Abstract
Fetal sheep with placental insufficiency-induced intrauterine growth restriction (IUGR) have lower hindlimb oxygen consumption rates (OCRs), indicating depressed mitochondrial oxidative phosphorylation capacity in their skeletal muscle. We hypothesized that OCRs are lower in skeletal muscle mitochondria from IUGR fetuses, due to reduced electron transport chain (ETC) activity and lower abundances of tricarboxylic acid (TCA) cycle enzymes. IUGR sheep fetuses (n = 12) were created with mid-gestation maternal hyperthermia and compared with control fetuses (n = 12). At 132 ± 1 days of gestation, biceps femoris muscles were collected, and the mitochondria were isolated. Mitochondria from IUGR muscle have 47% lower State 3 (Complex I-dependent) OCRs than controls, whereas State 4 (proton leak) OCRs were not different between groups. Furthermore, Complex I, but not Complex II or IV, enzymatic activity was lower in IUGR fetuses compared with controls. Proteomic analysis (n = 6/group) identified 160 differentially expressed proteins between groups, with 107 upregulated and 53 downregulated mitochondria proteins in IUGR fetuses compared with controls. Although no differences were identified in ETC subunit protein abundances, abundances of key TCA cycle enzymes [isocitrate dehydrogenase (NAD+) 3 noncatalytic subunit β (IDH3B), succinate-CoA ligase ADP-forming subunit-β (SUCLA2), and oxoglutarate dehydrogenase (OGDH)] were lower in IUGR mitochondria. IUGR mitochondria had a greater abundance of a hypoxia-inducible protein, NADH dehydrogenase 1α subcomplex 4-like 2, which is known to incorporate into Complex I and lower Complex I-mediated NADH oxidation. Our findings show that mitochondria from IUGR skeletal muscle adapt to hypoxemia and hypoglycemia by lowering Complex I activity and TCA cycle enzyme concentrations, which together, act to lower OCR and NADH production/oxidation in IUGR skeletal muscle.
Keywords: electron transport chain, mitochondria, placental insufficiency, proteomics, tricarboxylic acid cycle
INTRODUCTION
Intrauterine growth-restricted (IUGR) fetuses have reduced lean mass and impaired glucose metabolism compared with normally growing counterparts (13). Many cases of IUGR are caused by reductions in placental function, leading to fetal nutrient restriction, resulting in fetal hypoglycemia, hypoxemia, and maladaptive sarcopenia (63, 70, 78, 85). Moreover, it has been proposed that nutrient and oxygen restriction to the fetus during development is associated with permanent alterations in whole-body metabolism and a fixed functional capacity of vital organs in postnatal life (5, 66). Our ovine model of hyperthermia-induced placental insufficiency successfully recapitulates several aspects of the human IUGR phenotype (52). These maladaptations to IUGR in utero can eventually lead to complications later in life, as these adults are more prone to develop metabolic syndrome (73).
Normally, the oxidation of glucose and lactate accounts for >50% of whole-fetus oxygen consumption, underscoring the importance of these substrates in fetal metabolism (35). When placental transport capacity for glucose and oxygen is limited, the IUGR fetus prioritizes growth of neurological tissues at the expense of peripheral tissue growth through metabolic and endocrine adaptations (6, 51, 68). This is evidenced in the IUGR fetus by lower hindlimb skeletal muscle mass and weight-specific oxygen consumption rates (OCRs), even though hindlimb weight-specific glucose uptake is similar to uptake in control fetuses (74). Body weight-specific glucose utilization rates are also similar between IUGR and control fetuses, despite lower fractional glucose oxidation rates that have been shown to persist in skeletal muscle of IUGR lambs (14, 54, 87). Importantly, higher glucose extraction efficiencies and expression levels of glycolytic enzymes maintain normal hindlimb glucose-oxygen quotients, but further reduction in the lactate-oxygen quotient indicates greater lactate output per mole of oxygen consumed (72, 74, 81). These findings imply that IUGR fetuses have lower skeletal muscle oxidative metabolic capacity.
Mitochondria, the site of cellular respiration and oxidative phosphorylation, are central to metabolism. Within the mitochondrial matrix, the conversion of pyruvate, the primary metabolite from glucose, into acetyl-CoA is the first step in a series of redox steps that ultimately lead to the production of NADH and FADH2 within the tricarboxylic acid (TCA) cycle (67). Although the TCA cycle does not directly consume oxygen, it is considered aerobic, because the NADH and FADH2 produced by the acetyl-CoA metabolism must transfer their electrons to the electron transport chain (ETC) at Complexes I and II, respectively, to regenerate NAD+ and FADH for further revolutions of the TCA cycle (1, 41). Regeneration of these redox cofactors through oxidation at the ETC relies upon the terminal electron transfer to oxygen at Complex IV (COX4) (21, 57, 89). Thus the TCA cycle is indirectly dependent upon oxygen for function. If sufficient electron transfer does not occur within the ETC (e.g., post-translational modification of ETC subunits), then mitochondrial oxygen consumption rates decrease; however, this leads to alterations in cellular metabolism and substrate selection that hinder TCA cycle function (2, 15, 19, 28, 75). As such, the TCA cycle and the ETC are linked, and a decrease of the mitochondrial metabolism to conserve oxygen would concomitantly lower glucose metabolism.
Given that the IUGR fetuses have less lean body mass than their appropriately grown counterparts, it is not surprising that previous studies have shown that IUGR skeletal muscle metabolically adapts in utero (14, 24, 74, 87, 88). Glucose metabolism and oxygen consumption are linked through the TCA cycle and subsequent oxidative phosphorylation, and previous findings in IUGR models with placental restriction indicate reduced mitochondrial function in the fetal skeletal muscle (24, 61). However, mitochondrial metabolic capacity has not yet been examined comprehensively in the skeletal muscle of IUGR fetuses.
The objective of this study was to uncover differences between IUGR and control skeletal muscle mitochondrial metabolism by the following: 1) measuring OCRs and ETC subunit activities on isolated mitochondria as a means to quantitatively measure ETC function and 2) using a proteomic-based approach to measure differences in protein abundances within the mitochondria-proteome between IUGR and control fetuses. Because IUGR fetuses have previously been reported to possess lower hindlimb OCRs, we tested the hypothesis that OCRs are lower in skeletal muscle mitochondria from IUGR fetuses due to reduced ETC activity and lower abundances of TCA cycle enzymes.
MATERIALS AND METHODS
Fetal sheep model of IUGR.
Studies on pregnant Columbia-Rambouillet ewes were approved by the University of Arizona Institutional Animal Care and Use Committee and performed at the Agricultural Research Complex, which is accredited by the American Association for Accreditation of Laboratory Animal Care International. The experimental design of this study was performed in accordance with the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines (47). Columbia-Rambouillet cross-bred ewes with singleton pregnancies were purchased from the University of Arizona Sheep Unit and transported to the laboratory at 35 ± 2 days of gestation age (dGA). The ewes were 2–4 yr of age with unknown parity. Singleton fetuses were determined by ultrasonography before group assignment. Ewes were assigned by a simple randomization method into one of two groups: control and IUGR. Placental insufficiency-induced IUGR fetuses (n = 15) were created by exposing pregnant ewes to elevated ambient temperatures (40°C for 12 h; 35°C for 12 h; dew point 22°C) from 40 ± 1 dGA to 91 ± 1 dGA (term 149 dGA), as described previously (56). Control fetuses (n = 12) were from ewes maintained 22 ± 1°C, which were fed alfalfa pellets to the average ad libitum feed intake of ewes in the IUGR group. Water and salt were available to ewes ad libitum. After the hyperthermic exposure, all ewes were maintained in a thermoneutral environment alongside ewes in the control group. Three IUGR fetuses were lost before surgery, making the final animal numbers for the experimental groups: control, n = 12, and IUGR, n = 12.
Surgical preparation and fetal physiological studies.
At day 123 ± 1, each fetus underwent a sterile, surgical procedure to place indwelling, polyvinyl arterial, and venous catheters for blood sampling and infusion, as described previously (54). Animals were allowed to recover for at least 5 days before in vivo physiological experimentation to determine rates of fetal glucose, oxygen, and lactate umbilical uptakes. Fetal catheters for blood sampling were placed in the abdominal aorta via hindlimb pedal arteries and the umbilical vein. Infusion catheters were placed in the femoral veins via the saphenous veins. Maternal catheters were placed in the femoral artery and vein for arterial sampling and venous infusions. All catheters were tunneled subcutaneously to the ewe’s flank, exteriorized through a skin incision, and kept in a plastic mesh pouch sutured to the ewe’s skin. At induction of all surgical procedures, ewes received an intramuscular injection of penicillin G procaine-injectable suspension (24,000 units/kg; Agri-Cillin, Huvepharma, Inc., Peachtree City, GA) and intravenous injection of Ketofen (2 mg/kg; Zoetis, Kalamazoo, MI). Ewes were given postoperative analgesics intravenously (10 mg · kg−1 · day−1 phenylbutazone; VetOne, Boise, ID) for 3 days. The vascular catheters were flushed daily with heparinized saline solution (100 U/mL heparin in 0.9% NaCl solution; Vedco, Inc., St. Joseph, MO).
Rates of umbilical (net fetal) uptake for oxygen, glucose, and lactate were measured at 130 ± 1 days of gestation. Umbilical blood flows were measured by steady-state tritiated water diffusion and normalized to fetal weight for the study (New England nucleotides; PerkinElmer Life Sciences, Boston, MA). After a priming bolus of 50 μCi 3H2O, the intravenous 3H2O infusion (0.83 μCi/min) began, 60 min before sampling commenced. Four sets of blood samples were collected at 10-min intervals to determine umbilical blood flow, blood gasses, blood oximetry, plasma metabolites, and hormone concentration. At the start of tracer infusion, an infusion of maternal blood (10 mL/h) was given to the fetus to avoid fetal anemia from sampling. Two control and two IUGR animals were not included in the physiological studies, due to failure of catheter patency. The animal numbers for blood gas, hormone, and metabolite collection were the following: control, n = 10, and IUGR, n = 10.
Arterial blood gases and oximetry for each sample were measured with an ABL825 (Radiometer, Copenhagen, Denmark), and values were temperature corrected to 39.1°C. Plasma glucose and lactate concentrations were measured with the Yellow Springs Instruments (YSI) model 2700 Select Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Additionally, plasma concentrations of insulin (ovine insulin ELISA; ALPCO Diagnostics, Windham, NH; intra- and interassay coefficients of variation <6%; sensitivity 0.14 ng/mL) and norepinephrine (noradrenaline ELISA; Labor Diagnostika Nord GmbH & Co., KG, Germany; intra- and interassay coefficients of variation <14%; sensitivity 35 pg/mL) were measured.
Tissue collection.
At day 132 ± 1, the ewe and the fetus were euthanized with an intravenous administration of sodium pentobarbital (86 mg/kg) and phenytoin sodium (11 mg/kg; Euthasol; Virbac Animal Health). Fetal organs and skeletal muscles [biceps femoris (BF)] were dissected and weighed. The tissue that was not used immediately for mitochondrial isolations was then snap frozen in liquid nitrogen and stored at −80°C for in vitro experiments.
The large muscle groups of the body (e.g., biceps femoris, latissimus dorsi, etc.) have a proportionally greater impact on glucose metabolism due to mass compared with smaller muscles. Additionally, these large muscle groups are also comprised of mixed fiber types (25, 34, 39, 86). Therefore, we chose the biceps femoris (BF) muscle as the representative muscle group for this study.
Skeletal muscle moisture content.
Tissue water content was quantified utilizing the BF muscle. BF muscle (300 mg; n = 12/group) was placed in a drying oven, set at a temperature of +40°C until reaching constant weight. Muscles were then reweighed (dry weight), and the percentage of water mass was calculated using the following equation: (wet weight − dry weight)/wet weight × 100.
Isolation of mitochondria.
Fresh BF muscle from each fetus was cleaned of fascia, 3 grams of cleaned BF was minced and homogenized, and mitochondria were isolated by density gradient centrifugation, according to established protocols using “Isolation Buffer 1” and “Isolation Buffer 2” (29). Isolation Buffer 1 consists of a final concentration of the following: 67 mM sucrose, 50 mM Tris/HCl, 50 mM 1 M KCl, 10 mM EDTA, and 0.02% w/v BSA in distilled water and adjusted to pH 7.4. Isolation Buffer 2 consists of a final concentration of the following: 250 mM sucrose, 3 mM EGTA, and 1 mM Tris in distilled water and adjusted to pH 7.4. Oxygen consumption rates were measured in “Experimental Buffer,” which was prepared by mixing the following: 10 mM Tris/HCl, 5 mM MgCl2, 2 mM inorganic phosphate (Pi), 0.02 mM EGTA, and 250 mM sucrose in distilled water and adjusted to pH 7.4.
Mitochondrial oxygen consumption rate.
Following isolation, mitochondria were incubated on ice in Isolation Buffer 2 for 30 min before OCR measurements to ensure sufficient washout of respiratory substrates (29). For each OCR analysis, pelleted mitochondria were resuspended in 1:3 (v/v) Experimental Buffer that was prewarmed to 39.1°C. The resulting solution was divided evenly into three separate chambers of a Fluorescence Lifetime Micro Oxygen Monitoring System (Instech Laboratories, Inc., Plymouth Meeting, PA), which was prewarmed to 39.1°C, as previously described (69).
Partial pressure of O2 (Po2) in each chamber was recorded over time using fiber optic sensors and NeoFox viewer software (Instech Laboratories, Inc.). Oxygen consumption rates (OCRs; nanomole O2 per minute) were determined from the slope of Po2 disappearance over time for State 3 and State 4 and were calculated using the average of triplicate measurements for each condition. Maximum Complex I-linked respiration (State 3) was measured in the presence of 5 mM glutamate, 5 mM malate, and 100 µM ADP. For each mitochondria preparation, OCRs were recorded for 5 min before the addition of ADP (+glutamate/malate, −ADP; State 4Low ADP) to ensure sufficient use/washout of respiratory substrates for each sample before the addition of 100 µM ADP, State 3 measurements. To measure OCR in the absence of oxidative phosphorylation (State 4Oligomycin), an ATP synthase inhibitor, oligomycin A (5 µM final concentrations) was added after State 3 measurements (11). All OCRs at State 4Low ADP were not different of their respective State 4Oligomycin measurements. The respiratory control ratio (RCR) was calculated as the ratio of State 3-to-State 4Oligomycin OCRs (11).
Integrity of the mitochondria preparations of each sample was determined by the addition of 100 µM cytochrome c during State 3 OCR measurements and confirmed by less than a 16% increase in OCRs (Sigma). Following OCR analysis, the isolated mitochondria were collected from each chamber, and protein concentrations were measured with a Pierce Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher) for OCR normalization to protein concentration.
In-solution tryptic digestion of mitochondria.
To determine changes in the mitochondrial proteome associated with IUGR, 50 μg of mitochondrial isolates was supplemented with dithiothreitol (DTT) at a final concentration of 5 mM and incubated at 56°C for 30 min. Samples were cooled to room temperature for 10 min and incubated with 15 mM acrylamide for 30 min at room temperature while protected from light. The samples were supplemented with additional DTT at a final concentration of 5 mM and incubated in the dark for 15 min to quench the alkylation reaction. Prechilled, 100% acetone (6 volumes) was added to the samples and incubated for 1 h at −20°C to precipitate proteins, followed by centrifugation at 16,000 g for 10 min at 4°C. Prechilled, 90% acetone (400 μL) was added to the protein pellet and vortexed, followed by centrifugation at 16,000 g for 5 min at 4°C. The remaining acetone was removed, and the protein pellets were air dried for 2–3 min. The protein pellet was resuspended in 50 μL of digestion buffer [50 mM NH4HCO3, 1% sodium deoxycholate (SDC)] and sonicated for 5 min. Lys-C (1 μg) was added to each sample and incubated at 37°C for 2 h while shaking at 300 rpm. Afterward, 50 μL of 50 mM ammonium bicarbonate and 2 μg of trypsin were added to each sample and incubated at 37°C overnight while shaking at 300 rpm. Formic acid (FA; 14.7 µL; 40%)/1% heptafluorobutyric (HFBA) was added to each sample and incubated for 10 min (final concentration is 4% FA/0.1% HFBA) to simultaneously stop trypsin digestion and cause precipitation of the SDC contained in the digestion buffer. The SDC was pelleted by centrifuging at 12,000 g for 10 min, and the peptide-containing solution was extracted. The samples were desalted with Pierce Peptide Desalting Spin Columns, per the manufacturer’s protocol [Thermo Fisher Scientific; catalog number (cat. no.) 89852], and the peptides were dried by vacuum centrifugation. The dried peptides were resuspended in 20 μL of 0.1% FA (v/v), and the peptide concentration was determined with the Pierce Quantitative Colorimetric Peptide Assay Kit, per the manufacturer’s protocol (Thermo Fisher Scientific; cat. no. 23275). The final sample (600 ng) was analyzed by mass spectrometry.
Mass spectrometry and data processing.
HPLC-electrospray ionization-tandem mass spectrometry (ESI-MS/MS) was performed in positive ion mode on a Thermo Scientific Orbitrap Fusion Lumos Tribrid mass spectrometer fitted with an EASY-Spray Source (Thermo Scientific, San Jose, CA). NanoLC was performed using a Thermo Scientific UltiMate 3000 Rapid Separation Liquid Chromatography (RSLC)nano System with an EASY Spray C18 LC column (Thermo Scientific; 50 cm × 75 μm inner diameter, packed with PepMap RSLC C18 material, 2 µm; cat. no. ES803); loading phase for 15 min at 0.300 μL/min; and mobile phase, linear gradient of 1–34% Buffer B in 119 min at 0.220 μL/min, followed by a step to 95% Buffer B over 4 min at 0.220 μL/min, hold 5 min at 0.250 μL/min, and then a step to 1% Buffer B over 5 min at 0.250 μL/min and a final hold for 10 min (total run 159 min); Buffer A = 0.1% FA/H2O; Buffer B = 0.1% FA in 80% acetonitrile. All solvents were liquid chromatography-mass spectrometry grade. Spectra were acquired using XCalibur, version (v)2.3 (Thermo Fisher Scientific).
Label-free quantitative proteomics.
Progenesis QI for proteomics software (version 2.4; Nonlinear Dynamics Ltd., Newcastle upon Tyne, UK) was used to perform ion intensity-based, label-free quantification, similar to that previously described (71). In brief, in an automated format, .raw files were imported and converted into two-dimensional maps (y-axis = time; x-axis = mass-to-charge ratio (m/z)], followed by selection of a reference run for alignment purposes. An aggregate dataset containing all peak information from all samples was created from the aligned runs, which were then further narrowed down by selecting only +2, +3, and +4 charged ions for further analysis. The samples were then grouped in control versus IUGR. A peak list of fragment ion spectra was exported in Mascot generic file (.mgf) format and searched against the Ovis aries UniProt database (27,372 entries) using Mascot (version 2.6; Matrix Science, London, UK). The search variables that were used were the following: 10 parts per million (ppm) mass tolerance for precursor ion masses and 0.5 Da for product ion masses; digestion with trypsin; a maximum of two missed tryptic cleavages; and variable modifications of oxidation of methionine and phosphorylation of serine, threonine, and tyrosine; peak detection incorporating one mass unit of 13C (carbon 13). The resulting Mascot .xml file was then imported into Progenesis, allowing for peptide/protein assignment, whereas peptides with a Mascot ion score of <25 were not considered for further analysis. Precursor ion-abundance values for peptide ions were normalized to all proteins. For quantification, proteins must have possessed at least one or more unique, identifying peptides.
The subcellular localization database, COMPARTMENTS, was used to identify mitochondrial proteins using a confidence score of 3 or greater (9). Of those mitochondrial-specific proteins, comparisons were made to identify differential expression (DE) between groups. Perseus software platform was used to generate principal component analysis (PCA) plots and heatmaps to compare and visualize differences between samples, and a pseudo-count of one was added to the abundance scores to avoid a logarithm of zero (83). Volcano plot visualizations were constructed to summarize protein abundance between the two experimental groups. The figure was generated using statistical software R (v3.3.3) and plotting fold change (x-axis, log2 scale) against P value (y-axis, −log10 scale), obtained from conducting a two-sample t test on the log (base 2)-normalized abundances.
Database for Annotation, Visualization and Integrated Discovery (DAVID) was used to identify the significant biological themes within Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for DE proteins (Supplemental Tables S2–S5; all Supplemental Tables is available at https://doi.org/10.6084/m9.figshare.11872449) (3, 20, 37, 38, 42–44). Graphs for the individual, significantly enriched annotations within GO subontologies [Biological Process (BP), Cellular Component (CC), and Molecular Function (MF)] and KEGG pathways were plotted as fold enrichments against log10-adjusted P values. Pathways were summarized to reduce redundant, nonphysiologically relevant terms.
Enzymatic assessment of mitochondrial function.
Complex I, II, and IV activities were measured in biceps femoris muscle from the same subset of 12 animals used for proteomic analysis (n = 6/group). Measurements were recorded, in triplicate, with the Complex I (cat. no. ab109721; Abcam), Complex II (cat. no. ab109908; Abcam), and Complex IV (cat. no. ab109909; Abcam) Enzyme Activity Kits, and all were analyzed using a SpectraMax M2 plate reader (Molecular Devices, San Jose, CA). Skeletal muscle (100 mg) was homogenized in cold phosphate-buffered saline (PBS), and the protein concentration was measured using a Pierce BCA Protein Assay Kit. Samples were adjusted to recommended protein concentrations, and the proteins were extracted from the samples using 10× detergent, provided by the manufacturer. Samples were then centrifuged at 12,000 g for 20 min at 4°C. Sample concentrations were verified to be ~5 mg/mL. Samples were loaded into the wells at a concentration of 100 µg/200 µL (Complex I assay), 60 µg/50 µL (Complex II), or 10 µg/200 µL (Complex IV), along with appropriate positive and negative controls.
For each assay, plates were incubated at recommended temperatures, and activity was extrapolated by optical density in a kinetic mode: Complex I, 1-min intervals at 450 nm for 30 min; Complex II, 20-s intervals at 600 nm for 60 min; and Complex IV, 1-min intervals at 550 nm for 120 min. The linear rate of absorbance was then calculated and normalized to whole protein concentration.
Western blot analysis.
Mitochondrial protein expression was evaluated in the same subset of 12 animals used for proteomic analysis (n = 6/group). Protein lysates were prepared from biceps femoris muscle in ice-cold CelLytic MT Buffer (Sigma-Aldrich, St. Louis, MO) containing additional protease and phosphatase inhibitors: 0.5 mM phenylmethane sulfonyl fluoride (Thermo Fisher Scientific), 2 µg/mL aprotinin (Sigma-Aldrich), 2.5 µg/mL leupeptin (Sigma-Aldrich), 100 µM DTT, and 5 mM sodium vanadate (Thermo Fisher Scientific). Protein concentrations were measured with a Pierce BCA Protein Assay Kit. Protein (50 µg) from each animal was separated on a 15% polyacrylamide gel and transferred onto PVDF membranes (Bio-Rad, Hercules, CA). The membranes were blocked in 5% (w/v) nonfat milk in Tris-buffered saline, 0.1% Tween (TBST), solution for 30 min at room temperature and then incubated overnight at 4°C with primary antibodies in 5% nonfat milk in TBST. The primary antibodies used are as follows: anti-Complex IV (COX4; 1:3,000; RRID:AB_879754), anti-isocitrate dehydrogenase (NAD+) 3 noncatalytic subunit β (IDH3B; 1:3,000; RRID:AB_2819360), anti-NADH dehydrogenase 1α subcomplex 4-like 2 (NDUFA4L2; 1:3,000; RRID:AB_2761150), anti-oxoglutarate dehydrogenase (OGDH; 1:3,000; RRID:AB_2156766), anti-succinate-CoA ligase ADP-forming subunit-β (SUCLA2; 1:3,000; RRID:AB_2819117), and Total Oxidative Phosphorylation (OXPHOS) Rodent Western Blot (WB) Antibody Cocktail (1:3,000; RRID:AB_2629281). Specificity of antibodies was verified by the presence of a single band at the expected molecular weight, and Total OXPHOS antibody mixture was used, as previously described (26). Goat anti-rabbit or anti-mouse IgG secondary antibodies conjugated with horseradish peroxidase (1:15,000; Bio-Rad; RRID:AB_11125142) were detected with Thermo Scientific SuperSignal West Pico Chemiluminescent Substrate and exposed to Blue Lite Autorad Film (VWR, Radnor, PA). Protein concentrations were quantified using photographed images and densitometric analyses (Scion Image Software, Frederick, MD). Protein concentrations were normalized to citrate synthase (CS; 1:3,000; Santa Cruz; RRID:AB_2813783). All samples were evaluated in triplicate, and data are presented relative to control values.
Measurements for skeletal muscle mitochondria density.
Mitochondrial DNA (mtDNA) was copurified with genomic DNA (nDNA) from 100 mg of biceps femoris from the same subset of 12 animals used for proteomic analysis (n = 6/group). DNA concentration and purity were determined from absorbance measurements with a NanoDrop ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE). Total DNA (12.5 ng) was used per reaction. DNA was amplified using a QuantiTect SYBR Green PCR Kit (Qiagen, Venlo, Netherlands) and a CFX Connect Real-Time PCR Detection System (Bio-Rad). Optimal annealing temperatures for primer pairs were determined. Primer specificity was confirmed with nucleotide sequencing of the cloned PCR products (pCR 2.1-TOPO vector; Thermo Fisher Scientific). Primer efficiencies and sensitivities were measured with serial cDNA dilutions. All primers had efficiency ≥93%. After an initial 15-min incubation at 96°C, all reactions went through 40 cycles of denaturation (96°C for 30 s), annealing temperature (60°C for 30 s), and extension and read (72°C for 10 s). Primers synthesized for PCR are as follows for mtDNA and nDNA, respectfully: NADH-ubiquinone oxidoreductase subunit 1 (ND1), forward (F): 5′-CCAGCATGACCCCTAGCAAT-3′, reverse (R): 5′-AGAATAGGGCGAATGGTCCG-3′, and ribosomal protein S15 (RPS15), forward (F): 5′-TGAGCAACTGATGCAGCTATACA-3′, reverse (R): 5′-AAGGTCTTGCCGTTGTAGACG-3′. mtDNA copy number was calculated using a standard curve and normalizing to nDNA copy number. Gene analysis was performed adhering to Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (17).
Citrate synthase (CS) activity was measured, in triplicate, in whole biceps femoris muscle with the Citrate Synthase Assay Kit (cat. no. CS0720; Sigma-Aldrich), as described previously (18). Rates for CS are reported as nanomole per minute per milligram of muscle protein.
Statistical analysis.
Significant differences (P < 0.05) between groups (control and IUGR) for morphometric data were determined with an ANOVA and post hoc least-significant difference test using general linear model procedures in JMP software (version 14.0.0; SAS Institute, Cary, NC). Data for OCRs, enzyme assays, immunoblots, and DNA ratio were analyzed using unpaired t test between groups after testing for the homogeneity of variance. Normality and skewness were ensured using a D’Agostino-Pearson normality test. The variance between groups for each condition (e.g., morphometry, OCR, immunoblot, etc.) was similar between groups in all conditions.
The male-to-female ratio (male:female) for OCR experiments and biometric measurements (n = 12/group) was 5:7 for both IUGR and control groups. The male-to-female ratio for blood flow studies (n = 10/group) was 4:6 for both IUGR and control groups. The male-to-female ratio for the first six animals per group used in the proteomic analysis, enzymatic activities, mitochondria density comparison, and immunoblots was 3:3 for both IUGR and control groups. When data were analyzed to include fetal sex as a variable in our ANOVA, we did not find significant differences, and sex was, therefore, not included in the model. Data are presented as means ± standard error (SE).
RESULTS
Placental insufficiency lowers glucose uptake and fetal weight.
Blood oxygen, plasma glucose, and plasma insulin concentrations were lower in IUGR fetuses than controls (Table 1). Plasma norepinephrine concentrations were 5.6-fold higher in IUGR fetuses compared with controls, similar to our previous studies and other models of IUGR (52, 53, 56, 60, 72). Plasma lactate concentrations were not different between groups. Weight-specific umbilical blood flow and umbilical (net fetal) oxygen uptakes were not different between groups. In IUGR fetuses, rates of umbilical glucose and lactate uptakes were 24% and 46% lower than controls, respectively.
Table 1.
Metabolic and hormone concentrations and umbilical uptakes for control and IUGR fetuses
| Measurement | Control (10) | IUGR (10) |
|---|---|---|
| Male-to-female ratio, male:female | 4:6 | 4:6 |
| Umbilical blood flow, mL · min−1 · kg−1 | 200.9 ± 24.2 | 167.6 ± 8.6 |
| Blood O2 content, mM | 3.4 ± 0.1 | 1.1 ± 0.1* |
| Umbilical oxygen uptake, µmol · min−1 · kg−1 | 355.1 ± 16.4 | 318.7 ± 14.0 |
| Plasma glucose, mM | 1.03 ± 0.04 | 0.63 ± 0.05* |
| Umbilical glucose uptake, µmol · min−1 · kg−1 | 30.4 ± 0.8 | 23.2 ± 1.2* |
| Plasma lactate, mM | 2.15 ± 0.12 | 2.73 ± 0.39 |
| Umbilical lactate uptake, µmol · min−1 · kg−1 | 19.3 ± 2.2 | 10.4 ± 1.8* |
| Plasma norepinephrine, pg/mL | 770 ± 140 | 4,340 ± 1,020* |
| Plasma insulin, ng/mL | 0.37 ± 0.02 | 0.15 ± 0.02* |
Values are expressed as means ± SE. Significant differences (P < 0.05) between groups were determined by ANOVA and post hoc least-significant difference test using general linear model procedures. IUGR, intrauterine growth restriction.
P < 0.05, difference between groups.
Fetal and placental masses were 48% and 56% lower, respectively, in IUGR fetuses compared with control fetuses (Table 2). Gestational age at necropsy was not different between the groups. Total BF muscle weight was 55% lower in IUGR fetuses than control fetuses; however, BF moisture content was not different between groups (Table 2). Although brain weight was lower in IUGR fetuses compared with controls, brain weight relative to fetal body weight was 68% higher in IUGR fetuses.
Table 2.
Body and organ masses of control and IUGR fetuses at necropsy
| Measurement | Control (12) | IUGR (12) |
|---|---|---|
| Male-to-female ratio, male:female | 5:7 | 5:7 |
| Age at necropsy, days | 132 ± 1 | 132 ± 1 |
| Fetal weight, kg | 3.33 ± 0.21 | 1.73 ± 0.12* |
| Placental weight, g | 425 ± 43.4 | 188 ± 47.2* |
| Brain weight, g | 51.9 ± 1.4 | 43.0 ± 1.5* |
| Brain weight, g/fetal weight, kg | 15.2 ± 0.8 | 25.6 ± 2.8* |
| Total biceps femoris weight, g | 34.5 ± 1.3 | 15.6 ± 1.6* |
| Total biceps femoris weight, g/fetal weight, kg | 10.9 ± 0.4 | 8.94 ± 0.3* |
| Biceps femoris moisture content, % | 77.7 ± 2.1 | 75.8 ± 2.6 |
Values are expressed as means ± SE. Significant differences (P < 0.05) between groups were determined by ANOVA and least-significant difference test using general linear model procedures. IUGR, intrauterine growth restriction.
P < 0.05, difference between groups.
Lower mitochondrial oxygen consumption rates in IUGR skeletal muscle.
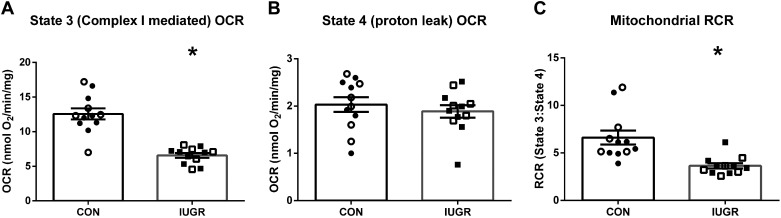
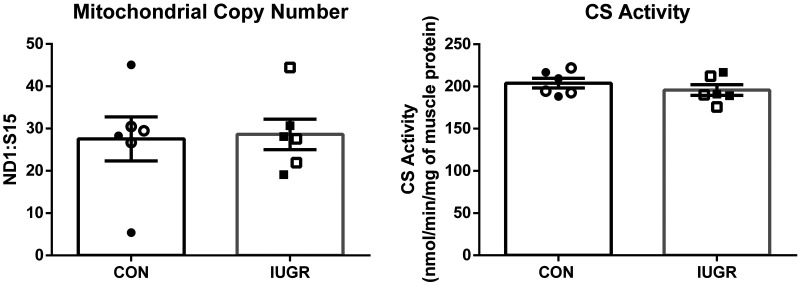
Mitochondrial respiration rates in State 3 conditions were 47% lower in mitochondria isolated from the IUGR skeletal muscle than controls (Fig. 1A). No differences were observed between groups for State 4Oligomycin conditions (Fig. 1B). The RCR was 40% lower for IUGR mitochondria compared with control fetuses (Fig. 1C). In the biceps femoris muscle, mitochondrial DNA copy number and CS activity were not different between groups (Fig. 2).
Fig. 1.
Reduced mitochondrial oxygen consumption rates (OCRs) in intrauterine growth restriction (IUGR) skeletal muscle. Mitochondria isolated from biceps femoris muscle of IUGR (n = 12) and control (CON; n = 12) fetuses were evaluated. Oxygen consumption rates (OCRs) normalized to mitochondrial protein concentrations were measured at State 3 conditions (Complex I mediated; A) and State 4 conditions (proton leak; B). C: the respiratory control ratio (RCR; State 3:State 4 ratio) is presented. The data were analyzed using unpaired t test between groups, and data are presented as the means ± SE, with *P < 0.05 denoting group differences.
Fig. 2.
Mitochondrial density was unaffected in intrauterine growth restriction (IUGR) skeletal muscle. Left: mitochondrial DNA copy number to nuclear DNA copy number in the biceps femoris muscle is represented as a ratio of NADH-ubiquinone oxidoreductase subunit 1 (ND1):(RPS15) genes for control (CON; n = 6) and IUGR (n = 6) fetuses. Right: citrate synthase (CS) activity normalized to milligrams of whole biceps femoris tissue is presented for CON and IUGR fetuses. The data were analyzed using unpaired t test between groups, and data are presented as the means ± SE; there were no significant differences observed between groups.
Lower Complex I electron transport chain activity in IUGR mitochondria.
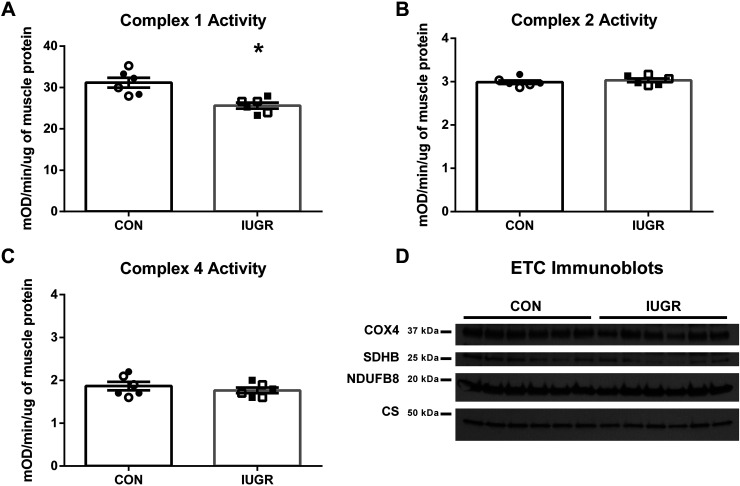
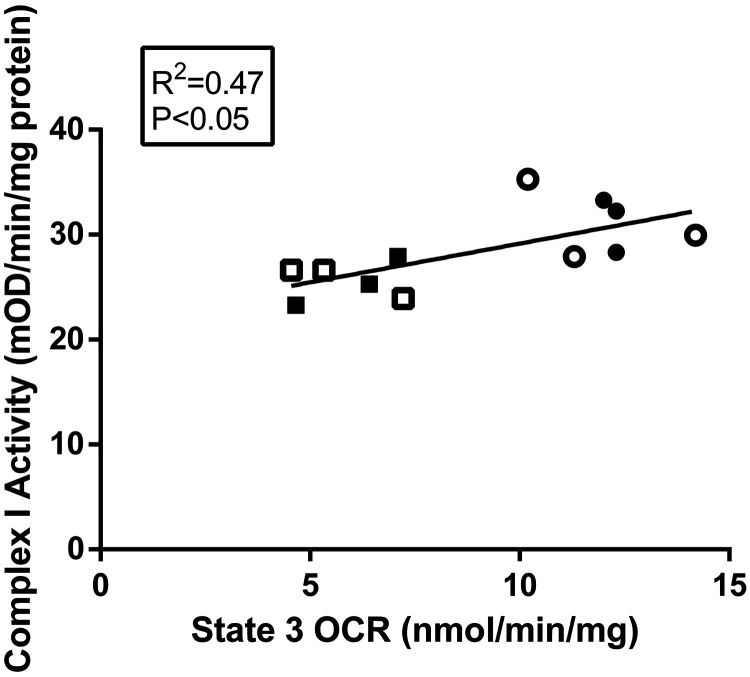
In a subset of animals (n = 6/group), function and expression of the individual complexes in the ETC were evaluated. Complex I activity was 18% lower in IUGR muscle compared with control muscle (Fig. 3A). No differences in activity between groups were found for Complexes II or IV (Fig. 3, B and C). To assess total ETC expression, representative OXPHOS (Complexes I–V) proteins were evaluated by immunoblot analysis on whole BF muscle lysates; however, there were no differences in the abundances of representative OXPHOS proteins between groups (Fig. 3D). Furthermore, Complex I activity was positively associated with State 3 OCRs for both groups (Fig. 4).
Fig. 3.
Reduced electron transport chain activity rates, but not complex abundances in intrauterine growth restriction (IUGR) mitochondria. Complex I (A), Complex II (B), and Complex IV (C) activities of the electron transport chain (ETC) were determined for biceps femoris muscle of IUGR and control (CON) fetuses. D: representative immunoblots are presented for Complex I [NADH dehydrogenase 1β subcomplex subunit 8 (NDUFB8)], Complex II [succinate dehydrogenase B (SDHB)], and Complex IV (COX4) subunits and citrate synthase (CS) for whole biceps femoris muscle protein lysate. No differences in protein concentrations were observed between groups by immunoblot analysis. For each group, males are represented by open symbols, and females are represented by closed symbols. The data were analyzed using unpaired t test between groups after testing for equal variance, and the data are presented as the means ± SE. *P < 0.05 denotes significance differences between groups. mOD denotes milli optical density.
Fig. 4.
Correlation of mitochondrial oxygen consumption rates (OCRs) and State 3 Complex I activity. State 3 oxygen consumption rates (OCRs) plotted against Complex I activity rates for the subset of intrauterine growth restriction (IUGR; squares, n = 6) and control (CON; circles, n = 6). For each group, males are represented by open symbols, and females are represented by closed symbols. Linear regression was performed with JMP 14, and R2 and P values are indicated. mOD denotes milli optical density.
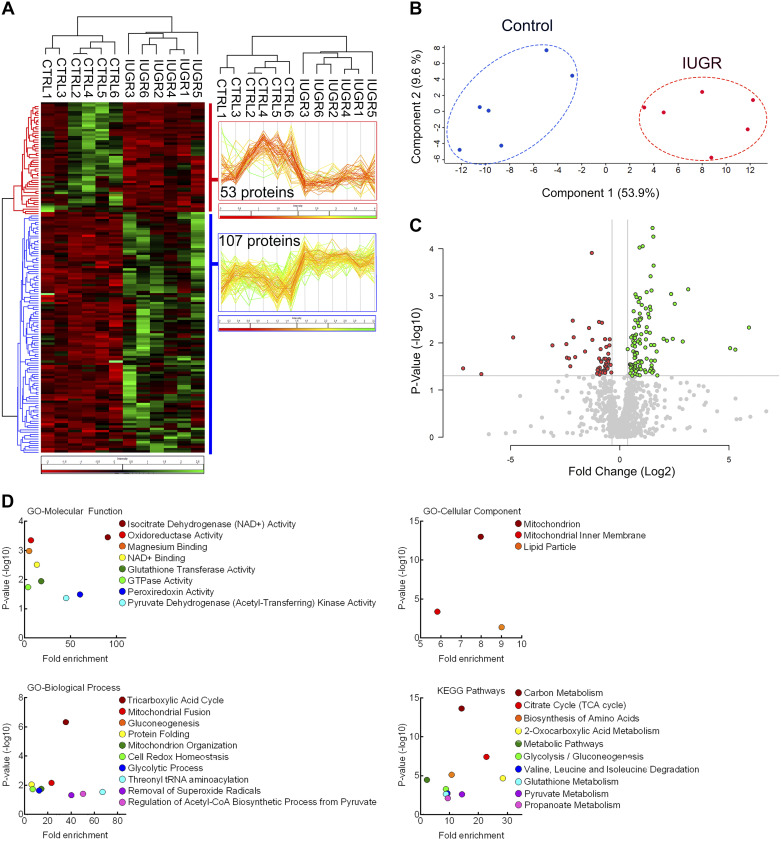
IUGR mitochondria proteomic profiles identify mechanisms for Complex I inhibition.
Within our 12 samples, proteomic analysis identified 2,691 total, unique proteins. Of those, 1,112 were identified as mitochondrial-specific proteins by a confidence of 3 or greater using COMPARTMENTS (Supplemental Table S1) (9). Within the mitochondrial-specific proteins, organic, hierarchical clustering of the DE proteins grouped the control and IUGR samples showing 107 upregulated proteins and 53 downregulated proteins in IUGR mitochondria compared with control mitochondria (Fig. 5, A–C). Enriched GO terms for the differentially expressed proteins were found in all three GO subontologies [Biological Process (BP), Cellular Component (CC), and Molecular Function (MF)], as well as KEGG pathways (Fig. 5D). GO-BP and KEGG pathways each had 10 enriched pathways, and both overlap in the following terms: “TCA cycle,” “Glycolysis/Gluconeogenesis,” and “Pyruvate metabolism” (Supplemental Tables S2 and S5). GO-CC and GO-MF each had three and nine enriched pathways, respectively (Supplemental Tables S3 and S4).
Fig. 5.
Proteomic analysis reveals 160 differentially expressed proteins in intrauterine growth restriction (IUGR) skeletal muscle mitochondria. A: expression patterns for the differentially expressed (DE) proteins are present in the heatmap that shows organic, hierarchical clustering of the significant DE proteins between control (CTRL) and IUGR mitochondria. Lower protein abundances are shown in red, and higher protein abundances are shown in green for IUGR versus control comparison. Hierarchical clustering of the 107 upregulated (blue) and 53 downregulated (red) for control versus IUGR comparison is displayed on either side of the heatmap. B: a principal component analysis (PCA) plot shows distinct separation between control (blue) and IUGR (red) mitochondria protein abundances. C: for the control and IUGR comparison, the volcano plot for log2 fold change plotted against log10 adjusted P values identifies all significant DE proteins with lower (red) and higher (green) protein abundances of IUGR mitochondria. D: the P values for each Gene Ontology (GO) term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway for all DE proteins (both up- and downregulated) for the control vs. IUGR comparison were graphed against their individual fold enrichment scores. The protein list for each GO term or KEGG pathway can be found in Supplemental Tables S2–S5.
KEGG pathway analysis of the 160 DE proteins showed 17 proteins were connected to “Carbon metabolism”: 7 proteins were upregulated, and 10 proteins were downregulated in IUGR mitochondria compared with controls (Supplemental Table S5). Furthermore, KEGG pathway analysis identified eight proteins connected to TCA cycle, and all eight of these proteins were downregulated in IUGR mitochondria, and all eight proteins were found within the Carbon Metabolism pathway (Supplemental Table S5). Additionally, of these eight downregulated proteins, five overlap with “2-Oxocarboxylic acid metabolism” (Supplemental Table S5).
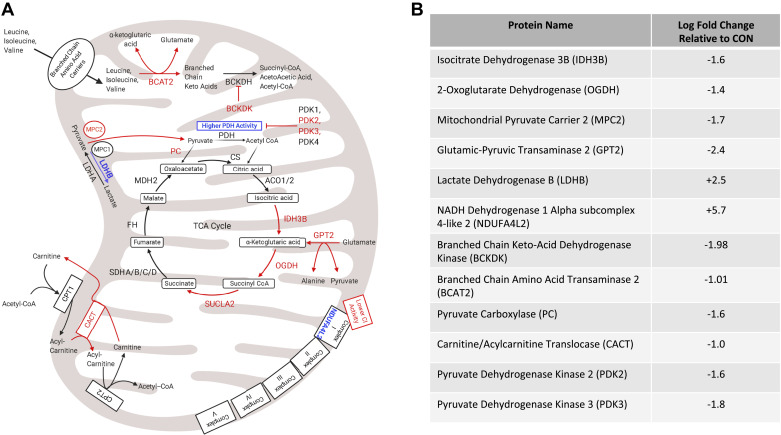
Proteomic analysis also showed a downregulation of proteins involved in pyruvate flux into the TCA cycle in IUGR mitochondria. These proteins include mitochondrial pyruvate carrier 2 (MPC2), pyruvate dehydrogenase (PDH) kinases 2 and 3 (PDK2,3), and pyruvate carboxylase (PC; Fig. 6). Additionally, the results identified lower abundances of enzymes involved in amino acid metabolism, such as branched chain aminotransferase 2 (BCAT2) and branched chain keto acid dehydrogenase kinase (BCKDK) in IUGR mitochondria compared with controls (Fig. 6).
Fig. 6.
Changes in mitochondrial metabolic enzymes in response to intrauterine growth restriction (IUGR) relative to control (CON). A: the schematic outlines major mitochondrial enzymes and their processes in the tricarboxylic acid (TCA) cycle (ETC), fatty acid oxidation, or branched chain amino acid metabolism, created using BioRender.com. In the schematic, lower protein abundances (red) and greater protein abundances (blue) for IUGR mitochondria are indicated, whereas enzymes that are not different are in black text. Proposed decreased metabolic fluxes in IUGR mitochondria are shown with red arrows. B: fold changes are presented for each differentially expressed (DE) protein of interest. ACO1/2, aconitate hydratase 1/2; BCKDH, branched-chain α-keto acid dehydrogenase; CPT1,2, carnitine palmitoyltransferase 1,2; CS, citrate synthase; FH, fumarate hydratase; LDHA, lactate dehydrogenase A; MDH2, malate dehydrogenase 2; MPC1, mitochondrial pyruvate carrier 1; PDH, pyruvate dehydrogenase; PDK1,4, pyruvate dehydrogenase kinases 1,4; SDHA–D, succinate dehydrogenase A–D; SUCLA2, succinate-CoA ligase ADP-forming subunit β.
No upregulated proteins in IUGR mitochondria were directly involved in the TCA cycle, amino acid metabolism, or β-oxidation. However, two upregulated proteins in IUGR mitochondria were identified as potential regulatory factors for metabolism: NDUFA4L2 and lactate dehydrogenase B (LDHB), which are centrally located in mitochondrial metabolism (Fig. 6).
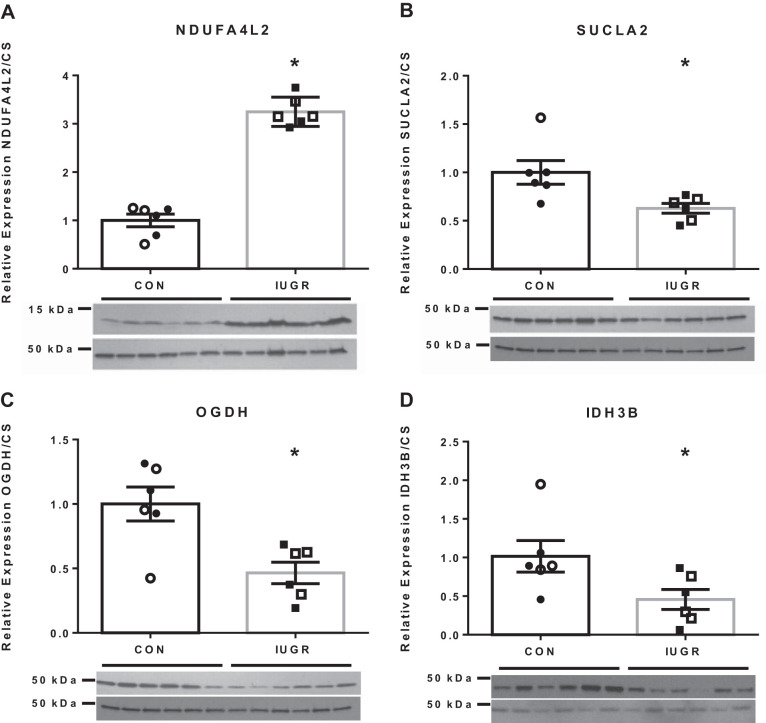
Immunoblot confirms reductions in TCA cycle enzymes and elevated NDUFA4L2.
NADH dehydrogenase 1α subcomplex 4-like 2 (NDUFA4L2) was selected as an upregulated protein in IUGR mitochondria that also affects ETC function at Complex I. NDUFA4L2 concentrations were threefold higher in IUGR fetuses compared with controls (Fig. 7A).
Fig. 7.
Immunoblots of differentially expressed (DE) proteins confirm proteomic results. Representative immunoblots are presented for NADH dehydrogenase 1α subcomplex 4-like 2 (NDUFA4L2; A), succinate-CoA ligase ADP-forming subunit β (SUCLA2; B), oxoglutarate dehydrogenase (OGDH; C), and isocitrate dehydrogenase (NAD+) 3 noncatalytic subunit β (IDH3B; D). The relative abundances to citrate synthase (CS) are shown for control (CON) and intrauterine growth restriction (IUGR; n = 6/group). For each group, males are represented with open symbols, and females are represented by closed symbols. The data were analyzed using unpaired t test between groups after testing for equal variance, and the data are presented as the means ± SE. *P < 0.05 denotes significance differences between groups.
TCA cycle enzymes, isocitrate dehydrogenase (NAD+) 3 noncatalytic subunit β (IDH3B), succinate-CoA ligase ADP-forming subunit-β (SUCLA2), and oxoglutarate dehydrogenase (OGDH) were validated with immunoblots (Fig. 7, B–D). IDH3B, SUCLA2, and OGDH abundances were 55%, 38%, and 54% lower in IUGR fetuses, respectively, compared with control (Fig. 7, B–D).
DISCUSSION
To adapt to low nutrient and oxygen conditions in utero, skeletal muscle mitochondria from IUGR fetuses have lower maximal Complex I-mediated respiratory rates and reduced Complex I activity compared with controls. The reductions in Complex I activity in IUGR mitochondria were associated with greater NDUFA4L2 concentrations, because abundances of other representative ETC proteins remain unchanged. Induction of NDUFA4L2 during hypoxia has been shown to decrease cellular respiration by reducing the functional capacity of Complex I (23, 50, 79). ETC function and subsequent energy production rely on adequate supplies of reducing equivalents, primarily from the TCA cycle (1, 84). Interestingly, our proteomic and Western analyses show that mitochondria isolated from skeletal muscle of IUGR fetuses have lower concentrations of TCA cycle enzymes compared with controls. Lower OCR, Complex I activity, and TCA cycle enzyme abundances in IUGR skeletal muscle mitochondria may serve as an avenue for substrate (e.g., pyruvate) conservation in IUGR skeletal muscle in response to nutrient and oxygen restriction induced by placental insufficiency.
Mitochondrial oxygen consumption.
Glucose metabolism and oxygen consumption are entwined via mitochondrial metabolism. IUGR fetuses are not only hypoxemic, hypoglycemic, and smaller than control fetuses, but they also exhibit decreased rates of growth, protein accretion, as well as lower whole-fetus glucose oxidation rates (14, 22, 27, 52, 64, 70, 74, 80, 82). In the present study, mitochondria from IUGR skeletal muscle have lower State 3 (Complex I-mediated) OCRs compared with controls, supporting previous observations of globally reduced energy status in their skeletal muscle and lower fetal hindlimb oxygen consumption rates (24, 74). The reduction in State 3 OCR of IUGR mitochondria was independent of Complex II and IV activities, but OCRs correlated with lower Complex I activity (Fig. 4). State 4 OCRs (proton leak) were not different between groups, thus showing that the lower NADH-coupled respiration in IUGR mitochondria was due to lower State 3 OCRs (Fig. 1). Furthermore, the defect in IUGR State 3 OCR was also independent of ETC proteins, because abundances examined by immunoblots and proteomic analyses were not different for several protein subunits of the ETC complexes. Together, these findings indicate that lower mitochondria respiration rates in IUGR skeletal muscle are, in part, related to inhibition of Complex I of the ETC.
Complex I-mediated oxygen consumption, which accounts for the majority of mitochondrial oxygen consumption, relies upon an adequate supply of O2, NADH, Pi, and ADP (77). Consisting of 45 subunits, Complex I is the largest, most complicated, and least understood component of the ETC, and its activity is regulated in response to nutrient and oxygen availability (30, 76). The regulation of Complex I activity has yet to be fully elucidated, but it appears to be governed by its active/dormant (A/D) transition state in response to substrate and oxygen supply: the A form operates at physiological temperatures and substrates, but Complex I transitions into the D form if oxygen/substrates become limiting (4, 31, 49). The mechanism behind the Complex I A/D transition is unknown, but it may be due to post-translational modifications or a decrease in core catalytic subunit expression (45, 77, 90). Intriguingly, our proteomic analysis of isolated mitochondria indicates that neither of these mechanisms is responsible for reduced State 3 OCRs in IUGR mitochondria. However, we observed greater NDUFA4L2 abundance in skeletal muscle mitochondria from IUGR fetuses, which has been shown to inhibit Complex I activity and may serve as a potential adaptive response mechanism to conditions of placental insufficiency and IUGR (23, 50, 79).
The expression of NDUFA4L2 is stimulated by hypoxia-inducible factor 1α (HIF-1α), the expression of which is a primary adaptive response to decreases in available oxygen (50, 79). Although IUGR fetuses are hypoxemic, increased prolyl hydroxylase domain expression, which opposes the actions of HIF-1α, is observed in IUGR tissues; however, increased prolyl hydroxylase domain expression is matched by increased HIF-1/2α activity in IUGR tissues, which most likely represents a new metabolic set point in response to chronic hypoxia in IUGR fetuses (10, 33, 58, 59, 65). Whereas the physiological function of NDUFA4L2 remains to be fully elucidated, when it is expressed, NDUFA4L2 is inserted into Complex I and reduces reactive oxygen species (ROS) generation and oxygen consumption by decreasing electron flux (50, 79). Furthermore, due to its strategic position downstream of NADH production, increased NDUFA4L2 expression would reduce OCRs regardless of fuel origin. Importantly, increased NDUFA4L2 expression would keep total substrate oxidation lower, thereby conserving substrates for vital tissues at the expense of skeletal muscle metabolism.
IUGR fetal skeletal muscle is depleted in purines, ribose, oxygen, glucose metabolites, and key amino acids, indicating a lower energy status (24, 74). However, a depletion of skeletal muscle metabolites may be consequential to, and not a cause of, decreased skeletal muscle mass in IUGR fetuses. One mechanism likely to reduce accretion rates and metabolism is a reduction in skeletal muscle OCR. Since the rate of oxygen consumption by mitochondria in vivo is determined by the phosphorylation potential [log(ATP/ADP × Pi)], electrons will only be donated to oxygen if ADP is concomitantly phosphorylated to ATP (7, 32, 40, 48). As such, the rate of oxidative phosphorylation is coupled to the rate of ATP utilization. Therefore, a reduction in OCR will reduce ATP production and force established fetal muscle fibers to reduce energy (ATP) expenditure, of which a significant portion is devoted to protein synthesis (8, 46, 55). Taken together, the adaptation of IUGR skeletal muscle to a hypoxic, nutrient-restricted environment decreases its need for energy and metabolic requirements at the expense of its growth.
Although Complex I activity and State 3 (Complex I-mediated) OCRs were highly correlated (Fig. 4), the magnitude of reduction in IUGR fetuses was not equivalent for each measurement (18% lower Complex I activity vs. 47% lower State 3 OCRs in IUGR fetuses compared with controls). The measurements of Complex I activity demonstrate a lower rate in the NADH redox capability; however, the ability of Complex I to transfer protons to the intermembrane space or transfer electrons within Complex I (or between Complex I and coenzyme Q) was not investigated. Alterations in either of these processes may explain the discrepancy between State 3 OCR and Complex I activity measurements in this study. Interestingly, in vivo experiments show that weight-specific hindlimb oxygen uptakes are 29% lower in IUGR fetuses compared with controls (74). Although these in vivo hindlimb OCRs are higher than the State 3 OCRs for isolated mitochondria, this result is expected, because the in vivo measurements include Complex II activity (FADH redox), which also contributes to the total OCR. In contrast, the in vitro State 3 OCRs for isolated mitochondria were performed in the absence of Complex II activity.
The link between the electron transport chain and energy conservation.
Due to the low energy status of IUGR skeletal muscle, it is not surprising that the TCA cycle enzyme abundances are lower in IUGR mitochondria (Fig. 6) (24). A decrease in OCRs would result in a reduction in electron flow through the ETC, and this would subsequently inhibit the flow of glycolytic substrates through the mitochondrial metabolism, since oxidative phosphorylation is dependent upon reducing equivalents, namely NADH. Consequently, reduced ETC function reduces NADH oxidation, increases the NADH:NAD+ ratio, and inhibits key TCA cycle enzymes, such as IDH and OGDH, both of which, along with CS, represent the rate-limiting steps of the TCA cycle (16). Interestingly, both IDH and OGDH are downregulated in IUGR mitochondria (Fig. 7). Although NADH and ATP only allosterically inhibit IDH and OGDH, the downregulation of these proteins in our dataset could represent adaptation to a lower energy requirement in IUGR skeletal muscle (24).
Intriguingly, IUGR mitochondria appear to conserve glutamate by decreasing the abundances of enzymes that use glutamate or glutamate derivatives, such as BCAT2, glutamic-pyruvic transaminase 2 (GPT2), OGDH, and BCKDK (Fig. 6). Because amino acid oxygen quotients are lower in the IUGR hindlimb, these fetuses may conserve amino acids rather than use them in energy-producing pathways, such as the TCA cycle. Although these data are consistent with current studies, speculation on amino acid metabolism in IUGR skeletal muscle mitochondria is beyond the scope of this study (24, 74).
Due to their relative mass, larger muscle groups have a proportionally greater impact on the glucose metabolism and are composed of mixed fiber types (25, 34, 39). The BF was chosen as the representative muscle group for this study. However, the IUGR BF muscle has fewer type I fibers compared with control BF muscle, and type 1 fibers are known to have higher mitochondrial density compared with type II fibers, which indicates that changes in muscle composition might impact mitochondrial content (86). The citrate synthase activity assay, along with other measurements in whole BF muscle (DNA ratio and immunoblots for mitochondrial ETC proteins) performed in this study, serves as a proxy for mitochondrial density. Furthermore, with the use of both immunoblot and proteomic measurements, no differences in citrate synthase abundances were observed between groups. This implies that mitochondrial density in the biceps femoris is similar between groups, and the abundance of citrate synthase per mitochondria is similar between groups. Together, these measurements do not support reductions in mitochondrial content in the BF muscle of IUGR fetuses. This might reflect that in the IUGR sheep fetus, there is a smaller proportion of type 1 fibers to total fiber number, and the 5% reduction in this population, which is <20% in the BF muscle, does not lead to major declines in mitochondrial density (86).
Pyruvate metabolism and OCR.
Although we found a decrease in the abundances of various TCA cycle enzymes, we did not find a significant increase in the abundances of the enzymes that inhibit pyruvate catabolism into acetyl-CoA. In fact, the abundances of pyruvate dehydrogenase, pyruvate dehydrogenase phosphatase 1, and pyruvate dehydrogenase phosphatase 2 were not different between groups (Supplemental Table S1). Furthermore, the abundances of pyruvate dehydrogenase kinases were either lower or unchanged in IUGR mitochondria compared with controls, supporting our previous findings (72) (Fig. 6 and Supplemental Table S1). However, we did find lower pyruvate carboxylase (PC) abundance in IUGR mitochondria, which may be due to the decrease in demand of NADH by the ETC.
Increased, or normal, pyruvate catabolism in IUGR skeletal muscle runs contrary to speculation of IUGR glucose metabolism and activation of the Cori cycle (14, 80). Intuitively, increased pyruvate catabolism should increase substrate available to the ETC, although greater NDUFA4L2 expression is expected to limit the capacity of IUGR skeletal muscle to oxidize NADH. Remarkably, we show lower mitochondrial pyruvate carrier 2 (MPC2) abundance in IUGR mitochondria compared with controls. MPC2 and mitochondrial pyruvate carrier 1 (MPC1) are highly conserved pyruvate transporters and are thought to be the primary pyruvate transporters on the inner mitochondrial membrane (12, 36). Regulation of MPC2 is yet to be fully understood; however, it is hypothesized that acetylation of MPC2 occurs under hypoxia, thereby reducing oxygen consumption under stress (62, 84). Although IUGR skeletal muscle has increased PDH activity, this may be a result of decreased pyruvate flux across the mitochondrial membrane due to decreased MPC2 abundance (72). Therefore, the increase in PDH activity in IUGR skeletal muscle mitochondria may act to maintain pyruvate flux to meet minimal skeletal muscle energy requirements. The reduction in MPC2, along with the reduction in ETC function via NDUFA4L2, may represent a coadaptive response by IUGR skeletal muscle mitochondria as a coping mechanism to a low nutrient environment.
Perspectives.
Together, the lower respiratory rates, lower Complex I activity, and lower TCA cycle enzyme abundances in IUGR mitochondria indicate that there is a lower energy homeostasis of IUGR skeletal muscle compared with controls. Proteomic and immunoblot analysis of isolated mitochondria showed greater NDUFA4L2 abundance in IUGR mitochondria, which inhibits Complex I activity. These findings have major implications in the treatment of IUGR, as these data indicate that IUGR skeletal muscle adapts to a low nutrient, in utero environment by lowering mitochondrial metabolic capacity.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK-084842 (to Principal Investigator S. W. Limesand); National Heart, Lung, and Blood Institute Grant T32 HL007249-42 (to A. L. Pendleton; Principal Investigator C. C. Gregorio); and National Institute of Food and Agriculture Postdoctoral Fellowship Award Numbers 2015-03545 and T32 HL007249 (to L. E. Camacho; Principal Investigator C. C. Gregorio).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.P. and S.W.L. conceived and designed research; A.L.P., A.T.A., A.C.K., M.A.D., L.E.C., K.D., M.J.A., P.R.L., and S.W.L. performed experiments; A.L.P., K.D., M.J.A., P.R.L., and S.W.L. analyzed data; A.L.P., A.T.A., A.C.K., M.A.D., L.E.C., K.D., M.J.A., P.R.L., R.M.L., and S.W.L. interpreted results of experiments; A.L.P., K.D., M.J.A., P.R.L., R.M.L., and S.W.L. prepared figures; A.L.P. and S.W.L. drafted manuscript; A.L.P., A.T.A., A.C.K., M.A.D., L.E.C., K.D., M.J.A., P.R.L., R.M.L., and S.W.L. edited and revised manuscript; A.L.P., A.T.A., A.C.K., M.A.D., L.E.C., K.D., M.J.A., P.R.L., R.M.L., and S.W.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nathan R. Steffens and Mandie M. Dunham for their technical assistance.
REFERENCES
- 1.Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 68: 475–478, 2014. doi: 10.1007/s12013-013-9750-1. [DOI] [PubMed] [Google Scholar]
- 2.Aretz I, Hardt C, Wittig I, Meierhofer D. An impaired respiratory electron chain triggers down-regulation of the energy metabolism and de-ubiquitination of solute carrier amino acid transporters. Mol Cell Proteomics 15: 1526–1538, 2016. doi: 10.1074/mcp.M115.053181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babot M, Birch A, Labarbuta P, Galkin A. Characterisation of the active/de-active transition of mitochondrial complex I. Biochim Biophys Acta 1837: 1083–1092, 2014. doi: 10.1016/j.bbabio.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJP, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31: 1235–1239, 2002. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 6.Beltrand J, Verkauskiene R, Nicolescu R, Sibony O, Gaucherand P, Chevenne D, Claris O, Lévy-Marchal C. Adaptive changes in neonatal hormonal and metabolic profiles induced by fetal growth restriction. J Clin Endocrinol Metab 93: 4027–4032, 2008. doi: 10.1210/jc.2008-0562. [DOI] [PubMed] [Google Scholar]
- 7.Berg JM, Tymoczko JL, Stryer L. The regulation of cellular respiration is governed primarily by the need for ATP. In: Biochemistry. New York: Freeman, 2002. [Google Scholar]
- 8.Bier D. The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington, DC: National Academies Press, 1999. [PubMed] [Google Scholar]
- 9.Binder JX, Pletscher-Frankild S, Tsafou K, Stolte C, O’Donoghue SI, Schneider R, Jensen LJ. COMPARTMENTS: unification and visualization of protein subcellular localization evidence. Database (Oxford) 2014: bau012, 2014. doi: 10.1093/database/bau012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botting KJ, McMillen IC, Forbes H, Nyengaard JR, Morrison JL. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J Am Heart Assoc 3: e000531, 2014. doi: 10.1161/JAHA.113.000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337: 96–100, 2012. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown LD. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J Endocrinol 221: R13–R29, 2014. doi: 10.1530/JOE-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 309: R920–R928, 2015. doi: 10.1152/ajpregu.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubber P, Hartounian V, Gibson GE, Blass JP. Abnormalities in the tricarboxylic acid (TCA) cycle in the brains of schizophrenia patients. Eur Neuropsychopharmacol 21: 254–260, 2011. doi: 10.1016/j.euroneuro.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton K, Krebs HA. The free-energy changes associated with the individual steps of the tricarboxylic acid cycle, glycolysis and alcoholic fermentation and with the hydrolysis of the pyrophosphate groups of adenosinetriphosphate. Biochem J 54: 94–107, 1953. doi: 10.1042/bj0540094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin Chem 55: 611–622, 2009. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 18.Camacho LE, Chen X, Hay WW Jr, Limesand SW. Enhanced insulin secretion and insulin sensitivity in young lambs with placental insufficiency-induced intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 313: R101–R109, 2017. doi: 10.1152/ajpregu.00068.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capitanio D, Fania C, Torretta E, Viganò A, Moriggi M, Bravatà V, Caretti A, Levett DZH, Grocott MPW, Samaja M, Cerretelli P, Gelfi C. TCA cycle rewiring fosters metabolic adaptation to oxygen restriction in skeletal muscle from rodents and humans. Sci Rep 7: 9723, 2017. doi: 10.1038/s41598-017-10097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbon S, Douglass E, Dunn N, Good B, Harris NL, Lewis SE, Mungall CJ, Basu S, Chisholm RL, Dodson RJ, Hartline E, Fey P, Thomas PD, Albou LP, Ebert D, Kesling MJ, Mi H, Muruganujan A, Huang X, Poudel S, Mushayahama T, Hu JC, LaBonte SA, Siegele DA, Antonazzo G, Attrill H, Brown NH, Fexova S, Garapati P, Jones.; The Gene Ontology Consortium . The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res 47: D330–D338, 2019. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerretelli P, Gelfi C. Energy metabolism in hypoxia: reinterpreting some features of muscle physiology on molecular grounds. Eur J Appl Physiol 111: 421–432, 2011. doi: 10.1007/s00421-010-1399-5. [DOI] [PubMed] [Google Scholar]
- 22.Cetin I, Radaelli T, Taricco E, Giovannini N, Alvino G, Pardi G. The endocrine and metabolic profile of the growth-retarded fetus. J Pediatr Endocrinol Metab 14, Suppl 6: 1497–1505, 2001. [PubMed] [Google Scholar]
- 23.Chaillou T, Hynynen H, Ferreira D, Pironti G, Kenne E, Andersson DC, Ruas JL, Tavi P, Lanner JT. NDUFA4L2—connecting metabolic signals and mitochondrial function in cardiac and skeletal muscle. Free Radic Biol Med 100: S186, 2016. doi: 10.1016/j.freeradbiomed.2016.10.511. [DOI] [Google Scholar]
- 24.Chang EI, Wesolowski SR, Gilje EA, Baker PR II, Reisz JA, D’Alessandro A, Hay WW Jr, Rozance PJ, Brown LD. Skeletal muscle amino acid uptake is lower and alanine production is greater in late gestation intrauterine growth restricted fetal sheep hindlimb. Am J Physiol Regul Integr Comp Physiol 317: R615–R629, 2019. doi: 10.1152/ajpregu.00115.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahmane R, Djordjevič S, Smerdu V. Adaptive potential of human biceps femoris muscle demonstrated by histochemical, immunohistochemical and mechanomyographical methods. Med Biol Eng Comput 44: 999–1006, 2006. doi: 10.1007/s11517-006-0114-5. [DOI] [PubMed] [Google Scholar]
- 26.Darby JRT, Sorvina A, Bader CA, Lock MC, Soo JY, Holman SL, Seed M, Kuchel T, Brooks DA, Plush SE, Morrison JL. Detecting metabolic differences in fetal and adult sheep adipose and skeletal muscle tissues. J Biophotonics 13: e201960085, 2020. doi: 10.1002/jbio.201960085. [DOI] [PubMed] [Google Scholar]
- 27.Economides DL, Nicolaides KH. Blood glucose and oxygen tension levels in small-for-gestational-age fetuses. Am J Obstet Gynecol 160: 385–389, 1989. doi: 10.1016/0002-9378(89)90453-5. [DOI] [PubMed] [Google Scholar]
- 28.Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 7: 254–261, 2004. doi: 10.1016/j.pbi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2: 287–295, 2007. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 30.Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia. Redox Biol 12: 208–215, 2017. doi: 10.1016/j.redox.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galkin A, Moncada S. Modulation of the conformational state of mitochondrial complex I as a target for therapeutic intervention. Interface Focus 7: 20160104, 2017. doi: 10.1098/rsfs.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giesen J, Kammermeier H. Relationship of phosphorylation potential and oxygen consumption in isolated perfused rat hearts. J Mol Cell Cardiol 12: 891–907, 1980. doi: 10.1016/0022-2828(80)90058-9. [DOI] [PubMed] [Google Scholar]
- 33.Ginouvès A, Ilc K, Macías N, Pouysségur J, Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIF and protects cells from necrosis. Proc Natl Acad Sci USA 105: 4745–4750, 2008. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouzi F, Maury J, Molinari N, Pomiès P, Mercier J, Préfaut C, Hayot M. Reference values for vastus lateralis fiber size and type in healthy subjects over 40 years old: a systematic review and metaanalysis. J Appl Physiol (1985) 115: 346–354, 2013. doi: 10.1152/japplphysiol.01352.2012. [DOI] [PubMed] [Google Scholar]
- 35.Hay WW Jr, Myers SA, Sparks JW, Wilkening RB, Meschia G, Battaglia FC. Glucose and lactate oxidation rates in the fetal lamb. Proc Soc Exp Biol Med 173: 553–563, 1983. doi: 10.3181/00379727-173-41686. [DOI] [PubMed] [Google Scholar]
- 36.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ERS, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337: 93–96, 2012. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18: 111–129, 1973. doi: 10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 40.Kammermeier H. Phosphorylation potential and free energy of ATP. Adv Organ Biol 4: 159–169, 1998. doi: 10.1016/S1569-2590(08)60085-3. [DOI] [Google Scholar]
- 41.Kamzolova SV, Shishkanova NV, Morgunov IG, Finogenova TV. Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res 3: 217–222, 2003. doi: 10.1016/S1567-1356(02)00188-5. [DOI] [PubMed] [Google Scholar]
- 42.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28: 27–30, 2000. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40, D1: D109–D114, 2012. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42, D1: D199–D205, 2014. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kien CL, Rohrbaugh DK, Burke JF, Young VR. Whole body protein synthesis in relation to basal energy expenditure in healthy children and in children recovering from burn injury. Pediatr Res 12: 211–216, 1978. doi: 10.1203/00006450-197803000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korzeniewski B. What regulates respiration in mitochondria? Biochem Mol Biol Int 39: 415–419, 1996. doi: 10.1080/15216549600201451. [DOI] [PubMed] [Google Scholar]
- 49.Kotlyar AB, Vinogradov AD. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim Biophys Acta 1019: 151–158, 1990. doi: 10.1016/0005-2728(90)90137-S. [DOI] [PubMed] [Google Scholar]
- 50.Lai RKH, Xu IMJ, Chiu DKC, Tse APW, Wei LL, Law CT, Lee D, Wong CM, Wong MP, Ng IOL, Wong CCL. NDUFA4L2 fine-tunes oxidative stress in hepatocellular carcinoma. Clin Cancer Res 22: 3105–3117, 2016. doi: 10.1158/1078-0432.CCR-15-1987. [DOI] [PubMed] [Google Scholar]
- 51.Larciprete G, Valensise H, Di Pierro G, Vasapollo B, Casalino B, Arduini D, Jarvis S, Cirese E. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet Gynecol 26: 258–262, 2005. doi: 10.1002/uog.1980. [DOI] [PubMed] [Google Scholar]
- 52.Limesand SW, Camacho LE, Kelly AC, Antolic AT. Impact of thermal stress on placental function and fetal physiology. Anim Reprod 15, Suppl 1: 886–898, 2018. doi: 10.21451/1984-3143-AR2018-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limesand SW, Rozance PJ, Brown LD, Hay WW Jr. Effects of chronic hypoglycemia and euglycemic correction on lysine metabolism in fetal sheep. Am J Physiol Endocrinol Metab 296: E879–E887, 2009. doi: 10.1152/ajpendo.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 55.Lindqvist LM, Tandoc K, Topisirovic I, Furic L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr Opin Genet Dev 48: 104–111, 2018. doi: 10.1016/j.gde.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macko AR, Yates DT, Chen X, Shelton LA, Kelly AC, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology 157: 2104–2115, 2016. doi: 10.1210/en.2015-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mason HS. Mechanisms of oxygen metabolism. Science 125: 1185–1188, 1957. doi: 10.1126/science.125.3259.1185. [DOI] [PubMed] [Google Scholar]
- 58.McGillick EV, Orgeig S, Morrison JL. Structural and molecular regulation of lung maturation by intratracheal vascular endothelial growth factor administration in the normally grown and placentally restricted fetus. J Physiol 594: 1399–1420, 2016. doi: 10.1113/JP271113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGillick EV, Orgeig S, Morrison JL. Regulation of lung maturation by prolyl hydroxylase domain inhibition in the lung of the normally grown and placentally restricted fetus in late gestation. Am J Physiol Regul Integr Comp Physiol 310: R1226–R1243, 2016. doi: 10.1152/ajpregu.00469.2015. [DOI] [PubMed] [Google Scholar]
- 60.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35: 730–743, 2008. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 61.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol 587: 4199–4211, 2009. doi: 10.1113/jphysiol.2009.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagampalli RSK, Quesñay JEN, Adamoski D, Islam Z, Birch J, Sebinelli HG, Girard RMBM, Ascenção CFR, Fala AM, Pauletti BA, Consonni SR, de Oliveira JF, Silva ACT, Franchini KG, Leme AFP, Silber AM, Ciancaglini P, Moraes I, Dias SMG, Ambrosio ALB. Human mitochondrial pyruvate carrier 2 as an autonomous membrane transporter. Sci Rep 8: 3510, 2018. doi: 10.1038/s41598-018-21740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicolaides KH, Economides DL, Soothill PW. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161: 996–1001, 1989. doi: 10.1016/0002-9378(89)90770-9. [DOI] [PubMed] [Google Scholar]
- 64.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Coe AM, Rodeck CH. Maternal-fetal glucose gradient in normal pregnancies and in pregnancies complicated by alloimmunization and fetal growth retardation. Am J Obstet Gynecol 161: 924–927, 1989. doi: 10.1016/0002-9378(89)90753-9. [DOI] [PubMed] [Google Scholar]
- 65.Orgeig S, McGillick EV, Botting KJ, Zhang S, McMillen IC, Morrison JL. Increased lung prolyl hydroxylase and decreased glucocorticoid receptor are related to decreased surfactant protein in the growth-restricted sheep fetus. Am J Physiol Lung Cell Mol Physiol 309: L84–L97, 2015. doi: 10.1152/ajplung.00275.2014. [DOI] [PubMed] [Google Scholar]
- 66.Osmond C, Barker DJP. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 108, Suppl 3: 545–553, 2000. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277: 30409–30412, 2002. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 68.Padoan A, Rigano S, Ferrazzi E, Beaty BL, Battaglia FC, Galan HL. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am J Obstet Gynecol 191: 1459–1464, 2004. doi: 10.1016/j.ajog.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 69.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng 98: 1071–1082, 2007. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Farrazzi E, Buscaglia M, Battaglia FC. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med 328: 692–696, 1993. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- 71.Parker SS, Krantz J, Kwak EA, Barker NK, Deer CG, Lee NY, Mouneimne G, Langlais PR. Insulin induces microtubule stabilization and regulates the microtubule plus-end tracking protein network in adipocytes. Mol Cell Proteomics 18: 1363–1381, 2019. doi: 10.1074/mcp.RA119.001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pendleton AL, Humphreys LR, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Increased pyruvate dehydrogenase activity in skeletal muscle of growth-restricted ovine fetuses. Am J Physiol Regul Integr Comp Physiol 317: R513–R520, 2019. doi: 10.1152/ajpregu.00106.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roseboom TJ. Epidemiological evidence for the developmental origins of health and disease: effects of prenatal undernutrition in humans. J Endocrinol 242: T135–T144, 2019. doi: 10.1530/JOE-18-0683. [DOI] [PubMed] [Google Scholar]
- 74.Rozance PJ, Zastoupil L, Wesolowski SR, Goldstrohm DA, Strahan B, Cree-Green M, Sheffield-Moore M, Meschia G, Hay WW Jr, Wilkening RB, Brown LD. Skeletal muscle protein accretion rates and hindlimb growth are reduced in late gestation intrauterine growth-restricted fetal sheep. J Physiol 596: 67–82, 2018. doi: 10.1113/JP275230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senthilnathan P, Padmavathi R, Magesh V, Sakthisekaran D. Modulation of TCA cycle enzymes and electron transport chain systems in experimental lung cancer. Life Sci 78: 1010–1014, 2006. doi: 10.1016/j.lfs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 76.Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr 10: 67–83, 2016. doi: 10.4137/CMPed.S40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma LK, Lu J, Bai Y. Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr Med Chem 16: 1266–1277, 2009. doi: 10.2174/092986709787846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soothill PW, Nicolaides KH, Campbell S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J (Clin Res Ed) 294: 1051–1053, 1987. doi: 10.1136/bmj.294.6579.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I, López-Bernardo E, Perales-Clemente E, Martínez-Ruiz A, Enríquez JA, Aragonés J, Cadenas S, Landázuri MO. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting Complex I activity. Cell Metab 14: 768–779, 2011. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thorn SR, Regnault TRH, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thorn SR, Rozance PJ, Friedman JE, Hay WW, Brown LD. Evidence for increased Cori cycle activity in IUGR fetus (Abstract). Pediatr Res 74: 481, 2013. [Google Scholar]
- 83.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 13: 731–740, 2016. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 84.Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell 56: 425–435, 2014. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J 427: 333–347, 2010. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 86.Yates DT, Cadaret CN, Beede KA, Riley HE, Macko AR, Anderson MJ, Camacho LE, Limesand SW. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive type I fibers near term. Am J Physiol Regul Integr Comp Physiol 310: R1020–R1029, 2016. doi: 10.1152/ajpregu.00528.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yates DT, Camacho LE, Kelly AC, Steyn LV, Davis MA, Antolic AT, Anderson MJ, Goyal R, Allen RE, Papas KK, Hay WW Jr, Limesand SW. Postnatal β2 adrenergic treatment improves insulin sensitivity in lambs with IUGR but not persistent defects in pancreatic islets or skeletal muscle. J Physiol 597: 5835–5858, 2019. doi: 10.1113/JP278726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yates DT, Clarke DS, Macko AR, Anderson MJ, Shelton LA, Nearing M, Allen RE, Rhoads RP, Limesand SW. Myoblasts from intrauterine growth-restricted sheep fetuses exhibit intrinsic deficiencies in proliferation that contribute to smaller semitendinosus myofibres. J Physiol 592: 3113–3125, 2014. doi: 10.1113/jphysiol.2014.272591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJA. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem 265: 16330–16336, 1990. [PubMed] [Google Scholar]
- 90.Zickermann V, Kerscher S, Zwicker K, Tocilescu MA, Radermacher M, Brandt U. Architecture of complex I and its implications for electron transfer and proton pumping. Biochim Biophys Acta 1787: 574–583, 2009. doi: 10.1016/j.bbabio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]