Abstract
Purpose
To perform complex preimplantation genetic tests (PGT) for aneuploidy screening, Robertsonian translocation, HLA-matching, and X-linked hyper IgM syndrome (XHIGM) caused by a novel mutation c.156 G>T of CD40LG gene.
Methods
Reverse transcription PCR (RT-PCR) and Sanger sequencing were carried out to confirm the causative variant of CD40LG gene in the proband and parents. Day 5 and D6 blastocysts, obtained by in vitro fertilization (IVF) with intracytoplasmic sperm injection, underwent trophectoderm (TE) biopsy and whole genomic amplification (WGA) and next generation sequencing (NGS)-based PGT to detect the presence of a maternal CD40LG mutation, aneuploidy, Robertsonian translocation carrier, and human leukocyte antigen (HLA) haplotype.
Results
Sanger sequencing data of the genomic DNA showed that the proband has a hemizygous variant of c. 156 G>T in the CD40LG gene, while his mother has a heterozygous variant at the same position. Complementary DNA (cDNA) of CD40LG amplification and sequencing displayed that no cDNA of CD40LG was found in proband, while only wild-type cDNA of CD40LG was amplified in the mother. PGT results showed that only one of the six tested embryos is free of the variant c.156 G>T and aneuploidy and having the consistent HLA type as the proband. Meanwhile, the embryo is a Robertsonian translocation carrier. The embryo was transplanted into the mother’s uterus. Amniotic fluid testing results are consistent with that of PGT. A healthy baby girl was delivered, and the peripheral blood testing data was also consistent with the testing results of transplanted embryo.
Conclusions
The novel mutation of c. 156 G>T in CD40LG gene probably leads to XHIGM by nonsense-meditated mRNA decay (NMD), and complex PGT of preimplantation genetic testing for monogenic disease (PGT-M), aneuploidy (PGT-A), structural rearrangement (PGT-SR), and HLA-matching (PGT-HLA) can be performed in pedigree with both X-linked hyper IgM syndrome and Robertsonian translocation.
Keywords: XHIGM, CD40LG, Robertsonian translocation, HLA, PGT
Introduction
Hyper IgM syndrome (HIGM) is a rare immunodeficiency disorder characterized by normal or increased serum IgM and decreased IgG, IgA, and IgE due to defective immunoglobulin class switching. X-linked HIGM (XHIGM) is the most frequent type with defects in immunoglobulin class switch recombination Ig-CSR previously shown to be caused by mutations in the genes encoding CD40 ligand (CD40LG) and regarded as a combined T and B cells immunodeficiency disease [1, 2]. Patients with HIGM are highly susceptible to recurrent sinopulmonary infections, Pneumocystis jiroveci pneumonia (PJP), and chronic diarrhea due to Cryptosporidium infection that may lead to sclerosing cholangitis. They are also prone to intermittent or persistent neutropenia, autoimmune diseases, and malignancies [3]. Most patients with XHIGM developed symptoms in infancy. Therapy has included a long-term and continuous injection of immune globulin to avoid infections [4]. Hematopoietic stem cell transplantation (HSCT) is a healing therapeutic choice if HLA-matched donors are available [5] though it is too difficult to find one in a short time. Preimplantation genetic testing (PGT) is an acceptable option to deliver a histocompatible sibling to facilitate curative hematopoietic stem cell transplantation (HSCT) [6]. Here we study a pedigree of which the proband is a XHIGM patient, affected with a novel mutation of c.156G>T in the CD40LG gene, and his mother is a carrier both of Robertsonian translocation and XHIGM. PGT-A and PGT-SR were performed to screen aneuploidy and distinguish Robertsonian translocation carrier embryos from normal embryos. PGT-M and PGT-HLA were performed to select a HLA-compatible embryo free of XHIGM. Finally, a healthy baby girl was delivered.
Materials and methods
Patients
The proband was susceptible to infections from 1 year old. Clinical tests found that the proband was neutropenic and having low level of natural killer cells. Serum immunoglobulin levels were as follows: IgG, 397 mg/dL (reference value, 500–1200 mg/dL); IgA, 75 mg/dL (reference value, 23–190 mg/dL); IgE, < 17.10 (reference value, 0–60 IU/mL); and IgM, 195 mg/dL (reference value, 50–199 mg/dL). The proband was initially diagnosed of XHIGM.
Mutation analysis of the CD40LG gene
Genomic DNA was extracted from peripheral blood lymphocytes using commercially available kits (ZEESAN) according to manufacturer’s instructions. Then, CD40LG was amplified with primers previously reported [7] for Sanger sequencing. PCR was set up in a final volume of 25 μL, including1.25 U Taq polymerase (Thermo Scientific), 0.2 μM primers, 200 μM dNTP, and 50 ng DNA template. A preheating step at 94 °C for 3 min was carried out. The following cycling conditions for PCR were 35 cycles of denaturation (94 °C for 30 s), annealing (60 °C for 30 s), and extension (72 °C for 30 s). A final extension step consisting of 5 min at 72 °C was carried out.
RT-PCR
Total RNA was extracted from the peripheral blood according to the protocol of total RNA extraction reagent kit (Takara). Complementary DNA was synthesized according to the protocol of PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara). Specific primers for the mRNA sequence of CD40LG and ACTB were designed. The forward primer of CD40LG is 5′-ACAACCAAACTTCTCCCCGA-3′, and the reverse primer of CD40LG is 5′-TCTCCTGTGTTGCATCTCTGT-3′, with a 214 bp PCR product. The forward primer of ACTB is 5′-CAGAGCCTCGCCTTTGCCGA-3′, and the reverse primer of ACTB is 5′-ATGCCGGAGCCGTTGTCGAC-3′, with a 101 bp PCR product.
G-band karyotype
Culturing, harvesting, and metaphase preparation of the peripheral blood sample and amniotic fluid were carried out as previously described [8]. Whenever possible, at least 25 mitoses were analyzed (median number of analyzed mitoses in this material, 25). Nomenclature was according to the International System for Human Cytogenomic Nomenclature 2016 (ISCN, 2016).
IVF-TE biopsy and whole genome amplification
Informed consent was signed for the PGT cycle, and the PGT was approved by the Ethics Committee of the Reproductive Medical Hospital Affiliated to Shandong University. The couples were treated following 2 standard superovulation protocols described previously [9]. About 3–5 cells were obtained by laser-assisted trophectoderm (TE) biopsy from day 5 embryos. Obtained cells were washed and transferred to a PCR tube containing 2.5 μL phosphate buffer saline (PBS) under strictly sterile and DNA-free conditions against contamination. Whole genome amplification (WGA) was performed following the protocol of the SurePlex™ Kit (Illumina). The WGA product was used for the subsequent tests including CD40LG mutation analysis, linkage analysis, aneuploidy analysis, Robertsonian translocation carrier screening, and HLA haplotyping.
PGT-M
Single nucleotide polymorphisms (SNP) with high frequency in the genome from the 1000 Genomes Project which are located mainly 1 Mb upstream and downstream of the CD40LG gene were chosen as markers for linkage analysis. WGA product was purified, labeled, and amplified with SNP primers of the commercial kit (Beijing Zhongyi Kangwei Medical Equipment Co., Ltd.). The multiplex PCR products were sequenced on the Personal Genome Machine (PGM) (Life Technologies) as previously described methods [10]. Embryo’s variant c.156 G>T of CD40LG gene was confirmed by Sanger sequencing. PCR was set up as above described in the mutation analysis of the CD40LG gene.
PGT-A
WGA product was purified, labeled, and amplified with the commercial kit (Beijing Zhongyi Kangwei Medical Equipment Co., Ltd.) and sequenced to screen aneuploidy on the Personal Genome Machine (PGM) (Life Technologies) as previously described methods [11].
PGT-SR
SNP markers which locate nearby the centromere of the chromosome 13 and the chromosome 21 in the genomes of Southern Han Chinese (CHS) and Han Chinese in Beijing (CHB) from the 1000 Genomes Project were chosen as markers for linkage analysis and haplotyping. WGA product was purified, labeled, and amplified with SNP primers supplied by the commercial kit (Beijing Zhongyi Kangwei Medical Equipment Co., Ltd.). The multiplex PCR products of the SNPs were sequenced on the Personal Genome Machine (PGM) (Life Technologies), and data was analyzed as previously described methods [12].
PGT-HLA
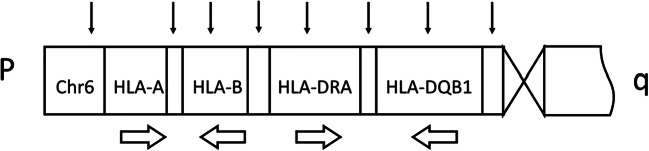
SNP markers for linkage analysis (Fig. 1) and haplotyping of HLA-A, HLA-B, HLA-DRA, and HLA-DQB1 locate upstream of the HLA-A, between the HLA-A and HLA-B, between the HLA-B and HLA-DRA, between the HLA-DRA and HLA-DQB1, and upstream of HLA-DQB1 in the genomes of Southern Han Chinese (CHS) and Han Chinese in Beijing (CHB) from the 1000 Genomes Project. WGA product was purified, labeled, and amplified with SNP primers of the commercial kit (Beijing Zhongyi Kangwei Medical Equipment Co., Ltd.). The multiplex PCR products of the SNPs associated with HLA were sequenced on the Personal Genome Machine (PGM) (Life Technologies) and analyzed with software supplied by the kit.
Fig. 1.
The distribution of the SNPs used for haplotyping of HLA. Chr6, chromosome 6; p, the short arm of chromosome; q, the long arm of chromosome; black thin arrow represent the distribution of the SNPs selected for HLA haplotyping. Broad hollow arrow represents the direction of transcription
Prenatal and postnatal testing
Amniotic fluid was collected for karyotype, HLA haplotyping, and mutation analysis of CD40LG via methods described above. The peripheral blood of the newborn was collected for HLA haplotyping and mutation analysis of CD40LG.
Results
Mutation analysis of the CD40LG gene
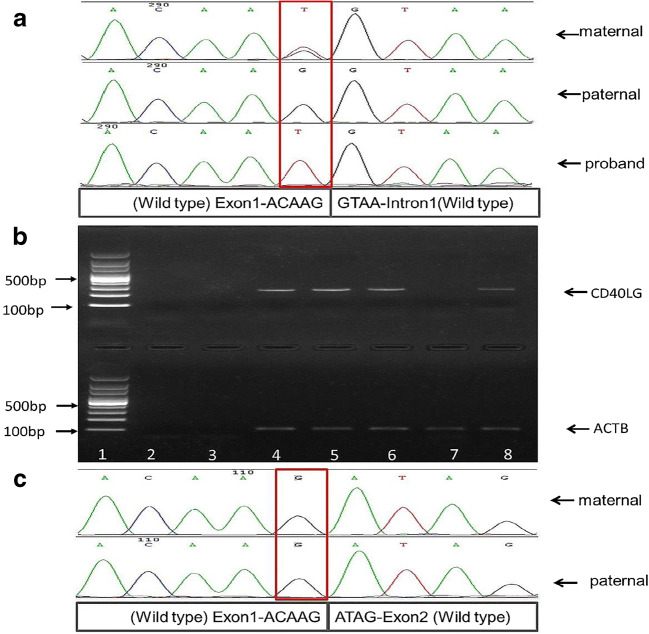
Sanger sequencing results showed that the proband has a hemizygous variant of c. 156 G>T in the CD40LG gene. The maternal variation is heterozygous and the paternal is normal (Fig. 2a).
Fig. 2.
Genomic and RT-PCR results. a Mutation analysis results of the genomic sequence of CD40LG. The proband is hemizygous of the variation c.156 G>T. The maternal variation is heterozygous and the paternal is normal. b Agarose gel electrophoresis of the cDNA product of CD40LG and ACTB. Lane 1, 100 bp DNA ladder; lane 2, blank control; lane 3, peripheral blood genomic DNA; lane 4, female control peripheral blood cDNA; lane 5, male control peripheral blood cDNA; lane 6, maternal cDNA; lane 7, cDNA of the proband; lane 8, paternal cDNA. There is no cDNA amplification of the CD40LG in the proband (lane 7), while the maternal (lane 6) and paternal (lane 8) cDNA were amplified. c Sanger sequencing results of CD40LG cDNA. The target base of c.156 in maternal cDNA sequence is wild-type G without the presence of the variant form T. The paternal target base is wild-type G as well
RT-PCR
Reverse transcription PCR and cDNA Sanger sequencing results showed that the proband has no cDNA transcription product of CD40LG, while the mother has only the wild-type target fragment (Fig. 2b–c).
G-band karyotype
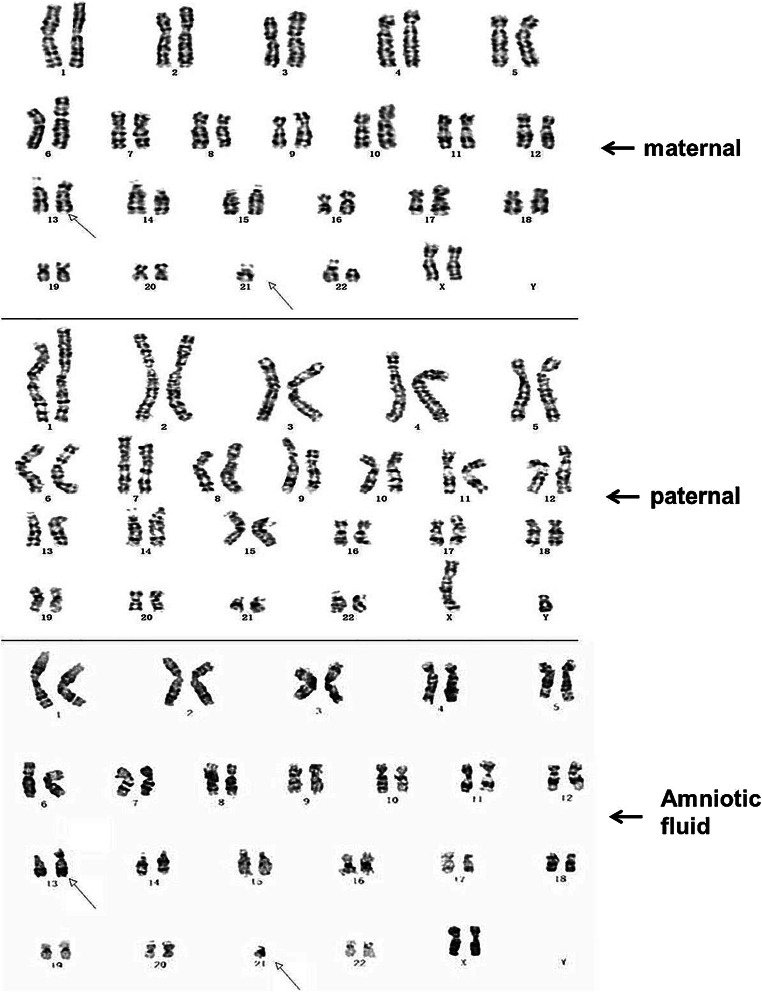
The maternal karyotype is 45, XX, der (13;21) (q10;q10), 22 ps, while the paternal karyotype is 46, XY, and the karyotype of the amniotic fluid was 45, XX, der (13;21) (q10;q10) (Fig. 3).
Fig. 3.
G-band karyotype of the pedigree. Maternal karyotype is 45, XX, der (13;21) (q10;q10), 22 ps. The paternal karyotype is 46, XY. The karyotype of the amniotic fluid was 45, XX, der (13;21) (q10;q10)
PGT results
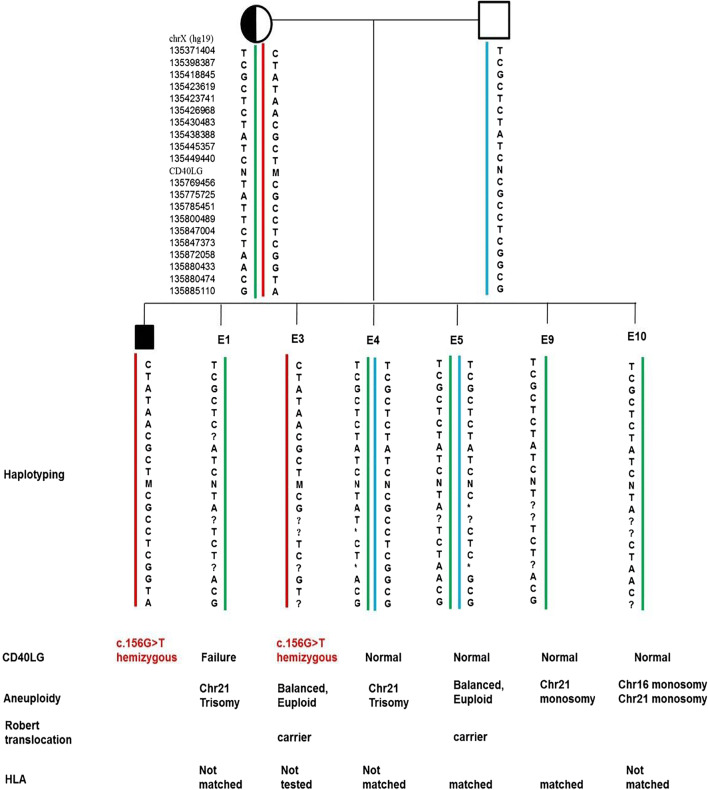
Preimplantation test results showed that only one of the six tested embryos is free of the novel variant c.156G>T in CD40LG and aneuploidy, meanwhile HLA-matched to the proband (Fig. 4).
Fig. 4.
PGT results of the pedigree. M, mutational type of CD40LG; N, wild type of CD40LG; ?, failure of amplification; *, allele dropout
Prenatal and postnatal testing and pregnant outcome
Finally, a healthy girl was born. The results of the amniotic fluid and peripheral blood of the newborn are in accordance with each other and the PGT results of the transplanted embryo.
Discussion
This study describes a complex preimplantation genetic test for Robertsonian translocation, HLA, and X-linked hyper IgM syndrome (XHIGM) caused by a novel mutation of CD40LG gene.
Hyper-immunoglobulin M (HIGM) syndrome is a heterogeneous group of primary immunodeficiency disorders, characterized by recurrent infections associated with decreased serum levels of IgG, IgA, and IgE and normal to increased serum levels of IgM [13]. XHIGM is the most common type of HIGM caused by mutations in CD40LG [14]. Neutropenia is the most common hematologic manifestation and could also be the first clinical finding in XHIGM [15]. In this study, the proband developed infection and neutropenia during the course of disease. Initial diagnosis of X-HIGM was made in the proband based on relative elevated serum IgM levels and decreased levels of IgG, IgA, and IgE. Mutation analysis of the proband revealed c.156 G>T in CD40LG. RT-PCR and cDNA sequencing results showed that no cDNA of CD40LG was amplified from the proband’s peripheral blood sample and only wild-type cDNA was found in the proband’s mother. It was predicted that variation c.156G>T which is at the exon-intron boundary of the CD40LG gene affects splicing of the mRNA and leads to a premature termination codon and nonsense-mediated mRNA decay (NMD) of the proband’s mRNA [16, 17]. This results is analogous to the previous report of which a c.156G>A mutation in CD40LG resulted in altered splicing and less than 1% of wild-type mRNA produced [18]. Meanwhile, c.156G>C, another variant at the same position of CD40LG, was reported in a XHIGM patient which was predicted to be probably damaging [19]. Above results and reports together demonstrate that the variation of c.156 G>T in CD40LG is probably damaging and causing the XHIGM.
PGT can provide couples at risk with the option of avoiding an affected pregnancy and having progeny free of XHIGM. PGT with HLA typing provides the possibility of having access to HLA-identical stem cell transplantation through selection and transfer of those unaffected embryos which are also HLA-matched to the sibling. PGT for the detection of XHIGM performed together with HLA typing for couples with children affected by genetic disorders that require HLA-identical stem cell transplantation therapy has been reported [20]. PGT for distinguishing a balanced Robertsonian translocation carrier embryo from a truly normal embryo in parallel with comprehensive chromosome screening has also been reported [21–23]. Complex preimplantation genetic tests for Robertsonian translocation, HLA, and X-linked hyper IgM syndrome (XHIGM) caused by a novel mutation of CD40LG gene has not been reported previously. For complex PGT including PGT-M, PGT-A, PGT-SR, and PGT-HLA, more DNA is required. As the biopsied TE cells are fewer (3–5 cells), WGA can provide sufficient DNA templates for the combined performance of PGT-M, PGT-A, PGT-SR, and PGT-HLA. SNP markers were applied to prevent misdiagnosis due to ADO [24] and ensure the accuracy of PGT including PGT-M, PGT-HLA, and PGT-SR. Meanwhile, NGS-based PGT-A for aneuploidy screening was used in the aneuploidy screening of embryos considering that NGS was validated as an effective platform for copy number variation in trophectoderm (TE) biopsies [25] and is potentially useful for detecting segmental aberrations on embryo biopsied [26] and low level mosaic aneuploidies on TE biopsies which were previously concluded as euploid by aCGH [27].
In conclusion, the variant of c.156G>T in CD40LG probably cause XHIGM due to the mechanism of nonsense-mediated mRNA decay. For pedigree with complex situation as the above described, NGS-based PGT-M, PGT-A, PGT-SR, and PGT-HLA can be used together to help the family. In this study, the family is expected to benefit by the birth of a healthy sibling for their affected son, along with the potential of curing their affected child through the option of having a HSCT treatment with fully compatible donors.
Acknowledgments
We thank the family for their participation in this study.
Funding information
The research was funded by the National Key Research and Development Program of China (2018YFC1003100, 2018YFC1004900), Natural Science Foundation of Shandong Province (ZR2018PH006, ZR2018MC014), and Key Research and Development Program of Shandong Province (2017G006035).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This project was approved by the Ethics Committee of the Reproductive Medical Hospital Affiliated to Shandong University.
Statement of informed consent
The couple signed informed consent forms for ICSI treatment and PGT.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, Roberts RL, Noelle RJ, Ledbetter JA, Francke U, Ochs HD. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-G. [DOI] [PubMed] [Google Scholar]
- 2.Korthauer U, Graf D, Mages HW, Briere F, Padayachee M, Malcolm S, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 3.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, Stiehm ER, Conley ME. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine. 2003;82:373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 4.Bayrakci B, Ersoy F, Sanal O, Kilic S, Metin A, Tezcan I. The efficacy of immunoglobulin replacement therapy in the long-term follow-up of the B-cell deficiencies (XLA, HIM, CVID) Turk J Pediatr. 2005;47:239–246. [PubMed] [Google Scholar]
- 5.Kato T, Tsuge I, Inaba J, Kato K, Matsuyama T, Kojima S. Successful bone marrow transplantation in a child with X-linked hyper-IgM syndrome. Bone Marrow Transplant. 1999;23:1081–1083. doi: 10.1038/sj.bmt.1701753. [DOI] [PubMed] [Google Scholar]
- 6.Bick SL, Bick DP, Wells BE, Roesler MR, Strawn EY, Lau EC. Preimplantation HLA haplotyping using tri-, tetra-, and pentanucleotide short tandem repeats for HLA matching. J Assist Reprod Genet. 2008;25:323–331. doi: 10.1007/s10815-008-9233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu L, Wang X, Wang Y, Wang J. Identification of two novel mutations in patients with X-linked primary immunodeficiencies. Fetal Pediatr Pathol. 2015;34:91–98. doi: 10.3109/15513815.2014.969414. [DOI] [PubMed] [Google Scholar]
- 8.Han L, Zhao FL, Sun QF, Wang P, Wang XA, Guo F, Fu BH, Lü YM. Cytogenetic analysis of peripheral blood lymphocytes, many years after exposure of workers to low-dose ionizing radiation. Mutat Res Genet Toxicol Environ Mutagen. 2014;771:1–5. doi: 10.1016/j.mrgentox.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Porat S, Savchev S, Bdolah Y, Hurwitz A, Haimov-Kochman R. Early serum beta-human chorionic gonadotropin in pregnancies after in vitro fertilization: contribution of treatment variables and prediction of long-term pregnancy outcome. Fertil Steril. 2007;88:82–89. doi: 10.1016/j.fertnstert.2006.11.116. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Diao Z, Xu Z, Zhou J, Yan G, Sun H. The clinical application of NGS-based SNP haplotyping for PGD of Hb H disease. Syst Biol Reprod Med. 2017;63:212–217. doi: 10.1080/19396368.2017.1296501. [DOI] [PubMed] [Google Scholar]
- 11.Sachdeva K, Discutido R, Albuz F, Almekosh R, Peramo B. Validation of next-generation sequencer for 24-chromosome aneuploidy screening in human embryos. Genet Test Mol Biomarkers. 2017;21:674–680. doi: 10.1089/gtmb.2017.0108. [DOI] [PubMed] [Google Scholar]
- 12.Treff NR, Thompson K, Rafizadeh M, Chow M, Morrison L, Tao X, Garnsey H, Reda CV, Metzgar TL, Neal S, Jalas C, Scott RT, Jr, Forman EJ. SNP array-based analyses of unbalanced embryos as a reference to distinguish between balanced translocation carrier and normal blastocysts. J Assist Reprod Genet. 2016;33:1115–1119. doi: 10.1007/s10815-016-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Notarangelo LD, Duse M, Ugazio AG. Immunodeficiency with hyper-IgM (HIM) Immunodefic Rev. 1992;3:101–121. [PubMed] [Google Scholar]
- 14.Lee WI, Torgerson TR, Schumacher MJ, Yel L, Zhu Q, Ochs HD. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005;105:1881–1890. doi: 10.1182/blood-2003-12-4420. [DOI] [PubMed] [Google Scholar]
- 15.Leven EA, Maffucci P, Ochs HD, Scholl PR, Buckley RH, Fuleihan RL, Geha RS, Cunningham CK, Bonilla FA, Conley ME, Ferdman RM, Hernandez-Trujillo V, Puck JM, Sullivan K, Secord EA, Ramesh M, Cunningham-Rundles C. Hyper IgM syndrome: a report from the USIDNET registry. J Clin Immunol. 2016;36:490–501. doi: 10.1007/s10875-016-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anna A, Monika G. Splicing mutations in human genetic disorders: examples, detection, and confirmation. J Appl Genet. 2018;59:253–268. doi: 10.1007/s13353-018-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurosaki T, Maquat LE. Nonsense-mediated mRNA decay in humans at a glance. J Cell Sci. 2016;129:461–467. doi: 10.1242/jcs.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawat A, Mathew B, Pandiarajan V, Jindal A, Sharma M, Suri D, Gupta A, Goel S, Karim A, Saikia B, Minz RW, Imai K, Nonoyama S, Ohara O, Giliani SC, Notarangelo LD, Chan KW, Lau YL, Singh S. Clinical and molecular features of X-linked hyper IgM syndrome - an experience from North India. Clin Immunol. 2018;195:59–66. doi: 10.1016/j.clim.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verlinsky Y, Rechitsky S, Sharapova T, Laziuk K, Barsky I, Verlinsky O, Tur-Kaspa I, Kuliev A. Preimplantation diagnosis for immunodeficiencies. Reprod BioMed Online. 2007;14:214–223. doi: 10.1016/S1472-6483(10)60790-0. [DOI] [PubMed] [Google Scholar]
- 21.Shamash J, Rienstein S, Wolf-Reznik H, Pras E, Dekel M, Litmanovitch T, Brengauz M, Goldman B, Yonath H, Dor J, Levron J, Aviram-Goldring A. Preimplantation genetic haplotyping a new application for diagnosis of translocation carrier's embryos- preliminary observations of two robertsonian translocation carrier families. J Assist Reprod Genet. 2011;28:77–83. doi: 10.1007/s10815-010-9483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zeng Y, Ding C, Cai B, Lu B, Li R, Xu Y, Xu Y, Zhou C. Preimplantation genetic testing of Robertsonian translocation by SNP array-based preimplantation genetic haplotyping. Prenat Diagn. 2018;38:547–554. doi: 10.1002/pd.5258. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Zhang Z, Niu W, Yang Q, Yao G, Shi S, Jin H, Song W, Chen L, Zhang X, Guo Y, Su Y, Hu L, Zhai J, Zhang Y, Dong F, Gao Y, Li W, Bo S, Hu M, Ren J, Huang L, Lu S, Xie XS, Sun Y. Mapping allele with resolved carrier status of Robertsonian and reciprocal translocation in human preimplantation embryos. Proc Natl Acad Sci U S A. 2017;114:E8695–EE702. doi: 10.1073/pnas.1715053114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bakker PI, McVean G, Sabeti PC, Miretti MM, Green T, Marchini J, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng H, Jin H, Liu L, Liu J, Wang WH. Application of next-generation sequencing for 24-chromosome aneuploidy screening of human preimplantation embryos. Mol Cytogenet. 2015;8:38. doi: 10.1186/s13039-015-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vera-Rodriguez M, Michel CE, Mercader A, Bladon AJ, Rodrigo L, Kokocinski F, et al. Distribution patterns of segmental aneuploidies in human blastocysts identified by next-generation sequencing. Fertil Steril. 2016;105:1047–55 e2. doi: 10.1016/j.fertnstert.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Chow JFC, Yeung WSB, Lee VCY, Lau EYL, Ng EHY. Evaluation of preimplantation genetic testing for chromosomal structural rearrangement by a commonly used next generation sequencing workflow. Eur J Obstet Gynecol Reprod Biol. 2018;224:66–73. doi: 10.1016/j.ejogrb.2018.03.013. [DOI] [PubMed] [Google Scholar]