Keywords: GPCR, incretin, intestine, mesenteric lymph, neuropeptide
Abstract
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released from enteroendocrine cells (EECs) in response to nutrient ingestion and lower blood glucose levels by stimulation of insulin secretion and thus are defined as incretins. GLP-1 receptor (GLP-1R) expression has been identified on enteric neurons that include intrinsic afferent neurons, extrinsic spinal, and vagal sensory afferents but has not been shown to have an incretin effect through these nerves. GLP-1 and GIP enter the mesenteric lymphatic fluid (MLF) after a meal via the interstitial fluid (IF) from local tissue secretion and/or blood capillaries. We tested if MLF could induce diet-dependent intransient increases in intracellular calcium ([Ca2+]i) in cultured sensory neurons. Postprandial rat MLF, collected from the superior mesenteric lymphatic duct, induced a significant twofold higher intransient increase in [Ca2+]i in primary-cultured sensory neurons than MLF from fasted rats. Inhibition of transient receptor potential vanilloid 1 (TRPV1) and TRPV1 and ankyrin 1 cation channels (TRPA1) with ruthenium red eliminated the difference. Substance P (SP) (a peptide that stimulates insulin secretion) sensor cells cocultured with sensory neurons showed both the GLP-1R agonist exendin-4 (Ex-4) and GIP induced transient increases in [Ca2+]i directly coupled to SP secretion in the sensory nerves. Ex-4-induced release of SP required expression of either TRPA1 or TRPV1. These data identify unrecognized actions of GLP-1 and GIP as incretins by acting as neurolymphocrines and suggest a mechanism for sensory nerves to respond to the postprandial state through MLF.
NEW & NOTEWORTHY Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are secreted upon eating to lower blood sugar. GLP-1 and GIP were found to induce the secretion of substance P (SP) from cultured sensory nerves. SP enhances insulin secretion. Mesenteric lymphatic fluid (MLF) also stimulates sensory neurons in a diet-dependent manner. These studies identify new actions of GLP-1 and GIP as incretins and suggest a mechanism for sensory nerves to respond to diet through MLF.
INTRODUCTION
The concept that dietary factors initiate secretion of chemical signaling molecules from the intestinal mucosa into the circulation to initiate peripheral organ responses led to discovery of the first hormone, secretin (8). Subsequent observations revealed that some chemical mediators released in response to diet lowered blood glucose. These mediators were named incretins (51a). Currently, the complement of hormones released by chemosensory enteroendocrine cells (EEC) in the gastrointestinal mucosa in response to diet include secretin, cholecystokinin, enteroglucagon glucagon-like peptide 1 (GLP-1), GIP, peptide YY, and somatostatin, to list a few. These peptide hormones target peripheral tissues, including the pancreas and visceral afferent nerves.
Of the many characterized gut peptides, only GLP-1 and GIP meet criteria of incretins, through their potentiation of glucose-stimulated insulin secretion (22). GLP-1 and GIP activate the GLP-1 receptor (GLP-1R) and GIP receptors (GIPR), members of the B1 family of G protein-coupled receptors (GPCRs) (5). Consistent with their incretin functions, GLP-1R and GIPR occur in the endocrine pancreas (32, 78) and in a variety of tissues and have multiple biological effects other than incretin actions (5, 68). Enteric neurons innervate the intestinal mucosa. These include intrinsic primary afferent neurons (IPANs), extrinsic spinal, and vagal sensory afferent nerves, and vagal afferents (20, 49. 69, 83), which have cell bodies in the dorsal root ganglia (DRGs) and nodose ganglia (vagal afferents) (52).
Enteric vagal afferents’ response to changes in feeding versus fasting serves as a key neuronal pathway that modulates feeding-induced metabolic and behavioral responses. However, it has not been shown that GLP-1 acts as a nutrient-responsive incretin via enteric vagal afferents (83). Extra vagal pathways, such as those of spinal sensory nerves, have not been reported as responsive to the postprandial state. The demonstration that dietary-induced changes in MLF stimulated sensory nerves presented the possibility that molecules present in MLF might activate sensory nerves (64). Peptidergic neurons, such as those that release SP, have been previously reported to innervate the endothelial cells that comprise the mucosal lymphatic lacteals (36, 37, 41). Although those nerves were shown to innervate lymphatic vessels and lacteals, their functions were unknown.
MLF comprises molecules from exogenous origins such as those derived from the intestinal lumen from the diet and molecules from endogenous local cellular environments of the interstitial fluid (IF) (82). GLP-1 and GIP have been shown to accumulate in MLF after a meal at up to 10-fold higher concentrations than found in blood (19). The physiological significance of this accumulation or the biological significance of MLF incretins has not been established. The possible role that sensory afferents have in enhancing GLP-1 or GIP-mode of action as an incretin has not been explored.
Apart from peptide hormones (19, 54), MLF also contains potentially biologically active proteins including unique proteins not found in the serum that display changes in concentration in response to feeding (48, 58). Peptidergic nerves such as those that release SP innervate or are in close association with the endothelial cells that comprise the mucosal lymphatic lacteals (36, 37, 41, 64). Although a number of these nerves would appear to contact luminal contents of the lymphatic vessels, the significance of this observation is unknown. Establishing the activation of those sensory nerves by molecules in lymph, such as those from the diet or other extracellular paracrine agents, would suggest a novel mechanism by which peripheral nerves and the central nervous system (CNS) could monitor and respond to peripheral and environmental signals.
Neurons expressing transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) cation channels display a “sensory-effector” function with the release of stored neuropeptides, such as SP (43). Among its many effects, SP shares some biological actions with GLP-1 such as enhancing insulin secretion (25, 47, 76). Therefore, we explored whether GLP-1 and GIP could exert their effects as incretins through postprandial changes in the MLF. Here, we report that GLP-1 and GIP induce the release of SP from dispersed primary cultured mouse DRG sensory neurons and this response for GLP-1 is dependent on expression of both TRPV1 and TRPA1 channels. Similar to GLP-1 and GIP, MLF was also found to induce a transient increase in intracellular calcium concentration ([Ca2+]i) of sensory neurons, which was dependent on functional TRPV1 channel and on the postprandial state of the animal. These studies suggest a pathway for sensory afferents to respond to diet and expand the mechanisms by which GLP-1 and GIP can act as incretins, through the release of SP, as part of a neurolymphocrine system.
EXPERIMENTAL PROCEDURES
Compounds.
Ex-4, a potent agonist of the GLP-1R, was purchased from AnaSpec. GIP was purchased from Phoenix Pharmaceuticals, Inc. All other chemicals were purchased from Sigma-Aldrich.
Animals.
Wild-type (WT) C57BL/6 mice (6–10 wk) were from Jackson Laboratory. An equal number of males and females were used as controls for all experiments. Procedures were carried out according to the guidelines of the National Institutes of Health Animal Research and were approved by Institutional Animal Care and Use Committees of the University of California at Berkeley and the University of Cincinnati. GLP-1R knockout (KO) and GIPR KO mice on a C57BL/6 background (67) were provided by Dr. Daniel Drucker. TRPA1 KO and TRPV1 KO mice (6, 13) were gifts from Dr. David Julius. TRPA1/V1 double-mutant mice were generated as described (7). Briefly, TRPV1–/– and TRPA1–/– animals were crossed resulting in TRPV1+/– TRPA1+/– progeny, which were then crossed to yield WT and double-KO siblings for analyses.
Plasmid constructs.
The GLP-1R expression vector was constructed by subcloning PCR amplified human GLP-1R open reading frame into pcDNA 3.1 (Addgene). PCR amplification was carried out using a cDNA clone from GE Healthcare (CloneId: 8327594, Accession: BC112126) as the template (forward primer, 5′-GGCCGGCCGCCCGCCATGGCCGGC-3′; reverse primer, 5′-GGAAGATCTTCCCCAGGGTCGGCTGCAGGAGGC-3′). The NK1R expression vector was constructed by subcloning PCR amplified human NK1R open reading frame into pcDNA 3.1 (Addgene). A cDNA clone from GE Healthcare (CloneID: 30915310; Accession No. BC074912) was used as a template for the PCR amplification (forward primer, 5′-CGCCAAGCTTCACCATGGATAACGTCCTCCC-3′; reverse primer, 5′-CAAAGGCCGCGGGGCCAAGGAGAGCACATTGG-3′). The constructs were verified by DNA sequencing (DNA Sequencing Facility, University of California, Berkeley, CA).
Immunocytochemistry.
Gastrointestinal tissues were from adult female (n = 3) and male (n = 3) C57BL/6 mice. Tissues were immersion-fixed for 1–2 days at 4°C. For cryostat sections, tissues were incubated in 20–25% sucrose in PBS for 24 h at 4°C, embedded in OCT compound (Miles), and sectioned at 10 μm. Sections were processed after mounting on slides. Before immunostaining, tissue sections were incubated in an aqueous solution of 0.3% Sudan black in 70% ethanol for 5 min, rinsed, and processed as previously described (64). Tissues were incubated with one of the following primary antibodies: GLP-1R, mouse 1:200 (44) Developmental Studies Hybridoma Bank, Iowa, Iowa City: substance P, rat 1:400, Cuello et. al. (18); TRPV1, guinea pig 1:1,000, Julius and colleagues (79); or podoplanin, Syrian hamster 1:400, Farr et. al. (24) Developmental Studies Hybridoma Bank, Iowa; LYVE-1 rabbit 1:200 (Abcam). Tissues were then washed and incubated with secondary antibodies conjugated to goat anti-rabbit or rabbit anti-rat, mouse, or guinea pig IgG conjugated to FITC, Rhodamine Red X, Texas Red, horseradish peroxidase (Jackson ImmunoResearch), Alexa-488, or Alexa-568 (1:200 to 1:1,500 dilution, at room temperature for 2 h; Molecular Probes). For simultaneous detection of two antigens, specimens were incubated with primary antibodies against both antigens and then secondary antibodies labeled with contrasting fluorophores. Sections were then washed and mounted in ProLong Diamond Antifade Mountants (ThermoFisher) and then coverslipped. Nonspecificity was assessed by analysis of staining by omission of primary antisera.
Confocal microscopy.
Specimens were observed using a Zeiss 710 laser scanning confocal microscope or a Leica TCS-SP confocal microscope. The following objectives were used: Zeiss Fluar 20 (NA 1.0), Plan Apo 40 (NA 1.4), 100 (NA 1.3), Leica 10 (NA 0.4), 20 (NA 0.7), and 100 (NA 1.4). Images were collected at a zoom of 1–2 and typically 10–20 optical sections were taken at intervals of 0.5–1.0 µm as previously described (17). Images were processed to adjust contrast and brightness using Adobe PhotoShop CS6 (Adobe Systems, Mountain View, CA) and digitally colored to represent the appropriate fluorophores (17). Images of stained and control slides were collected and processed identically.
Structured illumination microscopy.
Imaging was performed in the Biological Imaging Facility at University of California, Berkeley using a Carl Zeiss Elyra PS.1 microscope with a ×63/1.4 and ×100 1.47 objective lenses. Excitation wavelengths were 405 nm, 488 nm, 561 nm, and 642 nm. Fluorescence emission was collected through appropriate dichroic mirrors and single-color filters (BP 495–550 nm, BP 570–620 nm, and LP 655 nm). Structured illumination microscopy (SIM) processing and channel alignment were done using ZEN Black, with three-dimensional (3D) rendering, colocalization, and isosurface modeling using Imaris v9 (Oxford Instruments). Processing and filtering settings were kept constant and image intensity was preserved with the raw image scale option in Zen. Colocalization analysis was performed using Huygens Pro 18.10 (Scientific Volume Imaging, The Netherlands) to generate a colocalization map using the Costes method of threshold estimation with Manders coefficients of x = 0.833 and y = 0.465. Images presented are representative of a minimum of five fields viewed per replicate with at least two technical replicates, and the experiment was conducted in at least three biological replicates.
MLF extraction.
MLF was extracted as previously described (64). Briefly, MLF was obtained from a cannula placed in the superior mesenteric lymphatic duct from isoflurane anesthetized and overnight fasted rats. They were presented with 3-ml bolus of either dextrin (1.1 g in 3ml 0.9% NaCl representing fasted lymph) or lipid-rich Ensure (representing fed lymph, Abbott Laboratories). Ensure is a mixed meal consisting of fat, carbohydrate, and protein. Sixty minutes before the Ensure infusion, MLF was collected for the fasted value. MLF was taken at various time points after Ensure was infused into the duodenum and placed on ice. MLF collected at 120 min after the nutrient infusion (at 1:50 dilution) induced the highest increase in [Ca2+]i mobilization in DRG neurons. Therefore, we used this collection time to represent fed MLF.
Cell culture conditions and transfection.
The nontumorigenic rat mucosal epithelial cells (hBRIE 380i cells; Ref. 4) used for this study were as previously described (15). They were well-characterized subclones expressing enterocyte phenotypes. Experiments were performed using hBRIE 380i cells of passage 11–16. Cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM; Gibco) with 10% bovine calf serum (BCS; Hyclone), 100 U/ml penicillin, and 100 g/ml streptomycin (Invitrogen) as additional supplements at 37°C in 5% CO2-95% air. For experiments, cells were plated on circular optical borosilicate glass coverslips (18 mm, 0.13 to 0.17 mm; FisherScientific) coated with poly-l-lysine (Sigma-Aldrich) and placed in 12-well plate at ~80% confluency on the day of use. For transient transfection, cells were trypsinized, resuspended in IMDM, and incubated with the plasmid DNA (3 μg plasmid DNA/1 million cells) in a volume between 0.2 and 0.7 ml at RT for 5 min. Transfection was carried out by electroporation using a Gene Pulser (Bio-Rad), in 0.4-mm cuvette at 0.25 kV and 960°F. Immediately after electroporation, 1 mL of IMDM-10% BCS was added to the cuvette. Cells were then plated on circular coverslips to reach ~80% confluency in 16 h.
Neuronal cell culture and transfection.
DRG were dissected and dispersed as previously described (57) with some modifications. DRG were removed from T1-T13, L1-L6, and S1-S4 segments of both sides of the spine from 4 to 8-wk-old mice. They were dissociated with 0.125% collagenase P (Boehringer) in CMF Hank’s solution (Gibco) at 37°C for 40–60 min, pelleted (5 min at 750 g), and resuspended in 0.25% trypsin (Invitrogen) at 37°C for 2 min. DRG were triturated gently with a fire-polished Pasteur pipette in culture medium (DMEM, Gibco) with 5% Equine Serum (Hyclone), 5% FBS (Hyclone), 0.1 mg/ml penicillin-streptomycin (Invitrogen), and 2 mM l-glutamine (Invitrogen) and then centrifuged at 750 g for 5 min using an Eppendorf 5702 centrifuge. Cells were resuspended in culture medium and plated onto coverslips coated with poly-l-lysine (PLL, Sigma-Aldrich). Cell cultures were maintained in a 5% vol/vol CO2 incubator at 37°C. Transient transfections were carried out using Lipofectamine 2000 (Invitrogen), following the provider’s protocols for neuronal cells grown at 60–80% confluency. Cultures were examined 1–2 days after plating by Ca2+ microfluorimetry.
Mouse primary DRG neurons-hBRIE 380i coculture sensor system.
DRG neurons were cocultured with hBRIE 380i cells that were transiently transfected with the NK1R receptor. The coculture served as a functional assay that could be used to demonstrate the direct coupling of a sensory activation by GLP-1 and GIP to a secretory response. HBRIE 380i cells are nontumorigenic rat small intestinal epithelial cell lines and are ideal for these studies as they can grow adjacent to sensory neurons without forming layers over these cells (4). Cells were selected that showed no endogenous activity for the secretagoges Ex-4, GLP-1, GIP, capsaicin (Cap), and allyl isothiocyanate (AITC) by sequential cloning. Cells were then conditioned to grow in the culture sensory cell media and substratum.
HBRIE 380i cells were transiently transfected to heterologously express NK1R and found to respond to SP starting at 0.5 nM and reaching a plateau at 100 nM. They were plated on the same glass coverslips as the DRG neurons ~16 h post-DRG dispersion and plating. The coculture was maintained in the DRG neuronal culture medium at 37°C in 5% CO2-95% air. The hBRIE380i-NK1R cells could thus act as sensors for SP secretion from individual and adjacent neurons by displaying changes in intercellular calcium as measured by fura-2 AM Approximately 40 h after DRG neuron and 16 h after hBRIE380i-NK1R coculture plating, cells were preloaded with fura-2 AM and visualized by microscopy.
Calcium mobilization assay.
Calcium imaging experiments where performed as previously described (64) with some modifications. For fura-2 AM measurement of [Ca2+]i mobilization, hBRIE 380i cells and DRG neurons were incubated at 37°C in HBSS (Gibco), 0.1% BSA (Hyclone), and 20 mM HEPES (Gibco) at pH 7.4 for 50–60 min. Fura-2 AM (Invitrogen) was used at 2 μM and supplemented with 0.01% Pluronic F-127 (wt/vol, Invitrogen). Coverslips were washed three times with Krebs-Ringer solution and were mounted in an open chamber at 37°C.
Fluorescence of individual cells was measured using a Nikon Diaphot inverted microscope with a ×40 Neofluar [1.4 numerical aperture (NA)] or a long-working-distance water immersion ×40 (0.75 NA) lens. A charge-coupled device camera collected emission (>510 nm) images during alternate excitation at 340 ± 5 and 380 ± 5 nm with a filter wheel. Excitation and emission were controlled by Sutter instrument LAMBDA 10–2 (UV box) (Sutter Instruments, Novato, CA), video camera (PIC-III, Sutter Instrument), and a video microscopy acquisition program (Imaging Workbench 5.2).
Test substances were directly added to the chamber. Each coverslip received a maximum of 6 sequential treatments, initiated with vehicle control, followed by an agonist of GLP-1R Ex-4 (50 nM), GIP (50 nM), SP (250 nM), Cap (1 µM), or AITC (1 µM). DRG neurons received a KCl (100 mM) treatment at termination of the experiment and hBRIE 380i cells received 10 mM calcium at termination. Postexperimentally, neurons were selected based on their response to KCl using ANOVA followed by Tukey’s post hoc tests. They were identified as neurons if the average ratio during two 10-s time intervals before the addition of KCl were above the number obtained using ANOVA followed by Tukey’s post hoc tests. Once identified, those neurons were further selected based on their response to specific treatments by the same statistical procedure. They were deemed to be a treatment responsive if the average ratio during two 10-s time intervals before the addition of the stimulus was above the number obtained using ANOVA followed by Tukey’s post hoc tests. The same statistical procedure, ANOVA followed by Tukey’s post hoc tests, was employed when comparing different treatments. Statistics were performed using Microsoft Excel. Statistical significance was assessed by one-way ANOVA, followed by Tukey’s post hoc test. All graphs displaying fura-2 AM ratios have been normalized to the baseline ratio F340/F380.
Statistical analysis.
All statistical tests were performed using Microsoft Excel. Values are reported as the means ± SE (where n = number of mice used) for [Ca2+]i mobilization imaging experiments where multiple independent days of imaging were performed. A one-way single factor ANOVA followed by the Tukey’s post hoc tests and the Student’s t test (where appropriate) were used. Efforts were made to ensure that equal numbers of mice of each genotype were used for each experiment (where appropriate) and that treatment and control groups were of identical or near-identical size and age. Significance was labeled as follows: NS, not significant, P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
The demonstration that MLF can induce increases in [Ca2+]i in TRPV1-expressing sensory neurons from primary cultured mouse DRG suggests that sensory nerves, which are closely associated with lacteals, express receptors activated by molecules in the MLF and IF (64). Both vagal afferents and DRG axons target specific locations in the myenteric plexus, submucosa, mucosa, and vascular vessels (71, 87). We collected lymph samples from the superior mesenteric lymph vessel whose tributaries, such as those from lacteals, are innervated or in close proximity to IPANs, as well as from extrinsic neurons that are exposed to MLF. Peptidergic neurons such as those that release calcitonin gene-related peptide (CGRP) and SP have been shown to innervate lacteals (37–39, 64, 65). We examined expression of GLP-1R and SP immunoreactivity localized to areas exposed to IF and MLF. We located immunoreactivity for SP at neuro-like fibers of the muscularis mucosae and in mucosal cells of the villi, as described (31, 40, 45, 63) (Fig. 1A). Mucosal cells expressed GLP-1R as previously described (69). In addition, GLP-1R and SP colocalized in cells closely associated with lacteals.
Fig. 1.
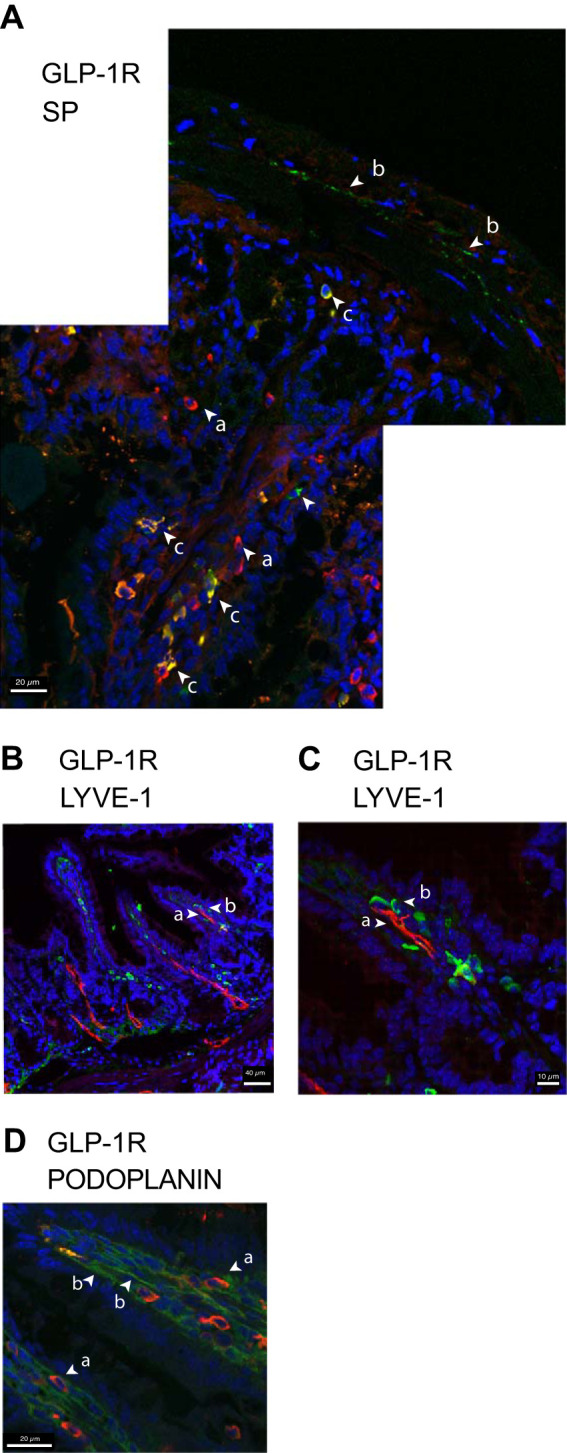
Immunocytochemical localization of glucagon-like peptide-1 receptor (GLP-1R), substance P (SP), and transient receptor potential vanilloid 1 (TRPV1) in mouse distal small intestinal lacteals and in nerve fibers. A: a low-magnification confocal image montage of jejunal mucosa demonstrating GLP-1R (red, rhodamine labeled) on cells along the length of mucosal villus (arrows a). GLP-1R and SP (green, FITC labeled) cells colocalize mid-villus and occasionally in regions of the crypt region (yellow, arrows c), and SP immunoreactivity was detected in nerve-like fibers (arrows b). B and C: immunocytochemical localization of GLP-1R at the inner luminal surface of the villar lacteal endothelium. Maximum intensity projection villar LYVE-1 (red, rhodamine labeled) is shown localized primarily to an inner central area of the lacteal endothelium (arrow a) compared with the pattern of expression of GLP-1R (green, FITC labeled, arrow b). D: maximum intensity projection of a mucosal villus demonstrating differential distribution GLP-1R (red, rhodamine labeled, arrows a) in relation to podoplanin. In contrast to LYVE-1, podoplanin appears to be immunolocalized to the outer surface of the endothelial lacteal (green, FITC labeled, arrows b) where GLP-1R is more closely associated. DAPI-stained nuclei are blue. The Object Pearson and Manders colocalizations were determined using HuygensPro software Images and are representative of a minimum of 5 fields viewed of a minimum of 5 replicate samples of tissue sections per animal (n = 6) with at least 2 technical replicates per set of samples.
We used the lymphatic endothelial cell markers LYVE-1 and podoplanin to further examine the relationship between GLP-1R and the lacteal (Fig. 1, B and C). Volumetric rendering of SIM images showed GLP-1R was positioned between the podoplanin expressing outer surface of the luminal facing lymphatic endothelium (Fig. 1D) and the central lacteal vessel that expressed LYVE-1 (Fig. 1C). This places GLP-1R in direct contact with MLF. In addition to mucosa epithelial cells, GLP-1R distribution coincides with GLP-1R modulation of intestinal intraepithelial lymphocyte (IEL-GLP-1R) signaling and transcript expression (86). Nevertheless, GLP-1R distribution in the intestine previously identified by immununocytochemical (ICC) techniques were ambiguous, likely due to differential specificity of the antibodies used (69) and low expression of the receptor. The nature of family B-GPCR might limit ICC techniques, which limits conclusions that this approach alone supports.
Coexpression of GLP1-R and TRPV1 in cells within the lacteals led us to test whether sensory neurons would respond to MLF from fed animals. We tested activation of mouse primary cultured DRG neurons in response to MLF from rats taken at various time points after lipid-rich Ensure was instilled into the duodenum. Before the infusion, rats cannulated at the superior mesenteric lymphatic duct were fasted overnight. Sixty minutes before the bolus infusion, MLF was collected for fasted values (Fig. 2A). MLF (from both fed and fasted rats) transiently induced [Ca2+]i in about two-thirds (60%) of total cultured DRG neurons (KCl sensitive) (Fig. 2B). Approximately one-third (28%) of the neurons were MLF responsive to either Cap or AITC, one-third of which (14% of total neurons) were responsive to both Cap and AITC. A proportion of DRG neurons stimulated by MLF might not be sensory neurons. We further explored if sensory neurons would show a differential response to MLF taken from pre- or postprandial animals. Sensory nerves exposed to MLF from fasted animals showed attenuated responses to Cap and AITC compared with their responses when exposed to postprandial MLF (Fig. 3). This indicates that MLF/IF can modulate sensory nerve response to TRPV1 and TRPA1 channel-activating agents.
Fig. 2.
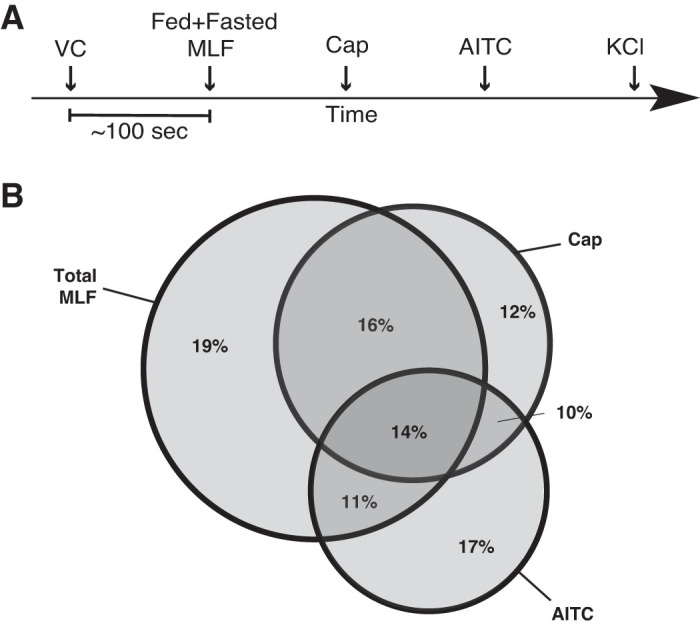
Mesenteric lymphatic fluid (MLF) induces a transient increase in intracellular calcium concentration ([Ca2+]i) in sensory neurons. A: timeline used for fura-2 AM calcium imaging assay on primary cultured dorsal root ganglia (DRG) neurons. Dispersed DRG neurons were plated on 18-mm round borosilicate glass coverslips and cultured for 18 h. Fifty to sixty minutes before microscopy, cells were loaded with 2 µM fura-2 AM After being preloaded with fura-2 AM, cultured cells were treated with a vehicle control (VC; 1× Ringer solution) followed by (in 100-s intervals) fed and/or fasted MLF (at 120 min after food intake for fed, 0 min for fasted), capsaicin (Cap; 1 μM), allyl isothiocyanate (AITC; 1 μM), and KCl (100 mM). Neurons were exposed to total MLF (1:50 dilution) taken from fed or fasted rats combined (n = 3). B: Venn diagram comparing the percentage of Cap and AITC-responsive neurons that displayed transient increase in [Ca2+]i in response to MLF. Sixty percent of the total neurons responded to MLF of which 30% and 25% of the MLF responsive neurons reacted to Cap and AITC, respectively. Twenty-eight percent of the Cap and AITC responding neurons were not MLF responsive. Among the MLF responsive sensory neurons, 16% and 11% were only Cap or AITC responsive respectively. Greater than 400 neurons were examined in 3 independent cultures from 4 mice.
Fig. 3.
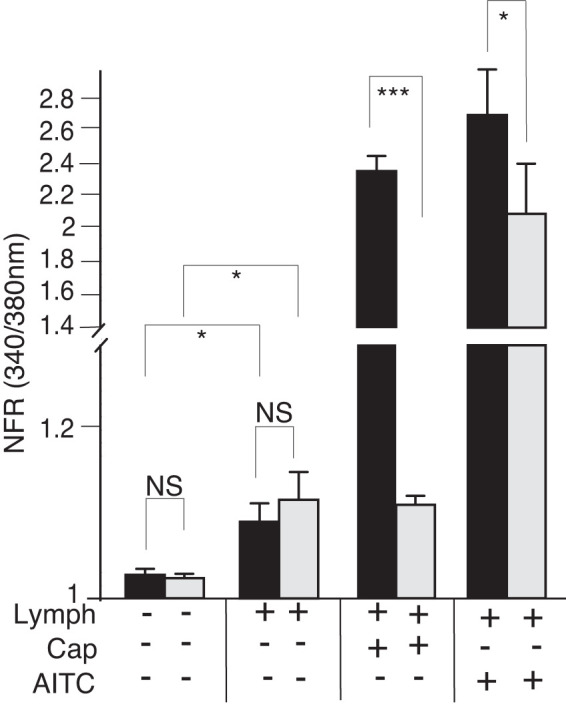
Mesenteric lymphatic fluid (MLF) induces transient increases in intracellular calcium concentration ([Ca2+]i) and modulates the [Ca2+]i response to capsaicin (Cap) and allyl isothiocyanate (AITC) in sensory neurons. Primary cultured dorsal root ganglia (DRG) were dispersed, plated on 18 coverslips, and treated with MLF from fasted (gray bars) or fed (black bars) animals, Cap, or allyl isothiocyanate (AITC) as described in Fig. 2. MLF added to total neuronal population of cells significantly induced transient increases in [Ca2+]i. Significant differences in transient increases in [Ca2+]i in response to postprandial MLF were seen in sensory neurons responsive to Cap and AITC. Additionally, both Cap and AITC transient increases in [Ca2+]i were significantly enhanced in the presence of fed lymph. Greater than 400 neurons were examined in 3 independent cultures from 3 mice. Bars are means ± SE (n = 3 animals). NFR, normalized fura-2 AM response; NS, not significant. *P < 0.05, ***P < 0.001. Statistical analysis was by ANOVA using Tukey's post hoc test.
Fed MLF compared with fasted MLF did not appear to induce differences in the transient [Ca2+]i increase when data were compiled from the total DRG neurons. This was in contrast to differences observed in Cap- and AITC-responsive sensory nerves. Therefore, we explored the importance of TPV1 and TRPA1 activity for the observed fed and fasted MLF response. We preincubated sensory neuron cultures with ruthenium red (RR), which blocks both the TRPV1 and TRPA1 channel pores. We then recorded the fura-2 response in cells exposed to fed and fasted MLF in the presence of RR. RR treatment significantly attenuated the MLF transiently induced [Ca2+]i responses to MLF from both fasted and postprandial animals (Fig. 4)
Fig. 4.
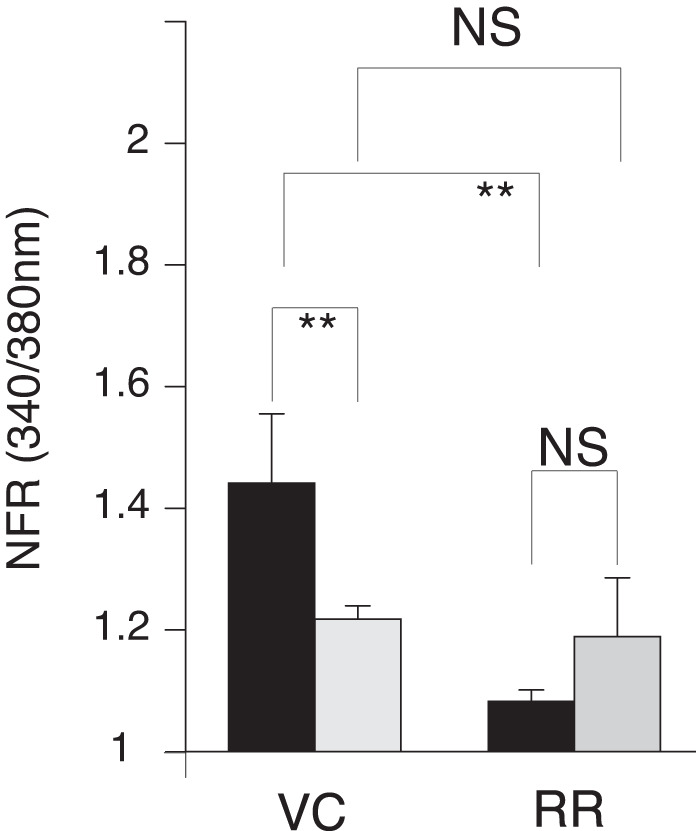
Postprandial mesenteric lymphatic fluid (MLF) enhancement of transient increases in intracellular calcium concentration ([Ca2+]i) in sensory nerves is abolished with the transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) antagonist ruthenium red (RR). After being preloaded with fura-2 AM (10 µM), dispersed mouse primary cultured dorsal root ganglia (DRG) neurons were rested in a Ringer solution containing 10 µM of the TRPV1 and TRPA1 pore blocker RR for 10 min before fura-12 imaging. MLF, capsaicin (Cap), and allyl isothiocyanate (AITC) were applied to the neurons as outlined in Fig. 2 and each contained 10 µM RR. To determine the importance of TRPV1 and TRPA1 on the postprandial MLF response of the sensory nerves, the transient increases in [Ca2+]i in response to MLF from fed (black bars) or fasted (gray bars) rats was measured in the presence of RR. Blockage of channels was verified by the addition of Cap or AITC. Inhibition of both TRPV1 and TRPA1 channels abolished the differences in transient [Ca2+]i observed in fed and fasted MLF of the vehicle controls (VC). Greater than 400 neurons were examined in 3 independent cultures from 4 mice. Bars are means ± SE. NFR, normalized fura-2 AM response; NS, not significant. **P < 0.01. Statistical analysis was by ANOVA using Tukey's post hoc test.
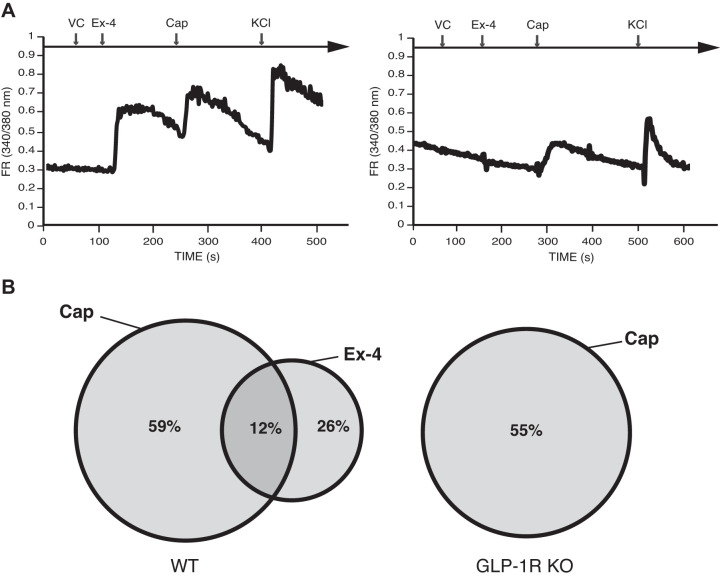
Given dietary-induced changes in lymph, we tested if GLP-1 acting as an incretin hormone in postprandial lymph induced a transient [Ca2+]i increase in sensory nerves. Fifty nanomoles of Ex-4, a potent GLP-1R agonist, induced a transient increase in [Ca2+]i in primary cultured mouse DRG sensory neurons responsive to Cap and AITC (Fig. 5A). Fifty-nine percent of cultured DRG neurons responded only to Ex-4, whereas 12% responded to both Ex-4 and Cap (Fig. 5B). Ex-4 did not produce a transient increase in [Ca2+]i in GLP-1R KO DRG neurons. The DRG from GLP-1R KO animals, however, contained more Cap-responsive neurons than those from WT animals (55% vs. 38%) (Fig. 5B).
Fig. 5.
Exendin-4 (Ex-4) induces a transient increase in intracellular calcium concentration ([Ca2+]i) in dorsal root ganglia (DRG) neurons. A: representative tracing a single mouse primary cultured DRG neuron treated sequentially with vehicle controls (VC) (Krebs-Ringer solution), 50 nM Ex-4, 10 µM capsaicin (Cap), 10 µM allyl isothiocyanate (AITC), and 100 mM KCl. Left: tracing is from wild-type (WT) animals. Right: tracing from glucagon-like peptide-1 receptor (GLP-1R) knockout (KO) animals. The trace represents an average of 15 responsive cells; among the 200 neurons examined in 3 independent cultures from 3 animals per genotype. B: the Venn diagram illustrates that 12% of the Cap-responsive neurons also respond to Ex-4. Twenty-six percent of the Ex-4 responding sensory neurons were non-Cap responsive, which would indicate that Ex-4 induces a transient increase in [Ca2+]i populations of nonsensory neurons. FR, fura-2 AM response.
Our ICC data revealed a population of cells that coexpressed GLP1-R and SP. This caused us to question whether Ex-4 in cells induced SP release in cells coexpressed GLP-1 and SP. In addition, TRPV1 and TRPA1 have been associated with release of SP in gastric tissues and sensory neurons (29, 74). The use of DRG neurons as a model for GLP-1-induced activation of sensory nerves has limitations. DRG neurons represent a highly heterogeneous group of neurons. Because various cell types might express GLP-1R in the villus, it is necessary to identify those cells in which Ex-4-induced increases in transient [Ca2+]i also produce SP secretion. Previous studies measuring neuropeptide secretion from the media of primary DRG neuron cultures did not account for the specific cell subtypes that release neuropeptides. In addition, the assay methodology was often not sufficiently sensitive to measure low levels of secretory products. Moreover, detection of transient changes in [Ca2+]i of a single sensory neuron does not directly translate to the secretion of peptides from that neuron. Finally, expression of receptor protein or transcript does not always indicate functional activity.
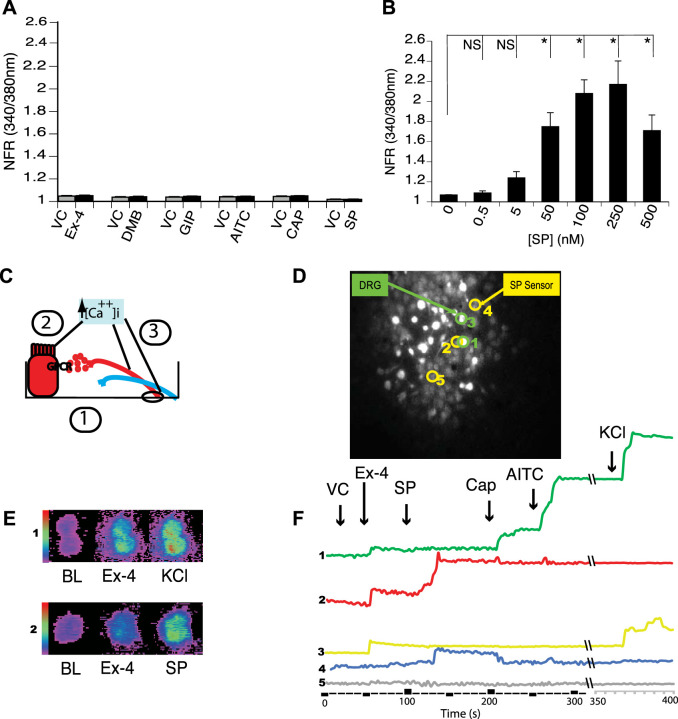
We, therefore, developed a functional assay to determine if GLP-1 stimulation of sensory neurons that express TRPV1 and TRPA1 directly stimulates SP secretion. HBRIE 380i cells transfected to heterologously express the SP NK1R could be cultured with primary dispersed mouse DRG neurons and act as SP sensors that would respond to the SP released from individual and adjacent neurons by displaying changes in [Ca2+]i (measured using fura-2 AM) (Fig. 6). Nontumorigenic hBRIE 380i cells that displayed no endogenous responses to Ex-4, GLP-1, GIP, Cap, AITC, and SP (Fig. 6A) were subcloned to grow under culture conditions used for DRG neurons. The subcloned cells were transiently transfected to heterologously express NK1R. They responded to SP starting at 0.5 nM and approached a plateau at 100 nM (Fig. 6B). Dispersed DRG and hBRIE 380i-NK1R cocultures were preloaded with fura-2 AM and visualized by microscopy for changes in [Ca2+]i in response to a variety of stimulants. Figure 6, D–F, shows representative response plots using the SP sensor bioassay. Transient increases in [Ca2+]i in response to 50 nM Ex-4 occurred in both sensory neurons responsive to AITC and Cap, which is a SP-sensor response (Fig. 6F). These transient increases in [Ca2+]i also occurred in neurons that did not display SP-sensor response (Fig. 6F), indicating that GLP-1 affects peptidergic sensory and nonsensory neurons. The Ex-4-induced SP-sensor response suggests that a number of GLP-1 effects could be mediated through SP and other neuropeptides secreted from sensory neurons.
Fig. 6.
Dispersed primary cultured dorsal root ganglia (DRG) neurons and hBRIE 380i cells coculture bioassay for determining a substance P (SP) functional phenotype of individual sensory nerves. A: hBRIE 380i cells that displayed no endogenous activity to all stimulants were selected by sequential cloning. These cells were further conditioned for growth in media used for the coculture with sensory nerves. HBRIE 380i subclones that did not show a transient increase in intracellular calcium concentration ([Ca2+]i) in response to either exendin-4 (Ex-4) (50 nM), glucagon-like peptide-1 receptor agonist DMB (50 nM), glucose-dependent insulinotropic polypeptide (GIP; 50 nM), allyl isothiocyanate (AITC; 1 µM), capsaicin (Cap; 1 µM), or SP (250 nM) (black bars), compared with the vehicle control (VC) (gray bars). Bars are means ± SE (n = 3 cultures). B: hBRIE 380i cells were transfected to heterologously express the NK1R receptor and then were stimulated using different concentrations of SP to determine a dose response. The SP-sensors significantly responded to SP in the range from 5 nM to 250 nM. Bars are means ± SE (n = 3 independent cultures). NFR, normalized fura-2 AM response; NS, not significant. *P < 0.05. C: diagram illustrating sensor cell model (1). Dispersed mouse primary cultured DRG neurons were plated on 18 mm round borosilicate glass coverslips and cocultured with SP sensors. Fifty to sixty minutes before microscopy, cells were loaded with 2 µM fura-2 AM. Next, the cultured cells were treated with VC, followed by Ex-4, and lastly with SP to identify sensor cells, Cap to identify cells expressing transient receptor TRPV1, AITC to identify cells with TRPA1, and KCl to identify nerves. Activation was determined by changes in a transient increase in [Ca2+]i in (2, 3). Sensor cells (2) were activated by secreted SP from adjacent nerves. D and F: example of the bioassay for SP secretion by single neurons using the coculture of neuronal cells with SP sensor cells. Cell 1 was a neuron (KCl responsive) that released SP in response to Ex-4 by expressing both TRPA1 and TRPV1 channels. The SP response was detected by sensor cell 2 in the presence of Ex-4 (red). Cell 3 was a nerve that responded to Ex-4 but did not release SP as indicated by sensor cell 4 and was not responsive to TRPA1 and TRPV1 agonists. Cell 5 was a nontransfected hBRIE 380i cell. E: the range of detection in the transient increase in [Ca2+]i visualized colorimetrically for the DRG neurons (panel 1) and SP-sensors (panel 2) when treated with Ex-4 and SP.
We observed a significant increase in the SP-sensor response in detectors adjacent to DRG sensory neurons that reacted to Ex-4 with a transient increase in [Ca2+]i (Fig. 7A). Consistent with observations of the increased number of DRG sensory neurons from the GLP-1R KO animals that responded to Cap, we observed enhancement of the increase in [Ca2+]i corresponding to increases in SP-sensor response with Cap treatment (Fig. 7B). This suggests that GLP-1 attenuates Cap-induced neuropeptide secretion. This is consistent with the increase in SP-sensor response caused by Ex-4 that we observed in TRPV1 KO DRG neurons (Fig. 8A). Attenuation of the transient increase in [Ca2+]i in DRG neurons parallels the attenuation in SP secretion by Ex-4 in the TRPA1 KO DRG neurons (Fig. 8B). No significant difference in the transient increase in [Ca2+]i was observed in DRG neuronal [Ca2+]i response or in the SP-sensor response in sensory neurons from TRPA1/TRPV1 double KO animals. Thus Ex-4-induced SP secretion depends on expression of either or both TRPA1 and TRPV1 channels (Fig. 9).
Fig. 7.
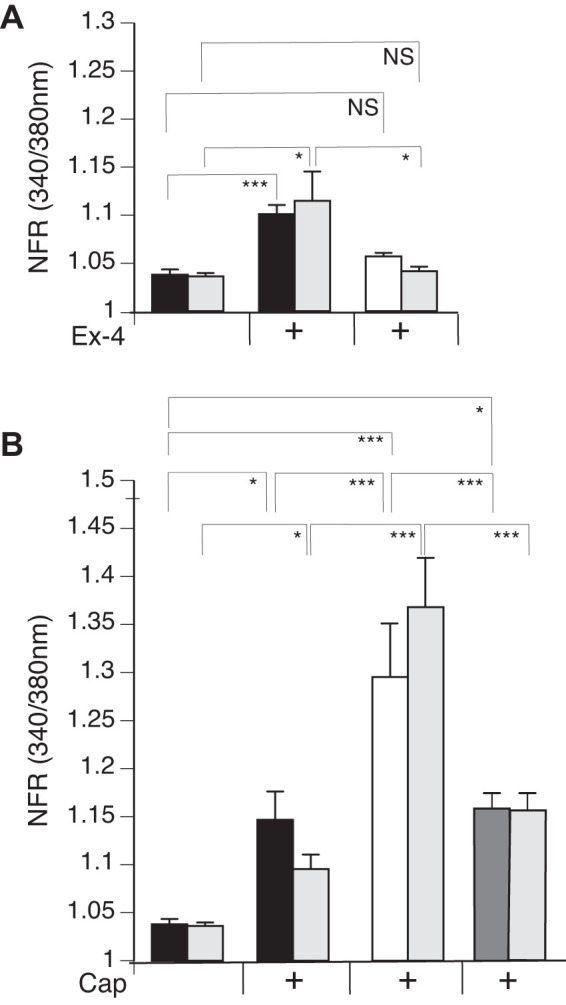
Exendin-4 (Ex-4) treatment of dorsal root ganglia (DRG) neurons significantly induces release of substance P (SP) and attenuates SP release in response to capsaicin (Cap). Mouse primary cultured DRG neurons from wild-type (WT) (black bars) and glucagon-like peptide-1 receptor (GLP-1R) gene knockout (KO) (white bars) animals were cocultured with SP sensors (gray bars) and exposed to 50 nM Ex-4 followed by Cap and KCl. Concentrations of the treatments were as described in Fig. 6. For GLP-1R rescue, DRG from GLP-1R KO animals were transiently transfected with GLP-1R. A: Ex-4 induced a significant transient increase in the [Ca2+]i in the WT (black bars) but not in GLP-1R KO (gray bars). The sensor response corresponded to the DRG neuron calcium response, showing significance in the WT and no significant difference in the GLP-1R KO. B: Cap, in the presence of Ex-4, significantly stimulated SP response in the WT and GLP-1R KO rescue (white bars) but not in the GLP-1R KO. Greater than 400 neurons were examined in 3 independent cultures from 4 mice. y-Axis is the ratiometric calcium imaging of fura-2 AM-loaded cells (at 340/380 nm) normalized to the ratio obtained from cells treated with Ringer solution only. Bars are means ± SE (n = 4 animals). NS, not significant; NFR, normalized fura-2 AM response. *P < 0.05, ***P < 0.001. Statistical analysis was done by ANOVA using Tukey's post hoc test.
Fig. 8.
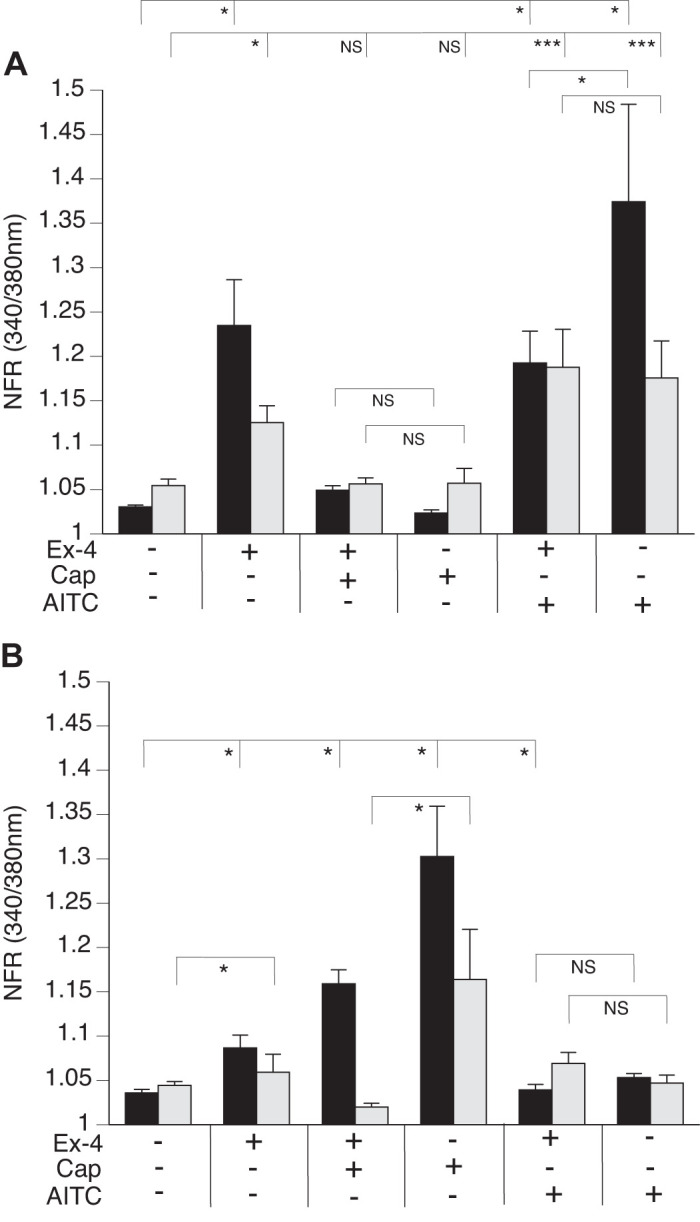
Exendin-4 (Ex-4) induced substance P (SP) release from dorsal root ganglia (DRG) neurons occurs in sensory neurons that express transient receptor potential vanilloid 1 (TRPV1) or transient receptor potential ankyrin 1 (TRPA1) channels. DRG from TRPV1 knockout (KO) (A) and TRPA1 KO (B) animals were cocultured with SP sensors (gray bars) and exposed to 50 nM Ex-4, followed by capsaicin (Cap), allyl isothiocyanate (AITC), and KCl. Concentrations of the treatments were as described in Fig. 6. A: TRPV1 KO neurons (black bars) produce a significant increase in Ex-4 calcium response and also a corresponding SP-sensor (gray bars) response. Cap treatment, however, does not result in significant difference in either DRG neurons or SP-sensor intracellular calcium concentration ([Ca2+]i). Attenuation in the transient increase in the [Ca2+]i of DRG neurons calcium, but not in SP, is observed with AITC treatment. B: TRPA1 KO neurons (black bars) also show a transient increase in the [Ca2+]i in response to Ex-4 which corresponds to SP secretion (gray bars). Greater than 400 neurons were examined in 3 independent cultures from 3 mice for each genotype. Bars are means ± SE (n = 3 animals). NFR, normalized fura-2 AM response; NS, not significant. *P < 0.05, ***P < 0.001. Statistical analysis was by ANOVA using Tukey's post hoc test.
Fig. 9.
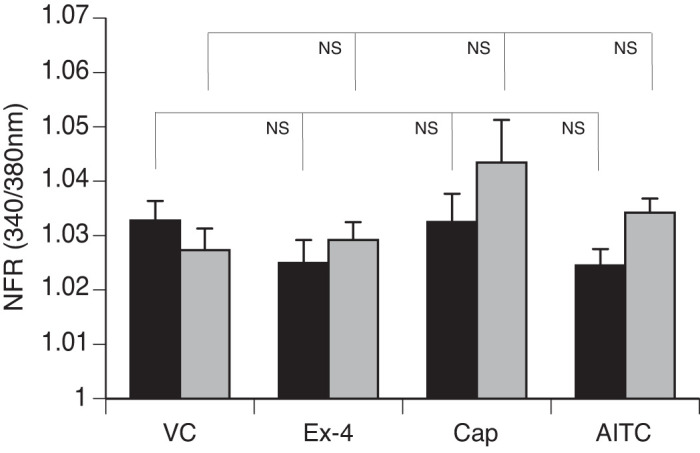
Exendin-4 (Ex-4)-induced substance P (SP) release does not occur in sensory neurons from transient receptor potential ankyrin 1 (TRPA1)/transient receptor potential vanilloid 1 (TRPV1) double knockout (KO) dorsal root ganglia (DRG). Mouse primary cultured DRG neurons (black bars) from TRPA1/TRPV1 double KO animals were cocultured with SP sensors (gray bars) and exposed to 50 nM Ex-4, followed by capsaicin (Cap), allyl isothiocyanate (AITC), and KCl. Concentrations of the treatments were as described in Fig. 6. Greater than 400 neurons were examined in 3 independent cultures from 4 mice. Bars are means ± SE (n = 4 animals). NFR, normalized fura-2 AM response; NS, not significant. Statistical analysis was by ANOVA using Tukey's post hoc test.
Because GIP also occurs in postprandial MLF, we examined if GIP-like GLP-1 also produces a SP response. GIP-responsive neurons induced SP secretion (Fig. 10). Unlike GLP-1, however, GIP enhanced rather than attenuated Cap-induced SP release from peptidergic sensory neurons.
Fig. 10.
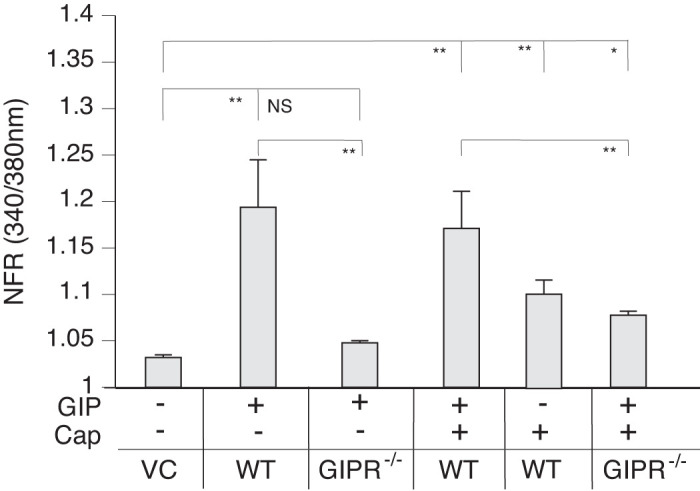
Glucose-dependent insulinotropic polypeptide (GIP) induces substance P (SP) release from capsaicin-responsive sensory neurons. The graph depicts SP-sensor (gray bars) response. Mouse primary cultured dorsal root ganglia (DRG) neurons from wild-type (WT) and GIP knockout (KO) animals were cocultured with SP sensors and exposed to 50 nM GIP. GIP induced an increased calcium response in SP-sensors adjacent to capsaicin (Cap)-responsive sensory neurons in DRGs from WT animals (gray bars) but not from GIP KO animals. GIP enhanced the Cap-induced SP secretion. Greater than 400 neurons were examined in 3 independent cultures from 4 mice. Bars are means ± SE (n = 4 animals). NFR, normalized fura-2 AM response; NS, not significant. *P < 0.05, **P < 0.01. Statistical analysis was by ANOVA using Tukey's post hoc test.
DISCUSSION
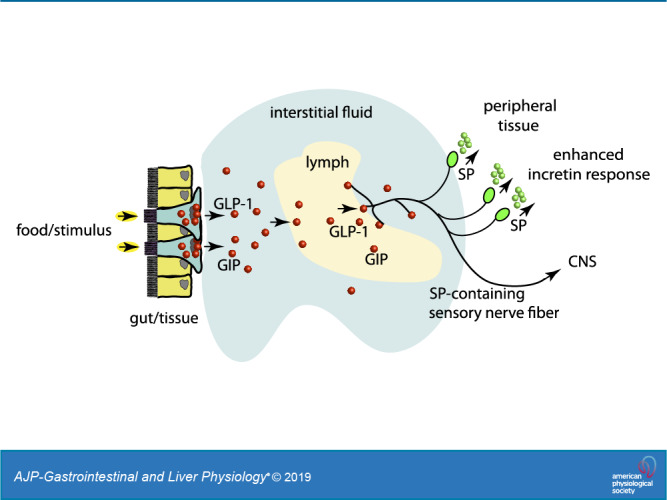
In this study, we have shown that GLP-1 and GIP induce SP secretion from sensory nerves, and that sensory nerves respond to the postprandial state when in contact with MLF. This supports the concept of the lymphatic vasculature and its associated IF as regulators of metabolism in response to exogenous and endogenous signal such as nutrients and hormones, respectively. MLF provided a unique model for these studies because it contains molecules derived from exogenous sources and from the local IF. Furthermore, the MLF composition can be manipulated experimentally in a physiological context, such as the luminal presentation of the diet.
Traditionally, the lymphatic system has been viewed as a passive network of endothelial lined capillaries and collecting vessels that contribute critically to maintaining hydrostatic pressure, lipid transport, antigen presentation, and return of extravasated plasma proteins to the circulation (11, 66, 82). The lymphatic microvasculature affords convergence of molecules from local IF pools to activate enteric nerves to release neuropeptides, including SP.
We collected MLF from the superior mesenteric lymphatic duct, which is proximate to IPANs and extrinsic neurons (26, 28), many of which are exposed to lymphatic and interstitial fluid. Gastrointestinal tissues are extensively innervated by mesenteric nerves, including sympathetic and parasympathetic nerve fibers and vagal and spinal afferent nerve fibers that arise from the nodose ganglia and DRG (23, 27, 77). Afferent vagal signaling contributes critically to dietary-induced secretion of gut hormones, such as CCK and GLP-1 (10, 51, 60). Stimulation of sensory nerve activity by MLF in response to diet has been mostly unexplored. Sensory nerves, whose cell bodies originate in the DRG, have not been shown to be responsive to postprandial conditions. Characterization of the GPCR LPA5, however, suggested this possibility (64). CGRP-positive nerves of lacteals coexpress LPA5 in regions in contact with MLF. MLF from fed animals induces a transient [Ca2+]i increase in primary cultured DRG neurons that express LPA5-related peptide (CGRP). Similar to mucosal lacteals, the media layer of peripheral lymphatic vessel walls is richly innervated by nerves containing CGRP and SP neuropeptides (36, 37). These lymphatic vessel walls are close to the lumen of the MLF collecting vessels, where they contact MLF or other molecules in the lymph derived from the interstitium.
Transcriptional profile studies have shown that vagal afferents express relatively few gut peptide receptors, which are typically low or absent in other types of peripheral afferents (14). Since GIPR was not found to be expressed in afferent vagal nerves, some investigators have concluded that GIP acts as a classical hormone (23). Similarly, GLP-1R activation on vagal afferents has not been shown to produce nutrient signals by GLP-1 but rather accounted for gastric stretch receptors responses (83). Therefore, vagal GLP-1R activation does not appear to contribute GLP-1 effects as an incretin. There still remains some question as to the physiological importance of gut peptides signaling in vagal afferents (9).
In primary cultured DRG, we observed significant transient increases in [Ca2+]i in response to both GLP-1R and GIP-R activation. This coincided with the transient increases in [Ca2+] observed for insulin-induced secretion by activation of GLP-1R and the G-coupled proteins (21, 59, 70, 88). The GLP-1-induced increase in [Ca2+]i depended on expression of TRPV1 or TPA1 neurons of the DRG. An enhanced [Ca2+]i response occurs with stimulation of both channels. Our observations contrast with one report whereby GLP-1 did not induce transient increases in [Ca2+]i in primary cultured DRG sensory neurons (3). Acute treatment with Ex-4 or GLP-1 also did not affect Cap sensitivity. These observations prompted the conclusion that the GLP-1R did not participate in signaling involving TRPV1 (3). These results are unexpected because in other cells, the GLP-1R couples to Gαs, Gαq, Gαi, and Gαo (30, 35, 59), whose pathways correlate with increase in [Ca2+]i. For example, in pancreatic β-cells GLP-1 activates a cAMP-regulated Ca2+ signaling pathway (22, 35). Therefore, GLP-1 action might be mediated either via a direct coupling of the GLP-1 receptor with different G proteins or via activation of a single G protein that modulates various functions through different subunits (16). B family GPCRs such as GLP-1R and GIPR can be pleiotropically coupled with multiple signaling pathways that are important for receptor function (2). The lack of a transient increase in [Ca2+]i in primary cultured DRG, in response to GLP-1 in a previous study (3), could reflect differences in in vitro conditions that favor particular subpopulations of DRG neurons in vivo (62). This exemplifies the importance of linking receptor activation and signaling to a specific downstream effector response such as secretion.
Although the brain and the afferent vagus nerve ganglia are major sites of GLP-1R and GIPR expression, ICC and transcript analyses are suggestive that these receptors also occur in neuronal fibers, cell bodies of the small intestine and colon, and the DRG (3, 61, 73). However, it remains to be determined if the localization and transcript data correlate with receptor activity. Recently, deep single-cell RNA sequencing of colon-projecting sensory neurons from mouse thoracic and lumbar spine have revealed new distinct sensory neuron subtypes of mucosal afferents that express LPA5. This has led to the speculation that these neurons might represent subtypes of mucosal afferents that sense and transduce luminal contents (34) although these studies did not explore the possibility for GLPR-1R or GIPR expression. Neurons associated with mesenteric lymphatic vessels that express CGRP receptor also have been implicated as part of a complex neuroendocrine-mediated regulation of chylomicron trafficking through enterocytes (72).
Cap infusion induces SP and the subsequent release of insulin, glucagon, and pancreatic exocrines (75, 76). Stimulation of porcine vagal nerves, however, has not been reported to cause SP release (76). Therefore, vagal afferents do not appear to contribute to the modulation of insulin secretion by SP. The GLP-1-induced secretion of SP from DRG argues for involvement of sensory neurons in regulation of the pancreatic endocrine and exocrine secretory response. This is consistent with the observation that TRPV1 activation (and presumably SP secretion) can mediate glucose-induced insulin secretion (89). It is possible that GLP-1 exerts insulinotropic action not only through direct action on beta cells but may be partially dependent on sensory nerves (1).
Up to 70% of retrograde labeled small to medium pancreatic primary afferent neurons in both the thoracolumbar DRGs and the nodose ganglions NGs show TRPV1 immunoreactivity (53). Hence, the majority of pancreatic primary sensory neurons express TRPV1. TRPV1 has been found to be expressed in a majority of vagal C-fiber afferents and vagal neurons innervate virtually all visceral tissues (50, 81). This suggests the possibility that vagal and thoracolumbar pancreatic primary afferents could work in parallel to reflexes initiated by vagal afferent activation.
Sensory nerves of the DRG such as those containing CGRP have cell bodies that project to the distal intestine (33). Thus MLF-induced local release of SP from DRG sensory neurons suggests a similar pathway whereby GLP-1 and GIP from lacteals exert effects on sensory nerves of pancreatic islets. These effects would rely on canonical pathways, such as vagal afferents, as well as sensory pathways involving spinal nerves and the CNS (85) in response to exogenous and endogenous stimuli.
Both TRPV1 and TRPA1 have central roles as noxious- and chemo-sensors (46). SP from gastrointestinal tissues correlates to expression and activation of TRPV1 and TRPA1 (29, 74, 84). Our demonstration that Ex-4 does not induce SP secretion in TRPV1 and TRPA1 double KO neurons supports a conclusion that TRPA1 and TRPV1 coexpression allows the two channels to interact and modulate each other’s activities (56). This might be exemplified by an accumulated transient increase of [Ca2+]i leading to SP secretion. This regulation could have significant physiological importance for GLP-1 as a modulator of TRP proteins on peptidergic neurons, such as those that secrete SP, and the potential synergy between GIP/GLP-1 and SP on end organ response.
Endogenous SP enhances insulin secretion and exocrine pancreatic secretion (25, 47, 75). The presence of GLP-1 and GIP in the MLF/IF and their induced release of SP expand insight into the effector response of these incretin hormones. The mode of action of GLP-1 and GIP on sensory neurons is not likely confined to SP release. At least 12 neuropeptides are present in TRPV1-positive sensory neurons (55, 80). These include somatostatin, vasoactive intestinal polypeptide, cholecystokinin, galanin, and corticotrophin-releasing factor, which all have established systemic effects. The proposition that multiple neuropeptides can be simultaneously released from TRPV1-activated sensory nerves further expands the potential mode of action of GLP-1 and GIP beyond the effects of SP. The local MLF/IF-induced neuropeptide release from sensory nerves suggests the likelihood of a shared pathway for molecules in the IF and MLF, such as GLP-1 and GIP. This allows their effects on peripheral tissues as well as the CNS (85) in a pathway independent of the vascular circulation through a neurolymphocrine system. An example of another peptide potentially acting through this pathway is CCK. CCK is an important satiety factor that stimulates the vagus nerve to influence central feeding centers. The CCK receptor (CCKa-R) is expressed by both viscero- and somatosensory primary sensory neurons (12). Stimulation of sensory nerves by lymphatic fluid containing CCK in addition to vagal afferents would support a role for this receptor as a mediator both of CCK-induced satiety and in sensory processing at the spinal level.
Lymphatic fluid contains a vast number of factors that can act as ligands to receptors expressed on nerves. We chose to examine the effects of GLP-1 and GIP on the sensory nerves of DRG because these peptides increase in concentration in MLF in response to feeding. They also have a biological effect that depends on the postprandial state. These studies were not meant to indicate that GIP and GLP-1 in the MLF are responsible for postprandial effects on sensory nerves, but rather as a first step in showing that GLP-1 and GIP could expand their incretin mode of action through sensory nerves that are in contact with the MLF. It was also critical to establish the potential importance of sensory nerves in transducing gut luminal signals and in sensing the postprandial state.
The presence of a protein or transcript for a GPCR does not necessarily indicate functional activity of the receptor. Given the considerable cross talk among GPCRs and signaling pathways, changes in intracellular signaling molecules alone are not sufficient to assume a secretory response. We, therefore, utilized a model that provided a functional assay for these receptors that coupled stimuli with secretion in an individual cell. While the SP sensors can distinguish if GLP-1 is acting on a neuronal cell body or neighboring cell such as a satellite cell, we cannot always rule out the possibility of neurites from neighboring cells contributing to a signal in areas of higher cell density.
Future studies on the identification of specific receptor activation, such as GLP-R, on specific afferent neurons, which produces a physiological event, will likely require development of a combination of genetic animal models designed to test these responses in vivo. There are factors that make these studies challenging such as the large number of molecules in lymph; interaction cross talk between GPCRs, between TPA proteins, and between signaling pathways; and the need to show specificity. Characterization of peripheral neurons associated with lymphatic vessels could reveal a system for mediating biological signals from lymphatic and interstitial fluid to peripheral tissue through a neurolymphocrine network that parallels the endocrine system.
GRANTS
This study was funded in part by National Institute of Food and Agriculture (CA-B-NTS-0230-H to G. Aponte) and the National Institutes of Health (DK-119135, DK-058630; to P. Tso).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.W.A. conceived and designed research; F.M., A.L.G., P.T., and G.W.A. performed experiments; F.M. and G.W.A. analyzed data; F.M. and G.W.A. interpreted results of experiments; F.M. and G.W.A. prepared figures; G.W.A. drafted manuscript; F.M. and G.W.A. edited and revised manuscript; F.M. and G.W.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Diana M Bautista, David Julius, Daniel Drucker, Terry Machen, and Christian Schwarzer for providing valuable suggestions and animals to help make these studies possible. We also thank Aye Hlaing for valuable contributions to the laboratory.
REFERENCES
- 1.Ahrén B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol 286: R269–R272, 2004. doi: 10.1152/ajpregu.00423.2003. [DOI] [PubMed] [Google Scholar]
- 2.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC) (5th ed). Br J Pharmacol 164: S1–324, 2011. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand U, Yiangou Y, Akbar A, Quick T, MacQuillan A, Fox M, Sinisi M, Korchev YE, Jones B, Bloom SR, Anand P. Glucagon-like peptide 1 receptor (GLP-1R) expression by nerve fibres in inflammatory bowel disease and functional effects in cultured neurons. PLoS One 13: e0198024, 2018. doi: 10.1371/journal.pone.0198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aponte GW, Keddie A, Halldén G, Hess R, Link P. Polarized intestinal hybrid cell lines derived from primary culture: establishment and characterization. Proc Natl Acad Sci USA 88: 5282–5286, 1991. doi: 10.1073/pnas.88.12.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci 11: 772–779, 2008. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 28: 325–353, 1902. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20, Suppl 1: 64–72, 2008. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst 31: 191–201, 1990. doi: 10.1016/0165-1838(90)90185-L. [DOI] [PubMed] [Google Scholar]
- 11.Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr Physiol 9: 207–299, 2018. doi: 10.1002/cphy.c180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broberger C, Holmberg K, Shi TJ, Dockray G, Hökfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 903: 128–140, 2001. doi: 10.1016/S0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- 13.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 14.Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman GS, Roberson DP, Ghasemlou N, Piccoli C, Ahat E, Wang V, Cobos EJ, Stucky CL, Ma Q, Liberles SD, Woolf CJ. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3: e04660, 2014. doi: 10.7554/eLife.04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol 292: G98–G112, 2007. doi: 10.1152/ajpgi.00295.2006. [DOI] [PubMed] [Google Scholar]
- 16.Clapham DE, Neer EJ. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature 365: 403–406, 1993. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 17.Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490: 239–255, 2005. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- 18.Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 76: 3532–3536, 1979. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 293: R2163–R2169, 2007. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 20.Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol 13: 954–958, 2013. doi: 10.1016/j.coph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113: 546–593, 2007. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Egerod KL, Petersen N, Timshel PN, Rekling JC, Wang Y, Liu Q, Schwartz TW, Gautron L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab 12: 62–75, 2018. doi: 10.1016/j.molmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, Aruffo A. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J Exp Med 176: 1477–1482, 1992. doi: 10.1084/jem.176.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu XW, Sun AM. Stimulative effect of substance P on insulin secretion from isolated rat islets under normobaric oxygen incubation. Zhongguo Yao Li Xue Bao 10: 69–73, 1989. [PubMed] [Google Scholar]
- 26.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 817: 39–71, 2014. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 27.Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72: 143–164, 2004. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54: 1–18, 1998. doi: 10.1016/S0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 29.Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D, Santucci M, Gerard NP, Lucattelli M, Lungarella G, Fischer A, Grady EF, Bunnett NW, Geppetti P. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic Biol Med 43: 581–589, 2007. doi: 10.1016/j.freeradbiomed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Hällbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochim Biophys Acta 1546: 79–86, 2001. doi: 10.1016/S0167-4838(00)00270-3. [DOI] [PubMed] [Google Scholar]
- 31.Heitz P, Polak JM, Timson DM, Pearse AG. Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochemistry 49: 343–347, 1976. doi: 10.1007/BF00496138. [DOI] [PubMed] [Google Scholar]
- 32.Heller RS, Aponte GW. Intra-islet regulation of hormone secretion by glucagon-like peptide-1-(7–36) amide. Am J Physiol Gastrointest Liver Physiol 269: G852–G860, 1995. doi: 10.1152/ajpgi.1995.269.6.G852. [DOI] [PubMed] [Google Scholar]
- 33.Hibberd TJ, Kestell GR, Kyloh MA, Brookes SJ, Wattchow DA, Spencer NJ. Identification of different functional types of spinal afferent neurons innervating the mouse large intestine using a novel CGRPα transgenic reporter mouse. Am J Physiol Gastrointest Liver Physiol 310: G561–G573, 2016. doi: 10.1152/ajpgi.00462.2015. [DOI] [PubMed] [Google Scholar]
- 34.Hockley JRF, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G, Smith ES. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68: 633–644, 2019. doi: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holz GG 4th, Leech CA, Habener JF. Activation of a cAMP-regulated Ca(2+)-signaling pathway in pancreatic beta-cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem 270: 17749–17757, 1995. doi: 10.1074/jbc.270.30.17749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hukkanen M, Konttinen YT, Terenghi G, Polak JM. Peptide-containing innervation of rat femoral lymphatic vessels. Microvasc Res 43: 7–19, 1992. doi: 10.1016/0026-2862(92)90003-8. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa S, Kasahara D, Iwanaga T, Uchino S, Fujita T. Peptidergic nerve terminals associated with the central lacteal lymphatics in the ileal villi of dogs. Arch Histol Cytol 54: 311–320, 1991. doi: 10.1679/aohc.54.311. [DOI] [PubMed] [Google Scholar]
- 38.Ichikawa S, Okubo M, Uchino S, Hirata Y. The intimate association of nerve terminals with the lacteal endothelium in the canine duodenal villi observed by transmission electron microscopy of serial sections. Arch Histol Cytol 53, Suppl: 137–146, 1990. doi: 10.1679/aohc.53.Suppl_137. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa S, Shiozawa M, Iwanaga T, Uchino S. Immunohistochemical demonstration of peptidergic nerve fibers associated with the central lacteal lymphatics in the duodenal villi of dogs. Arch Histol Cytol 54: 241–248, 1991. doi: 10.1679/aohc.54.241. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa K, Ozaki T. Distribution of several gut neuropeptides and their effects on motor activity in muscularis mucosae of guinea-pig proximal colon. J Auton Nerv Syst 64: 91–100, 1997. doi: 10.1016/S0165-1838(97)00019-2. [DOI] [PubMed] [Google Scholar]
- 41.Ito Y, Magari S, Sakanaka M. Immunoelectron-microscopic localization of peptidergic nerve fibers around lymphatic capillaries in the rat liver. Arch Histol Cytol 53, Suppl: 199–208, 1990. doi: 10.1679/aohc.53.Suppl_199. [DOI] [PubMed] [Google Scholar]
- 43.Jensen CB, Pyke C, Rasch MG, Dahl AB, Knudsen LB, Secher A. Characterization of the glucagonlike peptide-1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology 159: 665–675, 2018. doi: 10.1210/en.2017-00812. [DOI] [PubMed] [Google Scholar]
- 44.Jensen EP, Poulsen SS, Kissow H, Holstein-Rathlou NH, Deacon CF, Jensen BL, Holst JJ, Sorensen CM. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol 308: F867–F877, 2015. doi: 10.1152/ajprenal.00527.2014. [DOI] [PubMed] [Google Scholar]
- 45.Jessen KR, Saffrey MJ, Van Noorden S, Bloom SR, Polak JM, Burnstock G. Immunohistochemical studies of the enteric nervous system in tissue culture and in situ: localization of vascoactive intestinal polypeptide (VIP), substance-P and enkephalin immunoreactive nerves in the guinea-pig gut. Neuroscience 5: 1717–1735, 1980. doi: 10.1016/0306-4522(80)90091-3. [DOI] [PubMed] [Google Scholar]
- 46.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 29: 355–384, 2013. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 47.Kaneto A, Kaneko T, Kajinuma H, Kosaka K. Effects of substance P and neurotensin infused intrapancreatically on glucagon and insulin secretion. Endocrinology 102: 393–401, 1978. doi: 10.1210/endo-102-2-393. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan K, Dwivedi P, Davidson S, Yang Q, Tso P, Siems W, Hill HH Jr. Monitoring dynamic changes in lymph metabolome of fasting and fed rats by electrospray ionization-ion mobility mass spectrometry (ESI-IMMS). Anal Chem 81: 7944–7953, 2009. doi: 10.1021/ac901030k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kedees MH, Guz Y, Grigoryan M, Teitelman G. Functional activity of murine intestinal mucosal cells is regulated by the glucagon-like peptide-1 receptor. Peptides 48: 36–44, 2013. doi: 10.1016/j.peptides.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Kim SH, Hadley SH, Maddison M, Patil M, Cha B, Kollarik M, Taylor-Clark TE. Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5HT3 and Tac1 expression. eNeuro 7: ENEURO.0494-19.2020, 2020. doi: 10.1523/ENEURO.0494-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krieger JP, Langhans W, Lee SJ. Vagal mediation of GLP-1's effects on food intake and glycemia. Physiol Behav 152: 372–380, 2015. doi: 10.1016/j.physbeh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 51a.La Barre J. Sur les possibilites d’un traitement du diabete par l’incretine. Bull Acad R Med Belg 12: 620–634, 1932. [Google Scholar]
- 52.Lai NY, Mills K, Chiu IM. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J Intern Med 282: 5–23, 2017. doi: 10.1111/joim.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lázár BA, Jancsó G, Oszlács O, Nagy I, Sántha P. The insulin receptor is colocalized with the TRPV1 nociceptive ion channel and neuropeptides in pancreatic spinal and vagal primary sensory neurons. Pancreas 47: 110–115, 2018. doi: 10.1097/MPA.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 54.Lu WJ, Yang Q, Sun W, Woods SC, D’Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol 293: G963–G971, 2007. doi: 10.1152/ajpgi.00146.2007. [DOI] [PubMed] [Google Scholar]
- 55.Maggi CA. The Pharmacological Modulation of Neurotransmitter Release. New York: Harcourt Brace, 1993. [Google Scholar]
- 56.Masuoka T, Kudo M, Yamashita Y, Yoshida J, Imaizumi N, Muramatsu I, Nishio M, Ishibashi T. TRPA1 channels modify TRPV1-mediated current responses in dorsal root ganglion neurons. Front Physiol 8: 272, 2017. doi: 10.3389/fphys.2017.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 58.Mittal A, Middleditch M, Ruggiero K, Buchanan CM, Jullig M, Loveday B, Cooper GJ, Windsor JA, Phillips AR. The proteome of rodent mesenteric lymph. Am J Physiol Gastrointest Liver Physiol 295: G895–G903, 2008. doi: 10.1152/ajpgi.90378.2008. [DOI] [PubMed] [Google Scholar]
- 59.Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 140: 1132–1140, 1999. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
- 60.Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst 61: 149–154, 1996. doi: 10.1016/S0165-1838(96)00071-9. [DOI] [PubMed] [Google Scholar]
- 61.Okawa T, Kamiya H, Himeno T, Seino Y, Tsunekawa S, Hayashi Y, Harada N, Yamada Y, Inagaki N, Seino Y, Oiso Y, Nakamura J. Sensory and motor physiological functions are impaired in gastric inhibitory polypeptide receptor-deficient mice. J Diabetes Investig 5: 31–37, 2014. doi: 10.1111/jdi.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25: 345–357, 2000. doi: 10.1016/S0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 63.Pearse AG, Polak JM. Immunocytochemical localization of substance P in mammalian intestine. Histochemistry 41: 373–375, 1975. doi: 10.1007/BF00490081. [DOI] [PubMed] [Google Scholar]
- 64.Poole DP, Lee M, Tso P, Bunnett NW, Yo SJ, Lieu T, Shiu A, Wang JC, Nomura DK, Aponte GW. Feeding-dependent activation of enteric cells and sensory neurons by lymphatic fluid: evidence for a neurolymphocrine system. Am J Physiol Gastrointest Liver Physiol 306: G686–G698, 2014. doi: 10.1152/ajpgi.00433.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popper P, Mantyh CR, Vigna SR, Maggio JE, Mantyh PW. The localization of sensory nerve fibers and receptor binding sites for sensory neuropeptides in canine mesenteric lymph nodes. Peptides 9: 257–267, 1988. doi: 10.1016/0196-9781(88)90258-6. [DOI] [PubMed] [Google Scholar]
- 66.Possenti L, Casagrande G, Di Gregorio S, Zunino P, Costantino ML. Numerical simulations of the microvascular fluid balance with a non-linear model of the lymphatic system. Microvasc Res 122: 101–110, 2019. doi: 10.1016/j.mvr.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 113: 635–645, 2004. doi: 10.1172/JCI200420518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pujadas G, Drucker DJ. Vascular biology of glucagon receptor superfamily peptides: mechanistic and clinical relevance. Endocr Rev 37: 554–583, 2016. doi: 10.1210/er.2016-1078. [DOI] [PubMed] [Google Scholar]
- 69.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290, 2014. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 70.Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, Bockaert J, Bertrand G, Dalle S. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem 285: 1989–2002, 2010. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ratcliffe EM. Molecular development of the extrinsic sensory innervation of the gastrointestinal tract. Auton Neurosci 161: 1–5, 2011. doi: 10.1016/j.autneu.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis RB, Ding S, Nielsen NR, Pawlak JB, Blakeney ES, Caron KM. Calcitonin-receptor-like receptor signaling governs intestinal lymphatic innervation and lipid uptake. ACS Pharmacol Transl Sci 2: 114–121, 2019. doi: 10.1021/acsptsci.8b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63: 1224–1233, 2014. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci 19: 1811–1818, 2004. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt P, Poulsen SS, Hilsted L, Rasmussen TN, Holst JJ. Tachykinins mediate vagal inhibition of gastrin secretion in pigs. Gastroenterology 111: 925–935, 1996. doi: 10.1016/S0016-5085(96)70060-4. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt PT, Tornøe K, Poulsen SS, Rasmussen TN, Holst JJ. Tachykinins in the porcine pancreas: potent exocrine and endocrine effects via NK-1 receptors. Pancreas 20: 241–247, 2000. doi: 10.1097/00006676-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Tan LL, Bornstein JC, Anderson CR. The neurochemistry and innervation patterns of extrinsic sensory and sympathetic nerves in the myenteric plexus of the C57Bl6 mouse jejunum. Neuroscience 166: 564–579, 2010. doi: 10.1016/j.neuroscience.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 78.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89: 8641–8645, 1992. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998. doi: 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 80.Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin 26: 286–294, 2005. doi: 10.1111/j.1745-7254.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Kollarik M, Ru F, Sun H, McNeil B, Dong X, Stephens G, Korolevich S, Brohawn P, Kolbeck R, Undem B. Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One 12: e0185985, 2017. doi: 10.1371/journal.pone.0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92: 1005–1060, 2012. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 83.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166: 209–221, 2016. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Y, Jia J, Xie C, Wu Y, Tu W. Transient receptor potential ankyrin 1 and substance P mediate the development of gastric mucosal lesions in a water immersion restraint stress rat model. Digestion 97: 228–239, 2018. doi: 10.1159/000484980. [DOI] [PubMed] [Google Scholar]
- 85.Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and β cell preservation. Prog Biophys Mol Biol 107: 248–256, 2011. doi: 10.1016/j.pbiomolbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, Johnson-Henry KC, Yeung W, Surette MG, Bang KW, Sherman PM, Drucker DJ. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes 64: 2537–2549, 2015. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 87.Zagorodnyuk VP, Brookes SJ, Spencer NJ. Structure-function relationship of sensory endings in the gut and bladder. Auton Neurosci 153: 3–11, 2010. doi: 10.1016/j.autneu.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 88.Zhang QH, Hao JW, Li GL, Ji XJ, Yao XD, Dong N, Yao YM. Proinflammatory switch from Galphas to Galphai signaling by Glucagon-like peptide-1 receptor in murine splenic monocyte following burn injury. Inflamm Res 67: 157–168, 2018. [DOI] [PubMed] [Google Scholar]
- 89.Zhong B, Ma S, Wang DH. TRPV1 Mediates glucose-induced insulin secretion through releasing neuropeptides. In Vivo 33: 1431–1437, 2019. doi: 10.21873/invivo.11621. [DOI] [PMC free article] [PubMed] [Google Scholar]