Abstract
Microvascular endothelial dysfunction, a precursor to atherosclerotic cardiovascular disease, increases with aging. Endothelium-derived hyperpolarizing factors (EDHFs), which act through K+ channels, regulate blood flow and are important to vascular health. It is unclear how EDHFs change with healthy aging. To evaluate microvascular endothelial reliance on K+ channel-mediated dilation as a function of age in healthy humans. Microvascular function was assessed using intradermal microdialysis in healthy younger (Y; n = 7; 3 M/4 W; 26 ± 1 yr) and older adults (O; n = 12; 5 M/7 W; 64 ± 2 yr) matched for V̇o2peak (Y: 39.0 ± 3.8, O: 37.6 ± 3.1 mL·kg−1·min−1). Participants underwent graded local infusions of: the K+ channel activator Na2S (10−6 to 10−1 M), acetylcholine (ACh, 10−10 to 10−1 M), ACh + the K+ channel inhibitor tetraethylammonium (TEA; 25 or 50 mM), and ACh + the nitric oxide synthase-inhibitor l-NAME (15 mM). Red blood cell flux was measured with laser-Doppler flowmetry and used to calculate cutaneous vascular conductance (CVC; flux/mean arterial pressure) as a percentage of each site-specific maximum (%CVCmax, 43°C+28 mM sodium nitroprusside). The %CVCmax response to Na2S was higher in older adults (mean, O: 51.7 ± 3.9% vs. Y: 36.1 ± 5.3%; P = 0.03). %CVCmax was lower in the ACh+TEA vs. the ACh site starting at 10−5 M (ACh: 34.0 ± 5.7% vs. ACh+TEA: 19.4 ± 4.5%; P = 0.002) in older and at 10−4 M (ACh: 54.5 ± 9.4% vs. ACh+TEA: 31.2 ± 6.7%; P = 0.0002) in younger adults. %CVCmax was lower in the ACh+l-NAME vs. the ACh site in both groups starting at 10−4 M ACh (Y: P < 0.001; O: P = 0.02). Healthy active older adults have enhanced K+ channel-dependent endothelial vasodilatory mechanisms, suggesting increased responsiveness to EDHFs with age.
Keywords: aging, endothelium-derived hyperpolarizing factors, microvascular function, skin blood flow, vasodilation
INTRODUCTION
Aging is associated with increased risk of cardiovascular disease (5). Endothelial dysfunction underlies atherosclerotic cardiovascular disease development (42, 44), making it a critical therapeutic target in the prevention of cardiovascular disease. Vascular dysfunction is characterized by reduced endothelium-dependent vasodilation and is typically assessed by measuring the endothelial release of vasodilatory factors, including nitric oxide (NO). While there is clear evidence that NO-mediated vasodilation is reduced with aging (22, 23, 28), the role(s) of non-NO mediators, such as endothelium-derived hyperpolarizing factors (EDHFs) in altered endothelial vasodilatory function with aging are less well understood.
EDHFs act through K+ channels to induce vascular smooth muscle hyperpolarization, resulting in vasodilation (36). The majority of this response occurs via calcium-activated potassium channels (KCa) (13, 30). Previous work from our laboratory showed a functional role for EDHFs, and more specifically H2S, in the microcirculation in younger men and women (29). We also demonstrated that middle-aged hypertensive adults lack functional H2S-dependent vasodilation (18). However, it remains unclear how primary healthy human aging affects reliance on these signaling pathways. Limited evidence in rodents indicates that EDHF-dependent vasodilation may be reduced with aging (10, 14, 31, 32); however, how healthy aging influences EDHF-dependent dilation in humans is still largely unknown. There is a complex interaction among vasoactive mediators (NO, prostaglandins, and EDHFs) and in the presence of reduced NO and/or prostaglandin bioavailability, there may be an upregulation in EDHF-dependent vasodilatory mechanisms (4, 9, 15, 17, 34).
In humans, the cutaneous microcirculation can be used as a model to evaluate vascular endothelial function (25). Cutaneous microvascular dysfunction is associated with dysfunction in multiple other vascular beds, may lead to conduit artery dysfunction, and is predictive of cardiovascular morbidity and mortality (11, 25, 28). Cutaneous intradermal microdialysis can be used to evaluate microvascular endothelial and vascular smooth muscle cell function. This in vivo localized bioassay delivers pharmacological agents to the cutaneous vasculature (25), allowing for isolation of vasoreactivity associated with individual signaling factors. Using this approach, we pharmacologically assessed endothelium-dependent vasodilation in response to the endothelium-dependent agonist acetylcholine with nonspecific K+ channel blockade. Additionally, we directly assessed K+ channel-mediated vasodilation using the K+ channel activator Na2S. As a secondary aim, we evaluated the contribution of NO to endothelium-dependent dilation, to determine whether compensatory changes in NO signaling occurred with alterations in K+ channel signaling. We hypothesized that healthy older adults would have reduced endothelial NO-dependent dilation but a partially compensatory upregulation of K+-dependent vasodilatory responses compared with younger adults.
METHODS
All study protocols were approved by the Pennsylvania State University’s Institutional Review Board and the Federal Drug Administration (IND120058). Participants completed three study visits, including a screening, a treadmill V̇o2max test, and an experimental intradermal microdialyisis protocol.
Participants.
After informed consent, participants completed a series of initial screenings, including a health history questionnaire, resting blood pressure, assessment of current physical activity status (International Physical Activity Questionnaire), and blood chemistries, including a lipid panel (Quest Diagnostics).
Participants were recruited according to two age cohorts: younger (18–30 yr) and older (60–80 yr). All participants were nonsmokers, did not have known cardiovascular disease or metabolic conditions, and were not taking any medications known to influence endothelial function (e.g., statins and antihypertensive medications). Additionally, participants had resting blood pressures <140/<90 mmHg, low-density lipoprotein cholesterol <180 mg/dl, and hemoglobin A1c <6.5%. All older women reported that they were postmenopausal and were not on hormone replacement therapy.
Participants completed a graded treadmill test to measure peak aerobic capacity (V̇o2peak) using indirect calorimetry (Parvo Medics, Parvo, UT). Before the test, participants refrained from eating or consuming any caffeine or alcohol for 3 h and refrained from vigorous physical activity for 12 h. Participants self-selected speed, and treadmill grade was increased by 2% every 2 min until volitional fatigue (speed was increased if necessary). As not all participants met the criteria for a maximal exercise test (1a), values are reported as V̇o2peak. One younger and one older participant completed a submaximal cycle ergometer test, and estimated V̇o2peak was calculated on the basis of heart rate and workload.
Microvascular assessment of endothelium-dependent vasodilation.
Participants refrained from vigorous exercise, caffeine, and alcohol for 12 h before experimental protocols. With the exception of two young women who had a contraceptive implant (Nexplanon), premenopausal women were measured during days 1–8 of the menstrual cycle or during the placebo phase of oral contraceptive treatment.
Four intradermal microdialyisis fibers (CMA Linear 30 Probe; 55 kDa cut-off) were placed in the ventral forearm, as previously described (23, 24, 39). Our laboratory has used intradermal microdialysis (Harvard Apparatus, Holliston, MA) to evaluate microvascular endothelial function in similar participant populations (23, 24, 39). Sites were randomly assigned as the K+-channel activator Na2S (10−6 to 10−1 M), acetylcholine (ACh, 10−10 to 10−1 M), ACh + the K+-channel blocker tetraethylammonium [TEA; 25 mM or 50 mM (19)], and ACh + NG-nitro-l-arginine methyl ester [l-NAME, 15 mM (2)]. Personal communications (Brunt, University of Colorado) confirmed that 50 mM TEA has similar inhibitory effects as 25 mM TEA; the higher concentration of TEA was used in one older and one younger participant. The same older and younger participant did not undergo a Na2S dose response due to logistical considerations. Pharmacological agents were prepared the morning of each experiment and were dissolved in lactated Ringer solution. Na2S was prepared with HCl and lactated Ringer solution to ensure a neutral pH (18). Perfusates were filtered (0.2-μm membrane; Acrodisc) and wrapped in aluminum to avoid photodegradation.
During the ~60- to 90-min needle trauma-induced hyperemia (i.e., increased blood flow) period, the ACh+TEA and ACh+l-NAME sites were pretreated with each inhibitor; lactated Ringer was perfused through the other two fibers. Pharmacological agents were perfused at a rate of 2 μL/min (Hive controller and microinfusion pumps; BASi, West Lafayette, IN) throughout the experiment. Local heating units were placed over each microdialysis site (Temperature Monitor SH02, Moor Instruments, Devon, UK), and local skin temperature was fixed at 33°C throughout the experiment. Laser-Doppler flowmeters (Moor Instruments, Devon, UK) were placed into each local heating unit to continuously measure red blood cell flux.
Experiments did not begin until blood flow was no longer decreasing and was stable (i.e., resolution of hyperemia). Following this, baseline skin blood flow (~10 min) was measured, after which increasing doses (in 5-min intervals) of Na2S, ACh, or ACh plus site-specific inhibitors were perfused through the respective fibers. After the final dose, maximal skin blood flow was induced at each site by increasing local skin temperature to 43°C and then perfusing 28 mM sodium nitroprusside (SNP; USP, Rockville, MD). Inducing maximal blood flow using local heat and SNP has been previously used by our laboratory group and others (9, 18, 29) and allows for normalization of blood flow for comparisons across treatment sites. Throughout the study, brachial artery blood pressure was measured every 5 min (Cardiocap; GE Healthcare, Milwaukee, WI) on the noninstrumented arm.
Data analysis.
Laser-Doppler flux (LDF) was continuously measured at 40 Hz during experiments and stored offline (PowerLab and Laboratory Chart, ADInstruments). Cutaneous vascular conductance (CVC) was calculated as LDF/mean arterial pressure. Within each site, CVC was normalized to maximal blood flow (%CVCmax) by dividing the CVC value for a specific dose by the maximal value obtained throughout the protocol (2, 39). %CVCmax was evaluated at 1-min intervals, and the final minute of each dose was used in the subsequent analysis. If the final minute of a dose included a movement artifact or other anomalies, the nearest 1-min segment was used.
Statistical analysis.
Statistical significance was accepted as P ≤ 0.05. Differences in participant characteristics were evaluated using independent t tests or an equivalent nonparametric test. ACh and Na2S concentrations were log transformed. As no differences in %CVCmax were seen within or between groups during baseline and the first four concentrations of each dose-response protocol (e.g., ACh: 10−10 to 10−7 M), analyses were performed on the six highest concentrations of each dose-response to increase the degrees of freedom for statistical analyses. Dose-response data were not modeled using pharmacological curve-fitting, as this would have eliminated data due to either poor model fit (i.e., low r2) or because a parameter (e.g., logEC50) could not be calculated. By expressing data as a percentage of maximal dilation, we were not constrained by the assumptions of curve modeling and could evaluate whether there were differences in blood flow at each concentration of our pharmacological agent of choice, better matching our question of interest (43). Differences in %CVCmax during dose responses were evaluated using repeated-measure ANOVAs (within-participant factors: treatment and concentration; between-participant factor: group). A priori specific planned comparisons were performed with a Bonferroni correction.
We evaluated the difference in area under the curve (AUC) for the highest six doses (0.01 to 0.1 M) of Na2S between younger and older adults with an independent Student’s t-test. We evaluated the difference in area between the ACh vs. ACh + TEA or l-NAME sites for the highest six doses of ACh (10−6 to 10−1 M) in younger vs. older adults using an independent t-test.
RESULTS
Participant characteristics.
Participants were healthy and had similar cardiovascular disease risk profiles. By study design, the age difference between groups was significant (P < 0.001) (Table 1). Older participants also had higher total cholesterol (P < 0.001), high-density lipoprotein cholesterol (P = 0.001), low-density lipoprotein cholesterol (P = 0.006), and hemoglobin A1c (P < 0.001), compared with younger participants, but were not clinically elevated above normal, with the exception of total cholesterol. Weight, body mass index, blood pressure, V̇o2peak, and time spent in moderate-to-vigorous physical activity did not differ between groups (all P > 0.05)
Table 1.
Subject characteristics by age group
| Younger | Older | |
|---|---|---|
| n (M/W) | 7 (3/4) | 12 (5/7) |
| Age, yr | 26 ± 1 | 64 ± 2* |
| Weight, kg | 68.3 ± 3.3 | 70.6 ± 4.3 |
| BMI, kg/m2 | 24.4 ± 1.1 | 24.0 ± 1.0 |
| SBP, mmHg | 114 ± 4 | 119 ± 2 |
| DBP, mmHg | 70 ± 3 | 75 ± 2 |
| Total cholesterol, mg/dl | 151 ± 11 | 205 ± 6* |
| HDL-C, mg/dl | 53 ± 1 | 72 ± 6* |
| LDL-C, mg/dl | 82 ± 10 | 116 ± 6* |
| HbA1C, % | 4.9 ± 0.1 | 5.4 ± 0.1* |
| V̇o2peak, mL·kg−1·min−1 | 39.0 ± 3.8 | 37.6 ± 3.1 |
| MVPA, MET − min/wk | 1,904 ± 692 | 5,488 ± 1,725 |
Values are expressed as means ± SE. BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; M, men; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity; SBP, systolic blood pressure; W, women.
P < 0.05.
Microvascular responses.
In response to Na2S perfusion (Fig. 1), both groups demonstrated increasing %CVCmax with increasing Na2S concentrations (P < 0.001); however, older adults had higher %CVCmax compared with younger adults from 0.02 M Na2S (P = 0.0005) to 0.1 M Na2S (P = 0.0002). Area under the curve was significantly greater in older versus younger adults (younger: 31.4 ± 5.6 vs. older: 47.6 ± 3.4 AU, P = 0.019)
Fig. 1.
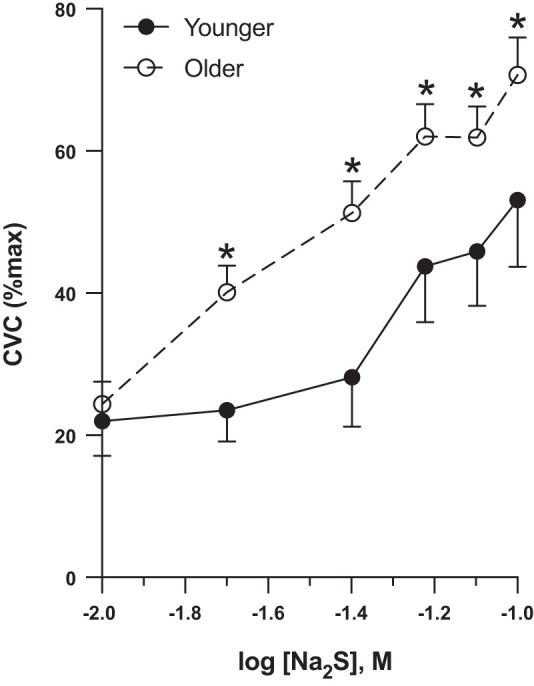
K+ channel-dependent stimulation. In response to K+ channel stimulation via a Na2S dose response, older adults (n = 11, 4 M/7 W) had higher blood flow responses compared with younger adults (n = 6, 3 M/3 W). Differences were evaluated between groups using a repeated measures ANOVA and post hoc testing. Data are expressed as means ± SE, CVC, cutaneous vascular conductance; M, men; W, women. *P < 0.05.
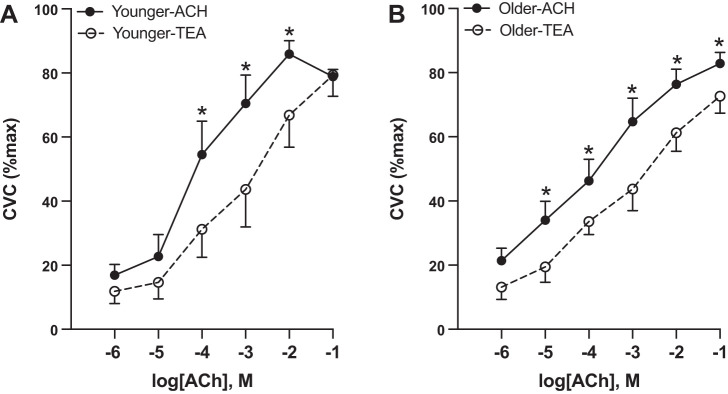
There was no group difference in %CVCmax in the ACh site (all P > 0.05), except older adults had a higher %CVCmax at 10−5 M ACh (P = 0.04). There was no difference in AUC between younger versus older adults (Y: 281.5 ± 25.5 vs. O: 273.4 ± 23.6 AU; P = 0.83). At the ACh+TEA perfusion site (Fig. 2), overall %CVCmax was lower compared with ACh alone (P = 0.006). There was an interaction between treatment and concentration (P = 0.043), such that there was less of a blood flow response to increasing concentrations of ACh in the ACh+TEA site. There was no difference in %CVCmax between groups for the ACh+TEA site at any concentration of ACh (all P > 0.05). In younger participants, %CVCmax was lower in the ACh+TEA compared with the ACh site from 10−4 M (P = 0.0002) to 10−2 M ACh (P = 0.002). In older adults, %CVCmax was lower in the TEA versus the ACh site from 10−5 M (P = 0.002) to 10−1 M ACh (P = 0.03). There was no difference in area between the curves in younger versus older adults (younger: 79.6 ± 51.5 vs. older: 72.5 ± 21.6 AU; P = 0.89).
Fig. 2.
K+ channel-dependent dilation. In response to K+ inhibition via tetraethylammonium (TEA), younger adults (n = 7, 3 M/4 W) had similar blood flow responses in the control (acetylcholine, ACh) and the K+-channel inhibited site until 10−4 M ACh (A). Older adults (n = 12, 5 M/7 W) had lower blood flow responses in the K+-channel inhibited site starting at 10−5 M ACh (B). Differences were evaluated between groups and sites using a repeated-measures ANOVA and post hoc testing. Data are expressed as means ± SE. CVC, cutaneous vascular conductance; M, men; W, women. *P < 0.05.
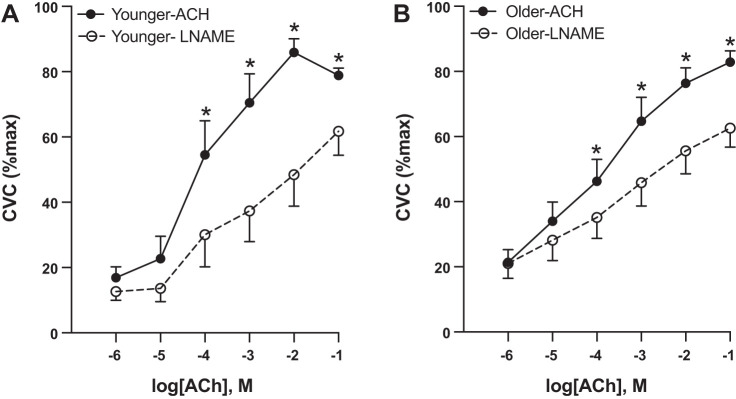
ACh coperfused with l-NAME (Fig. 3) resulted in overall lower %CVCmax compared with the ACh-alone site (P = 0.004), and there was an interaction between concentration and treatment, such that there was less of a blood flow response to ACh in the ACh+l-NAME site (P = 0.003). There was no difference in %CVCmax between groups in the ACh+l-NAME site (P = 0.56), but %CVCmax was higher in older adults at 10−5 M ACh (P = 0.01). In both younger and older participants, %CVCmax was significantly lower in the ACh+l-NAME versus the ACh site starting at 10−4 M ACh (younger: P < 0.001, older: P = 0.02) and at higher concentrations. There was no difference in area between the curves in younger versus older adults (younger: 114.8 ± 55.2 versus older: 66.8 ± 29.0 AU, P = 0.41).
Fig. 3.
Nitric oxide-dependent dilation. In response to nitric oxide (NO) inhibition via NG-nitro-l-arginine methyl ester (l-NAME), younger (n = 7, 3 M/4 W; A) and older adults (n = 12, 5 M/7 W; B) had lower blood flow in the l-NAME vs. the control (acetylcholine, ACh) site starting at 10−4 M ACh. Differences were evaluated between groups and sites using a repeated-measures ANOVA and post hoc testing. Data are expressed as means ± SE. CVC, cutaneous vascular conductance; M, men; W, women. *P < 0.05.
DISCUSSION
The principle finding from this study was that healthy older adults had increased vasodilatory sensitivity (defined as a higher response at a lower concentration) to direct K+ channel activation. In contrast to our initial hypothesis, ACh-dependent endothelial function was similar between healthy older and younger participants. However, there were subtle differences in the downstream mechanisms mediating this response, including an increase in TEA-sensitive K+ channel-mediated vasodilation at a lower concentration of ACh in older adults. Collectively, these data suggest that in healthy, physically active, and aerobically fit older adults, there is increased responsiveness to K+-dependent endothelial dilatory mechanisms.
The majority of evidence evaluating endothelial dysfunction has assessed the ability of endothelial cells to release NO; however, endothelial cells release a variety of dilator molecules that collectively subserve vascular function and maintain vascular health. Endothelium-derived hyperpolarizing factors (EDHFs) induce vascular smooth muscle hyperpolarization predominantly through KCa channel-dependent mechanisms, although other K+ channels, including KATP and inwardly rectifying K+ (KIR) channels, are also involved in vasodilation in humans (16, 19, 20, 36). Prominent EDHFs include hydrogen sulfide (H2S) and hydrogen peroxide (H2O2); however, there are many other signaling factors (e.g., K+) that are also EDHFs. NO-dependent dilation is reduced with aging and/or cardiovascular disease risk factors, but it may be partially restored with health-enhancing behaviors (e.g., exercise and salt restriction) (8, 27, 38).
The present article examined how K+ channel-dependent dilatory mechanisms (i.e., EDHFs) are influenced by healthy aging. Our data demonstrate enhanced end-organ responsiveness to K+ channel stimulation in healthy older compared with younger adults, as evidenced by greater dilation at lower concentrations of Na2S. K+ channel-mediated vasodilation is related to vascular endothelial health (1, 45). In rodents, aging is associated with a reduction in the number and function of KCa channels (3, 21, 47), along with a reduction in sensitivity to KCa channel activation (10). We have previously shown that otherwise healthy hypertensive adults maintain vascular smooth muscle sensitivity to K+ channel stimulation via Na2S, but lack a functional contribution of H2S to direct endothelial stimulation (18). By contrast, the current study shows that fit, healthy normotensive older adults have enhanced vascular smooth muscle responsiveness to K+ channel stimulation, with a modest increase in K+ channel endothelium-dependent vasodilation. This enhanced responsiveness may be due to the high fitness and physical activity in the older adults, which in rodents counteracts functional alterations in K+ channels (3).
There is a complex interaction among multiple endothelium-derived vasodilator mechanisms that allows for maintenance of endothelium-dependent vasodilation. For example, with reduced NO and/or prostaglandin bioavailability, there is an upregulation in EDHF-dependent vasodilatory mechanisms, particularly in the microcirculation (4, 9, 15, 17, 34). Furthermore, with atherosclerotic development or risk factors for cardiovascular disease, there is a shift from NO-mediated dilation to greater reliance on EDHF-mediated dilation (e.g., H2O2) (6, 35). Although there is strong evidence for diminished NO-dependent vasodilation with aging, how EDHFs are influenced by aging has been less clear. Our data suggest that in this cohort of healthy physically active older adults, there may be a modest increase in K+ channel-dependent (i.e., EDHF-dependent) vasodilation, with no change in NO-dependent dilation. Our data also suggest that ACh-dependent vasodilation is well maintained in physically active and aerobically fit older adults.
Although it is generally accepted that there is a reduction in endothelial function with advanced age (38), whether this is reflected in ACh-mediated responses is less clear. Some studies show maintained ACh-mediated vasodilation with aging (24), while others show a reduction (40). In cross-sectional and longitudinal studies, age-related reductions in ACh-dependent vasodilation were attenuated or eliminated with exercise training (12, 38, 40). High physical activity and fitness also increase NO in humans and EDHF-dependent vasodilation in rodents (7, 26, 40). Furthermore, regular exercise may counteract the negative influence of aging on functional changes in K+ channels (3). Therefore, the maintained vasodilatory responses that we show in older adults across all ACh dose responses and the enhanced responsiveness to K+ channel stimulation may be due to their low cardiovascular risk profile and their relatively high fitness (older adults: ~90th percentile for V̇o2max, younger adults ~45th percentile for V̇o2max) and physical activity patterns. This is of particular interest as it indicates that physical activity and high cardiorespiratory fitness may mitigate or ameliorate the age-related alteration in endothelial function previously reported in the literature and may do so through K+ channel-mediated mechanisms. Future research is needed to evaluate the influence of aging on K+ channel-mediated dilation in inactive older adults.
Despite maintained ACh-dependent dilation, we observed enhanced vasodilation in response to K+ channel-dependent stimulation in older adults. With disease such as hypertension, we have shown reduced ACh-dependent dilation, and maintained K+ channel-dependent dilation (18). It is presumed that the maintained sensitivity in hypertensive adults may be a compensatory adaptation due to overall reduced endothelium-dependent dilation. Although speculative, we hypothesize that the enhanced K+ channel-mediated dilation that we see in active older adults may represent a compensatory adaptation, to allow for maintenance of endothelium-dependent dilation on the backdrop of age-associated alterations in endothelium function that may occur in less healthy and less active older adults (e.g., reduced ACh-mediated dilation).
Limitations.
Although this study provides important mechanistic insight into endothelial vasodilator capacity in healthy adults, it has several limitations. The sample size for the study was relatively small. Additionally, this study evaluated the influence of primary aging on K+ channel-mediated vasodilatory mechanisms in the context of high physical activity and cardiorespiratory fitness. By recruiting highly active and fit older adults, we were able to infer that any group differences are likely to be age-specific without the influence of training status and changes in cardiovascular disease risk factors, such as blood pressure and cholesterol, that typically occur with aging. Although this is a strength of the current study, results are not generalizable to the general elderly population or to those with higher cardiovascular disease risk or overt disease. Findings should be replicated in a larger and more diverse cohort of participants, particularly in older adults who are less physically active. The similar risk factor profile between our younger and older groups allowed us to evaluate the influence of healthy aging on microvascular outcomes; however, this study was cross-sectional, and further work is needed to determine longitudinal changes in these signaling pathways.
This study represents an important first step in evaluating whether healthy aging influences K+ channel-dependent dilation; however, it was underpowered to evaluate sex differences. The influence of sex on K+ channel-mediated dilatory mechanisms is unclear. Some evidence indicates that EDHFs may be influenced by estrogens (41, 48), while at least one study suggests that estrogen may not influence EDHFs (37). Therefore, an important next step will be to evaluate whether reliance on K+ channel-dependent mechanisms differs by sex.
Finally, TEA is efficacious at blocking KCa-mediated vasodilation, which is the primary mechanism through which EDHFs act (13, 30); however, it does not block KATP-mediated vasodilation, through which H2S can also induce vasodilation (46). We have previously shown that the vasodilatory response to Na2S was almost completely abolished in the presence of TEA (29); therefore, we do not believe that concomitantly blocking KATP channels would have a large effect on our study outcomes. Additionally, there are signaling pathways other than NO and EDHFs, which may be influenced by healthy aging (e.g., prostaglandins). Future investigation is needed to determine the role of these signaling pathways in K+ channel-dependent dilation.
Perspectives and Significance
Overall, our data suggest that in healthy, physically active, and aerobically fit older adults, there is an upregulation in K+ channel-dependent vasodilator function. This may represent a compensatory adaptation to allow for maintenance of endothelium-dependent vasodilation in the presence of other age-related alterations in endothelial function. These findings may have important implications for future research, as they suggest that K+ channel-dependent dilatory mechanisms may be molecular targets to maintain endothelium-dependent dilation with age.
GRANTS
This study was supported by the National Institutes of Health (NIH) Grants HL-093238, NIH T32-AG049676, and NCATS-UL1-TR002014, as well as an American College of Sports Medicine Foundation Doctoral Student Research Grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.L.K. and L.M.A. conceived and designed research; C.S. and C.W.B. performed experiments; C.S. and C.W.B. analyzed data; C.S., C.W.B., W.L.K., and L.M.A. interpreted results of experiments; C.S. prepared figures; C.S. and L.M.A. drafted manuscript; C.S., C.W.B., W.L.K., and L.M.A. edited and revised manuscript; C.S., C.W.B., W.L.K., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank study participants, Registered Nurse Susan K. Slimak, Sean Shank, and other members of the Thermoregulatory and Microvascular Physiology Laboratory at Penn State University for contributions to this project.
REFERENCES
- 1.Al-Magableh MR, Kemp-Harper BK, Hart JL. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens Res 38: 13–20, 2015. doi: 10.1038/hr.2014.125. [DOI] [PubMed] [Google Scholar]
- 1a.American College of Sports Medicine ACSM’s Guidelines for Exercise Testing and Prescription. New York: Wolters Kluwer, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Alba BK, Stanhewicz AE, Kenney WL, Alexander LM. Acute dairy milk ingestion does not improve nitric oxide-dependent vasodilation in the cutaneous microcirculation. Br J Nutr 116: 204–210, 2016. doi: 10.1017/S0007114516001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albarwani S, Al-Siyabi S, Baomar H, Hassan MO. Exercise training attenuates ageing-induced BKCa channel downregulation in rat coronary arteries. Exp Physiol 95: 746–755, 2010. doi: 10.1113/expphysiol.2009.051250. [DOI] [PubMed] [Google Scholar]
- 4.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996. doi: 10.1161/01.CIR.94.12.3341. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018.] doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 6.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 112: 5, 2017. doi: 10.1007/s00395-016-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol 586: 3511–3524, 2008. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Brunt VE, Fujii N, Minson CT. Endothelial-derived hyperpolarization contributes to acetylcholine-mediated vasodilation in human skin in a dose-dependent manner. J Appl Physiol (1985) 119: 1015–1022, 2015. doi: 10.1152/japplphysiol.00201.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chennupati R, Lamers WH, Koehler SE, De Mey JGR. Endothelium-dependent hyperpolarization-related relaxations diminish with age in murine saphenous arteries of both sexes. Br J Pharmacol 169: 1486–1499, 2013. doi: 10.1111/bph.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Waard GA, Fahrni G, de Wit D, Kitabata H, Williams R, Patel N, Teunissen PF, van de Ven PM, Umman S, Knaapen P, Perera D, Akasaka T, Sezer M, Kharbanda RK, van Royen N; Oxford Acute Myocardial Infarction (OxAMI) Study investigators . Hyperaemic microvascular resistance predicts clinical outcome and microvascular injury after myocardial infarction. Heart 104: 127–134, 2018. doi: 10.1136/heartjnl-2017-311431. [DOI] [PubMed] [Google Scholar]
- 12.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 13.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 14.Fujii K, Ohmori S, Tominaga M, Abe I, Takata Y, Ohya Y, Kobayashi K, Fujishima M. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol Heart Circ Physiol 265: H509–H516, 1993. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- 15.Fujii N, Meade RD, Minson CT, Brunt VE, Boulay P, Sigal RJ, Kenny GP. Cutaneous blood flow during intradermal NO administration in young and older adults: roles for calcium-activated potassium channels and cyclooxygenase? Am J Physiol Regul Integr Comp Physiol 310: R1081–R1087, 2016. doi: 10.1152/ajpregu.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf) 219: 152–161, 2017. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 17.Goto K, Kansui Y, Oniki H, Ohtsubo T, Matsumura K, Kitazono T. Upregulation of endothelium-derived hyperpolarizing factor compensates for the loss of nitric oxide in mesenteric arteries of Dahl salt-sensitive hypertensive rats. Hypertens Res 35: 849–854, 2012. doi: 10.1038/hr.2012.36. [DOI] [PubMed] [Google Scholar]
- 18.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 69: 902–909, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearon CM Jr, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Endothelium-dependent vasodilatory signalling modulates α1 -adrenergic vasoconstriction in contracting skeletal muscle of humans. J Physiol 594: 7435–7453, 2016. doi: 10.1113/JP272829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594: 2261–2273, 2016. doi: 10.1113/JP270593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilgers RHP, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2275–H2284, 2007. doi: 10.1152/ajpheart.00949.2006. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev 35: 119–125, 2007. doi: 10.1097/jes.0b013e3180a02f85. [DOI] [PubMed] [Google Scholar]
- 23.Holowatz LA, Thompson CS, Kenney WL. l-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 574: 573–581, 2006. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Zhang H, Tan X, Hu M, Shen B. Exercise restores impaired endothelium-derived hyperpolarizing factor-mediated vasodilation in aged rat aortic arteries via the TRPV4-KCa2.3 signaling complex. Clin Interv Aging 14: 1579–1587, 2019. doi: 10.2147/CIA.S220283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney WL, Edward F. Adolph Distinguished Lecture: Skin-deep insights into vascular aging. J Appl Physiol (1985) 123: 1024–1038, 2017. doi: 10.1152/japplphysiol.00589.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutz JL, Greaney JL, Santhanam L, Alexander LM. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J Physiol 593: 2121–2129, 2015. doi: 10.1113/JP270054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung SWS, Vanhoutte PM. Endothelium-dependent hyperpolarization: age, gender and blood pressure, do they matter? Acta Physiol (Oxf) 219: 108–123, 2017. doi: 10.1111/apha.12628. [DOI] [PubMed] [Google Scholar]
- 31.Ma N, Liu HM, Xia T, Liu JD, Wang XZ. Chronic aerobic exercise training alleviates myocardial fibrosis in aged rats through restoring bioavailability of hydrogen sulfide. Can J Physiol Pharmacol 96: 902–908, 2018. doi: 10.1139/cjpp-2018-0153. [DOI] [PubMed] [Google Scholar]
- 32.Mantelli L, Amerini S, Ledda F. Roles of nitric oxide and endothelium-derived hyperpolarizing factor in vasorelaxant effect of acetylcholine as influenced by aging and hypertension. J Cardiovasc Pharmacol 25: 595–602, 1995. doi: 10.1097/00005344-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol 279: H459–H465, 2000. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 35.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation 123: 2244–2253, 2011. doi: 10.1161/CIRCULATIONAHA.110.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozkor MA, Quyyumi AA. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol Res Pract 2011: 156146, 2011. doi: 10.4061/2011/156146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahimian R, Chan L, Goel A, Poburko D, van Breemen C. Estrogen modulation of endothelium-derived relaxing factors by human endothelial cells. Biochem Biophys Res Commun 322: 373–379, 2004. doi: 10.1016/j.bbrc.2004.07.137. [DOI] [PubMed] [Google Scholar]
- 38.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol (1985) 112: 791–797, 2012. doi: 10.1152/japplphysiol.01257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. doi: 10.1161/01.CIR.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 41.Tang Z, Wang Y, Zhu X, Ni X, Lu J. Exercise increases cystathionine-γ-lyase expression and decreases the status of oxidative stress in myocardium of ovariectomized rats. Int Heart J 57: 96–103, 2016. doi: 10.1536/ihj.15-099. [DOI] [PubMed] [Google Scholar]
- 42.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 43.Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol (1985) 111: 1703–1709, 2011. doi: 10.1152/japplphysiol.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 45.Xue H, Zhou S, Xiao L, Guo Q, Liu S, Wu Y. Hydrogen sulfide improves the endothelial dysfunction in renovascular hypertensive rats. Physiol Res 64: 663–672, 2015. doi: 10.33549/physiolres.932848. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou E, Qing D, Li J. Age-associated endothelial dysfunction in rat mesenteric arteries: roles of calcium-activated K+ channels (KCa). Physiol Res 59: 499–508, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Tang Z, Cong B, Du J, Wang C, Wang L, Ni X, Lu J. Estrogens increase cystathionine-γ-lyase expression and decrease inflammation and oxidative stress in the myocardium of ovariectomized rats. Menopause 20: 1084–1091, 2013. doi: 10.1097/GME.0b013e3182874732. [DOI] [PubMed] [Google Scholar]