Abstract
Purpose
To evaluate the association of objectively measured physical activity (PA) and sedentary behaviour before and during in vitro fertilization (IVF) with controlled ovarian stimulation (COS) and pregnancy outcomes.
Methods
This longitudinal study involved 107 infertile women undergoing IVF treatment. PA and sedentary behaviour were measured for 14 consecutive days using accelerometry as follows: (1) before IVF treatment, (2) during IVF at the implantation time, immediately after embryo transfer, and (3) after positive pregnancy test. Total screen time was assessed by questionnaires. COS results were measured as the number of oocytes and embryos obtained, and the study outcomes included positive hCG, clinical pregnancy, and live birth.
Results
Compared with baseline activity levels, women significantly reduced their PA and increased sedentary behaviour during IVF (p ≤ 0.001). Higher average PA, light PA, and ratio between breaks in every ≥ 30-min blocks of sedentary time showed positive associations, while sedentary time, number, and time accumulated in blocks of ≥ 30 min of sedentary time associated negatively with oocyte and embryo counts (all p < 0.05). Women with high total screen time during non-work days (≥ 7 h) obtained 4.7 oocytes (p = 0.005) and 2.8 embryos (p = 0.008) less in COS. PA and sedentary behaviour before and during IVF did not affect the positive hCG, clinical pregnancy, and live birth outcomes.
Conclusion
Our study results suggest that higher time spent in PA and lower time spent in sedentary behaviour before entering assisted reproduction is associated with better COS outcomes, while activity levels before and during IVF do not affect the implantation, pregnancy, and live birth outcomes.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01864-w) contains supplementary material, which is available to authorized users.
Keywords: Controlled ovarian stimulation, In vitro fertilization, Physical activity, Pregnancy outcome, Sedentary behaviour
Introduction
Infertility is a growing medical and global public health concern [1], with a prevalence of 12–15% of couples in childbearing age [2]. Infertility is experienced by an estimated 48.5 million couples worldwide, and is steadily increasing with the trend to delay the time of first pregnancy in developed countries, and now also in developing countries [3]. Even though the development of assisted reproductive technologies (ARTs) and the constant improvements in the treatment protocols have helped many infertile couples to achieve pregnancy, the rates of pregnancy and live births among all ART-treated couples still remain ~ 30% per treatment cycle [4]. Ways to improve ARTs outcomes have become a critical topic for both clinicians and couples undergoing infertility treatment.
Several of the most influential factors of infertility treatment, such as female age and genetic factors, are non-modifiable, while there is emerging evidence that modifiable factors, like smoking, weight, nutrition, or physical activity (PA) among others, can influence assisted reproduction [5, 6]. In fact, comparative studies indicate that PA intervention may be as effective as other clinical interventions used for improving reproductive health outcomes, while the type, intensity, frequency, and duration of optimal PA remain unclear [7]. The American Congress of Obstetricians and Gynaecologists recommends pregnant women to engage in moderate intensity exercise for at least 30 min most days per week [8], and suggests for overweight women to lose weight and become more physically active prior to pregnancy [9]. There are, however, no specific guidelines of PA for women attempting conception or women undergoing infertility treatment. In fact, no consensus has been reached regarding the effect of PA before/during ART on pregnancy outcome [10]. The few studies of female PA and infertility treatment success have produced mixed results, where higher PA has been associated with decreased embryo implantation and live birth in in vitro fertilization (IVF) [11], no effect [5, 1, 2], or others have reported beneficial effect of PA on IVF outcomes [10, 13–16]. Nevertheless, in all these studies, PA was measured using self-reported questionnaires, which are prone to recall bias and reliance problems [6], and clearly, more studies evaluating objectively PA effects on assisted reproduction are needed.
High levels of sedentary behaviour, any waking behaviour characterized by a very low energy expenditure while in a sitting or reclining posture, on the other hand, have been associated with many detrimental health consequences [17], but its effect on infertility treatment outcomes remains unexplored. Another important unexplored question to clinical practice is what PA levels to recommend for women after embryo transfer, during the 2 weeks of critical time of embryo implantation and early pregnancy establishment. Clarifying the role of PA and sedentary behaviour in infertility treatment, using objective methods for assessing PA and sedentary behaviour, may help to provide the long-sought-after PA recommendations for women undergoing infertility treatment.
The objective of this study was to examine the associations of objectively measured PA and sedentary behaviour before and during assisted reproduction with IVF treatment outcomes.
Materials and methods
This longitudinal study was carried out among infertile women in reproductive age, entering IVF/ICSI (intracytoplasmic sperm injection) treatment cycle and receiving fresh embryo transfer (ET) at Elite Clinic and Tartu University Hospital’s Women’s Clinic, Estonia from January 2013 to December 2016. IVF treatment cycles with donor oocytes were excluded. After a detailed explanation, in total, 107 women agreed to participate in the study (see Fig. 1 for the study design). Power calculation analyses showed that sample size of 100 participants with an alpha error of 5% and a power of 90% would be enough to detect an association of an effect size of 10% variance explained of the study outcomes. At enrolment, all participants were asked to fill out a questionnaire of general characteristics, and reproductive health. Patient’s measurements of weight, height, and waist and hip circumference were obtained by an assistant nurse. The BMI was calculated as weight (kg)/height2 (m).
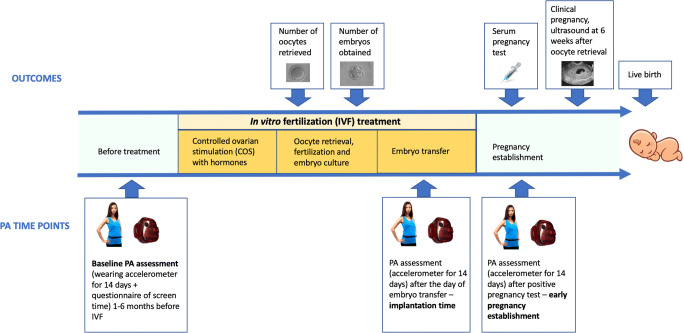
Fig. 1.
Study design. Three time points for physical activity (PA)/sedentary behaviour and 5 time points for infertility treatment outcomes were assessed in this longitudinal study. PA and sedentary behaviour were measured for 14 consecutive days using accelerometry at each of the three time points: (1) before IVF treatment, (2) during IVF at the implantation time, immediately after embryo transfer, and (3) after positive pregnancy test. Total screen time was measured at the baseline, before any IVF treatment, using a questionnaire. The study outcomes included the number of oocytes and embryos obtained after COS, and positive hCG test, clinical pregnancy, and live birth
IVF treatment protocol was conducted according to the gonadotropin-releasing hormone (GnRH) antagonist or agonist protocols. All patients started recombinant FSH (Gonal F, Merck Serono, Italy; or Bemfola, Finox Biotech AG, Switzerland) injections on day 2–7 of menses, continuing daily for 9 ± 2–3 days until 3 follicles achieved 18 mm of diameter, and human chorionic gonadotrophin (hCG) (Merck Serono, Italy) was administered. The controlled ovarian stimulation (COS) follow-up included 3–4 ultrasound assessments of endometrium and follicular growth. Final follicular maturation was achieved using 250 mcg of hCG followed by oocyte retrieval 36 h later.
Patients who received IVF or ICSI were both included (later indicated in conjunction as ‘IVF’). Embryo transfer (ET) was done after COS on days 2 to 5. A serum pregnancy test, referred to as positive hCG, was performed on 14 ± 2 days after ET and considered to be positive if β-hCG > 10 mLU/mL. The ultrasound evaluation for defining clinical pregnancy was performed 6 weeks after oocyte retrieval, and was considered positive in the presence of at least one intrauterine gestational sac and cardiac activity on ultrasound. Live birth was abstracted from medical records.
All women were Caucasians. Patients did not receive any restrictions or recommendations for PA during the infertility treatment. The study was approved by the Research Ethics Committee of the University of Tartu, and written informed consent was obtained from all subjects before their participation.
Assessment of PA and sedentary behaviour
PA and sedentary behaviour were measured for 14 consecutive days using accelerometry at each of the three time points as follows: (1) before IVF treatment, (2) during IVF at the implantation time, immediately after embryo transfer, and (3) after positive pregnancy test (see Fig. 1 for the study design).
Uniaxial accelerometers GT1M (ActiGraph LLC, FL, USA) were placed on participants’ waists to objectively assess PA and sedentary activities’ time. Detailed information on accelerometer assessment is described elsewhere [18]. Briefly, patients were asked to wear accelerometers for 14 consecutive days and to only remove them for swimming, bathing, and sleeping. Three measurements of PA and sedentary behaviour, up to 14 days each, were performed within the study as follows: (1) one to 6 months before IVF, termed as baseline activity; (2) after ET, starting from the day after the embryo transfer (embryo implantation time); and (3) after positive serum pregnancy test, from the next day after the test (early pregnancy establishment) (Fig. 1).
ActiLife software version 6.10.2 (ActiGraph LLC, FL, USA) was used to process accelerometer data. Accelerometer’s output consists in a score calculated in a minute basis intended to measure movement, usually referred to as ‘activity counts’. Non-wear time was defined using algorithm proposed by Choi et al. [19, 20], which consists of 90 min of consecutive 0 cpm with an allowance of 2 min of activity when it is placed between two 30-min windows of 0 cpm. This algorithm outperformed other algorithms on the detection of non-wear time according to our recent systematic review on accelerometry’s data analyses methods [21]. Only women wearing the accelerometer for at least 10 h/day for 4 or more days were included into the analysis (N = 98) [21].
PA is usually classified into different intensity categories, i.e., different levels of energy expenditure required to perform certain PA, usually measured as metabolic equivalents. Metabolic equivalents are usually measured as oxygen consumption relative to body weight per minute (ml/kg/min). In this regard, all activities occurring below 1.5 metabolic equivalents in a seated or reclined posture are considered sedentary behaviour. PA requiring energy expenditure between 1.5 and 3 metabolic equivalents is considered of light intensity, and between 3 and 6 metabolic equivalents is considered moderate PA. Vigorous PA included the activities requiring hard physical effort and they require more than 6 metabolic equivalents.
Accelerometers are unable to measure the energy expenditure required to perform PA, but the existing linear relationship between movement/accelerations, expressed as cpm, and metabolic equivalents allow to estimate PA intensity using validated cpm cut-points [22]. In this regard, we calculated time per day spent at different intensities of PA using the Freedson’s cut-points [22]. Briefly, sedentary time, light PA, moderate PA, and vigorous PA were considered when cpm accumulation was < 100 cpm, 100–1951 cpm, 1952–5724 cpm and > 5724 cpm, respectively [22]. Moderate-to-vigorous PA (MVPA) was calculated by summing the time spent at moderate and vigorous PA. Average physical activity, expressed as mean cpm, was computed as the sum of counts per all valid days divided by the total wear time in minutes in these days. Beyond, as maintained time in certain behaviours can be important of health outcomes [23], the number and time spent in bouts of at least 10 min of MVPA and 30 min of sedentary time were computed. An interruption up to 2 min below or above the threshold (i.e. < 1952 cpm for MVPA and > 100 cpm for sedentary time, respectively) was allowed to consider bouts of activity. A variable of sedentary breaks was also computed as the total number of ‘breaks’ during continuous sedentary bouts. Finally, we computed a ratio between the number of breaks divided by the total time accumulated in 30 min (or longer) bouts of sedentary time.
In addition to the objectively measured PA and sedentary time, the women filled in a questionnaire, where different screen times (TV watching, DVD, computer) were assessed by asking women to indicate the total screen time spent during the last 5 work days and total screen time spent during the last non-work days (e.g. weekend). These questions were obtained from previous large-scale surveys and have shown good reliability, validity, and relation with health outcomes [24].
Statistical analyses
Descriptive characteristics of the sample and baseline PA/sedentary behaviour were calculated. The intra-subject changes in PA/sedentary behaviour from baseline (before starting infertility treatment) to the implantation period (2 weeks onwards from embryo transfer) were tested using repeated measured analysis of variance (ANOVA) models. Associations of baseline PA/sedentary variables with oocytes and embryos obtained after hormonal stimulation were examined using linear regression models, after adjustment for a set of potential confounders selected based on existing clinical knowledge: accelerometer registered time, age, body mass index, educational level, smoking, infertility diagnosis, infertility duration, and the amount of follicle stimulation hormone administered. Finally, the associations of PA/sedentary levels at different time points (i.e. baseline, after embryo transfer, and after positive serum hCG pregnancy test) or changes from baseline to implantation period with different pregnancy outcomes (i.e. positive hCG, clinical pregnancy, and live birth) were examined using binary logistic regression models after adjustment for the same set of confounders. Further, we tested whether PA/sedentary levels at the three different time points varied between women who became pregnant and those who did not (positive hCG, positive clinical pregnancy, and live birth) using one-way analysis of covariance (ANCOVA) models, with the PA/sedentary variables as dependent variables (one per model), the pregnancy groups (Yes vs. No) as fixed factor, and the same confounders previously selected. Next, we performed isotemporal substitution modelling (also called reallocation modelling, which is becoming more frequently used in the field of sport sciences [25, 26]. The isotemporal concept is based on the assumption that the sum of time spent in sedentary activities, plus those of light intensity, plus those of moderate-to-vigorous intensity (MVPA) result in the total waking time registered by the accelerometer, so that if a woman increases the time spent in one of the components (e.g. light PA), the time spent in another component (e.g. sedentary time) must be reduced proportionally if the rest of components remain unchanged. Statistically, this is handled with linear regression models entering all the activity/sedentary variables (e.g. total registered time, light PA, and MVPA) except one (e.g. sedentary time), plus all the potential confounders.
All analyses were performed using the IBM-SPSS software, version 20.0. Armonk, NY, USA. The level of significance was set at p < 0.05 for all the analyses.
Results
Characteristics
Characteristics of the study sample are presented in Table 1. In total, 107 women agreed to participate in this longitudinal study, but as 6 women cancelled the IVF treatment, the final number of women for the analyses remained 101. Majority of women (70%) underwent infertility treatment because of male factor infertility, unexplained infertility, tubal factor infertility, advanced age, or because of no partner. Seven per cent of women were infertile due to polycystic ovary syndrome (PCOS) and 23% due to endometriosis. The average time of infertility in the study group was 4.6 years. Following IVF/ICSI, 43.6% of women had a positive hCG test, 39.6% of women had clinical pregnancy and finally 28.7% of women gave live birth. As twin pregnancy was only reported for six (15%) out of 40 women with clinical pregnancy, we were unable to perform any analyses to assess the associations between PA/sedentary patterns and twinning rate. Spare embryos from 50 women were frozen for future embryo transfers, but as most of them are still being stored, we were not able to calculate the PA and sedentary behaviour effects on cumulative pregnancy rate.
Table 1.
Characteristics of the study population (patients undergoing in vitro fertilization, IVF)
| n | Mean | SD | |
| Age (years) | 101 | 33.5 | 4.1 |
| Height (cm) | 101 | 167.1 | 5.5 |
| Weight (kg) | 101 | 66.9 | 12.8 |
| BMI (kg/m2) | 101 | 23.9 | 4.4 |
| Infertility duration (years) | 101 | 4.6 | 3.5 |
| Total FSH dose (IU) | 100 | 1560.7 | 575.0 |
| Oocytes retrieved | 101 | 11.6 | 7.1 |
| Embryos obtained | 101 | 6.6 | 4.5 |
| Embryos transferred | 101 | 1.5 | 0.6 |
| Frequency | % | ||
| Weight status (UW/NW/OW/OB) | 101 | 7/61/23/10 | 6.9/60.4/22.8/9.9 |
| Smoking (0/1/2) | 101 | 57/36/8 | 56.4/35.6/7.9 |
| Education (university) | 101 | 65 | 64.4 |
| Diagnosis (0/1/2) | 101 | 71/7/23 | 70.3/6.9/22.8 |
| Menstrual cycle (regular) | 101 | 87 | 86.1 |
| IVF protocol (agonist) | 101 | 72 | 71.3 |
| IVF/ICSI (IVF) | 101 | 39 | 38.6 |
| ET stage (2-3 day) | 101 | 59 | 62.8 |
| hCG (positive) | 101 | 44 | 43.6 |
| Clinical pregnancy (positive) | 101 | 40 | 39.6 |
| Live birth (yes) | 101 | 29 | 28.7 |
BMI body mass index, ET embryo transfer, FSH follicle stimulating hormone, ICSI intracytoplasmic sperm injection, IVF in vitro fertilization, UW underweight if BMI < 18.5 kg/m2, NW normal-weight if BMI between 18.5 and 24.9 kg/m2, OW overweight if BMI between 25.0 and 29.9 kg/m2, and OB obese if BMI ≥ 30 kg/m2
ET – embryo transfer
SD standard deviation
Smoking was coded as 0 = never, 1 = formerly, and 2 = currently
Educational level was coded as 0 = under university and 1 = university
Diagnosis group 0—infertility is due to male factor infertility, unexplained infertility, tubal factor, female age, or no partner; Diagnosis group 1—infertility is due to PCOS (including male factor infertility and PCOS; tubal factor and PCOS); Diagnosis group 2—infertility is due to endometriosis (including women with endometriosis and spouses’ male factor infertility)
Menstrual cycle was coded as regular vs. irregular
IVF protocol was coded as agonist vs. antagonist
IVF/ICSI was coded as IVF vs. ICSI
ET stage was coded as 2-3 day vs. blastocyst
hCG test and clinical pregnancy were coded as positive vs. negative
Live birth was coded as yes vs. no.
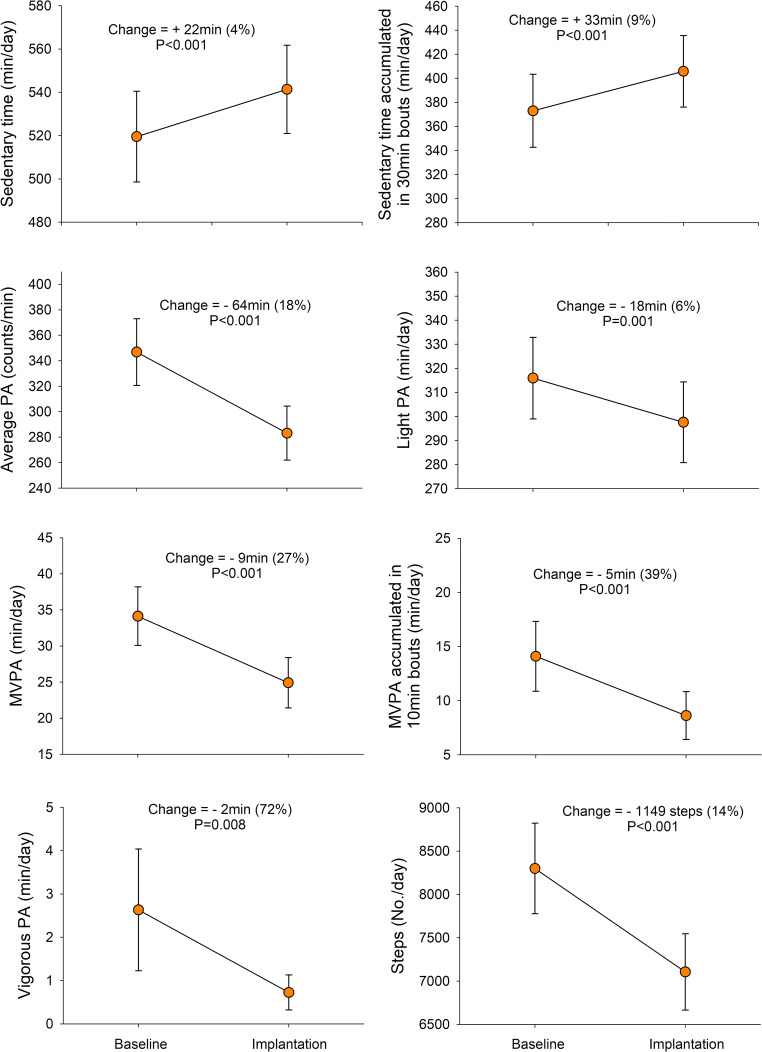
Baseline PA and sedentary behaviour among women before infertility treatment are presented in Supplementary Table 1. The compliance wearing the accelerometer was high, i.e. although the minimum required to be included in the analyses was 4 days with ≥ 10 wearing hours (N = 98), among those included, 90% of them had 10 valid days or more registered (N = 88). When comparing the baseline activity levels of the infertile women, i.e. before IVF, with their PA levels during the IVF treatment, specifically at the time of embryo implantation, the women significantly reduced their PA levels and increased sedentary time as follows: average PA reduced 18% (p < 0.001), light PA reduced 6% (p = 0.001), MVPA reduced 27% (p < 0.001), MVPA accumulated blocks of 10 min reduced 39% (p < 0.001), vigorous PA reduced 72% (p = 0.008), and number of steps per day reduced 14% (p < 0.001), while daily total sedentary time increased 4% (p < 0.001) and sedentary time accumulated in 30-min blocks increased 9% (p < 0.001) (Fig. 2).
Fig. 2.
Changes in physical activity and sedentary behaviour from baseline levels to the infertility treatment procedure, at the period of embryo implantation (14 days registered after embryo transfer) (N = 79 women with valid accelerometer data at both time points). PA, physical activity; MVPA, moderate-to-vigorous PA. P values report the significance of the intra-subject changes tested by repeated measured analysis of variance (ANOVA). The ranges (min. and max.) of the scale used in the Y axes are based on the percentile 25 and 75 of the present study sample
Relationships between baseline PA and sedentary behaviour with COS outcomes
Linear regression analysis of the associations of PA/sedentary variables with oocytes and embryos obtained after COS are summarized in Table 2. The average PA (p = 0.04), light PA (p = 0.03), and ratio between breaks in every 30-min sedentary time (p = 0.002) showed positive association with the number of oocytes obtained in COS, while sedentary time (p = 0.02), number of 30-min blocks of sedentarism (p = 0.02), and time accumulated in ≥ 30-min blocks of sedentarism (p = 0.01) associated negatively with the oocyte count in COS (Table 2).
Table 2.
Associations of baseline physical activity and sedentary time with controlled ovarian stimulation (COS) outcome – oocytes retrieved and embryos developed (N = 97)
| Oocytes | Embryos | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UnStnd. B | Lower CI | Upper CI | Stnd. B | p value | UnStnd. B | Lower CI | Upper CI | Stnd. B | p value | |
| Average PA (counts/min) | 0.01 | 0.00 | 0.02 | 0.21 | 0.04 | 0.01 | 0.00 | 0.01 | 0.19 | 0.07 |
| Sedentary time (hours/day) | − 1.29 | − 2.36 | − 0.22 | − 0.29 | 0.02 | − 0.77 | −1.46 | −0.08 | −0.27 | 0.03 |
| Light PA (hours/day) | 1.25 | 0.13 | 2.37 | 0.23 | 0.03 | 0.79 | 0.07 | 1.50 | 0.23 | 0.03 |
| Moderate PA (min/day) | 0.04 | − 0.05 | 0.12 | 0.09 | 0.40 | 0.01 | −0.05 | 0.06 | 0.03 | 0.78 |
| Vigorous PA (min/day) | 0.06 | − 0.19 | 0.31 | 0.05 | 0.64 | 0.06 | −0.10 | 0.22 | 0.08 | 0.44 |
| MVPA (min/day) | 0.04 | − 0.04 | 0.12 | 0.11 | 0.30 | 0.01 | −0.04 | 0.06 | 0.05 | 0.61 |
| 10 min bouts of MVPA (No./day) | 0.64 | − 1.94 | 3.22 | 0.06 | 0.62 | − 0.57 | −2.21 | 1.07 | −0.08 | 0.49 |
| Time accumulated in 10 min bouts of MVPA (min/day) | − 0.01 | − 0.11 | 0.10 | − 0.01 | 0.93 | − 0.03 | −0.09 | 0.04 | −0.09 | 0.45 |
| 30 min bouts of sedentarism (No./day) | − 0.88 | − 1.59 | − 0.18 | − 0.27 | 0.02 | −0.58 | −1.03 | −0.13 | −0.28 | 0.01 |
| Time accumulated in 30 min bouts of sedentarism (hours/day) | − 0.87 | − 1.55 | − 0.19 | − 0.28 | 0.01 | − 0.47 | −0.91 | −0.03 | −0.24 | 0.04 |
| Ratio breaks:sedentary time in 30 min bouts (No./hours each day) | 9.79 | 3.74 | 15.85 | 0.34 | 0.002 | 5.12 | 1.18 | 9.05 | 0.28 | 0.01 |
| Steps (1000/day) | 0.21 | − 0.36 | 0.77 | 0.08 | 0.47 | 0.06 | −0.30 | 0.42 | 0.03 | 0.75 |
Linear regression models adjusted for accelerometer registered time, age, body mass index, educational level (university vs. below university), smoking (never, before but not now and currently), infertility diagnosis (see Table 1 for coding), infertility duration (years), and follicle stimulation hormone administered. Exploratory additional adjustments for treatment protocol (agonist/antagonist) or infertility centre did not alter the results
PA physical activity, MVPA moderate-to-vigorous PA, CI confidence intervals, UnStnd. unstandardized beta coefficient B, Stnd. B standardized beta coefficient
Regarding the obtained embryos in COS and IVF, light PA (p = 0.03) and ratio between breaks in every 30-min sedentary time (p = 0.01) showed positive association with the number of embryos obtained, while sedentary time (p = 0.03), number of 30-min blocks of sedentarism (p = 0.01), and time accumulated of 30-min blocks of sedentarism (p = 0.04) associated negatively with the number of obtained embryos.
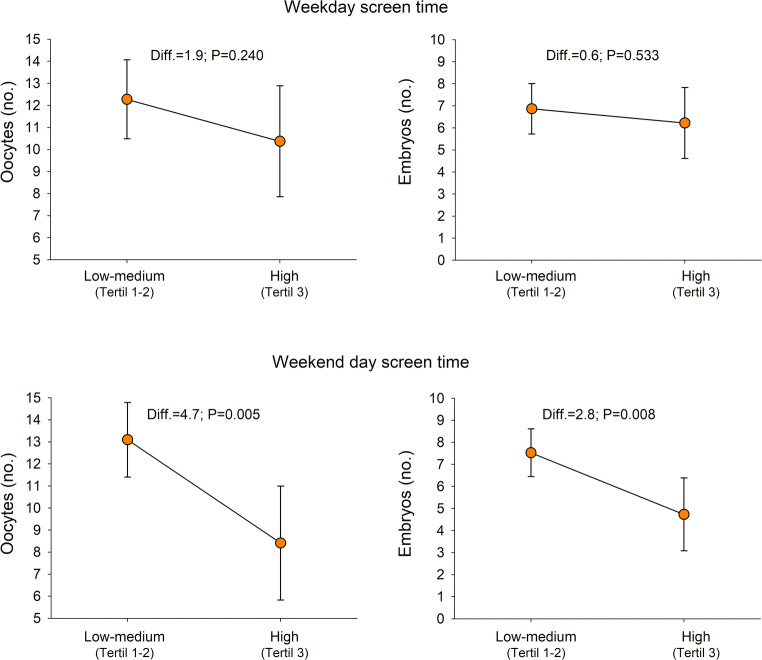
When assessing the self-reported total screen time spent during work days and non-work days, the women with high self-reported total screen time during non-work days (≥ 7 h TV, DVD, computer screen time) obtained 4.7 oocytes (p = 0.005) and 2.8 embryos (p = 0.008) less in COS than women with low-medium screen time (Fig. 3).
Fig. 3.
Differences in the number of oocytes and embryos obtained after controlled ovarian stimulation according to different levels of self-reported screen time in work days and during non-work days (N = 98). Analysis of covariance (ANCOVA) models with oocytes or embryos as dependent variables (in separate models), groups of screen time as fixed factors, and a set of potential confounders as covariates: age, body mass index, educational level (university vs. below university), smoking (never, before but not now and currently), infertility diagnosis (see Table 1 for coding), infertility duration (years), and follicle stimulation hormone administered. High screen time was defined as belonging to the 3rd tertile, while low-middle screen time was defined as belonging to 1st or 2nd tertiles. High screen time in the non-work days (i.e. 3rd tertile) was equivalent to watch screens (TV, DVD, computers) for 7 h or more during the non-work days, i.e. about 3–4 h per day. When high screen time was defined using the 4th quartile instead of 3rd tertile, the results were consistent
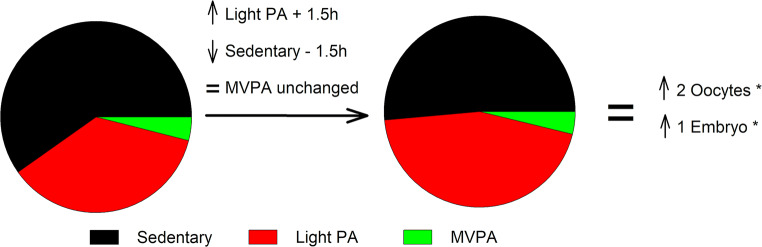
Next, we performed an isotemporal analysis of the time spent in different intensities of baseline PA and sedentarism in association with the number of oocytes and embryos obtained in COS (Fig. 4). This analysis predicted that if a woman increased her daily light PA for 1.5 h, thereby reducing her daily sedentary behaviour for 1.5 h, she would obtain 1.8 oocytes and 1.2 embryos more under COS (p = 0.03 and p = 0.03, respectively).
Fig. 4.
Graphical illustration of the accelerometer isotemporal analyses showing the association of the time spent in different intensities of physical and sedentarism activities with oocytes and embryos obtained after stimulation in our study group (N = 98). The interpretation of this model is that by a given value of MVPA and registered time, an increase in, for example, 1.5 h in light PA accumulated during the whole day (slow walking, slow biking, some houseworks, etc.) should be mirrored by a decrease in sedentary time (the only variable left out of the model), which in turn would result in the change in the dependent variable indicated by the unstandardized regression coefficient. In our study, the regression coefficient for oocytes and embryos of the described models were 1.8 (P = 0.03) and 1.2 (P = 0.03), respectively (these values were rounded to the closest entire number in the figure for simplicity)
Relationships between PA and sedentary behaviour with pregnancy outcomes
Logistic regression analysis of possible associations of PA/sedentary behaviour at different time points (at baseline and during IVF) with IVF outcomes such as positive hCG, clinical pregnancy, and live birth are presented in Tables 3 and 4. No statistically significant association of different PA and sedentary indicators with IVF outcomes were detected.
Table 3.
Associations of baseline physical activity and sedentary time with in vitro fertilization (IVF) outcomes (N = 97)
| Baseline physical and sedentary activities | Positive hCG | Clinical pregnancy | Live birth | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (14 days registered) | OR | Lower CI |
Upper CI |
OR | Lower CI |
Upper CI |
OR | Lower CI |
Upper CI |
| Average PA (counts/min) | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 |
| Sedentary time (hours/day) | 1.09 | 0.79 | 1.52 | 1.06 | 0.76 | 1.48 | 1.08 | 0.75 | 1.57 |
| Light PA (hours/day) | 0.95 | 0.68 | 1.34 | 1.01 | 0.71 | 1.43 | 0.96 | 0.65 | 1.41 |
| Moderate PA (min/day) | 0.99 | 0.97 | 1.02 | 0.99 | 0.96 | 1.01 | 0.99 | 0.96 | 1.02 |
| Vigorous PA (min/day) | 0.93 | 0.82 | 1.04 | 0.93 | 0.83 | 1.05 | 0.95 | 0.83 | 1.09 |
| MVPA (min/day) | 0.99 | 0.96 | 1.01 | 0.98 | 0.95 | 1.01 | 0.99 | 0.96 | 1.02 |
| 10-min bouts of MVPA (No./day) | 1.07 | 0.51 | 2.26 | 0.80 | 0.36 | 1.77 | 0.86 | 0.35 | 2.11 |
| Time accumulated in 10 min bouts of MVPA (min/day) | 0.99 | 0.96 | 1.02 | 0.99 | 0.95 | 1.02 | 0.99 | 0.95 | 1.03 |
| 30-min bouts of sedentarism (No./day) | 1.06 | 0.85 | 1.31 | 1.05 | 0.84 | 1.31 | 1.17 | 0.90 | 1.52 |
| Time accumulated in 30-min bouts of sedentarism (hours/day) | 1.05 | 0.85 | 1.30 | 1.03 | 0.83 | 1.28 | 1.06 | 0.83 | 1.34 |
| Ratio breaks:sedentary time in 30-min bouts (No./hours each day) | 0.72 | 0.11 | 4.55 | 0.84 | 0.13 | 5.54 | 2.26 | 0.28 | 18.58 |
| Steps (1000/day) | 0.93 | 0.79 | 1.11 | 0.91 | 0.77 | 1.09 | 0.95 | 0.78 | 1.16 |
Binary logistic regression models adjusted for accelerometer registered time, age, body mass index, educational level (university vs. below university), smoking (never, before but not now and currently), infertility diagnosis (see Table 1 for coding), infertility duration (years), and follicle stimulation hormone administered. Additional adjustment for the treatment protocol (agonist/antagonist), infertility centre and transferred embryo stage did not change the results
PA physical activity, MVPA moderate-to-vigorous PA, OR odds ratio, CI confidence intervals
Table 4.
Associations of physical activity and sedentary time during IVF (during 14 days after embryo transfer, ET) with treatment outcomes (N = 79)
| Physical and sedentary activities after ET | Positive hCG | Clinical pregnancy | Live birth | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | Lower CI |
Upper CI |
OR | Lower CI |
Upper CI |
OR | Lower CI |
Upper CI |
|
| Average PA (counts/min) | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 |
| Sedentary time (hours/day) | 1.11 | 0.75 | 1.64 | 1.09 | 0.73 | 1.63 | 1.22 | 0.79 | 1.88 |
| Light PA (hours/day) | 0.94 | 0.63 | 1.41 | 0.99 | 0.65 | 1.50 | 0.87 | 0.56 | 1.35 |
| Moderate PA (min/day) | 0.98 | 0.94 | 1.01 | 0.96 | 0.92 | 1.00 | 0.95 | 0.90 | 1.01 |
| Vigorous PA (min/day) | 1.14 | 0.85 | 1.51 | 1.16 | 0.87 | 1.57 | 1.33 | 0.97 | 1.82 |
| MVPA (min/day) | 0.98 | 0.94 | 1.01 | 0.96 | 0.92 | 1.00 | 0.95 | 0.90 | 1.01 |
| 10-min bouts of MVPA (No./day) | 1.17 | 0.40 | 3.44 | 0.83 | 0.27 | 2.55 | 0.73 | 0.20 | 2.61 |
| Time accumulated in 10-min bouts of MVPA (min/day) | 0.99 | 0.94 | 1.05 | 0.98 | 0.93 | 1.04 | 0.98 | 0.92 | 1.05 |
| 30-min bouts of sedentarism (No./day) | 1.12 | 0.86 | 1.46 | 1.14 | 0.86 | 1.50 | 1.31 | 0.96 | 1.81 |
| Time accumulated in 30-min bouts of sedentarism (hours/day) | 1.11 | 0.87 | 1.41 | 1.10 | 0.85 | 1.41 | 1.18 | 0.90 | 1.55 |
| Ratio breaks:sedentary time in 30-min bouts (No./hours each day) | 1.60 | 0.31 | 8.37 | 1.63 | 0.28 | 9.34 | 2.92 | 0.38 | 22.50 |
| Steps (1000/day) | 1.03 | 0.96 | 1.10 | 0.83 | 0.61 | 1.12 | 0.73 | 0.51 | 1.04 |
Binary logistic regression models adjusted for accelerometer registered time, age, body mass index, educational level (university vs. below university), smoking (never, before but not now and currently), infertility diagnosis (see Table 1 for coding), infertility duration (years), and follicle stimulation hormone administered. Additional adjustment for the treatment protocol (agonist/antagonist), infertility centre, and transferred embryo stage did not change the results
PA physical activity, MVPA moderate-to-vigorous PA, OR odds ratio, CI confidence intervals
Next, we analysed whether changes in PA/sedentary behaviour from baseline to implantation were associated with pregnancy outcomes. When the analyses of Tables 3 and 4 were conducted using changes in PA and sedentary time from baseline to implantation period, the results were consistent to those shown in the tables, i.e. no significant associations were observed. Likewise, no significant association was found when entering into the model activity/sedentary behaviour of a person in a specific time point plus the change observed over time. Moreover, those women with proven positive hCG pregnancy tests were asked to wear the accelerometer for 14 more days to test whether the PA and sedentary behaviour at that period of pregnancy had any relation with the risk of miscarriage and early pregnancy establishment. We observed no statistically significant associations (data not shown).
In further exploratory analyses, we tested whether PA and sedentary behaviour at the different time points (baseline, after ET, after positive hCG test) differed between women who became pregnant and those who did not using ANCOVA models and adjusting for the same set of confounders as in the logistic regression models indicated in Tables 2, 3, and 4. The results showed no significant differences in PA and sedentary behaviour between women who had a successful pregnancy and those who did not (in all cases P > 0.1, data not shown). Further, no associations between IVF outcomes and total screen time spent during work days and non-work days were detected (data not shown).
Discussion
To the best of our knowledge, this is the first study where PA and sedentary behaviour is objectively measured for 14 days before any infertility treatment and for 4 weeks during IVF procedure, 14 days after ET and 14 days after positive pregnancy test, in order to closely observe how the activity levels relate to IVF outcomes. In our cohort of infertile women, higher baseline light PA and lower baseline sedentary behaviour were associated with higher number of oocytes and embryos obtained in IVF, while PA and sedentary behaviour before and during IVF did not affect the implantation, pregnancy, and live birth outcomes in fresh embryo transfers. These associations suggest that lifestyle changes such as increase in PA and reduction of sedentary time may positively influence ovarian hyperstimulation under ART protocols. Indeed, a recent systematic review and meta-analysis concluded that PA may be an affordable and feasible alternative or complementary therapy to fertility treatments [7]. In our study, increased PA and reduced sedentary time support obtaining higher number of oocytes and embryos per COS at ovarian puncture, leading to more embryos available for spare embryo freezing. In fact, a recent study shows that higher oocyte yield is independently associated with more embryos obtained and higher cumulative live birth rates [27]. Nevertheless, due to the limited number of patients enrolled in our study, we cannot directly prove that higher PA and decreased sedentary behaviour are related to higher cumulative pregnancy rate combining the pregnancies from fresh and all frozen-thawed embryo transfers. This seems highly likely and should be demonstrated in larger patient cohorts. Our study findings also suggest that PA (at the levels observed in our study) during the most vulnerable period of establishing a pregnancy, e.g., after embryo transfer and until confirmation of a clinical pregnancy, is not harmful for the IVF procedure to succeed.
The mechanisms through which PA affect fecundability could be as follows: (1) PA may influence ovarian function by altering production of oestrogens and other steroid hormones via the hypothalamic-pituitary-ovarian-axis [28]; (2) PA can impact reproductive function through its ability to regulate energy balance and affect BMI, which, in turn, are correlated with the reproductive system [29]; (3) PA may influence lipid profiles and inflammation [30]; (4) moderate PA levels have been shown to increase the expression of antioxidant enzymes throughout the body [31]; (5) PA may improve the ART outcome through insulin sensitization, which has been shown to have an effect on ovarian response to clomiphene citrate during ovulation induction [32]; and (6) PA can help to relieve stress and anxiety, which have been shown to affect negatively ARTs [33, 34]. On the other hand, extreme levels of exercise and being underweight may result in disruption of menstrual cyclicity and increase risk for amenorrhea and subfertility, mostly via disruption of hypothalamic-pituitary endocrine axis [35]. However, we lack the information on thresholds for the amount and intensity of activity to achieve optimal fertility.
Our study results demonstrate that women who were engaged in light PA before entering infertility treatment obtained more oocytes and embryos after the stimulation than women who were less active. Also a recent study has associated light PA prior to pregnancy with improved fecundity among overweight/obese women with previous pregnancy loss [36]. Different types of PA (e.g. household, recreational, work, transportation) or exercise (e.g. aerobic, strength) may have different impacts on fertility. Vigorous PA for instance has been believed to be detrimental, while moderate PA could have beneficial effects on fertility [6]. To our knowledge, only two previous studies have assessed the influence of PA on COS, and no association between PA and the number of oocytes and embryos obtained was found [12, 15], although the number of oocytes and embryos tended to be lower among inactive women [12]. In both of these studies, PA was measured using self-reported questionnaires, which are more biased than objectively measured PA [13, 37] which could explain these differences.
Based on the current study, we believe that the public health message is to encourage light PA among infertile women by reducing the time spent for sedentary behaviours. Indeed, according to the recently published 2018 Physical Activity Guidelines Advisory Committee Scientific Report, the current evidence supports that, among individuals with low levels of activity, replacing sedentary behaviour with light-intensity PA is associated with a better systemic health [23]. Our study results demonstrate that women with high sedentary behaviour (> 8 h per day) and 30-min period blocks of sedentarism (> 7) before treatment associate with a lower number of oocytes and embryos in IVF. Furthermore, women who broke more frequently their sedentary behaviour obtained better COS outcomes than women who did less breaks. Sedentary behaviour for prolonged periods of time has been identified as an important public health concern, associated with a range of health issues [38–40], and is another potential modifiable risk factor for infertility. Indeed, in a recent study, sedentary behaviour was strongly associated with female infertility [41]. Even further, our isotemporal analysis predicted that if a woman decreased her daily sedentary behaviour for 1.5 h a day and thereby increased 1.5 h light PA, she would obtain 2 oocytes and 1 embryo more in COS. In our cohort of 101 women, we obtained in total ~ 1200 oocytes, while by increasing PA and reducing sedentary behaviour, this number could grow up to ~ 1400. A previous extensive study analysing the reproductive potential of 207,000 oocytes in fresh and frozen embryo transfers yielded 4.5% live birth per oocyte [42], indicating that by exploiting these extra 200 oocytes in IVF, the birth of nearly 10 babies is expected.
Another intriguing result in our study is the link observed between TV/screen watching and assisted reproduction outcomes, which has not been studied before. We found that total screen time at non-work days was strongly associated with the number of oocytes and embryos obtained in COS. The effect is even stronger than sedentary behaviour measured by accelerometry, which could be explained by the fact that not all sedentary behaviours seem to be equally harmful for health, and TV watching seems to be the most harmful [17]. In fact, screen time during work days (which tends to be more work-related) was not associated with COS outcomes among our cohort of women, while the screen time during non-work days should be more volunteer behaviour and more likely to be TV based. TV watching has been associated with detrimental health consequences [43, 44], including lower sperm concentration and lower total sperm count in young men [45].
In our study, women while undergoing IVF significantly reduced PA and increased sedentary behaviour, which means that few women engaged in vigorous activity during IVF and therefore we cannot adequately assess whether high-intensity PA during treatment affects pregnancy outcomes. In fact, we did not detect any associations of objectively measured PA and sedentary behaviour before and during IVF treatment with IVF outcomes such as positive hCG, clinical pregnancy, and live birth. Our study results are in line with the only previous study that has objectively assessed PA, where women significantly reduced their PA and increased sedentary behaviour levels during IVF and no association of PA on IVF outcomes was detected [13]. Although they measured objectively PA during IVF, for 1 week, and the activity levels 1 year before entering IVF were assessed with self-reported questionnaires [13]. In line with our objectively measured data are a number of previous studies demonstrating that bed rest after ET in IVF programs does not influence pregnancy outcomes and is unnecessary [46–50]. Our results contribute to the existing knowledge suggesting that, at least within the levels of PA observed in our participants, higher levels of PA during this critical period of implantation of the embryo had no negative impact on the changes to become pregnant, supporting the notion that there is no evidence to suggest to avoid PA during this period. Nevertheless, we did not find very high/extreme levels of PA within our sample; therefore, we cannot know how these extreme doses of PA could affect successful implantation.
Limitations and strengths
First, the sample may not be generalizable and should be confirmed in other populations. Next, the accelerometer used in the study required removal while in the water, meaning that water-related activities could not be recorded. Our observational study design does not allow to draw conclusions on causality. Likewise, despite the inclusion of a broad set of potential confounders in this study, there is always the possibility that other confounding factors not included in the analyses could influence the results. There are, however, several strengths in our study that should be highlighted. Firstly, that PA and sedentary behaviour were measured objectively using accelerometry and that we measured activities for 14 consecutive days during three critical periods. Also, our unique study design should be acknowledged, where we included only fresh embryo transfers, and measured PA before any infertility treatment and 4 weeks during the treatment, which enabled us to closely observe the periods of embryo implantation and early pregnancy establishment. Further, the confirmation of the study findings with self-reported method, screen time measure, strengthens our conclusions.
Conclusions
Infertility treatment is costly in time, money, and emotional stress; therefore, it is of utmost importance to identify factors which dictate the success of infertility treatment, especially the factors that could be modifiable (e.g. lifestyle factors). This is the first study to objectively measure PA before and during infertility treatment and we show that PA and sedentary behaviour can influence ovarian stimulation outcomes in IVF protocol. Our results suggest that increase in light PA and reduction of sedentary behaviour and screen watching before IVF may help infertile women to improve their results in COS. On the other hand, no positive or negative association of PA with pregnancy outcomes in fresh embryo transfers, including during the critical period of embryo implantation, was observed. Thus, we conclude that PA does not seem to affect the quality of the oocytes (as measured by the achieved pregnancies), but rather the quantity of the oocytes obtained (as measured by COS parameters). Whether the increased number of oocytes collected in more active women is transformed into improved pregnancy rate from cumulative fresh and frozen embryo transfers should be proven by the forthcoming studies.
Electronic supplementary material
(DOCX 21 kb).
Acknowledgements
We thank infertile women for participating in the study.
Author’s contributions
All authors contributed to the study design, execution, and interpretation of the study. DS, EM, MN, JM, AE, AS, AS, and AS were responsible for the data collection; FBO and JHM were responsible for the data analysis; and DS and SA drafted the first version of the manuscript. All authors revised critically the manuscript and approved the final version of the manuscript.
Funding information
The study was supported by Estonian Ministry of Education and Research (grant IUT34-16); Enterprise Estonia (grant EU48695); the European Commission Horizon 2020 research and innovation program under grant agreement 692065 project WIDENLIFE); MSCA-RISE-2015 project MOMENDO (grant no 691058), and University of Tartu base funding; Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and European Regional Development Fund (FEDER): grants RYC-2016-21199 and ENDORE SAF2017-87526-R; Programa Operativo FEDER Andalucía (B-CTS-500-UGR18) and by the University of Granada Plan Propio de Investigación 2016 -Excellence actions: Unit of Excellence on Exercise and Health (UCEES) - and Plan Propio de Investigación 2018 - Programa Contratos-Puente, and the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades, European Regional Development Funds (ref. SOMM17/6107/UGR); and Karolinska Institutet Foundation grant (Ref: 2011FoBi1184).
Compliance with ethical standards
Ethical approval
The study was approved by the Research Ethics Committee of the University of Tartu.
Statement of informed consent
Written informed consent was obtained from all subjects before their participation.
Footnotes
Key message
Higher levels of light physical activity and shorter sedentary behaviour and screen-watching times before entering infertility treatment associate with better ovarian stimulation outcomes under IVF treatment protocols.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. 2010;93:16.e1–16.e10. doi: 10.1016/j.fertnstert.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 2.McLaren JF. Infertility evaluation. Obstet Gynecol Clin N Am. 2012;39:453–463. doi: 10.1016/j.ogc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Datta J, Palmer MJJ, Tanton C, Gibson LJJ, Jones KGG, Macdowall W, et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum Reprod. 2016;0:1–11. [DOI] [PMC free article] [PubMed]
- 4.Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, Dimitriadis E. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12:654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- 5.Gaskins AJ, Williams PL, Keller MG, Souter I, Hauser R, Chavarro JE, EARTH Study Team Maternal physical and sedentary activities in relation to reproductive outcomes following IVF. Reprod BioMed Online. 2016;33:513–521. doi: 10.1016/j.rbmo.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evenson KR, Hesketh KR. Studying the complex relationships between physical activity and infertility. Am J Lifestyle Med. 2016;10:232–234. doi: 10.1177/1559827616641379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mena GP, Mielke GI, Brown WJ. The effect of physical activity on reproductive health outcomes in young women: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:542–564. doi: 10.1093/humupd/dmz013. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Committee Obstetric Practice ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 9.Good Health Before Pregnancy: Preconception Care [Internet]. Women’s Heal. Care Physicians. 2017. p. https://www.acog.org/Patients/FAQs/Good-Health-Bef. Available from: https://www.acog.org/Patients/FAQs/Good-Health-Before-Pregnancy-Preconception-Care
- 10.Rao M, Zeng Z, Tang L. Maternal physical activity before IVF/ICSI cycles improves clinical pregnancy rate and live birth rate: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2018;16:11. doi: 10.1186/s12958-018-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris SN, Missmer SA, Cramer DW, Powers RD, McShane PM, Hornstein MD. Effects of lifetime exercise on the outcome of in vitro fertilization. Obstet Gynecol. 2006;108:938–945. doi: 10.1097/01.AOG.0000235704.45652.0b. [DOI] [PubMed] [Google Scholar]
- 12.Ramezanzadeh F, Kazemi A, Yavari P, Nasr-Esfahani MH, Nejat S, Rahimi-Foroshani A, Saboor-Yaraghi A. Impact of body mass index versus physical activity and calorie intake on assisted reproduction outcomes. Eur J Obstet Gynecol Reprod Biol. 2012;163:52–56. doi: 10.1016/j.ejogrb.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Evenson KR, Calhoun KC, Herring AH, Pritchard D, Wen F, Steiner AZ. Association of physical activity in the past year and immediately after in vitro fertilization on pregnancy. Fertil Steril. 2014;101:1047–1054.e5. doi: 10.1016/j.fertnstert.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira RC, Halpern G, de Cássia Savio Figueira R, de Almeida Ferreira Braga DP, Iaconelli A, Jr, Borges E, Jr, et al. Physical activity, obesity and eating habits can influence assisted reproduction outcomes. Womens Health (Lond) 2010;6:517–524. doi: 10.2217/whe.10.40. [DOI] [PubMed] [Google Scholar]
- 15.Kucuk M, Doymaz F, Urman B. Effect of energy expenditure and physical activity on the outcomes of assisted reproduction treatment. Reprod BioMed Online. 2010;20:274–279. doi: 10.1016/j.rbmo.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Palomba S, Falbo A, Valli B, Morini D, Villani MT, Nicoli A, la Sala GB. Physical activity before IVF and ICSI cycles in infertile obese women: an observational cohort study. Reprod BioMed Online. 2014;29:72–79. doi: 10.1016/j.rbmo.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 18.Pärn T, Grau Ruiz R, Kunovac Kallak T, Ruiz JR, Davey E, Hreinsson J, Wånggren K, Salumets A, Sjöström M, Stavreus-Evers A, Ortega FB, Altmäe S. Physical activity, fatness, educational level and snuff consumption as determinants of semen quality: findings of the ActiART study. Reprod BioMed Online. 2015;31:108–119. doi: 10.1016/j.rbmo.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer Wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of Wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44:2009–2016. doi: 10.1249/MSS.0b013e318258cb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, Labayen I, Ruiz JR, Ortega FB. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47:1821–1845. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Committee 2018 Physical Activity Guidelines Advisory. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC; 2018.
- 24.Dunstan DW, Barr ELM, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N. Television viewing time and mortality: the australian diabetes, obesity and lifestyle study (ausdiab) Circulation. 2010;121:384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 25.Hansen BH, Anderssen SA, Andersen LB, Hildebrand M, Kolle E, Steene-Johannessen J, et al. Cross-sectional associations of reallocating time between sedentary and active Behaviours on Cardiometabolic risk factors in young people: an international Children’s Accelerometry database (ICAD) analysis. Sports Med. 2018;48:2401–2412. doi: 10.1007/s40279-018-0909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170:519–527. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venetis CA, Tilia L, Panlilio E, Kan A. Is more better? A higher oocyte yield is independently associated with more day-3 euploid embryos after ICSI. Hum Reprod. 2018. [DOI] [PubMed]
- 28.Tworoger SS, Missmer SA, Eliassen AH, Barbieri RL, Dowsett M, Hankinson SE. Physical activity and inactivity in relation to sex hormone, prolactin, and insulin-like growth factor concentrations in premenopausal women - exercise and premenopausal hormones. Cancer Causes Control. 2007;18:743–752. doi: 10.1007/s10552-007-9017-5. [DOI] [PubMed] [Google Scholar]
- 29.Redman LM. Physical activity and its effects on reproduction. Reprod BioMed Online. 2006;12:579–586. doi: 10.1016/s1472-6483(10)61183-2. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen TK, Williams MA, Lee I-M, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during Pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Cabrera M-C, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod. 2010;25:1005–1013. doi: 10.1093/humrep/dep466. [DOI] [PubMed] [Google Scholar]
- 33.Hämmerli K, Znoj H, Barth J. The efficacy of psychological interventions for infertile patients: a meta-analysis examining mental health and pregnancy rate. Hum Reprod Update. 2009;15:279–295. doi: 10.1093/humupd/dmp002. [DOI] [PubMed] [Google Scholar]
- 34.Frederiksen Y, Farver-Vestergaard I, Skovgård NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ Open. 2015;5:e006592. doi: 10.1136/bmjopen-2014-006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise LA, Rothman KJ, Mikkelsen EM, Sørensen HT, Riis AH, Hatch EE. A prospective cohort study of physical activity and time to pregnancy. Fertil Steril. 2012;97:1136–1142.e4. doi: 10.1016/j.fertnstert.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo LM, Whitcomb BW, Mumford SL, Hawkins M, Radin RG, Schliep KC, Silver RM, Perkins NJ, Kim K, Omosigho UR, Kuhr DL, Holland TL, Sjaarda LA, Schisterman EF. A prospective study of physical activity and fecundability in women with a history of pregnancy loss. Hum Reprod. 2018;33:1291–1298. doi: 10.1093/humrep/dey086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagströmer M, Bergman P, De Bourdeaudhuij I, Ortega FB, Ruiz JR, Manios Y, et al. Concurrent validity of a modified version of the international physical activity questionnaire (IPAQ-A) in European adolescents: the HELENA study. Int J Obes. 2008;32(Suppl 5):S42–S48. doi: 10.1038/ijo.2008.182. [DOI] [PubMed] [Google Scholar]
- 38.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackenzie K, Such E, Norman P, Goyder E. The development, implementation and evaluation of interventions to reduce workplace sitting: a qualitative systematic review and evidence-based operational framework. BMC Public Health. 2018;18:833. doi: 10.1186/s12889-018-5768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388:1311–1324. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 41.Foucaut A-M, Faure C, Julia C, Czernichow S, Levy R, Dupont C. Sedentary behavior, physical inactivity and body composition in relation to idiopathic infertility among men and women. Drevet JR, editor. PLoS One. 2019;14:e0210770. doi: 10.1371/journal.pone.0210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoop D, Ermini B, Polyzos NP, Haentjens P, De Vos M, Verheyen G, et al. Reproductive potential of a metaphase II oocyte retrieved after ovarian stimulation: an analysis of 23 354 ICSI cycles. Hum Reprod. 2012;27:2030–2035. doi: 10.1093/humrep/des131. [DOI] [PubMed] [Google Scholar]
- 43.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J-W, Zhao L-G, Yang Y, Ma X, Wang Y-Y, Xiang Y-B. Association between television viewing time and all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol. 2015;182:908–916. doi: 10.1093/aje/kwv164. [DOI] [PubMed] [Google Scholar]
- 45.Gaskins AJ, Mendiola J, Afeiche M, Jørgensen N, Swan SH, Chavarro JE. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med. 2015;49:265–270. doi: 10.1136/bjsports-2012-091644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharif K, Afnan M, Lashen H, Elgendy M, Morgan C, Sinclair L. Is bed rest following embryo transfer necessary? Fertil Steril. 1998;69:478–481. doi: 10.1016/s0015-0282(97)00534-7. [DOI] [PubMed] [Google Scholar]
- 47.Purcell KJ, Schembri M, Telles TL, Fujimoto VY, Cedars MI. Bed rest after embryo transfer: a randomized controlled trial. Fertil Steril. 2007;87:1322–1326. doi: 10.1016/j.fertnstert.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 48.Lambers MJ, Lambalk CB, Schats R, Hompes PGA. Ultrasonographic evidence that bedrest after embryo transfer is useless. Gynecol Obstet Investig. 2009;68:122–126. doi: 10.1159/000226283. [DOI] [PubMed] [Google Scholar]
- 49.Su TJ, Chen YC, Hung YT, Yang YS. Comparative study of daily activities of pregnant and non-pregnant women after in vitro fertilization and embryo transfer. J Formos Med Assoc. 2001;100:262–268. [PubMed] [Google Scholar]
- 50.Bar-Hava I, Kerner R, Yoeli R, Ashkenazi J, Shalev Y, Orvieto R. Immediate ambulation after embryo transfer: a prospective study. Fertil Steril. 2005;83:594–597. doi: 10.1016/j.fertnstert.2004.07.972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 21 kb).