Abstract
Purpose
Dexamethasone has been widely used to treat acute inflammatory diseases and endotoxic shocks in animal models. Meloxicam is one of the most commonly used anti-inflammatory agents in avian species. However, little is known about the effects of dexamethasone and meloxicam on lipopolysaccharide (LPS)-induced acute inflammatory response in birds. In the present study, LPS-challenged broiler chickens were used to investigate the comparative protective effects of meloxicam and dexamethasone on LPS-induced acute inflammatory responses.
Methods
Lipopolysaccharide (LPS)-induced acute lung injury (ALI) histopathological scores, selected serum acute phase reactants, inflammatory mediators, and gangliosides were evaluated in broiler chickens inoculated with E. coli LPS and simultaneously treated with two doses of meloxicam (0.5 and 2 mg/kg BW) and dexamethasone (2 and 4 mg/kg BW).
Results
LPS-induced ALI scores were not significantly different between the meloxicam-treated, dexamethasone-treated, and untreated positive control groups at 4 hours after LPS inoculation. Interleukin-6 concentrations were also statistically the same among the positive control, dexamethasone-treated, and meloxicam-treated groups at 3 and 12 hours after LPS inoculation. However, these anti-inflammatory drugs reduced adenosine deaminase, ceruloplasmin, lipid-bound sialic acid, protein-bound sialic acid, and total sialic acid in LPS-inoculated broiler chickens at 12, 24, and 48 hours after LPS inoculation in a drug- and dose-dependent manner. Ovotransferrin concentrations were not significantly different between positive control and treatment groups at 12 hours after LPS inoculation. However, twenty-four hours after LPS inoculation, all the treated groups, except the one treated with 0.5 mg/kg meloxicam, showed significantly lower concentrations of ovotransferrin as compared with the positive control group.
Conclusion
Our results showed that dexamethasone was more effective than meloxicam in inhibiting the LPS-induced response in broiler chickens by diminishing the serum levels of adenosine deaminase, ceruloplasmin, and gangliosides.
Keywords: lipopolysaccharide, meloxicam, dexamethasone, interleukin-6, acute phase proteins, chickens
Introduction
Lipopolysaccharide (LPS), or bacterial endotoxin, is a major pathogenic part of the outer membrane of Gram-negative bacteria and is considered to have an important role in the onset of Gram-negative sepsis, inflammation, shock, failure of multiple organs, and finally occurrence of high mortality.1 The inoculation of chickens with LPS has led to significant changes in the plasma proteome within 12-hour post infection.2 Inflammation is a complex homoeostatic process which is associated with many infectious diseases and consequently leads to a prominent systemic critical reaction known as acute phase response (APR).3,4 The APR, following the inflammatory process triggered by LPS from Gram-negative bacteria involves variations in the serum levels of different acute phase proteins (APPs), cytokines, enzymes, and metabolites with the aim of restoring the physiologic homeostasis of the host organism.5,6 Therefore, acute endotoxemia could be considered as an appropriate inflammation model for gaining insight into the inflammatory processes.7 Alterations in the circulating levels of APPs are associated with the onset and durability of LPS inflammation; accordingly, APPs have been widely used as appropriate markers for investigating the health status of humans and other mammals.8,9
Upon exposure to various inflammatory conditions, such as acute bacterial endotoxemia resulting from LPS inflammation, circulating leukocytes release pro-inflammatory cytokines, which are necessary for the recruitment of neutrophils to the site of inflammation and the regulation of different metabolic responses.10–12 These cytokines are involved in the regulation of the synthesis of APPs in the liver and have a mediating role in inducing the febrile response during inflammation and the development of the inflammation process by raising APPs.13
The need for gaining knowledge on different aspects of immune reactions of chickens during infections and different environmental or nutritional situations has led to the increased interest for investigations on APPs in chickens.14 Adenosine deaminase (ADA) is an enzyme that accelerates the conversion of adenosine to inosine and regulates extracellular adenosine and inosine concentrations in mammals.15 ADA, as an endogenous regulator of the innate immune system, is essential in the proliferation and differentiation of T lymphocytes. In addition, ADA is involved in controlling the magnitude of purinergic response under physiological conditions and, to a larger extent, pathological events, such as inflammation.16,17 Ovotransferrin, as a positive APP in chickens, increases during the APR triggered by a variety of experimentally induced inflammatory reactions and performs its bacteriostatic role by diffusing through the outer membrane of Gram-negative bacteria.18,19 Serum ovotransferrin is used as a biomarker of inflammatory diseases in chickens.20 Ceruloplasmin is a multifunctional antioxidant protein which accumulates and transports the copper within the body. The role of ceruloplasmin as an APP became evident by observing that its level increases in different infectious diseases.21
As a family of neuraminic acid derivatives, sialic acids are involved in many biological and pathological phenomena located at the end chain of many APPs. The majority of sialic acids found are either protein-bound or lipid-bound, and only a few of them are in free form.22 Sialic acids have a principal role in cell-to-cell identification and interaction, which mediate various cell-cell adhesion processes in the inflammation and immune response.23 Sialic acids concentration increases immediately following various bacterial and viral inflammatory responses associated with various diseases in humans and animals. Hence, the evaluation of sialic acids concentration may be helpful in the diagnosis and prognosis of inflammatory diseases.24–26
Non-steroidal anti-inflammatory drugs (NSAIDs) are extensively used as analgesics to alleviate pain and treat inflammatory musculoskeletal diseases and other inflammatory ailments, such as LPS endotoxemia. The NSAIDs mechanism of action is to inhibit prostaglandins and thromboxanes production as the major mediators in the inflammation process. This feature of NSAIDs makes them a more appropriate candidate, compared to their steroidal counterparts, to be applied in a targeted approach for treating inflammation.27
Meloxicam is a COX-2-selective NSAID in its therapeutic dose. However, the COX-2 specificity of meloxicam decreases at high doses and the drug could bind to COX-1 with some controversial effects.28 Pharmacokinetics and toxicological effects of meloxicam have been evaluated in chickens and other avian species.29 Empirical dose ranges of meloxicam (0.5–2.0 mg/kg BW) has been used for the various inflammatory conditions and postoperative pain managements in different bird species with no adverse effects on either the renal and intestinal organs or the immune and hematopoietic system of birds.29 These qualifications explain why meloxicam has become the most commonly used anti-inflammatory medication in avian species.30
Dexamethasone, as a synthetic derivative of cortisol, targets the phospholipid-arachidonate cascade to control inflammatory reactions.31 It has been shown that dexamethasone can modulate the production of cytokines and acute phase proteins in the acute phase of inflammation.32,33 Despite the limitations specified in the pharmacopeia regarding the use of dexamethasone as a steroidal drug, its use has been approved in few cases such as septic shock and Gram-negative endotoxemia with a dose range of 2–4 mg/kg in different avian species.30,34,35
To our knowledge, no report is available about the comparative effects of dexamethasone and meloxicam on magnitude of the acute inflammatory response induced by bacterial LPS in avian species. In the present study, based on the previously described LPS-induced inflammation model in broiler chicken,36–38 LPS-challenged broiler chickens were used to investigate the comparative protective effects of these anti-inflammatory compounds on LPS-induced acute inflammatory responses.
Materials and Methods
Animals
Fifty-four one-day-old Ross 308 male chicks were provided by a local commercial poultry farm (Fars province, Iran) and raised in an environmentally controlled poultry house in the Animal Research Unit of Shiraz University Veterinary School for five weeks under the standard environmental conditions and in compliance with the production parameters recommended by the broiler producer company.39 All the birds used in this experiment were handled in accordance with the technical regulations and the guidelines set out by the committee of animal ethics of Shiraz University, Iran. The protocols of the study were approved by the Ethics Committee of Shiraz University (IACUC no: 4687/63).
Experimental Group
The chickens, aged five weeks, were divided into six groups of 9 birds each. The mean body weight of all chickens was 1.65 ± 0.2 kg. The positive LPS group was intravenously (IV) injected with LPS of E. coli O55 B5 (Sigma-Aldrich, USA) at a dose of 0.5 mg/kg. The birds in the negative control group were injected with the same volume of pyrogen-free water.
The other four groups were treated with meloxicam 5% (Razak, Iran) and dexamethasone 0.2% (Razak, Iran), as anti-inflammatory drugs, in combination with LPS. Immediately after the IV inoculation of LPS, meloxicam and dexamethasone were intramuscularly (IM) injected in the pectoral muscles at two different doses, ie 0.5 mg/kg, 2 mg/kg and 2 mg/kg, 4 mg/kg, respectively.40
Histological Examination
Four hours after LPS administration, three chickens were randomly selected from each group and euthanized. Their upper right lung lobes were removed and stored in 10% buffered formalin for one week. The samples were dehydrated, embedded in paraffin, cut into 5-µm sections, and stained with hematoxylin and eosin (H&E) according to standard procedures. Four microscopic fields (100×), which contained at least a tertiary bronchus per field, per tissue sample for each bird were randomly chosen and observed by a light microscope. Five high-power fields (HPF), containing an interatrial septum, were randomly assigned to each field. All sections were scored according to the previously described criteria for LPS-induced acute lung injury,41 with some modifications for chicken lungs. In brief, the thickness of the interatrial septum, infiltration of inflammatory cells, and hemorrhage were scored in each HPF using a blinded approach. Acute lung injury scores (ALI scores) for each item was categorized according to the following scale: 0: minimum damage; 1: mild damage; 2: moderate damage; 3: severe damage; 4: maximum damage. Accordingly, the final ALI score for each field varied within a range from 0 to 12.
Serum Sampling
Blood was collected from the jugular vein at 3 h and 12 h after LPS injection to determine IL-6 concentration and at 12, 24, and 48 h after LPS injection to measure the adenosine deaminase, ceruloplasmin, ovotransferrin and, gangliosides (TSA, LBSA, PBSA). The sera were separated by centrifugation at 750g for 15 min and stored at −20 C until further use.
IL-6 and Ovotransferrin Assays
The serum levels of IL-6 and ovotransferrin were measured using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) and commercial chicken-specific kits (Shanghai Crystal Day Biotech, Shanghai, China). The sensitivity of IL-6 and ovotransferrin kits was 0.53 ng/L and 0.024 mg/mL, respectively. The intra-assay and inter-assay precision of these kits were CV < 8% and CV < 10%, respectively.
Gangliosides (TSA, LBSA, PBSA) Assay
Serum total sialic acid (TSA) concentration was determined by the thiobarbituric acid method. Lipid-bound sialic acid (LBSA) concentration was determined using the method described by Katopodis et al.42 Protein-bound sialic acid (PBSA) concentration was measured by subtracting LBSA from the serum TSA.
Adenosine Deaminase (ADA) Assay
ADA concentration was assessed by an enzymatic-calorimetric assay kit (Diazyme Laboratories, Gregg Court, California, USA).
Ceruloplasmin Determination
The measurement of the serum ceruloplasmin level was performed with the method suggested by Bestujeva and Kolb.43
Statistical Analysis
Results are expressed as mean ± SD. For the analysis of serum data, one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test, was used. In cases of a failed normality test, Kruskal–Wallis ANOVA was performed followed by Dunn’s post hoc test. For histopathological scoring, non-parametric Mann–Whitney U-test was performed. Statistical analyses were conducted with SPSS software (version 16.0, SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered significant.
Results
Histopathology
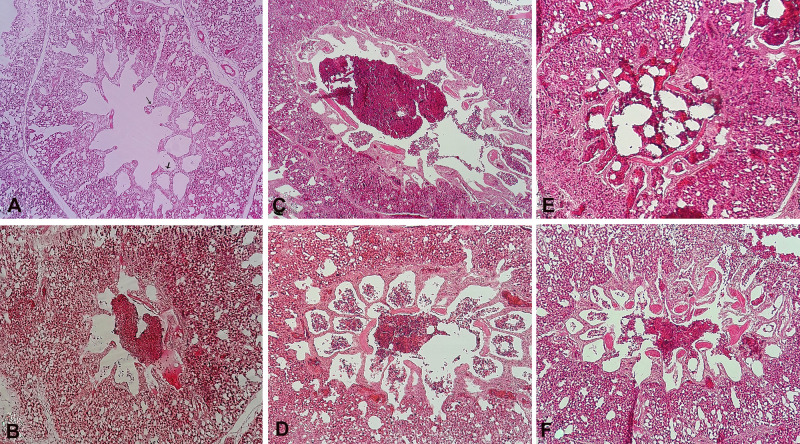
The histopathological examination of H&E-stained lungs showed that LPS treatment, compared to the control condition (negative control: Figure 1A), clearly stimulated a diffuse inflammatory response, severe hemorrhage, and thicker interatrial septum as observed in the positive control (Figure 1B), meloxicam-treated, and dexamethasone-treated groups (Figure 1C–F).
Figure 1.
The histopathological features of IV injection of LPS E. coli O55 B5 with or without meloxicam and dexamethasone treatment on lung injury (H&E, ×100). (A) Negative control: four hours after IV injection of pyrogen-free water, arrow: interatrial septum; (B) Positive control: four hours after IV injection of LPS E. coli O55 B5; (C) LPS+ meloxicam 0.5: four hours after IV injection of LPS E. coli O55 B5 and IM injection of 0.5 mg/kg meloxicam; (D) LPS+ meloxicam 2: four hours after IV injection of LPS E. coli O55 B5 and IM injection of 2 mg/kg meloxicam; (E) LPS+ dexamethasone 2: four hours after IV injection of LPS E. coli O55 B5 and IM injection of 2 mg/kg meloxicam; (F) LPS+ dexamethasone 4: four hours after IV injection of LPS E. coli O55 B5 and IM injection of 4 mg/kg meloxicam.
Abbreviations:E. coli, Escherichia coli; H&E, hematoxylin and eosin; IM, intramuscular; IV, intravenous; LPS, lipopolysaccharide.
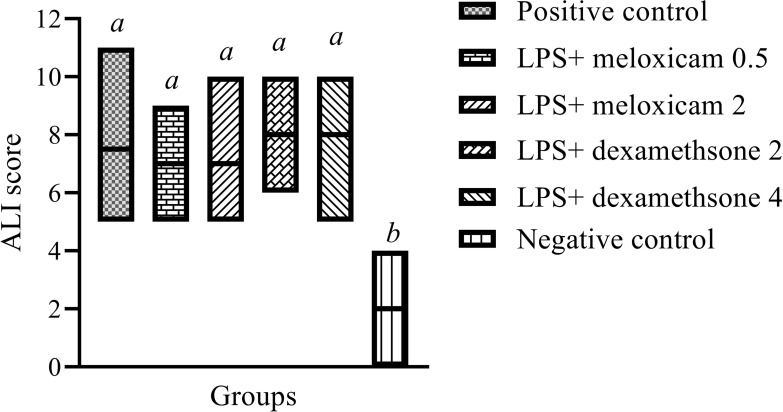
Lipopolysaccharide-induced ALI scores were not significantly different between the meloxicam-treated, dexamethasone-treated, and untreated positive control groups (P> 0.05) (Figure 2).
Figure 2.
Histopathological LPS-induced ALI scores in lung 4 hours after IV injection of LPS E. coli O55 B5 with or without meloxicam and dexamethasone treatment. For histopathological scoring, the results were expressed as box plots with median (minimum to maximum) and comparison was made using the non-parametric Mann–Whitney U-test.
Note:a,bColumns with different superscripts are statistically different at P<0.05.
Abbreviations: ALI, acute lung injury; E. coli, Escherichia coli; IV, intravenous; LPS, lipopolysaccharide.
Interleukin-6 and Acute Phase Proteins
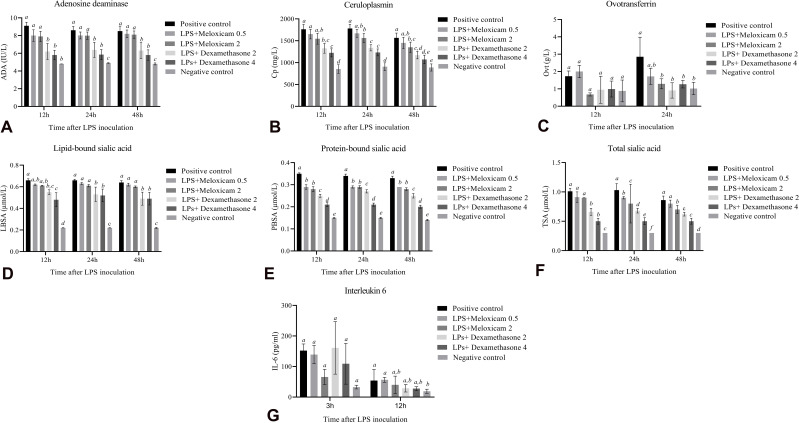
The concentration of ADA in the positive control at 12, 24, and 48 hours after LPS inoculation was significantly higher than that of the negative control (P≤0.05). Meloxicam treatment (0.5 and 2 mg/kg) had no significant effects on ADA concentration in comparison with the positive control group (P> 0.05). In contrast, dexamethasone treatment (2 and 4 mg/kg) significantly lowered the ADA concentration in comparison with the positive control and meloxicam-treated groups at 12, 24, and 48 hours after LPS inoculation (P≤0.05) (Figure 3A). Ceruloplasmin concentrations were not significantly different between the positive control group and 0.5 mg/kg meloxicam-treated group at 12, 24, and 48 hours after LPS inoculation (P> 0.05). However, 2 mg/kg meloxicam significantly decreased ceruloplasmin concentrations in comparison with the positive control group at the same time points mentioned above (P≤0.05). On the other hand, ceruloplasmin concentrations were significantly reduced in 2 and 4 mg/kg dexamethasone-treated groups compared with those of the positive control group (P≤0.05) (Figure 3B). Ovotransferrin concentrations were not significantly different between the positive control and treatment groups at 12 hours after LPS inoculation (P> 0.05). Twenty-four hours after LPS inoculation, all the treated groups, except the one treated with 0.5 mg/kg of meloxicam, had significantly lower concentrations of ovotransferrin compared with the positive control group (P≤0.05). Unfortunately, it was not possible to measure ovotransferrin level at 48 hours after inoculation because of inadequate serum storage (Figure 3C).
Figure 3.
The effects of meloxicam and dexamethasone treatments on the serum concentration of IL-6 (3 and 6 hours after IV injection of LPS E. coli O55 B5) and acute phase proteins (12, 24, and 48 hours after IV injection of LPS E. coli O55 B5). (A) ADA, (B) Cp, (C) Ovt, (D) LBSA, (E) PBSA, (F) TSA, and (G) IL-6. Data are expressed as means ± SD (n=9).
Note:a,b,c,d,e,fColumns with different superscripts are statistically different at P<0.05.
Abbreviations: ADA, adenosine deaminase; Cp, ceruloplasmin; E. coli, Escherichia coli; IL-6, interleukin-6; IV, intravenous; LBSA, lipid-bound sialic acid; LPS, lipopolysaccharide; Ovt, ovotransferrin; PBSA, protein-bound sialic acid; TSA, total sialic acid.
The injection of 0.5 and 2 mg/kg meloxicam did not significantly alter the lipid-bound sialic acid concentration compared with that of the positive control group (P> 0.05). On the other hand, the injection of 2 and 4 mg/kg dexamethasone significantly changed the lipid-bound sialic acid concentration in comparison with that of the positive control group at 12, 24, and 48 hours after LPS inoculation (P≤0.05) (Figure 3D). Different doses of meloxicam and dexamethasone significantly decreased the concentration of protein-bound sialic acid in comparison with that of the positive control group (P≤0.05). Dexamethasone was significantly more effective than meloxicam in decreasing the concentration of protein-bound sialic acid at 12, 24, and 48 hours after LPS inoculation (P≤0.05) (Figure 3E). The total sialic acid level was not significantly different between the positive control and meloxicam-treated groups at 12 hours after LPS inoculation (P> 0.05). However, dexamethasone significantly decreased the total sialic acid level in comparison with that of the positive control and meloxicam-treated groups at 12 hours after LPS inoculation (P≤0.05). Twenty-four hours after LPS inoculation, dexamethasone and meloxicam significantly reduced the total sialic acid level in a dose-dependent manner compared with that of the positive control group. Forty-eight hours after LPS inoculation, the effect of 0.5 mg/kg meloxicam treatment on the total sialic acid level was not significantly different from that of the positive control (P> 0.05). On the other hand, 2 mg/kg meloxicam and dexamethasone treatments significantly decreased the total sialic acid level in comparison with that in the positive control and 0.5 mg/kg meloxicam-treated groups (P≤0.05) (Figure 3F).
Interleukin-6 concentrations were not significantly different between the positive control and treatment groups at 3 and 12 hours after LPS inoculation (P> 0.05) (Figure 3G).
Discussion
The present study was designed to explore the extent that dexamethasone or meloxicam could modulate LPS-induced lung inflammation, serum APPs concentration, and IL-6 level in broiler chickens. Some special features of avian lungs, such as a single basal lamina and a thin squamous epithelium layer at the blood-gas interface, predispose their respiratory systems to bacterial pathogens and possibly LPS-induced acute lung injury.44 In some previous studies, E. coli-derived LPS were used to induce the model of acute lung injury in chickens.37,44,45 In the current experiment, LPS treatment in the positive control group clearly stimulated a diffuse inflammatory response, severe hemorrhage, and thicker interatrial septum, which resulted in higher ALI scores in the positive control group as compared with those in the negative control group. Ansari et al44 found indistinguishable margins between different parts of the pulmonary lobules, along with a narrowed lumen of the pulmonary atria and obvious congestion and heavy leukocytes infiltration of the pulmonary parenchyma 12 hour after LPS inoculation to broiler chickens. Intravenous LPS administration to the broiler chickens also caused a decrease in circulating white blood cells and appreciable sequestration of the leukocytes to the lungs.37 In our study, LPS-induced ALI scores were not significantly different among the dexamethasone-treated, meloxicam-treated, and untreated positive control groups. The effects of dexamethasone and meloxicam administrations on the LPS-induced acute lung injuries has not been studied previously in avian species. However, some previous evidence show that pre- and simultaneous treatment with dexamethasone can attenuate LPS-induced acute lung injury in mouse models.46,47 In addition, Dexamethasone inhibited LPS-induced hydrogen sulphide biosynthesis in a mouse model which can contribute to the anti-inflammatory effect of dexamethasone in endotoxic shock.48 Moreover, it has been shown that meloxicam could decrease endotoxin-induced acute lung injury in rabbits.49
Interleukin-6 (IL-6) is a multifunctional cytokine that plays a major role in regulating immune responses, acute phase reactions, and hematopoiesis. In accordance to our study, De Boever et al37 showed maximum levels of secreted IL-6 at 3 h after intravenous LPS administration in the broiler chickens. In the current study, dexamethasone and meloxicam administrations did not significantly affect the concentration of LPS-induced IL-6 in the serum of chickens at 3 and 12 hours after LPS injection. The effects of dexamethasone and meloxicam administrations on the LPS-induced IL-6 secretion had not been studied previously. However, De Boever et al38 showed that the administration of other anti-inflammatory drugs, including tepoxalin, sodium-salicylate, and ketoprofen did not influence the concentration of IL-6 in plasma 3 hours after LPS administration.
In a previous study, Adanin et al50 showed that the prevention of adenosine degradation could attenuate proinflammatory cytokine responses after LPS inoculation in rats. They suggested that the inhibition of ADA could be a novel therapeutic approach to control the systemic inflammatory response. In this study, serum ADA activity was significantly higher in the E. coli LPS-treated group than in the negative control group. Meloxicam administration was not associated with a significant effect on ADA concentration. However, the ADA concentration in the dexamethasone-treated groups was lower than that of the positive control and meloxicam-treated groups. Yazar et al51 indicated that dexamethasone could significantly decrease ADA levels in E. coli LPS endotoxemia in rats.
It is well documented that the concentration of sialic acid immediately rises following different inflammatory stimuli.52–54 In the present study, the concentrations of total sialic acid, lipid-bound sialic acid, and protein-bound sialic acid were significantly higher in the LPS-challenged groups than in the negative control group. The administration of meloxicam did not significantly affect LBSA level but reduced PBSA in comparison with the positive control group. Dexamethasone was more effective than meloxicam in reducing LBSA, PBSA, and TSA.
In this study, the ceruloplasmin concentration was significantly higher in LPS-inoculated chickens in comparison with that of non-inoculated birds. The increase in the ceruloplasmin concentration in the serum of chickens after LPS injection was consistent with the findings of Butler et al,21 Curtis and Butler,55 and Baert et al.36 Moreover, dexamethasone, as a corticosteroid, showed greater effects on ceruloplasmin concentrations than did meloxicam. Baert et al36 showed that different doses of sodium salicylate, as an NSAID, did not have significant effects on E. coli LPS endotoxemia in broiler chickens. Our findings are in accordance with that of a previous study, which showed that anti-inflammatory drugs, including tepoxalin, sodium salicylate, and ketoprofen, did not have significant effects on LPS-induced ceruloplasmin concentrations.38
Rath et al20 reported that the concentration of ovotransferrin in chickens increases during inflammatory processes and microbial infections. Furthermore, they suggested that ovotransferrin concentration could be considered a diagnostic marker of infection and inflammation. In our study, 24 h after LPS injection, ovotransferrin concentration in E. coli LPS-inoculated positive control group was significantly higher than that of the negative control group. Horvatić et al2 recorded the maximum concentration of ovotransferrin at 24 h after E. coli LPS inoculation in chickens. In our study, all the treated groups, except the one treated with 0.5 mg/kg meloxicam, showed significantly lower ovotransferrin concentrations compared with those of untreated positive control group.
Conclusion
Different doses of dexamethasone and meloxicam did not have significant effects on LPS-induced ALI scores and serum IL-6 levels. However, these anti-inflammatory drugs reduced adenosine deaminase, ceruloplasmin, ovotransferrin, lipid-bound sialic acid, protein-bound sialic acid, and total sialic acid in LPS-inoculated broiler chickens. Dexamethasone was more effective than meloxicam in the reducing of these LPS-induced markers.
Funding Statement
This work was supported by a grant from Shiraz University (grant number 97GCU4M271378).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Mayer H, Bhat UR, Masoud H, Radziejewska-Lebrecht J, Widemann C, Krauss JH. Bacterial lipopolysaccharides. Pure Appl Chem. 1989;61(7):1271–1282. doi: 10.1351/pac198961071271 [DOI] [Google Scholar]
- 2.Horvatić A, Guillemin N, Kaab H, et al. Quantitative proteomics using tandem mass tags in relation to the acute phase protein response in chicken challenged with Escherichia coli lipopolysaccharide endotoxin. J Proteomics. 2019;192:64–77. doi: 10.1016/j.jprot.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 3.Franco RF, Dejonge E, Dekkers PE, et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96(2):554–559. doi: 10.1182/blood.V96.2.554 [DOI] [PubMed] [Google Scholar]
- 4.Klasing KC, Korver DR. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. J Anim Sci. 1997;75(suppl 2):58–67. [Google Scholar]
- 5.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 6.Koppenol A, Everaert N, Buyse J, Delezie E. Challenge with lipopolysaccharides or Freund’s adjuvant? What is the best option to trigger acute phase protein production in broilers? Res Vet Sci. 2015;99:96–98. doi: 10.1016/j.rvsc.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 7.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24:7–11. doi: 10.1097/01.shk.0000191384.34066.85 [DOI] [PubMed] [Google Scholar]
- 8.Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc. 1989;48(3):347–354. doi: 10.1079/PNS19890050 [DOI] [PubMed] [Google Scholar]
- 9.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP. New insights into the biology of the acute phase response. J Clin Immunol. 1999;19(4):203–214. doi: 10.1023/A:1020563913045 [DOI] [PubMed] [Google Scholar]
- 10.Blackburn JW. Validity of acute phase proteins as markers of disease activity. J Rheumatol. 1994;42:9–13. [PubMed] [Google Scholar]
- 11.Kushner I, Gewurz H, Benson MD. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981;97(6):739–749. [PubMed] [Google Scholar]
- 12.Yoshioka M, Watanabe A, Shimada N, Murata H, Yokomizo Y, Nakajima Y. Regulation of haptoglobin secretion by recombinant bovine cytokines in primary cultured bovine hepatocytes. Domest Anim Endocrinol. 2002;23(3):425–433. doi: 10.1016/S0739-7240(02)00174-1 [DOI] [PubMed] [Google Scholar]
- 13.Takimoto T, Sato K, Akiba Y, Takahashi K. Role of chicken TL1A on inflammatory responses and partial characterization of its receptor. J Immunol. 2008;180(12):8327–8332. doi: 10.4049/jimmunol.180.12.8327 [DOI] [PubMed] [Google Scholar]
- 14.O’reilly EL, Eckersall PD. Acute phase proteins: a review of their function, behaviour and measurement in chickens. Worlds Poult Sci J. 2014;70(1):27–44. doi: 10.1017/S0043933914000038 [DOI] [Google Scholar]
- 15.Fox IH, Kelley WN. The role of adenosine and 2ʹ-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47(1):655–686. doi: 10.1146/annurev.bi.47.070178.003255 [DOI] [PubMed] [Google Scholar]
- 16.Aran JM, Colomer DO, Matutes ES, Vives-Corrons JL, Franco RA. Presence of adenosine deaminase on the surface of mononuclear blood cells: immunochemical localization using light and electron microscopy. J Histochem Cytochem. 1991;39(8):1001–1008. doi: 10.1177/39.8.1856451 [DOI] [PubMed] [Google Scholar]
- 17.Desrosiers MD, Cembrola KM, Fakir MJ, et al. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol. 2007;179(3):1884–1892. doi: 10.4049/jimmunol.179.3.1884 [DOI] [PubMed] [Google Scholar]
- 18.Sylte MJ, Suarez DL. Vaccination and acute phase mediator production in chickens challenged with low pathogenic avian influenza virus; novel markers for vaccine efficacy? Vaccine. 2012;30(20):3097–3105. doi: 10.1016/j.vaccine.2012.02.055 [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Newberry L, Clark FD, et al. Changes in serum ovotransferrin levels in chickens with experimentally induced inflammation and diseases. Avian Dis. 2002;46(1):122–131. doi: 10.1637/0005-2086(2002)046[0122:CISOLI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 20.Rath NC, Anthony NB, Kannan L, et al. Serum ovotransferrin as a biomarker of inflammatory diseases in chickens. Poult Sci. 2009;88(10):2069–2074. doi: 10.3382/ps.2009-00076 [DOI] [PubMed] [Google Scholar]
- 21.Butler EJ, Curtis MJ, Harry EG, Deb JR. Effect of Escherichia coli endotoxins on plasma para-phenylenediamine oxidase (caeruloplasmin) activity in the domestic fowl. J Comp Pathol. 1972;82(3):299–306. doi: 10.1016/0021-9975(72)90009-6 [DOI] [PubMed] [Google Scholar]
- 22.Crook M. The determination of plasma or serum sialic acid. Clin Biochem. 1993;26(1):31–38. doi: 10.1016/0009-9120(93)90014-W [DOI] [PubMed] [Google Scholar]
- 23.Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83(7):623–634. doi: 10.1016/S0300-9084(01)01303-7 [DOI] [PubMed] [Google Scholar]
- 24.Karagenc TI, Kiral FK, Seyrek K, Bildik A, Eren H. Detection of serum total sialic acid in cattle with natural tropical theileriosis. Rev Med Vet. 2005;156:578–582. [Google Scholar]
- 25.Nazifi S, Tabande MR, Hosseinian SA, Ansari-Lari M, Safari H. Evaluation of sialic acid and acute-phase proteins (haptoglobin and serum amyloids A) in healthy and avian infection bronchitis virus-infected chicks. Comp Clin Path. 2011;20(1):69–73. doi: 10.1007/s00580-009-0939-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wongkham S, Bhudhisawasdi V, Chau-in S, et al. Clinical significance of serum total sialic acid in cholangiocarcinoma. Clin Chim Acta. 2003;327(1–2):139–147. doi: 10.1016/S0009-8981(02)00371-6 [DOI] [PubMed] [Google Scholar]
- 27.Peters SM, Yancy H, Deaver C. In vivo characterization of inflammatory biomarkers in swine and the impact of flunixin meglumine administration. Vet Immunol Immunop. 2012;148(3–4):236–242. doi: 10.1016/j.vetimm.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 28.Montesinos A, Encinas T, Ardiaca M, Gilabert JA, Bonvehí C, Orós J. Pharmacokinetics of meloxicam during multiple oral or intramuscular dose administration to African grey parrots (Psittacus erithacus). Am J Vet Res. 2019;80(2):201–207. doi: 10.2460/ajvr.80.2.201 [DOI] [PubMed] [Google Scholar]
- 29.Gildersleve M Effect of age on the pharmacokinetics of meloxicam in ISA Brown chickens (Gallus gallus domesticus) [Doctoral dissertation]. Palmerston North, New Zealand: Massey University; 2015. [Google Scholar]
- 30.Guzman DSM, Hawkins MG, Murphy JP. Analgesia In: Samour J, editor. Avian Medicine. 3 ed. Mosby International Ltd; 2016:192–199. [Google Scholar]
- 31.Watteyn A, Wyns H, Plessers E, et al. Pharmacokinetics of dexamethasone after intravenous and intramuscular administration in broiler chickens. Vet J. 2013;195(2):216–220. doi: 10.1016/j.tvjl.2012.06.026 [DOI] [PubMed] [Google Scholar]
- 32.Glojnaric I, Cuzic S, Erakovic-Haber V, Parnham MJ. The serum amyloid A response to sterile silver nitrate in mice and its inhibition by dexamethasone and macrolide antibiotics. Int Immunopharmacol. 2007;7(12):1544–1551. doi: 10.1016/j.intimp.2007.07.031 [DOI] [PubMed] [Google Scholar]
- 33.Hirao S, Wada H, Nakagaki K, et al. Inflammation provoked by Mycoplasma pneumoniae extract: implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunol Med Microbiol. 2011;62(2):182–189. doi: 10.1111/j.1574-695X.2011.00799.x [DOI] [PubMed] [Google Scholar]
- 34.Ritchie BW, Harrison GJ. Formulary In: Ritchie BW, Harrison GJ, Harrison LR, editors. Avian Medicine: Principles and Application. Florida: Wingers Publishing, Inc.; 1994:461. [Google Scholar]
- 35.Abou-Madi N. Avian anesthesia. Vet Clin North Am Exot Anim Pract. 2001;4(1):147–167. doi: 10.1016/S1094-9194(17)30055-5 [DOI] [PubMed] [Google Scholar]
- 36.Baert K, Duchateau L, De Boever S, Cherlet M, De Backer P. Antipyretic effect of oral sodium salicylate after an intravenous E. coli LPS injection in broiler chickens. Br Poult Sci. 2005;46(2):137–143. doi: 10.1080/0071660500065151 [DOI] [PubMed] [Google Scholar]
- 37.De Boever S, Croubels S, Meyer E, et al. Characterization of an intravenous lipopolysaccharide inflammation model in broiler chickens. Avian Pathol. 2009;38(5):403–411. doi: 10.1080/03079450903190871 [DOI] [PubMed] [Google Scholar]
- 38.De Boever S, Neirinckx EA, Meyer E, et al. Pharmacodynamics of tepoxalin, sodium‐salicylate and ketoprofen in an intravenous lipopolysaccharide inflammation model in broiler chickens. J Vet Pharmacol Ther. 2010;33(6):564–572. doi: 10.1111/j.1365-2885.2010.01184.x [DOI] [PubMed] [Google Scholar]
- 39.Aviagen; 2015. Available from: http://en.aviagen.com/assets/Tech_Center/BB_Resources_Tools/Pocket_Guides/Ross-Broiler-Pocket-Guide-2015-EN.pdf. Accessed April21, 2020.
- 40.Bailey TA, Apo MM. Pharmaceutical products commonly used in avian medicine In: Samour J, editor. Avian Medicine. 3 ed. Mosby International Ltd; 2016:637–678. [Google Scholar]
- 41.Faller S, Zimmermann KK, Strosing KM, et al. Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med Gas Res. 2012;2(1):26. doi: 10.1186/2045-9912-2-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katopodis N, Hirshaut Y, Geller NL, Stock CC. Lipid-associated sialic acid test for the detection of human cancer. Cancer Res. 1982;42(12):5270–5275. [PubMed] [Google Scholar]
- 43.Bestujeva SV, Kolb VS. Determination of the activity of ceruloplasmin in the blood serum by the method of Revin In: Kolb VG, Kamishnikov VS, editors. Practical Book in Clinical Chemistry. 2nd ed. Minsk, Belarus: Military Publishing House of the USSR; 1982:290–291. [Google Scholar]
- 44.Ansari AR, Ge XH, Huang HB, et al. Effects of lipopolysaccharide on the histomorphology and expression of toll-like receptor 4 in the chicken trachea and lung. Avian Pathol. 2016;45(5):530–537. doi: 10.1080/03079457.2016.1168923 [DOI] [PubMed] [Google Scholar]
- 45.Peng LY, Yuan M, Song K, et al. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-κB pathway activation. Int Immunopharmacol. 2019;72:467–472. doi: 10.1016/j.intimp.2019.04.046 [DOI] [PubMed] [Google Scholar]
- 46.Yu Z, Ouyang JP, Li YP. Dexamethasone attenuated endotoxin-induced acute lung injury through inhibiting expression of inducible nitric oxide synthase. Clin Hemorheol Microcirc. 2009;41(2):117–125. doi: 10.3233/CH-2009-1162 [DOI] [PubMed] [Google Scholar]
- 47.Al-Harbi NO, Imam F, Al-Harbi MM, et al. Dexamethasone attenuates LPS-induced acute lung injury through inhibition of NF-κB, COX-2, and pro-inflammatory mediators. Immunol Invest. 2016;45(4):349–369. doi: 10.3109/08820139.2016.1157814 [DOI] [PubMed] [Google Scholar]
- 48.Li L, Whiteman M, Moore PK. Dexamethasone inhibits lipopolysaccharide‐induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J Cell Mol Med. 2009;13(8b):2684–2692. doi: 10.1111/j.1582-4934.2008.00610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuilian W, Jianxin W, Xinfu L. The role of MMP-2 mRNA in endotoxin-induced acute lung injury in rabbits and the effects of meloxican in its treatment. MJCPLA. 2006;11:10. [Google Scholar]
- 50.Adanin S, Yalovetskiy IV, Nardulli BA, Sam AD, Jonjev ZS, Law WR. Inhibiting adenosine deaminase modulates the systemic inflammatory response syndrome in endotoxemia and sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):1324–1332. doi: 10.1152/ajpregu.00373.2001 [DOI] [PubMed] [Google Scholar]
- 51.Yazar E, Bulbul A, Avci G, et al. Effects of enrofloxacin, flunixin meglumine and dexamethasone on disseminated intravascular coagulation, cytokine levels and adenosine deaminase activity in endotoxaemia in rats. Acta Vet Hung. 2010;58(3):357–367. doi: 10.1556/avet.58.2010.3.8 [DOI] [PubMed] [Google Scholar]
- 52.Mosleh N, Nazifi S, Alaeddini A. Changes in serum acute phase reactants, inflammatory mediators and gangliosides in Japanese quail (Coturnix japonica) with retained yolk sac. Pak Vet J. 2012;32:251–254. [Google Scholar]
- 53.Asasi K, Mohammadi A, Boroomand Z, Hosseinian SA, Nazifi S. Changes of several acute phase factors in broiler chickens in response to infectious bronchitis virus infection. Poult Sci. 2013;92(8):1989–1996. doi: 10.3382/ps.2012-02902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yazdani A, Asasi K, Nazifi S. Evaluation of acute-phase proteins and inflammatory mediators changes in native chickens experimentally infected with Salmonella typhimurium. Comp Clin Path. 2015;24(4):733–739. doi: 10.1007/s00580-014-1972-0 [DOI] [Google Scholar]
- 55.Curtis MJ, Butler EJ. Response of caeruloplasmin to Escherichia coli endotoxins and adrenal hormones in the domestic fowl. Res Vet Sci. 1980;28(2):217–222. doi: 10.1016/S0034-5288(18)32750-4 [DOI] [PubMed] [Google Scholar]