Abstract
Pandemic SARS-CoV-2 infection has rapidly developed into a socioeconomic and humanitarian catastrophe. Basic principles to prevent SARS-CoV-2 transmission are social distancing, face masks, contact tracing and early detection of SARS-CoV-2. To meet these requirements, virtually unlimited test capacities delivering results in a rapid and reliable manner are a prerequisite. Here, we provide and validate such a rapid, convenient and efficient kit-independent detection of SARS-CoV-2 RNA, termed COVID-quick-DET. This straightforward method operates with simple proteinase K treatment and repetitive heating steps with a sensitivity of 94.6% in head-to-head comparisons with kit-based isolation methods. This result is supported by data obtained from serially diluted SARS-CoV-2 virus stocks. Given its cost- and time-effective operation, COVID-quick-DET might be best suited for countries with general shortage or temporary acute scarcity of resources and equipment.
Keywords: COVID-19, SARS-CoV-2, Proteinase K, Heating, Kit-free RNA extraction
1. Results and discussion
The current pandemic coronavirus has spread rapidly over the world to become the major medical and socioeconomic burden of the current and past era. Infections and deaths continue to rise worldwide: at least 26.347.573 people have been infected so far, and 869.600 humans with Severe-Acute-Respiratory-Syndrome-Corona-Virus-2 (SARS-CoV-2) infection died (status 04.09.2020, 5 pm; (https://coronavirus.jhu.edu/map.html)). The exponential increase of the new corona virus disease (COVID-19) limits broad testing of patients and medical staff in industrialized and in particular economically less developed countries. The latter are currently faced with an overwhelming caseload (https://coronavirus.jhu.edu/map.html). For instance, in the African continent 29–44 million infected people are predicted for the next year (https://www.afro.who.int). Conversely, limited test capacities prevent (i) the correct assessment of the infection rate in a given population, (ii) the tracing of potentially infected individuals, and (iii) thus, the spread across the entire population. This is particularly important as almost 20 % of infected individuals are asymptomatic and transmission occurs unhindered (Mizumoto et al., 2020). Amongst others, South Korea has conducted most tests worldwide, considered as the basis for rapid disease control. Accordingly, a recent simulation calculated a required daily test capacity between 0.7–3.6 tests per thousand residents depending on the mitigation policies in place to contain the disease (Fiore et al., 2020). Current SARS-CoV-2 tests mostly depend on the costly isolation of viral RNA elements using viral RNA isolation kits from human specimen (Corman et al., 2020). However, those kits and recently even viral swab kits have become increasingly scarce, thus delaying the provision of SARS-CoV-2 RNA as a starting material for subsequent RT-qPCR (Corman et al., 2020). Long delivery times and uncertain deliveries per se, particularly into less urbanized country parts further complicate this situation (https://www.the-scientist.com/). Finally, kit-based RNA isolations represent a major expense factor within the worldwide commercial crisis caused by COVID-19, a particular obstacle in economically less developed countries.
Here, we provide a simple, short and fast protocol to extract SARS-CoV-2 RNA from throat swaps and other materials from the respiratory tract without the necessity to use viral RNA isolation kits (referred to as COVID-quick-DET). More than 40.000 patients and health care professionals have been so far routinely tested in our facility using customized total nucleic acid purification kits followed by subsequent SARS-CoV-2-RNA RT-qPCR (Corman et al., 2020; https://www.who.int/). Out of those, more than 230 specimens have been detected positive for SARS-CoV-2. To operate independently from RNA isolation kits and to accelerate sample processing, we developed COVID-quick-DET: First, a small amount of swab storage solution or the same amount of normal saline applied to resuspend the dry swab via vortexing (1 mL normal saline, 5 s) were used as an input for subsequent proteinase K treatment. Specifically, 3 μL proteinase K (20 mg/mL) was placed in the lid of an Eppendorf tube. 80−100 μl of the patient sample was pipetted into the Eppendorf tube. After closing the lids, all samples were pulse-spun for 2–5 seconds at 8.000 rpm. This was followed by an incubation of 3 min at 56 °C, then 3 min at 95 °C to inactivate the proteinase. Heating to 56 °C or higher has been previously shown to safely inactivate SARS coronavirus, which is structurally similar to SARS-CoV-2 (Darnell et al., 2004; Kariwa et al., 2006). After a short centrifugation step, 5 μL of the sample was then added to the PCR master mix and the RT-PCR is carried out. Primer and probe were published elsewhere by Corman et al. (Corman et al., 2020) to detect the E gene. Confirmation of positive results was provided by proof of ORF1b-nsp14 (Chu et al., 2020; https://www.who.int/).
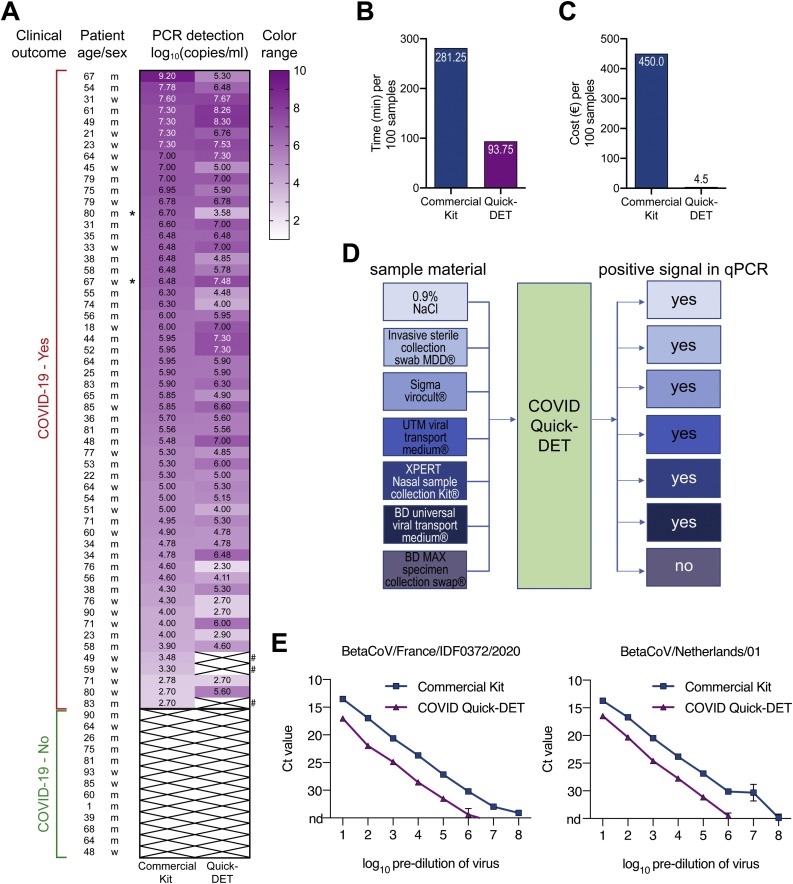
A head-to-head pilot comparison starting from the same SARS-CoV-2 positive specimens (n = 56; 54 nasopharyngeal swabs, 2 bronchoalveolar lavages) demonstrated that COVID-quick-DET generates similar SARS-CoV-2 gene equivalents as the custom nucleic acid purification kit. While three samples from asymptomatic individuals did not reach the detection limit, all symptomatic COVID-19 patients could be identified with COVID-quick-DET [Fisher’s exact test, two-tailed, not significant, P-value 0.2432; (Fig. 1 A, gene expression values); COVID-quick-DET sensitivity: 94.64 %]. We also tested 13 COVID-19 negative specimen with both methods and did not observe any false positive results using COVID-quick-DET method (Fisher’s exact test, two-tailed, not significant, P-value 1.000). We have applied the method for testing clinical staff: All of the approximately 600 employees tested were healthy at the time. All tests were negative (below the detection limit). To the best of our knowledge, none of them was positive when tested with a kit-based viral RNA isolation method during the following 2–3 weeks and without evident COVID-19 symptoms. In addition, COVID-quick-DET can be conducted in any laboratory using standard molecular biology laboratory reagents and provides RNA suitable for quantitative PCR analytics within appx. 90 min vs. 280 min kit-based per 100 samples (Fig. 1B).
Fig. 1.
(A) Heat map of SARS-CoV-2 gene equivalents (log10) for 56 COVID-19 patients using a commercial RNA isolation kit or COVID-quick-DET. The colors in the heat map represent gene expression levels (log10). White crossed boxes marked with “#” represent the samples giving discrepant results between COVID-quick-DET and the reference method. Samples taken from bronchoalveolar lavages are indicated by asterisk. (B) Time for RNA isolation per 100 samples in minutes. (C) Costs for RNA isolation per 100 samples in Euro. (D) Schematic workflow of tested material compatible with COVID-Quick-DET method and successful qPCR. (E) Detection of SARS-CoV-2 ORF1b-nsp14 in samples prepared by serial dilution of virus stocks of two strains in medium (French-left panel; Dutch-right panel). Viral RNA was either extracted by Qiagen Viral RNA Mini Kit (Qiagen #52906) or prepared by the COVID-quick-DET protocol before being subjected to RT-qPCR as described (Chu et al., 2020; https://www.who.int/). For direct comparison to COVID-quick-DET, RNA was eluted in the original sample volume without concentration using Qiagen Viral RNA Mini Kit. Ct 35 was set as cut-off. Reactions have been run in duplicates, values are mean ± SD. N.d. = not detected (Ct => 35).
Finally, we calculated costs per testing 100 individuals with either method and observed a 100-fold reduction (4.50€ vs. 450€; Fig. 1C), a relevant issue in light of an average testing rate of appx. 400.000 tests per week in the accredited laboratories of Germany (https://www.alm-ev.de). Finally, COVID-quick-DET operates equally effective in most material collection devices, and most importantly also in normal saline upon a dry Q-tip swab (Fig. 1D). However, COVID-quick-DET did not detect all SARS-CoV-2 true positive human specimen. To better define the detection threshold of our method, we performed serial dilutions of SARS-CoV-2 virus stocks of two strains in medium followed by viral RNA extraction either by COVID-quick-DET or by a commercial kit [BetaCoV/France/IDF0372/2020 (#014V-03890) and BetaCoV/Netherlands/01/NL/2020 (#010V-03903); (https://www.european-virus-archive.com/evag-portal/field_product_type/virus-55)]. Extracted RNA was subjected to RT-qPCR for SARS-CoV-2 ORF1b-nsp14 in the prepared samples. Confirming sensitivity observed for clinical samples, COVID-quick-DET operates reliably across a wide range of viral dilutions (>6 log units), however, the threshold is around 3–4 cycles below the commercial reference RNA extraction method (Fig. 1E). Accordingly, the detection limit of COVID-quick-DET is a cycle threshold (ct value) of around 31–33, corresponding to approximately 5.000–10.000 genome equivalents per milliliters.
Thus, COVID-quick-DET clearly has limitations: In our head-to-head tested cohort, this limitation would have led to 5.35 missed patients per 100 truly SARS-CoV-2 infected individuals. Moreover, we noticed that COVID-quick-DET works best on nasopharyngeal swabs but fails to deliver reliable results when applied to tracheal secretion, obtained during tracheotomy (data not shown). We hypothesize that the high protein content of this mucus/protein-rich specimen seriously affects PCR efficiency (Bai et al., 2018). However, in a situation with shortened resources such emergency protocols with spared costs and time are urgently needed. Ahead of the pandemic hitting economically less developed countries, COVID-quick-DET might be a solution to provide any test opportunity instead of none to those regions in the world. We believe that applying this simple and fast SARS-CoV-2 RNA sample protocol to many laboratories worldwide will contribute to circumvent shortage of commercially available viral RNA isolation kits for testing and identifying COVID-19 positive patients and medical staff. Thereby, COVID-quick-DET will offer a key test resource to developing and resource-limited countries but also less urbanized regions that are likely to become severely affected in the near future by COVID-19.
2. Conclusion
We provide a rapid, convenient and efficient kit-independent detection of SARS-CoV-2 RNA, circumventing shortage of viral RNA isolation kits for testing and identifying SARS-CoV-2 infected individuals.
CRediT authorship contribution statement
Detlef Michel: Conceptualization, Methodology, Investigation, Data curation, Validation, Writing - original draft, Writing - review & editing. Karin M. Danzer: Conceptualization, Writing - original draft, Writing - review & editing, Visualization, Supervision, Funding acquisition. Rüdiger Groß: Investigation. Carina Conzelmann: Investigation. Janis A. Müller: Investigation. Axel Freischmidt: Investigation. Jochen H. Weishaupt: Investigation. Sandra Heller: Visualization. Jan Münch: Supervision, Writing - review & editing. Manuela Michel: Investigation. Thomas Stamminger: Resources, Conceptualization, Software, Supervision, Project administration, Funding acquisition. Alexander Kleger: Conceptualization, Writing - original draft, Writing - review & editing, Visualization, Supervision, Funding acquisition. Markus Otto: Project administration, Conceptualization, Funding acquisition.
Declaration of Competing Interest
The authors do not have any conflict of interest
Acknowledgements
This study was supported by the COVID-19 research funding of the state Baden-Wuerttemberg to A.K., M.O., T.S., and J.M., and the H2020 Fight-nCoV programme to J.M. and by the Deutsche Forschungsgemeinschaft (DFG) Emmy Noether Research Group DA 1657/2-1 (KMD). R.G. and C.C. are part of and R.G. is funded by a scholarship from the International Graduate School in Molecular Medicine Ulm.
References
- Bai Y., Xiao Y., Suo Y., Shen Y., Shao Y., Zhang D., Zhou C. Enhancement of PCR sensitivity and yield using thiol-modified primers. Sci. Rep. 2018;8:14858. doi: 10.1038/s41598-018-33223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore V.G., DeFelice N., Glicksberg B.S., Perl O., Shuster A., Kulkarni K., O’Brien M., Pisauro M.A., Chung D., Gu X. 2020. Containment of Future Waves of COVID-19: Simulating the Impact of Different Policies and Testing Capacities for Contact Tracing, Testing, and Isolation. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(Suppl 1):119–123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
https://www.alm-ev.de, https://www.alm-ev.de/files/site-files/08%20Pressemitteilungen%20ALM/2020/Insights%20Praesentationen/200721-ALM-PK-Corona-Diagnostik-Insights-KW29.pdf.
https://coronavirus.jhu.edu/map.html.
https://www.afro.who.int, https://www.afro.who.int/news/new-who-estimates-190-000-people-could-die-covid-19-africa-if-not-controlled.
https://www.european-virus-archive.com/evag-portal/field_product_type/virus-55.
https://www.the-scientist.com/, https://www.the-scientist.com/news-opinion/rna-extraction-kits-for-covid-19-tests-are-in-short-supply-in-us-67250.
https://www.who.int/, https://www.who.int/docs/default-source/coronaviruse/peiris-protocol-16-1-20.pdf?sfvrsn=af1aac73_4.