Abstract
Diuretics and renin-angiotensin system blockers are often insufficient to control the blood pressure (BP) in salt-sensitive (SS) subjects. Abundant data support the proposal that the level of atrial natriuretic peptide may correlate with the pathogenesis of SS hypertension. We hypothesized here that increasing atrial natriuretic peptide levels with sacubitril, combined with renin-angiotensin system blockage by valsartan, can be beneficial for alleviation of renal damage in a model of SS hypertension, the Dahl SS rat. To induce a BP increase, rats were challenged with a high-salt 4% NaCl diet for 21 days, and chronic administration of vehicle or low-dose sacubitril and/or valsartan (75 μg/day each) was performed. Urine flow, Na+ excretion, and water consumption were increased on the high-salt diet compared with the starting point (0.4% NaCl) in all groups but remained similar among the groups at the end of the protocol. Upon salt challenge, we observed a mild decrease in systolic BP and urinary neutrophil gelatinase-associated lipocalin levels (indicative of alleviated tubular damage) in the valsartan-treated groups. Sacubitril, as well as sacubitril/valsartan, attenuated the glomerular filtration rate decline induced by salt. Alleviation of protein cast formation and lower renal medullary fibrosis were observed in the sacubitril/valsartan- and valsartan-treated groups, but not when sacubitril alone was administered. Interestingly, proteinuria was mildly mitigated only in rats that received sacubitril/valsartan. Further studies of the effects of sacubitril/valsartan in the setting of SS hypertension, perhaps involving a higher dose of the drug, are warranted to determine if it can interfere with the progression of the disease.
Keywords: atrial natriuretic peptide, sacubitril, salt-sensitive hypertension, valsartan
INTRODUCTION
Hypertension is becoming more prevalent in the United States and the world, and thus increases the risk of cardiovascular complications such as heart disease and stroke in the population. According to the Centers for Disease Control and Prevention, one in every three Americans suffers from high blood pressure (74). The likelihood of hypertension increases with a consistent consumption of high salt (58). A specific subgroup of individuals with hypertension classified as “salt sensitive” (SS) exhibit significant changes in blood pressure in response to salt intake and are at a higher risk for renal disease (14). For years, diuretics and the antagonism of the renin-angiotensin-aldosterone system (RAAS) have been recognized, although not universally effective, treatments for SS hypertension (70). There is a pressing need to develop new, potent, and multifarious treatments for the growing and diverse SS subpopulation.
One of the important factors that has been shown to play a role in SS hypertension is atrial natriuretic peptide (ANP). ANP is an osmoregulatory protein that is encoded by the Nppa gene; it has been associated with regulation of electrolyte homeostasis and blood pressure (43, 69). ANP lowers blood pressure by promoting salt excretion and is generally considered a counteractant that keeps the RAAS in check (42). ANP is synthesized by atrial and, to a lower extent, ventricular cardiomyocytes in a 151-amino acid pre-pro-peptide form (6), which is proteolytically cleaved by corin to yield active 28-amino acid-long ANP (8, 12, 23, 49). Interestingly, corin knockout as well as ANP knockout mice are hypertensive and salt sensitive (5, 28, 71).
ANP is a particularly interesting hormone in the context of SS hypertension, as its plasma concentration correlates with salt intake (2, 9, 47). Animal studies have shown that lack of ANP may result in SS hypertension and also leads to biventricular hypertrophy and cardiomyocyte enlargement (independently of a blood pressure increase) (39). Human studies have revealed that in response to a high salt intake, secretion of ANP may be blunted in Black SS individuals with hypertension (28, 59). There are abundant data supporting a pathogenic role for a low level of ANP in salt sensitivity; for instance, the Dallas Heart study showed that Black individuals had significantly lower natriuretic peptide levels than White and Hispanic individuals and concluded that this may lead to a greater susceptibility to salt retention and hypertension (18). Furthermore, a blunted ANP response to acute volume expansion has been reported in SS individuals, particularly after a 5-day-long high-salt diet (72). Most interestingly, information derived from the Framingham Offspring Cohort was able to predict SS hypertension by lower levels of circulating NH2-terminal ANP (31).
Therefore, ANP can be crucial for the condition of salt sensitivity, which makes it a compelling therapeutic target (14). However, ANP itself cannot be used as a treatment because of its short (<5 min) plasma half-life (50); thus, current ANP-related therapies are based on targeting the enzymes responsible for its degradation. Besides receptor-mediated degradation, ANP is cleared by the extracellular proteolytic enzymes neprilysin (NEP) and insulin-degrading enzyme (1, 50). Combinations of NEP inhibitors (such as sacubitril) and RAAS inhibitors [for instance, angiotensin receptor blockers (ARBs), such as losartan or valsartan] have been recently deemed successful in treating heart failure (19, 21). One such medication, ENTRESTO or LCZ-696 (1:1 combination of sacubitril and valsartan, also known as angiotensin receptor-NEP inhibitor), is approved by the Federal Drug Administration for heart failure treatment (15). It is important that NEP inhibitors should be administered together with a RAAS inhibitor: since NEP inhibitors increase circulating ANP and then lower blood pressure, this evokes a counteracting response from the RAAS, which needs to be prevented by an ARB or angiotensin-converting enzyme inhibitor (21). Medications based on ARBs and NEP inhibitors show potential for patients with chronic kidney disease (CKD) and might have an effect on hypertension (21, 27, 29, 62). Therefore, there is reasonable evidence to justify testing potential beneficial effects of increasing the circulating ANP levels using NEP inhibitors in renal disease, and especially in SS hypertension, where existing medications are often insufficient to properly control blood pressure. In this study, we focused on the effects of LCZ-696 on the development of renal damage in Dahl SS rats, an established rodent model mimicking major aspects of human SS hypertension.
MATERIALS AND METHODS
Animal procedures and the experimental protocol.
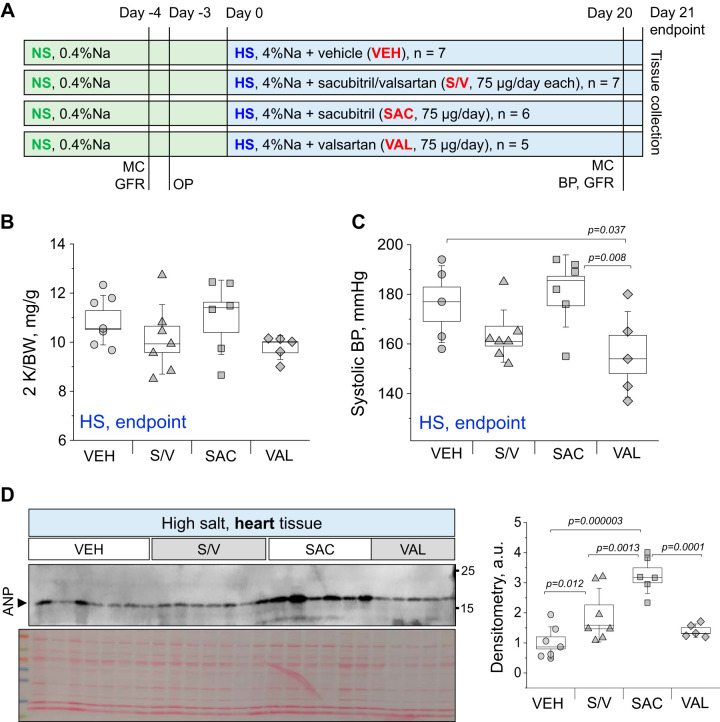
Male Dahl SS rats were obtained from Charles River Laboratories at 7 wk of age and were kept on a purified AIN-76A-based 0.4% NaCl diet (normal-salt diet, catalog no. 113755, Dyets) for a week. To induce SS hypertension at the age of 8 wk, rats were switched to a purified AIN-76A-based 4% NaCl diet (high-salt diet, Dyets) for 21 days. Figure 1A shows the experimental protocol schematic. The prospective groups were administered vehicle, sacubitril, valsartan, or a 1:1 mix of sacubitril/valsartan at 75 µg/day (each) via an osmotic pump (2ML4, Alzet, 2.5 µl/h) installed subcutaneously 3 days before the high-salt dietary challenge. Glomerular filtration rate (GFR) was measured a day before the osmotic pump surgery and at day 20 of high-salt diet; urine was collected in metabolic cages (Lab Products) for 24 h before the GFR measurements (following 24 h of metabolic cage adjustment). Animals were weighed the day of GFR measurements. All experimental procedures regarding Dahl SS rats were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and adhered to the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals.
Fig. 1.
Experimental protocol and basic end-point parameters. A: schematic representation of the experimental protocol. NS, normal-salt diet; HS, high-salt diet; MC, metabolic cage collections; GFR, glomerular filtration rate measurements; BP, blood pressure measurement; OP, osmotic pump installation. B and C: end-point two kidney-to-total body weight ratio (2 K/BW; B) and systolic blood pressure value (C) in vehicle (VEH)-, sacubitril/valsartan (S/V)-, sacubitril (SAC)-, and valsartan (VAL)-treated groups. D: Western blot analysis showing atrial natriuretic peptide (ANP) expression in end point-collected heart tissues from VEH-, S/V-, SAC-, and VAL-treated groups. a.u., arbitrary units. Each point on the graphs denotes data obtained from one animal. One-way ANOVA with a Holm-Sidak test was used for significance comparisons. P values are shown for comparisons where P < 0.05.
Blood pressure measurements, kidney flush, and tissue isolation.
Blood pressure measurements via tail-cuff plethysmography (IITC Life Science) were obtained from each rat immediately before the end-point kidney flush. At the completion of the high-salt diet challenge for 21 days, rats were surgically prepared for a kidney flush and arterial blood collection. Briefly, rats were anesthetized, and the descending aorta was catheterized as previously described (11). Kidneys were first flushed with PBS (2 mL/min per kidney) until blanched via a catheter in the abdominal aorta. The kidneys were then excised and decapsulated. Kidney tissues were then snap frozen in liquid nitrogen or stored in 10% formalin for histological assessment.
GFR measurements.
GFR was measured in unrestrained conscious rats using a high-throughput method featuring detection of fluorescent FITC-labeled inulin (TdB Consultancy, Uppsala, Sweden) clearance from blood. The method was adapted for rats from a protocol previously described for mice by Rieg (55) and previously published by us (24). Predialyzed 20 mg/mL of FITC-inulin solution in saline (2 µL/1 g body wt) was administered by a bolus tail vein injection to rats briefly anesthetized with isoflurane. Immediately after the injection, anesthesia was discontinued and animals were allowed to regain consciousness. Then, 10 µL of blood were collected 3, 5, 8, 16, 25, 40, 60, 80, 100, and 120 min after the injection by tail bleed. Next, plasma was separated, and inulin clearance was quantified by FITC intensity. Fluorescence measurements were performed using a NanoDrop 3300 Fluorospectrometer (ThermoFisher Scientific, Wilmington, DE). GFR was then calculated from the observed decrease in FITC fluorescence using a two-compartment model (the initial fast decay representing the redistribution of FITC-inulin from the intravascular compartment to the extracellular fluid and the slower phase reflecting clearance from plasma). GFR curves were approximated with a biexponential decay function using OriginPro 9.0 (OriginLab, Northhampton, MA) software, and GFR values (in mL/min) were obtained from the fitting parameters using the previously described equation (24).
Tissue processing, histological staining, and analysis.
Rat kidneys were fixed in zinc formalin, paraffin embedded, sectioned, and then mounted on slides following standard procedures. Slides were stained with Masson trichrome and imaged on a Nikon Eclipse Ti-2 microscope. Tissues were randomized and coded before being submitted for blocking, sectioning, and staining. A Nikon Plan Fluor optical lens of ×20 was used to assess glomeruli (0.50 numerical aperture, 2.1 working distance). Glomeruli were blindly scored on a scale of 0−4. A score of 0 represented a healthy glomerulus with no sclerosis. A score of 1 represented 1−25% mesangial expansion and sclerosis compared with a score of 2, which represented 26−50% mesangial expansion and sclerosis. A score of 3 was given if there was 51−75% mesangial expansion and sclerosis, and a score 4 represented 76−100% glomerular mesangial expansion and sclerosis. For the analysis of fibrosis, picrosirius red-stained kidneys were imaged with a Nikon Eclipse Ti-2 microscope for further use in digital analysis. Slides were incubated in a solution of 0.2% phosphomolybdic acid (RT 26357-01, EMS) for 3 min. Slides were then rinsed and transferred to a solution containing 0.1% sirius red in saturated picric acid (RT 26357-02, EMS) for 90 min. Slides were then immediately put into acidified water for 2 min. Fiji software (NIH) was used to determine the percentage of fibrosis: the region of interest (or the whole kidney) was selected, and the area of the region of interest was measured. Using the Color Deconvolution Plugin in ImageJ, the area of fibrosis was then identified using a Threshold tool, and the percentage of the total area was calculated (n = 5−7, ×10 images from each kidney were used for analysis). Protein casts were assessed from trichrome-stained slides scanned with a Perkin-Elmer Vectra Polaris Automated Quantitative Pathology Imaging System Slide scanner and then scored separately and blindly by two people on a scale of 0−4. A score of 0 represented a healthy kidney sample with no protein casts visible. A score of 1 represented a kidney sample with <5% protein casts compared with a score of 2, which represented 5−10% of the sample containing protein casts. A score of 3 was given to a sample with 11−15% protein casts, and a score of 4 was given to a kidney with >20% protein casts.
Urinalysis (electrolytes, creatinine, and protein) and plasma analysis (electrolytes and blood urea nitrogen).
For the assessment of proteinuria, urine samples were centrifuged at 1,000 g for 3 min to remove debris, and supernatants were used for the estimation of proteinuria by SDS-PAGE. Each urine sample (10 µL) was mixed with Laemmli buffer (2× with β-mercaptoethanol) at a ratio of 1:1 and heated at 90°C for 5 min. Samples were then loaded into wells of a Criterion 26-well gel (catalog no. 3450044) and run at 120 V using a High Current Bio-Rad PowerPac electrophoresis power supply for 1 h. BSA (5 µg) was used as a reference point. The gel was then stained with Coomassie blue solution [0.01% Coomassie brilliant blue R 250, 50% (vol/vol) methanol, and 10% (vol/vol) glacial acetic acid] for 30 min at room temperature, and an image was acquired with the LI-COR Odyssey imaging system. Analysis was performed in Fiji (NIH); values were adjusted for 24-h urinary flow rate.
Urine and plasma electrolytes were evaluated with a Carelyte analyzer from Diamond Diagnostics (to separate plasma, blood samples obtained from the abdominal aorta before kidney flush were centrifuged immediately after collection at 6,000 g for 5 min, snap frozen in LN2, and stored at −80°C). Plasma creatinine levels were measured using a Quantichrom Creatinine Assay Kit (DICT-500). A standard curve was created from the stock 50 mg/dL creatinine standard (6, 2, 1, 0.5, and 0 mg/dL). Creatinine concentrations were determined by measuring absorbance per the manufacturer’s instructions. Blood urea nitrogen and aldosterone levels were measured using a urea assay kit (KA1652, Abnova) and aldosterone ELISA (ADI900173, Enzo Life Sciences), respectively, according to the manufacturers’ instructions.
Western blot analysis.
After excision, kidneys were cut in 1- to 2-mm slices, and the cortical kidney pieces were pulse sonicated in RIPA buffer containing protease inhibitor cocktail (Roche) on ice and then spin cleared at 10,000 g for 10 min. The resulting supernatant was subjected to SDS-PAGE, transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) for probing with antibodies, and subsequently visualized by enhanced chemiluminescence (Thermo Scientific, Waltham, MA). The following antibodies were used: ANP antibody (PA5-79758, Invitrogen), goat anti-rabbit IgG secondary antibody, horseradish peroxidase (no. 31460, Invitrogen), α-smooth muscle actin antibody (no. 14-9760-82, Invitrogen), and anti-mouse IgG, horseradish peroxidase (W4021B, Promega). Western blot analysis was also performed to determine the presence of kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) in urine. Urine samples were prepared by mixing spin-cleared urine with 2× Laemmli buffer (with β-mercaptoethanol) at a 1:1 ratio; 15 μL of each sample were loaded on the gel. KIM-1 antibody (PA5-793452, Invitrogen) or NGAL antibody (PA5-46938, Invitrogen) were used followed by horseradish peroxidase-conjugated secondary antibodies (no. 31460, Invitrogen).
Statistical analysis.
One-way ANOVA with a Holm- Sidak test post hoc and one-way repeated-measures ANOVA with a Holm-Sidak test were used when applicable. Data are expressed as box plots, with whiskers being SDs, the box representing SE, and the line showing the median. Values of P < 0.05 were considered statistically significant. Origin 2019b was used for all statistical analysis.
RESULTS
Assessment of basic renophysiological parameters after drug administration.
Figure 1A shows the timeline of the experimental protocol (described in detail in methods). Body weight was measured in all groups before the start of the high salt challenge (on the normal-salt diet) and after 21 days on the high-salt diet. Body weight increased significantly at the end of the experiment compared with the normal-salt diet (P < 0.05 for all groups; see Supplemental Fig. 1S in the Supplemental Material, available online at https://doi.org/10.6084/m9.figshare.12049134.v1). End-point kidney-to-body weight and heart-to-body weight ratios and body weights were similar between groups (results shown in Fig. 1B and Supplemental Fig. S1, available online at https://doi.org/10.6084/m9.figshare.12049134.v1). Blood pressure was assessed at the end of the experimental protocol (Fig. 1C), and we found a significant decrease in systolic blood pressure in the valsartan-treated group versus the vehicle-treated group (155.8 ± 7.7 vs. 176.0 ± 6.9 mmHg, respectively). We confirmed a significant increase in the ANP level in animals treated with sacubitril and sacubitril/valsartan (see Fig. 1D for a Western blot for ANP conducted in heart tissue). End-point plasma electrolyte levels are provided in Supplemental Fig. S2 (https://doi.org/10.6084/m9.figshare.12049134.v1) and were found to be similar among the studied groups.
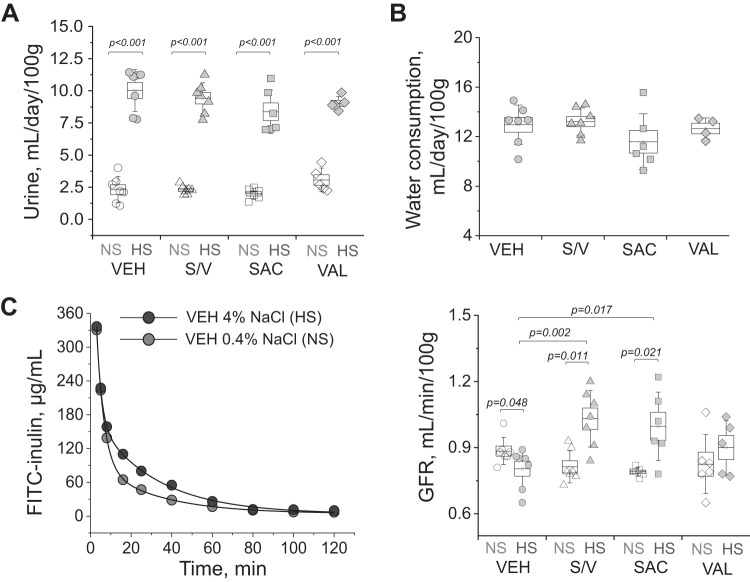
Twenty-four-hour urine flow rate was obtained in metabolic cages (following a 24-h adjustment period) 4 days prior to administration of the dietary challenge as well as on day 21 of the high-salt diet. As expected, all groups showed a statistically significant increase in urine production compared with the normal-salt time point on day 21 of the high salt challenge, while end point values were similar among groups (P < 0.001 for all groups; Fig. 2A). Daily water consumption recorded on day 21 of the high-salt diet was similar among the groups (Fig. 2B). GFR measured before the start of the dietary challenge (Fig. 2C) was similar among the groups (0.88 ± 0.02, 0.81 ± 0.03, 0.83 ± 0.05 and 0.79 ± 0.01 mL·min−1·100 g body wt−1 in the vehicle-, sacubitril/valsartan-, sacubitril-, and valsartan-treated groups, respectively). At the end of the protocol, we observed a decrease in GFR in the control group compared with the normal-salt diet (P = 0.048). Hyperfiltration was noted in the groups that were administered sacubitril (with or without valsartan, P = 0.002 and 0.017 vs. control, respectively), which was attenuated in the valsartan-treated group (0.80 ± 0.03, 1.03 ± 0.05, 0.90 ± 0.05, and 1.00 ± 0.06 mL·min−1·100 g body wt−1 in the vehicle-, sacubitril/valsartan-, sacubitril-, and valsartan-treated groups, respectively).
Fig. 2.
In vivo renal function comparison in experimental groups. A: 24-h urine flow (normalized to body weight) obtained from experimental animals before [normal-salt diet (NS)] and on day 20 after a switch to the high-salt diet (HS) and administration of vehicle (VEH), sacubitril/valsartan (S/V), sacubitril alone (SAC), and valsartan alone (VAL). B: body weight-normalized end-point water consumption in the studied experimental groups. C, left: representative curves of FITC-inulin elimination (VEH-treated animal on the normal-salt diet and at the end of the high-salt diet protocol). C, right, glomerular filtration rate (GFR) measured in experimental animals before (normal-salt diet) and on day 20 after a switch to the high-salt diet and administration of VEH, S/V, SAC, and VAL. Each point on the graphs denotes data obtained from one animal. One-way ANOVA with a Holm-Sidak test was used for significance comparisons. P values are shown for comparisons where P < 0.05.
Urinary osmolar and electrolyte excretion.
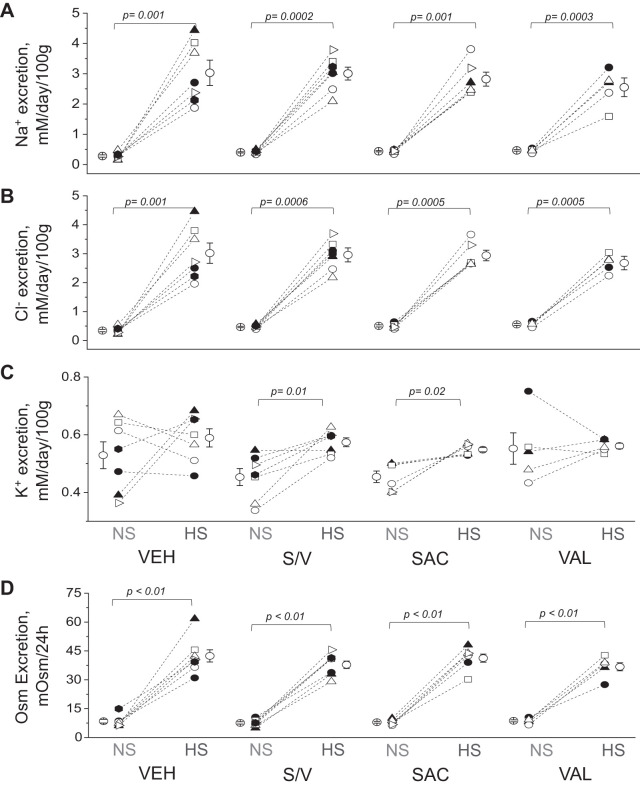
Urine samples obtained in metabolic cage experiments were used to determine electrolyte and osmolar excretion over a 24-h time period (Fig. 3, A−D). We found a significant increase in urinary Na+, Cl−, and osmolar excretion in all urine samples collected from rats fed a high-salt diet compared with a paired point before the dietary salt challenge. Among the groups fed the same diets, excretion values for Na+, Cl−, and osmoles were similar. Interestingly, urinary K+ excretion increased after the high salt challenge in the sacubitril/valsartan-treated group (P = 0.01) and sacubitril-treated group versus the vehicle-treated group (P = 0.02; Fig. 3C).
Fig. 3.
Electrolyte and osmole (Osm) excretion. A−D: excretion of Na+ (A), Cl− (B), K+ (C), and total Osm (D) excretion measured in urine samples collected for 24 h from experimental animals before [normal-salt diet (NS)] and on day 20 after a switch to the high-salt diet (HS) and administration of vehicle (VEH), sacubitril/valsartan (S/V), sacubitril alone (SAC), and valsartan alone (VAL). Each point on the graphs denotes data obtained from one animal. One-way ANOVA with a Holm-Sidak test was used for significance comparisons. Paired data (before-after high-salt diet) were compared using Student’s paired t test. P values are shown for comparisons where P < 0.05.
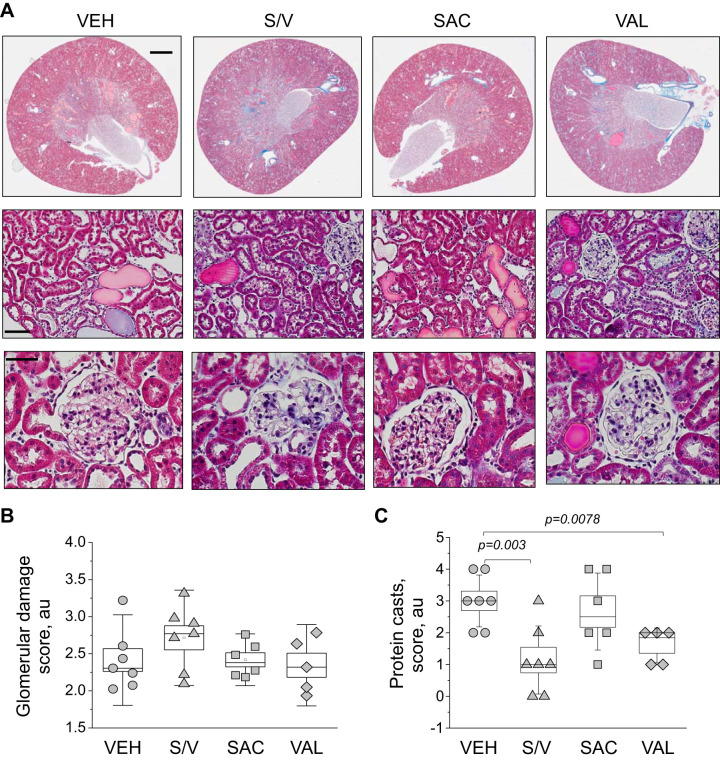
Renal damage.
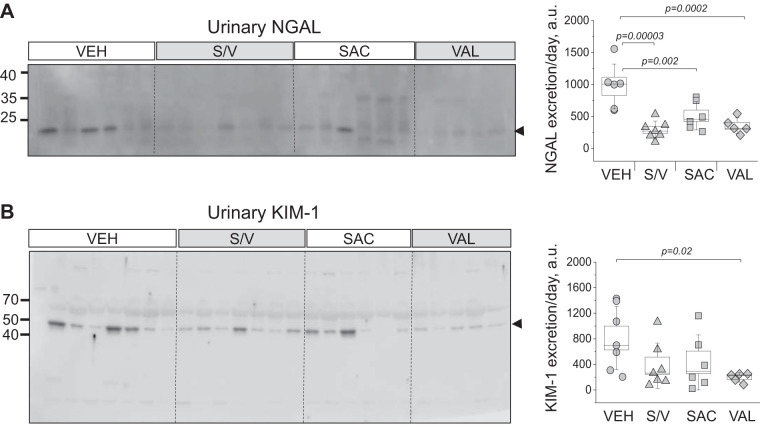
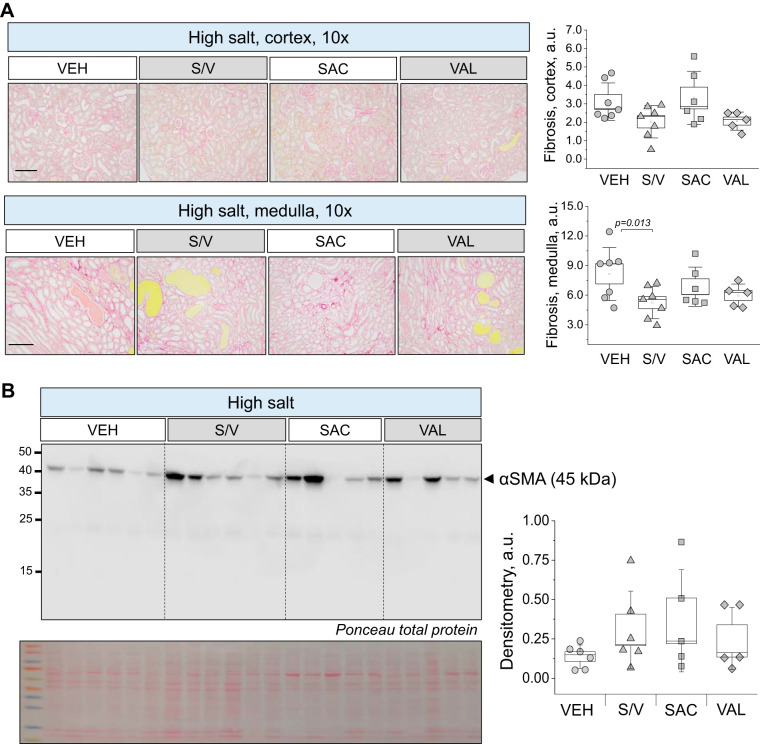
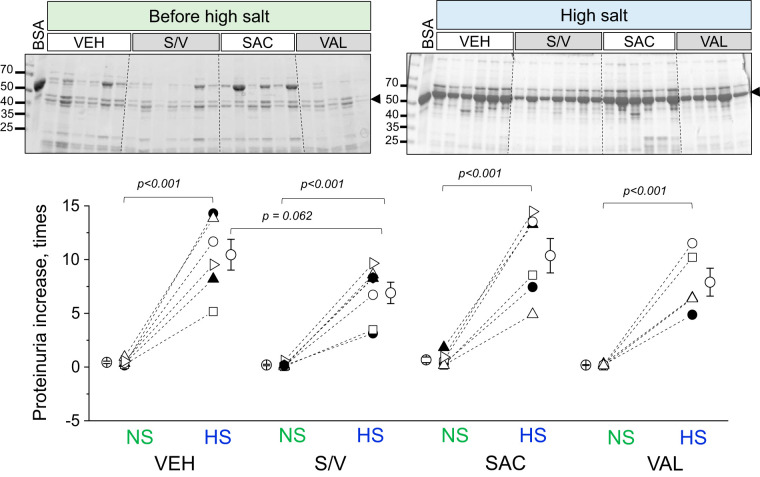
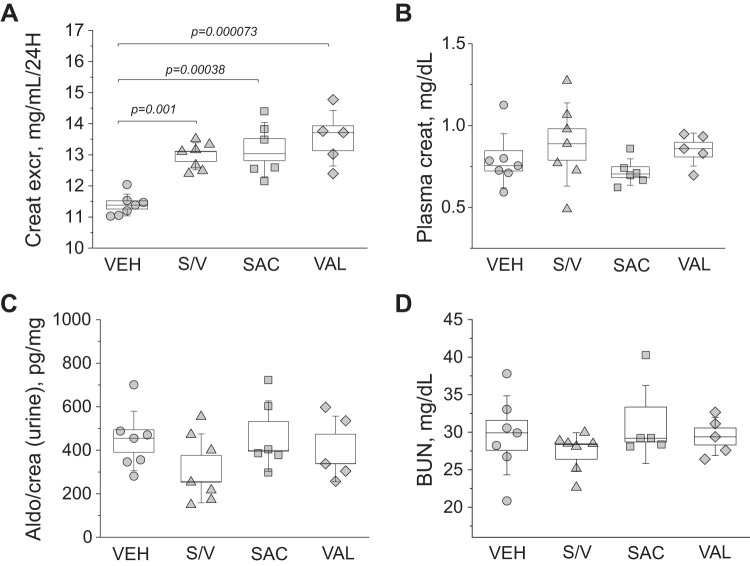
Analysis of renal damage markers in end-point urine samples using Western blots revealed interesting trends. We found a significant decrease in NGAL excretion from rats treated with sacubitril/valsartan, sacubitril, and valsartan versus vehicle-treated rats (data were normalized to urine flow rate; Fig. 4A), indicative of lower tubular damage in these groups. Furthermore, valsartan treatment also decreased KIM-1 excretion compared with the vehicle-treated group (P = 0.02; Fig. 4B). End-point tissues collected from all four groups (high-salt diet) were stained with Masson trichrome to assess glomerular damage and protein cast formation. Blinded glomerular damage scoring revealed similar glomerular lesions across all groups (Fig. 5, A and B). Protein cast analysis showed a dramatic attenuation of medullary and cortical protein cast formation in sacubitril/valsartan- and valsartan-treated groups compared with the control group (Fig. 5C). Picrosirius red staining (Fig. 6A) revealed a significant decrease in medullary, but not cortical, fibrosis of animals treated with sacubitril/valsartan (P = 0.013 vs. the vehicle-treated group). Next, we tested α-smooth muscle actin levels in the renal cortex, and the Western blot revealed a lot of variation in the treated groups versus the control group (Fig. 6B); therefore, no statistical significance was recorded. In accordance with the protein cast analysis, we observed an attenuation of end-point proteinuria in the sacubitril/valsartan-treated group versus the vehicle-treated group (P = 0.062 for the sacubitril/valsartan- vs. vehicle-treated group; Fig. 7, A and B). As shown in Fig. 8A, creatinine excretion was elevated in all treated groups versus the vehicle-treated group, whereas no differences in blood urea nitrogen were recorded (Fig. 8D); plasma creatinine was not different among the groups (Fig. 8B). The urinary aldosterone-to-creatinine ratio was assessed, and the values were similar among the groups (Fig. 8C).
Fig. 4.
Analysis of renal tubular damage markers in the urine. Left: Western blot analyses of urinary neutrophil gelatinase-associated lipocalin (NGAL; A) and kidney injury molecule-1 (KIM-1; B) levels obtained from experimental animals on day 20 after a switch to a high-salt diet and administration of vehicle (VEH), sacubitril/valsartan (S/V), sacubitril alone (SAC), and valsartan alone (VAL). Each lane on the Western blot represents a separate experimental animal. Right: summaries of densitometry values (normalized to 24-h urine flow). a.u., arbitrary units. One-way ANOVA with a Holm-Sidak test was used for significance comparisons. P values are shown for comparisons where P < 0.05.
Fig. 5.
Histological characterization of renal damage with Masson trichrome staining. A: representative images of renal tissues from experimental rats isolated at the end point of the experimental protocol [high-salt diet, upon administration of vehicle (VEH), sacubitril/valsartan (S/V), sacubitril alone (SAC), and valsartan alone (VAL)]. The top row shows scans of coronal midsections of kidneys stained with Masson trichrome (scale bar = 2 mm). The middle and bottom rows demonstrate representative ×10 images taken in the cortical area (scale bar = 100 μm) and enlarged images of glomeruli from the renal cortex (scale bar = 50 µm), respectively. B and C: graphs summarizing the analysis of glomerular damage scoring (B) and protein cast scoring (C). a.u., arbitrary units. One-way ANOVA with a Holm-Sidak test was used for significance comparisons. P values are shown if data were statistically significant. Each point on the graphs denotes data obtained from one animal except from B, where each point is an average of at least 100 glomeruli scored in renal tissue of individual animals. P values are shown for comparisons where P < 0.05.
Fig. 6.
Renal fibrosis analysis in the experimental groups. A, left: representative images of renal tissues stained with picrosirius red. Shown are images of the cortex and medulla (×10, scale bar = 100 μm) obtained from rats at the end point of the experimental protocol [high-salt diet, upon administration of vehicle (VEH), sacubitril/valsartan (S/V), sacubitril alone (SAC), and valsartan alone (VAL)]. A, right: analysis of the staining. a.u., arbitrary units. B, left: expression of α-smooth muscle actin (α-SMA) measured in the renal cortex of the animals at the end point of the experimental protocol. Each lane on the Western blot represents a separate experimental animal. B, right: summaries of densitometry values. Total protein staining (Ponceau) is below the Western image. One-way ANOVA with a Holm-Sidak test was used for significance comparisons. P values are shown for comparisons where P < 0.05.
Fig. 7.
End-point quantification of proteinuria. Top: Western blots obtained from urinary samples collected from experimental animals before [normal-salt diet (NS)] and on day 20 after a switch to the high-salt diet (HS) and administration of vehicle (VEH), sacubitril/valsartan (S/V), sacubitril alone (SAC), and valsartan alone (VAL). BSA (5 μg) was used as a loading control (first lane). Bottom: quantification was performed by normalizing end-point proteinuria values (corrected for urine flow) to the starting point. Each point on the graphs denotes data obtained from one animal. One-way ANOVA with a Holm-Sidak post hoc test was used for significance comparisons among groups on the same salt diet. Paired data (before-after high-salt diet) was compared using a Student’s paired t test. P values are shown for comparisons where P < 0.05.
Fig. 8.
Plasma and urinary creatinine, aldosterone, and blood urea nitrogen (BUN) levels. A−D: creatinine excretion (Creat excr; A, normalized to urine flow), plasma creatinine (creat) level (B), aldosterone-to-creatinine ratio (Aldo/crea; C, in the urine), and BUN (D). All data were obtained at the end point of the experimental protocol. One-way ANOVA with a Holm-Sidak post hoc test was used for significance comparisons among groups. Each point on the graphs denotes data obtained from one animal. P values are shown for comparisons where P < 0.05.
DISCUSSION
The RAAS is a crucial factor for the development of hypertension, as indicated by the successful use of angiotensin-converting enzyme inhibitors and ARBs to decrease blood pressure (13). However, a blunted RAAS is an essential characteristic of SS hypertension and is one of the reasons why ARBs are considered inferior to other treatments, such as Ca2+ channel blockers and diuretics, in the reduction of blood pressure in patients with this form of hypertension (51). Interestingly, the plasma concentration of ANP correlates with salt intake (2, 9, 47). Furthermore, animal studies have shown that a lack of ANP may result in SS hypertension (39), while human studies have revealed that in response to a high salt intake, secretion of ANP may be blunted in SS individuals with hypertension (28, 59). These observations clearly point to the fact that ANP plays a critical role in mitigating the development of SS hypertension (14). Taking into consideration the success of angiotensin receptor-NEP inhibitors to treat various cardiovascular complications, it was compelling to test the effects of these drugs in kidney disease and SS hypertension. Interestingly, the recent United Kingdom HARP-III trial, which assessed if NEP inhibition improved kidney function in CKD in the short to medium term, found no effect on renal function (21) (vs. an ARB control). In this study, we compared the effects of sacubitril (a NEP inhibitor), valsartan (an ARB), or their combination (angiotensin receptor-NEP inhibitor) in the Dahl SS rat, a well-established model of SS hypertension and associated renal damage.
We picked a relatively low dose of drugs for this study, an average of ~0.3 mg·kg−1·day−1 of each drug (0.6 mg/kg daily total for drug combination) was given to animals throughout the protocol. For humans, the recommended starting oral dose of LCZ-696 is 25−50 mg twice daily, which translates into ∼0.7−1.5 mg·kg−1·day−1 for a 70-kg person. Furthermore, we dispensed the drugs continuously, via an osmotic pump implanted subcutaneously. Various regimens for ARB/NEP inhibitor dosing have been reported. For instance, the sacubitril/valsartan combination was administered to Zucker obese rats at 68 mg·kg−1·day−1 (oral gavage for 10 wk) (19) or Sprague-Dawley rats that underwent a 5/6 nephrectomy at 60 mg/day (also by gavage) (27, 62). In a different subtotal nephrectomy study, Wistar rats received 30 mg/kg LCZ696 daily by gavage (64). In another study, a combination of irbesartan (ARB) and thiorphan (NEP inhibitor) was given to diabetic rats via an osmotic pump at 0.1 mg·kg−1·day−1 via an osmotic minipump (57). Therefore, the selected dose here is on the lower side of the range, although the route of administration should be taken into consideration.
We observed differential effects of ARB, NEP inhibitor, and the combination of the two on renal function and overall physiology (major experimental outcomes are shown in Table 1). Two main effects were largely driven by LCZ-696: a reduction in proteinuria and renal medullary fibrosis (however, renal protein cast formation was also found to be reduced in valsartan-treated animals). These findings are in accordance with studies that showed a reduction in proteinuria when ARBs were used together with NEP inhibitors versus ARB alone in kidney disease (19, 27, 57). Interestingly, the United Kingdom HARP-III trial demonstrated that over a 12-mo period, sacubitril/valsartan had similar effects on albuminuria to irbesartan (20). In the present study, sacubitril/valsartan in combination were able to lower renal medullary but not cortical, fibrosis (shown by picrosirius red staining). This finding is in line with data previously reported by others. In kidney disease, LCZ-696 has been reported to ameliorate oxidative stress, inflammation, and fibrosis beyond treatment with ARB alone (27). However, it is also possible that treatment with valsartan or sacubitril alone may attenuate renal fibrosis. In diabetic kidney disease, renal periarterial and tubulointerstitial fibrosis were reduced in all treatment groups (sacubitril/valsartan, valsartan, and an antihypertensive drug) to a similar extent (19). Although several studies have shown that LCZ-696 attenuates fibrosis in cardiac tissue (4, 36, 62), a recent commentary in the Journal of the American College of Cardiology, following the report by Zile et al (79), raised the question if LCZ-696 is truly antifibrotic (76) and suggested that the various markers of renal fibrosis might be affected differentially, depending on the severity of the damage and the underlying cause. To fully comprehend the mechanisms behind this complex clinical picture, a more thorough study focused on fibrosis-related outcomes is warranted.
Table 1.
Summary of the experimental outcomes
| Parameter | Sacubitril and Valsartan | Sacubitril | Valsartan |
|---|---|---|---|
| Driven by drug combination | |||
| Proteinuria | ↓, P = 0.061 | ↔ | ↔ |
| Renal medullary fibrosis | ↓, P = 0.013* | ↔ | ↓, P = 0.08 |
| Primarily sacubitril driven | |||
| Atrial natriuretic peptide level in heart tissue | ↑, P = 0.012* | ↑, P = 0.000003* | ↔ |
| Glomerular filtration rate | ↑, P = 0.002* | ↑, P = 0.017* | ↔ |
| Primarily valsartan driven | |||
| Urinary kidney injury molecule-1 excretion | ↓, P = 0.08 | ↔ | ↓, P = 0.02* |
| Systolic blood pressure | ↔ | ↔ | ↓, P = 0.008* |
| Renal protein casts | ↓, P = 0.003* | ↔ | ↓, P = 0.009* |
| Both sacubitril and valsartan driven | |||
| Urinary neutrophil gelatinase-associated lipocalin excretion | ↓, P = 0.00003* | ↓, P = 0.002* | ↓, P = 0.0002* |
| Creatinine excretion | ↑, P = 0.001* | ↑, P = 0.0004* | ↑, P = 0.000073* |
Shown is a summary of significant outcomes of the study driven by sacubitril only, valsartan only, both sacubitril and valsartan, and their combination. Outcomes with P < 0.05 and important outcomes with P > 0.05 (due to lower power) are shown. An increase, decrease, and no change (vs. the vehicle-treated group) are denoted as ↑, ↓, and ↔, respectively.
P < 0.05, outcome vs. vehicle (end point, on high-salt diet).
Overall, we found that the majority of the outcomes were driven by valsartan. However, we observed a mild increase in K+ excretion compared with baseline in the groups that were administered sacubitril compared with the starting point (no drug). There are multiple factors that might have contributed to this phenomenon. First, it is important to mention that there was no difference in K+ excretion when the independent groups were compared; therefore, this might be an artifact of the metabolic cage collections, especially since the rats are presumably in a steady state after 21 days of high-salt diet. On the one hand, there are known effects of ANP on Na+ and K+ transport. The actions of ANP along the nephron include inhibition of Na+-K+-ATPase, reducing apical Na+, K+, and protein organic cation transporters in the proximal tubule, decreasing Na+-K+-Cl− cotransporter activity in the thick ascending limb (63), and decreasing epithelial Na+ channel activity (17). In addition, ANP has been shown to dramatically reduce salt appetite (3, 26, 61), which, in turn, can affect K+ excretion (75). In a study by Vormfelde et al. (67), it was shown that carriers of low functional alleles of ANP excreted more K+ when given a diuretic than carriers of the higher functional alleles. However, if this is the case and the epithelial Na+ channel is being inhibited in sacubitril-treated groups and there no other confounding factors, the ANP increase should result in lower K+ excretion. We believe that further research is needed to explore the potentially exciting interaction between ANP and K+ transport in SS hypertension, in a study designed to specifically resolve this question in this setting.
With regard to the the effects of valsartan, first and foremost, systolic blood pressure was significantly reduced by valsartan only, largely unaffected by sacubitril, and was trended toward a decrease when sacubitril was administered together with valsartan. A study by Imanishi et al. (25) demonstrated that in patients with diabetes, ARBs reduce the salt sensitivity of blood pressure by decreasing renal oxidative stress. In the United Kingdom HARP-III trial in patients with CKD, compared with irbesartan, allocation to sacubitril/valsartan was able to reduce average systolic and diastolic blood pressures by 5.4 (95% confidence interval: 3.4−7.4) and 2.1 (95% confidence interval: 1.0−3.3) mmHg (20). This could be attributed to the overall higher effectiveness of valsartan versus irbesartan, since the United Kingdom HARP-III trial did not include valsartan-only or sacubitril-only groups. Nixon et al. (45) reported that in patients with essential hypertension, valsartan is more effective at lowering blood pressure than losartan and shows comparable efficacy to other ARBs. However, a study in SS Asian participants showed that sacubitril/valsartan resulted in significantly greater decreases in ambulatory blood pressure values compared with valsartan (70). Since there are significant genetic variations in factors that predispose humans (and animals) to salt sensitivity (14, 30, 33, 35, 44), the genetics must be taken into consideration when assessing the effectiveness of the drugs.
In addition to blood pressure, in our study, valsartan drove the alleviation of tubular damage, renal cortical fibrosis, and renal protein cast formation. Jing et al. (27) reported that in Sprague-Dawley rats that underwent a 5/6 nephrectomy, the degree of tubulointerstitial injury and glomerulosclerosis in LCZ-696-treated rats was significantly less compared with both the valsartan-alone and untreated groups. In diabetic nephropathy, KIM-1 was found to be reduced in rats treated with sacubitril/valsartan versus valsartan alone (19). In contrast, we showed that urinary NGAL was significantly reduced in all three treatment groups, whereas urinary KIM-1 excretion was only significantly lower in valsartan-treated animals. Although the present findings are generally in line with previously reported findings, we need to also assess it from the perspective of GFR and urinary flow. In humans with CKD, sacubitril/valsartan has been shown to improve estimated GFR compared with baseline (52). Furthermore, in a rat model of diabetic nephropathy, sacubitril/valsartan prevented hyperfiltration compared with valsartan alone (19). Our study demonstrates a sacubitril-driven improvement in GFR. While we saw a typical renal damage-driven decrease in GFR in control animals fed a high-salt diet (7), end-point GFR was higher in sacubitril- and sacubitril/valsartan-treated groups but not in the valsartan-treated group. We can assume that a high-salt diet would evoke faster filtration due to an increased salt load and water consumption, which later decreases due to renal tissue damage; thus, we can hypothesize that sacubitril attenuated the GFR decline evoked by a high-salt diet.
The importance of ANP has been established in inflammation-associated conditions in the kidney, heart, pancreas, and lungs (10, 22, 41, 46, 77). Among recent findings, it has been shown that ANP could downregulate IL-1β release by inhibiting the NLR family pyrin domain containing 3 inflammasome (37) and was able to attenuate inflammatory responses in an acute lung injury model (78). A linkage of ANP to the immune system, and later its role in innate immune functions as well as in the adaptive immune response, was proposed (40, 65, 66). However, the RAAS is also known to be an important regulator and effector of inflammation, and potential therapeutic use of RAAS inhibitors has been proposed in the treatment of inflammatory diseases (38, 48, 54, 60, 73). In SS hypertension, in particular, inflammation is a well-known player, and inhibition of angiotensin receptors has been repeatedly associated with decreased kidney inflammation in the setting (16, 32, 53, 56, 68). However, in a condition of hypernatremia, a widely used ARB, losartan, was not able to decreases the overexpression of the inflammatory markers, while ANP was deemed as a useful tool to regulate the expression of key components of the tubulointerstitial inflammation in the renal medulla (10). We speculate that modifications of the immune system and renal inflammation are important factors that could contribute to the observed renal outcomes, and the differential effects of sacubitril/valsartan and valsartan could be due to their effects on inflammation. More studies are required to support these speculations, and we believe that further research into the potential link between ANP and inflammation in the setting of SS hypertension will close an important gap in knowledge.
Interestingly, a recent report by Lunder et al. (34) showed that very low-dose fluvastatin-valsartan combination decreases parameters of inflammation and oxidative stress in patients with type 1 diabetes. Our data show that low-dose administration of sacubitril, valsartan, and the combination drug LCZ-696 have mild beneficial, although differential, effects on renal damage, fibrosis, proteinuria, tubular damage, and blood pressure. This shows the plausibility of repurposing LCZ-696 for treatment of renal damage induced by SS hypertension. Thus, our study opens new possibilities and sets the stage to explore if low-dose combination treatment could have clinical benefits for SS individuals. However, there is a need for more research studies and higher dosing, in different animal models and diverse genetic backgrounds, to completely close the existing gap in knowledge.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R00DK105160 (to D. V. Ilatovskaya), the Dialysis Clinic Inc Reserve Fund, the Medical University of South Carolina SCTR support program via NIH Grant UL1TR001450 (to D. V. Ilatovskaya), American Physiological Society (APS) Research Career Enhancement, and Lazaro J Mandel awards (to D. V. Ilatovskaya). In part, this work was supported by NIH Grant U54DA016511, Biomedical Laboratory Research and Development Service of the Veterans Office Office of Research and Development Award IK2BX003922, and the APS 2019 S&R Foundation Ryuji Ueno Award (all to K. Y. DeLeon-Pennell) as well as Cell and Molecular Imaging Shared Resource, Hollings Cancer Center, Medical University of South Carolina Grant P30CA138313 (to M. B. Gooz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.R.F. and D.V.I. conceived and designed research; I.P., M.D., R.F., A.S., M.T., V.V., Y.K., K.Y.D.-P., and D.V.I. performed experiments; I.P., M.D., R.F., A.S., M.T., V.V., Y.K., M.B.G., R.S.S., K.Y.D.-P., and D.V.I. analyzed data; I.P., M.D., R.F., A.S., V.V., M.B.G., R.S.S., K.Y.D.-P., W.R.F., and D.V.I. interpreted results of experiments; I.P., M.D., R.F., A.S., V.V., M.B.G., R.S.S., W.R.F., and D.V.I. prepared figures; Y.K., W.R.F., and D.V.I. drafted manuscript; I.P., M.D., R.F., A.S., M.T., V.V., Y.K., M.B.G., R.S.S., K.Y.D.-P., W.R.F., and D.V.I. edited and revised manuscript; I.P., M.D., R.F., A.S., M.T., V.V., Y.K., M.B.G., R.S.S., K.Y.D.-P., W.R.F., and D.V.I. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the Medical University of South Carolina Histology & Immunohistochemistry Laboratory for assistance with preparation of sample and staining of tissues. Mikhail V. Fomin (Medical University of South Carolina) is recognized for help with glomerular filtration rate sample measurements.
REFERENCES
- 1.Ando K, Umetani N, Kurosawa T, Takeda S, Katoh Y, Marumo F. Atrial natriuretic peptide in human urine. Klin Wochenschr 66: 768–772, 1988. doi: 10.1007/BF01726575. [DOI] [PubMed] [Google Scholar]
- 2.Angelis E, Tse MY, Pang SC. Interactions between atrial natriuretic peptide and the renin-angiotensin system during salt-sensitivity exhibited by the proANP gene-disrupted mouse. Mol Cell Biochem 276: 121–131, 2005. doi: 10.1007/s11010-005-3672-1. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn RE, Samson WK, Fulton RJ, Stricker EM, Verbalis JG. Central oxytocin and ANP receptors mediate osmotic inhibition of salt appetite in rats. Am J Physiol Regul Integr Comp Physiol 269: R245–R251, 1995. doi: 10.1152/ajpregu.1995.269.2.R245. [DOI] [PubMed] [Google Scholar]
- 4.Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail 12: e005565, 2019. doi: 10.1161/CIRCHEARTFAILURE.118.005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci USA 102: 785–790, 2005. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra S, Cherian D, Verghese PP, Jacob JJ. Physiology and clinical significance of natriuretic hormones. Indian J Endocrinol Metab 17: 83–90, 2013. doi: 10.4103/2230-8210.107869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowley AW Jr, Ryan RP, Kurth T, Skelton MM, Schock-Kusch D, Gretz N. Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension 62: 85–90, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crimmins DL, Kao JL. A 68 residue N-terminal fragment of pro-atrial natriuretic peptide is a monomeric intrinsically unstructured protein. J Biochem 150: 157–163, 2011. doi: 10.1093/jb/mvr046. [DOI] [PubMed] [Google Scholar]
- 9.Cuneo RC, Espiner EA, Crozier IG, Yandle TG, Nicholls MG, Ikram H. Chronic and acute volume expansion in normal man: effect on atrial diameter and plasma atrial natriuretic peptide. Horm Metab Res 21: 148–151, 1989. doi: 10.1055/s-2007-1009176. [DOI] [PubMed] [Google Scholar]
- 10.Della Penna SL, Rosón MI, Toblli JE, Fernández BE. Role of angiotensin II and oxidative stress in renal inflammation by hypernatremia: benefits of atrial natriuretic peptide, losartan, and tempol. Free Radic Res 49: 383–396, 2015. doi: 10.3109/10715762.2015.1006216. [DOI] [PubMed] [Google Scholar]
- 11.Domondon M, Polina I, Nikiforova AB, Sultanova RF, Kruger C, Vasileva VY, Fomin MV, Beeson GC, Nieminen AL, Smythe N, Maldonado EN, Stadler K, Ilatovskaya DV. Renal glomerular mitochondria function in salt-sensitive hypertension. Front Physiol 10: 1588, 2020. doi: 10.3389/fphys.2019.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong L, Wang H, Dong N, Zhang C, Xue B, Wu Q. Localization of corin and atrial natriuretic peptide expression in human renal segments. Clin Sci (Lond) 130: 1655–1664, 2016. doi: 10.1042/CS20160398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drenjančević-Perić I, Jelaković B, Lombard JH, Kunert MP, Kibel A, Gros M. High-salt diet and hypertension: focus on the renin-angiotensin system. Kidney Blood Press Res 34: 1–11, 2011. doi: 10.1159/000320387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council . Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68: e7–e46, 2016. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 15.Fala L. Entresto (sacubitril/valsartan): first-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. Am Health Drug Benefits 8: 330–334, 2015. [PMC free article] [PubMed] [Google Scholar]
- 16.Franco M, Martínez F, Rodríguez-Iturbe B, Johnson RJ, Santamaría J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol 291: F1281–F1287, 2006. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 17.Guo LJ, Alli AA, Eaton DC, Bao HF. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol 304: F930–F937, 2013. doi: 10.1152/ajprenal.00638.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial differences in natriuretic peptide levels: the Dallas Heart study. JACC Heart Fail 3: 513–519, 2015. doi: 10.1016/j.jchf.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habibi J, Aroor AR, Das NA, Manrique-Acevedo CM, Johnson MS, Hayden MR, Nistala R, Wiedmeyer C, Chandrasekar B, DeMarco VG. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker obese rat. Cardiovasc Diabetol 18: 40, 2019. doi: 10.1186/s12933-019-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, Kalra PA, McMurray JJV, Taal M, Wheeler DC, Landray MJ, Baigent C. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation 138: 1505–1514, 2018. doi: 10.1161/CIRCULATIONAHA.118.034818. [DOI] [PubMed] [Google Scholar]
- 21.Haynes R, Zhu D, Judge PK, Herrington WG, Kalra PA, Baigent C. Chronic kidney disease, heart failure and neprilysin inhibition. Nephrol Dial Transplant, 2019. doi: 10.1093/ndt/gfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houng AK, McNamee RA, Kerner A, Sharma P, Mohamad A, Tronolone J, Reed GL. Atrial natriuretic peptide increases inflammation, infarct size, and mortality after experimental coronary occlusion. Am J Physiol Heart Circ Physiol 296: H655–H661, 2009. doi: 10.1152/ajpheart.00684.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichiki T, Boerrigter G, Huntley BK, Sangaralingham SJ, McKie PM, Harty GJ, Harders GE, Burnett JC Jr. Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. Am J Physiol Regul Integr Comp Physiol 304: R102–R109, 2013. doi: 10.1152/ajpregu.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilatovskaya DV, Levchenko V, Pavlov TS, Isaeva E, Klemens CA, Johnson J, Liu P, Kriegel AJ, Staruschenko A. Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC). EBioMedicine 40: 663–674, 2019. doi: 10.1016/j.ebiom.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imanishi M, Okada N, Konishi Y, Morikawa T, Maeda I, Kitabayashi C, Masada M, Shirahashi N, Wilcox CS, Nishiyama A. Angiotensin II receptor blockade reduces salt sensitivity of blood pressure through restoration of renal nitric oxide synthesis in patients with diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 14: 67–73, 2013. doi: 10.1177/1470320312454764. [DOI] [PubMed] [Google Scholar]
- 26.Itoh H, Nakao K, Katsuura G, Morii N, Shiono S, Sakamoto M, Sugawara A, Yamada T, Saito Y, Matsushita A. Centrally infused atrial natriuretic polypeptide attenuates exaggerated salt appetite in spontaneously hypertensive rats. Circ Res 59: 342–347, 1986. doi: 10.1161/01.RES.59.3.342. [DOI] [PubMed] [Google Scholar]
- 27.Jing W, Vaziri ND, Nunes A, Suematsu Y, Farzaneh T, Khazaeli M, Moradi H. LCZ696 (sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res 9: 5473–5484, 2017. [PMC free article] [PubMed] [Google Scholar]
- 28.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267: 679–681, 1995. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 29.Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant 30: 738–743, 2015. doi: 10.1093/ndt/gfu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res 26: 521–525, 2003. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 31.Lieb W, Pencina MJ, Jacques PF, Wang TJ, Larson MG, Levy D, Kannel WB, Vasan RS. Higher aldosterone and lower N-terminal proatrial natriuretic peptide as biomarkers of salt sensitivity in the community. Eur J Cardiovasc Prev Rehabil 18: 664–673, 2011. doi: 10.1177/1741826710389406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X, Crowley SD. Inflammation in salt-sensitive hypertension and renal damage. Curr Hypertens Rep 20: 103, 2018. doi: 10.1007/s11906-018-0903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luft FC. Molecular genetics of salt-sensitivity and hypertension. Drug Metab Dispos 29: 500–504, 2001. [PubMed] [Google Scholar]
- 34.Lunder M, Janić M, Savić V, Janež A, Kanc K, Šabovič M. Very low-dose fluvastatin-valsartan combination decreases parameters of inflammation and oxidative stress in patients with type 1 diabetes mellitus. Diabetes Res Clin Pract 127: 181–186, 2017. doi: 10.1016/j.diabres.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Luzardo L, Noboa O, Boggia J. Mechanisms of salt-sensitive hypertension. Curr Hypertens Rev 11: 14–21, 2015. doi: 10.2174/1573402111666150530204136. [DOI] [PubMed] [Google Scholar]
- 36.Maslov MY, Foianini S, Mayer D, Orlov MV, Lovich MA. Synergy between sacubitril and valsartan leads to hemodynamic, antifibrotic, and exercise tolerance benefits in rats with preexisting heart failure. Am J Physiol Heart Circ Physiol 316: H289–H297, 2019. doi: 10.1152/ajpheart.00579.2018. [DOI] [PubMed] [Google Scholar]
- 37.Mezzasoma L, Antognelli C, Talesa VN. Atrial natriuretic peptide down-regulates LPS/ATP-mediated IL-1β release by inhibiting NF-κB, NLRP3 inflammasome and caspase-1 activation in THP-1 cells. Immunol Res 64: 303–312, 2016. doi: 10.1007/s12026-015-8751-0. [DOI] [PubMed] [Google Scholar]
- 38.Milanesi S, Verzola D, Cappadona F, Bonino B, Murugavel A, Pontremoli R, Garibotto G, Viazzi F. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J Cell Physiol 234: 10868–10876, 2019. doi: 10.1002/jcp.27929. [DOI] [PubMed] [Google Scholar]
- 39.Mishra S, Ingole S, Jain R. Salt sensitivity and its implication in clinical practice. Indian Heart J 70: 556–564, 2018. doi: 10.1016/j.ihj.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohapatra SS. Role of natriuretic peptide signaling in modulating asthma and inflammation. Can J Physiol Pharmacol 85: 754–759, 2007. doi: 10.1139/Y07-066. [DOI] [PubMed] [Google Scholar]
- 41.Najenson AC, Courreges AP, Perazzo JC, Rubio MF, Vatta MS, Bianciotti LG. Atrial natriuretic peptide reduces inflammation and enhances apoptosis in rat acute pancreatitis. Acta Physiol (Oxf) 222: e12992, 2018. doi: 10.1111/apha.12992. [DOI] [PubMed] [Google Scholar]
- 42.Nehme A, Zouein FA, Zayeri ZD, Zibara K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J Cardiovasc Dev Dis 6: 14, 2019. doi: 10.3390/jcdd6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB; Wellcome Trust Case Control Consortium . Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41: 666–676, 2009. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nierenberg JL, Li C, He J, Gu D, Chen J, Lu X, Li J, Wu X, Gu CC, Hixson JE, Rao DC, Kelly TN. Blood pressure genetic risk score predicts blood pressure responses to dietary sodium and potassium: the GenSalt study (Genetic Epidemiology Network of Salt Sensitivity). Hypertension 70: 1106−1112, 2017. doi: 10.1161/HYPERTENSIONAHA.117.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nixon RM, Müller E, Lowy A, Falvey H. Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach. Int J Clin Pract 63: 766–775, 2009. doi: 10.1111/j.1742-1241.2009.02028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nojiri T, Hosoda H, Tokudome T, Miura K, Ishikane S, Kimura T, Shintani Y, Inoue M, Sawabata N, Miyazato M, Okumura M, Kangawa K. Atrial natriuretic peptide inhibits lipopolysaccharide-induced acute lung injury. Pulm Pharmacol Ther 29: 24–30, 2014. doi: 10.1016/j.pupt.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Overlack A, Ruppert M, Kolloch R, Göbel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension 22: 331–338, 1993. doi: 10.1161/01.HYP.22.3.331. [DOI] [PubMed] [Google Scholar]
- 48.Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother 94: 317–325, 2017. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 49.Pemberton CJ, Siriwardena M, Kleffmann T, Ruygrok P, Palmer SC, Yandle TG, Richards AM. First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: initial assessment as cardiovascular biomarkers. Clin Chem 58: 757–767, 2012. doi: 10.1373/clinchem.2011.176990. [DOI] [PubMed] [Google Scholar]
- 50.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J 278: 1808–1817, 2011. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi H, Liu Z, Cao H, Sun WP, Peng WJ, Liu B, Dong SJ, Xiang YT, Zhang L. Comparative efficacy of antihypertensive agents in salt-sensitive hypertensive patients: a network meta-analysis. Am J Hypertens 31: 835–846, 2018. doi: 10.1093/ajh/hpy027. [DOI] [PubMed] [Google Scholar]
- 52.Quiroga B, de Santos A, Sapiencia D, Saharaui Y, Álvarez-Chiva V. Sacubitril/valsartan in chronic kidney disease, the nephrologist point of view. Nefrologia 39: 646–652, 2019. doi: 10.1016/j.nefro.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 54.Ranjbar R, Shafiee M, Hesari A, Ferns GA, Ghasemi F, Avan A. The potential therapeutic use of renin-angiotensin system inhibitors in the treatment of inflammatory diseases. J Cell Physiol 234: 2277–2295, 2019. doi: 10.1002/jcp.27205. [DOI] [PubMed] [Google Scholar]
- 55.Rieg T. A High-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp 2013: e50330, 2013. doi: 10.3791/50330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 57.Roksnoer LC, van Veghel R, van Groningen MC, de Vries R, Garrelds IM, Bhaggoe UM, van Gool JM, Friesema EC, Leijten FP, Hoorn EJ, Danser AH, Batenburg WW. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition compared with AT1 receptor blockade alone. Clin Sci (Lond) 130: 1209–1220, 2016. doi: 10.1042/CS20160197. [DOI] [PubMed] [Google Scholar]
- 58.Rust P, Ekmekcioglu C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. Adv Exp Med Biol 956: 61–84, 2017. doi: 10.1007/5584_2016_147. [DOI] [PubMed] [Google Scholar]
- 59.Rutledge DR, Sun Y, Ross EA. Polymorphisms within the atrial natriuretic peptide gene in essential hypertension. J Hypertens 13: 953–955, 1995. doi: 10.1097/00004872-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Satou R, Penrose H, Navar LG. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr Hypertens Rep 20: 100, 2018. doi: 10.1007/s11906-018-0900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stellar E, Epstein AN. Neuroendocrine factors in salt appetite. J Physiol Pharmacol 42: 345–355, 1991. [PubMed] [Google Scholar]
- 62.Suematsu Y, Jing W, Nunes A, Kashyap ML, Khazaeli M, Vaziri ND, Moradi H. LCZ696 (sacubitril/valsartan), an angiotensin-receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease. J Card Fail 24: 266–275, 2018. doi: 10.1016/j.cardfail.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol 308: F1047–F1055, 2015. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ushijima K, Ando H, Arakawa Y, Aizawa K, Suzuki C, Shimada K, Tsuruoka SI, Fujimura A. Prevention against renal damage in rats with subtotal nephrectomy by sacubitril/valsartan (LCZ696), a dual-acting angiotensin receptor-neprilysin inhibitor. Pharmacol Res Perspect 5: e00336, 2017. doi: 10.1002/prp2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vollmar AM. The role of atrial natriuretic peptide in the immune system. Peptides 26: 1086–1094, 2005. doi: 10.1016/j.peptides.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 66.Vollmar AM, Lang RE, Hänze J, Schulz R. A possible linkage of atrial natriuretic peptide to the immune system. Am J Hypertens 3: 408–411, 1990. doi: 10.1093/ajh/3.5.408. [DOI] [PubMed] [Google Scholar]
- 67.Vormfelde SV, Toliat MR, Nürnberg P, Brockmöller J. Atrial natriuretic peptide polymorphisms, hydrochlorothiazide and urinary potassium excretion. Int J Cardiol 144: 72–74, 2010. doi: 10.1016/j.ijcard.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 68.Wade B, Petrova G, Mattson DL. Role of immune factors in angiotensin II-induced hypertension and renal damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 314: R323–R333, 2018. doi: 10.1152/ajpregu.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wakui H, Tamura K, Masuda S, Tsurumi-Ikeya Y, Fujita M, Maeda A, Ohsawa M, Azushima K, Uneda K, Matsuda M, Kitamura K, Uchida S, Toya Y, Kobori H, Nagahama K, Yamashita A, Umemura S. Enhanced angiotensin receptor-associated protein in renal tubule suppresses angiotensin-dependent hypertension. Hypertension 61: 1203−1210, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang TD, Tan RS, Lee HY, Ihm SH, Rhee MY, Tomlinson B, Pal P, Yang F, Hirschhorn E, Prescott MF, Hinder M, Langenickel TH. Effects of sacubitril/valsartan (LCZ696) on natriuresis, diuresis, blood pressures, and NT-proBNP in salt-sensitive hypertension. Hypertension 69: 32−41, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08484. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int 82: 26–33, 2012. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Widecka K, Krzyzanowska-Swiniarska B, Celibała R, Gruszczyńska M, Goździk J, Ciechanowski K, Czekalski S. [Effect of intravenous sodium chloride load on levels of atrial natriuretic peptide (ANP) and 3′5′ guanosine monophosphate (cGMP) in plasma of patients with uncomplicated sodium-sensitive arterial hypertension maintained on different dietary sodium intake]. Pol Arch Med Wewn 89: 117–124, 1993. [PubMed] [Google Scholar]
- 73.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin ii-elicited hypertension. Hypertension 67: 163–170, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension 65: 54−61, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young DB, Jackson TE, Tipayamontri U, Scott RC. Effects of sodium intake on steady-state potassium excretion. Am J Physiol Renal Physiol 246: F772–F778, 1984. doi: 10.1152/ajprenal.1984.246.6.F772. [DOI] [PubMed] [Google Scholar]
- 76.Zannad F, Ferreira JP. Is sacubitril/valsartan antifibrotic? J Am Coll Cardiol 73: 807–809, 2019. doi: 10.1016/j.jacc.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Li M, Yang Y, Yan Y, Li J, Qu J, Wang J. NPR-A: a therapeutic target in inflammation and cancer. Crit Rev Eukaryot Gene Expr 25: 41–46, 2015. doi: 10.1615/CritRevEukaryotGeneExpr.2015012447. [DOI] [PubMed] [Google Scholar]
- 78.Zhu YB, Zhang YB, Liu DH, Li XF, Liu AJ, Fan XM, Qiao CH, Ling F, Liu YL. Atrial natriuretic peptide attenuates inflammatory responses on oleic acid-induced acute lung injury model in rats. Chin Med J (Engl) 126: 747–750, 2013. [PubMed] [Google Scholar]
- 79.Zile MR, O’Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, Packer M, McMurray JJV, Shi V, Lefkowitz M, Rouleau J. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol 73: 795–806, 2019. doi: 10.1016/j.jacc.2018.11.042. [DOI] [PubMed] [Google Scholar]