Abstract
In the central nervous system (CNS), nuclei of the brain stem play a critical role in the integration of peripheral sensory information and the regulation of autonomic output in mammalian physiology. The nucleus tractus solitarius of the brain stem acts as a relay center that receives peripheral sensory input from vagal afferents of the nodose ganglia, integrates information from within the brain stem and higher central centers, and then transmits autonomic efferent output through downstream premotor nuclei, such as the nucleus ambiguus, the dorsal motor nucleus of the vagus, and the rostral ventral lateral medulla. Although there is mounting evidence that sex and sex hormones modulate autonomic physiology at the level of the CNS, the mechanisms and neurocircuitry involved in producing these functional consequences are poorly understood. Of particular interest in this review is the role of estrogen, progesterone, and 5α-reductase-dependent neurosteroid metabolites of progesterone (e.g., allopregnanolone) in the modulation of neurotransmission within brain-stem autonomic neurocircuits. This review will discuss our understanding of the actions and mechanisms of estrogen, progesterone, and neurosteroids at the cellular level of brain-stem nuclei. Understanding the complex interaction between sex hormones and neural signaling plasticity of the autonomic nervous system is essential to elucidating the role of sex in overall physiology and disease.
Keywords: autonomic, estrogen, neurosteroid, progesterone, sex
INTRODUCTION
With the introduction of the National Institutes of Health’s policies on rigor and reproducibility, a new emphasis has been placed on understanding sex and thereby sex hormones as biological variables. For years, it was assumed that fluctuations in sex hormones associated with the ovarian cycle produced significant and ubiquitous variability in physiological parameters. Therefore, studying males exclusively allowed for reductions in physiological variability. This classic assumption was recently challenged by the identification that some experimental outcomes exhibit greater interindividual differences in males than interindividual differences in females (12). There is even an argument to be made that males represent a population more susceptible to disease involving dysregulated autonomic signaling, such as cardiovascular disease (79) and metabolic syndrome (73). In these latter cases, the opposite sex is likely an excellent model since females exhibit a protective mechanism against autonomic dysfunction that could be harnessed as a therapeutic. Therefore, more investigations are needed to understand how sex and sex hormones are affecting brain-stem autonomic signaling. This is particularly striking in the context of synaptic plasticity and the use of whole cell patch-clamping, which recently received renewed attention for the technique’s ability to investigate the intricacies of neuronal function (93). Perhaps not surprising then, recent reports using these experimental approaches provide clear evidence that sex hormones do have actions within fundamental signaling units of the autonomic nervous system (56, 62, 65).
FRAMEWORK FOR THE INVESTIGATION OF SEX AND REPRODUCTIVE HORMONES IN AUTONOMIC NEUROPHYSIOLOGY
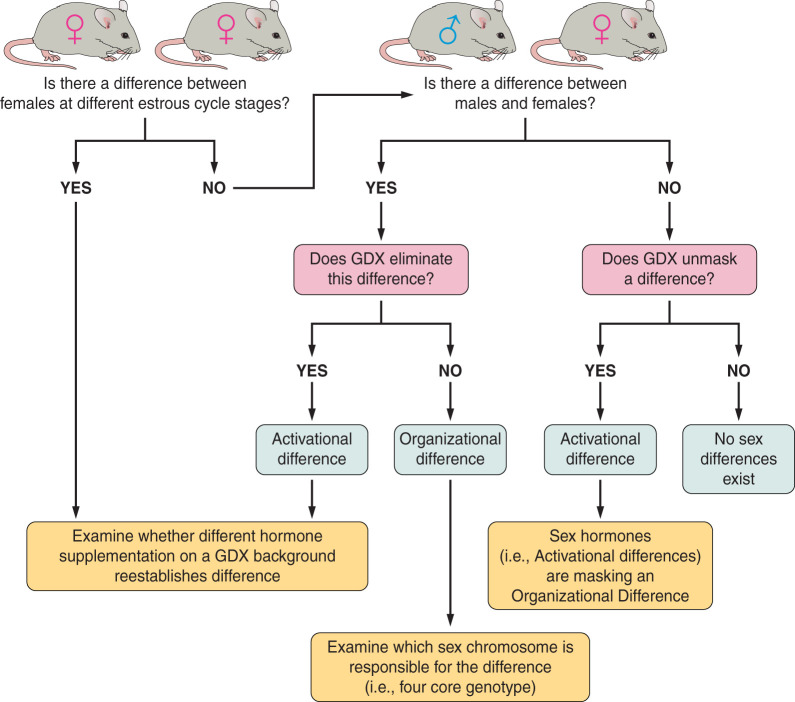
A brief discussion of experimental approaches used to determine sex differences is first presented here to provide a framework for sex differences in autonomic control (Fig. 1). However, those interested in a detailed discussion of experimental approaches should be directed to other reviews (11, 74). Sex differences are generally classified into two categories: organizational and activational. Activational differences are mediated by direct activity of sex steroids and are considered transient in nature, meaning that the presence of a steroid causes a reversible change. Investigations into sex differences may be started by simply comparing males and females. Often, however, sex differences are subtle, and, therefore, it is useful to separate females by their stage within the ovarian cycle. If these approaches indicate significant differences between males and females, follow-up experiments might include gonadectomized animals. If differences are abolished by gonadectomy, then gonadectomy with specific hormone supplementation can be used to determine which steroid is responsible for suspected activational differences.
Fig. 1.
A flowchart summarizing the framework of different experimental approaches and models used to determine sex differences. To determine whether a sex difference is present and to differentiate between an activational or organizational sex difference, a series of questions must be asked. After determining an effect of interest, a logical first question is to ask whether the effect varies between males and females. If so, one should proceed to comparing gonadectomized males and females. If the difference in effect disappears after males and females are gonadectomized, then this suggests an activational sex difference exists for which the cause may then be probed by hormone supplementation. If the difference in effect remains regardless of gonadectomy (GDX), then this suggests an organizational difference that must be tested using a method such as four-core genotype. Alternatively, one might be interested in whether the estrous cycle affects the responses of females to a known measurement. In this case, females would first be examined across different stages of the menstrual/estrous cycle. It would then be necessary to conduct an experiment in which gonadectomized females received hormone supplementation to mimic the different estrous stages and determine which stage correlates with the effect. Finally, if there is no difference in effect between males and females whether intact or gonadectomized, then the conclusion can be made that there is no sex difference.
If gonadectomized males and females maintain their differences in a measured outcome, then the role of organizational differences must be considered. Organizational differences are permanent in nature. These differences are mediated by chromosomal differences between the sexes (i.e., XX vs. XY). The best example of this might be the testicular testosterone surge that occurs early in perinatal development (117). The presence of a Y chromosome confers the sex-determining region Y (Sry) gene that is critical for the development of testes. Embryonic testes release testosterone during critical periods in development. This surge in testosterone has been suggested to masculinize the brain by causing permanent changes in neural circuitry (3, 67). Although the activity of a steroid is required, suggestive of an activational event, this surge leads to permanent changes in neuronal circuits even when testosterone levels return to normal. Therefore, the masculinization of the brain is classified as an organizational change. This masculinization of the brain is suggested as a mechanism for sex differences in postnatal development and maturation of the autonomic nervous system, especially its regulation of heart rate (15, 18) likely through differences in synaptic signaling (34). Organizational differences are best investigated using genetic model systems. One in particular, known as the four-core genotype, removes the Sry gene from the Y chromosome and places it on an autosomal chromosome (2). This model creates mice with the traditional sex chromosome to gonad development (i.e., XX with ovaries and XY with testes). However, the movement of the Sry gene to an autosomal chromosome creates two additional mice, XX with testes and XY with ovaries. Additional effects of sex chromosome “dosing” can be examined with models like the sex chromosome trisomy model (84).
Regardless of model(s) used, the interpretation of sex steroid signaling in any physiological system is challenging. The nuances associated with cycling hormone levels are difficult to model, especially since these hormones often interact with each other in a brain-region-specific way (66). This phenomenon has been confirmed with estrogen and progesterone in autonomic centers for hormone receptor expression (53, 77). Therefore, experimental approaches such as ovariectomy with single-hormone replacement can be seen as too reductionist, and genetic manipulations, to test activational and/or organizational effects, may have compensatory responses that complicate the interpretations and may not appropriately mimic normal physiological hormone activity. Importantly, it is possible for sex-specific adaptions to prevent overt changes, thus effectively “canceling each other out” (33). This potentially leads to the conclusion that no sex difference exists in a particular experimental outcome. However, these difficulties should not limit enthusiasm for this type of work since increasing our understanding of sex differences could lead to novel therapeutics for diseases associated with these systems.
SEX DIFFERENCES IN AUTONOMIC PHYSIOLOGY
The relationship between sex and autonomic regulation is complex (8, 57, 91) and highly dependent on age (8). For example, young women show a significantly blunted blood pressure response to autonomic blockade using the nicotinic receptor antagonist trimethaphan compared with young male counterparts (26), likely, in part, because young women lack the same relationship between mean sympathetic nerve activity and total peripheral resistance as men (50). However, as women age, these relationships skew toward more masculinized responses (8). These age-related changes are thought to mediate some of the sex differences in cardiovascular disease (i.e., young women show protection against cardiovascular diseases, yet there is a striking uptick in disease rates with menopause; Ref. 86). This decreased sympathetic tone is not limited to cardiovascular autonomic regulation; the counterregulatory response (CRR) to hypoglycemia is also blunted in women in part through blunted sympathetic activation (32, 98). However, complete autonomic blockade with trimethaphan significantly altered the CRR in women (51), indicating that the parasympathetic system likely plays a larger role compared with men. This conclusion is supported by preclinical models (59). Despite these sex differences in the CRR, there is no sex difference in the occurrence of hypoglycemia in patients with type 1 diabetes (32a), suggesting that women are likely protected from the effects of antecedent hypoglycemia on autonomic activity (32). Gastrointestinal function and disease is also markedly different between sexes and across the menstrual cycle (57) with notable differences in metabolic responses, including the use of different energy sources under normal, healthy conditions and during disease (72). Despite our understanding of the existence of sex differences throughout different autonomic functions, the neuromechanistic underpinning(s) of these differences is largely unexplored. Therefore, understanding the action of sex hormones on the regulation of autonomic circuits will be informative in our development of therapeutics to treat a wide array of autonomic dysfunction(s).
BRAIN-STEM AUTONOMIC CIRCUITS
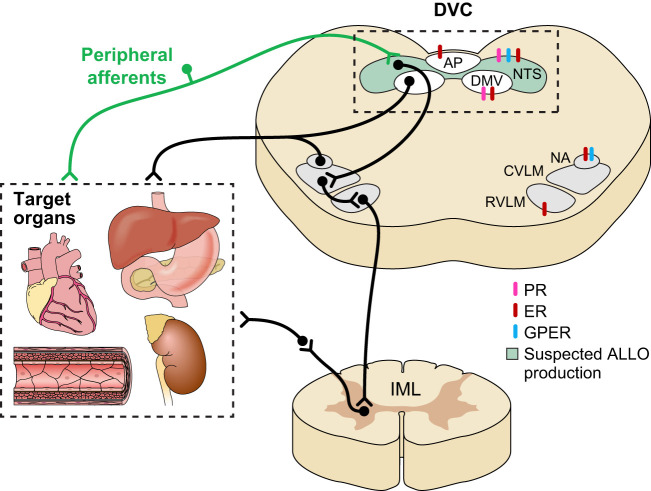
Viscerosensory input from various peripheral organs synapse within the brain stem’s nucleus tractus solitarius (NTS; Fig. 2). These sensory afferent fibers use glutamate as their primary neurotransmitter to carry a wide array of physiological information, including baroreceptors and chemoreceptors from the carotid bodies/aortic arch and mechanoreceptors and chemoreceptors from the gut. Serving as a relay station, the first-order NTS neurons integrate and encode the sensory information received from viscerosensory afferent terminals and send this information to upstream central nuclei, like the hypothalamus’s paraventricular nucleus, or to downstream autonomic motor nuclei, like the dorsal motor nucleus of the vagus (DMV), nucleus ambiguus (NA), and ventral lateral medulla (VLM). Both the DMV and NA comprise the motor limb of the parasympathetic nervous system. The NA is the ventral parasympathetic premotor nucleus that sends prominent projections to the heart (75) and is critical for baroreflex responses (25) and respiratory sinus arrhythmia (80). The DMV is the dorsal parasympathetic premotor nucleus that sends prominent projections to subdiaphragmatic viscera, including the stomach, intestine, liver, and arguably the pancreas. Both of these nuclei are cholinergic and receive glutamatergic and GABAergic projections (7, 19, 31, 41, 64, 87, 116), including direct inputs from the NTS (19, 41, 42, 64). Therefore, these motor neurons are significantly regulated by synaptic input, including vagal afferent terminals via second-order NTS neurons or possibly through direct afferent connections (94). In the generation of the baroreflex (a critical cardiovascular regulatory mechanism), NTS neurons project to the caudal (C)VLM, which, in turn, projects to the rostral (R)VLM. The RVLM sends prominent projections to the intermediolateral cell column of the spinal cord, and, therefore, these cells serve as premotor sympathetic neurons.
Fig. 2.
A schematic representation of the autonomic brain stem. Viscerosensory afferent neurons from vital organs, such as the heart, gastrointestinal system, pancreas, adrenal glands, kidneys, and blood vessels, synapse in the nucleus tractus solitarius (NTS). NTS neurons modulate descending autonomic output nuclei, like the dorsal motor nucleus of the vagus (DMV), nucleus ambiguus (NA), and the ventral lateral medulla (VLM). Although significant work must be done to determine the integrated nature of sex steroid modulation of autonomic signaling, all critical autonomic brain-stem regions express estrogen and progesterone receptors. Of recent interest is allopregnanolone, a metabolite of progesterone, which may be produced locally by the NTS. The extent of allopregnanolone’s role in modulating signals is unknown, but there is evidence that it is important in the regulation of GABAA receptor activity. Since GABAergic signaling is a key mechanism by which the NTS regulates DMV, rostral VLM (RVLM), and NA activity (and, in turn, peripheral organ system function), allopregnanolone could be a novel modulator of autonomic output. ALLO, allopregnanolone; AP, area postrema; CVLM, caudal VLM; DVC, dorsal vagal complex; ER, estrogen receptor; GPER, G protein-coupled estrogen receptor; IML, intermediolateral column; PR, progesterone receptor.
However, the autonomic brain stem does not exclusively rely on intact afferent connections for sensing the internal milieu. The NTS is part of a larger structure known as the dorsal vagal complex. In addition to the NTS, the dorsal vagal complex comprises area postrema and the DMV. Although the lipophilic nature of cholesterol-based steroid hormones allows them to readily penetrate the blood-brain barrier, area postrema is a circumventricular organ and resides outside of the blood-brain barrier. The fenestrated capillaries within the NTS allow for many types of large molecules to pass unimpeded (47), and indeed neurons within both area postrema and the NTS are capable of intrinsically sensing peripheral homeostatic signals (9, 13, 16, 82, 95, 118). Therefore, the dorsal vagal complex is a prime candidate to integrate sex hormone information into autonomic function with limited need for active/supported transport or high levels of steroid. Despite our current understanding of these circuits, all of this signaling circuitry undergoes robust experience-dependent plasticity (17, 24, 35) and more importantly may be uniquely susceptible to autonomic insults (35). Our limited understanding of the dynamic modulation of these autonomic brain-stem regions limits our potential to develop treatments to target these networks. Therefore, although brain-stem circuitry is critical in the regulation of multiple physiological homeostatic functions, significantly more work must be done to understand how sex and sex differences affect brain-stem autonomic signaling plasticity.
INFLUENCE OF REPRODUCTIVE HORMONES ON BRAIN-STEM AUTONOMIC CIRCUITS
Central Estrogen Receptors in Autonomic Physiology
Despite significant evidence for the role of sex and sex hormones in autonomic physiology, the mechanism(s) and neurocircuitry responsible for these differences are not well characterized under either healthy or disease conditions. Estrogen is a well-established mediator of activational sex differences. Traditionally, estrogens influence “long-term” signaling through two different nuclear estrogen receptors (ERα and ERβ) that serve as ligand-dependent transcriptional factors regulating gene expression (81). Emerging evidence also exists for a short-term or “rapid effect” of estrogen signaling. This type of signaling is thought to come, in part, from ERα and ERβ located at the plasma membrane (49) but also from a G protein-coupled estrogen receptor known as GPER (89). Both ERα and ERβ are expressed throughout the brain stem’s autonomic regulatory centers with prominent expression in the NTS (76, 108, 121) and RVLM (109) and exhibit sex differences in their expression patterns (101, 114). Although only a limited amount of research exists on GPER activity in brain-stem autonomic neurons, GPER expression has been identified in the NTS and NA (21, 78).
A well-established modulatory role for estrogen in the brain stem is illustrated by its impact on satiety signaling. Estrogen decreases food intake and thereby weight through an increased facilitation of satiety signaling (36), including an increased satiating potency of lipids (4); this effect correlates with increased c-fos expression in the NTS with lipid consumption, thereby suggesting the NTS as estrogen’s site of action (4, 37). Estradiol microinjections directly into the NTS increased the effectiveness of CCK to suppress feeding (111), whereas knockdown of ERα blunts CCK-dependent signaling (44). Estrogen also increases the density (27) and excitability (90) of vagal afferent terminals within the NTS, which are required for CCK-dependent satiation. Together, these data support the hypothesis that estrogen potentiates CCK-dependent vagal afferent signaling in the NTS and can modulate further information processing to upstream nuclei through its effect on the dorsal vagal complex. Estrogen’s anorectic actions are not limited to modulation of CCK signaling but also include other satiation factors like apolipoprotein A-IV (106) and leptin (28). For example, estrogen’s effects on apolipoprotein A-IV-dependent satiation require recruitment of steroid receptor coactivator-1 in the dorsal vagal complex (104) by ERα (105).
Estrogen’s modulation of vagally mediated homeostasis is also not limited to satiation (52). Central estrogen administration increases both vagal nerve activity and baroreflex sensitivity, and antagonizing ERα within the NA abolished this estrogen-induced increase in baroreflex sensitivity (96, 97). Similarly, microinjections of GPER agonists into the NA induce a bradycardia (22), suggesting that estrogen might work through multiple different receptor types to influence parasympathetic control of heart rate. Central estrogen administration, however, also decreases sympathetic tone (96, 97). With the use of comparisons between male and female rats, females have shown reduced sympathetic nerve activation after experimentally induced hypertension (120), and this protective effect in females can be abolished by knockdown of ERβ within the RVLM (107, 122). Taken together, these data indicate that estrogen receptors modulate autonomic responses via brain-stem circuitry through a complex interaction between multiple receptor types and different cellular locations, rather than just direct effects on afferent terminals and/or their synaptic connections within the NTS.
Despite evidence implicating estrogen in the modulation of autonomic responses, only a limited number of studies have been done to determine the direct mechanistic actions of estrogen on autonomic neuronal excitability. In nonautonomic brain regions, estrogen typically activates L-type voltage-gated calcium channels, resulting in an increase in neuronal excitability (100). With the use of comparisons between males and females in different ovarian cycle stages, estrogen has been suggested to inhibit DMV motor neuron firing (56), and direct estrogen application also inhibited the firing frequency of unidentified NTS neurons (121). A similar decrease in excitability exists after estrogen application in the RVLM through a reduction in L-type calcium channel number (115), whereas sex differences in sympathetic nerve activity after the induction of experimental hypertension (120) have been suggested to be mediated through sex differences in the postsynaptic membrane expression of excitatory glutamatergic receptors (113). Whether the mechanism(s) of action is on L-type calcium channels or glutamate receptors (or both), estrogen likely decreases RVLM neuron activity. Conversely, GPER activation depolarizes cardiac vagal motor neurons within the NA (22), and differential effects of estrogen receptors on calcium responses have been shown in area postrema (83). These data together suggest that the brain stem might be unique in estrogen mechanism(s) of action and/or in its number of nuclei in which estrogen reduces neuronal excitability. Moreover, the autonomic brain stem contains considerable cell-type-specific estrogen actions.
Central Progesterone Receptors in Autonomic Physiology
Although progesterone receptors were first discovered in the brain in 1973 (99), the direct action of progesterone receptors in neuronal signaling plasticity is significantly understudied compared with estrogen. Traditionally, transcriptional actions of progesterone occur through progesterone receptors with two isoforms (PR-A and PR-B) that are derived from distinct promotor regions of a single gene (29, 60). However, similar to estrogen, progesterone also has rapid, gene expression-independent signaling actions. Several diverse receptor types have been suggested to mediate the rapid, nontranscriptional actions of progesterone. Some of these include unique membrane-bound progesterone receptors (mPR; Ref. 123), classic PR-A/B receptors that also activate intracellular signaling pathways, and even progesterone binding directly to oxytocin receptors (46). PR-A/B receptors are expressed in several brain-stem regions, including the NTS and DMV (40, 61). Although, to our knowledge, no investigations have looked for their presence in the brain stem, mPRs have been identified in at least the hypothalamus (6). Given the diverse range of putative mediators of progesterone’s nontranscriptional actions, the suggested intercellular signaling cascades are equally diverse. However, significant evidence exists that mPRs ultimately converge on intracellular calcium stores for short-term effects (6). Although these effects remain to be studied in the brain stem, the ability of progesterone receptors to mobilize calcium in other tissues does suggest an ability to modulate neuronal activity (103).
A role for progesterone in the brain stem has not been shown for modulation of cardiovascular function, but progesterone appears to regulate brain-stem control of respiratory function. The importance of the progesterone in respiratory function was first identified when progesterone microinjection into the NTS produced significant, dose-dependent increases in respiratory frequency that were blocked when progesterone receptors were antagonized (10). However, the neuronal substrate for this progesterone-receptor-dependent hyperventilation remains unclear and likely is state-dependent. For example, progesterone did not affect NTS neuronal discharge under normal conditions (112) but did blunt NTS neuronal responses to hypoxia (85). In addition, since the magnitude of hyperventilation is increased in the presence of estrogen, suggesting that estrogen can increase the gain of progesterone-induced hyperventilation (23, 55). Since respiratory centers in the brain stem contribute to cardiorespiratory coupling (20, 80), it is likely that progesterone modulates cardiovascular autonomic regulation in some form through its effects on brain-stem circuitry. Additionally, given the importance of oxytocin receptor signaling in the dorsal vagal complex (87, 88), the ability of progesterone receptors to translocate oxytocin receptors to the membrane in the hypothalamus remains a potential mechanism of action of progesterone receptors in the brain stem (102).
Central Allopregnanolone in Autonomic Physiology
Progesterone has also been implicated in an additional type of neuromodulation through its catalyzation by 5α-reductase into the neurosteroid, allopregnanolone (39, 69). Traditionally, neurosteroids are considered positive allosteric modulators of the γ-aminobutyric-A (GABAA) receptors responsible for fast inhibitory neurotransmission (39), and their actions on GABAA receptor signaling have been established in several regions of the brain, including the hypothalamus (38). Many autonomic brain-stem nuclei, including the NA (14), NTS (42, 45), DMV (7, 31, 43), and RVLM (30, 63), are tightly regulated by GABAA receptor-mediated inhibition, and allopregnanolone is known to concentrate in the brain stem in both males and females (110). Allopregnanolone increases inhibition of hippocampal neurons across the estrous cycle and during pregnancy (68, 70, 119), and similar changes in GABAA receptor inhibition have been demonstrated in the brain stem, at least for the DMV (65). The effects of allopregnanolone on respiratory circuits have been confirmed through GABAA receptors (92). Therefore, these endogenous modulators of GABAergic inhibition may play a critical role in regulating both autonomic nervous system function and dysfunction.
In addition to identifying a progesterone-receptor-dependent facilitation of respiratory drive, the seminal work by Bayliss et al. (10) also identified a significant drop in blood pressure that was not eliminated by progesterone receptor antagonism. Follow-up investigations have since suggested that these effects are mediated not by progesterone receptor activation but through progesterone’s catalyzation to allopregnanolone (71). This allopregnanolone-induced decrease in blood pressure associates with a blunted baroreflex response (54, 71) through a brain-stem pathway (54). Since exogenous allopregnanolone application attenuates vagal afferent transmission in NTS neurons through GABAA receptor activation, which results in decreased afferent-stimulation-induced firing within NTS neurons (62), allopregnanolone-mediated inhibition of afferent signaling is a potential mechanism for allopregnanolone’s blunting of the baroreflex. In addition to direct effects on GABAA receptors, 5α-reductase-dependent neurosteroids increase membrane insertion of GABAA receptors (1). Although this has not been confirmed as a functional change resulting from neurosteroid activity, a similar increase in membrane insertion of GABAA receptors has been proposed for diabetes-induced plasticity of GABAergic neurotransmission in DMV neurons (17). Allopregnanolone may also exert opposing effects on postsynaptic GABAA receptor activity depending on extracellular GABA concentration (48), suggesting that further work is needed to determine the complex interplay between allopregnanolone and GABAA receptors in autonomic circuits.
Although allopregnanolone readily and rapidly crosses the blood-brain barrier when injected into the systematic circulation (58), evidence also exists for its local production within the brain (39). The notion of locally produced allopregnanolone is supported by the observation that a progesterone-mediated decrease in blood pressure was not blocked by the antagonism of progesterone since this experiment would require the conversion of progesterone to allopregnanolone locally (10). Together, these data support the NTS as a likely location of progesterone conversion to allopregnanolone. Although we do not currently know the exact location of 5α-reductase activity within the brain stem, there is a compelling case for the role of allopregnanolone in the regulation of at least parasympathetic nervous system output and reflex responses.
CONCLUSIONS
The new National Institute of Health's emphasis on understanding sex as a biological variable has promoted the need to understand its role in autonomic control. Despite abundant evidence that sex plays a role in signal processing within autonomic brain-stem circuits, considerable research must be done to further our understanding of mechanism(s) of action for regulating many aspects of physiology, including neural excitability of autonomic brain circuitry. Given the prominent sex differences in physiological responses and diseases that are regulated by the autonomic nervous system, these types of investigations are critical to our development of therapeutics for diseases, including those associated with the cardiovascular and metabolic system. In terms of effects of major sex steroids, estrogen, progesterone, and the progesterone derivative allopregnanolone are implicated in the regulation of neural communication within the autonomic brain stem. Specifically, current evidence suggests that estrogen promotes vagally mediated responses (i.e., satiation and baroreflex) while restraining sympathetic drive. It does this through a wide array of effects on neuronal excitability within individual autonomic brain-stem regions, which likely depend on whether a region promotes or inhibits vagal responses. Progesterone, on the other hand, has received limited attention, making it difficult to assess its role in overall autonomic drive. However, progesterone receptors are present within key autonomic nuclei and, therefore, do likely alter intracellular calcium flux, leading to changes in neuronal responsivity. Exciting new data also point to a potential role for allopregnanolone to influence the activity of autonomic circuits, likely through GABAA receptor function. Modulation of allopregnanolone within the NTS results in transient hypotension, suggesting a role of neurosteroids in the regulation of blood pressure that could include the maintenance of critical inhibitory signaling within the dorsal vagal complex. However, our understanding of the role of sex steroids in autonomic circuits is still limited, and much more work must be done.
Perspectives and Significance
In summary, this review aims to discuss our understanding of the role sex hormones play in autonomic brain-stem circuits. Sex differences exist in a wide array of the autonomic nervous system’s regulatory function, including the control of cardiovascular, gastrointestinal, and metabolic systems. Although our understanding of mechanism(s) is limited, both estrogen and progesterone play a role in establishing these sex differences, and their exact role in altered neuronal excitability is specific to each autonomic brain region. This review also suggests a novel contributor to the hormone milieu, allopregnanolone. Taken together, significantly more research must be done to elucidate how sex hormones influence autonomic circuities. These studies should continue to include single-hormone replacement but expand to include genetic models and understudied hormones like progesterone and allopregnanolone.
GRANTS
This work was supported by American Heart Association Scientist Development Grant 16SDG26590000 to C. R. Boychuk. This work was also supported by the NIH Jointly Sponsored Predoctoral Training Program in the Neurosciences Training Grant T32 NS082145 (to S. Fedorchak) and NIH-sponsored Cardiovascular Training in Texas Training Grant T32 HL007446 (to E. L. Littlejohn).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.L.L., S.F., and C.R.B. prepared figures; E.L.L., S.F., and C.R.B. drafted manuscript; E.L.L., S.F., and C.R.B. edited and revised manuscript; E.L.L., S.F., and C.R.B. approved final version of manuscript.
REFERENCES
- 1.Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci USA 111: 7132–7137, 2014. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30: 1–9, 2009. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7: 413–442, 1984. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 4.Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148: 5656–5666, 2007. doi: 10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- 6.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 147: 4151–4159, 2006. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 7.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol 570: 469–484, 2006. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayliss DA, Millhorn DE, Gallman EA, Cidlowski JA. Progesterone stimulates respiration through a central nervous system steroid receptor-mediated mechanism in cat. Proc Natl Acad Sci USA 84: 7788–7792, 1987. doi: 10.1073/pnas.84.21.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 12.Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ 7: 34, 2016. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake CB, Smith BN. Insulin reduces excitation in gastric-related neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol 303: R807–R814, 2012. doi: 10.1152/ajpregu.00276.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABAA receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J Neurophysiol 96: 3266–3272, 2006. doi: 10.1152/jn.00590.2006. [DOI] [PubMed] [Google Scholar]
- 15.Boychuk CR, Fuller DD, Hayward LF. Sex differences in heart rate variability during sleep following prenatal nicotine exposure in rat pups. Behav Brain Res 219: 82–91, 2011. doi: 10.1016/j.bbr.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Boychuk CR, Gyarmati P, Xu H, Smith BN. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarii. J Neurophysiol 114: 999–1007, 2015. doi: 10.1152/jn.00310.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boychuk CR, Halmos KC, Smith BN. Diabetes induces GABA receptor plasticity in murine vagal motor neurons. J Neurophysiol 114: 698–706, 2015. doi: 10.1152/jn.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boychuk CR, Hayward LF. Prenatal nicotine exposure alters postnatal cardiorespiratory integration in young male but not female rats. Exp Neurol 232: 212–221, 2011. doi: 10.1016/j.expneurol.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boychuk CR, Smith KC, Peterson LE, Boychuk JA, Butler CR, Derera ID, McCarthy JJ, Smith BN. A hindbrain inhibitory microcircuit mediates vagally-coordinated glucose regulation. Sci Rep 9: 2722, 2019. doi: 10.1038/s41598-019-39490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boychuk CR, Woerman AL, Mendelowitz D. Modulation of bulbospinal rostral ventral lateral medulla neurons by hypoxia/hypercapnia but not medullary respiratory activity. Hypertension 60: 1491–1497, 2012. doi: 10.1161/HYPERTENSIONAHA.112.197954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193: 311–321, 2007. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 22.Brailoiu GC, Arterburn JB, Oprea TI, Chitravanshi VC, Brailoiu E. Bradycardic effects mediated by activation of G protein-coupled estrogen receptor in rat nucleus ambiguus. Exp Physiol 98: 679–691, 2013. doi: 10.1113/expphysiol.2012.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodeur P, Mockus M, McCullough R, Moore LG. Progesterone receptors and ventilatory stimulation by progestin. J Appl Physiol (1985) 60: 590–595, 1986. doi: 10.1152/jappl.1986.60.2.590. [DOI] [PubMed] [Google Scholar]
- 24.Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil 21: 1309–e126, 2009. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Z, Zhang H, Yu J, Wurster RD, Gozal D. Attenuation of baroreflex sensitivity after domoic acid lesion of the nucleus ambiguus of rats. J Appl Physiol (1985) 96: 1137–1145, 2004. doi: 10.1152/japplphysiol.00391.2003. [DOI] [PubMed] [Google Scholar]
- 26.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494–498, 2005. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 27.Ciriello J, Caverson MM. Effect of estrogen on vagal afferent projections to the brainstem in the female. Brain Res 1636: 21–42, 2016. doi: 10.1016/j.brainres.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006. [Erratum in Diabetes 56: 2649, 2007.] doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 29.Conneely OM, Kettelberger DM, Tsai MJ, Schrader WT, O’Malley BW. The chicken progesterone receptor A and B isoforms are products of an alternate translation initiation event. J Biol Chem 264: 14062–14064, 1989. [PubMed] [Google Scholar]
- 30.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 31.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017: 208–217, 2004. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis SN, Shavers C, Costa F. Gender-related differences in counterregulatory responses to antecedent hypoglycemia in normal humans. J Clin Endocrinol Metab 85: 2148–2157, 2000. doi: 10.1210/jc.85.6.2148. [DOI] [PubMed] [Google Scholar]
- 32a.The DCCT Research Group Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 90: 450–459, 1991. doi: 10.1016/0002-9343(91)90605-W. [DOI] [PubMed] [Google Scholar]
- 33.De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145: 1063–1068, 2004. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 34.Dergacheva O. Chronic intermittent hypoxia alters neurotransmission from lateral paragigantocellular nucleus to parasympathetic cardiac neurons in the brain stem. J Neurophysiol 113: 380–389, 2015. doi: 10.1152/jn.00302.2014. [DOI] [PubMed] [Google Scholar]
- 35.Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol 592: 2799–2811, 2014. doi: 10.1113/jphysiol.2014.273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav 82: 35–41, 2004. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 281: R738–R746, 2001. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- 38.Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci 20: 3067–3075, 2000. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferando I, Mody I. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia 53, Suppl 9: 89–101, 2012. doi: 10.1111/epi.12038. [DOI] [PubMed] [Google Scholar]
- 40.Francis K, Meddle SL, Bishop VR, Russell JA. Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res 927: 18–26, 2002. doi: 10.1016/S0006-8993(01)03318-2. [DOI] [PubMed] [Google Scholar]
- 41.Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol 101: 1755–1760, 2009. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D, Smith BN. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res 1291: 40–52, 2009. doi: 10.1016/j.brainres.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904–914, 2010. doi: 10.1152/jn.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142: 4751–4757, 2001. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 45.Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol 464: 525–539, 2003. doi: 10.1002/cne.10831. [DOI] [PubMed] [Google Scholar]
- 46.Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 392: 509–512, 1998. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 47.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 259: R1131–R1138, 1990. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 48.Haage D, Johansson S. Neurosteroid modulation of synaptic and GABA-evoked currents in neurons from the rat medial preoptic nucleus. J Neurophysiol 82: 143–151, 1999. doi: 10.1152/jn.1999.82.1.143. [DOI] [PubMed] [Google Scholar]
- 49.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev 28: 726–741, 2007. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 50.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Havel PJ, Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 46: 801–807, 1997. doi: 10.2337/diab.46.5.801. [DOI] [PubMed] [Google Scholar]
- 52.Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond) 130: 9–18, 2016. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- 53.Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology 140: 3255–3263, 1999. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- 54.Heesch CM. Neurosteroid modulation of arterial baroreflex function in the rostral ventrolateral medulla. Auton Neurosci 161: 28–33, 2011. doi: 10.1016/j.autneu.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Hohimer AR, Hart MV, Resko JA. The effect of castration and sex steroids on ventilatory control in male guinea pigs. Respir Physiol 61: 383–390, 1985. doi: 10.1016/0034-5687(85)90080-5. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, Babic T, Travagli RA. Sex differences in GABAergic neurotransmission to rat DMV neurons. Am J Physiol Gastrointest Liver Physiol 317: G476–G483, 2019. doi: 10.1152/ajpgi.00112.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Y, Greenwood-Van Meerveld B, Johnson AC, Travagli RA. Role of estrogen and stress on the brain-gut axis. Am J Physiol Gastrointest Liver Physiol 317: G203–G209, 2019. doi: 10.1152/ajpgi.00144.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson IM, Birzniece V, Lindblad C, Olsson T, Bäckström T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res 934: 125–131, 2002. doi: 10.1016/S0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- 59.Karlsson S, Scheurink AJ, Ahrén B. Gender difference in the glucagon response to glucopenic stress in mice. Am J Physiol Regul Integr Comp Physiol 282: R281–R288, 2002. doi: 10.1152/ajpregu.2002.282.1.R281. [DOI] [PubMed] [Google Scholar]
- 60.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9: 1603–1614, 1990. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kastrup Y, Hallbeck M, Amandusson A, Hirata S, Hermanson O, Blomqvist A. Progesterone receptor expression in the brainstem of the female rat. Neurosci Lett 275: 85–88, 1999. doi: 10.1016/S0304-3940(99)00753-3. [DOI] [PubMed] [Google Scholar]
- 62.Kim S, Kim SM, Oh B, Tak J, Yang E, Jin YH. Allopregnanolone effects on transmission in the brain stem solitary tract nucleus (NTS). Neuroscience 379: 219–227, 2018. doi: 10.1016/j.neuroscience.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 63.Kubo T, Kihara M. Studies on GABAergic mechanisms responsible for cardiovascular regulation in the rostral ventrolateral medulla of the rat. Arch Int Pharmacodyn Ther 285: 277–287, 1987. [PubMed] [Google Scholar]
- 64.Lewin AE, Vicini S, Richardson J, Dretchen KL, Gillis RA, Sahibzada N. Optogenetic and pharmacological evidence that somatostatin-GABA neurons are important regulators of parasympathetic outflow to the stomach. J Physiol 594: 2661–2679, 2016. doi: 10.1113/JP272069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Littlejohn EL, Espinoza L, Lopez MM, Smith BN, Boychuk CR. GABAA receptor currents in the dorsal motor nucleus of the vagus in females: influence of ovarian cycle and 5α-reductase inhibition. J Neurophysiol 122: 2130–2141, 2019. doi: 10.1152/jn.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature 274: 276–278, 1978. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 67.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science 211: 1294–1302, 1981. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 68.Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 29: 9592–9601, 2009. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maguire J, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology 34, Suppl 1: S84–S90, 2009. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8: 797–804, 2005. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 71.Masilamani S, Heesch CM. Effects of pregnancy and progesterone metabolites on arterial baroreflex in conscious rats. Am J Physiol Regul Integr Comp Physiol 272: R924–R934, 1997. doi: 10.1152/ajpregu.1997.272.3.R924. [DOI] [PubMed] [Google Scholar]
- 72.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav 187: 20–23, 2018. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6: 14, 2015. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab 25: 1216–1230, 2017. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett 132: 217–221, 1991. doi: 10.1016/0304-3940(91)90305-D. [DOI] [PubMed] [Google Scholar]
- 76.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol 473: 270–291, 2004. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 77.Milner TA, Drake CT, Lessard A, Waters EM, Torres-Reveron A, Graustein B, Mitterling K, Frys K, Iadecola C. Angiotensin II-induced hypertension differentially affects estrogen and progestin receptors in central autonomic regulatory areas of female rats. Exp Neurol 212: 393–406, 2008. doi: 10.1016/j.expneurol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology 149: 3306–3312, 2008. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 124: 2145–2154, 2011. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res 93: 565–572, 2003. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- 81.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 81: 1535–1565, 2001. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 82.Orts-Del’Immagine A, Wanaverbecq N, Tardivel C, Tillement V, Dallaporta M, Trouslard J. Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. J Physiol 590: 3719–3741, 2012. doi: 10.1113/jphysiol.2012.227959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pamidimukkala J, Hay M. 17β-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol 285: H1515–H1520, 2003. doi: 10.1152/ajpheart.00174.2003. [DOI] [PubMed] [Google Scholar]
- 84.Park JH, Burns-Cusato M, Dominguez-Salazar E, Riggan A, Shetty S, Arnold AP, Rissman EF. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav 7: 609–617, 2008. doi: 10.1111/j.1601-183X.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pascual O, Morin-Surun MP, Barna B, Denavit-Saubié M, Pequignot JM, Champagnat J. Progesterone reverses the neuronal responses to hypoxia in rat nucleus tractus solitarius in vitro. J Physiol 544: 511–520, 2002. doi: 10.1113/jphysiol.2002.023994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history. A life course analysis of the original Framingham Heart Study cohort. Eur Heart J 23: 458–466, 2002. doi: 10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- 87.Piñol RA, Bateman R, Mendelowitz D. Optogenetic approaches to characterize the long-range synaptic pathways from the hypothalamus to brain stem autonomic nuclei. J Neurosci Methods 210: 238–246, 2012. doi: 10.1016/j.jneumeth.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piñol RA, Jameson H, Popratiloff A, Lee NH, Mendelowitz D. Visualization of oxytocin release that mediates paired pulse facilitation in hypothalamic pathways to brainstem autonomic neurons. PLoS One 9: e112138, 2014. doi: 10.1371/journal.pone.0112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7: 715–726, 2011. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qiao GF, Li BY, Lu YJ, Fu YL, Schild JH. 17β-Estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am J Physiol Cell Physiol 297: C654–C664, 2009. doi: 10.1152/ajpcell.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97: 1–37, 2017. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 92.Ren J, Greer JJ. Neurosteroid modulation of respiratory rhythm in rats during the perinatal period. J Physiol 574: 535–546, 2006. doi: 10.1113/jphysiol.2006.108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reyes AD. A breakthrough method that became vital to neuroscience. Nature 575: 38–39, 2019. doi: 10.1038/d41586-019-02836-6. [DOI] [PubMed] [Google Scholar]
- 94.Rinaman L, Card JP, Schwaber JS, Miselis RR. Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J Neurosci 9: 1985–1996, 1989. doi: 10.1523/JNEUROSCI.09-06-01985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts BL, Zhu M, Zhao H, Dillon C, Appleyard SM. High glucose increases action potential firing of catecholamine neurons in the nucleus of the solitary tract by increasing spontaneous glutamate inputs. Am J Physiol Regul Integr Comp Physiol 313: R229–R239, 2017. doi: 10.1152/ajpregu.00413.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res 879: 105–114, 2000. doi: 10.1016/S0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- 97.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17β-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci 84: 78–88, 2000. doi: 10.1016/S1566-0702(00)00196-X. [DOI] [PubMed] [Google Scholar]
- 98.Sandoval DA, Ertl AC, Richardson MA, Tate DB, Davis SN. Estrogen blunts neuroendocrine and metabolic responses to hypoglycemia. Diabetes 52: 1749–1755, 2003. doi: 10.2337/diabetes.52.7.1749. [DOI] [PubMed] [Google Scholar]
- 99.Sar M, Stumpf WE. Neurons of the hypothalamus concentrate [3H]progesterone or its metabolites. Science 182: 1266–1268, 1973. doi: 10.1126/science.182.4118.1266. [DOI] [PubMed] [Google Scholar]
- 100.Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA 105: 15148–15153, 2008. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123: 89–100, 2006. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 102.Schumacher M, Coirini H, Frankfurt M, McEwen BS. Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci USA 86: 6798–6801, 1989. doi: 10.1073/pnas.86.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol 113: 6–39, 2014. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Shen L, Liu Y, Tso P, Wang DQ, Davidson WS, Woods SC, Liu M. Silencing steroid receptor coactivator-1 in the nucleus of the solitary tract reduces estrogenic effects on feeding and apolipoprotein A-IV expression. J Biol Chem 293: 2091–2101, 2018. doi: 10.1074/jbc.RA117.000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen L, Liu Y, Wang DQ, Tso P, Woods SC, Liu M. Estradiol stimulates apolipoprotein A-IV gene expression in the nucleus of the solitary tract through estrogen receptor-α. Endocrinology 155: 3882–3890, 2014. doi: 10.1210/en.2014-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen L, Wang DQ, Lo CM, Tso P, Davidson WS, Woods SC, Liu M. Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology 151: 3163–3168, 2010. doi: 10.1210/en.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shih CD. Activation of estrogen receptor β-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci 16: 60, 2009. doi: 10.1186/1423-0127-16-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294: 76–95, 1990. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 109.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat 38: 185–196, 2009. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 110.Sze Y, Gill AC, Brunton PJ. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J Neuroendocrinol 30: e12644, 2018. doi: 10.1111/jne.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 149: 1609–1617, 2008. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tolchard S, Ingram CD. Electrophysiological actions of oxytocin in the dorsal vagal complex of the female rat in vitro: changing responsiveness during the oestrous cycle and after steroid treatment. Brain Res 609: 21–28, 1993. doi: 10.1016/0006-8993(93)90849-I. [DOI] [PubMed] [Google Scholar]
- 113.Van Kempen TA, Dodos M, Woods C, Marques-Lopes J, Justice NJ, Iadecola C, Pickel VM, Glass MJ, Milner TA. Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension. Neuroscience 307: 83–97, 2015. doi: 10.1016/j.neuroscience.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.VanderHorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-α and -β immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488: 152–179, 2005. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 115.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res 1094: 163–178, 2006. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Piñol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem α1 and β1 receptors. J Neurosci 34: 6182–6189, 2014. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106: 306–316, 1980. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 118.Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148: 1868–1881, 2007. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther 346: 146–160, 2013. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xue B, Badaue-Passos D Jr, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17β-estradiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol 296: H1577–H1585, 2009. doi: 10.1152/ajpheart.01255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xue B, Hay M. 17β-Estradiol inhibits excitatory amino acid-induced activity of neurons of the nucleus tractus solitarius. Brain Res 976: 41–52, 2003. doi: 10.1016/S0006-8993(03)02629-5. [DOI] [PubMed] [Google Scholar]
- 122.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-β in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension 61: 1255–1262, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100: 2237–2242, 2003. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]