Abstract
Extracts or active components from Acorus gramineus Aiton (EAAGA) have been clinically used for cognition impairment more than hundreds of years and are still used in modern times in China and elsewhere worldwide. Previous studies reported that EAAGA improves cognition impairment in animal models. Here, we conducted a preclinical systematic review to assess the current evidence of EAAGA for cognition impairment. We searched 7 databases up until June 2019. Methodological quality for each included studies was accessed according to the CAMARADES 10-item checklist. The primary outcome measures were neurobehavioral function scores evaluated by the Morris water maze test, electrical Y-maze test, step-down test, radial eight-arm maze test, and step-through test. The secondary outcome measures were mechanisms of EAAGA for cognition function. Finally, 34 studies involving 1431 animals were identified. The quality score of studies range from 1 to 6, and the median was 3.32. Compared with controls, the results of the meta-analysis indicated EAAGA exerted a significant effect in decreasing the escape latency and error times and in increasing the length of time spent in the platform quadrant and the number of platform crossings representing learning ability and memory function (all P < 0.01). The possible mechanisms of EAAGA are largely through anti-inflammatory, antioxidant, antiapoptosis activities, inhibition of neurotoxicity, regulating synaptic plasticity, protecting cerebrovascular, stimulating cholinergic system, and suppressing astrocyte activation. In conclusion, EAAGA exert potential neuroprotective effects in experimental cognition impairment, and EAAGA could be a candidate for cognition impairment treatment and further clinical trials.
1. Introduction
With the average life expectancy increasing, there is concern about the proportion of cognitive impairment in the global population, which results from degeneration of the brain and very high prevalence in elderly individuals [1]. The World Health Organization estimates that the number of people over the age of 60 will be around 2 billion in 2050, while the number of cognitive impairment patients is expected to rise rapidly along with the aging population worldwide [2, 3]. However, so far, clinical trials have not identified efficacious neuroprotective therapies for cognitive impairment patients [4]. Thus, given the huge translational gap between the animal studies and clinical trials, seeking or developing innovative neuroprotectants is urgently needed.
For more than a millennium, traditional Chinese medicine (TCM), a main form of complementary and alternative medicine, has been used in Asian countries, especially in China, Japan, and Korea, to alleviate various symptoms of cognitive deficits and to facilitate learning and memory [5]. Acorus gramineus Aiton (AGA) (record 2322 (http://www.theplantlist.org.)), the dry rhizomes of Acorus gramineus Solander (Shi Changpu), is listed officially in the Chinese Pharmacopoeia and used in oriental medicines for more than hundreds of years to treat neurological disorders. AGA possessed various pharmacological effects on the central nervous system, including neuroprotective effects [6, 7], central inhibitory effects [8], inhibitory effects on excitotoxic neuronal death [9], and stroke [10], and amelioration in learning and memory [5]. AGA may be effective for the improvement of amnesia [9]. AGA contains different extract fractions: volatile oil, composing mainly of β-asarone (63.2–81.2%), and α-asarone (8.8–13.7%) [11], as well as water extract, ethyl ether extract, ethyl acetate extract, N-butanol extract, and the defatted decoction fractions. AGA is often used as a component in some Chinese herbal formulas. Among 75 of the most famous Chinese herbal formulas characterized as improving intelligence both in ancient and modern time in China, more than half contain AGA, such as Kai-Xin-San [12] and Chong-MyungTang [13].
Systematic reviews are believed to be preferred; only data that from systematic reviews will be considered as the highest level of medical evidence basis for the levels of evidence from the Centre of Evidence-Based Medicine in Oxford [14]. Preclinical systematic reviews are a powerful approach to analyze and synthesize the results of an intervention from animal data into a useful document that can help to shape further basic research, optimize the experimental studies, and enhance the success rate of future clinical trials [15]. Thus, we conducted a preclinical systematic review to assess the current evidence of extracts or active components from Acorus gramineus Aiton (EAAGA) and active component for animal models of cognitive impairment.
2. Materials and Methods
2.1. Search Strategies
Experimental studies of EAAGA for cognitive impairment were identified in the databases, including PubMed, Embase, Web of Science, Wanfang database, China National Knowledge Infrastructure (CNKI), CBM, and VIP information database. All searches were performed from inception to June 2019. Studies about assessing the effectiveness of AAGA for improving cognitive function impairment in animals were identified. The search terms were as follows: (Acorus tatarinowii Schott OR Rhizoma acori graminei OR Acorus calamus OR Acorus gramineus Soland OR acorus gramineus aiton OR Acori graminei rhizoma OR Acori tatarinowii rhizoma OR grassleaf sweetfalg Rhizome) AND (cognitive function impairment OR amnesia OR dementia OR Alzheimer's disease).
2.2. Inclusion Criteria
Experimental studies on EAAGA for cognitive impairment models were included, regardless of publication status or animal species, gender, age, and methods of model establishment. The primary outcome measurements were Morris water maze test (MWM test), electric Y-maze test (EY-M test), radial eight-arm maze test (RAM test), Step down test (SD test), and/or Step through test (ST test). The secondary outcome measures were mechanisms of EAAGA for learning and/or memory function.
2.3. Exclusion Criteria
Exclusion criteria were prespecified as follows: (1) the article was a review, case report, comment, clinical trial, abstract, or editorial; (2) the article was a clinical or in vitro study; (3) the article was not a research about cognitive impairment model; (4) EAAGA was used as combination; (5) there was no control group; and (6) the article was a duplicate publication.
2.4. Data Extraction
The information of each included study was extracted: (1) author and publication year, animal model species, method of anesthesia, and random method; (2) characteristics of animals, including species, sex, animal number, and weight; (3) treatment information from treatment and control groups, including drug, dose, method of treatment, timing for initial treatment, frequency, and duration of treatment; and (4) outcome measures, sample size, and corresponding data including mean value, standard deviation, and intergroup differences. If outcomes were presented at different time points, we extracted data from the last time point. If studies utilized dose gradient of the drug, we extracted data from the highest dose of EAAGA and active component since the dose-response relationship. If the data were incomplete or presented in graphs, we tried to contact the authors for data needed or calculated using relevant software. Information of the mechanism studies of EAAGA and active component for cognitive impairment models among the included articles was extracted.
2.5. Quality Assessment
The methodological quality of included studies was evaluated by two independent reviewers using Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) 10-item checklist [16]. For calculating an aggregate quality score, each item of this scale was attributed one point.
2.6. Statistical Analysis
Meta-analysis was conducted via RevMan version 5.3. To estimate the effect of EAAGA on cognitive impairment, the random effects model and standard mean difference (SMD) with 95% confidence intervals (CIs) were calculated. Heterogeneity was assessed via I2 statistics test. If probability value was less than 0.05, the difference was considered statistically significant. In addition, to explore potential sources of high heterogeneity, subgroup analyses were performed according to animal species and models. Difference between groups was determined by partitioning heterogeneity and utilizing the χ2 distribution with degrees of freedom (df).
3. Results
3.1. Study Selection
We identified 2368 potentially relevant papers after systematical search from six databases. After removing duplicates, 1887 studies remained. By reading titles and abstracts, 1602 articles were excluded that were reviews, case reports, comments, abstracts, clinical trials, letters, or editorials. After reading the remaining 285 full-text articles, 228 studies were excluded for at least one of following reasons: (1) not an animal study; (2) the article was not a research about cognitive impairment; (3) the study did not access the effects of AGA or active component on the animal model of cognitive impairment; (4) EAAGA was not used as a monotherapy; and (5) lack of control group. Ultimately, 34 eligible articles [5, 6, 10, 11, 17–46] were selected (Figure 1).
Figure 1.
Flow diagram.
3.2. Characteristics of Included Studies
Sixteen studies [5, 6, 10, 11, 17–27, 37] were published in English, and 18 studies were in Chinese between 1999 and 2019. In total, 34 studies with 1431 animals were included. Ten species were referred, including Sprague-Dawley (SD) rat (n = 236, 16.49%), Wistar rats (n = 130, 9.08%), Kunming mice (n = 530, 37.04%), ICR mice (n = 236, 16.49%), NIH mice (n = 168, 11.74%), AβPP/PS1 double-transgenic mice (n = 26, 1.82%); APPswe/PS1dE9 double transgenic mice (n = 22, 1.54%), C57BL/6 mice (n = 24, 1.68%), senescence-accelerated prone-8 (SAMP8) mice (n = 26, 1.82%), and FMR1gene knock mice (n = 33, 2.31%). The weight of SD rats ranged from 200 g to 650 g, the weight of Wistar rats used ranged from 30 g to 250 g, and the weight of mice ranged from 17 g to 50 g. Twenty-two studies used male rodents, 1 study used female rodents, 5 study used both female and male rodents, and the remaining 6 studies did not provide gender details. Sodium pentobarbital was used to induce anesthesia in 8 studies, and chloral hydrate was used in 2 studies [20, 21], 1 study [41] used phenytoin sodium, 1 study [17] used CO2, and 1 study [10] used isoflurane, while the remaining 21 studies did not report the type of anesthetics. Cognitive impairment models were induced by lead [17], noise stress [18], LPS [19], amyloid beta 1-42 [11, 21, 26, 28, 29, 37, 41, 46], D-gal plus AlCl3 [22], scopolamine [5, 24, 30, 34–36, 42, 45], ethanol [5, 32, 34–36], sodium nitrite [5, 32], corticosterone [23], Ibotenic acid [25], chronic restraint stress [31], pentobarbital sodium [32], D-galactose [33, 38], AlCl3 [40], streptozotocin (STZ) [43], pent ylenetet razol (PTZ) [44], and NaNO2 [34–36]. As an intervention, fourteen studies [6, 17, 20, 22, 23, 26, 27, 32, 35, 37, 39, 41, 42, 46] used β-asarone, eight studies [18, 19, 21, 24, 33, 38, 40, 44] used α-asarone, three studies [10, 25, 44] utilized AGA, twelve studies [5, 11, 22, 28–32, 35, 36, 43, 45] used essential oil, seven studies [11, 28, 29, 33–36] researched water extract, four studies [11, 28, 29, 32] used defatted decoction, and one study [18] researched ethyl acetate extract. Normal distilled water control was used in 2 studies [17, 33]; Tween 80 control was used in 6 studies [5, 6, 18, 20, 27, 32]; normal saline control was used in 24 studies; 0.5% methylcellulose solution containing 1% Tween 80 control was used in 1 study [24], and 2% propylene glycol containing 2% polyethylene glycol stearate control was used in 1 study [43]. Neurobehavioral function indices as primary outcome measures were carried out by the Morris water maze test (MWM test) (n = 28), step-down test (SD test) (n = 6), electrical Y-maze test (EY-M test) (n = 3), step-through test (ST test) (n = 4), and radial eight-arm maze test (RAM test) (n = 3). The characteristics of the 34 studies are shown in Table 1.
Table 1.
Basic characteristics of the included studies.
| Study (years) | Species (sex, n = experimental/control group) | Weight | Random method | Model (method) | Anesthetic | Method of administration | Outcome index (time) | Intergroup differences | |
|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | ||||||||
| Yang et al. [17] | SD rats (mix, 7/7) | NR | NR | Chronic lead-induced dysmnesia model | CO2 | β-Asarone (2.5, 10, and 40 mg kg−1, ip); from 9 to 11 weeks old; once daily for 3 weeks | Distilled water (same volume, ip); from 9 to 11 weeks old; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.001 |
| (2) MWM test (swimming speed) | (2) P > 0.05 | ||||||||
| (3) MWM test (time spent in target quadrant) | (3) P < 0.05 | ||||||||
| (4) MWM test (times crossed the platform) | (4) P > 0.05 | ||||||||
| (5) Dendritic spine density | (5) P < 0.001 | ||||||||
|
| |||||||||
| Wei et al., 2013 | AβPP/PS1 double-transgenic mice (13/13) | NR | NR | AβPP/PS1 double-transgenic mice | NR | β-Asarone (7 and 21 mg kg−1, ig); onset the experiment; once daily for 4 months | Tween 80 (same volume, ig); onset the experiment; once daily for 4 months | (1) MWM test (escape latency) | (1) P < 0.001 |
| (2) Cell viability | (2) P < 0.05 | ||||||||
|
| |||||||||
| Sundaramahalingam et al. [18] | Wister strain albion rats (male, 6/6) | 200-220 g | NR | Noise stress induced memory impairment model | NR | α-Asarone (9 mg kg−1, ip); onset the experiment; once daily for 30 d | Tween 80 (same volume, ip); onset the experiment; once daily for 30 d | (1) RAM test (number of errors) | (1) P < 0.05 |
| (2) Hsp 70 mRNA levels | (2) P < 0.05 | ||||||||
| (3) Ache activity | (3) P < 0.05 | ||||||||
| (4) SOD/CAT/GPx activity | (4) P < 0.05 | ||||||||
| (5) VC/VE/GSH levels | (5) P < 0.05 | ||||||||
| (6) G6PD activity | (6) P < 0.05 | ||||||||
| Wister strain albion rats (male, 6/6) | 200-220 g | NR | Noise stress exposed rats | NR | Ethyl acetate extract (50 mg kg−1, ip); onset the experiment; once daily for 30 d | Tween 80 (same volume, ip); onset the experiment; once daily for 30 d | (1) RAM test (number of errors) | (1) P < 0.05 | |
| (2) Hsp 70 mRNA levels | (2) P < 0.05 | ||||||||
| (3) Ache activity | (3) P < 0.05 | ||||||||
| (4) SOD/CAT/GPx activity | (4) P < 0.05 | ||||||||
| (5) VC/VE/GSH levels | (5) P < 0.05 | ||||||||
| (6) G6PD activity | (6) P < 0.05 | ||||||||
|
| |||||||||
| Shin et al. [19] | C57BL/6 mice (male, 12/12) | 25-28 g | NR | LPS-induced cognitive handicap model | NR | α-Asarone (7.5, 15, and 30 mg kg−1, ig); 3 days before the LPS injection; once daily for 3 d | Normal saline (same volume, ig); 3 days before the LPS injection; once daily for 3 d | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) MWM test (times crossed the platform) | (2) P < 0.05 | ||||||||
| (3) TNF-α/IL-1β mRNA levels | (3) P < 0.05 | ||||||||
| (4) CA1 neurons count | (4) P < 0.05 | ||||||||
| (5) TUNEL-labeled cells count | (5) P < 0.05 | ||||||||
| (6) BACE1/Iba1 protein expressions | (6) P < 0.05 | ||||||||
|
| |||||||||
| Ma et al. [11] | Six-week-old NIH mice (male, 6/6) | 20-25 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Water extract (20 mg g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline; (same volume, ig); after the first MWM test; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) Aβ positive cells count | (2) P < 0.05 | ||||||||
| (3) DCx expression | (3) P < 0.05 | ||||||||
| (4) Nestin positive cells count | (4) P < 0.05 | ||||||||
| Six-week-old NIH mice (male, 6/6) | 20-25 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Essential oil (20 mg g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline; (same volume, ig); after the first MWM test; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.05 | |
| (2) Aβ positive cells count | (2) P < 0.05 | ||||||||
| (3) DCx expression | (3) P < 0.05 | ||||||||
| (4) Nestin positive cells count | (4) P < 0.05 | ||||||||
| Six-week-old NIH mice (male, 6/6) | 20-25 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Defatted decoction (20 mg g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline; (same volume, ig); after the first MWM test; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.05 | |
| (2) Aβ positive cells count | (2) P < 0.05 | ||||||||
| (3) DCx expression | (3) P < 0.05 | ||||||||
| (4) Nestin positive cells count | (4) P < 0.05 | ||||||||
|
| |||||||||
| Liu et al. [20] | APPswe/PS1dE9 double transgenic mice (male, 11/11) | NR | NR | APPswe/PS1dE9 double transgenic mice | Chloral hydrate | β-Asarone (21.2, 42.4, and 84.8 mg kg−1, ig); onset the experiment; once daily for 2.5 months | Tween 80 (same volume, ig); onset the experiment; once daily for 2.5 months | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) MWM test (time spent in target quadrant) | (2) P < 0.05 | ||||||||
| (3) MWM test (times crossed the platform) | (3) P < 0.05 | ||||||||
| (4) SYP/GluR1 expression | (4) P < 0.05 | ||||||||
|
| |||||||||
| Limón et al. [21] | Wistar rats (male, 8/8) | 230–250 g | NR | Aβ1-42-induced AD model | Chloral hydrate | α-Asarone (10 mg kg−1, i.h.); after injection of amyloid-β; once daily for 16 d | Normal saline; (same volume, ig); after injection of amyloid-β; once daily for 16 d | (1) RAM test (percentage of correct responses) | (1) P < 0.001 |
| (2) Nitrite levels | (2) P < 0.05 | ||||||||
|
| |||||||||
| Li et al. [22] | Wistar rats (female, 7/7) | 150–180 g | NR | D-gal and AlCl3 induced AD model | Sodium pentobarbital | β-Asarone (25, 50 and 100 mg kg−1, i.h.); 28 d after injection of AlCl3 and D-gal; once daily for 14 d | Normal saline; (same volume, i.h); after the first MWM test; once daily for 14 d | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) MWM test (time spent in target quadrant) | (2) P < 0.05 | ||||||||
| (3) MWM test (times crossed the platform) | (3) P < 0.05 | ||||||||
| (4) MWM test (swimming speed) | (4) P > 0.05 | ||||||||
| (5) ET-1, eNOS, and APP expression | (5) P < 0.05 | ||||||||
| (6) Lactic acid and pyruvic acid content | (6) P < 0.05 | ||||||||
| (7) Na+- K+ ATPase activity | (7) P < 0.05 | ||||||||
| (8) rCBF | (8) P < 0.05 | ||||||||
|
| |||||||||
| Zhang et al. [5] | Aged Kunming mice (male, 10/10) | 40-50 g | NR | Aged mice | NR | Essential oil (0.02, 0.04, and 0.08 g kg−1, orally); onset the experiment; once daily for 15 d | Tween 80 (same volume, orally); onset the experiment; once daily for 15 d | (1) SD test (escape latency) | (1) P < 0.01 |
| (2) SD test (number of errors) | (2) P < 0.05 | ||||||||
| Aged Kunming mice (male, 10/10) | 40-50 g | NR | Scopolamine-induced dysmnesia model | NR | Essential oil (0.02, 0.04, and 0.08 g kg−1, orally); onset the experiment; once daily for 15 d | Tween 80 (same volume, orally); onset the experiment; once daily for 15 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| Aged Kunming mice (male, 10/10) | 40-50 g | NR | Ethanol-induced dysmnesia model | NR | Essential oil (0.02, 0.04, and 0.08 g kg−1, orally); onset the experiment; once daily for 15 d | Tween 80 (same volume, orally); onset the experiment; once daily for 15 d | (1) SD test (escape latency) | (1) P < 0.01 | |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| Aged SD rats (male, 10/10) | 550-650 g | NR | Aged rats | NR | Essential oil (0.02, 0.04, and 0.08 g kg−1, orally); onset the experiment; once daily for 30 d | Tween 80 (same volume, orally); onset the experiment; once daily for 30 d | (1) EY-M test (number of errors) | (1) P < 0.01 | |
| (2) NE, DA and 5-HT level | (2) P < 0.01 | ||||||||
| (3) AChE activity | (3) P < 0.01 | ||||||||
| (4) P < 0.01 | |||||||||
| Aged SD rats (male, 10/10) | 550-650 g | NR | Sodium nitrite-induced dysmnesia model | NR | Essential oil (0.02, 0.04, and 0.08 g kg−1, orally); onset the experiment; once daily for 30 d | Tween 80 (same volume, orally); onset the experiment; once daily for 30 d | (1) EY-M test (number of errors) | (1) P < 0.05 | |
| (2) P < 0.01 | |||||||||
|
| |||||||||
| Lee et al. [10] | SD rats (male, 5/7) | 250-280 g | NR | MCAO-induced cognitive impairments model | Isoflurane | AGA (100 mg kg−1, po); after occlusion; once daily for 21 d | Normal saline; (same volume, i.h); after occlusion; once daily for 21 d | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) Cell density | (2) P < 0.05 | ||||||||
|
| |||||||||
| Lee et al. [23] | SD rats (male, 7/7) | 200-220 g | NR | Chronic corticosterone-exposed model | Sodium pentobarbital | β-Asarone (50, 100, and 200 mg kg−1, ip); 30 min prior to the CORT; once daily for 21 d | Normal saline; (same volume, ip); 30 min prior to the CORT; once daily for 21 d | (1) MWM test (swimming speed) | (1) P > 0.05 |
| (2) serum CORT levels | (2) P < 0.05 | ||||||||
| (3) BDNF and CREB expression | (3) P < 0.05 | ||||||||
| (4) Bax and Bcl-2 mRNAs expression | (4) P < 0.05 | ||||||||
|
| |||||||||
| Kumar et al. 2012 | ICR mice (8/8) | NR | NR | Scopolamine-induced amnesic model mode | NR | α-Asarone (3, 10, and 30 mg kg−1, po); 15 d before scopolamine injection; once daily for 15 d | 0.5% methylcellulose solution containing 1% Tween 80 (same volume, po); 15 d before scopolamine injection; once daily for 15 d | (1) SD test (escape latency) | (1) P < 0.01 |
| (2) AchE activity | (2) P < 0.001 | ||||||||
| (3) MDA levels | (3) P < 0.001 | ||||||||
| (4) SOD activity | (4) P < 0.05 | ||||||||
|
| |||||||||
| Kim et al. [25] | SD rats (male, 5/5) | 260–280 g | NR | Ibotenic acid-induced amnesia | Sodium pentobarbital | AGA (100 mg kg−1,ip); after surgery; once daily for 3 weeks | Saline (same volume, ip); after surgery; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.001 |
| (2) ChAT positive neurons count | (2) P > 0.05 | ||||||||
| (3) AchE neurons density | (3) P < 0.05 | ||||||||
|
| |||||||||
| Geng et al. [26] | SD rats (male, 20/20) | 220-240 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | β-Asarone (12.5, 25, or 50 mg kg−1, ig); 3 d after Aβ (1-42) hippocampus injection; once daily for 28 d | Saline (same volume, ip); 3 d after Aβ (1-42) hippocampus injection; once daily for 28 d | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) MWM test (times crossed the platform) | (2) P < 0.05 | ||||||||
| (3) Annexin V-positive cells | (3) P < 0.05 | ||||||||
| (4) Caspase-3 and Caspase-3 mRNA express | (4) P < 0.05 | ||||||||
| (5) Bcl-2 and Bcl-2 mRNA levels | (5) P < 0.05 | ||||||||
| (6) Bcl-w, and Bcl-w mRNA express | (6) P < 0.05 | ||||||||
| (7) P-JNK express | (7) P < 0.05 | ||||||||
|
| |||||||||
| Chen et al. [27] | SAMP8 mice (13/13) | NR | NR | SAMP8 mice | NR | β-Asarone (34 mg kg−1, ig); onset the experiment; once daily for 2 months | Tween 80 (same volume, ig); onset the experiment; once daily for 2 months | (1) MWM test (number of platform crossing) | (1) P < 0.05 |
| (2) MWM test (escape latency) | (2) P < 0.05 | ||||||||
| (3) LC3-positive cells | (3) P < 0.05 | ||||||||
| (4) Beclin express | (4) P < 0.05 | ||||||||
| (5) 1p62 express | (5) P < 0.05 | ||||||||
| (6) ROCK1 express | (6) P < 0.05 | ||||||||
| (7) GAP43, MAP2 and SYN expression | (7) P < 0.05 | ||||||||
| (8) GAP43, MAP2 and SYN positive cells | (8) P < 0.05 | ||||||||
| (9) Lipofuscin-positive cells | (9) P < 0.05 | ||||||||
|
| |||||||||
| Ma et al. [28] | NIH mice (male, 6/6) | 18-20 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Water extract (0.02 g g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline (same volume, ig) after the first MWM test; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) Beta-amyloid IOD | (2) P < 0.05 | ||||||||
| NIH mice (male, 6/6) | 18-20 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Defatted decoction (0.02 g g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline (same volume, ig) after the first MWM test; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.05 | |
| (2) Beta-amyloid IOD | (2) P < 0.05 | ||||||||
| NIH mice (male, 6/6) | 18-20 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Essential oil (0.02 g g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline (same volume, ig) after the first MWM test; once daily for 3 weeks | (1) MWM test (escape latency) | (1) P < 0.05 | |
| (2) Beta-amyloid IOD | (2) P < 0.05 | ||||||||
|
| |||||||||
| Tian et al. [29] | NIH mice (male, 6/6) | 18-20 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Water extract (0.02 g g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline (0.2 ml/10 g, ig); after surgery; once daily for 3 weeks | (1) MWM test (number of platform crossing) | (1) P < 0.05 |
| (2) NOS activity | (2) P < 0.05 | ||||||||
| NIH mice (male, 6/6) | 18- 0 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Defatted decoction (0.02 g g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline (0.2 ml/10 g, ig); after surgery; once daily for 3 weeks | (1) MWM test (number of platform crossing) | (1) P > 0.05 | |
| (2) MWM test (time spent in target quadrant) | (2) P > 0.05 | ||||||||
| (3) NOS activity | (3) P < 0.05 | ||||||||
| NIH mice (male, 6/6) | 18-20 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | Essential oil (0.02 g g−1, ig); after the first MWM test; once daily for 3 weeks | Normal saline (0.2 ml/10 g, ig); after surgery; once daily for 3 weeks | (1) MWM test (number of platform crossing) | (1) P < 0.05 | |
| (2) NOS activity | (2) P < 0.05 | ||||||||
|
| |||||||||
| Zhou et al. [30] | SD rats (male, 10/10) | 250 ± 20 g | NR | Scopolamine-induced AD model | NR | Essential oil (12 g kg−1, ig); onset the experiment; once daily for 21 d | NS (same volume, ig); onset the experiment; once daily for 21 d | (1) MWM test (escape latency) | (1) P < 0.01 |
| (2) MWM test (number of platform crossing) | (2) P < 0.01 | ||||||||
| (3) GFAP - positive cells | (3) P < 0.01 | ||||||||
| (4) SOD content | (4) P < 0.05 | ||||||||
| (5) MDA content | (5) P < 0.05 | ||||||||
|
| |||||||||
| Wang GM et al., 2017 | Kunming mice (mix, 12/12) | 5-6 weeks | NR | Chronic restraint stress-induced cognitive impairments mode | NR | Essential oil (4.5 g kg−1, ig), onset the experiment; twice daily for 28 d | NS (same volume, ig); onset the experiment; twice daily for 28 d | (1) MWM test (escape latency) | (1) P < 0.01 |
| (2) MWM test (number of platform crossing) | (2) P < 0.01 | ||||||||
| (3) Body mass | (3) P < 0.05 | ||||||||
| (4) Plasma cortisol levels | (4) P < 0.01 | ||||||||
|
| |||||||||
| Hu et al. [32] | Kunming mice (male, 11/11) | 18-20 g | NR | Sodium nitrite-induced amnesic model | NR | Essential oil (0.053 g kg−1, ig); 21 d before sodium nitrite injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before sodium nitrite injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.05 |
| (2) SD test (number of errors) | (2) P < 0.05 | ||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium nitrite-induced amnesic model | NR | Defatted decoction (5 g kg−1, ig); 21 d before sodium nitrite injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before sodium nitrite injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.05 | ||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium nitrite-induced amnesic model | NR | α-Asarone (0.024 g kg−1, ig); 21 d before sodium nitrite injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before sodium nitrite injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | |||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium nitrite-induced amnesic model | NR | β-Asarone (0.037 g kg−1, ig); 21 d before sodium nitrite injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before sodium nitrite injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Ethanol-induced amnesic model | NR | Essential oil (0.053 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Ethanol-induced amnesic model | NR | Defatted decoction (5 g kg−1, ig); 21d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) SD test (escape latency) | (1) P > 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.05 | ||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Ethanol-induced amnesic model | NR | α-Asarone (0.024 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.01 | |
| (2) SD test (number of errors) | |||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Ethanol-induced amnesic model | NR | β-Asarone (0.037 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium pentobarbital-induced amnesic model | NR | Essential oil (0.053 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) EY-M test (number of errors) | (1) P < 0.05 | |
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium pentobarbital-induced amnesic model | NR | Defatted decoction (5 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) EY-M test (number of errors) | (1) P < 0.05 | |
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium pentobarbital-induced amnesic model | NR | α-Asarone (0.024 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) EY-M test (number of errors) | (1) P < 0.05 | |
| Kunming mice (male, 11/11) | 18-20 g | NR | Sodium pentobarbital-induced amnesic model | NR | β-Asarone (0.037 g kg−1, ig); 21 d before ethanol injection; once daily for 21 d | Tween 80 (same volume, ig); 21 d before ethanol injection; once daily for 21 d | (1) EY-M test (number of errors) | (1) P < 0.05 | |
|
| |||||||||
| Chen et al. [33] | ICR mice (male, 10/10) | 18 ± 1 g | Random number table | D-gal-induced dementia model | NR | Water extract (70, 35, 17.5, or 8.75 mg kg−1, ig); 1 week after D-galactose injection; once daily for 7 weeks | Distilled water (same volume, ig); 1 week after D-galactose injection; once daily for 7 weeks | (1) MWM test (escape latency) | (1) P > 0.05 |
| (2) MWM test (number of platform crossing) | (2) P > 0.05 | ||||||||
| (3) MDA levels | (3) P > 0.05 | ||||||||
| (4) SOD activity | (4) P > 0.05 | ||||||||
|
| |||||||||
| Gu et al. [34] | ICR mice (male, 10/10) | 19.6 ± 1.5 g | Random number table | Scopolamine-induced dysmnesia model | NR | Water extract (70, 35, 17.5, or 8.75 mg kg−1, ig); onset the experiment; once daily for 2 weeks | NS (same volume, ig); onset the experiment; once daily for 2 weeks | (1) SD test (escape latency) | (1) P < 0.01 |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| ICR mice (male, 10/10) | 19.6 ± 1.5 g | Random number table | NaNO2-induced dysmnesia model | NR | Water extract (70, 35, 17.5, or 8.75 mg kg−1, ig); onset the experiment; once daily for 2 weeks | NS (same volume, ig); onset the experiment; once daily for 2 weeks | (1) SD test (escape latency) | (1) P < 0.01 | |
| (2) SD test (number of errors) | (2) P < 0.05 | ||||||||
| ICR mice (male, 10/10) | 19.6 ± 1.5 g | Random number table | Ethanol-induced dysmnesia model | NR | Water extract (70, 35, 17.5, or 8.75 mg kg−1, ig); onset the experiment; once daily for 2 weeks | NS (same volume, ig); onset the experiment; once daily for 2 weeks | (1) ST test (escape latency) | (1) P < 0.01 | |
| (2) ST test (number of errors) | (2) P < 0.01 | ||||||||
| (3) AchE activity | (3) P > 0.05 | ||||||||
| Wistar rats (male, 10/10) | 200 ± 25 g | NR | Scopolamine-induced dysmnesia model | NR | Water extract (35, 17.5, or 8.75 mg kg−1, ig); onset the experiment; once daily for 4 weeks | NS (same volume, ig); onset the experiment; once daily for 2 weeks | (1) MWM test (escape latency) | (1) P < 0.01 | |
| (2) MWM test (number of platform crossing) | (2) P < 0.01 | ||||||||
|
| |||||||||
| Wu et al., 2004 | Aged NIH mice (male, 10/10) | NR | NR | Aged mice | NR | Essential oil (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) AchE activity | (2) P < 0.05 | ||||||||
| (3) C-jun express | (3) P < 0.05 | ||||||||
| Aged NIH mice (male, 10/10) | NR | NR | Aged mice | NR | β-Asarone (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) MWM test (escape latency) | (1) P > 0.05 | |
| (2) MWM test (number of errors) | (2) P > 0.05 | ||||||||
| (3) AchE activity | (3) P > 0.05 | ||||||||
| (4) C-jun express | (4) P > 0.05 | ||||||||
| Aged NIH mice (male, 10/10) | NR | NR | Aged mice | NR | Water extract (0. 01075 ml g−1 g, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) MWM test (escape latency) | (1) P > 0.05 | |
| (2) AchE activity | (2) P > 0.05 | ||||||||
| (3) C-jun express | (3) P < 0.05 | ||||||||
| Kunming mice (male, 10/10) | NR | NR | Ethanol-induced dysmnesia model | NR | Water extract (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) ST test (escape latency) | (1) P < 0.05 | |
| Kunming mice (male, 10/10) | NR | NR | NaNO2-induced dysmnesia model | NR | Essential oil (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) SD test (escape latency) | (1) P > 0.05 | |
| (2) SD test(number of errors) | (2) P > 0.05 | ||||||||
| Kunming mice (male, 10/10) | NR | NR | NaNO2-induced dysmnesia model | NR | β-Asarone (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P > 0.05 | ||||||||
| Kunming mice (male, 10/10) | NR | NR | NaNO2-induced dysmnesia model | NR | Water extract (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P > 0.05 | ||||||||
| Kunming mice (male, 10/10) | NR | NR | Scopolamine-induced dysmnesia model | NR | Essential oil (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P > 0.05 | ||||||||
| Kunming mice (male, 10/10) | NR | NR | Scopolamine-induced dysmnesia model | NR | β-Asarone (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P > 0.05 | ||||||||
| Kunming mice (male, 10/10) | NR | NR | Scopolamine-induced dysmnesia model | NR | Water extract (0. 01075 ml g−1, ig); onset the experiment; twice daily for 10 d | NS (same volume, ig); onset the experiment; once daily for 10 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test(number of errors) | (2) P > 0.05 | ||||||||
|
| |||||||||
| Wen et al., 2009 | ICR mice (mix, 10/10) | 20 ± 2 g | NR | Ethanol-induced dysmnesia model | NR | Water extract (3 and 12 g kg−1, ig); onset the experiment; twice daily for 14 d | NS (same volume, ig); onset the experiment; twice daily for 14 d | (1) ST test (escape latency) | (1) P < 0.01 |
| ICR mice (mix, 10/10) | 20 ± 2 g | NR | NaNO2-induced dysmnesia model | NR | Essential oil (3 and 12 g kg−1, ig); onset the experiment; once daily for 14 d | NS (same volume, ig); onset the experiment; twice daily for 14 d | (2) EY-M test (number of errors) | (1) P < 0.05 | |
| ICR mice (mix, 10/10) | 20 ± 2 g | NR | Scopolamine-induced dysmnesia model | NR | Water extract (3 and 12 g kg-1, ig); onset the experiment; once daily for 14 d | NS (same volume, ig); onset the experiment; twice daily for 14 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.05 | ||||||||
| ICR mice (mix, 10/10) | 20 ± 2 g | NR | Scopolamine-induced dysmnesia model | NR | Essential oil (3 and 12 g kg−1, ig); onset the experiment; once daily for 14 d | NS (same volume, ig); onset the experiment; twice daily for 14 d | (1) SD test (escape latency) | (1) P < 0.05 | |
| (2) SD test (number of errors) | (2) P < 0.01 | ||||||||
| ICR mice (mix, 10/10) | 20 ± 2 g | NR | Scopolamine-induced dysmnesia model | NR | Water extract (3 and 12 g kg−1, ig); onset the experiment; once daily for 14 d | NS (same volume, ig); onset the experiment; twice daily for 14 d | (1) MWM test (escape latency) | (1) P < 0.01 | |
| ICR mice (mix, 10/10) | 20 ± 2 g | NR | Scopolamine-induced dysmnesia model | NR | Essential oil (3 and 12 g kg-1, ig); onset the experiment; once daily for 14 d | NS (same volume, ig); onset the experiment; twice daily for 14 d | (1) MWM test (escape latency) | (1) P < 0.01 | |
|
| |||||||||
| Yang et al. [37] | SD rats (male, 12/12) | 250 ± 30 g | NR | Aβ1-42-induced AD model | NR | β-Asarone (10, 20, and 30 mg kg−1, ig); after the model finished; twice daily for 28 d | NS (same volume, ig); after the model finished; once daily for 28 d | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) MWM test (number of platform crossing) | (2) P < 0.01 | ||||||||
| (3) Astrocyte activity | (3) P < 0.01 | ||||||||
|
| |||||||||
| Zhou et al. [38] | SD rats (male,10/10) | 200-250 g | NR | D-gal- and AlCl3-induced AD model | NR | α-Asarone (10, 25 mg kg−1, ip); after the after model finished; once daily for 28 d | NS (same volume, ip); after the model finished; once daily for 28 d | (1) MWM test (number of platform crossing) | (1) P < 0.01 |
| (2) Aβ and tau protein expression | (2) P < 0.01 | ||||||||
| (3) ACh levels | (3) P < 0.05 | ||||||||
| (4) AChE levels | (4) P > 0.05 | ||||||||
| (5) ChAT levels | (5) P > 0.05 | ||||||||
|
| |||||||||
| Jiang et al., 2007 | Kunming mice (mix, 10/10) | 18-20 g | NR | AlCl3-induced AD model | NR | β-Asarone (1.06, 2.12, and 4.24 mg 100 g−1, ig); after the model finished; once daily for 2 months | NS (same volume, ig); after the model finished; once daily for 2 months | (1) MWM test (number of errors) | (1) P < 0.01 |
| (2) SOD levels | (2) P < 0.01 | ||||||||
| (3) MAD levels | (3) P < 0.01 | ||||||||
|
| |||||||||
| Huang et al. [40] | FMR1gene knock mice (16/17) | 17-18 g | NR | Fragile X syndrome model | NR | α-Asarone (3, 6, 9, 12, 24 mg kg−1, ip); onset the experiment; once daily for 8 d | NS (same volume, ip); onset the experiment; once daily for 8 d | (1) SD test (number of errors) | (1) P > 0.05 |
| (2) P-Akt expression | (2) P < 0.05 | ||||||||
| (3) Akt expression | (3) P > 0.05 | ||||||||
|
| |||||||||
| Wang BL et al., 2017 | SD rats (male, 15/15) | 280 ± 20 g | Random number table | Aβ1-42-induced AD model | Phenytoin sodium | β-Asarone (10, 20, and 30 mg kg−1, ig); after the model finished; once daily for 4 weeks | NS (same volume, ig); after the model finished; once daily for 4 weeks | (1) MWM test (escape latency) | (1) P < 0.01 |
| (2) MWM test (number of platform crossing) | (2) P < 0.01 | ||||||||
| (3) HIF levels | (3) P < 0.05 | ||||||||
|
| |||||||||
| Guo et al. [42]. | Kunming mice (male, 11/11) | 25 ± 5 g | Random block allocation method | Scopolamine-induced AD model | NR | β-Asarone (21.2 mg kg−1, ig); after the model finished; once daily for 14 d | NS (same volume, ig); after the model finished; once daily for 14 d | (1) MWM test (escape latency) | (1) P < 0.01 |
|
| |||||||||
| Jiang et al. [43] | Wistar rats (mix, 8/8) | 250-300 g | NR | STZ-induced AD model | NR | Essential oil (5, 10 and 20 g kg-1, ig); onset the experiment; once daily for 20 d | Solvent (same volume, ig); onset the experiment; once daily for 20 d | (1) MWM test (escape latency) | (1) P < 0.01 |
| (2) SOD levels | (2) P < 0.01 | ||||||||
| (3) MAD levels | (3) P < 0.01 | ||||||||
|
| |||||||||
| Yang et al. [44] | Wistar rats (10/10) | 35 ± 5 g | NR | PTZ-induced epilepsy model | NR | α-Asarone (29 mg kg−1, ig); after PTZ injection; twice daily for 7 d | NS (same volume, ig); after PTZ injection; twice daily for 7 d | (1) MWM test (number of platform crossing) | (1) P < 0.05 |
| (2) MWM test (time spent in target quadrant) | (2) P < 0.05 | ||||||||
| Wistar rats (10/10) | 35 ± 5 g | NR | PTZ-induced epilepsy model | NR | AGA (2.35 g kg−1, ig); after PTZ injection; twice daily for 7 d | NS (same volume, ig); after PTZ injection; twice daily for 7 d | (1) MWM test (number of platform crossing) | (1) P < 0.05 | |
| (2) MWM test (time spent in target quadrant) | (2) P < 0.05 | ||||||||
|
| |||||||||
| Wang et al. [45] | ICR mice (mix, 10/10) | 20 ± 2 g | NR | Scopolamine-induced dysmnesia model | NR | Essential oil (100, 150, and 300 mg kg-1, ig); before the experiment; once daily for 7 d | NS (same volume, ig); before the experiment; once daily for 7 d | (1) MWM test (escape latency) | (1) P < 0.01 |
| (2) MWM test (number of platform crossing) | (2) P < 0.01 | ||||||||
| (3) MWM test (time spent in target quadrant) | (3) P < 0.01 | ||||||||
|
| |||||||||
| Ma et al. [46] | SD rats (male, 6/6) | 260-280 g | NR | Aβ1-42-induced AD model | Sodium pentobarbital | β-Asarone (12.5, 25, 50 mg kg−1, ig); after the model finished; once daily for 4 weeks | NS (same volume, ig); after the model finished; once daily for 4 weeks | (1) MWM test (escape latency) | (1) P < 0.05 |
| (2) GA P-43 mRNA levels | (2) P < 0.05 | ||||||||
| (3) SYP mRNA levels | (3) P < 0.05 | ||||||||
| (4) PSD-95 mRNA levels | |||||||||
Ach: acetylcholine; AchE: acetylcholinesterase; SD rats: Sprague-Dawley rats; NIH mice: National Institutes of Health mice; SAMP8 mice: senescence-accelerated mouseprone 8 mice; AD: Alzheimer's disease; AlCl3: aluminum trichloride; ChAT: acetylcholine transferase; D-gal: D-galactose; i.g.: intragastrical injection; i.p.: intraperitoneal injection; i.h.: hypodermic injection; MWM test: Morris water maze test; MCAO: middle cerebral artery occlusion; MDA: malondialdehyde; GSH-PX: glutathione peroxidase; NR: not report; SD test: step down test; STZ: streptozotocin; SOD: superoxide dismute; HIF: hypoxia-inducible factor; GSH-Px: glutathione peroxidase; NE: norepinephrine; 5-TH: 5-hydroxytryptamine; DA: dopamine; SYN/SYN: synaptophysin; NOS: nitric oxide synthase; Bcl-2: B-cell lymphoma/leukemia-2; MAP2: microtubule-associated protein 2; RAM: radial eight-arm maze; EY-M: electric Y-maze; Aβ1-42: amyloid beta 1-42; PTZ: pent ylenetet razol; NS: normal saline.
3.3. Study Quality
The quality scores of the 34 included studies varied from 1/10 to 6/10 with the average of 3.32. One study [40] got 1 point; 11 studies [29, 31–36, 38, 39, 42, 44] got 2 points; 9 studies [5, 6, 18, 24, 25, 27, 30, 43, 45] got 3 points; 4 studies got 4 points; 7 studies got 5 points; and 2 studies [20, 37] got 6 points. Thirty-four studies were published. Sixteen studies described control of temperature [6, 10, 17–26, 30, 37, 41, 45]. Random allocation was declared in 28 studies [5, 6, 11, 17, 19–23, 26–28, 30–39, 41–46]; 1 study [42] used random block allocation method, and 2 studies used the method of random digit table [34, 41]. Two studies [23, 37] described the use of blinded assessment of outcome. Thirteen studies did not use anesthetics with significant intrinsic neuroprotective activity, and the remaining 21 studies did not report the type of anesthetics [5, 6, 18, 19, 24, 27, 30–40, 42–45]. Sixteen studies reported compliance with animal welfare regulations [5, 10, 11, 17–22, 24, 27, 28, 37, 41, 43, 45]. Four studies mentioned statement of potential conflict of interests [11, 20, 28, 37]. None of the included studies reported allocation concealment, sample size calculation, and the utilization of animal or model with relevant comorbidities. The quality scores for the included studies are shown in Table 2.
Table 2.
Quality assessment of included studies.
| Study (years) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [17] | √ | √ | √ | √ | √ | 5 | |||||
| Wei et al., 2013 | √ | √ | √ | 3 | |||||||
| Sundaramahalingam et al. [18] | √ | √ | √ | 3 | |||||||
| Shin et al. [19] | √ | √ | √ | √ | 4 | ||||||
| Ma et al. [11] | √ | √ | √ | √ | √ | 5 | |||||
| Liu et al. [20] | √ | √ | √ | √ | √ | √ | 6 | ||||
| Limón et al. [21] | √ | √ | √ | √ | √ | 5 | |||||
| Li et al. [22] | √ | √ | √ | √ | √ | 5 | |||||
| Zhang et al. [5] | √ | √ | √ | 3 | |||||||
| Lee et al. [10] | √ | √ | √ | √ | 4 | ||||||
| Lee et al. [23] | √ | √ | √ | √ | √ | 5 | |||||
| Kumar et al., 2012 | √ | √ | √ | 3 | |||||||
| Kim et al. [25] | √ | √ | √ | 3 | |||||||
| Geng et al. [26] | √ | √ | √ | √ | 4 | ||||||
| Chen et al. [27] | √ | √ | √ | 3 | |||||||
| Ma et al. [28] | √ | √ | √ | √ | √ | 5 | |||||
| Tian et al. [29] | √ | √ | 2 | ||||||||
| Zhou et al. [30] | √ | √ | √ | 3 | |||||||
| Wang GM et al., 2017 | √ | √ | 2 | ||||||||
| Hu et al. [32] | √ | √ | 2 | ||||||||
| Chen et al. [33] | √ | √ | 2 | ||||||||
| Gu et al. [34] | √ | √ | 2 | ||||||||
| Wu et al., 2004 | √ | √ | 2 | ||||||||
| Wen et al., 2009 | √ | √ | 2 | ||||||||
| Yang et al. [37] | √ | √ | √ | √ | √ | √ | 6 | ||||
| Zhou et al. [38] | √ | √ | 2 | ||||||||
| Jiang et al., 2007 | √ | √ | 2 | ||||||||
| Huang et al. [40] | √ | 1 | |||||||||
| Wang BL et al., 2017 | √ | √ | √ | √ | √ | 5 | |||||
| Guo et al. [42] | √ | √ | 2 | ||||||||
| Jiang et al. [43] | √ | √ | √ | 3 | |||||||
| Yang et al. [44] | √ | √ | 2 | ||||||||
| Wang et al. [45] | √ | √ | √ | 3 | |||||||
| Ma et al. [46] | √ | √ | √ | √ | 4 |
1: peer-reviewed publication; 2: statements describing control of temperature; 3: randomization to treatment group; 4: allocation concealment; 5: blinded assessment of outcome; 6: avoidance of anesthetics with known notable intrinsic neuroprotective properties; 7: use of animals with relevant comorbidities; 8: sample size calculation; 9: compliance with animal welfare regulations; 10: declared any potential conflict of interest.
3.4. Effectiveness
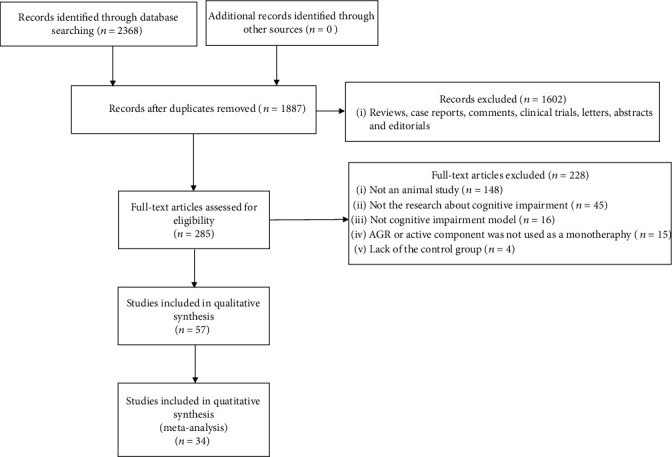
The Morris water maze test, including the probe test and the spatial test, was conducted in 28 studies [6, 10, 11, 17, 19, 20, 22, 23, 25–31, 33–39, 41–46]. Twenty-seven studies reported the spatial test using the escape latency as an outcome measure. Meta-analysis of 20 studies with 27 comparisons showed EAAGA significantly decreased the escape latency compared with the control (n = 490, SMD = −1.09, 95% CI [−1.37 to −0.82], P < 0.00001; heterogeneity: χ2 = 49.48, df = 26 (P = 0.004); I2 = 47%; Figure 2(a)). In the probe test, meta-analysis of 16 studies [17, 19, 20, 22, 26, 27, 29–31, 33, 34, 37, 38, 41, 44, 45] with 19 comparisons showed EAAGA were significant for increasing number of platform crossings (n = 398, SMD = 1.60, 95% CI [1.25 to 1.94], P < 0.00001; heterogeneity: χ2 = 34.29, df = 18 (P = 0.01); I2 = 47%; Figure 2(b)) compared with controls. Meta-analysis of 6 studies [17, 20, 22, 29, 34, 44] with 7 comparisons showed a significant effect of EAAGA in increasing the length of time spent in platform quadrant compared with control (n = 144, SMD = 1.78, 95% CI [0.90 to 2.67], P < 0.0001; heterogeneity: χ2 = 22.41, df = 6 (P = 0.001); I2 = 73%). As the values of I2 were greater than 50%, we sequentially omitting each study; two studies [20, 22] were removed and markedly reduced the heterogeneity (n = 86, SMD = 2.34, 95% CI [1.55 to 3.12], P < 0.00001; heterogeneity: χ2 = 6.57, df = 4 (P = 0.16); I2 = 39%; Figure 2(c)). Two studies [20, 22] used relatively large doses of β-asarone that might have potential toxic effects [47]. Meta-analysis of 3 studies [20, 23, 25] for increasing percentage of time in the platform quadrant (n = 44, SMD = 4.01, 95% CI [2.86 to 5.15], P < 0.00001; heterogeneity: χ2 = 0.03, df = 2 (P = 0.98); I2 = 0%; Figure 2(d)). Three studies [17, 22, 23] showed there were not a significant difference in improving the swimming velocity compared with controls.
Figure 2.
The forest plot in Morris water maze test. Effects of EAAGA for decreasing the escape latency (a) in spatial test, increasing crossing numbers (b), increasing exact time (c), and increasing percentage of time (d) in platform quadrant in probe test compared with control group.
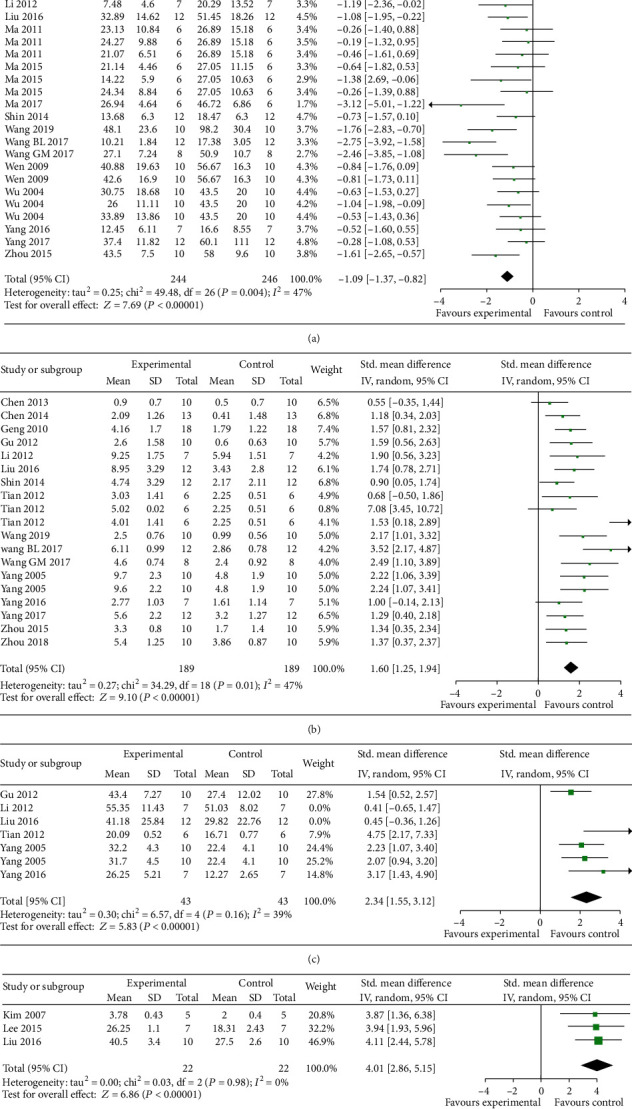
The step-down test, including the training test which represents learning ability and retention test which represents memory ability, was conducted in 6 studies [5, 32, 34–36, 40]. Meta-analysis of 5 studies with 19 comparisons showed EAAGA were significant for increasing right reaction latency in the retention test (n = 396, SMD = 1.15, 95% CI [0.87 to 1.43], P < 0.00001; heterogeneity: χ2 = 28.98, df = 18 (P = 0.05); I2 = 38%; Figure 3(a)) and 1 study [5] for increasing right reaction latency (P < 0.05) in the training test. Meta-analysis of 3 studies [32, 35, 36] with 16 comparisons showed EAAGA were significant for decreasing the error times (n = 336, SMD = −1.06, 95% CI [−1.30 to −0.83], P < 0.00001; heterogeneity: χ2 = 15.01, df = 15 (P = 0.45); I2 = 0%; Figure 3(b)) in the retention test and 1 study [5] for decreasing the error times (P < 0.05) in the training test.
Figure 3.
The forest plot in Step-down test. Effects of EAAGA for increasing right reaction latency in the retention test (a) and decreasing the error times in the retention test (b) compared with control group.
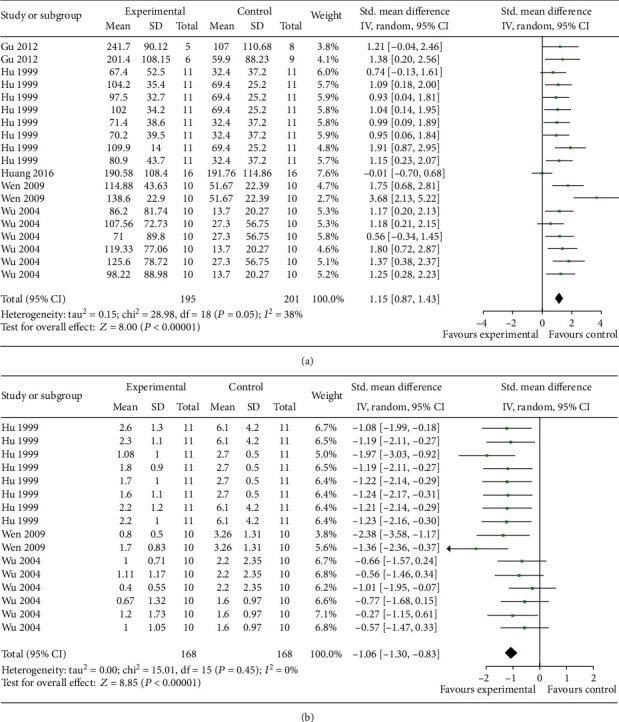
The electrical Y-maze test was conducted in 3 studies [5, 32, 36]. Meta-analysis of 3 studies showed EAAGA were significant for decreasing error reaction times (n = 168, SMD = −1.22, 95% CI [−1.56 to −0.88], P < 0.00001; heterogeneity: χ2 = 3.95, df = 7 (P = 0.79); I2 = 0%; Figure 4).
Figure 4.
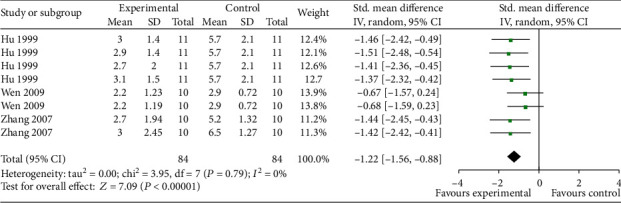
The forest plot in Electrical Y-maze test. Effects of EAAGA for decreasing error reaction times compared with control group.
The step-through test was conducted in 4 studies [24, 34–36]. Meta-analysis of 4 studies with 7 comparisons showed EAAGA were significant for decreasing latency in the retention test (n = 134, SMD = 1.26, 95% CI [0.81 to 1.71], P < 0.00001; heterogeneity: χ2 = 8.09, df =6 (P = 0.23); I2 = 26%; Figure 5(a)) and 2 studies [35, 36] with 5 comparisons showed EAAGA significantly decreased the number of errors in the retention test (n = 100, SMD = −1.02, 95% CI [−1.45 to −0.60], P < 0.00001; heterogeneity: χ2 = 1.05, df = 4 (P = 0.90); I2 = 0%; Figure 5(b)) compared with controls.
Figure 5.
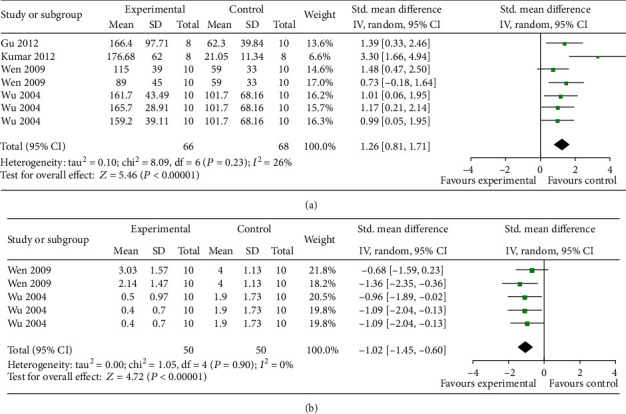
The forest plot in Step-through test. Effects of EAAGA for decreasing latency in the retention test (a) and decreasing the number of errors in the retention test (b) compared with control group.
The eight-arm maze test was conducted in 3 studies [10, 18, 21]. Meta-analysis of 2 studies [10, 21] showed EAAGA were significant for increasing number of correct choices (n = 25, SMD = 1.15, 95% CI [0.00 to 2.29], P = 0.05; heterogeneity: χ2 = 1.63, df = 1 (P = 0.20); I2 = 38%; Figure 6(a)) and 2 studies [10, 18] with 3comparisons showed EAAGA significantly decreased the number of errors in the training test (n = 33, SMD = −2.36, 95% CI [−3.36 to −1.37], P < 0.00001; heterogeneity: χ2 = 1.00, df = 2 (P = 0.61); I2 = 0%; Figure 6(b)) compared with controls.
Figure 6.
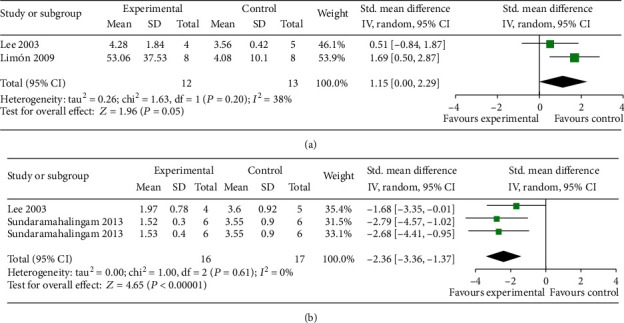
The forest plot in Eight-arm maze test. Effects of EAAGA for increasing correct choices (a) and decreasing the number of errors (b) compared with control group.
3.5. Neuroprotective Mechanisms
The mechanisms of neuroprotection of EAAGA on cognitive impairment were studied in 34 included articles [5, 6, 10, 11, 17–46] as follows: (1) reduction of oxidative reactions by increasing the activity of SOD [30, 35, 39, 41, 43] activity, while decreasing the activity of SOD and AChE [18, 24], decreasing the levels of MDA [24, 30, 33] and nitric oxide [21], decreasing the mRNA levels of hsp 70, increasing the levels of VC, VE, and GSH, and increasing the activity of CAT and G6PD [18]; (2) inhibition of apoptosis by increasing the mRNA levels of Bcl-2, BDNF, CREB [6, 23, 42], Bcl-w and Bcl-2 [26], and c-jun [35], decreasing the mRNA levels of Bax [23], increasing the expression of BDNF, CREB [23], Bcl-w, and Bcl-2 [26], decreasing the expression of caspase-3, p-JNK [26], and BACE1 [19], and preventing cell loss [10], Aβ, and Tau protein [38]; (3) repression of inflammatory reactions by decreasing the expression of TNF-α and IL-1β mRNA levels [19]; (4) repression of autophagy by decreasing LC3, ROCK, and beclin1 expression and increasing p62, GAP43, MAP2, and SYN expression [27]; (5) protection of cerebrovascular by increasing rCBF and the Na-K-ATP activity, decreasing pyruvic acid contents, and decreasing the mRNA levels of ET-1, eNOS, and APP [22]; (6) promotion of cognitive function by increasing the levels of 5-HT, NE, DA, and NE [5] and suppression of astrocyte activation [37]; (7) stimulation of cholinergic system by increasing AchE and ChAT neurons [25]; (8) improvement of memory impairments through regulation of synaptogenesis, which is mediated via Arc/Arg3.1 and Wnt pathway [17]; (9) neuroprotection through damage of Akt pathway [40]; (10) inhibition of neurotoxicity by decreasing the expression of DCx and nestin, decreasing nestin positive cells [11], decreasing Aβ plaques depositions, and decreasing NOS activity [29]; (11) regulation of synaptic plasticity by increasing the expression of SYP and GluR1 [20, 46] and decreasing the expression of GAP-43 and PSD-95 [46]; and (12) inhibition of chronic stress by decreasing plasma cortisol levels [41]. Characteristics of mechanism studies of EAAGA on experimental ischemic stroke are shown in Table 3 and Figure 7.
Table 3.
Characteristics of mechanism studies of EAAGA on cognition impairment.
| Study (years) | Model | Method of administration (experimental group versus control group) | Observations | Possible mechanisms |
|---|---|---|---|---|
| Yang et al. [17] | Chronic lead-induced dysmnesia model | β-Asarone versus distilled water | Attenuated memory deficits | Arc/Arg3.1 and Wnt pathway |
| Increased dendritic spine density | Increased dendritic spine density | |||
| Up-regulated NR2B, Arc/Arg3.1, and Wnt7a protein expression | ||||
|
| ||||
| Wei et al., 2013 | AβPP/PS1 double-transgenic mice | β-Asarone versus Tween 80 | Improved cognitive function | CaMKII/CREB/Bcl-2 signaling pathway |
| Prevents PC12 cell and cortical neuron damage | Inhibition of apoptosis | |||
| Inhibited the apoptosis of PC12 cells and cortical neurons | ||||
|
| ||||
| Sundaramahalingam et al. [18] | Noise stress induced memory impairment model | α-Asarone versus Tween 80 | Prevent memory impairment | Reduction of oxidative reactions |
| Decreased hsp 70 mRNA levels | ||||
| Decreased SOD and AChE activity | ||||
| Increased CAT and G6PD activity | ||||
| Increased VC, VE, and GSH levels | ||||
|
| ||||
| Shin et al. [19] | LPS-induced cognitive handicap mode | α-Asarone versus NS | Ameliorated memory deficits | Repression of inflammatory reactions |
| Reduced Iba1 protein expression | Inhibition of apoptosis | |||
| Reduced TNF-α and IL-1β mRNA | ||||
| Reduced BACE1 expression | ||||
| Increased CA1 neurons | ||||
| Reduced TUNEL-labeled cells | ||||
|
| ||||
| Ma et al. [11] | Aβ1-42-induced AD model | Water extract versus NS | Ameliorated memory deficits | Inhibition of neurotoxicity |
| Essential oil versus NS | Reduced Aβ positive cells | |||
| Defatted decoction versus NS | Decreased DCx and nestin expression | |||
| Decreased nestin positive cells | ||||
|
| ||||
| Liu et al. [20] | APPswe/PS1dE9 double transgenic mice | β-Asarone versus Tween 80 | Improved the learning and memory ability | Regulation of synaptic plasticity |
| Increased SYP and GluR1 expression | ||||
|
| ||||
| Limón et al. [21] | Aβ-induced AD model | α-Asarone versus NS | Ameliorated memory deficits | Reduction of oxidative reactions |
| Decreased NO levels | ||||
|
| ||||
| Li et al. [22] | D-gal- and AlCl3-induced AD model | β-Asarone versus NS | Improved the learning and memory ability | Protection of cerebrovascular |
| Increased rCBF and the activity of Na–K-ATP | ||||
| Decreased pyruvic acid contents | ||||
| Decreased ET-1, eNOS, and APP mRNA expression | ||||
|
| ||||
| Zhang et al. [5] | Aged mice | Essential oil versus Tween 80 | Improved cognitive function Increased 5-HT, NE, DA, and NE levels Decreased AChE activity |
Improvement of cognitive function |
| Scopolamine-induced dysmnesia model | Essential oil versus Tween 80 | |||
| Ethanol-induced dysmnesia model | Essential oil versus Tween 80 | |||
| Aged rats | Essential oil versus Tween 80 | |||
| Sodium nitrite-induced dysmnesia model | Essential oil versus Tween 80 | |||
|
| ||||
| Lee et al. [10] | MCAO/2 h-induced cognitive impairments model | AGA versus NS | Attenuated learning and memory deficits Increased cell density |
Inhibition of apoptosis |
|
| ||||
| Lee et al. [23] | Chronic corticosterone exposed | β-Asarone versus NS | Improved cognitive function Increased BDNF and CREB expression Increased BDNF, CREB, and Bcl-2 mRNAs levels Decreased Bax mRNAs levels Decreased serum levels of CORT |
Inhibition of apoptosis |
|
| ||||
| Kumar et al., 2012 | Scopolamine-induced amnesic model | α-Asarone versus vehicle | Improved cognitive function Increased of AchE activity Inhibition MDA expression and SOD levels Reduced SOD activity |
Reduction of oxidative reactions |
|
| ||||
| Kim et al. [25] | Ibotenic acid-induced amnesia | AGA versus NS | Ameliorated learning and memory deficits Increased ChAT positive neurons Increased AchE neurons |
Stimulation of cholinergic system |
|
| ||||
| Geng et al. [26] | Aβ1-42-induced AD rat model | β-Asarone versus NS | Ameliorated learning and memory deficits Increased Bcl-2, Bcl-w expression Increased Bcl-2 and Bcl-w mRNA levels Decreased cleavage of caspase-3 Reduced caspase-3 mRNA levels Decreased p-JNK expression |
Inhibition of apoptosis |
|
| ||||
| Chen et al. [27] | SAMP8 mice | β-Asarone versus NS | Improved cognitive function Reduced ROCK, beclin1, and LC3 expression Increased p62 expression Increased GAP43, MAP2, and SYN expression Increased GAP43-, MAP2-, and SYN-positive cells Decreased lipofuscin-positive cells |
Reduction of autophagy |
|
| ||||
| Ma et al. [28] | Aβ-induced AD model | Water extract versus NS | Ameliorated learning and memory deficits Decreased Aβ plaques depositions |
Improvement of cognitive function |
| Water extract without oil versus NS | ||||
| Essential oil versus NS | ||||
|
| ||||
| Tian et al. [29] | Aβ-induced AD model | Water extract versus NS | Ameliorated learning and memory deficits Decreased NOS activity |
Inhibition of neurotoxicity |
| Defatted decoction versus NS | ||||
| Essential oil versus NS | ||||
|
| ||||
| Zhou et al. [30] | Scopola-induced AD model | Essential oil versus NS | Ameliorated learning and memory deficits Decreased GFAP expression Decreased MDA levels Increased SOD levels |
Reduction of oxidative reactions |
|
| ||||
| Wang GM et al., 2017 | Chronic restraint stress-induced cognitive impairments mode | Essential oil versus NS | Ameliorated learning and memory deficits Increased body mass Decreased plasma cortisol levels |
Inhibition of chronic stress |
|
| ||||
| Hu et al. [32] | Sodium nitrite-induced amnesic model | Essential oil versus Tween 80 | Increased learning and memory deficits | Improvement of cognitive function |
| Defatted decoction versus Tween 80 | ||||
| α-Asarone versus Tween 80 | ||||
| β-Asarone versus Tween 80 | ||||
| Ethanol-induced amnesic model | Essential oil versus Tween 80 | Ameliorated learning and memory deficits | Improvement of cognitive function | |
| Defatted decoction versus Tween 80 | ||||
| α-Asarone versus Tween 80 | ||||
| β-Asarone versus Tween 80 | ||||
|
| ||||
| Chen et al. [33] | D-galactose-induced dementia model | Water extract versus distilled water | Ameliorated memory deficits Decreased MDA levels Increased SOD activity |
Reduction of oxidative reactions |
|
| ||||
| Gu et al. [34] | Scopolamine-induced dysmnesia mice | Water extract versus NS | Ameliorated memory deficits | Improvement of cognitive function |
| NaNO2-induced dysmnesia model | Water extract versus NS | Ameliorated memory deficits | ||
| 45% ethanol-induced dysmnesia mice | Water extract versus NS | Ameliorated memory deficits The AchE activity of mice brain was not influenced |
||
| Scopolamine-induced dysmnesia rat | Water extract versus NS | Ameliorated memory deficits | ||
|
| ||||
| Wu et al., 2004 | Aged mice | Essential oil versus NS | Ameliorated memory deficits Decreased AChE activity Increased c-jun mRNA levels |
Inhibition of apoptosis |
| Aged mice | β-Asarone versus NS | |||
| Aged mice | Water extract versus NS | |||
| Ethanol-induced dysmnesia model | Water extract versus NS | |||
| NaNO2-induced dysmnesia model | Essential oil versus NS | |||
| NaNO2-induced dysmnesia model | β-Asarone versus NS | |||
| NaNO2-induced dysmnesia model | Water extract versus NS | Ameliorated memory deficits | ||
| Scopolamine-induced dysmnesia model | Essential oil versus NS | |||
| Scopolamine-induced dysmnesia model | Essential oil versus NS | |||
| Scopolamine-induced dysmnesia model | Water extract versus NS | |||
|
| ||||
| Wen et al., 2009 | Ethanol-induced dysmnesia model | Water extract versus NS | Ameliorated memory deficits | Inhibition of apoptosis |
| NaNO2-induced dysmnesia model | Essential oil versus NS | |||
| Scopolamine-induced dysmnesia model | Water extract versus NS | |||
| Scopolamine-induced dysmnesia model | Essential oil versus NS | |||
| Scopolamine-induced dysmnesia model | Water extract versus NS | |||
| Scopolamine-induced dysmnesia model | Essential oil versus NS | |||
|
| ||||
| Yang et al. [37] | Aβ1-42-induced AD model | β-Asarone versus NS | Improved cognitive function Inhibited AQP4, IL-1β, and TNF-α expression Decreased Aβ deposition Alleviated hippocampal damage |
Suppression of astrocyte activation |
|
| ||||
| Zhou et al. [38] | D-gal- and AlCl3-induced AD model | α-Asarone versus NS | Improved cognitive function Decreased Aβ and Tau protein expression Increased ACh expression |
Inhibition of apoptosis |
|
| ||||
| Jiang et al. 2007 | AlCl3-induced AD model | β-Asarone versus NS | Improved cognitive function Increased SOD and GSH-Px levels Decreased MAD levels |
Reduction of oxidative reactions |
|
| ||||
| Huang et al. [40] | Fragile X syndrome model | α-Asarone versus NS | Improved cognitive function | Damage of Akt pathway |
|
| ||||
| Wang BL et al., 2017 | Aβ1-42-induced AD model | β-Asarone versus NS | Improved cognitive function Decreased HIF and MDA levels Increased SOD and CAT levels |
Reduction of oxidative reactions |
|
| ||||
| Guo et al. [42] | Scopolamine-induced AD model | β-Asarone versus NS | Improved cognitive function | Inhibition of apoptosis |
|
| ||||
| Jiang et al. [43] | STZ-induced AD model | Essential oil versus solvent | Improved cognitive function Decreased MDA levels Increased SOD levels |
Reduction of oxidative reactions |
|
| ||||
| Yang et al. [44] | PTZ-induced epilepsy model | AGA versus NS | Improved cognitive function | Inhibition of apoptosis |
| PTZ-induced epilepsy model | α-Asarone versus NS | Improved cognitive function | Inhibition of apoptosis | |
|
| ||||
| Wang et al. [45] | Aβ1-42-induced AD model | Essential oil versus NS | Improved cognitive function | Improvement of cognitive function |
|
| ||||
| Ma et al. [46] | D-gal- and AlCl3-induced AD model | β-Asarone versus NS | Improved cognitive function Decreased GA P-43 mRNA levels Increased SYP mRNA levels Decreased PSD-95 mRNA levels |
Regulation of synaptic plasticity |
Ach: acetylcholine; AchE: acetylcholinesterase; AD: Alzheimer's disease; AlCl3: aluminum trichloride; ChAT: acetylcholine transferase; D-gal: D-galactose; MCAO: middle cerebral artery occlusion; MDA: malondialdehyde; STZ: streptozotocin; SOD: superoxide dismute; HIF: hypoxia-inducible factor; SYN/SYN: synaptophysin; MAP2: microtubule-associated protein 2; Aβ1-42: amyloid beta 1-42; NS: normal saline; PTZ: pent ylenetet razol; GSH-Px: glutathione peroxidase; NE: norepinephrine; 5-TH: 5-hydroxytryptamine; DA: dopamine; NOS: nitric oxide synthase; Bcl-2: B-cell lymphoma/leukemia-2.
Figure 7.
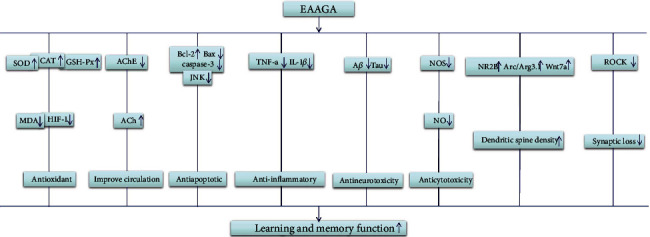
A schematic representation of possible mechanisms of EAAGA for improving learning and memory function. The possible mechanisms of different active ingredients are as follows: (1) AGA: the dry rhizomes of Acorus gramineus Solander can inhibit apoptosis and stimulate cholinergic system. (2) Essential oil: AGA contains up to 4.86% essential oil, which displayed antioxidation effects by decreasing the levels of MDA and increasing the levels of SOD, exhibited anticytotoxicity effects via decreasing NOS activity, exerted antineurotoxicity effects by decreasing Aβ plaques depositions, and improved cognitive function by decreasing the activity of AChE. (3) β-Asarone: a major component of essential oil (63.2–81.2%) displayed antioxidation effects by decreasing the levels of MDA and HIF, increasing the levels of SOD, CAT, and GSH-Px; exerted antiapoptotic activity through regulating CaMKII/CREB/Bcl-2 signaling pathway and decreasing the levels of Bax mRNAs, caspase-3 mRNA, and JNK; inhibited synaptic loss through reducing ROCK expression; mediated synaptogenesis via Arc/Arg3.1 and Wnt pathway; improved circulation by decreasing the activity of AChE; and exerted antineurotoxicity by decreasing Aβ plaques depositions. (4) α-Asarone: another major component of essential oil (8.8–13.7%) exerted antioxidation effects by increasing CAT, SOD, and GSH-Px.; displayed anti-inflammatory activity through reducing the expression of proinflammatory mediators; improved circulation via decreasing the activity of AChE; and exerted antineurotoxicity by decreasing Aβ plaques depositions. (5) Water extract: displayed antioxidation effects by decreasing the levels of MDA and increasing the levels of SOD, exerted antineurotoxicity by decreasing Aβ plaques depositions; and improved cognitive function by decreasing the activity of AChE. (6) Defatted decoction: exerted antineurotoxicity by decreasing Aβ plaques depositions and displayed anticytotoxicity effects via decreasing the activity of NOS.
4. Discussion
As far as we know, it is the first preclinical systematic review that determined the efficacy of EAAGA for learning and memory function. In the present study, 34 studies with 1431 animals showed that EAAGA significantly improve learning and memory function, suggesting the potential neuroprotective functions of EAAGA in cognitive function impairment. However, given methodological weaknesses, the overall available evidence from the present study should be interpreted cautiously.
Some limitations should be considered while interpreting this study. First, we only searched databases in Chinese and English. The absence of studies published in other languages may cause certain degree selective bias [48]. Second, the methodological quality of included studies showed some inherent drawback. Most of the research had methodological flaws in aspects of blinding, randomization, allocation concealment, sample size calculation, and lacking statement of potential conflict of interests [49, 50]. The studies without adequate sample sizes, allocation concealment, or randomization may result in inflated estimates of treatment efficacy [51, 52]. Lower quality trials could attribute to statistically significant 30–50% exaggeration of treatment efficacy [53]. Third, no study adopted animals with comorbidities, which would have created more relevant models for human pathology [49]. Thereby, the results should be interpreted cautiously.
The poor design of animal research hindered the translation of animal research into effective preclinical drug treatments for human disease [54, 55]. Thus, it is necessary to take a rigor experimental design to overcome methodology quality issues for further research. The Animal Research: Reporting of In Vivo Experiments (ARRIVE) [56, 57] is a reporting guideline consisting of a 20-item checklist that provides recommendations on Introduction, Methods, Results, and Discussion which were recommended to be utilized as guidelines when designing and reporting animal research on EAAGA for improving the cognitive function impediment. Meanwhile, many drugs that exerted significant effects in animal researches failed to translate into effective clinical drug treatments [58, 59]. One of the possible reasons is the application of drug doses and the timing of drug administration in animal models that are inapplicable for human disease [55]. In the present study, doses of EAAGA and timing for initial administration in animal models were inconsistent among the 34 included studies. Thus, we suggest further studies to determinate the optimal gradient doses and timing of administration in animal models of cognition impairment.
The present study showed that EAAGA had cognitive enhancing effects through different mechanisms as follows: (1) reduction of oxidative reactions by increasing the activity of SOD [30, 35, 39, 41, 43] activity, while decreasing the activity of SOD and AChE [18, 24], decreasing the levels of MDA [24, 30, 33] and nitric oxide [21], decreasing the mRNA levels of hsp 70, increasing the levels of VC, VE and GSH, and increasing the activity of CAT and G6PD [18]; (2) inhibition of apoptosis by increasing the mRNA levels of Bcl-2, BDNF, CREB [6, 23, 42], Bcl-w and Bcl-2 [26], and c-jun [35], decreasing the mRNA levels of Bax [23], increasing the expression of BDNF, CREB [23], Bcl-w, and Bcl-2 [26], decreasing the expression of caspase-3, p-JNK [26], and BACE1 [19], and preventing cell loss [10], Aβ, and Tau protein [38]; (3) repression of inflammatory reactions by decreasing the expression of TNF-α and IL-1β mRNA levels [19]; (4) repression of autophagy by decreasing LC3, ROCK, and beclin1 expression and increasing p62, GAP43, MAP2, and SYN expression [27]; (5) protection of cerebrovascular by increasing rCBF and the Na-K-ATP activity, decreasing pyruvic acid contents, and decreasing the mRNA levels of ET-1, eNOS, and APP [22]; (6) promotion of cognitive function by increasing the levels of 5-HT, NE, DA, and NE [5] and suppression of astrocyte activation [37]; (7) stimulation of cholinergic system by increasing AchE and ChAT neurons [25]; (8) improvement of memory impairments through regulation of synaptogenesis, which is mediated via Arc/Arg3.1 and Wnt pathway [17]; (9) neuroprotection through damage of Akt pathway [40]; (10) inhibition of neurotoxicity by decreasing the expression of DCx and nestin, decreasing nestin positive cells [11], and decreasing Aβ plaques depositions, decreased NOS activity [29]; (11) regulation of synaptic plasticity by increasing the expression of SYP and GluR1 [20, 46] and decreasing the expression of GAP-43 and PSD-95 [46]; and (12) inhibition of chronic stress by decreasing plasma cortisol levels [41]. However, cellular and molecular alteration mechanisms of EAAGA and active components for cognition impairment have not been clearly explored yet, which presented an exciting investigative direction of further studies. All 5 measuring methods for learning and memory ability were used in the 34 included studies, which showed that the measuring methods for cognition impairment were inconsistent. The diverse measuring methods for learning and memory ability need further study.
5. Conclusions
Although some factors such as study quality may undermine the validity, EAAGA exert potential neuroprotective effects in cognition impairment. In addition, AGA and active components may be a promising candidate for clinical trials.
Acknowledgments
This project was supported by the Young and MiddleAged University Discipline Leaders of Zhejiang Province, China (2013277) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (2015). We would like to thank LetPub (http://www.letpub.com) for providing linguistic assistance during the preparation of this manuscript. This work was supported by the grant from the National Natural Science Foundation of China (81573750/81473491/81173395/H2902).
Contributor Information
Guo-Qing Zheng, Email: gq_zheng@sohu.com.
Li-Ping Zhang, Email: zlphmx@163.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Thompson R. F. The neurobiology of learning and memory. Science. 1986;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 2.WHO and Alzheimer’s Disease. International Dementia: a Public Health Priority. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Calia C., Johnson H., Cristea M. Cross-cultural representations of dementia: an exploratory study. Journal of Global Health. 2019;9(1, article 011001) doi: 10.7189/jogh.09.011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parihar M.-S., Hemnani T. Alzheimer's disease pathogenesis and therapeutic interventions. Journal of Clinical Neuroscience. 2004;11(5):456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H., Han T., Yu C.-H., Rahman K., Qin L.-P., Peng C. Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. Journal of Pharmacy and Pharmacology. 2007;59(2):301–309. doi: 10.1211/jpp.59.2.0016. [DOI] [PubMed] [Google Scholar]
- 6.Wei G., Chen Y.-B., Chen D.-F., et al. β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AβPP/PS1 mice. Journal of Alzheimer's Disease. 2013;33(3):863–880. doi: 10.3233/JAD-2012-120865. [DOI] [PubMed] [Google Scholar]
- 7.Irie Y., Keung W.-M. Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid- β peptide. Brain Research. 2003;963(1-2):282–289. doi: 10.1016/S0006-8993(02)04050-7. [DOI] [PubMed] [Google Scholar]
- 8.Koo B.-S., Park K.-S., Ha J.-H., Park J.-H., Lim J.-C., Lee D.-U. Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biological & Pharmaceutical Bulletin. 2003;26(7):978–982. doi: 10.1248/bpb.26.978. [DOI] [PubMed] [Google Scholar]
- 9.Cho J., Joo N.-E., Kong J.-Y., Jeong D.-Y., Lee K.-D., Kang B.-S. Inhibition of excitotoxic neuronal death by methanol extract of Acori graminei rhizoma in cultured rat cortical neurons. Journal of Ethnopharmacology. 2000;73(1-2):31–37. doi: 10.1016/S0378-8741(00)00262-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee B., Choi Y., Kim H., et al. Protective effects of methanol extract of Acori graminei rhizoma and Uncariae Ramulus et Uncus on ischemia-induced neuronal death and cognitive impairments in the rat. Life Sciences. 2003;74(4):435–450. doi: 10.1016/j.lfs.2003.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y., Tian S., Sun L., et al. The effect of acori graminei rhizoma and extract fractions on spatial memory and hippocampal neurogenesis in amyloid beta 1-42 injected mice. CNS & Neurological Disorders Drug Targets. 2015;14(3):411–420. doi: 10.2174/1871527314666150225124348. [DOI] [PubMed] [Google Scholar]
- 12.Yan L., Xu S. L., Zhu K. Y., et al. Optimizing the compatibility of paired-herb in an ancient Chinese herbal decoction Kai-Xin-San in activating neurofilament expression in cultured PC12 cells. Journal of Ethnopharmacology. 2015;162:155–162. doi: 10.1016/j.jep.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Lee M.-R., Yun B.-S., Park S.-Y., et al. Anti-amnesic effect of Chong-Myung-Tang on scopolamine-induced memory impairments in mice. Journal of Ethnopharmacology. 2010;132(1):70–74. doi: 10.1016/j.jep.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Glasziou P., Vandenbroucke J.-P., Chalmers I. Assessing the quality of research. BMJ. 2004;328(7430):39–41. doi: 10.1136/bmj.328.7430.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy S.-P., Murphy A.-N. Pre-clinical systematic review. Journal of Neurochemistry. 2010;115(4):p. 805. doi: 10.1111/j.1471-4159.2010.06998.x. [DOI] [PubMed] [Google Scholar]
- 16.Macleod M. R., O'Collins T., Howells D. W., Donnan G. A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. doi: 10.1161/01.str.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q.-Q., Xue W.-Z., Zou R.-X., et al. β-Asarone rescues Pb-induced impairments of spatial memory and synaptogenesis in rats. PLoS One. 2016;11(12, article e0167401) doi: 10.1371/journal.pone.0167401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundaramahalingam M., Ramasundaram S., Rathinasamy S. D., Natarajan R. P., Somasundaram T. Role of Acorus calamus and a-asarone on hippocampal dependent memory in noise stress exposed rats. Pakistan Journal of Biological Sciences. 2013;16(16):770–778. doi: 10.3923/pjbs.2013.770.778. [DOI] [PubMed] [Google Scholar]
- 19.Shin J.-W., Cheong Y.-J., Koo Y.-M., et al. α-Asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomolecules & Therapeutics. 2014;22(1):17–26. doi: 10.4062/biomolther.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S., Yang C., Zhang Y., et al. Neuroprotective effect of β-asarone against Alzheimer’s disease: regulation of synaptic plasticity by increased expression of SYP and GluR1. Drug Design, Development and Therapy. 2016;10:1461–1469. doi: 10.2147/dddt.s93559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limón I. D., Mendieta L., Díaz A., et al. Neuroprotective effect of alpha-asarone on spatial memory and nitric oxide levels in rats injected with amyloid-β(25-35) Neuroscience Letters. 2009;453(2):98–103. doi: 10.1016/j.neulet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Li Z., Zhao G., Qian S., et al. Cerebrovascular protection of β-asarone in Alzheimer's disease rats: a behavioral, cerebral blood flow, biochemical and genic study. Journal of Ethnopharmacology. 2012;144(2):305–312. doi: 10.1016/j.jep.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Lee B., Sur B., Cho S.-G., et al. Effect of beta-asarone on impairment of spatial working memory and apoptosis in the hippocampus of rats exposed to chronic corticosterone administration. Biomolecules & Therapeutics. 2015;23(6):571–581. doi: 10.4062/biomolther.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar H., Kim B. W., Song S. Y., et al. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Bioscience, Biotechnology, and Biochemistry. 2014;76(8):1518–1522. doi: 10.1271/bbb.120247. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.-H., Hahm D.-H., Lee H.-J., Pyun K.-H., Shim I. Acori graminei rhizoma ameliorated ibotenic acid-induced amnesia in rats. Evidence-based Complementary and Alternative Medicine. 2009;6(4):457–464. doi: 10.1093/ecam/nem158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng Y., Li C., Liu J., et al. Beta-asarone improves cognitive function by suppressing neuronal apoptosis in the beta-amyloid hippocampus injection rats. Biological & Pharmaceutical Bulletin. 2010;33(5):836–843. doi: 10.1248/bpb.33.836. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Wei G., Nie H., et al. β-Asarone prevents autophagy and synaptic loss by reducing ROCK expression in asenescence-accelerated prone 8 mice. Brain Research. 2014;1552(2014):41–54. doi: 10.1016/j.brainres.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y.-X., Li G.-Y., Sun L.-Z., Tan L., Tian S.-M. Effects of the different extracted parts from Acori graminei rhizoma on learning and memory deficits in Aβ-injected mice. Chinese Journal of Neuroanatomy. 2011;27(5):521–526. [Google Scholar]
- 29.Tian S.-M., Ma Y.-X., Sun L.-Z., Tan L., Liu J., Li G.-Y. Effects of different fractions of Acori graminei rhizoma extracts on learning and memory abilities in A β - induced Alzheimer disease mice. Chinese Journal of Pathophysiology. 2012;28(1):159–162. [Google Scholar]
- 30.Zhou X.-J., Dai S.-J., Chen H.-S., Xie F.-R., Li C.-Y., Yang Y.-X. Effects of Acorus T atarinowii Schott volatile oil on learning and memory impairment rats induced by scopolamine and its possible mechanism. Journal of Gansu College of Traditional Chinese Medicine. 2015;32(1):1–6. [Google Scholar]
- 31.Wang G.-M., Li S.-L., Cai L., Liu W.-H. Shichangpu volatile oil microemulsion can improve the memory of mice with chronic stress. Journal of New Chinese Medicine. 2017;49(12):4–7. [Google Scholar]
- 32.Hu J.-G., Gu J., Wang Z.-W. Experimental study on the effects of shichiopu and its active components on learning and memory. Journal of Chinese Medicinal Materials. 1999;22(11):584–585. [Google Scholar]
- 33.Chen J., Gu F.-H., Liu X., Xu H.-Y., Yang P.-M., Dong W.-X. Effect of SIPI-SCPd on improving learning and memory of dementia mice caused by D-galactose. World Clinical Drugs. 2013;34(1):21–40. [Google Scholar]
- 34.Gu F.-H., Chen J., Xu H.-Y., Yang P.-M., Dong W.-X. Experimental study of SIPI-SCPd to ameliorate learning and memory fuctions in animals. World Clinical Drugs. 2012;33(3):150–155. [Google Scholar]
- 35.Wu B., Fang Y.-Q. Study on Acorus tatarinowii Schott chemical base and mechanism of enhancing intelligence. Chinese Archives of Traditional Chinese Medicine. 2004;22(9):1634–1641. [Google Scholar]
- 36.Wen Z.-J., Chen H.-W. The election for the effective fractions extracted from Acorus Gramineus on restoring consciousness and inducing resuscitation of model mice. Chinese Archives of Traditional Chinese Medicine. 2009;27(10):2203–2205. [Google Scholar]
- 37.Yang Y., Xuan L., Chen H., et al. Neuroprotective effects and mechanism of β-asarone against Aβ1–42-induced injury in astrocytes. Evidence-based Complementary and Alternative Medicine. 2017;2017:14. doi: 10.1155/2017/8516518.8516518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y., Jin H., Zou H.-X., Chen Y., Gao X.-F., Wang Q.-T. Improvement of α -asarone submicron emulsion on the spatial learning and memory ability of rats with Alzheimer’ s disease. West China Journal of Pharmaceutical Sciences. 2018;33(5):493–496. [Google Scholar]
- 39.Jiang Y., Fang Y.-Q., Zou Y.-Y. Effects of the β-asaroneon learning and memory ability and SOD, GSH-Px and MDA levels in AD mice. Chinese Journal of Gerontology. 2017;27(12):1126–1127. [Google Scholar]
- 40.Huang X.-Y., Chen X., Zhang W.-W. Effects of α - asarone on step-down test and expressions of P - Akt in hippocampus of Fmr1 knockout mouse. Journal of Clinical and Experimental Medicine. 2016;15(17):1662–1665. [Google Scholar]
- 41.Wang B.-L., Xuan L., Dai S.-J., Ji L.-T., Li C.-Y., Yang Y.-X. Protective effect of β-asarone on AD rat model induced by intracerebroventricular injection of Aβ1 -42 combined 2-VO and its mechanism. China Journal of Chinese Materia. 2017;42(24):4847–4854. doi: 10.19540/j.cnki.cjcmm.20171218.004. [DOI] [PubMed] [Google Scholar]
- 42.Guo J.-H., Chen Y.-B., Wei G., Nie H., Zhou Y., Chen S.-Y. Effects of active components of Rhizoma Acori Tatarinowii and their compatibility at different ratios on learning and memory abilities in dementia mice. Traditional Chinese Drug Research & Clinical Pharmacology. 2012;23(2):144–147. [Google Scholar]
- 43.Jiang Z.-K., Li X., Chen Z. Effect of volatile oil from acorus tatarinowii on learning and memory of streptozotocin induced dementia in rats. Chinese Journal of Gerontology. 2018;38(2):263–265. [Google Scholar]
- 44.Yang L.-B., Li S.-L., Huang Y.-Z., Liang J.-M., Wang Y.-H., Zhang S.-Q. Effect of Acorus gramineus and its active componentα -asarone on behavior and memory function of epileptic young rats. Chinese Traditional and Herbal Drugs. 2005;36(7):1035–1038. [Google Scholar]
- 45.Wang W. Q., Wang X., Wang W. Effects of Acortw tatarinowii Schott volatile oil on learning and memory impairment in mice and acute toxicity. Journal of Pharmaceutical Research. 2019;38(2):76–79. [Google Scholar]
- 46.Ma Y.-X., Li G.-Y., Liu J., et al. Effects of β-asarone on synaptic plasticity of hippocampal neurons in rats with Alzheimer's disease. Guangdong Medical Journal. 2017;38(10):1489–1492. [Google Scholar]
- 47.Unger P., Melzig M. F. Comparative study of the cytotoxicity and genotoxicity of alpha- and beta-asarone. Scientia Pharmaceutica. 2012;80(3):663–668. doi: 10.3797/scipharm.1204-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guyatt G.-H., Oxman A.-D., Montori V., et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. Journal of Clinical Epidemiology. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Landis S.-C., Amara S.-G., Asadullah K., et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altman D. G. Transparent reporting of trials is essential. The American Journal of Gastroenterology. 2003;108(8):1231–1235. doi: 10.1038/ajg.2012.457. [DOI] [PubMed] [Google Scholar]
- 51.Bebarta V., Luyten D., Heard K. Emergency medicine animal research: does use of randomization and blinding affect the results? Academic Emergency Medicine. 2003;10(>6):684–687. doi: 10.1197/aemj.10.6.684. [DOI] [PubMed] [Google Scholar]
- 52.Ospina M. B., Kelly K., Klassen T. P., Rowe B. H. Publication bias of randomized controlled trials in emergency medicine. Academic Emergency Medicine. 2006;13(1):102–108. doi: 10.1197/j.aem.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 53.Moher D., Pham B., Jones A., et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 54.Moher D., Avey M., Antes G., Altman D. G. The national institutes of health and guidance for reporting preclinical research. BMC Medicine. 2015;13(1):p. 34. doi: 10.1186/s12916-015-0284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baginskait J. Scientific Quality Issues in the Design and Reporting of Bioscience Research: A Systematic Study of Randomly Selected Original In Vitro, In Vivo and Clinical Study Articles Listed in the PubMed Database, in CAMARADES Monograph. Edinburgh, UK; 2012. http://www.dcn.ed.ac.uk/camarades/files/Camarades%20Monograph%20201201.pdf. [Google Scholar]
- 56.Kilkenny C., Browne W.-J., Cuthill I.-C., Emerson M., Altman D.-G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biology. 2010;8(6, article e1000412) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hooijmans C. R., de Vries R., Leenaars M., Curfs J., Ritskes-Hoitinga M. Improving planning, design, reporting and scientific quality of animal experiments by using the Gold Standard Publication Checklist, in addition to the ARRIVE guidelines. British Journal of Pharmacology. 2011;162(6):1259–1260. doi: 10.1111/j.1476-5381.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker D., Lidster K., Sottomayor A., Amor S. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies. PLoS Biology. 2014;12(1, article e1001756) doi: 10.1371/journal.pbio.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prager E.-M., Chambers K.-E., Plotkin J.-L., et al. Improving transparency and scientific rigor in academic publishing. Brain and Behavior: A Cognitive Neuroscience Perspective. 2019;9(1, article e01141) doi: 10.1002/brb3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]


