Abstract
Cerebral blood flow (CBF) is commonly inferred from blood velocity measurements in the middle cerebral artery (MCA), using nonimaging, transcranial Doppler ultrasound (TCD). However, both blood velocity and vessel diameter are critical components required to accurately determine blood flow, and there is mounting evidence that the MCA is vasoactive. Therefore, the aim of this study was to employ imaging TCD (ITCD), utilizing color flow images and pulse wave velocity, as a novel approach to measure both MCA diameter and blood velocity to accurately quantify changes in MCA blood flow. ITCD was performed at rest in 13 healthy participants (7 men/6 women; 28 ± 5 yr) with pharmaceutically induced vasodilation [nitroglycerin (NTG), 0.8 mg] and without (CON). Measurements were taken for 2 min before and for 5 min following NTG or sham delivery (CON). There was more than a fivefold, significant, fall in MCA blood velocity in response to NTG (∆−4.95 ± 4.6 cm/s) compared to negligible fluctuation in CON (∆−0.88 ± 4.7 cm/s) (P < 0.001). MCA diameter increased significantly in response to NTG (∆0.09 ± 0.04 cm) compared with the basal variation in CON (∆0.00 ± 0.04 cm) (P = 0.018). Interestingly, the product of the NTG-induced fall in MCA blood velocity and increase in diameter was a significant increase in MCA blood flow following NTG (∆144 ± 159 ml/min) compared with CON (∆−5 ± 130 ml/min) (P = 0.005). These juxtaposed findings highlight the importance of measuring both MCA blood velocity and diameter when assessing CBF and document ITCD as a novel approach to achieve this goal.
Keywords: cerebral blood flow, MCA, nitroglycerin, transcranial Doppler ultrasound
INTRODUCTION
A consistent blood flow is critical for normal cerebral function due to the brain’s unique inability to store energy coupled with a high metabolic demand. Cerebral blood flow (CBF) is regulated by both systemic and local mechanisms, both of which can be altered with aging and disease (4, 23, 25). Transcranial Doppler ultrasound (TCD) has long been established as a noninvasive method to assess and better advance our knowledge of cerebrovascular function (1, 32, 37). TCD utilizes the difference between emitted ultrasound waves and reflected waves from moving red blood cells within intracerebral vessels, known as the Doppler shift, to determine blood velocity (13). Such a Doppler approach only provides pulse-wave velocity without brightness (B-mode) imaging and therefore can determine blood velocity but does not provide any structural information. In contrast, duplex ultrasound of peripheral vessels facilitates both two-dimensional B-mode and pulse-wave velocity simultaneously to obtain both blood velocity and vessel diameter, facilitating the calculation of blood flow. However, currently, traditional duplex ultrasound cannot be employed in the brain due to interference from the skull.
Cerebral perfusion is commonly assessed by insonation of the middle cerebral arteries (MCAs) through the temporal window where the skull is the thinnest in adults. Indeed, the MCAs are the terminal branches of the internal carotid arteries, supplying 80% of the blood flow to the brain (23). Importantly, for intracranial arteries, typically the MCAs, TCD is often the method of choice, as even this thin area of the skull prohibits the utilization of duplex ultrasound and image-forming insonation of the vessel walls (B-mode). However, when using nonimaging TCD, blood velocity can only be a surrogate measure of blood flow if the assumption that the MCA is not vasoactive holds true. In contrast, imaging transcranial Doppler (ITCD), combining blood velocity measurements and color flow imaging, to visualize and assess diameter of the large intracranial vessels, can, potentially, facilitate the actual assessment of blood flow in the brain. Indeed, a limited number of studies in animals and humans (31, 38) have utilized color flow, with on-screen calipers, to measure the diameter of the MCA.
Recently, there has been a debate as to the impact of vasoactive stimuli on MCA diameter (7, 15). However, when studies were examined that have combined magnetic resonance imaging (MRI) to assess the cross-sectional area of the MCA and TCD, to measure changes in MCA velocity as consequence of altered blood gasses or blood pressure, there is clear evidence of an evolution in results. Specifically, the majority of such older studies, which are commonly cited as an indication of no change in MCA diameter, have significant experimental design limitations or employed lower resolution MRI (1.5 T) (14, 28, 33). Later MRI findings with improved field strength (i.e., 3 and 7 T), and the improved spatial resolution that this affords, document measurable changes in MCA diameter as a result of vasoactive stimuli (3, 11, 27, 35, 36). Interestingly, despite mounting evidence that the MCA is vasoactive, TCD is still routinely used to measure MCA velocity as a surrogate for CBF, without considering the impact of changes in diameter on actual blood flow or a simplistic one-line acknowledgment of the limitation of this approach.
Therefore, in this study, we employed a two-pronged approach to both improve upon the Doppler ultrasound method of assessing MCA diameter and blood velocity and, ultimately, determine MCA blood flow, as well as advance our understanding of the interrelationships between these variables. To achieve these aims, first, in the periphery, we validated ITCD, as a novel method to measure vessel diameter, which, in combination with blood velocity, can facilitate the quantification of MCA blood flow. Second, we utilized ITCD in conjunction with pharmaceutically induced vasodilation to demonstrate that by only measuring blood velocity in the MCA without a concomitant assessment of diameter would likely result in an inaccurate evaluation of MCA blood flow.
METHODS
Participants
A total of 23 young, healthy individuals volunteered to participate in this investigation, with 10 (9 men and 1 women) partaking in the validation of vessel diameter with color flow images protocol and 13 (7 men and 6 women) being assessed in the main MCA measurement protocol with and without nitroglycerin (NTG). All participants were, nonsmokers, normal weight, normotensive, and free of cardiovascular, metabolic, and neurological disorders. Before testing, protocol approval and written informed consent were obtained according to the University of Utah and Salt Lake City Veterans Affairs Medical Center (VAMC) Institutional Review Boards, in accordance with the principles outlined in the Declaration of Helsinki, except for registration in a database. Data collection took place at the Utah Vascular Research Laboratory located in the Salt Lake City VAMC’s Geriatric Research, Education, and Clinical Center. All participants refrained from both food and caffeine for at least 4 h and had not performed any exercise within the past 24 h.
Validation of Vessel Diameter Assessed With Color Flow Images
To verify that color flow imaging assessments of diameter reflect the more traditional B-mode assessment, in which the artery wall is clearly visible, we measured vessel diameter of the upper brachial, lower brachial, and radial arteries in 10 participants in both B-mode and with color flow images. Specifically, upon arrival to the laboratory participants assumed a supine position for the ultrasound measurements and rested for ~20 min in a dimly lit, temperature-controlled room. All data were collected, during one testing session, with the proximal and distal brachial artery and radial artery diameters recorded by Doppler Ultrasound (Logiq 7 and E9 XDclear, GE), using a linear array transducer probe (ML6-15) at an imaging frequency of 10 MHz and an insonation angle of ≤60°. Imaging frequencies and gain were optimized for vessel wall identification, and all diameter measurements were taken at peak r-wave velocity (systole). Color flow images were collected in these same vascular sites with the B-mode gain turned to zero.
MCA Assessment With and Without Pharmaceutically Induced Vasodilation
Upon arrival to the laboratory height and weight were measured and seated resting blood pressures were taken. Participants were subsequently moved to a supine position for all ultrasound measurements and baseline central hemodynamic assessments and rested for ~20 min in a dimly lit, temperature-controlled room. Data were collected during one testing session with two testing conditions: 1) CON and 2) pharmaceutically induced vasodilation (NTG). Condition orders were not randomized, and the CON condition always preceded NTG. However, participants were unaware of the order. A sham delivery was employed for the CON condition, where a laboratory assistant asked the participant to open his or her mouth in expectation of either receiving sublingual NTG tabs (2 tabs 0.4 mg Nitrostat; 0.8 mg total) or the investigator going through the same motion but without the drug. Of note, NTG has been documented in both animals and humans to significantly dilate the MCAs (12, 18, 27, 29), and this dose of NTG was based upon prior work by our group as it produces a robust dilation and facilitates the assessment of endothelial independent vasodilation in the periphery (8).
The CON condition was used as a time-matched control to account for basal variations over the same time course as the NTG condition. In each condition hemodynamics were measured continuously. Following the establishment of stable variables, for both CON and NTG, Doppler ultrasound measurements were taken for 2 min to determine a baseline and 5 min following.
Measurements During MCA Assessment With and Without NTG Protocol
MCA diameter, blood velocity, and blood flow.
Doppler ultrasound (Logiq E9 XDclear, GE) measurements of the MCA were performed, through the right transtemporal window at the M1 segment, with a matrix phased array transducer probe (M5S-D) operating in transcranial color flow mode, at an imaging frequency of 1.9 MHz. With the use of the transtemporal approach, the MCA was visualized by utilizing color-coded transcranial duplex sonography in the coronal anterior plane. Specifically, after the Circle of Willis was visualized, based on the branching of the anterior cerebral artery from the internal carotid artery, the M1 segment of the MCA was identified for interrogation (26). Then, with the use of anatomical motion (m)-mode, the diameter of this portion of the MCA was determined at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution could be achieved, and the central axis diameter was continuously recorded on the graphic motion display. This m-mode approach facilitates extra high-density images (304 pixels per inch), with a pixel size of 0.083 mm. The blood velocity was obtained using the same transducer with an imaging frequency of 3.6 MHz, operated at a high-pulsed repetition frequency mode (2–25 kHz) with a depth of 4–8 cm. Of note, pilot work revealed that the simultaneous assessment of both MCAs, with two separate Doppler ultrasound systems, was not possible due to signal interference between the two probes. These assessments were performed in sequence, with color flow images collected for 20 s, followed immediately by velocity measurements, for 12 s. This sequence of assessments was repeated during both the 2 min baseline recordings and following CON and NTG administration for 5 min. Diameters were assessed by a trained investigator, blinded to the condition. Four MCA diameter measurements, aligned with the ECG r-wave, were performed in each 20 s clip (1 diameter per 5 s) and averaged. MCA blood flow was calculated with these serial measurements using MCA diameter and mean velocity (Vmean): MCA blood flow (mL/min) = Vmean · π (vessel diameter/2)2 · 60. For each participant, peak NTG-induced MCA diameter was identified, and the corresponding blood velocity was used to calculate MCA blood flow at the maximum diameter. For the CON peak assessments, the same time points as those identified as the NTG peaks were selected. During each condition the probe position was stable, with the sample volume positioned in the center of the vessel and adjusted to cover the full width of the artery. The baseline data in the CON and NTG conditions were used to determine coefficients of variation (CV) for MCA diameter, blood velocity, and blood flow. Furthermore, a between investigator CV for the analysis of NTG-induced MCA diameter was calculated from two investigators reproducing the analysis in a blinded fashion (n = 3).
Central hemodynamics.
After anthropometric data were entered and calibrated to brachial blood pressure, heart rate (HR) and mean arterial pressure (MAP) were measured, and stroke volume (SV) and cardiac output (CO) were estimated with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). Specifically, beat-by-beat pressure waveforms assessed by photoplethysmography, from a finger cuff, were used to estimate SV, using the Modelflow method (Beatscope, Finapres Medical Systems), and in combination with HR were used to calculate CO. All Finapres variables were acquired at 200 Hz via a data acquisition system (AcqKnowledge, Biopac Systems, Goleta, CA).
Statistical Analysis
All statistical procedures were performed using SPSS (SPSS 22, Chicago, IL). Data were tested for normality and homogeneity using Shapiro-Wilk test and normal Q-Q plots. A linear regression was used to evaluate the relationship between vessel diameter assessed in B-mode and vessel diameter assessed with color flow images. Additionally, a Bland-Altman plot was used to examine agreement in diameter measurements between standard B-mode and color flow in peripheral radial and brachial arteries (6). Limits of agreement were established as 2 SD from the mean difference. Overestimation or underestimation of the mean diameter by both measurement methods was considered significant if the 95% confidence intervals (95% CIs) did not include 0. A linear regression of the mean difference between the two methods was conducted to statistically test for proportional bias. Differences in MAP were compared using repeated measures ANOVA. Delta MCA diameter, delta velocity, and delta blood flow were compared using paired sample t tests. Statistical significance was set at P < 0.05. Data are presented as means ± SD unless otherwise noted.
RESULTS
Comparison of Vessel Diameter Assessed with Both B-Mode and Color Flow Images
Vessel diameter assessed with both color flow imaging and B-mode ultrasound in the brachial, and radial arteries ranged from ~0.2–0.5 cm and were not significantly different from each other (P = 0.41). The values for vessel diameter attained by these two approaches exhibited excellent agreement and were, therefore, strongly related (r2 = 0.97, P < 0.001, y = 0.00 + 1.05 × x ± 0.02) (Fig. 1A). Furthermore, the Bland-Altman technique revealed no systematic bias between the color flow and B-mode-measured diameters in the radial and brachial arteries. The linear regression of the mean difference revealed no proportional bias (P = 0.238) (Fig. 1B).
Fig. 1.
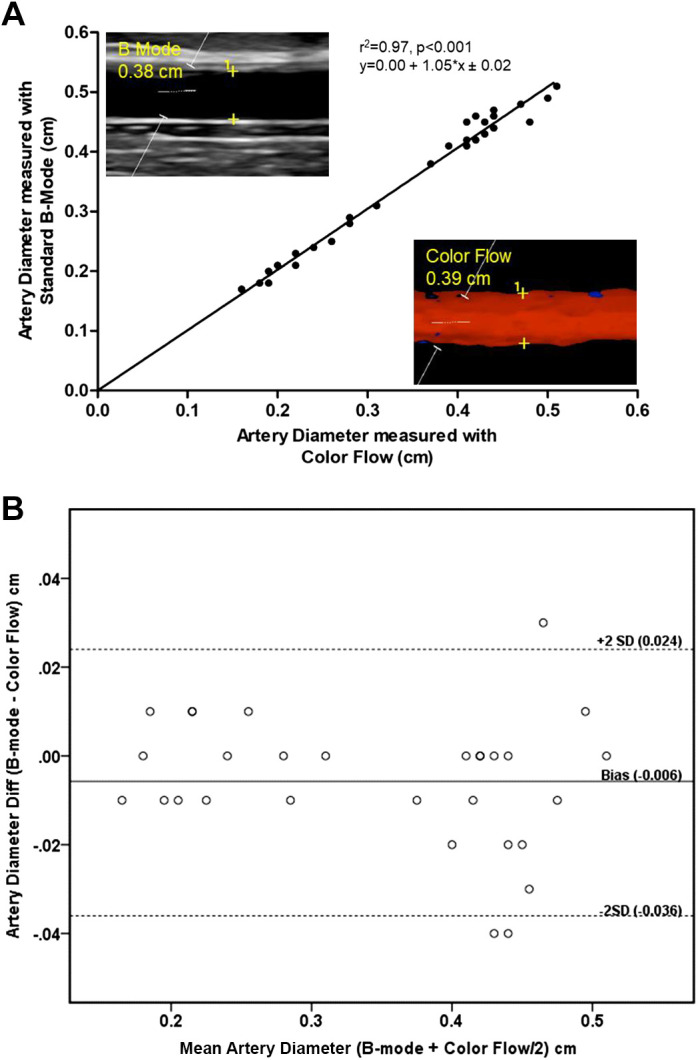
A comparison of peripheral vessel diameter determined by both color flow and B-mode ultrasound. A: linear regression between Standard B-mode and color flow measured diameters in the brachial and radial arteries (r2 = 0.97, P < 0.001). Inlaid, exemplar B-mode image illustrating the resolution of peripheral artery wall and a color flow image illustrating the assessment of wall diameter with color flow (without B-mode). B: Bland-Altman plot of vessel diameters performed with B-mode and color flow in the brachial and radial arteries. The y-axis indicates the difference between B-mode and color flow diameter, whereas the x-axis documents the average of their values. The bias represents the mean difference between the measurements, with values above zero indicating an overestimation and values below zero indicating an underestimation of B-mode relative to color flow diameter. Upper and lower lines represent bias ± 2 SD (n = 10).
Participants and Blood Pressure Response to NTG
All 13 participants completed the study protocol without any adverse effects during or following sublingual NTG. Participant characteristics and baseline hemodynamics are reported in Table 1. The effect of NTG and CON on blood pressure was as expected. Specifically, following NTG, there was a linear decline in mean arterial pressure (MAP) during the 5 min of recording with stable values during CON. The MAP at baseline (120-s avg.), during peak dilation (30-s avg.), and at the end of recording (30-s avg.) exhibited a significant time effect (P = 0.011), where the end MAP (90 ± 8 mmHg) was significantly lower than baseline MAP (95 ± 7 mmHg) (P = 0.002). Although tending to be lower than baseline, MAP during the peak diameter change (93 ± 2 mmHg) was not significantly attenuated. There were no changes in pressure during the CON with time matched averages to NTG for baseline, at peak dilation, and at the end of the measurements (baseline: 94 ± 9 mmHg; peak: 95 ± 10 mmHg; end: 94 ± 9 mmHg, P = 0.209).
Table 1.
Participant characteristics and baseline hemodynamics
| Characteristic | Study 1 | Study 2 |
|---|---|---|
| Sex (men/women) | 9/1 | 7/6 |
| Age, yr | 29 ± 4 | 28 ± 5 |
| Height, cm | 176.6 ± 7.5 | 174.5 ± 9.8 |
| Weight, kg | 77.1 ± 12.4 | 70.4 ± 12.5 |
| BMI, kg/m2 | 24 ± 4 | 23 ± 3 |
| Systolic BP, seated, mmHg | - | 108 ± 10 |
| Diastolic BP, seated, mmHg | - | 67 ± 9 |
| MAP, mmHg | - | 94 ± 9 |
| HR, beats/min | - | 57 ± 9 |
| SV, ml/beat | - | 89 ± 21 |
| CO, l/min | - | 5.1 ± 1.2 |
Values are means ± SD. BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure; HR, heart rate; SV, stroke volume; CO, cardiac output. Study 1: values of validation protocol; hemodynamics not measured. Study 2: values of middle cerebral artery protocol.
MCA Diameter, Blood Velocity, and Blood Flow Responses to Pharmaceutically Induced Vasodilation
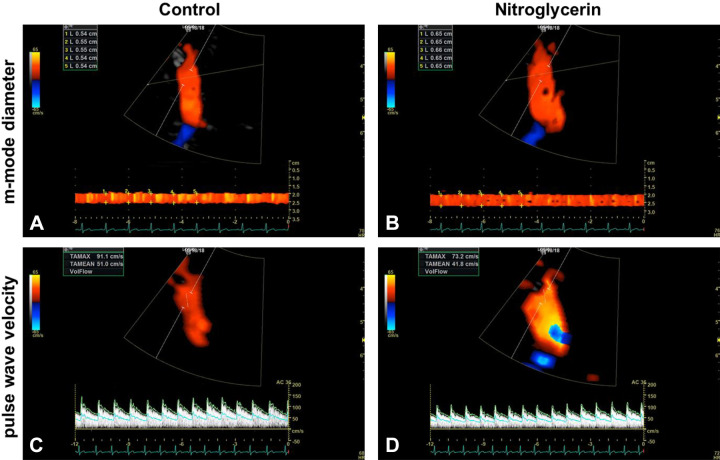
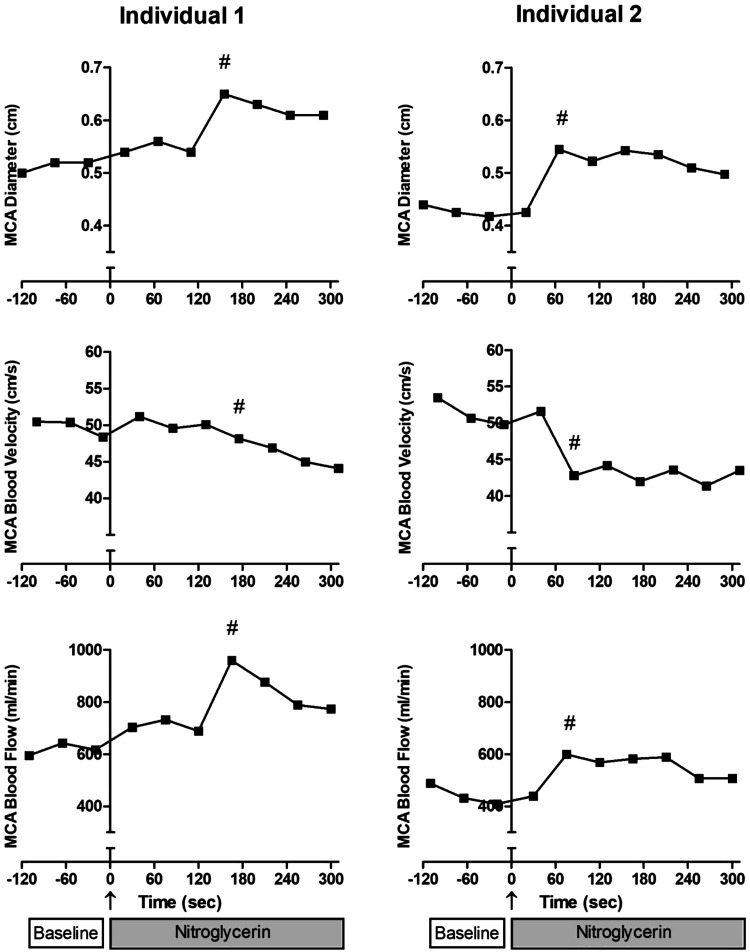
Representative MCA diameter and blood velocity screen displays are presented in Fig. 2. These images illustrate the diameter measurements across the edges of m-mode display at the r-wave and pulse-wave velocity during CON and NTG conditions. As illustrated in Fig. 3, NTG administration resulted in considerable individual variation in the time course for vessel diameter change, with peak diameter occurring at quite different time points between participants within the 5 min following the drug administration. In contrast, blood velocity, more uniformly, gradually declined over time. Peak NTG-induced blood flow (# symbols in Fig. 3, bottom) was identified and calculated using the peak NTG-induced diameter and the corresponding velocity measurement (# symbols in Fig. 3, top and middle), which was, of note, typically, also the greatest blood flow recorded.
Fig. 2.
Exemplar middle cerebral artery motion (m)-mode diameter and pulse-wave velocity images during control and nitroglycerin conditions. A: M-mode image during control condition with multiple r-wave diameter measurements across the m-mode display (diameter range = 0.54–0.55 cm). B: M-mode diameter image during nitroglycerin condition with multiple r-wave diameter measurements across the m-mode display (diameter range = 0.65–0.66 cm). C: pulse-wave velocity during placebo condition (TAmean = 51.0 cm/s). D: pulse-wave velocity during nitroglycerin condition (TAmean = 41.8 cm/s).
Fig. 3.
Exemplar middle cerebral artery (MCA) diameter, velocity, and blood flow responses to pharmaceutically induced vasodilation. Peak nitroglycerin (NTG)-induced diameter and the corresponding velocity measurement (#, top and middle) were used to identify and calculate peak NTG-induced MCA blood flow (#, bottom).
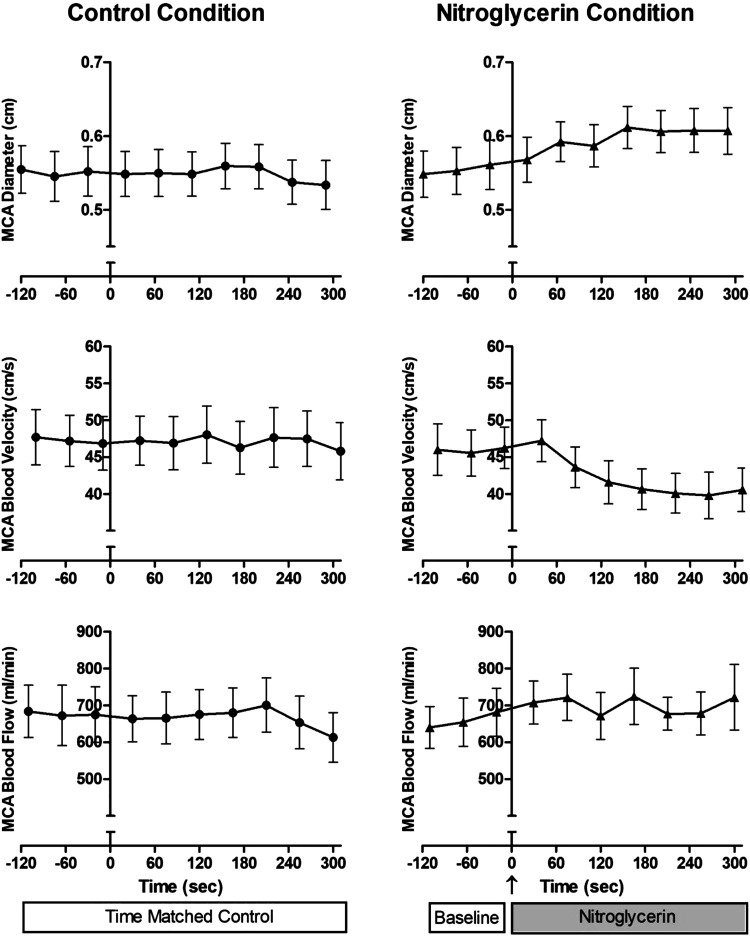
When considered as group mean responses, in CON conditions, MCA diameter, blood velocity, and blood flow across time appropriately represented the individual data that were relatively constant over time. In contrast, in the NTG condition, the individual temporal heterogeneity blurred the group effect of NTG over time. However, with NTG, there was still evidence of a gradual increase in MCA diameter, a fall in velocity, and a subsequent increase in blood flow over time following the drug delivery (Fig. 4).
Fig. 4.
Middle cerebral artery (MCA) diameter, blood velocity, and blood flow in control and nitroglycerin conditions over time. Note, individual temporal heterogeneity blurs the effect of nitroglycerin over time. Values are means ± SE.
Peak MCA Diameter, Blood Velocity, and Blood Flow Responses to Pharmaceutically Induced Vasodilation
In response to NTG, the MCA diameter, measured in color flow mode, increased considerably more in response to NTG compared with the basal variation in CON (∆0.09 ± 0.04 and ∆0.00 ± 0.04 cm, respectively, P = 0.018). Additionally, there was over a fivefold fall in MCA blood velocity compared with small fluctuations in CON (∆-4.95 ± 4.6 and ∆−0.88 ± 4.7 cm/s, respectively, P < 0.001). This NTG-induced increase in MCA diameter and fall in blood velocity resulted in in a significant increase in blood flow compared with minimal basal fluctuations in CON (∆144 ± 159 ml/min and ∆−5 ± 130 ml/min, respectively, P = 0.005) (Fig. 5).
Fig. 5.
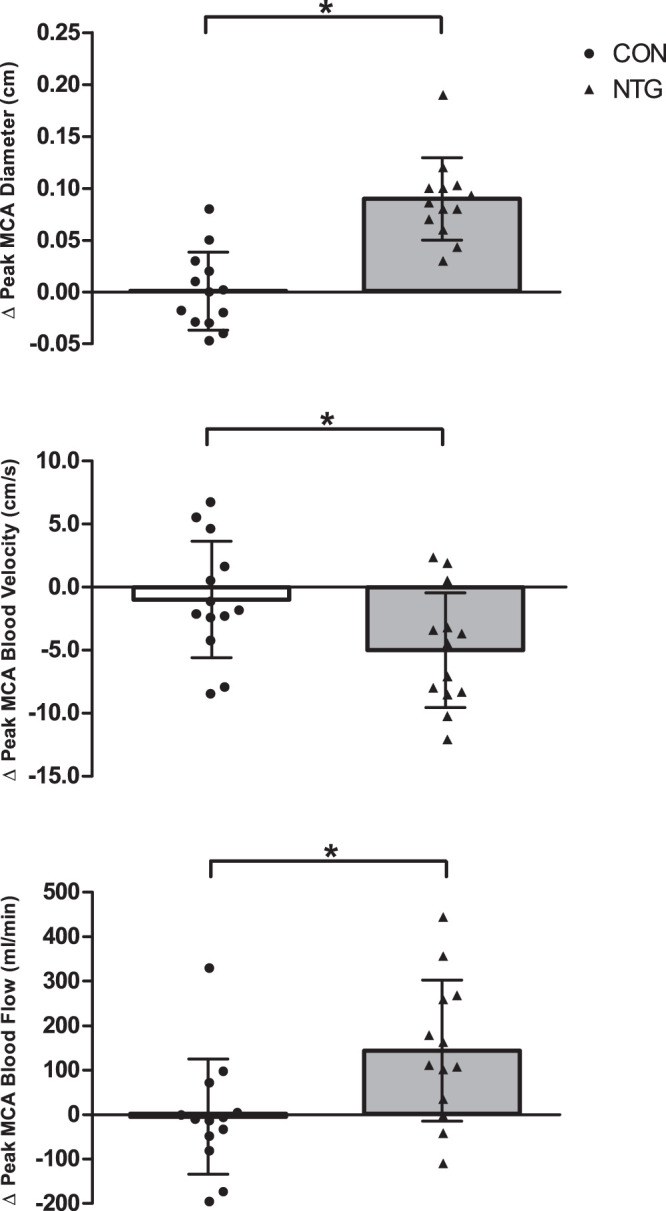
Delta peak middle cerebral artery diameter (MCA), blood velocity, and blood flow in control (CON) and with pharmaceutically induced vasodilation. Data are presented as both individual data and means ± SD. Data were compared using paired sample t test. *Significantly different between control and nitroglycerin (NTG) conditions (P < 0.05; n = 13).
Reproducibility of MCA Diameter, Blood Velocity, and Blood Flow Measurements and Analysis
The CV for the measurement and analysis, by the same investigator during two baseline periods, for MCA diameter was 2.5%, for blood velocity was 3.9%, and for blood flow was 5.3%. The between investigator CV for the analysis of NTG-induced MCA diameter was 5.6%.
DISCUSSION
The transcranial Doppler ultrasound assessment of blood velocity in the MCA is a commonly reported measure used to infer CBF and therefore utilized in cerebrovascular function tests. However, assuming blood velocity of the MCA is proportional to blood flow is likely inappropriate, in some circumstances, due to growing evidence that the MCA is vasoactive, which can exponentially influence blood flow calculations. Using ITCD ultrasound with color flow in anatomical m-mode to measure both MCA diameter and blood velocity is a novel method to quantify blood flow. Initially, in extracranial vessels, where the walls can be visualized, we documented that vessel diameter measurements using the color flow mode was strongly related to diameter measured using standard B-mode images and exhibited no systematic bias. In terms of the MCA, where the vessel walls cannot be insonated, utilization of ITCD in combination with a vasoactive stimulus, this study demonstrates the importance of directly measuring vessel diameter concomitantly with velocity to facilitate accurate blood flow calculations. Specifically, NTG induced over a fivefold fall in blood velocity and close to a 1-mm increase in MCA diameter that resulted in a significant increase in MCA blood flow compared with negligible baseline fluctuations during CON. These findings highlight the usefulness of this noninvasive, relatively simple, and low-cost method of measuring MCA diameter and blood velocity to calculate blood flow for future studies that aim to quantify cerebrovascular function.
Imaging TCD as a Novel Approach to Detect Changes in MCA Diameter
Utilizing ITCD in color flow mode has been documented in guideline papers to correctly identify the location of the MCA M1 segment to then appropriately measure blood velocity (21, 22). The first reports of color flow imaging of the MCA employed for measurements of blood flow was in the work of Taylor et al. (30, 31). This group studied newborn lambs utilizing color flow imaging to measure MCA vessel diameter, which improved the accuracy of relative blood flow measurements over mean blood velocity alone. In humans, an intriguing research paper investigating the effect of altitude on CBF suggested applying color flow mode to determine diameter when out in the field, where the use of MRI is not feasible (16). They found that the increase in MCA diameter (5.0 to 6.0 mm), rather than the blood velocity, was a major contributor to increased oxygen delivery within the first few hours of acute hypoxia exposure. An earlier laboratory validation of a form of ITCD, which compared color flow-measured MCA diameters to MRI-determined diameters during normoxia and hypoxia, found that MCA diameters are systematically larger in color mode, but the regression between color-mode diameter and MRI diameter measurements was strong (r2 = 0.67) (38). Of note, the methods described in the aforementioned studies that used color flow measurements are not sufficiently described and do not allow for a continuous measurement of MCA blood flow with sequential velocity and diameter data points. As illustrated in Fig. 1, the current ITCD approach performed in peripheral arm vessels exhibits both a strong relationship between color flow assessed vessel diameter and standard B-mode measurements and no systematic bias. Furthermore, when applied to the MCA, this ITCD approach revealed good reproducibility both across the assessment and analysis of the variables required to assess MCA blood flow (vessel diameter and blood velocity) and the NTG-induced change in MCA diameter analyzed by two investigators.
TCD Measurements as an Indicator of CBF
Current understanding of cerebrovascular physiology has been strongly influenced by Transcranial Doppler ultrasound because of its relatively low cost, portability, ease of use, high-sampling frequency, and noninvasive quality (37). Indeed, utilizing TCD as a tool for the assessment of CBF has been common across a wide array of physiological perturbations (e.g., end-tidal Pco2, NTG, exercise, etc.) and is still clinically relevant in terms of the assessment of cerebrovascular reactivity with aging and disease states. However, alterations in the diameter of the MCA will impact MCA blood flow in such a way that velocity changes will not parallel blood flow changes. Specifically, based on Poiseuille’s law and the continuity equation, with respect to fluid, assuming that velocity is unchanged, a 3% change in diameter would result in ~6% change in volumetric flow. Thus unknown changes in vessel diameter during TCD assessments will exponentially influence blood flow calculations.
A few select studies are obstinately cited to support the primary assumption of TCD, that middle cerebral blood velocity is proportional to CBF due to a constant MCA diameter (5, 14, 28, 33). In 2000, Serrador et al. (28) sought to directly examine the MCA diameter of 12 healthy participants with a 1.5-T MRI, while also assessing blood velocity with TCD, in response to an increase or decrease in end-tidal carbon dioxide partial pressure. The authors concluded that the MCA diameter was stable across a wide range of hyper- and hypocapnia, and therefore, TCD velocity was concluded to be a valid index of changes in total MCA blood flow. This conclusion is still referenced to date, even though it has been highlighted that in this prior study there was trend for a 4% dilation during hypercapnia (2). Similarly, a commonly referenced article by Valdueza et al. (33) reported no significant changes in the MCA diameter during hyperventilation as measured by 1.5-T MRI. In contrast, our data document a significant vasodilation of the MCA in response to NTG by simply measuring with Doppler ultrasound in color flow mode, and this, despite a drop in blood velocity, increased MCA blood flow (Fig. 5).
Evidence of MCA Vasodilation
Interestingly, just 2 yr following the often-cited article by Valdueza et al. (33), which reported no significant changes in the MCA diameter during hyperventilation measured by 1.5-T MRI, the same author explicitly stated that MCA blood velocities measured by TCD cannot be used to assume CBF. This change in stance was a consequence of their most recent findings of a range of calculated MCA vasodilation from −0.9% to 22% in response to maximal hypercapnia (34). Later MRI studies, using improved field strength (i.e., 3 and 7 T), with better resolution of the cross-sectional area of the MCA in response to hyper- and hypocapnic conditions, also now, more definitively, documented the capacity of the MCA to constrict and dilate (3, 9–11, 36). Specifically, with modest alterations in end-tidal Pco2, 19 healthy participants exhibited an average ~8% increase in MCA diameter when exposed to hypercapnia and a ~4% decrease in diameter following hyperventilation (10). These results reveal that MCA blood flow inferred by changes in TCD velocity can underestimate actual MCA blood flow by as much as 20%. Moreover, the same group conducted a follow-up time course study with similar methods for MCA measurements and changes in end-tidal CO2 and determined the same magnitude of effect on the vessel diameter with both high and low end-tidal CO2 levels (11).
When the effect of hypercapnia on MCA cross-sectional area was investigated, with the use of MRI, in both young and old healthy adults, it has been documented that the diameter increased by 9% and 4%, respectively (9). Another research group confirmed a similar finding of a ~7% increase in MCA diameter following hypercapnia measured by MRI at 7 T but found no significant changes during hypocapnia (36). Variability in the magnitude of responses may be due to different methodologies employed, resolution capacities, postimaging variability with manual measurements, and MRI acquisition parameters. The variability in these findings points to the limits of MRI as a gold standard measurement for MCA cross-sectional area changes but should not distract from the clear findings that MCA diameter is not constant. In the current study, NTG increased MCA diameter, with quite a variety of individual time courses for peak diameter change (Fig. 3). As a consequence of these varied time courses for MCA vasodilation and the clearly lowered MCA blood velocity, as a group over time, the true changes in MCA blood flow were blurred but certainly were not attenuated as the velocity data may suggest (Fig. 4).
Pharmaceutically Induced MCA Diameter Changes
NTG provides an exogenous source of nitric oxide that results in endothelial independent vasodilation. An elegant study design using the inhalation of xenon133 and single-photon emission computed tomography in combination with TCD found that 1 mg of sublingual NTG lowered MCA velocity by 25%, which represented a relative increase in MCA diameter of 15% (12). The current data also reveal a large and significant ~18% increase in MCA diameter in response to sublingual NTG (Fig. 5, top), which, despite a significant fall (~10%) in blood velocity (Fig. 5, middle), ultimately resulted in a large and significant increase (~28%) in MCA blood flow (Fig. 5, bottom). More recently, Schulz and colleagues utilized TCD to measure MCA blood velocity and employed a 7-T neruo-optimized MRI scanner to measure MCA diameter following sublingual NTG spray (27). With 0.4 mg of NTG spray, they documented a modest 11.5% increase in diameter, which, as a consequence of a concomitant decrease in velocity, resulted in an unchanged MCA blood flow. This contrasts with our findings that we observed an increase in blood flow with a lowering of velocity. However, it is important to point out that their MCA blood flow calculations were acquired by aligning two separate laboratory sessions, one in which TCD velocity was measured and the other where MRI cross sectional area was assessed. The different outcome in terms of MCA blood flow with this and the current study is likely the result of NTG dose (0.4 mg and 0.8 mg, respectively). Sublingual NTG tablets and oral spray are relatively equivalent, yet they can have variable bioavailability and, therefore, may result in different peak concentration across individuals. Thus it is also beneficial, as in the current study, to have sequential real-time measurements of diameter and velocity with ITCD to detect individual peak responses (17, 24). However, regardless, both this prior study and the current work highlight the observation that the measurement of MCA velocity without MCA diameter measurements would falsely represent the CBF response to oral NTG, with blood velocity indicating a fall in MCA blood flow while a combination of both blood velocity and MCA diameter reveals either no change in MCA blood flow or a significant increase.
Experimental Considerations
A limitation of this study is the lack of a direct comparison of the ITCD diameter with MRI, currently considered the gold standard of vessel diameter assessment. However, nitroglycerin-induced MCA vasodilation measured directly with MRI, and indirectly with middle cerebral artery blood velocity calculations, has been documented, on several occasions (12, 18, 27, 29), by others. Ultimately, ITCD may provide a more readily accessible method for detecting diameter change. Another limitation of this work is that the B-mode and color flow imaging comparison within the peripheral vessels was not also performed with NTG administration, which would have facilitated the assessment of the delta diameter with both techniques. Of note, during cerebral NTG assessment, there was variability in the peak MCA diameter response times between individuals, with peak diameter in 6/13 participants occurring at the end of the 5 min of assessment and 7/13 exhibiting a peak response between 1 and 4 min. Although the individual time course of the response to NTG was not homogeneous (Fig. 3), this does not detract from the overall findings, as documented by the delta peak response (Fig. 5).
It should also be noted that the current results were obtained in healthy young adults and the highly successful ITCD scanning (no subject exclusions due to challenging anatomy, etc.) could, potentially, be hindered in older adults, women, African Americans, and Asians, where acoustic window failure is increased by differences in the thickness and texture of the temporal bone (19, 20). Furthermore, end-tidal CO2, recognized to be related to MCA diameter, was not measured in this study. However, the vasodilatory stimulus utilized here, NTG, has been previously documented to have no effect on end-tidal CO2 (12, 27), and hence, this was unlikely to be a confounding factor. This study is also limited in that it focuses on MCA vasodilation and does not assess vasoconstriction of the MCA. Future studies warrant this assessment and could potentially address this by employing this method under hypoxic and hypercapnic conditions. Additionally, our method employs handheld sonography of the MCA, without traditional stabilizing headgear, requiring a trained sonographer to maintain insonation angle stable, and this poses a potential limitation. However, this was not an issue in the current study. Finally, these results are limited to the assessment of the MCA in healthy young participants in a supine position and may not be generalizable to other age groups, clinical populations, different body positions (e.g., seated and standing), and other experimental conditions (e.g., exercise and sit-to-stand maneuvers). Of note, smaller intracranial arteries were not investigated in this study, but, although likely more challenging to assess, could be of interest in the future. These experimental considerations warrant further investigation to fine tune the use of ITCD to assess both MCA blood velocity and MCA diameter to facilitate the accurate assessment of MCA blood flow.
Perspectives and Significance
There is growing evidence that clearly supports the notion that MCA diameter is not constant when exposed to physiologically relevant perturbations. The acknowledged limitation of TCD, failure to assess MCA diameter, can be relatively easily addressed by measuring the MCA diameter with ITCD rather than either assuming a constant diameter or that the discrepancy between MCA blood velocity and blood flow is negligible. As the assessment of cerebrovascular reactivity is still clinically relevant, this study provides useful methodological insight and highlights an approach to measure both MCA blood velocity and MCA diameter, with real-time resolution, to facilitate the accurate assessment of MCA blood flow.
GRANTS
This work was supported by the National Institutes of Health High Priority, Short-Term Project Award Grant R56-AG057584-01, Ruth L. Kirschtein Research Service Award Grant T32HL-139451, and Veterans Administration Rehabilitation Research and Development Service Grants E6910-R, E1697-R, E1433-P, E9275-L, and E1572-P.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.J., K.L.S., R.M.B., J.R.G., and R.S.R. conceived and designed research; C.L.J., K.L.S., R.M.B., J.R.H., and S.H.P. performed experiments; C.L.J., K.L.S., and R.M.B. analyzed data; C.L.J., K.L.S., R.M.B., and R.S.R. interpreted results of experiments; C.L.J., K.L.S., and R.S.R. prepared figures; C.L.J. drafted manuscript; C.L.J., K.L.S., R.M.B., J.R.H., S.H.P., J.R.G., and R.S.R. edited and revised manuscript; C.L.J., K.L.S., R.M.B., J.R.H., S.H.P., J.R.G., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57: 769–774, 1982. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Hoiland RL. Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol (1985) 117: 1081–1083, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khazraji BK, Shoemaker LN, Gati JS, Szekeres T, Shoemaker JK. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J Cereb Blood Flow Metab 39: 1204–1214, 2019. doi: 10.1177/0271678X18762880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birdsill AC, Carlsson CM, Willette AA, Okonkwo OC, Johnson SC, Xu G, Oh JM, Gallagher CL, Koscik RL, Jonaitis EM, Hermann BP, LaRue A, Rowley HA, Asthana S, Sager MA, Bendlin BB. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 21: 1313–1320, 2013. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 17: 913–915, 1986. doi: 10.1161/01.STR.17.5.913. [DOI] [PubMed] [Google Scholar]
- 6.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327: 307–310, 1986. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 7.Brothers RM, Zhang R. CrossTalk opposing view: The middle cerebral artery diameter does not change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4077–4079, 2016. doi: 10.1113/JP271884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broxterman RM, Witman MA, Trinity JD, Groot HJ, Rossman MJ, Park SY, Malenfant S, Gifford JR, Kwon OS, Park SH, Jarrett CL, Shields KL, Hydren JR, Bisconti AV, Owan T, Abraham A, Tandar A, Lui CY, Smith BR, Richardson RS. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension 74: 208–215, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 37: 344–355, 2017. doi: 10.1177/0271678X15626156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Coverdale NS, Lalande S, Perrotta A, Shoemaker JK. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am J Physiol Heart Circ Physiol 308: H1030–H1038, 2015. doi: 10.1152/ajpheart.00761.2014. [DOI] [PubMed] [Google Scholar]
- 12.Dahl A, Russell D, Nyberg-Hansen R, Rootwelt K. Effect of nitroglycerin on cerebral circulation measured by transcranial Doppler and SPECT. Stroke 20: 1733–1736, 1989. doi: 10.1161/01.STR.20.12.1733. [DOI] [PubMed] [Google Scholar]
- 13.DeWitt LD, Wechsler LR. Transcranial Doppler. Stroke 19: 915–921, 1988. doi: 10.1161/01.STR.19.7.915. [DOI] [PubMed] [Google Scholar]
- 14.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32: 737–742, 1993. doi: 10.1227/00006123-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Hoiland RL, Ainslie PN. CrossTalk proposal: The middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4073–4075, 2016. doi: 10.1113/JP271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imray C, Chan C, Stubbings A, Rhodes H, Patey S, Wilson MH, Bailey DM, Wright AD; Birmingham Medical Research Expeditionary Society . Time course variations in the mechanisms by which cerebral oxygen delivery is maintained on exposure to hypoxia/altitude. High Alt Med Biol 15: 21–27, 2014. doi: 10.1089/ham.2013.1079. [DOI] [PubMed] [Google Scholar]
- 17.Jensen KM, Mikkelsen S. Studies on the bioavailability of glyceryl trinitrate after sublingual administration of spray and tablet. Arzneimittelforschung 47: 716–718, 1997. [PubMed] [Google Scholar]
- 18.Kistler JP, Vielma JD, Davis KR, FitzGibbon S, Lees RS, Crowell RM. Effects of nitroglycerin on the diameter of intracranial and extracranial arteries in monkeys. Arch Neurol 39: 631–634, 1982. doi: 10.1001/archneur.1982.00510220029006. [DOI] [PubMed] [Google Scholar]
- 19.Krejza J, Swiat M, Pawlak MA, Oszkinis G, Weigele J, Hurst RW, Kasner S. Suitability of temporal bone acoustic window: conventional TCD versus transcranial color-coded duplex sonography. J Neuroimaging 17: 311–314, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kwon JH, Kim JS, Kang DW, Bae KS, Kwon SU. The thickness and texture of temporal bone in brain CT predict acoustic window failure of transcranial Doppler. J Neuroimaging 16: 347–352, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Lupetin AR, Davis DA, Beckman I, Dash N. Transcranial Doppler sonography. Part 1. Principles, technique, and normal appearances. Radiographics 15: 179–191, 1995. doi: 10.1148/radiographics.15.1.7899596. [DOI] [PubMed] [Google Scholar]
- 22.Martin PJ, Evans DH, Naylor AR. Measurement of blood flow velocity in the basal cerebral circulation: advantages of transcranial color-coded sonography over conventional transcranial Doppler. J Clin Ultrasound 23: 21–26, 1995. doi: 10.1002/jcu.1870230105. [DOI] [PubMed] [Google Scholar]
- 23.Nagata K, Yamazaki T, Takano D, Maeda T, Fujimaki Y, Nakase T, Sato Y. Cerebral circulation in aging. Ageing Res Rev 30: 49–60, 2016. doi: 10.1016/j.arr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Noonan PK, Benet LZ. Incomplete and delayed bioavailability of sublingual nitroglycerin. Am J Cardiol 55: 184–187, 1985. doi: 10.1016/0002-9149(85)90325-X. [DOI] [PubMed] [Google Scholar]
- 25.Ozturk ED, Tan CO. Human cerebrovascular function in health and disease: insights from integrative approaches. J Physiol Anthropol 37: 4, 2018. doi: 10.1186/s40101-018-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogge A, Doepp F, Schreiber S, Valdueza JM. Transcranial color-coded duplex sonography of the middle cerebral artery: more than just the M1 segment. J Ultrasound Med 34: 267–273, 2015. doi: 10.7863/ultra.34.2.267. [DOI] [PubMed] [Google Scholar]
- 27.Schulz JM, Al-Khazraji BK, Shoemaker JK. Sodium nitroglycerin induces middle cerebral artery vasodilatation in young, healthy adults. Exp Physiol 103: 1047–1055, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. doi: 10.1161/01.STR.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 29.Siepmann M, Kirch W. Effects of nitroglycerine on cerebral blood flow velocity, quantitative electroencephalogram and cognitive performance. Eur J Clin Invest 30: 832–837, 2000. doi: 10.1046/j.1365-2362.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor GA, Hudak ML. Color Doppler ultrasound of changes in small vessel diameter and cerebral blood flow during acute anemia in the newborn lamb. Invest Radiol 29: 188–194, 1994. doi: 10.1097/00004424-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Taylor GA, Short BL, Walker LK, Traystman RJ. Intracranial blood flow: quantification with duplex Doppler and color Doppler flow US. Radiology 176: 231–236, 1990. doi: 10.1148/radiology.176.1.2112768. [DOI] [PubMed] [Google Scholar]
- 32.Tymko MM, Ainslie PN, Smith KJ. Evaluating the methods used for measuring cerebral blood flow at rest and during exercise in humans. Eur J Appl Physiol 118: 1527–1538, 2018. doi: 10.1007/s00421-018-3887-y. [DOI] [PubMed] [Google Scholar]
- 33.Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhäupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol 18: 1929–1934, 1997. [PMC free article] [PubMed] [Google Scholar]
- 34.Valdueza JM, Draganski B, Hoffmann O, Dirnagl U, Einhäupl KM. Analysis of CO2 vasomotor reactivity and vessel diameter changes by simultaneous venous and arterial Doppler recordings. Stroke 30: 81–86, 1999. doi: 10.1161/01.STR.30.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Verbree J, Bronzwaer A, van Buchem MA, Daemen M, van Lieshout JJ, van Osch M. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab 37: 2921–2927, 2017. doi: 10.1177/0271678X16679419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Wilson MH, Edsell ME, Davagnanam I, Hirani SP, Martin DS, Levett DZ, Thornton JS, Golay X, Strycharczuk L, Newman SP, Montgomery HE, Grocott MP, Imray CH; Caudwell Xtreme Everest Research Group . Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia–an ultrasound and MRI study. J Cereb Blood Flow Metab 31: 2019–2029, 2011. doi: 10.1038/jcbfm.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]