Abstract
Purpose
We aimed to identify novel variants in TUBB8 and corresponding new abnormal phenotypes in oocytes/fertilization/ embryonic development responsible for female infertility.
Methods
Sanger sequencing of TUBB8 was performed in infertile women with abnormalities in oocyte maturation or embryonic development. The effects of the variants were evaluated in patients’ oocytes by morphological observations and immunofluorescence.
Results
We identified 34 novel variants of TUBB8 in 51 patients who were diagnosed with abnormalities in oocyte maturation or early embryonic development. We found a novel phenotype in which large polar bodies were present in three independent patients possibly associated with a recurrent variant. Moreover, we identified a novel type of TUBB8 variant consisting of an in-frame deletion-insertion, which has not been previously reported.
Conclusions
Our present study identified 34 novel variants in TUBB8 in 51 patients. These patients show oocyte maturation arrest, oocytes with large polar body, fertilization failure, early embryonic arrest or embryonic implantation failure. These results expand the kinds of variants and phenotypic spectrum of TUBB8 variants with regard to female infertility.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01830-6) contains supplementary material, which is available to authorized users.
Keywords: Female infertility, TUBB8, Novel variants, Novel phenotype
Introduction
Successful human reproduction initiates with the fusion of a functional oocyte with a sperm, and thus, defects in the process of oocyte maturation will cause female infertility [1, 2]. The application of assisted reproductive technology provides the unique opportunity to evaluate the morphology of human oocytes [3], and since the 1990s, several cases of infertility have been reported due to oocyte maturation arrest [4–6]. However, the underlying genetic factors behind this phenotype have remained largely unknown.
In 2016, we identified the first gene—tubulin beta 8 class VII (TUBB8) (MIM: 616768)—as being responsible for human oocyte maturation arrest in a dominant inheritance pattern [7], and the causal relationship between TUBB8 variants and oocyte maturation arrest was further confirmed in the following studies by us and others [8–12]. In addition, some cases harboring TUBB8 variants have been shown to follow a recessive inheritance pattern, and different variants in TUBB8 have been shown to cause phenotypic variability, including both fertilization and embryonic developmental problems [8–10, 13, 14]. According to previous data, TUBB8 is a major gene for patients with abnormalities in oocyte, fertilization, and embryonic development, and although a total of 48 variants have been identified, it is necessary to identify additional novel variants and potential new phenotypes caused by variants, which will provide for establishing comprehensive role of TUBB8 in early human reproductive process.
In the present study, we identified a total of 34 novel variants in 51 patients who were diagnosed with abnormalities in oocyte maturation or with early embryonic arrest. Notably, we observed a new kind of phenotype of large polar bodies in three independent patients sharing the same variant. We also identified a novel kind of TUBB8 variant consisting of an in-frame deletion-insertion. All of these findings expand the phenotypic and genotypic spectrum of TUBB8 variants, which will lay the foundation for future genetic counseling.
Materials and methods
Human subjects
The infertile female patients were recruited from the Reproductive Medicine Department of the Third Affiliated Hospital of Zhengzhou University, the Reproductive Medicine Center of the Shanghai Ninth Hospital affiliated with Shanghai Jiao Tong University, the Shanghai Ji Ai Genetics and IVF institute affiliated with the Obstetrics and Gynecology Hospital of Fudan University, the Reproductive Medicine Center of the Shaanxi Maternal and Child Care Service Center, and the Fertility Center of Shenzhen Zhongshan Urology Hospital. A total of 1255 patients with oocyte maturation arrest, fertilization failure, embryonic arrest, or implantation problems were used in this study. The healthy controls were recruited from the Shanghai Ninth Hospital affiliated with Shanghai Jiao Tong University. The studies of were approved by the Ethics Committee of the Medical College of Fudan University.
Sequence analysis of TUBB8
Genomic DNA samples from affected individuals, their family members, and healthy controls were extracted from peripheral blood using the QIAamp DNA Blood Mini Kit (Qiagen). Four exons were amplified by specific primers (Supplemental Table 1). The amplicons were sequenced by Sanger sequencing and were then aligned to the reference sequence of TUBB8 (Gene Bank: NM_177987.2) using the CodonCode software to identify rare variants. The genome Aggregation Database (gnomAD) (http://gnomad.broadinstitute.org/) was used to analyze the frequency of the corresponding variants, and PROVEN (http://provean.jcvi.org/) was used for in silico analysis.
Evaluation of oocyte and embryo phenotypes
The morphologies of the oocytes and embryos were evaluated by light microscopy. For immunofluorescence, oocytes were first fixed in 3.0% paraformaldehyde, then incubated in membrane permeabilizing solution for 20 min and blocking buffer for 2 h at room temperature. The oocyte spindles were stained with an anti-β-tubulin-FITC antibody (1:300 dilution, F2043, Sigma-Aldrich), and the DNA was labeled with Hoechst 33342 (1:600 dilution, BD). The oocytes were mounted on glass slides, and images were captured on a confocal laser-scanning microscope (Leica).
Results
Novel variants in TUBB8
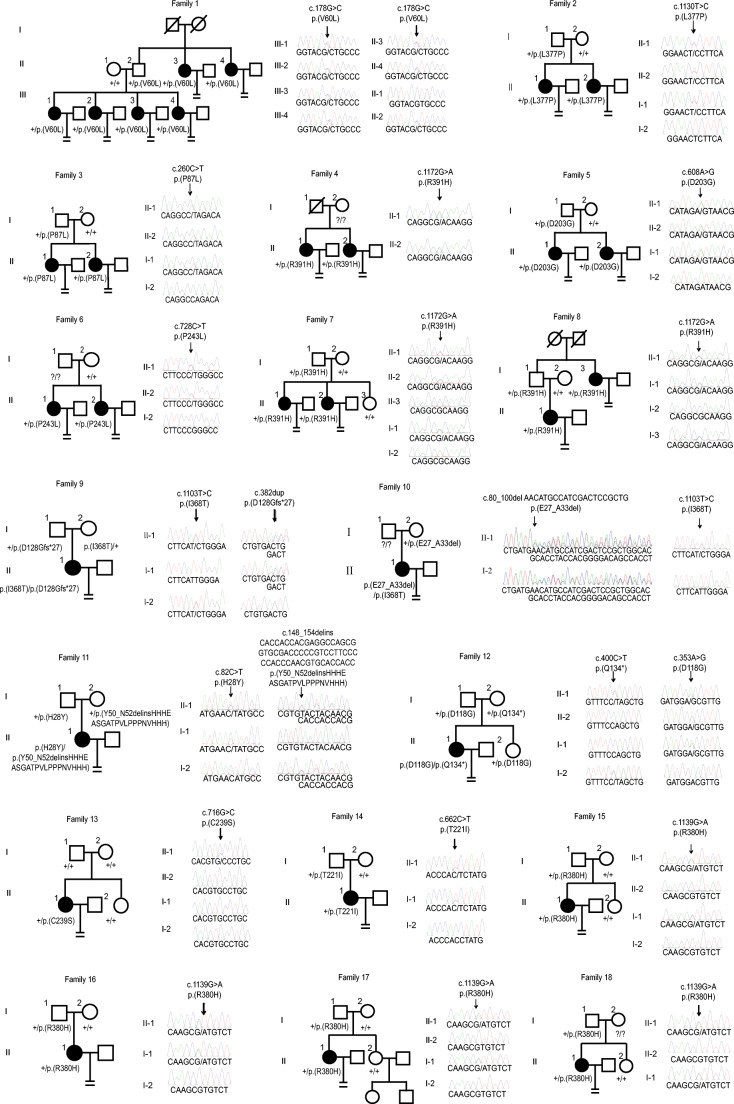
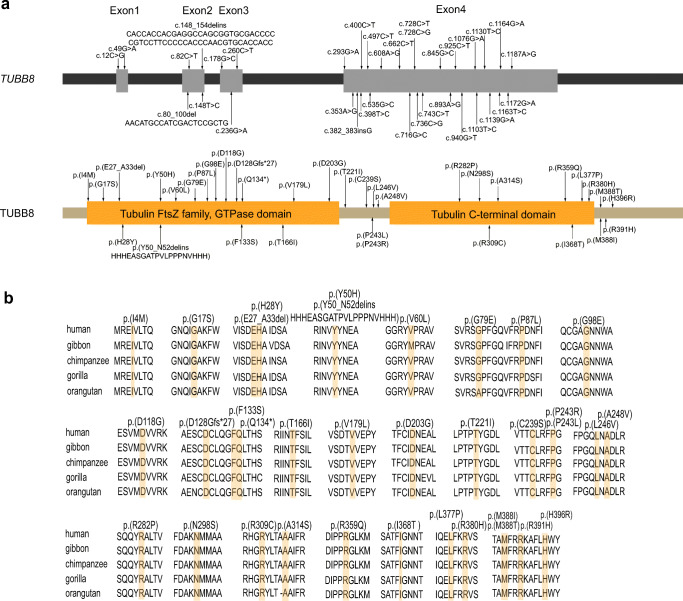
We identified a total of 34 novel variants in 51 patients from 39 families (Fig. 1), including 31 missense variants, 1 nonsense variant, 1 frameshift insertion variant, and a novel kind of in-frame deletion-insertion variant (family 11) (Supplemental Fig. 1). Most of the variants were heterozygous and were dominantly inherited or arose de novo. One family was identified with a homozygous variant (family 34), and five families were identified with compound heterozygous variants (families 9, 10, 11, 12, and 39). The positions of the variants are shown in Fig. 2a. In silico analysis suggested that most of the variants are deleterious to the function of TUBB8 as predicted by PROVEAN. Besides, the frequencies of the variants were ranged from 0.8 × 10−3 to 3.2 × 10−3 in the patients we recruited, which are much higher than that in the gnomAD database (< 10−4 or absent in the gnomAD) (Table 1), suggesting that the variants are likely pathogenic. The altered residues of these variants in TUBB8 are highly evolutionarily conserved among different primate species (Fig. 2b).
Fig. 1.
Pedigrees of 39 families with TUBB8 variants. Sanger sequencing conformation is shown to the right of the pedigrees. The “=” sign indicates infertility, black circles represent affected individuals, slashes indicate deceased individuals, and question marks indicate the absence of DNA samples. The “+” sign means wild type
Fig. 2.
The location and conservation of mutated residues in TUBB8. a The positions of all variants are indicated in the genomic structure of TUBB8, and the corresponding amino acids are shown on the TUBB8 protein. b Conservation analysis of altered amino acids among five primate species
Table 1.
TUBB8 variant spectrum in the 39 families with abnormalities of oocyte maturation and embryonic development
| Family | Genomic Position On Chr10(bp) | cDNA Change | Protein Change | Variant Type | Inheritance | PROVENa | gnomADb | gnomAD easb |
|---|---|---|---|---|---|---|---|---|
| 1 | 94,654 | c.178G>C | p.(V60L) | Missense | AD | N | NA | NA |
| 2 | 93,202 | c.1130T>C | p.(L377P) | Missense | AD | D | NA | NA |
| 3 | 94,572 | c.260C>T | P.(P87L) | Missense | AD | D | NA | NA |
| 4, 7, 8,33 | 93,160 | c.1172G>A | p.(R391H) | Missense | Unknown, AD | D | NA | NA |
| 5 | 93,724 | c.608A>G | p.(D203G) | Missense | AD | D | NA | NA |
| 6 | 93,604 | c. 728C>T | p.(P243L) | Missense | Unknown | D | NA | NA |
| 9 | 93,229 | c.1103T>C | p.(I368T) | Missense | AR | D | NA | NA |
| 93,950 | c.382 dup | p.(D128Gfs*27) | Frameshift insertion | NA | NA | NA | ||
| 10 | 93,229 | c.1103T>C | p.(I368T) | Missense | Unknown | D | NA | NA |
| 94810_94830 | c.80_100del AACATGCCATCGACTCCGCTG | p.(E27_A33del) | In-frame deletion | D | NA | NA | ||
| 111 | 94,828 | c.82C>T | p.(H28Y) | Missense | AR | D | 2.483 × 10−5 | 3.269 × 10−4 |
| 94,756_ 94,762 | c.148_154delinsCACCACCACGAGGCCAGCGGTGCGACCCCCGTCCTTCCCCCACCCAACGTGCACCACC | p.(Y50_N52delinsHHHEASGATPVLPPPNVHHH) | In-frame deletion-insertion | NA | NA | NA | ||
| 12 | 93,932 | c.400C>T | p.(Q134*) | Nonsense | AR | NA | 0 | 0 |
| 93,979 | c.353A>G | p.(D118G) | Missense | D | NA | NA | ||
| 13 | 93,616 | c.716G>C | p.(C239S) | Missense | De novo | D | NA | NA |
| 14 | 93,670 | c.662C>T | p.(T221I) | Missense | AD | D | 0 | 0 |
| 15,16,17,18, | 93,193 | c.1139G>A | p.(R380H) | Missense | AD; Unknown | D | 0 | 0 |
| 19 | 93,169 | c.1163T>C | p.(M388T) | Missense | De novo | D | NA | NA |
| 20 | 94,596 | c.236G>A | p.(G79E) | Missense | AD | D | NA | NA |
| 21 | 94,039 | c.293G>A | p.(G98E) | Missense | AD | D | NA | NA |
| 22 | 94,762 | c.148T>C | p.(Y50H) | Missense | AD | N | 0 | 0 |
| 23 | 93,589 | c.743C>T | p.(A248V) | Missense | AD | D | NA | NA |
| 24 | 93,439 | c.893A>G | p.(N298S) | Missense | AD | D | NA | NA |
| 25 | 93,392 | c.940G>T | p.(A314S) | Missense | AD | N | NA | NA |
| 26 | 93,145 | c.1187A>G | p.(H396R) | Missense | De novo | D | NA | NA |
| 27 | 93,604 | c.728C>G | p.(P243R) | Missense | Unknown | D | NA | NA |
| 28 | 93,835 | c.497C>T | p.(T166I) | Missense | Unknown | D | NA | NA |
| 29 | 95,167 | c.12C>G | p.(I4M) | Missense | Unknown | N | NA | NA |
| 30 | 93,168 | c.1164G>A | p.(M388I) | Missense | Unknown | N | NA | NA |
| 31 | 93,487 | c.845G>C | p.(R282P) | Missense | Unknown | D | NA | NA |
| 32 | 95,130 | c.49G>A | p.(G17S) | Missense | Unknown | N | 5.238 × 10-6 | 0 |
| 34 | 93,256 | c.1076G>A | p.(R359Q) | Missense | Unknown | N | 1.062 × 10-5 | 0 |
| 35, 37 | 93,596 | c.736C>G | p.(L246V) | Missense | Unknown | D | NA | NA |
| 36 | 93,407 | c.925C>T | p.(R309C) | Missense | Unknown | D | 3.265 × 10-5 | 5.081 × 10-5 |
| 38 | 93,797 | c.535G>C | p.(V179L) | Missense | Unknown | D | NA | NA |
| 39 | 93,934 | c.398T>C | p.(F133S) | Missense | Unknown | D | NA | NA |
| 94,828 | c.82C>T | p.(H28Y) | Missense | D | 2.483 × 10-5 | 3.269 × 10-4 |
AD, autosome dominant; AR, autosome recessive; N, neutral; D, deleterious; NA, not available
aVariant effect predicted by PROVEAN; bFrequency of corresponding variants in the total and East Asian (eas) population of gnomAD
Phenotypic spectrum of patients with TUBB8 variants
Consistent with our previous findings [8, 9, 14], patients with TUBB8 variants showed variable phenotypes in oocyte maturation or embryonic development. The specific clinical information regarding the oocytes retrieved from the affected individuals is given in Table 2. In the present study, we categorized the phenotypes of patients with novel TUBB8 variants as the following: (1) oocytes arrested at an immature stage, especially at the metaphase I (MI) stage (families 1, 2, 6, 9, 10, 19, 21, 26, 29, 30, 31, 37, and 38) (Fig. 3a), (2) oocytes that failed to be fertilized (families 3 and 13), (3) zygotes that failed to cleave (families 4, 7, 12, 15, 23, 28, and 39), (4) early embryonic arrest (families 8, 11, 16, 17, 20, 25, 27, 32, 33, 34, 35, and 36) (Fig. 3b), (5) embryos with implantation potential but failed to conceive after implantation (families 3, 5, 11, 13, 16, 27, 29, and 32), and (6) abnormal oocyte morphology at an immature stage (families 14, 15, 16, 17, and 22). Compared with the normal first polar body (PB1) oocytes (Fig. 3a), we observed a new phenotype of large polar bodies in affected individuals from families 15, 16, and 17 (Fig. 3c), and most of these abnormal PB1 oocytes either failed to be fertilized or were fertilized but resulted in early embryonic arrest (Table 2). Furthermore, all three independent individuals with large polar bodies shared the recurrent variant c.1139G>A, p.(R380H), implying the variant may have impact on morphology of the polar body. Oocytes from some of the patients were collected for immunostaining. For individual II-1 in family 1, the morphology of the oocyte spindle was abnormal compared with the healthy control (Fig. 3d). For patients in family 9 and family 21, the oocytes retrieved from individual II-1 in both families showed the complete absence of the oocyte spindle (Fig. 3d), which indicated the dysfunction associated with these variants. These findings further expand the phenotypic spectrum of patients with TUBB8 variants.
Table 2.
Clinical characteristics of the patients with TUBB8 variants
| Family | Age (years) | Duration of infertility (years) | Previous IVF and ICSI cycles | Total oocytes retrieved | GV oocytes | MI oocytes | Immature oocytes of unknown stage | PB1 oocytes | Oocytes with abnormal morphology | Fertilized oocytes | No. of embryos that could be cleaved | Usable embryos | Outcome of embryo transfer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 6 | 1 | 11 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | / |
| 2 | 32 | 3 | 1 | 7 | 0 | 4 | 0 | 2 | 1 | 2 | 0 | 0 | / |
| 3 | 38 | 8 | 3 | 44 | 0 | 0 | 44 | 0 | 0 | 9 | 0 | 1 | Failure |
| 4 | 33 | 6 | 2 | 26 | 0 | 0 | 26 | 0 | 0 | 15 | 3 | 0 | / |
| 5 | 29 | 7 | 3 | 40 | 0 | 0 | 40 | 0 | 0 | 26 | 12 | 4 | Failure |
| 6 | 36 | 12 | 3 | 39 | 0 | 0 | 39 | 0 | 0 | 0 | 0 | 0 | / |
| 7 | 27 | 6 | 2 | 29 | 3 | 13 | 0 | 13 | 0 | 7 | 1 | 0 | / |
| 8 | 30 | 7 | 3 | 51 | 0 | 0 | 51 | 0 | 0 | 24 | 16 | 3 | / |
| 9 | 30 | 4 | 2 | 13 | 0 | 9 | 0 | 2 | 2 | 0 | 0 | 0 | / |
| 10 | 26 | / | 1 | 10 | 0 | 9 | 0 | 1 | 0 | 1 | 0 | 0 | / |
| 11 | 35 | 9 | 4 | 31 | 0 | 0 | 20 | 11 | 0 | 11 | 8 | 2 | Failure |
| 12 | 31 | 7 | 3 | 51 | 0 | 1 | 50 | 0 | 44 | 44 | 0 | 0 | / |
| 13 | 31 | 4 | 2 | 17 | 0 | 0 | 5 | 12 | 0 | 2 | 1 | 1 | Failure |
| 14 | 34 | 6 | 2 | 21 | 0 | 2 | 2 | 0 | 17 | 2 | 0 | 0 | / |
| 15 | 23 | 3 | 1 | 11 | 0 | 0 | 0 | 4 | 7 | 4 | 0 | 0 | / |
| 16 | 40 | 13 | 3 | 21 | 0 | 8 | 0 | 8 | 5 | 7 | 6 | 4 | Failure |
| 17 | 30 | 6 | 1 | 9 | 0 | 4 | 0 | 0 | 5 | 4 | 4 | 0 | / |
| 19 | 37 | 15 | 2 | 59 | 14 | 45 | 0 | 0 | 0 | 0 | 0 | 0 | / |
| 20 | 33 | / | 2 | 37 | 2 | 5 | 0 | 28 | 2 | 19 | 19 | 3 | / |
| 21 | 29 | 5 | 1 | 5 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | / |
| 22 | 32 | / | 1 | 17 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | / |
| 23 | 31 | 4 | 3 | 22 | 0 | 0 | 22 | 0 | 0 | 4 | 0 | 0 | / |
| 25 | 29 | 6 | 1 | 6 | 0 | 0 | 0 | 6 | 0 | 6 | 2 | 0 | / |
| 26 | 32 | 4 | 2 | 25 | 4 | 13 | 0 | 8 | 0 | 4 | 1 | 0 | / |
| 27 | / | 9 | 3 | 29 | 0 | 0 | 0 | 29 | 0 | 26 | 12 | 1 | Failure |
| 28 | 27 | 4 | 1 | 15 | 0 | 0 | 15 | 0 | 0 | 8 | 4 | 0 | / |
| 29 | 29 | 3 | 1 | 12 | 5 | 5 | 0 | 2 | 0 | 2 | 2 | 2 | Failure |
| 30 | 28 | 5 | 2 | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | / |
| 31 | 26 | 7 | 2 | 24 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | / |
| 32 | 31 | / | 3 | 49 | 0 | 0 | 49 | 0 | 0 | 24 | 14 | 2 | Failure |
| 33 | 34 | 8 | 2 | 4 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | / |
| 34 | 32 | 6 | 2 | 24 | 0 | 0 | 24 | 0 | 0 | 12 | 8 | 0 | / |
| 35 | 37 | 9 | 2 | 18 | 0 | 0 | 0 | 18 | 0 | 11 | 11 | 0 | / |
| 36 | 25 | 2 | 1 | 20 | 0 | 0 | 20 | 0 | 0 | 17 | 14 | 0 | / |
| 37 | 30 | / | 2 | 39 | 0 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | / |
| 38 | 30 | 5 | 2 | 13 | 2 | 9 | 0 | 2 | 0 | 1 | 1 | 0 | / |
| 39 | 33 | 11 | 1 | 12 | 0 | 2 | 0 | 8 | 2 | 3 | 1 | 0 | / |
Families 18 and 24 had no clinical information
IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; GV, germinal vesicle; MI, metaphase I; PB1, first polar body; /, not available
Fig. 3.
Phenotypes of oocyte maturation and embryonic development problems in patients. a The morphologies of a normal mature PB1 oocyte and immature MI oocytes from patients in families 1, 9, and 21. b The morphologies of a normal 8-cell embryo and early-arrested embryos from patients in families 25 and 33. c The morphologies of abnormal mature PB1 oocytes from patients in families 15, 16, and 17. The black arrows indicate the first polar body. d Immunostaining results of a normal oocyte from a healthy control and the oocytes from the affected individuals. Oocytes were immunolabeled with an anti-β-tubulin antibody to visualize the oocyte spindle and counterstained with Hoechst 33342 to visualize the DNA. Scale bar is 50 μm
Discussion
In the present study, we identified a total of 34 novel variants in 51 patients from 39 families. Of these, 33 families had heterozygous variants, 1 family had a homozygous variant, and 5 families had compound heterozygous variants. The compound heterozygous variants include a new type of TUBB8 variant consisting of an in-frame deletion-insertion (family 11). The majority of variants were located in exon 4 of TUBB8. These results extend the spectrum of TUBB8 variants responsible for abnormalities in oocyte maturation and embryonic development.
In this study, we found a novel kind of phenotype in three independent patients (families 15, 16, and 17) presenting with oocytes containing large polar bodies. Importantly, all three patients with large polar bodies shared a same recurrent variant (c.1139G>A p.(R380H)), implying the possible role of this variant to the phenotype of large polar body. Future studies are needed to explore the mechanism of large polar bodies by constructing TUBB8 p.R380H overexpression transgenic mice. Combining the present study with previous reports, 14 of the identified variants are recurrent, and this will provide genetic markers for genetic testing and genetic counseling for patients with infertility due to problems with oocyte maturation or embryonic development.
Previously, we identified TUBB8 variants as major genetic determinants of human oocyte maturation arrest [7]. However, some of the oocytes retrieved from the patients could be matured to the PB1 stage, but most of these PB1 oocytes either failed to be fertilized or failed to form normal embryos [8, 9, 14]. TUBB8 is a highly conserved primate-specific β-tubulin isotype that is specifically expressed in oocytes and has a key role in meiotic spindle assembly. Thus, we speculate that the fertilization failure or embryonic arrest might at least partly result from nuclear maturation defects in PB1 oocytes. Due to the scarcity of human oocytes available for experimental purposes, the underlying mechanism needs to be further clarified in more patients’ oocytes or studied in transgenic mice with the corresponding TUBB8 variants.
In conclusion, we have further confirmed the critical role of TUBB8 during human oocyte maturation and embryonic development. Oocytes from patients with large polar bodies extend the phenotypic spectrum of TUBB8, and all of the novel variants we identified expand the variant spectrum of TUBB8. This will help to understand the comprehensive role of TUBB8 in early human reproductive process and provide genetic markers for future genetic counseling for more individualized treatments.
Electronic supplementary material
(DOCX 250 kb)
Acknowledgments
We thank the patients and their families for participating in this study, as well as the doctors for contributing to patient sample collection and for providing clinical support. We also thank Ke Qiao from the core facility of the Institute of Metabolic & Integrative Biology, Fudan University, for her excellent technical expertise in cell imaging techniques.
Authors’ contributions
YG, SX, Ling W, ZY, BL, JF, RS, JS, XS and YK contributed to the recruitment, characterization, and oocyte imaging of the patients. LZ, QS, and Lei W conceived and designed the research study. LZ and WW performed the exon sequencing, and LZ performed the immunostaining. BC, JM, Jie D, and QL organized the medical records. Zhihua Z, Zhou Z, and Jing D analyzed the data. LZ, QS, and Lei W wrote the initial draft of the manuscript. LJ and LH helped in improving the manuscript.
Funding information
This work was supported by the National Key Research and Development Program of China (2018YFC1003800, 2016YFC1000600, 2017YFC1001500), the National Natural Science Foundation of China (81725006, 81822019, 81771581, 81971450, 81771649 and 81971382), the project supported by Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), the Project of Shanghai Municipal Science and Technology Commission(19JC1411001), the Shanghai Rising Star Program (17QA1400200), the Natural Science Foundation of Shanghai (19ZR1444500, 17ZR1401900), Shuguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (18SG03), the capacity Building Planning Program for Shanghai Women and Children’s Health Service, and the collaborative innovation center project construction for Shanghai women and children’s health
Compliance with ethical standards
Ethical approval
This study was approved by the ethics committee of the Ninth Hospital of Shanghai Jiao Tong University and the institutional review board of the Medical College of Fudan University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lin Zhao and Yichun Guan contributed equally to this work.
Contributor Information
Lei Wang, Email: wangleiwanglei@fudan.edu.cn.
Qing Sang, Email: sangqing@fudan.edu.cn.
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 3.Grainger DA, Tjaden BL, Tatpati LL. Chapter 20-assisted reproductive technologies. In: Goldman MB, Troisi R, Rexrode KM, editors. Women and Health (Second Edition): Academic Press; 2013. p. 307–20.
- 4.Rudak E, Dor J, Kimchi M, Goldman B, Levran D, Mashiach S. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril. 1990;54(2):292–296. doi: 10.1016/S0015-0282(16)53706-6. [DOI] [PubMed] [Google Scholar]
- 5.Eichenlaub-Ritter U, Schmiady H, Kentenich H, Soewarto D. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod. 1995;10(9):2343–2349. doi: 10.1093/oxfordjournals.humrep.a136297. [DOI] [PubMed] [Google Scholar]
- 6.Hartshorne G, Montgomery S, Klentzeris L. A case of failed oocyte maturation in vivo and in vitro. Fertil Steril. 1999;71(3):567–570. doi: 10.1016/S0015-0282(98)00505-6. [DOI] [PubMed] [Google Scholar]
- 7.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, et al. Mutations in TUBB8 and Human Oocyte Meiotic Arrest. N Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, et al. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53(10):662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, et al. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32(2):457–464. doi: 10.1093/humrep/dew322. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Tong X, Luo L, Zheng S, Jin R, Fu Y, et al. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod Biomed Online. 2017;35(3):305–310. doi: 10.1016/j.rbmo.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Wang AC, Zhang YS, Wang BS, Zhao XY, Wu FX, Zhai XH, et al. Mutation analysis of the TUBB8 gene in primary infertile women with arrest in oocyte maturation. Gynecol Endocrinol. 2018;34(10):900–904. doi: 10.1080/09513590.2018.1464138. [DOI] [PubMed] [Google Scholar]
- 12.Xiang J, Wang W, Qian C, Xue J, Wang T, Li H, et al. Human oocyte maturation arrest caused by a novel missense mutation in TUBB8. J Int Med Res. 2018;46(9):3759–3764. doi: 10.1177/0300060518778638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan P, Zheng L, Liang H, Li Y, Zhao H, Li R, et al. A novel mutation in the TUBB8 gene is associated with complete cleavage failure in fertilized eggs. J Assist Reprod Genet. 2018;35(7):1349–1356. doi: 10.1007/s10815-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B, Wang W, Peng X, Jiang H, Zhang S, Li D, et al. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur J Hum Genet. 2019;27(2):300–307. doi: 10.1038/s41431-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 250 kb)