Abstract
Purpose
The fertility of women decreases with age because of factors such as an increased incidence of aneuploidies and—possibly—decreased mitochondrial activity in oocytes. However, the relationship between maternal aging and mitochondrial function of their embryos remains unknown. Here, we assessed the relationship between maternal age and mitochondrial functions in their oocytes and embryos
Methods
The relationships between maternal age and oxygen consumption rates (OCRs), mitochondrial DNA (mtDNA) copy numbers, or blastocyst development was investigated using 81 embryos donated from 63 infertility couples. The developmental rates from morulae to blastocysts were retrospectively analyzed using data of 105 patients.
Results
The OCRs of morulae decreased with maternal age (r2 = 0.48, P < 0.05) although there were no relationships between maternal age and mtDNA copy number in any stages. The more oxygen consumed at the morula stage, the shorter time was required for embryo development to the mid-stage blastocyst (r2 = 0.236, P < 0.05). According to the clinical data analysis, the developmental rate from morulae to blastocysts decreased with maternal age (P < 0.05, < 37 years, 81.1%, vs. ≥ 37 years, 64.1%).
Conclusions
The data of the present study revealed that mitochondrial function at the morula stage of human embryos decreased with their maternal age and a decrease of mitochondrial function led to slow-paced development and impaired developmental rate from morulae to blastocysts.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01869-5) contains supplementary material, which is available to authorized users.
Keywords: Maternal age, Mitochondrial function, Oxygen consumption rate
Introduction
The fertility of women decreases with age [1]. One of the main reasons for the poor development of embryos obtained from older women is an increased rate of chromosomal aberrations [2] caused by premature bivalent separation into univalents during meiosis [3]. Additionally, a decline in mitochondrial function in oocytes with advancing age has also been proposed as a reason for poor embryo developmental competence [4–7]. The adverse effects of maternal aging on oocyte mitochondria have been reported as morphological abnormalities such as mitochondrial swelling, vacuolization and alterations to cristae [8, 9], and a possible decrease in mitochondrial DNA (mtDNA) copy number [10, 11]. However, the effects of maternal aging on mitochondrial function, such as oxygen consumption rate (OCR) in embryos, are not well understood.
It has been shown that the mitochondrial function of preimplantation embryos increases as their development advances toward the blastocyst stage, especially after embryonic (zygotic) gene activation [12–15]. In mammalian cells, adenosine triphosphate (ATP) is mainly generated by the mitochondrial electron transport system. Thus, the measurement of the OCR of developing embryos could be used as an effective tool to assess mitochondrial function for evaluation of their normality and developmental potential [12–18], which may help to evaluate embryo development potential prior to implantation.
Here we aimed to explore the relationship between maternal age and mitochondrial function of developing embryos.
Materials and methods
To assess the relationship between maternal aging and mitochondrial function of their embryos, vitrified–warmed cleavage stage embryos on day 3 (68–70 h after ICSI) and blastocysts on day 5 (116–118 h after ICSI), donated from 53 couples, were measured for OCRs and mtDNA copy numbers (Supplementary Fig. 1). All the embryos were received after obtaining written informed consent.
To determine the relationship between OCRs of morulae and their developmental competence, we assessed the time required from morulae to the mid-stage blastocyst after the measurement of their OCRs. Time-lapse images were captured by light-emitting diode illumination six times per hour over 2 days using a Primovision system (Vitrolife Japan Corporation, Tokyo, Japan) as described [19]. Embryos were cultured individually in potassium simplex optimized medium containing amino acids [20] (KSOMaa) and 5% (v/v) synthetic serum substitute (SSS, 99193; Irvine Scientific, St. Ana, CA, USA) at 37 °C under 5% CO2, 5% O2, and 90% N2 with high humidity. Eighteen embryos donated from 10 couples were examined. Only embryos donated from women aged 34–36 years at OPU were used to avoid female age effects.
In a retrospective analysis of clinical data regarding developmental competence from the morula to blastocyst stages, OPU was performed on 105 patients between February 2014 and July 2015 (Tables 1 and 2). Embryos were cultured in a single-step medium (SAGE 1-Step™ 67010010, CooperSurgical/Origio, Målov Denmark or Continuous Single Culture®, 90164, Fujifilm, Tokyo, Japan). Blastocyst morphology was evaluated at 120 and 144 h after insemination according to Gardner and Lane [21]. Blastocysts that were scored at least 3AA, but not grade C, were regarded as morphologically good. The developmental rates were compared between two age groups of women (< 37 years vs. ≥ 37 years).
Table 1.
Maternal characteristics in retrospective study
| < 37 years | ≥ 37 years | |
|---|---|---|
| No. of patient | 49 | 56 |
| Maternal age (years) | 33.1 | 39 |
| Body mass index (kg/m2) | 20.3 | 20.6 |
| Number of previous OPU | 0.16 | 0.29 |
| Stimulation protocol | ||
| Agonist | 31 | 30 |
| Antagonist | 18 | 26 |
| Current smoker | 0 | 0 |
| Ex-smoker | 3 | 2 |
| Cause of infertility | ||
| Tubal | 8 | 7 |
| PCO | 2 | 4 |
| Endometriosis | 9 | 9 |
| Male | 11 | 12 |
| Amount of FSH dose (IU) | ||
| FSH | 1091 | 1304 |
| HMG | 880 | 928 |
| Number of ova | ||
| Retrieved | 18 | 15 |
| Mature | 16 | 13 |
| Fertilized | 13 | 10 |
| Fertilization rate | 81.3 | 76.9 |
| Number of | ||
| cIVF | 20 | 24 |
| ICSI | 29 | 32 |
| Proportion of ICSI | 59 | 57 |
Table 2.
Retrospective analysis of rates of development from morulae to blastocysts
| Sage | CSC | |||
|---|---|---|---|---|
| < 37 years | > 37 years | < 37 years | > 37 years | |
| Number of patients | ||||
| 35* | 29* | 16* | 29* | |
| Number of cultured | ||||
| 251 | 207 | 145 | 186 | |
| Number of morulae | ||||
| 174 | 117 | 106 | 134 | |
| Rate (%)† | 69.3a | 56.5a | 73.1 | 72.0 |
| Number of blastocysts on day 5 | ||||
| 135 | 75 | 92 | 86 | |
| Rate (%)‡ | 77.6a | 64.1a | 86.8b | 64.2b |
| Number of morphologically good blastocysts on day 5 | ||||
| 54 | 24 | 32 | 31 | |
| Rate (%)‡ | 31 | 20.5 | 30.2 | 23.1 |
| Number of blastocysts on day 6 | ||||
| 160 | 100 | 97 | 111 | |
| Rate (%)‡ | 92.2a | 85.5a | 91.5b | 82.8b |
| Number of morphologically good blastocysts on day 6 | ||||
| 65 | 35 | 42 | 34 | |
| Rate (%)‡ | 37.4 | 29.9 | 39.6b | 25.4b |
Embryos were cultured in SAGE 1-Step™ medium (Sage) or Continuous Single Culture® medium (CSC) as described in the Methods. *Embryos obtained from 3 patients were randomly divided and cultured in two media. †The rate of morulae per cultured fertilized ova, ‡The rate of blastocysts per morulae. abValues with same superscripts within each column differ (P < 0.05)
Ovarian stimulation and insemination
Patients were subjected to controlled ovarian stimulation according to their medical history as described [22]. Ovulation was induced by hCG administration when at least one leading follicle reached 18 mm in diameter. Transvaginal follicle aspiration was carried out 36 h after the hCG injection. Insemination was carried out by ICSI or coculture with their partner’s spermatozoa for conventional in vitro fertilization 40 h after hCG administration.
OCR measurements
Embryos were frozen by vitrification [23] on days 3 or 5 after ICSI. After warming, day 3 embryos were cultured in KSOMaa medium at 37 °C under 5% CO2, 5% O2, and 90% N2 with high humidity for 17–22 h. Day 5 blastocysts were cultured for 8 h, and then their OCR was assessed because vitrified-warmed embryos recovered their OCR to the same level before vitrification 8 h after warming [17]. Morulae on day 4 that reached the compaction stage from 17 to 22 h after warming were included in the analysis. Blastocysts on day 5 that reached the expanded stage with a tightly packed inner cell mass (ICM) and trophectoderm (TE) consisting of cohesive epithelium were included in the analysis. The OCR of samples was measured using scanning electrochemical microscopy (CRAS-1.0; Clino Ltd., Miyagi, Japan) as described [15, 17] at 37 °C under air. Briefly, each oocyte or embryo was transferred into a cone-shaped microwell filled with 5-mL human tubal fluid (HTF) medium buffered with 21-mM HEPES containing 2.7-mM glucose, 0.33-mM pyruvate, and 5% SSS (mHTF), where it sank to the bottom and remained at the lowest point. A platinum microdisk electrode was loaded with 5 mL of mHTF, and its tip potential was maintained at − 0.6 V versus the Ag/AgCl electrode with a potentiostat to monitor the local oxygen concentration. The microelectrode scanned along the z-axis from the edge of the sample, and the OCR was calculated with custom software based on the spherical diffusion theory [24]. OCR was measured at three points for each embryo and at the ICM and both TE sides of blastocysts. The mean value was shown as its OCR read because there was no difference in the OCR value between ICM and TE sides [17]. The OCR was also measured in the presence of mitochondrial toxins (mitotoxins) [15, 18]. Stock solutions of 1-mM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; C2920, Merck Millipore Co., Darmstadt, Germany) and 1-M sodium cyanide (380970, Sigma-Aldrich) were prepared in ethanol and water, respectively. During the recording of OCR by embryos in mHTF medium, either FCCP or cyanide was added to give final concentrations of 1 μM and 1 mM, respectively. Cyanide at 1 mM normally completely inhibits mitochondrial cytochrome c oxidase activity. The mitochondrial OCRs (mtOCRs) were calculated by subtracting the value obtained in the presence of cyanide from those obtained without any mitochondrial toxins. The maximum mitochondrial OCRs (maxOCRs) value was calculated by subtracting the values obtained in the presence of cyanide from those obtained in the presence of FCCP.
Mitochondrial DNA measurements
All specimens were placed in 2-μL DNase-free water droplet individually immediately after OCR measurement and cryopreserved at − 70 °C until assay [15]. Each sample was lysed in 4-μL aliquots of lysis buffer (20-mM Tris (# 252859, Merck Millipore Co.), 0.4 mg/ml proteinase K (# P2308, Merck Millipore Co.), 0.9% Nonidet P-40 (# 21-3277-2, Merck Millipore Co.), and 0.9% Tween 20 (# P1379, Merck Millipore Co.)) at 55 °C for 30 min, followed by heating at 95 °C for 5 min; the lysate was then diluted in DNase-free water to a final volume of 40 μL. A 4-μL aliquot of the lysate was added to 12.5-μL Quantifact SYBR Green master mix (Qiagen, Venlo, Limburg, the Netherlands), 8.5-μl DNase-free water, and 1 μM of each prime polymerase chain reaction (PCR) amplifications were run in triplicate in a Rotorgene Q thermal cycler (Qiagen) according to the following conditions: 95 °C for 5 min, 40 cycles of 95 °C for 5 s, and 60 °C for 10 s. SYBR green fluorescence was measured at the end of each cycle. The melting curve was analyzed to check the specificity of the PCR products. A standard curve was generated for each run using 10fold serial dilutions representing copies of the external standard (103 to 106 copies). The external standard used in the present study was the PCR product of the corresponding gene cloned into a vector using the Zero Blunt TOPO PCR cloning kit (Thermo Fisher Scientific, Waltham, MA, USA), and the product was confirmed by sequencing before use. The primers were designed using Primer3Plus software (http://sourceforge.net/projects/primer3/) and targeted the NADH-ubiquinone oxidoreductase chain 1 (ND1; 126 bp region from 3485 to 3610) and ND6 gene sequences (163 bp region from 14,407 to 14,549) of human mitochondria (NC012920.1).
Study approval
The study was performed in accordance with Declaration of Helsinki protocols 7th version. Prior to inclusion in the study, all donating couples gave their written informed consent. This study was approved by the local ethics Institutional Review Board of IVF Namba Clinic, the institutional review board of Hyogo College of Medicine, and the Japan Society of Obstetrics and Gynecology (Registry Nos 135 and 138).
Statistics
The relationship between maternal age and mtDNA/OCRs of embryos was assessed using Pearson’s correlation coefficient test after the confirmation of normal distribution by a Chi-square goodness-of-fit test. Data obtained from a retrospective clinical analysis regarding developmental competence was compared using Chi-square tests. P < 0.05 was considered statistically significant.
Results
Effect of maternal age on OCRs and mtDNA copy number
To assess the relationship between maternal age and mitochondrial function of their embryos, 30 morulae on day 4 after ICSI and 34 expanded blastocysts on day 5, donated from 24 and 29 couples, respectively, were used for study. All embryos used in this study were inseminated by ICSI to avoid the contamination of mtDNA from spermatozoa attached to the zona pellucida. Women whose infertility causes were reported as being endometriosis, premature ovarian insufficiency, or hormone imbalance were excluded.
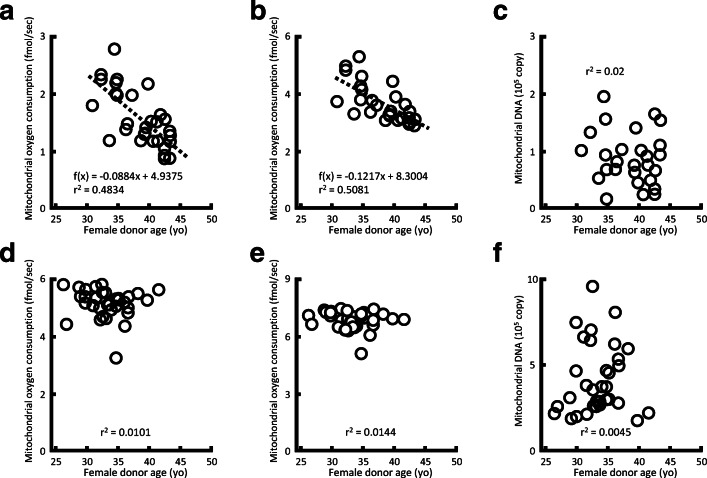
The mtOCRs and maxOCRs of morulae on day 4 after ICSI decreased with maternal age (mtOCRs, P < 0.05, r2 = 0.4834; maxOCRs, P < 0.05, r2 = 0.5081, Fig. 1a, b). However, there were no relationships between maternal age and mtOCRs or maxOCRs of their blastocysts on day 5 (Fig. 1d, e). Similarly, mtDNA copy numbers in different stages of developing embryos did not show any correlation with maternal age (Fig. 1c, f).
Fig. 1.
Relationship between maternal age and mitochondrial oxygen consumption rates (mtOCRs), maximum mitochondrial OCRs (maxOCRs), or mitochondrial DNA (mtDNA) numbers of embryos. (a, b) Relationships between maternal age and mtOCRs or maxOCRs of morulae on day 4 (n = 30) and (d, e) expanded blastocysts on day 5 (n = 34). (c) Relationships between maternal age and mtDNA copy number of their morulae on day 4 (n = 28) and (f) expanded blastocysts on day 5 (n = 34) are shown. The mtOCRs and maxOCRs of morulae on day 4 after ICSI decreased with maternal age (mtOCRs, P < 0.05, r2 = 0.4834; maxOCRs, P < 0.05, r2 = 0.5081) (c, g). Only embryos inseminated by ICSI were used to avoid contamination with mtDNA from spermatozoa on the zona pellucida
Relationship between OCRs of morulae and their development: do morulae with higher OCRs have higher developmental competence?
To address this, we captured time-lapse images from the morula to blastocyst stages following the OCR measurement of morulae without using mitotoxins. To avoid the effect of maternal age, we used eighteen embryos at 6–8 cell stage on day 3 donated from 10 couples among whom the woman’s age ranged from 34.0 to 35.9 years at oocyte pick-up. At the morula stage, the more oxygen consumed, the shorter time was required for embryo development to the mid-stage blastocyst (r2 = 0.236, P < 0.05; Fig. 2). Mid-stage was defined as when the blastocoel size reached half that of the blastocyst.
Fig. 2.
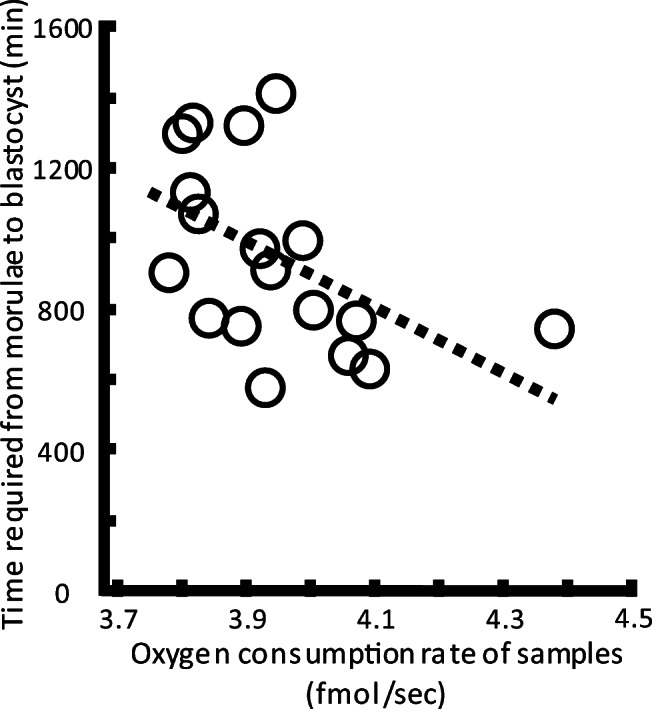
Relationship between OCRs of morulae and duration required to develop from morulae to mid-stage blastocysts. Morphological changes from morulae to blastocysts following measurement of OCRs of morulae cultured without mitotoxins were recorded every 10 min. To avoid the effect of maternal age, 6–8-cell stage embryos on day 3 were used from women aged 34–36 years at OPU. Eighteen embryos were donated from 10 couples. The more oxygen morulae consumed, the shorter was the duration required for embryo development between OCR measurement and mid-stage blastocysts (r2 = 0.236, P < 0.05). Mid-stage was defined as when the blastocoel reached half the size of the blastocyst
Retrospective analysis of developmental competence from morulae to blastocysts
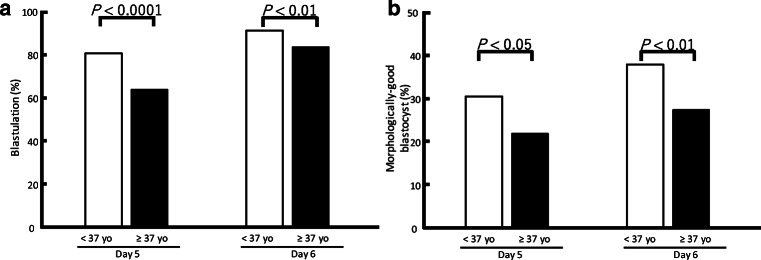
To determine how maternal age might affect morphological changes in embryos, the developmental rates to the blastocyst stage and the rate of formation of morphologically good blastocysts from morulae were compared retrospectively between two age groups of women (< 37 years n = 280 vs. ≥ 37 years n = 251). Retrospective analysis revealed that these development rates on day 5 after insemination decreased significantly with maternal age, being a blastulation rate of 81.1% in the younger vs. 64.1% in the older group (P < 0.01), and a 30.7% morphologically good blastocyst formation rate in the younger vs. 21.9% in the older group (P < 0.05; Fig. 3a, b). At day 6, the rates of development to the blastocyst and morphologically good blastocyst stages also significantly decreased with maternal age, being a 92.1% blastulation rate in the younger vs. 84.1% in the older group (P < 0.01) and a 38.2% morphologically good blastocyst formation rate in the younger vs. 27.5% in the older group (P < 0.01). These embryos were cultured in two commercially available media. The effects of maternal age were separately assessed in each medium. Irrespective of the culture medium, the adverse effect of maternal age was still observed in the morphologically good blastocyst formation rate (Table 2).
Fig. 3.
Retrospective analysis of developmental competence from morulae to blastocysts in the clinical data set. The rates of development from morulae to blastocysts (a) and to form morphologically good blastocysts (b) were compared retrospectively between two maternal age groups (< 37 years; n = 280; vs. ≥ 37 years, n = 251). Retrospective analysis revealed that the rates of development from morulae to blastocysts and to morphologically good blastocysts on day 5 after insemination decreased with maternal age. The rates of development from morulae to blastocysts and to morphologically good blastocysts on day 6 also decreased with maternal age. The data were for embryos cultured in two different commercially-available media (see Methods). Similar adverse effects of maternal age were observed in both culture media (Supplementary Figure)
Discussion
Here we found that the mitochondrial function of human morulae decreased with maternal age. Maternal age had no link with the copy number of mtDNA at both stages of embryo development or mtOCRs of blastocyst stage. The mtOCRs remain low until 8-cell stage in humans [15] and mice [14]. These data confirmed previous findings that mitochondrial biogenesis in humans is normally activated after embryonic gene activation at the 8-cell [15] or blastocyst stages [9, 25]. Moreover, the OCRs of human embryos rise dramatically following the 8-cell stage [15]. Accordingly, some degree of mitochondrial dysfunction in human embryos along with maternal aging could emerge after the 8-cell stage. Moreover, formation of the blastocoel cavity depends on cellular Na/K-ATPase activity and requires high levels of ATP [26]. These findings imply that morulae with reduced mitochondrial function might not form a blastocoel or have a delayed start as shown in Fig. 2. Subsequently, expanded blastocysts on day 5 had high mitochondrial function irrespective of maternal age (Fig. 1d, e) because embryos on day 4 without sufficient mitochondrial function could not reach the expanded blastocyst stage on the next day.
It has been generally believed that higher mtDNA content in TE cells correlates with a stressed state, such as aneuploidy and advanced maternal age [27–29]. This idea is supported by “quiet embryo hypothesis” which proposed that viable embryos have lower metabolism [30]. However, this hypothesis was based on metabolism before the start of blastulation which requires more energy [26]. Moreover, it has been shown that there was no correlation of mtDNA content in human blastocysts with their maternal age using an accurate quantitation of mtDNA copy number [31] consistent with our data using whole embryo. Therefore, time and further studies will tell whether the maternal age affect mtDNA content in their blastocysts.
To avoid the effect of cause of infertility on mtDNA content and OCRs, we excluded from the analysis women whose infertility causes were reported as being endometriosis, premature ovarian insufficiency, or hormone imbalance. However, embryos obtained from two different stimulation protocols (agonist and antagonist cycles) were included in the analysis. There were no differences in the OCR and mtDMA content of blastocysts between two different protocols (data not shown). Further studies should be required whether the patients’ background except for maternal age or stimulation protocol affects the OCR an mtDNA content of their embryos.
The data of the present study revealed that mitochondrial function at the morula stage of human embryos decreased with their maternal age and a decrease of mitochondrial function led to slow-paced development and impaired developmental rate from morulae to blastocysts.
Electronic supplementary material
Experimental design (PPTX 50 kb).
Acknowledgments
The authors thank Drs. M. Inoue, G. Udayanga, A. Takeshita, and T. Yamochi for their helpful comments, and James Cummins, PhD, from EDANZ Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Authors’ roles
N.M. and S.H. designed the experiment, interpreted the results, and wrote the manuscript with help from all authors. M.Y. measured OCRs. T.N., M.S., and Y.N. were involved in the analysis of clinical data. H.I. designed the qPCR procedures. A.F., H.S., and Y.M. supervised the project.
Funding information
Part of this work was supported by a grant from the Japan Agency for Medical Research and Development (17gk0110014h0002 and 18gk0110014h0003 to S.H. and Y.M.), and a grant from the Japan Society for the Promotion of Science (KAKENHI 17K08144 and 20K09674 to S.H.).
Compliance with ethical standards
Conflict of interest
The authors declare that they no conflict of interest exists.
Ethical approval
The study was performed in accordance with Declaration of Helsinki protocols 7th version. This study was approved by the local ethics Institutional Review Board of IVF Namba Clinic, the institutional review board of Hyogo College of Medicine, and the Japan Society of Obstetrics and Gynecology (Registry Nos 135 and 138).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol. 2018;29:327. doi: 10.3389/fendo.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Höög C, Kitajima TS. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nat Commun. 2015;6:7550. doi: 10.1038/ncomms8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartmann AK, Romão GS, Ramos Eda S, Ferriani RA. Why do older women have poor implantation rates? A possible role of the mitochondria. J Assist Reprod Genet. 2004;21:79–83. doi: 10.1023/B:JARG.0000027018.02425.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May-Panloup P, Chrétien MF, Jacques C, Vasseur C, Malthièry Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod. 2005;20:593–597. doi: 10.1093/humrep/deh667. [DOI] [PubMed] [Google Scholar]
- 7.Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller-Hocker J, Schafer S, Weis S, Munscher C, Strowitzki T. Morphological-cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996;2:951–958. doi: 10.1093/molehr/2.12.951. [DOI] [PubMed] [Google Scholar]
- 9.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;1:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11:843–846. doi: 10.1093/molehr/gah243. [DOI] [PubMed] [Google Scholar]
- 11.Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, Yoshimura Y. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–1375. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996;106:299–306. doi: 10.1530/jrf.0.1060299. [DOI] [PubMed] [Google Scholar]
- 13.Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62:1866–1874. doi: 10.1095/biolreprod62.6.1866. [DOI] [PubMed] [Google Scholar]
- 14.Ottosen LD, Hindkjaer J, Lindenberg S, Ingerslev HJ. Murine pre-embryo oxygen consumption and developmental competence. J Assist Reprod Genet. 2007;24:359–365. doi: 10.1007/s10815-007-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto S, Morimoto N, Yamanaka M, Matsumoto H, Yamochi T, Goto H, Inoue M, Nakaoka Y, Shibahara H, Morimoto Y. Quantitative and qualitative changes of mitochondria in human preimplantation embryos. J Assist Reprod Genet. 2017;34:573–580. doi: 10.1007/s10815-017-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnusson C, Hillensjo T, Hamberger L, Nilsson L. Oxygen consumption by human oocytes and blastocysts grown in vitro. Hum Reprod. 1986;1:183–184. doi: 10.1093/oxfordjournals.humrep.a136377. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka M, Hashimoto S, Amo A, Ito-Sasaki T, Abe H, Morimoto Y. Developmental assessment of human vitrified-warmed blastocysts based on oxygen consumption. Hum Reprod. 2011;26:3366–3371. doi: 10.1093/humrep/der324. [DOI] [PubMed] [Google Scholar]
- 18.Maezawa T, Yamanaka M, Hashimoto S, Amo A, Ohgaki A, Nakaoka Y, Fukuda A, Ikeda T, Inoue M, Morimoto Y. Possible selection of viable human blastocysts after vitrification by monitoring morphological changes. J Assist Reprod Genet. 2014;31:1099–1104. doi: 10.1007/s10815-014-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high potential embryos by culture in poly-(dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–337. doi: 10.1016/j.fertnstert.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Biggers JD, Racowsky C. The development of fertilized human ova to the blastocyst stage in KSOMAA medium: is a two-step protocol necessary? Reprod BioMed Online. 2002;5:133–140. doi: 10.1016/S1472-6483(10)61615-X. [DOI] [PubMed] [Google Scholar]
- 21.Gardner DK, Lane M. Embryo culture systems. In: Trounson AO, Gardner DK, editors. Handbook of in vitro fertilization. 2. Boca Raton: CRC Press; 1999. pp. 205–264. [Google Scholar]
- 22.Hashimoto S, Nakano T, Yamagata K, Inoue M, Morimoto Y, Nakaoka Y. Multinucleation per se is not always sufficient as a marker of abnormality to decide against transferring human embryos. Fertil Steril. 2016;106:133–139. doi: 10.1016/j.fertnstert.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Kuwayama M, Vajta G, Leda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod BioMed Online. 2005;11:608–14. [DOI] [PubMed]
- 24.Shiku H, Shiraishi T, Aoyagi S, Utsumi Y, Matsudaira M, Abe H, Hoshi H, Kasai S, Ohya H, Matsue T. Respiration activity of single bovine embryos entrapped in a cone-shaped microwell monitored by scanning electrochemical microscopy. Anal Chim Acta. 2004;522:51–58. doi: 10.1016/j.aca.2004.06.054. [DOI] [Google Scholar]
- 25.Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod. 2000;15(Suppl 2):148–159. doi: 10.1093/humrep/15.suppl_2.148. [DOI] [PubMed] [Google Scholar]
- 26.Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33:492–504. doi: 10.1002/mrd.1080330417. [DOI] [PubMed] [Google Scholar]
- 27.Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, Díaz-Gimeno P, Valbuena D, Simón C. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104:534–541. doi: 10.1016/j.fertnstert.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, Kokocinski F, Cohen J, Munne S, Wells D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241. doi: 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan Y, Yin X, Zhang S, Jiang H, Tan K, Li J, Xiong B, Gong F, Zhang C, Pan X, Chen F, Chen S, Gong C, Lu C, Luo K, Gu Y, Zhang X, Wang W, Xu X, Vajta G, Bolund L, Yang H, Lu G, Du Y, Lin G. Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience. 2014;3:30. doi: 10.1186/2047-217X-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- 31.Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, Viotti M. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2017;107:34–42. doi: 10.1016/j.fertnstert.2016.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental design (PPTX 50 kb).