Abstract
Purpose
The decision to undergo preimplantation genetic testing (PGT) entails a variety of personal and societal variables. Although PGT technology is widely accepted and used, few studies have queried the motives and concerns of PGT users; moreover, in-depth qualitative data regarding the PGT experience is scant.
Methods
In order to explore and analyze the experience, concerns, expectations, and attitudes toward the PGT technique and its implications, semi-structured interviews were conducted in a single tertiary medical center with 43 Israeli PGT users for HLA matching and autosomal dominant, autosomal recessive, and X-linked genetic disorders.
Results
The primary considerations in choosing PGT were prevention of birth of a child who would suffer a terminal or chronic disease as well as abrogation of a familial genetic condition. Religion played a decisive role in accepting PGT as an antenatal option. Regarding satisfaction with the PGT experience, many interviewees highlighted the need for greater attention to be given to potential stages of failure throughout the procedure and the need for emotional support.
Our clinical results regarding implantation rate and cumulative live birth rate are 38–40% and 27–30%, respectively.
Conclusion
This survey broadens understanding of the specialized needs of women, couples, and minority groups undergoing PGT and underscores the relevance of counseling services for PGT users.
Keywords: Preimplantation genetic testing, PGT users, Reproductive decision-making, Experiences, Qualitative research
Introduction
Recent advances in the field of molecular biology have led to the discovery of the genetic basis for various conditions. These developments, coupled with the rise of preimplantation genetic testing (PGT), first introduced in 1990 [1], have expanded antenatal options for mutation carriers who desire a biological child not affected by the genetic condition they carry. Moreover, since PGT obviates the need for pregnancy termination (TOP), for some sectors, this is the only relevant option for prenatal diagnosis (PND) [2, 3]. However, PGT is not an unencumbered solution. Among the issues are the complexities of the associated in vitro fertilization (IVF) procedure, especially for those with no fertility problems, the risk for misdiagnosis [4], the possible damage to the embryos following invasive manipulations [5], and several ethical concerns [6]. Thus, the decision to engage in PGT is complex and invariably is preceded by an intense decision-making experience.
In the last few years, major terminology changes were done, and the terms PGD and PGS have now been replaced with the umbrella term of “preimplantation genetic testing”, or PGT. PGT now encompasses all types of genetic testing on embryos. To differentiate the specific types of embryo testing, PGT is divided into three subtypes, defined as PGT for aneuploidies (PGT-A), PGT for monogenic/single gene defects (PGT-M), and PGT for chromosomal structural rearrangements (PGT-SR) [7].
Since in Israel, most PGT procedures performed are PGT-M, our research is focused on PGT-M.
In a review of the literature pertaining to PGT decision-making, three core issues were addressed: (1) cognitive appraisals, (2) emotional responses, and (3) moral judgments [8]. To many, PGT offered a sense of control with feelings of hope and relief, while acknowledging negative feelings of uncertainty, complex moral decision-making, stress, and cognitive dissonance [9–11].
Researchers have explored attitudes regarding theoretical PGT use in various countries and among various religious and ethnic groups [12–16].
Nonetheless, only scant empirical data have been collected that focus on the practical aspects of PGT use from people who have actually undergone PGT [17–23]. These earlier studies described emotional strain during the PGT journey [18, 20, 22], employing the term “emotional labor” to describe the PGT trajectory [19]. However, most of these studies were conducted more than a decade ago, when the technology of PGT was less widely acceptable [18] and the financial burden a major issue [18–20]. Moreover, half of these studies used self-administered questionnaires [14, 17, 18], and only a few used in-depth interviews with nine or ten PGT users [20, 22, 23] or at the most 18–21 PGT users [19, 21].
In Israel, the PGT procedure is well accepted by the medical community and therefore available in eight of the 26 national IVF clinics [24]. Since 2009, PGT is covered by national health insurance for at-risk couples with no limit on the number of IVF cycles required to give birth to two healthy children via PGT [25], and it is provided regardless of prior history of unassisted child birth(s). The widespread acceptance of PGT in Israel can be explained by epidemiological, social, and religious factors. Importantly, from an epidemiological standpoint, Israel has a high incidence of recessive genetic disease-associated mutation carriage among both Jewish and Arab populations because of founder effects and consanguinity [26]. As a result, large-scale (premarital and prenatal) screening tests are readily available and used by a large part of the population [27]. From a social perspective, in modern Israeli society, new reproductive technologies are widely accepted [28, 29], and prenatal genetic testing is quite popular [30]. Finally, PGT is often a preferable option for religious Jews [2, 31] and Muslims [3, 32] because it eliminates the possibility of TOP.
People at risk of transmitting genetic conditions to their offspring, who face dilemmas concerning their reproductive options based on the results of genetic testing, are introduced to PGT procedure early in their genetic counseling sessions. However, to date, less than optimal attention has been paid to the genetic counseling given to PGT candidates. Prior research on the needs and preferences of counselees regarding the process of genetic counseling, in general, has focused on obtaining information regarding the specific risks and options of each PGT couple and, to a lesser degree, general information pertaining to genetics; yet it has been noted that more sensitive communication during counseling was considered very important by a large percentage of counselees [33]. Moreover, prenatal genetic counseling, in several settings, was found to evoke anxiety and lead to misinterpretation of risk in some cases [34], whereas in other studies, prenatal counseling was seen as empowering counselees’ competence and their ability for self-determination [35].
Some studies underscored that, for many, the couple’s expectation to successfully achieve pregnancy was beyond that indicated by clinicians as realistic for PGT procedures [9]. Studies have emphasized the importance of proper communication by the medical team in order to establish realistic expectations from the procedure with its many uncertainties and thus create an increased sense of trust and respect for the medical authority [18, 19].
The purpose of this study, therefore, is to broaden our understanding of the decision-making processes of PGT users using a qualitative, in-depth survey of physical and emotional experiences in a relatively large cohort of couples undergoing PGT in a setting where the procedure is both socially accepted and covered financially by national health insurance.
Methods
Participants
The study population included women and men who received counseling regarding PGT at the PGT unit, Shaare Zedek Medical Center, Jerusalem, Israel. Other inclusion criteria for the study were age of at least 18 years and full comprehension of Hebrew, Arabic, or English. Excluded from the study were those who performed PGT for non-medical reason (e.g., social sex selection). In order to achieve adequate representation of population subgroups in the study, we used the purposive sampling technique [36] to ensure study participants representing the following broad set of sectors in the Israeli population: Jewish and Muslim; secular, religious, and ultra-orthodox; high school graduate and university graduate/post graduate; geographical residence in central urban locations and periphery non-urban locations; genetic disease carriers with disease symptoms (dominant trait) and disease carriers without disease symptoms (recessive and X-linked trait) including parents of children requiring human leukocyte antigen (HLA) matching.
Eligible subjects were first solicited by telephone to participate in the study. Persons who agreed to participate signed an informed consent form and scheduled an interview with the study coordinator, in the participants’ home or other convenient venue.
Instrumentation and procedure
Semi-structured, in-depth, face-to face interviews were carried out after receiving institutional review board (IRB) approval.
Male and female partners were not interviewed together during the same session; rather, each study participant was interviewed individually so as to protect the interviewee’s privacy, avoid influence of one dominant spouse, and allow for open discussion of sensitive issues.
The interviews lasted 45 min, on average, and were composed of three parts: (1) sociodemographic data such as genetic status of family members, followed by reproductive history including the usage of prenatal diagnosis in the past; (2) open-ended questions to explore the interviewee’s knowledge of different aspects of the PGT process (the risks involved, potential benefits, the specific disease condition for which PGT was being conducted, and knowledge of other prenatal diagnostic options); and (3) the decision-making process leading to the selection of PGT as the preferred PND method (i.e., the type of experts whose counsel was sought, the dynamics between the couple and other family members, and societal factors which may have directly/indirectly influenced important decisions). Attitudes regarding ethical issues were also explored in the final section of the interview.
Data preparation and analysis
Almost all interviews were audio-taped and later transcribed verbatim. Two participants refused audio-taping, and in those cases notes were taken by the interviewer.
Analysis was informed by the grounded theory approach [37, 38]. In this inductive approach, theory and conclusions emerged from the data, rather than from previously identified theoretical hypotheses. These methodologies were chosen to fill in existing gaps in our knowledge of PGT, specifically, from the point of view of PGT users while also contributing toward an in-depth understanding of the PGT process in certain cultural and ethnic contexts which have not yet been extensively explored in the literature. Final interviews did not reveal new major themes, and thus, saturation of themes [37] was applied. The quoted portions of the interview represent typical responses.
Results
Participant information
The PGT Unit database includes only the contact information of the female partner; therefore, male partners were enrolled into the study strictly by way of their respective female partners.
All participants received genetic counseling(s) regarding their genetic condition prior to the counseling in the PGT unit, so the counseling before the PGT process did not focus on description of the genetic condition under consideration and included mainly discussion regarding options for PND, information regarding IVF, ICSI, embryo biopsy, rates of pregnancy, risk of misdiagnosis, and recommendation for confirmatory PGT result testing via amniocentesis. The PGT counseling is given by one of two practitioners in the unit: a medical geneticist (head of the PGT unit) or trained genetic counselor.
Of the 34 invited couples, 27 (79%) agreed to participate. Those who did not participate provided the following reasons: the male partner or their religious authority (for example, a local rabbi) did not agree to participation, the interviewees lacked time for interview, the topic was too difficult to discuss, or the potential interviewee did not feel that their personal experiences would add new insight to the study.
The respondents who consented to participate are believed to represent the entire spectrum of PGT users in our PGT unit. Moreover, many of the features of the study cohort were similar to those who refused to participate in terms of age, ethnicity, geographical location, and referral reason for PGT testing.
Among the 27 couples who agreed to participate in the study, all females and 17 of their male partners were interviewed individually. However, one female’s interview was ineligible for analysis since it did not fully address the goals of this study (and therefore, it served as a pre-test). Thus, our final sample consisted of 43 participants: 26 females (60%) and 17 males (40%). The participants’ characteristics are presented in Table 1.
Table 1.
Characteristics of participants at the time of the interview
| Characteristics of participants |
Participants n = 43 n (%) |
|---|---|
| Sex | |
| Female | 26 (60) |
| Male | 17 (40) |
| Mean age (years) at time of the interview | |
| Female |
33.6; SD = 5.0; (range 21–44) |
| Male |
36.6; SD = 5.1; (range 29–50) |
| Education level | |
| High School | 12 (28) |
| College/university graduate | 22 (51) |
| College/university post graduate | 9 (21) |
| Religion | |
| Jewish | 37 (86) |
| Muslim | 4 (9) |
| Christian | 2 (5) |
| Religiosity (Jewish only n = 37) | |
| Secular | 15 (41) |
| Religious/Orthodox | 15 (41) |
| Ultra-Orthodox | 7 (19) |
SD standard deviation
Participants’ genetic and reproductive history
Seventeen interviewees (40%) were carriers of autosomal recessive diseases; fifteen interviewees (35%) had an autosomal dominant condition or were married to someone with a dominant condition; eight interviewees (19%) were carriers of X-linked disorders or were married to a carrier; and 3 (7%) interviewees were each parents of a child who needed HLA matching. Description of the genetic disorders for which participating couples performed PGT is detailed in Table 2.
Table 2.
Referrals for PGT by inheritance patterns
| Characteristics of referrals for PGT |
Couples n = 26 |
|---|---|
| Autosomal Dominant Conditions | |
| Myotonic dystrophy | 3 |
| Neuro-fibromatosis type 1 (NF1) | 2a |
| Achondroplasia | 1 |
| Autosomal dominant polycystic kidney (ADPKD) | 1 |
| Hereditary breast and ovarian cancer (HBOC, BRCA1) | 1 |
| Multiple endocrine neoplasia type 2 (MEN2) | 1 |
| Pseudohypoparathyroidism | 1 |
| Autosomal recessive conditions | |
| Non syndromic deafness (Connexin 26(GJB2)) | 3 |
| Cystic fibrosis | 2 |
| Familial dysautonomia | 1 |
| Leukodystrophy | 1 |
| Severe combined immunodeficiency (SCID) | 1 |
| Krabe + Pompe | 1b |
| Tay Sachs + Gaucher | 1b |
| X-linked conditions | |
| Fragile X | 2 |
| Incontinentia pigmenti | 1 |
| X-linked ichthyosis | 1 |
| HLA matching (Human leucocyte antigen) | 2 |
aIn one couple, both partners were diagnosed with Neuro-fibromatosis type 1bBoth partners were double heterozygotes
Overall, 20 (76%) couples gave birth to at least one child through PGT before their interviews. Almost all couples had complex medical and reproductive histories including at least one live or dead child who was diagnosed with a genetic condition, TOP(s) of genetically affected embryos, and/or multiple PGT cycles. The reproductive history of each couple is detailed in Table 3.
Table 3.
Reproductive history of couples at the time of the interview
| Reproductive history at the time of the interview |
Couples n = 26 n (%) |
|---|---|
| Fertility problems | |
| No | 18(68) |
| Yes | 8(32) |
| No. of children | |
| 0 | 4(15) |
| 1 | 8(31) |
| 2 | 3(12) |
| 3 | 8(31) |
| 4 | 2(8) |
| 9 | 1(4) |
| Affected child/deceased child (from the genetic condition) | |
| 0 | 16(62) |
| Affected | 8(31) |
| Deceased | 2a(8) |
| No. of TOP of affected embryo (from the genetic condition) | |
| 0 | 22(85) |
| 1 | 1(4) |
| 2 | 2(8) |
| 3 | 1(4) |
| No. of PGT cycles | |
| 0 | 2(8) |
| 1 | 13(50) |
| 2 | 2(8) |
| 3 | 2(8) |
| 4 | 3(12) |
| 5 | 1(4) |
| 7 | 2(8) |
| 12 | 1(4) |
| No. of children born after PGT | |
| 0 | 6(23) |
| 1 | 14(53) |
| 2 | 5b(19) |
| 3 | 1(4) |
TOP termination of pregnancyaOne of the couples had two children died from SCIDbAll couples had twins
The PGT procedure—clinical information
In most cases (90%), a single blastomere biopsy was performed on day three (at the 6–8 cell stage) and fresh transfer on day four when applicable. Only a small fraction (10–15%) of our embryos were grown to the blastocyst stage (day 5 or day 6). Frozen embryo transfer was performed in approximately 10% of cycles. The implantation rate was 38–40%, and the cumulative live birth rate was 27–30%.
Qualitative findings
The key themes are provided in Table 4.
Table 4.
Key themes
|
1) Primary motivation for performing PGT a) Prevention of genetic disorder in future offspring (1) Having control over reproductive reality (2) Prevention of suffering from future child b) PGT as the responsible step—parental sacrifice (1) Lack of support from society and state for disabled child c) Religious orientation | |
|
2) Perceived experience of PGT a) Positive (1) PGT as a source of control over genetic history (2) IVF stages allow awareness for early developmental stages of the fetus b) Negative (1) Emotional distress when facing failures (2) Lack of accurate information regarding IVF and PGT (3) Need for emotional support |
Considerations and motives in performing PGT
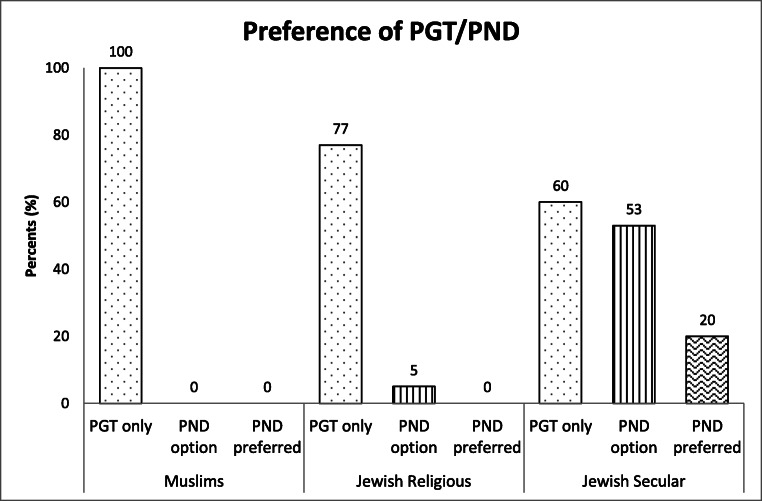
Most of the participants regarded PGT as their only possible reproductive choice. They did not report indecision regarding their choice of PGT; less than fourth of the participants mentioned PND (chorionic villus sampling (CVS) or amniocentesis) as an alternative for preventing their particular genetic condition. Three participants preferred the option of spontaneous pregnancy followed by PND in lieu of PGT. Major variations of preferences between different population groups were noticed, mainly according to religion and religious tendency (Fig. 1). One couple decided, after the PGT counseling, to remain child free, but for all other participants this option and other reproductive solutions such as adoption or use of donor gametes were not considered.
Fig. 1.
Preference of PGT/PND according to religion and religious tendency
The primary motivation for performing PGT was prevention of their genetic disorder in future offspring. Therefore, the decision to undergo PGT was perceived by the couples as the means to take control over the genetic status of their child as well as taking control over their reproductive reality:
“…at the end of the day, IVF is about us, and it’s about our choice and its under our control.. It’s my right to decide when to use it based on convenience but…I am not sure I really have this right, even if it’s for the benefit of my own child…. It’s a very very thin line between [my own autonomic decision and that of society] because I think that parents, or couples trying to get pregnant, see themselves in the moment wishing for a baby ... They see the here and now [of society] but our own personal needs as a couple and as individual human beings at our age may be different…so you have to look beyond it…” (30-year-old woman, carrier of a GJB2 mutation, causing non syndromic deafness).
The description/understanding of a life-lived “suffering” from a genetic disorder was variable. For some, suffering meant life with a handicap which leads to mockery at the hands of other children. Others described suffering as knowing throughout one’s life that he/she is at risk of a handicap, including of a late-onset disorder; and still, others considered a fertility problem in their future child as a problem that should be avoided.
Ten interviewees described engaging in PGT as parental sacrifice made by them to protect the future of their child:
“…it is not an easy thing to do but you don’t think about yourself, you think about the child and what he will experience [if] he is born as an abnormal child...” (40-year-old woman with Myotonic dystrophy).
Interviewees described their fertility history as painful and challenging in the context of TOP and/or IVF and PGT. These difficult emotional and physical events were considered worthwhile if they would result in the birth of healthy children who would not need to go through the same suffering experiences they had endured:
“I would not want to see my daughter performing fertility treatments because of a problem I could prevent. You would do anything for your child, you would give your right arm, and so going through fertility treatments is less than your right arm, with all due respect…. We want the best for our child. We don’t want him to suffer when he doesn’t have to. This is, in my point of view, the essence of parenthood” (36-year-old woman, carrier of Fragile X syndrome).
Others also considered the decision to perform PGT not as a choice but as a responsibility that they have undertaken for their yet unborn child:
“I think that this is what life is all about…. For your own child you wouldn’t save any financial or physical effort…From my point of view as a husband and as a man…there is no doubt that you have to [perform PGT for the sake of the child]” (30-year-old man, his spouse is a carrier of BRCA1 gene mutation).
Among participants and their partners, who were carriers of AD and XLI conditions, a prominent consideration was application of PGT as a preventative measure that would stop the transmission of genetic disease to their children and subsequent generations. Not only did these interviewees feel obligated to protect their children from a genetic condition or disease risk but they also were concerned with their children’s family planning. Given that a disease-causing allele existed in their family, one of the main goals of PGT would be to prevent transmission of that allele:
“… [My husband] has a mother who died [from the condition] and a sister who is now dead but needed a kidney transplant. It is obvious to him that he does not want to continue this chain” (38-year-old woman, her spouse is diagnosed with Autosomal dominant polycystic kidney).
Several participants explained the motive to use PGT by imagining the difficulties in the life of a child with an abnormality; these were mostly carriers of autosomal dominant conditions, a distinct group severely scarred by their genetic conditions who were strongly motivated to do whatever they could to control the risk and prevent the same experience in their offspring:
“I don’t want to have a child with NF because it is something you can easily identify on someone else. Why would I have a child with a blemish that he will carry on himself to school?” (31-year-old woman with Neurofibromatosis type 1 syndrome)
Several participants emphasized the role of society in making life harder for children with an abnormality.
“A child with impairment will not have a normal social life…He will be different and to be a different child is very very very complex… I don’t feel this now because I am living among grown-ups but if I was a child with these difficulties, I would definitely be laughed at. Definitely!” (34-year-old woman with Myotonic dystrophy)
Lack of support from the state establishment was also mentioned:
“…If, God forbid, something will happen to me or to my husband, a child with a genetic problem will be a burden on the state and the state will not take care of him as well as I would want …so if you can prevent it from happening altogether, it’s better” (35-year-old woman, carrier of Fragile X syndrome).
Religious orientation served as a powerful motive for the use of PGT. Among the participants declaring that they were religious (Orthodox or ultra-Orthodox), 17 interviewees reported PGT as the only option of PND for their genetic condition. They further explained that they would not choose the PND option because TOP in the case of an affected fetus would not be applicable for them:
“According to HALACHA [Jewish religious instruction] after the 10th week [of pregnancy] there is no such thing as pregnancy termination. There is no way [that I would do TOP]. Never” (35-year-old female carrier of Familial dysautonomia).
Perceived experience of PGT
Sixty-nine percent of the participants in this study were fertile and used IVF solely for the purpose of PGT. The fact of enduring IVF and its procedures to avoid passing on the genetic condition seemed like a comforting issue for them and gave them a sense of control, which was missing in their perceived personal genetic history:
“…If there is no fertility problem so there is no problem at all…I am OK with it. She [my wife] is OK with it, so the process should not bother us at all. It is only IVF… but if we were to have fertility problems then that means that I am not OK, [my wife] is not OK and the physiological situation is wrong and the psychological situation is wrong and everything is influenced negatively… It is like you are damaged goods. [On the other hand,] in our situation we have control over the IVF. It is unlike IVF for fertility issues that would require me to pray every day for a miracle…” (36-year-old man, spouse of a woman carrier of a Multiple endocrine neoplasia type 2 gene mutation).
Some women described the IVF procedure as a positive and unique experience that they could not have achieved with spontaneous pregnancy:
“I would not say that I was privileged but [the IVF staff] showed us the embryo right from the start, it was exciting. Who has this privilege? At least I comfort myself in these very special things; we also saw our eggs growing in the tube. It’s interesting” (30-year-old woman with Neurofibromatosis type 1, married to a man with Neurofibromatosis type 1).
Participants report tremendous emotional distress when things go wrong during the treatment cycles:
“We started the treatment and they collected seven eggs from me and nothing came out of it… Wow …that was difficult... very difficult. After all the anticipation and time invested, [and nothing to show for it]? And after all the pain that I went through, I cried and I cried…there was not one [healthy embryo]! And the ones that were normal didn’t develop [in culture]… I cried and cried and cried and cried” (43-year-old woman with Achondroplasia).
Several participants stressed that the deep disappointment they felt after an IVF cycle with no embryos to return was due to not being ready for failure at that particular stage of the procedure. Lack of accurate information regarding possible consequences of IVF and PGT testing in advance was mentioned as a major source of stress and led to a perceived feeling of lost control (which they had felt previously regarding IVF) and to mistrust of the medical team:
“The hardest thing for me was the cycle in which there was no [embryo] to transfer, because you take into account that a transferred embryo might not implant, but you can’t imagine a cycle would go by without a single embryo to transfer. I think it is something worth mentioning to people [in advance of the PGT process] that it can happen because we didn’t have it in mind at all. .. …that was my hardest ordeal in the process” (31-year-old woman carrier of Cystic fibrosis).
While some PGT users do not share their PGT experience with family and friends, others were much more communicative about it. Several subjects felt lonely in the experience and looked for emotional and practical support:
“I never met people who went through PGT. I think it is very very important to create some network of all PGT parents, from the first one until today, whoever is interested, and to keep us posted…” (39-year-old woman carrier of X-linked Ichthiosis).
Some interviewees stated that they would have liked to seek advice from care professionals such as a social worker or psychologist within the context of the IVF Unit. Others suggested that a woman performing PGT should have another woman with PGT experience involved in the process as a coach to help her understand how things work. Still other women viewed the internet as the most appropriate medium for support.
Ethical considerations regarding PGT
A wide range of ethical issues was discussed during the interviews. The acceptable applications of PGT have been reported elsewhere by us [39].
Most interviewees did not report difficulties with moral questions regarding the different aspects of PGT for themselves, the embryo, and society at large: ethical considerations did not influence their decision-making at all. These interviewees did not consider the selection of embryos by PGT as an ethical problem and when asked about this notion they explained their moral approach toward the procedure with arguments to justify their beliefs:
“I don’t see PGT as a problematic intervention. You are only taking some subset of possibilities which are derived from a particular couple’s embryos….[However, if you], add some external factor or try to manipulate [the embryos] in order to create …the best of everything, then it starts to become problematic [ethically], I think” (36-year-old man, carrier of Cystic fibrosis).
Disposal of embryos was also not seen as morally problematic: the early developmental stage in which the PGT selection takes place was the main justification for this attitude:
“It’s not that we are sorting healthy, happy, chubby fetuses with toes and fingers and we tell them ‘OK, you are not fine’. It’s not like stories from China, where one gives’ birth to a baby girl and then throws her in the garbage somewhere. … I think that there is no question of life when it’s at the eight cell stage” (30-year-old woman, carrier of a GJB2 mutation causing non syndromic deafness).
Most participants do not report any concerns regarding frozen embryos left over from the IVF process. Only two participants described strong emotions of care and devotion toward the embryos, one of these was Jewish and the other was Christian:
“…There are a few (frozen embryos) left and this scares me a lot...I feel like I left something behind ….For me it’s like they are children …” (32-year-old woman, carrier of severe combined immune deficiency).
Discussion
This study presents, for the first time to our knowledge, an in-depth qualitative analysis of a large sample of PGT users regarding their decision-making considerations and their experiences during the PGT process using an interview platform. The primary motivators to undergo PGT firstly devolved upon the clinical and psychological impact of the disease on a child affected by a disease with which most of the interviewees already had some experience, and secondly, to abrogate transmission of disease alleles to another generation. Devotion to religious doctrines proscribing termination of pregnancies plays a crucial role in accepting PGT as a feasible alternative. Despite genetic counseling that also outlines realistic expectations of a successful pregnancy with PGT, many interviewees noted the emotional/psychological distress when their personal expectations were not realized. Various modes of coping, support, and sharing of experiences were reported that helped mitigate the stress and disappointment. Generally, ethical issues were of less concern among these interviewees.
Previous studies with potential and actual PGT users found high acceptance levels of the PGT procedure with the primary reason for undergoing PGT being avoidance of suffering in future child(ren) [8, 11, 18, 19]. The results obtained in this study confirm these previous findings. Nevertheless, our participants also expressed their deep concern for family planning issues of the next generation and the desire to eliminate disease alleles from their lineage. Unexpectedly, among these interviewees, the difficulties of being a parent to a sick child were not raised. Hypothetically, one might ascribe this to a moral standard that lauds parents who sacrifice for their child rather than parents who think more selfishly. This notion is reinforced by the narrative of “parental sacrifice” which emerged from several descriptions in our interviews regarding the reproductive journey and PGT in particular.
Religiosity of PGT candidates has been mentioned in the literature as a factor that might reduce interest in PGT because it raises concerns regarding embryo status [10]. The present survey points to an apposed conclusion, as participants who were religious considered PGT to be the only possible option for a biological family. Prior research on this subject involved mostly Catholic and Christian subjects [18, 20] unlike the mostly Jewish and Muslim participants in the current study. Taken together, this may explain the discrepancy between the results of this study relative to previous studies given that Jewish and Muslim religious laws are considered to be more permissive for PGT intervention [3, 31] than Catholic/Christian scriptures [40]. As mentioned among our cohort, only one Jewish and one Christian interviewees were concerned with the fate of the unused frozen embryos.
The immense stress and disappointment many participants felt because of failure of conception at some stage of the PGT process has been reported before and has been attributed to the exaggerated hope of conception in otherwise fertile women [10]. Another reason for the high degree of stress and disappointment might be that PGT users assume that they are in control of the process. Hence, when things do not work out as planned, distress levels are high because a failed plan may be misconstrued as loss of control over family planning in general.
As opposed to other studies in which the moral/ethical aspects along the entire PGT journey were at the fore (e.g., interfering with a natural process; selecting embryos; destroying affected embryos; disposal of frozen embryos) [19, 41], most of our participants did appear not to wrestle with these issues: possibly the positive emphasis on families in the Israeli cultural context, for all ethnicities, offsets the dilemma and/or it has been dealt with by prioritizing having a biological child. While public debate regarding ethical considerations in assisted reproductive techniques (ART) in general and embryos’ status in particular hardly exist in Israel [28, 42], an intensive pro-reproductive cultural agenda [43] alongside the “quest for the perfect baby/babies” [44] is ascendant.
To our knowledge, this is the first study presenting a qualitative analysis of interviews of a large sample of PGT users in a permissive medical, cultural, religious setting that facilitates the use of PGT for those interested in it. One of the main strengths of our study was the diversity of the cohort which was composed of virtually all sectors of the Israeli population with diverse genetic conditions and at various points in the PGT journey. Moreover, this survey is the first of large cohorts of both Jewish and Muslim PGT users who are apparently avidly committed to PGT because of inherited genetic diseases [31] but whose reproductive motives for PGT and personal experiences have not been characterized.
Our clinical results regarding implantation rate and cumulative live birth rate (38–40% and 27–30%, respectively) are similar with ESHRE data collection [45].
Study limitations
The limitations of this study include a possible recall bias of motives and perceived experience due to different stages of participants in the PGT trajectory and a potential biased ascertainment toward those who coped best with the PGT processes and therefore agreed to be interviewed.
Since qualitative studies are inherently smaller in size, we felt that we needed a larger sample which would enable more expanded analysis options and thus more comprehensive understanding of this topic. In order to achieve these goals, we developed a detailed questionnaire, based on the themes that emerged from the interviews. The questionnaires, their analysis, and results are presented elsewhere (Zuckerman et al. under review). Finally, the Israeli context of our subjects, in which PGT is largely accepted, with minimal ethical considerations and covered by health insurance may not represent the attitudes of PGT candidates from other countries, which have different regulations, health system, and moral stance toward PGT.
Conclusion
Since there is growing demand for PGT worldwide, the group of PGT users will inevitably increase. Therefore, it is important to address imperfect features of the PGT experience where possible, to strengthen positive experiences while modifying problematic features which serve as sources of distress.
Among members in the clinical PGT setting, attention should be given to feelings of disappointment and consequent frustration during common “bumps in the road”. Fully informed genetic counseling, multidisciplinary professional support, and improved communication between PGT users themselves and medical staff are required to improve the PGT experience. Further research regarding PGT experiences in different settings both in PGT centers across Israel and elsewhere is needed as well.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Handyside AH, Kontogianni EH, Hardy K, Winston RML. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344(6268):768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 2.Grazi RV, Wolowelsky JB. Cultural concerns when counseling orthodox Jewish couples for genetic screening and PGD. J Genet Couns. 2015;24(6):878–881. doi: 10.1007/s10897-015-9860-6. [DOI] [PubMed] [Google Scholar]
- 3.Chamsi-Pasha H, Albar MA. Assisted reproductive technology: Islamic Sunni perspective. Hum Fertil (Camb) 2015;18(2):107–112. doi: 10.3109/14647273.2014.997810. [DOI] [PubMed] [Google Scholar]
- 4.Dreesen J, Destouni A, Kourlaba G, Degn B, Mette WC, Carvalho F, Moutou C, Sengupta S, Dhanjal S, Renwick P, Davies S, Kanavakis E, Harton G, Traeger-Synodinos J. Evaluation of PCR-based preimplantation genetic diagnosis applied to monogenic diseases: a collaborative ESHRE PGD consortium study. Eur J Hum Genet. 2014;22(8):1012–8. doi: 10.1038/ejhg.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Z, Wang Y, Lin J, Xu J, Ding G, Huang H. Genetic and epigenetic risks of assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 2017;44:90–104. doi: 10.1016/j.bpobgyn.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Harper J, Geraedts J, Borry P, Cornel MC, Dondorp WJ, Gianaroli L, Harton G, Milachich T, Kaariainen H, Liebaers I, Morris M, Sequeiros J, Sermon K, Shenfield F, Skirton H, Soini S, Spits C, Veiga A, Vermeesch JR, Viville S, de Wert G, Macek M, on behalf of ESHG, ESHRE and EuroGentest2 Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. Hum Reprod. 2014;29(8):1603–1609. doi: 10.1093/humrep/deu130. [DOI] [PubMed] [Google Scholar]
- 7.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershberger PE, Pierce PF. Conceptualizing couples’ decision making in PGD: emerging cognitive, emotional, and moral dimensions. Patient Educ Couns. 2010;81(1):53–62. doi: 10.1016/j.pec.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karatas JC, Strong KA, Barlow-Stewart K, McMahon C, Meiser B, Roberts C. Psychological impact of preimplantation genetic diagnosis: a review of the literature. Reprod BioMed Online. 2010;20(1):83–91. doi: 10.1016/j.rbmo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham J, Goldsmith L, Skirton H. The evidence base regarding the experiences of and attitudes to preimplantation genetic diagnosis in prospective parents. Midwifery. 2015;31(2):288–296. doi: 10.1016/j.midw.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Genoff Garzon MC, Rubin LR, Lobel M, Stelling J, Pastore LM. Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin Genet. 2018;94(1):22–42. doi: 10.1111/cge.13174. [DOI] [PubMed] [Google Scholar]
- 12.Winkelman WD, Missmer SA, Myers D, Ginsburg ES. Public perspectives on the use of preimplantation genetic diagnosis. J Assist Reprod Genet. 2015;32(5):665–675. doi: 10.1007/s10815-015-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olesen AP, Nor SN, Amin L. Attitudes toward pre-implantation genetic diagnosis (PGD) for genetic disorders among potential users in Malaysia. Sci Eng Ethics. 2016;22(1):133–146. doi: 10.1007/s11948-015-9639-z. [DOI] [PubMed] [Google Scholar]
- 14.Zierhut H, MacMillan ML, Wagner JE, Bartels DM. More than 10 years after the first ‘savior siblings’: parental experiences surrounding preimplantation genetic diagnosis. J Genet Couns. 2013;22(5):594–602. doi: 10.1007/s10897-013-9591-5. [DOI] [PubMed] [Google Scholar]
- 15.Chan JL, Johnson LNC, Sammel MD, DiGiovanni L, Voong C, Domchek SM, Gracia CR. Reproductive decision-making in women with BRCA1/2 mutations. J Genet Couns. 2017;26(3):594–603. doi: 10.1007/s10897-016-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallowell N, Badger S, Richardson S, Caldas C, Hardwick RH, Fitzgerald RC, Lawton J. High-risk individuals’ perceptions of reproductive genetic testing for CDH1 mutations. Familial Cancer. 2017;16(4):531–535. doi: 10.1007/s10689-017-9976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz MG, Fitzgerald L, Bankier A, Savulescu J, Cram DS. Issues and concerns of couples presenting for preimplantation genetic diagnosis (PGD) Prenat Diagn. 2002;22(12):1117–1122. doi: 10.1002/pd.498. [DOI] [PubMed] [Google Scholar]
- 18.Lavery SA, et al. Preimplantation genetic diagnosis: patients’ experiences and attitudes. Hum Reprod. 2002;17(9):2464–2467. doi: 10.1093/humrep/17.9.2464. [DOI] [PubMed] [Google Scholar]
- 19.Roberts C, Franklin S. Experiencing new forms of genetic choice: findings from an ethnographic study of preimplantation genetic diagnosis. Hum Fertil (Camb) 2004;7(4):285–293. doi: 10.1080/14647270400016449. [DOI] [PubMed] [Google Scholar]
- 20.Kalfoglou AL, Scott J, Hudson K. PGD patients’ and providers’ attitudes to the use and regulation of preimplantation genetic diagnosis. Reprod BioMed Online. 2005;11(4):486–496. doi: 10.1016/s1472-6483(10)61145-5. [DOI] [PubMed] [Google Scholar]
- 21.Dagan E, Birenbaum-Carmeli D, Friedman E, Feldman B. Performing and declining PGD: accounts of Jewish Israeli women who carry a BRCA1/2 mutation or partners of male mutation carriers. J Genet Couns. 2017;26(5):1070–1079. doi: 10.1007/s10897-017-0087-6. [DOI] [PubMed] [Google Scholar]
- 22.Haude K, McCarthy Veach P, LeRoy B, Zierhut H. Factors influencing the decision-making process and long-term interpersonal outcomes for parents who undergo preimplantation genetic diagnosis for Fanconi anemia: a qualitative investigation. J Genet Couns. 2017;26(3):640–655. doi: 10.1007/s10897-016-0032-0. [DOI] [PubMed] [Google Scholar]
- 23.Klitzman R. Challenges, dilemmas and factors involved in PGD decision-making: providers’ and patients’ views. Experiences and Decisions J Genet Couns. 2018;27(4):909–19. [DOI] [PubMed]
- 24.https://www.health.gov.il/Subjects/Med_Inst/IVF/Pages/IVF-list.aspx. Israeli Ministry of Health 2020.
- 25.http://call.health.gov.il/infocenter/index?page=content&id=EL7162. Israeli Ministry of Health. 2019.
- 26.Zlotogora J, Patrinos GP. The Israeli National Genetic database: a 10-year experience. Hum Genomics. 2017;11(1):5. doi: 10.1186/s40246-017-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlotogora J, Grotto I, Kaliner E, Gamzu R. The Israeli national population program of genetic carrier screening for reproductive purposes. Genet Med. 2016;18(2):203–206. doi: 10.1038/gim.2015.55. [DOI] [PubMed] [Google Scholar]
- 28.Shalev, C. and S. Gooldin, The uses and misuses of in vitro fertilization in Israel: some sociological and ethical considerations. Nashim: A Journal of Jewish Women's Studies & Gender Issues, 2006: p. 151–176.
- 29.Birenbaum-Carmeli D. Thirty-five years of assisted reproductive technologies in Israel. Reprod Biomed Soc Online. 2016;2:16–23. doi: 10.1016/j.rbms.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakst S, Romano-Zelekha O, Ostrovsky J, Shohat T. Determinants associated with making prenatal screening decisions in a national study. J Obstet Gynaecol. 2019;39(1):41–48. doi: 10.1080/01443615.2018.1463977. [DOI] [PubMed] [Google Scholar]
- 31.David BE, Weitzman GA, Hervé C, Fellous M. Genetic counseling for the orthodox Jewish couple undergoing preimplantation genetic diagnosis. J Genet Couns. 2012;21(5):625–630. doi: 10.1007/s10897-012-9502-1. [DOI] [PubMed] [Google Scholar]
- 32.Serour GI. Islamic perspectives in human reproduction. Reprod BioMed Online. 2008;17(Suppl 3):34–38. doi: 10.1016/s1472-6483(10)60328-8. [DOI] [PubMed] [Google Scholar]
- 33.Salemink S, Dekker N, Kets CM, van der Looij E, van Zelst-Stams WAG, Hoogerbrugge N. Focusing on patient needs and preferences may improve genetic counseling for colorectal cancer. J Genet Couns. 2013;22(1):118–124. doi: 10.1007/s10897-012-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tercyak KP, Johnson SB, Roberts SF, Cruz AC. Psychological response to prenatal genetic counseling and amniocentesis. Patient Educ Couns. 2001;43(1):73–84. doi: 10.1016/s0738-3991(00)00146-4. [DOI] [PubMed] [Google Scholar]
- 35.Lewis, C., H. Skirton, and R. Jones, Reproductive empowerment: the main motivator and outcome of carrier testing. J Health Psychol, 2011: p. 1359105311417193. [DOI] [PubMed]
- 36.Teddlie C, Yu F. Mixed methods sampling a typology with examples. J Mixed Methods Res. 2007;1(1):77–100. [Google Scholar]
- 37.Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis (introducing qualitative methods series) London: SAGE Publications; 2006. [Google Scholar]
- 38.Shkedi A. Words of meaning: qualitative research-theory and practice (Hebrew) Tel-Aviv: Tel-Aviv university Ramot; 2003. [Google Scholar]
- 39.Zuckerman S, Zeevi DA, Gooldin S, Altarescu G. Acceptable applications of preimplantation genetic diagnosis (PGD) among Israeli PGD users. Eur J Hum Genet. 2017;25(10):1113–1117. doi: 10.1038/ejhg.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benagiano G, Carrara S, Filippi V. Robert G Edwards and the Roman Catholic Church. Reprod BioMed Online. 2011;22(7):665–672. doi: 10.1016/j.rbmo.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Derks-Smeets IA, et al. Decision-making on preimplantation genetic diagnosis and prenatal diagnosis: a challenge for couples with hereditary breast and ovarian cancer. Hum Reprod. 2014;29(5):1103–1112. doi: 10.1093/humrep/deu034. [DOI] [PubMed] [Google Scholar]
- 42.Hashiloni-Dolev Y. Between mothers, fetuses and society: reproductive genetics in the Israeli-Jewish context. Nashim: A Journal of Jewish Women's Studies & Gender Issues. 2006;12(1):129–150. [Google Scholar]
- 43.Gooldin S. ‘Emotional rights’, moral reasoning, and Jewish-Arab alliances in the regulation of in-vitro-fertilization in Israel: theorizing the unexpected consequences of assisted reproductive technologies. Soc Sci Med. 2013;83:90–98. doi: 10.1016/j.socscimed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Remennick L. The quest for the perfect baby: why do Israeli women seek prenatal genetic testing? Sociology of health & illness. 2006;28(1):21–53. doi: 10.1111/j.1467-9566.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 45.Harper J, et al. The ESHRE PGD consortium: 10 years of data collection. Hum Reprod Update. 2012;18(3):234–247. doi: 10.1093/humupd/dmr052. [DOI] [PubMed] [Google Scholar]