Abstract
Investigating the cause of animal death is helpful to understand the reasons behind the interactions and conflicts between humans and animals. To further develop the cause of death investigation, we report a case of a Chinese spot-billed duck (Anas zonorhyncha) which hatched from a rescued duck and died 10 days after release. We inspected the duck’s cause of death using an interview of concerned people, external body examination, necropsy, and genetic examinations. Based on the fractures, the main cause of death was determined to be a traffic accident. Furthermore, molecular tests helped to detect raccoon DNA in the visible bite wounds. This case shows that molecular biological method is one of the methods of clarify the animals’ cause of death.
Keywords: alien animals, Anas zonorhyncha, cause-of-death, forensic science, Japan
Conflict between humans and animals has been a global issue. There are some reports of injuries caused by both humans [19, 38] and domestic animals [22, 27] to the wildlife. In addition, smuggling [3, 5, 10, 17, 24, 32, 36, 37] and niche conflict with alien animals [8] are well known effects of humans on wildlife. The “One Health” approach aims to achieve optimal health outcomes by recognizing the interconnections between humans, domestic animals, wildlife, and their shared environment [13, 23]. However, unified procedures to investigate the causes of death in wildlife are not currently followed globally; therefore, further technological development and research is needed. The cause of animal death must be more broadly examined, regardless of the animal involved or whether the death was caused by animals, humans, or any other factors.
Forensic veterinary science is a field which employs scientific methods for systematic examination of the cause of death in animals [30]. It is usually performed in a consistent manner, involving an interview with whom it might concern about the condition of the animal and the environment in which it was found by the forensic veterinarian or human with specialized knowledge. After the interview, an external body examination (superficial) and a necropsy are performed, and if necessary, pathological (including histopathological), toxicological, and genetic examinations are additionally performed [28, 34]. Forensic veterinary science may provide ways to present valuable evidence in lawsuits, and contribute to the field of wildlife medicine [6]. Investigations on the causes of death through a series of systematic checks reveal almost all pertinent evidence [28]. Collecting and collating evidence on the cause of death in wildlife becomes challenging owing to the variation in the elapsed time from death to its discovery. This makes the primary cause of death in wildlife relatively unclear compared to that in companion animals. The potential causes of death include injuries caused by humans intentionally such as abuse, unintentionally caused injuries such as traffic accidents, environmental pollution [2], and attacks from imported wildlife or domestic animals [25]. Cooper [6] described an outline for the forensic veterinary examination of wildlife, but did not specify the findings related to cause of death, or the evaluation methods for investigation [7]. In Japan, the government conducted a study to find the cause of death in Okinawa rails (Gallirallus okinawae) using forensic examinations, and found that the cause of death was domestic cat (Felis catus) bite wounds [20]. However, there are few previous reports highlighting the methods to identify the attacking species from an injury site, in a cause of death investigation. In each investigation, to accurately verify the cause of death in wild animals, it is necessary to accumulate as many findings as possible. To achieve this and to contribute to the legal and veterinary medicine fields, this case study reports the results of our investigation into the cause of death of a Chinese spot-billed duck (Anas poecilorhyncha), using an interview, external body examination, necropsy, and genetic examination.
We conducted an interview of multiple rehabilitators in Shiga prefecture. Based on the answers from our interview, the deceased duckling hatched from a rehabilitated duck (mother duck) in a wildlife rescue center in Shiga prefecture. After hatching, the duckling was reared by mother duck and the rehabilitators released it into the wild on August 25, 2018, when the duckling (duck) was 65 days old. The rehabilitators had recognized that the duck had no abnormal behavior. The duck was released at the shore of Lake Biwa in Takashima City, Shiga Prefecture, Japan (Lat. 35 N and long. 136 E). When the duck was released, an identification ring was attached to the duck’s tarsus. After release, the rehabilitators observed the duck in the neighboring area around the release site and did not report any abnormal behavior. On September 4, 2018 (10 days after the release), the duck was found dead on the roadside near the release site (Lat. 35 N and long. 136 E). Through the interview, it was determined that the area where the duck carcass was found had no high buildings (higher than two floors), and there were no blood stains on the road nearby. When the duck was discovered, its head was missing from the neck up. The surrounding area was searched, but the missing body part could not be found. Upon interviewing the rehabilitators, it was speculated that the raccoons (Procyon lotor) and domestic cats living in that area could have attacked the duck.
After the interview, the carcass was examined (Fig. 1). The carcass was transported at −20°C to Nippon Veterinary and Life Science University for inspection. First, an external body examination was performed. If the cause of a wound was difficult to determine, the wound size was measured and inspected to determine whether a genetic examination was necessary. During the external body examination, nutritional conditions were evaluated using the Keel Scoring Chart [35] and age of duck was evaluated using the tail and primary feathers. In the necropsy, sex was identified by observing the genitals of the duck carcass, and cause of death was listed based on any trauma or abnormalities in the internal organs of the carcass. A genetic examination was performed using the swabs collected from the surface of wounds. A negative control (NC) sample was also collected from a deep layer of the quadriceps femoris muscle. Deoxyribonucleic acid (DNA) was extracted from tissue of each wound using a kit (DNeasy Blood & Tissue kit™, Qiagen, Venlo, Netherlands). As a positive control (PC), a muscle sample from a domestic cat and a racoon which were previously obtained from each animal’s respective carcasses were used. In the area where the body was found, based on information from the interview, common duck predators were presumed to be domestic cats and raccoons. To identify the domestic cat and raccoon from the swab samples, the cytochrome b gene was targeted [9, 15, 29]. The primers used for DNA amplification (Table 1). All polymerase chain reactions (PCR) assays comprised <100 ng DNA, 1X GoTaq® colorless master mix (Promega, Madison, WI, USA), and 10 pM each primer. In the PCR protocol, after the pre-denaturation at 95°C for 3 min, denaturation step at 94°C for 2 min, an annealing steps at 55°C in raccoon and 48°C in domestic cat for 30 sec, and extension step of 30 sec at 75°C in total of 35 cycles, followed by a final extension of 7 min at 75°C. The target region was analyzed for its nucleotide sequence to identify the species it belonged to (samples were outsourced for analysis).
Fig. 1.
The dead body of the duck. This carcass was a Chinese spot-billed duck (Anas poecilorhyncha). It was collected 10 days after its release. The upper part from the neck was lost, and three bite wounds were found on the body surface (white arrows). White arrows indicate chest, belly, and limb bite wounds in order from the left. Swabs were collected for each bite point and neck wound, but the bite mark on the limb had already started decomposing; thus, a DNA test was not performed from it.
Table 1. Nucleotide sequence of primers.
| Cytochrome b of mitochondria (for raccoons) | |
| Forward | 5′- CCATCAGCACCCAAAGCT -3′ |
| Reverse | 5′- CGCTTAAACTTATGTCCTGTAACC -3′ |
| Cytochrome b of mitochondria (for domestic cats) | |
| Forward | 5′- CAATGGTCACAGGACATATAC -3′ |
| Reverse | 5′- GGTTTGGCAAGACATAAATAG -3′ |
The target regions of each species were amplified using a pair of gene specific primer.
In the external body examination, the Keel Score of the duck carcass was one out of five, and therefore, it was classified as “severely emaciated”. There were signs of bite marks on the left-side of its chest (Fig. 2), belly (Fig. 3), and limb (Fig. 4), but we were unable to identify the animal species responsible for the bites. The feathers on the duck’s back had been plucked and its neck had been bluntly cut.
Fig. 2.
Left chest (Chest). The wound is at the left chest (indicated by a white arrow). The wound size was determined as: 7.90 mm length, 5.30 mm width, and 6.95 mm depth.
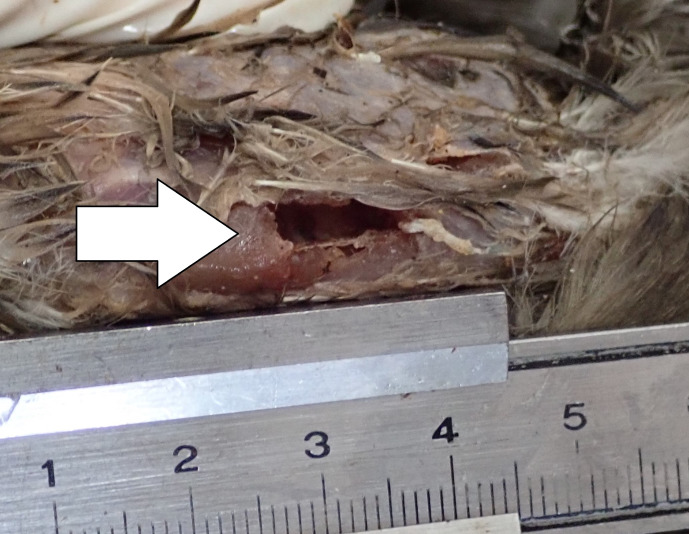
Fig. 3.
Left abdomen (Belly). The wound present at the left abdomen (indicated by a white arrow). The wound size was determined as: 12.0 mm length, 6.20 mm width, and 9.30 mm depth.
Fig. 4.
Left thigh side (Limb). The wound present in the left thigh (indicated by a white arrow). The wound size was determined as: 5.20 mm length, 3.60 mm width, 5.80 mm depth. The wound looked corrupt because the tissue color was greenish and melting.
In the necropsy, the duck had undeveloped testes and was deemed to be a young male. Although there were no notable visceral findings, three ruptures were identified in the lungs, the left kidney, and in the small intestine. The sternum and pelvis had commind fractures and the left rib had an oblique fracture.
As the species that bit the duck could not be identified from the bite and wound marks, a genetic examination was performed using the swab collected from the bite sites and a neck wound. Swabs from the bite sites at chest, belly, and wound at neck, were tested to identify the species. The bite mark on the left limb had already started decomposing; therefore, a DNA test was not performed from it. PCR amplified products at the neck wound and chest swabs (Fig. 5) matched with that for raccoons; we did not detect any samples that matched those of domestic cats (Fig. 6). Upon sequencing the gene products thought to be from raccoons, they were identified to be from a raccoon species in Tanabe City, Wakayama Prefecture and in Tomakomai City, Hokkaido, with a 95.55% concordance rate (GenBank accession numbers of: AB462048.1, AB462049.1).
Fig. 5.
PCR product of raccoon mitochondria. PCR amplification products of raccoons were identified at 610 bp. This figure shows the 100 bp size marker (M) showing negative control (NC), positive control (PC), swab from wound at each neck (Neck), chest (Chest), and belly (Belly) from left to right lane. The product at about 600 bp was confirmed from the neck and chest swab samples.
Fig. 6.
PCR product of domestic cat mitochondria. PCR amplification products of domestic cats can be identified at 180 bp. This figure shows the 100 bp size marker (M) showing positive control (PC), swab from wound at each neck (Neck), chest (Chest), and belly (Belly), and negative control (NC) from left to right lanes. In this result, no PCR amplification was seen in the target region in any of the three swabs. The band at 180 bp or less was considered a non-specific region according to Naka et al. [29], hence, this PCR amplification product was not considered in this survey.
In this case, the duck had severe fractures. Complex fractures in multiple locations are often seen in traffic accident victims, and in forensic veterinary science, the cause of death in bodies with these types of fractures is considered traffic accidents [10, 12, 40]. In bird traffic accidents, especially bird strikes with cars, birds often suffer from internal, and not external, damage; their bodies typically do not have injuries such as open wounds or blood congestion in their muscle or skin [14, 16]. Thus, in this case, the main cause of death was determined to be a traffic accident, and the involvement of the raccoon could occur after the duck’s death. In wildlife rescue cases, the most common cause of death is by a domestic cat [21]. If a bird is injured by a domestic cat, it is commonly scarred around its waist and neck [5]. In contrast, the duck’s neck in this case was missing, which could be associated with raccoons or foxes (Vulpes vulpes), whose body sizes and muscles are more developed than those of domestic cats [11]. However, the raccoons are also known to carry away parts of the body, such as the head [4, 31, 33, 39]. It is possible, based on our experience, that when they are not fasting (satiety), racoons carry a body part of a hunted animal to their burrow. The duck’s body condition might have been a problem with rehabilitation until the duck release. One of the subjects in forensic veterinary science is the verification of animal abuse [2, 11]; animal abuse includes domestic animals and wildlife [18].
Verification of animal abuse also contributes to the sanity of human societies, as animal abuse closely links to the occurrence of crime and domestic violence [1]. Therefore, it is necessary to further spread forensic veterinary science in Japan. Human forensic laboratories organize knowledge about cause of death and conduct specific analysis such as genetic tests [18, 20, 26], however there are few reports performing these tests in wild animal [17]. The cause is suggested that a forensic veterinary science needs to be used in combination with various disciplines such as anatomy, pathology, ecology, behavioral science, and physiology.
This study determined the cause of death in a rescued duck. In this case, the main cause of death was determined to be a traffic accident (unintentional human accident), but concomitantly, this case also highlighted the spread of alien species and the niche conflict with alien animals in Japan. Therefore, forensic veterinary science may not only be used to determine the cause of death in an individual, but also to obtain valuable knowledge on the environment and ecosystem surrounding the individual. In order to achieve the aims of the “One Health” approach, it is necessary to accumulate such findings on the causes of death in animals, using a versatile and systematic inspection protocol, such as the one used in this study.
Acknowledgments
We thank all the staff members of the Houchouzu NPO (Osaka and Shiga prefectures). We are grateful to T. Sakai from the Houchouzu NPO and T. Kato from the Department of Wildlife Medicine of the Nippon Veterinary and Life Science University of Tokyo prefecture for the providing the technical advice on raccoons. We are grateful to Y. Hamaya and M. Sakata from the Department of Wildlife Medicine of the Nippon Veterinary and Life Science University of Tokyo prefecture for investigating the gene samples. We also thank K. Uchida for advice about the ecology of raccoons.
REFERENCES
- 1.Arkow P.1999. The abuse of animals and human interpersonal violence. pp. 50–61. In: Child Abuse, Domestic Violence, and Animal Abuse: Linking the Circles of Compassion for Prevention and Intervention (Ascione, F. R. and Arkow, P. eds.), Purdue University Press, West Lafayette. [Google Scholar]
- 2.Arluke A., Luke C.1997. Physical cruelty toward animals in Massachusetts, 1975–1996. Soc. Anim. 5: 195–204. doi: 10.1163/156853097X00123 [DOI] [Google Scholar]
- 3.Brearley G., McAlpine C., Bell S., Bradley A.2012. Influence of urban edges on stress in an arboreal mammal: a case study of squirrel gliders in southeast Queensland. Aust. J. Ecol. 27: 1407–1419. [Google Scholar]
- 4.Buxton D. F., Goodman D. C.1967. Motor function and the corticospinal tracts in the dog and raccoon. J. Comp. Neurol. 129: 341–360. doi: 10.1002/cne.901290405 [DOI] [PubMed] [Google Scholar]
- 5.Camarós E., Cueto M., Lorenzo C., Villaverde V., Rivals F.2016. Large carnivore attacks on hominins during the Pleistocene: a forensic approach with a Neanderthal example. Archaeol. Anthropol. Sci. 8: 635–646. doi: 10.1007/s12520-015-0248-1 [DOI] [Google Scholar]
- 6.Cooper J. E.2013. Working with dead animals. pp. 237–324. In: Wildlife Forensic Investigation Principles and Practice (Cooper, J. E. and Cooper, M. E. eds.), CRC Press, Oxford. [Google Scholar]
- 7.Cooper J. E.2013. Working with live animals. pp. 181–216. In: Wildlife Forensic Investigation Principles and Practice (Cooper, J. E. and Cooper, M. E. eds.), CRC Press, Oxford. [Google Scholar]
- 8.Crowley S. L., Hinchliffe S., McDonald R. A.2017. Conflict in invasive species management. Front. Ecol. Environ. 15: 133–141. doi: 10.1002/fee.1471 [DOI] [Google Scholar]
- 9.Cullingham C. I., Kyle C. J., Pond B. A., White B. N.2008. Genetic structure of raccoons in eastern North America based on mtDNA: implications for subspecies designation and rabies disease dynamics. Can. J. Zool. 86: 947–958. doi: 10.1139/Z08-072 [DOI] [Google Scholar]
- 10.Decamp C. E., Braden T. D.2008. Sacroiliac fracture −Separation in the dog: A study of 92 cases. Vet. Surg. 14: 127–130. doi: 10.1111/j.1532-950X.1985.tb00841.x [DOI] [Google Scholar]
- 11.De Graff R., Yamasaki M.2001. New England wildlife, habitat, natural history, and distribution 1st ed., University Press of New England, Lebanon. [Google Scholar]
- 12.De Munnynck K., Van de Voorde W.2002. Forensic approach of fatal dog attacks: a case report and literature review. Int. J. Legal Med. 116: 295–300. doi: 10.1007/s00414-002-0332-9 [DOI] [PubMed] [Google Scholar]
- 13.Dunne G., Gurfield N.2009. Local veterinary diagnostic laboratory, a model for the one health initiative. Vet. Clin. North Am. Small Anim. Pract. 39: 373–384. doi: 10.1016/j.cvsm.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 14.EL-Sayed, A. F.2019. Air craft damage. In: Bird Strike in Aviation: Statistics, Analysis and Management (EL-Sayed, A. F ed.), Wiley-Blackwell, Hoboken. [Google Scholar]
- 15.Frantz A. C., Heddergott M., Lang J., Schulze C., Ansorge H., Runge M., Braune S., Michler F. F., Wittstatt U., Hoffmann L., Hohmann U., Michler B. A., Van den Berge K., Horsburgh G. J.2013. Limited mitochondrial DNA diversity is indicative of a small number of founders of the German raccoon (Procyon lotor) population. Eur. J. Wildl. Res. 59: 665–674. doi: 10.1007/s10344-013-0719-6 [DOI] [Google Scholar]
- 16.Gullone E., Clarke J. P.2010. Animal abuse, cruelty, and welfare: an Australian perspective. p. 314. In: The International Handbook of Animal Abuse and Cruelty: Theory, Research, and Application (Ascione, F. R. ed.), Purdue University Press, West Lafayette. [Google Scholar]
- 17.Gupta S. K., Thangaraj K., Singh L.2006. A simple and inexpensive molecular method for sexing and identification of the forensic samples of elephant origin. J. Forensic Sci. 51: 805–807. doi: 10.1111/j.1556-4029.2006.00154.x [DOI] [PubMed] [Google Scholar]
- 18.Hartup B. K.1996. Rehabilitation of native reptiles and amphibians in DuPage County, Illinois. J. Wildl. Dis. 32: 109–112. doi: 10.7589/0090-3558-32.1.109 [DOI] [PubMed] [Google Scholar]
- 19.Hawk D.2011. Society for wildlife forensic science. p. 18. In: Wildlife Forensics Methods and Applications (Huffman, J. E. and Wallace, J. R. ed.), Wiley-Blackwell, Hoboken. [Google Scholar]
- 20.Imazato H., Onuma M., Nagamine T., Nakaya Y.2012. Molecular species identification of predators of endangered species on Okinawa-Jima island. Mammal Study 37: 159–164. doi: 10.3106/041.037.0207 [DOI] [Google Scholar]
- 21.Ishibashi T., Okubo T., Nomura O., Yasuda T., Baba K., Suda O., Nakatsu S., Ohsio T., Sumita Y., Morita A.2007. The medical-examination chart total of Wild Birds. The 13th annual meeting of Japanese society of Zoo and wildlife medicine Announcement Abstract.
- 22.Japan Veterinary Medical Association Wildlife Countermeasure Review Committee.2016. Recognition of wildlife rehabilitation: Measures against wild animals entering a big conversion period: The 21st Annual Meeting of the Wildlife Medical Society Symposium organized by the Japan Wildlife Medical Society, Japanese journal of Zoo and Wildlife Medicine (in Japanese).
- 23.Jessup D. A.2004. The welfare of feral cats and wildlife. J. Am. Vet. Med. Assoc. 225: 1377–1383. doi: 10.2460/javma.2004.225.1377 [DOI] [PubMed] [Google Scholar]
- 24.Jobin R. M., Patterson D., Zhang Y.2008. DNA typing in populations of mule deer for forensic use in the Province of Alberta. Forensic Sci. Int. Genet. 2: 190–197. doi: 10.1016/j.fsigen.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 25.Maria A. C. B. E., Rego A. A. M. S., Maiorka P. C.2013. Necropsy findings in dogs that died during grooming or other pet service procedures. J. Forensic Sci. 58: 1189–1192. doi: 10.1111/1556-4029.12236 [DOI] [PubMed] [Google Scholar]
- 26.Molina-Lopez R. A., Valverdú N., Martin M., Mateu E., Obon E., Cerdà-Cuéllar M., Darwich L.2011. Wild raptors as carriers of antimicrobial-resistant Salmonella and Campylobacter strains. Vet. Rec. 168: 565. doi: 10.1136/vr.c7123 [DOI] [PubMed] [Google Scholar]
- 27.Munro H. M. C., Thrusfield M. V.2001. ‘Battered pets’: Munchausen syndrome by proxy (factitious illness by proxy). J. Small Anim. Pract. 42: 385–389. doi: 10.1111/j.1748-5827.2001.tb02486.x [DOI] [PubMed] [Google Scholar]
- 28.Munro R., Munro H.2011. Forensic veterinary medicine 2. postmortem investigation. In Pract. 33: 262–270. doi: 10.1136/inp.d3599 [DOI] [Google Scholar]
- 29.Naka H., Udagawa T., Omi T., Ochiai K., Yamamoto T., Takezawa Y., Hayama S.2015. Research on ecology elucidation by individual identification of Tsushima cat (Prionailurus bengalensis euptilurus) using fecal DNA analysis. The 158th meeting of the Japanese Society of Veterinary Science.
- 30.Newbery S., Munro R.2011. Forentic veterinary medicine 1. Investigation involving live animals. In Pract. 33: 220–227. doi: 10.1136/inp.d2876 [DOI] [Google Scholar]
- 31.Peppin L., McEwing R., Carvalho G. R., Ogden R.2008. A DNA-based approach for the forensic identification of Asiatic black bear (Ursus thibetanus) in a traditional Asian medicine. J. Forensic Sci. 53: 1358–1362. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Espona S., Pérez-Barbería F. J., McLeod J. E., Jiggins C. D., Gordon I. J., Pemberton J. M.2008. Landscape features affect gene flow of Scottish Highland red deer (Cervus elaphus). Mol. Ecol. 17: 981–996. doi: 10.1111/j.1365-294X.2007.03629.x [DOI] [PubMed] [Google Scholar]
- 33.Petras J. M., Lehman R. A. W.1966. Corticospinal fibers in the raccoon. Brain Res. 3: 195–197. doi: 10.1016/0006-8993(66)90077-1 [DOI] [PubMed] [Google Scholar]
- 34.Roscoe D. E., Stansley W.2011. Wildlife forensic pathology and toxicology in wound analysis and pesticide poisoning. pp. 109–127. In: Wildlife Forensics (Huffman, J. E. and Wallace, J. R. eds.), Wiley-Blackwell, Hoboken. [Google Scholar]
- 35.Scott D.2016. Handling and physical examination. p. 5. In: Raptor Medicine, Surgery and Rehabilitation, 2nd ed. CABI, Oxfordshire. [Google Scholar]
- 36.Wasser S. K., Joseph Clark W., Drori O., Stephen Kisamo E., Mailand C., Mutayoba B., Stephens M.2008. Combating the illegal trade in African elephant ivory with DNA forensics. Conserv. Biol. 22: 1065–1071. doi: 10.1111/j.1523-1739.2008.01012.x [DOI] [PubMed] [Google Scholar]
- 37.Wasser S. K., Clark B., Laurie C.2009. The ivory trail. Sci. Am. 301: 68–74, 76. doi: 10.1038/scientificamerican0709-68 [DOI] [PubMed] [Google Scholar]
- 38.Williams V. M., Dale A. R., Clarke N., Garrett N. K. G.2008. Animal abuse and family violence: survey on the recognition of animal abuse by veterinarians in New Zealand and their understanding of the correlation between animal abuse and human violence. N. Z. Vet. J. 56: 21–28. doi: 10.1080/00480169.2008.36800 [DOI] [PubMed] [Google Scholar]
- 39.Wirth F. P., O’Leary J. L., Smith J. M., Jenny A. B.1974. Monosynaptic corticospinal-motoneuron path in the raccoon. Brain Res. 77: 344–348. doi: 10.1016/0006-8993(74)90799-9 [DOI] [PubMed] [Google Scholar]
- 40.Zeleny M., Grusova K.2015. A car accident involving a restrained dog within the vehicle: a case report. Vet. Med. (Praha) 60: 399–402. doi: 10.17221/8389-VETMED [DOI] [Google Scholar]