Abstract
Alfalfa is the major fodder crop of Sultanate Oman, but salinity is a major problem in its cultivation. Therefore, thirty-four alfalfa (Medicago sativa L.) landraces of Oman were evaluated for morphology and forage yield response to different salinity levels viz. 1 (control), 3, 6, 9, and 12 dS m−1 under greenhouse conditions. The experiment was conducted under a completely randomized design. Different alfalfa landraces responded differently to the five salinity levels for plant height, number of branches, number of leaves, leaflet width, leaflet length, forage fresh weight, and forage dry matter yield. Salt stress caused a reduction in growth and dry matter yield of alfalfa landraces with exception of some, which responded positively to the salinity levels of 3 and 6 dS m−1 compared to control for the number of leaves per plant. Moreover, some landraces had better forage fresh weight and dry matter yield at 6 dS m−1 than 3 dS m−1. Alfalfa landraces OMA 257, OMA, 245, OMA 270, OMA 315, OMA 211, OMA 117, OMA 56, OMA 239, OMA 148, OMA 131, OMA 95, OMA 263, OMA 262, OMA 289 and OMA 220 were designated as salt tolerant based on their overall performance across salinity levels of 6, 9 and 12 dS m−1. However, the landraces OMA 305, OMA 100, OMA 211, OMA 148, OMA 60, OMA 248, OMA 9, OMA 88, and OMA 302 collected were sensitive to 6, 9 and 12 dS m−1 salinity stress. The study showed the variation of alfalfa landraces potential for salinity tolerance, and their potential for cultivation in saline areas and/or use in breeding programs aimed to develop salt tolerant alfalfa genotypes.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00856-5) contains supplementary material, which is available to authorized users.
Keywords: Alfalfa, Accessions, Branches, Forage yield, Leaflet, Salinity
Introduction
Worldwide, soil salinity is a limiting factor in terms of decreasing agricultural production. An FAO report of ‘FAO Land and Plant Nutrition Management Service’ declared 6% (400 Mha) of the world's land as salt affected or sodic (Parihar et al. 2015). Recent data indicated that of a total of 230 Mha irrigated land, 45 Mha (19.5%) are saline, while out of 1,500 Mha of dryland, 32 Mha (2.1%) is salt affected (Parihar et al. 2015). In many semi-arid parts of the world, salinity is threatening the sustainability of crop production.
Globally, salt stress is a major abiotic factor, which significantly decreases the growth and productivity of plants. In the Sultanate of Oman, salinity is also a major problem. The 38% (11.7 Mha) of the total area in Oman is salt affected (FAO 2008), which accounts for 4.97% (70, 000 ha) of agriculturally suitable land in the country (MAF 2014). In Oman, there exist two types of salinity. The first one is due to poor irrigation management in the areas where freshwater is supplied. This could be improved through improved drainage and better soil water management. The second type of salinity is owing to the uncontrolled extraction of fresh groundwater than available in the coastal areas. This imbalance in coastal aquifers allows the inflow of seawater which causes salinization of irrigation water sources.
In Oman, the salinity level of alfalfa fields varies from 1 to 15 dS m−1 (MAF 2012). Although much of the research into salinity tolerance in alfalfa has focused on germination and early growth (Yacoubi et al. 2013), a few studies evaluated alfalfa for forage yield. For instance, in a study of five ecotypes from Azarbaijan, salinity significantly reduced the forage yield of all five alfalfa ecotypes of which two ecotypes were identified as salt tolerant for producing better forage yield at salinity level of 20 dS m−1 (Monirifar and Barghi 2009).
Salinity stress affects plant growth by disturbing the water relations and ionic balance. Under salinity stress, the plants first face water stress that leads to a decrease in leaf expansion (Farooq et al. 2015, 2017), while long term salinity causes an ionic imbalance in plants with a reduction in photosynthesis (Farooq et al. 2017), carotenoids and chlorophyll. Salinity decreases the germination of crops (> 50%) by causing toxicity in the germinating embryos due to inhibited uptake of water. Moreover, it reduced the growth of plants (by more than 70%) due to ion toxicity or decreased photosynthesis (Farooq et al. 2017). In the transpiration stream the entry of salt cause ionic toxicity, which damages cells of transpiring leaves which in turn reduces the growth (Munns 1993; Al-Farsi et al. 2020). Furthermore, under salinity, carbon fixation decreased owing to the reduction in CO2 availability through stomata limitation for diffusion (Flexas et al. 2004; Li et al. 2010; Al-Farsi et al. 2020). In a study, it was found that salinity stress of 180 mM reduced the germination by 50% in Phaseolus species (Bayuelo-Jimenes et al. 2002). In legumes, stand establishment and later growth stages are more sensitive stages to salinity compared to the germination phase (Al-Mutata 2003).
In earlier studies, the screening of alfalfa accessions against salinity tolerance was done at germination and stand establishment stages (Wang et al. 2009; Yacoubi et al. 2013). However, in this study, the screening of different alfalfa accessions against salinity stress was performed based on the forage fresh and dry matter yield. The evaluation of alfalfa genotypes of the Sultanate of Oman for morphological characters and forage yield under different levels of salinity has rarely been explored. This study was, therefore, conducted to evaluate the response of local landraces of alfalfa at lateral growth stages to a varying degree of salinity stress and their tolerance level. It was hypothesized that; the different Omani alfalfa landraces would be tolerant to a varying degree of salinity stress. The specific objectives were (i) to select salt tolerant landraces of alfalfa at different levels of salinity and (ii) to recommend using these salt tolerant alfalfa landraces in breeding programs.
Materials and methods
The experimental site, treatments, and design
The experiment was carried out in a greenhouse (temperature 26 ± 2/18 ± 2 °C (day/night) at the Agriculture Research Station, Rumais, Sultanate of Oman. The seeds were planted on February 04, 2015, and harvested on July 25, 2015. Thirty-four local alfalfa landraces collected from different parts of Oman were screened at different levels of salinity stresses viz., 1 (control), 3, 6, 9, and 12 dS m−1 (Table 1). The study was conducted in a completely randomized design with four replications.
Table 1.
Alfalfa landraces used in the study and
source of origin
| S. No. | Accession No | Germplasm Source / Origin | Nature of Geographic location | Salinity range (dS m−1) |
|---|---|---|---|---|
| 1 | OMA 270 | A'Sharqiya, Oman | Interior Plain | 1.00 |
| 2 | OMA 245 | A'Sharqiya, Oman | Interior Plain | 1.10 |
| 3 | OMA 9 | Al-Dakhiliya, Oman | Interior Plain | 4.52 |
| 4 | OMA 117 | Al-Dhahirah, Oman | Interior mountain | 0.45 |
| 5 | OMA 224 | Al-Dakhiliya, Oman | Interior mountain | 0.30 |
| 6 | OMA 239 | Al-Buraimi, Oman | Interior Plain | 1.20 |
| 7 | OMA 262 | A'Sharqiya, Oman | Interior mountain | 0.52 |
| 8 | OMA 60 | Al-Dakhiliya, Oman | Interior mountain | 0.58 |
| 9 | OMA 282 | A'Sharqiya, Oman | Interior mountain | 0.38 |
| 10 | OMA 95 | Al-Dhahirah, Oman | Interior Plain | 0.36 |
| 11 | OMA 220 | Al-Dakhiliya, Oman | Interior mountain | 0.51 |
| 12 | OMA 248 | A'Sharqiya, Oman | Interior Plain | 1.10 |
| 13 | OMA 253 | A'Sharqiya, Oman | Interior Plain | 1.20 |
| 14 | OMA 263 | A'Sharqiya, Oman | Interior Plain | 1.00 |
| 15 | OMA 289 | A'Sharqiya, Oman | Interior mountain | 0.50 |
| 16 | OMA 315 | North Batinah, Oman | Coastal Plain | 3.50 |
| 17 | OMA 290 | A'Sharqiya, Oman | Interior mountain | 0.54 |
| 18 | OMA 211 | Al-Dakhiliya, Oman | Interior Plain | 0.80 |
| 19 | OMA 222 | Al-Dakhiliya, Oman | Interior Plain | 0.50 |
| 20 | OMA 268 | A'Sharqiya, Oman | Interior Plain | 0.53 |
| 21 | OMA 56 | Al-Dakhiliya, Oman | Interior Plain | 0.48 |
| 22 | OMA 257 | A'Sharqiya, Oman | Interior mountain | 0.30 |
| 23 | OMA 228 | Al-Dakhiliya, Oman | Interior mountain | 0.42 |
| 24 | OMA 41 | Al-Dakhiliya, Oman | Interior Plain | 1.30 |
| 25 | OMA 100 | Al-Dakhiliya, Oman | Interior Plain | 0.36 |
| 26 | OMA 141 | South Batinah, Oman | Coastal mountain | 0.38 |
| 27 | OMA 5 | Al-Dakhiliya, Oman | Interior Plain | 3.27 |
| 28 | OMA 88 | Al-Dhahirah, Oman | Interior Plain | 0.50 |
| 29 | OMA 305 | North Batinah, Oman | Coastal Plain | 3.00 |
| 30 | OMA 302 | A'Sharqiya, Oman | Interior mountain | 0.50 |
| 31 | OMA 148 | South Batinah, Oman | Coastal mountain | 0.38 |
| 32 | OMA 131 | South Batinah, Oman | Coastal mountain | 0.02 |
| 33 | OMA 195 | South Batinah, Oman | Coastal mountain | 1.00 |
| 34 | OMA 98 | Al-Dhahirah, Oman | Interior Plain | 1.16 |
Crop husbandry
Seeds of alfalfa landraces were sown (five seeds per pot) in soil filled pots (30 cm diameter and 40 cm height) (5 kg pot−1). The experimental soil was sandy loam with pH (7.1), electrical conductivity (2.17 dS m−1), total nitrogen (N; 0.031%), extractable phosphorus (P; 0.023 mg kg−1) and extractable potassium (K; 0.061 mg kg−1). Fertilizer N (urea), P (triple super sulphate) and K (potassium sulphate) were applied at 0.089:0.053:0.112 g pot−1 respectively according to national recommendations (N 200: P 120: K 250 kg ha−1). The whole amount of P and K, and half N were applied at planting while remaining N was applied at two equal splits 40 and 65 days after sowing. Until germination, the pots of each species were frequently irrigated with control water lightly and later three times a week until the harvest of seedlings. Salinity treatments were imposed after the first 45 days of emergence. Salinity treatments were prepared by diluting seawater (48.5 + 2 dS m−1) in 100-L iron drums and then salinity was imposed according to the treatment levels in each pot, and the electrical conductivity was measured using conductivity TDS meter (Hach, Loveland, CO).
Observations
At the blooming stage, the observations were recorded for each accession. Plant height (cm) was measured from the base to the top of the plants with a ruler. The same plants were used to count the number of branches and the number of leaves per plant. The leaflet length (mm), and leaflet width (mm) were measured with a ruler. At harvesting, the fresh weight (g)/plant was recorded, and dry matter yield was taken after oven drying the samples at 70 ± 2 °C (AOAC 2000).
Statistical analysis
Data were subjected to ANOVA using MSTAT-C software (Gomez and Gomez 1984) and treatment means were separated by HSD (honestly significant difference) at P ≤ 0.05. The genetic variation within and among the landraces was analyzed using the Principal Component Analysis (PCA) by XL-STAT-2020 (Addinsoft-2020. XLSTAT statistical and data analysis solution. New York, USA. https://www.xlstat.com) and the dendrogram was constructed with the PCA on dissimilarity (Euclidean distance) using Ward’s method.
Results
Salinity tolerance across all accessions for different characters
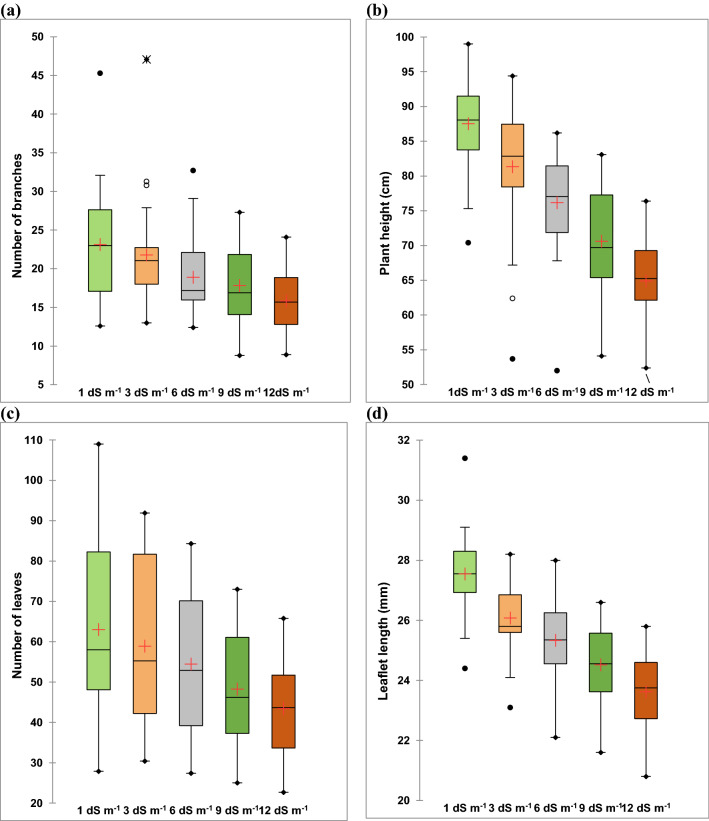
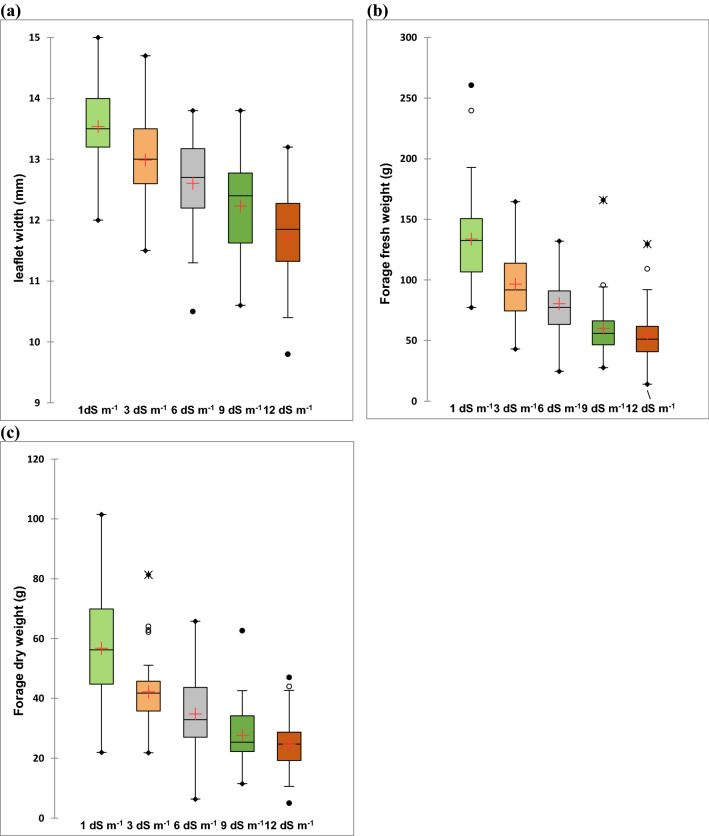
Salinity stress suppressed the growth of alfalfa accessions. With the increase in salinity levels from 1 to 12 dS m−1, the growth and forage yield of alfalfa accessions decreased significantly (Figs. 1, 2; Tables S1–S4). The highest reduction for all characters in all accessions was recorded at a salinity level of 12 dS m−1 (highest salt stress) followed by at 9 dS m−1. All the studied traits as plant height, number of branches per plant, number of leaves, leaflet length and width, forage fresh weight and forage dry matter yield were significantly reduced at the highest salt level compared to control (Figs. 1, 2; Tables S1–S4). However, some accessions responded positively at moderate salinity level (3 and 6 dS m−1) for the numbers of leaves per plant compared to control (Table S2) while, for forage fresh weight and forage dry matter yield, some accessions performed better at 6 dS m−1 than low salinity level of 3 dS m−1 (Tables S3 and S4).
Fig. 1.
Box plots showing the influence of salt stress on the (a) number of branches per plant; (b) plant height; (c) number of leaves and (d) leaflet length of 34 alfalfa accessions of Omani Origin. The box plots show the median, mean, first quartile, third quartile, minimum and maximum. Whiskers separate the sample from extreme data point. Data presented beyond whiskers represent outliers
Fig. 2.
Box plots showing the influence of salt stress on the (a) leaflet width; (b) forage fresh weight; and (c) forage dry weight of 34 alfalfa accessions of Omani origin. The box plots show the median, mean, first quartile, third quartile, minimum and maximum. Whiskers separate the sample from extreme data point. Data presented beyond whiskers represent outliers
Tolerant and sensitive genotypes of alfalfa based on morphology and forage yield at different salinity levels
The genotypes OMA 257, OMA 245, OMA, 270, OMA 315, OMA 211, OMA 117, OMA 56, OMA 239, OMA 148, OMA 131, OMA 95, OMA 263, OMA 262, OMA 289 and OMA 220 collected from different plains of Oman were tolerant against salinity stress of 6, 9 and 12 dS m−1 whereas the accessions OMA 305, OMA 100, OMA 211, OMA 148, OMA 60, OMA 248, OMA 9, OMA 88, and OMA 302 were sensitive at salt stress of 6, 9 and 12 dS m−1 for all the studied traits (Tables S1–S4).
Salinity tolerance of individual accessions for each character tested
Salinity tolerance of individual accessions was assessed based on the salinity levels for each growth character tested (Figs. 1, 2; Tables S1–S4). The genotype OMA 257 collected from the Interior mountain of A'Sharqiya had significantly higher numbers of branches per plant (32 branches) in comparison with all other accessions at salt stress of 6 dS m−1 (Table S1). The next superior accession OMA 315 (serial no.16) of North Batinah, collected from coastal plain had 27 branches at salt stress of 9 dS m−1 while at 12 dS m−1 the best accession was OMA 211, which had 24 branches compared to the rest of accessions (Table S1).
Regarding plant height, accession OMA 257 (serial no. 22) of A’Sharqiya collected from the mountain area had superior plant height (86.2 cm) followed by accession OMA 117 (85.1) collected from the interior mountain at salinity level of 6 dS m−1. At the salt level of 9 and 12 dS m−1, the accession OMA 257 and OMA 56 were the longest in length respectively compared to the rest of the accessions (Table S1). The three leaflet characters: the number of the leaves, width, and length varied amongst accessions to different extents (Tables S2-S3). One accession from Al-Buraimi (OMA 239, serial no. 6, collected from the interior plains) had the greatest leaflet number (84) followed by accession OMA 148 (serial no. 31) (78) of South Batinah collected from the coastal mountain at 6 dS m−1. The genotype OMA 131 had the maximum number of leaves (73 & 65) at 9 and 12 dS m−1 (Table S2).
The accessions varied much less for leaflet length and width (Tables 3–4) although accessions OMA 95 and OMA 239 (serial no. 10 & 6) had significantly longer leaflets (P < 0.05) at 6 dS m−1. The accessions OMA 263 and OMA 315 had the longest leaflets at salinity stress of 9 and 12 dS m−1, respectively compared to the rest of the accessions (Table S2). Regarding leaflet width, the accession OMA 262 (serial no. 7) had wider leaflets than all the other accessions at 6 dS m−1 while, at 9 and 12 dS m−1 the wider leaflets were recorded for accession OMA 289 (serial no. 15) and OMA 220 (serial no.11) (Table S3). There existed a significant variation for forage fresh weight and dry matter yield among the accessions (Table S3 and S4). However, the accession OMA 95 had maximum forage fresh weight (166 g) at 9 dS m−1 while at 6 and 12 dS m−1 the best performing accession was OMA 270 (Table S3). In respect of forage dry matter yield, the best performing accessions were OMA 117 (65.8 g), OMA 270 (62.6 g), and OMA 245 (47.0 g) at salinity stress of 6, 9 and 12 dS m−1, respectively (Table S4). The accession OMA 148 collected from the coastal mountain of Sultanate Oman was highly salt sensitive at all salinity levels (Table S4).
Cluster and principal component analyses
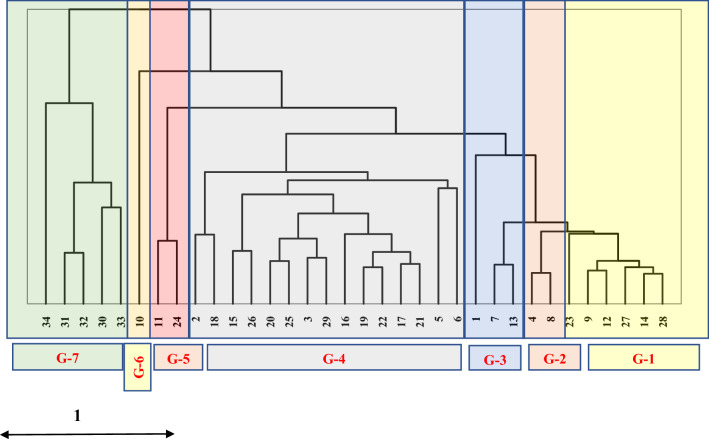
The cluster analysis showed a high level of genetic similarity among the 34 accessions of alfalfa (Fig. 3). The highest genetic similarity level was found between the accessions OMA 263 and OMA 88 (Fig. 3). However, the accessions OMA 95 and OMA 98 had the least genetic similarity (Fig. 3). All the 34 alfalfa accessions were grouped with 7 distinct groups. The genotypes OMA 88, OMA 263, OMA 5, OMA 248, OMA 282, and OMA 228 form one cluster. The biggest cluster consisted of the highest of 15 accessions namely OMA 239, OMA 224, OMA 56, OMA 290, OMA 257, OMA 222, OMA 315, OMA 305, OMA 9, OMA 100, OMA 268, OMA 141, OMA 289, OMA 211 and OMA 245 (Fig. 3). The genotypes OMA 270 and OMA 98 were genetically diverse. Moreover, accessions OMA 270 and OMA 95 were also diverse but less compared to genotypes OMA 270 and OMA 98 (Fig. 3).
Fig. 3.
Agglomerative hierarchical clustering of 34 alfalfa accessions of Sultanate Oman based on studied growth and forage yield traits under different salt stress. The dendrogram was constructed with XLSTAT-2020 on dissimilarity (Euclidean distance) using Ward’s method distances. The different colors of boxes showed the different group of accessions
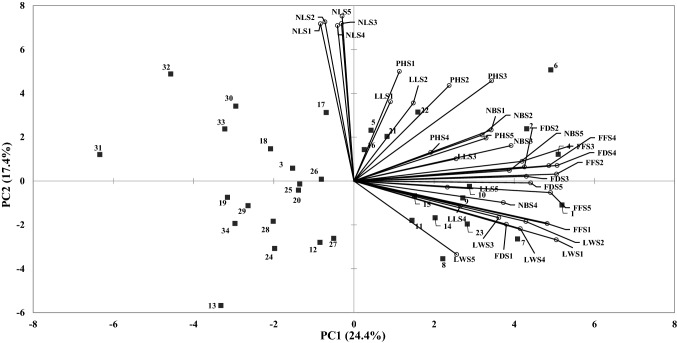
The biplot principal component analysis shows the interrelationship among the different tested traits of alfalfa accessions under varying levels of salinity stress. The total variation among the different accessions of alfalfa under varying salinity levels was 41.74% contributed by PC1 (17.4%) and PC2 (24.4%) (Fig. 4). The highest numbers of leaves per plant were obtained at salt stress of 12 dS m−1. The highest op vector, regarding plant height, was recorded at 6 dS m−1 in a positive quadrate. Accession OMA 245 collected from the interior plane of Oman was best performing for plant height at 12 dS m−1 while accessions OMA 268 and OMA 98 were salt sensitive. The highest op vector for the number of branches per plant was recorded at 6 dS m−1 indicating that the maximum number of branches per plant can be obtained at this salinity level. The accession OMA 270 was the best in the number of branches per plant at 9 dS m−1 salinity stress while accessions OMA 211, OMA 141, and OMA 195 were poor performers (Fig. 4). The highest op vector for forage fresh weight was recorded at 3 dS m−1 showed that maximum forage fresh weight can be obtained at 3 dS m−1. The accession OMA 95 was salt tolerant for forage fresh weight at 12 dS m−1 while OMA 141 and OMA 148 were salt sensitive for forage fresh weight (Fig. 4). The highest op vector for forage dry matter yield was at 9 dS m−1 which indicated that the maximum dry matter yield can be obtained at this salinity level. Among the different accessions, accession OMA 117 was salt tolerant for forage dry matter yield at 9 dS m−1 while accession OMA 100 and OMA 141 were salt sensitive for forage dry matter yield (Fig. 4).
Fig. 4.
Polygon view of GGE biplot showing relation of different traits of alfalfa accessions with salinity levels. S1 to S5 in each trait shows the salinity levels S1 (control), S2 (3 dS m−1), S3 (6 dS m−1), S4 (9 dS m−1) and S5 (12 dS m−1). NB = number of branches per plant; PH = plant height; NL = number of leaves; LL = leaflet length; LW = leaflet width; FF = forage fresh weight and FD = forage dry matter yield
Discussion
The results indicated a variable degree of tolerance of the Omani accessions of alfalfa to different levels of salinity stress. Only a few accessions showed differential tolerance for different traits under varying salinity levels. In most cases, salinity negatively impacted the growth parameters, however, in a few cases such as in respect of the number of leaves per plant, some accessions performed better under moderate salinity levels (viz. 3 and 6 dS m−1) compared to control (Fig. 1; Table S2) while, for forage fresh weight and dry matter yield some accessions’ performance was better at 6 dS m−1 compared to 3 dS m−1 (Fig. 2; Tables S3 & S4). Higher salt tolerance of different alfalfa accessions was due to osmoregulation and as an osmotic adjustment that occurs with the synthesis of organic solutes (polyols, amino acids, proteins, amides, and sugars) (Garg and Noor 2009) and through ion uptake (Farooq et al. 2015). Moreover, genes that take part in maintaining osmoregulation and homeostasis in plants under salinity stress in alfalfa have been explored (Nanjo et al. 1999; Ferreira et al. 2015). Furthermore, in a study, Al-Saady and Khan (2013) found that some alfalfa accessions showed higher dry matter stress index (dry weight of stressed compared to unstressed plants) under higher salinity levels (8 dS m−1) compared to lower salinity. This type of response is more common in halophytes, many of which require NaCl for optimal growth (Munns and Tester 2008) and some crops like sugar beet which is sensitive to salinity at germination and early seedling growth (Sadughi et al. 2015) but is tolerant at lateral stages.
In this study, salinity stress had a negative impact on all growth traits of alfalfa accessions at all levels of salt stress (3, 6, 9 and 12 dS m−1) however, salt level of 9 and 12 dS m−1 had a severe negative influence on all tested characters compared to moderate salinity stress of 3 and 6 dS m−1 (Figs. 1, 2; Tables S1–S4). Moreover, there exists a diverse behavior of tolerance against salinity stress in all accessions of alfalfa collected from different plains of Sultanate Oman as alfalfa is highly heterozygous and polyploid due to cross pollination, therefore there is tremendous variability in its population (Cornacchione and Suarez 2017). The accession OMA 315 of North Batinah collected from the coastal plain, OMA 257 of A'Sharqiya collected from Interior Mountain, OMA 131 of South Batinah, OMA 263 and OMA 289 of A'Sharqiya were highly tolerant at 9 dS m−1 (Tables S1–S4). Furthermore, some accessions as OMA 211, OMA 56, OMA 131, OMA 315 and OMA 220 were salt tolerant to higher salt stress of 12 dS m−1 as the roots of salt tolerant genotypes maintain osmotic balance through the transport of photosynthates to the stressed roots (Al-Niemi et al. 1992). Moreover, some genotypes which exclude salt from roots transport less salt to the upper parts of plants (Sandhu et al. 2017). Salt tolerance in plants is controlled by many ways including salt overly sensitive pathway (Qiu et al. 2002) by which sodium is excluded from the plant roots through the involvement of genes (Gupta and Huang 2014).
However, some accessions as OMA 305 of North Batinah, OMA 9, OMA 60, OMA 100, and OMA 211 of Al-Dakhiliya, OMA 148 of South Batinah, OMA 248 and OMA 302 of A'Sharqiya collected from interior plain and OMA 88 of Al-Dhahirah were sensitive to salinity stress. Moreover, these accessions were not significantly (P < 0.05) different from each other in leaflet length and leaflet width (Tables S2–S3). This indicates that there exists a significant potential for improving these accessions for their suitability under different forage production systems having varying degrees of salinity. This could be done by either producing pure seed or by selecting the clones of these alfalfa accessions under high salinity levels. Alternatively, lines or clones with salt tolerant genes could be further characterized through molecular marker techniques such as microsatellites. These lines could then be incorporated into poly-cross breeding involving lines of clones or seed samples as illustrated for the use of best clones of alfalfa in breeding programs by many earlier alfalfa researchers and breeders (Sleper and Poehlman 2006).
There was moderate variation (41.5%) among the different accessions of alfalfa under different salinity stress levels (Fig. 4). The highest number of leaves was obtained at 12 dS m−1 salinity stress whereas the highest plant height was at 6 dS m−1 in positive quadrate. The maximum dry matter yield was obtained at 9 dS m−1 (Fig. 4). At 12 dS m−1, the accessions OMA 245 and OMA 95 were the best performers for plant height and forage fresh weight respectively (Fig. 4). The accession OMA 117 was the best performer (salt tolerant) for forage dry matter yield at 9 dS m−1, while accessions OMA 100 and OMA 141 were poor performers (salt sensitive) (Fig. 4). The highest salinity (9 and 12 dS m−1) tolerance in accessions OMA 245, OMA 95, and OMA 117 showed that their parents could be halophytes as such response is more common in halophytes which require high NaCl for optimal growth (Munns and Tester 2008).
Thirty-four alfalfa accessions were clustered together into seven groups with group-4 as the biggest group which had clustered maximum accessions (Fig. 3). There was very low genetic diversity among the 34 accessions which showed that these accessions share a common gene pool and there is high gene flow among alfalfa accessions owing to breeding for the same trait. There was a high degree of genetic diversity among the accessions OMA 270, OMA 95, and OMA 98 (Fig. 3) which indicated that there was low gene flow among the parents for these genotypes.
To find salt tolerance in plants from physiological and genetic aspects is complex. On the other hand, investigating salt tolerance using existing genetic variation (Smýkal et al. 2015) and to exploit sources to develop new variation (Sharma et al. 2013) with poly traits breeding instead of the single trait have been reported to be effective (Duc et al. 2015). Another interesting option is the mass screening of genotypes to identify salt tolerant genotypes among the existing diverse germplasm to develop better performing genotypes (Farooq et al. 2017) as a variation for salt tolerance exists within and between the species of legumes (Moreno et al. 2000). In conclusion, in the present study, there exists a great potential of variation against salinity tolerance among the local accessions of alfalfa collected from diverse regions of the Sultanate of Oman which could be utilized in breeding and selecting suitable genotypes for specific regions of Oman for their better performance and forage production. The highly salt tolerant accessions such as OMA 245 (plant height), OMA 95 (forage fresh weight), and OMA 117 (forage dry matter yield) can be used for future breeding to develop better forage producing genotypes of alfalfa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Support from the Sultan Qaboos University (IG/AGR/CROP/19/02), Oman, and Ministry of Agriculture and Fisheries, Oman, to conduct this study is acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Mutata M. Effect of salinity on germination and seedling growth of chickpea (Cicer arietinum) genotypes. Int J Agric Biol. 2003;5:226–229. [Google Scholar]
- Al-Niemi TS, Campbell WF, Rumbaugh MD. Response of alfalfa cultivars to salinity during germination and post-germination growth. Crop Sci. 1992;32:976–980. doi: 10.2135/cropsci1992.0011183X003200040029x. [DOI] [Google Scholar]
- Al-Saady NA, Khan AJ. A study on germination rate, dry matter weight and amylase activity of L. (alfalfa) under induced NaCl stress. Adv Crop Sci Technol. 2013;1:108. doi: 10.4172/2329-8863.1000108. [DOI] [Google Scholar]
- AOAC (2000) Official Methods of Analysis. 17th Edition, The Association of Official Analytical Chemists, Gaithersburg, MD, USA. Methods 925.10, 65.17, 974.24, 992.16
- Bayuelo-Jimenes JS, Debouck DG, Lynch JP. Salinity tolerance in Phaseolus species during early vegetative growth. Crop Sci. 2002;42:2184–2192. doi: 10.2135/cropsci2002.2184. [DOI] [Google Scholar]
- Cornacchione MV, Suarez DL. Evaluation of alfalfa (Medicago sativa L.) populations’ response to salinity stress. Crop Sci. 2017;57:137–150. doi: 10.2135/cropsci2016.05.0371. [DOI] [Google Scholar]
- Duc G, Agrama H, Bao S, Berger J, Bourion V, De Ron AM, Gowda CLL, Mikic A, Millot D, Singh KB, Tullu A, Vandenberg A, Vaz Patto MC, Warkentin TD, Zong X. Breeding annual grain legumes for sustainable agriculture: new methods to approach complex traits and target new cultivar ideotypes. Crit Rev Plant Sci. 2015;34:381–411. doi: 10.1080/07352689.2014.898469. [DOI] [Google Scholar]
- Al-Farsi SM, Nawaz A, Rehman A, Nadaf SK, Al-Sadi AM, Siddique KH, Farooq M. Effects, tolerance mechanisms and management of salt stress in lucerne (Medicago sativa) Crop Pasture Sci. 2020 doi: 10.1071/CP20033. [DOI] [Google Scholar]
- Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, Migdadi HM, Alghamdi SS, Siddique KHM. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem. 2017;118:199–217. doi: 10.1016/j.plaphy.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Farooq M, Hussain M, Wakeel A, Siddique KHM. Salt stress in maize effects resistance mechanisms and management: a review. Agron Sustain Dev. 2015;35:461–481. doi: 10.1007/s13593-015-0287-0. [DOI] [Google Scholar]
- Ferreira JFS, Cornacchione MV, Liu X, Suarez DL. Nutrient composition, forage parameters, and antioxidant capacity of alfalfa (Medicago sativa L.) in response to saline irrigation water. Agriculture. 2015;5:577–597. doi: 10.3390/agriculture5030577. [DOI] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004;6:269–279. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- FAO (2008) AQUASTAT Country Profile – Oman. Food and Agriculture Organization of the United Nations (FAO). Rome, Italy. https://www.fao.org
- Garg N, Noor Z. Genotypic differences in plant growth, osmotic and antioxidative defence of Cajanus cajan (L.) Millsp modulated by salt stress. Arch Agron Soil Sci. 2009;55:3–33. doi: 10.1080/03650340802393303. [DOI] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2. New York: Wiley; 1984. [Google Scholar]
- Gupta B, Huang BR. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom. 2014;2014:1–18. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu G, Gao H, Sun G, Zhao H, Xie N. A comprehensive evaluation of salt-tolerance and the physiological response of Medicago sativa at the seedling stage. Acta Pratacult Sin. 2010;19:79–86. [Google Scholar]
- MAF (2014) Agriculture Statistics. Department of Agriculture Statistics, Directorate General of Planning and development, Ministry of Agriculture & Fisheries. Sultanate of Oman
- MAF (2012) Oman Salinity Strategy. Prepared by Ministry of Agriculture & Fisheries, Sultanate of Oman and International Center for Biosaline Agriculture, Dubai, The United Arab Emirates
- Monirifar H, Barghi M. Identification and selection for salt tolerance in alfalfa (Medicago sativa L.) ecotypes via physiological traits. Notulae Sci Biol. 2009;1:63–66. doi: 10.15835/nsb113498. [DOI] [Google Scholar]
- Moreno LS, Maiti RK, Gonzales AN, Star JV, Foroughbakhch R, Gonzales HG. Genotypic variability in bean cultivars (Phaseolus vulgaris L.) for resistance to salinity at the seedling stage. Indian Agric. 2000;44:1–12. [Google Scholar]
- Munns R. Physiological processes limiting plant growth in saline soil: some dogmas and hypotheses. Plant Cell Environ. 1993;16:15–24. doi: 10.1111/j.1365-3040.1993.tb00840.x. [DOI] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Ann Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 1999;461:205–210. doi: 10.1016/S0014-5793(99)01451-9. [DOI] [PubMed] [Google Scholar]
- Parihar P, Singh S, Singh R, Singh VP, Prasad SM. Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadughi M, Sharifan H, Pessarakli M. Effects of caspian sea water on sugar beet seed germination. J Plant Nutr. 2015;38:1685–1693. doi: 10.1080/01904167.2015.1042164. [DOI] [Google Scholar]
- Sandhu D, Cornacchione MV, Ferreira JF, Suarez DL. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci Rep. 2017;7:42958. doi: 10.1038/srep42958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Upadhyaya H, Varshney R, Gowda C. Pre-breeding for diversification of primary gene pool and genetic enhancement of grain legumes. Front Plant Sci. 2013;4:309. doi: 10.3389/fpls.2013.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleper DA, Poehlman JM. Breeding field crops. Oxford, UK: Blackwell Publishing; 2006. [Google Scholar]
- Smýkal P, Coyne CJ, Ambrose MJ, Maxted N, Schaefer H, Blair MW, Berger J, Greene SL, Nelson MN, Besharat N, Vymyslický T, Toker C, Saxena RK, Roorkiwal M, Pandey MK, Hu J, Li YH, Wang LX, Guo Y, Qiu LJ, Redden RJ, Varshney RK. Legume crops phylogeny and genetic diversity for science and breeding. Crit Rev Plant Sci. 2015;34:43–104. doi: 10.1080/07352689.2014.897904. [DOI] [Google Scholar]
- Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem. 2009;47:570–577. doi: 10.1016/j.plaphy.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Yacoubi R, Job C, Belghazi M, Chaibi W, Job D. Proteomic analysis of the enhancement of seed vigour in osmoprimed alfalfa seeds germinated under salinity stress. Seed Sci Res. 2013;23:99–110. doi: 10.1017/S0960258513000093. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.