Abstract
20-Hydroxyeicosatetraenoic acid (20-HETE) has been linked to blood pressure (BP) regulation via actions on the renal microvasculature and tubules. We assessed the tubular 20-HETE contribution to hypertension by generating transgenic mice overexpressing the CYP4A12-20-HETE synthase (PT-4a12 mice) under the control of the proximal tubule (PT)-specific promoter phosphoenolpyruvate carboxykinase (PEPCK). 20-HETE levels in the kidney cortex of male (967 ± 210 vs. 249 ± 69 pg/mg protein) but not female (121 ± 15 vs. 92 ± 11 pg/mg protein) PT-4a12 mice showed a 2.5-fold increase compared with wild type (WT). Renal cortical Cyp4a12 mRNA and CYP4A12 protein in male but not female PT-4a12 mice increased by two- to threefold compared with WT. Male PT-4a12 mice displayed elevated BP (142 ± 1 vs. 111 ± 4 mmHg, P < 0.0001), whereas BP in female PT-4a12 mice was not significantly different from WT (118 ± 2 vs. 117 ± 2 mmHg; P = 0.98). In male PT-4a12 mice, BP decreased when mice were transitioned from a control-salt (0.4%) to a low-salt diet (0.075%) from 135 ± 4 to 120 ± 6 mmHg (P < 0.01) and increased to 153 ± 5 mmHg (P < 0.05) when mice were placed on a high-salt diet (4%). Female PT-4a12 mice did not show changes in BP on either low- or high-salt diet. In conclusion, the expression of Cyp4a12 driven by the PEPCK promoter is sex specific, probably because of its X-linkage. The salt-sensitive hypertension seen in PT-4a12 male mice suggests a potential antinatriuretic activity of 20-HETE that needs to be further explored.
Keywords: 20-HETE, hypertension, kidney
INTRODUCTION
20-Hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) is a product of arachidonic acid metabolism by cytochrome P-450 enzymes, specifically CYP4A and CYP4F. 20-HETE has been extensively studied in the vasculature and renal structures and has been linked to pro- and antihypertensive mechanisms (2, 48, 49).
20-HETE is a vasoactive lipid whose production within the vasculature is primarily localized to the microcirculation. In renal microvessels, 20-HETE promotes vasoconstriction by sensitizing the response to constrictor stimuli and by impairing endothelium-dependent relaxation via endothelial nitric oxide synthase (eNOS) uncoupling. These actions along with 20-HETE’s ability to upregulate the renin-angiotensin system via transcriptional activation of the angiotensin-converting enzyme (ACE) constitute 20-HETE prohypertensive actions (16, 24, 34). On the other hand, the antihypertensive actions of 20-HETE are assigned to its ability to affect renal ion transport mechanisms. 20-HETE is produced in renal tubular segments, with proximal tubules being the major site of CYP4A/4F expression and 20-HETE biosynthesis (18, 27, 42). Early studies showed that 20-HETE inhibits proximal tubule Na+-K+-ATPase (26, 39) as well as the Na+-K+-2Cl− cotransporter (NKCC) (6) and the apical K+ channel (11) in the thick ascending limb. In a series of studies, Roman and colleagues (35–37, 41, 47), using pharmacological and genetic approaches, provided substantial evidence for a role of 20-HETE in the development of salt-sensitive hypertension. Also, in humans a deficiency in renal 20-HETE synthesis and/or action measured as decreased urinary excretion in response to salt loading has been linked to salt-sensitive hypertension (20). However, recent findings indicated that 20-HETE exerts actions along the nephron conducive to sodium retention. These actions include activation of the sodium-chloride cotransporter (NCC) in the distal tubule and the sodium-hydrogen exchanger (NHE3) in the proximal tubules (31, 38).
In a recent study, we reported (29) that the hypertension in Cyp4a14-knockout mice, which overexpress Cyp4a12 (the major murine 20-HETE synthase in mouse), is accompanied by reduced sodium excretion and that both the salt retention and hypertension are reversed by administration of 2,5,8,11,14,17-hexaoxanonadecan-19-yl 20-hydroxyicosa-6(Z),15(Z)-dienoate (20-SOLA), a 20-HETE antagonist. A limitation to the aforementioned report and others is the lack of targeted manipulation of 20-HETE levels to a specific tissue or cell type. To begin assessing the contribution of tubular 20-HETE to hypertension, we generated transgenic mice overexpressing Cyp4a12 under the control of the proximal tubule (PT)-specific promoter phosphoenolpyruvate carboxykinase (PEPCK). Here we show that increased levels of 20-HETE in the renal tubules lead to hypertension in male mice that appears to be salt sensitive.
METHODS
Animals.
All experimental protocols were approved by the institutional animal care and use committee of New York Medical College. Transgenic mice overexpressing Cyp4a12 within the proximal tubules were generated by crossbreeding floxed Cyp4a12 (Cyp4a12fl/fl) mice with Cre mice carrying the proximal tubule (PT)-specific promoter phosphoenolpyruvate carboxykinase (PEPCK) (32). The Cre mice were generously gifted by Dr. Volker Haase, Vanderbilt University, Nashville, TN. The PEPCK-Cre transgene was generated with a mutated version of the PEPCK promoter, which reduces PEPCK expression in the liver by 60% and increases PEPCK expression in the kidney by 10-fold in transgenic mice (32). The Cyp4a12fl/fl mice were generously gifted by Dr. Wolf-Hagen Schunck and Prof. Michael Bader from the Max-Delbrück-Centrum, Berlin, Germany. Eight- to twelve-week-old Cyp4a12fl/flPTCre+/− (PT-4a12) mice were used for the study, and their age-matched littermates, Cyp4a12fl/flPTCre−/−, served as control mice [wild type (WT)]. Male and female mice (8–12 wk old) were separated into groups depending on whether they were positive or negative for Cre. All mice were housed in metabolic cages (Hatteras Instruments, Cary, NC) with free access to food and water. Before measurements were taken, mice were allowed to acclimate to the metabolic cages for 5 days. At the end of experiments, mice were euthanized and tissues were harvested for RT-PCR, immunoblotting, and LC-MS/MS analysis of 20-HETE levels.
Blood pressure.
Blood pressure measurements were taken with a CODA noninvasive blood pressure system (Kent Scientific, Torrington, CT). All animals were allowed 4 days to acclimate to the system before measurements were collected. The CODA tail-cuff system is able to obtain values ±10% of the average blood pressure. Blood pressure was also measured by carotid artery catheterization. Briefly, mice were placed in an isoflurane induction chamber with the vaporizer and oxygen flow set at 5% and 500 mL/min, respectively. The anesthetized mice were then transferred to an operation table (37°C) and masked with an isoflurane nose cone, with isoflurane vaporizer and oxygen flow adjusted to 2% and 200 mL/min, respectively. After the neck area was shaved, a middle incision was made to expose the left common carotid artery through blunt dissection. A Scisense 1.2F solid-state pressure catheter (Transonic, Ontario, Canada) was placed in the left carotid artery for monitoring heart rate (HR) and arterial BP. The pressure catheter was connected to a SP200 pressure control unit. Hemodynamic parameters including systemic BP and HR were recorded on PowerLab (ADInstruments, Colorado Springs, CO) and analyzed with LabChart v8 software (ADInstruments).
Salt experiments.
Mice were placed in metabolic cages on a control diet containing 0.4% NaCl for 10 days. The mice were then placed on a low-salt diet containing 0.075% NaCl for 14 days, followed by an additional 12 days on a high-salt diet containing 4% NaCl. The different rodent chows were purchased from Envigo (Indianapolis, IN). Blood pressure, body weight, urine volume, and food and water consumption were measured every 2 days. Urinary sodium was measured with flame photometry and a Smartlyte electrolyte analyzer (Diamond Diagnostic, Holliston, MA) for baseline measurements and daily urine analysis, respectively.
20-HETE measurements.
20-HETE was quantified by LC-MS/MS-based lipidomics as previously described (10). Blood was withdrawn at the end of the experiment by aspiration from the inferior vena cava. Briefly, animals were anesthetized with isoflurane, and their abdomens were swabbed with 70% alcohol and opened by a midline longitudinal incision. Next, the caudal (inferior) vena cava was identified and punctured, and blood was drawn into a heparinized syringe. Blood was centrifuged at 2,000 rpm for 15 min to separate the plasma. Plasma samples were subjected to chloroform-methanol extraction and alkali hydrolysis for assessment of free and esterified 20-HETE. Cortical tissues were harvested into a tube containing HPLC-grade methanol, homogenized, and 20-HETE extracted as described below. Renal preglomerular arteries were microdissected and incubated in Krebs bicarbonate buffer, pH 7, 1 mM NADPH at 37°C for 1 h. Tissue incubations were terminated with 2 volumes of cold methanol, internal standards were added, and samples were kept at −80°C. Urine was mixed with two parts of methanol before LC-MS/MS. Eicosanoids were extracted with solid-phase Strata-X Polymeric Reversed Phase 60-mg cartridges (Phenomenex, Torrance, CA). Identification and quantification of 20-HETE were performed on a Shimadzu Triple Quadrupole Mass Spectrometer LCMS-8050 equipped with a Nexera UHPLC using multiple reaction monitoring mode. MS conditions were ionization mode: negative heated electrospray (HESI); applied voltage: −4.5 to approximately −3 kV; nebulizer gas: 3.0 L/min N2; drying gas: 5.0 L/min N2; heating gas: 12.0 L/min air; interface temperature: 400°C; desolvation line temperature: 100°C; heat block temperature: 500°C; and internal standard: d6 20-HETE. UHPLC conditions were as follows: analytical column measures were Zorbax Eclipse Plus C18 RRHD (50-mm length × 2.1-mm inner diameter, 1.8 µm); mobile phase A: 95% water-5% acetonitrile 0.05% acetic acid; mobile phase B: acetonitrile 0.05%; time program 40% B (0 min) → 75% B (3 min) → 85% B (7.5 min); flow rate: 0.4 mL/min; injection volume: 5 µL; column oven temperature: 40°C. Synthetic standards were used to obtain standard curves (0.5–500 pg) for each compound. These standard curves were used to calculate final concentrations of 20-HETE. All solvents were HPLC grade or higher.
RT-PCR.
RNA was isolated from mouse tissues and microdissected tubules with a RNeasy Mini Kit (QIAGEN catalog no. 74104) as per the manufacturer’s protocol. cDNAs were synthesized with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher catalog no. K1621). The qPCR primer sets for Cyp4a12 (forward: 5′-CCAGCATTACACGAACAGAGT-3′ and reverse: 5′-GATTTCTAGCTCCCTGGATTGG-3′) and β-actin (forward: 5′-GACTCATCGTACTCCTGCTTG-3′ and reverse: 5′-GATTACTGCTCTGGCTCCTAG-3′) were purchased from Integrated DNA Technologies (IDT, Coralville, Iowa). Primers were diluted to a concentration of 10 μM, and 0.5 μL was used for each 10-μL qPCR reaction along with 5 μL of Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA). Samples were run in triplicate on a Stratagene Mx3000P Real-Time PCR System (Agilent Technologies) with PCR conditions as follows: 50°C for 2 min; 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, then 60°C for 1 min; a dissociation stage of 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. Data were analyzed with the 2−ΔΔCT method (where CT is threshold cycle), with mouse tissue expression values normalized to β-actin.
Western blot.
At the end of the salt challenge diets, mice were euthanized by isoflurane overdose and tissues were snap frozen in liquid nitrogen and stored at −80°C. Tissues were homogenized in lysis buffer containing protease and phosphatase inhibitors (Roche Life Science). Tissue lysates were centrifuged at 3000 g for 15 min at 4°C. After centrifugation, the pellet was discarded and the supernatant was used for further analysis. Protein quantity of the supernatant was measured by Bradford assay. Lysates (50 µg protein) were then loaded onto a 10% SDS-PAGE gel and ran at 100 V for 1 h. Protein was then transferred to a PVDF membrane for 2 h with Bio-Rad transfer apparatus. After transfer, PVDF membranes were blocked with Li-Cor blocking buffer for 1 h at room temperature. The following antibodies were used: Cyp4a (Santa Cruz, sc-271983); NHE3 (Santa Cruz, sc-16103); phospho-NHE3 serine-605 (Santa Cruz, sc-53961); NCC (EMD Millipore, AB3553); phospho-NCC threonine-53 (PhosphoSolutions, P1311-53); beta-actin (Sigma, A5441); and SGK-1 (Abcam, Ab32374). Li-Cor secondary antibody was incubated on membranes for 2 h at room temperature. Proteins were visualized with Li-Cor Odyssey infrared imaging system version 3.0.21.
Statistical analysis.
All values are reported as means ± SE. Significance of difference in mean values was determined by unpaired Student’s t test, one-way ANOVA, or repeated-measures two-way ANOVA, followed by Tukey’s post hoc multiple comparison test. P < 0.05 was considered to be significant.
RESULTS
Male PT-4a12 mice display tubule-specific overexpression of CYP4A12-20-HETE-synthase and are hypertensive.
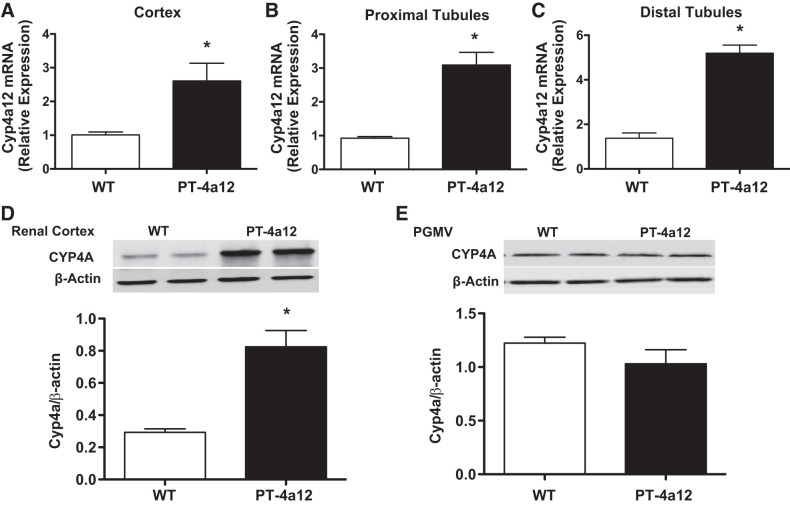
Isolated renal cortical tissue from male PT-4a12 mice demonstrated a 2.5-fold increase in cortical Cyp4a12 mRNA (Fig. 1A) and a 3-fold increase in CYP4A12 protein levels (Fig. 1D) compared with their WT littermates. Moreover, Cyp4a12 mRNA in microdissected proximal tubules from PT-4a12 mice was 3-fold higher compared with WT mice (Fig. 1B). The increased expression of Cyp4a12 was also detected in microdissected distal tubules (Fig. 1C). In contrast, no significant difference was observed in CYP4A protein levels in isolated preglomerular arteries of male PT-4a12 and WT mice (Fig. 1E), indicating that the Cre transgene directed Cyp4a12 overexpression to tubular structures.
Fig. 1.
A–C: Cyp4a12 mRNA in kidney cortex (A), proximal tubules (B), and distal tubules (C) from male wild-type (WT) and PT-4a12 mice. D and E: Cyp4a protein levels in renal cortex (D) and preglomerular microvessels (PGMV; E) from male WT and PT-4a12 mice. Results are means ± SE; n = 5 mice/group. *P < 0.05 vs. WT (unpaired Student’s t test).
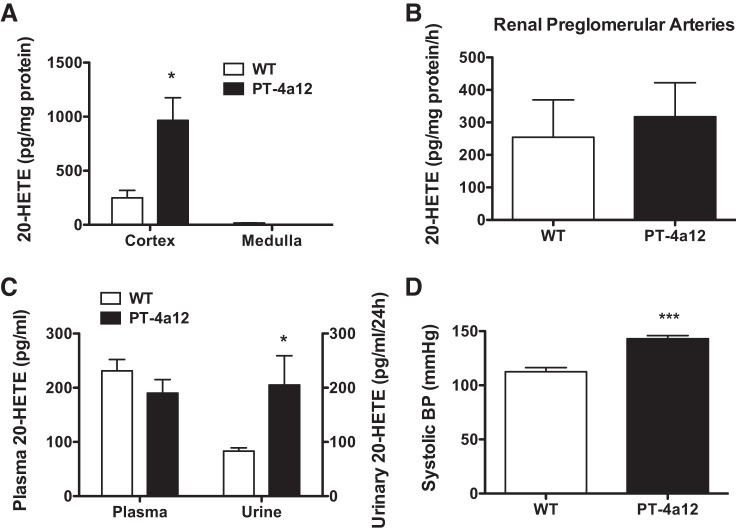
The increased expression of Cyp4a12 in tubular structures was associated with increased levels of 20-HETE in kidney cortex, which in PT-4a12 mice were fourfold higher than in WT littermates (Fig. 2A). In contrast, 20-HETE levels were barely detectable in kidney medulla of either PT-4a12 or WT mice (Fig. 2A), and its production in preglomerular arteries was not different between PT-4a12 and WT mice (Fig. 2B). In addition, urinary 20-HETE excretion was higher in PT-4a12 mice compared with WT littermates (205 ± 54 vs. 83 ± 6 pg·mL−1·24 h−1; P < 0.05), whereas plasma 20-HETE levels were not different (Fig. 2C).
Fig. 2.
A–C: 20-Hydroxyeicosatetraenoic acid (20-HETE) levels in cortex and medulla (A), preglomerular arteries (B), and plasma and urine (C) from male wild-type (WT) and PT-4a12 mice. D: systolic blood pressure (BP) in male WT and PT-4a12 mice. Results are means ± SE; n = 5 mice/group. *P < 0.05 and ***P < 0.001 vs. WT (unpaired Student’s t test).
Overexpression of Cyp4a12 and the accompanying 20-HETE increases in tubular structures led to a hypertensive phenotype in male PT-4a12 mice. As seen in Fig. 2D, male PT-4a12 mice had higher systolic blood pressure than their WT littermates (143 ± 3 vs. 112 ± 4 mmHg; P < 0.001).
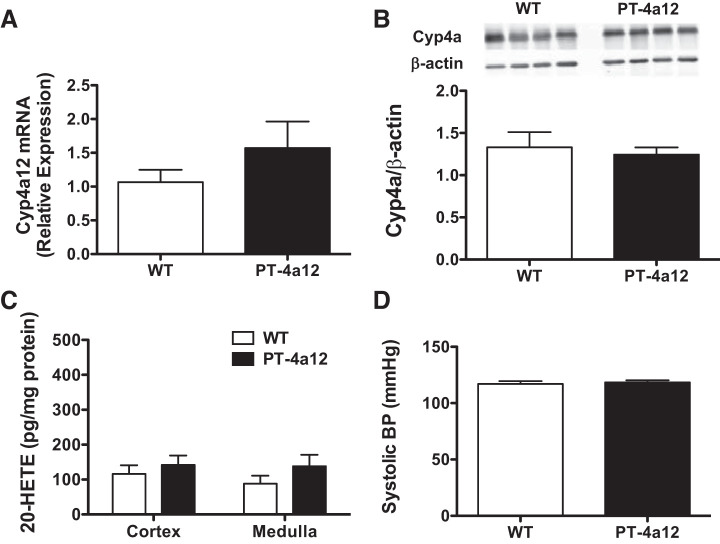
Unexpectedly, female PT-4a12 mice did not show the biochemical and phenotypic changes seen in male PT-4a12 mice. Cyp4a12 mRNA and CYP4A12 protein levels in cortical tissues from female PT-4a12 mice were not different from those in WT littermates (Fig. 3, A and B). Likewise, levels of 20-HETE in renal cortex from female PT-4a12 mice were not different from those in WT littermates (Fig. 3C). Finally, blood pressure of female PT-4a12 mice was not different from that of corresponding WT littermates (Fig. 3D). These results indicate that transgene expression was lacking in female mice, possibly because the PEPCK-Cre is integrated on the X-chromosome and is subject to X-chromosome inactivation (32).
Fig. 3.
A–C: renal cortical levels of Cyp4a12 mRNA (A) and Cyp4a protein (B) and 20-hydroxyeicosatetraenoic acid (20-HETE) content in cortex and medulla (C) from female wild-type (WT) and PT-4a12 mice. D: systolic blood pressure (BP) in 8- to 12-wk-old female WT and PT-4a12 mice. Results are means ± SE; n = 5 mice/group.
Systolic and diastolic blood pressure are increased in male PT-4a12 mice.
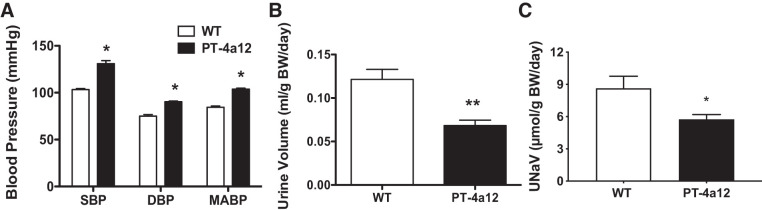
Blood pressure in male PT-4a12 mice was also measured by carotid artery catheterization. As seen in Fig. 4A, systolic, diastolic, and mean blood pressure were significantly higher in male PT-4a12 mice compared with their WT littermates. Systolic blood pressure measured by this method was similar to that obtained by the tail cuff method (Fig. 2D).
Fig. 4.
A: systolic (SBP), diastolic (DBP), and mean (MABP) blood pressure in male wild-type (WT) and PT-4a12 mice. B and C: urine volume (B) and urinary sodium excretion (UNaV; C) in male WT and PT-4a12 mice. Results are means ± SE; n = 5 mice/group. *P < 0.05 and **P < 0.01 vs. WT (unpaired Student’s t test). BW, body weight.
Water intake in male PT-4a12 mice was significantly lower compared with WT littermates (0.114 ± 0.006 vs. 0.142 ± 0.004 mL·g body wt−1·day−1; P < 0.01). Accordingly, urinary volume was lower in male PT-4a12 mice compared with their WT littermates (0.07 ± 0.01 vs. 0.12 ± 0.01 mL·g body wt−1·day−1; P = 0.0068) (Fig. 4B). Sodium excretion was also lower in male PT-4a12 mice compared with their WT littermates (5.75 ± 0.45 vs. 8.65 ± 1.11 µmol·g body wt−1·day−1; P = 0.026) (Fig. 4C). In female PT-4a12 mice urine volume and sodium excretion were not significantly different compared with WT littermates (not shown).
PT-4a12 mice display salt-sensitive hypertension.
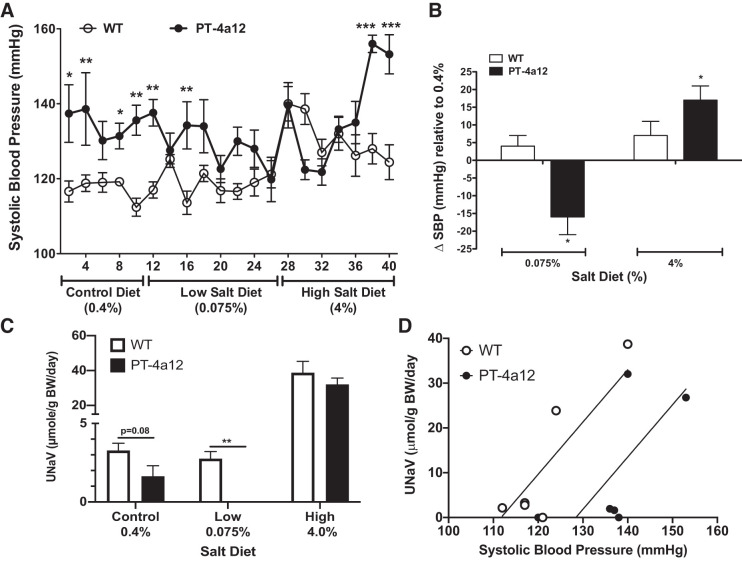
Next, we assessed whether dietary salt affects blood pressure in male PT-4a12 mice. Mice were placed on chow containing normal (0.4%), low (0.075%), or high (4%) sodium for 10, 14, and 12 days, respectively. Compared with blood pressure in male PT-4a12 mice on a normal-salt diet (0.4%), blood pressure at the end of 14 days on low-salt diet was lower (120 ± 6 vs. 136 ± 4 mmHg; P = 0.058), whereas blood pressure was significantly higher at the end of the 12 days on a high-salt diet (153 ± 5 mmHg; P = 0.028) (Fig. 5, A and B). At the last day of low- and high-salt diets, blood pressure in WT mice was not significantly different from that on a normal-salt diet (Fig. 5B). Female PT-4a12 mice showed changes in blood pressure similar to WT mice (not shown). Moreover, the low-salt- and high-salt-induced decrease and increase, respectively, in blood pressure seen in male PT-4a12 mice was significantly different from that in corresponding WT mice (Fig. 5B). These findings suggest that hypertension in male PT-4a12 mice is salt sensitive. Assessment of urinary sodium excretion at day 2 of low-salt diet showed that compared with WT PT-4a12 mice excreted less sodium. However, sodium excretion on day 2 of high-salt diet was not different between WT and PT-4a12 mice (Fig. 5C). This pattern was similar on the last day of each diet (not shown). The relationship between sodium excretion and blood pressure depicted in Fig. 5D indicated that the pressure-natriuresis curve in male PT-4a12 mice shifted to the right but its slope was not different from that of WT mice. The response of female PT-4a12 mice to salt challenges was not different from that of the WT mice (not shown).
Fig. 5.
A: effects of low- and high-salt diets on systolic blood pressure in male PT-4a12 and wild-type (WT) mice. B: changes in systolic blood pressure (SBP) compared with control-salt diet. Results are means ± SE of the difference between the last day of low- or high-salt diet and the last day of control-salt diet. C: urinary sodium excretion (UNaV) on day 2 of each diet. Results are means ± SE; n = 5 mice/group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. WT mice (1-way ANOVA). D: pressure-natriuresis curve depicting average systolic blood pressure vs. average sodium excretion (n = 5 mice/group). BW, body weight.
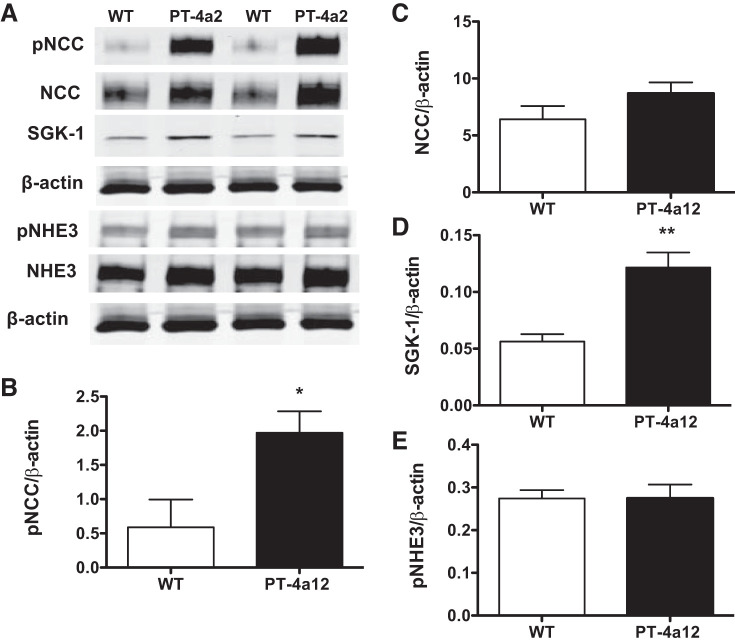
The mechanisms by which 20-HETE contributes to sodium retention are largely unexplored. Recent studies suggested that 20-HETE increases expression and activity of the sodium-chloride cotransporter (NCC), localized in the distal tubule, and may also activate the sodium-hydrogen exchanger (NHE3) in the proximal tubules (31, 38). As seen in Fig. 6, A and B, NCC phosphorylation at threonine 53, which is required for its activation (28), was significantly higher in kidneys from PT-4a12 mice compared with WT littermates, whereas total NCC was not changed significantly (Fig. 6C). Moreover, the level of serum- and glucocorticoid-inducible kinase-1 (SGK-1), which is known to activate numerous ion channels including NCC (21), was significantly increased in kidneys from PT-4a12 mice compared with WT littermates (Fig. 6D). We also assessed phosphorylation of NHE3 at serine 605, which has been reported to have an inhibitory effect on activity of NHE3 (52). There were no differences in the levels of phosphorylated (serine 605) or nonphosphorylated NHE3 between WT and PT-4a12 mice (Fig. 6E).
Fig. 6.
Western blot (A) and densitometry analysis of phosphorylated sodium-chloride cotransporter (pNCC; B), NCC (C), serum- and glucocorticoid-inducible kinase-1 (SGK-1; D), and phosphorylated sodium-hydrogen exchanger (pNHE3; E) in renal cortex from male PT-4a12 and wild-type (WT) mice. Results are means ± SE; n = 3 or 4 mice/group. *P < 0.05 and **P < 0.01 vs. WT (unpaired Student’s t test).
DISCUSSION
Manipulation of 20-HETE levels in animal models pointed to its involvement in promoting both pro- and antihypertensive mechanisms based on its ability to inhibit renal ion transport and promote vasoconstriction, respectively (7). To this end, global and vascular-specific overexpression of CYP4A/F-20-HETE synthase in rats and mice leads to increased blood pressure (15, 17, 40, 44, 50). On the other hand, selective targeting of the kidney with 20-HETE biosynthesis inhibitors produces hypertension and promotes salt-sensitive hypertension, suggesting that deficiency in renal 20-HETE is responsible, in part, for the salt sensitivity of blood pressure in Dahl SS rats (14, 43, 47). The underlying mechanism for 20-HETE antihypertensive actions is believed to be primarily related to 20-HETE-mediated inhibition of NKCC in the thick ascending limb of the loop of Henle (TALH) and K channels in the distal tubules, which consequently promotes natriuresis (1, 6). However, several studies suggested that 20-HETE has effects on ion transport in the proximal tubules, including inhibition of Na+-K+-ATPase and activation of NHE3, which also may contribute to blood pressure regulation (26, 30, 31, 33, 39). The present study is the first to demonstrate that targeted overexpression of Cyp4a12-20-HETE to renal tubules leads to hypertension that appears to be salt sensitive. It challenges the conventional paradigm for tubular 20-HETE that was extensively studied in Dahl SS rats and suggests that tubule-derived 20-HETE may have other effects that counteract this paradigm.
In the present study, targeting of Cyp4a12 to the proximal tubule was achieved by crossing PEPCK-Cre+/− mice with Cyp4a12fl/fl mice. According to Rankin et al. (32), PEPCK-Cre is expressed in most S1 and S2 segments and all S3 segments of the renal proximal tubule, with little or no expression in the early renal distal tubule or the medullary thick ascending limb of Henle. Indeed, male mice having both Cre-recombinase and Cyp4a12-flox genes (PEPCK-Cre+/−-Cyp4a12fl/fl) displayed increased levels of Cyp4a12 mRNA and protein in cortical tissues as well as in isolated proximal tubules. Interestingly, Cyp4a12 mRNA levels were also significantly elevated in microdissected distal tubules but not present in the renal vasculature. This pattern of Cyp4a12 overexpression was not observed in female PT-4a12 mice, and as indicated above, it is likely due to the X-linkage of PEPCK as reported (32). Overall, our results clearly indicate that expression driven by the PEPCK promoter in male PT-4a12 mice is confined to renal tubular structures but not solely to the proximal tubules. The significant upregulation of Cyp4a12 in the distal tubules of PT-4a12 mice may be the result of overflow of 20-HETE from the proximal tubule, which, in turn, acts as a positive feedback regulator of its biosynthetic enzyme through activation of PPARα (25, 53), a known transcriptional activator of CYP4A in animals (12, 13). The Cyp4a12 broad tubular expression observed in this study may have implications regarding the observed hypertensive phenotype of these transgenic mice (vide infra). Importantly, the increased tubular expression of Cyp4a12 in male PT-4a12 mice was accompanied with increased 20-HETE levels in cortical tissues and urine, with no changes in 20-HETE production in the renal vasculature, further confirming the tubular specificity of the PEPCK promoter.
The key finding in this manuscript is that male PT-4a12 mice are hypertensive and that the hypertension is salt sensitive. Compared with WT, male PT-4a12 mice displayed higher systolic and diastolic blood pressure that was accompanied by decreased urinary volume and urinary sodium. The hypertension in male PT-4a12 mice was salt sensitive, as systolic blood pressure decreased when mice were placed on a low-salt diet and increased when they were placed on a high-salt diet. Accordingly, the pressure-natriuresis curve shifted to the right to afford sodium balance at higher pressure. The mechanism of salt sensitivity as a consequence of targeting overexpression of the CYP4A12–20-HETE synthase to the proximal tubule is unclear. NHE3 is the major sodium transporter in this segment of the nephron and is responsible for ~50% reabsorption of filtered sodium in this segment (23). In animal models, increased NHE3 activity leads to hypertension, whereas proximal tubule-specific deletion of NHE3 attenuates ANG II-mediated hypertension (22). The effect of 20-HETE on NHE3 regulation is unclear; reports have assigned to 20-HETE both inhibitory and stimulatory effects on NHE3 (3, 30, 31, 53). In this study, we did not observe differences between WT and PT-4a12 mice in NHE3 expression or the degree of phosphorylated NHE3 at serine 605, which is considered necessary for NHE3 inactivation. However, we cannot exclude the possibility that NHE3 contributes to the salt conservation seen in the PT-4a12 mice, since its regulation is quite complex. As for the contribution of NCC, Savas et al. (38) demonstrated increased NCC activity in mice overexpressing the human CYP4A11 and suggested that the salt-sensitive hypertension displayed by these mice is due, in part, to NCC activation by 20-HETE. Interestingly, the PT-4a12 mice showed a significant overexpression of Cyp4a12 in the distal tubules, where NCC is primarily localized. Furthermore, the overexpression of CYP4A12-20-HETE synthase was associated with elevated SGK-1, which phosphorylates NCC and stimulates its activity (28). Increased activity of NCC may result in reduced sodium excretion and urine volume, which corresponds to elevated baseline systolic blood pressure measurement.
We cannot exclude the possibility that excess production of 20-HETE in the proximal tubule may also affect other segments along the nephron, such as the thick ascending limb of loop of Henle’s loop (TALH), where early studies showed that 20-HETE prevents K+ efflux and Na+ reabsorption by inhibiting the large-conductance 70-pS K+ channel and the Na+-K+-2Cl− cotransporter (NKCC2) (5, 6, 45). Accordingly, the antihypertensive mechanism of 20-HETE has been attributed in part to its ability to inhibit sodium retention at the level of the TALH. It is possible that the salt-sensitive blood pressure response and the adjusted sodium excretion are the consequences of an equilibrium developing between 20-HETE natriuretic and antinatriuretic bioactions. A similar argument has been proposed in a study by Wu et al. (51), in which they used mice overexpressing the human CYP4F2-20-HETE synthase, which are hypertensive, and showed that 20-HETE exerts natriuresis via downregulation of NKCC2 in renal adaptation to elevated Na+ intake.
In humans, the genetic variants of CYP4A11 and CYP4F2 are reportedly associated with salt-sensitive hypertension and hypertension, respectively (8, 19). The F434S variant (rs1126742) of CYP4A11 as well as the V433M variant (rs2108622) of CYP4F2 yields proteins with lower catalytic efficiency and reduced 20-HETE biosynthesis in vitro. However, whereas the salt-sensitive hypertension in subjects carrying the CYP4A11 rs1126742 allele is associated with reduced urinary 20-HETE, the hypertension in subjects carrying the CYP4F2 rs2108622 allele is associated with increased urinary 20-HETE (46). One explanation for the increased 20-HETE levels in subjects with the CYP4F2 rs2108622 allele is that there is a compensatory upregulation of CYP4A11 that leads to increased 20-HETE biosynthesis. The PT-4a12 mouse model somewhat mirrors the reported hypertension and increased urinary 20-HETE levels in subjects with the CYP4F2 rs2108622 allele and therefore presents a suitable animal model to study the antinatriuretic actions of tubular 20-HETE as well as its effects on renal hemodynamics. Both may represent the driving force for the hypertension and sodium conservation in this model.
Perspectives and Significance
Hypertension has become one of the most pressing public health challenges and remains the leading cause of deaths (10.4 million/yr) globally (9). Alarmingly, current treatments fail to control hypertension in more than a fifth of patients worldwide, making it critical to explore novel targets to lower BP (4). 20-HETE, a bioactive arachidonic acid metabolite, has been extensively studied for its role in BP regulation through actions on the microvasculature and the renal tubules. The precise role of 20-HETE in the renal tubules is unclear, with some studies indicating an antihypertensive role linked to inhibition of Na+-K+-ATPase, NKCC, and apical K channel whereas others point to a prohypertensive role linked to activation of NHE3 and NCC. We show that proximal tubule-targeted overexpression of Cyp4a12, the primary murine 20-HETE synthase, leads to increased proximal and distal tubular expression of CYP4A12 and elevated levels of urinary 20-HETE and to salt-sensitive hypertension. The mechanism by which 20-HETE contributes to salt sensitivity of hypertension may be related to its ability to activate NCC in the distal tubules (38). Further studies using the recently identified 20-HETE receptor, 20-HETE antagonists, and 20-HETE biosynthesis inhibitors will provide more mechanistic insights on 20-HETE-mediated antinatriuretic action.
GRANTS
This work was supported by National Institutes of Health Grants PO1 HL-034300 (to M. L. Schwartzman) and RO1 HL-139793 (to M. L. Schwartzman) and a diversity supplement to V. Garcia (HL-139793-1S).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L.S. conceived and designed research; A.G., K.A., S.H., S.K., and V.P. performed experiments; A.G., K.A., J.V.P., S.H., S.K., V.P., V.G., and M.L.S. analyzed data; J.V.P., V.P., V.G., A.N., and M.L.S. interpreted results of experiments; A.G., K.A., S.H., and S.K. prepared figures; A.G. and M.L.S. drafted manuscript; A.G., K.A., J.V.P., V.G., A.N., and M.L.S. edited and revised manuscript; K.A., J.V.P., S.H., V.G., A.N., and M.L.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the intellectual input of Dr. WenHui Wang and Dr. Daohong Lin regarding the assessment of salt sensitivity of hypertension. We also thank Dr. Xiaotong Su for technical assistance in performing microdissection of renal tubules.
REFERENCES
- 1.Amlal H, Legoff C, Vernimmen C, Paillard M, Bichara M. Na+-K+(NH4+)-2Cl- cotransport in medullary thick ascending limb: control by PKA, PKC, and 20-HETE. Am J Physiol Cell Physiol 271: C455–C463, 1996. doi: 10.1152/ajpcell.1996.271.2.C455. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila JH, Wang W, Falck JR. Arachidonic acid monooxygenase: genetic and biochemical approaches to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediat 120: 40–49, 2015. doi: 10.1016/j.prostaglandins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol 287: R58–R68, 2004. doi: 10.1152/ajpregu.00713.2003. [DOI] [PubMed] [Google Scholar]
- 4.Dzau VJ, Balatbat CA. Future of hypertension. Hypertension 74: 450–457, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13437. [DOI] [PubMed] [Google Scholar]
- 5.Escalante B, Erlij D, Falck JR, McGiff JC. Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol Cell Physiol 266: C1775–C1782, 1994. doi: 10.1152/ajpcell.1994.266.6.C1775. [DOI] [PubMed] [Google Scholar]
- 6.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science 251: 799–802, 1991. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 7.Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens 24: 37–46, 2015. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O’Donnell CJ, Brown NJ, Waterman MR, Capdevila JH. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation 111: 63–69, 2005. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 9.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1789–1858, 2018. https://oi.org/10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilani A, Pandey V, Garcia V, Agostinucci K, Singh SP, Schragenheim J, Bellner L, Falck JR, Paudyal MP, Capdevila JH, Abraham NG, Laniado Schwartzman M. High-fat diet-induced obesity and insulin resistance in CYP4a14−/− mice is mediated by 20-HETE. Am J Physiol Regul Integr Comp Physiol 315: R934–R944, 2018. doi: 10.1152/ajpregu.00125.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu R, Wei Y, Jiang H, Balazy M, Wang W. Role of 20-HETE in mediating the effect of dietary K intake on the apical K channels in the mTAL. Am J Physiol Renal Physiol 280: F223–F230, 2001. doi: 10.1152/ajprenal.2001.280.2.F223. [DOI] [PubMed] [Google Scholar]
- 12.Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol 75: 2263–2275, 2008. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Hardwick JP, Eckman K, Lee YK, Abdelmegeed MA, Esterle A, Chilian WM, Chiang JY, Song BJ. Eicosanoids in metabolic syndrome. Adv Pharmacol 66: 157–266, 2013. doi: 10.1016/B978-0-12-404717-4.00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoagland KM, Flasch AK, Roman RJ. Inhibitors of 20-HETE formation promote salt-sensitive hypertension in rats. Hypertension 42: 669–673, 2003. doi: 10.1161/01.HYP.0000084634.97353.1A. [DOI] [PubMed] [Google Scholar]
- 15.Holla VR, Adas F, Imig JD, Zhao X, Price E Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA 98: 5211–5216, 2001. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat 120: 9–16, 2015. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol 297: F875–F884, 2009. doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito O, Alonso-Galicia M, Hopp KA, Roman RJ. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol Renal Physiol 274: F395–F404, 1998. doi: 10.1152/ajprenal.1998.274.2.F395. [DOI] [PubMed] [Google Scholar]
- 19.Laffer CL, Gainer JV, Waterman MR, Capdevila JH, Laniado-Schwartzman M, Nasjletti A, Brown NJ, Elijovich F. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension 51: 767–772, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laffer CL, Laniado-Schwartzman M, Wang MH, Nasjletti A, Elijovich F. 20-HETE and furosemide-induced natriuresis in salt-sensitive essential hypertension. Hypertension 41: 703–708, 2003. doi: 10.1161/01.HYP.0000051888.91497.47. [DOI] [PubMed] [Google Scholar]
- 21.Lang F, Shumilina E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J 27: 3–12, 2013. doi: 10.1096/fj.12-218230. [DOI] [PubMed] [Google Scholar]
- 22.Li XC, Zhu D, Chen X, Zheng X, Zhao C, Zhang J, Soleimani M, Rubera I, Tauc M, Zhou X, Zhuo JL. Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) in the kidney attenuates Ang II (angiotensin II)-induced hypertension in mice. Hypertension 74: 526–535, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41: 175–193, 2005. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 25.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos 35: 1126–1134, 2007. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 26.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+,K+-ATPase. J Clin Invest 99: 1224–1230, 1997. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omata K, Abraham NG, Schwartzman ML. Renal cytochrome P-450-arachidonic acid metabolism: localization and hormonal regulation in SHR. Am J Physiol Renal Physiol 262: F591–F599, 1992. doi: 10.1152/ajprenal.1992.262.4.F591. [DOI] [PubMed] [Google Scholar]
- 28.Pacheco-Alvarez D, Cristóbal PS, Meade P, Moreno E, Vazquez N, Muñoz E, Díaz A, Juárez ME, Giménez I, Gamba G. The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 29.Pandey V, Garcia V, Gilani A, Mishra P, Zhang FF, Paudyal MP, Falck JR, Nasjletti A, Wang WH, Schwartzman ML. The blood pressure-lowering effect of 20-HETE blockade in Cyp4a14(−/−) mice is associated with natriuresis. J Pharmacol Exp Ther 363: 412–418, 2017. doi: 10.1124/jpet.117.243618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quigley R, Baum M, Reddy KM, Griener JC, Falck JR. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol Renal Physiol 278: F949–F953, 2000. doi: 10.1152/ajprenal.2000.278.6.F949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley R, Chakravarty S, Zhao X, Imig JD, Capdevila JH. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron, Physiol 113: 23–28, 2009. doi: 10.1159/000235774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro CM, Dubay GR, Falck JR, Mandel LJ. Parathyroid hormone inhibits Na+-K+-ATPase through a cytochrome P-450 pathway. Am J Physiol Renal Physiol 266: F497–F505, 1994. doi: 10.1152/ajprenal.1994.266.3.F497. [DOI] [PubMed] [Google Scholar]
- 34.Rocic P, Schwartzman ML. 20-HETE in the regulation of vascular and cardiac function. Pharmacol Ther 192: 74–87, 2018. doi: 10.1016/j.pharmthera.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 10: 63S–67S, 1997. doi: 10.1016/S0895-7061(97)00077-0. [DOI] [PubMed] [Google Scholar]
- 36.Roman RJ, Hoagland KM, Lopez B, Kwitek AE, Garrett MR, Rapp JP, Lazar J, Jacob HJ, Sarkis A. Characterization of blood pressure and renal function in chromosome 5 congenic strains of Dahl S rats. Am J Physiol Renal Physiol 290: F1463–F1471, 2006. doi: 10.1152/ajprenal.00360.2005. [DOI] [PubMed] [Google Scholar]
- 37.Roman RJ, Ma YH, Frohlich B, Markham B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension 21: 985–988, 1993. doi: 10.1161/01.HYP.21.6.985. [DOI] [PubMed] [Google Scholar]
- 38.Savas Ü, Wei S, Hsu MH, Falck JR, Guengerich FP, Capdevila JH, Johnson EF. 20-Hydroxyeicosatetraenoic acid (HETE)-dependent hypertension in human cytochrome P450 (CYP) 4A11 transgenic mice: normalization of blood pressure by sodium restriction, hydrochlorothiazide, or blockade of the type 1 angiotensin II receptor. J Biol Chem 291: 16904–16919, 2016. doi: 10.1074/jbc.M116.732297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature 314: 620–622, 1985. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension 56: 871–878, 2010. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stec DE, Deng AY, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension 27: 564–568, 1996. doi: 10.1161/01.HYP.27.3.564. [DOI] [PubMed] [Google Scholar]
- 42.Stec DE, Flasch A, Roman RJ, White JA. Distribution of cytochrome P-450 4A and 4F isoforms along the nephron in mice. Am J Physiol Renal Physiol 284: F95–F102, 2003. doi: 10.1152/ajprenal.00132.2002. [DOI] [PubMed] [Google Scholar]
- 43.Stec DE, Mattson DL, Roman RJ. Inhibition of renal outer medullary 20-HETE production produces hypertension in Lewis rats. Hypertension 29: 315–319, 1997. doi: 10.1161/01.HYP.29.1.315. [DOI] [PubMed] [Google Scholar]
- 44.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Lu M. Effect of arachidonic acid on activity of the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol 106: 727–743, 1995. doi: 10.1085/jgp.106.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51: 1393–1398, 2008. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 47.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302: R1209–R1218, 2012. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev 22: 1–12, 2014. doi: 10.1097/CRD.0b013e3182961659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol 24: 1288–1296, 2013. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J, Liu X, Lai G, Yang X, Wang L, Zhao Y. Synergistical effect of 20-HETE and high salt on NKCC2 protein and blood pressure via ubiquitin-proteasome pathway. Hum Genet 132: 179–187, 2013. doi: 10.1007/s00439-012-1238-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999. doi: 10.1074/jbc.274.7.3978. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y, Huang H, Chang HH, Du J, Wu JF, Wang CY, Wang MH. Induction of renal 20-hydroxyeicosatetraenoic acid by clofibrate attenuates high-fat diet-induced hypertension in rats. J Pharmacol Exp Ther 317: 11–18, 2006. doi: 10.1124/jpet.105.095356. [DOI] [PubMed] [Google Scholar]