Abstract
Agriculture plays an important role in a country’s economy. The sector is challenged by many stresses, which led to huge loss in plant productivity worldwide. The ever-increasing population, rapid urbanization with shrinking agricultural lands, dramatic change in climatic conditions, and extensive use of agrochemicals in agricultural practices that caused environmental disturbances confront mankind of escalating problems of food security and sustainability in agriculture. Escalating environmental problems and global hunger have led to the development and adoption of genetic engineering and other conventional plant breeding approaches in developing stress-tolerant varieties of crops. However, these approaches have drawn flaws in their adoption as the process of generating tolerant varieties takes months to years in bringing the technology from the lab to the field. Under such scenario, sustainable and climate-smart agricultural practices that avail bacterial usage open the avenues in fulfilling the incessant demand for food for the global population. Ensuring stability on economic fronts, bacteria minimizes plant salt uptake by trapping ions in their exopolysaccharide matrix besides checking the expression of Na+/H+ and high-affinity potassium transporters. Herein we describe information on salinity stress and its effect on plant health as well as strategies adopted by plant growth-promoting rhizobacteria (PGPR) in helping plants to overcome salinity stress and in mitigating loss in overall plant productivity. It is believed that acquisition of advanced knowledge of plant-beneficial PGPR will help in devising strategies for sustainable, environment-friendly, and climate-smart agricultural technologies for adoption in agriculture to overcome the constrained environmental conditions.
Keywords: plant productivity, phytohormones, rhizosphere, PGPR, soil salinity
Introduction
With rapid urbanization, the reduction in agricultural land left less space to expand the cultivation of plants. Under such circumstances, expansion in plant production relies on increasing the fertility of soils to ensure food for all under the current global food security scenario (Godfray et al., 2010). In this direction, soil quality and water availability play a pivotal role in sustainable agricultural productivity. Any disbalance of salt in soil and water leads not only to decline in plant productivity but also even to their abandonment as it progresses with change in the land pattern from fertile to a marginal one. Although primary salinity is natural in the environment, the contribution by anthropogenic sources such as urbanization and deforestation is worth noting as these result in enhancing loss of the cultivable capacity of soils (land degradation and disturbance in the physical and the biological properties of soil) that affect plant productivity worldwide. Enhancement in salt deposits in an agricultural field hampers the growth of crop plants. In the scenario of decreased availability of fertile land, studies were directed in adopting genetic engineering approaches to complement traditional breeding methods in the development of salt-tolerant crops of food and fiber (Rozema and Flowers, 2008; Zhu et al., 2011; Dodd and Perez-Alfocea, 2012; Joshi et al., 2015). Despite significant efforts, the complexity in understanding the biological aspects of salt-stress-induced changes (morphological, biochemical, and physiological) renders limited success in developing salinity-stress-tolerant plants.
To cope up with the limited success in bringing technology-driven transgenics from the lab to the field, alternative strategies, such as the introduction of salt-tolerant microbes, are explored for adoption in augmenting and, as such, enhancing the growth of crops in salt-affected soils (Dodd and Perez-Alfocea, 2012; Etesami and Beattie, 2017; Etesami, 2018). Among them, plant growth-promoting rhizobacteria (PGPR) constitutes an important class of microorganisms that were found effective in inducing systemic tolerance in plants to tolerate abiotic stresses (Dutta and Khurana, 2015; Etesami and Beattie, 2018). However, PGPR from hypersaline soils (halotolerant PGPR) expressing plant growth-promoting (PGP) traits were found least affected by environmental factors such as climate, soil characteristics, etc., and thus are more efficient in enhancing salt tolerance in plants than PGPR from non-saline habitats (Giongo et al., 2008; Upadhyay et al., 2009; Egamberdieva and Kucharova, 2009; Khan et al., 2016). As part of the plant–bacterial interaction at the rhizospheric plane, plants were found to dictate the growth of microbiota for driving adaptation to changing environmental conditions (Berendsen et al., 2012). While many excellent reviews discussed a range of diverse plant-beneficial traits of microbiota encompassing both bacteria and fungi (Qin et al., 2016; Ilangumaran and Smith, 2017; Etesami and Beattie, 2017, 2018; Backer et al., 2018; Egamberdieva et al., 2019), the present study is aimed at highlighting the importance of plant–bacterial interactions, with comprehensive inputs about the mechanistic insights that operate at the plant level in mitigating salt stress toward improvement in crop yield as part of the climate-smart agricultural practices geared for feeding the ever-increasing global population.
Soil Salinity and Plant Growth
Increase in the concentration of salts, preferably sodium chloride (NaCl; electrical conductance >4 dSm–1 or 40 mM), attributed to both natural (salts released by weathering of rocks, salt from seawater influx, air-borne salts from oceans, etc.) and anthropogenic (surface runoff and irrigation-based salt deposition year after year) sources, renders the soil no longer suitable for cultivation (Pitman and Lauchli, 2002; Rengasamy, 2002). Despite suitable soil water columns, excessive salinity raising the concentration in soil solutions deprive plants of using it via osmotic reduction. High soil salt concentrations induce its effects right from imbibition of water to seed germination and root elongation that together have a great effect on the yield of crop plants (Katembe et al., 1998; Kaymakanova, 2009). It has been observed that the pre-treatment of seeds with different PGPR promotes seed germination and seedling growth (Poupin et al., 2013; Rahmoune et al., 2017; Bakhshandeh et al., 2020). As part of the mechanism, it is believed that PGPR helps in maintaining the balance of hormones, e.g., auxin to cytokinin levels during germination and the early stages of plant development, thereby playing a critical role in dictating the genetic program that controls post-embryonic roots and shoot growth (Chu et al., 2019; Qessaoui et al., 2019).
At later stages of plant growth, soil salinity interferes with root turgor that led to reduction in water absorption, decrease in the plant water column that progresses through dehydration and osmotic stress, inhibition of the metabolic machinery, disturbance in the transpiration system, and, most importantly, interference with parameters pertaining to photosynthesis (Kaushal and Wani, 2015). Photosynthesis refers to a major attribute in dry matter and, as such, in plant productivity, showing a decrease in saline condition owing to the reduction in leaf turgor and reduced leaf surface area (Qin et al., 2010; Tanveer and Shah, 2017). It occurs either through (1) decreased stomatal opening and CO2 uptake, which in turn is associated with the reduction in stomatal conductance or (2) operation of a less-efficient Calvin cycle due to limited chlorophyll content (Lycoskoufs et al., 2005; Chaves et al., 2009). Stunted growth (seedling) with reduced biomass and leaf area are observed effects of salinity stress in the growth (vegetative stage) of plants (Takemura et al., 2000; Wang et al., 2003). PGPR employ different mechanisms in encouraging plant growth, prominently being nutrient availability and securing mineral assets such as phosphorus, phytohormone production, production of volatile compounds in controlling seed- and soil-borne phytopathogen, and synergism with other plant-beneficial microorganisms in enhancing resistance against different stresses (Bhattacharyya and Jha, 2012; Bhattacharyya et al., 2015; Bell et al., 2015; Kurepin et al., 2015; Bach et al., 2016; Yuan et al., 2016). Additionally, a limited canopy that prevents water loss by transpiration also constitutes a plant survival mechanism under high salt concentrations (Savé et al., 1994; Ruiz-Sánchez et al., 2000; Colmer et al., 2005; Cassaniti et al., 2009, 2012).
Salinity Stress, PGPR, and Plant Productivity: A Triangular Conjecture
Salinity is a stress of global magnitude, having a substantial effect on plant growth, and is accountable for a significant loss in their productivity. Exerting adverse effects on germination, vigor, and yield, it led to drastic reduction in plant productivity, as observed in plants growing in arid and semi-arid areas (Paul and Lade, 2014). With an increase in salt concentration, disturbance in the cellular ion balance led to an enhancement in reactive oxygen species (ROS) production, besides taking a huge toll in exerting ionic toxicity on the accumulation of Na+ and Cl– ions (Grover et al., 2011). ROS (free oxygen radicals, superoxide, and hydrogen peroxide) are capable of damaging cellular structures and damage of biomolecules (proteins, lipids, etc.) besides talking a huge toll on chlorophyll degradation and lipid peroxidation that are, in turn, associated with a reduction in photosynthetic activity, damage of cellular membranes, and ultimately proceeding with induction of programmed cell death (Apel and Hirt, 2004). Interfering in cellular enzymatic functions, the accumulation of Na+ and Cl– ions produces diverse effects on different physiological fronts and in its effect on the growth and the development of plants (Nunkaew et al., 2015; Acosta-Motos et al., 2017). Photosynthesis capacity is reduced due to the interference of these ions with the opening and the closing of the stomata and in exerting osmotic stress as reflected in plants through reduction in leaf area and chlorophyll content (Munns, 1992; Kang et al., 2014a). Suppression of plant growth, a phenomenon of disturbed metabolic activities as a result of nutritional and hormonal imbalance together with abscission and senescence, is observed once the intensity of salinity stress, together with temperature, crosses the limit (Glick, 2014; Paul and Lade, 2014; Hashem et al., 2015). The accumulation of Cl– ion leads to inhibition of nitrate reductase activity in the photosynthetic pathway (Azza Mazher et al., 2007; Nadeem et al., 2014). Elevation in ethylene (C2H4) levels progresses with drastic effects on plant health such as defoliation, senescence, etc. (Barnawal et al., 2014; Glick, 2014). Upon overcoming the storage capacity of cells, the accumulation of salts progresses to dehydration of cells, ultimately leading to plant death (Kang et al., 2014a).
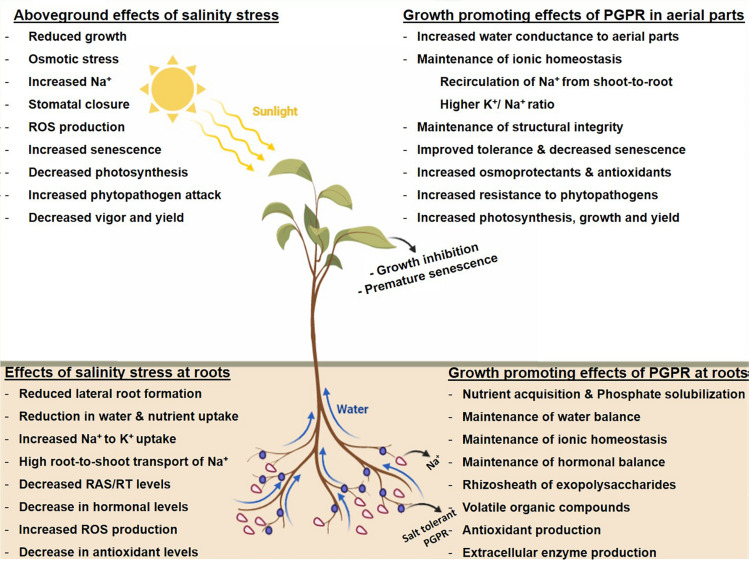
Constituting an excellent environment for them to flourish, plant-beneficial microorganisms play an important role in achieving sustainability in plant productivity under the current paradigm of climatic change. As part of the climate-smart agricultural practices, microorganisms improve nutrient availability to plants and, in return, get nutrients as root exudates from these plants (Patel et al., 2015; Hamilton et al., 2016; Singh and Strong, 2016). Halotolerant PGPR employs a wide range of strategies as adaption for survival under saline conditions and, in turn, executes a number of plant-beneficial mechanisms for improving the growth of crop plants growing under salinity stress (Figure 1). These include (1) making nutrients available to plants via solubilization of phosphorus and potassium, siderophore production for iron uptake, and fixation of atmospheric nitrogen (Etesami and Beattie, 2017; Etesami, 2018), (2) maintenance of water balance by changing the architecture of roots for hydraulic conductance (Arora et al., 2012), (3) selective uptake of K+ to Na+ ions for maintaining a high K+/Na+ ratio that indirectly reduces the intercellular accumulation of K+ to Na+ ions (Islam et al., 2016; Etesami, 2018), (4) exopolysaccharide (EPS)-mediated alleviation of salt stress by decreasing Na+ accumulation in roots and, as such, preventing their translocation to the leaves (Nunkaew et al., 2015; Qin et al., 2016; Etesami and Beattie, 2017), (5) production of volatile compounds and osmoprotectants that enhance the plants’ survival under salt stress (Creus et al., 2004; Timmusk et al., 2014), (6) protecting plants from oxidative stress by upregulating the activity of enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase as part of the antioxidant defense system (Islam et al., 2016), (7) maintenance of hormonal level for alleviation of salt stress (Etesami et al., 2014; Singh et al., 2015; Etesami and Beattie, 2017), (8) modulation in the expression of stress-responsive genes (Gond et al., 2015; Qin et al., 2016; Kaushal and Wani, 2016; Etesami and Beattie, 2017), and (9) production of extracellular enzymes that impart protection against phytopathogens competing with beneficial bacterial species for nutrients (Hariprasad et al., 2011; Dubey et al., 2014; Etesami, 2018).
FIGURE 1.
Salinity stress and tolerance mechanisms induced by plant growth-promoting rhizobacteria.
PGPR in the Alleviation of Salt Stress
Salinity stress adversely affects plant morphological, physiological, and biochemical functioning that, in turn, proves detrimental to plant health. Salt tolerance−a parameter quantified over given time−is survival, growth (vegetative), and biomass (harvestable) of the plant growing under salt stress to non-saline habitats (Munns, 2002b). The plants adopt either by inheriting genetic traits that impart salinity tolerance or by adopting a selectable mechanism of salt exclusion from the roots, thereby delaying salinity stress (Munns, 2002b; Zhu, 2007). A few (in particular, halophytes) conduct movement of accumulated salts via the xylem for precipitation at the leaf surface, while others have developed specialized structures (salt glands) in shoots, whereby salt is excreted on the surface for removal by wind or water (Ilangumaran and Smith, 2017). Additionally, plants undergo valuable interactions with bacterial species residing in the rhizospheric region, with an interaction pattern ranging from mutualism to antagonism. Colonization and successful establishment in the rhizospheric region are considered as a prerequisite for their interaction at the root surface. Traits that promote colonization of PGPR at the root surface include the availability of sufficient nutrients besides the property of being motile and capable of adherence (via pilli, surface-localized proteins, etc.) to plant roots (Jan et al., 2011). On one side where root exudates (organic acids, phenolics, sugars, amino acids, etc.) help microbes to flourish, it prompts changes (both physical and chemical) in plants related to defense, nutrient deficiency, and tolerance against heavy metals besides being important in eliciting strong responses against different abiotic stresses such as salinity as a mechanism of promoting plant growth (Jan et al., 2011; Nadeem et al., 2014; Rashid et al., 2016). Table 1 a detailed account of the growth-promoting attributes of PGPR in agroecosystems is given in the following discussion.
TABLE 1.
Plant growth-promoting rhizobacteria (PGPR)−plant interactions under salinity stress and plant beneficial effects recorded thereof.
| Sample number | Plant species | PGPR species inoculation | Effects observed | References |
| (1) | Maize (Zea mays) | Achromobacter xylosoxidans | Improved maize growth and productivity under drought stress | Danish et al., 2020 |
| B. licheniformis FMCH001 | Enhances plant water use efficiency via growth stimulation in both normal as well as in drought conditions | Akhtar et al., 2020 | ||
| Bacillus sps. | Induces plant response for defense enzymes, chlorophyll, proline, and soluble sugar under salt stress | Misra and Chauhan, 2020 | ||
| Bacillus sp. NBRI YN4.4 | Improves photosynthetic pigments and soluble sugar content and decreases proline level under stress conditions; also enhances soil enzymes dehydrogenase, alkaline phosphatase, and betaglucosidase, which help in improving soil health | Dixit et al., 2020 | ||
| Ochrobactrum sp. NBRISH6 | Helps in maintaining homeostasis through various mechanisms under deficit water stress condition | Mishra et al., 2020 | ||
| A. brasilense | Induced the development of a more extensive root system, regardless of growth medium nitrate concentration | Pii et al., 2019 | ||
| Burkholderia cenocepacia CR318 | Helps in the health and the growth of crop including phosphate and potassium solubilization and antimicrobial activity | You et al., 2020 | ||
| P. aeruginosa strain FB2 and B. subtilis strain RMB5 | Shows effectivity against a range of fungal phytopathogens | Ali et al., 2020 | ||
| Serratia liquefaciens KM4 | Maintenance of water balance, enhanced antioxidant enzyme activities, increased nutrient uptake | El-Esawi et al., 2018b | ||
| Pseudomonas sp., Arthrobacter sp., Bacillus sp., and members of other bacterial groups | Enhanced phosphate solubilization, IAA and ACC deaminase activity | Aslam and Ali, 2018 | ||
| A. brasilense Ab-V5 and Ab-V6, Rhizobium tropici CIAT 899 | Enhanced antioxidant enzyme activities | Fukami et al., 2018 | ||
| Bacillus aquimaris DY-3 | Maintenance of water balance, development of pigment system, enhanced antioxidant enzyme activities | Li and Jiang, 2017 | ||
| Bacillus amyloliquefaciens SQR9 | Enhanced solute accumulation, enhanced antioxidant enzyme activities, increased expression of salinity stress response genes | Chen et al., 2016 | ||
| Staphylococcus sciuri | Enhanced antioxidant enzyme activities | Akram et al., 2016 | ||
| Bacillus spp., Arthrobacter pascens | Phosphate solubilization, maintenance of water balance, increased antioxidant enzyme activities | Ullah and Bano, 2015 | ||
| Pantoea agglomerans | Increased expression of aquaporin genes | Gond et al., 2015 | ||
| P. syringae, P. fluorescens | Enhanced ACC deaminase activity | Zafar-ul-Hye et al., 2014 | ||
| Proteus penneri, P. aeruginosa, A. faecalis | Enhanced exopolysaccharide production | Naseem and Bano, 2014 | ||
| Azotobacter chroococcum | Enhanced growth, increased phosphate solubilization and K+/Na+ ratio | Rojas-Tapias et al., 2012 | ||
| Bacillus megaterium | Improved expression of ZmPIP isoforms | Marulanda et al., 2010 | ||
| Rhizobium, Pseudomonas spp. | Osmotic regulation | Bano and Fatima, 2009 | ||
| Pseudomonas spp., Enterobacter spp. | ACC deaminase activity | Nadeem et al., 2009 | ||
| Pseudomonas syringae, Enterobacter aerogenes, P. fluorescens | ACC deaminase activity | Nadeem et al., 2007 | ||
| Azospirillum brasilense | Maintenance of ion homeostasis, decreased nitrogenase activity | Hamdia et al., 2004 | ||
| (2) | Rice (Oryza sativa) | Bacillus aryabhattai, Achromobacter denitrificans, and Ochrobactrum intermedium | Helps to accumulate under salt stress and exhibits greater resistance to heavy metals | Sultana et al., 2020 |
| Klebsiella sp. PD3 | Degrades phenanthrene; also shows ACC deaminase activity and phosphate solubilization | Li X. et al., 2020 | ||
| Bacillus amyloliquefaciens SN13 | Induces metabolic and physiological parameters via different enzymes to reduce the impact of stress | Bisht et al., 2019 | ||
| Bacillus sp. JBS-28 | Promotes grain yields; also decreases arsenic accumulation in arsenic-contaminated soil and paddy fields | Aw et al., 2019 | ||
| Bacillus aryabhattai MS3 | Phosphate solubilization, enhanced siderophore and IAA production | Sultana et al., 2018 | ||
| Halobacillus dabanensis SB-26, Halobacillus sp. GSP 34 | Nitrogen fixation and IAA production | Rima et al., 2018 | ||
| Enterobacter sp. P23 | Growth promotion, phosphate solubilization, increased siderophore, and IAA production, reduction in ethylene production, enhanced antioxidant enzyme activities | Sarkar et al., 2018 | ||
| B. stratosphericus (NBRI 5Q and NBRI 7A) | Increased growth and biomass production, Phosphate solubilization, IAA production, enhanced ACC deaminase activity | Misra et al., 2017 | ||
| Thalassobacillus denorans (NCCP-58), Oceanobacillus kapialis (NCCP-76) | Increased germination and growth of root and shoot, developed pigment system, reduced Na+ ion accumulation | Shah et al., 2017 | ||
| Bacillus pumilus | Growth promotion, enhanced antioxidant enzyme production, reduced Na+ ion accumulation | Khan et al., 2016 | ||
| Bacillus and Citrobacter | Growth promotion, phosphate solubilization, IAA production | Habib et al., 2016 | ||
| Pseudomonas PF1 and TDK1 | Enhanced antioxidant enzyme production | Sen and Chandrasekhar, 2015 | ||
| Serratia sp., Pseudomonas sp. | Growth promotion, phosphate solubilization, IAA production | Nakbanpote et al., 2014 | ||
| Alcaligens sp., Bacillus sp., Ochrobactrum sp. | ACC deaminase activity | Bal et al., 2013 | ||
| P. pseudoalcaligenes, B. pumilus | Reduction in ROS production, delay of senescence | Jha and Subramanian, 2013 | ||
| B. amyloliquefaciens NBRISN13 (SN13) | Solute accumulation, enhanced expression of SOS1, EREBP, SERK1, and NADP-Me2 | Nautiyal et al., 2013 | ||
| (3) | Wheat (Triticum aestivum) | Variovorax paradoxus RAA3; Pseudomonas spp. DPC12, DPB13, DPB15, DPB16; Achromobacter spp. PSA7, PSB8; Ochrobactrum anthropi DPC9 | ACC deaminase activity; improves the growth of plants in water-stressed rain-fed environments | Chandra et al., 2019 |
| Planomicrobium chinense and Bacillus cereus with salicylic acid | Reduces moisture stress in plants | Khan and Bano, 2019 | ||
| Bacillus siamensis, Bacillus sp., and Bacillus methylotrophicus | ACC deaminase activity | Amna et al., 2019 | ||
| Bacillus subtilis | Induction of systemic resistance | Lastochkina et al., 2017 | ||
| Dietzia natronolimnaea | Enhanced expression of SOS-related genes, increased tissue-specific expression of ion transporters, modulation of ABA signaling cascade | Bharti et al., 2016 | ||
| Serratia marcescens CDP-13 | ACC deaminase activity, minimizes the salinity-induced oxidative damages to the plants | Singh and Jha, 2016 | ||
| Arthrobacter spp. SU18, B. aquimaris SU44, B. aquimaris SU8 | Root dry weight and shoot biomass | Upadhyay and Singh, 2015 | ||
| Azosprillium lipoferum, Pseudomonas fluorescens 169 | Development of pigment system | Saghafi et al., 2013 | ||
| Azospirillum | Development of pigment system, enhanced solute accumulation, increased seedling growth and plant yield | Nia et al., 2012 | ||
| Piriformo sporaindica, Azospirillum | Development of pigment system, enhanced solute accumulation, increased seedling growth | Zarea et al., 2012 | ||
| Azospirillum lipoferum | Growth and biomass accumulation | Bacilio et al., 2004 | ||
| (4) | Soybean (Glycine max) | Bradyrhizobium diazoefficiens USDA110, Bacillus velezensis S141 | Enhanced nodulation and N2-fixing efficiency by producing larger nodules | Sibponkrung et al., 2020 |
| Bradyrhizobium | Improves plant development and increases nodulation | Zeffa et al., 2020 | ||
| P. fluorescens LBUM677 | Enhances plant biomass, oil content, and lipid composition | Jiménez et al., 2020 | ||
| A. woluwensis, M. oxydans, A. aurescens, B. megaterium, and B. aryabhattai | Maintains osmotic balance and regulates salt tolerance | Khan et al., 2019 | ||
| L. adecarcoxylata LSE-1, Bradyrhizobium sp. LSBR-3 | Promotes plant growth with increased plant productivity | Kumawat et al., 2019 | ||
| Bacillus firmus SW5 | Development of root system, enhanced antioxidant enzyme levels | El-Esawi et al., 2018a | ||
| Bradyrhizobium japonicum USDA 110, P. putida TSAU1 | Development of root system with nodule formation, increased phosphate acquisition | Egamberdieva et al., 2017 | ||
| Pseudomonas simiae AU | Increased chlorophyll content, phosphate solubilization, IAA and siderophore production; decrease in Na+ accumulation at root surface | Vaishnav et al., 2016a | ||
| Bacillus thuriengenesis NEB17 | Increased PEPCO and RuBisCo expression, enhanced production of pyruvate kinase, proteins of photosystems I and II, isocitrate lyase, and antioxidant glutathione-S-transferase | Subramanian et al., 2016 | ||
| P. putida H-2-3 | Enhanced production of ABA, salicylic acid, and gibberellins | Kang et al., 2014b | ||
| P. fluorescens | Enhanced cytokinin production | Bhattacharyya and Jha, 2012 | ||
| Bradyrhizobium japonicum, Bacillus subtilis SU-12, Serratia proteamaculans | Exopolysaccharide production, antioxidant activity | Han and Lee, 2005 | ||
| (5) | Tomato (Solanum lycopersicum) | Bacillus subtilis Rhizo SF 48 | ACC deaminase activity; protects against oxidative damage and enhances plant growth against drought stress | Gowtham et al., 2020 |
| Funneliformis mosseae, Enterobacter sp. EG16, and Enterobacter ludwigii DJ3 | Enhances plant growth and tolerance to Cd in Cd-contaminated soil | Li Y. et al., 2020 | ||
| Leclercia adecarboxylata MO1 | IAA- and ACC-deaminase-producing abilities; improves plant tolerance to salinity stress | Kang et al., 2019 | ||
| Pseudomonas putida UW4 (ACC deaminase) | Increased shoot growth and expression of Toc GTPase | Yan et al., 2014 | ||
| Pseudomonas aeruginosa T15, Pseudomonas fluorescens NT1, Pseudomonas stutzeri C4 | Decreased ethylene levels, increased root and shoot length | Tank and Saraf, 2010 | ||
| Achromobacter piechaudii ARV8 | Enhanced induced systemic tolerance, enhanced ACC deaminase activity | Mayak et al., 2004 | ||
| (6) | Common bean (Phaseolus vulgaris) | Aneurinibacillus aneurinilyticus and Paenibacillus sp. | ACC deaminase activity | Gupta and Pandey, 2019 |
| Mycorrhizae, Bacillus subtilis, and Pseudomonas fluorescence | Controls the infection of Sclerotium rolfsii; also acts as biofertilizers | Mohamed et al., 2019 | ||
| Rhizobium | Increased nutrient content and dry weight | Yanni et al., 2016 | ||
| Pseudomonas chlororaphis TSAU13, Pseudomonas extremorientalis TSAU20 | Increased dry weight and root length | Egamberdieva, 2011 | ||
| Azospirillum brasilense, Rhizobium spp. | Enhanced root branching, increased secretion of flavonoids | Dardanelli et al., 2008 | ||
| (7) | Radish (Raphanus sativus) | Bacillus sp. CIK-516 | Improves plant growth and enhances Ni phytoextraction | Akhtar et al., 2018 |
| Lactobacillus sp., P. putida and Azotobacter chroococcum | Helps to mitigate salinity stress at the time of germination | Hussein and Joo, 2018 | ||
| Arthrobacter scleromae SYE-3 | Increased shoot length | Hong and Lee, 2017 | ||
| Staphylococcus kloosii, Kocuria erythromyxa | Increased chlorophyll content, increased shoot and root fresh and dry weight | Yildirim et al., 2008a | ||
| Bacillus spp. | Induction of plant growth | Yildirim et al., 2008b | ||
| (8) | Barley (Hordeum vulgare) | Hartmannibacter diazotrophicus | Growth induction, enhanced ACC deaminase activity, increased root and shoot dry weight | Suarez et al., 2015 |
| Curtobacterium flaccumfaciens | Promotes plant growth | Cardinale et al., 2015 |
Maintenance of Water Balance and Nutrient Acquisition
The hydration of cells, having a greater impact on physiological and metabolic processes, determines behavioral growth in plants. Hydraulic gradients in the xylem regulate water conductance from the roots to the leaves against an imbalance between the rate of transpiration and the available water absorbed from the soil (Passioura, 2010; Chavarria and dos Santos, 2012). The sustained transpiration of water from the leaf surface without any replenishment causes a reduction in xylem water potential that progressively leads to leaf dehydration, depending on the environmental conditions, stage of the growth of plant, canopy characteristics, and water quality as part of irrigation. The accumulation of salts at the root surface causes a transition in the root architecture (supresses lateral root formation) over time that influences the availability and uptake of soil nutrients. Salinity-induced osmotic stress proceeds with a decrease in diffusion and, as such, mass flow of nutrients as they are carried to the roots of plants by water (Zhu, 2001; Munns, 2002a; Ashraf, 2004; Sánchez-Blanco et al., 2004; Meloni et al., 2008; Franco et al., 2011; Chavarria and dos Santos, 2012). Under osmotic stress conditions, the aboveground plant parts undergo little photosynthetic activity and switch to the use of photo-assimilates, which causes a reduction in plant growth. All these events lead to a subsequent reduction in plant productivity (Chartzoulakis et al., 2002; Giri et al., 2003; Katerji et al., 2005; Bhatnagar-Mathur et al., 2007; Álvarez et al., 2012; Gómez-Bellot et al., 2013).
The inoculation of bacterial isolates to the roots of pepper plants resulted in an enhanced roots system, thereby increasing the ability of plants to uptake water from the surroundings (Marasco et al., 2013). The expression of aquaporins (water-conducting proteins) present in plasma and intracellular membrane determines the hydraulic conductance (L) at the root surface and, as such, the uptake of water from salinized soil for a plant (Moshelion et al., 2015; Qin et al., 2016). Plasma membrane intrinsic proteins (PIPs) constitute important aquaporins for a plant, which helps in its adaptation to changing environmental conditions (Marulanda et al., 2010; Moshelion et al., 2015). An expressional analysis of Zea mays roots inoculated with Bacillus megaterium and Pantoea agglomerans showed up-regulated PIP2 and ZmPIP1-1 genes that contribute to the increase in the L-values under salinity stress conditions (Gond et al., 2015). These studies reveal that PGP bacteria determine the resistance of plants to water stress irrespective of the nature of interaction in determining the specificity for growth-promoting activity. Plant−bacterial interactions at the root surface assist plants in maintaining the availability of water and helps in the acquisition of nutrients through nitrogen fixation, phosphate solubilizations, and siderophore production as part of their mechanism in fulfilling the nutritional requirements of plants (Beattie, 2015; Pii et al., 2015). Nitrogen−an essential nutrient that limits plant productivity−is often applied exogenously. However, inorganic fertilizers that compensate nitrogen deficiency often lead to a change in soil structure and, as such, composition of soil microflora (Rueda-Puente et al., 2003). Studies were performed on exploring the naturally occurring nitrogen fixers which have the potential for exploration toward plant growth promotion. Of the different interactions, the nitrogen-fixing assembly of rhizobia in the roots of legumes is an extensively studied relationship between plants and bacteria. In this symbiotic relationship, the rhizomes provide the legumes with nitrogen and, in return, get reduced carbon as nutrient and suitable environment for nitrogenase activity (Backer et al., 2018). Being a sensitive process, all stages of nitrogen fixation in leguminous plants were found to be prone to salinity effects, which result in a decrease in the nitrogen content of leguminous plants (de la Peña and Pueyo, 2012; Bruning and Rozema, 2013). In this regard, the commercial preparation of halotolerant free-living diazotrophs such as Azotobacter sp., Azospirillium sp., etc., proved beneficial than rhizobia in nitrogen fixation in a variety of crops worldwide, thereby found effective in increasing the yield of various cereal crops (Vessey, 2003; Bashan and de-Bashan, 2015; Sharma et al., 2016).
Phosphorus is a major essential macronutrient that constitutes another limiting nutrient for plants after nitrogen. The abundance of insoluble forms and the intensive agricultural practices in both saline and fertile soils deplete plants of this essential nutrient. On the second line, phosphate-solubilizing microorganisms (PSMs) convert and as such make non-soluble forms of phosphate to easily available soluble forms for efficient utilization by the plants (Backer et al., 2018). Compared to complementation with NPK fertilizers, the employment of phosphate-solubilizing bacteria was found effective in enhancing phosphate availability to plants without exacerbating the soil salinity levels (Etesami, 2018; Etesami and Beattie, 2018). The liberation of reactive forms of phosphate from organic compounds on utilizing enzyme phytase of PSMs constitutes another mode of phosphate availability to plants. Additionally, the production of hydrogen cyanide (HCN), which was earlier thought as a plant-protective mechanism, was found to be associated with an enhancement in phosphate availability to plants (Rijavec and Lapanje, 2016). Siderophore (iron-binding ligands) production is associated with the deprivation of pathogenic microorganisms of iron (a micronutrient) and making it available for use in respiration, photosynthesis, and nitrogen fixation by plants (Ahmed and Holmstrom, 2014; Saha et al., 2016).
Maintenance of Ionic Homeostasis
Alleviating the nutritional imbalance caused by a high influx of salt ions regulates the exchange of nutrients (both macro and micro) to minerals. Microbes increase nutrient availability to plants through the increased production of siderophores (metal chelation) and bringing changes in pH at the surface of rhizospheres (Dodd and Perez-Alfocea, 2012; Lugtenberg et al., 2013). Disturbance in ionic homeostasis is observed in crops that are poor excluders of Na+ (rice, beans, etc.) and sensitive to Cl– ions (citrus, soybean, etc.) grown in soils with high salt levels (Munns, 2002a; Tester and Davenport, 2003). Under salinity stress, the influx of Na+ into the roots undergoes translocation to the aerial parts via the xylem, with the final accumulation taking place at the leaf surface rather than at the roots (Tester and Davenport, 2003). As such, excluding Na+ from plants becomes difficult as only a small proportion of it undergoes recirculation to the roots via the phloem, thereby restricting it to the aerial parts, thus causing toxicity in plants. An increase in the concentration of Na+ disturbs the Na+/K+ ratio that progresses with the inhibition of cytosolic activities besides interfering with the activities of enzymes involved in respiration and photosynthesis (Baral et al., 2015; Jacoby et al., 2016). Considering the importance of Na+ homeostasis to the growth of plants, the regulatory network of Na+/H+ antiporter and high-affinity K+ transporters (HKT) is put to work for the efflux of Na+ ions from the cells throughout the plants (Tester and Davenport, 2003; Davenport et al., 2005). With localization on the plasma membrane, the Na+/H+ antiporter (also referred to as SOS1, salt overlay sensitive channel) efflux Na+ in response to its increasing cytosolic levels (Qiu et al., 2002). Also, the increase in plant Na+ level interferes with the uptake of K+ at the root surface via the low-affinity K+ uptake system. To increase salinity tolerance, plants activate high-affinity K+ transporters, thereby increasing the uptake of K+ over Na+ ions in plants (Rodríguez-Navarro and Rubio, 2006). Additionally, the activation of membrane-bound Ca2+ channels in response to a depolarization event generates a Ca2+ signal that indicates the occurrence of salt stress in plants. The Ca2+ signal is sensed by calcineurin B-like protein (CBL4; also referred to as SOS3) which undergoes complex formation with CBL-interacting protein kinase; CIPK24 (also referred to as SOS2) enables the phosphorylation of SOS1 for its activation, an event important in maintaining the Na+/K+ ratio by sustaining K+ transporters (Epstein, 1998; Halfter et al., 2000; Zhu, 2002).
Microbes minimize the accumulation of ions by increasing Na+ exclusion at the roots besides boosting the working affinity of K+ transporters that indirectly reduce their build-up in aerial parts, thereby contributing to the maintenance of ion homeostasis in plants. Besides promoting biofilm formation at the root surface that prevents the influx of Na+ into the roots, EPS production by PGPR strains traps cations in their matrix, thereby make it unavailable for uptake by the plants (Dodd and Perez-Alfocea, 2012). The inoculation of Aeromonas hydrophila and Bacillus sp. capable of producing EPS to the roots of wheat traps Na+ ions, thereby making it unavailable for accumulation at the leaf surface (Ashraf et al., 2004). The inoculation of B. subtilis GB03 to the roots of Arabidopsis thaliana results in the down-regulation of HKT1, thereby reducing the uptake of Na+ (Zhang et al., 2008; Qin et al., 2016). Restricting the uptake of Na+ at the root surface leads to induction in the expression of HKT1 in shoots for facilitating the recirculation of Na+ from the shoot toward the roots, which helps in maintaining a high K+/Na+ ratio in plants (Zhang et al., 2008; Qin et al., 2016; Ali et al., 2019). With the RNA interference-mediated mutation of Ca2+-dependent protein kinase, CPK12 increases the sensitivity of Arabidopsis thaliana to salt stress (Zhang et al., 2018). The inoculation of Azotobacter strains C5 and C9 increases the exclusion of Na+ and, in anticipation, enhances K+ uptake, which subsequently led to an increase in proline, polyphenol, and chlorophyll content in maize leaves grown under salt stress (Rojas-Tapias et al., 2012). While studying the short- and the long-term effects of salt stress on A. thaliana, the inoculation of Burkholderia phytofirmans PsJN was found to attribute tolerance to a high amount of salts via alteration in the expression of ion homeostasis-associated genes (HKT1, KT1, SOS1, and Na+/H+ exchanger NHX2) (Pinedo et al., 2015). Similarly, the inoculation of B. subtilis GB03 to Puccinella tenuiflora showed an upregulation in the expression of PtSOS1 and PtHKT1 with less Na+ accumulation under a high salt concentration (Niu et al., 2016).
Exopolysaccharide Production
Exopolysaccharide are homo- or hetero-polysaccharides produced by rhizobacteria that enable their survival under inhospitable conditions. Though polysaccharides vary in composition, glucose, galactose, and mannose are abundant monomers that, in association with other sub-unit components such as amino sugars, uronic acids, etc., form a capsule-like protective biofilm on the surface of cells (Upadhyay et al., 2011; Rossi and De Philippis, 2015). Formed under adverse conditions, the adsorption of EPS on soil via cation bridges and Van der waals forces stabilizes soil structure and aggregation (Sandhya et al., 2009). Binding soil particles to aggregates, EPS form an enclosed matrix that increases root-adhering soil per root tissue (RAS/RT), conferring protection against environmental fluctuations. The protective EPS capsule possesses strong water-holding capacity that helps in the nutrient uptake by plants besides maintaining a higher water potential around the plant roots, protecting the plant from desiccation and ensuring plant growth and survival under salinity stress (Upadhyay et al., 2011; Selvakumar et al., 2012; Balsanelli et al., 2014). In addition to its role in nodule formation in legume–rhizobia associations, it forms a protective biofilm around the roots, thereby imparting protection to plant against salinity stress (Stoodley et al., 2002; Skorupska et al., 2006). Additionally, EPS rhizosheaths around the plant roots get hold of Na+ ions, thereby make these unavailable to plants. The inoculation of Halomonas variabilis (HT1) and P. rifietoensis (RT4) under salinity stress stabilizes soil structures and aggregation, thereby increasing the growth of chickpea (Cicer arietinum var. CM-98) (Qurashi and Sabri, 2012). Exerting a capability to fight salt stress, the inoculation of Bacillus subtilis to Helianthus annus was found to downregulate the expression of HKT1/K+ transporter (Zhang et al., 2008). Pseudomonas aeruginosa inoculation reduces salt stress and promoted growth that led to an enhancement in yield in Helianthus annus (Tewari and Arora, 2014). EPS are also used as seed priming agents that promote seed germination and, as such, crop yield under salinity stress conditions (Tewari and Arora, 2014). The seed inoculation of Enterobacter sp. MN17 and Bacillus sp. MN54 of Chenopodium quinoa results in improved plant water relation following growth under a high salt (400mM NaCl) concentration (Yang et al., 2016). The inoculation of B. subtilis subsp. inaquosorum and Marinobacter lipolyticus SM19 significantly reduces the adverse effects of salinity stress in wheat (Atouei et al., 2019). Additionally, the inoculation of halotolerant Pseudomonas PS01 strain was found to be associated with the regulation of the expression of genes related to salt stress in A. thaliana (Chu et al., 2019).
Production of Volatile Organic Compounds
Volatile organic compounds (VOCs; lipophilic in nature) are low molecular weight compounds that serve as signals for development and systemic response within the same or neighboring plants (Choudhary et al., 2008; Niinemets, 2010). The PGPR-mediated production of VOCs induces a range of physiological changes in plants that stimulate its growth (increasing shoot biomass) besides inducing systemic resistance to disease and controlling the plant pathogens (Lee et al., 2012; Park et al., 2015; Tahir et al., 2017). VOCs promote the biosynthesis of osmo-protectants such as glycine betaine whose accumulation imparts protection to PS-II besides maintaining the enzymatic activity and the membrane integrity of cells under osmotic stress conditions (Mäkelä et al., 2000; Jagendorf and Takabe, 2001). The VOCs of B. subtilis reduce salt stress through an enhancement in the tissue-specific expression of the HKT1/K+ transporter that enhances nutrient uptake at the root surface while minimizing the influx of Na+ to the roots (Zhang et al., 2008). P. chlororaphis O6 production of 2R, 3R-butanediol prevents water loss by inducing stomatal closures in A. thaliana, thereby imparting tolerance to A. thaliana (Cho et al., 2008). The process is mediated by Aba-1 and OST-1 kinases of jasmonic acid, ethylene, and salicylic acid pathways in plants. An increase in the VOC level on priming wheat plants with B. thuringiensis AZP2 imparts self-protection to the plants that enhances survival (fivefold higher) with higher photosynthesis, resulting in increased biomass under salt stress conditions (Timmusk et al., 2014). VOCs produced by P. simiae up-regulates γ-glutamyl hydrolase, vegetative storage (regulating Na+ homeostasis), and RUBISCO large-chain (associated with an increase in chlorophyll content and, as such, photosynthesis) proteins that are considered important in eliciting induced systemic resistance in soybean (Glycine max) (Vaishnav et al., 2015). Butanoic acid released by Alcaligens faecalis strain JBCS1294 attribute salt tolerance to plants via reprogramming of auxin and gibberellin pathways (Bhattacharyya et al., 2015). A blend of 7-hexanol, 3-methylbutanol, and 2-undecanone was found effective in mimicking VOCs in attributing plant growth effects on inoculation with different bacterial species (Ledger et al., 2016).
Antioxidant Production
Reactive oxygen species (ROS; including superoxide O2–⋅, hydroxyl radical OH⋅, hydrogen peroxide H2O2, etc.), generated as a metabolic by-product in plants, functions primarily as a signaling molecule. Abnormality in the cellular metabolic process of plants growing under stress conditions enhances the production of ROS, which results in DNA damage, changes in redox state, abnormality in protein formation, denaturation of membranous proteins, lipid peroxidation, reduction in membrane fluidity, interference with enzymatic activity, and overall homeostasis of cell that progresses to cell damage and even to plant cell death (Miller et al., 2010; Halo et al., 2015). Under such conditions, both enzymatic (SOD, superoxide dismutase; CAT, catalase; APX, ascorbate peroxidase, etc.) and non-enzymatic antioxidants (GSH, glutathione; tocopherols; ascorbic acid, etc.) play a vital role in neutralizing the ROS and, as such, protect plant cells against oxidative stress (Kim et al., 2014; Kaushal and Wani, 2015). In this regard, PGPRs extend their antioxidant enzyme machinery as protection to plants against oxidative stress. Salt stress induction triggers adaptive response mechanisms, including the accumulation of compatible compounds (organic and inorganic) that decrease the hydraulic conductivity of membranes for reducing cellular osmotic stress (Hasegawa et al., 2000; Munns, 2002a; Abdul-Jaleel et al., 2007). The inoculation of Pseudomonas sp. to basil plants (Ocimum basilicum L.) grown under stress conditions results in increasing the CAT activity, while the application of a microbial consortia (Pseudomonas sp., B. lentus, and A. brasilense) results in enhancement in APX and GPX (Heidari and Golpayegani, 2011). Similarly, tomato seedlings inoculated with Enterobacter spp. showed an increase in APX activity (Sandhya et al., 2010), while the inoculation of PGPR to gladiolus showed an enhancement in SOD and CAT activities (Damodaran et al., 2013). The inoculation of PGPR to Solanum tuberosum grown under stress conditions results in an enhancement in the activity of APX, SOD, CAT, and glutathione reductase (Gururani et al., 2013). The inoculation of B. amyloliquefaciens NBRISN13 (SN13) of rice grown under salinity stress results in an enhancement in chlorophyll content and plant biomass besides increasing proline content and the expression of antioxidant enzymes such as CAT (Nautiyal et al., 2013). An up-regulation in stress-responsive genes associated with proline biosynthesis was observed on treating A. thaliana with Enterobacter sp. (Kim et al., 2014).
The inoculation of microbial consortia (A. nitroguajacolicus strain YB3 and YB5, P. jessenii R62, and P. synxantha R81) to IR-64 variety of rice grown under stress conditions induces and, as such, enhances SOD, peroxidase (POD), CAT, and APX levels (Gusain et al., 2015). A significant increase in the transcription of stress-responsive genes, AtRSA1 (associated with ROS detoxification) and AtWRKY8 (associated with maintenance of ion homeostasis), while reducing the expression of AtVQ9 (negative regulator of AtWRKY8), was observed on inoculating Paenibacillus youginensis-to A. thaliana seedlings (Sukweenadhi et al., 2015). The inoculation of maize seedling with B. amyloliquefaciens SQR9 improved the glutathione, POD, and CAT levels besides showing an enhancement in soluble sugar and chlorophyll content (Chen et al., 2016). The physiological effects of the treatment were assessed as enhancement in RBCL (related to photosynthesis), HKT1, and NHX-1, -2, and -3 genes. Modulation in the expression of complete gene families associated with abscisic acid (ABA) signaling, ion transport, SOS pathway, and antioxidants was observed on inoculating wheat with salt-tolerant Dietzia natronolimnaea (Bharti et al., 2016). The inoculation of soybean by P. simiae strain AU results in the enhancement of pyrroline-5-carboxylase synthase, associated with the synthesis of proline as part of tolerance to stress conditions (Vaishnav and Choudhary, 2019). The study goes well with previous reports regarding the enhancement in proline content during stress conditions (Ghosh et al., 2018; Patel et al., 2018). The inoculation of Azospirillum lipoferum FK1 of chickpea exhibited enhanced antioxidant enzyme levels besides demonstrating an increase in nutrient uptake and, as such, improvement in its growth and development (El-Esawi et al., 2019). The bacterial consortium of P. fluorescens S3, B. mojavensis S1, and B. pumilis mitigates salt-induced growth inhibition of barley through an enhancement in the water conductance and the nutrient uptake of plants. The inoculation of rice with Trichoderma asperellum and P. fluorescens results in an enhancement in the activity of POD, APX, SOD, and CAT that contributes to the alleviation of salt stress (Singh et al., 2020).
Enzymes and Metabolites of Bacterial Origin
Plant diseases are considered as a major constraint to crop yield. It has been observed that salinity stress contributes to an increase in the susceptibility of plants to attacks by different pathogens (Besri, 1993). As the usage of chemicals in the control of plant pathogens imparts deleterious effects, PGPR emerged as a potential substitute as a biological control strategy in the management of pathogen-associated diseases in plants (Compant et al., 2010; Etesami and Alikhani, 2018). PGPR-based mechanisms employed in the biological control of pathogens include the following:
-
(1)
Synthesis of cell-wall-degrading enzymes: The production of hydrolytic enzymes such as cellulases, glucanases, chitinases, protease, etc., hydrolyzing polymeric compounds such as cellulose, hemicellulose, chitin, cell wall proteins, etc., was found capable of inhibiting a variety of plant pathogens (Pal and Gardener, 2006; Mabood et al., 2014; Husson et al., 2017; Vaddepalli et al., 2017). Similarly, protease produced by different PGPR agents was found effective in reducing the infections of Fusarium sp. and M. phaseolina (Dunne et al., 1997; Gohel et al., 2004). The biocontrol potential of chitinase produced by Paenibacillus illinoissensis spp. provides protection against blight and damping off diseases in pepper (Capsicum annuum) caused by Phytophthora capsica and Rhizoctonia solani (Jung et al., 2003, 2005). Chitinase produced by B. suly reduces the infection severity of Fusarium sp. under greenhouse conditions (Hariprasad et al., 2011). The production of chitinases together with β-1,3-glucanases by PGPR such as B. subtilis BSK17 for utilizing them as a source of carbon is of prime importance as it forms a major enzyme group capable of degrading the chitin and laminarin components of fungal cell walls (Kumar et al., 2012; Dubey et al., 2014).
-
(2)
Synthesis of antimicrobial metabolites: With maximum reports from Bacillus and Pseudomonas genera, the production of a wide range of metabolites was found to restrict the growth of pathogens (Couillerot et al., 2009; Olanrewaju et al., 2017).
-
(3)
HCN production by Pseudomonas sp., Bacillus sp., Rhizobium, etc., was found capable of inhibiting cytochrome C oxidase along with other metalloenzymes (Nandi et al., 2017).
-
(4)
The synthesis of siderophores by different PGPR strains possessing a high affinity for Fe3+ ions chelates it and, as such, deprives pathogens of this essential mineral (Shen et al., 2013; Olanrewaju et al., 2017).
-
(5)
Prime plants for induction of induced systemic resistance that imparts a faster and stronger response to attacks by different pathogens (Olanrewaju et al., 2017).
Maintenance of Hormonal Balance
Phytohormones regulating plant growth and developmental processes attributes plants protection by imparting tolerance to cope up with diverse changes in the environment (Ryu and Cho, 2015). The exogenous application of phytohormones supplementing the internal hormonal pool was found effective in counteracting the deleterious effects of salt stress (Zahir et al., 2010). The exogenous application of indole-3-acetic acid (IAA) was found effective in stimulating the growth of roots and leaves, thereby alleviating salinity-induced reduction in plant productivity (Albacete et al., 2008; Dodd and Perez-Alfocea, 2012). Diminishing the endogenous hormonal level, metabolites, hormones, and enzymes produced by salt-tolerant (ST) PGPR complements the hormonal status of plants and, as such, contributes to the enhancement of salt tolerance in plants grown under salt stress (Egamberdieva and Kucharova, 2009; Ilangumaran and Smith, 2017). A common trait of PGPR, production of IAA, was found to increase the fitness of plants grown under salinity stress (Dodd et al., 2010; Tiwari et al., 2011). Tryptophan in root exudates is utilized by rhizobacteria for its conversion through multiple routes to IAA for it to be readily absorbed by plant roots (Spaepen and Vanderleyden, 2011; Ilangumaran and Smith, 2017). Complementing the endogenous IAA pool of plants, its function in plants depends on the internal IAA levels (ranging in function from promotion to inhibition of plant growth). Required for cell division and elongation in plants, the inoculation of ST-PGPR P. putida modulated internal IAA pools that resulted in an increase in the growth parameters in cotton plants grown under salinity stress (Yao et al., 2010; Egamberdieva et al., 2017). The inoculation of P. stutzeri, P. putida, and Stenotrophomonas maltophilia to Coleus plants was found to lead the production of IAA, cytokinin, and gibberellic acid (Patel and Saraf, 2017). The short-term treatment of Enterobacter sp. EJ01 increased the expression of salt stress-responsive genes such as late embryogenesis abundant (RAB18), DRE-binding protein (DREB2b), stress-inducible priming process (MPK3 and MPK6), etc., genes in Arabidopsis thaliana, while increasing the ROS scavenging activity of Solanum lycopersicum grown under salinity stress (Ilangumaran and Smith, 2017). The inoculation of halotolerants was found to be associated with an increase in the secretion of salicylic acid that leads to an enhancement in the growth of sunflower plant (Tewari and Arora, 2018). The inoculation of Leclerciaa decarboxylata MO1 in Solanum lycopersicum showed an improvement in chlorophyll fluorescence besides increasing sugar synthesis and the production of organic acids (Kang et al., 2019).
Cytokinin (CK) is another important class of phytohormones that assists plants in growth and development and in attributing resistance to different stresses (O’Brien and Benková, 2013). Though a common trait of PGPRs, they suffice plants of CK by either synthesizing it or altering its homeostasis in plants (Dodd et al., 2010; Pallai et al., 2012; Kapoor and Kaur, 2016). The inoculation of Pseudomonas sp. (P. aurantiaca and P. extremorientalis TSAU6 and TSAU20) results in alleviating the salinity-induced dormancy of wheat seeds besides enhancing their growth under salinity stress conditions (Egamberdieva, 2009). The inoculation of B. subtilis strain in Platycladus orientalis and lettuce plant showed an enhanced root-to-shoot signaling of CK, thereby improving plant growth under stress conditions (Arkhipova et al., 2007; Liu et al., 2013). The ability of PGPRs to synthesize CK highlights their importance in stimulating plant growth.
Gibberellins (GA) constitute another important class of phytohormones that play an important role in regulating cell division and elongation and in regulating meristematic activity at the roots and the leaves as part of its role in the developmental and physiological processes of plants (Wang et al., 2015; Guo et al., 2015; Martínez et al., 2016). Bottini et al. reported the production of gibberellin by PGPR strains of B. licheniformis, B. pumilis, and Azospirillium spp. (Bottini et al., 2004). Being a key factor associated with the inhibition of plant growth under stress conditions, PGPRs were found to enhance its levels in plants, thereby attributing a tolerance mechanism to plants for growth under salinity stress (Kang et al., 2014a; Martínez et al., 2016; Shahzad et al., 2016). Kang et al. (2014a) reported enhancement in the internal GA pools on inoculating plants with B. cereus MJ-1 and Promicromospora sp. SE188. A similar effect of regulating plant growth and development was observed on inoculating plants with B. aryabhattai SRB02 (Park et al., 2017). The inoculation of P. aeruginosa PM389 and ZNP1 together with B. endophyticus J13 and B. tequilensis J12 results in the alleviation of the stress-induced effects in A. thaliana (Ghosh et al., 2019).
Abscisic acid is a stress hormone primarily known for its role in the abscission of leaves and growth of plants. Synthesized under water deficit conditions, it triggers an adaptive response via the activation of a set of genes responsible for stress resistance as part of its survival strategy for the plants (Pliego et al., 2011; Sah et al., 2016). Its synthesis in the roots that occurs in response to low water potential triggers the growth of roots and the emergence of lateral roots, contributing to the enhancement in the uptake of water at the root surface (Vaishnav et al., 2016b). Simultaneously, its translocation from roots to leaves progresses with the control of the stomatal closure events toward regulation of water loss by reducing transpiration at the leaf surface (Yamaguchi-Shinozaki and Shinozaki, 1994; Dodd and Perez-Alfocea, 2012; Kaushal and Wani, 2015). PGPRs capable of producing ABA play an important role in plant–PGPR interactions (Dodd, 2003; Naz et al., 2009; Dodd et al., 2010). They either modulate the biosynthesis of ABA or regulate ABA-mediated signaling pathways in plants, thereby contributing to the growth and survival of plants under salinity stress. The inoculation of PGPRs often mitigate the sensitivity of plants to water scarcity by decreasing its accumulation at the roots and significantly altering its long-distance signaling, i.e., shoot-to-root or vice versa flow through the phloem and the xylem, respectively (Dodd and Perez-Alfocea, 2012; Jiang et al., 2012; Belimov et al., 2014). The inoculation of Phyllobacterium brassicacearum STM196 results in an enhancement of the ABA levels that reduces transpiration at the leaf surface and, as such, enhances salt stress tolerance in A. thaliana (Bresson et al., 2013). A few species of PGPR (Rhodococcus sp. and Novosphingobium sp.) inhabiting rhizospheric regions capable of metabolizing ABA under in vitro conditions represent another stress-relieving mechanism for plants (Belimov et al., 2014). The inoculation of plants with ABA-producing PGPRs (P. fluorescence Rt6M10, A. brasilense SP245, and B. licheniformis Rt4M10) results in enhancement in internal ABA pools, thereby increasing plant growth under salinity stress conditions (Salomon et al., 2014; Cohen et al., 2015). A study reported that PGPR stimulated the production of endogenous ABA in plants, relieving them of the effects of being grown under salinity stress (Forni et al., 2017). Both ABA synthesizing and metabolizing PGPRs are capable of modulating the internal ABA status of plants and, as such, are capable of relieving plants to show normal growth even under salinity stress conditions.
Apart from ABA, the synthesis of another stress hormone, ethylene, was found to improve tolerance or expedite senescence (Morgan and Drew, 1997). Ethylene, a gaseous hormone, significantly enhances the response of plants to stress conditions. Acting as a negative regulator of plant growth, ethylene induces its effects by reducing the growth of roots and modulating the nitrogen-fixing capability of plants (Ma et al., 2002; Mahajan and Tuteja, 2005; Gamalero and Glick, 2015). As ethylene-mediated inhibition of the auxin response factor constraints the growth of plants, secretion of 1-aminocyclopropane-1-carboxylase (ACC) deaminase by PGPR hampers its synthesis in plants (Glick et al., 2007). ACC deaminase secretion by PGPR metabolizes ACC (precursor of ethylene in plants) into α-ketoglutarate and ammonia besides altering the expression of genes encoding ACC synthase and ACC oxidase, which are involved in the synthesis of ethylene (Etesami and Beattie, 2017). ACC deaminase-producing strains of P. fluorescens and Enterobacter spp. produced a significant effect in increasing the yield of maize grown under salt stress conditions (Nadeem et al., 2009; Panwar et al., 2016). The inoculation of Pantoea dispera PSB3 to chickpea results in an enhancement in IAA and ACC deaminase production, which led to an improvement in pod size, seed weight, seed number, and altogether plant biomass (Panwar et al., 2016). The plants were also observed to have a higher K+/Na+ ratio, owing to a reduction in electrolyte leakage and a decreased uptake of Na+ besides leading to an increase in leaf water and chlorophyll content and enhancement in K+ uptake.
Conclusion and Future Perspectives
Though much progress has been made in understanding the different attributes of plant–microbe interactions and in formulating methodologies for crops grown under salinity stress, we still lag behind in achieving sustainability in plant productivity. With rising emphasis on environmental protection and sustainability in agriculture for food security, the timely mitigation of the adverse effects of different stresses, in a cost-effective manner, is required. For this to be realized, it becomes imperative to explore novel aspects of the plant-beneficial soil microbiota in relieving plants of stressful conditions. Microbiota from diversified environments needs characterization and exploration in terms of their acclimatization, in-depth knowledge of their ameliorative strategies for growth under stress conditions, and in acquiring knowledge of the intriguing mechanisms commonly employed in attributing plants with a potential to thrive in harsh edaphic conditions. As the efficiency of the microbiota depends on soil characteristics and plant species, a better understanding of plant–microbial interactions in the context of manipulation of stress-responsive genes in plants need further elucidation in terms of revealing their functionalities toward boosting plant defense and attaining enhancement in overall productivity. As the soil microbiota provides beneficial attributes to plants in withstanding salinity stress, newer prospects of understanding in the operational module of regulatory network-mediated plant defense in achieving tolerance against different stresses need to be undertaken in a timely manner. The same goes in terms of prospects of developing novel bioinoculants that could enhance the stability of crops grown under stress conditions and, as such, increase their productivity when grown in nutritionally poor agroecosystems. In addition to the screening and the optimization of PGPR strains for plant-beneficial characteristics under changing environmental conditions, the CRISPR/Cas approach in editing interactive networks of stress-responsive genes needs to be undertaken for their profound effect (metabolic, regulatory, and signaling) in overcoming stress and inducing tolerance in plants and their interacting partners toward attaining sustainability in agriculture production.
Author Contributions
AJ and SR conceived the idea. All authors contributed equally in generating the draft of the different sections and in the repeated editing of the contents present in the finalized version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Khursheed Ahmad, Mr. Asif Iqbal Jan, and Mr. Zaid Qureshi for their timely suggestion and extending help in getting the manuscript finalized.
References
- Abdul-Jaleel C., Manivannan P., Sankar B., Kishorekumar A., Gopi R., Somasundaram R., et al. (2007). Water deficit stress mitigation by calcium chloride in Catharanthus roseus: effects onoxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf. B 60 110–116. 10.1016/j.colsurfb.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Acosta-Motos J., Ortuño M., Bernal-Vicente A., Diaz-Vivancos P., Sanchez-Blanco M., Hernandez J. (2017). Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18 10.3390/agronomy7010018 [DOI] [Google Scholar]
- Ahmed E., Holmstrom S. J. (2014). Siderophores in environmental research: roles and applications. Microb. Biotechnol. 7 196–208. 10.1111/1751-7915.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M. J., Ullah S., Ahmad I., Rauf A., Mahmood Nadeem S., Yahya Khan M., et al. (2018). Nickel phytoextraction through bacterial inoculation in Raphanus sativus. Chemosphere 190 234–242. 10.1016/j.chemosphere.2017.09.136 [DOI] [PubMed] [Google Scholar]
- Akhtar S. S., Amby D. B., Hegelund J. N., Fimognari L., Großkinsky D. K., Westergaard J. C., et al. (2020). Bacillus licheniformis FMCH001 Increases Water Use Efficiency via Growth Stimulation in Both Normal and Drought Conditions. Front. Plant Sci. 11:297. 10.3389/fpls.2020.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram M. S., Shahid M., Tariq M., Azeem M., Javed M. T., Saleem S., et al. (2016). Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.). Front. Microbiol. 7:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albacete A., Ghanem M. E., Martínez-Andújar C., Acosta M., Sánchez-Bravo J., Martínez V., et al. (2008). Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 59 4119–4131. 10.1093/jxb/ern251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Maggio A., Bressan R. A., Yun D. J. (2019). Role and Functional Differences of HKT1-Type Transporters in Plants under Salt Stress. Int. J. Mol. Sci. 20:1059. 10.3390/ijms20051059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Hameed S., Shahid M., Iqbal M., Lazarovits G., Imran A. (2020). Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 232:126389. 10.1016/j.micres.2019.126389 [DOI] [PubMed] [Google Scholar]
- Álvarez S., Gómez-Bellot M. J., Castillo M., Bañón S., Sánchez-Blanco M. J. (2012). Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants. Environ. Exp. Bot. 78 138–145. 10.1016/j.envexpbot.2011.12.035 [DOI] [Google Scholar]
- Amna U. D., Din B., Sarfraz S., Xia Y., Aqeel Kamran M., Javed M. T., et al. (2019). Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC- deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 183:109466. 10.1016/j.ecoenv.2019.109466 [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Arkhipova T. N., Prinsen E., Veselov S. U., Martinenko E. V., Melentiev A. I., Kudoyarova G. R. (2007). Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 292 305–315. 10.1007/s11104-007-9233-5 [DOI] [Google Scholar]
- Arora N. K., Tewari S., Singh S., Lal N., Maheshwari D. K. (2012). “PGPR for protection of plant health under saline conditions,” in Bacteria in Agrobiology: Stress Management, ed. Maheshwari D. (Berlin: Springer), 239–258. 10.1007/978-3-662-45795-5_12 [DOI] [Google Scholar]
- Ashraf M. (2004). Some important physiological selection criteria for salt tolerance in plants. Flora 199 361–376. 10.1078/0367-2530-00165 [DOI] [Google Scholar]
- Ashraf M., Hasnain S., Berge O., Mahmood T. (2004). Inoculating wheat seedlings with exopolysaccharide- producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 40 157–162. [Google Scholar]
- Aslam F., Ali B. (2018). Halotolerant bacterial diversity associated with Suaeda fruticosa (L.) forssk. improved growth of maize under salinity stress. Agronomy 8:131 10.3390/agronomy8080131 [DOI] [Google Scholar]
- Atouei M. T., Pourbabaee A. A., Shorafa M. (2019). Alleviation of salinity stress on some growth parameters of wheat by exopolysaccharide-producing bacteria. Iran. J. Sci. Technol. Trans. A Sci. 43 2725–2733. 10.1007/s40995-019-00753-x [DOI] [Google Scholar]
- Aw X., Li Z., Wc L., Zh Y. (2019). The effect of plant growth-promoting rhizobacteria (PGPR) on arsenic accumulation and the growth of rice plants (Oryza sativa L.). Chemosphere 242:125136. 10.1016/j.chemosphere.2019.125136 [DOI] [PubMed] [Google Scholar]
- Azza Mazher A. M., Fatma El-Quesni E. M., Farahat M. M. (2007). Responses of ornamental plants and woody trees to salinity. World J. Agric. Sci. 3 386–395. [Google Scholar]
- Bach E., Seger G. D. S., Fernandes G. C., Lisboa B. B., Passaglia L. M. P. (2016). Evaluation of biological control and rhizosphere competence of plant growth promoting bacteria. Appl. Soil Ecol. 99 141–149. 10.1016/j.apsoil.2015.11.002 [DOI] [Google Scholar]
- Bacilio M., Rodriguez H., Moreno M., Hernandez J. P., Bashan Y. (2004). Mitigation of salt stress in wheat seedling by a gfp-tagged Azospirillum lipoferum. Biol. Fertil. Soils 40 188–193. [Google Scholar]
- Backer R., Rokem J. S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., et al. (2018). Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 9:1473. 10.3389/fpls.2018.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshandeh E., Gholamhosseini M., Yaghoubian Y., Pirdashti H. (2020). Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 90 123–136. 10.1007/s10725-019-00556-5 [DOI] [Google Scholar]
- Bal H. B., Nayak L., Das S., Adhya T. K. (2013). Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 366 93–105. 10.1007/s11104-012-1402-5 [DOI] [Google Scholar]
- Balsanelli E., de Baura V. A., Pedrosa F., de Souza E. M., Monteiro R. A. (2014). Exopolysaccharide biosynthesis enables mature biofilm formation on abiotic surfaces by Herbaspirillum seropedicae. PLoS One 9:e110392. 10.1371/journal.pone.0110392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano A., Fatima M. (2009). Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 45, 405–413. 10.1007/s00374-008-0344-9 [DOI] [Google Scholar]
- Baral A., Shruthi K. S., Mathew M. K. (2015). Vesicular trafficking and salinity responses in plants. IUBMB Life 67 677–686. 10.1002/iub.1425 [DOI] [PubMed] [Google Scholar]
- Barnawal D., Bharti N., Maji D., Chanotiya C. S., Kalra A. (2014). ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 171 884–894. 10.1016/j.jplph.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Bashan Y., de-Bashan L. E. (2015). “Inoculant preparation and formulations for azospirillum spp,” in Handbook for Azospirillum, eds Cassán F. D., Okon Y., Creus C. M. (Berlin: Springer; ), 469–485. 10.1007/978-3-319-06542-7_26 [DOI] [Google Scholar]
- Beattie G. A. (2015). Microbiomes: curating communities from plants. Nature 528 340–341. 10.1038/nature16319 [DOI] [PubMed] [Google Scholar]
- Belimov A. A., Dodd I. C., Safronova V. I., Dumova V. A., Shaposhnikov A. I., Ladatko A. G., et al. (2014). Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 74 84–91. 10.1016/j.plaphy.2013.10.032 [DOI] [PubMed] [Google Scholar]
- Bell C. W., Asao S., Calderon F., Wolk B., Wallenstein M. D. (2015). Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biol. Biochem. 85 170–182. 10.1016/j.soilbio.2015.03.006 [DOI] [Google Scholar]
- Berendsen R. L., Pieterse C. M. J., Bakker P. A. H. M. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci. 17 478–486. 10.1016/j.tplants.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Besri M. (1993). “Effects of salinity on plant diseases development,” in Towards the Rational use of High Salinity Tolerant Plants, eds Lieth H., Al Masoom A. A. (Dordrecht: Springer; ), 67–74. 10.1007/978-94-011-1860-6_8 [DOI] [Google Scholar]
- Bharti N., Pandey S. S., Barnawal D., Patel V. K., Kalra A. (2016). Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 6:34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar-Mathur P., Devi M. J., Reddy D. S., Lavanya M., Vadez V., Serraj R., et al. (2007). Stress inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water limiting conditions. Plant Cell Rep. 26 2071–2082. 10.1007/s00299-007-0406-8 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya D., Yu S. M., Lee Y. H. (2015). Volatile compounds from Alcaligenes faecalis JBCS1294 confer salt tolerance in Arabidopsis thaliana through the auxin and gibberellin pathways and differential modulation of gene expression in root and shoot tissues. Plant Growth Regul. 75 297–306. 10.1007/s10725-014-9953-5 [DOI] [Google Scholar]
- Bhattacharyya P. N., Jha D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28 1327–1350. 10.1007/s11274-011-0979-9 [DOI] [PubMed] [Google Scholar]
- Bisht N., Mishra S. K., Chauhan P. S. (2019). Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 143 937–951. 10.1016/j.ijbiomac.2019.09.154 [DOI] [PubMed] [Google Scholar]
- Bottini R., Cassán F., Piccoli P. (2004). Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 65 497–503. [DOI] [PubMed] [Google Scholar]
- Bresson J., Varoquaux F., Bontpart T., Touraine B., Vile D. (2013). P.G.P.R. The strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 200 558–569. 10.1111/nph.12383 [DOI] [PubMed] [Google Scholar]
- Bruning B., Rozema J. (2013). Symbiotic nitrogen fixation in legumes: Perspectives for saline agriculture. Environ. Exp. Bot. 92 134–143. 10.1016/j.envexpbot.2012.09.001 [DOI] [Google Scholar]
- Cardinale M., Ratering S., Suarez C., Zapata Montoya A. M., Geissler-Plaum R., Schnell S. (2015). Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 181 22–32. 10.1016/j.micres.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Cassaniti C., Leonardi C., Flowers T. J. (2009). The effects of sodium chloride ornamental shrubs. Sci. Hortic. 122 586–593. 10.1016/j.scienta.2009.06.032 [DOI] [Google Scholar]
- Cassaniti C., Romano D., Flowers T. J. (2012). “The response of ornamental plants to saline irrigation water,” in Irrigation Water Management, Pollution and Alternative Strategies, ed. Garcia-Garizabal I. (Rijeka: InTech; ), 132–158. [Google Scholar]
- Chandra D., Srivastava R., Gupta V. V. S. R., Franco C. M. M., Sharma A. K. (2019). Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can. J. Microbiol. 65 387–403. 10.1139/cjm-2018-0636 [DOI] [PubMed] [Google Scholar]
- Chartzoulakis K., Loupassaki M., Bertaki M., Androulakis I. (2002). Effects of NaCl salinity on growth, ion contentand CO2 assimilation rate of six olive cultivars. Sci. Hortic. 96 235–247. 10.1016/s0304-4238(02)00067-5 [DOI] [Google Scholar]
- Chavarria G., dos Santos H. P. (2012). Plant water relations: absorption, transport and control mechanisms. InTechOpen 5 105–132. [Google Scholar]
- Chaves M. M., Flexas J., Pinheiro C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103 551–560. 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu Y., Wu G., Veronican Njeri K., Shen Q., Zhang N., et al. (2016). Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Plant Physiol. 158 34–44. 10.1111/ppl.12441 [DOI] [PubMed] [Google Scholar]
- Cho S., Kang B., Han S., Anderson A., Park J.-Y., Lee Y.-H., et al. (2008). 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 21 1067–1075. 10.1094/mpmi-21-8-1067 [DOI] [PubMed] [Google Scholar]
- Choudhary D., Johri B., Prakash A. (2008). Volatiles as priming agents that initiate plant growth and defence responses. Curr. Sci. 94 595–604. [Google Scholar]
- Chu T. N., Tran B. T. H., Van Bui L., Hoang M. T. T. (2019). Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes 12:11. 10.1186/s13104-019-4046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. C., Bottini R., Pontin M., Berli F. J., Moreno D., Boccanlandro H., et al. (2015). Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 153 79–90. 10.1111/ppl.12221 [DOI] [PubMed] [Google Scholar]
- Colmer T. D., Muñi̇z R., Flowers T. J. (2005). Improving salt tolerance of wheat and barley: future prospects. Aust. J. Exp. Agric. 45 1425–1443. [Google Scholar]
- Compant S., Clément C., Sessitsch A. (2010). Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42 669–678. 10.1016/j.soilbio.2009.11.024 [DOI] [Google Scholar]
- Couillerot O., Prigent-Combaret C., Caballero-Mellado J., Moënne-Loccoz Y. (2009). Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 48 505–512. 10.1111/j.1472-765x.2009.02566.x [DOI] [PubMed] [Google Scholar]
- Creus C. M., Sueldo R. J., Barassi C. A. (2004). Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can. J. Bot. 82 273–281. 10.1139/b03-119 [DOI] [Google Scholar]
- Damodaran T., Sah V., Rai R., Sharma D., Mishra V., Jha S., et al. (2013). Isolation of salt tolerant endophytic and rhizospheric bacteria by natural selection and screening for promising plant growth-promoting rhizobacteria (PGPR) and growth vigour in tomato under sodic environment. Afr. J. Microbiol. Res. 7 5082–5089. [Google Scholar]
- Danish S., Zafar-ul-Hye M., Mohsin F., Hussain M. (2020). ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS One 15:e0230615. 10.1371/journal.pone.0230615 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dardanelli M. S., Fernández de Córdoba F. J., Espuny M. R., Rodríguez Carvajal M. A., Soria Díaz M. E., Gil Serrano A. M., et al. (2008). Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 40 2713–2721. 10.1016/j.soilbio.2008.06.016 [DOI] [Google Scholar]
- Davenport R., James R. A., Zakrisson-Plogander A., Tester M., Munns R. (2005). Control of sodium transport in durum wheat. Plant Physiol. 137 807–818. 10.1104/pp.104.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña T. C., Pueyo J. J. (2012). Legumes in the reclamation of marginal soils, from cultivar and inoculant selection to transgenic approaches. Agron. Sustain. Dev. 32 65–91. 10.1007/s13593-011-0024-2 [DOI] [Google Scholar]
- Dixit V. K., Misra S., Mishra S. K., Tewari S. K., Joshi N., Chauhan P. S. (2020). Characterization of plant growth-promoting alkalotolerant Alcaligenes and Bacillus strains for mitigating the alkaline stress in Zea mays. Antonie Van Leeuwenhoek 113 889–905. 10.1007/s10482-020-01399-1 [DOI] [PubMed] [Google Scholar]
- Dodd I. C. (2003). Hormonal interactions and stomatal responses. J. Plant Growth Regul. 22 32–46. 10.1007/s00344-003-0023-x [DOI] [Google Scholar]
- Dodd I. C., Perez-Alfocea F. (2012). Microbial amelioration of crop salinity stress. J. Exp. Bot. 63 3415–3428. 10.1093/jxb/ers033 [DOI] [PubMed] [Google Scholar]
- Dodd I. C., Zinovkina N. Y., Safronova V. I., Belimov A. A. (2010). Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 157 361–379. 10.1111/j.1744-7348.2010.00439.x [DOI] [Google Scholar]
- Dubey R. C., Khare S., Kumar P., Maheshwari D. K. (2014). Combined effect of chemical fertilisers and rhizosphere-competent Bacillus subtilis BSK17 on yield of Cicer arietinum. Arch. Phytopathol. Plant Protect. 47 2305–2318. 10.1080/03235408.2013.876744 [DOI] [Google Scholar]
- Dunne C., Crowley J. J., Moenne-Loccoz Y., Dowling D. N., de Bruijn F. J., O’Gara F. (1997). Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology 143 3921–3931. 10.1099/00221287-143-12-3921 [DOI] [PubMed] [Google Scholar]
- Dutta S., Khurana S. M. P. (2015). “Plant growth-promoting rhizobacteria for alleviating abiotic stresses in medicinal plants,” in Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants, eds Egamberdieva D., Shrivastava S., Varma A. (Berlin: Springer; ), 167–200. 10.1007/978-3-319-13401-7_8 [DOI] [Google Scholar]
- Egamberdieva D. (2009). Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 31 861–864. 10.1007/s11738-009-0297-0 [DOI] [Google Scholar]
- Egamberdieva D. (2011). Survival of Pseudomonas extremorientalis TSAU20 and P. chlororaphis TSAU13 in the rhizosphere of common bean (Phaseolus vulgaris) under saline conditions. Plant Soil Environ. 57 122–127. 10.17221/316/2010-pse [DOI] [Google Scholar]
- Egamberdieva D., Kucharova Z. (2009). Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils 45 561–573. [Google Scholar]
- Egamberdieva D., Wirth S., Bellingrath-Kimura S. D., Mishra J., Arora N. K. (2019). Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 10:2791. 10.3389/fmicb.2019.02791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D., Wirth S., Jabborova D., Räsänen L. A., Liao H. (2017). Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact. 12 100–107. 10.1080/17429145.2017.1294212 [DOI] [Google Scholar]
- El-Esawi M. A., Alaraidh I. A., Alsahli A. A., Alamri S. A., Ali H. M., Alayafi A. A. (2018a). Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 132 375–384. 10.1016/j.plaphy.2018.09.026 [DOI] [PubMed] [Google Scholar]
- El-Esawi M. A., Alaraidh I. A., Alsahli A. A., Alzahrani S. A., Ali H. M., Alayafi A. A., et al. (2018b). Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 19:3310. 10.3390/ijms19113310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Esawi M. A., Al-Ghamdi A. A., Ali H. M., Alayafi A. A. (2019). Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress related genes expression. Environ. Exp. Bot. 159 55–65. 10.1016/j.envexpbot.2018.12.001 [DOI] [Google Scholar]
- Epstein E. (1998). How calcium enhances plant salt tolerance. Science 280 1906–1907. 10.1126/science.280.5371.1906 [DOI] [PubMed] [Google Scholar]
- Etesami H. (2018). Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric. Ecosyst. Environ. 253 98–112. 10.1016/j.agee.2017.11.007 [DOI] [Google Scholar]
- Etesami H., Alikhani H. A. (2018). Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Eur. J. Plant Pathol. 150 497–506. 10.1007/s10658-017-1276-8 [DOI] [Google Scholar]
- Etesami H., Beattie G. A. (2017). “Plant-microbe interactions in adaptation of agricultural crops to abiotic stress conditions,” in Probiotics and Plant Health, eds Kumar V., et al. (Berlin: Springer; ), 163–200. 10.1007/978-981-10-3473-2_7 [DOI] [Google Scholar]
- Etesami H., Beattie G. A. (2018). Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 9:148. 10.3389/fmicb.2018.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H., Mirsyed Hosseini H., Alikhani H. A., Mohammadi L. (2014). Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J. Plant Growth Regul. 33, 654–670. 10.1007/s00344-014-9415-3 [DOI] [Google Scholar]
- Forni C., Duca D., Glick B. R. (2017). Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 410 335–356. 10.1007/s11104-016-3007-x [DOI] [Google Scholar]
- Franco J. A., Bañón S., Vicente M. J., Miralles J., Martínez-Sánchez J. J. (2011). Root development in horticultural plants grown under abiotic stress conditions-A review. J. Hortic. Sci. Biotechnol. 86 543–556. 10.1080/14620316.2011.11512802 [DOI] [Google Scholar]
- Fukami J., de la Osa C., Ollero F. J., Megías M., Hungria M. (2018). Coinoculation of maize with Azospirillum brasilense and Rhizobium tropici as a strategy to mitigate salinity stress. Funct. Plant Biol. 45 328–339. [DOI] [PubMed] [Google Scholar]
- Gamalero E., Glick B. R. (2015). Bacterial modulation of plant ethylene levels. Plant Physiol. 169 13–22. 10.1104/pp.15.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Gupta A., Mohapatra S. (2019). A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. Biotechnol. 35:90. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Sen S., Mohapatra S. (2018). Drought-mitigating Pseudomonas putida GAP-P45 modulates proline turnover and oxidative status in Arabidopsis thaliana under water stress. Ann. Microbiol. 68 579–594. 10.1007/s13213-018-1366-7 [DOI] [Google Scholar]
- Giongo A., Ambrosini A., Vargas L. K., Freire J. R. J., Bodanese-Zanettini M. H., Passaglia L. M. P. (2008). Evaluation of genetic diversity of bradyrhizobia strains nodulating soybean [Glycine max (L.) Merrill] isolated from South Brazilian fields. Appl. Soil Ecol. 38 261–269. 10.1016/j.apsoil.2007.10.016 [DOI] [Google Scholar]
- Giri B., Kapoor R., Mukerji K. G. (2003). Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 38 170–175. 10.1007/s00374-003-0636-z [DOI] [Google Scholar]
- Glick B. R. (2014). Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169 30–39. 10.1016/j.micres.2013.09.009 [DOI] [PubMed] [Google Scholar]
- Glick B. R., Cheng Z., Czarny J., Duan J. (2007). Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 119 329–339. 10.1007/978-1-4020-6776-1_8 [DOI] [Google Scholar]
- Godfray H. C. J., Beddington J. R., Crute I. R., Haddad L., Lawrence D., Muir J. F., et al. (2010). Food security: the challenge of feeding 9 billion people. Science 327 812–818. 10.1126/science.1185383 [DOI] [PubMed] [Google Scholar]
- Gohel V., Chaudhary T., Vyas P., Chhatpar H. S. (2004). Isolation and identification of marine chitinolytic bacteria and their potential in antifungal biocontrol. Indian J. Exp. Biol. 42 715–720. [PubMed] [Google Scholar]
- Gómez-Bellot M. J., Álvarez S., Castillo M., Bañón S., Ortuño M. F., Sánchez-Blanco M. J. (2013). Water relations, nutrient content and developmental responses of Euonymus plants irrigated with water of different degrees of salinity and quality. J. Plant Res. 126 567–576. 10.1007/s10265-012-0545-z [DOI] [PubMed] [Google Scholar]
- Gond S. K., Torres M. S., Bergen M. S., Helsel Z., White J. F., Jr. (2015). Induction of salt tolerance and up-regulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans. Lett. Appl. Microbiol. 60 392–399. 10.1111/lam.12385 [DOI] [PubMed] [Google Scholar]
- Gowtham H. G., Brijesh S., Mahadevamurthy M., Aiyaz M., Nagaraj Amruthesh K. (2020). Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 234:126422. 10.1016/j.micres.2020.126422 [DOI] [PubMed] [Google Scholar]
- Grover M., Ali S. Z., Sandhya V., Rasul A., Venkateswarlu B. (2011). Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 27 1231–1240. 10.1007/s11274-010-0572-7 [DOI] [Google Scholar]
- Guo H., Wang Y., Liu H., Hu P., Jia Y., Zhang C., et al. (2015). Exogenous GA3 application enhances xylem development and induces the expression of secondary wall biosynthesis related genes in Betula platyphylla. Int. J. Mol. Sci. 16 22960–22975. 10.3390/ijms160922960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Pandey S. (2019). ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 10:1506. 10.3389/fmicb.2019.01506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururani M. A., Upadhyaya C. P., Strasser R. J., Yu J. W., Park S. W. (2013). Evaluation of abiotic stress tolerance in transgenic potato plants with reduced expression of PSII manganese stabilizing protein. Plant Sci. 198 7–16. 10.1016/j.plantsci.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Gusain Y. S., Singh U. S., Sharma A. K. (2015). Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr. J. Biotechnol. 14 764–773. 10.5897/ajb2015.14405 [DOI] [Google Scholar]
- Habib S. H., Kausar H., Saud H. M., Ismail M. R., Othman R. (2016). Molecular characterization of stress tolerant plant growth promoting rhizobacteria (PGPR) for growth enhancement of rice. Int. J. Agric. Biol. 18 184–191. 10.17957/ijab/15.0094 29653435 [DOI] [Google Scholar]
- Halfter U., Ishitani M., Zhu J. K. (2000). The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. U.S.A. 97 3730–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halo B. A., Khan A. L., Waqas M., Al-Harrasi A., Hussain J., Ali L., et al. (2015). Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J. Plant Interact. 10 117–125. 10.1080/17429145.2015.1033659 [DOI] [Google Scholar]
- Hamdia M. A. E. S., Shaddad M. A. K., Doaa M. M. (2004). Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 44 165–174. 10.1023/b:grow.0000049414.03099.9b [DOI] [Google Scholar]
- Hamilton C. E., Bever J. D., Labbé J., Yang X. H., Yin H. F. (2016). Mitigating climate change through managing constructed microbial communities in agriculture. Agric. Ecosyst. Environ. 216 304–308. 10.1016/j.agee.2015.10.006 [DOI] [Google Scholar]
- Han H. S., Lee K. D. (2005). Physiological responses of soybean-inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res. J. Agric. Biol. Sci. 1 216–221. [Google Scholar]
- Hariprasad P., Divakara S. T., Niranjana S. R. (2011). Isolation and characterization of chitinolytic rhizobacteria for the management of Fusarium wilt in tomato. Crop Prot. 30 1606–1612. 10.1016/j.cropro.2011.02.032 [DOI] [Google Scholar]
- Hasegawa P. M., Bressan R. A., Zhu J. K., Bhnert H. J. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51 463–499. [DOI] [PubMed] [Google Scholar]
- Hashem A., Abd Allah E. F., Alqarawi A. A., Aldubise A., Egamberdieva D. (2015). Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 10 230–242. 10.1080/17429145.2015.1052025 [DOI] [Google Scholar]
- Heidari M., Golpayegani A. (2011). Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 11 57–61. 10.1016/j.jssas.2011.09.001 [DOI] [Google Scholar]
- Hong S. H., Lee E. Y. (2017). Phytostabilization of salt accumulated soil using plant and biofertilizers: field application. Int. Biodeterior. Biodegraf. 124 188–195. 10.1016/j.ibiod.2017.05.001 [DOI] [Google Scholar]
- Hussein K. A., Joo J. H. (2018). Plant growth-promoting rhizobacteria improved salinity tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 28 938–945. 10.4014/jmb.1712.12027 [DOI] [PubMed] [Google Scholar]
- Husson E., Hadad C., Huet G., Laclef S., Lesur D., Lambertyn V., et al. (2017). The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies. Green Chem. 19 4122–4131. 10.1039/c7gc01471f [DOI] [Google Scholar]
- Ilangumaran G., Smith D. L. (2017). Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front. Plant Sci. 8:1768. 10.3389/fpls.2017.01768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam F., Yasmeen T., Arif M. S., Ali S., Ali B., Hameed S., et al. (2016). Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 80 23–36. 10.1007/s10725-015-0142-y [DOI] [Google Scholar]
- Jacoby R. P., Che-Othman M. H., Millar A. H., Taylor N. L. (2016). Analysis of the sodium chloride-dependent respiratory kinetics of wheat mitochondria reveals differential effects on phosphorylating and non-phosphorylating electron transport pathways. Plant Cell Environ. 39 823–833. 10.1111/pce.12653 [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Takabe T. (2001). Inducers of glycinebetaine synthesis in barley. Plant Physiol. 127 1827–1835. 10.1104/pp.010392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan A. T., Azam M., Ali A., Haq Q. M. R. (2011). Novel approaches of beneficial Pseudomonas in mitigation of plant disease: an appraisal. J. Plant Interact. 6 195–205. 10.1080/17429145.2010.541944 [DOI] [Google Scholar]
- Jha Y., Subramanian R. B. (2013). Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline condition. Chil. J. Agric. Res. 73 213–219. 10.4067/s0718-58392013000300002 27315006 [DOI] [Google Scholar]
- Jiang F., Chen L., Belimov A. A., Shaposhnikov A. I., Gong F., Meng X., et al. (2012). Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum. J. Exp. Bot. 63 6421–6430. 10.1093/jxb/ers301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez J. A., Novinscak A., Filion M. (2020). Pseudomonas fluorescens LBUM677 differentially increases plant biomass, total oil content and lipid composition in three oilseed crops. J. Appl. Microbiol. 128 1119–1127. 10.1111/jam.14536 [DOI] [PubMed] [Google Scholar]
- Joshi R., Mangu V. R., Bedre R., Sanchez L., Pilcher W., Zandkarimi H., et al. (2015). “Salt adaptation mechanisms of halophytes: improvement of salt tolerance in crop plants,” in Elucidation of Abiotic Stress Signaling in Plants, ed. Pandey G. I. K. I. (New York, NY: Springer; ), 243–279. 10.1007/978-1-4939-2540-7_9 [DOI] [Google Scholar]
- Jung W. J., An K. N., Jin Y. L., Park R. D., Lim K. T., Kim K. Y., et al. (2003). Biological control of damping off caused by Rhizoctonia solani using chitinase producing Paenibacillus illinoisensis KJA-424. Soil Biol. Biochem. 35 1261–1264. 10.1016/s0038-0717(03)00187-1 [DOI] [Google Scholar]
- Jung W. J., Jin Y. L., Kim K. Y., Park R. D., Kim T. H. (2005). Changes in pathogenesis-related proteins in pepper plants with regard to biological control of phytopthora blight with Paenibacillus illinoisensis. Biocontrol 50 165–178. 10.1007/s10526-004-0451-y [DOI] [Google Scholar]
- Kang S. M., Khan A. L., Waqas M., You Y. H., Kim J. H., Kim J. G., et al. (2014a). Plant growth promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant. Interact. 9 673–682. 10.1080/17429145.2014.894587 [DOI] [Google Scholar]
- Kang S. M., Radhakrishnan R., Khan A. L., Kim M. J., Park J. M., Kim B. R., et al. (2014b). Gibberellin secreting rhizobacterium, Pseudomonas putida H-2- 3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 84 115–124. 10.1016/j.plaphy.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Kang S. M., Shahzad R., Bilal S., Khan L., Park Y.-G., Lee K.-E., et al. (2019). Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 19:80. 10.1186/s12866-019-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R., Kaur M. (2016). Cytokinins production by fluorescent Pseudomonas isolated from rhizospheric soils of Malus and Pyrus. Afr. J. Microbiol. Res. 10 1274–1279. 10.5897/ajmr2016.8211 [DOI] [Google Scholar]
- Katembe W. J., Ungar I. A., Mitchell J. P. (1998). Effect of Salinity on Germination and Seedling Growth of two Atriplex species (Chenopodiaceae). Ann. Bot. 82 167–175. 10.1006/anbo.1998.0663 [DOI] [Google Scholar]
- Katerji N., van Hoorn J. W., Mastrorilli M., Hamdy A. (2005). “Crop sensitivity to salinity,” in Non-ConventionalWater Use: WASAMED Project, eds Hamdy A., ElGamal F., Lamaddalena N., Bogliotti C., Guelloubi R. (Bari: CIHEAM/EU DG Research; ), 43–51. [Google Scholar]
- Kaushal M., Wani S. P. (2015). Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 66 35–42. 10.1007/s13213-015-1112-3 [DOI] [Google Scholar]
- Kaushal M., Wani S. P. (2016). Rhizobacterial-plant interactions: strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 231 68–78. 10.1016/j.agee.2016.06.031 [DOI] [Google Scholar]
- Kaymakanova M. (2009). Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.). Biotechnol. Biotechnol. Equip. 23 326–329. 10.1080/13102818.2009.10818430 [DOI] [Google Scholar]
- Khan A., Zhao X. Q., Javed M. T., Khan K. S., Bano A., Shen R. F., et al. (2016). Bacillus pumilus enhances tolerance in rice (Oryza sativa L.) to combined stresses of NaCl and high boron due to limited uptake of Na+. Environ. Exp. Bot. 124 120–129. 10.1016/j.envexpbot.2015.12.011 [DOI] [Google Scholar]
- Khan M. A., Asaf S., Khan A. L., Arjun A., Rahmatullah J., Sajid A., et al. (2019). Halotolerant Rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. Biomed Res. Int. 2019:9530963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Bano A. (2019). Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS One 14:e0222302. 10.1371/journal.pone.0222302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Jang Y. J., Lee S. M., Oh B. T., Chae J. C., Lee K. J. (2014). Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by upregulation of conserved salinity responsive factors in plants. Mol. Cells 37:109. 10.14348/molcells.2014.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Dubey R. C., Maheshwari D. K. (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 167 493–499. 10.1016/j.micres.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Kumawat K. C., Sharma P., Singh I., Sirari A., Gill B. S. (2019). Co-existence of Leclercia adecarboxylata (LSE-1) and Bradyrhizobium sp. (LSBR-3) in nodule niche for multifaceted effects and profitability in soybean production. World J. Microbiol. Biotechnol. 35:172. [DOI] [PubMed] [Google Scholar]
- Kurepin L. V., Park M. J., Lazarovits G., Bernards M. A. (2015). Burkholderia phytormans-induced shoot and root growth promotion is associated with endogenous changes in plant growth hormone levels. Plant Growth Regul. 75 199–207. 10.1007/s10725-014-9944-6 [DOI] [Google Scholar]
- Lastochkina O., Pusenkova L., Yuldashev R., Babaev M., Garipova S., Blagova D., et al. (2017). Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 121 80–88. 10.1016/j.plaphy.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Ledger T., Rojas S., Timmermann T., Pinedo I., Poupin M. J., Garrido T., et al. (2016). Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 7:1838. 10.3389/fmicb.2016.01838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Farag M. A., Park H. B., Kloepper W. J., Lee S. H., Ryu C. M. (2012). Induced resistance by a long-chain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS One 7:e48744. 10.1371/journal.pone.0048744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Q., Jiang X. W. (2017). Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ. J. Plant Physiol. 64 235–241. 10.1134/s1021443717020078 [DOI] [Google Scholar]
- Li X., Peng D., Zhang Y., Ju D., Guan C. (2020). Klebsiella sp. PD3, a phenanthrene (PHE)-degrading strain with plant growth promoting properties enhances the PHE degradation and stress tolerance in rice plants. Ecotoxicol. Environ. Saf. 201:110804. 10.1016/j.ecoenv.2020.110804 [DOI] [PubMed] [Google Scholar]
- Li Y., Zeng J., Wang S., Lin Q., Ruan D., Chi H., et al. (2020). Effects of cadmium-resistant plant growth-promoting rhizobacteria and Funneliformis mosseae on the cadmium tolerance of tomato (Lycopersicon esculentum L.). Int. J. Phytoremediat. 22 451–458. 10.1080/15226514.2019.1671796 [DOI] [PubMed] [Google Scholar]
- Liu F., Xing S., Ma H., Du Z., Ma B. (2013). Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 97 9155–9164. 10.1007/s00253-013-5193-2 [DOI] [PubMed] [Google Scholar]
- Lugtenberg B. J., Malfanova N., Kamilova F., Berg G. (2013). Plant growth promotion by microbes. Mol. Microb. Ecol. Rhizosphere 1-2, 559–573. [Google Scholar]
- Lycoskoufs I. H., Savvas D., Mavrogianopoulos G. (2005). Growth, gas exchange, and nutrient status in pepper (Capsicum annuum L.) grown in recirculating nutrient solution as affected by salinity imposed to half of the root system. Sci. Hortic. 106 147–161. 10.1016/j.scienta.2005.02.022 [DOI] [Google Scholar]
- Ma W., Penrose D. M., Glick B. R. (2002). Strategies used by rhizobia to lower plant ethylene levels and increase nodulation. Can. J. Microbiol. 48 947–954. 10.1139/w02-100 [DOI] [PubMed] [Google Scholar]
- Mabood F., Zhou X., Smith D. L. (2014). Microbial signaling and plant growth promotion. Can. J. Plant Sci. 94 1051–1063. 10.4141/cjps2013-148 [DOI] [Google Scholar]
- Mahajan S., Tuteja N. (2005). Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 444 139–158. 10.1016/j.abb.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Mäkelä P., Kärkkäinen J., Somersalo S. (2000). Effect of glycinebetaine on chloroplast ultrastructure, chlorophyll and protein content, and Rubisco activities in tomato grown under drought or salinity. Biol. Plant 43 471–475. 10.1023/a:1026712426180 [DOI] [Google Scholar]
- Marasco R., Rolli E., Vigani G., Borin S., Sorlini C., Ouzari H., et al. (2013). Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 8:e26741. 10.4161/psb.26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C., Espinosa-Ruiz A., Prat S. (2016). Gibberellins and plant vegetative growth. Annu. Plant Rev. 49 285–322. 10.1002/9781119210436.ch10 [DOI] [Google Scholar]
- Marulanda A., Azcon R., Chaumont F., Ruiz-Lozano J. M., Aroca R. (2010). Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232 533–543. 10.1007/s00425-010-1196-8 [DOI] [PubMed] [Google Scholar]
- Mayak S., Tirosh T., Glick B. R. (2004). Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 42 565–572. 10.1016/j.plaphy.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Meloni D. A., Gulotta M. R., Martínez C. A. (2008). Salinity tolerance in Schinopsis quebracho colorado: seed germination, growth, ion relations and metabolic responses. J. Arid. Environ. 72 1785–1792. 10.1016/j.jaridenv.2008.05.003 [DOI] [Google Scholar]
- Miller G., Suzuki N., Ciftci-Yilmaz S., Mittler R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Khan M. H., Misra S., Dixit V. K., Gupta S., Sateesh S. T., et al. (2020). Drought tolerant Ochrobactrum sp. inoculation performs multiple roles in maintaining the homeostasis in Zea mays L. subjected to deficit water stress. Plant Physiol. Biochem. 150 1–14. 10.1016/j.plaphy.2020.02.025 [DOI] [PubMed] [Google Scholar]
- Misra S., Chauhan P. S. (2020). ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S., Dixit V. K., Khan M. H., Kumar Mishra S., Dviwedi G., Yadav S., et al. (2017). Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt tolerant indigenous plant growth promoting rhizobacteria. Microbiol. Res. 205 25–34. 10.1016/j.micres.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Mohamed I., Eid K. E., Abbas M. H. H., Salem A. A., Ahmed N., Ali M., et al. (2019). Use of plant growth promoting Rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environ. Saf. 171 539–548. 10.1016/j.ecoenv.2018.12.100 [DOI] [PubMed] [Google Scholar]
- Morgan P. W., Drew M. C. (1997). Ethylene and plant responses to stress. Physiol. Plant. 100 620–630. 10.1034/j.1399-3054.1997.1000325.x 11841302 [DOI] [Google Scholar]
- Moshelion M., Halperin O., Wallach R., Oren R. A. M., Way D. A. (2015). Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water-use efficiency, growth and yield. Plant Cell Environ. 38 1785–1793. 10.1111/pce.12410 [DOI] [PubMed] [Google Scholar]
- Munns R. (2002a). Comparative physiology of salt and water stress. Plant Cell Environ. 25 239–250. 10.1046/j.0016-8025.2001.00808.x [DOI] [PubMed] [Google Scholar]
- Munns R. (2002b). Salinity, Growth and Phytohormones. Berlin: Springer. [Google Scholar]
- Munns R. A. (1992). Leaf elongation assay detects an unknown growth inhibitor in xylem sap from wheat and barley. Aust. J. Plant Physiol. 19 127–135. [Google Scholar]
- Nadeem S. M., Ahmad M., Zahir Z. A., Javaid A., Ashraf M. (2014). The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 32 429–448. 10.1016/j.biotechadv.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Nadeem S. M., Zahir Z. A., Naveed M., Arshad M. (2007). Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol. 53 1141–1149. 10.1139/w07-081 [DOI] [PubMed] [Google Scholar]
- Nadeem S. M., Zahir Z. A., Naveed M., Arshad M. (2009). Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol. 55 1302–1309. 10.1139/w09-092 [DOI] [PubMed] [Google Scholar]
- Nakbanpote W., Panitlurtumpai N., Sangdee A., Sakulpone N., Sirisom P., Pimthong A. (2014). Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J. Plant Interact. 9 379–387. 10.1080/17429145.2013.842000 [DOI] [Google Scholar]
- Nandi M., Selin C., Brawerman G., Fernando W. G. D., de Kievit T. (2017). Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol. Control 108 47–54. 10.1016/j.biocontrol.2017.02.008 [DOI] [Google Scholar]
- Naseem H., Bano A. (2014). Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 9 689–701. 10.1080/17429145.2014.902125 [DOI] [Google Scholar]
- Nautiyal C. S., Srivastava S., Chauhan P. S., Seem K., Mishra A., Sopory S. K. (2013). Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 66 1–9. 10.1016/j.plaphy.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Naz I., Bano A., Ul-Hassan T. (2009). Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. Afr. J. Biotechnol. 8 5762–5766. [Google Scholar]
- Nia S. H., Zarea M. J., Rejali F., Varma A. (2012). Yield and yield components of wheat as affected by salinity and inoculation with Azospirillum strains from saline or non-saline soil. J. Saudi Soc. Agric. Sci. 11 113–121. 10.1016/j.jssas.2012.02.001 [DOI] [Google Scholar]
- Niinemets U. (2010). Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends Plant Sci. 15 145–153. 10.1016/j.tplants.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Niu S. Q., Li H. R., Pare P. W., Aziz M., Wang S. M., Shi H. Z., et al. (2016). Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 407 217–230. 10.1007/s11104-015-2767-z [DOI] [Google Scholar]
- Nunkaew T., Kantachote D., Nitoda T., Kanzaki H., Ritchie R. J. (2015). Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 115 334–341. 10.1016/j.carbpol.2014.08.099 [DOI] [PubMed] [Google Scholar]
- O’Brien J. A., Benková E. (2013). Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 4:451. 10.3389/fpls.2013.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju O. S., Glick B. R., Babalola O. O. (2017). Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 33:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K. K., Gardener B. M. (2006). Biological control of plant pathogens. Plant Health Instr. 1–25. 10.1094/PHI-A-2006-1117-02 [DOI] [Google Scholar]
- Pallai R., Hynes R. K., Verma B., Nelson L. M. (2012). Phytohormone production and colonization of canola (Brassica napus L.) roots by Pseudomonas fluorescens 6-8 under gnotobiotic conditions. Can. J. Microbiol. 58 170–178. 10.1139/w11-120 [DOI] [PubMed] [Google Scholar]
- Panwar M., Tewari R., Gulati A., Nayyar H. (2016). Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol. Plant. 38:278. [Google Scholar]
- Park Y. G., Mun B. G., Kang S. M., Hussain A., Shahzad R., Seo C. W., et al. (2017). Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS One 12:e0173203. 10.1371/journal.pone.0173203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. S., Dutta S., Ann M., Raaijmakers J. M., Park K. (2015). Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 461 361–365. 10.1016/j.bbrc.2015.04.039 [DOI] [PubMed] [Google Scholar]
- Passioura J. B. (2010). Scaling up: the essence of effective agricultural research. Funct. Plant Biol. 37 585–591. [Google Scholar]
- Patel J. S., Singh A., Singh H. B., Sarma B. K. (2015). Plant genotype, microbial recruitment and nutritional security. Front. Plant Sci. 6:608. 10.3389/fpls.2015.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Trivedi G., Saraf M. (2018). Iron biofortification in mungbean using siderophore producing plant growth promoting rhizobacteria. Environ. Sustain. 1 357–365. 10.1007/s42398-018-00031-3 [DOI] [Google Scholar]
- Patel T., Saraf M. (2017). Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J. Plant Interact. 12 480–487. 10.1080/17429145.2017.1392625 [DOI] [Google Scholar]
- Paul D., Lade H. (2014). Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron. Sustain. Dev. 34 737–752. 10.1007/s13593-014-0233-6 [DOI] [Google Scholar]
- Pii Y., Aldrighetti A., Valentinuzzi F., Mimmo T., Cesco S. (2019). Azospirillum brasilense inoculation counteracts the induction of nitrate uptake in maize plants. J. Exp. Bot. 70 1313–1324. 10.1093/jxb/ery433 [DOI] [PubMed] [Google Scholar]
- Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growthpromoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 51 403–415. 10.1007/s00374-015-0996-1 [DOI] [Google Scholar]
- Pinedo I., Ledger T., Greve M., Poupin M. J. (2015). Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 6:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Lauchli A. (2002). “Global impact of salinity and Agricultural ecosystems,” in Salinity: Environment - Plants - Molecules, eds Lauchli A., Luttage U. (Amsterdam: Kluwer Academic Publishers; ), 3–20. 10.1007/0-306-48155-3_1 [DOI] [Google Scholar]
- Pliego C., Ramos C., Vicente A., Cazorla F. M. (2011). Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant Soil 340 505–520. 10.1007/s11104-010-0615-8 [DOI] [Google Scholar]
- Poupin M. J., Timmermann T., Vega A., Zuñi̇ga A., González B. (2013). Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS One 8:e69435. 10.1371/journal.pone.0069435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qessaoui R., Bouharroud R., Furze J. N., El Aalaoui M., Akroud H., Amarraque A., et al. (2019). Applications of New Rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci. Rep. 9:12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Dong W. Y., He K. N., Yu Y., Tan G. D., Han L., et al. (2010). NaCl salinity-induced changes in water status, ion contents and photosynthetic properties of Shepherdia argentea (Pursh) Nutt. Seedlings. Plant Soil Environ. 56 325–332. 10.17221/209/2009-pse [DOI] [Google Scholar]
- Qin Y., Druzhinina I. S., Pan X., Yuan Z. (2016). Microbially mediated plant salt tolerance and microbiome- based solutions for saline agriculture. Biotechnol. Adv. 34 1245–1259. 10.1016/j.biotechadv.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Qiu Q. S., Guo Y., Dietrich M. A., Schumaker K. S., Zhu J. K. (2002). Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. U.S.A. 99 8436–8441. 10.1073/pnas.122224699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurashi A. W., Sabri A. N. (2012). Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 43 1183–1191. 10.1590/s1517-83822012000300046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmoune B., Abdelkader M., Khelifi-Slaoui M., Khelifi L., Strueh A., Erban A., et al. (2017). Isolation and characterization of three new PGPR and their effects on the growth of Arabidopsis and Datura plants Isolation and characterization of three new PGPR and their effects on the growth of Arabidopsis and Datura plants. J. Plant Interact. 12 1–6. 10.1080/17429145.2016.1269215 [DOI] [Google Scholar]
- Rashid M. A., Mujawar L. H., Shahzad T., Almeelbi T., Ismail I. M. I., Oves M. (2016). Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 183 26–41. 10.1016/j.micres.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Rengasamy P. (2002). Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Aust. J. Exp. Agric. 42 351–361. [Google Scholar]
- Rijavec T., Lapanje A. (2016). Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front. Microbiol. 7:1785. 10.3389/fmicb.2016.01785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima F. S., Biswas S., Sarker P. K., Islam M. R., Seraj Z. I. (2018). Bacteria endemic to saline coastal belt and their ability to mitigate the effects of salt stress on rice growth and yields. Ann. Microbiol. 68 525–535. 10.1007/s13213-018-1358-7 [DOI] [Google Scholar]
- Rodríguez-Navarro A., Rubio F. (2006). High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 57 1149–1160. 10.1093/jxb/erj068 [DOI] [PubMed] [Google Scholar]
- Rojas-Tapias D., Moreno-Galván A., Pardo-Díaz S., Obando M., Rivera D., Bonilla R. (2012). Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 61 264–272. 10.1016/j.apsoil.2012.01.006 [DOI] [Google Scholar]
- Rossi F., De Philippis R. (2015). Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 5 1218–1238. 10.3390/life5021218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozema J., Flowers T. (2008). Ecology. Crops for a salinized world. Science 322 1478–1480. 10.1126/science.1168572 [DOI] [PubMed] [Google Scholar]
- Rueda-Puente E., Castellanos T., Troyo-Diéguez E., Díaz de León-Alvarez J. L., Murillo-Amador B. (2003). Effects of a Nitrogen-Fixing Indigenous Bacterium (Klebsiella pneumoniae) on the Growth and Development of the Halophyte Salicornia bigelovii as a New Crop for Saline Environments. J. Agron. Crop Sci. 189 323–332. 10.1046/j.1439-037x.2003.00051.x [DOI] [Google Scholar]
- Ruiz-Sánchez M. C., Domingo R., Torrecillas A., Pérez-Pastor A. (2000). Water stress preconditioning to improve drought resistance in young apricot plants. Plant Sci. 156 245–251. 10.1016/s0168-9452(00)00262-4 [DOI] [PubMed] [Google Scholar]
- Ryu H., Cho Y. G. (2015). Plant hormones in salt stress tolerance. J. Plant Biol. 58 147–155. 10.1007/s12374-015-0103-z [DOI] [Google Scholar]
- Saghafi K., Ahmadi J., Asgharzadeh A., Bakhtiari S. (2013). The effect of microbial inoculants on physiological responses of two wheat cultivars under salt stress. Int. J. Adv. Biol. Biomed. Res. 1 421–431. [Google Scholar]
- Sah S. K., Reddy K. R., Li J. (2016). Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7:571. 10.3389/fpls.2016.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha M., Sarkar S., Sarkar B., Sharma B. K., Bhattacharjee S., Tribedi P. (2016). Microbial siderophores and their potential applications: a review. Environ. Sci. Pollut. Res. 23 3984–3999. 10.1007/s11356-015-4294-0 [DOI] [PubMed] [Google Scholar]
- Salomon M. V., Bottini R., de Souza Filho G. A., Cohen A. C., Moreno D., Gil M., et al. (2014). Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, induce abscisic acid accumulation and synthesis of defense related terpenes in In vitro cultured grapevine. Physiol. Plant. 151 359–374. 10.1111/ppl.12117 [DOI] [PubMed] [Google Scholar]
- Sánchez-Blanco M. J., Rodríguez P., Olmos E., Morales M. A., Torrecillas A. (2004). Differences in the effects of simulated sea aerosol on water relations, salt content, and leaf ultrastructure of rock-rose plants. J. Environ. Qual. 33 1369–1375. [DOI] [PubMed] [Google Scholar]
- Sandhya V., Ali S. K. Z., Grover M., Reddy G., Venkateswarlu B. (2009). Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 46 17–26. 10.1007/s00374-009-0401-z [DOI] [Google Scholar]
- Sandhya V., Ali S. Z., Grover M., Reddy G., Venkateswarlu B. (2010). Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant statusand plant growth of maize under drought stress. Plant Growth Regul. 62 21–30. 10.1007/s10725-010-9479-4 [DOI] [Google Scholar]
- Sarkar A., Ghosh P. K., Pramanik K., Mitra S., Soren T., Pandey S., et al. (2018). A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Microbiol. Res. 169 20–32. 10.1016/j.resmic.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Savé R., Olivella C., Biel C., Adillón J., Rabella R. (1994). Seasonal patterns of water relationships, photosynthetic pigments and morphology of Actinidia deliciosa plants of the Haywards and Tomouri cultivars. Agronomie 2 121–126. 10.1051/agro:19940207 [DOI] [Google Scholar]
- Selvakumar G., Panneerselvam P., Ganeshamurthy A. N. (2012). “Bacterial mediated alleviation of abiotic stress in crops,” in Bacteria in Agrobiology: Stress Management, ed. Maheshwari D. K. (Berlin: Springer-Verlag; ), 205–224. 10.1007/978-3-662-45795-5_10 [DOI] [Google Scholar]
- Sen S., Chandrasekhar C. N. (2015). Effect of PGPR on enzymatic activities of rice (Oryza sativa L.) under salt stress. Asian J. Plant Sci. Res. 5 44–48. [Google Scholar]
- Shah G., Jan M., Afreen M., Anees M., Rehman S., Daud M. K., et al. (2017). Halophilic bacteria mediated phytoremediation of salt-affected soils cultivated with rice. J. Geochem. Explor. 174 59–65. 10.1016/j.gexplo.2016.03.011 [DOI] [Google Scholar]
- Shahzad R., Waqas M., Khan A. L., Asaf S., Khan M. A., Kang S. M., et al. (2016). Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 106 236–243. 10.1016/j.plaphy.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Sharma S., Kulkarni J., Jha B. (2016). Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 7:1600. 10.3389/fmicb.2016.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Hu H., Peng H., Wang W., Zhang X. (2013). Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genomics 14:271. 10.1186/1471-2164-14-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibponkrung S., Kondo T., Tanaka K., Tittabutr P., Boonkerd N., Yoshida K.-I., et al. (2020). Co-Inoculation of Bacillus velezensis Strain S141 and Bradyrhizobium Strains Promotes Nodule Growth and Nitrogen Fixation. Microorganisms 8:678. 10.3390/microorganisms8050678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P., Singh V., Gupta V. K., Shukla R., Prabha R., Sarma B. K., et al. (2020). Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci. Rep. 10:4818. 10.1038/s41598-020-61140-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. S., Strong P. J. (2016). Biologically derived fertilizer: a multifaceted bio-tool in methane mitigation. Ecotoxicol. Environ. Saf. 124 267–276. 10.1016/j.ecoenv.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Singh R. P., Jha P. N. (2016). The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS One 11:e0155026. 10.1371/journal.pone.0155026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Shelke G. M., Kumar A., Jha P. N. (2015). Biochemistry and genetics of ACC deaminase: a weapon to “stress ethylene” produced in plants. Frontiers in microbiology 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupska A., Janczarek M., Marczak M., Mazur A., Król J. (2006). Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb. Cell Fact. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaepen S., Vanderleyden J. (2011). Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 3:a001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P., Sauer K., Davies D. G., Costerton J. W. (2002). Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56 187–209. 10.1146/annurev.micro.56.012302.160705 [DOI] [PubMed] [Google Scholar]
- Suarez C., Cardinale M., Ratering S., Steffens D., Jung S., Montoya Z. M. A., et al. (2015). Plant growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl. Soil Ecol. 95 23–30. 10.1016/j.apsoil.2015.04.017 [DOI] [Google Scholar]
- Subramanian S., Ricci E., Souleimanov A., Smith D. L. (2016). A proteomic approach to lipo-chitooligosaccharide and thuricin 17 effects on soybean germination unstressed and salt stress. PLoS One 11:e0160660. 10.1371/journal.pone.0160660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukweenadhi J., Kim Y. J., Choi E. S., Koh S. C., Lee S. W., Kim Y. J., et al. (2015). Penibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought and salt stress. Microbiol. Res. 172 7–15. 10.1016/j.micres.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Sultana S., Paul S. C., Karim M. M. (2018). Salinity intrusion and coastal agriculture: adaptation strategies using salt-tolerant plant-growth promoting rhizobacteria for sustainable food security. Reg. Probl. 21 58–61. 10.31433/1605-220x-2018-21-3(1)-58-61 [DOI] [Google Scholar]
- Sultana S., Paul S. C., Parveen S., Alam S., Rahman N., Jannat B., et al. (2020). Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 66 144–160. 10.1139/cjm-2019-0323 [DOI] [PubMed] [Google Scholar]
- Tahir H. A. S., Gu Q., Wu H., Niu Y., Huo R., Gao X. (2017). Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 7:40481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura T., Hanagata N., Sugihara K., Baba S., Karube I., Dubinsky Z. (2000). Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat. Bot. 68 15–28. 10.1016/s0304-3770(00)00106-6 [DOI] [Google Scholar]
- Tank N., Saraf M. (2010). Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 5 51–58. 10.1080/17429140903125848 [DOI] [Google Scholar]
- Tanveer M., Shah A. N. (2017). An insight into salt stress tolerance mechanisms of Chenopodium album. Environ. Sci. Pollut. Res. Int. 24 16531–16535. 10.1007/s11356-017-9337-2 [DOI] [PubMed] [Google Scholar]
- Tester M., Davenport R. (2003). Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91 503–527. 10.1093/aob/mcg058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari S., Arora N. K. (2014). Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stressamelioration in sunflower under saline conditions. Curr. Microbiol. 69 484–494. 10.1007/s00284-014-0612-x [DOI] [PubMed] [Google Scholar]
- Tewari S., Arora N. K. (2018). Role of salicylic acid from Pseudomonas aeruginosa PF23EPS+ in growth promotion of sunflower in saline soils infested with phytopathogen Macrophomina phaseolina. Environ. Sustain. 1 49–59. 10.1007/s42398-018-0002-6 [DOI] [Google Scholar]
- Timmusk S., El-Daim I. A. A., Copolovici L., Tanilas T., Kännaste A., Behers L., et al. (2014). Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9:e96086. 10.1371/journal.pone.0096086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. (2011). Phytochemical screening and Extraction: a review. Int. Pharm. Sci. 1 98–106. [Google Scholar]
- Ullah S., Bano A. (2015). Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 61 307–313. 10.1139/cjm-2014-0668 [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Singh J., Singh D. (2011). Exopolysaccharide-producing plant growthpromoting rhizobacteria under salinity condition. Pedosphere 21 214–222. 10.1016/s1002-0160(11)60120-3 [DOI] [Google Scholar]
- Upadhyay S. K., Singh D. P. (2015). Effect of salt-tolerant plant growth promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 17 288–293. 10.1111/plb.12173 [DOI] [PubMed] [Google Scholar]
- Upadhyay S. K., Singh D. P., Saikia R. (2009). Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr. Microbiol. 59 489–496. 10.1007/s00284-009-9464-1 [DOI] [PubMed] [Google Scholar]
- Vaddepalli P., Fulton L., Wieland J., Wassmer K., Schaeffer M., Ranf S., et al. (2017). The cell wall- localized atypical β-1, 3 glucanase ZERZAUST controls tissue morphogenesis in Arabidopsis thaliana. Development 144 2259–2269. 10.1242/dev.152231 [DOI] [PubMed] [Google Scholar]
- Vaishnav A., Choudhary D. K. (2019). Regulation of Drought-Responsive Gene Expression in Glycine max L. Merrill is Mediated Through Pseudomonas simiae Strain AU. J. Plant Growth Regul. 38 333–342. 10.1007/s00344-018-9846-3 [DOI] [Google Scholar]
- Vaishnav A., Kumari S., Jain S., Varma A., Choudhary D. K. (2015). Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J. Appl. Microbiol. 119 539–551. 10.1111/jam.12866 [DOI] [PubMed] [Google Scholar]
- Vaishnav A., Kumari S., Jain S., Varma A., Tuteja N., Choudhary D. K. (2016a). PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 56 1274–1288. 10.1002/jobm.201600188 [DOI] [PubMed] [Google Scholar]
- Vaishnav A., Varma A., Tuteja N., Choudhary D. K. (2016b). “PGPR-mediated amelioration of crops under salt stress,” in Plant-Microbe Interaction: An Approach to Sustainable Agriculture, eds Choudhary D., Varma A., Tuteja N. (Singapore: Springer; ). [Google Scholar]
- Vessey J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255 571–586. [Google Scholar]
- Wang G. L., Que F., Xu Z. S., Wang F., Xiong A. S. (2015). Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 15:290. 10.1186/s12870-015-0679-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Altman A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218 1–14. 10.1007/s00425-003-1105-5 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6 251–264. 10.1105/tpc.6.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. M., Smith M. D., Glick B. R., Liang Y. (2014). Effects of ACC deaminase containing rhizobacteria on plant growth and expression of Toc GTPases in tomato (Solanum lycopersicum) under salt stress. Botany 92 775–781. 10.1139/cjb-2014-0038 [DOI] [Google Scholar]
- Yang A., Akhtar S. S., Iqbal S., Amjad M., Naveed M., Zahir Z. A., et al. (2016). Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 43 632–642. [DOI] [PubMed] [Google Scholar]
- Yanni Y., Zidan M., Dazzo F., Rizk R., Mehesen A., Abdelfattah F., et al. (2016). Enhanced symbiotic performance and productivity of drought stressed common bean after inoculation with tolerant native rhizobia in extensive fields. Agric. Ecosyst. Environ. 232 119–128. 10.1016/j.agee.2016.07.006 [DOI] [Google Scholar]
- Yao L., Wu Z., Zheng Y., Kaleem I., Li C. (2010). Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 46 49–54. 10.1016/j.ejsobi.2009.11.002 [DOI] [Google Scholar]
- Yildirim E., Donmez M. F., Turan M. (2008a). Use of bioinoculants in ameliorative effects on radish plants under salinity stress. J. Plant Nutr. 31 2059–2074. 10.1080/01904160802446150 [DOI] [Google Scholar]
- Yildirim E., Turan M., Donmez M. F. (2008b). Mitigation of salt stress in radish (Raphanus sativus L.) by plant growth promoting rhizobacteria. Rom. Biotechnol. Lett. 13 3933–3943. [Google Scholar]
- You M., Fang S., MacDonald J., Xu J., Yuan Z. C. (2020). Isolation and characterization of Burkholderia cenocepacia CR318, a phosphate solubilizing bacterium promoting corn growth. Microbiol. Res. 233:126395. 10.1016/j.micres.2019.126395 [DOI] [PubMed] [Google Scholar]
- Yuan S. F., Li M. Y., Fang Z. Y., Liu Y., Shi W., Pan B., et al. (2016). Biological control of tobacco bacterial wilt using Trichoderma harzianum amended bio-organic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae. Biol. Control 92 164–171. 10.1016/j.biocontrol.2015.10.013 [DOI] [Google Scholar]
- Zafar-ul-Hye M., Muhammad H., Zahir F., Ahmad Z., Hussain M., Hussain A. (2014). Application of ACC-deaminase containing rhizobacteria with fertilizer improves maize production under drought and salinity stress. Int. J. Agric. Biol. 16 591–596. [Google Scholar]
- Zahir Z. A., Shah M. K., Naveed M., Akhter M. J. (2010). Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. J. Microbiol. Biotechnol. 20 1288–1294. 10.4014/jmb.1002.02010 [DOI] [PubMed] [Google Scholar]
- Zarea M. J., Hajinia S., Karimi N., Goltapeh E. M., Rejali F., Varma A. (2012). Effect of Piriformospora indica and Azospirillumstrains from saline or non-saline soil on mitigation of the effects of NaCl. Soil Biol. Biochem. 45 139–146. 10.1016/j.soilbio.2011.11.006 [DOI] [Google Scholar]
- Zeffa D. M., Fantin L. H., Koltun A., de Oliveira A. L. M., Nunes M. P. B. A., Canteri M. G., et al. (2020). Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: a meta-analysis of studies from 1987 to 2018. PeerJ 8:e7905. 10.7717/peerj.7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kim M. S., Sun Y., Dowd S. E., Shi H., Paré P. W. (2008). Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 21 737–744. 10.1094/mpmi-21-6-0737 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Deng C., Deng S., Li N., Zhao C., et al. (2018). The Arabidopsis Ca2+-Dependent Protein Kinase CPK12 is involved in plant response to salt stress. Int. J. Mol. Sci. 19:4062. 10.3390/ijms19124062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Qu L., Hong X., Sun X. (2011). Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid. Based Complement. Altern. Med. 2011:615032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. K. (2001). Plant salt tolerance. Trends Plant Sci. 6 66–71. [DOI] [PubMed] [Google Scholar]
- Zhu J. K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. K. (2007). Plant Salt Stress. Hoboken, NJ: John Wiley & Sons. [Google Scholar]