Abstract
Purpose
This systematic review and meta-analysis aimed to compare pregnancy outcomes between immediate frozen embryo transfer (FET) performed within the first menstrual cycle after oocyte retrieval and delayed FET following subsequent cycles.
Methods
PubMed, EMBASE, and Web of Science were searched for eligible studies through January 2020. The main outcome measures were clinical pregnancy rate (CPR), live birth rate (LBR), and pregnancy loss rate (PLR). The effect size was estimated as risk ratio (RR) with 95% confidence interval (CI) using a random effects model. Inter-study heterogeneity was assessed by the I2 statistic.
Results
Twelve retrospective cohort studies involving 18,230 cycles were included. The pooled results revealed no significant differences between delayed and immediate FET in CPR (RR 0.94, 95% CI 0.87–1.03; I2 = 67.9%), LBR (RR 0.94, 95% CI 0.85–1.03; I2 = 67.5%), and PLR (RR 1.05, 95% CI 0.87–1.26; I2 = 42.7%). Subgroup analyses of freeze-all cycles showed a marginal decrease of CPR in delayed FET (RR 0.93, 95% CI 0.86–1.00; I2 = 53.6%), but no significant changes were observed regarding LBR (RR 0.93, 95% CI 0.85–1.02; I2 = 65.2%) and PLR (RR 1.09, 95% CI 0.84–1.41; I2 = 59.1%). No statistical differences were found in effect estimates among other subgroup analyses by ovarian stimulation protocol, trigger agent, endometrial preparation regimen, and embryo stage.
Conclusion
Timing of the first FET after oocyte retrieval was not significantly associated with pregnancy outcomes. This finding refutes the current common practice to delay FET after oocyte retrieval and reassures patients who wish to proceed with FET at their earliest convenience. Due to the high heterogeneity and observational nature of included studies, further randomized controlled trials are needed to confirm the results.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01857-9) contains supplementary material, which is available to authorized users.
Keywords: Frozen embryo transfer, Oocyte retrieval, Pregnancy outcome, Meta-analysis
Introduction
Controlled ovarian stimulation (COS) induces multifollicular development in one cycle and is an essential determinant of in vitro fertilization (IVF) success. In the case of a failed fresh embryo transfer (ET) attempt, cryopreservation of surplus embryos following COS has been widely used as a safe and efficient approach to increase cumulative pregnancy rates per oocyte retrieval [1, 2]. In addition, the practice of a freeze-all strategy is also on a constant increase in recent years [3, 4], which correlates with the improved embryo cryopreservation method by vitrification, incorporation of preimplantation genetic testing for aneuploidy, as well as promising results of preserved or even higher live birth rate and reduced risk of ovarian hyperstimulation syndrome compared to fresh ET [5–10].
One of the debatable issues that arise in daily clinical practice is the optimal time interval between COS and subsequent FET. Some physicians opt to delay the start of FET for at least one menstrual cycle in order to minimize any conceivable residual effect of COS on endometrial receptivity and ovarian function [11, 12]. Contrarily, others choose to perform FET immediately in the first menstrual cycle following oocyte retrieval to avoid unnecessary prolongation of time to pregnancy and reduce stress and anxiety of subfertile women who are eager to conceive as soon as possible. Thus far, a number of observational studies have assessed pregnancy outcomes in delayed versus immediate FET after a failed fresh ET or a freeze-all cycle, but the results are varied and inconclusive [13–24].
The aim of the present study is to comprehensively evaluate the effect of FET timing following oocyte retrieval on pregnancy outcomes through a systematic review and meta-analysis.
Materials and methods
This meta-analysis was performed in adherence to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guideline [25] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [26].
Search strategy
A systematic database search of PubMed (https://www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://www.embase.com), and Web of Science (https://www.webofknowledge.com) was conducted to identify all relevant literatures published through January 2020. The following terms were applied in our search: “time” or “timing” or “time interval” or “delayed” or “immediate” and “frozen embryo transfer” or “frozen-thawed embryo transfer” or “cryopreserved embryo transfer” or “cryo-thawed embryo transfer” and “oocyte retrieval” or “ovarian stimulation” or “ovum pick-up” or “in vitro fertilization” or “IVF” or “OPU.” Study language was limited to English, with no restriction imposed on the year of publication. Moreover, the reference lists of retrieved relevant reports were manually scrutinized for any additional studies.
Study selection
Studies were considered eligible if they satisfied the following four criteria: (i) cohort study design, (ii) the subjects were women undergoing first FET after a failed fresh ET or a freeze-all cycle, (iii) the exposure of interest was the time interval between COS and subsequent FET, and (iv) the outcomes included clinical pregnancy rate (CPR), live birth rate (LBR), or pregnancy loss rate (PLR). Studies were excluded if they (i) compared pregnancy outcomes between delayed and immediate cycles in consecutive fresh IVF/ICSI treatments and (ii) were published as reviews, editorials, commentaries, case reports, conference abstracts, or trial protocols. In the case of duplicate studies with overlapping populations, we retained the most recent version containing a larger sample size.
Studies were selected in a two-step process to ensure the correct identification. Firstly, titles and abstracts of all initially retrieved records were screened by two independent reviewers (J.H. and J.L.). Secondly, a decision on inclusion was made after evaluation of the full manuscripts that were likely to meet the eligible criteria. Any disagreements were resolved by group discussion with a third investigator (Y.K.).
Data extraction and quality assessment
The same two reviewers (J.H. and J.L.) independently performed data extraction with a standardized and pilot-tested form. The following information were recorded for each included study: the first author’s name, year of publication, country of origin, cohort design, study duration, population characteristics, definition of immediate/delayed transfer, cycle number, age, ovarian stimulation protocol, trigger agent, endometrial preparation regimen, embryo developmental stage, and outcome parameters. Data from different subgroups in the same study were also extracted for possible synthesis. The corresponding author was contacted by e-mail when information was missing or unclear in the original article.
The methodological quality of eligible studies was evaluated using the Newcastle-Ottawa Scale (NOS) [27]. This scale assigns a maximum of nine points to each study based on the following three broad aspects: the selection of subjects and assessment of exposure (4 points), comparability of study groups (2 points), and outcome ascertainment and follow-up adequacy (3 points). Studies scoring 7–9 points were regarded to have a high quality and a low risk of bias. Each study received a score from one of the two reviewers (J.H. and J.L.), and discrepancies were resolved by consensus with a third investigator (Y.K.).
Statistical analysis
Data for pregnancy outcomes were collected as dichotomous variables, and the results were expressed as risk ratio (RR) with 95% confidence interval (CI). Because the true effect size should not be assumed to be the same across individual studies, meta-analysis was performed using a random effects model to incorporate both within- and between-study variability [28]. The degree of heterogeneity was quantified by the I2 statistic, and we considered values of < 25%, 25–50% and > 50% to represent low, moderate, and high heterogeneity, respectively [29]. Publication bias was examined by means of funnel plots and Egger’s linear regression test, with P < 0.10 indicating a statistical significance [30].
Considering the clinical diversity of included studies, we further conducted five subgroup analyses to separately assess the effect of FET timing on pregnancy outcomes as follows: (i) study population (failed fresh ET, freeze-all and mixed), (ii) ovarian stimulation protocol (agonist-based, antagonist-based, and mixed), (iii) trigger agent (agonist only, hCG included and mixed), (iv) endometrial preparation regimen (artificial, natural, and mixed), and (v) embryo developmental stage (blastocyst, cleavage, and mixed or unclear). The statistical difference of the effects among subgroups was examined by interaction test. A sensitivity analysis by removing one study at a time was also performed to evaluate the stability of results and explore potential sources of heterogeneity.
We used the Stata software (version 16.0; StataCorp LP, College Station, Texas) for all analyses. Statistical significance was set at P < 0.05 except where otherwise specified.
Results
Literature search and study characteristics
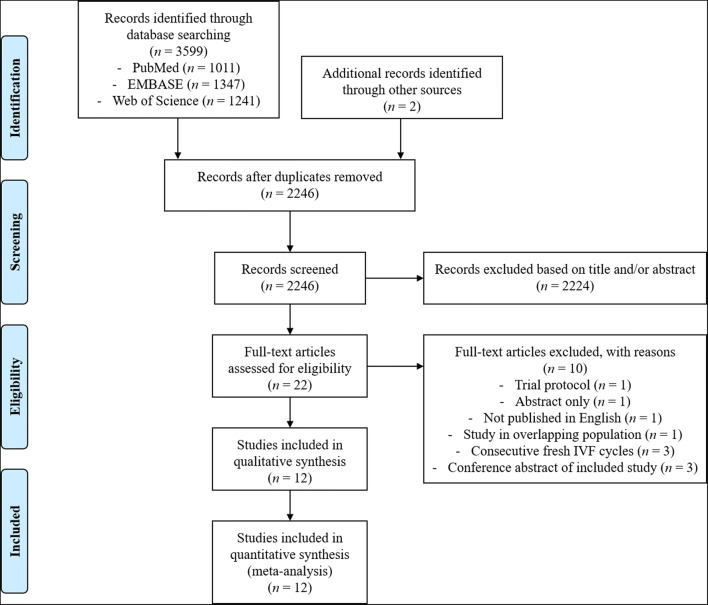
The initial literature search yielded 3599 records from electronic databases and another two articles through manual search. After removal of duplicates and screening of the titles and/or abstracts, 22 articles were further assessed for eligibility based on full-text review and 12 studies were finally included in the meta-analysis [13–24]. The process of study selection is detailed in Fig. 1.
Fig. 1.
The flow diagram of study selection. IVF, in vitro fertilization
Table 1 presents the main characteristics of the 12 included studies. These studies were published between 2016 and 2020 and were conducted in 10 different countries. All cohorts were retrospective, with the number of FET cycles varying from 129 to 4994. Seven studies focused on women undergoing first FET after a freeze-all cycle [13, 14, 17, 19, 20, 22, 23], three on women following a failed fresh ET [16, 21, 24], and two on women in both cases [15, 18]. In most studies [13, 14, 17–19, 22, 23], immediate transfer was defined as FET performed within the first menstrual cycle following oocyte retrieval (“cycle 1”), while delayed transfer referred to FET that took place after one or more menstrual cycles (“cycle ≥ 2”). The other five studies used the different time interval from oocyte retrieval to the start of FET (< 22/≥ 22 days) [16, 21], from oocyte retrieval to FET (< 50/≥ 50 days) [24], or from embryo cryopreservation to FET (25–35/50–70 days) [15] for classification of immediate and delayed transfer. Overall, the included studies were at low risk of bias with a NOS score of 8 (four studies) or 9 (eight studies) (Table S1).
Table 1.
Main characteristics of included studies in the meta-analysis
| Study | Country | Cohort design | Duration | Population | Definition of immediate/delayed transfer | Sample size (cycle) | Age (years) | Ovarian stimulation protocol | Trigger agent | Endometrial preparation | Embryo stage | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [13] | France | Retrospective, single-center | 2012–2015 | Women undergoing first FET after a freeze-all cycle | Within the first menstrual cycle/after one or more menstrual cycles following oocyte retrieval (cycle 1/≥ 2) | 474 | Mean, immediate, 33.7; delayed, 33.9 | GnRH antagonist, long agonist or short agonist | GnRH agonist or hCG | Artificial cycle | Blastocyst | CPR, LBR, PLR |
| [14] | China | Retrospective, single-center | 2014–2017 | Women undergoing first FET after a freeze-all cycle | Cycle 1/≥ 2 | 4404 | Mean, immediate, 31.6; delayed, 31.9 | GnRH antagonist or long agonist | hCG | Artificial or natural cycle | Cleavage, morula or blastocyst | CPR, LBR, PLR |
| [15] | Australia | Retrospective, multi-center | 2000–2014 | Women undergoing first FET after a failed fresh ET or a freeze-all cycle | 25–35/50–70 days from embryo cryopreservation to FET | 4994 | Median, immediate, 36.0; delayed, 35.5 | GnRH antagonist, long agonist or short agonist | hCG | Artificial or natural cycle | - | CPR, LBR, PLR |
| [16] | Israel | Retrospective, single-center | 2009–2016 | Women undergoing first FET after a failed fresh ET | ≤ 22/> 22 days from oocyte retrieval to the start of FET cycle | 198 | Mean, immediate, 32.8; delayed, 34.1 | GnRH antagonist, long agonist or short agonist | hCG | Natural or modified natural cycle | Cleavage or blastocyst | CPR, LBR |
| [17] | China | Retrospective, single-center | 2013–2016 | Women undergoing first FET after a freeze-all cycle | Cycle 1/≥ 2 | 2998 | Mean, immediate, 30.6; delayed, 30.9 | Short agonist or progestin-primed ovarian stimulation | GnRH agonist, hCG or both | Artificial or modified natural cycle | Cleavage or blastocyst | CPR, LBR, PLR |
| [18] | USA | Retrospective, single-center | 2013–2016 | Women undergoing first FET after a failed fresh ET or a freeze-all cycle | Cycle 1/≥ 2 | 344 | Mean, immediate, 33.7; delayed, 33.5 | GnRH antagonist or long agonist | GnRH agonist, hCG or both | Artificial or natural cycle | Blastocyst | CPR, LBR, PLR |
| [19] | Spain | Retrospective, single-center | 2012–2014 | Women undergoing first FET after a freeze-all cycle | Cycle 1/≥ 2 | 512 | Mean, immediate; 34.7; delayed, 35.3 | GnRH antagonist or long agonist | GnRH agonist or hCG | Artificial cycle | Cleavage or morula | CPR, LBR, PLR |
| [20] | Turkey | Retrospective, single-center | 2015–2016 | Women undergoing first FET after a freeze-all cycle | 32–46/≥47 days from oocyte retrieval to FET | 1121 | ≤ 42 | GnRH antagonist | GnRH agonist, hCG or both | Artificial cycle | Blastocyst | LBR |
| [21] | Belgium | Retrospective, single-center | 2010–2014 | Women undergoing first FET after a failed fresh ET | ≤ 22/> 22 days from oocyte retrieval to the start of FET cycle | 1183 | Mean, immediate, 32.4; delayed, 32.5 | GnRH antagonist | hCG | Artificial, natural or modified natural cycle | Cleavage or blastocyst | CPR, LBR |
| [22] |
Belgium/ Vietnam |
Retrospective, two-center | 2010–2015 | Women undergoing first FET after a freeze-all cycle | Cycle 1/≥ 2 | 333 | Mean, immediate, 30.9; delayed, 31.8 | GnRH antagonist | GnRH agonist | Artificial cycle | Cleavage or blastocyst | CPR, PLR |
| [23] | China | Retrospective, single-center | 2016–2018 | Women undergoing first FET after a freeze-all cycle | Cycle 1/≥ 2 | 1540 | Mean, immediate, 31.4; delayed, 31.0 | GnRH antagonist, long agonist, ultra-long agonist, short agonist, ultra-short agonist or mild stimulation | GnRH agonist or hCG | Artificial, natural or stimulated cycle | Cleavage | CPR, LBR, PLR |
| [24] | Israel | Retrospective, single-center | 2010–2015 | Women undergoing first FET after a failed fresh ET | < 50/≥ 50 days from oocyte retrieval to FET | 129 | Mean, immediate, 29.9; delayed, 29.6 | Long agonist | hCG | Artificial cycle | Cleavage or blastocyst | CPR, LBR, PLR |
CPR, clinical pregnancy rate; ET, embryo transfer; FET, frozen embryo transfer; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; LBR, live birth rate; PLR, pregnancy loss rate
Meta-analysis of CPR
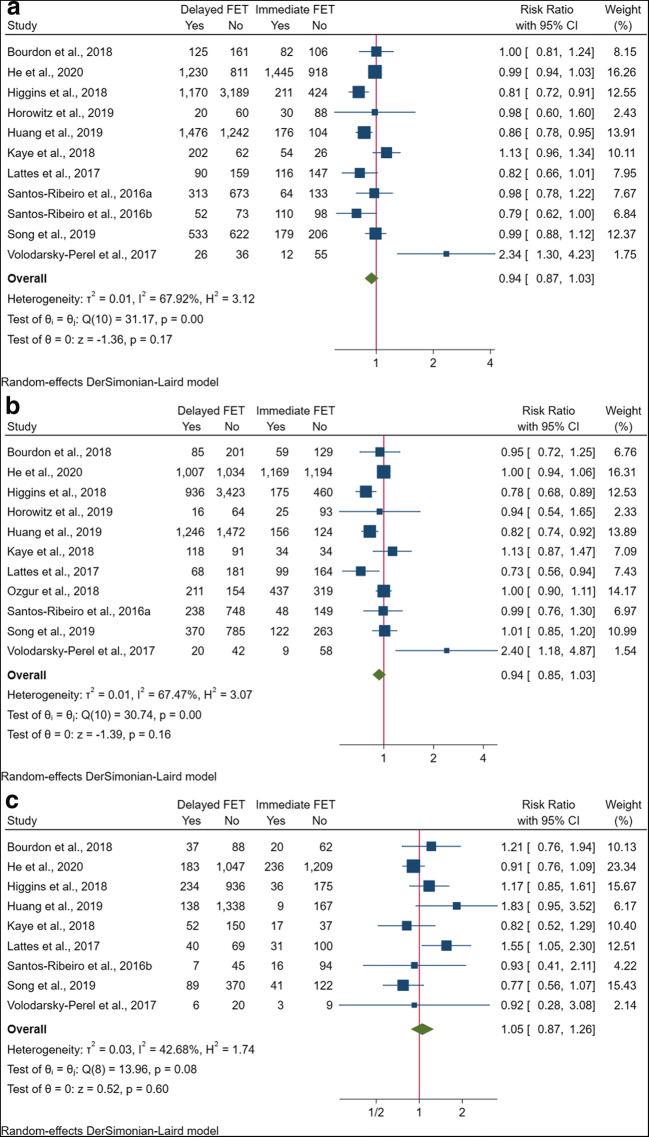
Eleven studies of 11,709 cycles reported data on the association between FET timing and CPR. In the majority of studies [13–19, 21, 22, 24], clinical pregnancy was clearly defined as the ultrasound visualization of gestational sac with or without cardiac activity at around 7 weeks of gestation, while the study by Song et al. [23] did not provide a detailed description. The pooled results revealed no significant difference in CPR between delayed and immediate FET after oocyte retrieval (RR 0.94, 95% CI 0.87–1.03; I2 = 67.9%) (Fig. 2a). In subgroup analysis, the combination of the six studies that focused on freeze-all cycles showed a marginally significant decrease of CPR in delayed FET (RR 0.93, 95% CI 0.86–1.00), whereas no such effects were observed in failed fresh ET (RR 1.24, 95% CI 0.77–1.99) and mixed cycles (RR 0.95, 95% CI 0.68–1.33) (Table S2). No significant changes were found in the effect estimates among other subgroup analyses by ovarian stimulation protocol, trigger agent, endometrial preparation regimen, and embryo stage (Table S2).
Fig. 2.
Forest plots of pregnancy outcomes for delayed versus immediate frozen embryo transfer after oocyte retrieval. a Clinical pregnancy rate. b Live birth rate. c Pregnancy loss rate. CI, confidence interval; FET, frozen embryo transfer
Meta-analysis of LBR
Eleven studies totaling 17,897 cycles investigated the effect of FET timing on LBR. Live birth was differently described as the delivery of a viable infant beyond 20 [15, 20, 23], 24 [13, 16, 17, 21, 24], or 28 [14] weeks of gestation, and was not defined in detail in two studies [18, 19]. The combination of all these studies showed that FET timing was not significantly associated with LBR (RR 0.94, 95% CI 0.85–1.03; I2 = 67.5%) (Fig. 2b). This pooled result did not change when subgroup analyses were performed on failed fresh ET (RR 1.21, 95% CI 0.75–1.95), freeze-all (RR 0.93, 95% CI 0.85–1.02), and mixed cycles (RR 0.92, 95% CI 0.64–1.32) (Table S2). Similarly, analyses by ovarian stimulation protocol, trigger agent, endometrial preparation regimen, and embryo stage showed no relevantly differential effect (Table S2).
Meta-analysis of PLR
Nine studies included data on PLR according to the timing of FET after oocyte retrieval, comprising a total of 15,728 transfer cycles. Pregnancy loss was defined as miscarriage occurring before 10 [13], 12 [17], or 20 [18, 19, 24] weeks of gestational age, and was not described in the other four studies [14, 15, 22, 23]. Most studies calculated PLR as the cycle number of pregnancy loss per clinical pregnancy, while Lattes et al. [19] evaluated it on the basis of positive pregnancy test. The pooled RR of PLR for delayed versus immediate FET was 1.05 (95% CI 0.87–1.26; I2 = 42.7%) (Fig. 2c). The subgroup analysis of freeze-all cycles presented consistent results (RR 1.09, 95% CI 0.84–1.41) with the combined effects of failed fresh ET (RR 0.92, 95% CI 0.28–3.08) and mixed cycles (RR 1.02, 95% CI 0.72–1.44) (Table S2). Further subgroup analyses by other confounders also showed no significant changes (Table S2).
Sensitivity analysis
On excluding the study by Volodarsky-Perel et al. [24], delayed FET was found to be marginally associated with a decrease in CPR compared with immediate FET (RR 0.93, 95% CI 0.87–1.00) (Fig. S1a), while no significant differences were found in LBR (RR 0.92, 95% CI 0.85–1.00) and PLR (RR 1.06, 95% CI 0.87–1.28) (Fig. S1 b–c). Removal of any other individual studies did not modify the estimates substantially, with pooled RR of CPR, LBR, and PLR ranging from 0.92 to 0.96, 0.92 to 0.96, and 0.98 to 1.11, respectively (Fig. S1 a–c).
Publication bias
The funnel plot was visually symmetric for each outcome (Fig. S2). In addition, regression-based Egger test did not reach statistical significance (P = 0.110, 0.137 and 0.494 for CPR, LBR, and PLR, respectively), indicating no obvious publication bias of included studies.
Discussion
To our knowledge, this is the first meta-analysis to assess the association between timing of FET after oocyte retrieval and pregnancy outcomes. Based on a total of 18,230 cycles from 12 retrospective cohort studies, we found no significant difference in CPR, LBR, and PLR between immediate FET within the first menstrual cycle and delayed FET following subsequent cycles.
In sensitivity analysis, removal of the study by Volodarsky-Perel et al. [24] resulted in a marginal yet significant change of the pooled estimate of CPR. This study supported postponement of first FET after a failed fresh ET using the long agonist protocol, which, from their speculation, may cause deeper suppression on the hypothalamic-pituitary-ovarian axis than the antagonist regimen and could lead to more small sustained lutein cysts with secretory activity in the follicular phase of the immediate FET cycle. However, when introducing the COS protocol for adjustment, several other studies failed to show a significant impact of the GnRH analogue used for stimulation [14, 16, 19]. A further subgroup analysis of agonist- and antagonist-based regimens also presented comparable pregnancy outcomes between delayed and immediate FET. These results suggest that the detrimental influence of COS on endometrial receptivity may end clinically following the first withdrawal bleeding regardless of the stimulation protocol.
Intriguingly, the deferral of FET after oocyte retrieval appears to be marginally associated with a lower CPR in freeze-all cycles, while this relationship is absent in failed fresh ET. One possible explanation for the discrepancy may be the different psychological status in these two situations. Women undergoing IVF treatment are generally desired to get pregnant as soon as possible, yet delayed FET in a freeze-all cycle may add to their anxiety and stress that could pose an adverse influence on pregnancy chance [31–33]. Contrarily, women who had already a failed fresh ET attempt may shoulder a greater psychological burden and thus need a longer time to recover from emotional distress. Nonetheless, it is also reasonable to argue that women undergoing non-elective freeze-all cycles may have decreased stress with their timeline expectations managed through physician communication in advance. Therefore, further research is warranted to examine this explanation since none of the included studies compared the anxiety, depression, and self-motivation level of these women. On the other hand, there could be some differences in the patient and stimulation characteristics of a freeze-all versus a fresh ET cycle, which may possibly influence pregnancy outcomes in later FET cycles. Embryo quality may also differ in FET after freeze-all and failed ET cycles, as embryos of higher quality are often preferentially transferred in a prior fresh cycle. However, as the decrease in CPR barely reached statistical significance and the number of studies in each subgroup was limited with a high heterogeneity, this result may also be coincidental and should be interpreted with caution.
Several included studies have also raised concerns that the effect of FET timing after COS may differ by the ovulation trigger agent and endometrial preparation regimen [13, 16, 18, 19, 22]. Indeed, compared to trigger with hCG that has a prolonged half-life, the luteal phase following agonist trigger is often shortened and disrupted [34] and may thus affect immediate FET in the first menstrual cycle. Patients undergoing natural cycle for FET may also be different from those receiving artificial endometrial preparation [16], in which exogenous steroids are prescribed to overtake or correct ovarian and endometrial function that might be still recovering to pre-stimulation status in an adjacent FET cycle. Nevertheless, both multivariable regression analyses among individual studies and subgroup analyses in this review showed no significant differential effects of these factors, implying further the lack of scientific evidence for deferring FET.
Findings from the present study should provide guidance to both clinical practice and research design. According to a recent web-based survey from 179 IVF centers in 58 countries, over 60% of physicians prefer to delay FET after oocyte retrieval with a failed fresh ET [35]. In randomized controlled trials (RCTs) designed to compare pregnancy outcomes between fresh and frozen embryo transfer, patients in the cryopreservation arm also had to wait for 2 months [36] or one menstrual cycle [8–10] to perform their first FET. Herein, our results refute the current common practice to delay FET in fear of the carryover effect of COS on an adjacent cycle, and reassure physicians and patients who wish to proceed with FET at their earliest convenience.
Several limitations have to be acknowledged of this meta-analysis. Firstly, all included studies were retrospective cohorts, and therefore, the quality of evidence was initially graded as low for the inherent methodological drawbacks. To date, only a two-center RCT was found to be carried out in China [37]. According to the preliminary results presented at the 35th annual meeting of the European Society of Human Reproduction and Embryology [37], the immediate FET group had a significantly higher ongoing pregnancy rate than the delayed group in both intention to treat analysis (47.0% (170/362) versus 39.2% (142/362)) and per protocol analysis (49.3% (170/345) versus 41.5% (142/342)). However, concern has been raised regarding the adequacy of the randomization process in this study given that important baseline characteristics such as female age varied significantly between the two groups, an event which could limit the validity of the findings of this RCT. Secondly, we observed a high degree of inter-study heterogeneity, which was presumed to be at partially caused by differences in the study population and cycle characteristics. Few studies reported pregnancy outcomes according to the standards by the International Committee for Monitoring Assisted Reproductive Technology (ICMART) [38], and the varied definitions of FET timing in some studies may possibly lead to misclassification of delayed and immediate transfer. In this regard, caution should be taken when generalizing the review finding to individual clinics with various combinations of ovarian stimulation, ovulation trigger, endometrial preparation, and embryo transfer strategies. Finally, bias may have been introduced because studies published as conference abstracts and in languages other than English were excluded from the meta-analysis.
In conclusion, our systematic review and meta-analysis demonstrated comparable pregnancy outcomes for immediate FET performed within the first menstrual cycle after oocyte retrieval versus delayed FET following subsequent cycles. This finding would not only provide reassuring evidence for patients who seek to proceed with their FET after COS without postponement but may also be useful for physicians in future clinical trial design. Due to the limited number, high heterogeneity and observational nature of included studies, further large RCTs are needed to confirm the results.
Electronic supplementary material
(PNG 206 kb)
(PNG 158 kb)
High Resolution (PNG 158 kb) (TIF 2454 kb)
(DOCX 29 kb)
Acknowledgments
The authors would like to express sincere gratitude to Dr. Hengye Huang, Department of Epidemiology, Shanghai Jiao Tong University School of Public Health, for her assistance in statistical analysis.
Authors’ contributions
J.H. and Y.K. contributed to the conception of the study. J.H. and J.L. performed the literature search, data extraction, and study quality assessment. J.H., J.L. and X.L. were involved in statistical analysis. J.H., N.S., and R.C. contributed to the interpretation of the results. J.H. was responsible for the manuscript drafting. All authors approved the final manuscript after critical revision for intellectual content.
Funding information
This study was funded by the National Key Research and Development Program of China (2018YFC1003000), National Natural Science Foundation of China (81771533) and Elite Group Project of Shanghai Ninth People’s Hospital (JY201801).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Doody KJ. Cryopreservation and delayed embryo transfer-assisted reproductive technology registry and reporting implications. Fertil Steril. 2014;102(1):27–31. doi: 10.1016/j.fertnstert.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 2.Polyzos NP, Drakopoulos P, Parra J, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including approximately 15,000 women. Fertil Steril. 2018;110(4):661–670.e661. doi: 10.1016/j.fertnstert.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 4.De Geyter C, Calhaz-Jorge C, Kupka MS, et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020(1):hoz038. doi: 10.1093/hropen/hoz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–497. doi: 10.1093/humrep/dev339. [DOI] [PubMed] [Google Scholar]
- 6.Bosch E, De Vos M, Humaidan P. The Future of Cryopreservation in Assisted Reproductive Technologies. Front Endocrinol (Lausanne) 2020;11:67. doi: 10.3389/fendo.2020.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Xiao X, Zhang J, Wang W, Wu J, Peng L, Wang X. Clinical outcomes of frozen embryo versus fresh embryo transfer following in vitro fertilization: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2018;298(2):259–272. doi: 10.1007/s00404-018-4786-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, Tian L, Hao C, Yang D, Zhou F, Shi J, Xu Y, Li J, Yan J, Qin Y, Zhao H, Zhang H, Legro RS. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H, Ma X, Ren H, Wang Y, Zhang D, Wang B, Liu F, Wu Q, Wang Z, Bai H, Li Y, Zhou Y, Sun M, Liu H, Li J, Zhang L, Chen X, Zhang S, Sun X, Legro RS, Chen ZJ. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi: 10.1056/NEJMoa1705334. [DOI] [PubMed] [Google Scholar]
- 10.Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, Tan J, Liang X, Cao Y, Wang Z, Qin Y, Zhao H, Zhou Y, Ren H, Hao G, Ling X, Zhao J, Zhang Y, Qi X, Zhang L, Deng X, Chen X, Zhu Y, Wang X, Tian LF, Lv Q, Ma X, Zhang H, Legro RS, Chen ZJ. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–1318. doi: 10.1016/S0140-6736(18)32843-5. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril. 2011;96(2):516–518. doi: 10.1016/j.fertnstert.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Bourdon M, Santulli P, Maignien C, Pocate-Cheriet K, Alwohaibi A, Marcellin L, Blais S, Chapron C. The interval between oocyte retrieval and frozen-thawed blastocyst transfer does not affect the live birth rate and obstetrical outcomes. PLoS One. 2018;13(10):e0206067. doi: 10.1371/journal.pone.0206067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Zheng H, Du H, et al. Delayed frozen embryo transfer failed to improve live birth rate and neonatal outcomes in patients requiring whole embryo freezing. Reprod Biol Endocrinol. 2020;18(1):1. doi: 10.1186/s12958-019-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins C, Healey M, Jatkar S, Vollenhoven B. Interval between IVF stimulation cycle and frozen embryo transfer: is there a benefit to a delay between cycles? Aust N Z J Obstet Gynaecol. 2018;58(2):217–221. doi: 10.1111/ajo.12696. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz E, Mizrachi Y, Farhi J, Shalev A, Raziel A, Weissman A. Modified natural-cycle cryopreserved embryo transfer: is a washout period needed after a failed fresh cycle? Reprod BioMed Online. 2019;39:439–445. doi: 10.1016/j.rbmo.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Lu X, Xie Q, Lin J, Cai R, Kuang Y. Timing of frozen-thawed embryo transfer after controlled ovarian stimulation in a non-elective freeze-all policy. Ann Transl Med. 2019;7(23):752. doi: 10.21037/atm.2019.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye L, Marsidi A, Rai P, Thorne J, Nulsen J, Engmann L, Benadiva C. Frozen blastocyst transfer outcomes in immediate versus delayed subsequent cycles following GnRH agonist or hCG triggers. J Assist Reprod Genet. 2018;35(4):669–675. doi: 10.1007/s10815-017-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lattes K, Checa MA, Vassena R, Brassesco M, Vernaeve V. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod. 2017;32(2):368–374. doi: 10.1093/humrep/dew306. [DOI] [PubMed] [Google Scholar]
- 20.Ozgur K, Bulut H, Berkkanoglu M, Humaidan P, Coetzee K. Frozen embryo transfer can be performed in the cycle immediately following the freeze-all cycle. J Assist Reprod Genet. 2018;35(1):135–142. doi: 10.1007/s10815-017-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos-Ribeiro S, Siffain J, Polyzos NP, et al. To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt? Fertil Steril. 2016;105(5):1202–1207.e1201. doi: 10.1016/j.fertnstert.2015.12.140. [DOI] [PubMed] [Google Scholar]
- 22.Santos-Ribeiro S, Polyzos NP, Lan VT, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod. 2016;31(11):2541–2548. doi: 10.1093/humrep/dew194. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Xiang S, Sun Z. Frozen embryo transfer at the cleavage stage can be performed within the first menstrual cycle following the freeze-all strategy without adversely affecting the live birth rate: a STROBE-compliant retrospective study. Medicine (Baltimore) 2019;98(38):e17329. doi: 10.1097/MD.0000000000017329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volodarsky-Perel A, Eldar-Geva T, Holzer HE, Schonberger O, Reichman O, Gal M. Cryopreserved embryo transfer: adjacent or non-adjacent to failed fresh long GnRH-agonist protocol IVF cycle. Reprod BioMed Online. 2017;34(3):267–273. doi: 10.1016/j.rbmo.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O’Connell D et al The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 14 Jan 2020
- 28.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assist Reprod Genet. 2013;30(1):35–41. doi: 10.1007/s10815-012-9904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gameiro S, Boivin J, Domar A. Optimal in vitro fertilization in 2020 should reduce treatment burden and enhance care delivery for patients and staff. Fertil Steril. 2013;100(2):302–309. doi: 10.1016/j.fertnstert.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Cesta CE, Viktorin A, Olsson H, et al. Depression, anxiety, and antidepressant treatment in women: association with in vitro fertilization outcome. Fertil Steril. 2016;105(6):1594–1602.e1593. doi: 10.1016/j.fertnstert.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Humaidan P, Papanikolaou EG, Kyrou D, Alsbjerg B, Polyzos NP, Devroey P, Fatemi HM. The luteal phase after GnRH-agonist triggering of ovulation: present and future perspectives. Reprod BioMed Online. 2012;24(2):134–141. doi: 10.1016/j.rbmo.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 35.IVF-Worldwide. Survey results: frozen-thawed embryo transfer. Available at: http://ivf-worldwide.com/survey/frozen-thawed-embryo-transfer/results-frozen-thawed-embryotransfer.html. Assessed 23 June 2019
- 36.Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27(7):357–363. doi: 10.1007/s10815-010-9412-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Li H, Sun X, Yang J, et al. Comparison of the ongoing pregnancy rate of immediate versus delayed frozen-thawed embryo transfer following a stimulated IVF cycle: a prospective randomized controlled trial. Hum Reprod. 2019;34(Supplement_1):i29–i31. [Google Scholar]
- 38.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, van der Poel S, International Committee for Monitoring Assisted Reproductive Technology. World Health Organization The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod. 2009;24(11):2683–2687. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 206 kb)
(PNG 158 kb)
High Resolution (PNG 158 kb) (TIF 2454 kb)
(DOCX 29 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.