Abstract
The aim of this study was to determine whether serum symmetric dimethylarginine (SDMA) and cystatin C (CysC) levels can be utilized as more accurate markers of early kidney dysfunction in dogs. Forty-one client-owned dogs with chronic kidney disease (CKD), which were clinically stable, and ten beagles as healthy controls were included. All dogs underwent physical examination, systemic blood pressure measurement, complete blood cell count, and plasma biochemistry analyses. Frozen serum was used for SDMA and CysC analyses. Data analysis was performed using Kruskal Wallis, Pearson’s correlation, Bland-Altman plots, and receiver operating characteristic curve. SDMA and CysC levels were significantly higher in patients with CKD at various International Renal Interest Society (IRIS) stages than in the healthy controls. In particular, CysC level was the only biomarker that could indicate the earliest stage of CKD (IRIS stage I). Similar to these results, CysC level showed better sensitivity and specificity compared to the other biomarkers in early CKD dogs.
Keywords: canine, creatinine, cystatin C, glomerular filtration rate, symmetric dimethylarginine
Chronic kidney disease (CKD) is defined as the progressive loss of kidney function over times. In order to determine kidney function, Glomerular filtration rate (GFR) has long been considered the golden standard [11]. However, a direct measurement of GFR is a time-consuming and labor-intensive process. Therefore, it is not routinely used in clinical practice [5]. As an indirect marker, such as plasma creatinine (CREA), serum symmetric dimethylarginine (SDMA), and Cystatin C (CysC) levels are measured to substitutions of kidney function.
CREA is chosen by the International Renal Interest Society (IRIS) to evaluate and monitor kidney function [15]. CREA levels can be affected by age, sex, ethnicity, dietary protein intake, and lean body mass. Consequently, the sensitivity of serum CREA level for the early stage of IRIS is limited. Although CREA metabolism and measurement and its limitations have been reviewed in human medicine, similar reviews are lacking in veterinary medicine [4] Therefore, in this research CREA and the other indirect kidney factors are studied its correlations.
SDMA is mainly filtered out from the kidneys so considered an accurate and sensitive biomarker for estimating GFR in humans with coronary diseases and assessing renal dysfunction in humans [12]. SDMA level is not influenced by muscle mass, but some non-renal changes can affect the accuracy of its measurement [14]. SDMA serum concentration can be useful to detect CKD in cat on average 17 months and dogs on average 9.8 months before CREA increase above the reference rage [7, 8]. Whereas muscle mass, diet, inflammation, diabetes, and estrogen therapy have no significant impacts on SDMA concentrations, obesity, sex, and age have effects on SDMA levels [9, 12].
CysC is a cysteine protease inhibitor that is filtered by the glomerulus and unaffected by non-renal factors such as inflammation, age, and sex [11]. With greater sensitivity, serum CysC level has been replaced CREA as a marker of GFR in humans [11]. In veterinary medicine, measuring CysC concentration is also useful as an endogenous marker of indirect GFR than plasma CREA concentration in dogs [1, 13, 18]. Dogs with CKD show significantly higher CysC concentrations than healthy dogs [1, 13, 18] and dogs with various non-renal diseases (e.g., immune-mediated, endocrine, dermatologic, cardiologic, or neoplastic diseases [2, 3, 10, 18].
The aim of this study was to evaluate the CysC concentrations in various staged CKD dogs and compare its efficacy as using a biomarker of kidney dysfunction to those of CREA and SDMA in the same group. CysC measurement has not been widely used as much as CREA in small animal veterinary practices; however, with growing interest its application, this study would be meaningful for the first step.
MATERIALS AND METHODS
Animals
Ten healthy beagles were included as controls. They were cared for and used in accordance with the guidelines of the Institutional Animal Care and Use Committee at Chonnam National University. A total of 41 client-owned dogs were included for the study. They presented to the hospital from 2017 to 2018 for medical check-ups, new diagnoses, or treatment of current disorders. Only dogs with CKDs which were clinically stable were included in the study. Pre- and post-renal conditions were ruled out. In particular, the IRIS stage I patient group was classified as non-azotemic after dehydration correction, reduction of urine specific gravity, SDMA, and CREA concentrations of sequentially collected sample were classified by the IRIS staging system. Additionally, structural abnormalities (degenerative changes, renal calculi, etc.) were also included. Unfortunately, IRIS stage IV patients died during inpatient treatment. All ten healthy beagles were three-year-old males. 15 dogs were <5 years of age, 10 dogs were 5–10 years of age, and 24 dogs were >10 years of age (Table 1). Of the total 49 dogs, 10 were beagles; 10 were Shih Tzu; 7 were Maltese; 6 were Yorkshire terriers; 3 were Miniature poodles; 2 were of each of following breeds: American cocker spaniel, dachshund, English cocker spaniel, Pomeranian, Miniature schnauzer, and mixed; 1 was a Boston terrier. The characteristics of these 49 dogs are presented in Table 1. For the accurate evaluation of SDMA, all subject body condition scores are presented in Table 2.
Table 1. The signalments for 49 dogs in the study.
| Total (n=49) | |||
|---|---|---|---|
| n | % | ||
| Gender | |||
| Intact male | 11 | 22.4 | |
| Castrated male | 13 | 26.5 | |
| Intact female | 5 | 10.2 | |
| Spayed female | 20 | 40.8 | |
| Age | |||
| <5 years | 15 | 30.6 | |
| 5–10 years | 10 | 20.4 | |
| >10 years | 24 | 49.0 | |
| Breeds | |||
| American Cocker Spaniel (10.0 kg a)) | 2 | 4.1 | |
| Beagle (11.24 kg a)) | 10 | 20.4 | |
| Boston Terrier (10.2 kg b)) | 1 | 2.0 | |
| Dachshund (8.3 kg a)) | 2 | 4.1 | |
| English Cocker Spaniel (9.85 kg a)) | 2 | 4.1 | |
| Maltese (3.73 kg a)) | 7 | 14.3 | |
| Mixed (6.4 kg a)) | 2 | 4.1 | |
| Pomeranian (3.9 kg a)) | 2 | 4.1 | |
| Miniature Poodle (3.38 kg a)) | 3 | 6.1 | |
| Miniature Schnauzer (7.65 kg a)) | 2 | 4.1 | |
| Shih Tzu (5.482 kg a)) | 10 | 20.4 | |
| Yorkshire Terrier (2.86 kg a)) | 6 | 12.2 | |
a) Mean body weight; b) body weight.
Table 2. Physical data in the study.
| Variable (unit) | Healthy control (n=10) |
International Renal Interest Society stage | |||
|---|---|---|---|---|---|
| I (n=18) |
II (n=7) |
III (n=13) |
IV (n=1) |
||
| BW (kg) | 11.2 ± 0.93 | 5.20 ± 2.50a) | 6.61 ± 1.86a) | 4.90 ± 3.44a) | 6.20 ± 0.00 |
| BP (mmHg) | 132 ± 8.83 | 150 ± 21.6a) | 135 ± 18.8 | 153 ± 41.7 | 170 ± 0.00 |
| HR (beats per min) | 123 ± 12.7 | 144 ± 15.8a) | 121 ± 30.6 | 147 ± 21.9a) | 148 ± 0.00 |
| BT (°C) | 38.8 ± 0.45 | 38.4 ± 0.41a) | 38.5 ± 0.94 | 37.9 ± 0.85a) | 37.1 ± 0.00 |
| BCS (1–9) | 3.00 ± 0.00 | 4.70 ± 1.32a) | 5.29 ± 1.25a) | 4.54 ± 1.85a) | 6.00 ± 0.00 |
a) P<0.05 healthy controls vs. IRIS groups by t-test. BW, body weight; BP, blood pressure; HR, heart rate; HR, heart rate; BT, body temperature; BCS, body condition score.
Categorization of concurrent diseases
The 39 dogs were categorized according to the affected organs for the convenience of statistical analysis (Table 3). All malignant tumors were classified as cancer, regardless of origin.
Table 3. Concurrent diseases in the dogs with chronic kidney disease (CKD).
| Diagnosis | n | % | |
|---|---|---|---|
| Neoplasia | HAS | 2 | 4.9 |
| LSA | 2 | 4.9 | |
| Melanoma | 1 | 2.4 | |
| MGT | 1 | 2.4 | |
| HCC | 1 | 2.4 | |
| SCC | 1 | 2.4 | |
| Endocrine disorder | HAC | 12 | 29.3 |
| Iatrogenic HAC | 2 | 4.9 | |
| Hypothyroidism | 2 | 4.9 | |
| Urinary disease | Cystitis | 5 | 12.2 |
| Bladder calculus | 7 | 17.1 | |
| Urethral calculus | 1 | 2.4 | |
| Kidney stone | 2 | 4.9 | |
| CKD | 41 | 100 | |
| AKI | 3 | 7.3 | |
| Cardiac disease | CHF | 14 | 34.1 |
| Gastrointestinal disease | Pancreatitis | 10 | 24.4 |
| Megaesophagus | 1 | 2.4 | |
| Hematological disease | IMHA | 1 | 2.4 |
| Neural disease | MUE | 1 | 2.4 |
| Respiratory disease | Bronchitis | 1 | 2.4 |
| Dermatological disease | AD | 2 | 4.9 |
| Otitis externa | 2 | 4.9 | |
| Ophthalmological disease | Uveitis | 1 | 2.4 |
HAS, hemangiosarcoma; LSA, lymphoma; MGT, mammary gland tumor; HCC, hepatocellular carcinoma; SCC, squamous cell carcinoma; HAC, hyperadrenocorticism; AKI, acute kidney injury; CHF, chronic heart failure; IMHA, immune mediated hemolytic anemia; MUE, meningitis of unknown etiology; AD, atopic dermatitis.
Blood analysis (complete blood cell count and serum biochemistry)
All dogs underwent physical examination, a complete blood count (CBC), and plasma biochemical profiles were evaluated. Venous blood was collected in a heparinized syringe for immediate blood gas analysis and the remaining blood samples were stored in an EDTA or lithium-heparin tube. EDTA whole blood was evaluatedand biochemical profiles were measured. The CBC and biochemical profiles of the healthy control dogs were within the normal reference range.
Analysis of CREA, SDMA, and CysC concentrations
To determine CREA levels, 4 ml of blood was collected from the jugular vein into a lithium-heparin tube. Plasma was immediately separated by centrifugation at 4,000 × g for 5 min at room temperature and CREA concentrations were measured with Procyte Dx Hematology Analyzer (IDEXX Laboratories, Inc., Westbrook, ME, USA). To measure SDMA and CysC levels, whole blood was taken from either the jugular vein directly into sterile vacutainer tubes (Franklin Lakes, Bergen, NJ, USA). The collected tubes were placed in the upright position in the rack and the blood samples were allowed to be clotted at room temperature for 20 min. By following centrifugation in 1,500 × g for 10 min at 4°C, the supernatants were stored at −80°C or dry ice until the tests. SDMA concentrations were measured using the Chemistry analyzer AU480 method (Beckman Coulter, Inc., Brea, CA, USA) and CysC was recalibrated to the standardized. CysC measurement was used by the Roche enzymatic method (Hoffmann-La Roche, Ltd., Basel, Switzerland). This assay method was the Particle-Enhanced Turbidimetric Immune-Assay (PETIA) [6].
Statistical analysis
All values are expressed as the mean and standard deviation (SD). Statistical analyses were conducted twice with three different software programs: SPSS for Windows 12.0K (SPSS Inc., Chicago, IL, USA), GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA), and MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium). A probability value less than 0.05 (P<0.05) was considered statistically significant. The data represent the results of Kruskal Wallis and Dunn’s multiple comparisons tests. The relationships between the kidney markers (CREA, SDMA, and CysC levels) were measured using Pearson’s coefficient of bivariate correlation and Bland-Altman plots. The optimal cut-off points for the biomarkers were determined using receiver operating characteristic (ROC) curves, selecting for the highest sensitivity or percentage of correct classification at a specificity >90%. Sensitivity, specificity, likelihood ratios, and area under the curves (AUC) were also calculated.
RESULTS
Patient population
Of the dogs with CKD, 46.2% were IRIS stage I, 17.9% were IRIS stage II, 33.3% were IRIS stage III, and 2.6% were IRIS stage IV (Table 4). The control dogs had no evidence of CKD.
Table 4. Population of the study and International Renal Interest Society (IRIS) stages in chronic kidney disease (CKD).
| Group | n | % | |
|---|---|---|---|
| Healthy control | 10 | 20.4 | |
| CKD | 39 | 79.6 | |
| IRIS stage | I | 18 | 46.2 |
| II | 7 | 17.9 | |
| III | 13 | 33.3 | |
| IV | 1 | 2.6 | |
Characteristics of animal participants
Body weight, blood pressure, heart rate, body temperature, and body condition score (BCS) of the healthy and IRIS stage dogs are presented in Table 2. Compared to that in the healthy controls, blood pressure was higher in IRIS stage I, III, IV dogs. BCS was significantly higher in all IRIS stage dogs than in the healthy controls. Body weight and heart rate had no relation.
CBC and serum-chemistry findings in healthy and IRIS stage dogs
The CBC values of the healthy controls were within the normal reference range in Table 5. Hematocrit was significantly lower in IRIS stage III compared to that in the healthy controls and IRIS stage I. Platelet was increased in IRIS stage I and IRIS stage II compared to that in the healthy controls. No change was observed in WBC in either group of dogs studied.
Table 5. The complete cell count values in the study.
| Variable (unit) | Healthy control (n=10) |
International Renal Interest Society stage | |||
|---|---|---|---|---|---|
| I (n=18) |
II (n=7) |
III (n=13) |
IV (n=1) |
||
| HCT (%) | 44.8 ± 4.77 | 41.9 ± 7.40 | 38.3 ± 8.78 | 34.9 ± 10.8a,b) | 32.0 ± 0.00 |
| WBC (K/µl) | 11.3 ± 1.78 | 10.9 ± 4.58 | 9.6 ± 4.24 | 12.9 ± 8.43 | 20.4 ± 0.00 |
| PLT (K/µl) | 310 ± 59.7 | 472 ± 181 | 424 ± 114a) | 351 ± 212 | 806 ± 0.00 |
a) P<0.05 healthy controls vs IRIS groups by t-test. b) P<0.05 IRIS stage I vs IRIS stages II, III, and IV by t-test. HCT, hematocrit; WBC, white blood cell; PLT, platelet.
The serum biochemistry analysis and urinalysis results are shown in Table 6. The BUN, CREA, calcium, and phosphorus values of the healthy controls were normal. BUN was significantly increased in IRIS stage III compared to that in the healthy controls and IRIS stage I. CREA was also significantly increased in IRIS stage II and III compared to that in the healthy controls and IRIS stage I. As CREA is used in IRIS classification, its increase is stage-dependent. No change was observed in calcium in either group of dogs studied. On the other hand, phosphorus was significantly increased in IRIS stage III compared with that of the IRIS stage I. USG was significantly decreased in IRIS stage III and IV compared to that in the healthy control and IRIS stage I and II. No statistical significance was observed in urinalysis.
Table 6. The serum biochemistry analysis and urinalysis in the study.
| Variable (unit) | Healthy control (n=10) |
International Renal Interest Society stage | |||
|---|---|---|---|---|---|
| I (n=18) |
II (n=7) |
III (n=13) |
IV (n=1) |
||
| BUN (mg/dl) | 16.9 ± 2.08 | 17.1 ± 11.3 | 19.0 ± 8.11 | 62.2 ± 40.2a,b) | 111 ± 0.00 |
| CREA (mg/dl) | 0.82 ± 0.12 | 0.74 ± 0.28 | 1.53 ± 0.18a,b) | 2.43 ± 8.02 | 4.50 ± 0.00 |
| Ca (mg/dl) | 9.48 ± 0.18 | 9.04 ± 1.04 | 9.09 ± 0.87 | 9.62 ± 1.32 | 9.80 ± 0.00 |
| PHOS (mg/dl) | 4.39 ± 1.27 | 4.09 ± 0.77a) | 4.16 ± 1.03 | 5.58 ± 2.86 | 16.1 ± 0.00 |
| USG | 1.031 ± 0.011 | 1.022 ± 0.013 | 1.020 ± 1.012 | 1.011 ± 0.004 | 1.000 ± 0.000 |
a) P<0.05 healthy controls vs. IRIS groups by t-test. b) P<0.05 IRIS stage I vs. IRIS stages II, III, and IV by t-test.BUN, blood urea nitrogen; CREA, creatinine; Ca, total calcium; PHOS, phosphorus; USG, urine specific gravity.
Levels of CREA, SDMA, and CysC in dogs with CKD
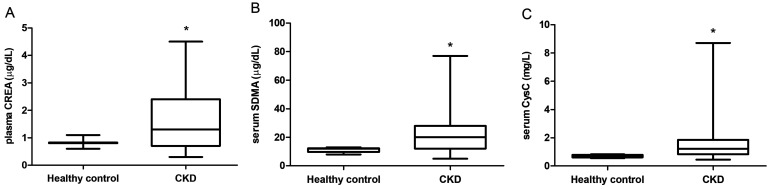
CREA and SDMA and CysC levels were significantly elevated in CKD patients than in healthy controls (Fig. 1). For CREA, the mean concentration was 0.82 ± 0.38 µg/dl in healthy controls and 1.52 ± 0.16 µg/dl in CKD patients (Fig. 1A). For SDMA, the mean concentration was 11.3 ± 0.53 µg/dl in healthy controls and 21.3 ± 2.02 µg/dl in CKD patients (Fig. 1B). The mean concentration of CysC was 0.69 ± 0.02 mg/l in healthy controls and 1.66 ± 0.24 mg/l in CKD patients (Fig. 1C).
Fig. 1.
Boxplot of biomarker levels in healthy dogs (n=10) and dogs with chronic kidney disease (CKD) (International Renal Interest Society (IRIS) stages I, II, III, and IV; n=39). *P<0.05 vs. the healthy controls.
Correlation of CREA, SDMA, and CysC levels in dogs with CKD
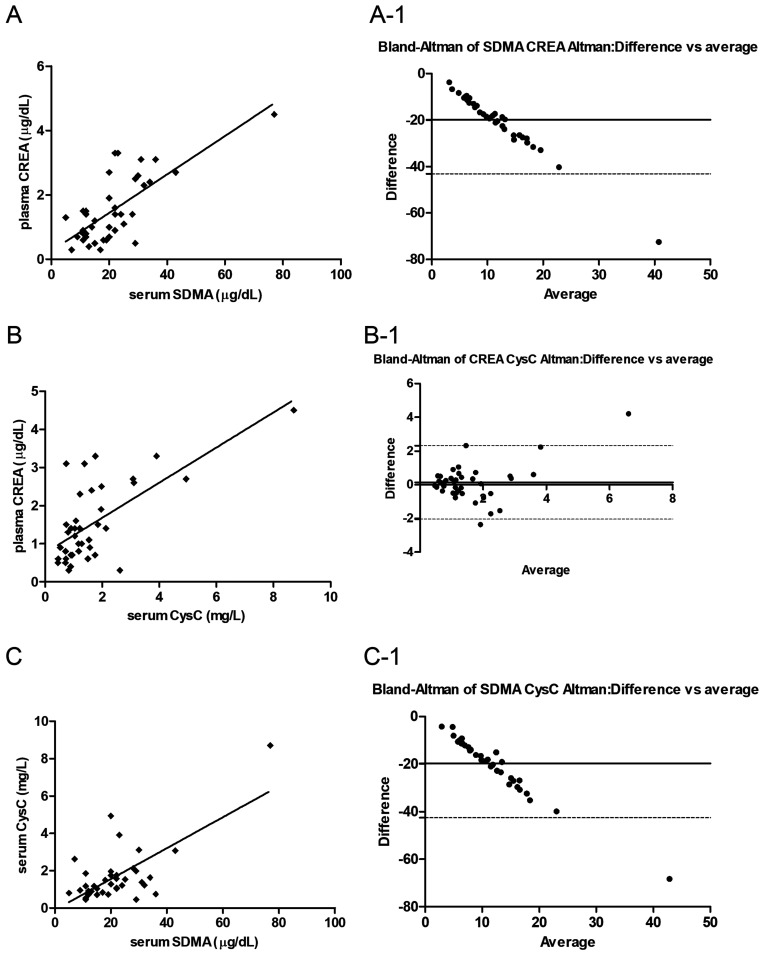
CREA and SDMA and CysC concentrations were plotted for patients with CKD using a scatter plot (Fig. 2). CREA concentration strongly correlated with SDMA (Fig. 2A). The serum levels of CysC were also strongly correlated with CREA (Fig. 2B) as well as SDMA (Fig. 1C) levels. As the dogs with CKD became more azotemic, CREA and SDMA and CysC data points shifted to the upper right quadrant. Bland-Altman plots revealed the data of CREA and CysC levels with minimal bias (Fig. 2B-1) for the majority of measured variables which demonstrated a strong agreement among the protocols.
Fig. 2.
Correlations of plasma creatinine (CREA) and serum symmetric dimethylarginine (SDMA) levels (A), serum cystatin C (CysC) and plasma CREA levels (B), and serum CysC and serum SDMA levels (C) in dogs with chronic kidney disease (CKD) (International Renal Interest Society (IRIS) stages I, II, III, and IV; n=39). The solid line indicates the power trendline of the data. The dotted central horizontal full lines (A-1, B-1, C-1) represent the mean of the differences (=bias) between the two methods. The two dotted horizontal lines represent the upper and lower 95% limits of agreement (=bias ± 1.96×SD).
Comparison of CREA, SDMA, and CysC levels among dogs according to IRIS stage of CKD
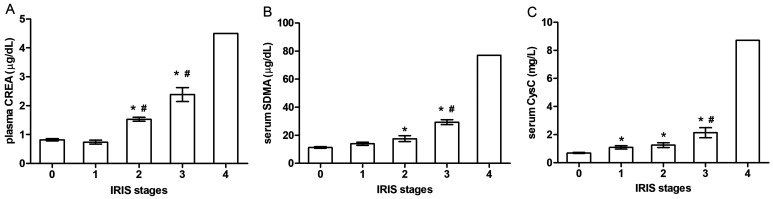
The results of all three markers for each IRIS stage are presented in Fig. 3. The mean CREA concentrations were 0.82 ± 0.03 µg/dl in the healthy controls, 0.73 ± 0.06 µg/dl in IRIS stage I, 1.52 ± 0.06 µg/dl in IRIS stage II, 2.38 ± 0.24 µg/dl in IRIS stage III, and 4.50 ± 0.00 µg/dl in IRIS stage IV (Fig. 3A). The mean SDMA concentrations were 11.3 ± 0.53 µg/dl in the healthy controls, 13.9 ± 1.11 µg/dl in IRIS stage I, 17.5 ± 2.13 µg/dl in IRIS stage II, 29.3 ± 1.72 µg/dl in IRIS stage III, and 77.0 ± 0.00 µg/dl in IRIS stage IV (Fig. 3B). The mean CysC concentrations were 0.69 ± 0.02 mg/l in the healthy controls, 1.09 ± 0.12 mg/l in IRIS stage I, 1.25 ± 0.17 mg/l in IRIS stage II, 2.14 ± 0.35 mg/l in IRIS stage III, and 8.71 ± 0.00 mg/l in IRIS stage IV (Fig. 3C). CREA and SDMA concentrations were significantly higher in IRIS stages II and III than in the healthy controls, whereas the CREA and SDMA concentrations were not significant between the healthy controls and IRIS stage I (Fig. 3A and 3B). However, the mean CysC concentration was significantly higher in IRIS stages I, II, and III than in the healthy controls (Fig. 3C).
Fig. 3.
Concentrations of plasma creatinine (CREA), serum symmetric dimethylarginine (SDMA), and serum cystatin C (CysC) in healthy dogs and dogs with chronic kidney disease (CKD). 0: healthy controls (n=10), 1: International Renal Interest Society (IRIS) stage I (n=18), 2: IRIS stage II (n=7), and 3: IRIS stage III (n=13). *P<0.05 healthy controls vs. IRIS stages I, II, and III. #P<0.05 IRIS stage I vs. IRIS stages II and III.
Sensitivity and specificity of CREA, SDMA, and CysC levels in dogs with CKD
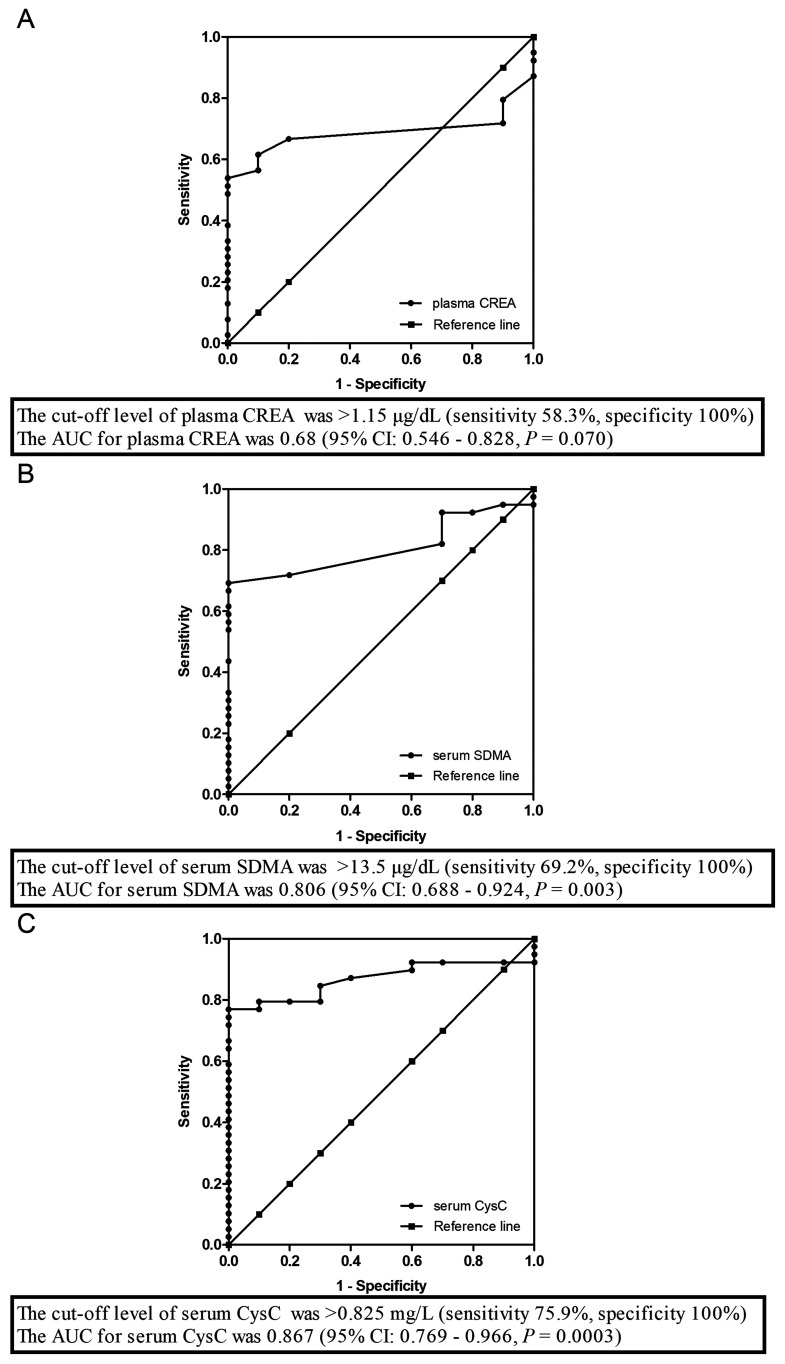
The sensitivity and specificity of the biomarkers for detecting CKD in dogs were evaluated. The area under the ROC curve (AUC), sensitivity, and specificity results are shown in Fig. 4. The cut-off level of CREA for detecting CKD in dogs was >1.15 µg/dl (sensitivity 58.3%, specificity 100%). The AUC for CREA was 0.68 (95% CI: 0.546–0.828, P=0.07; Fig. 4A). The cut-off level of SDMA for detecting CKD dogs was >13.5 µg/dl (sensitivity 69.2%, specificity 100%). The AUC for SDMA was 0.806 (95% CI: 0.688–0.924, P=0.003; Fig. 4B). The cut-off level of CysC for detecting CKD dogs was >0.825 mg/l (sensitivity 76.9%, specificity 100%). The AUC for CysC was 0.867 (95% CI: 0.769–0.966, P=0.0003; Fig. 4C).
Fig. 4.
Nonparametric receiver operating characteristic (ROC) plots of the sensitivity and specificity of plasma creatinine (CREA) (A), serum symmetric dimethylarginine (SDMA) (B), and serum cystatin C (CysC) (C) levels for detecting kidney damage in 39 dogs with chronic kidney disease (CKD).
Therefore, the data collected in this study among the CKD patients showed that CysC had the highest sensitivity for CKD, followed by SDMA and CREA.
Comparison of the sensitivity and specificity of CREA, SDMA, and CysC levels between the healthy controls and dogs in IRIS stage I
The sensitivity and specificity of the CKD biomarkers were compared between the healthy controls and patients in IRIS stage I (Fig. 5). The cut-off level of CREA for detecting stage I CKD dogs was ≤0.95 µg/dl (sensitivity 22.2%, specificity 90%). The AUC of CREA was 0.619 (95% CI: 0.409–0.829, P=0.302). The cut-off level of SDMA for detecting stage I CKD dogs was >13.5 µg/dl (sensitivity 50.0%, specificity 100%). The AUC of serum SDMA was 0.669 (95% CI: 0.470–0.868, P=0.143). The cut-off level of CysC for detecting stage I CKD dogs was >0.825 mg/l (sensitivity 66.7%, specificity 100%). The AUC of CysC was 0.802 (95% CI: 0.636–0.968, P=0.009).
Fig. 5.
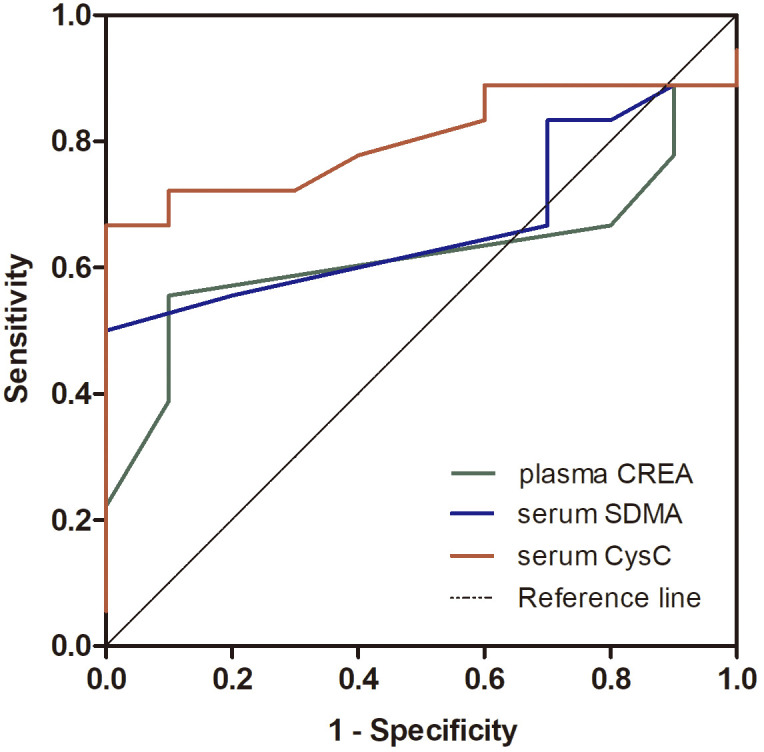
Nonparametric receiver operating characteristic (ROC) plots of the comparison of the sensitivity and specificity of plasma creatinine (CREA), serum symmetric dimethylarginine (SDMA), and serum cystatin C (CysC) levels for detecting kidney damage between healthy and International Renal Interest Society (IRIS) stage I dogs (n=18).
The results suggested that CysC show the best sensitivity followed by SDMA and, CREA in comparison between healthy controls and IRIS stage I CKD group.
DISCUSSION
The following were the main results of this study: 1. CysC level was significantly higher in dogs with CKD than in healthy controls; 2. CysC concentration strongly correlated with CREA and SDMA concentrations; 3. CysC was the most effective biomarker for detecting early stage CKD (IRIS stage I); and 4. CysC was a more sensitive marker of early or late stage CKD than CREA or SDMA. The clinical significance of this study can be summarized as follows. First, CysC significantly correlated with IRIS stage I and stage II, whereas CREA and SDMA did not. Second, in this study, CysC showed higher sensitivity than the conventional indicators CREA and SDMA in CKD patients.
CREA, SDMA and CysC levels were significantly higher in dogs with CKD than in the healthy controls in this study. Similarly, a study on humans reported a comparison of eGFR equations of CREA, SDMA, and CysC and their application as markers of kidney function [17]. Several veterinary studies also reported that the levels of kidney biomarkers CREA, SDMA, and CysC were higher in dogs with CKD than in healthy dogs [2, 9, 11], however, the results were not the same as those reported by Pelander et al. for CysC [16]. It has reported that CysC is not a superior biomarkers than others, whereas the present study has showed that CysC is a beneficial biomarker for detecting IRIS stage I compared to CREA or SDMA. The sensitivity and specificity of CysC were higher than other factors. Additional research still is needed to validate the role of CysC in canine CKD.
We investigated the relationship between CREA and SDMA concentrations in dogs with CKD throughout IRIS stages. We found a significant positive correlation between SDMA and CREA levels which has been reported previously in dogs with CKD in literature [9]. CysC level was correlated highly with CREA and SDMA levels by Pearson’s correlation coefficient in the present study. The correlation between CysC and CREA levels was highly plotted in the Bland-Altman method. It showed that the correlations among multiple markers of kidney function in dogs with CKD. Steubl et al. [17] also stated that markers such as CREA and CysC exhibit a hyperbolic correlation to eGFR in human patients with CKD.
A recent study investigated the use of serum CREA, SDMA, and CysC levels to detect reduced GFR in clinically stable dog. Adjusting the cutoff for CysC to correspond to diagnose, the sensitivity is 90% (0.49 mg/dl) and the specificity 72%, which were lower than both of CREA and SDMA [16]. Whereas showed that CysC was higher sensitivity and specificity compared to other factors. CREA and SDMA are insufficient for detecting IRIS stage I in the previous study [16] and also the present study, while serum levels of the biomarker CysC are showed increased in IRIS stage I in the present study. To the best of our knowledge, the present study was assessed that CysC levels in dogs with CKD to determine its efficacy for detecting early stage CKD compared to that of CREA and SDMA.
The results of the present study indicate that CysC is a beneficial biomarker for detecting IRIS stage I compared to CREA or SDMA. Earlier detection will allow the initiation of reno-protective interventions to slow the progression of kidney disease sooner, ultimately extending the life expectancy of dogs with CKD.
The present study has some limitations. The number of population was not enough to evaluate the performance of these biomarkers. Further, GFR was not directly measured, three biomarkers, CREA, SDMA, and CysC, were used to estimate GFR, and we were not able to assess GFR for our canine patients with CKD. Further studies are needed.
CONFLICT OF INTERST
We have no conflicts of interest.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (NRF-2016R1D1A3B04934798).
REFERENCES
- 1.Almy F. S., Christopher M. M., King D. P., Brown S. A.2002. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J. Vet. Intern. Med. 16: 45–51. doi: 10.1111/j.1939-1676.2002.tb01605.x [DOI] [PubMed] [Google Scholar]
- 2.Antognoni M. T., Siepi D., Porciello F., Fruganti G.2005. Use of serum cistatin C determination as a marker of renal function in the dog. Vet. Res. Commun. 29Suppl 2: 265–267. doi: 10.1007/s11259-005-0058-5 [DOI] [PubMed] [Google Scholar]
- 3.Antognoni M. T., Siepi D., Porciello F., Rueca F., Fruganti G.2007. Serum cystatin-C evaluation in dogs affected by different diseases associated or not with renal insufficiency. Vet. Res. Commun. 31Suppl 1: 269–271. doi: 10.1007/s11259-007-0044-1 [DOI] [PubMed] [Google Scholar]
- 4.Braun J. P., Lefebvre H. P., Watson A. D.2003. Creatinine in the dog: a review. Vet. Clin. Pathol. 32: 162–179. doi: 10.1111/j.1939-165X.2003.tb00332.x [DOI] [PubMed] [Google Scholar]
- 5.Ghys L., Paepe D., Smets P., Lefebvre H., Delanghe J., Daminet S.2014. Cystatin C: a new renal marker and its potential use in small animal medicine. J. Vet. Intern. Med. 28: 1152–1164. doi: 10.1111/jvim.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghys L. F., Paepe D., Lefebvre H. P., Reynolds B. S., Croubels S., Meyer E., Delanghe J. R., Daminet S.2016. Evaluation of cystatin c for the detection of chronic kidney disease in cats. J. Vet. Intern. Med. 30: 1074–1082. doi: 10.1111/jvim.14256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall J. A., Yerramilli M., Obare E., Yerramilli M., Jewell D. E.2014. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J. Vet. Intern. Med. 28: 1676–1683. doi: 10.1111/jvim.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall J. A., Yerramilli M., Obare E., Yerramilli M., Almes K., Jewell D. E.2016. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J. Vet. Intern. Med. 30: 794–802. doi: 10.1111/jvim.13942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hokamp J. A., Nabity M. B.2016. Renal biomarkers in domestic species. Vet. Clin. Pathol. 45: 28–56. doi: 10.1111/vcp.12333 [DOI] [PubMed] [Google Scholar]
- 10.Jensen A. L., Bomholt M., Moe L.2001. Preliminary evaluation of a particle-enhanced turbidimetric immunoassay (PETIA) for the determination of serum cystatin C-like immunoreactivity in dogs. Vet. Clin. Pathol. 30: 86–90. doi: 10.1111/j.1939-165X.2001.tb00263.x [DOI] [PubMed] [Google Scholar]
- 11.Linnetz E. H., Graves T. K.2010. Glomerular filtration rate in general small animal practice. Compend. Contin. Educ. Vet. 32: E1–E5, quiz E6. [PubMed] [Google Scholar]
- 12.Martens-Lobenhoffer J., Bode-Böger S. M.2015. Amino acid N-acetylation: metabolic elimination of symmetric dimethylarginine as symmetric N(α)-acetyldimethylarginine, determined in human plasma and urine by LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 975: 59–64. doi: 10.1016/j.jchromb.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Miyagawa Y., Takemura N., Hirose H.2009. Evaluation of the measurement of serum cystatin C by an enzyme-linked immunosorbent assay for humans as a marker of the glomerular filtration rate in dogs. J. Vet. Med. Sci. 71: 1169–1176. doi: 10.1292/jvms.71.1169 [DOI] [PubMed] [Google Scholar]
- 14.Nabity M. B., Lees G. E., Boggess M. M., Yerramilli M., Obare E., Yerramilli M., Rakitin A., Aguiar J., Relford R.2015. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection. J. Vet. Intern. Med. 29: 1036–1044. doi: 10.1111/jvim.12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo-Marín L., Martínez-Subiela S., Pastor J., Tvarijonaviciute A., Garcia-Martinez J. D., Segarra S., Cerón J. J.2017. Evaluation of various biomarkers for kidney monitoring during canine leishmaniosis treatment. BMC Vet. Res. 13: 31. doi: 10.1186/s12917-017-0956-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelander L., Häggström J., Larsson A., Syme H., Elliott J., Heiene R., Ljungvall I.2019. Comparison of the diagnostic value of symmetric dimethylarginine, cystatin C, and creatinine for detection of decreased glomerular filtration rate in dogs. J. Vet. Intern. Med. 33: 630–639. doi: 10.1111/jvim.15445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steubl D., Block M., Herbst V., Nockher W. A., Schlumberger W., Satanovskij R., Angermann S., Hasenau A. L., Stecher L., Heemann U., Renders L., Scherberich J.2016. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 95: e3011. doi: 10.1097/MD.0000000000003011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehner A., Hartmann K., Hirschberger J.2008. Utility of serum cystatin C as a clinical measure of renal function in dogs. J. Am. Anim. Hosp. Assoc. 44: 131–138. doi: 10.5326/0440131 [DOI] [PubMed] [Google Scholar]