Abstract
Background
A threshold Clinical Frailty Scale (CFS) of 5 (indicating mild frailty) has been proposed to guide ICU admission for UK patients with coronavirus disease 2019 (COVID-19) pneumonia. However, the impact of frailty on mortality with (non-COVID-19) pneumonia in critical illness is unknown. We examined the triage utility of the CFS in patients with pneumonia requiring ICU.
Methods
We conducted a retrospective cohort study of adult patients admitted with pneumonia to 170 ICUs in Australia and New Zealand from January 1, 2018 to September 31, 2019. We classified patients as: non-frail (CFS 1–4) frail (CFS 5–8), mild/moderately frail (CFS 5–6),and severe/very severely frail (CFS 7–8). We evaluated mortality (primary outcome) adjusting for site, age, sex, mechanical ventilation, pneumonia type and illness severity. We also compared the proportion of ICU bed-days occupied between frailty categories.
Results
1852/5607 (33%) patients were classified as frail, including1291/3056 (42%) of patients aged >65 yr, who would potentially be excluded from ICU admission under UK-based COVID-19 triage guidelines. Only severe/very severe frailty scores were associated with mortality (adjusted odds ratio [aOR] for CFS=7: 3.2; 95% confidence interval [CI]: 1.3–7.8; CFS=8 [aOR: 7.2; 95% CI: 2.6–20.0]). These patients accounted for 7% of ICU bed days. Vulnerability (CFS=4) and mild frailty (CFS=5) were associated with a similar mortality risk (CFS=4 [OR: 1.6; 95% CI: 0.7–3.8]; CFS=5 [OR: 1.6; 95% CI: 0.7–3.9]).
Conclusions
Patients with severe and very severe frailty account for relatively few ICU bed days as a result of pneumonia, whilst adjusted mortality analysis indicated little difference in risk between patients in vulnerable, mild, and moderate frailty categories. These data do not support CFS ≥5 to guide ICU admission for pneumonia.
Keywords: COVID-19, frailty, intensive care unit, mortality, observational study, pneumonia, respiratory failure
Editor's key points.
-
•
Clinical Frailty Scale (CFS) scores indicating mild frailty have been proposed to guide ICU admission for UK patients with coronavirus disease 2019 (COVID-19) pneumonia.
-
•
However, the impact of frailty on mortality with (non-COVID-19) pneumonia is unknown.
-
•
The authors examined the relationship between CFS and mortality in patients requiring ICU for (non-COVID-19) pneumonia, using a large Australian and New Zealand database on critical care admissions.
-
•
Only CFS scores ≥7 were associated with higher (adjusted) mortality.
-
•
These data suggest that CFS scores ≥5 alone are not useful for guiding the allocation of critical care resources.
Frailty, a multiply determined state of decline in health imparting vulnerability to stress, is present in 30% of patients admitted to the ICU.1 It is associated with higher mortality, longer ICU and hospital lengths of stay, increased discharge to residential care, and greater health costs.1, 2, 3, 4 During the coronavirus disease 2019 (COVID-19) pandemic, patient frailty has been advocated as a criterion to triage ICU admission in the presence of pneumonia.5 The Clinical Frailty Scale (CFS),6 a widely used, simple, and valid frailty screening measure in use in critical illness, is being used for this purpose. Specifically, in the UK, the National Institute for Health and Care Excellence initially recommended that a CFS ≥5, corresponding to mild frailty, be considered as a threshold above which ICU admission could be potentially initially be declined. This guidance was intended to help ration ICU resources should demand for beds exceeds supply.5 This advice was subsequently revised to advise caution in applying the CFS in younger patients and in those with stable long-term disabilities, and hence to apply it only in patients aged >65 yr.7
However, evidence supporting triage recommendations for pneumonia requiring critical care support is lacking. Although the commonest complication of COVID-19 disease resulting in ICU admission is hypoxic respiratory failure secondary to pneumonia, the impact of frailty on outcomes amongst patients with pneumonia admitted to the ICU is unclear, and the relevance of the 65-yr-old cut-off advocated for considering the CFS currently is unknown.
Accordingly, we conducted a multicentre retrospective cohort study of patients admitted with pneumonia to ICUs in Australia and New Zealand to evaluate the association of frailty with in-hospital mortality and other outcomes. In doing so, our aim was to provide data to inform guidelines on the use of the CFS as a triage tool and on the appropriateness of the triage threshold of a CFS ≥5 recommended in current guidelines both overall and in those aged >65 yr.
Methods
Study design
This was a retrospective cohort study, using previously described methods.8 Population based data were collected by the Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcome and Resource Evaluation, which manages the ANZICS Adult Patient Database (APD), including data on ≥80% of all patients admitted to ICUs in Australia and New Zealand.9 Data are subject to automated validity checks, with data collectors undergoing regular quality assurance review and training. Data dictionary use is mandated. This clinical registry is used for benchmarking amongst contributing ICUs. Ethics approval was provided by the Alfred Hospital Human Research Ethics Committee (584/18).
Inclusion criteria
All patients with pneumonia aged ≥16 yr admitted to an ICU between January 1, 2018 and September 31, 2019 were included.
Exclusion criteria
We excluded patients who were admitted to the ICU for the sole purpose for organ donation or palliative care. In the event of multiple ICU admissions during a hospital stay, only the first was included.
Data collection
Patient characteristic data were age, sex, diagnosis (defined by the Acute Physiology and Chronic Health Evaluation [APACHE] III-j diagnosis codes), medical treatment limitation order (either patient initiated or because of medical futility), and illness severity scores (APACHE II and III-j10 and Australian and New Zealand Risk of Death [ANZROD] scores).11 Patients were defined as having pneumonia using APACHE III-j admission diagnoses of bacterial or viral pneumonia.
Explanatory variable: frailty
The Canadian Study of Health and Aging CFS was used for frailty measurement, a 9-point judgement-based categorical scale, which is inclusive—everyone has a place on it. This scale has demonstrated validity and reliability in frailty assessment in ICU patients and other populations.2 , 6 The modified eight-category CFS is the most utilised frailty assessment in the critically ill.12 This scale ranges from a CFS=1 (very fit), 2 (well), 3 (managing well), 4 (vulnerable), 5 (mildly frail), 6 (moderately frail), 7 (severely frail) to 8 (very severely frail).6 Frailty scores were both dichotomised as non-frail (CFS 1–4) or frail (CFS 5–8) according to accepted definitions,2 with the frail cohort further considered in terms of mild/moderate frailty (CFS 5–6) and severe/very severe frailty (CFS 7–8). Frailty is a non-mandatory variable in the ANZICS APD, collected since 2017, and is defined as the patient's level of physical function 2 months before hospital admission.
Primary outcome
The primary outcome was in-hospital mortality.
Secondary outcomes
We assessed the following secondary outcomes: discharge to nursing home/chronic care, survival to community discharge, organ support within the ICU (mechanical and noninvasive ventilation, renal replacement therapy, vasoactive infusion, and extracorporeal membrane oxygenation), and ICU bed day occupancy.
Statistical analysis
Data were examined for normality and compared between groups using χ2 tests (equal proportion), Student's t-tests (parametric data), or Wilcoxon rank-sum tests, reporting results as n (%), means (standard deviation), and medians (inter-quartile range [IQR]), respectively.
The relationship between frailty and outcome was determined fitting hierarchical logistic regression using the PROC GLIMMIX procedure with patients nested within sites and site treated as a random effect with results reported as marginal odds ratios (ORs) (95% confidence interval [CI]). Model covariates include age, gender, pneumonia type (viral or bacterial), mechanical ventilation, and illness severity, with heterogeneity determined by fitting first-order interactions with frailty. Patient illness severity was measured by the ANZROD score, a locally derived highly discriminatory score for mortality in Australian and New Zealand ICU patients.9 , 11 , 13 Whilst ANZROD ordinarily includes patient age, to model age separately, the age component within ANZROD was removed. As frailty is an ordinal score with eight categories, it was initially modelled as a categorical variable and then secondly as a continuous variable, where evidence of linearity was observed to exist. After a significant interaction between CFS and age, exploratory analyses were performed on patients dividing age into 10 yr blocks.
Predefined subgroup analysis was performed on patients determined by pneumonia type (viral vs bacterial), age (>65 yr), and mechanical ventilation. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA), and a two-sided P-value of 0.05 was used to indicate statistical significance.
Results
Subject characteristics
Over the study period, 9353 patients with pneumonia aged ≥16 yr were admitted to 170 ICUs; 671 were excluded (574 readmissions, 38 palliative care admissions, and 59 missing hospital outcome), of whom 5607/8682 had complete frailty data. In total, 1852/5607 (33%) patients were classified as frail, which increased in frequency with advancing age (Fig. 1 ). Patients classified as frail were older with higher illness severity scores (Table 1 ). Patients with frailty also had more chronic co-morbidities (particularly chronic respiratory disease) and were more likely to have treatment limitations in place on admission to the ICU (Table 1; Supplementary Tables 2 and 3).
Fig 1.
Distribution of Clinical Frailty Scale (CFS) scores, stratified by age.
Table 1.
Baseline patient characteristics of included patients. ANZROD, Australian and New Zealand Risk of Death; APACHE, Acute Physiology and Chronic Health Evaluation; IQR, inter-quartile range; sd, standard deviation.
| Characteristic | All patients (n=5607) | Frail patients (n=1852) | Non-frail patients (n=3755) | P-value |
|---|---|---|---|---|
| Age (yr), mean (sd) | 63.8 (17.6) | 70 (15.6) | 60.8 (17.7) | <0.0001 |
| Sex (men) | 3041 (54.2%) | 968 (52.3%) | 2073 (55.2%) | 0.038 |
| Any co-existing condition | 2377 (42.4%) | 1115 (60.2%) | 1262 (33.6%) | <0.0001 |
| Chronic respiratory | 1150 (20.5%) | 617 (33.3%) | 533 (14.2%) | <0.0001 |
| Chronic cardiovascular | 592 (10.6%) | 300 (16.2%) | 292 (7.8%) | <0.0001 |
| Metastatic cancer | 217 (3.9%) | 104 (5.6%) | 113 (3.0%) | <0.0001 |
| Immunosuppression | 573 (10.2%) | 210 (11.3%) | 363 (9.7%) | 0.05 |
| Chronic renal failure | 266 (4.7%) | 138 (7.5%) | 128 (3.4%) | <0.0001 |
| Chronic liver disease | 90 (1.6%) | 39 (2.1%) | 51 (1.4%) | 0.036 |
| Liver cirrhosis | 85 (1.5%) | 39 (2.1%) | 46 (1.2%) | 0.011 |
| APACHE II score, mean (sd) | 17.4 (6.8) | 19.2 (6.4) | 16.5 (6.8) | <0.0001 |
| APACHE III-j score, mean (sd) | 56.7 (21.5) | 61.5 (19.6) | 54.3 (21.9) | <0.0001 |
| ANZROD, median (IQR) | 8.0% (3.6–19.1%) | 14.6% (6.7–29.4%) | 6.1% (2.9–13.5%) | <0.0001 |
| ANZROD (age component removed), median (IQR) | 7.0 (3.5–15.8) | 11.6 (5.4–23.8) | 5.6 (3.0–11.8) | <0.0001 |
| Treatment limitations on admission | 1051 (18.7%) | 710 (38.3%) | 341 (9.1%) | <0.0001 |
| Hospital admission source | ||||
| Home | 4263 (76.0%) | 1378 (74.4%) | 2885 (76.8%) | 0.045 |
| Chronic care/palliative care/nursing home | 95 (1.7%) | 76 (4.1%) | 19 (0.5%) | <0.0001 |
| Transfer from other acute hospital | 1144 (20.4%) | 360 (19.4%) | 784 (20.9%) | 0.21 |
| Mental health | 37 (0.7%) | 23 (1.2%) | 14 (0.4%) | <0.0001 |
| Rehabilitation | 8 (0.2%) | 4 (0.2%) | 4 (0.1%) | 0.45 |
| Missing | 60 (1.1%) | 11 (0.6%) | 49 (1.3%) | 0.015 |
| Viral pneumonia | 1261 (22.5%) | 352 (19.0%) | 909 (24.2%) | <0.0001 |
Primary outcome
Frail patients were twice as likely to die both in the ICU (unadjusted mortality: 12% [229/1852] vs 6% [229/3755]; P<0.001) and in hospital (21% [391/1852] vs 9% [322/3755]; P<0.001) (Table 2 ).
Table 2.
Univariable and multivariable ICU and hospital outcomes by Clinical Frailty Scale status. Multivariable analyses adjusting for site, age, sex, pneumonia type, and illness severity. CI, confidence interval; IQR, inter-quartile range; OR, odds ratio.
| Clinical frailty scale | Number of patients | ICU mortality, n (%) | Hospital mortality, n (%) | Adjusted hospital mortality, OR (95% CI) | Discharge to nursing home/chronic care, n (%) | Adjusted discharge to nursing home/chronic care, OR (95% CI) | Discharge to home, n (%) | ICU bed days, median (IQR) | Hospital bed days, median (IQR) |
|---|---|---|---|---|---|---|---|---|---|
| Non-frail | 3755 | 229 (6.1) | 322 (8.6) | 49 (1.3) | 2757 (73.4) | 3.0 (1.7–6.0) | 8.2 (5.0–14.2) | ||
| 1 | 216 | 5 (2.3) | 6 (2.8) | Reference | 1 (0.5) | Reference | 185 (85.6) | 2.6 (1.7–5.5) | 6.2 (4.1–9.8) |
| 2 | 911 | 26 (2.9) | 38 (4.2) | 0.80 (0.32–1.98) | 4 (0.4) | 0.64 (0.07–5.84) | 737 (80.9) | 3.0 (1.6–6.2) | 7.7 (4.8–13.0) |
| 3 | 1438 | 91 (6.3) | 115 (8.0) | 1.02 (0.43–2.42) | 20 (1.4) | 1.41 (0.18–10.89) | 1048 (72.9) | 3.0 (1.7–5.9) | 8.5 (5.2–14.6) |
| 4 | 1190 | 107 (9.0) | 163 (13.7) | 1.61 (0.68–3.82) | 24 (2) | 1.72 (0.22–13.23) | 787 (66.1) | 3.1 (1.7–6.0) | 8.9 (5.7–15.3) |
| Frail | 1852 | 229 (12.4) | 391 (21.1) | 111 (6.0) | 981 (53.0) | 3.0 (1.7–5.7) | 9.6 (5.5–17.0) | ||
| 5 | 723 | 71 (9.9) | 112 (15.5) | 1.62 (0.67–3.90) | 27 (3.7) | 3.12 (0.4–24.02) | 405 (56.0) | 3.1 (1.8–6.0) | 9.3 (5.7–16.2) |
| 6 | 722 | 92 (12.8) | 154 (21.3) | 2.05 (0.85–4.91) | 44 (6.1) | 5.03 (0.66–38.45) | 392 (54.3) | 3.0 (1.8–5.4) | 10.0 (5.5–426) |
| 7 | 344 | 48 (14.0) | 95 (27.6) | 3.20 (1.31–7.81) | 36 (10.5) | 10.57 (1.38–80.95) | 161 (46.8) | 3.0 (1.6–5.7) | 9.8 (5.7–17.8) |
| 8 | 63 | 18 (28.6) | 30 (47.6) | 7.18 (2.59–19.9) | 4 (6.3) | 6.59 (0.69–62.91) | 23 (36.5) | 2.9 (1.6–6.2) | 7.3 (4.0–14.0) |
Primary outcome: hierarchical logistic regression analysis
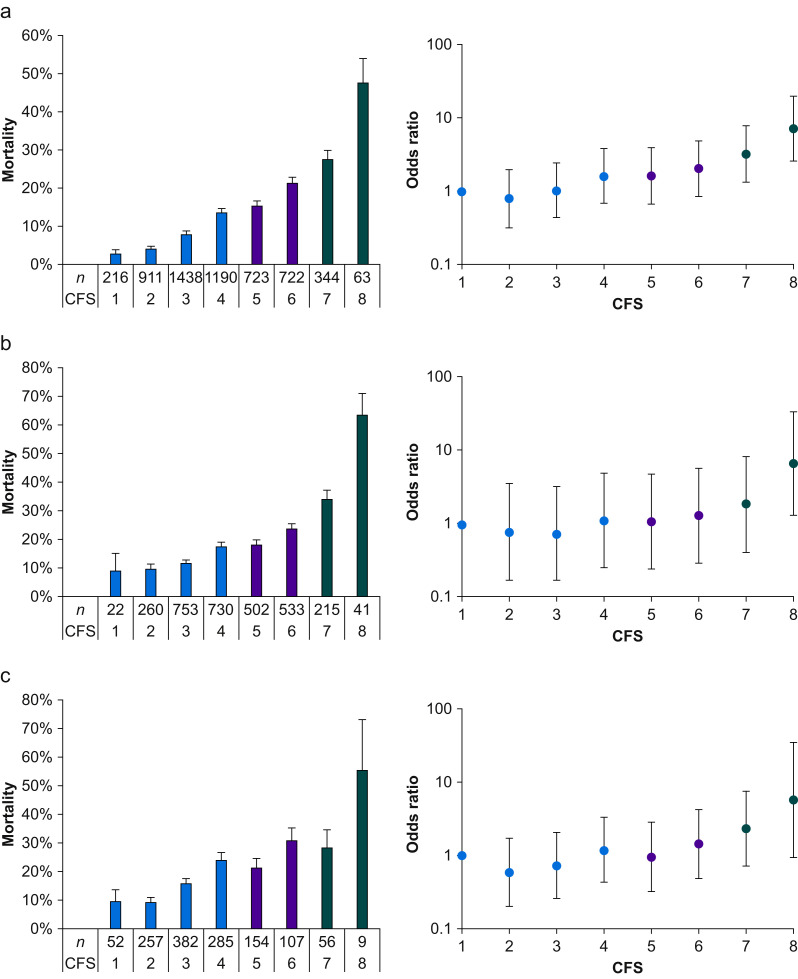
When considered as a continuous variable, frailty remained significantly associated with hospital mortality, after adjustment for site, age, sex, pneumonia type, mechanical ventilation, and illness severity (OR: 1.30 for each point increase in CFS; 95% CI: 1.22–1.38; P<0.001). Whilst the unadjusted risk of death increased with higher CFS, after multivariable adjustment, similar risk was evident in CFS Categories 4–6. (Fig. 2 ). The relationship between frailty and mortality remained independent of sex, pneumonia type, mechanical ventilation, patient severity, and age (Supplementary Tables 2 and 5).
Fig 2.
Hospital mortality according to Clinical Frailty Scale (CFS) score. ∗Error bars for mortality are standard error of the mean, for odds ratio are 95% confidence interval. (Data in Table 2 [whole cohort], Supplementary Table 7 [patients ≥65 yr], and Supplementary Table 9 [patients mechanically ventilated]). (a) Whole cohort, (b) age >65 yr, and (c) mechanically ventilated.
When considered by CFS category, the highest mortality was observed in patients with CFS=7 (28% [95/344]) and CFS=8 (48% [30/63]; Table 2). After adjustment, only these two highest frailty categories were associated with an increased risk of death over lower categories (Table 2, Fig. 2). These mortality differences remained in patients aged >65 yr (Fig. 2; Supplementary Tables 7 and 8; Supplementary Fig. 1), and in patients who required mechanical ventilation (Fig. 2; Supplementary Tables 9 and 10; Supplementary Fig. 1).
Secondary outcomes
ICU bed occupancy
Patients with CFS=7–8 accounted for 7% of all ICU bed days, compared with 24% of all ICU bed days for patients with CFS=5–6 (Fig. 3 ).
Fig 3.
Total ICU bed days occupied stratified by Clinical Frailty Scale (CFS). ∗Error bars are standard error of the mean.
Discharge to nursing home/chronic care
A total of 6% (111/1852) of patients with frailty vs 1% (49/3755) of patients without frailty were discharged to a nursing home/chronic care (P<0.001); this was a new discharge location in 3% (59/1852) of patients with frailty vs 1% (33/3755) of patients without frailty, who previously resided at home (P<0.001) (Table 2; Supplementary Table 4). On multivariable analysis, the association with new chronic care discharge persisted (OR for each point increase in CFS=1.38; 95% CI: 1.19–1.60; P<0.001, with no difference in slope between viral and bacterial pneumonia subtypes) (Supplementary Table 6). Overall, 82% (1179/1445) of all patients with CFS=5–6 were discharged alive from hospital, with 3% (42/1445) newly admitted to a nursing home/chronic care residential location. In mechanically ventilated patients, 75% (195/261) of all patients with CFS=5–6 were discharged alive from hospital (Supplementary Table 12).
Organ support
Patients with frailty were less likely to be mechanically ventilated in the ICU (19% vs 28%; P<0.001), but more likely to undergo noninvasive ventilation (55% vs 43%; P<0.001; Table 3 ). There was no difference in renal replacement therapy, but fewer patients with frailty underwent vasoactive infusions or extracorporeal membrane oxygenation (Table 3).
Table 3.
Process of care and complications by Clinical Frailty Scale status. P<0·001 for all categories, apart from renal replacement therapy (P=0.31).
| Clinical frailty scale | Number of patients (n=5607) | Invasive ventilation, n (%) (n=5559) | Noninvasive ventilation, n (%) (n=5075) | Renal replacement therapy, n (%) (n=4951) | Vasoactive infusion, n (%) (n=5052) | Extracorporeal membrane oxygenation, n (%) (n=4955) |
|---|---|---|---|---|---|---|
| Non-frail | 3755 | 976 (75.0) | 1469 (43.2) | 151 (4.5) | 1232 (36.1) | 30 (0.9) |
| 1 | 216 | 52 (24.2) | 66 (34.4) | 5 (2.6) | 54 (28.0) | 8 (4.2) |
| 2 | 911 | 257 (28.5) | 335 (40.7) | 29 (3.6 | 262 (31.8) | 11 (1.4) |
| 3 | 1438 | 382 (26.9) | 553 (42.3) | 63 (4.9) | 509 (38.8) | 8 (0.6) |
| 4 | 1190 | 285 (24.2) | 515 (47.7) | 54 (5.1) | 407 (37.7) | 3 (0.3) |
| Frail | 1852 | 326 (25.0) | 920 (54.9) | 73 (4.5) | 504 (30.7) | 3 (0.2) |
| 5 | 723 | 154 (21.4) | 360 (54.2) | 29 (4.5) | 213 (32.5) | 2 (0.3) |
| 6 | 722 | 107 (14.9) | 346 (53.3) | 30 (4.9) | 194 (30.8) | 1 (0.2) |
| 7 | 344 | 56 (16.3) | 180 (58.8) | 12 (4) | 83 (27.3) | 0 (0) |
| 8 | 63 | 9 (14.3) | 34 (60.7) | 2 (3.9) | 14 (26.4) | 0 (0) |
Sensitivity analyses
Compared with patients with missing frailty scores, patients with complete frailty data were older (median [IQR] age: 67 [53–77] vs 66 [52–76] yr) with lower APACHE III-j scores (mean: 57 vs 59; Supplementary Table 1). Mortality in hospital and ICU, bed days, and ANZROD (median: 8.0% vs 8.5%) were similar between included and non-included patients.
Discussion
In this population-level retrospective cohort study, we found that one-third of patients with pneumonia admitted to Australian and New Zealand ICUs were categorised as frail. Although these patients were twice as likely to die in the ICU and hospital, the adjusted increased risk of death was only observed in those with severe and very severe frailty. When considered as separate categories, lesser degrees of mild/moderate frailty (CFS=5–6) were not strongly associated with mortality; four out of five patients in these categories were discharged from hospital alive, with half discharged directly home. Patients categorised as vulnerable had a similar risk of in-hospital mortality as those categorised as mildly frail.
Based on the June 19, 2020 UK Intensive Care National Audit and Research Centre COVID-19 report, 90.4% of all COVID-19 patients admitted to UK critical care units were able to live without assistance in daily activities before ICU admission compared with only 73.6% of patients with viral pneumonia (non-COVID-19) admitted to UK critical care units from 2017 to 2019.14 These data suggest that ICU triage guidelines based on frailty are likely already being used in UK practice (integral to the definition of a CFS ≥5 is the requirement for assistance with activities of daily living). Although COVID-19 patients with a CFS ≥5 remain eligible for ICU admission under the guidelines, particularly subsequent to initial ward management and deterioration, these data imply that few of these COVID-19 affected patients with frailty are currently admitted to UK ICUs. Our findings also indicate that the increased mortality risk for ICU patients with pneumonia in the mild frailty category, who would potentially be declined ICU admission based on UK COVID-19 ICU triage recommendations, was essentially identical to the risk in the vulnerable category who would not be declined admission. Our finding that the association between frailty and mortality was less pronounced in patients aged >65 yr further suggests caution in the use of the CFS in ICU triage in older patients with pneumonia—the target group of this guidance.
Our findings are concordant with a previous report suggesting comparatively better outcomes in critical illness for patients with mild frailty than higher categories.2 , 12 However, they contrast with a recent European study of 1100 patients undergoing review by a rapid response team, in which 67% (193/287) of patients categorised as CFS 5–6 either died or remained hospitalised at 30 days, similar to outcomes for patients in higher frailty categories.15 The relevance of this study to patients with pneumonia or with COVID-19 admitted to the ICU is unclear. In our study, relatively few patients were discharged to chronic care facilities both overall and in patients aged >65 yr. Coupled with mortality findings, these data suggest overall acceptable outcomes for patients with pneumonia, particularly at lower levels of frailty. Patients with a CFS ≥5 accounted for almost a third of ICU bed days, whereas patients with severe and very severe frailty only accounted for 7% of ICU bed days. These findings imply that adjusting the CFS threshold used for triage purposes to exclude only patients with severe or very severe frailty would result in relatively few patients with pneumonia being declined ICU admission on frailty grounds. Moreover, it would only be useful if ICU demand did not substantially exceed the number of available beds.
Alternative approaches to ICU triage during the COVID-19 pandemic have been advocated or implemented outside of the UK, incorporating both initial patient assessment and review of ICU progress over time. One widely cited triage protocol is the Ottawa triage protocol, based on Sequential Organ Failure Assessment (SOFA) score cut-offs, which, in a simulated pandemic model, has been shown to potentially increase ICU bed availability by 53%.16 , 17 A similar protocol utilising progressively more restrictive ‘tiers’ of criteria for non-initiation and withdrawal of intensive care, depending on resource scarcity, has been developed in Minnesota.18 More recently, ANZICS has released guidance to support the development of local admission policies, in the event that ICU demand outstrips supply during the COVID-19 pandemic.19 Other studies have demonstrated overall poorer outcomes with frailty in very old critically ill patients.8 , 20 , 21 Taken together, these studies suggest that measurement of frailty is important, but should perhaps be best considered in the context of other variables in making triage decisions.
The risk of death in our study for patients with a CFS of 4 was essentially identical to that observed with a CFS ≥5. Thus, one approach to substantially reduce ICU occupancy might be to reduce the ICU admission threshold to a CFS ≥4. Our data suggest that this approach would reduce total ICU bed occupancy by half, at the expense of not admitting many more patients with expected favourable outcomes. Alternatively, if triaging patient admissions purely on expected mortality, a threshold of a CFS ≥7 is more supported by our data. If multiple vulnerable or frail patients were being considered for the same ICU bed, our data suggest that factors other than the CFS (such as dynamic SOFA score assessments) should also be used to determine which patient is admitted to the ICU. Given that outcomes for CFS 4 and 5 patients were similar in our study, this would arguably be more equitable than the current approach being advocated by the National Institute for Health and Care Excellence.5
Our study has numerous strengths, including the scale and scope of the registry used, which includes critically ill patients drawn from all states and territories in a bi-national contemporary cohort at a population level. The population studied was not restricted beyond admissions for palliative care, and thus represents a comprehensive overview of the prevalence and impact of frailty in ICU in Australia and New Zealand. A further strength is the frailty measure chosen, with the CFS having become the dominant tool for frailty assessment in critically ill patients. We have previously demonstrated that this scale has acceptable inter-individual variation in the critically ill population, and correlates well with more comprehensive frailty measurement scales.22 , 23
The major limitation of our study is that we studied patients with non-COVID pneumonia. As pulmonary infection with severe acute respiratory syndrome coronavirus 2 presents a novel disease, this presents challenges for extrapolating our findings from critically ill patients in Australia and New Zealand to patients with COVID-19 in the UK. However, reports of COVID-19 patients requiring critical care from Lombardy, Italy24 and Wuhan, China25 suggest that the characteristics of patients included in our study are similar to those seen in COVID-19 patients. We cannot preclude the possibility that the relationship between frailty and outcomes is different for patients with COVID-19 compared with other pneumonias. However, our contemporary cohort of patients with a range of bacterial and viral pneumonias found no evidence that the aetiology of pneumonia affected the association between frailty and outcomes. Emerging data have suggested that different phenotypes of COVID-19 might have different ventilator requirements.26 , 27 However, we found no evidence that the relationship between frailty and outcomes was different for patients who were ventilated compared with those who were not. Frailty scores were missing for 35% of patients; extrapolation of our results to the whole population and to overall ICU bed-day occupancy could have hence been biased. Sensitivity analyses, however, revealed no major clinically significant differences between included and non-included patients, with no differences in either ICU or hospital length of stay likely to have changed percentages of bed-day occupancy within frailty categories. It would have been useful to have data on post-hospitalisation outcomes because it is possible that frailty may have been associated with late mortality as found in other studies.2 , 22 We did not define the diagnostic criteria for bacterial or viral pneumonia beyond that of the APACHE III-j diagnostic codes. It is possible that misclassification thus occurred, although we note that this is a common limitation of registry studies. Data collectors used medical record review to determine the CFS scores, in contrast to prior studies.2 , 6 Our prior work, however, has shown that CFS derivation from retrospective medical ICU chart review is feasible.23 Moreover, additional prior research has also demonstrated that CFS scores assigned in this manner correlate well with those determined through patient interview.28 We did not mandate formal training of frailty measurement in participating ICUs. Inaccurate scoring was hence possible; however, all data collection in the APD is conducted with reference to a data dictionary, with explicit instructions and definitions about the CFS measurement.
Conclusions
Frailty is common in patients with pneumonia admitted to the ICU and is associated with in-hospital mortality. Mild frailty (CFS=5) was not more strongly associated with mortality than vulnerability (CFS=4), suggesting that CFS ≥5 may not define a suitable triage threshold based purely on expected mortality. Given only patients with severe and very severe frailty (CFS ≥7) had a higher risk of death over lower categories, with little extra ICU capacity resulting from exclusion of these patients, we advocate that factors other than the CFS (e.g. dynamic illness severity scoring) should also be considered in determining which patient is admitted to the ICU.
Authors' contributions
Study design: all authors
Study supervision: RB, DP
Literature search: JND
Data analysis: MB, EP, DP
Data interpretation: JND, RB, PJY, KR, DP
Writing/revision of paper: all authors
Acknowledgements
This research was conducted during the tenure of a Health Research Council of New Zealand Clinical Practitioner Fellowship held by PJY. The Medical Research Institute of New Zealand is supported by Independent Research Organisation funding from the Health Research Council of New Zealand.
Handling editor: Gareth Ackland
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.07.049.
Declarations of interest
KR reports personal fees from Clinical Cardio Day, Cape Breton University; CRUIGM Montreal; speaker at Jackson Laboratory, Bar Harbor, ME; speaker at MouseAGE, Rome Italy; Lundbeck; Frontemporal Dementia Study Group; and Sun Life Insurance, Japan, outside the submitted work. KR is President and Chief Science Officer of DGI Clinical, which, in the last 5 yr has contracts with pharma and device manufacturers (Baxter, Baxalta, Shire, Hollister, Nutricia, Roche, and Otsuka) on individualised outcome measurement. In 2017, he attended an advisory board meeting with Lundbeck. Otherwise, any personal fees are for invited guest lectures and academic symposia, received directly from event organisers, chiefly for presentations on frailty. He is Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research and with additional funding from the Alzheimer Society of Canada and several other charities, and, in its first phase (2013–8), from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Canadian Institutes of Health Research, QEII Health Sciences Centre Foundation, Capital Health Research Fund, and the Fountain Family Innovation Fund of the QEII Health Sciences Centre Foundation.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Muscedere J., Waters B., Varambally A. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw S.M., Stelfox H.T., McDermid R.C. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Maguet P., Roquilly A., Lasocki S. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 4.Fernando S.M., McIsaac D.I., Perry J.J. Frailty and associated outcomes and resource utilization among older ICU patients with suspected infection. Crit Care Med. 2019;47:e669–e676. doi: 10.1097/CCM.0000000000003831. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence . 2020. COVID-19 rapid guideline: critical care in adults.https://www.nice.org.uk/guidance/ng159 Available from: [PubMed] [Google Scholar]
- 6.Rockwood K., Song X., MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence, 2020, Available from: https://www.nice.org.uk/news/article/nice-updates-rapid-covid-19-guideline-on-critical-care (accessed 12 April 2020).National Institute for Health and Care, 2020
- 8.Darvall J.N., Bellomo R., Paul E. Frailty in very old critically ill patients in Australia and New Zealand: a population-based cohort study. Med J Aust. 2019;211:318–323. doi: 10.5694/mja2.50329. [DOI] [PubMed] [Google Scholar]
- 9.Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation . 2018. ReportIntensive care resources and activity in Australia and New Zealand, adult patient database: activity report 2017/2018. Carlton. [Google Scholar]
- 10.Knaus W.A., Wagner D.P., Draper E.A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 11.Paul E., Bailey M., Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care. 2013;28:935–941. doi: 10.1016/j.jcrc.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw M., Majumdar S.R., Rolfson D.B., Ibrahim Q., McDermid R.C., Stelfox H.T. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20:175. doi: 10.1186/s13054-016-1338-x. [erratum appears in Crit Care 2016; 20:223] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilcher D., Paul E., Bailey M., Huckson S. The Australian and New Zealand Risk of Death (ANZROD) model: getting mortality prediction right for intensive care units. Crit Care Resusc. 2014;16:3–4. [PubMed] [Google Scholar]
- 14.Intensive Care National Audit & Research Centre . 2020. ICNARC report on COVID-19 in critical care.https://www.icnarc.org/DataServices/Attachments/Download/da19fd54-70b2-ea11-9127-00505601089b Available from: [Google Scholar]
- 15.So R.K.L., Bannard-Smith J., Subbe C.P., Jones D.A., van Rosmalen J., Lighthall G.K. The association of clinical frailty with outcomes of patients reviewed by rapid response teams: an international prospective observational cohort study. Crit Care. 2018;22:227. doi: 10.1186/s13054-018-2136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christian M.D., Hawryluck L., Wax R.S. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175:1377–1381. doi: 10.1503/cmaj.060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung W.K., Myburgh J., Seppelt I.M. A multicentre evaluation of two intensive care unit triage protocols for use in an influenza pandemic. Med J Aust. 2012;197:178–181. doi: 10.5694/mja11.10926. [DOI] [PubMed] [Google Scholar]
- 18.Hick J.L., O’Laughlin D.T. Concept of operations for triage of mechanical ventilation in an epidemic. Acad Emerg Med. 2006;13:223–229. doi: 10.1197/j.aem.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Warrillow S., Austin D., Cheung W. ANZICS guiding principles for complex decision making during the COVID-19 pandemic. Crit Care Resusc. 2020;22:98–102. doi: 10.51893/2020.2.sa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaatten H., De Lange D.W., Morandi A. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years) Intensive Care Med. 2017;43:1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 21.Guidet B., de Lange D.W., Boumendil A. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46:57–69. doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darvall J.N., Greentree K., Braat M.S., Story D.A., Lim W.K. Contributors to frailty in critical illness: multi-dimensional analysis of the Clinical Frailty Scale. J Crit Care. 2019;52:193–199. doi: 10.1016/j.jcrc.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Darvall J.N., Boonstra T., Norman J. Retrospective frailty determination in critical illness from review of the ICU clinical record. Anaesth Intensive Care. 2019;47:343–348. doi: 10.1177/0310057X19856895. [DOI] [PubMed] [Google Scholar]
- 24.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shears M., Takaoka A., Rochwerg B. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. 2018;45:197–203. doi: 10.1016/j.jcrc.2018.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.