Abstract
Purpose
To report the first live birth after frozen-thawed ovarian transplantation in Turkey and the second case for an acute lymphoblastic leukemia (ALL) survivor in the world.
Methods
A 19-year-old patient underwent ovarian tissue cryopreservation (OTC) before cord blood transplantation in 2010. She was diagnosed as ALL with a bone marrow biopsy revealing 90% blast ALL-L2 type, and karyotype analyses indicated reciprocal translocation at t(9;22)(q34;q11). The patient received the Berlin-Frankfurt-Munster (BFM) protocol, and complete remission was achieved before fertility preservation. Serum AMH level was measured as 1.5 ng/ml, and 12 antral follicles were counted on ultrasound. She was informed about fertility preservation options and decided to proceed with OTC, with her signed consent before cord blood transplantation in April 2011. Ovarian tissue transplantation (OTT) was performed in 2017 when the patient was menopausal with serum FSH levels > 100 IU/ml and estradiol < 20 pg/ml and hematologically in molecular remission. Detailed molecular analysis, standard histology, and immunohistochemistry demonstrated that the thawed tissue is free of malignant cells.
Results
Six months following OTT, she had spontaneous menstruation with serum FSH 11 IU/ml and estradiol 53 pg/ml. Two consecutive IVF cycles yielded three top-quality embryos. Following three embryo transfer cycles, one fresh and two frozen, a healthy term live birth was achieved. Frozen-thawed-transplanted tissues were extracted during caesarean delivery upon the patient’s request after a total period of 25 months in vivo, and histopathological evaluation revealed that the tissue was free of leukemic infiltration.
Conclusion
The authors report the first pregnancy and live birth in Turkey and the second live birth in the world following transplantation of frozen-thawed ovarian tissue in a leukemia survivor. As the transplanted tissues were removed during caesarean delivery, histological findings prove the functionality and the malignant-free status of the transplanted tissue during the grafted period.
Keywords: Fertility preservation, Ovarian transplantation, Acute lymphoblastic leukemia, Live birth
Introduction
Despite increasing cancer incidence, cancer-related death rates have decreased thanks to the development of early diagnosis and effective treatment modalities [1]. Therefore, issues regarding the quality of life of cancer survivors have become a fundamental part of contemporary multidisciplinary cancer treatment. Infertility, a major long-term side effect of cytotoxic treatments, which occurs due to ovarian damage or damage to the endocrine organs or uterus, is manifested most commonly by premature ovarian failure (POF) and early menopause. Especially, alkylating chemotherapeutic agents such as cyclophosphamide and busulfan and pelvic radiotherapy can cause permanent loss of fertility. In addition, surgery for endometriosis or other benign ovarian tumors and treatment protocols including cytotoxic agents for some hematological or immune diseases are also associated with loss of fertility [2].
Fertility preservation is an indispensable form of preventive treatment in order to maintain future fertility before destructive treatments. Therefore, ovarian tissue cryopreservation (OTC) and re-transplantation is no longer accepted as an experimental procedure [3] and is an established routine fertility preservation technique in many countries such as Belgium, Denmark, Australia, Portugal, France, Germany, Spain, Sweden, Israel, the UK, and the USA [4, 5]. Induction of puberty following ovarian tissue transplantation has also been demonstrated in a patient previously treated for Ewing sarcoma [6].
With increasing live births and advancement in technology, the label of “experimental status” has been debated heavily and recently been removed [7–9]. Indeed, some studies have argued that ovarian cryopreservation and autologous re-transplantation may be an alternative to postmenopausal hormone replacement therapy (HRT) and could be used for further possible indications other than fertility preservation [10, 11]. According to recent reports, after autologous transplantation of frozen-thawed ovarian tissue, long-term endocrine functions were restored in 67 to 93% of patients, indicating that the hormone-producing units were preserved well [9, 12, 13]. Unlike oocyte freezing, OTC theoretically allows the preservation of hundreds of primordial follicles at once, without the need for controlled ovarian stimulation. In fact, ovarian stimulation and oocyte cryopreservation can be performed in prepubertal girls, but oocyte pick up may technically be difficult, and OTC still seems to be the gold standard procedure in this age group [14].
Since the exact number of cases that underwent OTC or OTC plus re-transplantation is unknown, the effectiveness of the procedure cannot be calculated definitely [13]. However, many clinical programs contribute to the development of the technique by creating their own networks and opening their data for national and international access [15]. Although more than 130 live births have been reported with this method in literature [2], the technique still needs to be validated in terms of avoiding reintroduction of malignant cells into the patient during ovarian tissue re-transplantation [16]. Theoretically, it is not possible to detect exactly the presence of malignant cells in tissues that have been scheduled for transplantation; however, harvested ovarian tissue pieces can be evaluated before freezing or after thawing in order to detect residual malignant cells indirectly by using molecular techniques and prior fixation. To date, not a single case has been reported in humans, indicating a malignant relapse after OTC and re-transplantation due to malignant cell residue in the tissue [17]. Nevertheless, as concern regarding reintroduction of malignancy after transplantation continues, many papers have been published to describe ways to detect and eliminate malignant cells. [16–24]. The best alternative for tissue re-transplantation is discovering efficient and robust techniques for in vitro maturation (IVM) or artificial ovary applications [25, 26]. Although OTC is indicated before the treatment of leukemia, some authors do not recommend auto-transplantation because of the potential risk for disease recurrence [27]. This paper reports a healthy live birth after OTC and re-transplantation in a patient, who is a survival of acute lymphocytic leukemia (ALL). Pregnancy and live birth were established after extensive tissue assessment for malignant contamination and successful transplantation, and transplanted tissues were removed during caesarian section due to the aforementioned theoretical concerns and according to the patient’s demand. The current case is the first live birth reported from Turkey following OTC and the second in terms of ALL survivors in the world [21].
Case report
Material and methods
Ethical approval was received from the Ankara University Ethics Committee (approval no: 3083) for leukemic patients undergoing ovarian tissue cryopreservation and transplantation, and all the undergone procedures received written and signed consent from the patient.
A 19-year-old patient was referred to our clinic in April 2010 for fertility preservation. A year before, she was diagnosed with ALL after bone marrow biopsy, as she had presented with symptoms of bleeding, fever, and frequent infections. Her bone marrow biopsy revealed 90% blast ALL-L2 type, and karyotype analyses indicated reciprocal translocation at t(9;22)(q34;q11). Initially, the patient received the Berlin-Frankfurt-Munster (BFM) induction and consolidation protocol: vincristine, daunorubicin, L-asparaginase, and methotrexate, and complete remission was achieved. Then, the patient was referred to our fertility preservation unit before she was scheduled to receive myeloablative conditioning chemotherapy for cord blood transplantation, which included FluCy-TBI in April 2011 (Flu—fludarabine, Cy—cyclophosphamide, TBI—total body irradiation). At the referral, serum AMH level was measured as 1.5 ng/ml, and 12 antral follicles were counted on ultrasound. She was informed about fertility preservation options and gave her signed consent to proceed with OTC, as there was not enough time for controlled ovarian stimulation prior to scheduled chemotherapy and she did not approve transvaginal oocyte collection, as she was not married.
Cryopreservation procedure
Unilateral ovarian tissue resection was performed by laparoscopic surgery, and three quarters of the left ovary were removed. The tissue was transferred to the embryology laboratory, where the medullar layer was removed in a 3-(N-morpholino)propanesulfonic acid (MOPS) buffered gamete handling solution (G-MOPS, Vitrolife AB, Gothenburg, Sweden) immediately and the cortex layer was cut into 20 fragments of 0.5 × 1 cm-sized, 1 mm-thick pieces. Each cortical fragment was loaded into a cryovial (CBS™ High Security Tube, Cryo Bio System, L’Aigle, France) containing 1 mL of cryopreservation medium with cryoprotectant agents (CPA), consisting of 1.5 M dimethylsulfoxide (DMSO) (D2650; Sigma-Aldrich, St. Louis, MO), 0.2 M sucrose (S9378; Sigma-Aldrich) dissolved in gamete handling solution supplemented with 10% human serum albumin. Tissue fragments were equilibrated in ice on a rocking stage for 15 min and placed into a computer-controlled slow rate freezer machine (Kryo 360-1.7, Planer Limited, Middlesex, UK). The slow freezing procedure started at 4 °C, and with a rate of 2 °C/min, the temperature was decreased to – 9 °C. After 10 min of soaking, manual seeding was performed, and further cooling at a rate of 0.3 °C/min down to − 40 °C was achieved. In the final step, the samples reached − 140 °C at a rate of 10 °C/min and then were transferred into liquid nitrogen.
Thawing procedure
After cord blood transplantation, the patient developed ovarian failure with amenorrhea and her serum FSH rose to 179 mIU/mL and AMH levels were below 0.1 ng/ml. Upon marriage, 7 years after the initial diagnosis, she applied to our center with the request of re-transplantation of her ovarian tissues to conceive. Upon decision to re-transplant the frozen ovarian tissues, one of the cortical strips was thawed in advance, to uncover any residual malignant cells, using histopathological techniques and polymerase chain reaction (PCR). For thawing, the vial containing the cortical tissue was removed from the nitrogen tank, allowed to stand at room temperature for about 1 min, and then transferred into a 37 °C water bath. After full thawing was achieved, the vial content was unloaded onto a culture dish and the sample tissue was passed through media, containing CPA in gradually decreasing concentrations. The contents of the vial were initially put into medium containing 1.0 M DMSO, 0.2 M sucrose, and subsequently, media containing 0.5 M DMSO, 0.2 M sucrose; only 0.2 M sucrose and 0.1 M sucrose, respectively, were then transferred into CPA-free gamete manipulation medium. All media was prepared in gamete manipulation medium containing 10% human serum albumin and in each, the tissue was incubated with gentle agitation for 5 min at room temperature. In order to detect minimal residual disease (MRD), the entire thawed sample tissue was evaluated by means of immunohistochemistry and PCR. CD3 (marker for T lymphocyte), CD20 (marker for B lymphocyte), CD34 (marker for hematopoietic stem cells and blood vessels), PAX5 (marker for subtypes of B-progenitor ALL), TDT (marker expressed on the surface of leukemic cells), and inhibin (for detection of functional follicles) expressions were analyzed by immunohistochemistry on paraffin embedded slides taken from the whole tissue. Additionally, hematoxylin-eosin staining was performed on two paraffin sections, in order to overview the tissue histology. Another piece from the thawed ovarian fragment was analyzed using molecular qPCR analysis for the detection of the presence of the BCR/ABL p190 fusion gene with a suitable kit (ipsogen® BCR-ABL1 Mbcr IS-MMR, Qiagen GmbH, Hilden, Germany) by using fusion gene cDNA specific primer, m-bcr:BCR-ABL p190 (e1a2) (Düzen Laboratories, Ankara, Turkey).
Follicle survival and methods to detect malignant cells
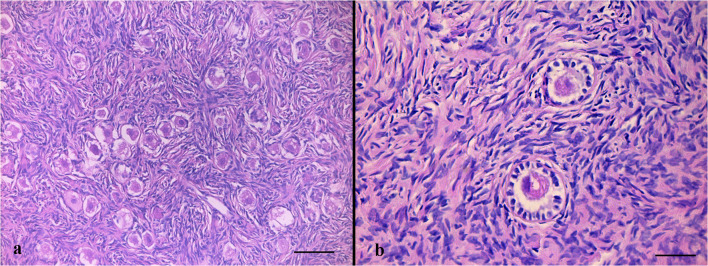
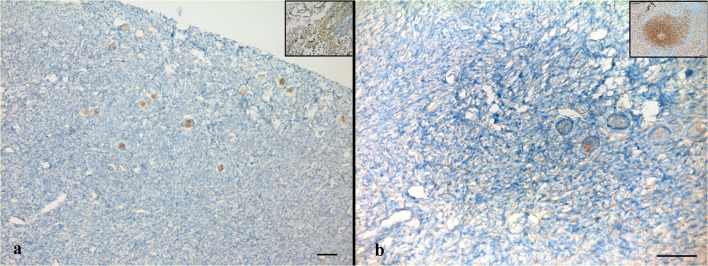
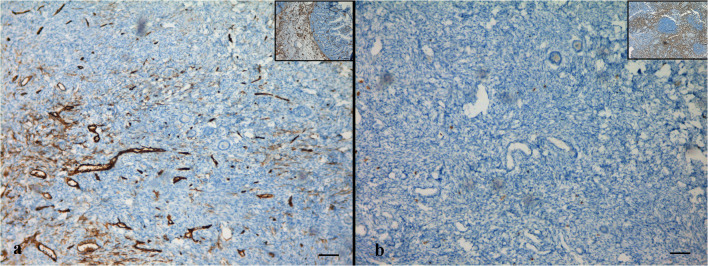
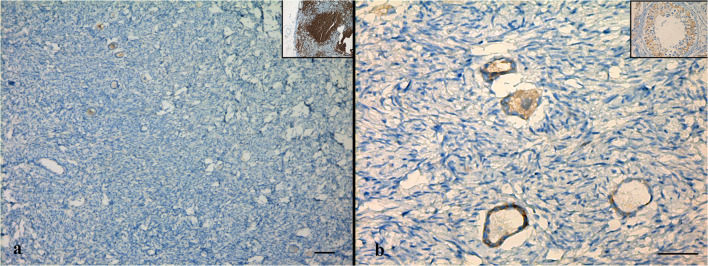
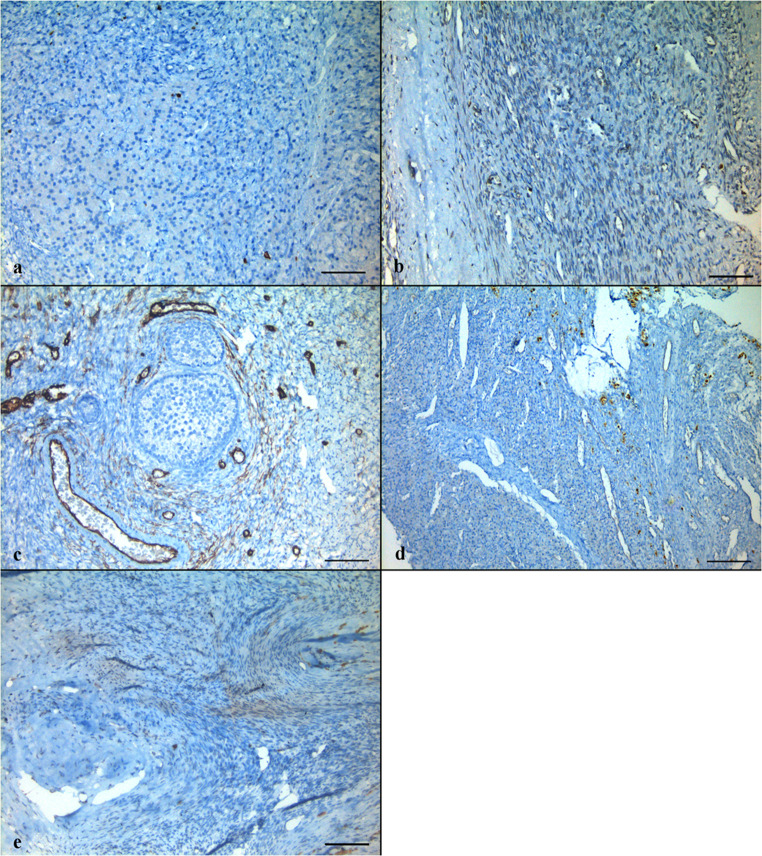
The cortical strips thawed prior to re-transplantation underwent detailed evaluation in order to examine follicular and stromal appearance and to detect residual lymphoblastic infiltration. In hematoxylin-eosin staining, follicular and stromal structures of the ovarian cortex were assessed as intact. There were an abundant number of primordial follicles, most of which contained an undamaged oocyte surrounded by a single layer of squamous follicle cells. Some primordial follicles were displayed as shrunken or with vacuolated cytoplasm with irregular oolemma, despite an intact follicular structure. A small number of healthy primary follicles were observed in their early stage of development with a single layer of cuboidal follicle cells surround the growing oocytes (Fig. 1a, b). Immunohistochemical analyses were performed by labeling the paraffin sectioned ovarian cortex tissue with the aforementioned markers together with positive control tissues. Accordingly, TDT and PAX5 labeling revealed a negative staining pattern (Fig. 2a, b). With CD34, there was positive staining on the vascular structures in the ovarian stroma, but there was no appearance suggesting blastic cell infiltration (Fig. 3a). CD3 showed a small number of lymphocytes in one area without a specific appearance of infiltration (Fig. 3b). CD20 staining was negative (Fig. 4a). A rare positivity in inhibin expression was found in the follicle epithelial cells and stroma (Fig. 4b). No BCR/ABL p190 fusion gene was detected in the qPCR evaluation.
Fig. 1.
Hematoxylin-eosin stained sections of the frozen-thawed ovarian cortex display an abundant number of primordial follicles, most of which contain an intact primary oocyte (a). A small number of healthy primary follicles was observed (b). Scale bars indicate 100 μm in Fig. 1a, 50 μm in Fig. 1b
Fig. 2.
Immunohistochemical labeling of cortical tissue with TDT (a) and PAX5 (b) reveal negative staining. Inlets show positive control staining (a: bone marrow, b: lymph node). Scale bars indicate 100 μm
Fig. 3.
Immunohistochemical labeling of cortical tissue with CD34 (a) and CD3 (b). Inlets show positive control staining (a: colon, b: lymph node). Scale bars indicate 100 μm
Fig. 4.
Immunohistochemical labeling of cortical tissue with CD20 (a) and inhibin (b). Inlets show positive control staining (a: lymph node, b: testis). Scale bars indicate 100 μm
Ovarian transplantation
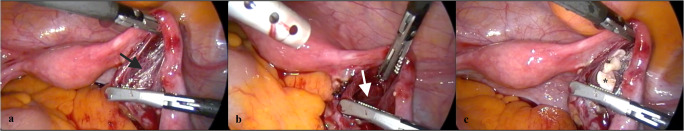
One week before ovarian tissue re-transplantation surgery, a pouch opening was performed by laparoscopy between the peritoneal leaves, just below the right fallopian tube in the mesoovarium (Fig. 5a) in order to prepare a transplant niche (Fig. 5b) and to induce angiogenesis. In December 2017, ovarian transplantation was performed laparoscopically. Briefly, the patient was prepared for the transplantation in the operating room, and simultaneously, 9 additional ovarian tissue fragments were thawed as described above. The total number of thawed cortical fragments was 10, so half of the frozen tissues were thawed for this current transplantation procedure. Thawed fragments were transported to the operating room in a MOPS-buffered gamete-handling medium immediately, and with the help of spoon forceps, the ovarian cortex fragments were inserted into the pre-prepared cavity in the right side mesoovarium (Fig. 5c). The pouch lips were closed using 5/0 vicryl suture after the ovarian cortex fragments were aligned so that both sides of the tissues would be in contact with the mesoovarium wall.
Fig. 5.
Images taken during laparoscopic ovarian tissue re-transplantation surgery. One week before transplantation, the right mesovarium was explored (a, black arrow) and a transplant niche was prepared in the form of a pouch (b, white arrow). A week later, upon thawing, nine cortical strips (asterisk) were transferred laparoscopically (c).
Results
Follow-up, controlled ovarian stimulation
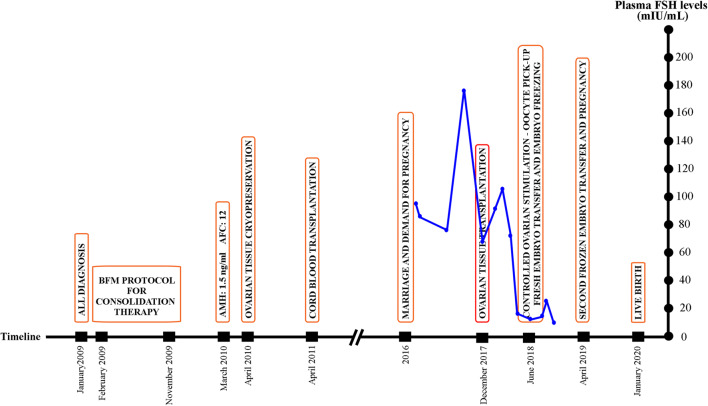
Endocrine follow-ups while the patient was on HRT showed gradually decreasing serum FSH and LH and increasing estradiol levels: In January 2018, FSH = 92 IU/ml, LH = 28 IU/ml, E2 < 10 pg/ml; in February 2018; FSH = 107 IU/ml, E2 < 10 pg/ml; in March 2018, FSH = 76 IU/ml, LH = 41 IU/ml; E2 = 29 pg/ml; in April 2018, FSH = 18 IU/ml, LH = 9.4 IU/ml, E2 = 78 ng/ml (Fig. 11)
Fig. 11.
Graph of plasma FSH measurements (navy dots and lines) of the patient presented for fertility preservation from the time of referral to the pregnancy with a timeline of significant events (orange rectangles) starting from the diagnosis. The red rectangle indicates the day of ovarian transplantation
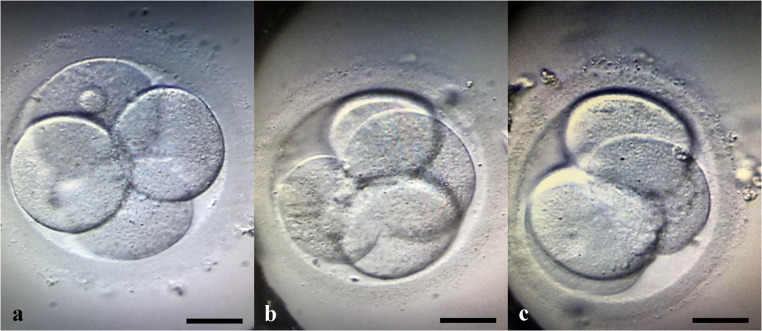
Transvaginal ultrasonography performed in March 2018 revealed an atrophic left ovary and a 5-mm follicle in the right ovarian region. In June 2018, the patient had spontaneous menstruation without medication for the first time, and her endocrine values were as follows: FSH = 18 IU/ml, LH = 9.4 IU/ml, E2 = 78 pg/ml. The first IVF attempt was in June 2018. On her second day of menstruation, FSH level was 11 IU/ml, LH was 2.79 IU/ml, estradiol was 53 pg/mL, and serum AMH was 0.4 ng/ml. Two consecutive controlled ovarian hyperstimulation cycles with GnRH antagonist protocol (Cetrotide, Merck, Darmstadt, Germany) were performed, using a combination of highly purified hMG 75 IU/day (Menopur, Ferring, Saint-Prex, Switzerland) and recombinant FSH 150 IU/day (Gonal F, Merck), and a transvaginal oocyte pick up was performed 35 h after dual trigger using 250 mg of recombinant hCG (Ovitrelle250, Merck) and 1 mg of leuprolide acetate (Lucrin, Abbott, Chicago, IL) [28]. Follicle aspiration was performed from the grafted tissues, as the ovaries of the patient were already atrophic and invisible by ultrasound. A total of 10 oocytes were collected, 7 were mature, and intracytoplasmic sperm injection was performed using normozoospermic ejaculate of the patient’s husband, yielding 3 grade 1 embryos [29]. While the first fresh (Fig. 6a) and frozen embryo transfers (Fig. 6b) were unsuccessful, a second frozen embryo transfer, with a top quality Day 3 embryo (Fig. 6c, Day 2 pictures are displayed), performed in April 2019 resulted in pregnancy. An HRT cycle was chosen to prepare the endometrium since the patient experienced some irregular cycles following the oocyte retrieval procedures. For this purpose, increasing doses of estradiol hemihydrate tablets (Estrofem, Novo Nordisk A/S, Bagsværd, Denmark) were used, starting from Day 2 of the menstrual cycle. The initial dose was 2 mg/day and was increased up to 6 mg/day until the endometrial lining was trilaminar in appearance and reached 8 mm in thickness. Then, 80 mg of 8% micronized progesterone was started twice a day (Crinone gel 8%, Merck), and continued up to the 9th week of pregnancy.
Fig. 6.
Three grade-1 embryos resulted in pregnancy and live birth cumulatively from oocytes derived from transplanted ovarian cortical tissue. Micrographs were obtained on day 2 of embryo development. Scale bars indicate 30 μm.
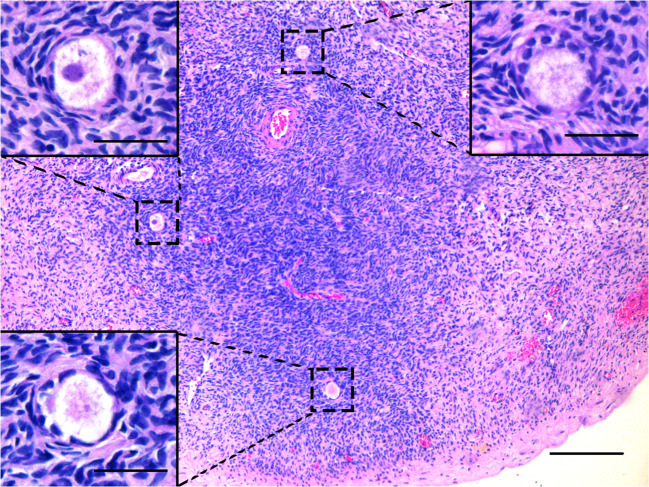
A healthy 2980-g newborn with Apgar scores of 8 and 10 was delivered by caesarean section on the 38th week of pregnancy, in January 2020. The mother and newborn were discharged as healthy on Day 1 postoperative. During caesarean delivery, all the transplanted ovarian fragments were removed, upon the patient’s request, and sent for histopathological analysis for any possible blastic infiltration. Hematoxylin-eosin staining revealed healthy primordial (Fig. 7) and growing follicles (Fig. 8), as well as corpora albicans and luteum with hemosiderin-laden macrophage groups (Fig. 9). Notably, immunohistochemical staining against CD3, CD20, CD34, PAX5, and TDT antigens revealed no blastic infiltration in the removed ovarian tissues (Fig. 10).
Fig. 7.
Hematoxylin-eosin staining of ovarian tissue that was frozen-thawed, re-transplanted, and finally removed during the caesarian section. Dashed rectangles show healthy primordial follicles and inlets demonstrate magnified images. Scale bars indicate 50 μm.
Fig. 8.
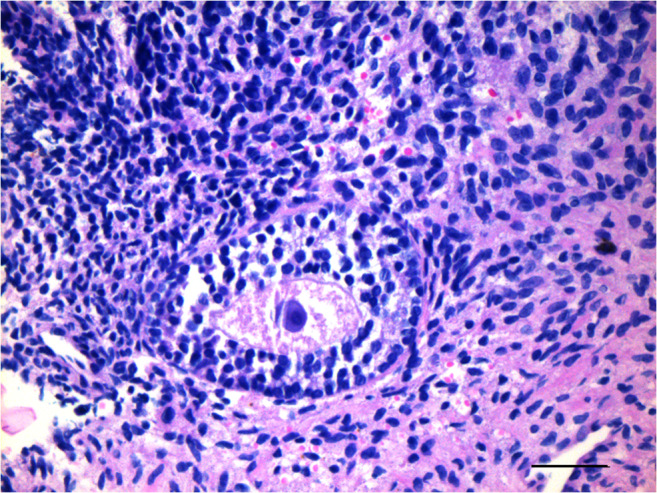
Hematoxylin-eosin stained ovarian section demonstrates a growing pre-antral follicle with a healthy primary oocyte and its zona pellucida. Scale bar indicates 50 μm.
Fig. 9.
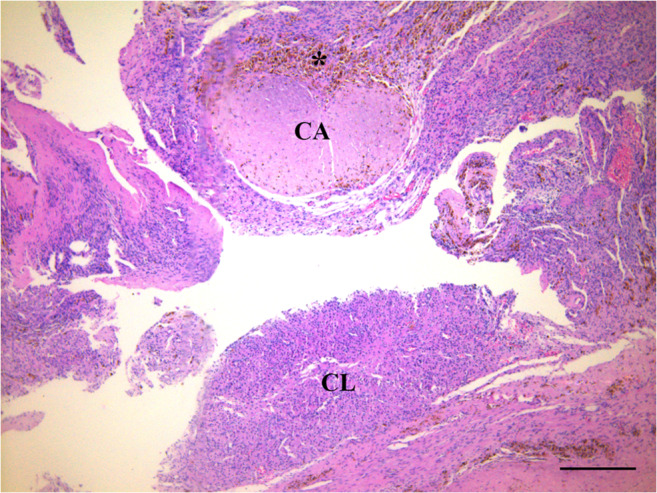
Hematoxylin-eosin stained ovarian section demonstrates corpus albicans (CA) and corpus luteum (CL) with hemosiderin-laden macrophages (asterisk). Scale bar indicates 50 μm.
Fig. 10.
Immunohistochemical staining against CD3 (a), CD20 (b), CD34 (c), PAX5 (d), and TDT (e) antigens reveal no blastic infiltration in removed ovarian tissues. Scale bars indicate 50 μm.
Discussion
OTC is no longer labeled as experimental [3], and it is widely used as the gold standard fertility preservation technique in many countries worldwide [4, 13]. Despite some groups reporting the safety and efficacy of ovarian stimulation in peri-pubertal children at risk of ovarian failure due to cancer treatment [30], oocyte pick up procedures may be technically compelling in these young girls, and there is no alternative way to protect future fertility other than OTC in many countries. Additionally, it is reported that puberty can be induced following OTC and re-implantation [6, 31] and that live birth is possible, even when the ovarian tissue is cryopreserved before puberty [32]. With more than 130 live births [2], close to 93% endocrine recovery and 30% pregnancy rates published in some reports [9, 33], there is still the theoretical risk of malignant cell transplantation and efficiency data of the entire procedure is insufficient [2]. According to the questionnaire results of a large number of patient series from Belgium, it was revealed that there was a 33% live birth rate among 21 patients (3.9% of the total) who underwent auto-transplantation [34]. An interesting report comparing the oocyte versus ovarian cryopreservation by Diaz-Garcia et al. indicated that the usage rate of cryopreserved material was 4.8% in oocyte and 6.2% in ovarian cryopreservation, and their pregnancy and live birth rates were comparable [35].
Here, the authors report the first live birth after OTC and re-transplantation nationally, as OTC was legalized in the last decade in Turkey. Ovarian tissue cryopreservation started at the Ankara University School of Medicine in 2004 with a grant from the “State Planning Organization of Turkey.” Ethical approval was provided for patients undergoing ovarian tissue cryopreservation and transplantation. Approximately 300 cryopreservation procedures for both malignant and nonmalignant diseases and 6 cases of ovarian transplantation have been performed thus far in the same institution.
Before ovarian re-transplantation, the patient was in complete menopause, assessed by extremely high FSH and low estradiol values and atrophic ovaries because of ovarian failure. The FSH values increased dramatically and returned to normal reference ranges, after a fluctuation period following re-transplantation. It was demonstrated that transplanted grafts might function similarly to poor responder ovaries. Since follicular growth dynamics may be different in patients with decreased ovarian reserve who also had irregular menstrual cycles, it may not be infrequent to see fluctuations of hormone levels in such a group of patients with increased FSH or decreased estradiol levels [36] (Fig. 11).
This case is also important in terms of being the second published healthy live birth following ovarian transplantation in a patient with ALL [21]. Another interesting fact of this report is that the authors were obliged to remove the transplanted tissues during caesarean section and therefore were able to examine them after 25 months of in vivo grafting. By histologic assessment, it was proven that the grafted tissue maintained primordial follicles and sustained folliculogenesis. Notably, no blastic infiltration was found in the removed tissues. Since leukemia is a malignancy originating from bone marrow, it has the potential to infiltrate all tissues with circulation, and the risk of distant metastasis is very high. In this respect, it has been argued by some groups that ovarian re-transplantation is unsafe for the purpose of fertility preservation in leukemia patients [27]. On the other hand, there are contradictory reports presenting lack of malignant leukemia cells in the cryopreserved ovarian tissue if the patient is in complete remission at that time [18]. Disease transfer was demonstrated after transplantation of leukemia-infected tissues into immune-deficient mice [27]. Although this xenotransplantation model does not accurately reflect the condition of patients with leukemia, it emphasizes the need for more reliable laboratory tests in order to detect MRD before the transplantation of these tissues [16–19, 22]. However, it is debated to what extent these tissue fragments will represent the whole cohort of transplanted tissues [21]. Since immunohistochemical tests can show tissue integrity and lymphoblastic infiltration to a certain point, further molecular tests such as PCR, next generation sequencing, or flow cytometry are mandatory, as leukemic cells expressing specific markers of the disease will improve screening for MRD [17]. In the present case, leukemic cells contained the Philadelphia chromosome as a reference in PCR analysis and no leukemic cells were found in the thawed-sample tissue.
Data is limited on the timing of the ovarian biopsy and especially information on the hematologic malignancies regarding the risk of metastasis. A paper from Dolmans et al. [27] reported that an induction or consolidation therapy prior to OTC has no or minimal effect on the MRD present in the vessels or the stroma of the grafted ovary in leukemia patients. They concluded that a single cycle of chemotherapy is not enough to eliminate the leukemic cells from the biopsied ovary. On the other hand, the corner stone report by Greve et al. revealed that ovarian tissues lack malignant contamination when patients of leukemia are in complete remission [18]. Even though there is lack of evidence, it seems better to cryopreserve ovarian tissue in leukemia patients after consolidation therapy, prior to stem cell transplantation, in order to minimize the leukemic cells inside the tissue. Furthermore, even though some reports demonstrated no significant decrease in follicular density following induction chemotherapy [18], it should be noted that the ovarian reserve and effectiveness of assisted reproductive technologies may diminish with each round of chemotherapy administered [37]. In theory, it may be advantageous to perform ovarian tissue harvesting after the first round of chemotherapy in order to clear any neoplastic cells residing in the ovary. In the current case, since the patient had already underwent previous consolidation chemotherapy, it might have influenced the presence of leukemic cells in the ovarian cortex.
It was reported that when “immune-deficient mice” were used, only some of the PCR-positive tissues were able to induce leukemia [27]. Therefore, a positive result obtained with PCR does not necessarily translate into disease recurrence [21]. Indeed, the new immune system that is constituted following hematopoietic stem cell transplantation, unlike from an innate immune system, would have competent immune responses against any possible circulating tumor cells. Experimental studies are running to transplant these tissues in fibrin carriers [23] and artificial ovaries or to isolate primordial follicles for IVM [26]. However, isolation of primordial follicles and effective IVM techniques are not performed routinely, and none of these treatments is sufficient to be offered as an alternative method to auto-transplantation [21].
In this case, a peritoneal pouch was prepared laparoscopically in the mesoovarium a week before the re-transplantation. Despite not being standard and unproven, we believe that this process reduced the time of the re-transplantation surgery and provided a vascularized niche for the re-transplanted ovarian cortical tissues. The re-transplanted tissues were removed during caesarean delivery upon request of the patient, due to the patient’s concerns about the recurrence of leukemia. Thus, the frozen-thawed ovarian tissues remained in the patient for 25 months. According to the immunohistochemical examinations, there was no leukemic activity in the tissues removed, and no pathological findings were observed in the patient’s hematological follow-ups during this period. In addition, histological examination of the removed tissues showed that, despite a decrease in primordial follicle density, the transplanted tissues were functional with well-preserved stromal integrity and continued folliculogenesis and hormone-releasing units such as corpus luteum. We think that removal of tissues is an ethically challenging decision because the removal of them, with the concern of leukemic activation, will bring the patient back to menopause. The decision was made upon the patient’s personal demands and the presence of the ten remaining cryopreserved ovarian tissues remain for further use.
Conclusion
Here we report the first case of a live birth after ovarian cryopreservation and re-transplantation in Turkey. We have been carrying out ovarian tissue cryopreservation procedure since 2005 and accepting tissue biopsies from other institutions all over the country as a pilot center at the Ankara University Medical Faculty, Center for Assisted Reproduction and Infertility. There have been almost 300 patients from whom ovarian tissues were cryopreserved for both malignant and nonmalignant diseases in the fertility preservation program. The presented case is the second re-transplantation procedure, and the clinic has achieved restoration of endocrine function in all of the six patients who underwent ovarian re-transplantation. All the patients’ follow-ups are still in progress [38].
Although this case report is remarkable, one should be cautious about the risks of ovarian tissue transplantation as the procedure is case-dependent and cannot be extrapolated to all leukemia patients. This case report implies significant importance because it is the first national pregnancy and live birth following auto-transplantation and the second published case among leukemia survivors worldwide. Despite highly debated, depending on the prognosis and the patient’s request, removing transplanted tissues after conception may be an alternative strategy that increases safety.
Acknowledgments
Part of the data according to current case report was presented as a poster in the 6th World Congress of the International Society for Fertility Preservation, November 14–16, 2019.
Authors’ contributions
Sonmezer M was responsible for gynecologic and endocrine follow-up, surgeries, analysis, manuscript drafting, and critical discussion. Ozkavukcu S was responsible for tissue handling, embryo manipulation, analysis, manuscript drafting, and critical discussion. Sukur YM was responsible for gynecologic and endocrine follow-up, surgeries, and manuscript drafting. Kankaya D was responsible for pathological evaluations and critical discussion. Arslan O was responsible for hematological follow-up, analysis, manuscript drafting, and critical discussion.
Data availability
Data obtained in this manuscript is available.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Text has been prepared using Microsoft Word application.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.Epi-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive Medicine. Electronic address aao Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112(6):1022–1033. doi: 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of human ovarian tissue: a review. Transfus Med Hemother. 2019;46(3):173–181. doi: 10.1159/000499054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirot C, Brugieres L, Yakouben K, Prades-Borio M, Marzouk F, de Lambert G, et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment. Twenty years of experience at a single center. Acta Obstet Gynecol Scand. 2019;98(5):630–637. doi: 10.1111/aogs.13616. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RA, Hindmarsh PC, Wallace WH. Induction of puberty by autograft of cryopreserved ovarian tissue in a patient previously treated for Ewing sarcoma. Eur J Cancer. 2013;49(13):2960–2961. doi: 10.1016/j.ejca.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Dolmans M-M, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104(5):1097–1098. doi: 10.1016/j.fertnstert.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Forman EJ. Ovarian tissue cryopreservation: still experimental? Fertil Steril. 2018;109(3):443–444. doi: 10.1016/j.fertnstert.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Meirow D, Ra'anani H, Shapira M, Brenghausen M, Chaim SD, Aviel-Ronen S, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 10.von Wolff M, Dittrich R, Stute P. Transplantation of ovarian tissue to postpone menopause–is it really more advantageous for women's health than menopause hormone therapy? Reprod BioMed Online. 2015;31(6):827. doi: 10.1016/j.rbmo.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen SG, Andersen CY. Cryopreservation of ovarian tissue: opportunities beyond fertility preservation and a positive view into the future. Front Endocrinol. 2018;9:347. doi: 10.3389/fendo.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod (Oxford, England) 2016;31(9):2031–2041. doi: 10.1093/humrep/dew165. [DOI] [PubMed] [Google Scholar]
- 13.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34(3):325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril. 2016;105(1):6–12. doi: 10.1016/j.fertnstert.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 15.von Wolff M, Andersen CY, Woodruff TK, Nawroth F. FertiPROTEKT, Oncofertility Consortium and the Danish Fertility-Preservation Networks - what can we learn from their experiences? Clin Med Insights Reprod Health. 2019;13:1179558119845865. doi: 10.1177/1179558119845865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolmans M-M, Masciangelo R. Risk of transplanting malignant cells in cryopreserved ovarian tissue. Minerva Ginecol. 2018;70(4):436–443. doi: 10.23736/s0026-4784.18.04233-8. [DOI] [PubMed] [Google Scholar]
- 17.Zver T, Alvergnas-Vieille M, Garnache-Ottou F, Ferrand C, Roux C, Amiot C. Minimal residual disease detection in cryopreserved ovarian tissue by multicolor flow cytometry in acute myeloid leukemia. haematologica. 2014:haematol. 2014.113373. [DOI] [PMC free article] [PubMed]
- 18.Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M, Ralfkiær E, Andersen CY. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120(22):4311–4316. doi: 10.1182/blood-2012-01-403022. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Serrano M, Novella-Maestre E, Roselló-Sastre E, Camarasa N, Teruel J, Pellicer A. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Hum Reprod. 2009;24(9):2238–2243. doi: 10.1093/humrep/dep196. [DOI] [PubMed] [Google Scholar]
- 20.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising "ex vivo" method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN, Schiff E, Orvieto R, Meirow D. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril. 2018;109(1):48–53. doi: 10.1016/j.fertnstert.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Soares M, Saussoy P, Maskens M, Reul H, Amorim CA, Donnez J, Dolmans MM. Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients. Br J Haematol. 2017;178(2):231–239. doi: 10.1111/bjh.14657. [DOI] [PubMed] [Google Scholar]
- 23.Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, van Ruymbeke E, Champagne SD, Donnez J, Amorim CA. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet. 2018;35(1):41–48. doi: 10.1007/s10815-017-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolmans MM, Amorim CA. Construction and use of artificial ovaries. Reproduction (Cambridge, England). 2019. 10.1530/rep-18-0536. [DOI] [PubMed]
- 25.Salama M, Anazodo A, Woodruff TK. Preserving fertility in female patients with hematological malignancies: a multidisciplinary oncofertility approach. Ann Oncol. 2019;30(11):1760–1775. doi: 10.1093/annonc/mdz284. [DOI] [PubMed] [Google Scholar]
- 26.Salama M, Woodruff TK. From bench to bedside: current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet Gynecol Scand. 2019;98(5):659–664. doi: 10.1111/aogs.13552. [DOI] [PubMed] [Google Scholar]
- 27.Dolmans M-M, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 28.Seval MM, Ozmen B, Atabekoglu C, Sukur YE, Simsir C, Kan O, et al. Dual trigger with gonadotropin-releasing hormone agonist and recombinant human chorionic gonadotropin improves in vitro fertilization outcome in gonadotropin-releasing hormone antagonist cycles. J Obstet Gynaecol Res. 2016;42(9):1146–1151. doi: 10.1111/jog.13021. [DOI] [PubMed] [Google Scholar]
- 29.Alpha Scientists in Reproductive M, Embryology ESIGo The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod (Oxford, England) 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 30.Oktay K, Bedoschi G. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in turner syndrome or cancer treatments. J Pediatr Adolesc Gynecol. 2014;27(6):342–346. doi: 10.1016/j.jpag.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. doi: 10.1016/S0140-6736(11)61781-9. [DOI] [PubMed] [Google Scholar]
- 32.Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod (Oxford, England) 2015;30(9):2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 33.Beckmann MW, Lotz L, Toth B, Baston-Büst DM, Fehm T, Frambach T, Germeyer A, Goeckenjan M, Häberlin F, Henes M, Hirchenhain J, Hübner S, Korell M, Krüssel JS, Müller A, Reinsberg J, Schwab R, Seitz S, Sütterlin M, van der Ven H, van der Ven K, Winkler-Crepaz K, Wimberger P, von Wolff M, Liebenthron J, Dittrich R. Concept paper on the technique of cryopreservation, removal and transplantation of ovarian tissue for fertility preservation. Geburtshilfe Frauenheilkd. 2019;79(01):53–62. doi: 10.1055/a-0664-8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadoul P, Guilmain A, Squifflet J, Luyckx M, Votino R, Wyns C, et al. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod (Oxford, England) 2017;32(5):1046–1054. doi: 10.1093/humrep/dex040. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I, et al. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril. 2018;109(3):478–85.e2. doi: 10.1016/j.fertnstert.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Sönmezer M, Cil AP, Oktay K. Ongoing pregnancies from early retrieval of prematurely developing antral follicles after DHEA supplementation. Reprod BioMed Online. 2009;19(6):816–819. doi: 10.1016/j.rbmo.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Oktay K, Oktem O, Reh A, Vahdat L. Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol. 2006;24(24):4044–4046. doi: 10.1200/jco.2006.06.9823. [DOI] [PubMed] [Google Scholar]
- 38.Ozkavukcu S, Sonmezer M, Atabekoglu C, Berker B, Ozmen B. Ovarian cryopreservation (OC) and orthotopic re-transplantation: experiences of a pilot center in Turkey. Fertil Steril. 2013;100(3):S170. doi: 10.1016/j.fertnstert.2013.07.1470. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data obtained in this manuscript is available.