Abstract
Obese patients who often present metabolic dysfunction-associated fatty liver disease (MAFLD) are at risk of severe presentation of coronavirus disease 2019 (COVID-19). These patients are more likely to be hospitalized and receive antiviral agents and other drugs required to treat acute respiratory distress syndrome and systemic inflammation, combat bacterial and fungal superinfections and reverse multi-organ failure. Among these pharmaceuticals, antiretrovirals such as lopinavir/ritonavir and remdesivir, antibiotics and antifungal agents can induce drug-induced liver injury (DILI), whose mechanisms are not always understood. In the present article, we hypothesize that obese COVID-19 patients with MAFLD might be at higher risk for DILI than non-infected healthy individuals or MAFLD patients. These patients present several concomitant factors, which individually can favour DILI: polypharmacy, systemic inflammation at risk of cytokine storm, fatty liver and sometimes nonalcoholic steatohepatitis (NASH) as well as insulin resistance and other diseases linked to obesity. Hence, in obese COVID-19 patients, some drugs might cause more severe (and/or more frequent) DILI, while others might trigger the transition of fatty liver to NASH, or worsen pre-existing steatosis, necroinflammation and fibrosis. We also present the main mechanisms whereby drugs can be more hepatotoxic in MAFLD including impaired activity of xenobiotic-metabolizing enzymes, mitochondrial dysfunction, altered lipid homeostasis and oxidative stress. Although comprehensive investigations are needed to confirm our hypothesis, we believe that the current epidemic of obesity and related metabolic diseases has extensively contributed to increase the number of cases of DILI in COVID-19 patients, which may have participated in presentation severity and death.
Keywords: COVID-19, Fatty liver, Drug hepatotoxicity, Cytochrome P450
Highlights
-
•
Obesity and fatty liver (MAFLD) enhance the risk of severe presentation of COVID-19.
-
•
Hospitalized COVID-19 patients may be treated with drugs at risk of liver toxicity.
-
•
Obese COVID-19 patients with MAFLD may be at high risk of drug-induced hepatotoxicity.
-
•
Increased hepatotoxicity is due to concomitant underlying risk factors of liver injury.
-
•
Risk factors include obesity, MAFLD, polypharmacy, severe inflammation and hypoxemia.
1. COVID-19, obesity and related metabolic diseases
Since late 2019, the emergence of a new coronavirus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV-2) has become a worldwide threat for human health. This coronavirus is responsible for a self-limiting disease of the upper airways, called coronavirus disease 2019 (COVID-19), which may result in intensive care unit (ICU) admission and fatalities [1]. Several studies suggested that obesity, hypertension and diabetes greatly increase the risk of severe and prolonged COVID-19 presentation [[2], [3], [4]]. However, it is still unclear why obesity and related metabolic disorders enhance COVID-19 severity. It has been postulated that the chronic pro-inflammatory state associated with these metabolic diseases may play a role, at least in part due to an activation of the renin-angiotensin system [[5], [6], [7]]. Hence, this pre-existing pro-inflammatory background seems to favour the cytokine storm, which may result in the multi-organ failure observed in severe COVID-19 [[8], [9], [10]]. Importantly, metabolic dysfunction-associated fatty liver disease (MAFLD, the term now replacing nonalcoholic fatty liver disease or NAFLD), which is present in the majority of obese people, was also found to be associated with severe COVID-19 [11,12].
2. COVID-19, liver function tests and histology
Liver function tests (LFTs) classically include the measurement in plasma (or serum) of alanine and aspartate aminotransferases (ALT and AST, reflecting hepatocyte cytolysis), alkaline phosphatase and γ-glutamyltransferase (ALP and GGT, reflecting cholestasis) as well as bilirubin and albumin (reflecting alterations of specific hepatic biological functions). A prolonged prothrombin time (PT) can also reflect an alteration of the hepatic synthesis of different coagulation factors. Abnormal LFTs is observed in up to 54% of COVID-19 patients, although severe liver dysfunction seems rare and mostly associated with poor disease outcome [13]. The most frequent abnormal LFT in COVID-19 patients is an elevation in ALT and AST, which is commonly mild or moderate (i.e. <5 times the upper reference limit) [[13], [14], [15]]. Patients admitted to the ICU are more likely to present high levels of ALT, AST and bilirubin as well as prolonged PT [1,13,15].
Different factors seem to cause abnormal LFTs and liver dysfunction in COVID-19 patients. SARS CoV-2 by itself may play a role because its receptor ACE2 (angiotensin-converting enzyme 2) was detected in cholangiocytes and to a much lesser degree in hepatocytes, whereas SARS CoV-2 RNA was found in liver samples [13,14]. Hence, elevation in ALP and GGT in some COVID-19 patients may reflect viral-induced cholangiocyte injury [14]. However, the direct cytopathic effects of the virus have been questioned by some authors [15,16]. The cytokine storm may also be involved since different pro-inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α) can be harmful to the liver [13,17,18]. Prolonged hypoxia due to acute respiratory distress syndrome (ARDS), shock and blood hyperviscosity may play a role in critically ill patients [13,18]. The role of pre-existing liver disease such as MAFLD and cirrhosis should be considered in some patients [1,13,14]. Finally, drug-induced liver injury may be involved [1,13,14,19], as discussed below in Section 4.
Post-mortem liver histology performed in some COVID-19 patients showed different liver lesions including lobular focal necrosis, apoptosis, microvesicular and macrovacuolar steatosis, inflammatory infiltrates in the portal areas and sinusoidal dilatation [1,[14], [15], [16]]. However, these lesions are not specific and may be observed in different settings such as MAFLD, sepsis or DILI [14,16]. Nonetheless, the presence of microvesicular steatosis in some patients suggests severe mitochondrial dysfunction, which can be induced (or favoured) by numerous factors including congenital enzymatic deficiencies and drugs [20].
3. Treatments in COVID-19 patients
COVID-19 patients can be treated by two types of drugs. On the first hand, various drugs with anti-SARS CoV-2 properties are currently being tested although their clinical benefit is still uncertain, and sometimes controversial. The main drugs currently under investigation include lopinavir/ritonavir combination, remdesivir and hydroxychloroquine, which can be associated with azithromycin (Table 1 ). Regarding the association of the protease inhibitors (PIs) lopinavir/ritonavir, it should be mentioned that the second PI is used to enhance lopinavir pharmacological effect by inhibiting cytochrome P450 3A4 (CYP3A4) activity [21]. Readers are invited to peruse recent reviews regarding the pharmacological properties of these different drugs [[22], [23], [24], [25]]. These reviews also discuss the possible effectiveness of other drugs with anti-SARS CoV-2 effects.
Table 1.
Liver toxicity of the main drugs with anti-SARS-CoV-2 properties and/or anti-inflammatory effects currently tested in COVID-19 patients.
| Drugs | Pharmacological class | Hepatotoxicity | Mechanisms of hepatotoxicity |
|---|---|---|---|
| Lopinavir/ritonavir | Antiretroviral protease inhibitors (PIs) | Ritonavir can induce mild to moderate (<6 N) elevation in aminotransferases in ∼44% of patients. Mostly presence of cytolysis but cholestasis, steatosis and fibrosis can also be present. Lopinavir seems less hepatotoxic than ritonavir. References: [29,30,88,98] |
Mechanisms of PI-induced hepatic toxicity seem to be multiple with involvement of mitochondrial dysfunction, oxidative stress and endoplasmic reticulum stress. PIs could also be hepatotoxic via indirect mechanisms, in particular by inducing lipodystrophy and insulin resistance. References: [62,80,82,89] |
| Remdesivir | Antiviral nucleoside analogue | Mild to moderate (<6 N) elevation in aminotransferases in ∼22% of patients. References: [28,99] |
Currently unknown. |
| Hydroxychloroquine | Antimalarial agent with antiviral, anti-inflammatory and immune-regulatory effects | Mild to moderate (<6 N) elevation in aminotransferases in ∼11% of patients. Rare occurrence of acute liver injury, sometimes with jaundice. References: [29,30,90,98] |
Possible role of oxidative stress and lipid peroxidation. Possible role of lysosomal membrane permeabilization and release of cathepsin, thus activating the pro-apoptotic protein Bax. Hence, Bax-induced mitochondrial membrane permeabilization may occur secondary to lysosomal destabilization. References: [62,91,92] |
| Azithromycin | Macrolide antibiotic with antiviral properties. Possible synergistic antiviral effect in association with hydroxychloroquine | Mild to moderate (<6 N) elevation in aminotransferases in ∼40% of patients. Rare cases of severe hepatotoxicity with hepatocellular injury and jaundice or with chronic cholestatic liver failure. References: [29,30,93,98] |
Possible role of mitochondrial dysfunction and oxidative stress. Cholestasis is not related to the inhibition of liver canalicular bile salt export pump (BSEP). References: [[94], [95], [96], [97]] |
| Tocilizumab | Interleukin-6 (IL-6) receptor antagonist (monoclonal antibody) | Elevation in aminotransferases and acute liver injury, sometimes severe. Reference: [100] |
Currently unknown. |
On the other hand, COVID-19 patients may receive drugs routinely used during the course of viral illnesses with possible onset of bacterial and fungal superinfections, ARDS, systemic inflammation and even multi-organ failure. These drugs include antibiotics such as macrolides (other than azithromycin) and beta-lactams, antifungal agents such as azole derivatives, immunomodulatory agents such as corticosteroids (e.g. dexamethasone, methylprednisolone) and monoclonal antibodies such as the anti-interleukin-6 (IL-6) receptor tocilizumab (Table 1) or the anti-IL-1 receptor anakinra [22,23,25,26]. Anticoagulant treatments, mostly heparin and low-molecular-weight heparin, the sympathomimetic amine norepinephrine and different sedative agents (e.g. midazolam, propofol, fentanyl) can also be required in critically ill COVID-19 patients. Notably, although acetaminophen (paracetamol) is rarely prescribed as antipyretic agent in ICU patients, this drug is frequently used prior to hospitalization [27]. Hence, clinicians should not overlook the possibility of major acetaminophen self-administration in COVID-19 patients. This analgesic and antipyretic drug will be discussed below.
4. Drug-induced hepatotoxicity in COVID-19
Although liver injury is not the primary cause of death in COVID-19, elevated serum ALT and AST levels are often observed in hospitalized patients [1,[13], [14], [15],17,18,28]. Hepatotoxicity may represent the first cause of liver injury [[13], [14], [15],17,18,28], although it should be underlined that assessing DILI in a clinical setting where abnormal LFTs can be induced by pre-existing liver diseases and COVID-19 is a particularly difficult issue. Many drugs prescribed in these patients are potentially hepatotoxic. For instance, lopinavir, ritonavir, remdesivir, hydroxychloroquine, azithromycin and tocilizumab can induce liver injury, sometimes even severe (Table 1). Other drugs such as carbapenems (e.g. imipenem, meropenem), azole derivatives (e.g. ketoconazole, fluconazole), corticosteroids and propofol can also be hepatotoxic [29,30]. DILI and other adverse effects in COVID-19 patients may be precipitated by acute cardiovascular failure and kidney injury, resulting in drug overdose by reducing drug metabolism and elimination. Notably, acetaminophen-induced liver injury can be favoured by increased hepatic CYP2E1 activity [30], which is often observed in individuals with heavy alcohol consumption or in patients suffering from MAFLD, as discussed in section 7.1.
Clearly, future investigations will be required to determine which of these drugs could be involved in the elevated serum aminotransferases commonly observed in COVID-19 patients. It should be pointed out that accountability assessment in cases with suspected DILI is mainly based on chronological and clinical criteria as well as the elimination of other causes of liver injury [31]. Drug accountability can be evaluated by different methods such as the Roussel Uclaf Causality Assessment Method (RUCAM) [31,32]. Of note, other methodological approaches are required for causality assessment of suspected DILI in patients with pre-existing nonalcoholic steatohepatitis (NASH) [33].
5. Hypothesis: are Covid-19 patients with pre-existing MAFLD at higher risk for DILI?
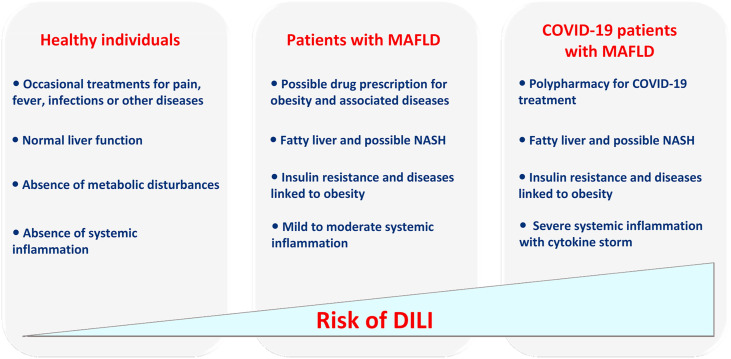
Herein, we hypothesize that obese COVID-19 patients with MAFLD may be at higher risk for DILI than non-infected healthy individuals or MAFLD patients. This assumption is based on the fact that these patients present several concomitant factors, which individually can favour DILI (Fig. 1 ). First, as previously mentioned, many drugs prescribed in COVID-19 patients are potentially harmful for the liver, and thus polypharmacy is expected to enhance the risk of hepatotoxicity. Noteworthy, numerous drug-drug interactions potentially exist with the pharmaceuticals prescribed in COVID-19 patients, thus enhancing the risk of toxicity [34]. Second, the cytokine storm potentially associated with severe COVID-19 can favour DILI. It has been shown that different pro-inflammatory cytokines such as TNF-α, IL1-β and IL-6 can significantly enhance the hepatotoxicity potential of numerous drugs including antibiotics [35,36]. Third, MAFLD might even further enhance the risk of DILI. There are increasing clinical reports (sometimes confirmed by experimental studies) suggesting that some drugs can be considered as more hepatotoxic in obese patients with MAFLD, when compared with lean individuals [[37], [38], [39], [40], [41], [42], [43]].
Fig. 1.
According to our hypothesis, obese COVID-19 patients with metabolic dysfunction-associated fatty liver disease (MAFLD) may be at higher risk for drug-induced liver injury (DILI) than non-infected healthy individuals or MAFLD patients. This higher risk may be secondary to different factors including multiple drug administration (i.e. polypharmacy) to treat COVID-19, pre-existing fatty liver and possibly non-alcoholic steatohepatitis (NASH), altered activity of cytochromes P450 and other xenobiotic-metabolizing enzymes secondary to MAFLD and insulin resistance (as illustrated in Fig. 2) and the cytokine storm reflecting severe systemic inflammation.
DILI in MAFLD seems to occur as two distinct clinical situations [38,39,43]. First, some drugs such as acetaminophen and some antibiotics may cause more severe and/or more frequent acute liver injury (Table 2 ) [[43], [44], [45]]. Regarding, acetaminophen, it should be pointed out that its hepatotoxicity is mainly observed after overdose but some cases of liver injury (sometimes severe) may also occur with recommended therapeutic dosing [39,46,47]. Other pharmaceuticals including corticoids, antiretroviral agents and methotrexate seem to trigger the transition of simple fatty liver to NASH or worsen pre-existing steatosis, necroinflammation and fibrosis [39,48,49]. The mechanisms whereby some pharmaceuticals can be more hepatotoxic in MAFLD are presented in section 7. Lastly, it should be underlined that not all drugs may be at risk in MAFLD. For instance, statins and amiodarone-induced hepatotoxicity does not seem to be more frequent in MAFLD patients [43].
Table 2.
Examples of drugs shown (or suspected) to induce more frequent and/or more severe acute hepatitis in the context of obesity and metabolic dysfunction-associated fatty liver disease (MAFLD). Further information is provided in previous articles [[37], [38], [39], [40], [41], [42], [43]].
| Drug | Therapeutic class |
|---|---|
| Acetaminophen (paracetamol) Fosinopril Halothane Haloperidol Isoflurane Losartan Omeprazole Piperacillin-Tazobactam Sorafenib Telithromycin Ticlopidine Troglitazone |
Analgesic, antipyretic Antihypertensive Volatile anesthetic Antipsychotic Volatile anesthetic Antihypertensive Proton pump inhibitor Antibiotic Anticancer Antibiotic Inhibitor of platelet aggregation Antidiabetic (PPARγa agonist) |
Abbreviation: PPARγ, peroxisome proliferator activated receptor gamma.
6. How to test this hypothesis?
Because our hypothesis is predicting a non-established fact, cohort or registry studies are required in order to determine whether obese COVID-19 patients present increased risk of DILI. However, it should be kept in mind that hepatotoxicity is a rare event since DILI occurs at the most in about 1 per 10,000 treated patients [43]. This clinical approach can be complemented by experimental investigations in appropriate experimental models of MAFLD. Recently, we set up a cellular model of MAFLD by using metabolically competent HepaRG cells incubated with stearic acid (100 μM) for 7 days [50] or with a mixture of stearic and oleic acids (150 μM each) for 14 days [[51], [52], [53]]. These in vitro models of MAFLD were characterized by reduced CY3A4 activity and increased CYP2E1 activity, thus reproducing what has been reported in clinical studies [44,[54], [55], [56]], as discussed below. In addition, these models unveiled increased cytotoxicity of acetaminophen [50], troglitazone [52] and a mixture of benzo[a]pyrene and ethanol [51,53] in MAFLD cells compared with non-steatotic cells. Regarding acetaminophen [50], these in vitro investigations confirmed previous studies carried out in obese mice with MAFLD [44,45,57]. In contrast, we found that ritonavir was less cytotoxic in MAFLD cells compared with the non-steatotic cells [52]. Nonetheless, this cellular model of MAFLD is useful to determine whether drugs such as lopinavir, remdesivir, hydroxychloroquine and azithromycin are more toxic in this dysmetabolic state. The HepaRG cell model will also be useful to perform mechanistic studies in particular by investigating mitochondrial dysfunction, de novo lipogenesis, reactive oxygen species (ROS) overproduction and endoplasmic reticulum (ER) stress [[51], [52], [53],58], as discussed below.
Importantly, the above-described in vitro models of DILI using cells incubated with fatty acids should be refined in order to tally with the COVID-19 context. To this end, steatotic HepaRG cells could be cultured in the presence of SARS CoV-2-like particles. However, it should be mentioned that these cells express low levels of ACE2 and transmembrane serine protease 2 (TMPRSS2), which is also involved in the viral cellular entry [5,13]. Hence, HepaRG cells will need to be engineered to stably express these proteins. It will also be important to perform HepaRG culture in the presence of different pro-inflammatory cytokines and low oxygen levels to mimic the cytokine storm and hypoxia, respectively. Although these in vitro models are difficult to set up, they may provide invaluable information regarding drug-induced hepatotoxicity in the setting of COVID-19.
7. How can drugs be more hepatotoxic in MAFLD?
The mechanisms whereby drugs and xenobiotics are more hepatotoxic in MAFLD are not fully understood and are currently the matter of extensive in vitro and in vivo investigations due to the worldwide epidemic of obesity [39,40,44,[51], [52], [53], [54]]. These mechanisms are currently known only for a few compounds [39,43]. Hence, the mechanisms of DILI in MAFLD proposed herein represent the state of the art.
7.1. Acute liver injury in MAFLD
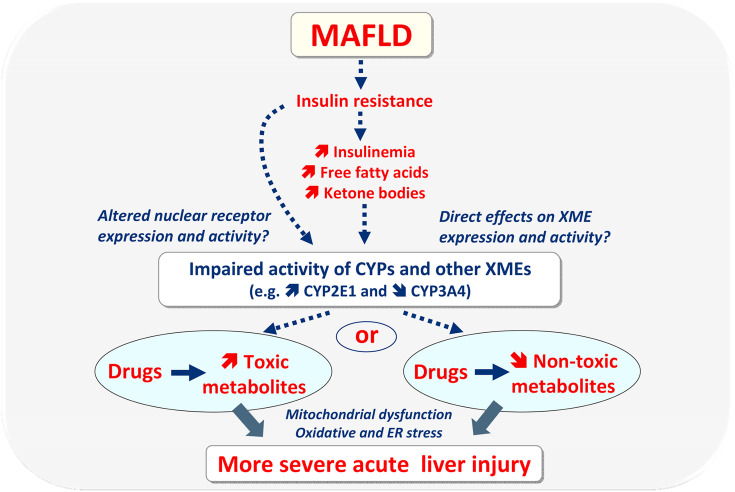
Some drugs may induce more severe acute liver injury in MAFLD because this disease is associated with altered activity of different xenobiotic-metabolizing enzymes (XMEs) including cytochromes P450 (CYPs) [44,[54], [55], [56]]. This can increase the generation of toxic metabolites, or conversely impair different detoxification pathways (Fig. 2 ) [[54], [55], [56],59]. For instance, human MALFD is often associated with increased CYP2E1 activity and reduced CYP3A4 activity and also with higher glucuronide formation, at least with some drugs such as lorazepam and acetaminophen [44,[54], [55], [56]]. Although acetaminophen undergoes higher glucuronide formation in MAFLD, its increased hepatotoxicity in this metabolic disease seems primarily linked to enhanced CYP2E1 activity, which generates greater hepatic amounts of the highly toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) [44,50,57].
Fig. 2.
Some drugs can cause more severe (and/or more frequent) acute liver injury in individuals with metabolic dysfunction-associated fatty liver disease (MAFLD), which is often associated with obesity and insulin resistance. Such enhanced risk in MAFLD is in relation to altered activity of cytochromes P450 (CYPs) and other xenobiotic-metabolizing enzymes (XMEs), which can increase the generation of toxic metabolites, or conversely impair detoxification pathways. In turn, higher hepatic concentrations of toxic metabolites or of the parent drugs can induce more severe acute liver injury through mitochondrial dysfunction, oxidative stress and endoplasmic reticulum (ER) stress, which can already be present in MAFLD. All these deleterious events may lead to hepatocyte necrosis or apoptosis (not shown). It is still unclear why MAFLD is associated with impaired activity of CYPs and other XMEs. Insulin resistance may play a role directly, or indirectly via hyperinsulinemia, increased free fatty acids and hyperketonemia. These events may directly affect XMEs or impair the expression and activity of nuclear receptors regulating XME transcription.
Increased generation of toxic metabolites or higher concentrations of the parent drugs can trigger cell death by necrosis and apoptosis. Regardless of the involved drugs, cell death commonly involves mitochondrial dysfunction. Severe impairment of oxidative phosphorylation (OXPHOS) causes ATP shortage and necrosis. Alternatively, in cells with enough ATP reserve, drug-induced mitochondrial dysfunction can induce the release of pro-apoptotic factors such as cytochrome c and apoptosis inducing factor (AIF), thus leading to caspase activation and apoptosis. These mitochondrial events in necrosis and apoptosis are associated with ROS overproduction and oxidative stress as well as ER stress (Fig. 2), which can already be present in MAFLD [40,60,61]. Hence, drugs can worsen pre-existing impairment of mitochondrial respiratory chain (MRC) and OXPHOS, ROS overproduction and different cellular stresses. Readers are invited to peruse previous reviews to get further information on drug-induced mitochondrial dysfunction and cell death [[62], [63], [64], [65]].
The mechanisms whereby MAFLD is frequently associated with CYP2E1 induction are still unclear. Some investigations suggest the role of insulin resistance and fatty acids such as stearic acid (Fig. 2) [50,66]. In keeping with the role of specific lipid derivatives and possibly free fatty acids, previous investigations reported that accumulation of hepatic triglycerides is not sufficient by itself to enhance CYP2E1 activity [57]. Interestingly, ketone bodies may also play a significant role in hepatic CYP2E1 induction [66]. Acetone and acetoacetate have been shown to increase CYP2E1 protein expression in rodent liver by reducing CYP2E1 protein degradation [66,67]. In addition, acetoacetate enhances translation of Cyp2e1 mRNA in the rat [67]. However, although both mitochondrial fatty acid oxidation (mtFAO) and ketogenesis are deemed to be enhanced in early MAFLD [60,68], ketogenesis per se seems to be progressively impaired during the evolution of the disease towards NASH [68,69]. Hence, the role of ketone bodies in CYP2E1 induction may only be transient in MAFLD. Finally, high blood glucose concentration may increase CYP2E1 expression in liver, even though conflicting results have been reported [66].
Much less is known regarding the mechanisms whereby MAFLD is commonly associated with lower expression and activity of CYP3A4. Previous investigations in the HepaRG cell line also suggested the role of stearic acid [50], but it is still unknown why this long-chain saturated fatty acid can concomitantly decrease and increase the expression and activity of CYP3A4 and CYP2E1, respectively. Another study pointed to a role of the fibroblast growth factor 21-pregnane X receptor (PXR) pathway [70], but recent investigations showed that the nuclear receptor PXR is not involved in MAFLD-associated CYP3A4 downregulation [71].
In contrast to CYP2E1 which metabolizes only a few drugs in addition to acetaminophen [54], CYP3A4 plays a major role in the metabolism of numerous pharmaceuticals including ritonavir and other antiretroviral PIs, remdesivir, hydroxychloroquine, midazolam and other benzodiazepines, fentanyl and different antibiotics [21,52,[72], [73], [74], [75]]. Hence, MAFLD-associated reduction in CYP3A4 activity impairs the biotransformation of several drugs prescribed in COVID-19 patients. The risk of drug-induced hepatotoxicity will be enhanced or reduced depending on whether CYP3A4 is involved in drug detoxication or in the generation of toxic reactive metabolites (Fig. 2). For instance, troglitazone is suspected to have induced severe hepatotoxicity in MAFLD due to lower CYP3A4-mediated biotransformation of this antidiabetic drug [52]. Finally, it should be pointed out that numerous drugs such as ritonavir, different antibiotics such as macrolides and fluoroquinolones and several antifungal azole derivatives such as ketoconazole and itraconazole are potent CYP3A4 inhibitors [73,76]. Hence, these drugs could significantly reinforce MAFLD-associated impairment of CYP3A4 activity.
7.2. Drug-induced aggravation of MAFLD
As previously mentioned, different drugs can aggravate pre-existing liver lesions classically observed in MAFLD including fatty liver, necroinflammation and fibrosis, or trigger the transition of simple fatty liver to NASH (Table 3 ) [38,39,43,48,49]. Regardless of the pathologic situation, previous investigations with several drugs and other xenobiotics strongly suggest a significant role of mitochondrial dysfunction, ROS overproduction and ER stress (Fig. 3 ) [38,39,43,51,53].
Table 3.
Example of drugs shown (or suspected) to aggravate pre-existing metabolic dysfunction-associated fatty liver disease (MAFLD), or to favour the transition of pre-existing fatty liver to nonalcoholic steatohepatitis (NASH). Further information is provided in previous articles [[38], [39], [40], [41], [42], [43]].
| Drug | Therapeutic class |
|---|---|
| Androgenic steroids Benzbromarone Corticosteroids Irinotecan Methotrexate NRTIs1 (e.g. stavudine, didanosine) Pentoxifylline Phenobarbital Protease inhibitors (e.g. lopinavir, ritonavir) Raloxifene Rosiglitazone Tamoxifen Tetracycline |
Anabolic Uricosuric Anti-inflammatory Anticancer Immunosuppressant, antimetabolite Antiretroviral Hemorrheologic agent, anti-TNF-α1 Antiepileptic Antiretroviral Anti-osteoporotic Antidiabetic (PPARγ1 agonist) Anticancer Antibiotic |
Abbreviations: NRTI, nucleoside reverse transcriptase inhibitor; PPARγ, peroxisome proliferator activated receptor gamma; TNF-α, tumour necrosis factor-alpha.
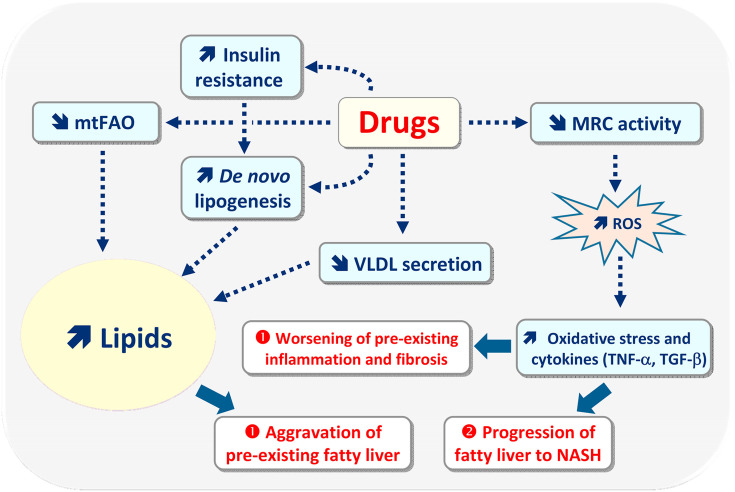
Fig. 3.
In patients with metabolic dysfunction-associated fatty liver disease (MAFLD), some drugs can worsen pre-existing fatty liver, necroinflammation and fibrosis (situation ➊), or induce a faster progression of simple fatty liver to nonalcoholic steatohepatitis (NASH) (situation ➋). Aggravation of pre-existing fatty liver can be secondary to drug-induced inhibition of mitochondrial fatty acid oxidation (mtFAO) and to impairment of very low-density lipoprotein (VLDL) secretion. Drugs can also enhance de novo lipogenesis either directly by activating different lipogenic nuclear receptors (not shown), or indirectly via insulin resistance and associated hyperinsulinemia. Oxidative stress and secondary overproduction of pro-inflammatory cytokines (e.g. TNF-α) and profibrotic cytokines (e.g. TGF-β) seem to play a role in the worsening of pre-existing inflammation and fibrosis, or in the faster progression of fatty liver to NASH. Oxidative stress and increased production of deleterious cytokines are secondary to reactive oxygen species (ROS), that are generated in excess via drug-induced impairment of mitochondrial respiratory chain (MRC) activity. In MAFLD, ROS overproduction can also be due to the induction of cytochrome P450 2E1.
Exacerbation of pre-existing fatty liver could involve mtFAO inhibition but also impairment of very low-density lipoprotein (VLDL) secretion and increased de novo lipogenesis (DNL), namely enhanced synthesis of fatty acids from carbohydrates (Fig. 3) [[38], [39], [40],43].
Many drugs can impair mtFAO by different mechanisms including direct inhibition of mtFAO enzymes such as carnitine palmitoyltransferase 1 (CPT1) and different acyl-CoA dehydrogenases as well as direct impairment of MRC and OXPHOS [20,63,77,78]. OXPHOS can also be impaired indirectly by alteration of mitochondrial DNA (mtDNA) homeostasis such as inhibition of mtDNA replication or translation [20,77,79]. All these adverse effects are not mutually exclusive. For instance, some drugs such as acetaminophen, amiodarone and tamoxifen can directly inhibit both mtFAO and MRC [20,77,78]. In addition, acetaminophen and troglitazone can impair MRC activity directly or indirectly through mtDNA depletion [77,79]. Finally, it should be mentioned that drug-induced inhibition of mtFAO could not only worsen pre-existing steatosis but also necrosis since this metabolic pathway is a major source of energy in hepatocytes [77,80].
Impairment of VLDL secretion can be caused by direct inhibition of the activity of microsomal triglyceride transfer protein (MTTP, also referred to as MTP), an ER enzyme playing a key role in apolipoprotein B (apoB) lipidation [81]. Alternatively, drugs can impair VLDL secretion by inducing ER stress and subsequent degradation of mRNAs of key proteins involved in VLDL assembly including MTTP, apoB and apoC3 [58]. Of note, ER stress can be induced by numerous pharmaceuticals such as PIs (e.g. ritonavir, lopinavir, indinavir) and other antiretroviral agents, acetaminophen and several nonsteroidal anti-inflammatory drugs (NSAIDs) including diclofenac and indomethacin [58,82].
Drug-induced enhanced hepatic DNL has been reported (or greatly suspected) with different drugs such as antiretroviral agents, amiodarone, benzbromarone, corticosteroids, indomethacin, rosiglitazone, sulindac and tetracyclines [39,43,58]. For some of these drugs (e.g. amiodarone, rosiglitazone), mechanistic studies suggested an activation of lipogenic transcription factors such as peroxisome proliferator activated receptor gamma (PPARγ) and sterol regulatory element binding protein-1c (SREBP1c) [39,43,58]. With other drugs (e.g. antiretroviral agents, corticosteroids) activation of DNL could be secondary to insulin resistance and concomitant hyperinsulinemia, which in turn activates SREBP1c in the liver [38,43,80].
Much less is known regarding the mechanisms whereby drugs can exacerbate pre-existing inflammation and fibrosis. Previous data with some drugs and other xenobiotics suggested an important role of ROS overproduction and subsequent oxidative stress, which in turn can favour the production of pro-inflammatory cytokines (e.g. TNF-α, IL1-β, IL-6) and transforming growth factor-β (TGF-β), a key pro-fibrotic cytokine (Fig. 3) [[38], [39], [40],83]. In the setting of drug-induced toxicity and MAFLD, ROS overproduction can be secondary to the dysfunctional mitochondria, which can produce large amounts of superoxide anion at the level of MRC complexes I and III [40,60,80]. Alternatively, ROS overproduction can be caused by CYP2E1 induction because this enzyme can produce superoxide anion and hydrogen peroxide during its catalytic cycle [54,84]. Finally, because CYP2E1 is located in the ER but also in mitochondria, ROS overproduction in these organelles can be secondary to MRC impairment and increased mitochondrial CYP2E1 expression [54,84,85].
8. Concluding remarks
Hospitalized COVID-19 patients are often polymedicated, which can greatly increase the risk of drug-drug interactions and drug-induced adverse events. Comorbidities such as obesity, MAFLD and diabetes may additionally enhance the risk of hepatotoxicity. Even though liver injury is not the primary cause of death in COVID-19 patients, hepatic dysfunction could undoubtedly worsen the patient’s condition, for instance by reducing the synthesis of coagulation proteins including factors II, V, VII, X, and fibrinogen [86]. However, it should be emphasized that severe liver disease with impaired coagulation factor synthesis is rare in the setting of COVID-19, which actually is mostly associated with mild-to-moderate elevation of ALT and AST [[13], [14], [15]].
Because a vaccine against SARS CoV-2 has not been marketed yet, antiviral drugs and other pharmaceuticals such as antibiotics and corticosteroids will still be administered to COVID-19 patients in the next few months. Thus, it would be important to identify drugs responsible for a specific increased risk of liver injury in COVID-19 patients, especially in those with obesity and related metabolic diseases. To this end, well-designed prospective clinical investigations should show that LFTs are normal or slightly abnormal in COVID-19 patients before their hospitalization in order to demonstrate that hepatic dysfunction occurring after admission is induced by the virus or triggered by drugs and not related to any significant pre-existing liver disease. Experimental investigations will also be required to confirm that MAFLD can significantly enhance the risk of drug-induced hepatotoxicity and investigate the involved mechanisms. Although rodent models of obesity and MAFLD can be used to reach these aims [43], lipid-laden hepatocytes can be more useful for preliminary drug screening [52,87]. However, these in vitro models should evolve towards more complex experimental systems with steatotic cells cultured not only with drugs but also in the presence of SARS CoV-2-like particles, pro-inflammatory cytokines and hypoxia. All these comprehensive scientific investigations should allow to refute or confirm the hypothesis that obese COVID-19 patients with pre-existing MAFLD might be at higher risk for DILI than non-infected healthy individuals or MAFLD patients. Nevertheless, regardless of the results, data from such clinical and experimental studies will undoubtedly benefit to COVID-19 patients but also to the scientific community. Finally, beyond the COVID-19 crisis, the worldwide epidemic of obesity is expected to increase the number of cases of DILI and possibly other drug-related adverse events.
Author contributions
Bernard Fromenty drafted the original manuscript. Pierre-Jean Ferron, Thomas Gicquel, Bruno Mégarbane and Bruno Clément critically amended the manuscript. All the authors have read and approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the Agence Nationale de Sécurité du Médicament et des produits de santé (ANSM) for the financial support granted for the PREVITOX network. We are also grateful to INSERM (Institut National de la Santé et de la Recherche Médicale) for its constant financial support.
References
- 1.Jothimani D., Venugopal R., Abedin M.F., Kaliamoorthy I., Rela M. COVID-19 and liver. J. Hepatol. 2020:S0168–S8278. doi: 10.1016/j.jhep.2020.06.006. 20)30377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., Chang B.P., Chau K.H., Choi J.J., Gavin N., Goyal P., Mills A.M., Patel A.A., Romney M.-L.S., Safford M.M., Schluger N.W., Sengupta S., Sobieszczyk M.E., Zucker J.E., Asadourian P.A., Bell F.M., Boyd R., Cohen M.F., Colquhoun M.I., Colville L.A., de Jonge J.H., Dershowitz L.B., Dey S.A., Eiseman K.A., Girvin Z.P., Goni D.T., Harb A.A., Herzik N., Householder S., Karaaslan L.E., Lee H., Lieberman E., Ling A., Lu R., Shou A.Y., Sisti A.C., Snow Z.E., Sperring C.P., Xiong Y., Zhou H.W., Natarajan K., Hripcsak G., Chen R. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020 doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajifathalian K., Kumar S., Newberry C., Shah S., Fortune B., Krisko T., Ortiz-Pujols S., Zhou X.K., Dannenberg A.J., Kumar R., Sharaiha R.Z. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York city. Obesity. 2020 doi: 10.1002/oby.22923. oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., the LICORN and the Lille COVID-19 and Obesity study group. Caizzo R., Caplan M., Cousin N., Duburcq T., Durand A., El kalioubie A., Favory R., Garcia B., Girardie P., Goutay J., Houard M., Jaillette E., Kostuj N., Ledoux G., Mathieu D., Moreau A.S., Niles C., Nseir S., Onimus T., Parmentier E., Préau S., Robriquet L., Rouze A., Six S., Verkindt H. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020;16:297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engin A.B., Engin E.D., Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ. Toxicol. Pharmacol. 2020;78 doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omarjee L., Janin A., Perrot F., Laviolle B., Meilhac O., Mahe G. Targeting T-cell senescence and cytokine storm with rapamycin to prevent severe progression in COVID-19. Clin. Immunol. 2020;216 doi: 10.1016/j.clim.2020.108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J. Hepatol. 2020;73:2051–2061. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G., Mantovani A., Byrne C.D., Wang X.-B., Yan H.-D., Sun Q.-F., Pan K.-H., Zheng K.I., Chen Y.-P., Eslam M., George J., Zheng M.-H. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 13.Bertolini A., van de Peppel I.P., Bodewes F.A., Moshage H., Fantin A., Farinati F., Fiorotto R., Jonker J.W., Strazzabosco M., Verkade H.J., Peserico G. Abnormal liver function tests in COVID-19 patients: relevance and potential pathogenesis. Hepatology. 2020 doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alqahtani S.A., Schattenberg J.M. Liver injury in COVID-19: the current evidence. United European Gastroenterol. J. 2020;8:509–519. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J., Fan J.-G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J. Clin. Transl. Hepatol. 2020;8:1–5. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philips C.A., Ahamed R., Augustine P. SARS-CoV-2 related liver impairment – perception may not be the reality. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.025. S0168827820303445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Z., Chen L., Li J., Cheng X., Yang J., Tian C., Zhang Y., Huang S., Liu Z., Cheng J. Clinical features of COVID-19-related liver functional abnormality. Clin. Gastroenterol. Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;428–430 doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeckmans J., Rodrigues R.M., Demuyser T., Piérard D., Vanhaecke T., Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch. Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fromenty B., Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol. Ther. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 21.Li F., Lu J., Ma X. CPY3A4-Mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab. Dispos. 2012;40:18–24. doi: 10.1124/dmd.111.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlow A., Landolf K.M., Barlow B., Yeung S.Y.A., Heavner J.J., Claassen C.W., Heavner M.S. Review of emerging pharmacotherapy for the treatment of coronavirus disease. Pharmacotherapy. 2019;40:416–437. doi: 10.1002/phar.2398. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mégarbane B., Scherrmann J. Hydroxychloroquine and azithromycin to treat patients with COVID-19: both friends and foes? J. Clin. Pharmacol. 2020;60:808–814. doi: 10.1002/jcph.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA Apr. 2020;13 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 26.Filocamo G., Mangioni D., Tagliabue P., Aliberti S., Costantino G., Minoia F., Bandera A. Use of anakinra in severe COVID-19: a case report. Int. J. Infect. Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Morales A.J., Cardona-Ospina J.A., Murillo-Muñoz M.M. Gastroenterologists, hepatologists, COVID-19 and the use of acetaminophen. Clin. Gastroenterol. Hepatol. 2020;18:2142–2143. doi: 10.1016/j.cgh.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrido I., Liberal R., Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment. Pharmacol. Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biour M., Ben Salem C., Chazouillères O., Grangé J.-D., Serfati L., Poupon R. [Drug-induced liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs] Gastroenterol. Clin. Biol. 2004;28:720–759. doi: 10.1016/S0399-8320(04)95062-2. [DOI] [PubMed] [Google Scholar]
- 30.LiverTox Clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD) 2012 http://www.ncbi.nlm.nih.gov/books/NBK547852/ accessed August 18, 2020. [PubMed] [Google Scholar]
- 31.Meunier L., Larrey D. Drug-induced liver injury: biomarkers, requirements, candidates, and validation. Front. Pharmacol. 2019;10:1482. doi: 10.3389/fphar.2019.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int. J. Mol. Sci. 2015;17:14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regev A., Palmer M., Avigan M.I., Dimick-Santos L., Treem W.R., Marcinak J.F., Seekins D., Krishna G., Anania F.A., Freston J.W., Lewis J.H., Sanyal A.J., Chalasani N. Consensus: guidelines: best practices for detection, assessment and management of suspected acute drug-induced liver injury during clinical trials in patients with nonalcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2019;49:702–713. doi: 10.1111/apt.15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge C., Marra F., Marzolini C., Boyle A., Gibbons S., Siccardi M., Burger D., Back D., Khoo S. Drug interactions: a review of the unseen danger of experimental COVID-19 therapies. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa340. dkaa340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosgrove B.D., King B.M., Hasan M.A., Alexopoulos L.G., Farazi P.A., Hendriks B.S., Griffith L.G., Sorger P.K., Tidor B., Xu J.J., Lauffenburger D.A. Synergistic drug–cytokine induction of hepatocellular death as an in vitro approach for the study of inflammation-associated idiosyncratic drug hepatotoxicity. Toxicol. Appl. Pharmacol. 2009;237:317–330. doi: 10.1016/j.taap.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharanek A., Burban A., Ciriaci N., Guillouzo A. Pro-inflammatory cytokines enhance dilatation of bile canaliculi caused by cholestatic antibiotics. Toxicol. In Vitro. 2019;58:51–59. doi: 10.1016/j.tiv.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Tarantino G., Conca P., Basile V., Gentile A., Capone D., Polichetti G., Leo E. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol. Res. 2007;37:410–415. doi: 10.1111/j.1872-034X.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 38.Fromenty B. Drug-induced liver injury in obesity. J. Hepatol. 2013;58:824–826. doi: 10.1016/j.jhep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Massart J., Begriche K., Moreau C., Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J. Clin. Transl. Res. 2017;3(Suppl 1):212–213. doi: 10.18053/jctres.03.2017S1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bessone F., Dirchwolf M., Rodil M.A., Razori M.V., Roma M.G. Review article: drug-induced liver injury in the context of nonalcoholic fatty liver disease - a physiopathological and clinical integrated view. Aliment. Pharmacol. Ther. 2018;48:892–913. doi: 10.1111/apt.14952. [DOI] [PubMed] [Google Scholar]
- 41.Lammert C., Imler T., Teal E., Chalasani N. Patients with chronic liver disease suggestive of nonalcoholic fatty liver disease may Be at higher risk for drug-induced liver injury. Clin. Gastroenterol. Hepatol. 2019;17:2814–2815. doi: 10.1016/j.cgh.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Gao P., Niu J. Metabolic comorbidities and risk of development and severity of drug-induced liver injury. BioMed Res. Int. 2019;2019:8764093. doi: 10.1155/2019/8764093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allard J., Le Guillou D., Begriche K., Fromenty B. Drug-induced liver injury in obesity and nonalcoholic fatty liver disease. Adv. Pharmacol. 2019;85:75–107. doi: 10.1016/bs.apha.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Michaut A., Moreau C., Robin M.-A., Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171–e179. doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- 45.García-Román R., Francés R. Acetaminophen-induced liver damage in hepatic steatosis. Clin. Pharmacol. Ther. 2020;107:1068–1081. doi: 10.1002/cpt.1701. [DOI] [PubMed] [Google Scholar]
- 46.Claridge L.C., Eksteen B., Smith A., Shah T., Holt A.P. Acute liver failure after administration of paracetamol at the maximum recommended daily dose in adults. BMJ. 2010;341:c6764. doi: 10.1136/bmj.c6764. –c6764. [DOI] [PubMed] [Google Scholar]
- 47.Bouvet R., Cauchois A., Baert A., Fromenty B., Morel I., Turlin B., Gicquel T. Fatal acetaminophen poisoning with hepatic microvesicular steatosis in a child after repeated administration of therapeutic doses. Forensic Sci. Int. 2020;310 doi: 10.1016/j.forsciint.2020.110258. [DOI] [PubMed] [Google Scholar]
- 48.Meunier L., Larrey D. Chemotherapy-associated steatohepatitis. Ann. Hepatol. 2020 doi: 10.1016/j.aohep.2019.11.012. S1665268120300041. [DOI] [PubMed] [Google Scholar]
- 49.Mori S., Arima N., Ito M., Ueki Y., Abe Y., Aoyagi K., Fujiyama S. Incidence, predictive factors, and severity of methotrexate-related liver injury in rheumatoid arthritis: a longitudinal cohort study. Rheumatol. Adv. Pract. 2020 doi: 10.1093/rap/rkaa020. rkaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michaut A., Le Guillou D., Moreau C., Bucher S., McGill M.R., Martinais S., Gicquel T., Morel I., Robin M.-A., Jaeschke H., Fromenty B. A cellular model to study drug-induced liver injury in nonalcoholic fatty liver disease: application to acetaminophen. Toxicol. Appl. Pharmacol. 2016;292:40–55. doi: 10.1016/j.taap.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucher S., Le Guillou D., Allard J., Pinon G., Begriche K., Tête A., Sergent O., Lagadic-Gossmann D., Fromenty B. Possible involvement of mitochondrial dysfunction and oxidative stress in a cellular model of NAFLD progression induced by benzo[a]pyrene/ethanol coexposure. Oxid. Med. Cell. Longev. 2018:4396403. doi: 10.1155/2018/4396403. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Guillou D., Bucher S., Begriche K., Hoët D., Lombès A., Labbe G., Fromenty B. Drug-induced alterations of mitochondrial DNA homeostasis in steatotic and nonsteatotic HepaRG cells. J. Pharmacol. Exp. Ther. 2018;365:711–726. doi: 10.1124/jpet.117.246751. [DOI] [PubMed] [Google Scholar]
- 53.Bucher S., Tête A., Podechard N., Liamin M., Le Guillou D., Chevanne M., Coulouarn C., Imran M., Gallais I., Fernier M., Hamdaoui Q., Robin M.-A., Sergent O., Fromenty B., Lagadic-Gossmann D. Co-exposure to benzo[a]pyrene and ethanol induces a pathological progression of liver steatosis in vitro and in vivo. Sci. Rep. 2018;8:5963. doi: 10.1038/s41598-018-24403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aubert J., Begriche K., Knockaert L., Robin M.A., Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Brill M.J.E., Diepstraten J., van Rongen A., van Kralingen S., van den Anker J.N., Knibbe C.A.J. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012;51:277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Cobbina E., Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) – pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017;49:197–211. doi: 10.1080/03602532.2017.1293683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aubert J., Begriche K., Delannoy M., Morel I., Pajaud J., Ribault C., Lepage S., McGill M.R., Lucas-Clerc C., Turlin B., Robin M.-A., Jaeschke H., Fromenty B. Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db mice. J. Pharmacol. Exp. Ther. 2012;342:676–687. doi: 10.1124/jpet.112.193813. [DOI] [PubMed] [Google Scholar]
- 58.Allard J., Bucher S., Massart J., Ferron P.-J., Le Guillou D., Loyant R., Daniel Y., Launay Y., Buron N., Begriche K., Borgne-Sanchez A., Fromenty B. Drug-induced hepatic steatosis in absence of severe mitochondrial dysfunction in HepaRG cells: proof of multiple mechanism-based toxicity. Cell Biol. Toxicol. 2020 doi: 10.1007/s10565-020-09537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuckerman M., Greller H.A., Babu K.M. A review of the toxicologic implications of obesity. J. Med. Toxicol. 2015;11:342–354. doi: 10.1007/s13181-015-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Begriche K., Massart J., Robin M.-A., Bonnet F., Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 62.Pessayre D., Fromenty B., Berson A., Robin M.-A., Lettéron P., Moreau R., Mansouri A. Central role of mitochondria in drug-induced liver injury. Drug Metab. Rev. 2012;44:34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- 63.Pessayre D., Mansouri A., Berson A., Fromenty B. Mitochondrial involvement in drug-induced liver injury. Handb. Exp. Pharmacol. 2010;196:311–365. doi: 10.1007/978-3-642-00663-0_11. [DOI] [PubMed] [Google Scholar]
- 64.Ramachandran A., Visschers R.G.J., Duan L., Akakpo J.Y., Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J. Clin. Transl. Res. 2018;4:75–100. doi: 10.18053/jctres.04.201801.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrade R.J., Chalasani N., Björnsson E.S., Suzuki A., Kullak-Ublick G.A., Watkins P.B., Devarbhavi H., Merz M., Lucena M.I., Kaplowitz N., Aithal G.P. Drug-induced liver injury. Nat. Rev. Dis. Primers. 2019;5:58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 66.Massart J., Begriche K., Fromenty B. Cytochrome P450 2E1 should not be neglected for acetaminophen-induced liver injury in metabolic diseases with altered insulin levels or glucose homeostasis. Clin. Res. Hepatol. Gastroenterol. Jun. 2020;19 doi: 10.1016/j.clinre.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 67.Abdelmegeed M.A., Carruthers N.J., Woodcroft K.J., Kim S.K., Novak R.F. Acetoacetate induces CYP2E1 protein and suppresses CYP2E1 mRNA in primary cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 2005;315:203–213. doi: 10.1124/jpet.105.084608. [DOI] [PubMed] [Google Scholar]
- 68.Sunny N.E., Bril F., Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol. Metabol. 2017;28:250–260. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Fletcher J.A., Deja S., Satapati S., Fu X., Burgess S.C., Browning J.D. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woolsey S.J., Beaton M.D., Mansell S.E., Leon-Ponte M., Yu J., Pin C.L., Adams P.C., Kim R.B., Tirona R.G. A fibroblast growth factor 21–pregnane X receptor pathway downregulates hepatic CYP3A4 in nonalcoholic fatty liver disease. Mol. Pharmacol. 2016;90:437–446. doi: 10.1124/mol.116.104687. [DOI] [PubMed] [Google Scholar]
- 71.Zeng H., Lin Y., Gong J., Lin S., Gao J., Li C., Feng Z., Zhang H., Zhang J., Li Y., Yu C. CYP3A suppression during diet-induced nonalcoholic fatty liver disease is independent of PXR regulation. Chem. Biol. Interact. 2019;308:185–193. doi: 10.1016/j.cbi.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 72.Guengerich F.P. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 73.Josephson F. Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition: drug-drug interactions in the treatment of HIV infection. J. Intern. Med. 2010;268:530–539. doi: 10.1111/j.1365-2796.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- 74.Jallouli M., Galicier L., Zahr N., Aumaître O., Francès C., Le Guern V., Lioté F., Smail A., Limal N., Perard L., Desmurs-Clavel H., Le Thi Huong D., Asli B., Kahn J.-E., Pourrat J., Sailler L., Ackermann F., Papo T., Sacré K., Fain O., Stirnemann J., Cacoub P., Leroux G., Cohen-Bittan J., Sellam J., Mariette X., Blanchet B., Hulot J.S., Amoura Z., Piette J.C., Costedoat-Chalumeau N. Plaquenil Lupus Systemic Study Group, Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis Rheum. 2015;67:2176–2184. doi: 10.1002/art.39194. [DOI] [PubMed] [Google Scholar]
- 75.Yang K. What do we know about remdesivir drug interactions? Clin. Transl. Sci. 2020:12815. doi: 10.1111/cts.12815. cts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pea F., Furlanut M. Pharmacokinetic aspects of treating infections in the intensive care unit: focus on drug interactions. Clin. Pharmacokinet. 2001;40:833–868. doi: 10.2165/00003088-200140110-00004. [DOI] [PubMed] [Google Scholar]
- 77.Fromenty B. Inhibition of mitochondrial fatty acid oxidation in drug-induced hepatic steatosis. Liver Research. 2019;3:157–169. doi: 10.1016/j.livres.2019.06.001. [DOI] [Google Scholar]
- 78.Labbe G., Pessayre D., Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 2008;22:335–353. doi: 10.1111/j.1472-8206.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 79.Fromenty B. Alteration of mitochondrial DNA homeostasis in drug-induced liver injury. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.110916. [DOI] [PubMed] [Google Scholar]
- 80.Begriche K., Massart J., Robin M.-A., Borgne-Sanchez A., Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011;54:773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Lettéron P., Angela S., Abdellah M., Bernard F., Dominique P. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology. 2003;38:133–140. doi: 10.1053/jhep.2003.50309. [DOI] [PubMed] [Google Scholar]
- 82.Foufelle F., Fromenty B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol. Res. Perspect. 2016;4 doi: 10.1002/prp2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell C., Robin M.-A., Mayeuf A., Mahrouf-Yorgov M., Mansouri A., Hamard M., Couton D., Fromenty B., Gilgenkrantz H. Protection against hepatocyte mitochondrial dysfunction delays fibrosis progression in mice. Am. J. Pathol. 2009;175:1929–1937. doi: 10.2353/ajpath.2009.090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knockaert L., Fromenty B., Robin M.-A. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity: mitochondrial CYP2E1. FEBS J. 2011;278:4252–4260. doi: 10.1111/j.1742-4658.2011.08357.x. [DOI] [PubMed] [Google Scholar]
- 85.Guengerich F.P. Cytochrome P450 2E1 and its roles in disease. Chem. Biol. Interact. 2020;322 doi: 10.1016/j.cbi.2020.109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lescot T., Karvellas C., Beaussier M., Magder S. Acquired liver injury in the intensive care unit. Anesthesiology. 2012;117:898–904. doi: 10.1097/ALN.0b013e318266c6df. [DOI] [PubMed] [Google Scholar]
- 87.Luo Y., Rana P., Will Y. Palmitate increases the susceptibility of cells to drug-induced toxicity: an in vitro method to identify drugs with potential contraindications in patients with metabolic disease. Toxicol. Sci. 2012;129:346–362. doi: 10.1093/toxsci/kfs208. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y., Lin Z., Liu Z., Harris S., Kelly R., Zhang J., Ge W., Chen M., Borlak J., Tong W. A unifying ontology to integrate histological and clinical observations for drug-induced liver injury. Am. J. Pathol. 2013;182:1180–1187. doi: 10.1016/j.ajpath.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 89.Hoss S.E., Bahr G.M., Echtay K.S. Lopimune-induced mitochondrial toxicity is attenuated by increased uncoupling protein-2 level in treated mouse hepatocytes. Biochem. J. 2015;468:401–407. doi: 10.1042/BJ20150195. [DOI] [PubMed] [Google Scholar]
- 90.Falcão M.B., Pamplona de Góes Cavalcanti L., Filgueiras Filho N.M., Antunes de Brito C.A. Case report: hepatotoxicity associated with the use of hydroxychloroquine in a patient with COVID-19. Am. J. Trop. Med. Hyg. 2020;102:1214–1216. doi: 10.4269/ajtmh.20-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boya P., Gonzalez-Polo R.A., Poncet D., Andreau K., Vieira H.L., Roumier T., Perfettini J.L., Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- 92.Jamshidzadeh A., Niknahad H., Kashafi H. Cytotoxicity of chloroquine in isolated rat hepatocytes. J. Appl. Toxicol. 2007;27:322–326. doi: 10.1002/jat.1194. [DOI] [PubMed] [Google Scholar]
- 93.Martinez M.A., Vuppalanchi R., Fontana R.J., Stolz A., Kleiner D.E., Hayashi P.H., Gu J., Hoofnagle J.H., Chalasani N. Clinical and histologic features of azithromycin-induced liver injury. Clin. Gastroenterol. Hepatol. 2015;13:369–376. doi: 10.1016/j.cgh.2014.07.054. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang K., Köck K., Sedykh A., Tropsha A., Brouwer K.L.R. An updated review on drug-induced cholestasis: mechanisms and investigation of physicochemical properties and pharmacokinetic parameters. J. Pharm. Sci. 2013;102:3037–3057. doi: 10.1002/jps.23584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y., Hafey M.J., Duong H., Evers R., Cheon K., Holder D.J., Galijatovic-Idrizbegovic A., Sistare F.D., Glaab W.E. Antibiotic-induced elevations of plasma bile acids in rats independent of bsep inhibition. Toxicol. Sci. 2017 doi: 10.1093/toxsci/kfx015. kfx015. [DOI] [PubMed] [Google Scholar]
- 96.Woodhead J.L., Yang K., Oldach D., MacLauchlin C., Fernandes P., Watkins P.B., Siler S.Q., Howell B.A. Analyzing the mechanisms behind macrolide antibiotic-induced liver injury using quantitative systems toxicology modeling. Pharm. Res. (N. Y.) 2019;36:48. doi: 10.1007/s11095-019-2582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang X., Baucom C., Elliott R.L. Mitochondrial toxicity of azithromycin results in aerobic glycolysis and DNA damage of human mammary epithelia and fibroblasts. Antibiotics. 2019;8:110. doi: 10.3390/antibiotics8030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olry L., Meunier B., Délire D., Larrey Y., Horsmans H., Le Louët. Drug-induced liver injury and COVID-19 infection: the rules remain the same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leegwater E., Strik A., Wilms E.B., Bosma L.B., Burger D.M., Ottens T.H., van Nieuwkoop C. Drug-induced liver injury in a COVID-19 patient: potential interaction of remdesivir with P-glycoprotein inhibitors. Clin. Infect. Dis. Jun. 2020;28 doi: 10.1093/cid/ciaa883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muhović D., Bojović J., Bulatović A., Vukčević B., Ratković M., Lazović R., Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. May. 2020;17 doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]