Abstract
Medicinal importance of Embelia ribes Burm f. is known since ancient time. Its berries are the main ingredient in Vidanga’ or ‘Baibidanga’—a component of ayurvedic formulations and possess medicinal properties such as antihelmintic, anticancer, neuroprotective and antidiabetic. Studies were conducted on phytochemicals, antioxidant activities, extraction efficiency of embelin from ten genotypes. Methanolic extract of berries from Nagavelli accession exhibited the highest total phenolic content (18.18 ± 0.14 mg GAE/g DW); whereas, ethanolic extract showed highest total flavonoid content (8.35 ± 0.20 mg RE/g DW). The antioxidant activities (AOA) were assessed and noted that ethanolic and methanolic extracts of berries from Nagavelli (NAG) accession revealed highest activities in terms of DPPH radical scavenging activity (67.48 ± 0.17%) and FRAP (66.73 ± 0.60 mg Fe(II)/g DW), respectively. In AOA analysis, berries extracted with different solvents were positively correlated with TPC. Principal component analysis revealed TPC and TFC were the most influencing components for strong antioxidant activities in E. ribes. Reverse phase high performance liquid chromatography (RP-HPLC) was used to quantify embelin content and its optimize extraction using various methods. In the preliminary studies, berries from NAG accession revealed highest (1.770%) embelin content. Further, berries from NAG accession were subjected to various extraction methods and found three fold increase (5.08%) in embelin content in microwave assisted extraction (90 s). Present study suggested that NAG accession found to be a promising source of natural antioxidants and embelin that can be used in pharmaceutical industries.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00859-2) contains supplementary material, which is available to authorized users.
Keywords: Embelia ribes, Embelin, RP-HPLC, Antioxidant, Total phenolic content, Total alkaloid content
Introduction
Genus Embelia belongs to family Primulaceae consist of about 130 species (Angiosperm Phylogeny Group IV 2016). Embelia ribes Burm. f. found in semi-evergreen to evergreen forests of India at altitudes above 400–1200 m. It is reported to be vulnerable and red-listed species in Western Ghats of India (Ravikumar and Ved 2000; Rajashekaran 2001; Mhaskar et al. 2011). E. ribes possess various medicinal properties such as analgesic, antitumor, anti-inflammatory, antidiabetic, anticancer, antihelmintic, anit-obese, antioxidant, and neuroprotective. It is also used against brain disorders, sore throat, fungal infections and to support the digestive functions (Chitra et al. 1994; Bhandari et al. 2002; Xu et al. 2005; Vinutha et al. 2007; Ansari et al. 2008; Ansari and Bhandari 2008, 2009; Singh et al. 2009; Choudhary et al. 2012; Sharanbasappa et al. 2017). E. ribes is considered as promising target in generating novel drugs (Souravi and Rajasekharan 2014). Berries of E. ribes have been recognized for the marker compound called embelin (Soumya et al. 2011). Embelin is reported to be non-peptidic, cell-permeable, XIAP inhibitory and represents an entirely new anticancer agent that targets the BIR3 domain of XIAP (Nikolovska et al. 2004). Embelin and its derivatives act as a PKC inhibitor, antiplatelet, antidiabetic and antithromobotic. It also used in the treatment associated with several neurodegenerative disorders (Jiun et al. 2019; Xiaole et al. 2020; Francesco et al. 2020). Analysis of embelin from the berries of E. ribes has been studied using HPLC by previous researchers (Soumya et al. 2011; Nagamani et al. 2013). Extraction of embelin was dependent on the solvent used and it was found that recovery was highest in polar solvents compared to non-polar (Latha 2007).
Very little information is available on the antioxidant activities and embelin content of the genotypes collected from the different localities. Therefore, the aim of the present study was focused on the comparative account of phytochemicals, antioxidant activities, quantification and optimized production of embelin content using different extraction methods from ten genotypes of E. ribes.
Materials and methods
Collection of plant material and sample preparation
Plant materials were collected from the ten different geographical areas of Western Ghats, India during June to July 2017 (Table 1). Voucher specimens (VVK01 to VVK10) were deposited in the Herbarium (SUK), Department of Botany, Shivaji University, Kolhapur, Maharashtra, India. Further, finely powdered berries from all the accession were extracted with methanol, ethanol, petroleum ether and distilled water. One gram of powered berries from each accession was extracted in 10 ml of 95% of each solvent for 90 s at 180 W using microwave assisted extraction separately. The extracts were filtered through a 0.22 µm nylon filter (Axiva filters), the volume of the extract was adjusted to 10 ml with each solvent, stored at 4 °C and used for phytochemicals and antioxidant analysis.
Table 1.
Geographical details of ten different accessions of Embelia ribes
| Sr. no. | Localities | Voucher number | Forest type | Latitude (N) | Longitude (E) | Elevation (m) |
|---|---|---|---|---|---|---|
| 1 | Amba, Kolhapur district, Maharashtra (AMB) | VVK 01 | Semi evergreen | 14° 31.466′ | 074° 33.691′ | 767.79 |
| 2 | Bisle Ghats, Border of Hassan and Dakshin kannada district, Karnataka (BIS) | VVK 02 | Semi evergreen | 13° 06.662′ | 075° 29.302′ | 573.38 |
| 3 | Charmadi Ghats, Dakshina kannada district, Karnataka (CHA) | VVK 03 | Semi evergreen | 16° 28.448′ | 073° 34.609 | 895.19 |
| 4 | Devimane Ghats, Uttara kannada district, Karnataka (DEV) | VVK 04 | Tropical wet evergreen | 14° 31. 278′ | 074° 33.066′ | 619 |
| 5 | Dicholi, Satara district, Maharashtra (DIC) | VVK 05 | Semi evergreen | 17° 26.552′ | 073° 43.409 | 710.18 |
| 6 | Jogfalls, border of Shimoga and Uttar kannada Karnataka (JOG) | VVK 06 | Dense evergreen | 16° 40.559′ | 074° 15.327′ | 610.81 |
| 7 | Kogar, Shimoga district, Karnataka (KOG) | VVK 07 | Dense evergreen | 14° 02.898′ | 074° 43.396′ | 548.64 |
| 8 | Koyana, Satara district, Maharashtra (KOY) | VVK 08 | Semi evergreen | 17° 46.547′ | 073° 42.366′ | 749.50 |
| 9 | Nagavelli, Shimoga district, Karnataka (NAG) | VVK 09 | Semi evergreen | 14° 02. 973′ | 074° 43.201′ | 541.32 |
| 10 | Talakaveri, Kodagu district, Karnataka (TAL) | VVK 10 | Dense evergreen | 12° 22.696′ | 075° 35.116 | 975.96 |
Phytochemical analysis
All the assays for phytochemical and antioxidant analysis of the extracts were performed in triplicates.
Total phenolic content (TPC)
TPC of the extracts was estimated by using Folin–Ciocalteu (Singleton and Rossi 1965). Briefly, 125 µl of extract was mixed with 1.8 ml of Folin–Ciocalteu reagent and kept for 5 min at 25 °C. Further, 1.2 ml of 15% Na2CO3 was added to the reaction mixture and kept for 90 min at room temperature. The absorbance of the reaction was measured at 765 nm. The concentration of the TPC was determined as milligram of gallic acid equivalent per gram dry weight (mg GAE/g DW) by using an equation obtained from the calibration curve.
Total flavonoid content (TFC)
TFC was quantified by using the aluminium chloride method (Chang et al. 2002). The extract (0.5 ml) was mixed with 1.5 ml methanol, 0.1 ml of 10% aluminium chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. The mixture was vortexed and the reaction was kept at the room temperature for 30 min and absorbance of reaction mixture was measured at 415 nm. The concentration of the TFC was determined as milligram of rutin equivalent per gram dry weight (mg RE/g DW).
Total alkaloid content (TAC)
TAC of the extracts was measured using 1, 10-phenanthroline method (Singh et al. 2004). Plant extract (100 µl) was mixed with 1 ml of 0.05 M of 1, 10- phenanthroline in ethanol,1 ml of 0.025 M FeCl3 in 0.5 M HCl and volume was adjusted to 10 ml by adding distilled water. The reaction mixture was incubated for 30 min in water bath at 70 °C. Above reaction mixture excluding plant extract, substituted by distilled water served as a blank. The absorbance was measured at 510 nm against reagent blank. The concentration of total alkaloid was determined by milligram colchicine equivalent per gram of dry weight (mg CE/g DW). All the analyses were performed in triplicates.
DPPH radical scavenging activity
The DPPH radical scavenging activity of various extracts of E. ribes were estimated by using modified and stable 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay (Jagtap et al. 2010). DPPH solution was prepared by dissolving 2.5 mg of DPPH in 100 ml of chilled methanol. Plant extract (100 μl) was allowed to react with 3 ml of DPPH solution. The reaction mixture was vortexed, kept in the dark at room temperature for 30 min and absorbance was recorded at 517 nm. A control sample without extract was also analyzed. Percent inhibition/radical scavenging activity (% RSA) was calculated using following formula:
where A = absorbance at 517 nm.
Ferric reducing antioxidant power assay (FRAP)
FRAP assay was carried out according to method described by Benzie and Strain (1996) with some modifications. FRAP reagent formed by assimilation of the acetate buffer (0.3 M, pH 3.6), 2, 4, 6-tripyridyl-s-triazine (TPTZ, 10 mM)) in 40 mM HCl and FeCl3. 6H2O (20 mM) in 10:1:1 ratio prior to use and heated to 37 °C in water bath for 10 min. The extracts were allowed to react with 2.7 ml of the FRAP reagent and the final volume of the reaction was adjusted to 3 ml with distilled water. Further, reaction mixture was kept in dark for 30 min and the absorbance was recorded at 593 nm. The results were expressed as milligram Fe(II) equivalent per gram dry weight.
Analysis of embelin by RP-HPLC
Preparation of sample and standard solutions
Preliminary screening of embelin content from the berries of all the genotypes was performed using continuous shaking extraction method (CSE). Extraction of embelin was carried using previous methods (Latha 2007; Nagamani et al. 2013). One gram of finely ground powered berries from all the accessions was extracted separately in 10 ml of 95% methanol using CSE at a fixed duration (110 ± 2 rpm, 360 min at 30 ± 1 °C). Standard embelin was weighed accurately and dissolved in methanol to obtain a standard stock solution (mg/ml). The stock solution was serially diluted to obtain working concentrations (250–1500 μg/ml) and used to obtain calibration curve (r2 value = 0.99, Supplementary Fig. 1). All the extracts were filtered through 0.22 µm nylon filter (Axiva filters), condensed and adjusted to 10 ml using 95% methanol and subjected to RP-HPLC analysis.
Chromatographic conditions
RP-HPLC analysis was performed on Jasco Chromatographic system (Model no. LC–2000 Plus) equipped with quaternary pump, auto sampler and UV detector (UV 2070). The separation was performed using Hiber C18 column (5 μm, 250–4.6 mm). The built-in ChromNAV software system was used for data processing. Mobile phase consisting of methanol: water: acetic acid: tetrahydrofuran (85: 15: 3: 0.1 v/v/v/v) was used for the separation with an injection of 20 μl. A flow rate of 2 ml per min was used with 20 min retention time. Detection of standard and samples was carried out at 288 nm. The qualitative analyses of embelin from 10 different samples were performed by comparing their retention time with those of standard. Identified embelin was confirmed by spiking with known concentration of respective standard. A calibration curve of embelin was used for quantification and the values were expressed in percentage. Prior to RP-HPLC analysis, the system suitability was assessed by triplicate injections of standard solutions and extracts. The peak areas of three independent injections of standard solutions and extracts were considered to evaluate repeatability of method.
Optimized extraction of embelin using different extraction methods
Based on the preliminary experiments, accession with highest embelin content was used further. Several extraction techniques employed in the present study were as follows:
Continuous shaking extraction (CSE)
One gram powder of berries was subjected separately to CSE using 10 ml of 95% methanol and placed on orbital shaker (REMI, India) equipped with temperature sensor. The shaking speed was adjusted to 110 ± 2 rpm at controlled temperature (30 ± 1 °C) for 3, 6, 9 and 12 h.
Soxhlet extraction (SE)
Dried powder of berries (1 g) was put into 100 ml Soxhlet thimble. The apparatus was fitted with 250 ml round bottom flask containing 100 ml of 95% methanol. The extraction temperature was controlled at 60 °C with a regulator. The flask was heated for 120, 180, 240 and 300 min. The solvent was refluxed until a given time was accomplished.
Ultrasonic extraction (UE)
Ultrasonic extraction was performed on an ultrasonicator (Revotek, India) at a working frequency of 30 kHz. One gram of dried powder was extracted with 95% methanol (10 ml) in 100 ml conical flask. The sonication was done for 10, 20, 30 and 40 min at room temperature.
Microwave assisted extraction (MAE)
One gram material was put into a 100 ml conical flask and 10 ml of 95% methanol was added to it. These flasks were exposed for 30, 60, 90 and 120 s in a microwave oven (Samsung, India) at 180 W and excess boiling was avoided by cooling at regular interval of 15 s.
All the above extracts were subjected to RP-HPLC analysis as mentioned earlier.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS ver. 16.0. Data was reported as mean ± standard deviation (SD) and subjected to one-way analysis of variance (ANOVA). Significant differences between mean values were determined by Duncan’s multiple range test (p < 0.05). The Pearson correlation coefficient was determined among TPC, TFC, TAC, DPPH and FRAP at p < 0.01. The Principal Component Analysis (PCA) performed based on phytochemicals (TAC, TPC and TFC) and antioxidant activities (DPPH and FRAP). PCA analysis was executed by calculating the eigenvectors and eigen values from the Eigen program in PAST software ver. 3.01.
Results and discussion
Phytochemical assays
Total phenolic content
The berries from NAG accession showed highest TPC (18.18 ± 0.14 mg GAE/g DW) in methanolic extract followed by KOY > DEV > TAL > JOG > AMB > KOG > DIC > CHA > BIS accessions. Among the studied localities, BIS accession showed lowest TPC (0.96 ± 0.36 mg GAE/g DW) in the petroleum ether extract (Table 2). It was also found that methanol was the best solvent for extraction of phenolic compounds among all the solvents. Methanolic extract of berries showed highest TPC followed by ethanol, water and petroleum ether extracts. These results are in agreement with the earlier reports with 13.16 ± 0.32 GAE/g DW of TPC from berries of E. ribes (Shadma and Naheed 2014). In the present study, we found the influence of different solvent for the recovery of TPC. Similar results have been reported in wheat and Salacia chinensis L. (Zhou and Yu 2004; Chavan et al. 2012). It seems that the TPC differ according to the plant species and the solvent used for the extraction (Liu et al. 2007). TPC, TFC and TAC from methanol, ethanol, petroleum ether and water extracts of E. ribes berries collected from ten different accessions presented in Table 2.
Table 2.
Averages of TPC, TFC, TAC, DPPH and FRAP from various solvent extract of Embelia ribes berries collected from different localities
| Localities | Solvent | TPCa mg GAE g−1 DW |
TFCb mg RE g−1 DW |
TACc mg CE g−1 DW |
DPPH % inhibition |
FRAPd mg Fe(II)E g−1 DW |
|---|---|---|---|---|---|---|
| Amba (AMB) | Methanol | 13.13 ± 0.30a | 4.84 ± 0.03b | 10.44 ± 0.34a | 38.27 ± 1.37b | 45.07 ± 0.72a |
| Ethanol | 11.87 ± 0.22b | 5.92 ± 0.03a | 8.66 ± 0.16b | 49.46 ± 0.17a | 42.34 ± 0.96b | |
| Pet. ether | 2.71 ± 0.16d | 2.45 ± 0.14c | 6.00 ± 0.16d | 3.94 ± 0.28c | 10.09 ± 0.21d | |
| Water | 10.52 ± 0.08c | 1.00 ± 0.11d | 8.00 ± 0.19c | 4.83 ± 0.79c | 27.67 ± 0.10c | |
| Bisle Ghats (BIS) | Methanol | 8.14 ± 0.63a | 2.52 ± 0.05b | 6.72 ± 0.19a | 29.80 ± 0.13b | 29.24 ± 0.10a |
| Ethanol | 7.22 ± 0.36b | 3.58 ± 0.10a | 5.77 ± 0.09b | 43.17 ± 0.15a | 26.62 ± 0.72b | |
| Pet. ether | 0.96 ± 0.36c | 1.01 ± 0.06c | 3.83 ± 0.33d | 0.54 ± 0.34d | 8.42 ± 0.21d | |
| Water | 6.98 ± 0.14b | 0.23 ± 0.05d | 4.77 ± 0.09c | 1.43 ± 0.30c | 18.34 ± 0.10c | |
| Charmadi Ghats (CHA) | Methanol | 9.35 ± 0.08a | 3.39 ± 0.08b | 7.83 ± 0.16a | 31.91 ± 1.23b | 37.98 ± 1.16a |
| Ethanol | 8.00 ± 0.38b | 4.17 ± 0.10a | 6.00 ± 0.16b | 45.29 ± 0.35a | 32.70 ± 1.66b | |
| Pet. ether | 1.11 ± 0.25c | 1.68 ± 0.29c | 4.88 ± 0.25c | 0.98 ± 0.38c | 8.63 ± 0.06d | |
| Water | 7.51 ± 0.22b | 0.43 ± 0.08d | 5.83 ± 0.16b | 2.02 ± 0.41c | 19.56 ± 0.60c | |
| Devimane Ghats (DEV) | Methanol | 15.51 ± 0.22a | 6.11 ± 0.05b | 13.16 ± 0.57a | 54.80 ± 0.27b | 57.07 ± 1.58a |
| Ethanol | 13.23 ± 0.29b | 6.84 ± 0.03a | 11.94 ± 0.09b | 60.62 ± 0.61a | 51.78 ± 1.58b | |
| Pet. ether | 3.58 ± 0.22c | 3.64 ± 0.23c | 8.77 ± 0.09d | 8.68 ± 0.31d | 12.19 ± 0.39d | |
| Water | 12.84 ± 0.36b | 2.19 ± 0.12d | 10.22 ± 0.16c | 11.82 ± 1.39c | 34.62 ± 0.16c | |
| Dicholi (DIC) | Methanol | 11.15 ± 0.30a | 3.72 ± 0.03b | 8.77 ± 0.19a | 34.24 ± 1.14b | 38.33 ± 0.21a |
| Ethanol | 9.55 ± 0.58b | 4.86 ± 0.34a | 6.89 ± 0.33c | 46.11 ± 0.27a | 36.89 ± 0.96b | |
| Pet. ether | 1.60 ± 0.29d | 2.01 ± 0.14c | 5.11 ± 0.09d | 1.78 ± 0.10c | 9.08 ± 0.26d | |
| Water | 8.77 ± 0.22c | 0.66 ± 0.06d | 6.05 ± 0.25b | 2.61 ± 0.30c | 20.40 ± 0.60c | |
| Jogfalls (JOG) | Methanol | 13.67 ± 0.43a | 5.09 ± 0.30b | 11.16 ± 0.16a | 40.60 ± 0.48b | 48.04 ± 0.06a |
| Ethanol | 12.07 ± 0.38b | 6.13 ± 0.16a | 9.33 ± 0.16b | 50.97 ± 0.23a | 44.65 ± 0.62b | |
| Pet. ether | 2.90 ± 0.43c | 2.74 ± 0.12c | 7.05 ± 0.09d | 6.71 ± 0.46c | 11.28 ± 0.16d | |
| Water | 11.49 ± 0.14b | 1.52 ± 0.11d | 8.83 ± 0.16c | 7.12 ± 0.59c | 31.27 ± 0.06c | |
| Kogar (KOG) | Methanol | 12.12 ± 0.74a | 4.17 ± 0.05b | 9.72 ± 0.19a | 37.22 ± 0.47b | 44.23 ± 0.96a |
| Ethanol | 10.76 ± 0.38b | 5.68 ± 0.12a | 7.66 ± 0.19b | 47.34 ± 0.11a | 41.75 ± 0.24b | |
| Pet. ether | 2.42 ± 0.30c | 2.23 ± 0.17c | 5.94 ± 0.25d | 2.70 ± 0.10c | 9.74 ± 0.10d | |
| Water | 9.98 ± 0.16b | 0.80 ± 0.12d | 6.50 ± 0.16c | 3.19 ± 0.74c | 25.01 ± 0.60c | |
| Koyana (KOY) | Methanol | 17.16 ± 0.14a | 6.98 ± 0.08a | 13.88 ± 0.28a | 60.43 ± 1.31a | 62.47 ± 0.96a |
| Ethanol | 14.15 ± 0.22b | 7.00 ± 0.05a | 12.27 ± 0.34b | 64.34 ± 0.05a | 58.49 ± 0.62b | |
| Pet. ether | 3.87 ± 0.16d | 4.19 ± 0.12b | 9.50 ± 0.16d | 9.48 ± 4.34b | 12.92 ± 0.21d | |
| Water | 13.52 ± 0.29c | 2.80 ± 0.06c | 10.77 ± 0.09c | 13.33 ± 1.87b | 35.95 ± 0.27c | |
| Nagavelli (NAG) | Methanol | 18.18 ± 0.14a | 7.98 ± 0.14b | 14.38 ± 0.82a | 65.28 ± 0.58b | 66.73 ± 0.60a |
| Ethanol | 15.32 ± 3.20bc | 8.35 ± 0.20a | 13.95 ± 0.57a | 67.48 ± 0.17a | 64.36 ± 0.36b | |
| Pet. ether | 4.75 ± 0.44d | 5.03 ± 0.20c | 10.94 ± 2.21b | 10.65 ± 0.46c | 14.11 ± 0.16d | |
| Water | 14.06 ± 0.83c | 3.37 ± 0.08d | 11.22 ± 1.34b | 14.44 ± 0.41d | 37.98 ± 0.16c | |
| Talakaveri (TAL) | Methanol | 14.25 ± 0.25a | 5.76 ± 0.10b | 12.00 ± 0.16a | 41.8 ± 0.68b | 53.45 ± 0.62a |
| Ethanol | 12.60 ± 0.65b | 6.47 ± 0.05a | 10.38 ± 0.19b | 57.05 ± 0.37a | 46.75 ± 0.36b | |
| Pet. ether | 3.05 ± 0.29c | 3.07 ± 0.08c | 7.50 ± 0.16d | 7.94 ± 0.18c | 11.98 ± 0.36d | |
| Water | 12.02 ± 0.08b | 1.84 ± 0.06d | 9.66 ± 0.16c | 9.07 ± 0.69c | 33.29 ± 0.12c |
Values are mean of three parallel experiments (mean ± SD)
The superscripts with different letters indicate significantly different values (Duncan multiple rang test, PB ≤ 0.05)
aGallic acid equivalent per gram dry weight
bRutin equivalent per gram dry weight
cColchicine equivalent per gram dry weight
dFeSO4 equivalent per gram dry weight
Total flavonoid content
Ethanolic extract of berries showed highest TFC followed by methanol, petroleum ether and water extracts. The results revealed that berries collected from NAG accession had the highest amount of TFC (8.35 ± 0.20 mg RE/g DW) in ethanolic extract, followed by KOY > DEV > TAL > JOG > AMB > KOG > DIC > CHA > BIS accessions. Among the studied accessions, BIS accession showed lowest TFC (0.23 ± 0.05 mg RE/g DW) in berries extracted with water (Table 2). In the present study, we found that ethanol was the most suitable solvent for extraction of the flavonoids among all the solvents used. The TFC in berries of E. ribes was higher than bark of E. ribes as compared to those reported in the literature with 1.35 mg QE/g of TFC (Sulaiman and Balachandran 2012). Similar kind of results were noted from the fruit pulp of Salacia chinensis L. extracted with different solvents (Chavan et al. 2012).
Total alkaloid content
Variation in TAC was observed in berries of E. ribes from different accessions. The results revealed that berries collected from NAG accession have the highest amount of TAC (14.38 ± 0.82 mg CE/g DW) in methanolic extract, followed by KOY > DEV > TAL > JOG > AMB > KOG > DIC > CHA > BIS accessions (Table 2). Among the studied accessions, BIS accession showed lowest TAC (3.83 ± 0.33 mg CE/g DW) in petroleum ether extract. It was also seen that methanol found to be the best solvent for extraction of the alkaloid among all the solvents. Methanolic extract of berries showed higher TAC followed by ethanol, water and petroleum ether extracts. Our finding revealed TAC was depend on the solvent used. The TAC was found to maximum in methanolic extract of Urginea wightii and U. indica with 3.101 ± 0.056 and 2.897 ± 0.319 µg/ml of TAC as compared to petroleum ether extract (Raj et al. 2017). Similar results for TAC in different plant parts of Anogeissus latifolia have been reported by Patil and Gaikwad (2011).
DPPH radical scavenging activity
DPPH radical scavenging activity of different solvent extracts of E. ribes berries from ten different accessions are shown in Table 2. Ethanolic extract of berries from NAG accession exhibited considerably higher DPPH radical scavenging activity (67.48 ± 0.17%) compared to other extracts. This trend was similar to that of TFC. Lowest DPPH radical scavenging activity was found in petroleum ether extract of berries (0.54 ± 0.34%) from the BIS accession. It was observed that highest antioxidant activity was reported in ethanolic extract of E. ribes berries among all the solvents used. Similar results for DPPH radical scavenging activity with 71.67 ± 0.81 and 69.57 ± 0.71% inhibition was observed in berries of E. ribes (Neelam et al. 2011; Shadma and Naheed 2014).
Ferric reducing antioxidant power assay (FRAP)
FRAP radical-scavenging activity of E. ribes berries from ten different accessions are shown in Table 2. The highest FRAP activity was observed in methanolic extract of berries form NAG accession with 66.73 ± 0.60 mg Fe(II)/g DW as compared to ethanol, water and petroleum ether extracts. Lowest FRAP activity was found in petroleum ether extract of berries with 8.42 ± 0.21 mg Fe(II)/g DW from BIS accession. Similar results for FRAP assay have been reported with 422 ± 2.15 and 398 ± 2.16 mM Fe(II)/g DW from the berries of E. ribes (Neelam et al. 2011; Shadma and Naheed 2014). Overall, NAG accession accounted with the higher value of antioxidant activities.
The correlation analysis showed strong positive correlation between TPC and FRAP with r-value 0.939 followed by TFC and DPPH i.e. 0.909 (Table 3). Correlation between TPC X FRAP and TFC X DPPH indicated r values greater than 0.9 (p < 0.01). TPC and TFC present in the berries were responsible for the strongest antioxidant activities (DPPH and FRAP). Many workers have been correlated the antioxidant activities of the plant extract with the total phenolic content (Shan et al. 2005; Cai et al. 2006; Li et al. 2008). The phytochemicals such as total phenolics and flavonoids have shown very high association with dietary antioxidants (Singh et al. 2018). In the present study positive correlation was found between the TPC and AOA in all the localities of E. ribes.
Table 3.
Correlation between antioxidant activities (DPPH and FRAP) and studied phytochemicals (TPC, TTC, and TFC) from the berries of E. ribes
| Studied parameters | TPC | TFC | TAC | DPPH | FRAP |
|---|---|---|---|---|---|
| TPC | 0.597** | 0.790** | 0.707** | 0.939** | |
| TFC | 0.597** | 0.752** | 0.909** | 0.784** | |
| TAC | 0.790** | 0.752** | 0.634** | 0.801** |
**Correlation is significant at the 0.01 level (2-tailed)
Principal component analysis (PCA)
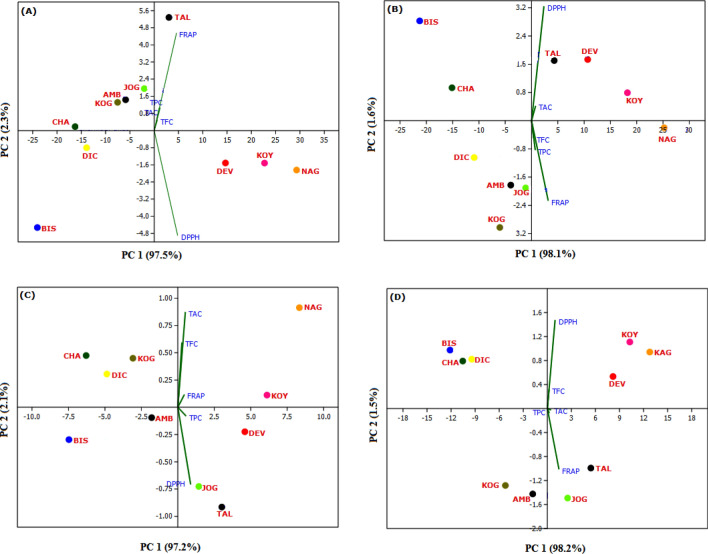
Principal Component Analysis (PCA) was performed to recognize the relationship between phytochemicals (TPC, TFC, and TAC) and antioxidant activities (DPPH and FRAP) of methanol, ethanol, petroleum ether and water extracts of ten different accessions of E. ribes (Fig. 1). PCA scatter plot of methanolic extracts exhibited 97.5 and 2.3% of total variance for first (PC1) and second (PC2) principal component, respectively (Fig. 1a). All the variables such as TPC, TFC, TAC and FRAP were placed at positive side of PC1 with loading values of 0.139, 0.177, 0.068, 0.59, respectively; whereas, DPPH was located at positive side of PC1 with loading value 0.773. The variables viz. TPC, TFC and TAC were positively correlated with FRAP activity. Both the PC1 and PC2 were resolved all methanolic extracts of TAL accession with high TPC, TFC, TAC and strong FRAP activity (Fig. 1a).
Fig. 1.
Principal component analysis (scores and loading plots) based on different phytochemicals analysed from the extracts of ten Embelia ribes genotypes and their antioxidant activities. A methanol, B ethanol, C water, D petroleum ether, TPC total phenolic content, TFC total flavonoid content, TAC total alkaloid content, DPPH 2-diphenyl-1-picrylhydrazyl (DPPH) assay, FRAP ferric reducing antioxidant power assay, AMB Amba, BIS Bisle Ghats, CHA Charmadi Ghats, DEV Devimane Ghats, DIC Dicholi, JOG Jogfalls, KOG Kogar, KOY Koyana, NAG Nagavelli, TAL Talakaveri
PCA of ethanolic extract demonstrated 99.7% of total variance (Fig. 1b). PC1 denoted 98.1% variance, wherein PC2 showed 1.6% of variance. The variables DPPH and TAC were positively associated with each other for both PC1 and PC2 with loading values of 0.173 and 0.551, respectively. However, the variables TFC, TPC and FRAP were negatively associated with loading value 0.07, 0.173 and 0.791, respectively. PCA analysis of ethanolic extract showed that KOY, DEV and TAL accessions were positively positioned on both PC1 and PC2 with significant TAC and strong DPPH activity (Fig. 1b). Similarly, PCA of water extract exhibited 97.2 and 2.1% of total variance for PC1 and PC2, respectively (Fig. 1c). The all variables such as TAC, TFC and FRAP were loaded at positive side of PC1 with loading values of 0.375, 0.103, 0.416, respectively; whereas, TPC and DPPH was loaded at negative side of PC2 with loading value 0.254 and 0.782, respectively. Variables viz. TAC and TFC were positively correlated with FRAP activity, wherein TPC was positively correlated with strongest DPPH activity. PCA depicted that NAG and KOY accessions were placed along the positive side of both principle components with high TAC, TFC and strong FRAP activity (Fig. 1c).
PCA of petroleum ether extract showed 99.7% of total variance (Fig. 1d). PC1 donated 98.2% variance, wherein PC2 showed 1.5% variance. The variables TFC and DPPH were positively associated with loading values of 0.087 and 0.817, respectively. However, the variables TPC, TAC and FRAP were negatively associated with loading value 0.250, 0.271 and 0.435, respectively. PCA analysis of petroleum ether extract showed that KOY, NAG and DEV accessions were positively positioned on both PC1 and PC2 with significant TFC and strong DPPH activity (Fig. 1d). PCA results showed that the TPC and TFC were most influencing components for the strongest antioxidant activities in different solvent extracts of E. ribes. In the present work, PCA was found to be the most efficient tool for the delineation and characterization of different accessions based on various phytochemicals and antioxidant activities. Four accessions viz. TAL, DEV, KOY and NAG showed positive correlation with phytochemicals and antioxidant activities. The present investigation concurs with the earlier researchers and revealed the discrimination of different genotypes, plant parts etc. (Ghadage et al. 2017; Ghane et al. 2018; Attar and Ghane 2019; Yadav et al. 2020). Ghadage et al. (2017) and Yadav et al. (2020) have been also found that studied phytochemicals are the known compounds that are potent antioxidants.
Preliminary screening of genotypes and embelin content
RP-HPLC analysis showed variation in the embelin content among the different genotypes as shown in Supplementary Table 1. Separation of standard embelin was observed using methanol: water: acetic acid: tetrahydrofuran (85: 15: 3: 0.1 v/v/v/v) at 7.43 ± 0.3 min (Fig. 2). The yield of embelin content assessed ranged from 1.005 ± 0.02 to 1.770 ± 0.01%. Preliminary analysis revealed that among all the genotypes, NAG accession revealed highest (1.707%) embelin content; however, lowest (1.005%) was noted from the BIS accession (Supplementary Table 1).
Fig. 2.
HPLC chromatograph of standard and berries of Nagavelli genotype extracted using microwave assisted extraction (MAE)
RP-HPLC analysis of optimized extraction of embelin using different extraction methods
The berries collected from NAG accession of E. ribes were used for quantification of embelin content by using various extraction methods. The variable response was recorded for embelin content extracted with several extraction methods. The yield of embelin assessed from NAG accession ranges from 1.23 ± 0.01 to 5.08 ± 0.09% (Table 4, Fig. 2). Among the extraction methods employed, MAE with 90 s (5.08%) was found to be superior followed by UE for 30 min (2.98%), SE for 300 min (1.80%) and CSE for 360 min (1.77%) (Table 4). Sudhakar et al. (2005) have been reported 4.33 and 3.96% of embelin content in E. ribes and E. robusta, respectively. Embelin content was also quantified by HPLC method in various samples of E. ribes collected from different geographical regions. The percentage of embelin estimated in present study i.e. 5.08% was comparatively higher than the earlier reports (Nagamani et al. 2013). The different extraction procedure affected the embelin content from the same locality. It was depicted that the extraction conditions could affect the yield of embelin content. Our findings on MAE are not in lined with the earlier report (Latha 2007). MAE has been the finest selection method due to its high efficiency in terms of extraction yield, low level of energy and solvent consumption, fast extraction and higher yield (Alara et al. 2018; Sweeta et al. 2019). The extraction methods have been known to alter the level of polyphenols in A. heyneanus (Pai et al. 2011), C. nutans (Mustapa et al. 2015) and S. chinensis (Ghadage et al. 2017).
Table 4.
Embelin content from berries of Nagavelli accession with respect to different extraction methods and extraction durations
| Sr. no | Extraction methods | Time intervals | Embelin content (%) |
|---|---|---|---|
| 1 | Continuous shaking extraction (CSE) | 180 min | 1.52 ± 0.01k |
| 360 min | 1.77 ± 0.01j | ||
| 540 min | 1.47 ± 0.02l | ||
| 720 min | 1.34 ± 0.01n | ||
| 2 | Soxhlet extraction (SE) | 120 min | 1.23 ± 0.01i |
| 180 min | 1.36 ± 0.01m | ||
| 240 min | 1.37 ± 0.07m | ||
| 300 min | 1.80 ± 0.02o | ||
| 3 | Ultrasonication extraction (UE) | 10 min | 1.83 ± 0.03h |
| 20 min | 2.44 ± 0.04g | ||
| 30 min | 2.98 ± 0.01d | ||
| 40 min | 2.67 ± 0.01f | ||
| 4 | Microwave assisted extraction (MAE) | 30 s | 2.75 ± 0.01e |
| 60 s | 3.42 ± 0.02c | ||
| 90 s | 5.08 ± 0.09a | ||
| 120 s | 3.47 ± 0.02b |
Values are mean of three parallel experiments (mean ± SD). The superscripts with different letters indicate significantly different values (Duncan multiple rang test, PB ≤ 0.05)
In the present investigation MAE for 90 s was found to be the most suitable extraction method for extraction of embelin content as compared to other methods.
Conclusion
Solvent used in the extraction significantly influenced amount of TPC, TFC, TAC and antioxidant activities from the ten different genotypes of E. ribes. From the phytochemical analysis, Nagavelli (NAG) showed promising antioxidant potential in terms of phytochemicals (TAC, TPC and TFC) and antioxidant activities (DPPH and FRAP). Furthermore, PCA revealed that TPC and TFC were the most influencing components to exhibit strong antioxidant activities. Among all the methods employed, microwave assisted extraction with 90 s proved to be fastest, simple and reproducible method for the highest embelin content. Nagavelli accession yielded the highest, 5.08 ± 0.01% of embelin content in MAE compared to other extraction methods. The present investigation on phytochemicals, antioxidants and optimized production of embelin content from Nagavelli accession revealed that it could be a promising candidate for the phamaceutical utilization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the Head, Department of Botany, Shivaji University, Kolhapur for providing the necessary facilities. We are thankful to Prof. S. R. Yadav, Dr. M. M. Lekhak, Dr. Ravikumar and Dr. Sharad Kamble for their help in current research. VVK is thankful to UGC, New Delhi for financial support under UGC-BSR Fellowship in Sciences for Students (No. F.25-1/2013-14 (BSR)/7-163/2007(BSR)).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alara OR, Abdurahman NH, Mudalip SKA, Olalere OA. Microwave assisted extraction of Vernonia amygdalina leaf for optimal recovery of total phenolic content. J Appl Res Med Aromat Plants. 2018;10:16–24. doi: 10.1016/j.jarmap.2018.04.004. [DOI] [Google Scholar]
- Angiosperm Phylogeny Group IV (APG IV) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc. 2016;181:1–20. doi: 10.1111/boj.12385. [DOI] [Google Scholar]
- Ansari MN, Bhandari U. Antihyperhomocysteinemic activity of ethanol extracts of Embelia ribes Burm. f. in Albino rats. Pharm Biol. 2008;46:283–287. doi: 10.1080/13880200701741146. [DOI] [Google Scholar]
- Ansari MN, Bhandari U. Effect of ethanolic extracts of Embelia ribes Burm. F. fruits on isoproterenol induced myocordial infarction in Albino rats. Pharm Biol. 2009;46:928–932. doi: 10.1080/13880200802367254. [DOI] [PubMed] [Google Scholar]
- Ansari MN, Bhandari U, Islam F, Tripathi CD. Evaluation of antioxidant and neuroprotective effect of ethanolic extracts of Embelia ribes Burm. f. in focal cerebral ischemia/reperfusion- induced oxidative stress in rats. Fund Clin Pharmocol. 2008;22:305–314. doi: 10.1111/j.1472-8206.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- Attar UA, Ghane SG. In vitro antioxidant, antidiabetic, antiacetylcholine esterase, anticancer activities and RP-HPLC analysis of phenolics from the wild bottle gourd (Lagenaria siceraria (Molina) Standl.) S Afr J Bot. 2019;125:360–370. doi: 10.1016/j.sajb.2019.08.004. [DOI] [Google Scholar]
- Benzie IF, Strain JJ. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extracts of Embelia ribes Burm. f. on dyslipidemia in diabetic rats. Int J Exp Diabetes Res. 2002;3:159–162. doi: 10.1080/15604280290013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YZ, Sun M, Xing J, Luo Q, Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Chang CY, Wen H, Chern J. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chavan JJ, Jagtap UB, Gaikwad NB, Dixit GB, Bapat VA. Total phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) fruit pulp. J Plant Biochem Biot. 2012;22:409–413. doi: 10.1007/s13562-012-0169-3. [DOI] [Google Scholar]
- Chitra M, Sukumar E, Suja V, Devi CSS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40:109–113. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- Choudhary HS, Bhandari U, Khanna G. Preventive effect of embelin from Embelia ribes on lipid metabolism and oxidative stress in high fat diet induced obesity in rats. Planta Med. 2012;78:651–657. doi: 10.1055/s-0031-1298379. [DOI] [PubMed] [Google Scholar]
- Francesco C, Miriam R, Sarjit K, Emmanuel GV, Nora M, Stuart B, Joanna DS, Fabio G, Jens ZP, Sandra I. Antioxidant properties of embelin in cell culture. Electrochemistry and theoretical mechanism of scavenging. Potential scavenging of superoxide radical through the cell membrane. Antioxidants. 2020;9:382. doi: 10.3390/antiox9050382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadage DM, Kshirsagar PR, Pai SR, Chavan JJ. Extraction efficiency, phytochemical profiles and antioxidative properties of different parts of Saptarangi (Salacia chinensis L.)—an important underutilized plant. Biochem Biophys Rep. 2017;12:79–90. doi: 10.1016/j.bbrep.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghane SG, Attar UA, Yadav PB, Lekhak MM. Antioxidant, antidiabetic, acetylcholine esterase inhibitory potential and estimation of alkaloid (lycorine and galanthamine) from Crinum sp.—an important source of anticancer and antialzheimer drug. Ind Crops Prod. 2018;125:168–177. doi: 10.1016/j.indcrop.2018.08.087. [DOI] [Google Scholar]
- Jagtap UB, Panaskar SN, Bapat VA. Evaluation of antioxidant capacity and phenol content in Jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Food Hum Nutr. 2010;65:99–104. doi: 10.1007/s11130-010-0155-7. [DOI] [PubMed] [Google Scholar]
- Jiun YL, Ray JC, Li TH, Tzu YL, Wan JL, Kuan HL. Embelin as a novel inhibitor of PKC in the prevention of platelet activation and thrombus formation. Clin Med. 2019;8:1724. doi: 10.3390/jcm8101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latha C. Microwave-assisted extraction of embelin from Embelia ribes. Biotechnol Lett. 2007;29:319–322. doi: 10.1007/s10529-006-9243-z. [DOI] [PubMed] [Google Scholar]
- Li HB, Wong CC, Cheng KW, Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci Technol. 2008;41:385–390. doi: 10.1016/j.lwt.2007.03.011. [DOI] [Google Scholar]
- Liu X, Dong M, Chen X, Jiang M, Lv X, Yan G. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Gingko biloba. Food Chem. 2007;105:548–554. doi: 10.1016/j.foodchem.2007.04.008. [DOI] [Google Scholar]
- Mhaskar M, Joshi S, Chavan B, Joglekar A, Barve N, Patwardhan A. Status of Embelia ribes Burm f. (Vidanga), an important medicinal species of commerce from northern Western Ghats of India. Curr Sci. 2011;100:547–552. [Google Scholar]
- Mustapa AN, Martin A, Mato RB, Cocero KJ. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind Crops Prod. 2015;74:83–94. doi: 10.1016/j.indcrop.2015.04.035. [DOI] [Google Scholar]
- Nagamani V, Sabitha RA, Satyakala M, Chandrashekar RGNV. High performance liquid chromatography (HPLC) analysis of embelin in different samples of Embelia ribes Burm. F.—a threatened medicinal plant of India. J Med Plants Res. 2013;7:1761–1767. doi: 10.5897/JMPR2013.4447. [DOI] [Google Scholar]
- Neelam J, Shaily G, Ramawat KG. Evaluation of antioxidant properties and total phenolic content of medicinal plants used in diet therapy during postpartum healthcare in Rajasthan. Int J Pharm Pharm Sci. 2011;3:248–253. [Google Scholar]
- Nikolovska CZ, Xu L, Hu Z, Tomita Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, Lippman ME, Yang D, Wang S. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure based computational screening of a traditional herbal medicine three dimensional structure database. J Med Chem. 2004;47:2430–2440. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- Pai SR, Nimbalkar MS, Pawar NV, Dixit GB. Optimization of extraction techniques and quantification of betulinic acid (BA) by RP-HPLC method from Ancistrocladus heyneanus Wall. Ex Grah. Ind Crops Prod. 2011;34:1458–1464. doi: 10.1016/j.indcrop.2011.05.006. [DOI] [Google Scholar]
- Patil UH, Gaikwad DK. Seasonal dynamics in the nutritional and antinutritional status of stem bark of Anogeissus latifolia. Int J Appl Biol Pharm Tech. 2011;2:370–378. [Google Scholar]
- Raj SM, Kameshwari MNS, Tharasaraswathi KJ, Shubharani R. Qualitative and quantitative analysis of phytochemicals in two different species of Urginea. Int J Pharm Biol Sci. 2017;8:5433–5438. [Google Scholar]
- Rajashekaran PE. Biodiversity of threatened species of medicinal plants in India. In: Hosette BB, Venkateshwarulu M, editors. Trends in wild life biodiversity conservation and management. India: Daya Publishing House; 2001. pp. 104–125. [Google Scholar]
- Ravikumar K, Ved DK (eds) (2000) Hundred red listed medicinal plants of conservation concern in Southern India. Foundation for revitalization of local health traditions (FRLHT), Bangalore, pp 45-47. https://trove.nla.gov.au/version/40758549
- Shadma S, Naheed A. Antioxidant properties and total phenolic content of herbs used in postpartum diet therapy in Patna (Bihar), India. Int J Pharm Biol Sci. 2014;9:17–20. doi: 10.9790/3008-09231720. [DOI] [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agr Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Sharanbasappa D, Veeresh PV, Satrasala N, Shivsharan BD. Antidiabetic activity of Embelia ribes, embelin and its derivatives: a systematic review and meta-analysis. Biomed Pharmacother. 2017;86:195–204. doi: 10.1016/j.biopha.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Singh SP, Shukla S, Yadav HK. Multivariate analysis in relation to breeding system in Opium poppy (Papaver somniferum L.) Genetika. 2004;36:111–120. doi: 10.2298/GENSR0402111S. [DOI] [Google Scholar]
- Singh D, Singh R, Singh P, Gupta RS. Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin Pharmacol Toxicol. 2009;105:243–248. doi: 10.1111/j.1742-7843.2009.00429.x. [DOI] [PubMed] [Google Scholar]
- Singh BK, Arti Koley TK, Maurya Singh PM, Singh B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. Sativus Schubl. & Martens) Physiol Mol Biol Plants. 2018;24:899–907. doi: 10.1007/s12298-018-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Soumya NM, Ranjith A, Arumughan C. RP-HPLC-DAD method for the estimation of embelin as marker in Embelia ribes and its polyherbal formulations. Biomed Chromatogr. 2011;25:600–605. doi: 10.1002/bmc.1489. [DOI] [PubMed] [Google Scholar]
- Souravi K, Rajasekharan PE. Ethnopharmacological Uses of Embelia ribes Burm. F.—a review. Int J Pharm Biol. 2014;9:23–30. [Google Scholar]
- Sudhakar RS, Unnikrishnan KP, Ravindran PN, Indira B. Determination of embelin in Embelia ribes and Embelia tsjeriamcottam by HPLC. Indian J Pharm Sci. 2005;67:734–736. [Google Scholar]
- Sulaiman CT, Balachandran I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J Pharm Sci. 2012;74:258–260. doi: 10.4103/0250-474x.106069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeta A, Nour HA, Rosli MY, Fahim F. Microwave-assisted extraction of saponin, phenolic and flavonoid compounds from Trigonella foenum-graecum seed based on two level factorial design. J Appl Res Med Aromat Plants. 2019;14:1–10. doi: 10.1016/j.jarmap.2019.100212. [DOI] [Google Scholar]
- Vinutha B, Prashanth D, Salma K, Sreeja SL, Padmaja PD. Screening of selected Indian medicinal plants for acetylcholiesterase inhibitory activity. J Ethnopharmacol. 2007;109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Xiaole C, Min G, Rongchao J, Weiqian DH, Xiaowen T, Yuling L, Denggao Z, Kun Z, Wenhua C, Xi Z, Zhaojun S, Panpan W. Design, synthesis and a-glucosidase inhibition study of novel embelin derivatives. J Enzyme Inhib Med Chem. 2020;35:565–573. doi: 10.1080/14756366.2020.171538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Cui J, Fu H, Proksch P, Lin W, Li M. Embelin derivatives and their anticancer activity through microtubule disassembly. Planta Med. 2005;71:944–948. doi: 10.1055/s-2005-871250. [DOI] [PubMed] [Google Scholar]
- Yadav PB, Lekhak UM, Ghane SG, Lekhak MM. Phytochemicals, antioxidants, estimation of cardiac glycoside (Scillaren A) and detection of major metabolites using LC-MS from Drimia species. S Afr J Bot. 2020 doi: 10.1016/j.sajb.2020.05.002. [DOI] [Google Scholar]
- Zhou K, Yu L. Antioxidant properties of bran extracts from Trego wheat grown at different locations. J Agr Food Chem. 2004;52:1112–1117. doi: 10.1021/jf030621m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.