Abstract
Background
As an important member of platinum-based chemotherapeutic drugs, cisplatin is effective and is commonly used in the treatment of esophageal cancer. However, repeated use of cisplatin usually causes severe side-effects on patients. Novel approaches should be explored to increase the sensitivity of cancer cells to cisplatin.
Methods
The expression level of miR-183 in esophageal cancer tissues and cell lines was measured by quantitative reverse transcriptase real-time PCR (qRT-PCR). The sensitivity of EC cell lines to cisplatin was evaluated by CCK-8 assay and flow cytometry. Luciferase reporter assay was used to confirm the association between miR-183 and FOXO1. The apoptosis pathway of EC cells was tested by Western blot assay.
Results
The expression level of miR-183 was increased in esophageal cancer patients’ tumor tissues and esophageal cancer cell lines. However, knockdown of miR-183 was found to enhance the effect of cisplatin on inducing the apoptotic cell death of esophageal cancer cells. In the mechanism research, we proved that FOXO1 was the target of miR-183 in esophageal cancer cells. Inhibition of miR-183 increased the expression of FOXO1 to promote the expression of Bim and Noxa. As Bim and Noxa acted as key pro-apoptotic proteins in mitochondrial apoptosis, inhibition of miR-183 enhanced the cisplatin-induced apoptosis pathway in esophageal cancer.
Conclusion
Knockdown of miR-183 enhanced the cisplatin-induced apoptosis in esophageal cancer through an increase of FOXO1 expression.
Keywords: cisplatin, miR-183, esophageal cancer, FOXO1
Introduction
Esophageal cancer (EC) is the fourth most common gastrointestinal cancer and a leading cause of mortality in the world.1,2 Despite surgery being the most effective treatment for early stage and localized cancers, chemotherapy is the only option for patients with advanced EC to prolong their life.3,4 Cisplatin is an effective chemotherapeutic agent that is used for the treatment of EC.5,6 However, a substantial proportion of EC patients do not benefit from cisplatin because of the drug resistance.7,8 Mechanisms which determine cisplatin sensitivity in EC are required to be identified.
Cisplatin is a platinum-based drug that cross-links with DNAs and thus induces apoptotic cell death of cancer cells.9,10 However, EC cells usually develop some strategies to exhibit low sensitivity to cisplatin-induced apoptosis.11,12 Thus, reducing the resistance of EC cells to apoptosis signals is an important strategy for sensitizing the cisplatin treatment.
FOXO1 is a well-known tumor suppressor that regulates DNA repair and cell cycle transition.13,14 As an important transcription factor, FOXO1 facilitates cell apoptosis through promoting the transcriptional activities of Bim and Noxa which are the key pro-apoptotic proteins in the mitochondrial apoptosis pathway.15,16 Previous studies have reported that downregulation of FOXO1 induces tumorgenesis, cancer development, and chemoresistance.17,19 Therefore, FOXO1 is a potential target for improving the cancer therapy.
MicroRNAs (miRNAs) are endogenous RNAs with 19–25 nucleotides in length. Although miRNAs are single-stranded and non-coding, they function as gene suppressors through interaction with targeted mRNAs.20,21 Previous research has proved that dysregulation of miRNA is associated with tumorigenesis and tumor development, because numerous miRNA-regulated genes are involved in various physiological processes including cell proliferation, differentiation, metabolism, and apoptosis.22,23 Moreover, studies also demonstrate that aberrant expression of miRNA is responsible for drug resistance of various cancers.24,25 Among these deregulated miRNAs, miR-183 has been reported to be involved in tumor growth and is responsible for poor prognosis of cancer patients.26,27 However, the association between miR-183 and cisplatin sensitivity of EC is still unclear. In the present study, we mainly explored the role of miR-183 in cisplatin-induced apoptosis in esophageal cancer.
Materials and Methods
Cell Lines and Patients’ Specimens
EC and paired paracancerous tissues were obtained from 25 patients who underwent resection between April 2017 and May 2019 at Fujian Medical University Union Hospital. We have obtained written informed consent from all of the patients. The experimental protocols were approved by the ethics committee of Fujian Medical University Union Hospital (No. 2019-ZQN-64). Human EC cell lines EC9706 and TE-9 were cultured under the condition recommended by the vendor (Cell Bank of Shanghai Institute of Cell Biology, Shanghai, China). Cells were kept in a 5% CO2 incubator at 37°C.
Quantitative Reverse Transcriptase Real-Time PCR (qRT-PCR)
Relative expression of miR-183 and FOXO1 was measured by using qRT-PCR analysis. Briefly, total RNAs in EC patients’ resected tissues and cell lines were isolated by using Trizol Reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instruction. For detection of miR-183, cDNAs were synthesized with a One Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa, China). For detection of FOXO1, cDNA was synthesized by using M-MLV Reverse Transcriptase (Thermo Fisher Scientific, Inc.) following the manufacturer’s instruction. Subsequently, relative expression of miR-183 and FOXO1 was detected by using SYBR Premix Ex Taq (TaKaRa). Fold change of miR-183 was normalized to U6 snRNA and fold change of FOXO1 was normalized to GAPDH with the comparative cycle threshold method (2−ΔΔCT).
Plasmid Construction
For enforced expression of FOXO1, the eukaryotic expression vector (pcDNA3.1 plasmid containing FOXO1 open reading frame; Thermo Fisher Scientific, Inc.) was conducted. Briefly, an open reading frame of FOXO1 gene was amplified by using the following primers: Forward, FOXO1 XhoI, 5ʹ-ATCTCGAGATGGCCGAGGCGCCTCAGGT-3ʹ, and reverse, FOXO1 BamHI, 5ʹ-ACGGATCCGCCTGACACCCAGCTATGTGTC-3ʹ. The recombinant pcDNA3.1 plasmid carrying FOXO1 open reading frame was named as FOXO1 plasmid. For luciferase reporter assay, the pMIR Firefly luciferase reporter with FOXO1 3ʹ UTR (pMIR-REPORT™ miRNA Expression Reporter Vector carrying wild type of FOXO1 3ʹ UTR fragment with the predicted miR-183 site; Ambion; Thermo Fisher Scientific, Inc.) was conducted. Briefly, a 3ʹ UTR fragment of FOXO1 gene was amplified by using the following primers: Forward, FOXO1 SpeI, 5ʹ-ACCGCATCGTGTGCTGCTGTAGATAAGG-3ʹ, and reverse, FOXO1 HindIII, 5ʹ-ACGAAGCTTTTGATAACAGGCTACTTGG-3ʹ. The recombinant pMIR Firefly luciferase reporter carrying wild type FOXO1 3ʹ UTR fragment was named as pMIR-FOXO1-wt. To mutate the seed region of the miR-183-binding sites (GUGCCAUU to GUGATAUU), the QuikChange Site-Directed Mutagenesis kit (Stratagene, Missouri, TEX, USA) was used based on the pMIR-FOXO1-wt following the manufacturer’s protocol. The conducted pMIR Firefly luciferase reporter carrying mutant type FOXO1 3ʹ UTR fragment was named as pMIR-FOXO1-mt.
Cell Transfection
FOXO1 plasmid, hsa-miR-183 mimic (miR-183), hsa-miR-183 antisense-oligonucleotide (anti-miR-183), negative control oligonucleotide (NCO) (GenePharma Co. Ltd, Shanghai, China), and FOXO1 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were transfected into cells by using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instruction. Twenty-four hours after transfection, cells were collected for the following experiments.
Cell Viability Assay
Transfected EC cells were seeded into 96-well plates. After cell adherence on plates, the corresponding cells were treated with medium containing cisplatin. After 48 hours incubation, cell viability was detected by CCK-8 according to the manufacturer’s instruction. The absorbance values of each well were determined by using a microplate reader (Sunrise Microplate Reader, TECAN, Switzerland). Results were expressed as the absorbance relative to the NCO group. Half inhibiting concentration (IC50) of cisplatin to EC9706 and TE-9 was evaluated according to the cell viability curve.
Luciferase Reporter Assay
Recombinant pMIR-reporter (2 μg/mL), renilla luciferase pRL-TK plasmid (100 ng/mL, Promega, USA) and miR-183 mimic (or anti-miR-183) were co-transfected into cells by using Lipofectamine 2000. Forty-eight hours after transfection, luciferase activities were measured by using a Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA) according to the manufacturer’s instruction. Relative firefly luciferase activities were determined by normalization to Renilla luciferase activities in each well.
Western Blot Analysis
Protein samples were prepared and mixed with SDS loading buffer. Equal amounts of proteins were then electrophoresized by 10% SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked in 5% non-fat milk before incubation with primary antibodies (Cell Signaling Technologies, Danvers, MA, USA). Subsequently, the membranes were washed and probed with appropriate secondary antibodies conjugated to horseradish peroxidase. Protein bands were detected by using an enhanced chemiluminescent substrate (Thermo Fisher Scientific, Inc.).
Flow Cytometry
Cells were collected and washed twice with cold PBS. For detection of mitochondrial membrane potential (MMP, ΔΨ), cells were stained with 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl imidacarbo cyanine iodide (JC-1, Molecular Probes; Waltham, MA, USA) as an indicator. Cells emitting red fluorescence were considered as cells with high MMP, and cells emitting green fluorescence were considered as cells with low MMP. For measurement of apoptotic rate, an Annexin V-FITC Apoptosis Detection Kit (Sigma Aldrich, St. Louis, MO, USA) was used according to the manufacturer’s instruction. The Annexin V-positive cells were considered as the apoptotic cells. All of the cells were analyzed by using FACSAria flow cytometry (BD Biosciences, San Jose, CA, USA).
In vivo Experiment
Anti-miR-183-EC9706 was conducted to stably express miR-183 antisense nucleotide sequence by using a lentiviral-based system (Genechem Co., Ltd., Shanghai, China). For tumourigenesis assays, 5×106 of anti-miR-183-EC9706 or control EC9706 cells were inoculated subcutaneously into the BALB/c nude mice (4~5 weeks old). After xenografts reached 0.5 cm in diameter, animals were treated with cisplatin i.p. twice a week (2 mg/kg) until the mice were killed 25 days post-injection. Subsequently, the tumors were taken out for the following experiments. Collagenase type III was used to purify the tumor tissue cells. The animal experiments were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, issued by the National Institute of Health. Experiment protocols were approved by the ethics committee of Fujian Medical University Union Hospital (JN.No20190515S0150117).
Statistical Analysis
Three independent experiments were performed to obtain the data. Data values were presented as means±standard deviation (SD). Statistical differences between groups were analyzed by One-way analysis of variance (ANOVA) using SPSS 15.0 software. Differences were considered significant when P-values were less than 0.05.
Results
miR-183 is Overexpressed in EC Patients’ Tumor Tissues and Cell Lines
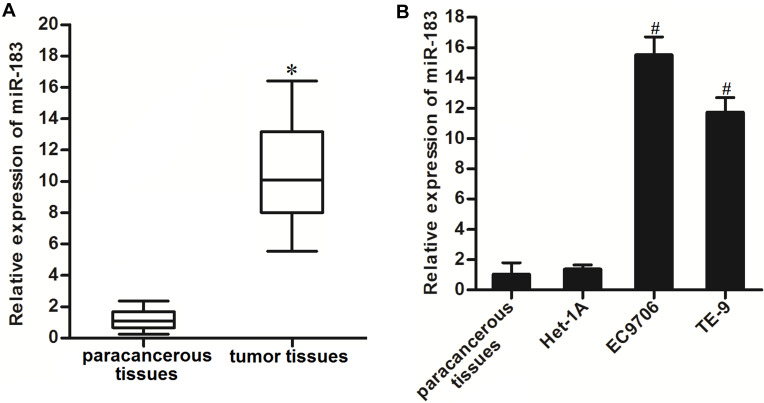
To investigate the potential role of miR-183 in EC, the expression levels of miR-183 in EC patients’ tumor tissues and cell lines were tested by using qRT-PCR analysis. As shown in Figure 1A, obviously a higher level of miR-183 was observed in EC patients’ tumor tissues compared to the paracancerous tissues. Furthermore, the expression level of miR-183 was found to be upregulated in EC9706 and TE-9 EC cell lines compared to the normal tissue cells and Het-1A which was human normal esophageal epithelial cell line (Figure 1B). These results suggested that an increase of miR-183 was a frequent event in EC.
Figure 1.
Overexpression of miR-183 in EC tissues and cell lines. (A) Expression level of miR-183 in EC patients’ tumor tissues and paracancerous tissues was tested by qRT-PCR analysis. (B) QRT-PCR analysis was performed to test the expression level of miR-183 in paracancerous tissues, normal EC cell line Het-1A, and EC cell lines EC9706 and TE-9.
Notes: Data was expressed as mean±SD. *P<0.05 vs paracancerous tissues. #P<0.05 vs Het-1A.
Abbreviations: EC, esophageal cancer; qRT-PCR, quantitative reverse transcriptase real-time PCR.
Knockdown of miR-183 Increases the Sensitivity of EC Cells to Cisplatin
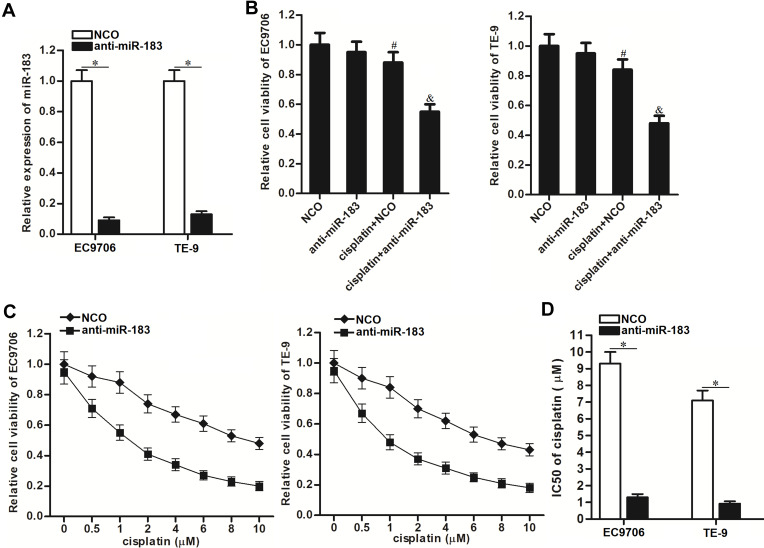
To explore the influence of miR-183-overexpression on the sensitivity of EC cells to cisplatin, we knocked down the miR-183 by using the miR-183 antisense-oligonucleotide (anti-miR-183). As shown in Figure 2A, transfection with anti-miR-183 significantly decreased the cellular level of miR-183 in EC9706 and TE-9 cell lines. Next, we treated the NCO-transfected or anti-miR-183-transfected EC9706 and TE-9 cells with cisplatin at the concentration of 1 μM, because 1 μM cisplatin just induced relatively slight cell death of EC9706 and TE-9 (Figure 2B). Results of the CCK-8 assay revealed that the anti-miR-183-transfected EC9706 and TE-9 EC cells exhibited obvious higher sensitivity to cisplatin compared to the NCO-transfected EC9706 and TE-9 cells (Figure 2B). To detect the effect of anti-miR-183 on changing the IC50 of cisplatin to EC cells, we treated the anti-miR-183-transfected EC9706 and TE-9 cells with different concentrations of cisplatin. We observed that anti-miR-183 can enhance the cytotoxicity of various concentrations of cisplatin against EC (Figure 2C). Specifically, IC50 of cisplatin to NCO-transfected EC9706 was 6.4-fold higher than that to anti-miR-183-transfected EC9706, and the IC50 of cisplatin to NCO-transfected TE-9 was 7.1-fold higher than that to anti-miR-183-transfected TE-9 (Figure 2D). These data indicated that knockdown of miR-183 can increase the sensitivity of EC cells to cisplatin.
Figure 2.
Anti-miR-183 increased the cisplatin sensitivity of EC cells. (A) Detection of miR-183 expression in EC9706 and TE-9 EC cells after they were transfected with anti-miR-183 (50 pmol/mL). (B) CCK-8 assays were performed to evaluate the cell viability of EC9706 and TE-9 cells after they were treated with anti-miR-183 (50 pmol/mL) and cisplatin (1 μM). (C) Cell viability curve of NCO or anti-miR-183-transfected EC9706 and TE-9 cells under the treatment of different concentrations of cisplatin. (D) Transfection with anti-miR-183 decreased the IC50 of cisplatin to EC9706 and TE-9 EC cells.
Notes: Data was expressed as mean±SD. *P<0.05. #P<0.05 vs NCO group. &P<0.05 vs cisplatin+NCO group.
Abbreviations: EC, esophageal cancer; NCO, negative control oligonucleotide; IC50, half inhibiting concentration; CCK-8, Cell Counting Kit-8.
Anti-miR-183 Sensitizes EC Cells to Cisplatin-Induced Apoptosis
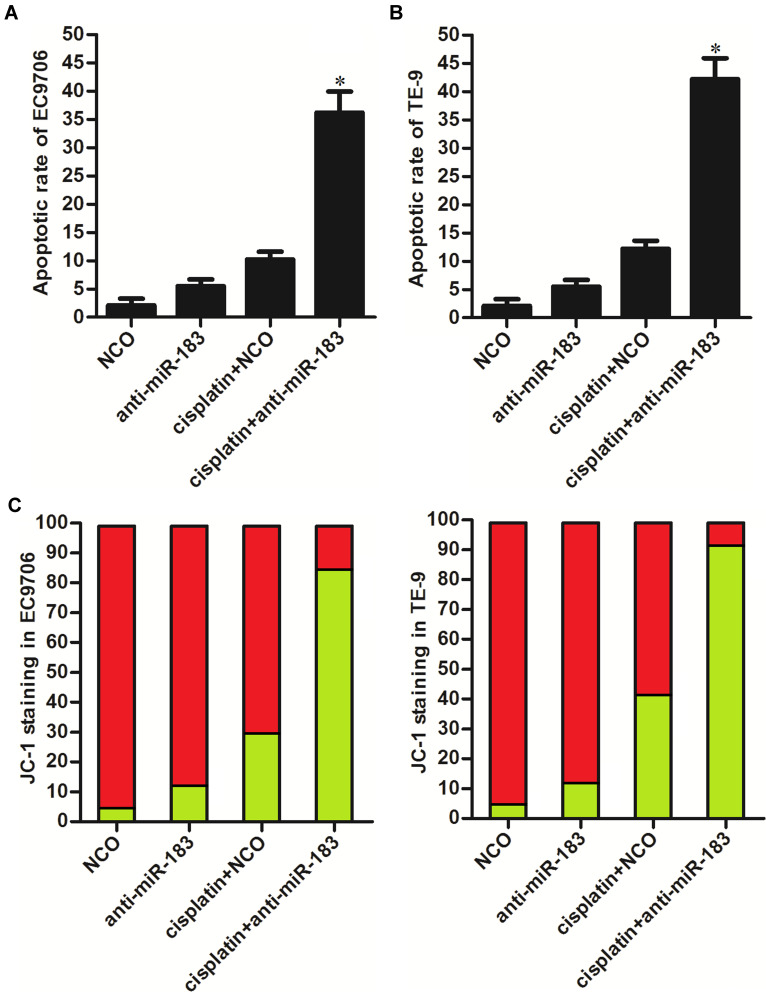
To deeply investigate the role of anti-miR-183 in cisplatin treatment in EC, we next tested the apoptosis pathway. As shown in Figure 3A and B, under the treatment of equal concentration of cisplatin, apoptotic rate of anti-miR-183-transfected EC9706 and TE-9 cells was significantly higher than the NCO-transfected EC9706 and TE-9 cells. Furthermore, we found that transfection with anti-miR-183 promoted the collapse of mitochondrial membrane potential (MMP, ΔΨ) induced by cisplatin (Figure 3C). These data showed that transfection with anti-miR-183 can sensitize EC cells to cisplatin-induced apoptosis through the mitochondrial pathway.
Figure 3.
Anti-miR-183 sensitized EC cells to cisplatin-induced apoptosis. (A) Cisplatin-induced (1 μM) apoptosis was enhanced by anti-miR-183 in EC9706 cells. (B) Cisplatin-induced (1 μM) apoptosis was enhanced by anti-miR-183 in TE-9 cells. (C) Cisplatin-induced (1 μM) collapse of MMP was enhanced by anti-miR-183 in EC9706 and TE-9 cells.
Notes: Data was expressed as mean±SD. *P<0.05 vs cisplatin+NCO group.
Abbreviations: EC, esophageal cancer; NCO, negative control oligonucleotide.
FOXO1 is the Target of miR-183 in EC
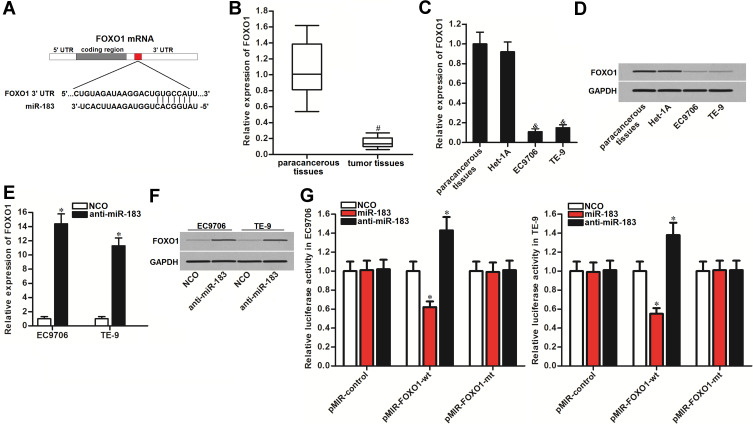
To explain how anti-miR-183 promoted cisplatin-induced apoptosis, we next searched the potential mRNA target of miR-183 in EC cells. According to the public databases of TargetScan, miRanda, and PicTar, FOXO1, a reported tumor suppressor in multiple cancers,28,30 was commonly predicted to be the potential target of miR-183. The seed region of 3ʹUTR of FOXO1 mRNA paired with miR-183 is shown in Figure 4A. Contrary to the overexpression of miR-183 in EC tissues (Figure 1A), expression of FOXO1 was found to be decreased in EC tissues (Figure 4B). Furthermore, FOXO1 was overexpressed in EC cell lines at both the mRNA level (Figure 4C) and protein level (Figure 4D). We therefore speculated that FOXO1 might be the target of miR-183 in EC. After transfection with anti-miR-183 in EC9706 and TE-9, we observed that expression of FOXO1 was increased at the mRNA level (Figure 4E) and protein level (Figure 4F). Furthermore, results of luciferase reporter assay showed that co-transfection with miR-183 mimic decreased the luciferase activities of FOXO1 3ʹ UTR reporter in EC9706 and TE-9 cells. On the contrary, anti-miR-183 increased the luciferase activities of the FOXO1 3ʹ UTR reporter (Figure 4G). Taken together, we demonstrated that FOXO1 was the target of miR-183. Overexpression of miR-183 induced reduction of FOXO1 in EC.
Figure 4.
FOXO1 is the target of miR-183 in EC. (A) Seed region of FOXO1 mRNA 3ʹ UTR paired with miR-183. (B) Expression level of FOXO1 in EC patients’ tumor tissues and paracancerous tissues was tested by qRT-PCR analysis. (C) mRNA level of FOXO1 in paracancerous tissues, Het-1A, TE-9, and EC9706 cells. (D) Protein level of FOXO1 in paracancerous tissues, Het-1A, TE-9, and EC9706 cells. (E) Anti-miR-183 increased the expression of FOXO1 in EC9706 and TE-9 cells at the mRNA level. (F) Anti-miR-183 increased the expression of FOXO1 in EC9706 and TE-9 cells at the protein level. (G) Effect of miR-183 mimic and anti-miR-183 on changing the luciferase activities of FOXO1 3ʹ UTR reporters in EC9706 and TE-9 cells.
Notes: Data was expressed as mean±SD. *P<0.05 vs NCO group. #P<0.05 vs paracancerous tissues. &P<0.05 vs Het-1A cell line.
Abbreviations: EC, esophageal cancer; NCO, negative control oligonucleotide; FOXO1, Forkhead box O 1; 3ʹ UTR, 3ʹ untranslated region.
Anti-miR-183 Enhances Cisplatin-Induced Cell Death Through the FOXO1 Pathway in EC
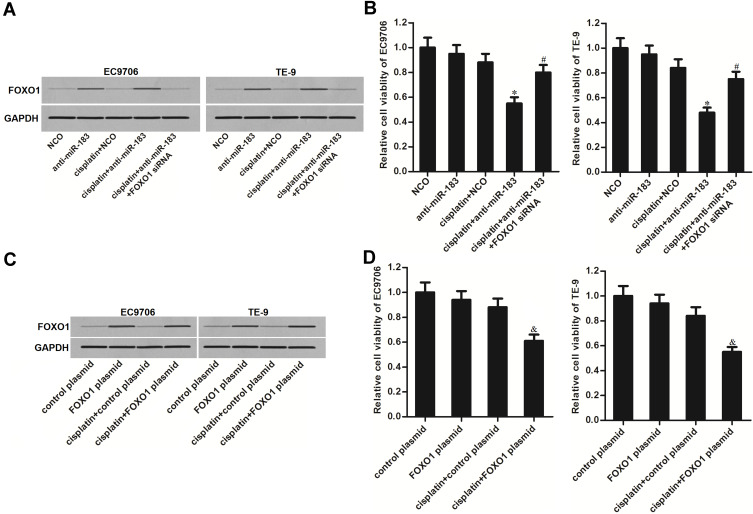
Given that FOXO1 was the target of miR-183, we next investigated whether the sensitization of anti-miR-183 on cisplatin was dependent on the upregulation of FOXO1. We thus co-transfected the EC9706 and TE-9 cells with FOXO1 siRNA to antagonize the effect of anti-miR-183. As shown in Figure 5A, FOXO1 siRNA suppressed the expression of FOXO1 in EC cells even though they were transfected with anti-miR-183. As a result, we found that FOXO1 siRNA significantly increased the cell viability of EC9706 and TE-9 cells which were co-treated with cisplatin and anti-miR-183 (Figure 5B). On the other hand, we directly increased the expression of FOXO1 through transfection with FOXO1 plasmid (Figure 5C). As a result, we found that treatment with FOXO1 plasmid also can enhance the cytotoxicity of cisplatin, similarly with anti-miR-183 (Figure 5D). Taken together, these data indicated that anti-miR-183 enhanced cisplatin-induced cell death through the FOXO1 pathway in EC.
Figure 5.
Anti-miR-183 increased the sensitivity of EC cells to cisplatin through the FOXO1 pathway. (A) Effect of FOXO1 siRNA (50 pmol/mL) and anti-miR-183 (50 pmol/mL) on changing the FOXO1 expression in EC9706 and TE-9 cells. (B) Transfection with FOXO1 siRNA (50 pmol/mL) decreased the cell death of EC9706 and TE-9 cells which were co-treated with anti-miR-183 (50 pmol/mL) and cisplatin (1 μM). (C) Effect of FOXO1 plasmid (2 μg/mL) on changing the FOXO1 expression in EC9706 and TE-9 cells. (D) Transfection with FOXO1 plasmid (2 μg/mL) increased the cell death of EC9706 and TE-9 cells which were treated with cisplatin (1 μM).
Notes: Data was expressed as mean±SD. *P<0.05 vs cisplatin+NCO group. #P<0.05 vs cisplatin+anti-miR-183 group. &P<0.05 vs cisplatin+control plasmid group.
Abbreviations: EC, esophageal cancer; NCO, negative control oligonucleotide; FOXO1, Forkhead box O 1; siRNA, small interfering RNA.
Anti-miR-183 Sensitizes EC Cells to Cisplatin-Induced Apoptosis Through the FOXO1 Pathway
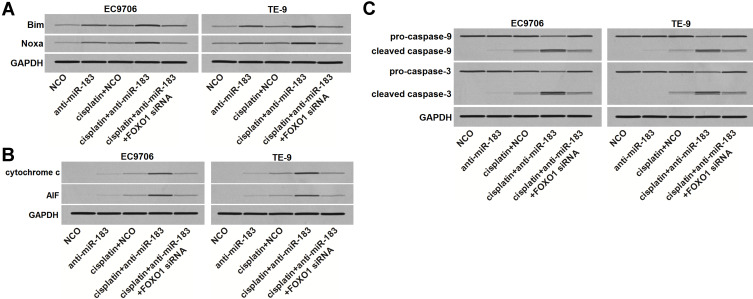
Bim and Noxa expression is directly transactivated by FOXO1.15,16 Given that, we next investigated the effect of anti-miR-183 on regulating the Bim and Noxa expression in cisplatin-treated EC cells. As shown in Figure 6A, despite the fact that anti-miR-183 cannot increase the expression of Bim and Noxa in cisplatin-free EC cells, it obviously promoted the expression of Bim and Noxa in cisplatin-treated EC cells. However, knockdown of FOXO1 by using FOXO1 siRNA abolished the effect of anti-miR-183 on Bim and Noxa. Bim and Noxa are important pro-apoptosis proteins that induce mitochondrial apoptosis.15,16 Given that, we tested the release of cytochrome c and apoptosis inducing factor (AIF) from mitochondria. As shown in Figure 6B, we found that transfection with anti-miR-183 obviously increased the level of cytochrome c and AIF in cytosol of EC cells which were treated with cisplatin. Meanwhile, we also observed obvious activation of caspase-9 and caspase-3 in the cisplatin and anti-miR-183 co-treated EC cells (Figure 6C). However, knockdown of FOXO1 by using FOXO1 siRNA abolished the promotion of anti-miR-183 on the cisplatin-dependent apoptosis pathway of EC cells (Figure 6B and C). Taken together, we demonstrated that anti-miR-183 can sensitize EC cells to cisplatin-induced apoptosis through the FOXO1 pathway.
Figure 6.
Anti-miR-183 sensitized EC cells to cisplatin-induced apoptosis through the FOXO1 pathway. (A) Anti-miR-183 (50 pmol/mL) increased the expression of Bim and Noxa in cisplatin-treated (1 μM) EC9706 and TE-9 cells, whereas the effect of anti-miR-183 was abolished by FOXO1 siRNA (50 pmol/mL). (B) Anti-miR-183 (50 pmol/mL) increased the release of cytochrome c and AIF in cisplatin-treated (1 μM) EC9706 and TE-9 cells, whereas the effect of anti-miR-183 was abolished by FOXO1 siRNA (50 pmol/mL). (C) Anti-miR-183 (50 pmol/mL) enhanced the cleavage of caspase-9 and caspase-3 in cisplatin-treated (1 μM) EC9706 and TE-9 cells, whereas the effect of anti-miR-183 was abolished by FOXO1 siRNA (50 pmol/mL).
Abbreviations: EC, esophageal cancer; FOXO1, Forkhead box O 1; siRNA, small interfering RNA.
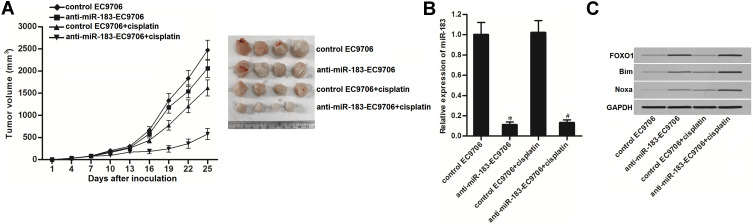
Knockdown of miR-183 Enhances the Anti-Tumor Effect of Cisplatin on EC in vivo
Control EC9706 or anti-miR-183-EC9706 were used to establish the human EC model on mice. We then found that the miR-183-inhibited EC tumors were more sensitive to cisplatin treatment compared to the control EC tumors (Figure 7A). Subsequently, we measured the expression of miR-183 and FOXO1 in the purified tumor tissues. We confirmed the knockdown of miR-183 in the anti-miR-183-EC9706 cells (Figure 7B). In contrast, the anti-miR-183-EC9706 tumors showed an obviously higher protein level of FOXO1. Furthermore, under the equal administration of cisplatin, the anti-miR-183-EC9706 tumors expressed a higher level of Bim and Noxa compared to the control tumors (Figure 7C). We thus demonstrated that knockdown of miR-183 can enhance the anti-tumor effect of cisplatin on EC in vivo.
Figure 7.
Knockdown of miR-183 enhanced anti-tumor effect of cisplatin on EC in vivo. (A) Anti-miR-183-EC9706 or control-EC9706 cells were inoculated subcutaneously into the BALB/c nude mice (4~5 weeks old) followed by treatment with cisplatin i.p. twice a week (2 mg/kg). Twenty-five days post-inoculation, mice were sacrificed and the tumors were resected. (B) Expression level of miR-183 in the purified tumors was detected by qRT-PCR analysis. (C) Western blot analysis was used to test the protein level of FOXO1, Bim and Noxa in the purified tumors.
Notes: Data was expressed as mean±SD. *P<0.05 vs control-EC9706 cells. #P<0.05 vs control-EC9706+cisplatin cells.
Abbreviations: EC, esophageal cancer; qRT-PCR, quantitative reverse transcriptase real time PCR; FOXO1, Forkhead box O 1.
Discussion
Cisplatin is one kind of platinum-based drug that induces apoptotic cell death in a wide range of cancers such as breast cancer, pancreatic cancer, gastric cancer, ovarian cancer, lung cancer, and esophageal cancer.31,36 Despite cisplatin-based treatment being effective for EC, cisplatin-resistance is still a common incidence during the course of chemotherapy.37 Factors that determine cisplatin sensitivity of EC are required to be identified.
Recent studies have reported that the expression profile of miRNA is closely associated with chemosensitivity. MiRNAs function as chemoresistant modulators and can be considered as potential therapeutic targets during cisplatin therapy.38,39 Among these cancer-related miRNAs, miR-183 has been reported to be responsible for tumorigenesis and drug resistance in cancers including EC.26,27,40,41 Consistent with these previous reports, we observed significant upregulation of miR-183 in EC cell lines and patients' tumor tissues. Moreover, we found that the cellular level of miR-183 partially determined the sensitivity of cisplatin to EC cells. Our data indicated that treatment with miR-183 antisense-oligonucleotide can be considered as a promising adjuvant therapy in vitro and in vivo.
FOXO1 is a well-known tumor suppressor that inhibits the occurrence of chemoresistance of cancers. However, cancerous cells usually reduce the expression level of FOXO1 to promote the cell proliferation and inhibit the cell apoptosis.42,43 In this study, we also observed the significant downregulation of FOXO1 in EC cells. Furthermore, we proved that the absence of FOXO1 was responsible for the reduction of cisplatin sensitivity to EC. Thus, FOXO1 can be considered as a novel target for improving the chemotherapy of cancer.
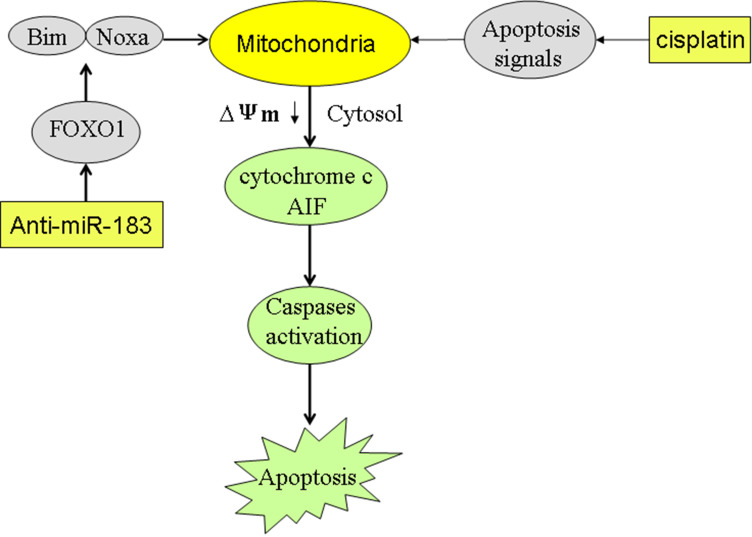
In our mechanism research, we proved that downregulation of FOXO1 in EC cells was induced by overexpression of miR-183. Furthermore, our data indicated that suppression of miR-183 can significantly increase the expression of FOXO1. Subsequently, FOXO1 promoted the expression of Bim and Noxa which were pro-apoptotic proteins. Under the apoptotic signaling caused by cisplatin, Bim and Noxa enhanced the dysfunction of mitochondria. As a result, mitochondria-derived apoptotic inducers such as cytochrome c and AIF44,45 were released from mitochondria into the cytosol of EC cells. Eventually, caspases-dependent apoptosis was triggered (Figure 8). In summary, we proved that knockdown of miR-183 can enhance the cisplatin-induced apoptosis in esophageal cancer through an increase of FOXO1 expression. Intervention on the miR-183/FOXO1 axis may represent a promising strategy for improving the chemotherapy of cancers.
Figure 8.
Schema of the predicted mechanisms implicated in EC cells response to cisplatin and anti-miR-183. Suppression of miR-183 significantly increased the expression of FOXO1. Subsequently, FOXO1 promoted the expression of Bim and Noxa which were pro-apoptotic proteins. Under the apoptotic signaling caused by cisplatin, Bim and Noxa enhanced the dysfunction of mitochondria. As a result, mitochondria-derived apoptotic inducers such as cytochrome c and AIF were released from mitochondria into the cytosol of EC cells. Eventually, caspases-dependent apoptosis was triggered.
Acknowledgments
Thanks are due to the whole contributors who assisted with this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac [DOI] [PubMed] [Google Scholar]
- 2.Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J. Esophageal carcinoma: towards targeted therapies. Cell Oncol (Dordr). 2020;43:195–209. doi: 10.1007/s13402-019-00488-2 [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. 2013;61:330–335. doi: 10.1007/s11748-013-0246-0 [DOI] [PubMed] [Google Scholar]
- 4.Ku GY. Systemic therapy for esophageal cancer: chemotherapy. Chin Clin Oncol. 2017;6:49. doi: 10.21037/cco.2017.07.06 [DOI] [PubMed] [Google Scholar]
- 5.Sawayama H, Ogata Y, Ishimoto T, et al. Glucose transporter 1 regulates the proliferation and cisplatin sensitivity of esophageal cancer. Cancer Sci. 2019;110:1705–1714. doi: 10.1111/cas.13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Jia J, Lu M, et al. Nimotuzumab plus paclitaxel and cisplatin as a 1st-line treatment for esophageal cancer: long term follow-up of a Phase II study. J Cancer. 2019;10:1409–1416. doi: 10.7150/jca.28659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu K, Hu Y, Yan K, et al. microRNA-10b confers cisplatin resistance by activating AKT/mTOR/P70S6K signaling via targeting PPARγ in esophageal cancer. J Cell Physiol. 2020;235:1247–1258. doi: 10.1002/jcp.29040 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Che W, Wang W, Su G, Zhen T, Jiang Z. CDKN3 promotes tumor progression and confers cisplatin resistance via RAD51 in esophageal cancer. Cancer Manag Res. 2019;11:3253–3264. doi: 10.2147/CMAR.S193793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xie C, Li A, et al. PKI-587 enhances chemosensitivity of oxaliplatin in hepatocellular carcinoma through suppressing DNA damage repair pathway (NHEJ and HR) and PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:5134–5149. [PMC free article] [PubMed] [Google Scholar]
- 10.Seo SU, Min KJ, Woo SM, Kwon TK. Z-FL-COCHO, a cathepsin S inhibitor, enhances oxaliplatin-mediated apoptosis through the induction of endoplasmic reticulum stress. Exp Mol Med. 2018;50:107. doi: 10.1038/s12276-018-0138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H, Mao Y, Ju Q, et al. C-terminal binding protein‑2 mediates cisplatin chemoresistance in esophageal cancer cells via the inhibition of apoptosis. Int J Oncol. 2018;53:167–176. doi: 10.3892/ijo.2018.4367 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Pan T, Li L, et al. Knockdown of TGIF attenuates the proliferation and tumorigenicity of EC109 cells and promotes cisplatin-induced apoptosis. Oncol Lett. 2017;14:6519–6524. doi: 10.3892/ol.2017.7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086 [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Qin L, Li S. miR-411 contributes the cell proliferation of lung cancer by targeting FOXO1. Tumour Biol. 2016;37:5551–5560. doi: 10.1007/s13277-015-4425-8 [DOI] [PubMed] [Google Scholar]
- 15.Cao D, Di M, Liang J, Shi S, Tan Q, Wang Z. MicroRNA-183 in cancer progression. J Cancer. 2020;11:1315–1324. doi: 10.7150/jca.39044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Pan X, Gui Y, et al. Upregulation of miR-183-5p predicts worse survival in patients with renal cell cancer after surgery. Cancer Biomark. 2019;24:153–158. doi: 10.3233/CBM-182047 [DOI] [PubMed] [Google Scholar]
- 17.Goto T, Takano M, Hirata J, Tsuda H. The involvement of FOXO1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers. Br J Cancer. 2008;98:1068–1075. doi: 10.1038/sj.bjc.6604279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang C, Xu M, Zhao Z, Chen J, Zhang L. MicroRNA-96 expression induced by low-dose cisplatin or doxorubicin regulates chemosensitivity, cell death and proliferation in gastric cancer SGC7901 cells by targeting FOXO1. Oncol Lett. 2018;16:4020–4026. doi: 10.3892/ol.2018.9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin Y, Li L, Wang F, et al. Knockdown of Mir-135b sensitizes colorectal cancer cells to oxaliplatin-induced apoptosis through increase of FOXO1. Cell Physiol Biochem. 2018;48:1628–1637. doi: 10.1159/000492284 [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 21.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittgen TD. Regulation of microRNA processing in development, differentiation and cancer. J Cell Mol Med. 2008;12:1811–1819. doi: 10.1111/j.1582-4934.2008.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He H, Tian W, Chen H, Jiang K. MiR-944 functions as a novel oncogene and regulates the chemoresistance in breast cancer. Tumour Biol. 2016;37:1599–1607. doi: 10.1007/s13277-015-3844-x [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Tang Q, Li S, Yang X. Inhibition of HAX-1 by miR-125a reverses cisplatin resistance in laryngeal cancer stem cells. Oncotarget. 2016;7:86446–86456. doi: 10.18632/oncotarget.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Gui LS, Zhao XL, Zhu LL, Li QW. FOXO1 is a tumor suppressor in cervical cancer. Genet Mol Res. 2015;14:6605–6616. doi: 10.4238/2015.June.18.3 [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Quan H, Wang S, Li X, Che X. MiR-183 promotes growth of non-small cell lung cancer cells through FoxO1 inhibition. Tumour Biol. 2015;36:8121–8126. doi: 10.1007/s13277-015-3550-8 [DOI] [PubMed] [Google Scholar]
- 28.Yu JJ, Wu YX, Zhao FJ, Xia SJ. miR-96 promotes cell proliferation and clonogenicity by down-regulating of FOXO1 in prostate cancer cells. Med Oncol. 2014;31:910. doi: 10.1007/s12032-014-0910-y [DOI] [PubMed] [Google Scholar]
- 29.Chi HC, Chen SL, Cheng YH, et al. Chemotherapy resistance and metastasis-promoting effects of thyroid hormone in hepatocarcinoma cells are mediated by suppression of FoxO1 and Bim pathway. Cell Death Dis. 2016;7:e2324. doi: 10.1038/cddis.2016.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valis K, Prochazka L, Boura E, et al. Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res. 2011;71:946–954. doi: 10.1158/0008-5472.CAN-10-2203 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Xie T. Ghrelin inhibits cisplatin-induced MDA-MB-231 breast cancer cell apoptosis via PI3K/Akt/mTOR signaling. Exp Ther Med. 2020;19:1633–1640. doi: 10.3892/etm.2019.8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong F, Liu X, Zhou Y, et al. Downregulation of METTL14 increases apoptosis and autophagy induced by cisplatin in pancreatic cancer cells. Int J Biochem Cell Biol. 2020;122:105731. doi: 10.1016/j.biocel.2020.105731 [DOI] [PubMed] [Google Scholar]
- 33.Tian H, Wang W, Meng X, et al. ERas enhances resistance to cisplatin-induced apoptosis by suppressing autophagy in gastric cancer cell. Front Cell Dev Biol. 2020;7:375. doi: 10.3389/fcell.2019.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai Y, Zhao XJ, Li F, et al. Truncated bid regulates cisplatin response via activation of mitochondrial apoptosis pathway in ovarian cancer. Hum Gene Ther. 2020;31:325–338. doi: 10.1089/hum.2019.206 [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Zhao X, Yang W. MiR-647 promotes cisplatin-induced cell apoptosis via downregulating IGF2 in non-small cell lung cancer. Minerva Med. 2019. doi: 10.23736/S0026-4806.19.06240-2 [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Li W, Xia Z, et al. Targeting MCL-1 sensitizes human esophageal squamous cell carcinoma cells to cisplatin-induced apoptosis. BMC Cancer. 2017;17:449. doi: 10.1186/s12885-017-3442-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou S, Jin W, Xiao W, et al. Integrin α5 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Am J Cancer Res. 2019;9:2774–2788. [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Y, Chen YJ, Yan YB, et al. MiR-186 bidirectionally regulates cisplatin sensitivity of ovarian cancer cells via suppressing targets PIK3R3 and PTEN and upregulating APAF1 expression. J Cancer. 2020;11:3446–3453. doi: 10.7150/jca.41135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H, Yang J, Yang S. MicroRNA-103a-3p potentiates chemoresistance to cisplatin in non-small cell lung carcinoma by targeting neurofibromatosis 1. Exp Ther Med. 2020;19:1797–1805. doi: 10.3892/etm.2020.8418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang XJ, Zhang DL, Fu C, Wei BZ, Li GJ. MiR-183 modulates multi-drug resistance in hepatocellular cancer (HCC) cells via miR-183-IDH2/SOCS6-HIF-1α feedback loop. Eur Rev Med Pharmacol Sci. 2016;20:2020–2027. [PubMed] [Google Scholar]
- 41.Yang M, Liu R, Xiajun L, et al. miRNA-183 suppresses apoptosis and promotes proliferation in esophageal cancer by targeting PDCD4. Mol Cells. 2014;37(12):873–880. doi: 10.14348/molcells.2014.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Q, Yang X, Zhou X, et al. MiR-3174 promotes proliferation and inhibits apoptosis by targeting FOXO1 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2020;S0006–291X(20):30645–30649. doi: 10.1016/j.bbrc.2020.03.152 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Zhang J, Zhang D, Ni W, Xiao H, Zhao B. Down-regulation of LINC00472 promotes osteosarcoma tumorigenesis by reducing FOXO1 expressions via miR-300. Cancer Cell Int. 2020;20:100. doi: 10.1186/s12935-020-01170-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Zhou Z, Zhu C, Cai Z, et al. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol Lett. 2018;15:7319–7327. doi: 10.3892/ol.2018.8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Y, Li B, Zhang H, et al. L-asparaginase induces in AML U937 cells apoptosis via an AIF-mediated mechanism. Front Biosci (Landmark Ed). 2014;19:515–527. doi: 10.2741/4222 [DOI] [PubMed] [Google Scholar]