Abstract
Rhizobacteria are known to ameliorate salinity stress through a wide variety of mechanisms including the production of aminocyclopropane-1-carboxylate deaminase (ACCD). Application of ACCD positive halophilic rhizobacteria ameliorate soil salinity along with its plant growth promotion activity. An effect of the inoculation of ACCD and antioxidant positive and halophilic Enterobacter sp. PR14 was reported on the seed germination and growth of rice and millet seedlings grown in saline and alkaline soil was evaluated. The rhizobacterial strain grew well over a high level of NaCl (15–90 M); at a wide range of pH (5–9); and produced a wide variety of plant growth-promoting (PGP) traits viz. indole-acetic acid (13 µg mL−1), ACCD (5.20 M mg−1 h−1), phosphate solubilization (0.99 g mL−1) and antioxidant enzymes such as superoxide dismutase (5.143 IU mg−1 protein), catalase (0.43 IU mg−1 protein) and glutathione (19.077 µg mg−1 protein) during log phase (30 h) of its growth. The stress with alkaline pH (9) and high salinity (90 M) caused a further increase in the synthesis of PGP traits, ACCD, and antioxidant enzymes. The combined application of Enterobacter sp. PR14, ammonium sulfate (as a substitute of ACC), and NaCl (30 M) resulted in a further increase in the seed germination and vigor in rice and millets vis-à-vis control and other treatments. After 15 days of growth, 61.72% more seed germination in rice and millet and 63.15% increase in sorghum was recorded over the control, and after 30 days of growth, 99.67%, 30%, and 54%, root length 50%, 30% and 54% shoot length in rice, sorghum and millet were observed respectively. A significant increase of 38.13%, 30.75%, and 16.36% in dry weight of rice, sorghum, and millet shoots was recorded respectively. Enterobacter sp PR 14, showing multiple plant growth-promoting traits has a great potential to be used as an efficient bioinoculant for growth promotion of rice and millets under alkaline and saline conditions.
Keywords: Alkalinity, Ammonium sulfate, Enterobacter sp., Finger millet, Growth promotion, PGP traits, Rice, Salinity, Sorghum
Introduction
Rice is a principal cereal crop of the world and millets especially sorghum and finger millets are the important crops in India. The production and quality of rice and millet, however, have been severely affected due to increasing soil salinity (Kumar et al. 2018; Egamberdieva et al. 2017). The use of halophilic plant growth-promoting rhizobacteria (PGPR) like Enterobacter sp., etc. have been proposed as the best alternative for promoting plant growth under saline and alkaline soil (Paulina et al. 2020). Halophilic PGPR improves crop yield as well as ameliorate salinity (Sayyed et al. 2015). Under the salt stress, they produce an aminocyclopropane-1-carboxylate deaminase (ACCD) enzyme that decreases plant ethylene levels causing more ramification of roots. More roots absorb more nutrients and thus leads to good growth and more crop yield (Egamberdieva et al. 2017). Halophilic ACCD positive PGPR produces a better PGP effect in the presence of salt levels (Egamberdieva et al. 2017). However, the role of ACCD positive PGPRs help in salinity stress tolerance has not been studied adequately.
Enterobacter sp. PR14 used in the present study was earlier reported to produce multiple biocontrol traits and grow well in the presence of high concentrations of various heavy metal ions (Sagar et al. 2020). In this study, this isolate was evaluated for its ACCD activity, salinity tolerance, pH tolerance, production of PGP traits and antioxidant enzymes and its ability to promote plant growth in rice and millets in presence of ammonia sulfate (as a substitute of ACC) under NaCl (15 and 30 M) stress.
Materials and methods
Bacterial culture and plant seeds
The bacterial culture used in the present study was previously isolated from the model organic farm of the Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad, India. This isolate possessing multiple plant beneficial traits has been identified as Enterobacter sp. PR14 (Sagar et al. 2020). Drought-tolerant rice, cv. Sahbhagi (Oryza sativa) and Kharif south varieties of sorghum, SCV 20 (Sorghum bicolor); and Finger Millets CO 14 (Eleusine coracana) were procured from a local market.
Screening for the production of plant PGP traits and ACCD by Enterobacter sp. PR14
Screening of Enterobacter sp. PR14 for phosphate (P) solubilization and quantitative estimation of P solubilization was performed on Pikovoskaya’s agar and in Pikovoskaya’s broth according to the method of Nautiyal (1999). Production of IAA and ACCD was carried out in Sabouraud’sand in minimal medium (MM) according to the methods of Brick et al. (1991) and Penrose and Glick (2003) respectively. The ACCD activity was defined as the amount of α-keto-butyrate produced per mg of protein per h.
Screening for antioxidant enzymes of Enterobacter sp. PR14
Superoxide dismutase (SOD), catalase (CAT), and reduced glutathione oxidase (GSH) act as antioxidant enzymes and protect the plants from damage by oxidative stress (Fazeli-Nasab and Sayyed 2019). The screening and production of ACCD, CAT, and GSH in Enterobacter sp. PR 14 was performed according to the method of Marklund and Marklund (1974), Beers and Sizer (1952), and Salbitani et al. (2017) respectively. One unit of SOD was defined as the amount of the enzyme needed to inhibit 50% of the autoxidation of pyrogallol, one unit of catalase was defined as a µM of H2O2 decomposed per min and one unit of GSH was defied as µM of GSH reduced per min.
Effect of incubation period on growth and the production of PGP traits and antioxidant enzymes in Enterobacter sp. PR14
To ascertain the exact time required for optimum growth, P solubilization, IAA production, and ACCD activity, the log phase culture (30 h) of Enterobacter sp. PR14 was separately grown in Pikovoskaya’s broth, Sabouraud’s broth, and MM respectively at 28 °C for 48 h. Samples were withdrawn after every 6 h interval and subjected to the estimation of growth, soluble P, IAA, and ACCD activity (Nautiyal 1999; Brick et al. 1991; Penrose and Glick 2003).
To decide the exact time required for the optimum growth and biosynthesis of SOD, CAT and GSH, the growth and the activities of these enzymes were estimated up to 48 h. Samples were withdrawn after every 6 h interval and subjected to the estimation of growth, SOD, CAT and GSH activity (Marklund and Marklund 1974; Beers and Sizer 1952; and Salbitani et al. 2017).
Evaluation of the effect of NaCl and pH on the growth of Enterobacter sp. PR 14
The ability of Enterobacter sp. PR 14 to grow under salinity i.e. high concentration of NaCl was studied by separately growing it in nutrient broth (NB) containing varying amounts of NaCl (15–100 M), pH of broth was maintained to 7.0 with phosphate buffer (pH 7). For determining the effect of pH, Enterobacter sp. PR 14, was grown in NB prepared with different pH in the range of 5–10 at 28 °C for 30 h. The extent of growth at a higher concentration of NaCl and the alkaline pH medium was taken as its potential to grow under the stresses of high salinity and alkaline conditions (Damodaran et al. 2013).
Effect of Enterobacter sp. PR14 inoculation on growth of rice, and millets under salinity stress
The seeds of rice, cv. Sahbhagi (Oryza sativa), sorghum SCV 20 (Sorghum bicolor), and finger millet C014 (Eleusine coracana) were surface disinfected and different treatments as mentioned below-
T0 (Control), T1 (Enterobacter sp. PR14 + 15 M NaCl), T2 (Control 30 M NaCl), T3 (Enterobacter sp. PR14 + 30 M NaCl), T4 (Control 15 M NaCl + ACC), T5 (Enterobacter sp. PR14 + 15 M NaCl + ACC), T6 (Control 30 M NaCl + ACC), T7 (Enterobacter sp. PR14 + 30 M NaCl + ACC). Seed bacterization (T1, T3, and T5) was performed with log phase culture (30 h) of Enterobacter sp. PR14 (104 cfu mL−1).
Treated seeds (10 seeds of each plant) were placed on germination papers in Petri plates at 25 °C for 30 days and daily irrigated with NaCl water (15 and 30 M) (Nandakumar et al. 2001). Non-bacterized seeds (T0) served as control. All the treatments were performed in three replicates and the average of triplicates was considered. After 15 days of incubation, the percent seed germination was recorded and root growth (length), shoot growth (length), and shoot dry weight, were studied after 15 and 30 days of sowing (DOS) under the in-vitro condition.
Effect of ammonium sulfate (substitute of ACC) on the growth of rice, and millets under salinity stress
The effect of Enterobacter sp. PR14 on the growth parameters of rice, sorghum, and finger millet was studied as described above, except for the irrigation of seeds, ammonium sulfate (1% w/v) along with 15 and 30 M of NaCl was used. All the treatments were performed in three replicates. After 15 days of sowing (DOS), the percent seed germination, and after 30 DOS, root growth (length) and shoot growth (length) and dry weight were recorded under the in-vitro condition.
Statistical analysis
Data given in this report is the average of three replicates that was statistically analyzed using students’ t-test. P values of ≤ 0.05 were taken as statistically significant values (Parker 1979).
Results and discussion
Production of plant beneficial metabolites by Enterobacter sp. PR14
Enterobacter sp. PR14 produced a copious amount of IAA (13 µg mL−1), ACCD (9.2 µM mg−1 h−1), and exhibited good P solubilization (0.99 g mL−1). Members of Enterobacteriaceae especially those that are adapted to abiotic stress, produce a wide variety of PGP metabolites (Ahmad and Khan 2010) such as siderophores (Sayyed et al. 2015; 2019a, b), exopolysaccharides (EPS), phytohormones and various stress-tolerant enzymes (Reshma et al. 2018). Sagar et al. (2019) reported the production of various PGP traits and ACCD in E.cloacae PR4. Jabborova et al. (2020) reported the production of various PGPR traits in halophilic endophytes.
Production of ACCD is due to the presence of ACC in MM and the production of ACCD in PGPR has been documented as one of the important mechanisms of tolerance of salinity, drought and other abiotic stresses (Sayyed et al. 2019a, b). The enzyme lowers the level of ACC that is exuded by plant roots and at the suboptimal level of ACC limits the level of ethylene in the plant roots is limited. Reduced level of ethylene increases root length, and larger roots absorb more nutrients, and thus increases crop yield (Sayyed et al. 2019a, b).
Screening for antioxidant enzymes of Enterobacter sp. PR14
Enterobacter sp. PR14 exhibited good activities of various antioxidant enzymes under the stresses of NaCl (100 M) and alkaline pH (9) (Table 1). Alkaline pH (9) and high salinity stress resulted in a significant (p < 0.001) increase in ACCD and antioxidant enzymes’ activities. Alkaline pH and salinity (90 M) enhanced ACCD activity from 0.112 to 0.910 and 0.115 to 0.910 µM mg−1 h−1 respectively, SOD activity from 3.061. to 11.955 and 3.061 to 8.489 IU mg−1 protein respectively, CAT activity from 0.0008 o 0.0011 and 0.0008 to 0.0048 IU mg−1 protein respectively and GSH activity from 12.314 to 22.127 and 12.314 to 40.533 IU mg−1 protein respectively (Table 1). Acidic pH (5) to neutral pH (7) and in the absence of salinity stress caused fewer activities of these enzymes (Table 1). Banerjee et al. (2015) reported high SOD and CAT activities in E.cloacae under the stresses of heavy metal ions. Sheikh et al. (2016) reported enhanced activities of SOD and CAT in ACCD positive Enterobacter sp. UPMR18 under salinity stress. Reshma et al. (2018) reported the production of antioxidant enzymes in fluorescent Pseudomonas. Production of antioxidant enzymes in Enterobacter sp PR14 is due to its halophilic physiology as halophiles produce these enzymes to grow under high salinity stress (Fazeli-Nasab and Sayyed 2019). Salinity stress creates oxidative stress that damages the cell membranes and cell structures. However, under stress conditions, the presence of ACCD and antioxidant enzyme-producing PGPR activates a defensive system in the plants and removes the free radicals produced due to salinity or other abiotic stress (Abbas et al. 2013).
Table 1.
Effect of different levels of NaCl and pH on growth and activities of ACCD and antioxidant enzymes in Enterobacter sp. PR14
| Factor | ACCD activity (µM mg−1 h−1) | Antioxidant enzymes | ||
|---|---|---|---|---|
| SOD (IU mg−1 protein) | Catalase (IU mg−1 protein) | GSH (µg mg−1 protein) | ||
| Control | 0.112 (0.01) | 3.061 (0.01) | 0.0008 (0.01) | 12.314 (0.01) |
| pH | ||||
| 5 | 0.124 (0.01) | 3.061 (0.01) | 0.0008 (0.01) | 12.314 (0.01) |
| 6 | 0.132 (0.01) | 4.005 (0.01) | 0.001 (0.01) | 17.485* (0.01) |
| 7 | 0.520 (0.01) | 5.143 (0.01) | 0.005 (0.01) | 19.077 (0.01) |
| 8 | 0.791 (0.01) | 8.462 (0.01) | 0.010 (0.01) | 21.019 (0.01) |
| 9 | 0.910* (0.01) | 11.954*** (0.01) | 0.0043 (0.01) | 22.127*** (0.01) |
| 10 | 0.701 (0.01) | 7.994 (0.01) | 0.0011 (0.01) | 19.132 (0.01) |
| NaCl (M) | ||||
| 0 | 0.115 (0.01) | 3.061 (0.01) | 0.0008 (0.01) | 12.314 (0.01) |
| 15 | 0.117 (0.01) | 3.312 (0.01) | 0.0008 (0.01) | 14.115 (0.01) |
| 30 | 0.213 (0.01) | 3,891 (0.01) | 0.0010 (0.01) | 15.412 (0.01) |
| 45 | 0.273 (0.01) | 3.311 (0.01) | 0.0013 (0.01) | 16.115 (0.01) |
| 60 | 0.326 (0.01) | 4.019 (0.01) | 0.0022 (0.01) | 18.317 (0.01) |
| 75 | 0.391 (0.01) | 4.245 (0.01) | 0.0024 (0.01) | 20.116 (0.01) |
| 90 | 0.910** (0.01) | 8.489** (0.01) | 0.0048** (0.01) | 40.533*** (0.01) |
| 100 | 0.503 (0.01) | 6.013 (0.01) | 0.0037 (0.01) | 34.219 (0.01) |
Figures in the parentheses are standard deviation
The influence of NaCl and pH was measured in terms of the extent of growth of Enterobacter sp. PR14 in NB prepared with increasing values of NaCl concentrations and pH values
SOD activity was measured in terms of 50% inhibition of autoxidation of in a reaction mixture containing pyrogallol and cell homogenate. Catalase activity was determined as the breakdown of H2O2 following the addition of cell homogenate in H2O2. Amount of GSH produced by Enterobacter sp. PR14 was determined from the calibration curve of GSH
*p < 0.05; **P < 0.01, ***p < 0.001
Effect of incubation period on growth and the production of PGP traits and antioxidant enzymes in Enterobacter sp. PR14
The growth curve (Fig. 1), revealed that Enterobacter sp. PR14 required lag period of 4 h, remained in an active logarithmic phase from 6 onwards to 30 h, reached a stationary phase of growth after 30 h, and in decline phase from 36 h onwards. The bacterium started secreting the PGP traits, ACCD (Fig. 1), and antioxidant enzymes (Fig. 2) from 12 h and continued up to 48 of growth, however, the maximum amounts of PGP traits, ACCD, and antioxidant enzymes were observed during active log phase of 30 h growth. An apparent decline in the amount of these metabolites occurred after 30 h growth. Cessation of growth and synthesis of PGP metabolites and antioxidant enzymes may be due to the reduced availability of nutrients, accumulation of toxic wastes, and changes in the pH of the medium. Sayyed et al (2015; 2019a, b), Wani et al (2016) and Reshma et al (2018) reported the production of exopolysaccharides, siderophore, and antioxidant enzymes during log-phase growth of Enterobacter sp RZS5, Achromobacter sp, and Pseudomonas sp. respectively.
Fig. 1.
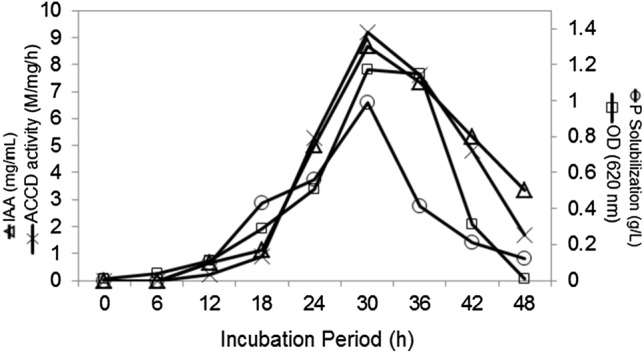
Effect of incubation period on growth and production of PGP traits and ACCD activity in Enterobacter PR14. Enterobacter sp PR14 was grown in Pikovoskaya’s broth, Sabouraud’s broth and MM at 28 °C for 48 h and samples withdrawn at every 6 h interval were subjected for the estimation of cell density (absorbance at 610 nm), ACCD activity and the amount of soluble P and IAA produced in the media
Fig. 2.
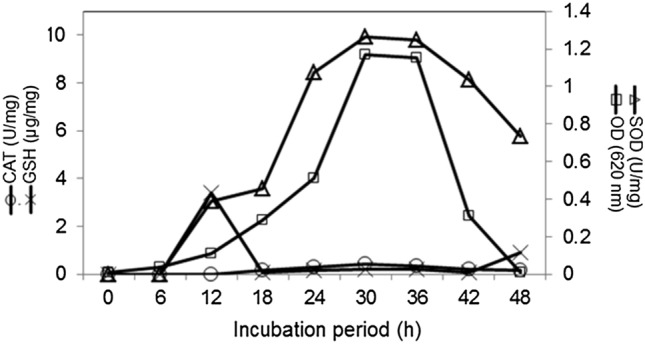
Effect of incubation period on growth and production of antioxidant enzymes in Enterobacter PR14. A 100 µL of cell homogenate of Enterobacter sp PR14 was separately added in 100 µL of pyrogallol and H2O2 and SOD activity was measured as prevention of autooxidation of pyrogallol, catalase activity was measured as a shift in absorbance of the reaction mixture at 240 nm and GSH activity was measured as the amount of reduced glutathione (GSH) produced in a reaction 412 nm
Evaluation of the effect of NaCl and pH on the growth of Enterobacter sp. PR 14
Enterobacter sp. PR14; grew well in NB prepared with varying levels of NaCl (15–90 M). The optimum growth of the isolate occurred at 90 M of NaCl. The growth of the organism in the absence of salt was comparatively less (Table 1). The luxurious growth of organisms in the presence of high levels of NaCl indicates its halophilic nature and the ability to withstand salinity stress. Halophiles are known to flourish more at higher salt level (1–3 M) as they have the potential of balancing the osmotic pressure of the environment by accumulating KCl or other compatible solutes and through the adaptation of the intracellular enzymes to protect their enzymes and proteins, halophiles require higher salt concentration (Oren 2008). Enterobacter sp. PR14 grew well over a wide range of pH (5–9); optimum growth occurred at pH 9 and higher salinity (90 M). This reflected the alkalophilic and halophilic nature of Enterobacter sp. PR14.
Effect of inoculation of Enterobacter sp. PR14 on the growth of rice and millets under salinity stress
Bacterization of seeds of rice, sorghum, and finger millet with Enterobacter sp. PR14 helped in the amelioration of salinity and promoted more growth of rice and millets under salinity. A significant (p ≤ 0.05) increase in all growth parameters of test plants was evident over the control plants (Table 2). Enterobacter sp. known to promote plant growth through direct and indirect mechanisms (Sayyed et al. 2015; Sagar et al. 2019; 2020).
Table 2.
Effect of various treatments on seed germination and growth of Rice, Sorghum and Finger millet after 15 and 30 DAS
| Treatment | % Germination 15 DAS | Root length (cm) 15 DAS | Root length (cm) 30 DAS | Shoot length (cm) 15 DAS | Shoot length (cm) 30 DAS | Shoot dry weight (g plant−1) 30 DAS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice | Sorghum | Millet | Rice | Sorghum | Millet | Rice | Sorghum | Millet | Rice | Sorghum | Millet | Rice | Sorghum | Millet | Rice | Sorghum | Millet | |
| T0 Control + 15 M NaCl | 50 | 60 | 50 | 2 | 3 | 2 | 3.1 | 7.1 | 2 | 2 | 4 | 3 | 11 | 12 | 10 | 9.0 | 13.1 | 9.1 |
| T1 PR14 + 15 M NaCl | 80 | 95 | 80 | 10 | 6 | 11 | 11.2 | 18.3 | 11 | 10 | 8 | 8 | 30 | 24 | 24 | 20.0 | 33.0 | 24.0 |
| T2 Control + 30 M NaCl | 50 | 62 | 42 | 1 | 1 | 2 | 4.6 | 3.3 | 2 | 1 | 2 | 1 | 9 | 8 | 6 | 6.2 | 24.2 | 27.2 |
| T3 PR14 + 30 M NaCl | 52 | 80 | 52 | 6 | 3 | 6 | 17.1 | 3.5 | 6 | 4 | 3 | 5 | 18 | 11 | 15 | 22.6 | 35.6 | 30.6 |
| T4 Control + ACC + 15 M NaCl | 50 | 60 | 51 | 2 | 2 | 2 | 12.9 | 7.3 | 2 | 1 | 4 | 3 | 20 | 14 | 11 | 18.0 | 23.0 | 20.0 |
| T5 PR14 + ACC + 15 M NaCl | 81 | 95 | 81 | 10 | 5 | 10 | 31.1 | 14.2 | 10 | 9 | 6 | 8 | 22 | 18 | 24 | 23.6 | 42.6 | 55.6 |
| T6 Control + ACC + 30 M NaCl | 51 | 61 | 42 | 1 | 1 | 2 | 22.9 | 4.5 | 2 | 1 | 1 | 2 | 14 | 15 | 8 | 21.3 | 33.2 | 40.9 |
| T7 PR14 + ACC + 30NaCl | 53 | 81 | 51 | 6 | 3 | 5 | 27.3 | 11.2 | 5 | 4 | 3 | 5 | 32 | 33 | 33 | 43.8 | 59.8 | 66.7 |
Test plants used were drought-tolerant rice variety, South Kharif variety of sorghum SCV 20, and finger millets CO 14 (E. coracana)
Seeds of test plants were bacterized (T1, T3, and T5) with log phase culture (30 h) of Enterobacter sp. PR14 (104 cfu mL−1)
Bacterized as well non-bacterized seeds were grown on germination papers in Petri plates at 25 °C for 30 days and irrigated daily with NaCl water (15 and 30 M). Seed germination was calculated as percent seed germination 15 DAS while root length and shoot lengths were measured 15 and 30 DAS. Shoot dry weight was measured 30 DAS
Rice, sorghum, and millet seeds bacterized with Enterobacter sp. PR14 and grown under 15 and 30 M of NaCl exhibited a significant improvement in seed germination and overall vigor. (Table 2). The combined application of Enterobacter sp. PR14, ammonium sulfate, and NaCl (30 M) resulted in a further increase in seed germination and vigor in rice and millets as compared to the control. Sheikh et al. (2016) reported enhanced plant growth in rice inoculated with ACCD and antioxidant enzyme-producing Enterobacter sp. Enhanced plant growth and salinity tolerance in rice and millets bacterized with halophilic PGPR that secretes a wide variety of plant beneficial metabolites have been reported by Uzzman et al. (2015), Jaemsaeng et al. (2018) and Niu et al. (2018). Shahnaz et al. (2020) reported the plant growth promotion in rice by salt-tolerant PGPR that produces EPS.
Sayyed et al. (2019a, b) claimed that the inoculation of ACCD positive PGPR induces physiological and biochemical changes in the plants that help alleviate salinity stress. Plant growth promotion under salinity stress is due to the synthesis of many stress-fighting enzymes like SOD, catalase, and GSH (Fazeli-Nasab and Sayyed 2019). Resistance to salinity stress in rice and millets following the inoculation with Enterobacter sp. PR14 is due to the significant rise in the amount of ACCD and antioxidant enzymes that resulted in a lower level of ACC. The lower level of ACCD helps in the good root ramification and thus better absorption of nutrients under salinity stress (Fazeli-Nasab and Sayyed 2019). Mitra et al. (2018) reported the plant growth-promoting effect in rice seedling under abiotic stress due to the inoculation of Enterobacter sp.
Siddikee et al. (2010) reported the inoculation of ACCD positive halophilic bacteria resulted in 47% more growth of test plant over the control plant. Good growth of test plants under alkaline pH and salinity stress is due to the secretion of copious amounts of reduced glutathione that in turn results in the synthesis of good amounts of antioxidant enzymes. Thus the combined effects of ACCD and antioxidant enzymes work as an excellent defense against oxidative stress (Salbitani et al 2017; Mirza et al. 2017).
Effect of ammonium sulfate (substitute of ACC) on the growth of rice and millets under salinity stress
Replacement of ammonium sulfate as a substitute for ACC resulted in more seed germination and more roots and shoot length in rice and millets under salt stress. Bacterization of rice, sorghum and finger millet with Enterobacter PR14 resulted in 80% and 51% shoot up in seed germination and 80% rise in root length and 51% increase in shoot length and 54% rise in shoot biomass vis-à-vis control treatment.
Presence of even low (1 M) stress of NaCl retards seed germination and root and shoot elongation, however, Enterobacter sp. PR14 bacterized seeds promoted seed germination, and plant vigor under high (30 M) NaCl stress. A significant increase of 90%, 50%, and 80% in shoot length in rice, normal sorghum, and finger millets was evident respectively over the control seeds.
Sagar et al. (2019) reported enhanced seed germination and plant growth in rice plants inoculated with Enterobacter cloacae PR4 in the presence of ammonium sulfate as a substitute for ACC. Kruasuwan and Thamchaipen et al. (2018) reported the ACCD activity of 0.1 μM α-ketobutyrate mg/h in endophytic Enterobacter sp. EN-21 and have observed the bacterium to be effective in salinity stress tolerance.
Conclusion
The dynamic and multifarious halophilic bacteria are vital for crop plants for the adaptation of rice and millet to salinity stress. Halophilic Enterobacter sp. PR14 capable of producing an array of PGP traits, growing under high stress of NaCl (90 M) and over a wide range of pH (5–9), producing substantial amounts of ACCD and antioxidant enzymes and promoting the growth of rice and millets in the presence of ammonia sulfate (as a substitute of ACC) makes it the best suitable bioinoculant for promotion of growth and yield in rice and millets with an aided potential of combating salinity stress. Thus the halophilic ACCD and antioxidant positive Enterobacter sp. PR14 can serve as an effective bio-inoculants in for rice and millets under alkaline salinity stress.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding this work through research group No. RG-1436-025.
Compliance with ethical standards
Conflicts of interest
All the authors declare no conflict of interest.
Consent for publication
All the authors have approved the paper and given their consent to publish this research.
Data availability and materials
All the data is available in the manuscript file.
Human and animal participants
Studies on human or animal subjects were not conducted.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas T, Pervez MA, Ayyub CM, Ahmad R. Assessment of morphological, antioxidant, biochemical, and ionic responses of salt-tolerant and salt-sensitive okra (Abelmoschus esculentus) under saline regime. Pak J Life Soc Sci. 2013;11(2):147–153. [Google Scholar]
- Ahmad M, Khan MS. Plant growth-promoting activities of phosphate solubilizing Enterobacter asburiae as influenced by fungicides. Eur Asia J Biosci. 2010;4:88–95. doi: 10.5053/ejobios.2010.4.0.11. [DOI] [Google Scholar]
- Banerjee G, Pandey S, Ray AK, Kumar R. Bioremediation of heavy metals by a novel bacterial strain enterobacter cloacae and its antioxidant enzyme activity, flocculant production, and protein expression in presence of lead, cadmium, and nickel. Water Air Soil Pollut. 2015;226:91. doi: 10.1007/s11270-015-2359-9. [DOI] [Google Scholar]
- Beers RF, Jr Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Brick JM, Bostock RM, Silverstone SE. Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Env Microbiol. 1991;57:535–538. doi: 10.1128/AEM.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran T, Sah V, Rai RB, Sharma DK, Mishra VK, Jha SK, Kannan R. Isolation of salt-tolerant endophytic and rhizospheric bacteria by natural selection and screening for promising plant growth-promoting rhizobacteria (PGPR) and growth vigor in tomato under sodic environment. Afr J Microbiol Res. 2013;7:5082–5089. doi: 10.5897/AJMR2013.6003. [DOI] [Google Scholar]
- Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Berg G, Liao H. Coordination between Bradyrhizobium and root colonizing Pseudomonas alleviates salt stress in soybean (Glycine max L.) through altering root system architecture and improving nodulation. J Plant Interact. 2017;12(1):100–107. doi: 10.1080/17429145.2017.1294212. [DOI] [Google Scholar]
- Fazeli-Nasab B, Sayyed RZ. Plant growth-promoting rhizobacteria and salinity stress: a journey into the soil. In: Sayyed RZ, Reddy MS, Antonious S, editors. Plant growth-promoting rhizobacteria for sustainable stress management. Singapore: Springer; 2019. pp. 21–34. [Google Scholar]
- Habib SH, Kausar H, Saud HM. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in Okra through ROS-scavenging enzymes. BioMed Res Intl. 2016;2016:6284547. doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants. 2017;23(2):249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabborova D, Annapurna K, Fayzullaeva M, Sulaymonov K, Kadirova D, Jabbarov Z, Sayyed RZ. Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc) Annals of Phytomed. 2020;9(1):1–6. doi: 10.21276/ap.2020.9.1.14. [DOI] [Google Scholar]
- Jaemsaeng R, Jantasuriyarat C, Tamchaipenet A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD) producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci Rep. 2018;31(8):1950. doi: 10.1038/s41598-018-19799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruasuwan W, Tamchaipenet A. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt–stress response in sugarcane. J Plant Growth Regul. 2018;37:849–858. doi: 10.1007/s00344-018-9780-4. [DOI] [Google Scholar]
- Kumar A, Tomer V, Kaur A, Kumar V, Guta K. Millets: A solution to agrarian and nutritional challenges. Agric Food Secur. 2018;7:31. doi: 10.1186/s40066-018-0183-3. [DOI] [Google Scholar]
- Marklund SL, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mitra S, Pramanik K, Sarkar A, Ghosh PK, Soren T, Maiti K. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Saf. 2018;156:183–196. doi: 10.1016/j.ecoenv.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Nandakumar R, Babu S, Viswanathan R, Raguchander T, Samiyappan R. Induction of systemic resistance in rice against sheath blight disease by plant growth-promoting rhizobacteria. Soil Biol Biochem. 2001;33:603–612. doi: 10.1016/S0038-0717(00)00202-9. [DOI] [Google Scholar]
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Niu X, Song L, Xiao Y, Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid and their potential in alleviating drought stress. Front Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Aquat Biosyst. 2008 doi: 10.1186/1746-1448-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RE (1979) Continuous distribution: tests of significance, In: Arnold BS (ed) Introductory statistics for biology, London, pp 18–42
- Paulina C, Amoozegar MA, Ventosa A. Halophiles and their biomolecules: recent advances and future applications in biomedicine. Mar Drugs. 2020;18(1):33. doi: 10.3390/md18010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Reshma P, Naik MK, Aiyaz M, Niranjana SR, Chennappa G, Shaikh SS, Sayyed RZ. Induced systemic resistance by 2,4 diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. Indian J Exp Biol. 2018;56(3):207–212. [Google Scholar]
- Sagar A, Shukla PK, Ramteke PW, Sayyed RZ. Stimulation of seed germination and growth parameters of rice var. Sahbhagi by Enterobacter cloacae (KP226569) in presence of ammonia sulfate as a substitute of 1-aminocyclopropane-1-carboxylate. In: Sayyed RZ, Reddy MS, Antonious S, editors. Plant growth-promoting rhizobacteria (PGPR): prospects for sustainable agriculture. Singapore: Springer; 2019. pp. 117–124. [Google Scholar]
- Sagar A, Riyazuddin R, Shukla PK, Ramteke PW, Sayyed RZ (2020) Heavy metal stresses tolerance in Enterobacter sp. PR14 is mediated by the plasmid. Indian J Exp Biol 58:115–121. https://nopr.niscair.res.in/handle/123456789/53518
- Salbitani G, Bottone C, Carfagna S. Determination of reduced and total glutathione content in extremophilic microalga Galdieria phlegrea. Bio-Protocol. 2017;7:e2372. doi: 10.21769/BioProtoc.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed RZ, Patel PR, Shaikh SS (2015) Plant growth promotion and root colonization by EPS producing Enterobacter sp. RZS5 under heavy metal contaminated soil. Indian J Exp Biol 53(2):116–123. https://hdl.handle.net/123456789/30443 [PubMed]
- Sayyed RZ, Seifi S, Patel PR, Shaikh SS, Jadhav HP, El Enshasy H. Siderophore production in groundnut rhizosphere isolate, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Env Sustain. 2019;1(3):295–301. doi: 10.1007/s42398-019-00070-4. [DOI] [Google Scholar]
- Sayyed RZ, Ilyas N, Tabassum B, Hashem A, Abdallah EF, Jadhav HP. Plausible role of plant growth-promoting rhizobacteria in future climatic scenario. In: Sobti RC, Arora NK, Kothari R, editors. Environmental biotechnology: for sustainable future. Singapore: Springer; 2019. pp. 175–197. [Google Scholar]
- Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol. 2010;20(11):1577–1584. doi: 10.4014/jmb.1007.07011. [DOI] [PubMed] [Google Scholar]
- Sultana S, Paul SC, Parveen S, Alam S, Rahman N, Jannat B, Hoque S, Rahman MT, Karim MM. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can J Microbiol. 2020;66(2):144–160. doi: 10.1139/cjm-2019-0323. [DOI] [PubMed] [Google Scholar]
- Uzzaman T, Sikder RK, Asif MI, Mehraj H, Jamal Uddin AFM. Growth and yield trial of sixteen rice varieties under system of rice intensification. Sci Agric. 2015;11(2):81–89. doi: 10.15192/PSCP.SA.2015.11.2.8189. [DOI] [Google Scholar]
- Wani SJ, Shaikh SS, Sayyed RZ. Statistical-based optimization and scale-up of the siderophore production process on laboratory bioreactor. Biotech. 2016;6:69. doi: 10.1007/s13205-016-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data is available in the manuscript file.


