. [Google Scholar]
- Funami T., Kataoka Y., Omoto T., Goto Y., Asai I., Nishinari K. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2a. Functions of guar gums with different molecular weights on the gelatinization behavior of corn starch. Food Hydrocolloids. 2005;19:15–24. [Google Scholar]
- Funami T., Kataoka Y., Omoto T., Goto Y., Asai I., Nishinari K. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2b. Functions of guar gums with different molecular weights on the retrogradation behavior of corn starch. Food Hydrocolloids. 2005;19:25–36. [Google Scholar]
- Fuongfuchat A., Jamieson A.M., Blackwell J., Gerken T.A. Rheological studies of the interaction of mucins with alginate and polyacrylate. Carbohydrate Research. 1996;284:85–99. doi: 10.1016/0008-6215(95)00396-7. [DOI] [PubMed] [Google Scholar]
- Gamel T.H., Abdel-Aal E.-S.M., Ames N.P., Duss R., Tosh S. Enzymatic extraction of beta-glucan from oat bran cereals and oat crackers and optimization of viscosity measurement. Journal of Cereal Science. 2014;59:33–40. [Google Scholar]
- Gao A., Nishinari K. Effect of degree of acetylation on gelation of konjac glucomannan. Biomacromolecules. 2004;5:175–185. doi: 10.1021/bm034302f. [DOI] [PubMed] [Google Scholar]
- Gao F., Yang C.X., Mo W., Liu Y.W., He Y.Q. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clinical and Investigative Medicine. 2008;31:E106–E116. doi: 10.25011/cim.v31i3.3467. [DOI] [PubMed] [Google Scholar]
- Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L.G.S., Guedes G.M.M., Fonseca X.M.Q.C., Pereira-Neto W.A., Castelo-Branco D.S.C.M., Sidrim J.J.C. Antifungal activity of different molecular weight chitosans against planktonic cells and biofilm of Sporothrix brasiliensis. International Journal of Biological Macromolecules. 2020;143:341–348. doi: 10.1016/j.ijbiomac.2019.12.031. [DOI] [PubMed] [Google Scholar]
- Garcia M.P.M., Gómez-Guillén M.C., López-Caballero M.E., Barbosa-Cánovas G.V. 1st ed. Taylor & Francis; 2016. Edible films and coatings. Fundamentals and applications. [Google Scholar]
- Gidley M.J., Bulpin P.V. Aggregation of amylose in aqueous systems: The effect of chain length on phase behavior and aggregation kinetics. Macromolecules. 1989;22:341–346. [Google Scholar]
- Garzón-Porras A., Bertuzzi D.L., Lucas K., da Silva L.C.E, de Oliveira M.G., Ornelas C. Nitric oxide releasing polyamide dendrimer with anti-inflammatory activity. ACS Applied Polymer Materials. 2020;2:2027–2034. [Google Scholar]
- Gidley M., Nishinari K. Physico-chemistry of (1,3)-β-glucans. In: Bacic A., Fincher G., Stone B., editors. Chemistry, biochemistry, and biology of 1-3 beta glucans and related polysaccharides. Elsevier; 2009. pp. 47–118. Chap.2.2. [Google Scholar]
- Gidley M.J., Yakubov G.E. Functional categorisation of dietary fibre in foods: Beyond ‘soluble’ vs ‘insoluble’. Trends in Food Science & Technology. 2019;86:563–568. [Google Scholar]
- Gong B., Cheng L., Gilbert R.G., Li C. Distribution of short to medium amylose chains are major controllers of in vitro digestion of retrograded rice starch. Food Hydrocolloids. 2019;96:634–643. [Google Scholar]
- Goubet I., Le Quere L.J., Voilley J.A. Retention of aroma compounds by carbohydrates: Influence of their physiochemical characteristics and of their physical state. A review. Journal of Agricultural and Food Chemistry. 1998;46:1981–1990. [Google Scholar]
- Gravelle A.J., Barbut S., Quinton M., Marangoni A.G. Towards the development of a predictive model of the formulation- dependent mechanical behaviour of edible oil-based ethylcellulose oleogels. Journal of Food Engineering. 2014;143:114–122. [Google Scholar]
- Grundy M.M.-L., Edwards C.H., Mackie A.R., Gidley M.J., Butterworth P.J., Ellis P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. British Journal of Nutrition. 2016;116:816–833. doi: 10.1017/S0007114516002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Bellissimo N., Rousseau D. Role of gel structure in controlling in vitro intestinal lipid digestion in whey protein emulsion gels. Food Hydrocolloids. 2017;69:264–272. [Google Scholar]
- Guo X., Ye X., Sun Y., Wu D., Wu N., Hu Y. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. Journal of Agricultural and Food Chemistry. 2014;62:1088–1095. doi: 10.1021/jf404717y. [DOI] [PubMed] [Google Scholar]
- Gupta S., Khan S., Muzafar M., Kushwaha M., Yadav A.K., Gupta A.P. Encapsulation: Entrapping essential oil/flavors/aromas in food. In: Grumezescu A.M., editor. Encapsulations. Elsevier Inc. Ltd; London: 2016. pp. 229–268. [Google Scholar]
- Gupta R.C., Lall R., Srivastava A., Sinha A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Frontiers in Veterinary Science. 2019;6:192. doi: 10.3389/fvets.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurpilhares D.B., Cinelli L.P./, Simas N.K., Pessoa A., Jr., Sette L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chemistry. 2019;280:175–186. doi: 10.1016/j.foodchem.2018.12.023. [DOI] [PubMed] [Google Scholar]
- Hakansson B., Nyden M., Soderman O. The influence of polymer molecular-weight distributions on pulsed field gradient nuclear magnetic resonance self-diffusion experiments. Colloid & Polymer Science. 2000;278:399–405. [Google Scholar]
- Harasym J., Suchecka D., Gromadzka-Ostrowska J. Effect of raw material size reduction by freeze-milling on beta-glucan recovery process from oat bran. Journal of Cereal Science. 2015;61:119–125. [Google Scholar]
- Harnkarnsujarit N. Glass-transition and non-equilibrium states of edible films and barriers. In: Bhandari B., Roos Y.H., editors. Non-equilibrium states and glass transitions in foods processing effects and product-specific implications. Woodhead Publ; 2017. pp. 349–377. [Google Scholar]
- Hatakeyama H., Hatakeyama T. Interaction between water and hydrophilic polymers. Thermochimica Acta. 1998;308:3–22. [Google Scholar]
- Hatakeyama T., Nakamura K., Takahashi M., Hatakeyama H. Phase transitions of gellan-water systems. Progress in Colloid and Polymer Science. 1998;114:98–101. [Google Scholar]
- Hatakeyama T., Quinn F.X. 2nd ed. John Wiley; Chichester: 1999. Thermal analysis, fundamentals and applications to polymer science. [Google Scholar]
- Haug A., Larsen B., Smidsrød O. A study of the constitution of alginic acid by partial acid hydrolysis. Acta Chemica Scandinavica. 1966;20:183–190. [Google Scholar]
- Haug A., Larsen B., Smidsrød O. Alkaline degradation of alginate. Acta Chemica Scandinavica. 1967;21:2859–2870. [Google Scholar]
- Haug A., Smidsrød O. Fractionation of alginates by precipitation with calcium and magnesium ions. Acta Chemica Scandinavica. 1965;19:1221–1226. [Google Scholar]
- Hemker H.C. A century of heparin: Past, present and future. Journal of Thrombosis and Haemostasis. 2016;14:2329–2338. doi: 10.1111/jth.13555. [DOI] [PubMed] [Google Scholar]
- Heng L., van Koningsveld G.A., Gruppen H., van Boekel M.A.J.S., Vincken J.P., Roozen J.P. Protein–flavor interactions in relation to development of novel protein foods. Trends in Food Science & Technology. 2004;15:217–224. [Google Scholar]
- Hjerde T., Stokke B.T., Smidsrød O., Christensen B.E. Free-radical degradation of triple-stranded scleroglucan by hydrogen peroxide and ferrous ions. Carbohydrate Polymers. 1998;37:41–48. [Google Scholar]
- Hort J., Hollowood T.A. Controlled continuous flow delivery system for investigating taste-aroma interactions. Journal of. Agricultural and Food Chemistry. 2004;52:4834–4843. doi: 10.1021/jf049681y. [DOI] [PubMed] [Google Scholar]
- Hossain K.S., Nishinari K. Chain release behavior of gellan gels. Progress in Colloid and Polymer Science. 2009;136:177–186. [Google Scholar]
- Hou C., Zhao X., Tian M., Zhou Y., Yang R., Gu Z. Impact of water extractable arabinoxylan with different molecular weight on the gelatinization and retrogradation behavior of wheat starch. Food Chemistry. 2020;318:126477. doi: 10.1016/j.foodchem.2020.126477. [DOI] [PubMed] [Google Scholar]
- Huang L., Takahashi R., Kobayashi S., Kawase T., Nishinari K. Gelation behavior of native and acetylated konjac glucomannan. Biomacromolecules. 2002;3:1296–1303. doi: 10.1021/bm0255995. [DOI] [PubMed] [Google Scholar]
- Hu B., Han L., Gao Z., Zhang K., Al-Assaf S., Nishinari K. Effects of temperature and solvent condition on phase separation induced molecular fractionation of gum Arabic/hyaluronan aqueous mixtures. International Journal of Biological Macromolecules. 2018;116:683–690. doi: 10.1016/j.ijbiomac.2018.05.073. [DOI] [PubMed] [Google Scholar]
- Hu X., Sheng X., Li X.L., Liu L., Zheng, Chen X. Effect of dietary oat β-glucan on high-fat diet induced obesity in HFA mice. Bioactive Carbohydrates and Dietary Fiber. 2015;5:79–85. [Google Scholar]
- Inouchi N. Study on structures and physical properties of endosperm starches of rice and other cereals. Journal of Applied Glycoscience. 2010;57:13–23. [Google Scholar]
- Iqbal D., Marchetti R., Aman A., Silipo A., Qader S.A.U., Molinaro A. Enzymatic and acidic degradation of high molecular weight dextran into low molecular weight and its characterizations using novel diffusion-ordered NMR spectroscopy. International Journal of Biological Macromolecules. 2017;103:744–750. doi: 10.1016/j.ijbiomac.2017.05.073. [DOI] [PubMed] [Google Scholar]
- Isobe S., Noguchi A. High moisture extrusion with a twin-screw extruder (part II): Injection molding of soy protein. Nippon Shokuhin Kogyo Gakkaishi. 1988;35:471–479. (in Japanese with English summary and figure captions) [Google Scholar]
- Iwamoto M., Kurachi M., Nakashima T., Kim D., Yamaguchi K., Oda T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Letters. 2005;579:4423–4429. doi: 10.1016/j.febslet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Jeng K.G., Hou R.C.W., Wang J., Ping L. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p-38 mitogen-activated protein kinase and nuclear factor-κB. Immunology Letters. 2005;97:101–106. doi: 10.1016/j.imlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J.A., Kendall C.W.C., McKeown-Eyssen G., Josse R.G., Silverberg J., Booth G.L. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes. Journal of American Medical Association. 2008;300:2741–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J.A., Reynolds D., Slavin B., Leeds A.R., Jenkins A.L., Jepson E.M. Dietary fiber and blood lipids: Treatment of hypercholesterolemia with guar crispbread. American Journal of Clinical Nutrition. 1980;33:575–581. doi: 10.1093/ajcn/33.3.575. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Woelever T.M., Leeds A.R., Gassuli M.A., Haisman P., Dilawari J. Dietary fibres, fibre analogues, and glucose tolerance : Importance of viscosity. British Medical Journal. 1978;1:1392–1394. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Li H., Shi J., Xu Z. Depolymerized konjac glucomannan: Preparation and application in health care. Journal of Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19:505–514. doi: 10.1631/jzus.B1700310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian W., Tu L., Wu L., Xiong H., Pang J., Sun Y. Physicochemical properties and cellular protection against oxidation of degraded Konjac glucomannan prepared by γ-irradiation. Food Chemistry. 2017;231:42–50. doi: 10.1016/j.foodchem.2017.03.121. [DOI] [PubMed] [Google Scholar]
- Jones J.M. Dietary fiber future directions: Integrating new definitions and findings to inform nutrition research and communication. Advances in Nutrition. 2013;4:8–15. doi: 10.3945/an.112.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A.D., Jensen S.L., Ziegler G., Pandeya A., Buleon A., Svensson B. Structural and physical effects of aroma compound binding to native starch granules. Starch /Stärke. 2012;64:461–469. [Google Scholar]
- Kandagal P.B., Ashoka S., Seetharamappa J., Shaikh S.M.T., Jadegoud Y., Ijare O.B. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. Journal of Pharmaceutical and Biomedical Analysis. 2006;41:393–399. doi: 10.1016/j.jpba.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Ohta K., Miyamoto H., Nakamura S. Preparation and solution properties of pullulan fractions as standard samples for water-soluble polymers. Carbohydrate Polymers. 1984;4:335–356. [Google Scholar]
- Kawai K. Iontophoresis/electroporation application (Mesoporation) as a transdermal drug delivery means and its dermatological application. Drug Delivery System. 2012;27―3:164–175. [Google Scholar]
- Kawai S., Nitta Y., Nishinari K. Model study for large deformation of physical polymeric gels. The Journal of Chemical Physics. 2008;128:134903. doi: 10.1063/1.2894845. [DOI] [PubMed] [Google Scholar]
- Kawata M., Okamoto A., Endo T., Tsukamoto Y. Viscoelasticity of synovial fluids and additive effect of hyaluronate. In: Nishinari K., editor. Hydrocolloids - Part 2 fundamentals and applications in food, biology, and medicine. Elsevier Science B.V; 2000. pp. 343–348. [Google Scholar]
- Kerckhoffs D.A.J.M., Hornstra G., Mensink R.P. Cholesterol- lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. American Journal of Clinical Nutrition. 2003;78:221–227. doi: 10.1093/ajcn/78.2.221. [DOI] [PubMed] [Google Scholar]
- Kiriyama S., Ebihara K., Ikegami S., Innami S., Katayama Y., Takehisa F. Searching for the definition, terminology and classification of dietary fiber and the new proposal from Japan. Journal of Japan Association for Dietary Fiber Research. 2006;10:11–24. [Google Scholar]
- Klinkesorn U., Sophanodora P., Chinachoti P., McClements D.J. Stability and rheology of corn oil- in-water emulsions containing maltodextrin. Food Research International. 2004;37:851–859. [Google Scholar]
- Kobayashi Y., Okalnoto A., Nishnan K. Viscoelasticity of hyaluronic acid with different molecular weights. Biorheology. 1994;31:235–244. doi: 10.3233/bir-1994-31302. [DOI] [PubMed] [Google Scholar]
- Kofuji K., Aoki A., Tsubaki K., Konishi M., Isobe T., Murata Y. Antioxidant activity of β-glucan. ISRN Pharmaceutics. 2012:5. doi: 10.5402/2012/125864. Article ID 125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama K. Oral sensing of food properties. Journal Of Texture Studies. 2015;46:138–151. [Google Scholar]
- Kohyama K., Sasaki T. Differential scanning calorimetry and a model calculation of starches annealed at 20 and 50 °C. Carbohydrate Polymers. 2006;63:82–88. [Google Scholar]
- Kojima T., Tabata K., Itoh W., Yanaki T. Molecular weight dependence of the antitumor activity. Agricultural & Biological Chemistry. 1986;50:231–232. [Google Scholar]
- Koliandris A.L., Morris C., Hewson L., Hort J., Taylor A.J., Wolf B. Correlation between saltiness perception and shear flow behaviour for viscous solutions. Food Hydrocolloids. 2010;24:792–799. [Google Scholar]
- Kong H., Yang J., Zhang Y., Fang Y., Nishinari K., Phillips G.O. Synthesis and antioxidant properties of gum Arabic-stabilizedselenium nanoparticles. International Journal of Biological Macromolecules. 2014;65:155–162. doi: 10.1016/j.ijbiomac.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Kusova A.M., Sitnitsky A.E., Idiyatullin B.Z., Bakirova D.R., Zuev Y.F. The effect of shape and concentration on translational diffusion of proteins measured by PFG NMR. Applied Magnetic Resonance. 2018;49:35–51. [Google Scholar]
- Kuz’mina N.E., Moiseev S.V., Krylov V.I., Yashkir V.A., Merkulov V.A. Determination of the parameters of molecular-weight distribution of hydroxyethyl starches by diffusion-ordered NMR spectroscopy. Journal of Analytical Chemistry. 2015;70:843–849. [Google Scholar]
- Kwong M.G.Y., Wolever T.M.S., Brummer Y., Tosh S. Attenuation of glycemic responses by oat beta-glucan solutions and viscoelastic gels is dependent on molecular weight distribution. Food & Function. 2013;4:401–408. doi: 10.1039/c2fo30202k. [DOI] [PubMed] [Google Scholar]
- Lagrain B., Wilderjans E., Glorieux C., Delcour J.A. Importance of gluten and starch for structural and textural properties of crumb from fresh and stored bread. Food Biophysics. 2012;7:173–181. [Google Scholar]
- Lahaye M., Robic A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8:1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- Lahrsen E., Liewert I., Alban S. Gradual degradation of fucoidan from Fucus vesiculosus and its effect on structure, antioxidant and antiproliferative activities. Carbohydrate Polymers. 2018;192:208–216. doi: 10.1016/j.carbpol.2018.03.056. [DOI] [PubMed] [Google Scholar]
- Lahrsen E., Schoenfeld A.-K., Alban S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydrate Polymers. 2018;189:162–168. doi: 10.1016/j.carbpol.2018.02.035. [DOI] [PubMed] [Google Scholar]
- Lambris J.D., Reid K.B., Volanakis J.E. The evolution, structure, biology and pathophysiology of complement. Immunology Today. 1999;20:207–211. doi: 10.1016/s0167-5699(98)01417-0. [DOI] [PubMed] [Google Scholar]
- Langrish T.A.G., Chan W.C., Kota K. Comparison of maltodextrin and skim milk wall deposition rates in a pilot-scale spray dryer. Powder Technology. 2007;179:84–89. [Google Scholar]
- Lapčik L., Jr., Lapčik L., de Smedt S., Demeester J., Chabreček J. Hyaluronan: Preparation, structure, properties, and applications. Chemical Reviews. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- Lapis T.J., Penner M.H., Lim J. Evidence that humans can taste glucose polymer. Chemical Senses. 2014;39:737–747. doi: 10.1093/chemse/bju031. [DOI] [PubMed] [Google Scholar]
- Lapis T.J., Penner M.H., Lim J. Humans can taste glucose oligomers independent of the hT1R2/hT1R3 sweet taste receptor. Chemical Senses. 2016;41:755–762. doi: 10.1093/chemse/bjw088. [DOI] [PubMed] [Google Scholar]
- Laurent T.C., Fraser J.R. Hyaluronan. Federation of American Societies for Experimental Biology Journal. 1992;6:2397–2402. [PubMed] [Google Scholar]
- Lazaridou A., Biliaderis C.G. Cryogelation of cereal β-glucans: Structure and molecular size effects. Food Hydrocolloids. 2004;18:933–947. [Google Scholar]
- Lazaridou A., Biliaderis C.G., Izydorczyk M.S. Molecular size effects on rheological properties of oat β- glucans in solutions and gels. Food Hydrocolloids. 2003;17:693–712. [Google Scholar]
- Lazaridou A., Biliaderis C.G., Micha-Screttas M., Steele B.R. A comparative study on structure–function relations of mixed linkage (1 →3), (1 →4) linear β-D-glucans. Food Hydrocolloids. 2004;18:837–855. [Google Scholar]
- LeBlanc J. Nutritional implications of cephalic phase thermogenic responses. Appetite. 2000;34:214–216. doi: 10.1006/appe.1999.0283. [DOI] [PubMed] [Google Scholar]
- LeBlanc J., Labrie A. A possible role for palatability of the food in diet-induced thermogenesis. International Journal of Obesity. 1997;21:1100–1103. doi: 10.1038/sj.ijo.0800520. [DOI] [PubMed] [Google Scholar]
- Leceta I., Guerrero P., de la Caba K. Functional properties of chitosan based films. Carbohydrate Polymers. 2013;93:339–346. doi: 10.1016/j.carbpol.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Leclere L., Van Cutsem P., Michiels C. Anti-cancer activities of pH- or heat-modified pectin. Frontiers in Pharmacology. 2013;4:1–8. doi: 10.3389/fphar.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Baek M.H., Cha D.S., Park H.J., Lim S.T. Freeze-thaw stabilization of potato starch gel by polysaccharide gums. Food Hydrocolloids. 2002;16:345–352. [Google Scholar]
- Lemieszek M., Rzeski W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemporary Oncology. 2012;16:285–289. doi: 10.5114/wo.2012.30055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H., Slade L. ‘Collapse’ phenomena-A unifying concept for interpreting the behaviour of low moisture foods. In: Mitchell J.R., Blanshard J.M.V., editors. Food structure - its creation and evaluation. Butterworths; London: 1987. pp. 149–180. [Google Scholar]
- Liao H., Ai W., Zhang K., Nakauma M., Funami T., Fang Y. Mechanisms of oligoguluronate modulating the calcium-induced gelation of alginate. Polymer. 2015;74:166–175. [Google Scholar]
- Li R., Feke D.L. Rheological and kinetic study of the ultrasonic degradation of xanthan gum in aqueous solution: Effects of pyruvate group. Carbohydrate Polymers. 2015;124:216e221. doi: 10.1016/j.carbpol.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Li J., Li B., Geng P., Song A.X., Wu J.Y. Ultrasonic degradation kinetics and rheological profiles of a food polysaccharide (konjac glucomannan) in water. Food Hydrocolloids. 2017;70:14–19. [Google Scholar]
- Lim J., Pullicin A.J. Oral carbohydrate sensing: Beyond sweet taste. Physiology & Behavior. 2019;202:14–25. doi: 10.1016/j.physbeh.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Lin L., Guo K., Zhang L., Zhang C., Liu Q., Wei C. Effects of molecular compositions on crystalline structure and functional properties of rice starches with different amylopectin extra-long chains. Food Hydrocolloids. 2019;88:137–145. [Google Scholar]
- Litwiniuk M., Krejner A., Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28:78–88. [PubMed] [Google Scholar]
- Liu Y., Yuan Y., Duan S., Li C., Bin Hu B., Liu A. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. International Journal of Biological Macromolecules. 2020;155:249–259. doi: 10.1016/j.ijbiomac.2020.03.217. [DOI] [PubMed] [Google Scholar]
- Lombry C., Dujardin N., Preat V. Transdermal delivery of macromolecules using skin electroporation. Pharmaceutical Research. 2000;17:32–37. doi: 10.1023/a:1007510323344. [DOI] [PubMed] [Google Scholar]
- Loret C., Schumm S., Pudney P.D.A., Frith W.J., Fryer P.J. Phase separation and molecular weight fractionation behaviour of maltodextrin/agarose mixtures. Food Hydrocolloids. 2005;19:557–565. [Google Scholar]
- Mackie A., Bajka B., Rigby N. Roles for dietary fibre in the upper GI tract: The importance of viscosity. Food Research International. 2016;88:234–238. [Google Scholar]
- Mahendran T., Williams P.A., Phillips G.O., Al-Assaf S., Baldwin T.C. New insights into the structural characteristics of the arabinogalactan-protein (AGP) fraction of gum Arabic. Journal of Agricultural and Food Chemistry. 2008;56:9269–9276. doi: 10.1021/jf800849a. [DOI] [PubMed] [Google Scholar]
- Mao P., Zhao M., Zhang F., F, Fang Y., Phillips G.O. Phase separation induced molecular fractionation of gum Arabic-sugar beet pectin systems. Carbohydrate Polymers. 2013;98:699–705. doi: 10.1016/j.carbpol.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Marangoni A., Garti N. 2nd ed. Academic Press; 2018. Edible oleogels. Structure and health implications. [Google Scholar]
- Martinez M.M., Li C., Okoniewska M., Mukherjee I., Vellucci D., Hamaker B. Slowly digestible starch in fully gelatinized material is structurally driven by molecular size and A and B1 chain lengths. Carbohydrate Polymers. 2018;197:531–539. doi: 10.1016/j.carbpol.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Mat D.J.L., Souchon I., Michon C., Le Feunteun S. Gastro-intestinal in vitro digestions of protein emulsions monitored by pH stat: Influence of structural properties and interplay between proteolysis and lipolysis. Food Chemistry. 2020;311:125946. doi: 10.1016/j.foodchem.2019.125946. [DOI] [PubMed] [Google Scholar]
- Matsukawa S., Ando I. A study of self-diffusion of molecules in polymer gel by pulsed-gradient spin-echo 1H NMR. Macromolecules. 1996;29:7136–7140. [Google Scholar]
- Ma F., Wang R., Li X., Kang W., Bell A.E., Zhao D. Physical properties of mucilage polysaccharides from Dioscorea opposita Thunb. Food Chemistry. 2020;311:126039. doi: 10.1016/j.foodchem.2019.126039. [DOI] [PubMed] [Google Scholar]
- Maytin E.V. Hyaluronan: More than just a wrinkle filler. Glycobiology. 2016;26:553–559. doi: 10.1093/glycob/cww033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Zhu P., Wang M., Wang F., Wang N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocolloids. 2019;96:663–670. [Google Scholar]
- McAllister J.W., Schmidt P.W., Dorfman K.D., Lodge T.P., Bates F.S. Thermodynamics of aqueous methylcellulose solutions. Macromolecules. 2015;48:7205–7215. [Google Scholar]
- McGhee J.D., von Hippel P.H. Theoretical aspects of DNA-protein interactions : Cooperative and non-cooperative binding of large ligands to a one-dimensional homogeneous lattice. Journal of Molecular Biology. 1974;86:469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- McKim J.M., Willoughby J.A., Sr., Blakemore W.R., Weiner M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Critical Reviews in Food Science and Nutrition. 2019;59(19):3054–3073. doi: 10.1080/10408398.2018.1481822. [DOI] [PubMed] [Google Scholar]
- McRorie J. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy. Nutrition Today. 2015;50:82–89. doi: 10.1097/NT.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRorie J.W., McKeown N.M. Evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. Journal of the Academy of Nutrition and Dietetics. 2017;17:251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Medi B.M., Layek B., Singh J. Electroporation for dermal and transdermal drug delivery. In: Dragicevic N., Maibach H.I., editors. Percutaneous penetration enhancers. Physical methods in penetration enhancement. 2017. pp. 105–122. [DOI] [Google Scholar]
- Meng X., Liang H., Luo L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydrate Research. 2016;424:30–41. doi: 10.1016/j.carres.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Mi Y., Chin Y.X., Cao W.X., Chang Y.G., Lim P.E., Chang H.X. Native κ-carrageenan induced-colitis is related to host intestinal microecology. International Journal of Biological Macromolecules. 2020;147:284–294. doi: 10.1016/j.ijbiomac.2020.01.072. [DOI] [PubMed] [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C.…Brodkorb A. A standardised static in vitro digestion method suitable for food - an international consensus. Food & Function. 2014;5:1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Mitchell J.R. Rheology of gels. Journal of Texture Studies. 1980;11:315–337. [Google Scholar]
- Miyaki T., Kawasaki H., Kuroda M., Miyamura N., Kouda T. Effect of a kokumi peptide, γ-glutamyl – valyl - glycine, on the sensory characteristics of chicken consommι. Flavour. 2015;4:17. [Google Scholar]
- Miyamura N., Jo S., Kuroda M., Kouda T. Flavour improvement of reduced-fat peanut butter by addition of a kokumi peptide, γ-glutamyl-valyl-glycine. Flavour. 2015;4:16. [Google Scholar]
- Miyoshi E., Takaya T., Nishinari K. Effects of glucose, mannose and konjac glucomannan on the gel-sol transition in gellan gum aqueous solutions by rheology and DSC. Polymer Gels and Networks. 1998;6:273–290. [Google Scholar]
- Mizuno T. Development of antitumor polysaccharides from mushroom fungi. Food and Food Ingredient Japanese Journal. 1996;167:69–87. [Google Scholar]
- Mo L., Chen Y., Li W., Guo S., Wang X., An H. Anti-tumor effects of (1 → 3)-β-D-glucan from Saccharomyces cerevisiae in S180 tumor-bearing mice. International Journal of Biological Macromolecules. 2017;95:385–392. doi: 10.1016/j.ijbiomac.2016.10.106. [DOI] [PubMed] [Google Scholar]
- Moran L.L., Yin Y., Cadwallader K.R., Padua G.W. Testing tools and physical, chemical, and microbiological characterization of microencapsulated systems. In: Gaonkar A.G., Vasisht N., Khare A.R., Sobel R., editors. Microencapsulation in the food industry. Elsevier Inc. Ltd; USA: 2014. pp. 323–352. [Google Scholar]
- Moritaka H., Yamanaka K., Kobayashi N., Ishihara M., Nishinari K. Effects of the gel size before ingestion and agarose molecular weight on the textural properties of a gel bolus. Food Hydrocolloids. 2019;89:892–900. [Google Scholar]
- Morris E.R. Rheological and organoleptic properties of food hydrocolloids. In: Nishinari K., Doi E., editors. Food hydrocolloids: Structures, properties and functions. Plenum Press; New York: 1993. pp. 201–210. [Google Scholar]
- Morris E.R., Cutler A.N., Ross-Murphy S.B., Rees D.A., Price J. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydrate Polymers. 1981;1:5–21. [Google Scholar]
- Morris E.R., Nishinari K., Rinaudo M. Gelation of gellan - a review. Food Hydrocolloids. 2012;28:373–411. [Google Scholar]
- Motoki M., Seguro K. Transglutaminase and its use for food processing. Trends in Food Science & Technology. 1998;9:204–210. [Google Scholar]
- Mozaffarian D., Katan M.B., Ascherio A., Stampfer M.J., Willett W.C. Trans fatty acids and cardiovascular disease. The New England Journal of Medicine. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- Mudgil D., Barak S., Patel A., Shah N. Partially hydrolyzed guar gum as a potential prebiotic source. International Journal of Biological Macromolecules. 2018;112:207–210. doi: 10.1016/j.ijbiomac.2018.01.164. [DOI] [PubMed] [Google Scholar]
- Murray B. Pickering emulsions for food and drinks. Current Opinion in Food Science. 2019;27:57–63. [Google Scholar]
- Nakamura S., Tobolsky A.V. Viscoelastic properties of plasticized amylose films. Journal of Applied Polymer Science. 1967;11:1371–1386. [Google Scholar]
- Nakata Y., Amitani K., Norisuye T., Kitamura S. Translational diffusion coefficient of cycloamylose in aqueous sodium hydroxide. Biopolymers. 2003;69:508–516. doi: 10.1002/bip.10393. [DOI] [PubMed] [Google Scholar]
- Nesmelova I.V., Melnikova D.L., Ranjan V., Skirda V.D. Translational diffusion of unfolded and intrinsically disordered proteins. Progress in Molecular Biology and Translational Science. 2019;166:85–108. doi: 10.1016/bs.pmbts.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Newman R., Vilarde N., Clavé P., Speyer R. Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD) Dysphagia. 2016;31:232–249. doi: 10.1007/s00455-016-9696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguo K., Huggins C.E., Truby H., Sinclair A.J., Clarke R.E., Bonham M.P. No effect of saturated fatty acid chain length on meal-induced thermogenesis in overweight men. Nutrition Research. 2018;51:102–110. doi: 10.1016/j.nutres.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Nieto-Nieto T.V., Wang Y.X., Ozimek L., Chen L.Y. Effects of partial hydrolysis on structure and gelling properties of oat globular proteins. Food Research International. 2014;55:418–425. [Google Scholar]
- Nijenhuis K. te. Investigation into the ageing process in gels of gelatin/water systems by the measurement of their dynamic moduli. Part I – Phenomenology. Colloid & Polymer Science. 1981;259:522–535. [Google Scholar]
- Niki R., Kohyama K., Sano Y., Nishinari K. Rheological study on the rennet-induced gelation of casein miceiles with different sizes. Polymer Gels and Networks. 1994;2:105–118. [Google Scholar]
- Nishimura T., Egusa A. “Koku’ of foods and the tastiness – the definition and the determinants. Food Chemical Monthly. 2014;352:25–31. 2014-8. (In Japanese) [Google Scholar]
- Nishimura T., Goto S., Miura K., Takakura Y., Egusa A., Wakabayashi H. Umami compounds enhance the intensity of retronasal sensation of aromas from model chicken soups. Food Chemistry. 2016;196:577–583. doi: 10.1016/j.foodchem.2015.09.036. [DOI] [PubMed] [Google Scholar]
- Nishinari K. Rheology of physical gels and gelling processes. Reports on progress in Polymer Physics in Japan. 2000;43:163–192. [Google Scholar]
- Nishinari K. Konjac glucomannan. In: Doxastakis G., Kiosseoglou V., editors. Novel macromolecules in food systems. Elsevier Science B.V; 2000. pp. 309–330. [Google Scholar]
- Nishinari K. Some thoughts on the definition of a gel. Progress in Colloid and Polymer Science. 2009;136:87–94. [Google Scholar]
- Nishinari K., Fang Y. Sucrose release from polysaccharide gels - a Review. Food & Function. 2016;7:2130–2146. doi: 10.1039/c5fo01400j. [DOI] [PubMed] [Google Scholar]
- Nishinari K., Fang Y. Relation between structure and rheological/thermal properties of agar. A mini-review on the effect of alkali treatment and the role of agaropectin. Food Structure. 2017;13:24–34. [Google Scholar]
- Nishinari K., Gao S. Konjac glucomannan. In: Biliaderis C.G., Izydorczyk M.S., editors. Functional food carbohydrates. CRC Press; 2007. pp. 97–125. Chap.3. [Google Scholar]
- Nishinari K., Hofmann K.E., Moritaka H., Kohyama K., Nishinari N. Gel-sol transition of methylcellulose. Macromolecular Chemistry and Physics. 1997;198:1217–1226. [Google Scholar]
- Nishinari K., Kohyama K., Williams P.A, Phillips G.O., Burchard W., Ogino K. Solution properties of pullulan. Macromolecules. 1991;24:5590–5593. [Google Scholar]
- Nishinari K., Koide S., Ogino K. On the temperature dependence of elasticity of thermoreversible gels. Journal de Physique. 1985;46:793–797. [Google Scholar]
- Nishinari K., Koide S., Williams P.A., Phillips G.O. A zipper model approach to the thermoreversible gel-sol transition. Journal de Physique. 1990;51:1759–1768. [Google Scholar]
- Nishinari K., Miyoshi E., Takaya T., Williams P.A. Rheological and DSC studies on the interaction between gellan gum and konjac glucomannan. Carbohydrate Polymers. 1996;30:193–207. [Google Scholar]
- Nishinari K., Mo Y., Takahashi R., Kubota K., Okamoto A. Conformational and rheological properties of hyaluronan. In: Kennedy J.F., Phillips G.O., Williams P.A., Hascall V.C., editors. HYALURONAN volume 1 - chemical, biochemical and biological aspects. Woodhead Publishing Ltd; 2000. pp. 89–98. [Google Scholar]
- Nishinari K., Takemasa M., Brenner T., Su L., Fang Y., Hirashima M. The food colloid principle in the design of elderly food. Journal of Texture Studies. 2016;47:284–312. [Google Scholar]
- Nishinari K., Takemasa M., Fang Y., Hossain K.S., Tsumura Y., Sone Y. Effects of xyloglucan with different molar masses on glucose in blood. Food Hydrocolloids. 2020 (in press) [Google Scholar]
- Nishinari K., Takemasa M., Su L., Michiwaki Y., Mizunuma H., Ogoshi H. Effect of shear thinning on aspiration - toward making solutions for judging the risk of aspiration. Food Hydrocolloids. 2011;25:1737–1743. [Google Scholar]
- Nishinari K., Takemasa M., Zhang H., Takahashi R. Storage plant polysaccharides: Xyloglucans, galactomannans, glucomannans. In: Kamerling J.P., editor. Vol. 2. Elsevier; 2007. pp. 614–652. (Comprehensive glycoscience). [Google Scholar]
- Nishinari K., Turcanu M., Nakauma M., Fang Y. Role of fluid cohesiveness in safe swallowing. Npj Science of Food. 2019;3:5. doi: 10.1038/s41538-019-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinari K., Watase M., Hatakeyama T. Effects of polyols and sugars on the structure of water in concentrated gelatin gels as studied by low temperature differential scanning calorimetry. Colloid & Polymer Science. 1997;275:1078–1082. [Google Scholar]
- Nishinari K., Watase M., Ogino K. On the temperature dependence of elasticity of agarose gels. Makromolekulare Chemie. 1984;185:2663–2668. [Google Scholar]
- Nishizawa Y. Executive summary of the Japan osteoporosis society guide for the use of bone turnover markers in the diagnosis and treatment of osteoporosis (2018 edition) Clinica Chimica Acta. 2019;498:101–107. doi: 10.1016/j.cca.2019.08.012. [DOI] [PubMed] [Google Scholar]
- Norisuye T. Triple-stranded helical structure of schizophyllan and its antitumor activity in aqueous solution. Makromolekulare Chemie, Suppl. 1985;14:105–118. [Google Scholar]
- Nunes R.W., Martin J.R., Johnson J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polymer Engineering & Science. 1982;22:205–228. [Google Scholar]
- Nyström M., Qazi W.M., Bülow M., Ekberg O., Stading M. Effects of rheological factors on perceived ease of swallowing. Applied Rheology. 2015;25:40–48. [Google Scholar]
- Oduah E.I., Linhardt R.J., Sharfstein S.T. Heparin: Past, present, and future. Pharmaceuticals. 2016;9:38. doi: 10.3390/ph9030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oe M., Tashiro T., Yoshida H., Nishiyama H., Masuda Y., Maruyama K. Oral hyaluronan relieves knee pain: A review. Nutrition Journal. 2016;15:11. doi: 10.1186/s12937-016-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E., Takahashi R., Yajima H., Nishinari K. Effects of molar mass on the coil to helix transition of sodium-type gellan gums in aqueous solutions. Food Hydrocolloids. 2006;20:378–385. [Google Scholar]
- Ohara A., Kodama H. Correlation between enthalpy relaxation and mechanical response on physical aging of polycarbonate in relation to the effect of molecular weight on ductile-brittle transition. Polymer. 2019;181:121720. [Google Scholar]
- Ohsu T., Amino Y., Nagasaki H., Yamanaka T., Takeshita S., Hatanaka T. Involvement of the calcium-sensing receptor in human taste perception. Journal of Biological Chemistry. 2010;285:1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu-Ogura Y., Matsushita M., Bariuan J.V., Nagaya K., Tsubota A., Saito M. Association of circulating exosomal miR-122 levels with BAT activity in healthy humans. Scientific Reports. 2019;9:13243. doi: 10.1038/s41598-019-49754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onogi S., Matsumoto T., Kamei E. Effects of molecular weight and its distribution on fractural behavior of polystyrene. Polymer Journal. 1972;3:531–537. [Google Scholar]
- Pan Y., Wu Z., Xie Q.-T., Li X.-M., Meng R., Zhang B. Insight into the stabilization mechanism of emulsions stabilized by Maillard conjugates: Protein hydrolysates-dextrin with different degree of polymerization. Food Hydrocolloids. 2020;99:105347. [Google Scholar]
- Park S.Y., Marsh K.S., Rhim J.W. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. Journal Of Food Science. 2002;67:194–197. [Google Scholar]
- Pengzhan Y., Ning L., Xiguang L., Gefei Z., Quanbin Z., Pengcheng L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta) Pharmacological Research. 2003;48:543–549. doi: 10.1016/s1043-6618(03)00215-9. [DOI] [PubMed] [Google Scholar]
- Peschel D., Zhang K., Fischer S., Groth T. Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives. Acta Biomaterialia. 2012;8:183–193. doi: 10.1016/j.actbio.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Petrusan J.-I., Rawel H., Huschek G. Protein-rich vegetal sources and trends in human nutrition: A review. Current Topics in Peptide & Protein Research. 2016;17:1–19. [Google Scholar]
- Pinterits A., Arntfield S.D. Improvement of canola protein gelation properties through enzymatic modification with transglutaminase. LWT - Food Science and Technology, l. 2007;41:128–138. [Google Scholar]
- Pomin V.H. Sulfated glycans in inflammation. European Journal of Medicinal Chemistry. 2015;92:353–369. doi: 10.1016/j.ejmech.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Powell D.A., Morris E.R., Gidley M.J., Rees D.A. Conformations and interactions of pectins. II. Influences of residue sequence on chain association in calcium pectate gels. Journal of Molecular Biology. 1982;155:517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Prawitwong P., Takigami S., Phillips G.O. Effects of γ-irradiation on molar mass and properties of Konjac mannan. Food Hydrocolloids. 2007;21:1362–1367. [Google Scholar]
- Puolanne E. Lawrie's meat science. Elsevier; 2017. Developments in our understanding of water-holding capacity in meat; pp. 167–190. (Chapter 8) [Google Scholar]
- Qiu S., Yadav M.P., Liu Y., Chen H., Tatsumi E., Yin L. Effects of corn fiber gum with different molecular weights on the gelatinization behaviors of corn and wheat starch. Food Hydrocolloids. 2016;53:180–186. [Google Scholar]
- Raman M., Doble M. κ-Carrageenan from marine red algae, Kappaphycus alvarezii– a functional food to prevent colon carcinogenesis. Journal of Functional Foods. 2015;15:354–364. [Google Scholar]
- Reumer P., Luft B., Borchard W. Compound formation and glassy solidification in the system gelatin-water. Colloid & Polymer Science. 1985;263:519–529. [Google Scholar]
- Rinaudo M. Chitin and chitosan: Properties and applications. Progress in Polymer Science. 2006;31:603–632. [Google Scholar]
- Roberts D.D., Elmore J.S., Langley K.R., Bakker J. Effects of sucrose, guar gum, and carboxymethylcellulose on the release of volatile flavor compounds under dynamic conditions. Journal of Agricultural and Food Chemistry. 1990;44:1321–1326. [Google Scholar]
- Rochas C., Rinaudo M., Landry S. Role of the molecular weight on the mechanical properties of kappa carrageenan gels. Carbohydrate Polymers. 1990;12:255–266. [Google Scholar]
- Rochas C., Rinaudo M., Vincedon M. Spectroscopic characterisation and conformation of oligo kappa carrageenans. International Journal of Biological Macromolecules. 1983;5:111–115. [Google Scholar]
- Rodriguez-Canto W., Cerqueira M.A., Chel-Guerrero L., Pastrana L.M., Aguilar-Vega M. Delonix regia galactomannan-based edible films: Effect of molecular weight and k-carrageenan on physicochemical properties. Food Hydrocolloids. 2020;103:105632. [Google Scholar]
- Rodriguez-Canto W., Chel-Guerrero L., Fernandez V.V.A., Aguilar-Vega M. Delonix regia galactomannan hydrolysates: Rheological behavior and physicochemical characterization. Carbohydrate Polymers. 2019;206:573–582. doi: 10.1016/j.carbpol.2018.11.028. [DOI] [PubMed] [Google Scholar]
- Roos Y., Karel M. Water and molecular weight effects on glass transitions in amorphous carbohydrates and carbohydrate solutions. Journal of Food Science. 1991;56:1676–1681. [Google Scholar]
- Ruiz-Aceituno L., Hernandez-Hernandez O., Kolida S., Moreno F.J., Methven L. Sweetness and sensory properties of commercial and novel oligosaccharides of prebiotic potential. LWT - Food Science and Technolog. 2018;97:476–482. [Google Scholar]
- Sahari J., Sapuan S.M., Zainudin E.S., Maleque M.A. Thermo-mechanical behaviors of thermoplastic starch derived from sugar palm tree (Arenga pinnata) Carbohydrate Polymers. 2012;92:1711–1716. doi: 10.1016/j.carbpol.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Saifullah M., Shishir M.R.I., Ferdowsi R., Rahman M.R.T., Vuong Q.V. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends in Food Science & Technology. 2019;86:230–251. [Google Scholar]
- Sato K. The presence of food-derived collagen peptides in human body-structure and biological activity. Food & Function. 2017;8:4325–4330. doi: 10.1039/c7fo01275f. [DOI] [PubMed] [Google Scholar]
- Saunders P.R., Ward A.G. Mechanical properties of degraded gelatins. Nature. 1955;176:26–27. [Google Scholar]
- Shang Q., Sun W., Shan X., Jiang H., Cai C., Hao J. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicology Letters. 2017;279:87–95. doi: 10.1016/j.toxlet.2017.07.904. [DOI] [PubMed] [Google Scholar]
- Shibata M., Hirotsuka H., Mizutani Y., Takahashi H., Kawada T., Matsumiya Isolation and characterization of key contributors to the “kokumi” taste in soybean seeds. Bioscience, Biotechnology, and Biochemistry. 2017;81:2168–2177. doi: 10.1080/09168451.2017.1372179. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Brenner T., Liao R., Matsukawa S. Diffusion of probe polymer in gellan gum solutions during gelation process studied by gradient NMR. Food Hydrocolloids. 2012;26:28–32. [Google Scholar]
- Shishir M.R.I., Xie L., Sun C., Zheng X., Chen W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology. 2018;78:34–60. [Google Scholar]
- Sjöö, M & Nilsson, L. (eds.) (2918). Starch in food: Structure, function and applications, 2nd ed., Woodhead Publ.
- Smidsrød O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discussions of the Chemical Society. 1974;57:263–274. p.278. [Google Scholar]
- Smidsrød O., Haug A. Properties of poly(1,4-hexauronates) in the gel state. II. Comparison of gels of different chemical composition. Acta Chemica Scandinavica. 1972;26:79–88. [Google Scholar]
- Sokolova E.V., Kravchenko A.O., Sergeeva N.V., Davydova V.N., Bogdanovich L.N., Yermak I.M. Effect of carrageenans on some lipid metabolism components in vitro. Carbohydrate Polymers. 2020;230:115629. doi: 10.1016/j.carbpol.2019.115629. [DOI] [PubMed] [Google Scholar]
- Song X., Chen Y., Zhao G., Sun H., Che H., Leng X. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydrate Polymers. 2020;231:115689. doi: 10.1016/j.carbpol.2019.115689. [DOI] [PubMed] [Google Scholar]
- Song S., Zhang B., Wu S., Huang L., Ai C., Pan J. Structural characterization and osteogenic bioactivity of a sulfated polysaccharide from pacific abalone (Haliotis discus hannai Ino) Carbohydrate Polymers. 2018;182:207–214. doi: 10.1016/j.carbpol.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Sonnendecker C., Thürmann S., Przybylski C., Zitzmann F., Heinke N., Krauke Y. Large-ring cyclodextrins as chiral selectors for enantiomeric Pharmaceuticals. Angewandte Chemie International Edition. 2019 doi: 10.1002/anie.201900911. [DOI] [PubMed] [Google Scholar]
- Sperling L.H. 4th ed. John Wiley & Sons; 2005. Introduction to physical polymer science. [Google Scholar]
- Sperry P.R. A simple quantitative model for the volume restriction flocculation of latex by water soluble polymers. Journal of Colloid and Interface Science. 1982;87:375–384. [Google Scholar]
- Steele C.M., Alsanei W.A., Ayanikalath S., Barbon C.E.A., Chen J., Cichero J.A.Y. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia. 2015;30:2–26. doi: 10.1007/s00455-014-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenner R., Matubayasi N., Shimizu S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocolloids. 2016;54:284–292. [Google Scholar]
- Stokke B.T., Smidsrød O., Zanetti F., Strand W., Skjåk-Bræk G. Distribution of uronate residues in alginate chains in relation to alginate gelling properties - 2: Enrichment of β-D-mannuronic acid and depletion of α-L-guluronic acid in sol fraction. Carbohydrate Polymers. 1993;21:39–46. [Google Scholar]
- Stone B.A. Chemistry of β –glucans. In: Bacic A., Fincher G., Stone B., editors. Chemistry, biochemistry, and biology of 1-3 beta glucans and related polysaccharides. 2nd ed. Elsevier; Amsterdam: 2009. pp. 5–46. [Google Scholar]
- Sun Y., Yang B., Wua Y., Liu Y., Gu X., Zhang H. Structural characterization and antioxidant activities of kappa-carrageenan oligosaccharides degraded by different methods. Food Chemistry. 2015;178:311–318. doi: 10.1016/j.foodchem.2015.01.105. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Al-Assaf S., Williams P.A., Kubota K., Okamoto A., Nishinari K. Asymmetrical-flow field-flow fractionation with on-line multiangle light scattering detection.1.Application to wormlike chain analysis of weakly stiff polymer chains. Biomacromolecules. 2003;4:404–409. doi: 10.1021/bm025706v. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Tokunou H., Kubota K., Ogawa E., Oida T., Kawase T. Solution properties of gellan gum: Change in chain stiffness between single- and double-stranded chains. Biomacromolecules. 2004;5:516–523. doi: 10.1021/bm034371u. [DOI] [PubMed] [Google Scholar]
- Takemasa M., Yuguchi Y., Kitamura S. Size and shape of cycloamylose estimated using column chromatography coupled with small-angle X-ray scattering. Food Hydrocolloids. 2020 doi: 10.1016/j.foodhyd.2020.105948. (in press) [DOI] [Google Scholar]
- Tamaru S., Igura N., Shimoda M. Effectiveness of water-air and octanol-air partition coefficients to predict lipophilic flavor release behavior from O/W emulsions. Food Chemistry. 2018;239:712–717. doi: 10.1016/j.foodchem.2017.06.127. [DOI] [PubMed] [Google Scholar]
- Tanaka F. Cambridge University Press; 2011. Polymer physics. [Google Scholar]
- Tan N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester R.F., Al-Ghazzewi F.H. Beneficial health characteristics of native and hydrolysed konjac (Amorphophallus konjac) glucomannan. Journal of the Science of Food and Agriculture. 2016;96:3283–3291. doi: 10.1002/jsfa.7571. [DOI] [PubMed] [Google Scholar]
- Tester R.F., Al-Ghazzewi F.H. Glucomannans and nutrition. Food Hydrocolloids. 2017;68:246–254. [Google Scholar]
- Tester R., Al-Ghazzewi F., Shen N., Chen Z., Chen F., Yang J. The use of konjac glucomannan hydrolysates to recover healthy microbiota in infected vaginas treated with an antifungal agent. Beneficial Microbes. 2012;3:61–66. doi: 10.3920/BM2011.0021. [DOI] [PubMed] [Google Scholar]
- Thu B., Smidsrød O., Skjåk Braæk G. Alginate gels - some structure-function correlations relevant to their use as immobilization matrix for cells. In: Wijffels R.H., Buitelaar R.M., Bucke C., Tramper J., editors. Immobilized cells: Basics and applications. Elsevier Science B.V; 1996. pp. 19–30. [Google Scholar]
- Tirtaatmadja V., McKinley G.H., Cooper-White J.J. Drop formation and breakup of low viscosity elastic fluids: Effects of molecular weight and concentration. Physics of Fluids. 2006;18(4) 043101-043101-18. [Google Scholar]
- Tobacman J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environmental Health Perspectives. 2001;109:983–994. doi: 10.1289/ehp.01109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita M. Gelation mechanism and percolation. Food Hydrocolloids. 1989;3:263–274. [Google Scholar]
- Tolg C., Yuan H., Flynn S.M., Basu K., Ma J., Tse K.C. Hyaluronan modulates growth factor induced mammary gland branching in a size dependent manner. Matrix Biology. 2017;63:117–132. doi: 10.1016/j.matbio.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Tolstoguzov V. Some thermodynamic considerations in food formulation. Food Hydrocolloids. 2003;17:1–23. [Google Scholar]
- Tomasetti C., Lu Li L., Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330–1334. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh S. The research legacy of Peter J. Wood. Bioactive Carbohydrates and Dietary Fiber. 2013;2:170–180. [Google Scholar]
- Tsuda K., Inden T., Yamanaka K., Ikeda Y. Effect of partially hydrolyzed guar gum on elevation of blood glucose after sugar intake in human volunteers. Journal of Japan Association for Dietary Fiber Research. 1998;2:15–22. [Google Scholar]
- Turcanu M. Politehnica University of Bucharest; 2017. Rheological characterization and modelling of fluids used in biomedical engineering. PhD dissertation. [Google Scholar]
- Turcanu M., Siegert N., Secouard S., Brito-de la Fuente E., Balan C., Gallegos C. An alternative elongational method to study the effect of saliva on thickened fluids for dysphagia nutritional support. Journal of Food Engineering. 2018;228:79–83. [Google Scholar]
- Turcanu M., Tascon L.F., Balan C., Gallegos C. 9th international symposium on advanced topics in electrical engineering. 2015. Capillary breakup extensional properties of whole human saliva; pp. 269–274. [Google Scholar]
- Turley E.A., Noble P.W., Bourguignon L.Y. Mini-review: Signaling properties of hyaluronan receptors. Journal of Biological Chemistry. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Ubbink J., Schoonman A. Kirk-Othmer encyclopedia of chemical technology. John Wiley & Sons, Inc; New York, NY: 2013. Flavor delivery systems; pp. 1–35. [Google Scholar]
- Ueno M., Hiroki T., Takeshita S., Jiang Z., Kim D., Yamaguchi K. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohydrate Research. 2012;352:88–93. doi: 10.1016/j.carres.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Veerman C., Sagis L.M.C., van der Linden E. Gels at extremely low weight fractions formed by irreversible self-assembly of proteins. Macromolecular Bioscience. 2003;2003(3):243–247. [Google Scholar]
- Viel S., Capitani D., Mannina L., Segre A. Diffusion-ordered NMR spectroscopy: A versatile tool for the molecular weight determination of uncharged polysaccharides. Biomacromolecules. 2003;4:1843–1847. doi: 10.1021/bm0342638. [DOI] [PubMed] [Google Scholar]
- Walstra P. CRC Press; 2003. Physical chemistry of foods. [Google Scholar]
- Wang K., Arntfield S.A. Modification of interactions between selected volatile flavor compounds and salt-extracted pea protein isolates using chemical and enzymatic approaches. Food Hydrocolloids. 2016;61:567–577. [Google Scholar]
- Wang Q., Ellis P. Oat β-glucan: Physicochemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. British Journal of Nutrition. 2014;112:S4–S13. doi: 10.1017/S0007114514002256. [DOI] [PubMed] [Google Scholar]
- Wang P., Hou C., Zhao X., Tian M., Gu Z., Yang R. Molecular characterization of water-extractable arabinoxylan from wheat bran and its effect on the heat-induced polymerization of gluten and steamed bread quality. Food Hydrocolloids. 2019;87:570–581. [Google Scholar]
- Wang H., Zhang X., Wang S., Li H., Lu Z., Shi J. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food and Function. 2018;9:3916–3929. doi: 10.1039/c8fo00209f. [DOI] [PubMed] [Google Scholar]
- Wang T., Liu L., Voglmeir J. Chemoenzymatic synthesis of ultralow and low-molecular weight heparins. BBA -Proteins and Proteomics. 2020;1868:140301. doi: 10.1016/j.bbapap.2019.140301. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou W. Non-equilibrium states and glass transitions in bakery products. In: Bhandari B., Roos Y.H., editors. Non-equilibrium states and glass transitions in foods. Processing effects and product-specific implications. Woodhead Publ; 2017. pp. 63–87. [Google Scholar]
- Warner R.D. Lawrie's meat science. Elsevier; 2017. The eating quality of meat – IV Water-holding capacity and juiciness; pp. 419–459. (Chapter 14) [Google Scholar]
- Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology. 2002;10:13–32. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Lan H.-L., Yamaguchi K., Konosu S. Role of extractive components of scallop in its characteristic taste development (Taste-active components of scallop Part II) Nippon Shokuhin Kogyo Gakkaishi. 1990;37:439–445. [Google Scholar]
- Watase M., Nishinari K. Rheological properties of agarose gels with different molecular weights. Rheologica Acta. 1983;22:580–587. [Google Scholar]
- Watase M., Nishinari K. Large deformation of hydrogels of poly(vinyl alcohol), agarose and kαppα‐carrageenan. Makromolekulare Chemie. 1985;186:1081–1086. [Google Scholar]
- Watase M., Nishinari K., Hatakeyama T. DSC study on properties of water in concentrated agarose gels. Food Hydrocolloids. 1985;2:427–438. [Google Scholar]
- Wellinghoff S., Baer E. The mechanism of crazing in polystyrene. Journal of Macromolecular Science, Part B. 1975;11:367–387. [Google Scholar]
- Welsh E.J., Rees D.A., Morris E.R., Madden J.K. Competitive inhibition evidence for specific intermolecular interactions in hyaluronate solutions. Journal of Molecular Biology. 1980;138:375–382. doi: 10.1016/0022-2836(80)90293-4. [DOI] [PubMed] [Google Scholar]
- West D.C., Hampson I.N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- White D.R., Hudson P., Adamson J.T. Dextrin characterization by high performance anion-exchange chromatography-pulsed amperometric detection and size-exclusion chromatography-multi-angle light scattering-refractive index detection. Journal of Chromatography A. 2003;997:79–85. doi: 10.1016/s0021-9673(03)00626-5. [DOI] [PubMed] [Google Scholar]
- de Wijk R.A., Prinz J.F., Engelen L., Weenen H. The role of alpha-amylase in the perception of oral texture and flavour in custards. Physiology & Behavior. 2004;83:81–91. doi: 10.1016/j.physbeh.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Wilcox M.D., Brownlee I.A., Richardson J.C., Dettmar P.W., Pearson J.P. The modulation of pancreatic lipase activity by alginates. Food Chemistry. 2014;146:479–484. doi: 10.1016/j.foodchem.2013.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolever T., Tosh S., Gibbs A., Brand-Miller J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. American Journal of Clinical Nutrition. 2010;92:723–732. doi: 10.3945/ajcn.2010.29174. [DOI] [PubMed] [Google Scholar]
- Wood P.J., Beer M.U., Butler G. Evaluation of role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. British Journal of Nutrition. 2000;84:19–23. [PubMed] [Google Scholar]
- Wood P.J., Braaten J.T., Scott F.W., Riedel K.D., Wolynetz M.S., Collins M.W. Effect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. British Journal of Nutrition. 1994;72:731–743. doi: 10.1079/bjn19940075. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2015. Guideline: Sugars intake for adults and children.http://apps.who.int/iris/bitstream/10665/149782/1/9789241549028_eng.pdf Geneva. [PubMed] [Google Scholar]
- Wouters A.G.B., Rombouts I., Fierens E., Brijs K., Delcour J.A. Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Comprehensive Reviews in Food Science and Food Safety. 2016;15:786–800. doi: 10.1111/1541-4337.12209. [DOI] [PubMed] [Google Scholar]
- Wu X., Nishinari, Gao Z., Zhao M., Zhang K., Fang Y. Gelation of β-lactoglobulin and its fibrils in the presence of transglutaminase. Food Hydrocolloids. 2016;52:942–951. [Google Scholar]
- Wu L., Sun J., Su X., Yu Q., Yu Q., Zhang P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydrate Polymers. 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Wu S., Wang X., Bao Y., Zhang C., Liu H., Li Z. Molecular insight on the binding of monascin to bovine serum albumin (BSA) and its effect on antioxidant characteristics of monascin. Food Chemistry. 2020;315:126228. doi: 10.1016/j.foodchem.2020.126228. [DOI] [PubMed] [Google Scholar]
- Xu Y., Gao Y., Liu F., Niu X., Wang L., Li X. Sulfated modification of the polysaccharides from blackcurrant and their antioxidant and α-amylase inhibitory activities. International Journal of Biological Macromolecules. 2018;109:1344–1354. doi: 10.1016/j.ijbiomac.2017.11.164. [DOI] [PubMed] [Google Scholar]
- Xu Y., Guo Y., Duan S., Wei H., Liu Y., Wang L. Effects of ultrasound irradiation on the characterization and bioactivities of the polysaccharide from blackcurrant fruits. Ultrasonics Sonochemistry. 2018;49:206–214. doi: 10.1016/j.ultsonch.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Xu Y., Niu X., Liu N., Gao Y., Wang L., Xu G. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chemistry. 2018;243:26–35. doi: 10.1016/j.foodchem.2017.09.107. [DOI] [PubMed] [Google Scholar]
- Xu X., Wu X., Wang Q., Cai N., Zhang H., Jiang Z. Immunomodulatory effects of alginate oligosaccharides on murine macrophage RAW264.7 cells and their structure-activity relationships. Journal of Agricultural and Food Chemistry. 2014;62:3168–3176. doi: 10.1021/jf405633n. [DOI] [PubMed] [Google Scholar]
- Yadav M.P., Fishman M.L., Chau H.K., Johnston D., Hicks K.B. Molecular characteristics of corn fiber gum and their influence on its emulsifying properties. Cereal Chemistry. 2007;84:175–180. [Google Scholar]
- Yamatoya K., Kuwano K., Suzuki J., Mitamura T., Sekiya K. Effect of hydrolyzed guar gum on frequency and feeling of defecation in humans. Bulletin of Applied Glycoscience. 1995;41:251–257. [Google Scholar]
- Yang C., Chung D., Shin I.S., Lee H., Kim J., Lee Y. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. International Journal of Biological Macromolecules. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Yang J., Vittori N., Wang W., Shi Y., Hoeflinger J.L., Miller M.J. Molecular weight distribution and fermentation of mechanically pre-treated konjac enzymatic hydrolysates. Carbohydrate Polymers. 2017;159:58–65. doi: 10.1016/j.carbpol.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Yao X., Nie K., Chen Y., Jiang F., Kuang Y., Yan H. The influence of non-ionic surfactant on lipid digestion of gum Arabic stabilized oil-in-water emulsion. Food Hydrocolloids. 2018;74:78–86. [Google Scholar]
- Yao J., Zhou Y., Chen X., Ma F., Li P., Chen C. Effect of sodium alginate with three molecular weight forms on the water holding capacity of chicken breast myosin gel. Food Chemistry. 2018;239:1134–1142. doi: 10.1016/j.foodchem.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Nishinari K. Dynamic viscoelastic study on the gelation of konjac glucomannan with different molecular weights. Food Hydrocolloids. 1999;13:227–233. [Google Scholar]
- You S.G., Yang C., Lee H.Y., Lee B.Y. Molecular characteristics of partially hydrolyzed fucoidans from sporophyll of Undaria pinnatifida and their in vitro anticancer activity. Food Chemistry. 2010;119:554–559. [Google Scholar]
- Yu G., Chen Y., Bao Q., Jiang Z., Zhu Y., Ni H. A low-molecular-weight ascophyllan prepared from Ascophyllum nodosum: Optimization, analysis and biological activities. International Journal of Biological Macromolecules. 2020;153:107–117. doi: 10.1016/j.ijbiomac.2020.02.334. [DOI] [PubMed] [Google Scholar]
- Yu W., Zou W., Dhital S., Wu P., Gidley M.J., Fox G.P. The adsorption of alpha-amylase on barley proteins affects the in vitro digestion of starch in barley flour. Food Chemistry. 2018;241:493–501. doi: 10.1016/j.foodchem.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Zaritzky N.E. Chemical and physical deterioration of frozen foods. In: Skibsted L.H., Risbo J., L Andersen M., editors. Chemical deterioration and physical instability of food and beverages. Woodhead Publ. Ltd; 2010. pp. 561–607. [Google Scholar]
- Zasypkin D.Y., Braudo E.E., Tolstoguzov V.B. Multicomponent biopolymer gels. Food Hydrocolloids. 1997;11:159–170. [Google Scholar]
- Zetzl A.K., Gravelle A.J., Kurylowicz M., Dutcher J.R., Barbut S., Marangoni A.G. Microstructure of ethylcellulose oleogels and its relationship to mechanical properties. Food Structure. 2014;2:27–40. [Google Scholar]
- Zhai H., Gunness P., Gidley M.J. Barley β-glucan effects on emulsification and in vitro lipolysis of canola oil are modulated by molecular size, mixing method, and emulsifier type. Food Hydrocolloids. 2020;103:105643. [Google Scholar]
- Zhang B., Bai B., Pan Y., Li X.M., Cheng J.S., Chen H.Q. Effects of pectin with different molecular weight on gelatinization behavior, textural properties, retrogradation and in vitro digestibility of corn starch. Food Chemistry. 2018;264:58–63. doi: 10.1016/j.foodchem.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang L., Shi Z., Shangguan W., Fang Y., Nishinari K., Phillips G.O. Emulsification properties of sugar beet pectin after modification with horseradish peroxidase. Food Hydrocolloids. 2015;43:107–113. [Google Scholar]
- Zhang M., Cui S.W., Cheung P.C.K., Wang Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends in Food Science & Technology. 2007;18:4–19. [Google Scholar]
- Zhang B., Hu B., Nakauma M., Funami T., Nishinari K., Draget K.I. Modulation of calcium-induced gelation of pectin by oligoguluronate as compared to alginate. Food Research International. 2019;116:232–240. doi: 10.1016/j.foodres.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Zhang J., Jin H., Zhang W., Ding C., O'Keeffe S., Ye M. Sour sensing from the tongue to the brain. Cell. 2019;179:392–402. doi: 10.1016/j.cell.2019.08.031. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kong H., Fang Y., Nishinari K., Phillips G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioactive Carbohydrates and Dietary Fiber. 2013;1:53–71. [Google Scholar]
- Zhang T., Lan Y., Zheng Y., Liu F., Zhao D., Mayo K.H. Identification of the bioactive components from pH-modified citrus pectin and their inhibitory effects on galectin-3 function. Food Hydrocolloids. 2016;58:113–119. [Google Scholar]
- Zhang L., Li X., Xu X., Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydrate Research. 2005;340:1515–1521. doi: 10.1016/j.carres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang X., Liu C., Li J. The degradation, antioxidant and antimutagenic activity of the mucilage polysaccharide from Dioscorea opposita. Carbohydrate Polymers. 2016;150:227–231. doi: 10.1016/j.carbpol.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yoshimura M., Nishinari K., Williams M.A.K., Foster T.J., Norton I.T. Gelation behaviour of konjac glucomannan with different molecular weights. Biopolymers. 2001;59:38–50. doi: 10.1002/1097-0282(200107)59:1<38::AID-BIP1004>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhai X., Zhao G., Ren F., Leng X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydrate Polymers. 2015;134:158–166. doi: 10.1016/j.carbpol.2015.07.065. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Hu X., Guo Q., Cui S.W., Xian Y., You S. Physicochemical properties and regulatory effects on db/db diabetic mice of β-glucans extracted from oat, wheat and barley. Food Hydrocolloids. 2014;37:60–68. [Google Scholar]
- Zheng Y., Li L., Feng Z., Wang H., Guihua Tai G. Preparation of individual galactan oligomers, their prebiotic effects, and use in estimating galactan chain length in pectin-derived polysaccharides. Carbohydrate Polymers. 2018;199:526–533. doi: 10.1016/j.carbpol.2018.07.048. [DOI] [PubMed] [Google Scholar]
- Zhong K., Tong L., Liu L., Zhou X., Liu X., Zhang Q. Immunoregulatory and antitumor activity of schizophyllan under ultrasonic treatment. International Journal of Biological Macromolecules. 2015;80:302–308. doi: 10.1016/j.ijbiomac.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Zhuang C., Gu W., Zhao Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Carbohydrate Polymers. 2019;212:197–205. doi: 10.1016/j.carbpol.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Zhou F., Zhao M., Su G., Sun W. Binding of aroma compounds with myofibrillar proteins modified by a hydroxyl-radical-induced oxidative system. Journal of Agricultural and Food Chemistry. 2014;62:9544–9552. doi: 10.1021/jf502540p. [DOI] [PubMed] [Google Scholar]
- Zhu F., Liu P. Starch gelatinization, retrogradation, and enzyme susceptibility of retrograded starch: Effect of amylopectin internal molecular structure. Food Chemistry. 2020;316:126036. doi: 10.1016/j.foodchem.2019.126036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.