Abstract
Although reduced social attention and increased nonsocial attention have been reported in individuals with autism spectrum disorder (ASD), the studies have relied on predominantly male samples and have been underpowered to examine sex differences. These processes may differ for females with ASD, who have been shown to be dissimilar to males in social motivation and nonsocial features, including circumscribed interests (CI). The goal of this study was to compare social and nonsocial visual attention between males and females with ASD on a validated eye-tracking paradigm. Eighty-five school-aged (6–10 years) males and females with and without ASD completed a paired preference task of face and object stimuli (half of which related to common CI). After covarying for chronological and mental age, the presence of concurrently presented CI images reduced prioritization and attention to faces for males more than females, replicating previous findings. ASD females maintained comparable attention patterns to typically developing females, suggesting that previous findings of reduced social attention and increased attention to CI-related objects in autism may be specific to males. These findings are also inconsistent with the “extreme male brain” theory of autism. The more normative orienting and attention to social stimuli for females with ASD may indicate distinct phenotypic characteristics relative to males and possibly serve as a protective effect.
Keywords: social motivation, circumscribed interests, eye-tracking, females
Lay Summary:
As autism is more commonly diagnosed in males, less is known about females with autism. Two areas of interest include the interests held by individuals with autism and how socially motivated they are. We used eye tracking as a way to understand these two areas. Our data reveal that elementary school-aged females (6–10 years) with autism attended to faces comparatively to females without autism, suggesting that (1) they were more socially motivated than males with autism and (2) the images of common interests were less motivating to them.
Introduction
Despite changes in diagnostic criteria and improved screening and diagnosis, the sex ratio in autism spectrum disorder (ASD) has remained constant and weighted toward males [Christensen et al., 2016;Loomes, Hull, & Mandy, 2017]. As a result, considerably less is known about females with ASD and the majority of published research includes largely, or in some cases exclusively, male samples. The goal of this study is to employ eye-tracking methodology to compare attention to social and nonsocial images (half overlapping with commonly reported interests in ASD) in primary/elementary school-aged males and females with and without ASD.
Behavioral and Clinical Phenotype of ASD Females
A growing body of research suggests subtle differences between the male and female phenotype of ASD that may contribute to differential trajectories and outcomes [Van Wijngaarden-Cremers et al., 2014]. Two areas of relevance for this study include higher social motivation in ASD females [Sedgewick, Hill, Yates, Pickering, & Pellicano, 2016] and more typical/fewer circumscribed interests (CI) [Hiller, Young, & Weber, 2014, 2016; Sutherland, Hodge, Bruck, Costley, & Klieve, 2017].
For example, Sedgewick et al. [2016] examined sex differences in social motivation between adolescent males and females with ASD using a mixed-methods approach. ASD females displayed comparable rates of motivation to females with no ASD diagnosis and ASD males reported the lowest rates of social motivation. In two qualitative studies, Cook, Ogden, and Winstone [2018] and Vine Foggo and Webster [2017] reported that ASD females demonstrate a motivation for friendships, enjoyment from friendships, and an understanding of the factors that contribute to successful friendships. ASD females also have been reported to display more behaviors conducive to typical friendships than ASD males, such as understanding of friendships and empathy, staying in close proximity to peers, and weaving in and out of social activities [Dean et al., 2014;Dean, Harwood, & Kasari, 2017;Head, McGillivray, & Stokes, 2014]. Together these studies suggest that ASD females may have a social advantage over their male counterparts and demonstrate more normative social patterns.
These differences may, in part, be mediated by differences in social and nonsocial interests held by ASD males and females. In two linked studies, Hiller et al. found that females with ASD presented with fewer restricted and repetitive behaviors (RRBs) and different CI [Hiller et al., 2014, 2016] than males with ASD. For example, ASD females were less likely to have a strong interest in screen time and gaming [Hiller et al., 2014]. Similarly, parents were less likely to report an interest in wheeled toys as a concern in females later diagnosed with ASD, with interests rated as seemingly random and repetitive behaviors with toys such as figurines and dolls rated as more common in females [Hiller et al., 2016]. Harrop, Green, and Hudry [2017] also reported more engagement with toys rated as female (dolls, tea sets, etc.) in preschool-aged females with ASD, mirroring trends observed in typical development [e.g., Maccoby & Jacklin, 1978;Servin, Bohlin, & Berlin, 1999]. These differences were also found in a large survey of parents of school-aged children with ASD, with differences in the types of interests held by males and females with ASD replicating what is reported in typical development [Sutherland et al., 2017]. Thus, both males and females with ASD tend to pursue sex-typical interests; however, because these interests are potentially more social for females, this may suggest that differences in both social motivation and CI between males and females with ASD could contribute to distinct developmental processes and trajectories.
Social Motivation Theory of ASD
The social motivation of ASD theory hypothesizes that some of the social and nonsocial behaviors and difficulties observed in ASD result from a lack of motivation toward social stimuli from a very early age [Chevallier et al., 2015;Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998;Dawson, Webb, & McPartland, 2005]. The theory accounts for a number of the social deficits observed in ASD, such as failure to orient to social stimuli, impairments in social cognition, fewer meaningful friendships, and greater interaction failures later in life. Complementing this viewpoint are studies of increased nonsocial motivation in ASD [Benning et al., 2016;Sasson, Dichter, & Bodfish, 2012;Unruh et al., 2016] that may contribute to the development of nonsocial features of ASD, such as RRBs and CI. However, the emerging evidence of social and nonsocial phenotypic differences for girls with ASD suggest that the social motivation theory, and specifically reported social and nonsocial attentional differences in ASD, may not be present to the same degree to females. Further, the extreme male brain (EMB) theory of ASD [Baron-Cohen, 2002], which posits that ASD represents an extreme extension of sex differences observed in typical development, would also hypothesize that ASD females should demonstrate social and nonsocial attentional patterns more similar to males with ASD than typically developing (TD) females. The current study offers a direct test of these hypotheses by comparing social and nonsocial attention between ASD males and females.
Using eye tracking to understand social motivation and interests
Eye-tracking studies have been used extensively in ASD research to quantify differences and atypicalities in visual attention to social stimuli [Fletcher-Watson, Leekam, Benson, Frank, & Findlay, 2009;Klin, Jones, Schultz, Volkmar, & Cohen, 2002;Pelphrey et al., 2002; Sasson, Elison, Turner-Brown, Dichter, & Bodfish, 2011;Sasson & Touchstone, 2014]. These studies range from infants at risk of ASD [Chawarska, Macari, Powell, DiNicola, & Shic, 2016], to young children [Jones, Carr, & Klin, 2008; Klin, Lin, Gorrindo, Ramsay, & Jones, 2009], school-aged children [Sasson, Turner-Brown, Holtzclaw, Lam, & Bodfish, 2008], and adults with ASD [Pelphrey et al., 2002]. The altered patterns of attention to social information demonstrated in these studies are assumed to reflect a difference in the way that individuals process information and interact with their social environment.
Eye tracking also has been applied to understand how the presence of nonsocial information impacts attention in ASD [Sasson et al., 2011;Sasson & Touchstone, 2014; Unruh et al., 2016]. In a series of studies, Sasson and colleagues observed attentional biases in individuals with ASD when presented with images representing social and nonsocial stimuli (including images that overlapped with common CI). During a passive viewing visual exploration task, school-aged children with ASD explored fewer images, demonstrated perseverative attention to the images they did view, and had a more detail-oriented profile of attention than TD controls [Sasson et al., 2008]. However, these effects were driven by children focusing attention to images of commonly occurring CI in ASD (e.g., trains and computers; [South, Ozonoff, & McMahon, 2005; Turner-Brown, Lam, Holtzclaw, Dichter, & Bodfish, 2011].
Using a preferential viewing paradigm (a paradigm where images are paired and the resulting patterns of visual orientation and attention shed light on the relative preference between two stimulus types), Sasson and Touchstone [2014] reported similar attention patterns to faces between preschool-aged children with and without ASD when presented alongside images unrelated to common CI. However, when faces were paired with images of common CI in ASD, children with ASD attended less to the face and prioritized nonsocial information. Similar findings were reported by Unruh et al. [2016] using a similar paradigm with adolescents with ASD. As a result of the low numbers of females in these studies, sex differences were not reported.
In a recent study, Chawarska et al. [2016] reported enhanced attention to social stimuli, including faces, in female infants at high risk for ASD compared to high-risk male infants, suggesting an altered profile of ASD risk in female probands. Ketelaars et al. [2017] examined fixation patterns to video clips in high-functioning adult females with ASD, reporting atypicalities in social attention relative to non-ASD female controls. However, unlike previous studies with majority male samples, no hyper-focus to the mouth was reported. While eye tracking has not been applied to examine sex differences preferences in ASD, it has been widely applied to examine sex differences in typical development, particularly in infancy. Preferential viewing paradigms have shown that infant, including neonate, females fixate more to faces [Gluckman & Johnson, 2013] and images of dolls [Alexander, Wilcox, & Woods, 2009;Jadva, Hines, & Golombok, 2010], whereas infant males fixate more to images of objects, including cars [Jadva et al., 2010].
Study Aims and Hypotheses
The aim of this study was to apply a validated preferential viewing paradigm [Sasson & Touchstone, 2014;Unruh et al., 2016] to examine patterns of attention in primary/elementary school-aged females and males with ASD and matched TD controls. The primary objective is to examine whether previous findings extend to a balanced sample of ASD males and females. The use of eye tracking allowed us to examine whether the differences reported in the behavioral phenotype of ASD females [e.g., Hiller et al., 2014, 2016;Sedgewick et al., 2016;Sutherland et al., 2017] are mirrored in their fixation patterns to social and nonsocial stimuli.
Based on previous behavioral literature, we expected that ASD females would prioritize social stimuli and fixate more to social stimuli than ASD males. We predicted that attention to nonsocial stimuli would be comparable between ASD males and females, but ASD males would show a preference for CI-related objects. Collectively, these hypotheses propose that previous findings of reduced social attention and increased nonsocial attention to CI-related objects in ASD are driven by a male-specific pattern that does not extend to the same degree to females with ASD. These predictions would be inconsistent with the EMB theory of ASD, which would instead predict the attentional patterns of ASD females would align with those of ASD males.
Methods
Participants
Eighty-seven children aged 6–10 years were recruited to the study: 54 children with a diagnosis of ASD (27 female and 27 males) and 33 children who were TD (16 females and 17 males). This study focused on primary/elementary school-aged children (6–10 years) with ASD because of the findings of a recent analysis that reported divergence in RRBs in females ages six and over [Van Wijngaarden-Cremers et al., 2014].
ASD children were recruited via research registries at the University of North Carolina at Chapel Hill. Registry participants are referred through collaborative relationships with the TEACCH Autism Program, the Carolina Institute for Developmental Disabilities, and other North Carolina clinics, agencies, and advocacy organizations t provide services for individuals with ASD. To acquire a sufficient sample of female children with ASD, families traveled from across the state to participate, and diagnostic protocols sometimes varied by TEACCH clinic. However, to qualify for the University of North Carolina (UNC) research registries, all children had undergone extensive observations and a comprehensive clinical evaluation, including a range of gold-standard diagnostic measures (such as ADOS and/or CARS). Parents also complete a comprehensive developmental history interview (e.g., the ADI). Evaluation results are entered in the registry database for the purpose of vetting referrals to studies. Exclusion criteria included the presence of seizures and acute medical or genetic conditions. As all children recruited into our ASD group were from the UNC Autism Registry that requires for inclusion confirmed clinical diagnoses of ASD, we did not repeat gold-standard diagnostic measures. However, to obtain a standardized clinical metric across the sample, all parents complete the Social Communication Questionnaire (SCQ) [Rutter, Bailey, & Lord, 2003].
TD children were recruited via research registries, social media, and word of mouth. Parents completed the SCQ [Rutter et al., 2003] to screen for ASD. No children had a diagnosis of a developmental or psychiatric disorder. We also excluded subjects with an immediate family member with an ASD diagnosis. TD children were selected to match on sex and if they were aged between 6 and 10 years. We did not purposefully match on developmental age due to the low-cognitive demands in the simple passive-viewing tasks and did not want to reduce any maturational effects that may be observed in the data. However, all subjects completed the differential ability scales (DAS-II) [Elliot, 2007] and any differences in developmental age were controlled for in the analysis. See Table 1 for subject characteristics.
Table 1.
Sample Characteristics
| Boys | Girls | Diagnosis (ASD vs.TD) Effects | ||||
|---|---|---|---|---|---|---|
| ASD | TD Controls | ASD | TD Controls | |||
| (n = 23) | (n = 16) | (n = 22) | (n = 16) | F | P | |
| CA (months) | 114.09 (9.96) | 93.50 (17.85) | 102.73 (17.66) | 94.87 (18.04) | 15.00 | <0.01** |
| MA (months) | 120.51 (26.36) | 124.30 (40.88) | 98.55 (34.61) | 115.36 (24.26) | 4.38 | 0.04* |
| SCQ score | 14.48 (5.92) | 3.50 (2.58) | 13.59 (4.97) | 2.00 (2.92) | 67.33 | <0.01** |
| RBS-R score | 26.26 (13.83) | 3.00 (2.96) | 34.95 (22.61) | 5.00 (8.91) | 36.57 | <0.01** |
| Current interests | 12.74 (5.82) | 11.88 (6.15) | 13.86 (6.09) | 15.50(7.51) | 0.04 | 0.83 |
| CI intensity | 14.43 (3.42) | 9.94 (2.23) | 14.95 (3.75) | 9.06 (3.55) | 36.71 | <0.01** |
Note. Table 1 reports descriptive statistics for the sample by sex and diagnosis. A series of ANOVAs were run to examine diagnostic (ASD vs. TD) differences in descriptive variables. Mean (SD) unless otherwise noted. SCQ, social communication questionnaire; RBS-R, repetitive behavior scales-revised; CI, circumscribed interests.
P = 0.05.
P = 0.01.
Children in both groups had normal or corrected vision. Children were reimbursed with a gift card for their participation in a wider study. Parents provided informed written consent and, when developmentally appropriate, children provided written assent to participate. The research protocol was approved by the Institutional Review Board at UNC-Chapel Hill.
Eye-Tracking Stimuli
Stimuli and protocols for this study represented those reported previously by Sasson and Touchstone [2014]. Subjects were shown 20 slides of paired social and nonsocial (object) images (Fig. 1). Social images composed of 20 color photographs of adult actors displaying one of five basic emotions (happy, sad, angry, fearful, and neutral). Photos were taken with permission from a subset of The Penn Emotion Recognition Task [Gur et al., 2002;Kohler et al., 2003;Sasson et al., 2010], a standardized test of facial emotion recognition ability. Four faces of each emotion were included; half were high intensity and half low intensity. The faces did not repeat, were split evenly between males and females, and included a range of ethnicities. Twenty object images were paired with social images. Half represented frequently occurring CI in ASD [South et al., 2005;Turner-Brown et al., 2011] that capture the attention of individuals, mainly males, with ASD [Sasson et al., 2008;Sasson et al., 2011]. These included trains, vehicles, airplanes, clocks, and blocks. Ten images of objects not typically related to common CI were also included (non-CI objects). These included furniture, clothing, tools, musical instruments, and plants. The object and face images were resized to the same approximate surface area and to an equivalent size (approximately 20% of the total viewing area). Social images were paired with either a CI or non-CI object. The pairing with emotions and intensity was counterbalanced as was the positioning (left vs. right) of stimuli types. The total run time was 2 min and 30 sec.
Figure 1.
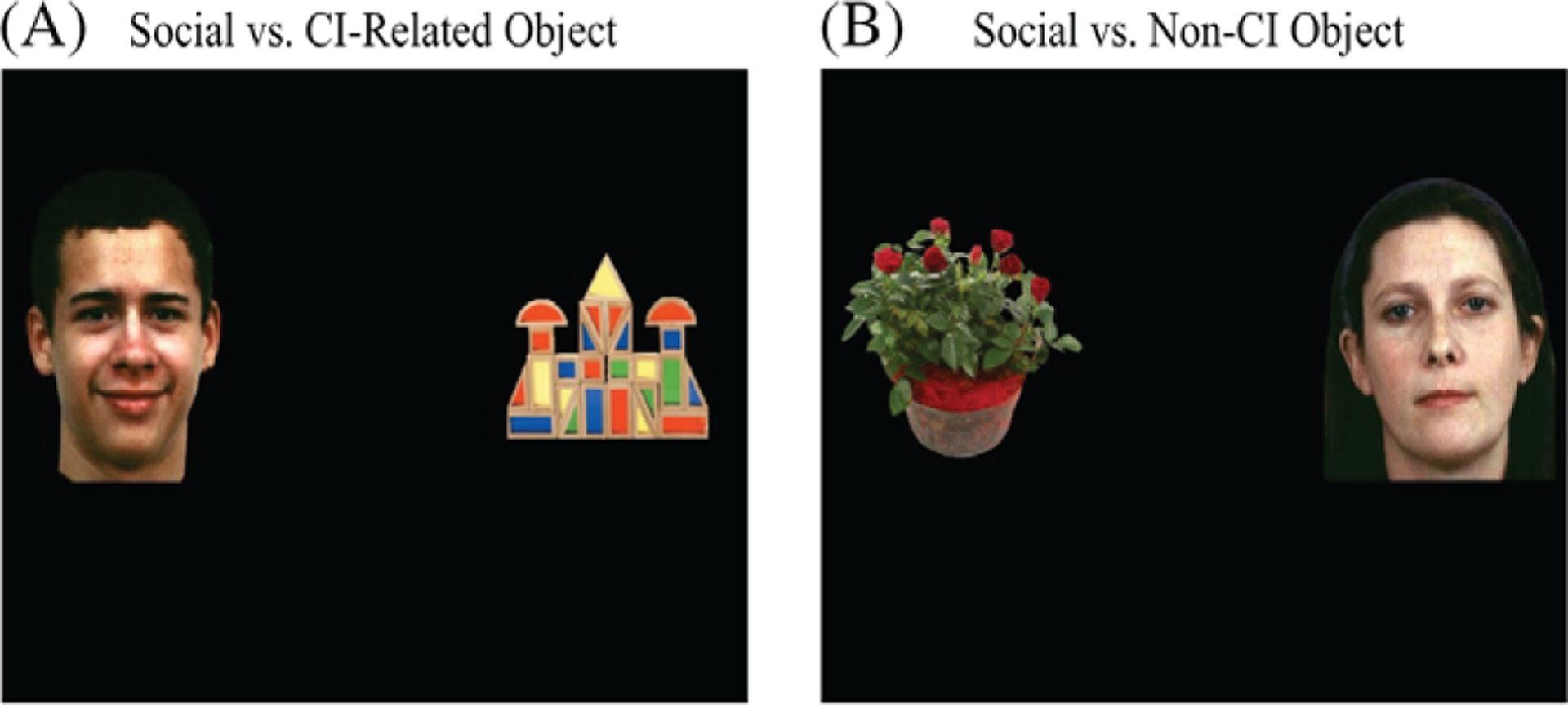
Example of paired-preference stimuli.
Eye-Tracking Procedure
The procedure implemented in the study followed the same employed by Sasson and Touchstone [2014]. Testing occurred in a single session at the Carolina Institute for Developmental Disabilities at UNC-Chapel Hill. Eye-tracking data were collected using a Tobii Pro 60XL eye-tracker, which uses the Pupil Center Correction Reflection method to record eye movements from both eyes at a sampling rate of 60 Hz with spatial accuracy of approximately 0.5°. Children were tested individually and sat either by themselves on a chair or on a cushion/booster seat to ensure a distance of approximately 60 cm from a 24” widescreen computer monitor. Raw eye tracking data were aggregated into fixations by Tobii Studio software using a fixation criterion of gaze remaining within a radius of 30 pixels for a minimum of 100 ms, as is consistent with previous research on visual attention to CI-related images. A five-point calibration procedure was completed prior to testing and repeated if quality was poor. Children were told that they would see pictures of faces and objects on the screen and they could look at them any way they wanted. Paired images were displayed one at a time for 5 sec each in a random order. Prior to each trial, an attention-getting animation (with sound) appeared at the center of the screen to reorient attention and to ensure all scanning patterns began equidistant between the paired stimuli.
Of the 87 children recruited to the study, two ASD males did not complete the eye-tracking task due to inattention/behavioral issues, leaving an initial sample of 85 children.
Cognitive and Parent-Report Measures
AH children completed the DAS-II [Elliot, 2007]. The DAS-II is an established measure of cognitive abilities from 30 months to 17 years, 11 months. It has been implemented in several studies of children with ASD [e.g., Bishop, Guthrie, Coffing, & Lord, 2011;Joseph, Tager-Flusberg, & Lord, 2002]. In this study, we administered six scales that comprise the core battery to derive nonverbal, verbal, and spatial ability scores and age equivalents. These core subscales are comparable across the early and school years’ protocols, and both protocols allow for out-of-level testing, allowing for administration based on ability level. The DAS-II has good internal, test-retest, and interrater reliabilities. The majority (n = 80) completed the school years form (ages 7–17); however, three children with ASD and one control completed the early years protocol.
Parents also completed the Repetitive Behavior Scale-Revised (RBS-R) [Bodfish, Symons, & Lewis, 1999] and the interests scale [Bodfish, 2003]. The RBS-R is a parent-report rating scale of RRB that rates the occurrence of a behavior on a 4 point-Likert scale from (0) does not occur to (3) occurs frequently and/or is severe. The RBS-R generates six subscale scores, which were summed to create a total score for analysis. The interests scale measures the presence and severity of CI. The scale consists of a checklist of interests that are summed to provide a score of current and past interests in their child. Seven additional questions ask the parent to select the child’s primary interest and rate the severity of this interest (frequency, social involvement, interference, and accommodation). Higher scores indicate more interference/greater severity, with scores ranging from 0 to 23.
Data Analysis
We analyzed the same dependent variables (DVs) as Sasson and Touchstone to measure social attention: (1) Preference, the percentage of on screen time devoted to faces and objects, which quantifies the distribution of attention between social and nonsocial stimuli; (2) Prioritization, the latency to first fixate on the face, which captures social orienting; and (3) Duration, fixation time per visit to the face, which assesses how long social images maintain the subject’s attention before shifting to the object (or out of areas of interest). We also calculated detail orientation, the average number of discrete fixations the participant makes on each stimulus type, relative to total time on the image. This was based on the findings of Unruh et al. [2016] who found that adolescents with ASD who completed a similar paired-preference task were more detail oriented than TD controls.
To reduce Type I error and based on Sasson and Touchstone’s preliminary analysis, which did not reveal group differences or interactions between facial intensity (extreme vs. mild) and emotion, we did not include these variables in our analysis.
Only children who spent at least 20% of the total paradigm time attending to the full screen were included in our analysis. This resulted in a final sample of 77 children; 45 ASD (23 male;22 female) and 32 TD (16 male; 16 female). Preliminary analysis revealed there were no effects of diagnosis or sex on total attention to the full screen. Despite targeted recruitment, there was a significant effect of diagnosis on chronological age (CA) between the groups (F = 15.00, P < 0.01) with our ASD groups being older than the TD groups (Table 1). There was a significant effect of sex on mental age (MA) as indexed by the DAS-II (F = 4.38, P = 0.04) with males having slightly higher MA than females, particularly in the ASD group (Table 1). This was expected given reports of ASD females requiring co-occurring intellectual disability and/or additional behavioral problems to reach threshold for diagnosis [Dworzynski, Ronald, Bolton, & Happe, 2012]. Both CA and MA were controlled for in the analysis—a common strategy in ASD and eye-tracking research [Chevallier et al., 2015;Fletcher-Watson et al., 2009;Sasson & Touchstone, 2014].
Our analysis was completed in two stages: First, we analyzed data from the whole sample to examine the effects of sex and diagnosis. Analysis of social attention between groups and sex was conducted using separate repeated measures ANOVAs on each variable type, with object type (CI and non-CI) as the within-subject variable and group (ASD, TD) and sex (male, female) as the between group variables. These analyses controlled for CA and MA (relationships between CA, MA, and each DV are presented in Supporting Information). Next, to test the hypothesis that, contrary to the EMB theory of ASD, females and males with ASD would differ in their social and nonsocial attentional patterns, we directly compared DVs between these two groups. This was done to provide a more sensitive index of these potential differences that could be obscured in the initial analyses as a result of a lack of power to detect a threeway interaction between task condition, sex, and diagnosis necessary to reveal that the ASD group differs by sex in their task performance. These analyses controlled for CA and MA because of the differences between the ASD males and females (CA: t = 2.67, P = 0.01;MA: t = 2.40, P = 0.02).
All analyses employed a Bonferroni correction to reduce the likelihood of Type I errors. Findings with conventional significance (P = 0.05) are reported. We also note several results that trended (P <0.10) toward significance to provide an index of potential effects that could be obscured due to lack of power due to the challenges of obtaining a large sample size of females with ASD. These results should be interpreted with caution.
Results
Preference
Faces.
For preference to faces, there was no main effects of condition (F = 0.75, P = 0.38) and only slight trends toward effects for both diagnosis (F = 2.67, P = 0.10) and sex (F = 2.56, P = 0.10). The ASD groups as a whole looked less to faces and females overall looked to faces more than males (Fig. 2). There was a condition*sex interaction (F = 12.18, P < 0.01, η2 = 0.15) indicating that the presence of CI images reduced fixation time to faces for males but not females (t = −2.89, P < 0.01;Fig. 2). When comparing the males and females in the ASD sample, there were no main effects of condition or sex. As with the whole group analysis, there was a significant condition*sex interaction in the ASD group (F = 7.60, P = 0.01, η2 = 0.16). Post hoc analyses revealed that ASD females fixated longer than ASD males to faces when paired with CI-related images (t = −2.49, P = o.01), suggesting that in contrast to their male counterparts, females with ASD did not reduce their fixation time to faces when CI objects were concurrently shown. These results mimic prior findings for males but not females with ASD.
Figure 2.
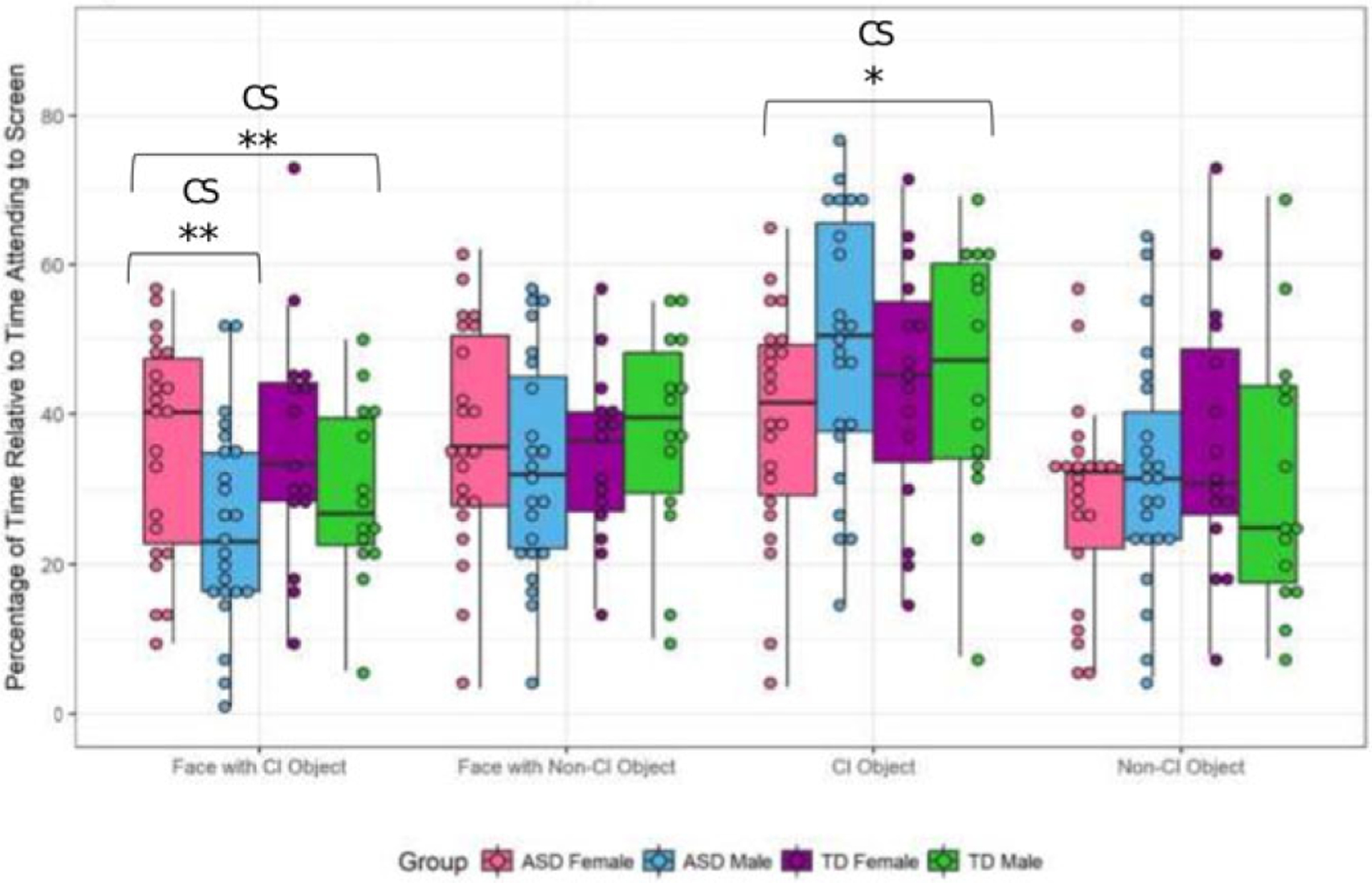
Preference to faces and objects. Whole Sample: Faces: There was a condition*sex interaction (F = 12.18, P < 0.01), with females fixating longer to faces when paired with a CI-related object (t = −2.89, P < 0.01). Objects: The main effect of condition approached significance (F = 3.94, P = 0.08), with longer overall fixations to CI-related objects. There was a condition*sex interaction (F = 3.94, P = 0.05), with males preferring to fixate to CI-related objects. ASD Sample: Faces: There was a condition*sex interaction (F = 7.60, P < 0.01), with females fixating longer to faces when paired with a CI-related object (t = −2.49, P = 0.01). Objects: Sex and condition*sex effects both approached significance (P = 0.07), with malesattending more to objects overall and CI-related objects in particular.*P ≤ 0.05; **P ≤ 0.01;C = condition effect;S = sex effect;CS = condition*sex interaction.
Objects.
For preference for objects (see Fig. 2), there was no main effect of diagnosis (F = 1.48, P = 0.23) or sex (F =0.17, P = 0.68), although the main effect of condition trended toward significance (F = 3.17, P = 0.08, η2 = 0.4) with a tendency for participants to fixate more on CI compared to non-CI objects. A significant condition*sex interaction (F = 3.94, P = 0.05, η2 = 0.05) emerged, driven by males showing a greater preference for CI-related objects (Fig. 2), though post hoc analyses were not significant. When directly comparing males and females with ASD, there was no main effect of condition, though the effect of sex approached significance with ASD males attending more to objects (F = 3.35, P = 0.07, η2 = 0.07). The sex*condition interaction also approached significance (F = 3.46, P = 0.07, η2 = 0.08) with ASD males demonstrating greater fixation to CI (t = 2.03, P = 0.04) but not non-CI objects (t = 0.84, P = 0.41).
Prioritization
Faces.
For prioritization, there were no main effects of condition, but there was a main effect of sex (F = 4.50, P = 0.04, η2 = 0.06) with females being faster to attend to faces than males (Fig. 3). A trend emerged for a group effect, with the ASD group being slower to orient to faces than the TD group (F = 3.14, P = 0.08, η2 = 0.04). There was also a trend toward a condition*sex interaction (F = 3.00, P = 0.08, η2 = 0.04). When directly comparing males and females in the ASD group, there was a slight trend toward ASD males being slower than females to attend to faces irrespective of condition (F = 2.47, P = 0.09, η2 = 0.06). There was no main effect of condition. Despite descriptive differences between ASD females and males in the CI related condition (Fig. 3), the condition* sex interaction was not significant (F = 2.47, P = 0.12, η2 = 0.06).
Figure 3.
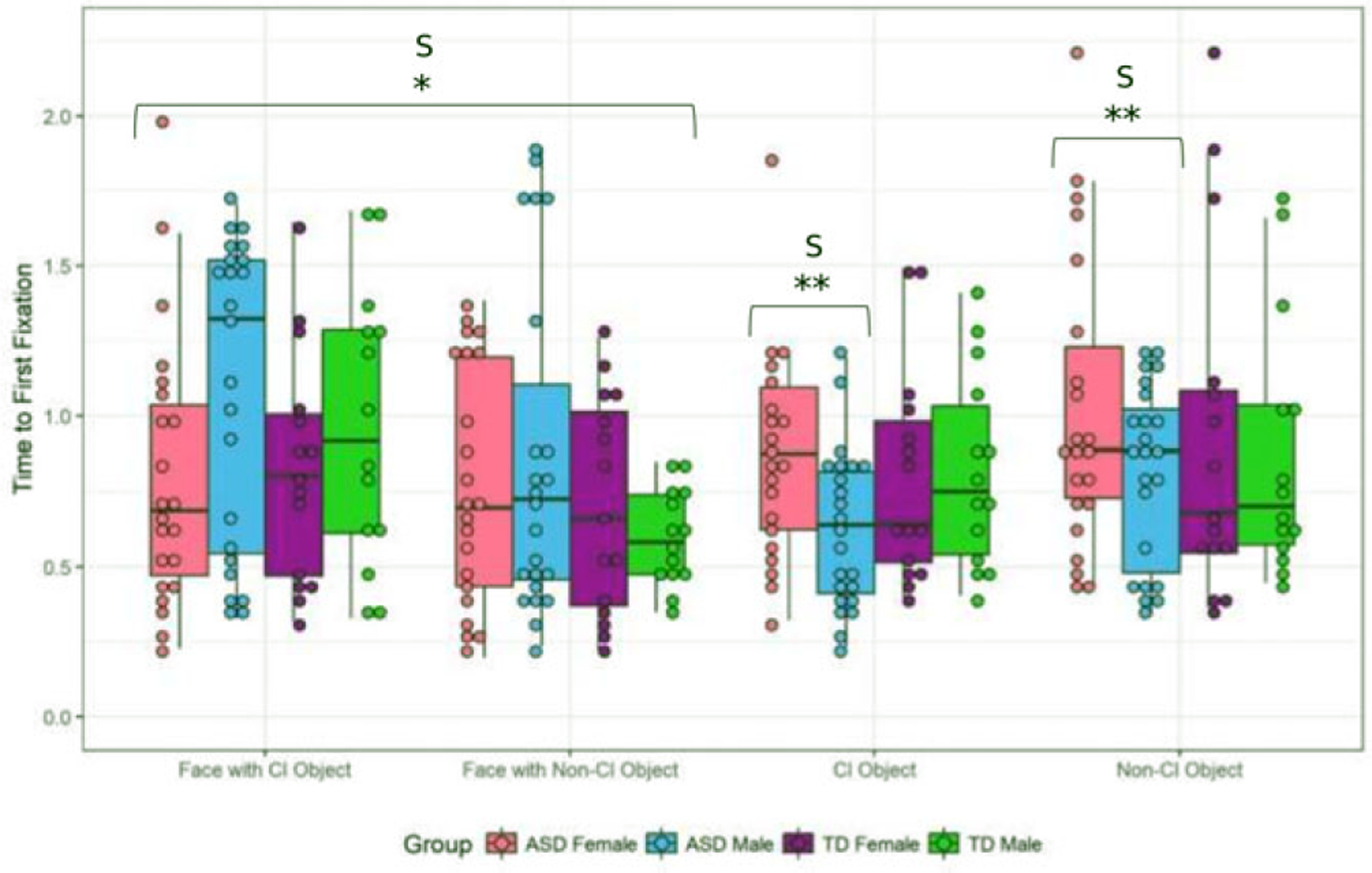
Prioritization of faces and objects. Whole Sample: Faces: There was a main effect of sex (F = 4.50, P = 0.04), with females being faster to attend to faces. There was a trend toward the ASD group being slower to attend to faces (F = 3.14, P = 0.08) and a trend toward a condition*sex interaction (F = 3.00, P = 0.08). Objects: There were no sex, condition, or diagnosis effects. ASD Sample: Faces: There were no significant trends between males and females with ASD in the time to first attend to faces;however, there was a trend toward males being slower (F = 2.47, P = 0.09). Objects: Males were faster to attend to objects than females (F = 5.74, P = 0.02).*P ≤ 0.05;**P ≤ 0.01; C = condition effect;S = sex effect;CS = condition*sex interaction.
Objects.
There were no main effects of condition, diagnosis or sex on the prioritization of objects in the whole sample (all P > 0.05;Fig. 3). But direct comparison of males and females with ASD revealed males were significantly faster to attend to objects than females (F = 5.74, P =0.02, η2 = 0.12).
Duration
Faces.
There were no effects of condition, diagnosis, or sex on the mean fixation duration to faces (all P > 0.05; Fig. 4). However, there was a condition*sex interaction (F = 4.55, P = 0.03, η2 = 0.06). While females had longer fixations to the face in the CI-related condition (Fig. 4), the post hoc analysis did not reach significance (t = −1.48, P =0.14).
Figure 4.
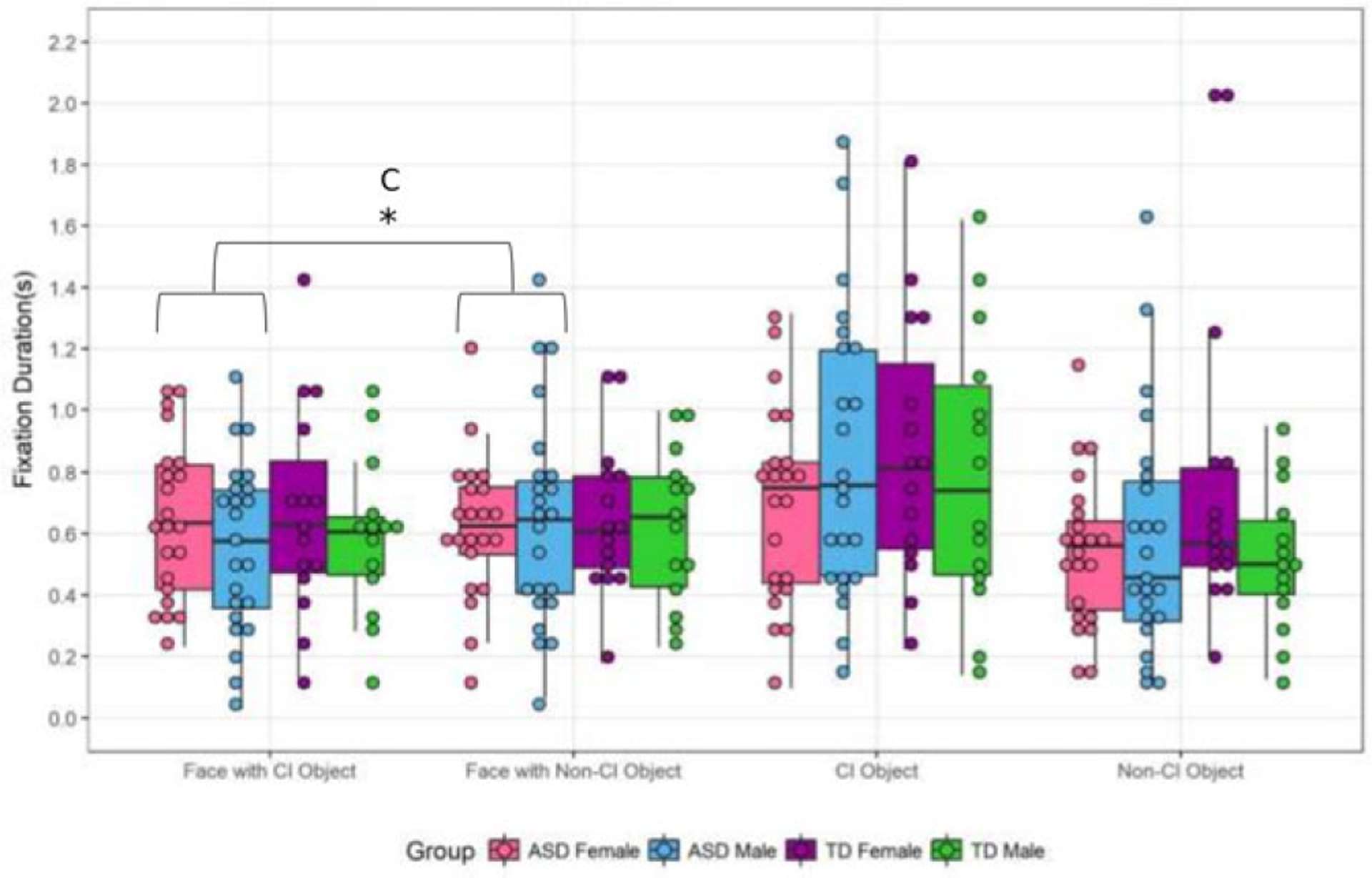
Fixation duration to faces and objects. Whole Sample: Faces: There a condition*sex interaction in mean fixation durations to faces (F = 4.55, P = 0.03). Post hoc tests were not significant. Objects: There was a condition*sex interaction (F = 4.32, P = 0.04). Post hoc tests were not significant. ASD Sample: Faces: There was a main effect of condition (F = 7.34, P = 0.01), with marginally longer fixations to faces in the non-CI condition. Objects: There were no condition or sex effects in the ASD group.*P ≤ 0.05;**P ≤ 0.01;C = condition effect;S = sex effect;CS = condition*sex interaction.
Despite the ASD groups appearing similar to one another and across conditions (Fig. 4), there was an effect of condition on mean fixation duration to faces (F = 7.34, P = 0.01, η2 = 0.15) with marginally longer fixations to faces in the non-CI condition. There was no main effect of sex, and despite a strong condition*sex interaction (F = 10.36, P < 0.01, η2 = 0.20), post hoc tests were not significant.
Objects.
For objects, there were no conditions, diagnosis, or sex main effects (all P > 0.05). There was a condition*sex interaction for duration for objects (F = 4.32, P = 0.04, η2 = 0.06), but post hoc analysis did not reach significance. There were no effects of condition and sex in the ASD group.
Detail Orientation
Faces.
There was no effect of condition or diagnosis on detail orientation to faces, but there was an effect of sex (F = 4.12, P = 0.04, η2 = 0.05), with males demonstrating greater detail orientation than females (Fig. 5). No interactions were significant. In the ASD group, there were no main effects of sex or condition, but there was a significant condition*sex interaction (F = 3.80, P = 0.05, η2 = 0.08). The post hoc analyses were not significant.
Figure 5.
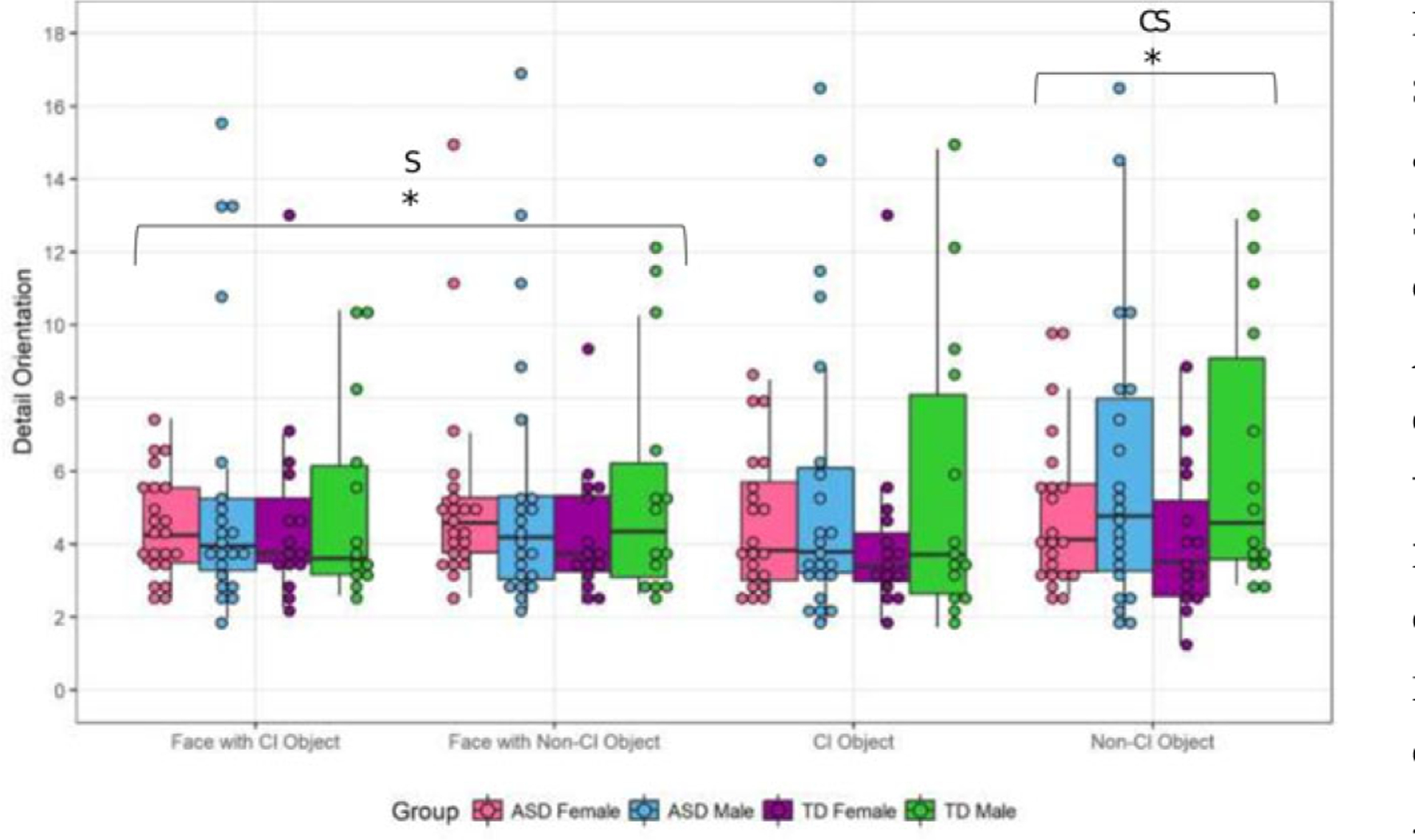
Detail orientation to faces and objects. Whole Sample: Faces: There a main effect of sex (F = 4.12, P = 0.04), with males being more detail orientated to faces than females. Objects: There was a trend toward a main effect for sex (F = 3.94, P = 0.09), with males being more detail orientated to objects. There was a condition*sex effect (F = 3.94, P = 0.05), with males being more detail orientated to objects in the non-CI condition (t = 2.50, P = 0.01). ASD Sample: Faces: There was a condition*sex interaction (F = 3.80, P = 0.05). Post hoc tests were not significant. Objects: There was a condition*sex interaction (F = 4.55, P = 0.04), with a trend toward males being more detail orientated to non-CI objects.Three outliers were removed from this figure.*P ≤ 0.05;**P ≤ 0.01;C = condition effect;S = sex effect;CS = condition*sex interaction.
Objects.
For objects, there was no main effect of condition or diagnosis, but there was a slight trend toward a main effect of sex (F = 2.84, P = 0.09, η2 = 0.04) with males being more detail orientated to objects overall (Fig. 5). There was a condition*sex interaction (F = 3.94, P = 0.05, η2 = 0.05). Males were more detail oriented to non-CI objects (t = 2.50, P = 0.01;Fig. 5). In the ASD group, there were no effects of condition or sex, but there was a condition*sex effect (F = 4.55, P = 0.04, η2 = 0.10). Post hoc analyses were not significant.
Discussion
The aim of this study was to examine whether patterns of social and nonsocial attention previously reported for school-aged children with ASD using overwhelmingly male samples [Sasson et al., 2008; Sasson & Touchstone, 2014;Unruh et al., 2016] extend to females with autism. Results indicated that the reduced social attention and increased nonsocial attention in ASD to objects commonly related to CI found in previous studies using the same paired preference paradigm [Sasson & Touchstone, 2014;Unruh et al., 2016] did not extended to females with ASD in our study. In general, our findings demonstrate more typical social and nonsocial attention in females with ASD relative to their male counterparts, suggesting a potential female protective effect in their preference and prioritization of social stimuli that may relate to their broader social phenotypic differences.
Sasson and Touchstone [2014] and Unruh et al. [2016] reported reduced fixation to faces in children and adolescents with autism when presented alongside CI-related objects. Here, however, females with ASD fixated longer to faces than ASD males when they were paired with CI related images, suggesting that the salience advantage afforded images of CI relative to faces reported previously may not occur to the same degree, if at all, for females with autism. In general, although many values reported here for males with ASD were comparable to those in Sasson and Touchstone [2014], group effects largely disappeared when females with ASD were included. Thus, prior studies may have inadvertently overextended findings from predominantly male samples to ASD in general, and at the same time underplayed the contribution of biological sex to their findings.
Indeed, the current study found evidence for strong sex differences regardless of diagnosis, with males but not females reducing their gaze to faces when paired with CI-related object, including in the ASD group. This suggests that prior findings may have been driven by their male-dominated samples, and that with a more equal inclusion of females, the effects of diagnosis are reduced while revealing a strong effect of biological sex. For instance, social attention for females with ASD mimicked social attention for TD females regardless of the type of object simultaneously displayed. They fixated to faces more and objects less, and oriented to faces faster, than males with ASD. These findings are largely inconsistent with the predictions of the EMB theory of ASD [Baron-Cohen, 2002] that presume phenotypic similarity between males and females with ASD and adds to a growing literature indicating that ASD in females manifests differently than in males. Our eye-tracking data complements parent reports of more typical CI [Hiller et al., 2014;Sutherland et al., 2017] and self-report of greater social motivation in ASD females [Cook et al., 2018;Vine Foggo & Webster, 2017].
The intact preference and prioritization of social stimuli in females with ASD found here could be indicative of a female protective effect. Greater interest and attention to social relative to nonsocial information from an early age could increase social learning opportunities and social abilities for females, leading to differences in phenotypic development compared to males with ASD. In turn, these skills could relate to under- or delayed identification of autism and improved “camouflaging” of characteristics seen later in development for females with ASD [Dean et al., 2017;Parish-Morris et al., 2017]. Indeed, it is possible that the findings reported here indicate a camouflaging ability to selectively engage with social stimuli rather than being truly motivated by it. Such a distinction is impossible to discern in the current study, and would require additional self-report and/or physiological data to conclusively determine. A recent event-related potential (ERP) study found that females with ASD actually had attenuated neural responses to faces compared to both ASD males and TD controls, suggestive of greater social impairment [Coffman, Anderson, Naples, & McPartland, 2015]. It is possible that potential dissociations exist between attention, processing and arousal, which can be explored through the coregistration of eye-gaze with ERP data, pupilometry or other physiological measures of arousal, such as heart rate. However, the fact that the current task was entirely passive viewing in which the only instructions were to look at the images any way that they wanted suggests that female participants voluntarily attended to social information rather than did so performatively. Regardless, these processes could be clarified in future studies through downward extensions of this paradigm to younger females on the spectrum and longitudinal data collection to examine whether these protective effects are observed early in development or potentially learned through social interactions and a greater ability to camouflage.
Future research also must consider the modification of paradigms to include interests commonly observed in TD females to understand whether the findings of previous majority male studies are applicable to ASD females. Indeed, it remains an open possibility that the sex differences reported here between ASD males and females may have been driven in part by the nature of the CI-related images included in the task. The CI represented were derived from a prior report of the most frequently occurring CI for children with autism, but this study also relied on predominantly male samples [South et al., 2005]. Thus, it is unclear if females with ASD would also demonstrate reduced social attention in the presence of CI-related images selected to represent the CI content more common for females with ASD [Hiller et al., 2014, 2016]. Future research should ask parents and, whenever possible, children how engaging they find the CI and non-CI images or whether the images reflect their interests. By testing this possibility, future research can determine whether the findings reported here of robust social attention in girls with ASD persists when nonsocial content that is more relevant and salient for them is included. If not, it would suggest similar attentional processes in girls and boys with autism that are revealed only when sex-typical nonsocial stimuli are included.
These findings also may have clinical relevance. In ASD, diagnostic criteria often reflect a lack of interest in social engagement with others; however, this clinical characterization may not fully hold for females with ASD who appear as interested in faces as their typically developing counterparts. This proclivity towards a social interest with others, either because of a biological proclivity or learned gender socialization processes, could contribute to the under or misdiagnosis of females with ASD. Further, there are treatment implications because many applied behavior analysis-based treatments focus early on the child attending to other’s faces, and this may not be a needed treatment target for females with ASD.
Limitations
This study has some limitations that are worthy of discussion. Although we included a relatively large sample of ASD females within a small age window, our sample size remains relatively small compared to studies of school aged children. As a result of the challenges of recruiting a large sample of females with ASD, some effects may have been underpowered. As a result, we included reports of both statistically significant and some trending results. However, any reported “trends” should be interpreted with caution, but may serve to guide future research questions within larger samples. Additionally, because all children in our ASD sample had received a local, clinical diagnosis of ASD that varied in timing and the gold standard ASD diagnostic tool(s) administered, we did not use scores from these measures, but instead included the SCQ as a parent-report measure to discriminate the ASD and TD groups that may lose some validity when applied to older children [Corsello et al., 2007]. It is unclear if the sample composition would differ if other diagnostic measures were used. Also, the TD groups were younger than the ASD groups, and while this was controlled for in the analysis, results may have differed if age was more tightly matched between groups.
It is noted that for certain variables (namely duration), groups appeared more similar than dissimilar, particularly when faces were paired with non-CI images (Fig. 5). Yet, after controlling for CA and MA, there were a number of significant effects and interactions. These effects could be driven by a handful of subjects or through the inclusion of CA and MA in our analysis that may inflate differences between our groups. However, the brief passive-viewing task used in this study requires minimal cognitive resources, and performance by our ASD females was more normative than ASD males despite being lower on MA, suggesting that their more typical gaze patterns relative to males was not cognitively driven. We also reran all analyses in a smaller, CA-matched sample and the overall pattern of significant and nonsignificant effects did not differ (see Supporting Information). Thus, given that MA and CA were covaried in all analyses, and the minimal relationships between MA and CA and our DVs (see Supporting Information), the group and sex differences reported here likely are not the product of differences in MA or CA.
It is unclear how findings would differ within more ecologically valid paradigms than the static image task administered here. Using different types of eye-tracking paradigms, Chevallier et al. [2015] reported the largest group effects for dynamic, social scenes. As females with ASD have been reported to be more socially motivated [Sedgewick et al., 2016] and have a greater ability to camouflage within social settings [Dean et al., 2017; Parish-Morris et al., 2017], a dynamic paradigm may reveal stronger findings than a static, less naturalistic one like the task used here. Relatedly, the current study only included generic CI-related images and not those tailored to the specific CI (if any) of the participants. Previous studies using individualized CI-related images have reported clear group differences between ASD and controls [Foss-Feig et al., 2016], and the lack of including personalized CI here is particularly relevant for females with ASD who may not have found the included CI images to be as salient relative to their male counterparts.
Conclusions
This study represents one of the first applications of eye-tracking to examine sex differences in ASD. Using a validated paired-preference paradigm, females with ASD demonstrated similar levels of prioritization and preference to social stimuli, irrespective of the category of paired object image. These findings suggest that females with ASD may be more motivated by social stimuli than their male peers and raise questions about the relevance of common CI-related stimuli for females and the EMB Theory of ASD. Our findings have implications for how motivated ASD females when studied using the same paradigms and frameworks as ASD males and using the same assessment and intervention protocols, calling for the need to recognize biological sex and gender in clinical practices and research designs.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by a North Carolina Translational and Clinical Sciences (NC TraCS) Pilot Grant (2KR691506) and Autism Science Foundation Accelerator Grant (16-003A) awarded to Clare Harrop. The research reported was also supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (R01HD082127 and P30-HD03110) and the National Center for Advancing Translational Sciences (UL1TR001111) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of NC TraCS, the Autism Science Foundation, or the National Institutes of Health. Portions of this data have been presented at the International Meeting For Autism Research 2017 (San Francisco, CA), the NIH Future Research Leaders Conference (Bethesda, MD), and the Triangle Neuroscience Conference (Raleigh, NC). We thank the families whose participation made this study possible.
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Appendix S1: Supplementary Material
Contributor Information
Desiree Jones, Allied Health Sciences, University of North Carolina at Chapel Hill, Carrboro, North Carolina.
Shuting Zheng, School of Education, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Sallie W. Nowell, Allied Health Sciences, University of North Carolina at Chapel Hill, Carrboro, North Carolina
Brian A. Boyd, Juniper Gardens Children’s Project, University of Kansas, Kansas City, Kansas
Noah Sasson, School of Behavioral and Brain Sciences, University of Texas at Dallas, Richardson, Texas.
References
- Alexander GM, Wilcox T, & Woods R (2009). Sex differences in infants’ visual interest in toys. Archives of Sexual Behavior, 38, 427–433. 10.1007/s10508-008-9430-1. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S (2002). The extreme male brain theory of autism. Trends in Cognitive Sciences, 6, 248–254. 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Benning SD, Kovac M, Campbell A, Miller S, Hanna EK, Damiano CR, … Dichter GS. (2016). Late positive potential ERP responses to social and nonsocial stimuli in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46, 3068–3077. 10.1007/s10803-016-2845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, & Lord C (2011). Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. American Journal on Intellectual and Developmental Disabilities, 116, 331–343. 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish J (2003). Interests scale. NC, USA: University of North Carolina at Chapel Hill. [Google Scholar]
- Bodfish J, Symons F, & Lewis M (1999). The repetitive behavior scale-revised: Morgantown, NC, USA: Western Carolina Center Research Reports. [Google Scholar]
- Chawarska K, Macari S, Powell K, DiNicola L, & Shic F (2016). Enhanced social attention in female infant siblings at risk for autism. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 188.e1-195.e1. 10.1016/j.jaac.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Parish-Morris J, McVey A, Rump KM, Sasson NJ, Herrington JD, & Schultz RT (2015). Measuring social attention and motivation in autism spectrum disorder using eye-tracking: Stimulus type matters. Autism Research, 8, 620–628. 10.1002/aur.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, … Yeargin-Allsopp M (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveillance Summaries, 65, 1–23. 10.15585/mmwr.ss6503a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman MC, Anderson LC, Naples AJ, & McPartland JC (2015). Sex differences in social perception in children with ASD. Journal of Autism and Developmental Disorders, 45, 589–599. 10.1007/s10803-013-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A, Ogden J, & Winstone N (2018). Friendship motivations, challenges and the role of masking for girls with autism in contrasting school settings. European Journal of Special Needs Education, 1–14. 10.1080/08856257.2017.1312797. [DOI] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal BL, & Lord C (2007). Between a ROC and a hard place: Decision making and making decisions about using the SCQ. Journal of Child Psychology and Psychiatry, 48, 932–940. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, & Brown E (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28, 479–485. 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27, 403–424. 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dean M, Harwood R, & Kasari C (2017). The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism, 21, 678–689. 10.1177/1362361316671845. [DOI] [PubMed] [Google Scholar]
- Dean M, Kasari C, Shih W, Frankel F, Whitney R, Landa R, … Harwood R (2014). The peer relationships of girls with ASD at school: Comparison to boys and girls with and without ASD. Journal of Child Psychology and Psychiatry, 55, 1218–1225. 10.1111/jcpp.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happe F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Child and Adolescent Psychiatry, 51, 788–797. 10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Elliot CD (2007). Differential ability scales-Second edition (DAS-II). San Antonio, TX: Pearson. [Google Scholar]
- Fletcher-Watson S, Leekam SR, Benson V, Frank MC, & Findlay JM (2009). Eye-movements reveal attention to social information in autism spectrum disorder. Neuropsychologia, 47, 248–257. 10.1016/j.neuropsychologia.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, McGugin RW, Gauthier I, Mash LE, Ventola P, & Cascio CJ (2016). A functional neuroimaging study of fusiform response to restricted interests in children and adolescents with autism spectrum disorder. Journal of Neurodevelopmental Disorders, 8, 15 10.1186/s11689-016-9149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman M, & Johnson SP (2013). Attentional capture by social stimuli in young infants. Frontiers in Psychology, 4, 527 10.3389/fpsyg.2013.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R, Schroeder L, Turner T, McGrath C, Chan R, Turetsky B, et al. (2002). Brain activation during facial emotion processing. NeuroImage, 16, 651. [DOI] [PubMed] [Google Scholar]
- Harrop C, Green J, & Hudry K (2017). Play complexity and toy engagement in preschoolers with autism spectrum disorder: Do girls and boys differ? Autism, 21, 37–50. 10.1177/1362361315622410. [DOI] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, & Stokes MA (2014). Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism, 5, 19 10.1186/2040-2392-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2014). Sex differences in autism spectrum disorder based on DSM-5 criteria: Evidence from clinician and teacher reporting. Journal of Abnormal Child Psychology, 42, 1381–1393. 10.1007/s10802-014-9881-x. [DOI] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2016). Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism, 20, 75–84. 10.1177/1362361314568899. [DOI] [PubMed] [Google Scholar]
- Jadva V, Hines M, & Golombok S (2010). Infants’ preferences for toys, colors, and shapes: Sex differences and similarities. Archives of Sexual Behavior, 39, 1261–1273. 10.1007/s10508-010-9618-z. [DOI] [PubMed] [Google Scholar]
- Jones W, Carr K, & Klin A (2008). Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry, 65, 946–954. 10.1001/archpsyc.65.8.946 [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, & Lord C (2002). Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 43, 807–821. 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaars MP, In’t Velt A, Mol A, Swaab H, Bodrij F, & van Rijn S (2017). Social attention and autism symptoms in high functioning women with autism spectrum disorders. Research in Developmental Disabilities, 64, 78–86. 10.1016/j.ridd.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz RT, Volkmar FR, & Cohen D (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59, 809–816. 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, & Jones W (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459, 257–261. 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. (2003). Facial emotion recognition in schizophrenia: Intensity effects and error pattern. The American Journal of Psychiatry, 160, 1768–1774. [DOI] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What Is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 466–474. 10.1016/j.jaac.2017. [DOI] [PubMed] [Google Scholar]
- Maccoby EE, & Jacklin CN (1978). The psychology of sex differences. Stanford, CA: Stanford University Press. [Google Scholar]
- Parish-Morris J, Liberman MY, Cieri C, Herrington JD, Yerys BE, Bateman L, … Schultz RT (2017). Linguistic camouflage in girls with autism spectrum disorder. Molecular Autism, 8, 48 10.1186/s13229-017-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, & Piven J (2002). Visual scanning of faces in autism. Journal of Autism and Developmental Disorders, 32, 249–261. 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The social communication questionnaire. Torrance, CA, USA: Western Psychological Services. [Google Scholar]
- Sasson NJ, Dichter GS, & Bodfish JW (2012). Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. PLoS One, 7, e42457 10.1371/journal.pone.0042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, & Bodfish JW (2011). Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders, 41, 242–247. 10.1007/s10803-010-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Pinkham AE, Richard J, Hughett P, Gur RE, & Gur RC (2010). Controlling for response biases clarifies sex and age differences in facial affect recognition. Journal of Nonverbal Behavior, 34, 207–221. [Google Scholar]
- Sasson NJ, & Touchstone EW (2014). Visual attention to competing social and object images by preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44, 584–592. 10.1007/s10803-013-1910-z. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, & Bodfish JW (2008). Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research, 1, 31–42. 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgewick F, Hill V, Yates R, Pickering L, & Pellicano E (2016). Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. Journal of Autism and Developmental Disorders, 46, 1297–1306. 10.1007/s10803-015-2669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin A, Bohlin G, & Berlin L (1999). Sex differences in 1-, 3-, and 5-year-olds’ toy-choice in a structured play-session. Scandinavian Journal of Psychology, 40, 43–48. 10.1111/1467-9450.00096. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, & McMahon WM (2005). Repetitive behavior profiles in asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders, 35, 145–158. 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Hodge A, Bruck S, Costley D, & Klieve H (2017). Parent-reported differences between school-aged girls and boys on the autism spectrum. Autism, 21(6), 785–794. 10.1177/1362361316668653. [DOI] [PubMed] [Google Scholar]
- Turner-Brown LM, Lam KS, Holtzclaw TN, Dichter GS, & Bodfish JW (2011). Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism, 15, 437–456. 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh KE, Sasson NJ, Shafer RL, Whitten A, Miller SJ, Turner-Brown L, & Bodfish JW (2016). Social orienting and attention is influenced by the presence of competing nonsocial information in adolescents with autism. Frontiers in Neuroscience, 10, 586 10.3389/fnins.2016.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, & Van der Gaag RJ (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 44, 627–635. 10.1007/s10803-013-1913-9. [DOI] [PubMed] [Google Scholar]
- Vine Foggo RS, & Webster AA (2017). Understanding the social experiences of adolescent females on the autism spectrum. Research in Autism Spectrum Disorder, 35, 74–85. 10.1016/j.rasd.2016.11.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


