Abstract
We previously reported a novel diagnostic method using follicle-sinus complexes (FSCs) in the muzzle skin for postmortem diagnosis of rabies in dogs. However, whether this method works in other animal species remains unclear. Here, FSCs were collected from a wolf, a red fox, 2 bats, and a cat, and examined for the presence of viral antigen, viral mRNA, and viral particles. Viral antigen and viral mRNA were confirmed in Merkel cells (MCs) in FSCs of all species. Electron microscopy performed using only samples from wolf and cat confirmed viral particles in MCs of FSCs. These results suggested that this novel diagnostic method using FSCs might be useful for detection of rabies not only in domestic but also wild animals.
Keywords: follicle-sinus complex, Merkel cell, postmortem diagnosis, wild animal
Rabies is an enzootic disease that poses a serious public health and economic problem in developing countries. Dogs constitute the main reservoir of the disease, followed by cats and other wild animals [13]. Currently, the most commonly used method for definite diagnosis of rabies in animals is the direct fluorescent antibody test (dFAT) using fresh brain sample [8, 15]. This method is rapid and sensitive; however, the sensitivity of the method decreases and it becomes unsuitable once the brain samples are decomposed under warm climate [1, 3, 5]. Actually, it is extremely difficult to collect fresh brain samples from the field, as infected animals are often found after death and carcasses are easily decomposed under warm climate. In addition, sampling of brain is laborious, and carries a high risk of exposure to viruses. Therefore, alternative specimens for diagnosis of rabies by low-cost, simple collection with low virus-exposure risk are needed in rabies-endemic countries.
Recently, we reported that follicle-sinus complexes (FSCs) in the muzzle skin could be a useful diagnostic material of rabid dogs [9,10,11]. Each FSC is known to be equipped with more than 2,000 sensory nerve endings [4]; therefore, it was thought that FSCs could be utilized as an alternative postmortem diagnostic material in dogs. However, their usefulness as a diagnostic material in different species, including wild and domestic animals, has not been studied. In the present study, FSCs were collected from wild animals and cats and subjected to histopathology, immunohistochemistry, in situ hybridization, and electron microscopy analyses for the development of novel diagnostic methods for rabies.
Formalin-fixed muzzle skin samples of 5 rabid animals, including 1 cat (Felis catus) from the Research Institute for Tropical Medicine (RITM) in the Philippines, 1 Mongolian wolf (Canis lupus chanco), and 1 red fox (Vulpes vulpes) from the Institute of Veterinary Medicine of Mongolian University, and 2 bats (Tadarida brasiliensis) from the Luis Pasteur Zoonosis Institute of Argentina, were submitted to our laboratory. The cat (3 years old, male) was free-roaming and had contact with the other animals both within and outside the household. The cat was euthanized because it showed primary clinical symptoms, such as restlessness and unprovoked aggressiveness. No information on the rabies vaccination status of this cat was available. The wolf was reported to have attacked tourists in the Mongolian national park and was euthanized by park staff. The red fox was found dead in Mongolia. The 2 bats from Argentina were euthanized because they showed symptoms, such as disorientation disorder.
Muzzle skin samples were re-fixed in 10% neutral-buffered formalin at 25°C for more than 72 hr. Subsequently, muzzle skins, including FSCs, were cut into transverse or longitudinal sections as recommended in our previous study [10]. Transverse sections were made at the level of the ring sinus of FSCs identified via stereoscopic microscopy (MZ7.5; Leica, Morrisville, NC, USA). Samples were embedded in paraffin, and sectioned at a thickness of 3 µm. Sections were stained with hematoxylin and eosin and serial sections were subjected to immunohistochemistry.
For detection of the rabies viral antigen in tissues, sections were stained with rabbit polyclonal rabies anti-phosphoprotein antibody (anti-P) using the polymer method as previously described [2, 12]. For identification of the cell type, the following primary antibodies were used: mouse monoclonal cytokeratin 20 (CK 20; Nichirei Biosciences, Tokyo, Japan) and mouse monoclonal cytokeratin CAM5.2 (CAM5.2; Becton-Dickinson, San Jose, CA, USA), as markers of Merkel cells (MCs) [6, 7]. Briefly, tissue sections were treated with 0.25% trypsin at 25°C for 30 min for anti-P antibodies, microwaved at 170 W for 10 min for anti-CK 20 antibodies, and treated with proteinase K for 10 min for anti-CK 20 and CAM5.2 antibodies. To remove endogenous peroxidase activity, all tissue sections were immersed in 0.3% H2O2 in methanol. To block nonspecific reactions, all sections were treated with 10% normal goat serum. Sections were incubated overnight with primary antibodies at 4°C in a humidified chamber (1:1,200 for anti-P, prediluted CK 20, and CAM5.2). Detection of primary antibodies was performed using Histofine® Simple StainTM MAX PO (rabbit) (Nichirei Biosciences) for anti-P and Histofine® Simple StainTM MAX PO (mouse) (Nichirei Biosciences) for anti-CK 20 and CAM5.2. Antibodies were visualized using 3-3′-diaminobenzidine (Nichirei Biosciences). Finally, slides were counterstained with hematoxylin.
To confirm the merge of viral antigen-positive cells and MCs, double immunofluorescence staining of a single tissue section was performed using immunofluorescent antibodies. FITC conjugated goat anti-rabbit IgG (H+L) (Southern Biotechnology Associates, Inc., Birmingham, AL, USA) and Alexa Fluor 546 conjugated goat anti-mouse IgG (H+L) (Thermo Fisher, Waltham, MA, USA) were used as secondary antibodies at 1:200 dilutions for the detection of the viral antigen (anti-P) and MCs (CAM5.2). In addition, Alexa Fluor 488 conjugated goat anti-chicken IgY (H+L) (Thermo Fisher) and Alexa Fluor 546 conjugated goat anti-rabbit IgG (H+L) (Thermo Fisher) were used as secondary antibodies at 1:200 dilutions for the detection of the viral antigen (anti-P) and MCs (CK 20), respectively. Finally, slides were counterstained and mounted with ProLongTM Gold Antifade Mountant with DAPI (Thermo Fisher).
To detect viral RNAs in FSCs, in situ hybridization was performed using paraffin blocks. RNAscope® probe-V-RABV-gp1 (NC 001542.1, region 59-1482) (ACD#456781) and RNAscope® 2.5 HD reagent kit-RED (Advanced Cell Diagnostics, ACD, Newark, CA, USA) were used as previously described [10, 14].
Transmission electron microscopy (TEM) was performed to observe viral particles in the MCs. TEM was only performed on the samples from wolf and cat, as autolysis had proceeded in the fox and bat samples. Paraffin-embedded tissue samples were cut into 1-mm blocks, deparaffinized, and dehydrated in acetone series. Tissue pieces were washed with phosphate-buffered saline, post-fixed for 12 hr at 25°C in 1% buffered osmium tetroxide, and embedded in epoxy resin. Approximately 70-nm-thick sections were stained with uranyl acetate and lead citrate and examined using a transmission electron microscope (H-7650, Hitachi, Tokyo, Japan).
Morphologically, no differences were observed in the structure of FSCs in each animal. We did not observe any histopathological findings and inclusion (Negri) bodies in any of the rabid animals.
Immunohistochemically, viral antigen-positive cells were shown to be concentrated in a part of the outer root sheath at the level of the ring sinus in the FSCs in all species (Fig. 1). Most of these cells were demonstrated to exhibit signals of both CK 20 and CAM5.2 antibodies following double immunofluorescence staining (Fig. 2). Occasionally, we observed viral antigen positivity in the peripheral nerves surrounding the FSCs. Despite sample conditions being poor in the case of the samples from the fox and bats, we could still confirm viral antigen positivity. In situ hybridization signals (Fig. 3) were detected in the cytoplasm of MCs of FSCs in all species, similar to those observed by immunohistochemistry.
Fig. 1.
Viral antigens were concentrated in a part of the outer root sheath of the follicle-sinus complexes at the level of the ring sinus. a: wolf, b: cat, c: red fox, d: bat. HS: hair shaft, OS: outer root sheath. Immunohistochemistry. Bar=100 µm.
Fig. 2.
Transverse sections of follicle-sinus complexes (FSCs) in the muzzle skin of wolf. CAM 5.2 (green, 2a), viral antigen (red, 2b), and double-positive signals (merged images, yellow, 2c), and CK 20 (red, 2d), viral antigen (green, 2e), and double-positive signals (merged images, yellow, 2f) are visible in a part of the outer root sheath of FSCs. OS: outer root sheath, RS: ring sinus. Immunofluorescence staining. Bar=50 µm.
Fig. 3.
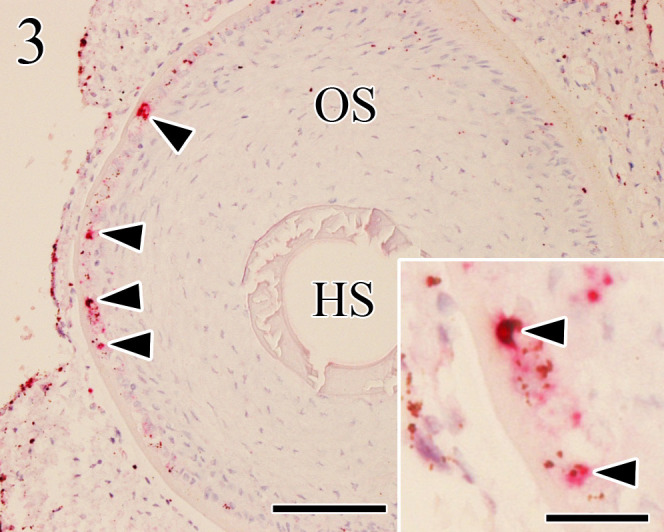
Positive signals (arrowheads) of in situ hybridization appear in the cytoplasm of Merkel cells of wolf follicle-sinus complexes (FSCs). In situ hybridization. HS: hair shaft, OS: outer root sheath. Bar=100 µm (insert, bar=25 µm).
Observation by electron microscopy revealed the presence of many matrix-like and viral-associated structures (Fig. 4) in the cytoplasm of MCs, as well as viral particles (approximately 150 to 200 nm in length, 70 nm wide) in the rough endoplasmic reticulum (Fig. 5), nerve endings, and matrix of MCs of FSCs from the samples of the cat and wolf.
Fig. 4.
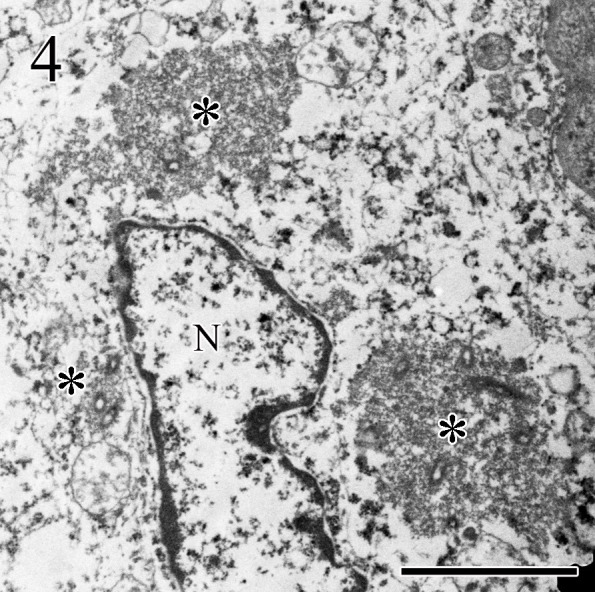
Three matrix structures (*) and viral-associated structures are observed in the cytoplasm of Merkel cells of follicle-sinus complexes of wolf. N: nuclear. Transmission electron microscopy. Bar=2 µm.
Fig. 5.

Bullet-shaped viral particles (arrowhead) are observed in the rough endoplasmic reticulum of Merkel cells of follicle-sinus complexes of wolf. Mi: mitochondria, N: nuclear. Transmission electron microscopy. Bar=500 nm.
In the present study, we investigated whether viral antigens, viral genome, and viral particles could be detected in MCs in various animal species. Accordingly, viral antigens were detected in MCs in all species, and this finding was further strengthened by the observed double positivity against viral antigen and MC markers in double immunofluorescence staining. Moreover, viral mRNA and viral particles were also observed by in situ hybridization and electron microscopy. Therefore, it was suggested that MCs of FSCs of the muzzle skin are target cells for viral infection by rabies in wolf, cat, fox, and bat. These results were similar to those of our previously reported study on rabid dogs [9,10,11], and it was thus concluded that our novel diagnostic method using FSCs might be a very useful alternative method for diagnosis of rabies not only in domestic but also wild animals. However, the number of animals examined in this study was very small. Therefore, further sample correction and objective evaluation using large sample numbers are required before application of this diagnostic method in the field.
Acknowledgments
This study was carried out with help from the Research Institute for Tropical Medicine (RITM) in the Philippines, Mongolian University of Life Sciences in Mongolia, and the Luis Pasteur Zoonosis Institute in Argentina. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Kakenhi No. 26450410), Heiwa Nakajima Foundation and AMED/JICA, SATREPS, Japan.
REFERENCES
- 1.Albas A., Ferrari C. I., da Silva L. H., Bernardi F., Ito F. H.1999. Influence of canine brain decomposition on laboratory diagnosis of rabies. Rev. Soc. Bras. Med. Trop. 32: 19–22. doi: 10.1590/S0037-86821999000100004 [DOI] [PubMed] [Google Scholar]
- 2.Boonsriroj H., Manalo D. L., Kimitsuki K., Shimatsu T., Shiwa N., Shinozaki H., Takahashi Y., Tanaka N., Inoue S., Park C. H.2016. A pathological study of the salivary glands of rabid dogs in the Philippines. J. Vet. Med. Sci. 78: 35–42. doi: 10.1292/jvms.15-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David D.2012. Role of the RT-PCR method in ante-mortem & post-mortem rabies diagnosis. Indian J. Med. Res. 135: 809–811. [PMC free article] [PubMed] [Google Scholar]
- 4.Halata Z.1993. Sensory innervation of the hairy skin (light- and electronmicroscopic study. J. Invest. Dermatol. 101Suppl: 75S–81S. doi: 10.1016/0022-202X(93)90505-C [DOI] [PubMed] [Google Scholar]
- 5.Kamolvarin N., Tirawatnpong T., Rattanasiwamoke R., Tirawatnpong S., Panpanich T., Hemachudha T.1993. Diagnosis of rabies by polymerase chain reaction with nested primers. J. Infect. Dis. 167: 207–210. doi: 10.1093/infdis/167.1.207 [DOI] [PubMed] [Google Scholar]
- 6.Moll I., Roessler M., Brandner J. M., Eispert A. C., Houdek P., Moll R.2005. Human Merkel cells-aspects of cell biology, distribution and functions. Eur. J. Cell Biol. 84: 259–271. doi: 10.1016/j.ejcb.2004.12.023 [DOI] [PubMed] [Google Scholar]
- 7.Narisawa Y., Hashimoto K., Nihei Y., Pietruk T.1992. Biological significance of dermal Merkel cells in development of cutaneous nerves in human fetal skin. J. Histochem. Cytochem. 40: 65–71. doi: 10.1177/40.1.1370310 [DOI] [PubMed] [Google Scholar]
- 8.OIE2013. Manual of diagnostic tests and vaccines for terrestrial animals. pp. 1185–1191. 7th ed., World Organisation for Animal Health, Paris. [Google Scholar]
- 9.Shimatsu T., Shinozaki H., Kimitsuki K., Shiwa N., Manalo D. L., Perez R. C., Dilig J. E., Yamada K., Boonsriroj H., Inoue S., Park C. H.2016. Localization of the rabies virus antigen in Merkel cells in the follicle-sinus complexes of muzzle skins of rabid dogs. J. Virol. Methods 237: 40–46. doi: 10.1016/j.jviromet.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 10.Shiwa N., Nakajima C., Kimitsuki K., Manalo D. L., Noguchi A., Inoue S., Park C. H.2018. Follicle sinus complexes (FSCs) in muzzle skin as postmortem diagnostic material of rabid dogs. J. Vet. Med. Sci. 80: 1818–1821. doi: 10.1292/jvms.18-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiwa N., Yamashita H., Tomioka K., Kimitsuki K., Manalo D. L., Inoue S., Park C. H.2019. Statistical analysis of the usefulness of follicle-sinus complexes as a novel diagnostic material for canine rabies. J. Vet. Med. Sci. 81: 182–185. doi: 10.1292/jvms.18-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoji Y., Inoue S., Nakamichi K., Kurane I., Sakai T., Morimoto K.2004. Generation and characterization of P gene-deficient rabies virus. Virology 318: 295–305. doi: 10.1016/j.virol.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Singh R., Singh K. P., Cherian S., Saminathan M., Kapoor S., Manjunatha Reddy G. B., Panda S., Dhama K.2017. Rabies-epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review. Vet. Q. 37: 212–251. doi: 10.1080/01652176.2017.1343516 [DOI] [PubMed] [Google Scholar]
- 14.Wang F., Flanagan J., Su N., Wang L. C., Bui S., Nielson A., Wu X., Vo H. T., Ma X. J., Luo Y.2012. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 14: 22–29. doi: 10.1016/j.jmoldx.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization.2013. WHO expert consultation on rabies. Second report. World Health Organ. Tech. Rep. Ser. 982: 1–139. [PubMed] [Google Scholar]




