Abstract
Ex situ conservation of Japanese rock ptarmigans began in 2015 with the aim of reintroducing artificially raised birds into their original habitat. However, the current raising method in captivity seems insufficient in terms of the survivability of artificially raised birds in natural conditions. Feeding management is one potential reason for such insufficiency. In this study, we performed a comprehensive analysis of the hydrophilic metabolites by LC-MS/MS for the cecal feces of Japanese rock ptarmigans under in situ and ex situ conservation to reveal their gut chemical environment. We also analyzed the developmental processes of cecal microbiomes both in situ semi-wild and ex situ captive individuals. Metabolites of nucleic acid were rich in the in situ individuals, and free amino acids were rich in the ex situ individuals. The differences in the microbiome composition between in situ and ex situ individuals were also pronounced; major genera of in situ individuals were not detected or few in ex situ individuals. The alpha diversity of the cecal microbiome of semi-wild chicks at 1 week of age was almost the same as that of their hens, while it was very low in captive individuals. Sub-therapeutic use of oxytetracycline, a diet rich in protein and energy, and isolation from adult birds are considered to be causes for these great differences in gut chemical and microbiological environment between in situ and ex situ individuals.
Keywords: in situ and ex situ conservation, metabolome, microbiome, rock ptarmigans
The Japanese rock ptarmigan (Lagopus muta japonica) is the southernmost subspecies of rock ptarmigan L. muta that adapts to severe environments, such as the arctic tundra and alpine areas [25]. Although the rock ptarmigan is categorized as a Least Concern species on the Red List of the International Union for the Conservation of Nature (IUCN), the small populations in the southern part of the distribution raises concerns of extinction [25]. Due to their rapid population decline in the past decade, both in situ and ex situ national conservation programs for Japanese rock ptarmigans were started in 2013 [24]. The ex situ conservation program is based on the establishment of “founders” to produce offspring ready for re-introduction to the wild. Accordingly, in 2015 and 2016, eggs from wild nests on Mt. Norikura were collected for hatching artificially in several Japanese zoos.
To date, so-called reintroduction programs have been conducted for various endangered species. Captive-reared animals are less likely to survive than wild conspecifics [1, 3, 4, 6, 21, 30]. Difference in the digestive efficiency among captive and wild individuals is one of the potential reasons for this failure, because the functional efficiency of the digestive system is vital to survival [6]. Captive animals are usually fed diets very different from their own natural foods, which potentially leads to maladaptation to their natural foods when these birds are released into the wild environment [22]. The alteration of the gut microbiome in captivity is a cause for such maladaptation to natural foods in reintroduced individuals. The situation is distinct in the case of herbivores, as herbivores depend completely on their gut microbiome for the digestion of plant polymers with the degradation of plant secondary metabolites that are often harmful to the host digestion [18, 19, 32, 37]. Since Japanese rock ptarmigans are herbivorous birds relying mainly on the leaves of alpine plants [16], acclimatization to captive food, mainly consisting of formula feeds for poultry chickens and pelleted foods for rabbits, may lead to the serious modification of the gut microbiome.
In this study, we focused on the biochemical characteristics of the intestinal environment of captive-reared Japanese rock ptarmigans in comparison with wild individuals. The biochemical information of intestinal contents can indicate the actual problem of captive food and be used as criteria for improving captive food, particularly for preparation for re-introduction to the wild. In addition, we analyzed the development of the gut microbiome in captive individuals as compared with those of wild chicks. It has been well known for a long time that the bacterial compositions of gut microbiomes significantly differ between wild and captive animals [35], and this is particularly true for rock ptarmigans [36]. However, the developmental process of the gut microbiome in captivity has not previously been the focus of study. Japanese rock ptarmigans have a specific way—the intentional coprophagy by chicks of the hen’s cecal feces of transferring the essential gut bacteria from mothers to chicks [17]. The absence of this specific coprophagy and routine dose of antimicrobials for young chicks may impair the proper development of the gut microbiome in captivity. This study demonstrates the significance of these two factors for the proper development of the gut microbiome and raises points that should be improved in the present captive feeding system.
MATERIALS AND METHODS
Birds and diet
Chicks and hens in in situ and ex situ conservation programs were subjected to cecum feces collection. The in situ protection methodology was defined as the so-called “cage protection method”, which protects wild chicks from predation and severe monsoon climate using a temporary cage settled in an alpine area [17]. In 2017, 3 cages were settled near a mountain lodge (2,900 a.s.l.) on Mt. Kitadake (35°40´ N, 138°14´ E), located in the Southern Japanese Alps. Although these chicks were raised under human management, they were fed the same food that wild chicks consume [17]. Thus, we refer to them as semi-wild chicks, hereafter. In total, 20 chicks from 3 families were subjected to cage protection in this study. The periods of protection of the first, second, and third cages were from July 5 to August 5 (32 days; from 2 days old to 33 days old), from July 5 to August 5 (32 days; from 3 days old to 34 days old), and from July 8 to August 5 (29 days; from 4 days old to 29 days old). We specified the hatching date of the chicks in the first cage by observing the nest. The hatching dates of chicks in the other cages were estimated by the size of the chicks. The sex of chicks was indistinguishable during the study.
The ex situ protection methodology was based on the artificial raising of chicks in zoos to create so-called “founders”. In this study, eggs were collected on Mt. Norikura in June 2016. Twelve eggs were collected from 6 nests from the wild population on Mt. Norikura (36°06´ N, 137°33´ E), and 4 eggs each were transported to the Ueno Zoo, the Toyama Family Park Zoo, and the Omachi Alpine Museum. The chicks in the two zoos were fed ad libitum diets based on a formula feed for poultry chicks (Flame-up for “Yo-su”, FEED ONE Co., Ltd., Yokohama, Japan), a pelleted feed for rabbit (RM-4, Funabashi Farm, Chiba, Japan) and a pelleted feed for rabbit (GB-1, GB-2 or GB-3, Funabashi Farm) with the mixture ratio 2:1:1. This concentrate feed was offered to chicks four times per day at 10 g/chick/time. Feed refusals were recovered and discarded. The diet was further supplemented with fresh Japanese mustard spinach ad libitum and 1 or 2 blueberry fruits per bird and 1 mealworm (Tenebrionidae) per bird per day. Water was available continuously. In addition to the antimicrobials contained in the poultry formula feed (colistin sulfate 50 g-potency/ton, avilamycin 5 g-potency/ton, and virginiamycin 3 g-potency/ton), Oxytetracycline-HCl (Okitera-Suiyo-San, 10 mg/dl in water, Kyoritsu Pharmaceutical Industries, Nara, Japan) was supplied to chicks in their drinking water for 7 days after hatching. The feeding protocol was fixed by the Japan Association of Zoos and Aquariums (JAZA) and approved by the Ministry of the Environment before the collection of eggs. The daily feed for an adult individual was RM-4 (40 g) and fresh Japanese mustard spinach (240 g).
In the Omachi Alpine Museum, a “home-made” complex feed was offered to the chicks ad libitum. The diet was mainly composed of natural leaves of oak (Quercus crispula Blum and Q. serrate), wheat flour, and soya meal. This diet was supplemented with formula feed for poultry chicks, mealworms, fresh vegetables, and fruits, as shown above. Oxytetracycline-HCl was also dosed as at the two zoos.
Collection of feces
For HPLC tandem mass spectrometry (LC-MS/MS) analyses and ion-exclusion HPLC analyses, fresh cecal feces were collected from 3 adult males at 1 year of age (CA, 4 samples), 2 chicks at 1 month of age (CC, 2 samples, sexes were indistinguishable) at the Toyama Family Park Zoo, and 3 adult hens at 1, 2 and 3 years old under semi-wild condition which is in situ protection on Mt. Kitadake as indicated above (SWA, 4 samples). Feces were immediately frozen with the aid of dry ice. Dry ice was produced on site with a dry ice maker (Bel-Art Frigimat Junior Dry Ice Maker, H-B Instruments, Trappe, PA, USA).
For microbiome analyses, fresh cecal feces of semi-wild chicks (SWC) were collected daily in the cages until their release at the age of 28 days. Cecum feces of chicks were distinguished from the cecum feces of hens by their size but were not identified by their host. On the other hand, cecum feces of captive chicks were collected at 7, 14, 21, 28 and 90 days in the Ueno Zoo, Toyama Family Park Zoo, and Omachi Alpine Museum. Details of the sampling procedures for both wild and captive birds are given in our previous paper [36]. Although 90 days was the maximum age of captive birds in the microbiome analysis, this is the age at which most of chicks attain adult size and become independent from their hen [25]. Hence, we assumed that the cecal microbiomes of individuals 90 days old can be considered to be adult cecal microbiomes.
LC-MS/MS analyses and ion-exclusion HPLC analyses
Hydrophilic metabolites in feces were extracted by the method of [23]. The method was optimized for metabolite analyses of intestinal lactic acid bacteria. Feces (approximately 100 mg) were diluted fivefold with Dulbecco’s Phosphate-Buffered Saline (D-PBS) (Thermo Fisher Scientific, Waltham, MA, USA) containing 2-morpholinoethanesulfonic acid (0.1 mmol/l) as an internal standard. They were extracted two times by intense mixing for 1 min and allowed to stand for 5 min on the icebox. 2 min after extraction, the upper aqueous portion without precipitation at the bottom was collected and centrifuged (15,000 × g for 5 min at 4°C), and then 200 µl of supernatant was centrifugally filtered through a 3-kDa cutoff filter (Amicon Ultra, Merck Millipore, Burlington, MA, USA). For analyzing metabolites in cecal feces, the filtrates were diluted tenfold with water and analyzed by liquid chromatography-mass spectrometry (LC-MS/MS). The LC-MS/MS analysis was performed using a Nexera X2 system (Shimadzu, Kyoto, Japan) equipped with two LC-30 AD pumps, a DGU-20A5R degasser, an SIL-30 AC autosampler, a CTO-20 AC column oven, and a CBM-20A control module, coupled with an LCMS-8060 triple quadrupole mass spectrometer (Shimadzu). A pentafluorophenylpropyl column (Discovery HS F5, 150 mm × 2.1 mm, 3 µm; Sigma-Aldrich, St. Louis, MO, USA) was used for the separation of metabolites. The mobile phase was composed of A: 0.1% (v/v) formic acid in water and B: 0.1% (v/v) formic acid in acetonitrile. The flow rate, column temperature, and injection volume were set as 0.25 ml/min, 40°C, and 3 µl, respectively. The gradient program for mobile phase B was as follows: 0 min, 0%; 2 min, 0%; 5 min, 25%; 11 min, 35%; 15 min, 95%; 20 min, 95%; 20.1 min, 0%; and 25 min, 0%. The mass spectrometer was equipped with an electrospray ionization (ESI) source under the following conditions: nebulizing gas flow, 3 l/min; heating gas flow, 10 l/min; interface temperature, 300°C; desolvation line temperature, 250°C; heating block temperature, 400°C; drying gas flow, 10 l/min; interface voltage for positive mode, +4.5 kV; and interface voltage for negative mode, −3.5 kV. Collision-induced dissociation gas pressure was set to 230 kPa. Peak selection, integration, and principal component analyses (PCA) were performed using Traverse MS software (Reifycs Inc., Tokyo, Japan). The peak area value of each metabolite was normalized to that of the internal standard (2-morpholinoethanesulfonic acid).
Ion-exclusion HPLC was used to analyze the short-chain fatty acids in cecal feces. The HPLC analysis was performed in accordance with the method described in a previous report by [14]. The analysis platform consisted of a Prominence system (Shimadzu) equipped with two LC-20 AD pumps, a DGU-20A3R degasser, an SIL-20 AC autosampler, a CTO-20 AC column oven, a CBM-20A control module, and a CDD-10AVP conductivity detector. Tandem ion exclusion columns (Shim-pack SCR-102H, 300 mm × 8.0 mm, 7 µm; Shimadzu) with a guard column (50 mm × 6.0 mm) were used for the separation of short-chain fatty acids. The mobile phase was composed of 5 mmol/lp-toluenesulfonic acid in water and delivered at a flow rate of 0.8 ml/min. The pH buffering solution was composed of 5 mmol/l p-toluenesulfonic acid, 20 mmol/l Bis-Tris, and 0.1 mmol/l ethylenediaminetetraacetic acid in water and delivered at a flow rate of 0.8 ml/min. The column temperature and injection volume were set as 45°C and 10 µl, respectively.
Quadruple analyses were done per sample. Principal component analysis (PCA) was done for the signal intensities of metabolites. Tukey’s test was used to analyze the average concentration of each metabolite in semi-wild, captive adults, and captive chicks with R (version 3.4.4).
DNA extraction and 16S rRNA deep sequencing
DNA was extracted using a tissue DNA kit of the QuickGene-Mini80 system (KURABO Industries, Tokyo, Japan) from fecal samples stored in buffer, as indicated previously [36]. Briefly, feces were recovered from the storage buffer and washed with PBS to remove the residual buffer. Fecal samples of about 20 to 50 µg were suspended in MDT buffer with glass beads. After bead disruption, the extraction steps followed the standard protocol for DNA extraction as indicated by the manufacturer. Obtained DNA solutions were measured for their concentration and quality by NanoDrop 1000 (Thermo Fisher Scientific) and a Quant-iT dsDNA HS assay kit using a Qubit® fluorometer (Invitrogen, Carlsbad, CA, USA).
The construction of 16S rRNA amplicon libraries and the following sequencing was done at BGI Japan (Kobe, Japan) with Illumina MiSeq technology, as indicated previously [17, 34].
The Shannon-Wiener algorithm was applied to evaluate the diversity of the cecal microbiome at each age (1, 2, 3, and 4 weeks of age and adult). The analysis was made with R (version 3.4.4).
Ethics statement
Fecal sampling was performed in a non-invasive manner in both in situ and ex situ situations. All sampling methods and access to the Japanese rock ptarmigans were approved by the Ministry of Environment (ERTDF 4-1604) and by the Agency for Cultural Affairs and the Ministry of Education, Science, Sport and Culture (27-4-365).
RESULTS
Chemical condition of cecal feces in semi-wild and captive individuals
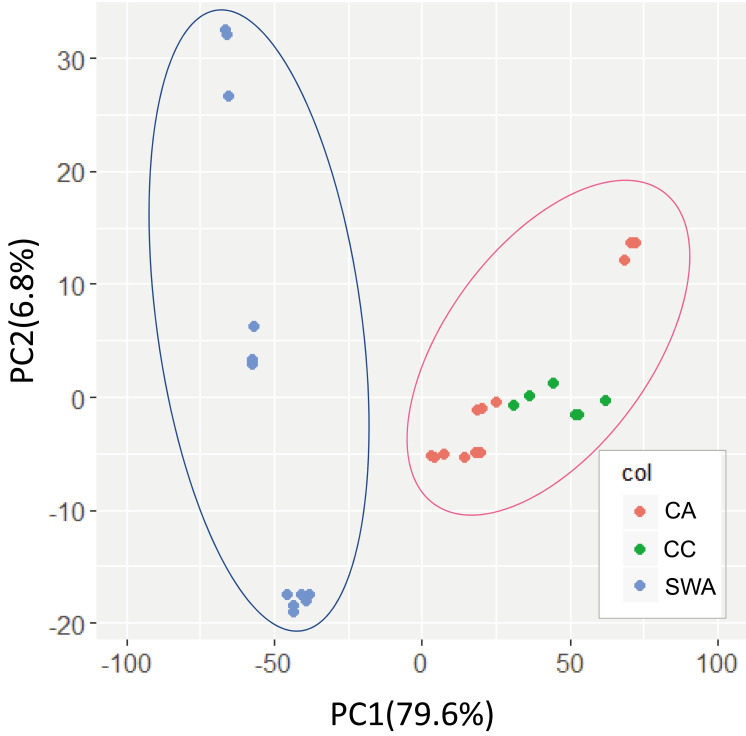
By LC-MS/MS analyses, 56 compounds and 60 compounds were detected, respectively, for feces of SWA, CA and CC. Detection profiles of the metabolites were separated for the 2 groups by PCA, and the separation was induced by the biased distribution of nucleic acid base metabolites and free amino acids (Fig. 1, Table 1). In the feces of SWA, methionine sulfone and some nucleic acid metabolites, including adenine, cytidine, uridine, and allantoin, were detected in high concentrations. In contrast, 10 amino acids, such as phenylalanine, leucine, valine, tyrosine, and methionine, were high in the feces of CA and CC. In addition, kynurenine, ophthalmic acid, nicotinic acid, fumaric acid, and taurocholic acid were also higher in the feces of CC and CA than in those of SWA (Table 1). Although the concentrations of symmetric dimethylarginine and inosine were not different between SWA and CA, those in CC were higher than in SWA and CA.
Fig. 1.
Principal component analyses for hydrophilic metabolites in the cecal feces of Japanese rock ptarmigans. SWA, semi-wild adult individuals; CA, captive adult individuals; CC, captive chicks.
Table 1. Hydrophilic metabolites in the cecal feces significantly different between captive and semi-wild Japanese rock ptarmigans.
| Metaborite | SWA | CA | CC | |
|---|---|---|---|---|
| Higher in semi-wild individuals than captive individuals | ||||
| Adenine | 3.17 × 101 ± 1.20 × 101 a) | 2.36 × 100 ± 6.038 × 10−1 b) | 1.77 × 100 ± 1.05 × 100 b) | |
| Allantoin | 5.54 × 100 ± 3.14 × 10−1 a) | 4.59 × 100 ± 2.310 × 10−1 b) | 4.71 × 100 ± 3.19 × 10−1 b) | |
| Cytidine | 8.76 × 101 ± 1.45 × 101 a) | 4.92 × 101 ± 1.124 × 101 b) | 6.04 × 101 ± 1.52 × 101 a, b) | |
| Methionine sulfone | 4.86 × 10−1 ± 1.51 × 10−2 a) | 4.42 × 10−1 ± 1.344 × 10−2 b) | 4.54 × 10−1 ± 3.71 × 10−2 a, b) | |
| Uridine | 8.51 × 101 ± 2.61 × 101 a) | 3.04 × 101 ± 7.631 × 100 b) | 3.37 × 101 ± 1.18 × 101 b) | |
| Higher in captive adult than semi wild individuals | ||||
| Adenosine 3′,5′-cyclic monophosphate | 1.95 × 100 ± 7.69 × 10−1 a) | 7.86 × 100 ± 1.63 × 100 b) | 4.71 × 100 ± 2.35 × 100 b) | |
| Asymmetric dimethylarginine | 3.60 × 100 ± 8.59 × 10−1 a) | 1.52 × 101 ± 3.70 × 100 b) | 2.15 × 101 ± 3.75 × 100 b) | |
| Citrulline | 4.00 × 10−1 ± 2.35 × 10−1 a) | 4.19 × 100 ± 2.49 × 100 a) | 2.78 × 100 ± 4.30 × 10−1 a, b) | |
| Dopamine | 2.89 × 10−1 ± 2.12 × 10−1 a) | 2.18 × 100 ± 7.63 × 10−1 b) | 1.68 × 100 ± 2.43 × 10−1 b) | |
| FMN | 2.30 × 10−1 ± 1.51 × 10−2 a) | 6.22 × 10−1 ± 1.69 × 10−1 a) | 4.24 × 10−1 ± 1.29 × 10−1 a, b) | |
| Glutamine | 2.96 × 100 ± 9.44 × 10−1 a) | 1.33 × 101 ± 5.72 × 100 b) | 1.52 × 101 ± 3.06 × 10−1 b) | |
| Guanosine 3′,5′-cyclic monophosphate | 6.23 × 10−1 ± 5.27 × 10−1 a) | 5.77 × 100 ± 1.77 × 100 b) | 6.37 × 100 ± 2.19 × 100 b) | |
| Histidine | 8.38 × 100 ± 1.29 × 100 a) | 1.33 × 101 ± 1.43 × 101 a) | 3.53 × 101 ± 1.44 × 101 a, b) | |
| Isoleucine | 9.52 × 101 ± 6.31 × 101 a) | 6.44 × 102 ± 1.93 × 102 b) | 8.09 × 102 ± 1.33 × 102 b) | |
| Kynurenine | 0.00 × 100 ± 0.00 × 100 a) | 6.43 × 10−1 ± 1.94 × 10−1 b) | 5.87 × 10−1 ± 8.20 × 10−2 b) | |
| Leucine | 1.09 × 102 ± 6.96 × 101 a) | 7.97 × 102 ± 1.93 × 102 b) | 9.45 × 102 ± 8.28 × 101 b) | |
| Lysine | 1.91 × 101 ± 9.53 × 100 a) | 7.97 × 102 ± 7.35 × 101 a) | 1.33 × 102 ± 2.64 × 101 a, b) | |
| Methionine | 2.07 × 101 ± 7.23 × 100 a) | 2.31 × 102 ± 9.52 × 101 b) | 2.35 × 102 ± 7.33 × 101 b) | |
| Nicotinic acid | 3.97 × 101 ± 3.38 × 101 a) | 1.21 × 102 ± 4.29 × 101 b) | 9.36 × 101±7.17 × 100 a, b) | |
| Phenylalanine | 2.22 × 102 ± 6.22 × 101 a) | 9.83 × 102 ± 2.34 × 102 b) | 1.16 × 103 ± 3.76 × 101 b) | |
| Tryptophan | 1.42 × 101 ± 4.23 × 100 a) | 1.21 × 102 ± 5.00 × 101 b) | 1.49 × 102 ± 4.19 × 100 b) | |
| Tyrosine | 1.42 × 101 ± 1.65 × 101 a) | 2.77 × 102 ± 1.39 × 102 b) | 4.01 × 102 ± 6.18 × 101 b) | |
| Valine | 1.04 × 102 ± 3.94 × 101 a) | 5.92 × 102 ± 2.90 × 102 b) | 7.27 × 102 ± 6.22 × 101 b) | |
| Xanthine | 1.18 × 101 ± 1.07 × 101 a) | 4.23 × 101 ± 1.49 × 101 b) | 2.35 × 101 ± 2.87 × 100a, b) | |
| Higher in captive chicks than semi wild and captive adult individuals | ||||
| Arginine | 1.29 × 102 ± 3.57 × 101 a, b) | 2.19 × 102 ± 3.80 × 101 a) | 3.49 × 102 ± 1.28 × 102 b) | |
| Argininosuccinic acid | 6.23 × 10−1 ± 1.81 × 10−1 a) | 7.49 × 10−1 ± 2.69 × 10−1 a) | 2.43 × 100 ± 6.79 × 10−1 b) | |
| Cholic acid | 2.00 × 10−2 ± 2.35 × 10−2 a) | 7.17 × 10−1 ± 6.00 × 10−1 a) | 5.31 × 100 ± 3.65 × 100 b) | |
| Fumaric acid | 7.26 × 10−2 ± 8.39 × 10−2 a) | 5.45 × 10−2 ± 8.70 × 10−2 a) | 2.92 × 10−1 ± 5.72 × 10−2 b) | |
| Inosine | 3.17 × 101 ± 8.82 × 100 a) | 2.00 × 101 ± 8.91 × 100 a) | 6.49 × 101 ± 1.40 × 101 b) | |
| Ophthalmic acid | 0.00 × 100 ± 0.00 × 100 a) | 8.29 × 10−2 ± 1.22 × 10−1 a) | 4.67 × 10−1 ± 9.74 × 10−2 b) | |
| Succinic acid | 7.90 × 100 ± 1.82 × 100 a) | 6.30 × 100 ± 2.66 × 100 a) | 4.11 × 101 ± 2.05 × 101 b) | |
| Symmetric dimethylarginine | 1.62 × 100 ± 3.47 × 10−1 a) | 6.30 × 100 ± 1.65 × 100 a) | 2.30 × 101 ± 4.59 × 100 b) | |
| Other pattern | ||||
| Adenylsuccinic acid | 1.58 × 100 ± 5.37 × 10−1 a) | 0.00 × 100 ± 0.00 × 100 b) | 2.88 × 100 ± 1.18 × 10−1c) | |
| Adenosine monophosphate | 7.43 × 100 ± 5.95 × 100 a, b) | 1.42 × 100 ± 3.98 × 10−1 a) | 1.83 × 101 ± 4.98 × 100 b) | |
| No significant difference | ||||
| 4-Hydroxyproline | 3.79 × 10−1 ± 2.57 × 10−1 a) | 7.93 × 10−1 ± 3.35 × 10−1 a) | 5.58 × 10−1 ± 1.22 × 10−1 a) | |
| Acetylcarnitine | 9.71 × 100 ± 1.38 × 101 a) | 3.48 × 100 ± 1.54 × 100 a) | 2.41 × 100 ± 3.38 × 10−1 a) | |
| Adenosine | 3.20 × 102 ± 2.40 × 102 a) | 1.88 × 101 ± 6.07 × 100 a) | 1.93 × 101 ± 1.91 × 101 a) | |
| Alanine | 9.95 × 10−1 ± 3.92 × 10−1 a) | 4.82 × 100 ± 3.84 × 100 a) | 4.82 × 100 ± 1.37 × 100 a) | |
| Aspartic acid | 2.78 × 100 ± 9.72 × 10−1 a) | 2.76 × 100 ± 9.16 × 10−1 a) | 3.46 × 100 ± 7.78 × 10−1 a) | |
| Carnitine | 2.95 × 101 ± 2.19 × 101 a) | 2.43 × 101 ± 9.46 × 100 a) | 2.91 × 101 ± 8.49 × 100 a) | |
| Choline | 2.50 × 101 ± 2.79 × 100 a) | 1.50 × 101 ± 6.45 × 100 a) | 2.78 × 101 ± 9.18 × 100 a) | |
| Citric acid | 1.29 × 102 ± 2.52 × 102 a) | 2.90 × 100 ± 3.33 × 10−1 a) | 2.44 × 100 ± 1.22 × 100 a) | |
| Creatine | 2.79 × 100 ± 3.18 × 100 a) | 6.00 × 100 ± 5.23 × 100 a) | 2.12 × 100 ± 9.49 × 10−1 a) | |
| Creatinine | 1.56 × 100 ± 1.47 × 100 a) | 5.87 × 100 ± 4.65 × 100 a) | 3.11 × 100 ± 4.56 × 10−2 a) | |
| Dopa | 2.50 × 101 ± 2.10 × 101 a) | 4.93 × 10−1 ± 9.31 × 10−2 a) | 7.75 × 10−1 ± 2.91 × 10−2 a) | |
| Epinephrine | 1.41 × 100 ± 1.23 × 100 a) | 3.74 × 100 ± 1.91 × 100 a) | 5.01 × 100 ± 1.12 × 100 a) | |
| Glutamic acid | 3.81 × 101 ± 1.36 × 101 a) | 5.33 × 101 ± 1.57 × 101 a) | 5.14 × 101 ± 2.62 × 100 a) | |
| Glycine | 3.19 × 10−1 ± 7.68 × 10−2 a) | 6.56 × 101 ± 3.41 × 10−1 a) | 7.02 × 10−1 ± 1.28 × 10−2 a) | |
| Guanine | 6.28 × 10−1 ± 6.25 × 10−1 a) | 7.46 × 10−1 ± 1.25 × 10−1 a) | 3.33 × 10−1 ± 1.84 × 10−1 a) | |
| Guanosine | 5.62 × 101 ± 4.01 × 101 a) | 3.60 × 101 ± 8.61 × 100 a) | 3.70 × 101 ± 2.19 × 101 a) | |
| Guanosine monophosphate | 1.14 × 100 ± 6.16 × 10−1 a) | 5.14 × 10−1 ± 4.37 × 10−1 a) | 1.38 × 100 ± 5.44 × 10−1 a) | |
| Hypoxanthine | 2.75 × 101 ± 1.90 × 101 a) | 1.92 × 101 ± 7.03 × 100 a) | 3.53 × 101 ± 4.36 × 100 a) | |
| Lactic acid | 0.00 × 100 ± 0.00 × 100 a) | 0.00 × 100 ± 0.00 × 100 a) | 1.46 × 101 ± 2.07 × 101 a) | |
| Orotic acid | 6.28 × 10−1 ± 4.88 × 10−1 a) | 2.82 × 100 ± 3.00 × 100 a) | 2.55 × 100 ± 1.05 × 100 a) | |
| Pantothenic acid | 2.39 × 101 ± 1.46 × 101 a) | 1.56 × 101 ± 1.16 × 101 a) | 3.01 × 101 ± 9.16 × 100 a) | |
| Proline | 1.37 × 101 ± 5.81 × 100 a) | 3.18 × 101 ± 2.70 × 101 a) | 2.00 × 101 ± 3.44 × 10−1 a) | |
| Derine | 6.28 × 10−1 ± 1.48 × 10−1 a) | 1.44 × 100 ± 1.13 × 100 a) | 1.39 × 100 ± 3.03 × 10−1 a) | |
| Taurocholic acid | 0.00 × 100 ± 0.00 × 100 a) | 4.75 × 10−1 ± 3.77 × 10−1 a) | 0.00 × 100 ± 0.00 × 100 a) | |
| Threonine | 9.85 × 10−1 ± 2.75 × 10−1 a) | 2.95 × 100 ± 1.94 × 100 a) | 3.46 × 100 ± 4.24 × 10−1 a) | |
| Thymidine | 3.27 × 100 ± 2.97 × 100 a) | 7.64 × 100 ± 2.53 × 100 a) | 2.17 × 100 ± 2.41 × 10−1 a) | |
| Thymine | 4.58 × 10−1 ± 5.57 × 10−1 a) | 4.73 × 100 ± 4.35 × 100 a) | 1.51 × 100 ± 1.35 × 10−1 a) | |
| Tracil | 6.71 × 100 ± 5.62 × 100 a) | 9.54 × 100 ± 5.94 × 100 a) | 5.76 × 100 ± 1.08 × 100 a) | |
| Uric acid | 3.76 × 101 ± 1.03 × 101 a) | 7.39 × 101 ± 4.47 × 101 a) | 1.16 × 101 ± 2.38 × 100 a) | |
Values (means with standard deviations) are relative signal intensities. SWA, semi-wild adult individuals; CA, captive adult individuals; CC, captive chicks. For details, see text. a, b, c) Means with different superscripts differ significantly at P<0.05. See details in the text.
Organic acid profiles as determined by an ion-exclusion HPLC were also different between SWA and captive individuals (CA and CC) (Table 2). Propionic acid was significantly higher in the SWA, in contrast to other acids. On the other hand, succinic acid was higher in the cecal feces of CC than in those of SWA and CA. Butyric acid was significantly higher in CC than in SWA.
Table 2. Concentration of organic acids (mainly SCFAs) determined by an ion-exclusion High Performance Liquid Chromatography in cecal feces of Japanese rock ptarmigans.
| Metaborite | SWA | CA | CC |
|---|---|---|---|
| Succinic acid | 5.96 × 101 ± 1.10 × 10−1 a) | 6.28 × 10−1 ± 1.42 × 10−1 b) | 2.73 × 100 ± 1.25 × 100 a) |
| Lactic acid | 0.00 × 100 ± 0.00 × 100 a) | 0.00 × 100 ± 0.00 × 100 a) | 1.54 × 100 ± 2.17 × 100 a) |
| Acetic acid | 1.94 × 101 ± 4.13 × 100 a) | 1.18 × 101 ± 5.81 × 100 a) | 1.65 × 101 ± 8.83 × 10−1 a) |
| Propionic acid | 4.76 × 100 ± 1.39 × 100 a) | 9.19 × 10−1 ± 4.10 × 10−1 a) | 5.77 × 10−1 ± 3.87 × 10−1 b) |
| Butyric acid | 9.17 × 10−1 ± 3.64 × 10−1 a, b) | 1.41 × 100 ± 1.02 × 100 a) | 2.81 × 100 ± 2.29 × 10−1 b) |
Values (means with standard deviations) are expressed as mmol/kg digesta. SWA, CA, and CC mean semi-wild adult individuals, captive adult individuals, and captive chicks of Japanese rock ptarmigans, respectively. a, b) Means with different superscripts differ significantly at P<0.05. See details in the text.
Development of microbiome between captive and semi-wild chicks and adults
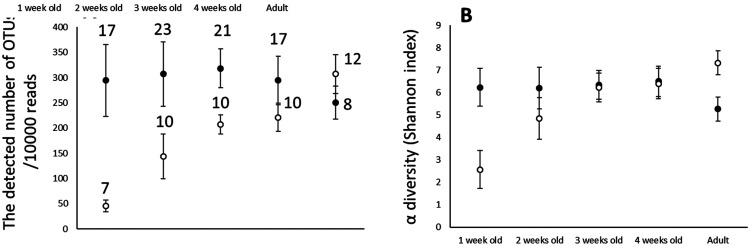
On average, 29,025 reads (min. 8,965 and max. 63,199) were obtained for 136 samples. The total number of OTUs (Operational taxonomic units) detected in captive individuals (CA and CC) (11,755 OTUs) was higher than that in semi-wild individuals (SWA and SWC) (6,949 OTUs). However, in a genus-level comparison, the diversity of cecal microbiomes was higher in semi-wild individuals than in captive individuals (306 genera vs. 183 genera). The same is true for a family-level comparison (107 families vs. 44 families). If OTU numbers are normalized by the total reads (the number of OTUs/10,000 reads), the values show that SWC at 1 week of age already have almost the same level of OTUs/10,000 reads, while those of CC at 1 week of age were quite low. The alpha diversity shows the same tendency as normalized OTU numbers (Fig. 2). These diversity metrics were quite different between both environments. The number of OTUs/10,000 reads detected and alpha diversity of CC gradually increased with growth and reached the same level as SWC at 4 weeks of age.
Fig. 2.
The change of detected number of Operational taxonomic units (OTUs)/10,000 reads (A) and α diversity of captive and semi-wild Japanese rock ptarmigan with growing (B). ●, Semi-wild individuals (SWA and SWC); ○, captive individuals (CA and CC). The number above plot is sample size and error bar mean Standard deviation.
The numbers of common OTUs, which were defined as OTU in cecal microbiomes continuously detected throughout ages (e.g., from 1 week of age to adult) of each group, were 217 (1.8% of the total number of detected OTUs) and 656 (9.4% of the total number of detected OTUs), respectively, for captive and semi-wild individuals. The proportion of these common OTUs was larger than 70% in SWC of all ages (Supplementary Fig. 1). On the other hand, it decreased with growth (from 95% to 45%) in CC. The top 30 OTUs of adult cecal microbiomes (SWA) were all commonly shared by chicks of the semi-wild group (SWC). On the other hand, 13 of the top 30 OTUs of CA cecal microbiomes were not detected CC at 1-week-old (Supplementary Table 1).
The most dominant phylum in both captive and semi-wild individuals was Firmicutes, followed by Proteobacteria in captive individuals and by Actinobacteria in semi-wild individuals (Supplementary Tables 2, 3). The top 2 phyla occupied over 90% of the total reads in all ages of captive and semi-wild individuals. At genus-level analyses, genera-compositions of the phyla Firmicutes, Actinobacteria, and Proteobacteria were different between captive and semi-wild individuals (Supplementary Tables 2, 3). The most prevalent genus in the adult cecal microbiome was detected in SWC at 1 week old. Although the proportion of Olsenella increased and unclassified genus in Ruminococcus decreased with growth in SWC, the proportion of other genera in Firmicutes and Actinobacteria did not change greatly. In contrast to SWC, the most prevalent genus in adult cecal microbiomes was not detected in CC at 1 week of age. In CC, the genera-composition at 1 week of age was different from those of other ages. In fact, the most common genera appeared after 2 weeks of age. It is noteworthy that the OTU belonging to the genus Salmonella was observed only from captive individuals.
DISCUSSION
Chemical composition of cecal feces
The clear separation of metabolite profiles between semi-wild (SWA) and captive individuals (CA, CC) was mainly due to differences in free amino acids and nucleic acid metabolites (Fig. 1, Table 1). Free amino acids were higher in captive individuals than in semi-wild individuals (Table 1). In contrast, nucleic acid metabolites were higher in semi-wild individuals than in captive individuals. Since feed composition directly affects the intestinal environment, higher free amino acid in cecal feces suggests that captive birds were fed a diet richer in protein as compared to the food in their own natural habitat. The cecum of rock ptarmigans, like other hindgut fermenters, is the chamber for the fermentation of indigestible food materials, such as fiber. Since amino acids are absorbed from the small intestine after protein digestion, higher free amino acid in the cecum suggests that the excess protein flowed into the cecum, where bacteria degrade them into free amino acids. Amino acids are usually degraded rapidly into ammonia and carboxylic acids by gut bacteria [26]. It is possible that the amount of protein is too great for the capacity of cecal bacteria in captivity. While higher nucleic acid metabolites in semi-wild individuals suggest that the natural food resources were relatively protein-limited as compared to the artificial diet of captive birds because nucleic acid is decomposed and is used by microbial protein synthesis as potential nitrogen resources in the ruminant [20]. These “non-protein nitrogen” resources are of importance under protein-limited feeding conditions [20]. The RM-4, main food of captive individuals, containing about 18% protein on dry matter basis. Although it has not been reported protein values of natural food resource of Japanese rock ptarmigan, our preliminary analyses indicated that crude protein levels (% on dry matter basis) of fresh leaves of alpine plants were 7.8, 5.2, 5.9, 4.0, and 7.0 respectively for Empetrum nigrum var. japonicum, Arcterica nana, Vaccinium vitis-idaea, Vaccinium ovalifolium, Oxytropis japonica. Our findings strongly suggest that the diet offered to Japanese rock ptarmigans is characterized as having an excess of protein as compared with the natural foods to which Japanese rock ptarmigans are adapted. The weight of captive Japanese rock ptarmigans at 30 days old (280 g vs. 150 g) was nearly twice as much as that of their wild counterparts (Y. Akiba, personal communication). This big difference in body weight between captive and semi-wild individuals was presumably caused by lack of physical activities in addition to the ad libitum feeding of nutrient rich formula feed under the captive condition.
Differences in the developmental process and composition of microbiomes between captive and wild Japanese rock ptarmigans
The normalized numbers of OTUs and alpha diversity of captive chicks (CC) at 1 week of age were very poor (Fig. 2) because they had no opportunity of coprophagy, the primary way of transmitting essential gut microbiome in rock ptarmigans [17] in addition to the daily dose of antibiotics. The major OTUs in semi-wild individuals belonging to genera Olsenella, Slackia, Shuttleworthia, and Megasphaera are widely seen in wild Japanese rock ptarmigans [34]. In contrast, these OTUs were not seen in captive individuals (Supplementary Table 3). On the other hand, the major bacterial genera found in captive individuals, such as Ruminococcus, Coprococcus, and Clostridium in Firmicutes, and several genera belonging to Proteobacteria are common in the gut of chickens and domestic animals [5, 9, 15, 38]. Captive-reared chicks acquired bacteria from the surrounding artificial environment, not from their hens, which makes their cecal microbiome very different from semi-wild chicks.
As suggested from the gut metabolome, high-protein diets affected the development of the gut microbiome. It is well known that the counts of Bacteroides spp. and Clostridium spp. were increased by diets high in beef, in the case of humans [12]. An animal protein–rich diet was also reported to increase the number of Bacteroides spp., Alistipes spp., Bilophila spp., and Ruminococcus spp. in humans [29].
The difference in short-chain fatty acids (SCFAs) between semi-wild (SWA) and captive individuals (CA, CC) (Table 2) was induced by the modification of the gut microbiome. A high level of coexistence of lactic acid bacteria such as Olsenella spp. and lactate utilizers such as Megasphaera spp. may lead to higher propionate levels in the guts of wild Japanese rock ptarmigans [34]. Megasphaera spp. are well known as potent butyrate producers [13], and its type strain can convert lactate to equimolar butyrate in the rat model [11]. However, this bacterium has the acrylate pathway [10], and wild strains can produce propionate better than butyrate by consuming lactic acid [7]. The loss of lactic acid utilizers such as Megasphaera spp. can lead to the smaller production of propionate in the cecum. Since propionic acid becomes a precursor of gluconeogenesis in the liver after absorption, propionic acid is important for the herbivore in glucose-limiting conditions [41]. The loss of lactate-utilizing propionate producers may limit the adaptation of artificially reared Japanese rock ptarmigans to natural feeding conditions. In addition to the major phyla in wild individuals, the loss of Synergistes spp. from captive individuals may impair their adaptation to wild plant consumption because species in this phylum are considered to impart the detoxification of plant glycoside [2]. The disappearance of Synergistes spp. in captivity is also seen in other subspecies of rock ptarmigans [28, 36] and captive capercaillies [40]. Therefore, grouses or ptarmigans easily loose this particular group of bacteria in captivity.
Problems with the current captivity method
Our results indicated that the current method of feeding Japanese rock ptarmigans in captivity should be improved in many ways. The feeding system is characterized by (1) isolation from hens, (2) a continuous prophylactic dose of antibiotics, and (3) the feeding of nutrient-rich foods, particularly protein. These artificial conditions impaired the proper development of the cecal microbiome and its functionality. Nutrient-rich food induces captive individuals to become overweight, which may further elicit other symptoms such as diarrhea, perosis, and even sudden death caused by opportunistic pathogens [8].
Although therapeutic intervention can be effective for infectious diseases, daily doses of antibiotics disturbs the healthy development of the gut microbiome. In fact, many OTUs in the cecal microbiome of 1-week-old captive chicks were not seen in those of their wild counterparts (Supplementary Tables 2, 3). It seems impossible to release these artificially raised birds into their own natural habitat because it is not an easy task for the released birds to readapt to the natural environment, particularly to the natural food items, without a properly developed gut microbiome. Accordingly, the establishment of the wild-type gut microbiome in captivity becomes important as a short-term goal of the current ex situ conservation of Japanese rock ptarmigans [33].
Infectious disease was the primary cause of death of Japanese rock ptarmigans at the Omachi Alpine Museum during the 20 years between 1970 and 1990, the period prior to the endorsement of a national conservation program [27]. Captive Japanese rock ptarmigan chicks still died apparently from infection with opportunistic pathogens Pseudomonas aeruginosa, Streptococcus lutetiensis, and Salmonella spp. even with much greater pathogen control in the present conservation program (N. Miyano, Y. Akiba, personal communications). Obviously, their original habitat, alpine areas, is less contaminated by pathogens than are zoos. However, a poorly developed gut microbiome, especially early in life, allowed opportunistic infections. Establishing a wild-type microbiome may also be effective for the prophylaxis of infectious diseases, as several strains of Lactobacillus apodemi isolated from wild Japanese rock ptarmigans showed potent antibacterial activity toward P. aeruginosa actually isolated from dead Japanese rock ptarmigan chicks in a zoo [33]. Probiotic/symbiotic approach using the health beneficial bacteria isolated from wild Japanese rock ptarmigans has a potential to develop raising method of captive Japanese rock ptarmigans without antibiotics.
The importance of reconstructing the gut microbiome was demonstrated recently in the reintroduction program of captive animals [31, 39]. In the case of Japanese rock ptarmigans, intentional coprophagy of chicks toward their mothers’ cecal feces can be used to reconstruct the wild-type microbiome in a captive environment. Moreover, the food of captive individuals should be improved, not only to allow the proper growth of birds, but also to induce the proper development of their gut microbiomes. Feed protein level is one of the important points to be addressed. The feeding of cytotoxic and anti-nutritional substances must also be considered for the proper development of the gut microbiome, at least for their future reintroduction.
Supplementary Material
Acknowledgments
This work was financially supported by the Environment Research and Technology Development Fund (ERTDF 4-1604 and 4-1903). The authors are thankful for the contributions of Mr. Y. Takahashi, Ms. N. Uno, Dr. N. Koike, and Dr. H. Watabe (Ueno Zoo); Mr. S. Horiguchi, Dr. Y. Akiba, Mr. H. Murai, and Mr. Y. Ishihara (Toyama Family Park Zoo); and Ms. K. Uchida, Mr. S. Sato, and Mr. N. Miyano (Omachi Alpine Museum) for sample collection. The kind support of the Kitadake Sanso Lodge is acknowledged.
REFERENCES
- 1.Aaltonen K., Bryant A. A., Hostetler J. A., Oli M. K.2009. Reintroducing endangered Vancouver Island marmots: survival and cause-specific mortality rates of captive-born versus wild-born individuals. Biol. Conserv. 142: 2181–2190. doi: 10.1016/j.biocon.2009.04.019 [DOI] [Google Scholar]
- 2.Allison M. J., Mayberry W. R., Mcsweeney C. S., Stahl D. A.1992. Synergistes jonesii, gen. nov., sp. nov.: a rumen bacterium that degrades toxic pyridinediols. Syst. Appl. Microbiol. 15: 522–529. doi: 10.1016/S0723-2020(11)80111-6 [DOI] [Google Scholar]
- 3.Blythe R. M., Smyser T. J., Johnson S. A., Swihart R. K.2015. Post-release survival of captive-reared Allegheny woodrats. Anim. Conserv. 18: 186–195. doi: 10.1111/acv.12158 [DOI] [Google Scholar]
- 4.Brittas R., Marcstrom V., Kenward R. E., Karlbom M.1992. Survival and breeding success of reared and wild ring-necked pheasants. J. Wildl. Manage. 56: 368–376. doi: 10.2307/3808836 [DOI] [Google Scholar]
- 5.Bryant M. P., Robinson I. M.1961. Some nutritional requirements of the genus Ruminococcus. Appl. Microbiol. 9: 91–95. doi: 10.1128/AEM.9.2.91-95.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champagnon J., Guillemain M., Elmberg J., Massez G., Cavallo F., Gauthier-Clerc M.2012. Low survival after release into the wild: assessing “the burden of captivity” on Mallard physiology and behaviour. Eur. J. Wildl. Res. 58: 255–267. doi: 10.1007/s10344-011-0573-3 [DOI] [Google Scholar]
- 7.Chen L., Shen Y., Wang C., Ding L., Zhao F., Wang M., Fu J., Wang H.2019. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front. Microbiol. 10: 162. doi: 10.3389/fmicb.2019.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlhausen K. E., Doroud L., Firl A. J., Polkinghorne A., Eisen J. A.2018. Characterization of shifts of koala (Phascolarctos cinereus) intestinal microbial communities associated with antibiotic treatment. PeerJ 6: e4452. doi: 10.7717/peerj.4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujisawa T.2016. Taxonomy of human intestinal Gram-positive bacteria—focus on anaerobes belonging to the phyla Firmicutes and Actinobacteria. J. Intestinal Microbiol 30: 177–190(in Japanese with an English summary). [Google Scholar]
- 10.Gonzalez-Garcia R. A., McCubbin T., Navone L., Stowers C., Nielsen L. K., Marcellin E.2017. Microbial propionic acid production. Fermentation (Basel) 3: 21. doi: 10.3390/fermentation3020021 [DOI] [Google Scholar]
- 11.Hashizume K., Tsukahara T., Yamada K., Koyama H., Ushida K.2003. Megasphaera elsdenii JCM1772T normalizes hyperlactate production in the large intestine of fructooligosaccharide-fed rats by stimulating butyrate production. J. Nutr. 133: 3187–3190. doi: 10.1093/jn/133.10.3187 [DOI] [PubMed] [Google Scholar]
- 12.Hentges D. J., Maier B. R., Burton G. C., Flynn M. A., Tsutakawa R. K.1977. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 37: 568–571. [PubMed] [Google Scholar]
- 13.Holdeman L. V., Moore W. E. C., Cato E. P.1977. Anaerobe Laboratory Manual 4th ed. Virginia Polytechnic Institute and State University, Blacksburg. [Google Scholar]
- 14.Hoshi S., Sakata T., Mikuni K., Hashimoto H., Kimura S.1994. Galactosylsucrose and xylosylfructoside alter digestive tract size and concentrations of cecal organic acids in rats fed diets containing cholesterol and cholic acid. J. Nutr. 124: 52–60. doi: 10.1093/jn/124.1.52 [DOI] [PubMed] [Google Scholar]
- 15.Isaacson R., Kim H. B.2012. The intestinal microbiome of the pig. Anim. Health Res. Rev. 13: 100–109. doi: 10.1017/S1466252312000084 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi A., Nakamura H.2011. Seasonal change of food items of the Japanese rock ptarmigan. Jpn. J. Ornithol. 60: 200–215(in Japanese with an English summary). doi: 10.3838/jjo.60.200 [DOI] [Google Scholar]
- 17.Kobayashi A., Tsuchida S., Ueda A., Yamada T., Murata K., Nakamura H., Ushida K.2019. Role of coprophagy in the cecal microbiome development of an herbivorous bird Japanese rock ptarmigan. J. Vet. Med. Sci. 81: 1389–1399. doi: 10.1292/jvms.19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohl K. D., Weiss R. B., Cox J., Dale C., Dearing M. D.2014. Gut microbes of mammalian herbivores facilitate intake of plant toxins. Ecol. Lett. 17: 1238–1246. doi: 10.1111/ele.12329 [DOI] [PubMed] [Google Scholar]
- 19.Kohl K. D., Connelly J. W., Dearing M. D., Forbey J. S.2016. Microbial detoxification in the gut of a specialist avian herbivore, the Greater Sage-Grouse. FEMS Microbiol. Lett. 363: fnw144. doi: 10.1093/femsle/fnw144 [DOI] [PubMed] [Google Scholar]
- 20.Leng R. A., Nolan J. V.1984. Nitrogen metabolism in the rumen. J. Dairy Sci. 67: 1072–1089. doi: 10.3168/jds.S0022-0302(84)81409-5 [DOI] [PubMed] [Google Scholar]
- 21.Liu B., Li L., Lloyd H., Xia C., Zhang Y., Zheng G.2016. Comparing post-release survival and habitat use by captive-bred Cabot’s Tragopan (Tragopan caboti) in an experimental test of soft-release reintroduction strategies. Avian Res. 7: 19. doi: 10.1186/s40657-016-0053-2 [DOI] [Google Scholar]
- 22.Liukkonen-Anttila T., Saartoala R., Hissa R.2000. Impact of hand-rearing on morphology and physiology of the capercaillie (Tetrao urogallus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 125: 211–221. doi: 10.1016/S1095-6433(99)00174-9 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M., Kibe R., Ooga T., Aiba Y., Kurihara S., Sawaki E., Koga Y., Benno Y.2012. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2: 233. doi: 10.1038/srep00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Environment Japan. 2012. Protection and propagation program of Japanese rock ptarmigan. Ministry of Environment, Japan. Tokyo, Japan. [Google Scholar]
- 25.Nakamura H.2007. Rock Ptarmigan Lagopus mutus japonicus. Jpn. J. Ornithol. 56: 93–114(in Japanese with an English summary). doi: 10.2326/jjo.56.93 [DOI] [Google Scholar]
- 26.Nugent J. H. A., Mangan J. L.1981. Characteristics of the rumen proteolysis of fraction I (18S) leaf protein from lucerne (Medicago sativa L). Br. J. Nutr. 46: 39–58. doi: 10.1079/BJN19810007 [DOI] [PubMed] [Google Scholar]
- 27.Omachi Alpine Museum.1992. Rock Ptarmigan−Life History and Challenge of Captivity. Shinano Mainichi Shinbun Press, Nagano (in Japanese). [Google Scholar]
- 28.Salgado-Flores A., Tveit A. T., Wright A. D., Pope P. B., Sundset M. A.2019. Characterization of the cecum microbiome from wild and captive rock ptarmigans indigenous to Arctic Norway. PLoS One 14: e0213503. doi: 10.1371/journal.pone.0213503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R. K., Chang H. W., Yan D., Lee K. M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T. H., Bhutani T., Liao W.2017. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15: 73. doi: 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokos C. K., Birtsas P. K., Tsachalidis E. P.2008. The aims of galliforms release and choice of techniques. Wildl. Biol. 14: 412–423. doi: 10.2981/0909-6396-14.4.412 [DOI] [Google Scholar]
- 31.Trevelline B. K., Fontaine S. S., Hartup B. K., Kohl K. D.2019. Conservation biology needs a microbial renaissance: a call for the consideration of host-associated microbiota in wildlife management practices. Proc. Biol. Sci. 286: 20182448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuchida S., Ohara Y., Kuramochi K., Murata K., Ushida K.2017. Effective degradation of phenolic glycoside rhododendrin and its aglycone rhododendrol by cecal feces of wild Japanese rock ptarmigans. Jpn. J. Zoo Wildl. Med. 22: 41–45. doi: 10.5686/jjzwm.22.41 [DOI] [Google Scholar]
- 33.Tsuchida S., Kobayashi A., Murata K., Ushida K.2018. Isolation of cecal bacteria from wild Japanese rock ptarmigans and their functionalities. Proc. 14th Intl. Grouse Symp., Utah State University, Logan.
- 34.Ueda A., Kobayashi A., Tsuchida S., Yamada T., Murata K., Nakamura H., Ushida K.2018. Cecal microbiome analyses on wild Japanese rock ptarmigans (Lagopus muta japonica) reveals high level of coexistence of lactic acid bacteria and lactate-utilizing bacteria. Microorganisms 6: 77. doi: 10.3390/microorganisms6030077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uenishi G., Fujita S., Ohashi G., Kato A., Yamauchi S., Matsuzawa T., Ushida K.2007. Molecular analyses of the intestinal microbiota of chimpanzees in the wild and in captivity. Am. J. Primatol. 69: 367–376. doi: 10.1002/ajp.20351 [DOI] [PubMed] [Google Scholar]
- 36.Ushida K., Segawa T., Tsuchida S., Murata K.2016. Cecal bacterial communities in wild Japanese rock ptarmigans and captive Svalbard rock ptarmigans. J. Vet. Med. Sci. 78: 251–257. doi: 10.1292/jvms.15-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vispo C., Karasov W. H.1997. The interaction of avian gut microbes and their host: an elusive symbiosis. pp.116–155. In: Gastrointestinal Microbiology (Mackie R. I. and White B. A. eds.), Chapman and Hall Microbiology Series, Springer, Boston. [Google Scholar]
- 38.Wei S., Morrison M., Yu Z.2013. Bacterial census of poultry intestinal microbiome. Poult. Sci. 92: 671–683. doi: 10.3382/ps.2012-02822 [DOI] [PubMed] [Google Scholar]
- 39.West A. G., Waite D. W., Deines P., Bourne D. G., Digby A., McKenzie V. J., Taylor M. W.2019. The microbiome in threatened species conservation. Biol. Conserv. 229: 85–98. doi: 10.1016/j.biocon.2018.11.016 [DOI] [Google Scholar]
- 40.Wienemann T., Schmitt-Wagner D., Meuser K., Segelbacher G., Schink B., Brune A., Berthold P.2011. The bacterial microbiota in the ceca of Capercaillie (Tetrao urogallus) differs between wild and captive birds. Syst. Appl. Microbiol. 34: 542–551. doi: 10.1016/j.syapm.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 41.Young J. W.1977. Gluconeogenesis in cattle: significance and methodology. J. Dairy Sci. 60: 1–15. doi: 10.3168/jds.S0022-0302(77)83821-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.