Current scientific literature increasingly documents a correlation between assisted reproductive technologies (ART) and epigenetic errors in the children born, attributed to errors in methylation affecting the embryonic genome [1–5]. The majority of the epigenetic anomalies observed to date have been largely associated with IVF/ICSI procedures themselves, rather than problems intrinsic to gametes as a result of male and/or female infertility etiology [2, 5]. For example, a recent paper describing a high incidence of four major imprinting disorders in Japanese babies born after ART suggests that the origin of these disorders may be in the period immediately following fertilization under currently used culture conditions [3, 5]; as this is the stage during which the zygote genome undergoes demethylation, any disruption to methylation maintenance makes this a likely possibility. Whether controlled ovarian hyperstimulation (COH) is linked to epigenetic errors in the offspring remains ambiguous. Although a negative correlation has been described in the mouse [6], bovine embryos provide a more appropriate model for studies of this kind until data become available for the human [7, 8]. In terms of fundamental processes involved with DNA methylation, there is greater homology between bovine and human DNA methyltransferases (DNMTs) than between mouse and human DNMTs structures. Moreover, the time course of oocyte maturation as well as imprinted gene expression and methylation patterns are conserved between humans and cattle during early development, compared with human and mouse [8], thus whether COH imparts a negative impact on the oocyte epigenome remains controversial [9].
However, the influence of culture conditions on epigenetic chromatin remodeling during pre-implantation development has become increasingly clear. In the early years of this century, well before the role of epigenetics and imprinting disorders in human ARTs was appreciated, RG Edwards voiced a suspicion that pre-implantation culture media could induce anomalies in the concepti [10]. Remarkably, despite the fact that biochemical and physiological mechanisms differ between human and mouse, and the bovine model appears to be more closely related to the human embryo [7], the mouse embryo assay (MEA) continues to be the accepted test for quality control of commercial IVF culture media, a dogma that has been contested [11]. The two features that differ most significantly are the duration of antral growth/maturation and the time to reach genomic activation, the period of maternal to zygotic transition (MZT). Further confirmation is provided in a paper that clearly demonstrated incorrect methylation processes in mouse embryos that were cultured in human IVF culture media; i.e., human IVF culture media do not support appropriate DNA methylation in murine embryos [12]. The authors concluded that all culture systems using commercial media that were tested resulted in the loss of imprinted methylation compared with in vivo-derived embryos; no significant differences were detected between different types of media, including sequential media. This data suggest that no commercial human IVF culture media would pass the MEA if appropriate methylation/epigenetics were taken into account.
Given this backdrop, the objective of this paper is to examine the biochemistry of DNA methylation in the context of current practice for the culture of human embryos. The physiological basis for this inquiry is the dynamic remodeling of chromatin and DNA methylation status that is differentially manifest in maternal and paternal genomes at discrete stages of pre-implantation development. Given the broadscale adoption of extended embryo culture to day 5/6 in human ART programs, close scrutiny regarding the impact of culture media on short- or long-term consequences for offspring born by IVF or ICSI is an urgent priority.
Two major sources of biochemical support are required in order to ensure a normal process of methylation/imprinting/epigenetics during pre-implantation embryo development:
Methyl donors allow regeneration/formation of S-adenosyl methionine (SAM), the universal cofactor for methylation; this includes providing methionine, an “essential amino acid” that has been removed from culture media because of so-called toxicity (see below [13–16]).
Protection against oxidative stress in order to avoid damage to DNA, whose integrity must be carefully safeguarded during the period when paternal genes are transmitted (Fig. 1). This protection is provided largely by a high level of glutathione (GSH) synthesis in the early embryo: γ-l-glutamyl-l-cysteinylglycine is the universal antioxidant molecule. Glutathione synthesis is also mandatory to enable correct swelling of the sperm head at the time of fertilization. Its synthesis requires cystine/cysteine as a precursor molecule, another “essential amino acid.” Cystine metabolism also leads to the formation of hypotaurine, a strong antioxidant; notably, the embryo is unable to synthesize hypotaurine during its early stages of development, and the molecule is instead incorporated from tubal secretions [17].
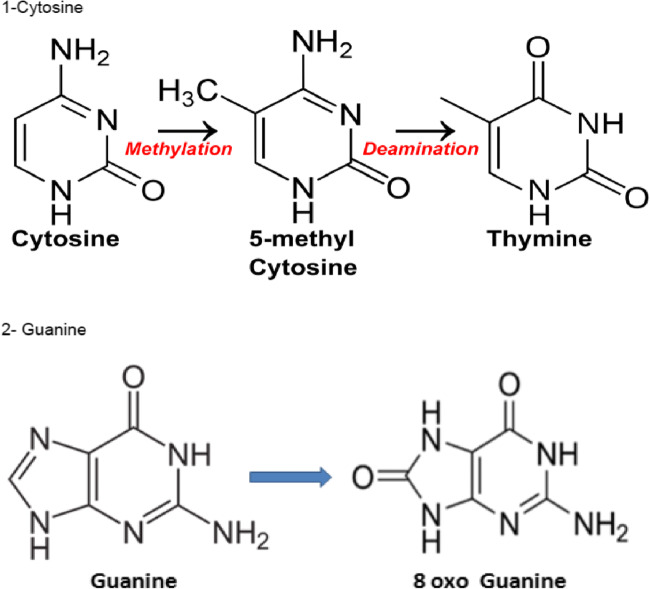
Fig. 1.
Potential oxidation of cytosine and guanine at the CpG sites
Errors in methylation are strongly linked to an imbalance in oxidative stress. Abnormally, low/inadequate concentrations of amino acids and the absence of “essential amino acids” may affect epigenetic processes: this is in complete agreement with the observations of Market-Velker that current commercial culture media is not capable of supporting correct methylation processes in pre-implantation embryos [12].
Biochemical aspects of culture media: amino acids, methylation, oxidative stress, and glucose
Methylation/imprinting/epigenesis
Two important questions must first be addressed.
Are active DNA methylation processes maintained during the first days of in vitro culture?
Does in vitro culture media meet the requirements of the pre-implantation embryo during this time?
As mentioned previously, methionine is the necessary fuel for methylation, and the methylation process must be protected against oxidative stress.
Methionine and methylation
Human oocytes/early embryos are readily able to incorporate methionine efficiently from the external milieu. In human embryos, as in mouse and bovine embryos, all of the enzymatic steps necessary for SAM synthesis are present [18] and expressed at high levels [19]. However, culture media designed for the early stages of embryo culture contain no or extremely low levels of methionine [20]. This is due to the concept that essential amino acids are toxic for mouse embryo culture described by Gardner and Lane [13], which has been contested [21]. The principle behind this concept is that essential amino acids should be eliminated from culture media in order to decrease/reduce ammonia production resulting from their in vitro metabolism [13, 16]. Ammonia is produced in culture media in vitro, but this can be removed by transamination of pyruvic acid to alanine, which can then be released into the culture medium [22]. Glutamine degradation has been reported as a major factor in the buildup of ammonia, but this is a minor feature at the basic pH of bicarbonate-buffered culture media currently in common use. Moreover, due to their dissociation factor (pK), the ammonium hydrogenocarbonate and carbonate produced, salts of a weak base (ammonium) and a weak acid (CO2), are highly unstable and immediately degraded to water, CO2, and NH3, eliminated in the gas flow. Carbamoyl phosphate synthetase (CSP1) is the first step in NH3 elimination, and this enzyme is highly expressed in the oocyte (100× background signal). Human IVF culture media do not support correct methylation in mouse embryos [12], and it is not surprising that the same observation can be made for human embryos. Methylation of DNA targets results in release of homocysteine (Hcy), and this must be recycled to methionine. Folates are mandatory for this process: this is present in only one commercial medium so far. Human oocytes express high levels of folate receptor 1 and folate transporter1 (SLC19A1), indicating that these molecules play an important role during the first 3 to 4 days of development, up to the onset of genomic activation (also known as the maternal to zygotic transition, MZT). Folates are at the center of a system that involves high molecular trafficking [19], and all of the enzymes involved in the folate and 1-carbon cycles are highly expressed in the oocyte.
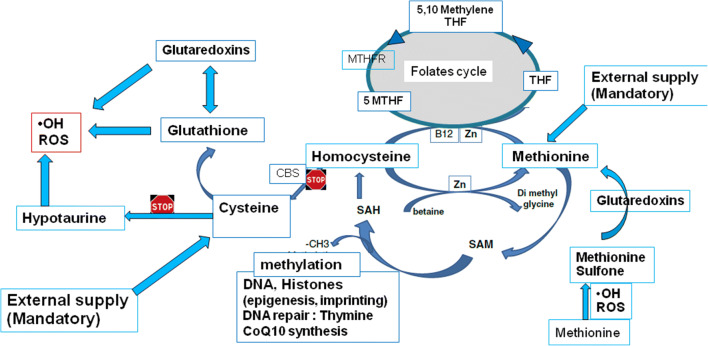
Correct DNA methylation processes also have a profound impact on genome stability: women carrying the C677T MTHFR SNP (methylenetetrahydrofolate reductase single-nucleotide polymorphism) generate pre-implantation embryos with high rates of aneuploidy and dramatically decreased viability due to their weak capacity to correctly metabolize folates. [23]. This confirms the central role of folates and may also explain the efficiency of “in vivo” treatment with 5MTHF (5-methyltetrahydrofolate) supplements, the direct substrate for MTHFR, before and during pregnancy. [24] The cystathionine beta-synthase (CBS) pathway, a biochemical side pathway that allows Hcy to be recycled to cysteine, is not expressed in human oocytes (Fig. 2): this means that conversion between the two sulfur amino acids cystine and methionine is not possible. In the pre-implantation embryo, methionine is protected from oxidation by two active systems that are highly expressed: methionine sulfoxide reductases and the glutaredoxins (see later).
Fig. 2.
1. Methionine (Met) is essential for the synthesis of SAM (S-adenosyl methionine) and downstream methylation reactions involved with epigenetic chromatin modifications. A fraction of Met is derived from the maternal oocyte pool, and its depletion following fertilization must be compensated in the culture medium for maintenance methylation to be sustained. Although Met is sensitive to spontaneous oxidation forming Met sulfone, oocyte-derived glutaredoxins (GRX) can restore Met to its non-oxidized state in the embryo during the first cleavage divisions. 2. Homocysteine cannot be recycled to cysteine: the two main sulfur amino acids cannot be linked. The CBS pathway is not expressed, and cysteine must be supplied externally to generate glutathione. Although the early embryo is able to synthesize glutathione, this is not the case for hypotaurine, as the CSD (cysteine sulfinate decarboxylase) enzyme is not expressed. Basically, protection against ROS depends on glutathione via or not the glutaredoxins (strongly expressed). 3. The BHMT pathway, betaine homocysteine methyltransferase is very marginally expressed. 4. Homocysteine recycling relies completely on the methionine synthase pathway, backed up by the folate cycle. The MTFR SNPs (methylenetetrahydrofolate reductase single-nucleotide polymorphism) can be a burden. Carriers of this SNP have a reduced ability to deliver 5MTHF (5 methylenetetrahhydrofolate), necessary as the fuel for MS to regenerate homocysteine [23, 24]
DNA methyltransferase and methylation
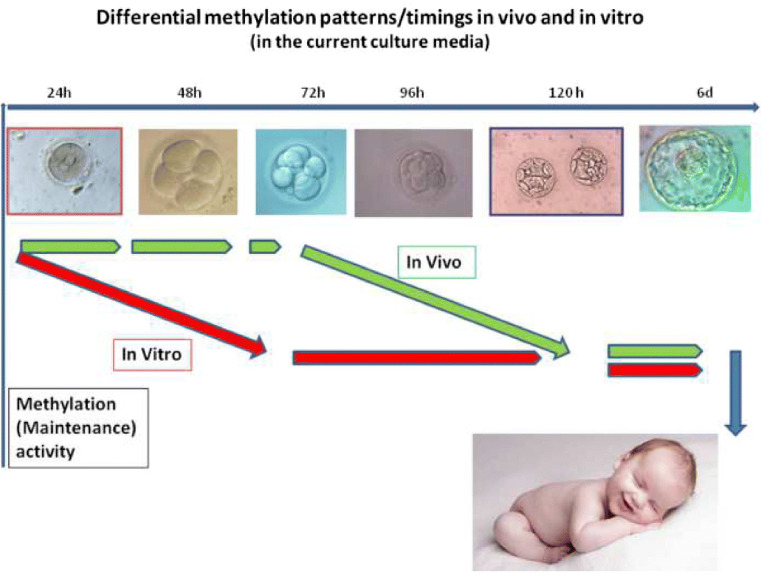
Rapid DNA demethylation of the zygote genome has been thought to occur immediately post-fertilization, and human IVF embryos apparently follow this scheme [25]. The paternal genome is rapidly demethylated first, followed by the passive demethylation of maternal genes during the subsequent cell cycle. However, paternal demethylated DNA seems to be immediately re-methylated [26]. Experiments in the mouse suggest that levels of methylation are stable up to and immediately after genomic activation [27–29], with methylcytosine levels remaining stable during this period. The DNA methyltransferase 1 (DNMT1) enzyme that is responsible for maintenance of DNA methylation is highly expressed in human oocytes: the polyA mRNA coding for its expression is one of the most abundant in the mature oocyte mRNA pool. DNMT3 is also expressed at an adequate level, indicating that active de novo methylation may occur, as has been observed in ruminants [26] and in mouse [27]: methylation anomalies in both maternal and paternal genes can be observed during early stages of in vitro development [6]. Methylation maintenance and to a lesser extent de novo methylation persist in human embryos until and immediately after MZT. These mechanisms are not adequately supported in vitro by currently available culture media. The anomalies subsequently observed in affected IVF children might be explained by the brief period of their development spent in vitro (Fig. 3). Human embryos produced in vitro using currently available media are not a good model for understanding human methylation/epigenesist/imprinting during pre-implantation development [30] (see Fig. 3). In common with most biochemical processes, oocyte DNA methylation capacity also decreases with age: another burden for human IVF embryos, as the ART population is usually in the older age group.
Fig 3.
Differential methylation patterns/timings in vivo and in vitro (current culture media)
Oxidative stress and methylation
Active and passive demethylation processes are known to take place in the pre-implantation embryo, with co-existing demethylation and maintenance of DNA methylation, although the relative importance of the 2 processes is still a matter of debate [31, 32]. A clear correlation between methylation anomalies and oxidative stress has been described [33], and IVF culture medium spontaneously generates free radicals during incubation, with no protection against oxidative stress [34] (Fig. 1).
An excess of oxygenated free radicals can lead to the formation of hydroxymethylcytosine, which may precipitate undue and abnormal demethylation processes. This may lead to oxidation of methylcytosine (MeC), causing active demethylation of some CpG sites, which are known to be crucially related to imprinting mechanisms [30–33]. Methionine restriction also increases mitochondrial oxidative stress [35].
Oxidative stress protection in the pre-implantation embryo: oxygen tension during culture
Oxidative stress is not a borderline problem during in vitro culture: as mentioned above, culture media spontaneously generate ROS [33], and there is no protection by antioxidants. Endogenous glutathione synthesis is mandatory, and this must be stimulated since a lack of cysteine sulfinate dehydrogenase (CSD) expression prevents the endogenous synthesis of hypotaurine. In vivo, hypotaurine uptake takes place from tubal secretions [17]. GSH is synthesized from cysteine in the cytosol, via the rate-limiting enzyme glutamate-cysteine ligase (GCL); this enzyme comprises a catalytic (GCLC) and a modifier (GCLM) subunit, both of which are expressed in the oocyte. Glutathione synthetase is also expressed, an enzyme that catalyzes the condensation of gamma-glutamylcysteine and glycine to form glutathione. Glutamic acid and glycine are important amino acids, which must be provided during early pre-implantation embryo development. Although the embryo has the capacity to synthesize both, uptake from the environment always consumes less time and energy than does biosynthesis. Glycine is found at millimolar concentrations in tubal fluid; in contrast, all other AAs are found at 10−1 millimolar concentrations. Cystine is the key component for glutathione synthesis, and cysteine/cystine is incorporated into the embryo by the alanine/serine/cysteine transporter 2 (SLC1A5), which is highly expressed both in the oocyte (30× the background signal) and in the pre-implantation embryo up to the time of genomic activation. A lack of cysteine may lead to the accumulation of serine and alanine. Cystine transport is activated by the solute carrier family 3 member 1 (SLC 3 A1, transport cystine and dibasic and neutral amino acids), which is also highly expressed in the oocyte (35-40 background signal). Elevated SLC3A1 expression accelerates cysteine uptake, with an accumulation of reduced glutathione (GSH). Cystine/cysteine is a basic “fuel” that should be provided in the culture of pre-implantation embryos at reasonable concentrations. Moreover, the early embryo is unable to divert some of its methionine to generate cysteine, as the cystathionine beta-synthase pathway required is not expressed (see Fig. 2).
Glutathione is essential at stages other than the time of fertilization. Glutaredoxins are redox enzymes of approximately one hundred amino acid residues (“light proteins”) that use glutathione as a cofactor. These proteins are thiol-disulfide oxidoreductases that use a glutathione-binding site and one or two active cysteines in their active site. They are highly expressed in oocytes, at levels between 250 and 450× background signal: they can reduce dehydroascorbic acid and, most importantly, methionine sulfoxide, an oxidation product of methionine. This mechanism also allows them to protect the imprinting/methylation process. Glutaredoxins are oxidized by oxidized substrates and are non-enzymatically reduced by reduced glutathione, which in turn is oxidized. Oxidized glutathione is then regenerated by glutathione reductase (GRX), expressed in oocytes at around 20× background signal. GRX needs NADPH generated by the pentose phosphate pathway: although removal of glucose from culture medium has been proposed as a means of avoiding the generation of free radicals released from its metabolism, reducing the concentration of glucose in the culture medium during the first stages of development is not a logical solution in terms of basic physiology and biochemistry. Glutathione peroxidase (GPX) is also highly expressed (50× background signal) at these stages: all of the components of the “glutathione chain” or “glutathione system” are crucial to maintain redox status and homeostasis in all cells, including, and especially, the embryo. Cysteine, produced via the synthesis of glutathione, as well as glutaredoxin (GRX) activity, protects appropriate methylation. This also allows the early embryo to maintain antioxidant protection as long as the basic fuel, i.e., cysteine/cystine is adequately provided in the culture media. This is not the case in the majority of culture media [20], possibly based upon the “essential AA theory” [13]. Not only the amino acid concentrations but their relative abundance/ratios are both of major importance because AA transporters are not specific for a single AA: they can transport the same AAs, but with different affinities, and this results in competition between them for the same transporter. The concentrations and the ratios of AA in the fallopian tube provide a good indication of the embryo’s potential requirements. Oxygen tension in the fallopian tube in vivo is around 7% O2; however, this is a highly protected environment with respect to oxidative stress [17], and a lower O2 concentration in culture media might be considered a “useful artifact.”
Oxidative stress and DNA repair
Several points merit consideration and review:
Damage to DNA as a result of oxidation is one of the major problems surrounding gamete and embryo quality/developmental competence. The “burden” is shared equally between male and female gametes [36]; oocytes have no more protection than do sperm cells.
The oocyte/early embryo does have the capacity to repair DNA damage, but this capacity has a finite limit [37, 38], which in oocytes decreases with maternal age. Damaged nuclear bases must be replaced, as “defective bricks make a defective wall.” Guanine is the base most sensitive to oxidation, and cytosine oxidation may lead to unwanted demethylation (Fig. 3). Guanine is the base that is most highly represented in telomeres, in the sequence TTAGGG; the immediate consequence of guanine oxidation could be telomere shortening, leading to cross-linking. However, all of the bases in DNA can be oxidized. The embryo is able to synthesize these bases, but external uptake/transport is also possible [39, 40], and this has the general feature of a lower requirement for time and energy. Supplementing culture media with nucleotide bases is thus of interest, although this is never/rarely done. Thymine synthesis requires methylation via SAM, additional justification for careful consideration of the role of methionine and methyl donors in IVF culture.
Conclusion
Anomalies related to methylation/epigenetics/imprinting are clearly a matter of controversies and questions in IVF babies. Early methylation and remodeling of the paternal genome could be at the epicenter of these problems [26, 41], but another area of interference with these processes now also raises concern. Environmental endocrine disruptor chemicals (EDCs), especially phthalates and bisphenols, are now found in body fluids of both men and women: blood, seminal plasma, follicular fluid, placenta, and amniotic fluid. These chemicals have effects on health and on problems related to fertility in particular. EDCs reduce the developmental capacity of gametes and embryos; time to pregnancy (TTP) is on the increase, without being linked to the couples’ wishes [42]. The structures of estradiol, bisphenol A, and Distilbene (a non-EDC) are sufficiently similar to create anxiety—it seems clear that EDCs can pass through the walls of the fallopian tubes and uterus and thus may have a direct impact on early embryos in these environments.
The major feature that raises concern is the effect of EDCs on methylation processes, either directly or via their ability to generate oxidative stress [43, 44]. These chemicals can induce health problems related to epigenetic disruption, and these epigenetic features may be subject to transgenerational transmission. It is important to bear in mind that IVF patients approach increasing age as the TTP increases, with the concomitant problem that gamete “protection” mechanisms are decreasing with maternal age. EDCs exacerbate problems associated with oxidative stress and methylation, increasing the negative pressure on reproductive systems.
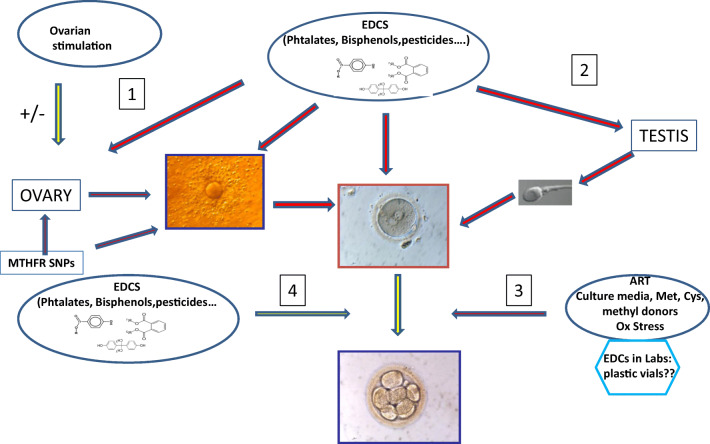
Returning to culture media: some formulations might have been previously accepted in terms of “safe” human embryo culture, but this no longer appears to be the case, especially in relation to the increased concentration of EDCs in body fluids (Fig. 4). Concentrations of the sulfur amino acid cysteine/cystine and methionine in media must be re-evaluated, and in no circumstances removed from their preparation. Methyl donors that facilitate homocysteine recycling are helpful supplements. Compounds that the embryo uses via uptake from the environment in vivo and is not able to synthesize should be added: examples include carnitine, which allows the beta-oxidation of lipids that are bound to albumin in in vitro conditions, and hypotaurine, a potent antioxidant that releases taurine, which acts as an important osmolyte. Adding free nucleotide bases might facilitate DNA repair. Although most of the biochemical pathways may be universal, the mouse embryo assay (MEA) has proved to be an inadequate basis for testing the efficacy of media for both human and mouse embryo culture, and steps should be taken to eliminate or redefine this test from IVF culture media quality control procedures. In this respect, it is important to acknowledge and bear in mind that both global methylation and specific methylation in imprinting control regions are decreased in the mouse embryo [45, 46] after in vitro culture. The pre-implantation embryo is submitted to multiple aggressions in vivo and in vitro (Fig. 4).
Fig. 4.
Factors affecting methylation of gametes and (early stage) embryos. 1. EDCs have a direct effect on the ovary and its capacity to allow appropriate methylation processes in the oocyte. Controlled ovarian stimulation has an effect on the quality of oocyte methylation in the mouse; this is less obvious in bovine. However, these specific problems may be exacerbated by EDcs, with their effects becoming apparent during the final stages of oocyte maturation, linked to their presence in follicular fluid. 2. It is common knowledge that EDCs affect the testis with an impact on sperm quality, up to the time that the sperm is ejaculated. 3. ART affects methylation of the early embryo (see text), due to the absence of methyl donors and agents that protect against oxidative stress in culture media. 4. In vivo, EDCs can also directly affect methylation processes via their presence in the fallopian tubes [41]
After 40 years of offering patients assisted reproductive techniques with increasingly complex technology and combinations of gamete donation, it is now time to focus on the negative impact of environmental oxidative stress and endocrine-disrupting chemicals on fertility. Nutritional support in the form of methyl donors that are able to enter the one-carbon cycle directly (see Fig. 1) has been shown to be efficacious in reversing to some extent the negative effects of OS and EDCs on male and female gametes as well as embryos [47], as observed in animal models. This strategy may also be effective for patients who carry MTHFR SNPs [24]. In conclusion, oxidative stress and disruption to DNA methylation errors represent two major concerns that should now be addressed in order to ensure the health of subsequent generations, whatever their mode of conception.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yves Ménézo, Email: yves.menezo@gmail.com.
Kay Elder, Email: kay.elder@gmail.com.
References
- 1.Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, Gaughan J, Coutifaris C, Sapienza C. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song S, Ghosh J, Mainigi M, Turan N, Weinerman R, Truongcao M, Coutifaris C, Sapienza C. DNA methylation differences between in vitro and in vivo conceived children are associated with ART procedures rather than infertility. Clin Epigenetics. 2015;7:41–48. doi: 10.1186/s13148-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiura H, Okae H, Miyauchi N, Sato F, Sato A, Van de Pette M, John RM, Kagami M, Nakai K, Soejima H, et al. Characterization of DNA methylation errors in patients with disorders conceived by assisted reproduction technologies. Hum Reprod. 2012;8:2541–2548. doi: 10.1093/humrep/des197. [DOI] [PubMed] [Google Scholar]
- 4.Choux C, Binquet C, Carmignac V, Bruno C, Chapusot C, Barberet J, Lamotte M, Sagot P, Bourc’his D, Fauque P. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: ART versus spontaneous conception without underlying infertility. Hum Reprod. 2018;33:331–340. doi: 10.1093/humrep/dex366. [DOI] [PubMed] [Google Scholar]
- 5.Hattori H, Hiura H, Kitamura A, Miyauchi N, Kobayashi N, Takahashi S, Okae H, Kyono K, Kagami M, Ogat T, et al. Association of four imprinting disorders and ART. Clin Epigenetics. 2019;11:21. doi: 10.1186/s13148-019-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Market-Velker BA, Zhang LS, Magri AC, Bonvissuto MRW. Mann dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19:36–51. doi: 10.1093/hmg/ddp465. [DOI] [PubMed] [Google Scholar]
- 7.Ménézo YJ, Hérubel F. Mouse and bovine models for human IVF. Reprod BioMed Online. 2002;4:170–175. doi: 10.1016/s1472-6483(10)61936-0. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Osorio N, Wang H, Rupinski J, Bridges SM, Memili E. Comparative functional genomics of mammalian DNA methyltransferases. Reprod BioMed Online. 2010;20:243–255. doi: 10.1016/j.rbmo.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Marshall KL, Rivera RM. The effects of superovulation and reproductive aging on the epigenome of the oocyte and embryo. Mol Reprod Dev. 2018;85:90–105. doi: 10.1002/mrd.22951. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RG, Ludwig M. Are major defects in children conceived in vitro due to innate problems in patients or to induced genetic damage? Reprod BioMed Online. 2003;7:131–138. doi: 10.1016/s1472-6483(10)61742-7. [DOI] [PubMed] [Google Scholar]
- 11.Menezo Y, Dale B, Elder K. Time to re-evaluate ART protocols in the light of advances in knowledge about methylation and epigenetics: an opinion paper. Hum Fertil (Camb) 2018;21:156–162. doi: 10.1080/14647273.2017.1317846. [DOI] [PubMed] [Google Scholar]
- 12.Market-Velker BA, Fernandes AD, Mann MR. Side-by-side comparison of five commercial media systems in a mouse model: suboptimal in vitro culture interferes with imprint maintenance. Biol Reprod. 2010;83:938–950. doi: 10.1095/biolreprod.110.085480. [DOI] [PubMed] [Google Scholar]
- 13.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. [PubMed] [Google Scholar]
- 14.Lane M, Gardner DK. Nonessential amino acids and glutamine decrease the time of the first three cleavage divisions and increase compaction of mouse zygotes in vitro. J Assist Reprod Genet. 1997;14:398–403. doi: 10.1007/BF02766148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane M, Gardner DK. Differential regulation of mouse embryo development and viability by amino acids. J Reprod Fertil. 1997;109:153–164. doi: 10.1530/jrf.0.1090153. [DOI] [PubMed] [Google Scholar]
- 16.Lane M, Hooper K, Gardner DK. Effect of essential amino acids on mouse embryo viability and ammonium production. J Assist Reprod Genet. 2001;18:519–525. doi: 10.1023/A:1016657228171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 18.Menezo Y, Khatchadourian C, Gharib A, Hamidi J, Greenland T, Sarda N. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci. 1989;44:1601–1609. doi: 10.1016/0024-3205(89)90455-4. [DOI] [PubMed] [Google Scholar]
- 19.Menezo Y, Lichtblau I, Elder K. New insights into human pre-implantation metabolism in vivo and in vitro. J Assist Reprod Genet. 2013;30:293–303. doi: 10.1007/s10815-013-9953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102:749–766. doi: 10.1016/j.fertnstert.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995;41:232–238. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- 22.Picton HM, Elder K, Houghton FD, Hawkhead JA, Rutherford AJ, Hogg JE, Leese HJ, Harris SE. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol Hum Reprod. 2010;16:557–569. doi: 10.1093/molehr/gaq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enciso M, Sarasa J, Xanthopoulou L, Bristow S, Bowles MF, Fragouli E, Delhanty J, Wells D. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum Genet. 2016;135:555–568. doi: 10.1007/s00439-016-1652-z. [DOI] [PubMed] [Google Scholar]
- 24.Servy EJ, Jacquesson-Fournols L, Cohen M, Menezo YMTHFR isoform carriers 5-MTHF (5-methyl tetrahydrofolate) vs. folic acid: a key to pregnancy outcome: a case series. J Assist Reprod Genet. 2018;35:1431–1435. doi: 10.1007/s10815-018-1225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ZD, Chan MM, Humm KC, Karnik R, Mekhoubad S, Regev A, Eggan K, Meissner A. DNA methylation dynamics of the human pre-implantation embryo. Nature. 2014;511:611–615. doi: 10.1038/nature13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JS, Jeong YS, Shin ST, Lee KK, Kang YK. Dynamic DNA methylation reprogramming: active demethylation and immediate remethylation in the male pronucleus of bovine zygotes. Dev Dyn. 2007;236:2523–2533. doi: 10.1002/dvdy.21278. [DOI] [PubMed] [Google Scholar]
- 27.Croteau S, Menezo Y. Methylation in fertilised and parthenogenetic preimplantation mouse embryos. Zygote. 1994;2:47–52. doi: 10.1017/s0967199400001751. [DOI] [PubMed] [Google Scholar]
- 28.Fulka H, Mrazek M, Tepla O, Fulka J., Jr DNA methylation pattern in human zygotes and developing embryos. Reproduction. 2004;128:703–708. doi: 10.1530/rep.1.00217. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto Y, Yoshida N, Suzuki T, Shimozawa N, Asami M, Matsuda T, Kojima N, Perry AC. Takada T DNA methylation dynamics in mouse preimplantation embryos revealed by mass spectrometry. Sci Rep. 2016;6:19134. doi: 10.1038/srep19134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menezo Y, Clement P, Dale B. DNA methylation patterns in the early human embryo and the epigenetic/imprinting problems: a plea for a more careful approach to human assisted reproductive technology (ART) Int J Mol Sci. 2019;20:1342. doi: 10.3390/ijms20061342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, Yang L, Zhang J, Li G, Ci W, Li W, Zhou Q, Aluru N, Tang F, He C, Huang X, Liu J. Programming and inheritance of parental DNA methylomes in mammals. Cell. 2014;157:979–991. doi: 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science. 2011;334:194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menezo YJ, Silvestris E, Dale B, Elder K. Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod BioMed Online. 2016;33:668–683. doi: 10.1016/j.rbmo.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Martın-Romero FJ, Miguel-Lasobras EM, Domınguez-Arroyo JA, Gonzalez-Carrera E, Alvarez IS. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod BioMed Online. 2008;17:652–661. doi: 10.1016/s1472-6483(10)60312-4. [DOI] [PubMed] [Google Scholar]
- 35.Lu S, Hoestje SM, Choo E, et al. Induction of caspase dependant and independant apoptosis in response to methionine restriction. Int J Oncol. 2003;22:415–420. [PubMed] [Google Scholar]
- 36.Lopes S, Jurisicova A. Casper RF Gamete-specific DNA fragmentation in unfertilized human oocytes after intracytoplasmic sperm injection. Hum Reprod. 1998;13:703–708. doi: 10.1093/humrep/13.3.703. [DOI] [PubMed] [Google Scholar]
- 37.Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18:357–365. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- 38.Menezo Y, Jr, Russo G, Tosti E, El Mouatassim S, Benkhalifa M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. J Assist Reprod Genet. 2007;24:513–520. doi: 10.1007/s10815-007-9167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada T, Tanikawa M, Iwabe T, Onohara Y, Mio Y, Terakawa N. Measurement of uptake and incorporation of nucleic acid precursors by preimplantation mouse embryos after development in vivo and in vitro. J Assist Reprod Genet. 1992;9:551–556. doi: 10.1007/BF01204253. [DOI] [PubMed] [Google Scholar]
- 40.Young RJ, Sweeney K, Bedford JM. Uridine and guanosine incorporation by the mouse one-cell embryo. Development. 1978;44:133–148. [PubMed] [Google Scholar]
- 41.Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007-2016) Fertil Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buck Louis GM, Smarr MM, Sun L, Chen Z, Honda M, Wang W, Karthikraj R, Weck J, Kannan K. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environ Res. 2018;163:64–70. doi: 10.1016/j.envres.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolinoy DC, Huang D, Jirtle R. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Waal E, Vrooman LA, Fischer E, Ord T, Mainigi MA, Coutifaris C, Schultz RM, Bartolomei MS. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet. 2015;24:6975–6985. doi: 10.1093/hmg/ddv400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vrooman LA, Rhon-Calderon EA, Chao OY, Nguyen DK, Narapareddy L, Dahiya AK, Putt ME, Schultz RM, Bartolomei MS. Assisted reproductive technologies induce temporally specific placental defects and the preeclampsia risk marker sFLT1 in mouse. Development. 2020;147(11):dev186551. doi: 10.1242/dev.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dattilo M, D’Amato G, Caroppo E, Menezo Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: mechanisms,clinical results, insights on gene-environment interactions and the role of diet. J Assist Reprod Genet. 2016;33:1633–1648. doi: 10.1007/s10815-016-0767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]