Abstract
Foot-and-mouth disease (FMD) is one of the most highly contagious animal diseases. In an effort to overcome the drawbacks of the currently used inactivated foot-and-mouth disease virus vaccine, a novel recombinant protein carrying foot-and-mouth disease virus VP1 GH loop epitope linked to vesicular stomatitis virus glycoprotein was expressed in a baculovirus system. Its antigenicity was confirmed with ELISA using monoclonal antibody against foot-and-mouth disease virus. Twice immunizations one month apart in field pigs resulted in a significant antibody increase compared to the glutathione S-transferase carrier containing the same epitope and the commercial vaccine. To my knowledge, this is the first report that the recombinant protein vaccine was superior to the current vaccine. Although further studies are required to examine their immunogenicity in a large number of animals, this study sheds light on the development of a novel recombinant protein vaccine that could be easily produced in a general laboratory as an alternative to the current FMD vaccine, which requires a biosafety level 3 containment facility for vaccine production.
Keywords: epitope, foot-and-mouth vaccine, glycoprotein, vesicular stomatitis virus
Foot-and-mouth disease (FMD) is one of the most highly contagious animal diseases, and the FMD virus (FMDV) rapidly replicates and spreads from infected animals via contact with susceptible animals and as an aerosol [6]. FMDV is the prototype member of the Aphthovirus genus of the picornaviridae family. The virus exists in seven different serotypes: O, A, C, Asia 1, and South African Territories 1 (SAT 1), SAT 2, and SAT 3, but a large number of subtypes have evolved within each serotype [11]. Since 2000, South Korea has experienced eleven FMD epidemics (March 2000, May 2002, January 2010, April 2010, November 2010, July 2014, December 2014, January 2016, February 2017, March 2018, and January 2019). Due to the extensive economic damage (approximately 3 billion dollars) in the 2011–2011 FMD outbreak, the South Korean government implemented a vaccination policy throughout the country for all FMD susceptible livestock.
Disadvantages of the current chemically inactivated FMDV vaccine include the risk of viral release during vaccine production, and problems in serological distinction between infected and vaccinated animals [5]. Thus, much effort has been made to develop alternative and safe vaccines utilizing the GH loop of capsid protein VP1 [1, 2, 4, 10, 11, 13, 15]. However, the immunogenicity of these recombinant vaccines was substantially lower than those of the traditional inactivated FMDV vaccines [12]. Vesicular stomatitis virus (VSV) glycoprotein is known to strongly elicit neutralization activity similar to those induced by the live virus [9]. In order to address the low immunogenicity of the peptide vaccine, one research group inserted a B cell target antigenic site into a VSV glycoprotein with the first 193 amino acids (aa) out of 517 aa as a candidate vaccine, resulting in limited efficacy compared to the conventional inactivated FMDV vaccine [3]. Another study showed that recombinant glycoprotein whole cell lysate was a better diagnostic antigen than the glycoprotein soluble fraction when applied in ELISA [7]. Therefore, in this study we constructed a recombinant protein vaccine using a whole VSV glycoprotein with 517 aa residues as a carrier to include the FMDV type O VP1 GH loop epitope corresponding to 129–169 aa and examined antigenicity and immunogenicity of this recombinant protein vaccine.
The FMDV type O VP1 GH loop epitope 129–169 aa was synthesized by replacing the 158th aa in proline with cysteine from the nucleotide sequence isolated in Jincheon, South Korea in 2014 (Bioneer, Daejeon, Korea); the sequence is shown in Table 1. The FMDV sequence was derived from O/SKR/JC/2014 (GenBank KX162590). The VSV glycoprotein originated from recombinant BacPAK8 (Clontech, Mountain View, CA, USA) containing the VSV (New Jersey) glycoprotein [7]. The VSV glycoprotein was excised and cloned into pFastBac HT-B vector according to the bac-to-bac system (Invitrogen, Carlsbad, CA, USA) using BamHI and XhoI. The recombinant VSV glycoprotein with FMDV epitope was constructed by inserting the FMDV VP1 GH loop epitope sequence between codons 160 and 161 of the VSV whole glycoprotein (517 aa). For recombinant VSV glycoprotein with FMDV epitope (VSV GP-VP1), 5 × 105 Sf9 cells were plated out per well in a 6-well plate. Twelve hours later, the cells were washed twice with serum and antibiotic free Grace media. The recombinant bacmid DNA was transfected using Cellfectin II (Invitrogen) according to the manufacturer’s instructions. The primary virus was harvested after 4 days and used for amplification; the virus titer was calculated by plaque assay. The optimal condition for expression of VSV GP-VP1 was determined by multiplicity of infection (MOI) tests. The Sf9 cells 2 × 104/well were cultured in a monolayer 24-well culture dish inoculated with baculovirus at a range of 0.05–10 MOI, culture supernatants were harvested every 24 hr for 6 days, and protein expression level was analyzed by SDS-PAGE and western blotting. The VSV GP-VP1 fused to a histidine affinity tag at its N-terminus were purified using nickel-nitrilotriacetic acid resin (Life Technologies, Rockford, AZ, USA) according to the manufacturer’s instructions. Briefly, Sf9 cells at a density of 1.5 × 106/ml in shaker flasks were infected with VSV GP-VP1 at 1.0 MOI and incubated with shaking for 96 hr at 27°C. The infected cells were harvested by centrifugation at 500×g for 5 min at 4°C. The pellet was resuspended in lysis buffer (20 mM Na2HPO4, 500 mM NaCl, pH 7.4, and 1 mM PMSF). After incubation for 15 min at 4°C, the samples were sonicated on ice and centrifuged at 10,000 g for 20 min. The insoluble fraction was resuspended in 50 ml of binding buffer (20 mM Tris, 100 mM NaH2PO4, pH 8.0, 8 M urea, and 20 mM imidazole) at 4°C. After centrifugation at 10,000 g for 20 min, the supernatants were filtered through a 0.45 µm membrane. The samples were loaded into a nickel-nitrilotriacetic acid column, washed with binding buffer (20 mM Tris, 100 mM NaH2PO4, pH 8.0, 8 M urea, and 20 mM imidazole). The final eluted samples in elution buffer (20 mM Tris, 100 mM NaH2PO4, pH 8.0, and 500 mM imidazole) were analyzed by 10% SDS-PAGE. Protein concentrations were determined according to the BCA method (Thermo Fisher Scientific, Rockford, AZ, USA).
Table 1. Amino acid sequences of the foot-and-mouth disease virus (FMDV) type O VP1 GH loop epitope insertion site within the vesicular stomatitis virus glycoprotein and glutathione S-transferase.
| Group | Amino acid sequence (N to C) |
|---|---|
| VSV GP-VP1a) | GP-WIVYNGNCKYTGGSLPNVRGDLQVLAPKAARCLPTSFNYGAIKDP-162 |
| GST-VP1b) | GST-VYNGNCKYTGGSLPNVRGDLQVLAPKAARCLPTSFNYGAIK |
Underlined sequences indicate VP1 129 to 169 amino acid residues from FMDV (GenBank: KX162590) isolated in 2014, South Korea, with displacement of proline to cysteine at residue 158 to enhance neutralization activity [13]. a) Recombinant vesicular stomatitis virus (VSV) glycoprotein fused to FMDV VP1 epitope (129–169). b) Recombinant glutathione S-transferase (GST) fused to FMDV VP1 epitope (129–169).
As a control antigen, the FMDV type O VP1 GH loop epitope 129–169 aa was cloned into pGEX 4T-1 (GE Healthcare, Piscataway, NJ, USA) using BamHI and XhoI. For recombinant glutathione S-transferase (GST)-VP1, E. coli strain BL21 was transformed with pGEX 4T-1 containing the FMDV type O VP1 epitope and grown overnight at 37°C with shaking. Cultures were diluted at a 1:10 ratio with fresh Luria Broth containing ampicillin and incubated for 3 hr at 37°C with shaking. Protein expression was induced by the addition of Isopropyl β-D-1-thiogalactopyranoside to a final concentration of 0.5 mM, and the cultures were incubated for 4 hr. Pellets were harvested from the culture by centrifugation at 3,000 × g for 10 min, resuspended in phosphate-buffered saline (PBS; 50 mM, pH 7.4) and disrupted by sonication at a 20% amplitude. The lysate was centrifuged at 3,000×g for 10 min, and the soluble protein fractions were collected and purified by affinity column chromatography using Glutathione Sepharose 4B (GE Healthcare) according to the manufacturer’s instructions. The GST control was also expressed by the above method using only pGEX 4T-1.
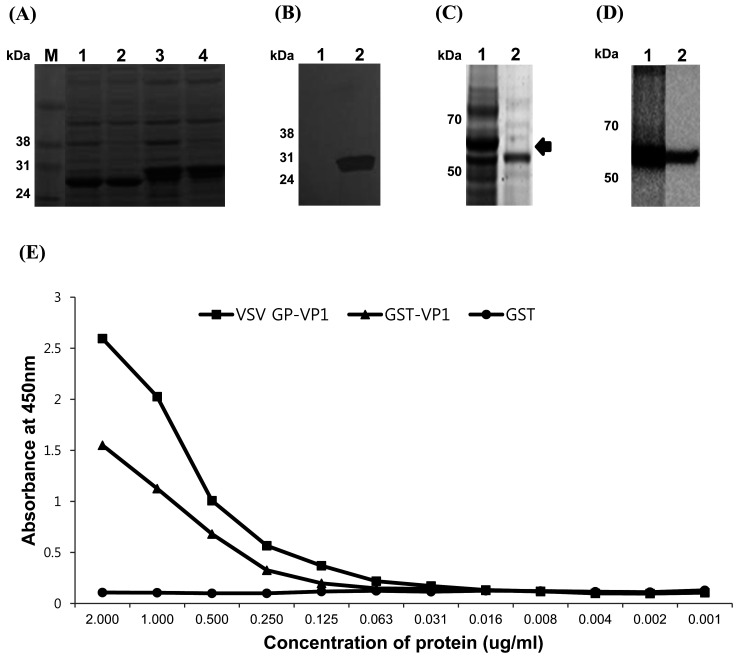
The resolved samples by SDS-PAGE were electro-transferred to a polyvinylidene difluoride membrane using an iBlotTM gel-transfer device (Invitrogen). The membranes were blocked with 2% (w/v) skim milk in Tris-buffered saline containing 0.1% of Tween 20 and incubated with GST antibody (GE Healthcare), monoclonal antibody (76.5E) against FMDV epitope, or antibody (NVSL, Ames, IA, USA) against VSV glycoprotein. This was followed by incubation with a goat anti-mouse horseradish peroxidase conjugated secondary antibody (Invitrogen) diluted to 1:5,000. Proteins were visualized with Pierce ECL Western Blotting Substrate (Invitrogen) using an Azure C600 device (Azure Biosystems, Dublin, CA, USA). SDS-PAGE showed that GST-VP1 was approximately 31 kDa compared to the GST control, which was 27 kDa and they were mainly expressed in the soluble fractions (Fig. 1A). In addition, FMDV type O VP1 epitope expression was confirmed by western blot using monoclonal antibody against FMDV type O VP1 neutralizing epitope, and there was no reactivity against the GST control (Fig. 1B).
Fig. 1.
Expression and antigenicity of recombinant vaccine candidates carrying the foot-and-mouth disease virus (FMDV) type O VP1 GH loop epitope. (A) SDS-PAGE analysis: Lane 1, total lysate of glutathione S-transferase; lane 2, soluble fraction of glutathione S-transferase (GST); lane 3, total lysate of GST-VP1; lane 4, soluble fraction of GST-VP1. (B) Western blot analysis with monoclonal antibody against type O FMDV: Lane 1, purified GST; lane 2, purified GST-VP1. (C) SDS-PAGE analysis: Lane 1, total lysate of recombinant glycoprotein fused to FMDV epitope; lane 2, soluble fraction of recombinant fused to FMDV epitope. The arrow indicates the band corresponding to the vesicular stomatitis virus glycoprotein-VP1. (D) Western blot analysis: Lane 1, total lysate of the vesicular stomatitis virus (VSV) GP-VP1 with monoclonal antibody against type O FMDV; lane 2, total lysate of the VSV GP-VP1 with monoclonal antibody against VSV glycoprotein. (E) Antigenicity of the VSV GP-VP1, GST-VP1, and GST control.
The VSV GP-VP1 was expressed in the insoluble fraction from insect cells with a molecular weight of around 64 kDa, consisting of VSV glycoprotein 517 aa, FMDV type O VP1 41 aa, and His tag 26 aa. Any protein band corresponding to the molecular weight (64 kDa) of VSV GP-VP1 in the soluble fraction was not observed by SDS-PAGE as shown in Fig. 1C. Western blot analysis showed that the VSV GP-VP1 reacted with monoclonal antibodies against FMDV capsid and VSV glycoprotein (Fig. 1D).
The antigenicity of the recombinant proteins was measured by ELISA using neutralizing monoclonal antibody against FMDV. Maxisorp ELISA 96-well plates (Nunc, Roskilde, Denmark) were coated with serially two-fold diluted recombinant proteins starting from 2 µg/ml in 0.05 M carbonate buffer (pH 9.6) overnight at 4°C. Plates were washed three times with PBS containing 0.05% Tween 20 (PBST), then incubated with 100 µl of monoclonal antibody (76.5E) against FMDV VP1 conjugated to horseradish peroxidase (0.5 µg/ml) diluted in diluent (PBST containing 5% skimmed milk) at room temperature for 1 hr. After washing the plates, 100 µl of 3,3′,5,5′-tetramethylbenzidine substrate was added. The color was developed for 15 min at room temperature, and the reaction was stopped by adding 50 µl of 0.5 M sulfuric acid. The optical densities of the samples were measured at 450 nm. All the recombinant proteins containing the FMDV epitope showed distinct reactivity in comparison to the GST control, which did not react with the antibody (Fig. 1E).
Animal experiments in this study were approved (APQA 2016-337) and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For immunogenicity test of vaccine candidate, growing pigs raised on a farm in Gyeongbuk province were examined in advance, and those negative by PrioCHECK FMDV Type O ELISA (Prionics, Lelystad, Netherlands) were selected for vaccination experiment. Briefly, a microtiter plate coated with non-infectious FMDV type O antigen was incubated with serum samples at room temperature. After washing, monoclonal antibody to FMDV type O, conjugated to horseradish peroxidase, was added to the plate and incubated at room temperature. The plate was developed with chromogen (TMB) substrate and stopped with the optical density being measured at 450 nm. The ELISA results were expressed as percentage inhibition (PI) values. If the PI value of the sample was more than 50%, the result was considered positive. If the PI was below 50%, the result was considered negative. Six pigs were vaccinated with the VSV GP-VP1 and six pigs with GST-VP1. One of the GST-VP1 vaccinated pigs died suddenly during the experiment. The recombinant protein immunogens (100 µg) were mixed with the ISA 206 adjuvant (Seppic, Paris, France) in a 1:1 ratio as recommended by the manufacturer’s instructions. A commercial vaccine, an oil-adjuvanted inactivated FMDV type O vaccine containing O1 Manisa and O 3039 strains (GCVP FMD Vaccine, South Korea) that had been imported from Merial Animal Health Ltd. and filled in the bottle at Green Cross Ltd. in South Korea was also immunized into four pigs with one dose (2 ml) according to the manufacturer’s instruction. Pigs were intramuscularly immunized twice with the recombinant proteins and commercial vaccine, four weeks apart. The blood samples were collected pre-immunization and 4 and 8 weeks after first immunization, allowed to clot at room temperature, and centrifuged (2,000×g, 10 min, 4°C). Sera samples were stored at −20°C until the serological test was performed. The sera collected from all the pigs were tested by FMDV type O ELISA for the detection of antibodies (IgM and IgG) against the FMDV type O capsid epitope. Before immunization, all pigs showed a PI level of approximately 20, regardless of experimental group. Out of them, only the VSV GP-VP1 group exhibited significant differences (P<0.05) after immunizations in comparison to the pre-immunization (Fig. 2). The GST-VP1 group and the commercial vaccine group did not show a significant difference from the pre-immunization.
Fig. 2.
Antibody responses elicited by various immunogens in pigs. Six pigs were vaccinated with the vesicular stomatitis virus glycoprotein-VP1, five pigs with glutathione S-transferase-VP1, and four pigs with commercial inactivated foot-and-mouth disease virus (FMDV) vaccine. Pigs were immunized twice intramuscularly, four weeks apart. The antibody responses were measured by FMDV type O ELISA, and the results were presented as average percentage inhibition (PI) (%). The statistical significance of differences between the booster immunization and pre-immunization stage was determined at a 95% confidence level, and the asterisks represent a statistically significant difference (P<0.05).
In this study, a novel recombinant protein carrying FMDV type O VP1 GH loop epitope linked to vesicular stomatitis virus glycoprotein was constructed and evaluated for its immunogenicity as an innovative vaccine candidate. Although a previous report introduced VSV glycoprotein as vaccine candidate, it resulted in limited efficacy compared to the conventional inactivated FMDV vaccine [3]. It was postulated that the low effect could be attributed to the fact that the partial outer fraction of the glycoprotein was used as the immunogen carrier instead of the complete VSV glycoprotein, and that the peptide size was not long enough to induce sufficient immunogenicity in cattle. Based on the previous results that extension of the FMDV epitope size from 140–160 aa to 129–169 aa increased the neutralizing index [14], and that the whole protein of the VSV glycoprotein was more antigenically effective than the soluble form [7], we inserted the FMDV type O VP1 129–169 codon into the VSV whole glycoprotein. The FMDV type O VP1 GH loop epitope was incorporated between the amino acids at positions 160 and 161 of the VSV glycoprotein [3]. The GST was employed as a control carrier because GST is the most frequently used fusion partner protein in recombinant protein expression systems. Since the massive FMD outbreaks in December 2010 in South Korea, the nationwide local veterinary services have carried out a serological test for the detection of the antibody elicited by vaccination using an FMDV type O ELISA kit every year. Therefore, in this study, the immunogenicity of the candidate vaccine was examined by FMDV type O ELISA to reflect the field situation. In addition, serological surveillance conducted every year in South Korea showed that pigs exhibited less immunogenicity in comparison to cattle with the same commercial FMD vaccine [8]. Therefore, a successful novel vaccine candidate should provide evidence of potential efficacy in local field pigs.
The VSV GP-VP1 groups showed higher immunogenicity than the GST-VP1 group in field pigs, considering that the FMDV type O VP1 epitope linked to VSV glycoproteins showed a significant increase compared to the pre-immunization stage. This might reflect the strong immunogenicity of the VSV glycoprotein as previously reported [9].
Theoretically, nationwide immunization of FMD susceptible livestock could result in a large difference in antibody prevalence among pig farms. Also, since this experiment was conducted at only one pig farm as a preliminary test, the low antibody prevalence induced by the recombinant protein and the commercial vaccine might be attributed to the characteristics of the experimental farm. In future studies, it will be necessary to experiment with a large number of pigs from a large number of farms. The relatively low immunogenicity of the recombinant proteins might also be due to the low immunogen concentration. While mouse or specific pathogen-free pigs were previously immunized with relatively low concentrations (20 or 100 µg) of immunogen [10, 14], local field pigs were immunized with a high concentration (500 µg) of immunogen [2]. Further study using various immunogen concentrations may be required.
In conclusion, a novel vaccine platform was constructed to carry FMDV type O VP1 GH loop epitope linked to a whole VSV glycoprotein as a novel vaccine candidate. To my knowledge, this is the first case to report that the recombinant protein vaccine was superior to the current inactivated FMDV vaccine. The findings of this study provide groundwork for the development of a novel recombinant protein vaccine that could be easily produced in a general laboratory as an alternative to the current FMD vaccine, which requires a biosafety level 3 containment facility for vaccine production.
Acknowledgments
This research was supported by grant no. N-1543386-2018-19–01 from the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
REFERENCES
- 1.Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F.1982. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature 298: 30–33. doi: 10.1038/298030a0 [DOI] [PubMed] [Google Scholar]
- 2.Cao Y., Li D., Fu Y., Bai Q., Chen Y., Bai X., Jing Z., Sun P., Bao H., Li P., Zhang J., Ma X., Lu Z., Liu Z.2017. Rational design and efficacy of a multi-epitope recombinant protein vaccine against foot-and-mouth disease virus serotype A in pigs. Antiviral Res. 140: 133–141. doi: 10.1016/j.antiviral.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 3.Capozzo A. V., Wilda M., Bucafusco D., de los Ángeles Lavoria M., Franco-Mahecha O. L., Mansilla F. C., Pérez-Filgueira D. M., Grigera P. R.2011. Vesicular stomatitis virus glycoprotein G carrying a tandem dimer of foot and mouth disease virus antigenic site A can be used as DNA and peptide vaccine for cattle. Antiviral Res. 92: 219–227. doi: 10.1016/j.antiviral.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 4.Cubillos C., de la Torre B. G., Jakab A., Clementi G., Borrás E., Bárcena J., Andreu D., Sobrino F., Blanco E.2008. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J. Virol. 82: 7223–7230. doi: 10.1128/JVI.00401-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doel T. R.2003. FMD vaccines. Virus Res. 91: 81–99. doi: 10.1016/S0168-1702(02)00261-7 [DOI] [PubMed] [Google Scholar]
- 6.Grubman M. J., Baxt B.2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17: 465–493. doi: 10.1128/CMR.17.2.465-493.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heo E. J., Lee H. S., Jeoung H. Y., Ko H. R., Kweon C. H., Ko Y. J.2010. Development of a blocking ELISA using a recombinant glycoprotein for the detection of antibodies to vesicular stomatitis New Jersey virus. J. Virol. Methods 164: 96–100. doi: 10.1016/j.jviromet.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 8.Park J. H.2013. Requirements for improved vaccines against foot-and-mouth disease epidemics. Clin. Exp. Vaccine Res. 2: 8–18. doi: 10.7774/cevr.2013.2.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley J. M., Emerson S. U., Wagner R. R.1972. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J. Virol. 10: 1231–1235. doi: 10.1128/JVI.10.6.1231-1235.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H. B., Piao D. C., Lee J. Y., Choi J. Y., Bok J. D., Cho C. S., Kang S. K., Choi Y. J.2017. Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate. Microb. Cell Fact. 16: 33. doi: 10.1186/s12934-017-0648-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason P. W., Grubman M. J., Baxt B.2003. Molecular basis of pathogenesis of FMDV. Virus Res. 91: 9–32. doi: 10.1016/S0168-1702(02)00257-5 [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez L. L., Barrera J., Kramer E., Lubroth J., Brown F., Golde W. T.2003. A synthetic peptide containing the consensus sequence of the G-H loop region of foot-and-mouth disease virus type-O VP1 and a promiscuous T-helper epitope induces peptide-specific antibodies but fails to protect cattle against viral challenge. Vaccine 21: 3751–3756. doi: 10.1016/S0264-410X(03)00364-5 [DOI] [PubMed] [Google Scholar]
- 13.Shao J. J., Wong C. K., Lin T., Lee S. K., Cong G. Z., Sin F. W. Y., Du J. Z., Gao S. D., Liu X. T., Cai X. P., Xie Y., Chang H. Y., Liu J. X.2011. Promising multiple-epitope recombinant vaccine against foot-and-mouth disease virus type O in swine. Clin. Vaccine Immunol. 18: 143–149. doi: 10.1128/CVI.00236-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C. Y., Chang T. Y., Walfield A. M., Ye J., Shen M., Chen S. P., Li M. C., Lin Y. L., Jong M. H., Yang P. C., Chyr N., Kramer E., Brown F.2002. Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine 20: 2603–2610. doi: 10.1016/S0264-410X(02)00148-2 [DOI] [PubMed] [Google Scholar]
- 15.Wang J. H., Liang C. M., Peng J. M., Shieh J. J., Jong M. H., Lin Y. L., Sieber M., Liang S. M.2003. Induction of immunity in swine by purified recombinant VP1 of foot-and-mouth disease virus. Vaccine 21: 3721–3729. doi: 10.1016/S0264-410X(03)00363-3 [DOI] [PubMed] [Google Scholar]