Abstract
Abstract
Soil salinity is a major abiotic stress that adversely affects crop growth, development and productivity worldwide. In this study, the individual and synergistic roles of putrescine (Put) and spermidine (Spd) in salinity stress tolerance of foxtail millet (Setaria italica L.) was assessed. In the present study, plants treated with combined biogenic amines Put + Spd possess very efficient antioxidant enzyme systems which help to control the uninhibited oxidation and protect the plants from oxidative damage by ROS scavenging. Additionally, lower concentration of Put + Spd under NaCl stress showed reduced hydrogen peroxide, electrolyte leakage and caspase-like activity than control. FTIR analysis underlying the ability of PAs induced tolerance and the chemical bonds of Put + Spd treated plants were reminiscent of control plants. Moreover, histochemical analysis with 2′,7′–dichlorofluorescein diacetate (DCF-DA), 3,3′–Diaminobenzidine (DAB) and nitrotetrazolium blue chloride (NBT) revealed that ROS accumulation was inhibited by combined PAs under salt stress condition. These results showed that Put + Spd significantly improve the endogenous PAs, which enhance high-salinity stress tolerance by detoxifying ROS. For the first time, the synergistic ROS scavenging ability of Put along with Spd was investigated upon salinity tolerance in C4 model foxtail millet crop. Overall, our findings illustrated the implication for improving salinity tolerance of agronomically important crop species.
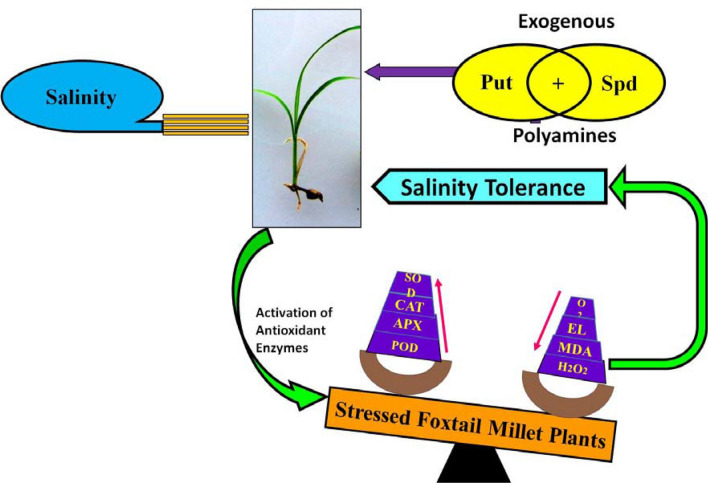
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00869-0) contains supplementary material, which is available to authorized users.
Keywords: Confocal laser scanning microscope, Fourier transform-infrared spectroscopy, Histochemical analysis, Polyamines, Reactive oxygen species, Salinity stress
Introduction
Salinity stress is detrimental to overall crop plant growth, production and yield, which cause economic losses, threatening global agriculture, particularly in steppes and arid zones (Panuccio et al. 2018). Salinization affects ~ 20% of world agricultural lands (Zhao et al. 2017). In general, under high salt stress, plants undergo several morphological, biochemical, physiological, and molecular level alterations which eventually lead to cell damage and growth reduction in plants (Satish et al. 2016; Sun et al. 2020). Salinity stress could induce generation of reactive oxygen species (ROS) in plants. ROS are harmful to macromolecules, cell signalling in proliferation which includes singlet oxygen (1O2), hydrogen peroxide (H2O2) and superoxide anion radical (O ¯2), that leads to membrane damage, degradation of protein, nucleic acid and eventually cell death (Huang et al. 2013; Kissoudis et al. 2014; Singh et al. 2019). The level of ROS in the stressed plants was regulated by the antagonism between the ROS producers and the scavengers (Asada 2006). Plants possess several stress-defence mechanisms which include the accumulation of compatible soluble solutes and the production of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and other peroxidases (POXs) to remove the excess ROS (Miller et al. 2010). These defence mechanisms can also be induced and/or enhanced by certain chemical compounds such as methyl jasmonate, sodium nitroprusside, melatonin, salicylic acid, strigolactone and some polyamines (PAs) (Yoon et al. 2009; Van Ha et al. 2014; Khan et al. 2015; Savvides et al. 2016). Hence, it is imperative to establish a new approach to enrich high salinity tolerance in plants.
The exogenous application of plant growth regulators is found to be effective in improving the resistance against various abiotic stresses viz., water deficit, water submergence and salinity (Liu et al. 2015; Satish et al. 2018). Nowadays, PAs have been considered as new plant growth regulators which are used to regulate plant growth and to enhance the competence of plants for abiotic stress tolerance (Liu et al. 2015; Sang et al. 2016). PAs are low-molecular weight aliphatic amines ubiquitously present in all the organisms with key functions such as the regulators of protein synthesis, DNA replication, cell division, growth and several biological activities (Roychoudhury et al. 2011). Spermidine (Spd), spermine (Spm) and putrescine (Put) are the most common natural PAs present in plants acting as signaling molecules in plant-environmental stresses (Tiburcio et al. 2014). Among these, Spd could be significantly active in plant stress signal transductions thus activating stress tolerance mechanism (Hu et al. 2012; Liu et al. 2015). Similarly, several researchers reported that Put can effectively reduce ROS accumulation and improve plant tolerance against stress induced toxicity (Mandal et al. 2013; Wang et al. 2013). Foliar spraying of Spd in plants has resulted in increased accumulation of PAs, proline, glutathione (GSH) and the antioxidant enzyme activities in stressed plants (Duan et al. 2008; Sun et al. 2020). Furthermore, the exogenous Spd found to improve stress tolerance to aging (Wang et al. 2000), drought (Nemeth et al. 2002; Zhou et al. 2015), salinity (Fariduddin et al. 2018), heat (Tang et al. 2018), chilling (Fu et al. 2019), heavy metal stress (Gong et al. 2016) and submergence (Liu et al. 2015) stresses in different plant species. However, the combination of PAs decreased the dosage of application and modulates stress tolerance. The efforts were made to study the antioxidant enzyme activities and ROS scavenging mechanism of PAs and synergistic effects of Put and Spd and their roles in foxtail millet salinity tolerance.
Foxtail millet (Setaria italica (L.) is an important small millet, serving as a staple food for many people in temperate, tropical and subtropical Asian and African countries (Sreenivasulu et al. 1999). Generally, foxtail millet plants are nutritionally high value crop possessing effective stress tolerance mechanisms to various abiotic stresses such as drought and salinity (Sreenivasulu et al. 2000; Zhang et al. 2005). Previously, Sudhakar et al. (2015) reported that PAs may be associated with the salinity stress tolerance in foxtail millet.
However, it is still unclear how the exogenous PAs improve the response in foxtail millet plants. Additionally, the synergistic effect of Put with Spd upon high salinity stress has not been studied so far. In the present study, we have investigated the effects of exogenous PAs on protection against salinity stress in foxtail millet by analyzing plant growth, physiological, biochemical and antioxidant enzymes activity. To the best of our knowledge, this is the first study to explore the synergistic effect of biogenic amines (Put and Spd) on salinity stress tolerance in foxtail millet. Perhaps, this study will aid in future for computational transcriptomic analyses of this important C4 model species.
Materials and Methods
Plant material and experimental conditions
The healthy foxtail millet seeds procured from Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India were surface sterilized according to Rathinapriya et al. (2018). Seeds were sown on MS medium (Murashige and Skoog 1962) buffered at pH 5.75 ± 0.5 with 3% w/v sucrose, 0.8% agar–agar type I (Himedia, Mumbai, India) and sodium chloride (NaCl; 200 mM) (Himedia, Mumbai, India). The rationale for 200 mM NaCl was previously described by Sudhakar et al. (2015). The autoclaved MS medium was supplemented with various concentrations of putresine (Put; 0.5, 1, 1.5 and 2 mM), spermidine (Spd; 0.5, 1, 1.5 and 2 mM) (Sisco Research Laboratories, Mumbai, India) individually or in combination Put + Spd (0.25 + 0.25, 0.5 + 0.5, 1 + 1 and 1.5 + 1.5 mM) respectively. To determine more suitable PAs (Put, Spd and Put + Spd) concentrations for modulating the growth of shoot and root, the preliminary assays were performed. Four-days-old germinated seedlings were placed in a growth chamber (Sanyo Versatile Environmental Test Chamber, Japan, Model No MLR351H) programmed with temperature 25 °C (± 1 °C), 16 h light (200 µM photons m−2 s−1), 8 h dark photoperiod and 70% relative humidity for 15 days. Assays were performed using 15 bottles (5 seeds per bottle) for each of the three independent biological repeats. Foxtail millet plants grown for 15 days on MS medium without NaCl and PAs are used as a control (unstressed) and in treated control, plants were exposed to 200 mM NaCl without PAs.
Effects of PAs on germination and growth parameters under salinity stress
The survival and growth of foxtail millet plants were assessed through germination percentage (GP) and the morphological factors such as fresh weight (FW), dry weight (DW), shoot and root length after 15 d of treatment. The germination rate was based on the percentage of emergence of seedling after 4 d of darkness incubation.
For DW measurement, plant samples were dried in oven at 70 °C for 48 h, then allowed to cool in room temperature (RT) and weighed.
The salinity induced physiological changes ameliorated by Put and Spd treated and untreated plants were assessed with leaf relative water content (RWC), leaf electrolyte leakage (EL) and caspase-like activity. RWC (%) was calculated by the following formula,
Leaf samples were allowed to dip overnight in deionized water for swelling, shortly air dried and weighed to determine the turgid weight (TW) (Sapeta et al. 2013). Electrolyte leakage (EL) was determined by the electrical conductivity method (Tabot and Adams 2013). The EL was calculated as:
where E1 is the initial electrical conductivity of the samples measured using a conductivity/TDS/ °C gauge (Cyberscan 200, Eutech Instruments, Singapore) and ET is the final electrical conductance of the solution. The caspase-like activity was assessed based on the protocol of Poborilova et al. (2013).
Photosynthetic pigment analysis
Based on the preliminary analyses, 1 mM of Spd, 1 mM of Put and 0.5 + 0.5 mM of Spd + Put were found to be effective and selected for further experiments. The total chlorophyll (a + b) and carotenoid content of the 200 mM NaCl with 1 mM Spd, 1 mM Put and Put + Spd (0.5 + 0.5 mM) treated plant leaves were measured according to Lichtenthaler and Buschmann (2001) method. Each sample of 0.1 g fresh leaves was weighed from the treated plants, finely ground and immersed in 98% methanol (Himedia, Mumbai, India). Samples were extracted by incubating at 4 °C for 24 h in complete darkness. The chlorophyll content of methanol extract was measured at 652.4 and 665.2 nm and carotenoid content was measured at 480 nm, respectively using UV–vis spectrophotometer (UV-2450, Shimadzu Analyticals, Japan). 98% methanol was used as a blank.
Determination of compatible soluble solute
Leaf protein content was quantified according to Bradford (1976) and bovine serum albumin (BSA) was used as a standard. Free proline content was extracted and determined by Bates et al. (1973) protocol on the basis of ninhydrin reaction and utilizing the l-Proline (Hi-Media, Mumbai, India) as a standard.
Determination of cell membrane damage
Hydrogen peroxide content in the treated plants was individually determined by the method described by Del Pozo and Lam (1998). Absorbance of H2O2 content was recorded with a UV–vis spectrophotometer (UV-2450, Shimadzu Scientific Instruments, Japan) at 390 nm. The H2O2 content was calculated through comparing its optical density value in the standard graph. Lipid peroxidation was detected by quantifying the total amount of malondialdehyde (MDA) contents measured by thiobarbituric acid (TBA) reaction method according to Heath and Packer (1968).
Assays of antioxidant enzyme activity and free radical production
In order to determine the reaction products of PAs oxidation, SOD, GR, APX, GPX and GSH were analyzed.
SOD activity was assayed according to Paoletti and Mocali (1990) by evaluating its ability to inhibit the oxidation of NADPH into NADP+ and it was measured by inhibition (50%) in the reduction of nitrobluetetrazolium chloride (NBT) at 340 nm for 5 min. CAT activity was determined by measuring the decomposition of H2O2 content at 240 nm for 3 min, as described by Aebi (1984). GR activity was determined as defined by Carlberg and Mannervik (1975). Enzyme assay was quantified in basic of oxidized glutathione decomposition of NADPH by monitoring the change in absorbance at 340 nm. APX assay was conducted according to Nakano and Asada (1981) and measured by estimating the rate of ascorbate oxidation at 290 nm for 3 min. Guaiacol peroxidase (GPX) activity was determined as described by Lin and Kao (1999). The enzyme activity was quantified in terms of tetraguaiacol formation in 1 min of increase in absorbance at 470 nm.
GSH level was measured by DTNB (5,5′-dithio-bis-[2-nitrobenzoic acid]) to form yellow coloured 5-thio-2-nitrobenzoic acid reaction method and the glutathione was used as a standard according to Sedlak and Lindsay (1968). Reaction mixture was then measured at 412 nm.
Assay of polyamine and diamine oxidase activity
Diamine oxidase (DAO) and polyamine oxidase (PAO) activity were assessed as described by Su et al. (2005) by quantifying the generation of H2O2 as a product of the oxidation of PAs. The enzyme reaction was initiated by the addition of Spd for PAO and Put for DAO determination and change in the optical density at 555 nm for 1 min was considered as one enzyme activity unit.
Histochemical detection of H2O2 and O ¯2
In situ H2O2 and O ¯2 production in foxtail leaves exposed to 200 mM NaCl, Put, Spd (1 mM) and Put + Spd (0.5 + 0.5 mM) were detected by the chromogenic substrate 3,3-diaminobenzidine (DAB) and nitrotetrazolium blue chloride (NBT) (Sisco Research Laboratories, Mumbai, India). Briefly, youngest leaves were excised from treated and control foxtail millet plants and immediately immersed into 0.1% DAB in 10 mM (pH 7.0) potassium phosphate solution for 5 h at RT (Mostofa et al. 2015). The DAB staining solution was decanted, replaced with an acetic acid: ethanol (1:3; v/v) for destaining of chlorophyll and photographed. The experiment was conducted using triplicates with the total of 20 seedlings for each treatment.
Analysis of H2O2 in confocal laser scanning microscopy
The production of ROS in the foxtail millet leaves treated with water control, 200 mM NaCl and Put + Spd were observed with 2′,7′–dichlorofluorescein diacetate (DCF-DA) staining as described by Rossi et al. (2017). Lower epidermis of young leaves were peeled and immersed in 10 mM Tris–HCl (pH 6.1) in dark for 2 h then the samples were transferred to DCF-DA (25 μM) solution for 20 min. The stained leaf samples were washed twice with Tris–HCl buffer and then images were visualized under confocal laser scanning microscopy (CLSM) (LSM, Jena, Carl Zeiss, Germany). The DCF-DA fluorescence intensities were analyzed using COMSTAT2 software procured from Dr. Claus Sternberg, DTU Systems Biology, Technical University of Denmark, Denmark.
FTIR analysis
Fourier transform-infrared spectroscopic (FTIR) analysis was performed to understand the metabolic changes of NaCl (200 mM), control (unstressed) and PAs (Put, Spd and Put + Spd) exposed foxtail millet plants. In the experiment, 100 mg of dried leaves were ground to a fine powder and compressed into pellets with KBr using Pelletization method. FTIR spectra was observed in the range of wavelengths 400 to 4000 cm−1 using Nicolet iS 5 FTIR instrument (Thermo Scientific, USA) and the peaks were identified using OMNIC software (Kannappan et al. 2017).
Statistical analysis
All the experiments were done in a completely randomized design with minimum of three replicates. Values in the text and tables were indicated Mean values ± SD of three independent experiments. Statistical analysis was done by one-way analysis of variance (ANOVA) using Duncan’s multiple range test, with Statistical Package for the Social Sciences software version 17.0 (SPSS, IBM Statistics) and taking p ≤ 0.05 as significant.
Results
PAs enhances salinity stress tolerance in foxtail millet
The role of PAs in the enhancement of salinity stress tolerance was investigated by measuring the GP and growth parameters of foxtail millet plants (Fig. 1) (Fig. S1). After 14 d incubation with Put, Spd (0.5, 1, 1.5 and 2 mM) and Put + Spd (0.25 + 0.25, 0.5 + 0.5, 1 + 1 and 1.5 + 1.5 mM), results revealed that the (Put + Spd; 0.5 + 0.5 mM) treated plants showed a 98.2% increased germination rate than single Put and Spd plants upon NaCl stress condition. PAs treatments upon salinity stress revealed significant variations in the foxtail millet seedlings growth and development, such as fresh weight, dry weight of shoots and roots and length of shoot and roots (Table 1). In 200 mM NaCl stress, a severe reduction of the shoot and root biomass was observed, wherein NaCl (200 mM) stress condition along with Put, Spd and Put + Spd, treatments alleviated the salinity stress mediated growth reduction in both shoots and roots. Fresh and dry weight of shoots and roots showed that PAs treatment increased the biomass content in foxtail millet plants (Table 1).
Fig. 1.

Polyamines ameliorates salinity tolerance in foxtail millet. Control (Unstressed), NaCl (Treated control; 200 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM) and NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM) (left to right)
Table 1.
Effect of exogenous Put, Spd and Put + Spd treatment on the growth parameters of 15 d old foxtail millet exposed to NaCl stress
| PAs concentration (mM) | Growth parameters (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| FW (mg) | DW (mg) | Length (cm) | |||||
| Shoot | Root | Shoot | Root | Shoot | Root | ||
| Control | 94.3 ± 0.7i | 23.2 ± 0.9i | 9.4 ± 0.6g | 3.1 ± 0.4e | 12.5 ± 0.8h | 5.4 ± 0.6ef | |
| NaCl | 200 mM | 38.6 ± 0.6a | 7.9 ± 0.3cd | 1.6 ± 0.7a | 0.3 ± 0.1a | 6.7 ± 0.2bc | 2.8 ± 0.8abcd |
| NaCl + Put | 0.5 | 76.8 ± 0.9c | 5.5 ± 0.6b | 2.4 ± 0.2abc | 0.6 ± 0.3ab | 7.9 ± 0.4cd | 3.3 ± 0.7abcd |
| 1 | 81.2 ± 0.4f | 6.0 ± 0.5b | 2.8 ± 0.8abcd | 0.9 ± 0.6abc | 8.4 ± 1.0def | 3.7 ± 0.5cd | |
| 1.5 | 79.3 ± 1.0de | 4.1 ± 0.2a | 2.2 ± 0.9ab | 0.7 ± 0.1abc | 6.8 ± 0.3bc | 2.6 ± 0.4abc | |
| 2 | 73.9 ± 1.1b | 3.8 ± 0.8a | 1.7 ± 0.7ab | 0.5 ± 0.2ab | 6.2 ± 0.6ab | 2.1 ± 0.9a | |
| NaCl + Spd | 0.5 | 80.4 ± 0.5ef | 9.4 ± 0.7e | 3.1 ± 0.4bcde | 1.1 ± 0.8abc | 8.2 ± 0.7de | 3.5 ± 0.3bcd |
| 1 | 85.3 ± 0.6g | 10.6 ± 0.3f | 3.7 ± 0.8cdef | 1.4 ± 0.9abcd | 9.3 ± 0.5ef | 4.0 ± 0.2de | |
| 1.5 | 78.9 ± 0.8de | 8.3 ± 0.5cde | 2.9 ± 0.7abcd | 1.3 ± 0.3abcd | 6.1 ± 0.9ab | 2.8 ± 0.4abcd | |
| 2 | 75.1 ± 0.9b | 7.2 ± 0.8c | 2.6 ± 0.3abcd | 0.9 ± 0.2abc | 5.3 ± 0.4a | 2.4 ± 0.7ab | |
| NaCl + Put + Spd | 0.25 + 0.25 | 84.8 ± 1.0g | 11.4 ± 0.6f | 4.3 ± 0.9ef | 1.8 ± 0.5cd | 9.5 ± 0.8f | 6.3 ± 0.4g |
| 0.5 + 0.5 | 88.5 ± 1.1h | 14.9 ± 0.9h | 5.1 ± 1.0f | 2.2 ± 0.8de | 11.0 ± 0.6g | 6.6 ± 0.3g | |
| 1 + 1 | 81.6 ± 1.2f | 12.9 ± 0.4g | 4.8 ± 0.9f | 1.5 ± 0.6bcd | 10.9 ± 0.7g | 5.9 ± 0.8fg | |
| 1.5 + 1.5 | 78.6 ± 0.4d | 8.8 ± 0.2de | 4.0 ± 1.0def | 1.0 ± 0.1bc | 8.6 ± 0.9def | 5.1 ± 0.6ef | |
Here, FW and DW represent fresh weight and dry weight of foxtail millet seedlings. Similarly, NaCl + Spd, NaCl + Put and NaCl + Put + Spd indicate spermidine (NaCl; 200 mM, Spd; 0.50 − 2.0 mM), putrescine (NaCl; 200 mM, Put; 0.50 − 2.0 mM) and putrescine + spermidine (NaCl; 200 mM, Put + Spd; 0.25 + 0.25, 0.50 + 0.50, 1.00 + 1.00 and 1.50 + 1.50 mM), respectively. Values are Mean ± standard deviation (SD) of independent triplicates (n = 3). Different superscripted letters (a–i) within the column indicates statistically significant differences among the treatments according to Duncan’s multiple range test (p ≤ 0.05). The optimum values for Put, Spd, Put+Spd are shown in bold for all the tested growth parameters
Similarly, the height of the shoot and root length measurement signifies that Put (1 mM) and Spd (1 mM) and Put + Spd (0.5 + 0.5 mM) application enrich the growth of salinity exposed foxtail millet than the other concentrations of Put and Spd exposed plants. These results evidenced that Put + Spd treatment significantly enhance the plant biomass and also influence plant growth recovery that leads tolerance to high salinity stress in treated plants. The 200 mM NaCl along with Put (1 mM) and Spd (1 mM) and Put + Spd (0.5 + 0.5 mM) concentrations were chosen for further experiments.
Effects PAs on physiological parameters
The physiological changes in foxtail millet leaves were analyzed to understand the mechanism underlying tolerance to NaCl stress condition under PAs treatments. Foxtail millet plants exposed to high salinity stressed condition showed increased EL and caspase-like activity and decreased in RWC in shoots compared to NaCl with exogenous PAs and water control treatments (Fig. 2). The RWC in salinity stressed leaf samples was progressively increased, whereas the reduction in EL and caspase-like activity was noticed by the application of Put, Spd and Put + Spd. However, the level of EL was significantly diminished in the control plants which were exposed to high salinity stress alone without any PAs applications. In comparison with the control (unstressed) plants, the EL (63%) and caspase-like activity (79%) increased, but decreased RWC (24%) upon NaCl exposure. Furthermore, in PAs treated plants, the EL and caspase activity was reduced by 48% and 57% in Put + Spd, 39% and 43% in Spd whereas 30% and 36% in Put respectively, as compared with NaCl exposed plants (Fig. 2a–c). These results firmly showed the regulatory roles of combined Put + Spd in high salinity stress.
Fig. 2.
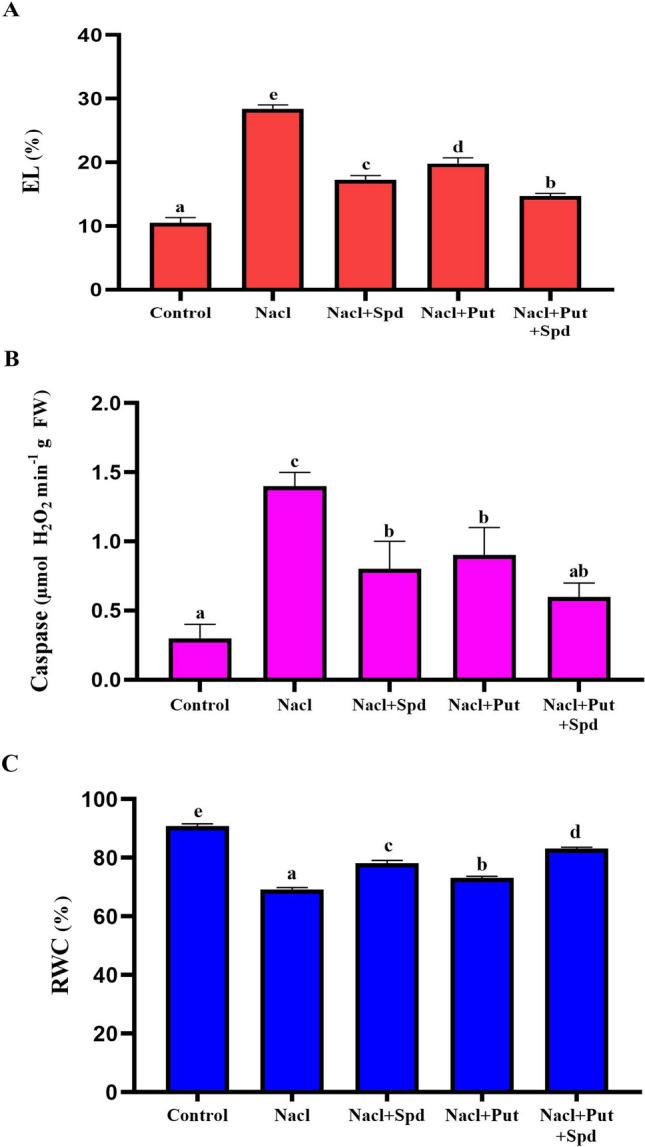
Effect of PAs on the physiological parameters in foxtail millet with or without NaCl stress. A. EL (Electrolyte leakage; %) and B. Caspase-like activity (µmol H2O2 min−1 g FW) C. RWC (Relative water content, %). Control (Unstressed), NaCl (200 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM) and NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM). The data correspond to the average of three replicates. Values represent the Mean ± SD (analyzed by SPSS version 17.0), bars with different letters are significantly different at p ≤ 0.05 based on Duncan’s multiple range test
PAs rescue the losses of chlorophyll, carotenoids and soluble protein
The synergistic effect of PAs on the photosynthetic pigments of foxtail millet under NaCl stress was determined in terms of total chlorophyll (a + b), carotenoids and soluble protein content. Salinity stress sharply increased the total chlorophyll (a + b) content, (54, 47 and 60%) (Fig. 3a, b) carotenoid content (50, 35 and 62%) and total soluble protein (15, 18 and 23%) in Spd, Put and Put + Spd supplements respectively, compared with the NaCl treated plants (Fig. 3c) (Fig. S2). The total chlorophyll (a + b), carotenoids and soluble protein contents were significantly reduced when the plants were exposed to NaCl alone (Fig. 3) (Fig. S2). The damaging effects of NaCl on the total chlorophyll (a + b), carotenoids and soluble protein contents were considerably declined by the exogenous application of combined PAs.
Fig. 3.
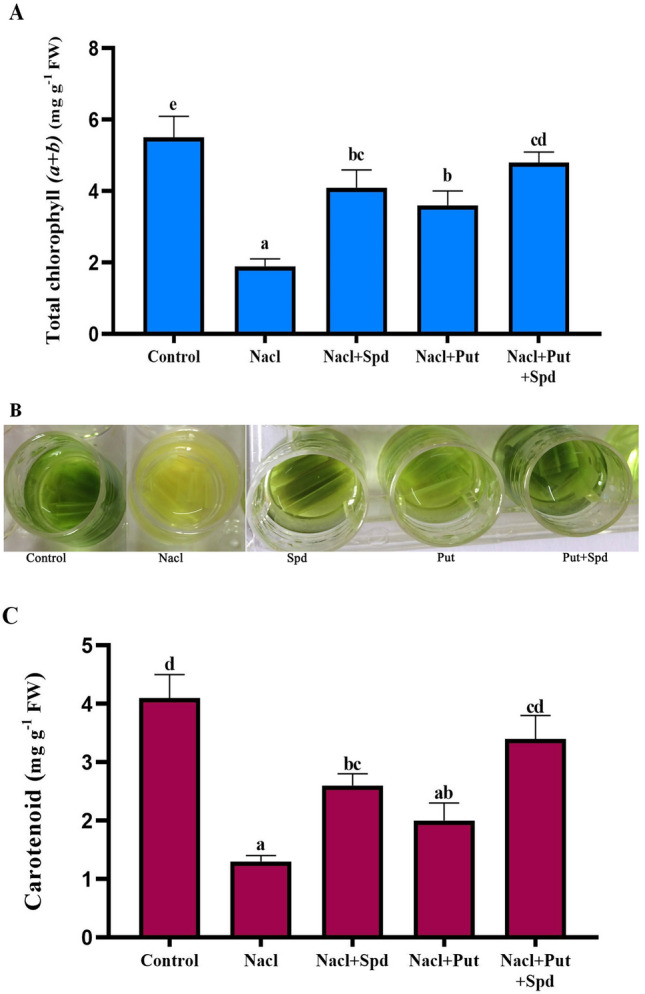
Effect of PAs on the photosynthetic pigments in foxtail millet seedlings upon NaCl stress. A. Total chl (a + b) (mg g−1 FW), B. Estimation of Chl and C. Carotenoids (mg g−1 FW). Here, Control (Unstressed), NaCl (200 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM) and NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM), respectively. The data correspond to the average of three replicates. Values represent the Mean ± SD (analyzed by SPSS version 17.0), bars with different letters are significantly different at p ≤ 0.05 based on Duncan’s multiple range test
Application of PAs inhibits the accumulation of proline
The role of PAs on osmoprotectant and ROS quencher was investigated by calculating the level of proline production under salinity stress. In this study, the proline contents were much higher in leaves of foxtail millet subjected to NaCl stress compared with the content in PAs treated and unstressed control plants. The addition of PAs decreased the proline accumulation by 22% in Put, 25% in Spd and 34% Put + Spd in comparison with NaCl treated plants, respectively (Fig. S3). These decreases were more significant in water control and NaCl with Put, Spd and Put + Spd treated plants. PAs applications are the most effective treatment, considerably controlling proline accumulation in salinity stressed foxtail millet plants. However, the combined Put + Spd application significantly regulates proline accumulation, which implies the synergistic effect of PAs on salinity stress.
PAs alleviates NaCl induced cell membrane damage
The damage in cell membrane was assessed by analyzing the MDA and H2O2 content which acts as prime indicators of oxidative stress. A drastic increase in the level of H2O2 and lipid peroxidation was noticed in NaCl (70 and 73%) compared with the control plants. The supplementation of Spd and Put reduced NaCl induced oxidative stress as evidenced by decreased levels, 44 and 45% in H2O2 and 44 and 39% in MDA respectively, when compared to the levels in plants treated only with NaCl stress (Fig. S4A, B). Moreover, co-application of Put + Spd to stressed plants resumed oxidative stress by increasing the level of H2O2 (52%) and MDA activity (58%) as compared with NaCl treated control. These results confirmed that Put + Spd increases salinity tolerance in foxtail millet plants by inhibiting the accumulation of toxic ROS contents.
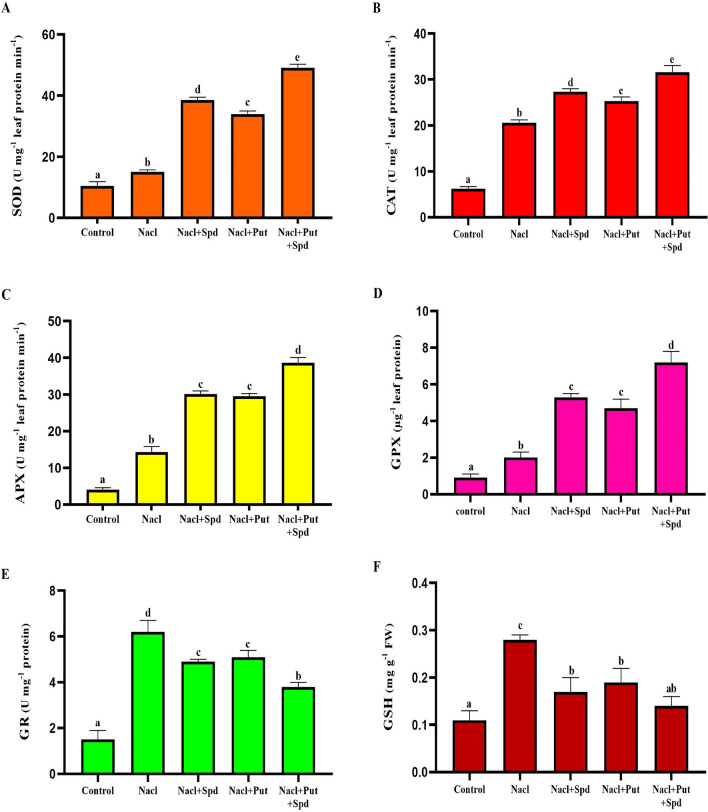
Assays of antioxidant enzyme activity and free radical production
In order to explore the potential regulatory roles of PAs in alleviation of salinity induced oxidative stress, various antioxidant enzymes viz. SOD, CAT, APX, GR, GPX and GSH activities were estimated (Fig. 4). In the present study, NaCl treatment stimulated significant increases in the SOD (31%), CAT (70%), APX (71%), GR (76%), GPX (55%) and GSH (61%) respectively, and resulted in a greater accumulation of H2O2, O ¯2 and MDA compared with the levels in unstressed control plants. This tendency is more obviously found in Spd than in Put, excluded for H2O2 content. SOD activity was considerably rising by 61, 56 and 69% in Spd, Put and Put + Spd treatments, whereas CAT activity increase by 25% in Spd, 19% in Put and 35% in co-treatment of Put + Spd as compared with the NaCl treated plants, which reveals the decrease in ROS (H2O2 and O ¯2) contents (Fig. 4a, b).
Fig. 4.
Effect of exogenous PAs on antioxidant enzymes in NaCl affected foxtail millet seedlings. A. Superoxide dismutase (SOD; U mg−1 leaf protein min−1), B. Catalase (CAT; U mg−1 leaf protein min−1), C. Ascorbate Peroxidase (APX; U mg−1 leaf protein min−1), D. Guaiacol peroxidase (GPX; µg−1 leaf protein), E. Glutathione Reductase (GR; U mg−1 protein) and F. Glutathione (GSH; mg g−1 FW). Here, Control (Unstressed), NaCl (200 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM) and NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM), respectively. The data correspond to the average of three replicates. Bars represent SD of the mean (n = 3). Different letters (a–e) indicate significant differences among the treatments at p ≤ 0.05, according to Duncan’s multiple range test
In comparison with NaCl treated control, APX and GPX activities raised (53 and 52%) and (62 and 57%) in NaCl + Spd and NaCl + Put treated plants. Meanwhile, the combined application of Put + Spd efficaciously increased the APX (63%) and GPX (72%) activities (Fig. 4c, d). The supplementation of PAs significantly reduced the activities of GR (39%) and GSH (50%) in NaCl + Put + Spd treatment when compared with the NaCl treatment (Fig. 4e, f). No remarkable changes in the level of antioxidant enzymes were noticed between the Put and Spd treated plants, however the synergistic effect of Put + Spd boost the antioxidant capability by enhancing the ROS scavenging mechanism in salinity stressed foxtail millet plants.
Assay of PAO and DAO activity
The generation of excess level of H2O2 through catalytic reaction was estimated by DAO and PAO, which play a key role in PAs homeostasis in plants. In the NaCl treated plants, DAO and PAO were severely increased by 62% and 81% when compared with the unstressed control plants. However, the DAO activity was gradually declined with the augmentation of Put (19%), Spd (22%) and Put + Spd (44%) treatment (Fig. S5A). Similarly, PAO activity was consistently decreased with 1 mM concentration of Put by 18%, Spd by 22% and Put + Spd (0.5 + 0.5 mM) registered a 29% decline as compared to salinity stressed plants (Fig. S5B). These results clearly specify the synergistic effect of Put + Spd enhancing salinity tolerance in foxtail millet.
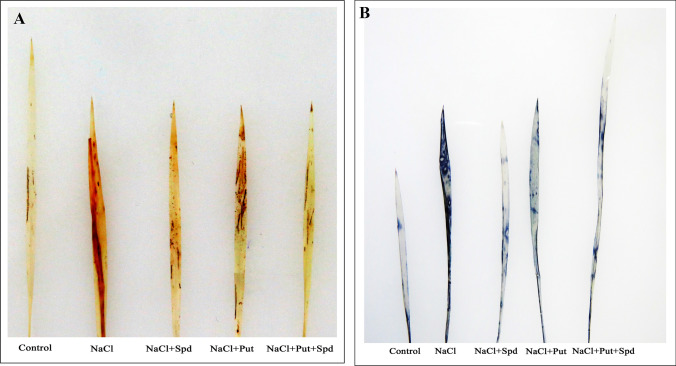
Histochemical detection of H2O2 and O ¯2
To elucidate whether the application of PAs in foxtail millet plants deals with ROS, detoxification induced by salinity stress were assessed by visualization of H2O2 and O ¯2 production using DAB and NBT staining. Foxtail millet plants were more extensively stained under NaCl stress condition than the controls (Fig. 5). The intensity of the DAB and NBT staining were considerably diminished in NaCl + Put and NaCl + Spd treated plants, respectively, signifying that PAs inhibited the accumulation of H2O2 and O ¯2 (Fig. 5a, b). Plants treated with NaCl + Put + Spd showed a minimal concentration of H2O2 and O ¯2, as similar to the control plants (Fig. 5a, b). These results mimic the role of Put + Spd in controlling the ROS homeostasis in salinity treated plants.
Fig. 5.
a Histochemical detection of H2O2 in salinity induced foxtail millet seedlings under PAs. DAB staining of H2O2 (Left to right) Here, Control (Unstressed), NaCl (200 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM), NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM). b Effect of PAs on histochemical detection of O∙−2 in leaves of foxtail millet seedlings under salinity stressed. NBT staining of O∙−2 (Left to right) Unstressed control, NaCl (200 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM), NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM)
CLSM analysis
The cell membrane damage was assessd by examining ROS levels in the leaf cells. The fluorescence intensity ratio of NaCl stressed plants is significantly higher than the unstressed control and PAs treated plants. Salinity stress generates increased ROS production within the leaf cells. The fluorescence intensity of the DCF-DA staining was considerably diminished in Put and Spd treated plants, respectively. Furthermore, the ROS production was profoundly reduced by the co-application of Put + Spd which was not statistically different from the unstressed control plants (Fig. 6). These observations precisely show that a combined application of PAs (Put + Spd) considerably ameliorates salinity tolerance in foxtail millet plants.
Fig. 6.
Analysis of cell membrane damage through confocal laser scanning microscopy in foxtail millet leaves treated with PAs (Left to right). a Unstressed control, b NaCl − 200 mM, c NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM), d NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM) and e NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM)
FTIR analysis
The cell wall damage as well as protein damage is the key indicator of stress response during any stress conditions in the plants. In this study, the molecular changes during the NaCl exposure was observed using FTIR analysis by comparing the functional group intensity variations among the control and treated plants. The amide I absorption region acts as key indicator which is particularly sensitive to salinity stress. The absorption regions such as 3050 to 2800, 1750 to 1250, and 1250 to 900 cm−1 bands corresponding to lipids, amides and carbohydrates, respectively, were significantly reduced in the NaCl treated plants; whereas these regions gradually enriched in Put, Spd and Put + Spd treatment. The enriched intensities of the mentioned regions suggested that the cell wall and protein damage were successfully renovated during these Put, Spd, Put + Spd treatment upon NaCl exposure. The absorption range around 3537 cm−1 indicates O–H and N–H stretching that predominantly found in chlorophyll protein and carbohydrates (Fig. 7). In FTIR analysis, the enriched intensities of these above mentioned regions clearly reveal that these two regions has been improved in Put, Spd and Put + Spd treatment, which stated that PAs enhances the salinity tolerance in foxtail millet plants.
Fig. 7.
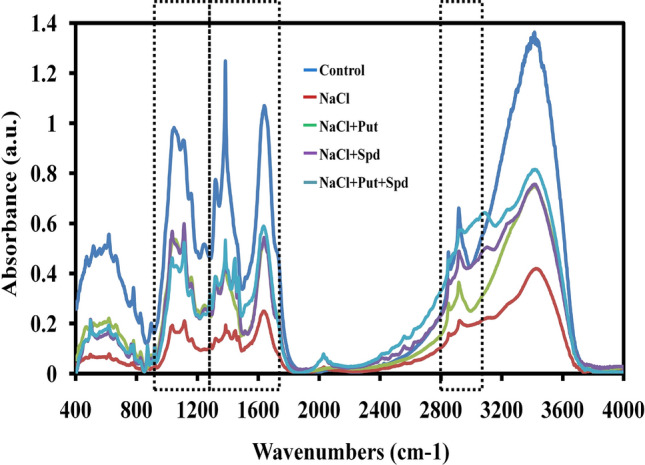
Analyses the metabolic profile of PAs treated foxtail millet under salinity stress through FTIR spectroscopy. The data correspond to the average of three replicates. Here, control (Unstressed), NaCl (200 mM), NaCl + Spd (NaCl; 200 mM, Spermidine; 1 mM), NaCl + Put (NaCl; 200 mM, Putrescine; 1 mM), NaCl + Put + Spd (NaCl + Putrescine + Spermidine; 200 + 0.5 + 0.5 mM), respectively
Discussion
Salinity is responsible for multidimensional stresses such as ionic, osmotic, oxidative stress and hormonal imbalances in plants also it affect the photosynthetic machinery, lipid and protein metabolic process (Rasool et al. 2013). Salinity stress alters the genome, epigenome, transcriptome and proteome of the plants in the form of physiological, morphological, and biochemical dynamism. Plants have in turn evolved many tangled tolerance mechanisms to withstand the salinity stress. However, these tolerant mechanisms vary among diverse plant species.
The salinity stress greatly suppressed the GP, FW, DW and plant height. It also affected photosynthetic pigments, RWC and soluble protein, indicating that NaCl impaired the photosynthetic mechanism and deactivate the enzyme activities (Parida and Das 2005; Hameed and Ashraf 2008; Hussain et al. 2015; Satish et al. 2016). Reduction in plant biomass acted as a reliable indicator for evaluating the degree of damage and the tolerance capability (Sun et al. 2020). This study showed that RWC, total chlorophyll (a + b), carotenoid, soluble protein, plant growth and biomass were significantly alleviated with PAs treatments. The photosynthetic pigments content have been significantly reduced upon high salinity stress exposure, this result coincide with the findings of Dąbrowski et al. (2017). The enrichment of chlorophyll biosynthesis was alleviated by exogenous application of PAs. However, the synergistic effect of Put with Spd upon high salinity stress in foxtail millet plants has not been studied so far.
Accumulation of proline, protein and imbalance water contents are the general responses to salinity stress (Zhang et al. 2014). In this study, protein and RWC were relatively lower and proline accumulation was higher in salinity treated plants when compared with the control foxtail millet which is highly correlated with the severity of the cell damage. These observations support the findings of Abdel-Latef (2005) and Wasti et al. (2012) in wheat and Zhang et al. (2014) in tomato. The application of Put and Spd restored the RWC without much accumulation of proline content which is inversely proportional to the NaCl stressed plants. This may be due to enhancement of the pathway for synthesis of protein and proline from glutamine or transforming the other amino acids into proline (Kong-Ngern et al. 2005; Zhang et al. 2014). In plant cells, the level of ROS was reported to be increased under high salinity stress condition (Borsani et al. 2005; Chawla et al. 2013). Similarly, in our study, the foxtail millet plants treated with NaCl showed a drastic oxidative stress by higher levels of H2O2 and O ¯2. Salinity induced oxidative damage by membrane lipid peroxidation were correlated with a significant increase in MDA level. Additionally, this study revealed encouraging results in plant defence against the toxicity of NaCl upon Put + Spd co-application.
In the present investigation, FTIR has been used to analyse the functional group intensity variations under PAs treatment which is associated with salinity stress tolerance. The FTIR exposes the molecular-vibrational transitions which offer characteristic details on molecular configurations in biological samples (Griffiths 1978). The lipids, amides and carbohydrates absorption regions were significantly reduced upon salinity stress while PAs treatment enriched these functional groups. Previously, studies have proven the deconvolution and analysis of two absorption regions such as amide I (1580–1700 cm−1) and pectin accumulation (1745 cm−1) as key indicators of stress levels (Griffiths 1978; Barth 2000; Yang and Yen 2002). Moreover, the amide I absorption region is particularly sensitive to salt stress (Yang and Yen 2002). The absorption regions such as 3050 to 2800, 1750 to 1250, and 1250 to 900 cm−1 bands corresponding to lipids, amides and carbohydrates, respectively, were higher in normal plants (Lahlali et al. 2014). The enriched intensities of these regions revealed that the amide and lipid regions has been enhanced in PAs treatment, which specified the protective action of membrane lipid, cell wall pectin, chlorophyll protein and carbohydrates, than that of NaCl stressed plants.
Several studies have revealed that Put is the main precursor of PAs biosynthesis in plants, which can significantly ameliorate the salinity stress. PAs are important regulators to overcome abiotic stress in plants through influencing the antioxidant activities (Parvin et al. 2014; Tajti et al. 2018). Furthermore, PAs have a pivotal role in complex signaling, cell proliferation, interacting with macromolecules, endogenous phytohormone, metabolic profiles and have potential to influence in various plant defence mechanisms at enzymatic and gene expression levels (Liu et al. 2015; Tajti et al. 2018). The application of PAs significantly increases the H2O2-scavenging activity through antioxidant enzymes production in salinity stressed plants, resulting in decline of H2O2 level. These results are in agreement with the findings of Mittler et al. (2004). The rise in these ROS-scavengers may accelerate the reduction of toxic ROS by converting H2O2 into the water to decrease excess H2O2 content (Mittler et al. 2004). In this study, exogenous treatment of Put and Spd strongly induces the activation of antioxidant enzymes and PAs biosynthesis can confer salinity tolerance in foxtail millet plants. Numerous studies illustrated that PAs could ameliorate antioxidant ability in various plant species such as rice (Roychoudhury et al. 2011), ginseng (Parvin et al. 2014), wheat (Rady and Hemida 2015), zoysiagrass (Li et al. 2016), finger millet (Satish et al. 2018), and cucumber (Duan et al. 2008; Wu et al. 2018). Our observations revealed that the synergistic effect of combined Put + Spd treatment enhances salinity tolerance in foxtail millet with their efficient elimination of ROS by scavenging system. Several reports (Nayyar and Chander 2004; Gong et al. 2016; Nahar et al. 2016; Paul et al. 2018; Chen et al. 2018) including this study, proved the regulatory roles of PAs on antioxidant defence, however PAs based ROS detoxification mechanism has still remained to be detected in plants. Based upon the above information, a schematic representation was portrayed in Fig. S6 which elucidates the biochemical mechanisms involving NaCl induced toxicity and the exogenous PAs (Put + Spd) mediated salinity stress tolerance in foxtail millet plants.
Conclusion
This is the first report demonstrating the salinity tolerance mechanism of combined PAs such as Put along with Spd in foxtail millet. The morphological, physiological and biochemical responses upon Put + Spd (0.5 + 0.5 mM) treatment showed higher adaptive mechanism than individually (1 mM Spd and 1 mM Put) treated plants. Further, the synergistic effect of Put + Spd confers salinity tolerance through inducing antioxidant enzymes and osmoprotectants. Overall, the exposure of combined PAs under NaCl stress coordinates the complex physiological and biochemical processes. Hence, the exogenous co-application of lower concentration of Put + Spd can be easily adopted to ameliorate the salinity stress in foxtail millet.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The author P. Rathinapriya (UGC order no: F.25-1/2013-14 (BSR)/7-326/2011 dt 30.05.2014) thank the University Grants Commission, New Delhi, India for financial support in the form of fellowship. The authors sincerely acknowledge the computational and Bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by DBT, GOI; File No. BT/BI/25/012/2012, BIF). The authors also thankfully acknowledge RUSA 2.0 [F. 24-51/2014-U, Policy (TN Multi-Gen), Dept of Edn, GOI], DST-FIST (Grant No. SR/FST/LSI-639/2015(C)), UGC-SAP (Grant No. F.5-1/2018/DRS-II (SAP-II)) and DST-PURSE (Grant No. SR/PURSE Phase 2/38 (G)) for providing instrumentation facilities.
Abbreviations
- APX
Ascorbate peroxidae
- CAT
Catalase
- CLSM
Confocal laser scanning microscope
- DAB
3,3-diaminobenzidine
- DAO
Diamine oxidase
- EL
Electrolyte leakage
- FTIR
Fourier transform-infrared spectroscopy
- GPX
Guaiacol peroxidise
- GR
Glutathione reductase
- GSH
Glutathione
- GSSG
Glutathione disulfide
- H2O2
Hydrogen peroxide
- MDA
Malondialdehyde
- NaCl
Sodium chloride
- NADH+
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NBT
Nitrotetrazolium blue chloride
- PAO
Polyamine oxidase
- PAs
Polyamines
- POD
Peroxidase
- Put
Putrescine
- ROS
Reactive oxygen species
- RWC
Relative water content
- SOD
Superoxide dismutase
- Spd
Spermidine
Author Contributions
Conceived and designed the experiments: PR & MR. Performed the experiments: PR MB KR RA RR. Analyzed the data: PR LS SP. Contributed reagents/materials/analysis tools: MR. Wrote the paper: PR. All the authors have read the manuscript and approved for publication.
Compliance with ethical standards
Conflict of interest
The authors don’t have any conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Latef AA (2005) Salt tolerance of some wheat cultivars. Ph.D. Thesis. South Valley Univ in Qena, Egypt, pp 1–159
- Aebi H. Catalase in vitro. Oxy Radic Biologic Sys. 1984;10:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A. The infrared absorption of amino acid side chains. Progress Biophys Mol Boil. 2000;74:141–173. doi: 10.1016/s0079-6107(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell. 2005;123:1279–1291. doi: 10.1016/j.cell.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik EB. Glutathione level in rat brain. J Biol Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- Chawla S, Jain S, Jain V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.) J Plant Biochem Biotechnol. 2013;22:27–34. [Google Scholar]
- Chen J, Fang J, Guo Z, Lu S. Polyamines and antioxidant defense system are associated with cold tolerance in centipede grass. Front Agr Sci Eng. 2018;5:129–138. [Google Scholar]
- Dąbrowski P, Kalaji MH, Baczewska AH, Pawluśkiewicz B, Mastalerczuk G, Borawska-Jarmułowicz B, Paunov M, Goltsev V. Delayed chlorophyll a fluorescence, MR 820: and gas exchange changes in perennial ryegrass under salt stress. J Luminescence. 2017;183:322–333. [Google Scholar]
- Del Pozo O, Lam E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr Biol. 1998;8:1129–1132. doi: 10.1016/s0960-9822(98)70469-5. [DOI] [PubMed] [Google Scholar]
- Duan J, Li J, Guo S, Kang Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol. 2008;165:1620–1635. doi: 10.1016/j.jplph.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Fariduddin Q, Khan TA, Yusuf M, Aafaqee ST, Khalil RR. Ameliorative role of salicylic acid and spermidine in the presence of excess salt in Lycopersicon esculentum. Photosynthetica. 2018;56:750–762. [Google Scholar]
- Fu Y, Zhang Z, Liu J, Chen M, Pan R, Hu W, Guan Y, Hu J. Seed priming with spermidine and trehalose enhances chilling tolerance of rice via different mechanisms. J Plant Growth Regul. 2019;10:1–11. [Google Scholar]
- Gong X, Liu Y, Huang D, Zeng G, Liu S, Tang H, Zhou L, Hu X, Zhou Y, Tan X. Effects of exogenous calcium and spermidine on cadmium stress moderation and metal accumulation in Boehmeria nivea (L.) Gaudich. Environ Sci Pollut Res. 2016;23:8699–8708. doi: 10.1007/s11356-016-6122-6. [DOI] [PubMed] [Google Scholar]
- Griffiths PR. Fourier transform infrared spectroscopy: recent developments. Appl Opt. 1978;17:1315–1317. doi: 10.1364/ao.17.001315. [DOI] [PubMed] [Google Scholar]
- Hameed M, Ashraf M. Physiological and biochemical adaptations of Cynodon dactylon (L.) Pers. from the salt range (Pakistan) to salinity stress. Flora. 2008;203:683–694. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stechiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang Y, Shi Y, Zhang Z, Zou Z, Zhang H, Zhao J. Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity–alkalinity mixed stress. Plant Physiol Biochem. 2012;57:200–209. doi: 10.1016/j.plaphy.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chen KL, Cheung CH, Chang JY. Autophagy induced by cathepsin S inhibition induces early ROS production, oxidative DNA damage, and cell death via xanthine oxidase. Free Radical Biol Medicine. 2013;65:1473–1486. doi: 10.1016/j.freeradbiomed.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Hussain T, Koyro HW, Huchzermeyer B, Khan MA. Eco-physiological adaptations of Panicum antidotale to hyperosmotic salinity: water and ion relations and anti-oxidant feedback. Flora. 2015;212:30–37. [Google Scholar]
- Kannappan A, Sivaranjani M, Srinivasan R, Rathna J, Pandian SK, Ravi AV. Inhibitory efficacy of geraniol on biofilm formation and development of adaptive resistance in Staphylococcus epidermidis RP62A. J Med Microbiol. 2017;66:1506–1515. doi: 10.1099/jmm.0.000570. [DOI] [PubMed] [Google Scholar]
- Khan MI, Nazir F, Asgher M, Per TS, Khan NA. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol. 2015;173:9–18. doi: 10.1016/j.jplph.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Kissoudis C, van de Wiel C, Visser RG, van der Linden G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front Plant Sci. 2014;5:207. doi: 10.3389/fpls.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong-Ngern K, Daduang S, Wongkham C, Bunnag S, Kosittrakun M, Theerakulpisut P. Protein profiles in response to salt stress in leaf sheaths of rice seedlings. Sci Asia. 2005;31:403–408. [Google Scholar]
- Lahlali R, Jiang Y, Kumar S, Karunakaran C, Liu X, Borondics F, Hallin E, Bueckert R. ATR–FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front Plant Sci. 2014;5:747. doi: 10.3389/fpls.2014.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin H, Zhang Q. The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance in zoysia grass (Zoysia japonica Steud) subjected to short-term salinity stress. Front Plant Sci. 2016;7:1221. doi: 10.3389/fpls.2016.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Proto Food Anal Chem. 2001;1:3–4. [Google Scholar]
- Lin CC, Kao CH. NaCl induced changes in ionically bounds peroxidase activity in roots of rice seedlings. Plant Soil. 1999;216:147–153. [Google Scholar]
- Liu JH, Wang W, Wu H, Gong X, Moriguchi T. Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci. 2015;6:10. doi: 10.3389/fpls.2015.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal C, Ghosh N, Maiti S, Das K, Gupta S, Dey N, Adak MK. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol Mol Biol Plants. 2013;19:91–103. doi: 10.1007/s12298-012-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Suzuki N, Ciftci-Yilmaz SU, Mittler RO. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Questions and future challenges. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mostofa MG, Rahman A, Ansary MM, Watanabe A, Fujita M, Tran LS. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep. 2015;5:14078. doi: 10.1038/srep14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum. 1962;15:473–497. [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 2016;126:245–255. doi: 10.1016/j.ecoenv.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nayyar H, Chander S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chick pea. J Agro Crop Sci. 2004;190:355–365. [Google Scholar]
- Nemeth M, Janda T, Horvath E, Paldi E, Szalai G. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci. 2002;162:569–574. [Google Scholar]
- Panuccio MR, Chaabani S, Roula R, Muscolo A. Bio-priming mitigates detrimental effects of salinity on maize improving antioxidant defense and preserving photosynthetic efficiency. Plant Physiol Biochem. 2018;132:465–474. doi: 10.1016/j.plaphy.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD (P) H. Oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants. Ecotoxicol Env Saf. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, Yang DC. Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene. 2014;537:70–78. doi: 10.1016/j.gene.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Paul S, Banerjee A, Roychoudhury A. Role of polyamines in mediating antioxidant defense and epigenetic regulation in plants exposed to heavy metal toxicity. Plants under metal and metalloid stress: responses, tolerance and remediation. Nature. 2018;5:229–247. [Google Scholar]
- Poborilova Z, Opatrilova R, Babula P. Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ Exp Bot. 2013;91:1–11. [Google Scholar]
- Rady MM, Hemida KA. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Env Saf. 2015;119:178–185. doi: 10.1016/j.ecoenv.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Rasool S, Hameed A, Azooz MM, Siddiqi TO, Ahmad P. Salt stress: causes, types and responses of plants. Ecophysiol Responses Plants Under Salt Stress. 2013;3:1–24. [Google Scholar]
- Rathinapriya P, Satish L, Rameshkumar R, Pandian S, Rency AS, Ramesh M. Role of activated charcoal and amino acids in developing an efficient regeneration system for Foxtail Millet (Setaria italica (L.) Beauv.) using leaf base segments. Physiol Mol Biol Plants. 2018;25:533–548. doi: 10.1007/s12298-018-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FR, Krapp AR, Bisaro F, Maiale SJ, Pieckenstain FL, Carrillo N. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant J. 2017;92:761–773. doi: 10.1111/tpj.13718. [DOI] [PubMed] [Google Scholar]
- Roychoudhury A, Basu S, Sengupta DN. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J Plant Physiol. 2011;168:317–328. doi: 10.1016/j.jplph.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sang T, Shan X, Li B, Shu S, Sun J, Guo SR. Comparative proteomic analysis reveals the positive effect of exogenous spermidine on photosynthesis and salinity tolerance in cucumber seedlings. Plant Cell Rep. 2016;35:1769–1782. doi: 10.1007/s00299-016-1995-x. [DOI] [PubMed] [Google Scholar]
- Sapeta H, Costa JM, Lourenco T, Maroco J, Van der Linde P, Oliveira MM. Drought stress response in Jatropha curcas: growth and physiology. Environ Experim Botany. 2013;85:76–84. [Google Scholar]
- Satish L, Rathinapriya P, Rency AS, Ceasar SA, Prathibha M, Pandian S, Rameshkumar R, Ramesh M. Effect of salinity stress on finger millet (Eleusine coracana (L.) Gaertn): histochemical and morphological analysis of coleoptile and coleorhizae. Flora. 2016;222:111–120. [Google Scholar]
- Satish L, Rency AS, Ramesh M. Spermidine sprays alleviate the water deficit-induced oxidative stress in finger millet (Eleusine coracana L. Gaertn.) plants. Biotech. 2018;8:63. doi: 10.1007/s13205-018-1097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvides A, Ali S, Tester M, Fotopoulos V. Chemical priming of plants against multiple abiotic stresses: mission possible? Trend Plant Sci. 2016;21:329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Singh M, Singh VP, Prasad SM. Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol Biochem. 2019;141:466–476. doi: 10.1016/j.plaphy.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Sreenivasulu N, Ramanjulu S, Ramachandra-Kini K, Prakash HS, Shekar-Shetty H, Savithri HS, Sudhakar C. Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of fox-tail millet with differential salt tolerance. Plant Sci. 1999;141:1–9. [Google Scholar]
- Sreenivasulu N, Grimm B, Wobus U, Weschke W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica) Physiol Planta. 2000;109:435–442. [Google Scholar]
- Su G, An Z, Zhang W, Liu Y. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. J Plant Physiol. 2005;162:1297–1303. doi: 10.1016/j.jplph.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Sudhakar C, Veeranagamallaiah G, Nareshkumar A, Sudhakarbabu O, Sivakumar M, Pandurangaiah M, Kiranmai K, Lokesh U. Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Rep. 2015;34:141–156. doi: 10.1007/s00299-014-1695-3. [DOI] [PubMed] [Google Scholar]
- Sun M, Wang T, Fan L, Wang H, Pan H, Cui X, Lou Y, Zhuge Y. Foliar applications of spermidine improve foxtail millet seedling characteristics under salt stress. Biol Plantarum. 2020;64:353–362. [Google Scholar]
- Tabot PT, Adams JB. Early responses of Bassia diffusa (Thunb.) Kuntze to submergence for different salinity treatments. South Afr J Bot. 2013;84:19–29. [Google Scholar]
- Tajti J, Janda T, Majláth I, Szalai G, Pal M. Comparative study on the effects of putrescine and spermidine pre-treatment on cadmium stress in wheat. Ecotoxicol Env Saf. 2018;148:546–554. doi: 10.1016/j.ecoenv.2017.10.068. [DOI] [PubMed] [Google Scholar]
- Tang S, Zhang H, Li L, Liu X, Chen L, Chen W, Ding Y. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct Plant Biol. 2018;45:911–921. doi: 10.1071/FP17149. [DOI] [PubMed] [Google Scholar]
- Tiburcio AF, Altabella T, Bitrián M, Alcázar R. The roles of polyamines during the lifespan of plants: from development to stress. Planta. 2014;240:1–18. doi: 10.1007/s00425-014-2055-9. [DOI] [PubMed] [Google Scholar]
- Van Ha C, Leyva-González MA, Osakabe Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Van Dong N, Yamaguchi-Shinozaki K. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci. 2014;111:851–856. doi: 10.1073/pnas.1322135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Manku S, Hall DG. Solid phase syntheses of polyamine toxins ho-416b and phtx-433. Use of an efficient polyamide reduction strategy that facilitates access to branched analogues. Organic Let. 2000;2:1581–1583. doi: 10.1021/ol005817b. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang W, Huang J. Putrescine mediates aluminum tolerance in red kidney bean by modulating aluminum-induced oxidative stress. Crop Sci. 2013;53:2120–2128. [Google Scholar]
- Wasti S, Mimouni H, Smiti S, Zid E, Ben Ahmed H. Enhanced salt tolerance of tomatoes by exogenous salicylic acid applied through rooting medium. J Integr Bio. 2012;16:200–207. doi: 10.1089/omi.2011.0071. [DOI] [PubMed] [Google Scholar]
- Wu J, Shu S, Li C, Sun J, Guo S. Spermidine-mediated hydrogenperoxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol Biochem. 2018;128:152–162. doi: 10.1016/j.plaphy.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Yang J, Yen HE. Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier transform infrared spectroscopy study. Plant Physiol. 2002;130:1032–1042. doi: 10.1104/pp.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JY, Hamayun M, Lee SK, Lee IJ. Methyl jasmonate alleviated salinity stress in soybean. J Crop Sci Biotechnol. 2009;12:63–68. [Google Scholar]
- Zhang JP, Wang MY, Bai YF, Jia JP, Wang GY. Rapid evaluation on drought tolerance of foxtail millet at seedling stage. J Plant Genet Resour. 2005;6:59–62. [Google Scholar]
- Zhang Y, Zhang L, Hu XH. Exogenous spermidine-induced changes at physiological and biochemical parameters levels in tomato seedling grown in saline-alkaline condition. Bot studies. 2014;55:1–8. doi: 10.1186/s40529-014-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Bai J, Lu Q, Zhang G. Effects of salinity on dynamics of soil carbon in degraded coastal wetlands: implications on wetland restoration. Phys Chem Earth Parts A/B/C. 2017;97:12–18. [Google Scholar]
- Zhou L, Zhou H, Peng Y, Zhang X, Ma X, Huang L, Yan Y. Exogenously applied spermidine improves drought tolerance in creeping bentgrass associated with changes in antioxidant defense, endogenous polyamines and phytohormones. J Plant Growth Regul. 2015;76:71–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.