Abstract
Statins and omega-3 supplementation have been recommended for cardiovascular disease prevention, but comparative effects have not been investigated. This study aimed to summarize current evidence of the effect of statins and omega-3 supplementation on cardiovascular events. A meta-analysis and a network meta-analysis of 63 randomized controlled trials were used to calculate pooled relative risks (RRs) and 95% confidence intervals (CIs) for the effects of specific statins and omega-3 supplementation compared with controls. Overall, the statin group showed significant risk reductions in total cardiovascular disease, coronary heart disease, myocardial infarction, and stroke; however, omega-3 supplementation significantly decreased the risks of coronary heart disease and myocardial infarction only, in the comparison with the control group. In comparison with omega-3 supplementation, pravastatin significantly reduced the risks of total cardiovascular disease (RR = 0.81, 95% CI = 0.72–0.91), coronary heart disease (RR = 0.75, 95% CI = 0.60–0.94), and myocardial infarction (RR = 0.71, 95% CI = 0.55–0.94). Risks of total cardiovascular disease, coronary heart disease, myocardial infarction, and stroke in the atorvastatin group were statistically lower than those in the omega-3 group, with RRs (95% CIs) of 0.80 (0.73–0.88), 0.64 (0.50–0.82), 0.75 (0.60–0.93), and 0.81 (0.66–0.99), respectively. The findings of this study suggest that pravastatin and atorvastatin may be more beneficial than omega-3 supplementation in reducing the risk of total cardiovascular disease, coronary heart disease, and myocardial infarction.
Keywords: Cardiovascular event, statin, omega-3, network meta-analysis
1. Introduction
Cardiovascular diseases (CVDs) are characterized by any disorders relating to the heart or blood vessels [1], including coronary heart disease (CHD), cerebrovascular disease, stroke, peripheral vascular disease, rheumatic and congenital heart diseases, and venous thromboembolism [2]. It has been reported that the burden of CVDs has remained high over the past decades, with 75% of overall CVD deaths occurring in low- to middle-income countries [3]. In general, the prevalence of CVDs is closely related to various risk factors, such as hypertension, overweight and obesity, dyslipidemia, and diabetes [4,5]. According to recent guidelines of the American College of Cardiology/American Heart Association (ACC/AHA) regarding the primary prevention of CVD, individuals aged 40 to 75 years with diabetes mellitus and low-density lipoprotein-cholesterol (LDL-C) ≥70 mg/dL or those with hypercholesterolemia (LDL-C ≥190 mg/dL) regardless of CVD risk are recommended with moderate to high-intensity statins [6,7]. Additionally, according to the European Society of Cardiology and the European Atherosclerosis Society (ESC/EAS) guidelines, high-risk individuals with LDL-C ≥190 mg/dL or triglyceride (TG) >200 mg/dL are also suggested with statin therapy [8,9].
Although findings from a meta-analysis of 19 randomized controlled trials (RCTs) showed the protective effect of statins on CVD events among a high-risk population compared with a placebo or non-statin interventions [10], updated evidence from recent RCTs is required. In addition, a recent meta-analysis of 13 RCTs found marine omega-3 supplementation to be associated with a decreased risk of CVD events in a dose-response manner [11], though the analysis was restricted to only certain RCTs [12,13,14,15,16]. Overall, the pairwise effects of statins and omega-3 supplementation on CVD prevention have not been investigated. Therefore, this study aims to systematically summarize current evidence of the effect of statins and omega-3 supplementation through a meta-analysis and to elucidate the comparative efficacy of statins and omega-3 supplementation in the prevention of cardiovascular events through a network meta-analysis (NMA) of RCTs.
2. Methods
2.1. Literature Search
We searched RCTs from previously published systematic reviews or meta-analyses of statin use or omega-3 supplementation in PubMed from its inception to January 2020. The search keywords were statin (statins or *statin), omega-3 (fish oil) supplementation (supplement or supplements), cardiovascular disease (cardiovascular diseases), systematic review, and meta-analysis.
2.2. Eligibility Criteria
Two review authors (T.H. and J.K.) independently performed the search and assessed the eligibility of studies. We included RCTs reporting the comparative efficacy of a statin versus a statin, a statin versus an omega-3 supplementation, a statin versus a control, or an omega-3 supplementation versus a control for the prevention of total CVD, CHD, myocardial infarction (MI), and stroke. We considered seven currently available HMG-CoA reductase inhibitors: atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin.
The following exclusion criteria were used: outcome data or full text not available; follow-up duration less than 1 year; source of omega-3 from dietary intake; or placebo containing omega-6. Studies investigating the effect of a high dose compared with a low dose of the same treatment or comparing the intervention with an active drug were also excluded. For each RCT, the following information was extracted: study name, publication year, mean or median age (years) and follow-up time (years); the percentages of subjects with a history of MI, hypertension, diabetes, and smoking; treatment name, sample size, number of each cardiovascular event, and baseline and achieved serum concentrations of total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), and LDL-C for each treatment arm.
2.3. Statistical Analysis
We first investigated the association between statins and omega-3 supplementation and the risk of total CVD, CHD, MI, and stroke in a meta-analysis of direct evidence. Subgroup analyses for statins and sensitivity analyses using both fixed-effect and random-effects models were also performed. The Higgins I2 metric [17] was used to measure heterogeneity across individual studies. Additionally, we performed meta-regression analysis to determine whether the effects of total statins and omega-3 supplementation on cardiovascular event prevention were modulated due to their lipid-lowering effect. In particular, the lipid lowering effect was identified as the absolute difference (mg/dL) in lipid changes (= achieved level—baseline level) between the intervention arm and the comparison arm. Weighted mean difference (WMD) and 95% CI were calculated for the pooled effect of statins and omega-3 supplementation on LDL-C reduction. We further applied a generalized additive model using the ‘mgcv’ package [18] to access the non-linear association between cardiovascular event prevention effect and LDL-C reduction in statins and omega-3 supplementation arm separately.
Relative risks (RRs) and 95% confidence intervals (CIs) for the comparative efficacy of statins, omega-3 supplementation, and control were computed in the NMA. As the prior probability was not established in the study hypothesis, we performed the NMA with the frequentist approach to combine direct and indirect evidence [19]. A funnel plot was utilized to assess publication bias [20], and a contribution plot was applied to examine the influence of direct estimates on network summary effects [21]. Stata SE version 14.0 (StataCorp, College Station, TX, USA) and R software (R Foundation for Statistical Computing, Vienna, Austria) (version 3.5.2) were used for all statistical analyses.
3. Results
3.1. Study Characteristics
In total, 1259 potentially relevant records were assessed (Figure 1); 11 meta-analyses [11,22,23,24,25,26,27,28,29,30,31] were reviewed, and eligible RCTs were extracted. Of the 387 extracted RCTs, 324 were excluded for the following reasons: unrelated topic (N = 82), insufficient data (N = 4), duplicate publication or population overlap (N = 115), inappropriate treatment (N = 19), non-target outcome (N = 51), other study design (N = 10), external source of omega-3 (N = 15), and duration of follow-up (N = 28). Ultimately, 45 RCTs of statins and 18 RCTs of omega-3 supplementation involving 264,516 adults were included in the meta-analysis and NMA [12,13,14,15,16,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88].
Figure 1.
Flow diagram of the systematic review.
Table 1 summarizes the characteristics of the included RCTs. The median age of the participants was 62.6 years, and the follow-up duration was 3.7 years; the rates of a history of MI, hypertension, diabetes, and smoking were 24.8%, 50.6%, 18.9%, and 38.6%, respectively. Additionally, the lipid profiles of TC, TG, HDL-C, and LDL-C of study participants from individual RCTs at baseline and differences in the change of serum lipid concentrations during the follow-up are presented in Table 2.
Table 1.
Characteristics of trials included in the final analysis.
| Study | Age (Years) | Follow-Up (Years) | MI (%) | Hypertension (%) | Diabetes (%) | Smoker (%) | Intervention Arm | Comparison Arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | N | Total CVD | CHD | MI | Stroke | Treatment | N | Total CVD | CHD | MI | Stroke | |||||||
| Manson 2019 (VITAL) [88] | 67.1 | 5.3 | 49.8 | 13.7 | 7.2 | EPA+DHA | 12,933 | 308 | 145 | 148 | 527 | Control | 12,938 | 370 | 200 | 142 | 567 | |
| Bhatt 2019 (REDUCE-IT) [86] | 64 | 4.9 | 58.6 | Icosapent Ethyl | 4069 | 250 | Control | 4090 | 355 | |||||||||
| Bowman 2018 (ASCEND) [87] | 63.3 | 7.4 | 0 | 49.4 | 100 | 53.8 | EPA+DHA | 7740 | 186 | 252 | Control | 7740 | 200 | 251 | ||||
| Yusuf 2016 (HOPE-3) [71] |
63.8 | 5.6 | 0 | 37.9 | 5.8 | 27.7 | Rosuvastatin | 6361 | 105 | 45 | 70 | Control | 6344 | 140 | 69 | 99 | ||
| Ford 2016 (WOSCOPS) [33] | 55 | 4.9 | 0 | 44 | Pravastatin | 3302 | 194 | Control | 3293 | 223 | ||||||||
| Izawa 2015 (ALPS-AMI) [82] |
66 | 2 | 63.2 | Pravastatin | 253 | 5 | Atorvastatin | 255 | 1 | |||||||||
| Hosomi 2015 (J-STAR) [32] |
4.9 | 76 | 23.4 | 53.6 | Pravastatin | 780 | 5 | Control | 785 | 12 | ||||||||
| Bonds 2014 (AREDS2) [79] |
74 | 4.8 | 7 | 5.9 | 13 | 56.6 | EPA+DHA | 2147 | 183 | 28 | 28 | 48 | Control | 2056 | 187 | 30 | 30 | 41 |
| Takano 2013 (PEARL) [43] |
62.6 | 3 | 25.1 | 45.3 | 27.4 | Pitavastatin | 288 | 3 | 8 | Control | 286 | 8 | 9 | |||||
| Roncaglioni 2013 (Risk & Prevention) [52] |
64 | 5 | 84.6 | 59.9 | 21.7 | EPA+DHA | 6239 | 620 | 310 | 80 | 80 | Control | 6266 | 630 | 324 | 90 | 60 | |
| Macchia 2013 (FORWARD) [14] |
66.1 | 91.4 | 12.9 | 42.2 | EPA+DHA | 289 | 4 | 1 | 3 | Control | 297 | 4 | 1 | 3 | ||||
| Yakusevich 2012 [35] | 65.7 | 1 | 9.8 | 77.6 | 9.8 | Simvastatin | 86 | 4 | 5 | Control | 97 | 5 | 7 | |||||
| Bosch 2012 (ORIGIN) [75] |
63.5 | 6.2 | 79.5 | 12.4 | EPA+DHA | 6281 | 2055 | 344 | 314 | Control | 6255 | 2087 | 316 | 336 | ||||
| Nicholls 2011 (SATURN) [83] |
57.6 | 2 | 24.4 | 70.4 | 15.3 | 32.3 | Atorvastatin | 519 | 11 | 2 | Rosuvastatin | 520 | 11 | 3 | ||||
| Emberson 2011 (MRC/BHF) [70] |
64 | 5 | 43.5 | 30.4 | 74.8 | Simvastatin | 10,269 | 444 | Control | 10,267 | 585 | |||||||
| Ostadal 2010 (FACS) [62] |
62.1 | 1 | 7.7 | 51.3 | 19.2 | 29.2 | Fluvastatin | 78 | 10 | 2 | 1 | Control | 78 | 21 | 4 | 3 | ||
| Kromhout 2010 (AlphaOmega) [60] |
69 | 3.4 | 100 | 89.7 | 21 | 16.9 | EPA+DHA | 2404 | 170 | 122 | 89 | 11 | Control | 2433 | 185 | 132 | 102 | 10 |
| Galan 2010 (SU.FOL.OM3) [67] |
61.4 | 4.7 | 46 | 72.8 | EPA+DHA | 1253 | 81 | 51 | 32 | 29 | Control | 1248 | 76 | 53 | 28 | 28 | ||
| Einvik 2010 (DO IT) [66] |
70 | 3 | 28 | 14.5 | 34 | EPA+DHA | 282 | 32 | 11 | 11 | Control | 281 | 36 | 9 | 9 | |||
| Dangour 2010 (OPAL) [13] |
75 | 2 | 3.9 | 55.9 | EPA+DHA | 434 | 7 | Control | 433 | 8 | ||||||||
| Chan 2010 (ASTRONOMER) [34] |
58 | 3.5 | 48.3 | Rosuvastatin | 134 | 35 | Control | 135 | 44 | |||||||||
| Rauch 2009 (OMEGA) [53] |
64 | 1 | 14.4 | 66.5 | 27 | 36.7 | EPA+DHA | 1919 | 199 | 547 | 87 | 27 | Control | 1885 | 165 | 568 | 78 | 13 |
| Mok 2009 (RCASS) [65] |
63 | 2 | 69.2 | 91.2 | 26.4 | Simvastatin | 113 | 2 | Control | 114 | 3 | |||||||
| Fellstrom 2009 (AURORA) [33] |
64.2 | 3.8 | 10.2 | 26.4 | 15.5 | Rosuvastatin | 1389 | 91 | 53 | Control | 1384 | 107 | 45 | |||||
| Tavazzi 2008 (GISSI-HF) [45] |
68 | 3.9 | 14.2 | 54.6 | 28.3 | 14.2 | EPA+DHA | 3494 | 1635 | 107 | 122 | Control | 3481 | 1687 | 129 | 103 | ||
| Tavazzi 2008 (GISSI-HF) [37] |
68 | 3.9 | 54.3 | 14.1 | Atorvastatin | 2285 | 61 | 82 | Control | 2289 | 70 | 66 | ||||||
| Ridker 2008 (JUPITER) [47] |
66 | 1.9 | 0 | 15.8 | Rosuvastatin | 8901 | 31 | 33 | Control | 8901 | 69 | 64 | ||||||
| Yokoyama 2007 (JELIS) [36] | 61 | 4.6 | 5.6 | 35.5 | 16.3 | 18.9 | EPA | 9326 | 262 | 71 | 166 | Control | 9319 | 324 | 93 | 162 | ||
| Kjekshus 2007 (CORONA) [55] | 73 | 2.7 | 60 | 63 | 29.5 | 8.6 | Rosuvastatin | 2514 | 131 | 126 | Control | 2497 | 154 | 138 | ||||
| Deedwania 2007 (SAGE) [81] | 72.5 | 1 | 45.9 | 64.5 | 23.2 | 59.4 | Atorvastatin | 446 | 16 | 1 | Pravastatin | 445 | 16 | 3 | ||||
| Nakamura 2006 (MEGA) [63] | 58.3 | 5.3 | 41.8 | 20.8 | 20.6 | Pravastatin | 3866 | 125 | 66 | 17 | 50 | Control | 3966 | 172 | 101 | 33 | 62 | |
| Knopp 2006 (ASPEN) [59] | 61 | 4 | 16.4 | 55.1 | 100 | 12.4 | Atorvastatin | 1211 | 297 | Control | 1199 | 313 | ||||||
| Brouwer 2006 (SOFA) [12] | 61.5 | 1 | 62.6 | 50.7 | 15.9 | 67 | EPA+DHA | 273 | 65 | 1 | Control | 273 | 62 | 3 | ||||
| Amarenco 2006 (SPARCL) [77] | 62.7 | 4.9 | 59 | Atorvastatin | 2365 | 530 | 123 | 43 | 265 | Control | 2366 | 687 | 204 | 82 | 311 | |||
| Yokoi 2005 (ATHEROMA) [40] | 59.3 | 3 | 45.5 | 42 | 18.8 | Pravastatin | 142 | 23 | 2 | 5 | Control | 146 | 25 | 4 | 4 | |||
| Wanner 2005 [41] | 65.7 | 4 | 17.6 | 100 | 40.4 | Atorvastatin | 619 | 205 | 93 | 60 | Control | 636 | 246 | 112 | 45 | |||
| Stone 2005 [42] | 1 | 38.9 | 62.4 | 16.5 | 69.5 | Atorvastatin | 96 | 1 | Control | 103 | 1 | |||||||
| Raitt 2005 [16] | 62.5 | 2 | 55.5 | 50.5 | EPA+DHA | 100 | 2 | 1 | 1 | Control | 100 | 5 | 3 | 3 | ||||
| Pedersen 2005 (IDEAL) [85] | 61.7 | 4.8 | 28 | 58.4 | 12 | 79.1 | Atorvastatin | 4439 | 1176 | 898 | 267 | 151 | Simvastatin | 4449 | 1370 | 1059 | 321 | 174 |
| Makuuchi 2005 (PCABG) [54] | 58.9 | 4.5 | 62 | 51.5 | 33.3 | 41.9 | Pravastatin | 152 | 26 | 1 | 4 | Control | 151 | 36 | 4 | 3 | ||
| Sever 2004 (ASCOT-LLA) [46] | 63 | 5 | 100 | 24.6 | 32.7 | Atorvastatin | 5168 | 389 | 89 | Control | 5137 | 486 | 121 | |||||
| Nissen 2004 (REVERSAL) [84] | 56.2 | 1.5 | 68.5 | 18.9 | 26.3 | Atorvastatin | 253 | 4 | 1 | Pravastatin | 249 | 7 | 1 | |||||
| Nakagawa 2004 (PCS) [64] | 55 | 5.4 | 59.2 | 17.5 | 67.5 | Pravastatin | 54 | 13 | 2 | 3 | Control | 66 | 19 | 3 | 4 | |||
| Koren 2004 (ALLIANCE) [58] | 61.2 | 4.5 | 57.8 | 22.1 | 19.5 | Atorvastatin | 1217 | 408 | 52 | 35 | Control | 1225 | 443 | 94 | 39 | |||
| Colhoun 2004 (CARDS) [74] | 62 | 3.9 | 100 | 65.3 | Atorvastatin | 1428 | 21 | Control | 1410 | 39 | ||||||||
| Cannon 2004 (PROVE IT-TIMI) [80] | 58.2 | 2 | 18.5 | 50.2 | 17.6 | Atorvastatin | 2099 | 139 | 21 | Pravastatin | 2063 | 153 | 21 | |||||
| Shepherd 2002 (PROSPER) [49] | 75.3 | 3.2 | 13.4 | 61.9 | 10.7 | 26.8 | Pravastatin | 2891 | 454 | 77 | 135 | Control | 2913 | 523 | 102 | 131 | ||
| Serruys 2002 (LIPS) [50] |
60 | 3.9 | 44.4 | 38.6 | 12 | 71.5 | Fluvastatin | 844 | 2 | Control | 833 | 1 | ||||||
| Sawayama 2002 (FAST) [51] | 66.3 | 2 | 39.6 | 25 | 57.8 | Pravastatin | 83 | 4 | 4 | Control | 81 | 11 | 11 | |||||
| Liem 2002 (FLORIDA) [56] | 60.5 | 1 | 11.5 | Fluvastatin | 265 | 21 | Control | 275 | 13 | |||||||||
| Davis 2002 (ALLHAT-LTT) [73] | 66.4 | 4.8 | 35.1 | 23.2 | Pravastatin | 5170 | 209 | Control | 5185 | 231 | ||||||||
| Arthros 2002 (GREACE) [78] | 58.5 | 3 | 81.2 | 42.9 | 19.6 | Atorvastatin | 800 | 21 | 9 | Control | 800 | 51 | 17 | |||||
| Nilsen 2001 [15] | 64 | 1.5 | 23.3 | 24.3 | 10.3 | 75.7 | EPA+DHA | 150 | 42 | 17 | Control | 150 | 36 | 12 | ||||
| Teo 2000 (SCAT) [39] |
61 | 4 | 70 | 36 | 11 | 82 | Simvastatin | 230 | 11 | 4 | Control | 230 | 10 | 7 | ||||
| Valagussa 1999 (GISSI-P) [44] | 59.4 | 3.5 | 12 | 35.6 | 14.8 | 77.2 | EPA+DHA | 2836 | 44 | Control | 2828 | 41 | ||||||
| Riegger 1999 [48] | 59.8 | 1 | 35.6 | 29.3 | 5.5 | 9.6 | Fluvastatin | 187 | Control | 178 | ||||||||
| Plehn 1999 (CARE) [57] |
5 | 100 | 42.7 | 14.1 | 16.1 | Pravastatin | 2081 | 92 | Control | 2078 | 124 | |||||||
| Tonkin 1998 (LIPID) [38] |
62 | 6.1 | 41.7 | 8.7 | 73.3 | Pravastatin | 4512 | 336 | 169 | Control | 4502 | 463 | 204 | |||||
| Downs 1998 (AFCAPS/TexCAPS) [72] | 58 | 5.2 | 21.9 | 2.3 | 12.4 | Lovastatin | 3304 | 194 | 163 | 57 | Control | 3301 | 255 | 215 | 95 | |||
| Bestehorn 1997 (CIS) [76] | 49.8 | 2.3 | 84.3 | Simvastatin | 129 | 1 | Control | 125 | 5 | |||||||||
| Jukema 1995 (REGRESS) [61] | 56 | 2 | 47.4 | 27.8 | 0.1 | 88 | Pravastatin | 450 | 59 | Control | 434 | 93 | ||||||
| Furberg 1995 (PLAC-I & II) [68] | 58 | 3 | 48.5 | 40.5 | 0.7 | 15.5 | Pravastatin | 281 | 14 | 9 | Control | 278 | 29 | 24 | ||||
| Furberg 1994 (ACAPS) [69] | 62 | 2.8 | 28.8 | 56.5 | Lovastatin | 460 | 5 | Control | 459 | 5 | ||||||||
CVD, cardiovascular disease; CHD, coronary heart disease; MI, myocardial infarction; EPA, eicosapentaenoic acid; and DHA, docosahexaenoic acid.
Table 2.
Lipid profiles of study participants.
| Study | Baseline Measurement | Change Difference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Arm | Comparison Arm | |||||||||||
| TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | |
| Manson 2019 (VITAL) [88] | ||||||||||||
| Bhatt 2019 (REDUCE-IT) [86] | 216.5 | 40.0 | 74.0 | 216.0 | 40.0 | 76.0 | −32.5 | 0 | −5.0 | |||
| Bowman 2018 (ASCEND) [87] | ||||||||||||
| Yusuf 2016 (HOPE-3) [71] | 201.5 | 128.8 | 44.7 | 127.8 | 201.3 | 126.5 | 44.9 | 127.9 | −21.2 | −34.6 | ||
| Ford 2016 (WOSCOPS) [33] | 272.0 | 162.0 | 44.0 | 192.0 | 272.0 | 164.0 | 44.0 | 192.0 | ||||
| Izawa 2015 (ALPS-AMI) [82] | 204.1 | 142.9 | 47.6 | 130.2 | 203.2 | 130.8 | 48.0 | 131.0 | −0.6 | |||
| Hosomi 2015 (J-STAR) [32] | 210.8 | 142.6 | 53.8 | 129.5 | 209.6 | 141.7 | 53.0 | 129.5 | −23.2 | −9.7 | 0.8 | −21.3 |
| Bonds 2014 (AREDS2) [79] | ||||||||||||
| Takano 2013 (PEARL) [43] | 203.2 | 50.7 | 125.2 | 201.2 | 50.8 | 125.5 | 2.0 | −31.5 | ||||
| Roncaglioni 2013 (Risk & Prevention) [52] | 215.6 | 150.0 | 50.9 | 131.8 | 216.4 | 150.0 | 51.2 | 132.5 | −0.5 | −8.1 | 0.6 | −0.4 |
| Macchia 2013 (FORWARD) [14] | ||||||||||||
| Yakusevich 2012 [35] | 211.5 | 102.7 | 85.1 | 201.9 | 91.2 | 86.2 | −29.4 | −11.5 | 8.9 | |||
| Bosch 2012 (ORIGIN) [75] | 189.0 | 46.0 | 112.0 | 190.0 | 46.0 | 112.0 | −1.1 | −14.5 | 0.1 | 0.6 | ||
| Nicholls 2011 (SATURN) [83] | 193.5 | 130.0 | 44.7 | 119.9 | 144.1 | 110.0 | 48.6 | 70.2 | −44.7 | −30.0 | 2.1 | −42.1 |
| Emberson 2011 (MRC/BHF) [70] | −32.9 | |||||||||||
| Ostadal 2010 (FACS) [62] | 212.7 | 208.8 | −56.2 | |||||||||
| Kromhout 2010 (AlphaOmega) [60] | 182.5 | 145.3 | 49.9 | 110.2 | 182.9 | 147.9 | 49.5 | 110.2 | ||||
| Galan 2010 (SU.FOL.OM3) [67] | 174.0 | 106.3 | 46.4 | 104.4 | 175.9 | 106.3 | 46.4 | 102.5 | ||||
| Einvik 2010 (DO IT) [66] | 243.6 | 150.6 | 54.1 | 158.1 | 239.8 | 150.6 | 54.1 | 154.7 | ||||
| Dangour 2010 (OPAL) [13] | ||||||||||||
| Chan 2010 (ASTRONOMER) [34] | 206.1 | 108.9 | 61.5 | 123.0 | 203.8 | 116.9 | 59.9 | 120.6 | 1.9 | −66.9 | ||
| Rauch 2009 (OMEGA) [53] | ||||||||||||
| Mok 2009 (RCASS) [65] | 226.2 | 118.7 | 45.6 | 151.6 | 227.0 | 125.8 | 44.9 | 150.4 | −54.5 | −11.5 | −50.7 | |
| Fellstrom 2009 (AURORA) [33] | 176.0 | 157.0 | 45.0 | 100.0 | 174.0 | 154.0 | 45.0 | 99.0 | −46 | −24.2 | 0 | −40.1 |
| Tavazzi 2008 (GISSI-HF) [45] | ||||||||||||
| Tavazzi 2008 (GISSI-HF) [37] | 122.2 | 121.0 | −0.1 | |||||||||
| Ridker 2008 (JUPITER) [47] | 186.0 | 118.0 | 49.0 | 108.0 | 185.0 | 118.0 | 49.0 | 108.0 | −19.0 | 0 | −54.0 | |
| Yokoyama 2007 (JELIS) [36] | 274.9 | 153.2 | 58.8 | 181.4 | 274.9 | 154.1 | 58.4 | 181.7 | 0 | −7.6 | 0 | |
| Kjekshus 2007 (CORONA) [55] | 178.0 | 48.0 | 137.0 | 176.0 | 47.0 | 136.0 | −42.0 | 2.0 | −63.0 | |||
| Deedwania 2007 (SAGE) [81] | 225.8 | 164.4 | 45.5 | 147.5 | 221.9 | 157.1 | 46.4 | 144.0 | −18.3 | −19.3 | −2.6 | −23.0 |
| Nakamura 2006 (MEGA) [63] | 242.5 | 127.5 | 57.6 | 156.6 | 242.5 | 127.5 | 57.6 | 156.6 | −20.5 | −14.2 | 19.3 | −20.7 |
| Knopp 2006 (ASPEN) [59] | 194.0 | 147.0 | 47.0 | 113.0 | 194.0 | 145.0 | 47.0 | 114.0 | −35.5 | −20.2 | 1.1 | −33.0 |
| Brouwer 2006 (SOFA) [12] | ||||||||||||
| Amarenco 2006 (SPARCL) [77] | 211.4 | 144.2 | 50.0 | 132.7 | 212.3 | 143.2 | 50.0 | 133.7 | −60.3 | −34.5 | 1.1 | −54.6 |
| Yokoi 2005 (ATHEROMA) [40] | 226.2 | 181.1 | 49.1 | 143.3 | 224.8 | 167.1 | 50.0 | 142.0 | −27.8 | −14.2 | 0.5 | −26.7 |
| Wanner 2005 [41] | 218.0 | 261.0 | 36.0 | 127.0 | 220.0 | 267.0 | 36.0 | 125.0 | −50.0 | |||
| Stone 2005 [42] | 228.0 | 149.0 | 44.0 | 149.0 | 230.0 | 151.0 | 43.0 | 151.0 | −35.9 | |||
| Raitt 2005 [16] | ||||||||||||
| Pedersen 2005 (IDEAL) [85] | 196.8 | 151.1 | 46.0 | 121.6 | 195.9 | 146.6 | 46.1 | 121.4 | −24.3 | −23.2 | −0.4 | −20.0 |
| Makuuchi 2005 (PCABG) [54] | 213.7 | 166.3 | 41.4 | 141.4 | 214.4 | 154.2 | 41.3 | 141.1 | −29.3 | −30.5 | 2.4 | −20.3 |
| Sever 2004 (ASCOT-LLA) [46] | 211.9 | 147.0 | 50.7 | 133.0 | 211.9 | 146.1 | 50.7 | 133.0 | −38.7 | −18.6 | 0.8 | 0 |
| Nissen 2004 (REVERSAL) [84] | 231.8 | 197.2 | 42.3 | 150.2 | 232.6 | 197.7 | 42.9 | 150.2 | −35.4 | −16.9 | −0.9 | −31.5 |
| Nakagawa 2004 (PCS) [64] | 200.1 | 141.9 | 43.0 | 128.7 | 200.5 | 143.9 | 43.3 | 128.3 | −17 | −7.9 | 0.4 | −15.3 |
| Koren 2004 (ALLIANCE) [58] | 226.0 | 197.0 | 40.0 | 147.0 | 225.0 | 198.0 | 41.0 | 146.0 | −20.0 | −12.0 | 0 | −16.0 |
| Colhoun 2004 (CARDS) [74] | 207.3 | 150.6 | 53.8 | 117.6 | 206.9 | 147.9 | 54.9 | 116.8 | −45.3 | −28.4 | 2.3 | −39.9 |
| Cannon 2004 (PROVE IT-TIMI) [80] | 181.0 | 158.0 | 38.0 | 106.0 | 180.0 | 154.0 | 39.0 | 106.0 | 0.3 | −33.0 | ||
| Shepherd 2002 (PROSPER) [49] | 220.4 | 132.9 | 50.3 | 146.9 | 220.4 | 132.9 | 50.3 | 146.9 | −34.8 | |||
| Serruys 2002 (LIPS) [50] | 200.0 | 160.0 | 38.0 | 131.0 | 199.0 | 160.0 | 37.0 | 132.0 | −1.0 | 0 | 0.3 | −49.9 |
| Sawayama 2002 (FAST) [51] | 251.5 | 168.7 | 56.7 | 160.7 | 255.2 | 135.8 | 56.5 | 171.6 | −1.8 | 15.3 | −43.1 | |
| Liem 2002 (FLORIDA) [56] | 204.9 | 150.6 | 46.4 | 135.3 | 208.8 | 141.7 | 46.4 | 139.2 | −45.4 | −29.2 | 1.8 | −40.9 |
| Davis 2002 (ALLHAT-LTT) [73] | 223.7 | 150.6 | 47.6 | 145.6 | 223.7 | 152.8 | 47.4 | 145.5 | −18.9 | 0.2 | 3.4 | −17.3 |
| Arthros 2002 (GREACE) [78] | 257.0 | 184.0 | 39.0 | 180.0 | 255.0 | 178.0 | 39.0 | 179.0 | −82.0 | −56.0 | 2.0 | −73.0 |
| Nilsen 2001 [15] | 229.7 | 145.3 | 41.8 | 231.6 | 137.3 | 44.9 | −11.3 | −52.9 | 4.8 | |||
| Teo 2000 (SCAT) [39] | 202.2 | 163.9 | 38.3 | 131.1 | 198.4 | 156.8 | 37.5 | 128.8 | −48.0 | −31.9 | 1.9 | −44.1 |
| Valagussa 1999 (GISSI-P) [44] | 210.2 | 162.6 | 41.5 | 137.3 | 211.6 | 161.9 | 41.7 | 138.5 | 2.6 | −6.3 | −0.2 | 4.2 |
| Riegger 1999 [48] | 289.0 | 189.0 | 53.0 | 198.0 | 284.0 | 183.0 | 56.0 | 193.0 | −36.4 | −37.9 | ||
| Plehn 1999 (CARE) [57] | 209.0 | 156.0 | 39.0 | 139.0 | 209.0 | 155.0 | 39.0 | 139.0 | 42.0 | 22.0 | −2.0 | 44.0 |
| Tonkin 1998 (LIPID) [38] | 218.0 | 142.0 | 36.0 | 150.0 | 218.0 | 138.0 | 36.0 | 150.0 | ||||
| Downs 1998 (AFCAPS/TexCAPS) [72] | 225.4 | 168.2 | 36.8 | 153.3 | 209.2 | 166.8 | 37.5 | 153.6 | −60.3 | −29.0 | 1.7 | −40.6 |
| Bestehorn 1997 (CIS) [76] | 240.3 | 44.3 | 164.5 | 243.4 | 43.6 | 167.4 | ||||||
| Jukema 1995 (REGRESS) [61] | 232.8 | 156.8 | 36.0 | 166.3 | 234.0 | 159.4 | 36.0 | 166.7 | −45.2 | −19.5 | 3.1 | −44.5 |
| Furberg 1995 (PLAC-I & II) [68] | 232.0 | 166.0 | 41.0 | 165.0 | 230.0 | 169.0 | 41.0 | 162.0 | ||||
| Furberg 1994 (ACAPS) [69] | 235.2 | 51.7 | 156.5 | 235.3 | 52.2 | 154.6 | ||||||
CVD, cardiovascular disease; CHD, coronary heart disease; MI, myocardial infarction; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. Data are presented as the mean or median serum lipid concentration. Change difference is defined as difference between lipid changes in the intervention arm versus lipid changes in the comparison arm. Change difference = (achieved level—baseline level) intervention arm—(achieved level—baseline level) comparison arm.
3.2. Meta-Analysis of Direct Estimates
In comparison with the control group, the pooled direct estimates of statins or omega-3 supplementation and the risk of CVD events are summarized in Table 3. Overall, the levels of significant findings were equivalent in both the fixed-effects and random-effect models, except for the association between atorvastatin or rosuvastatin and the risk of stroke. The total statin and omega-3 groups exhibited reduced risks of total CVD (RR = 0.89, 95% CI = 0.85–0.94), CHD (RR = 0.81, 95% CI = 0.75–0.89), MI (RR = 0.78, 95% CI = 0.78–0.85), and stroke (RR = 0.91, 95% CI = 0.85–0.98).
Table 3.
Pooled relative risk and 95% confidence interval from meta-analyses.
| N (I2) | Fixed-Effects Model | Random-Effects Model | |
|---|---|---|---|
| Total cardiovascular disease | |||
| Statins | 14 (30.0%) | 0.81 (0.78–0.85) | 0.81 (0.76–0.86) |
| Atorvastatin | 4 (55.5%) | 0.83 (0.78–0.88) | 0.83 (0.76–0.91) |
| Fluvastatin | 1 (NA) | 0.48 (0.24–0.94) | 0.48 (0.24–0.94) |
| Lovastatin | 1 (NA) | 0.76 (0.63–0.91) | 0.76 (0.63–0.91) |
| Pravastatin | 7 (28.8%) | 0.81 (0.74–0.89) | 0.77 (0.67–0.89) |
| Rosuvastatin | 1 (NA) | 0.80 (0.55–1.16) | 0.80 (0.55–1.16) |
| Omega-3 | 13 (0%) | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) |
| Overall | 27 (59.6%) | 0.92 (0.90–0.94) | 0.89 (0.85–0.94) |
| Coronary heart disease | |||
| Statins | 7 (0%) | 0.69 (0.62–0.77) | 0.70 (0.62–0.77) |
| Atorvastatin | 1 (NA) | 0.60 (0.49–0.75) | 0.60 (0.49–0.75) |
| Lovastatin | 1 (NA) | 0.76 (0.62–0.92) | 0.76 (0.62–0.92) |
| Pravastatin | 3 (0%) | 0.69 (0.56–0.84) | 0.69 (0.56–0.84) |
| Rosuvastatin | 1 (NA) | 0.75 (0.58–0.96) | 0.75 (0.58–0.96) |
| Simvastatin | 1 (NA) | 0.67 (0.11–3.95) | 0.67 (0.11–3.95) |
| Omega-3 | 10 (0%) | 0.90 (0.84–0.96) | 0.90 (0.85–0.96) |
| Overall | 17 (44.6%) | 0.84 (0.79–0.88) | 0.81 (0.75–0.89) |
| Myoca rdial infarction | |||
| Statins | 27 (51.4%) | 0.74 (0.69–0.79) | 0.69 (0.61–0.78) |
| Atorvastatin | 7 (73.6%) | 0.79 (0.71–0.87) | 0.70 (0.55–0.89) |
| Fluvastatin | 2 (42.6%) | 1.40 (0.76–2.56) | 1.18 (0.40–3.46) |
| Lovastatin | 2 (0%) | 0.62 (0.45–0.85) | 0.62 (0.45–0.85) |
| Pitavastatin | 1 (NA) | 0.37 (0.10–1.39) | 0.37 (0.10–1.39) |
| Pravastatin | 8 (3.1%) | 0.68 (0.60–0.77) | 0.66 (0.56–0.78) |
| Rosuvastatin | 4 (62.9%) | 0.74 (0.64–0.86) | 0.71 (0.55–0.91) |
| Simvastatin | 3 (11.2%) | 0.82 (0.43–1.56) | 0.85 (0.41–1.78) |
| Omega-3 | 15 (45.6%) | 0.88 (0.82–0.94) | 0.89 (0.80–0.99) |
| Overall | 42 (53.8%) | 0.81 (0.77–0.85) | 0.78 (0.72–0.85) |
| Stroke | |||
| Statins | 26 (33.3%) | 0.84 (0.80–0.89) | 0.85 (0.79–0.92) |
| Atorvastatin | 7 (64.9%) | 0.88 (0.79–0.98) | 0.88 (0.71–1.10) |
| Fluvastatin | 2 (11.4%) | 0.75 (0.17–3.31) | 0.77 (0.13–4.38) |
| Pravastatin | 9 (0%) | 0.88 (0.80–0.96) | 0.88 (0.80–0.96) |
| Rosuvastatin | 4 (68.8%) | 0.81 (0.70–0.95) | 0.80 (0.60–1.07) |
| Simvastatin | 3 (0%) | 0.76 (0.67–0.85) | 0.76 (0.67–0.85) |
| Omega-3 | 13 (36.1%) | 1.02 (0.94–1.10) | 0.88 (0.71–1.10) |
| Overall | 39 (46.2%) | 0.90 (0.86–0.94) | 0.91 (0.85–0.98) |
In subgroup analysis by statins, total statins were associated with decreased risks of total CVD, CHD, MI, and stroke, with RRs (95% CIs) of 0.81 (0.76–0.86), 0.70 (0.62–0.77), 0.69 (0.61–0.78), and 0.85 (0.79–0.82), respectively. Individually, atorvastatin and pravastatin had significant effects on all outcomes. Furthermore, rosuvastatin was associated with significantly reduced risks of CHD and MI, and lovastatin was associated with a significant risk reduction in total CVD. Fewer stroke events occurred in the simvastatin group than in the control group, and fluvastatin was observed to reduce total CVD. Moreover, omega-3 supplementation was associated with a 19% reduced risk of CHD (RR = 0.81, 95% CI = 0.75–0.89) and an 11% reduced risk of MI (RR = 0.89, 95% CI = 0.80–0.99).
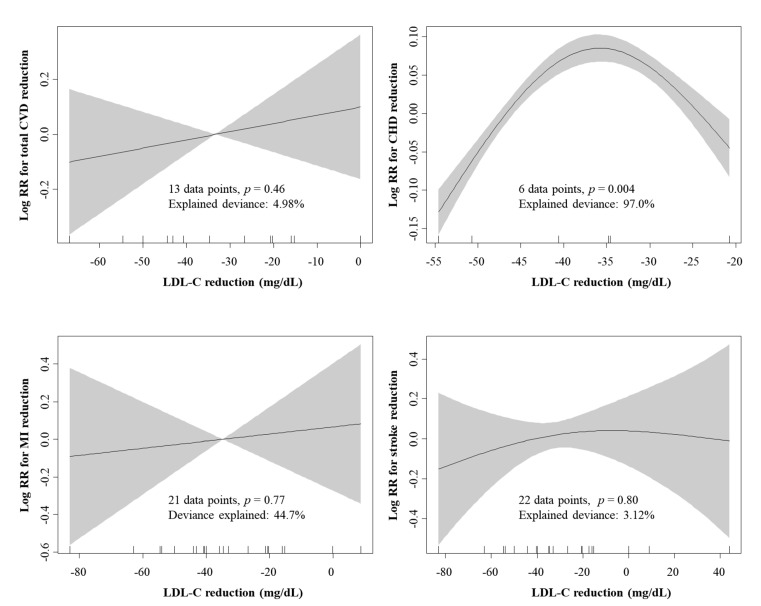
The meta-regression coefficients for the association between the lipid-lowering effect and risk reduction of cardiovascular events are presented in Table 4. The size of TC reduction achieved with overall statins and omega-3 supplementation was significantly associated with the size of total CVD and CHD reduction effect, with coefficients (p-values) of 0.0046 (<0.001) and 0.0042 (0.047), respectively. Additionally, total CVD and CHD reduction effect might be driven by per unit change in LDL-C level, with decreases of 0.0034 (p = 0.004) and 0.0044 (p = 0.047) in log (RRs) for total CVD and CHD per 1 mg/dL LDL-C lowering effect of statins and omega-3 supplementation, respectively. The combined meta-analysis of 20 effect sizes showed a significant reduction in LDL-C concentration following administration of statins (WMD, −33.63 mg/dL; 95% CI, −45.77 to −21.49 mg/dL; Figure A1), but not omega-3 supplementation (WMD, 0.12; 95% CI, −0.81 to 1.06 mg/dL; Figure A1). Relationships between cardiovascular event reduction and LDL-C lowering effects by subgroup for statins and omega-3 supplementation are presented in Figure A2 and Figure A3. CHD reduction effect of statins (p = 0.004) and stroke reduction effect of omega-3 supplementation (p = 0.02) were observed to be non-linearly associated with the LDL-C lowering effect, with the explained deviance of over 97%.
Table 4.
Change difference in lipid profiles in the association with risk reduction of cardiovascular events.
| Covariate | Total Cardiovascular Disease | Coronary Heart Disease | Myocardial Infarction | Stroke | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| TC | 0.0046 | <0.001 | 0.0042 | 0.047 | 0.0055 | 0.08 | −0.0111 | 0.53 |
| TG | 0.0026 | 0.51 | 0.0098 | 0.004 | 0.0009 | 0.89 | −0.0117 | 0.67 |
| HDL-C | −0.0116 | 0.18 | −0.0065 | 0.64 | −0.0198 | 0.35 | 0.0480 | 0.67 |
| LDL-C | 0.0034 | 0.004 | 0.0044 | 0.048 | 0.0048 | 0.09 | −0.0126 | 0.36 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
The coefficient from the meta-regression model represents the change in the log-transformed relative risk for every increase of one mg/dL in serum lipid concentrations.
3.3. Network Meta-Analysis of Direct and Indirect Estimates
Figure 2 presents the comparative effects among statins, omega-3 supplementation, and controls with regard to the risk of CVD events. Compared with findings from the meta-analysis of direct evidence, a significant effect of simvastatin (RR = 0.72, 95% CI = 0.56–0.92) on CHD was also observed, whereas the network estimates did not reveal any significant effects of pravastatin (RR = 0.88, 95% CI = 0.76–1.01), simvastatin (RR = 0.82, 95% CI = 0.65–1.03), or omega-3 supplementation (RR = 1.03, 95% CI = 0.91–1.17) on the risk of stroke.
Figure 2.
Network meta-analysis of statin, omega-3, and control in the risk of different cardiovascular events.
Pravastatin had a significantly lower risk of total CVD (RR = 0.81, 95% CI = 0.72–0.91), CHD (RR = 0.75, 95% CI = 0.60–0.94), and MI (RR = 0.71, 95% CI = 0.55–0.94) than omega-3 supplementation. The risks of total CVD, CHD, MI, and stroke in the atorvastatin group were lower than those in the omega-3 group, with RRs (95% CIs) of 0.80 (0.73–0.88), 0.64 (0.50–0.82), 0.75 (0.60–0.93), and 0.81 (0.66–0.99), respectively. Additionally, the risk of total CVD in the fluvastatin and lovastatin groups was lower than that in the omega-3 group, with RRs (95% CI) of 0.41 (0.18–0.95) and 0.77 (0.63–0.94), respectively.
Among statins, pravastatin, fluvastatin, lovastatin, and atorvastatin were found to be associated with lower risks of total CVD than simvastatin, with RRs (95% CIs) of 0.82 (0.69–0.96), 0.42 (0.18–0.97), 0.78 (0.62–0.98), and 0.81 (0.74–0.89), respectively. The risk of CHD in the atorvastatin group was also 19% lower than that in the simvastatin group (RR = 0.81, 95% CI = 0.73–0.90). Fluvastatin was associated with a higher MI risk than pravastatin, with RR = 2.21 and 95% CI = 1.04–4.69.
Data are presented as the relative risk and 95% confidence interval of row-defined intervention versus column-defined comparison for the risk of different cardiovascular events and with corresponding network plots of available direct comparison. The size of nodes is proportional to the number of studies including the respective treatments. The thickness of the edges is proportional to the mean control group risk for comparisons between control and active treatment groups.
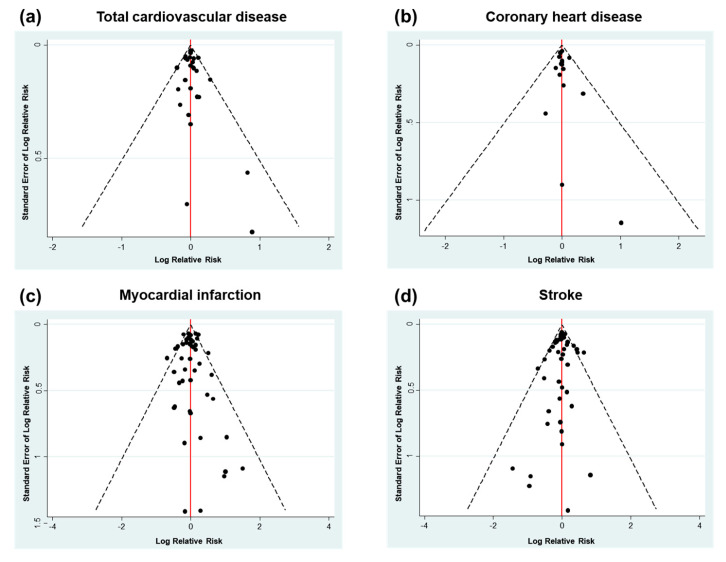
3.4. Publication Bias and Contribution Plot
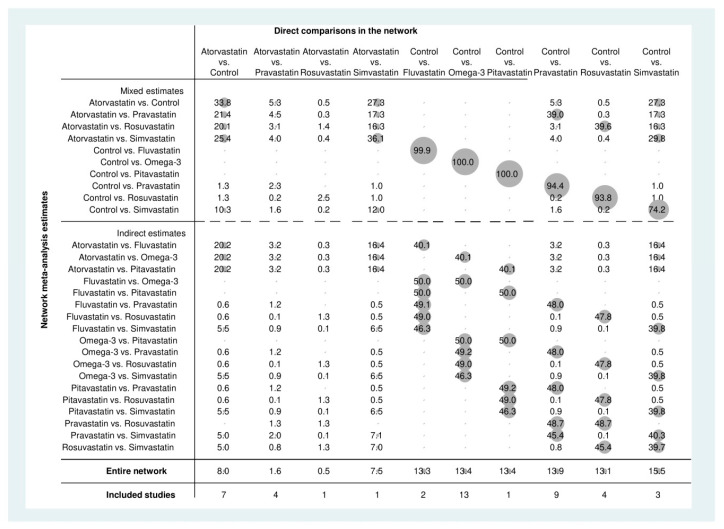
Funnel plot asymmetry indicating potential publication bias was not observed for different outcomes of CVD events (Figure 3). The contribution to mixed estimates and the influence on indirect estimates of direct estimates are presented in Figure A4, Figure A5, Figure A6 and Figure A7. Overall, most of the direct comparisons contributed nearly 100% to the same mixed comparisons in terms of total CVD (Figure A4) and CHD outcomes (Figure A5), except for control versus simvastatin. In contrast, approximately half of the direct comparisons did not contribute to the same mixed comparisons in terms of MI (Figure A6) and stroke outcomes (Figure A7).
Figure 3.
Funnel plot for publication bias according to (a) total cardiovascular disease, (b) coronary heart disease, (c) myocardial infarction, and (d) stroke.
3.5. Treatment Ranking
Fluvastatin had the highest probability of being the primary intervention for total CVD (85.4%) and stroke (42.2%), whereas atorvastatin and pitavastatin had the highest probabilities in terms of CHD (78.1%) and MI (72.7), respectively (Table 5). The surface under the cumulative ranking curve (SUCRA) values of cumulative ranking probability, which accounts for the uncertainty of spuriously high ranks, suggested better ranks for fluvastatin, atorvastatin, pitavastatin, and rosuvastatin in the prevention of total CVD (0.9), CHD (1.0), MI (0.9), and stroke (0.7).
Table 5.
Ranking probabilities and surface under the cumulative ranking (SUCRA) values of treatments according to different outcomes.
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | SUCRA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total cardiovascular disease | ||||||||||
| Control | 0 | 0 | 0 | 0 | 0.3 | 5.3 | 33.2 | 61.3 | 0.1 | |
| Omega-3 | 0 | 0 | 0 | 0.2 | 7.5 | 43.3 | 44.3 | 4.7 | 0.2 | |
| Simvastatin | 0 | 0 | 0.1 | 0.8 | 13.2 | 45.5 | 19.6 | 20.7 | 0.2 | |
| Pravastatin | 0.7 | 11.4 | 27.2 | 34.6 | 25.8 | 0.3 | 0 | 0 | 0.6 | |
| Fluvastatin | 85.4 | 7.1 | 1.7 | 1.5 | 2.1 | 0.6 | 0.3 | 1.3 | 0.9 | |
| Lovastatin | 3.6 | 32.0 | 30.1 | 17.5 | 15.6 | 1.0 | 0.2 | 0.1 | 0.7 | |
| Atorvastatin | 0.7 | 11.3 | 31.5 | 37.5 | 19.0 | 0 | 0 | 0 | 0.6 | |
| Rosuvastatin | 9.7 | 38.3 | 9.5 | 7.9 | 16.5 | 3.9 | 2.4 | 11.9 | 0.6 | |
| Coronary heart disease | ||||||||||
| Control | 0 | 0 | 0 | 0 | 0 | 2.5 | 97.5 | 0 | ||
| Omega-3 | 0 | 0 | 0.1 | 1.2 | 14.8 | 83.3 | 0.7 | 0.2 | ||
| Simvastatin | 0 | 26.8 | 26.1 | 24.6 | 18.8 | 3.4 | 0.3 | 0.6 | ||
| Pravastatin | 13.3 | 31.6 | 28.0 | 17.9 | 8.8 | 0.4 | 0 | 0.7 | ||
| Lovastatin | 3.2 | 12.3 | 21.7 | 29.5 | 29.1 | 3.9 | 0.3 | 0.5 | ||
| Atorvastatin | 78.1 | 15.9 | 4.9 | 1.2 | 0 | 0 | 0 | 1 | ||
| Rosuvastatin | 5.4 | 13.5 | 19.4 | 25.6 | 28.6 | 6.4 | 1.2 | 0.5 | ||
| Myocardial infarction | ||||||||||
| Control | 0 | 0 | 0 | 0 | 0.1 | 1.4 | 18.8 | 66.5 | 13.2 | 0.1 |
| Omega-3 | 0 | 0 | 0.1 | 0.8 | 7.4 | 33.0 | 49.5 | 8.5 | 0.7 | 0.3 |
| Simvastatin | 1.0 | 3.7 | 5.8 | 10.1 | 18.0 | 29.2 | 17.4 | 11.6 | 3.3 | 0.4 |
| Pravastatin | 8.4 | 30.1 | 28.2 | 19.0 | 10.6 | 3.3 | 0.4 | 0 | 0 | 0.7 |
| Fluvastatin | 0.2 | 0.8 | 0.7 | 0.9 | 2.0 | 3.6 | 5.4 | 7.4 | 78.9 | 0.1 |
| Lovastatin | 13.3 | 32.8 | 13.5 | 12.0 | 13.4 | 9.3 | 3.7 | 1.8 | 0.3 | 0.7 |
| Atorvastatin | 2.8 | 16.0 | 30.9 | 30.9 | 16.5 | 2.7 | 0.2 | 0 | 0 | 0.7 |
| Pitavastatin | 72.7 | 6.0 | 2.9 | 2.7 | 3.5 | 3.0 | 2.1 | 4.0 | 3.6 | 0.9 |
| Rosuvastatin | 2.1 | 10.6 | 17.9 | 23.6 | 28.5 | 14.4 | 2.6 | 0.3 | 0 | 0.6 |
| Stroke | ||||||||||
| Control | 0 | 0 | 0.1 | 1.2 | 13.3 | 38.1 | 37.2 | 10.1 | 0.2 | |
| Omega-3 | 0 | 0 | 0.4 | 2.3 | 8.0 | 21.7 | 39.6 | 28.0 | 0.2 | |
| Simvastatin | 10.9 | 23.4 | 23.1 | 19.5 | 13.0 | 6.6 | 2.4 | 1.1 | 0.7 | |
| Pravastatin | 1.9 | 8.0 | 16.8 | 27.3 | 28.2 | 14.7 | 2.2 | 0.7 | 0.5 | |
| Fluvastatin | 42.2 | 8.2 | 2.9 | 2.6 | 3.4 | 3.0 | 6.1 | 31.5 | 0.6 | |
| Atorvastatin | 5.5 | 18.2 | 28.3 | 25.2 | 16.3 | 5.6 | 0.6 | 0.2 | 0.6 | |
| Pitavastatin | 25.9 | 15.5 | 4.6 | 4.7 | 5.5 | 5.2 | 10.6 | 28.0 | 0.5 | |
| Rosuvastatin | 13.5 | 26.6 | 23.7 | 17.1 | 12.3 | 5.2 | 1.2 | 0.4 | 0.7 | |
4. Discussion
This meta-analysis and NMA of 63 RCTs investigated the effect of statins and omega-3 supplementation as well as their comparative efficacy of for the prevention of cardiovascular events. TG and HDL-C independent effects of total statins and omega-3 supplementation on the prevention of total CVD were observed. Furthermore, the effects of total statins and omega-3 supplementation on CHD, MI, and stroke were independent of the lipid-lowering effects of TC, TG, HDL-C, and LDL-C. Although the RCTs examined diverse populations, the findings were generally consistent for the significant risk reduction associated with statins in total CVD, CHD, MI, and stroke; conversely, a beneficial effect on stroke prevention was not observed for omega-3 supplementation. The findings were generally consistent in separate subgroup analyses of statins and omega-3 supplementation or in combined analyses of statins and omega-3 supplementation in terms of total CVD and CHD. Moreover, evidence was robust according to the Dersimonian–Laird methodology of the random-effects model, which accounts for between-study variability when at least two individual RCTs are included in pooled analysis.
The NMA of 264,516 adults showed that most statins and omega-3 supplementation were effective in reducing total CVD, CHD, and MI risks compared to the control group. Fluvastatin, atorvastatin, pitavastatin, and rosuvastatin were found to have the greatest effects on reducing total CVD, CHD, MI, and stroke, respectively.
Several guidelines have recommended statins for the primary and secondary prevention of CVD [89]. Statins can be classified as weak statins (simvastatin and pravastatin, compounds found in nature), strong (atorvastatin, pitavastatin, and rosuvastatin, synthetic molecules), and normal (fluvastatin and lovastatin) based on their ability to reduce cholesterol levels [90]. Yebyo et al. recently investigated the primary prevention effects of specific statins by NMA, though the date of the literature search was restricted to between 2013 and 2018, and the effect on total CVD and CHD was not investigated [31]. Statins were generally found to be associated with risk reductions in nonfatal MI (RR = 0.62, 95% CI = 0.53–0.72) and nonfatal stroke (RR = 0.83, 95% CI = 0.53–0.72) but not fatal MI or fatal stroke. In the current study, we found that statins significantly lowered the risks of combined fatal and nonfatal MI and stroke.
Several actions of omega-3 that overlap with the mechanisms of the pleiotropic effects of statins, including endothelial function improvement and antioxidant, anti-inflammatory, and antithrombotic effects, have been proposed [91,92]. Nonetheless, a previous meta-analysis also demonstrated no significant effects of omega-3 supplementation on total CVD (RR = 0.99, 95% CI = 0.89–1.09) and MI (RR = 0.81, 95% CI = 0.65–1.01) [93]. Rizos et al. also reported a null result for the effect of omega-3 supplementation on MI (RR = 0.89, 95% CI = 0.76–1.04) [94]. Aung et al. specified fatal and nonfatal CHD and different types of stroke, and the findings were still non-significant [28]. In contrast, Hu et al. recently updated findings from large-sample RCTs [11], including the A Study of Cardiovascular Events in Diabetes (ASCEND) trial [87], Vitamin D and Omega-3 Trial (VITAL) [88], and Reduction of Cardiovascular Events in Icosapent Ethyl-Intervention Trial (REDUCE-IT) that omega-3 supplementation significantly reduced the risk of total CVD (RR = 0.95, 95% CI = 0.92–0.98), MI (RR = 0.88, 95% CI = 0.83–0.94), and CHD (RR = 0.93, 95% CI = 0.89–0.96) [86]. Regardless a null finding for omega-3 supplementation in the prevention of stroke has consistently been reported [11,93,94].
This study had some intrinsic limitations. First, we did not specify the effect of statins and omega-3 supplementation on primary and secondary prevention of event outcomes, and primary prevention may be defined differentially in various studies. For example, Yebyo et al. categorized the population with CVD history was less than 10% of the total sample size as primary prevention [31], Naci et al. accepted a percentage up to 20% [22]. Additionally, the time frame in the identification of CVD history differed among RCTs. Therefore, we assumed that the effects of CVD and MI histories as well as other risk factors, such as hypertension, diabetes, and smoking, might not be significant. Second, arm-based data considering the sample size and number of events were included in the final analysis. Although the time to outcome for survival data is important in evaluating the effect of the intervention, the findings were not based on the contrast-based data of relative estimates because the hazard ratio was not available for all outcomes and in all RCTs. Third, there were only a few head-to-head RCTs of a statin versus a statin [80,81,82,83,84,85] and no RCT of a statin versus omega-3 supplementation. Last, due to the limitation of data availability, meta-regression for each type of statin as well as for omega-3 supplementation could not be performed separately.
Despite the abovementioned limitations, this study has important methodological strengths. To our knowledge, this is the first indirect comparison of statins versus omega-3 supplementation borrowing direct evidence of statins versus controls and omega-3 supplementation versus controls to provide quantitative evidence for the prevention of CVD events. The study was designed to minimize heterogeneity among controls from different RCTs. Although Abdelhamid et al. reported comprehensive evidence from 79 RCTs of omega-3 supplementation for the primary and secondary prevention of CVD [27], several RCTs were excluded from our analysis because of inappropriate control groups. The control group, which was defined as those with a lower intake of omega-3, might inappropriately develop an intermediary association with statins. RCTs in which the control group contained omega-6 were also excluded, as the debated effect of omega-6 intake has been reported [95,96,97,98,99]; thus, the inclusion of omega-6 in the control group might underestimate or overestimate the protective effect of omega-3. Moreover, robust findings were obtained by using different models in the meta-analysis and by comparing direct evidence from the meta-analysis and combined evidence from the NMA.
5. Conclusions
In summary, this study suggests that pravastatin and atorvastatin are more beneficial than omega-3 supplementation in reducing the risk of total CVD, CHD, and MI. Fluvastatin, atorvastatin, pitavastatin, and rosuvastatin showed the greatest effects on reducing total CVD, CHD, MI, and stroke, respectively.
Appendix A
Figure A1.
Meta-analysis for LDL-C lowering effect of statins and omega-3 supplementation. WMD, weighted mean difference; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol
Figure A2.
Non-linear association between cardiovascular event reduction and LDL-C lowering effects of statins. Cardiovascular events include total cardiovascular disease (CVD), coronary heart disease (CHD), myocardial infarction (MI), and stroke. RR, relative risk; CVD, cardiovascular disease; CHD, coronary heart disease; MI, myocardial infarction; LDL-C, low-density lipoprotein cholesterol.
Figure A3.
Non-linear association between cardiovascular event reduction and LDL-C lowering effects of omega-3 supplementation. Cardiovascular events include (a) total cardiovascular disease (CVD), (b) coronary heart disease (CHD), (c) myocardial infarction (MI), and (d) stroke. RR, relative risk; LDL-C, low-density lipoprotein cholesterol.
Figure A4.
Contribution plot for the total cardiovascular disease network. The size of the square is weighted as the percentage (as number) of the contribution of the direct estimate (horizontal axis) to the network estimate (vertical axis).
Figure A5.
Contribution plot for the coronary heart disease network. The size of the square is weighted as the percentage (as number) of the contribution of the direct estimate (horizontal axis) to the network estimate (vertical axis).
Figure A6.
Contribution plot for the myocardial infarction network. The size of the square is weighted as the percentage (as number) of the contribution of the direct estimate (horizontal axis) to the network estimate (vertical axis).
Figure A7.
Contribution plot for the stroke network. The size of the square is weighted as the percentage (as number) of the contribution of the direct estimate (horizontal axis) to the network estimate (vertical axis).
Author Contributions
Conceptualization, J.K. and T.H.; methodology, T.H. and J.K.; validation, J.K.; formal analysis, T.H.; data curation, T.H. and J.K.; writing-original draft preparation, T.H.; writing-review and editing, J.K.; project administration, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from National Cancer Center Korea (NO: 1910330).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Farley A., McLafferty E., Hendry C. The cardiovascular system. Nurs. Stand. 2012;27:35–39. doi: 10.7748/ns.27.9.35.s52. [DOI] [PubMed] [Google Scholar]
- 2.Stewart J., Manmathan G., Wilkinson P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017;6:2048004016687211. doi: 10.1177/2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Y., Guo Y., Zheng Y., Huang Z., Sun S., Kowal P., Shi Y., Wu F. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: Results from SAGE Wave 1. BMC Public Health. 2018;18:778. doi: 10.1186/s12889-018-5653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosiek A., Leksowski K. The risk factors and prevention of cardiovascular disease: The importance of electrocardiogram in the diagnosis and treatment of acute coronary syndrome. Ther. Clin. Risk Manag. 2016;12:1223–1229. doi: 10.2147/TCRM.S107849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KSoLA Dyslipidemia Fact Sheets in Korea. [(accessed on 21 December 2019)];2018 Available online: https://www.lipid.or.kr/file/Dyslipidemia%20Fact%20Sheets%20in%20Korea%202018.pdf.
- 6.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corra U., Cosyns B., Deaton C., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 10.Chou R., Dana T., Blazina I., Daeges M., Jeanne T.L. Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the US preventive services task force. J. Am. Med. Acad. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Hu F.B., Manson J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J. Am. Heart Assoc. 2019;8:e013543. doi: 10.1161/JAHA.119.013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwer I.A., Zock P.L., Camm A.J., Bocker D., Hauer R.N., Wever E.F., Dullemeijer C., Ronden J.E., Katan M.B., Lubinski A., et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: The Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. J. Am. Med. Acad. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 13.Dangour A.D., Allen E., Elbourne D., Fasey N., Fletcher A.E., Hardy P., Holder G.E., Knight R., Letley L., Richards M., et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: A randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2010;91:1725–1732. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 14.Macchia A., Grancelli H., Varini S., Nul D., Laffaye N., Mariani J., Ferrante D., Badra R., Figal J., Ramos S., et al. Omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: Results of the FORWARD (Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation) trial. J. Am. Coll. Cardiol. 2013;61:463–468. doi: 10.1016/j.jacc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Nilsen D.W., Albrektsen G., Landmark K., Moen S., Aarsland T., Woie L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am. J. Clin. Nutr. 2001;74:50–56. doi: 10.1093/ajcn/74.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Raitt M.H., Connor W.E., Morris C., Kron J., Halperin B., Chugh S.S., McClelland J., Cook J., MacMurdy K., Swenson R., et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: A randomized controlled trial. J. Am. Med. Acad. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Wood S. Package “mgcv”: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation, Version 1.8-31. [(accessed on 21 July 2020)]; Available online: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf.
- 19.Shim S., Yoon B.H., Shin I.S., Bae J.M. Network meta-analysis: Application and practice using Stata. Epidemiol. Health. 2017;39:e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 21.Chaimani A., Higgins J.P., Mavridis D., Spyridonos P., Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naci H., Brugts J.J., Fleurence R., Tsoi B., Toor H., Ades A.E. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: A network meta-analysis of placebo-controlled and active-comparator trials. Eur. J. Prev. Cardiol. 2013;20:641–657. doi: 10.1177/2047487313480435. [DOI] [PubMed] [Google Scholar]
- 23.Taylor F., Huffman M.D., Macedo A.F., Moore T.H.M., Burke M., Davey Smith G., Ward K., Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013;2013:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W., Zhang B. Statins for the prevention of stroke: A meta-analysis of randomized controlled trials. PLoS ONE. 2014;9:e92388. doi: 10.1371/journal.pone.0092388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balk E.M., Adams G.P., Langberg V., Halladay C., Chung M., Lin L., Robertson S., Yip A., Steele D., Smith B.T., et al. Omega-3 fatty acids and cardiovascular disease: An updated systematic review. Evid. Rep. Technol. Assess. Full Rep. 2016;223:1–1252. doi: 10.23970/AHRQEPCERTA223. [DOI] [PubMed] [Google Scholar]
- 26.Zhong P., Wu D., Ye X., Wu Y., Li T., Tong S., Liu X. Secondary prevention of major cerebrovascular events with seven different statins: A multi-treatment meta-analysis. Drug Des. Dev. Ther. 2017;11:2517–2526. doi: 10.2147/DDDT.S135785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelhamid A.S., Brown T.J., Brainard J.S., Biswas P., Thorpe G.C., Moore H.J., Deane K.H., AlAbdulghafoor F.K., Summerbell C.D., Worthington H.V., et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018;7:CD003177. doi: 10.1002/14651858.CD003177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., Geleijnse J.M., Rauch B., Ness A., Galan P., et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 trials involving 77917 individuals. J. Am. Med. Acad. Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielecka-Dabrowa A., Bytyci I., Von Haehling S., Anker S., Jozwiak J., Rysz J., Hernandez A.V., Bajraktari G., Mikhalidis D.P., Banach M. Association of statin use and clinical outcomes in heart failure patients: A systematic review and meta-analysis. Lipids Health Dis. 2019;18:188. doi: 10.1186/s12944-019-1135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tramacere I., Boncoraglio G.B., Banzi R., Del Giovane C., Kwag K.H., Squizzato A., Moja L. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: A systematic review and network meta-analysis. BMC Med. 2019;17:67. doi: 10.1186/s12916-019-1298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yebyo H.G., Aschmann H.E., Kaufmann M., Puhan M.A. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am. Heart J. 2019;210:18–28. doi: 10.1016/j.ahj.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Hosomi N., Nagai Y., Kohriyama T., Ohtsuki T., Aoki S., Nezu T., Maruyama H., Sunami N., Yokota C., Kitagawa K., et al. The Japan Statin Treatment Against Recurrent Stroke (J-STARS): A multicenter, randomized, open-label, parallel-group study. EBio Med. 2015;2:1071–1078. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford I., Murray H., McCowan C., Packard C.J. Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy: 20-year follow-up of West of Scotland Coronary Prevention Study. Circulation. 2016;133:1073–1080. doi: 10.1161/CIRCULATIONAHA.115.019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan K.L., Teo K., Dumesnil J.G., Ni A., Tam J., Investigators A. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 35.Yakusevich V.V., Malygin A.Y., Lychenko S.V., Petrochenko A.S., Kabanov A.V. The efficacy of high-dose simvastatin in acute period of ischemic stroke. Ration. Pharmacother. Card. 2012;8:4–16. doi: 10.20996/1819-6446-2012-8-1-4-16. [DOI] [Google Scholar]
- 36.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 37.Tavazzi L., Maggioni A.P., Marchioli R., Barlera S., Franzosi M.G., Latini R., Lucci D., Nicolosi G.L., Porcu M., Tognoni G., et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 38.Long-Term Intervention with Pravastatin in Ischaemic Disease Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 39.Teo K.K., Burton J.R., Buller C.E., Plante S., Catellier D., Tymchak W., Dzavik V., Taylor D., Yokoyama S., Montague T.J. Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: The Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT) Circulation. 2000;102:1748–1754. doi: 10.1161/01.CIR.102.15.1748. [DOI] [PubMed] [Google Scholar]
- 40.Yokoi H., Nobuyoshi M., Mitsudo K., Kawaguchi A., Yamamoto A., Investigators A.S. Three-year follow-up results of angiographic intervention trial using an HMG-CoA reductase inhibitor to evaluate retardation of obstructive multiple atheroma (ATHEROMA) study. Circ. J. 2005;69:875–883. doi: 10.1253/circj.69.875. [DOI] [PubMed] [Google Scholar]
- 41.Wanner C., Krane V., Marz W., Olschewski M., Mann J.F., Ruf G., Ritz E., German D., Dialysis Study I. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 42.Stone P.H., Lloyd-Jones D.M., Kinlay S., Frei B., Carlson W., Rubenstein J., Andrews T.C., Johnstone M., Sopko G., Cole H., et al. Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: The Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation. 2005;111:1747–1755. doi: 10.1161/01.CIR.0000160866.90148.76. [DOI] [PubMed] [Google Scholar]
- 43.Takano H., Mizuma H., Kuwabara Y., Sato Y., Shindo S., Kotooka N., Fujimatsu D., Kobayashi Y., Inoue T., Node K., et al. Effects of pitavastatin in Japanese patients with chronic heart failure: The Pitavastatin Heart Failure Study (PEARL Study) Circ. J. 2013;77:917–925. doi: 10.1253/circj.CJ-12-1062. [DOI] [PubMed] [Google Scholar]
- 44.Gissi P.I. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 45.Tavazzi L., Maggioni A.P., Marchioli R., Barlera S., Franzosi M.G., Latini R., Lucci D., Nicolosi G.L., Porcu M., Tognoni G., et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 46.Sever P.S., Dahlof B., Poulter N.R., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S.E., Kristinsson A., McInnes G.T., et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Drugs. 2004;64(Suppl. S2):43–60. doi: 10.2165/00003495-200464002-00005. [DOI] [PubMed] [Google Scholar]
- 47.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 48.Riegger G., Abletshauser C., Ludwig M., Schwandt P., Widimsky J., Weidinger G., Welzel D. The effect of fluvastatin on cardiac events in patients with symptomatic coronary artery disease during one year of treatment. Atherosclerosis. 1999;144:263–270. doi: 10.1016/S0021-9150(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 49.Shepherd J., Blauw G.J., Murphy M.B., Bollen E.L., Buckley B.M., Cobbe S.M., Ford I., Gaw A., Hyland M., Jukema J.W., et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/S0140-6736(02)11600-X. [DOI] [PubMed] [Google Scholar]
- 50.Serruys P.W., de Feyter P., Macaya C., Kokott N., Puel J., Vrolix M., Branzi A., Bertolami M.C., Jackson G., Strauss B., et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: A randomized controlled trial. J. Am. Med. Acad. 2002;287:3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 51.Sawayama Y., Shimizu C., Maeda N., Tatsukawa M., Kinukawa N., Koyanagi S., Kashiwagi S., Hayashi J. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST) J. Am. Coll. Cardiol. 2002;39:610–616. doi: 10.1016/S0735-1097(01)01783-1. [DOI] [PubMed] [Google Scholar]
- 52.Risk & Prevention Study Collaborative G., Roncaglioni M.C., Tombesi M., Avanzini F., Barlera S., Caimi V., Longoni P., Marzona I., Milani V., Silletta M.G., et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 53.Rauch B., Schiele R., Schneider S., Diller F., Victor N., Gohlke H., Gottwik M., Steinbeck G., Del Castillo U., Sack R., et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 54.Makuuchi H., Furuse A., Endo M., Nakamura H., Daida H., Watanabe M., Ohashi Y., Hosoda Y., Hosoda S., Yamaguchi H., et al. Effect of pravastatin on progression of coronary atherosclerosis in patients after coronary artery bypass surgery. Circ. J. 2005;69:636–643. doi: 10.1253/circj.69.636. [DOI] [PubMed] [Google Scholar]
- 55.Kjekshus J., Apetrei E., Barrios V., Bohm M., Cleland J.G., Cornel J.H., Dunselman P., Fonseca C., Goudev A., Grande P., et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 56.Liem A.H., van Boven A.J., Veeger N.J., Withagen A.J., Robles de Medina R.M., Tijssen J.G., van Veldhuisen D.J. Effect of fluvastatin on ischaemia following acute myocardial infarction: A randomized trial. Eur. Heart J. 2002;23:1931–1937. doi: 10.1053/euhj.2002.3291. [DOI] [PubMed] [Google Scholar]
- 57.Plehn J.F., Davis B.R., Sacks F.M., Rouleau J.L., Pfeffer M.A., Bernstein V., Cuddy T.E., Moye L.A., Piller L.B., Rutherford J., et al. Reduction of stroke incidence after myocardial infarction with pravastatin: The Cholesterol and Recurrent Events (CARE) study. The Care Investigators. Circulation. 1999;99:216–223. doi: 10.1161/01.CIR.99.2.216. [DOI] [PubMed] [Google Scholar]
- 58.Koren M.J., Hunninghake D.B., Investigators A. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: The alliance study. J. Am. Coll. Cardiol. 2004;44:1772–1779. doi: 10.1016/j.jacc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 59.Knopp R.H., d’Emden M., Smilde J.G., Pocock S.J. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: The Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 60.Kromhout D., Giltay E.J., Geleijnse J.M., Alpha Omega Trial G. n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 61.Jukema J.W., Bruschke A.V., van Boven A.J., Reiber J.H., Bal E.T., Zwinderman A.H., Jansen H., Boerma G.J., van Rappard F.M., Lie K.I., et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS) Circulation. 1995;91:2528–2540. doi: 10.1161/01.CIR.91.10.2528. [DOI] [PubMed] [Google Scholar]
- 62.Ostadal P., Alan D., Vejvoda J., Kukacka J., Macek M., Hajek P., Mates M., Kvapil M., Kettner J., Wiendl M., et al. Fluvastatin in the first-line therapy of acute coronary syndrome: Results of the multicenter, randomized, double-blind, placebo-controlled trial (the FACS-trial) Trials. 2010;11:61. doi: 10.1186/1745-6215-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura H., Arakawa K., Itakura H., Kitabatake A., Goto Y., Toyota T., Nakaya N., Nishimoto S., Muranaka M., Yamamoto A., et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): A prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 64.Nakagawa T., Kobayashi T., Awata N., Sato S., Reiber J.H., Nakajima H., Toyama Y.N., Hiraoka H., Kato O., Kirino M., et al. Randomized, controlled trial of secondary prevention of coronary sclerosis in normocholesterolemic patients using pravastatin: Final 5-year angiographic follow-up of the Prevention of Coronary Sclerosis (PCS) study. Int. J. Cardiol. 2004;97:107–114. doi: 10.1016/j.ijcard.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Mok V.C., Lam W.W., Chen X.Y., Wong A., Ng P.W., Tsoi T.H., Yeung V., Liu R., Soo Y., Leung T.W., et al. Statins for asymptomatic middle cerebral artery stenosis: The Regression of Cerebral Artery Stenosis study. Cerebrovasc. Dis. 2009;28:18–25. doi: 10.1159/000215939. [DOI] [PubMed] [Google Scholar]
- 66.Einvik G., Klemsdal T.O., Sandvik L., Hjerkinn E.M. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur. J. Cardiovasc. Prev. Rehabil. 2010;17:588–592. doi: 10.1097/HJR.0b013e328339cc70. [DOI] [PubMed] [Google Scholar]
- 67.Galan P., Kesse-Guyot E., Czernichow S., Briancon S., Blacher J., Hercberg S., Group S.F.O.C. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. Br. Med. J. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furberg C.D., Pitt B., Byington R.P., Park J.S., McGovern M.E. Reduction in coronary events during treatment with pravastatin. PLAC I and PLAC II Investigators. Pravastatin Limitation of Atherosclerosis in the Coronary Arteries. Am. J. Cardiol. 1995;76:60C–63C. doi: 10.1016/S0002-9149(99)80472-X. [DOI] [PubMed] [Google Scholar]
- 69.Furberg C.D., Adams H.P., Jr., Applegate W.B., Byington R.P., Espeland M.A., Hartwell T., Hunninghake D.B., Lefkowitz D.S., Probstfield J., Riley W.A., et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–1687. doi: 10.1161/01.CIR.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 70.Heart Protection Study Collaborative G., Jonathan E., Derrick B., Emma L., Sarah P., John D., Jane A., Rory C. C-reactive protein concentration and the vascular benefits of statin therapy: An analysis of 20,536 patients in the Heart Protection Study. Lancet. 2011;377:469–476. doi: 10.1016/S0140-6736(10)62174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yusuf S., Bosch J., Dagenais G., Zhu J., Xavier D., Liu L., Pais P., Lopez-Jaramillo P., Leiter L.A., Dans A., et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N. Engl. J. Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 72.Downs J.R., Clearfield M., Weis S., Whitney E., Shapiro D.R., Beere P.A., Langendorfer A., Stein E.A., Kruyer W., Gotto A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. J. Am. Med. Acad. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 73.Officers A., Coordinators for the A.C.R.G.T.A., Lipid-Lowering Treatment to Prevent Heart Attack T. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) J. Am. Med. Acad. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 74.Colhoun H.M., Betteridge D.J., Durrington P.N., Hitman G.A., Neil H.A., Livingstone S.J., Thomason M.J., Mackness M.I., Charlton-Menys V., Fuller J.H., et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 75.Investigators O.T., Bosch J., Gerstein H.C., Dagenais G.R., Diaz R., Dyal L., Jung H., Maggiono A.P., Probstfield J., Ramachandran A., et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 76.Bestehorn H.P., Rensing U.F., Roskamm H., Betz P., Benesch L., Schemeitat K., Blumchen G., Claus J., Mathes P., Kappenberger L., et al. The effect of simvastatin on progression of coronary artery disease. The Multicenter coronary Intervention Study (CIS) Eur. Heart J. 1997;18:226–234. doi: 10.1093/oxfordjournals.eurheartj.a015224. [DOI] [PubMed] [Google Scholar]
- 77.Karam J.G., Loney-Hutchinson L., McFarlane S.I. Stroke prevetion by aggressive reduction in cholestrol levels investigators. High-dose atorvastatin after stroke or transient ischemic attack: The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. J. Cardiometab. Syndr. 2008;3:68–69. doi: 10.1111/j.1559-4572.2008.07967.x. [DOI] [PubMed] [Google Scholar]
- 78.Athyros V.G., Papageorgiou A.A., Mercouris B.R., Athyrou V.V., Symeonidis A.N., Basayannis E.O., Demitriadis D.S., Kontopoulos A.G. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ’usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr. Med. Res. Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 79.Writing Group for the A.R.G., Bonds D.E., Harrington M., Worrall B.B., Bertoni A.G., Eaton C.B., Hsia J., Robinson J., Clemons T.E., Fine L.J., et al. Effect of long-chain omega-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: Results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. J. Am. Med. Acad. Intern. Med. 2014;174:763–771. doi: 10.1001/jamainternmed.2014.328. [DOI] [PubMed] [Google Scholar]
- 80.Cannon C.P., Braunwald E., McCabe C.H., Rader D.J., Rouleau J.L., Belder R., Joyal S.V., Hill K.A., Pfeffer M.A., Skene A.M., et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 81.Deedwania P., Stone P.H., Bairey Merz C.N., Cosin-Aguilar J., Koylan N., Luo D., Ouyang P., Piotrowicz R., Schenck-Gustafsson K., Sellier P., et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: Results of the Study Assessing Goals in the Elderly (SAGE) Circulation. 2007;115:700–707. doi: 10.1161/CIRCULATIONAHA.106.654756. [DOI] [PubMed] [Google Scholar]
- 82.Izawa A., Kashima Y., Miura T., Ebisawa S., Kitabayashi H., Yamamoto H., Sakurai S., Kagoshima M., Tomita T., Miyashita Y., et al. Assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial infarction—ALPS-AMI study. Circ. J. 2015;79:161–168. doi: 10.1253/circj.CJ-14-0877. [DOI] [PubMed] [Google Scholar]
- 83.Nicholls S.J., Ballantyne C.M., Barter P.J., Chapman M.J., Erbel R.M., Libby P., Raichlen J.S., Uno K., Borgman M., Wolski K., et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 84.Nissen S.E., Tuzcu E.M., Schoenhagen P., Brown B.G., Ganz P., Vogel R.A., Crowe T., Howard G., Cooper C.J., Brodie B., et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. J. Am. Med. Acad. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 85.Pedersen T.R., Faergeman O., Kastelein J.J., Olsson A.G., Tikkanen M.J., Holme I., Larsen M.L., Bendiksen F.S., Lindahl C., Szarek M., et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: A randomized controlled trial. J. Am. Med. Acad. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 86.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 87.Ascend Study Collaborative Group. Bowman L., Mafham M., Wallendszus K., Stevens W., Buck G., Barton J., Murphy K., Aung T., Haynes R., et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 88.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Albert C.M., Gordon D., Copeland T., et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grundy S.M. Primary prevention of cardiovascular disease with statins: Assessing the evidence base behind clinical guidance. Clin. Pharm. 2016;8 doi: 10.1211/CP.2016.20200568. [DOI] [Google Scholar]
- 90.Bird J.K., Calder P.C., Eggersdorfer M. The role of n-3 long chain polyunsaturated fatty acids in cardiovascular disease prevention, and interactions with statins. Nutrients. 2018;10:775. doi: 10.3390/nu10060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colussi G., Catena C., Novello M., Bertin N., Sechi L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017;27:191–200. doi: 10.1016/j.numecd.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 92.Gelosa P., Cimino M., Pignieri A., Tremoli E., Guerrini U., Sironi L. The role of HMG-CoA reductase inhibition in endothelial dysfunction and inflammation. Vasc. Health Risk Manag. 2007;3:567–577. [PMC free article] [PubMed] [Google Scholar]
- 93.Kwak S.M., Myung S.K., Lee Y.J., Seo H.G., Korean Meta-analysis Study G. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 94.Rizos E.C., Ntzani E.E., Bika E., Kostapanos M.S., Elisaf M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. J. Am. Med. Acad. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 95.Hooper L., Al-Khudairy L., Abdelhamid A.S., Rees K., Brainard J.S., Brown T.J., Ajabnoor S.M., O’Brien A.T., Winstanley L.E., Donaldson D.H., et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018;7:CD011094. doi: 10.1002/14651858.CD011094.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czernichow S., Thomas D., Bruckert E. n-6 Fatty acids and cardiovascular health: A review of the evidence for dietary intake recommendations. Br. J. Nutr. 2010;104:788–796. doi: 10.1017/S0007114510002096. [DOI] [PubMed] [Google Scholar]
- 97.Erkkila A., de Mello V.D., Riserus U., Laaksonen D.E. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Prog. Lipid Res. 2008;47:172–187. doi: 10.1016/j.plipres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Ramsden C.E., Hibbeln J.R., Majchrzak S.F., Davis J.M. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2010;104:1586–1600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DiNicolantonio J.J., O’Keefe J.H. Omega-6 vegetable oils as a driver of coronary heart disease: The oxidized linoleic acid hypothesis. Open Heart. 2018;5:e000898. doi: 10.1136/openhrt-2018-000898. [DOI] [PMC free article] [PubMed] [Google Scholar]