Abstract
Endothelial cells (ECs) from different human organs possess organ-specific characteristics that support specific tissue regeneration and organ development. EC specificity is identified by both intrinsic and extrinsic cues, among which the parenchyma and organ-specific microenvironment are critical contributors. These extrinsic cues are, however, largely lost during ex vivo cultures. Outstanding challenges remain to understand and reestablish EC organ specificity for in vitro studies to recapitulate human organ-specific physiology. Here, we designed an open microfluidic platform to study the role of human kidney tubular epithelial cells in supporting EC specificity. The platform consists of two independent cell culture regions segregated with a half wall; culture media are added to connect the two culture regions at a desired time point, and signaling molecules can travel across the half wall (paracrine signaling). Specifically, we report that in the microscale coculture device, primary human kidney proximal tubule epithelial cells (HPTECs) rescued primary human kidney peritubular microvascular EC (HKMEC) monolayer integrity and fenestra formation and that HPTECs upregulated key HKMEC kidney-specific genes (hepatocyte nuclear factor 1 homeobox B, adherens junctions-associated protein 1, and potassium voltage-gated channel subfamily J member 16) and endothelial activation genes (vascular cell adhesion molecule-1, matrix metalloproteinase-7, and matrix metalloproteinase-10) in coculture. Coculturing with HPTECs also promoted kidney-specific genotype expression in human umbilical vein ECs and human pluripotent stem cell-derived ECs. Compared with culture in HPTEC conditioned media, coculture of ECs with HPTECs showed increased upregulation of kidney-specific genes, suggesting potential bidirectional paracrine signaling. Importantly, our device is compatible with standard pipettes, incubators, and imaging readouts and could also be easily adapted to study cell signaling between other rare or sensitive cells.
Keywords: endothelial cells, human kidney tissue coculture, open microfluidics, paracrine signaling
INTRODUCTION
During human development, endothelial cells (ECs) acquire tissue-specific properties based on cues from the local microenvironment while organs undergo vascularization; in vitro cell culture systems often lack microenvironmental cues, making it challenging to maintain EC identity (20). Therefore, a better understanding of how to preserve EC morphological phenotypes and gene expression profiles in ex vivo environments is important to building more physiologically relevant EC-based disease models for pathological research. Specifically, the human kidney is a highly vascularized organ, and the peritubular capillaries play an important role in maintaining normal renal function of selective reabsorption and secretion, in addition to providing oxygen and nutrients to the tubules and surrounding cells (1, 27, 33). The loss of renal microvasculature integrity has been recognized as a classic finding contributing to progressive renal diseases, including tissue ischemia, tubular dysfunction, inflammation, and fibrosis (3, 27, 32, 36). Reabsorption occurs through both passive and active transport, from the proximal tubule epithelial cells lining the tubule, across the extracellular matrix (ECM) separating the tubules, through the ECs and into the adjacent peritubular capillaries (9).
The interplay between human kidney proximal tubule epithelial cells (HPTECs) and human kidney peritubular microvascular ECs (HKMECs) is a complex process largely regulated by soluble factors in the tubulointerstitium (27, 34, 38). The understanding of human kidney microvasculature injury and regeneration mechanisms has been of great interest in the past several decades, and a group of growth factors have been identified that mediate the intercellular interaction (52, 53, 55). Importantly, vascular endothelial growth factor (VEGF) is an angiogenic and vascular permeability factor that is critical to the survival and proliferation for ECs (23, 28). However, due to the challenge of isolating primary HKMECs from fresh tissues and the unavailability of an established HKMEC cell line, much of our understanding has come from cell culture models using nonrenal human cell lines, such as human umbilical vein ECs (HUVECs), or animal cells. For example, Kim et al. (28) showed that human kidney epithelial cells generate VEGF, which promoted branching angiogenesis of HUVECs in vitro; Zhao et al. (62) discovered that mouse renal proximal tubular epithelial cells helped mouse renal peritubular ECs maintain phenotypes in coculture. More recently, our group (33) successfully isolated and purified HKMECs and discovered that the significant difference between HKMECs and HUVECs in morphology, phenotype, and transcriptional profiling. Furthermore, we (37) reported that ECs from different human organs exhibit organ-specific gene expression profiles that correlate with specific cell functions, including metabolic rate, angiogenic potential, and barrier properties. However, compared with freshly isolated cells, most of organotypic gene expression is lost during in vitro expansion, which highlighted the importance of both using specific EC types and identifying ways to enhance organotypic properties during in vitro culture.
We sought to test whether soluble factors from HPTECs could be used to maintain kidney-specific gene expression and morphology in HKMECs cultured in vitro using a microfluidic coculture platform and further to understand if nonparenchymal tissues (i.e., nonkidney ECs) are plastic to develop organ specificity through paracrine signaling with parenchymal cells (i.e., kidney epithelial cells). Emerging as an alternative tool for coculture studies, microfluidic platforms reduce cell and reagent consumption and offer better control over the configuration of the cell culture regions. Despite the rapid development of microfluidic technologies, many microfluidic platforms require handling expertise and equipment such as pumps and valves, creating significant obstacles for general biological laboratories to adopt microfluidic cell culture devices (8, 48, 61). In the present study, our goal was to create a user-friendly microfluidic device not only suitable for our coculture objectives but also translatable to other cell culture schemes and easily adoptable by general biology researchers.
Here, we present an open microscale coculture platform that contains two cell culture chambers segregated by a polystyrene (PS) half wall. Paracrine signaling is initiated by connecting the two chambers with additional cell culture media, which overflows the half wall. We separately cultured HPTECs with three different endothelial cell types in the device and evaluated key EC organ-specific features under differential culture conditions. Our findings suggest that coculture with HPTECs supports maintenance of key organ-specific genes in primary human kidney ECs (HKMECs) and enhances kidney-specific gene expression in nonkidney-derived cells (HUVECs) and human pluripotent stem cell-derived ECs (hPSC-ECs) over 3 days in culture. This work also serves as a proof of concept to demonstrate that the presented device could become a simple and efficient platform to study paracrine signaling effects across different cell types.
MATERIALS AND METHODS
Open microfluidic coculture device fabrication.
Devices were fabricated using a Series 3 PCNC Mill (Tormach, Waunakee, WI) or a DATRONneo (Datron Dynamics, Milford, NH). The devices were designed with Solidworks 2017 × 64 (Solidworks, Waltham, MA) and converted to .TAP files with Sprutcam (Sprutcam, Naberezhnye Chelny, Russia) or to .simpl files with Fusion 360 (Autodesk, San Rafael, CA). Devices were milled from 2-mm-thick PS sheets (Goodfellow USA, Coraopolis, PA). After milling, the device was washed thoroughly with dish soap and water followed by sonicating with 70% ethanol at 68°C for 15 min and rinsed with DI water and then air dried. A piece of 1.2-mm PS plastic (Goodfellow USA) was cut (65 × 50 mm square), cleaned, and solvent bonded to the bottom surface of the device according to the protocol adapted from Young et al. (60). Briefly, a hot plate (HP60, Torrey Pines Scientific) was preheated to 65°C; the bottom of the milled device was mated to the top surface of a 1.2-mm PS square, and small amount of acetonitrile (ACN; A998-4, Fisher Chemicals) was pipetted gently through the hollow regions of the device until the liquid filled the entire mated area between the PS square and the device. A weight (2 kg) was placed on the top for ~30 s to facilitate the reaction between solvent and the polymer interface. The device was plasma treated for 5 min at 0.25 mbar and 70 W in a Zepto LC PC Plasma Treater (Diener Electronic, Ebhausen, Germany) using oxygen followed by 10 min of ultraviolet sterilization. An engineering drawing of the device with dimensions is included in Supplemental Fig. 1 in the Supplemental Material (available online at https://doi.org/10.6084/m9.figshare.12198513.v1. Original design files are included in the Supplemental Material.
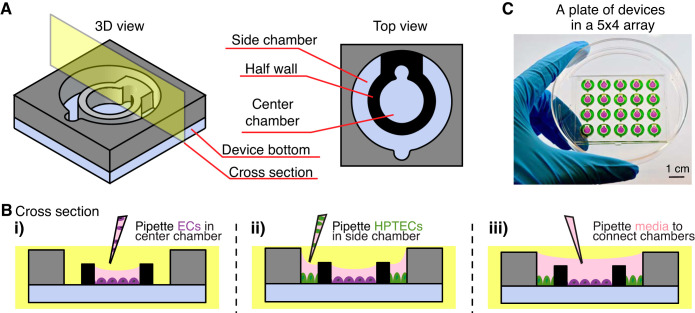
Fig. 1.
Coculture device design and operation. A: three-dimesional and top view of the device design. B: cross-sectional view of the device operation. Different cell types [endothelial cells (ECs) and human kidney proximal tubule epithelial cells (HPTECs)] are selectively seeded into the separated cell culture chambers (i and ii) and placed into paracrine signaling contact by the addition of shared media on top (iii). C: photograph of a plate of devices in the 5 × 4 array fitted inside a petri dish. Center and side chambers are loaded with purple and green dye, respectively, to visualize chamber segregation. The device loading process is shown in Supplemental Video S1.
Isolation and cell culture of HPTECs and HKMECs.
This work was approved by the University of Washington Institutional Review Board (IRB447773EA).
HPTECs and HKMECs were isolated from surgically dissected kidney tissues from fetal donors (donor A: 125 days, male; donor B: 113 days, male; donor C: 127 days, female; and donor D: 110 days, female), as we have previously reported (33). Kidneys were minced in serum-free EBM2 endothelial growth medium (Lonza) with 0.2 mg/mL Liberase and 100 U/mL DNase (Roche). The minced kidneys were incubated for 30 min at 37°C in a shaking water bath. The tissue homogenate was filtered through a 40-μm cell strainer. For HKMEC enrichment, epithelial cell adhesion molecule (EpCAM)-positive cells were depleted first from the cell suspension using EpCAM microbeads (Miltenyi Biotec). EpCAM-negative cells were cultured on gelatin-coated T-75 flasks in EBM2 media supplemented with antibiotic/antimycotic (Invitrogen), 10% FBS (Invitrogen), 10 μg/mL ECGS (Cell Biologics), 50 μg/mL heparin (Sigma), and 40 ng/mL VEGF (R&D Systems). Cells were cultured at 5% CO2 and 37°C until confluent, and HKMECs were sorted as the CD144-positive population on BD FACSAria II at the University of Washington South Lake Union Flow Cytometry Facility. After being sorted, HKMECs were cultured in EBM2 media supplemented with antibiotic/antimycotic (Invitrogen), 10% FBS (Invitrogen), 10 μg/mL ECGS (Cell Biologics), 50 μg/mL heparin (Sigma), and 20 ng/mL VEGF (R&D Systems) at 5% CO2. For HPTEC enrichment, EpCAM-positive cells were collected using EpCAM microbeads from the cell suspension. HPTECs were then cultured in T-75 flasks in DMEM-F-12 (Corning) supplemented with antibiotic/antimycotic (Invitrogen), ITS-X (Invitrogen), and 50 nM hydrocortisone (Sigma-Aldrich) at 5% CO2 and 37°C. Cells between passage 1 and 5 were used for coculture experiments.
HKMECs and HPTECs were plated on the center (17 μL) and side chamber (37 μL) of the coculture device at a density of 1 × 105 and 4.35 × 104 cells/cm2 with their own media, respectively. Twenty-four hours after the initial cell plating, the two chambers were connected using 100 μL HKMEC media with or without VEGF (20 mg/mL), which was replaced every 24 h. Throughout culturing, the devices were kept in a primary container [a 86 × 128-mm OmniTray or a 100 × 150-mm petri dish (Thermo Scientific)]. Sacrificial PBS droplets (total volume of 1 mL) were pipetted around the devices to mitigate evaporation. The primary container was placed in the center of a 245 × 245-mm BioAssay Dish (Corning) with 100 mL PBS. The peripheral of the primary container was wrapped tightly with moisten Kim Wipe. The secondary container was incubated at 5% CO2 and 37°C.
For HKMECs and HPTECs conditioned media culture experiment, HPTECs were plated on the side chambers of the device and recovered overnight. On the next day, HPTECs were fed with 100 μL HKMEC media supplemented with 20 mg/mL VEGF. HKMECs were plated on the center chambers of separate wells. After overnight recovery, HPTEC conditioned media were collected and filtered to remove any HPTEC debris, and 100 μL of conditioned media was added to the wells containing HKMECs. The conditioned media were collected and changed every 24 h.
Cell culture of HUVECs and hPSC-ECs.
HUVECs (Lonza) were cultured in EBM (Lonza) supplemented with the EGM Endothelial Cell Growth Medium SingleQuots Kit (Lonza) and used at passage 3–6. The coculture procedures for HUVEC-HPTEC were the same as those for the HKMEC-HPTEC coculture described above using HUVEC media. hPSCs (WTC line, a gift from Dr. Murry, University of Washington) were first differentiated to posterior-like ECs according to the protocol adapted from Palpant et al. (41) and Redd et al. (44) with low activin A and high bone morphogenetic protein 4 for 10 days. These cells were then trypsinized and plated into the coculture device (at the center well) to allow for attachment and growth into confluency. HPTECs were plated into the side well of the device on day 11 and cocultured with hPSC-ECs for an additional 2 days in hPSC-EC media (41). At day 13, five devices were fixed with paraformaldehyde for immunostaining, and an additional five devices were lysed for RNA collection.
Cell staining and imaging.
ECs in the study were washed with PBS, blocked with PBS containing 2% BSA for 1 h, and incubated with 0.1% Triton X-100 for 5 min. Cells were washed and incubated at 1:50 dilution overnight at 4°C with the following primary antibodies: mouse anti-plasmalemma vesicle-associated protein (PV1), rabbit anti-vascular endothelial cadherin (VECad), and sheep anti-von Willebrand factor (vWF; Abcam). Samples were washed and incubated at 1:200 dilution for 1 h at room temperature with the following secondary antibodies: goat anti-mouse 488, goat anti-rabbit 568, donkey anti-sheep 568, and Alexa Fluor 647 phalloidin (ThermoFisher). The secondary antibodies were removed, and a 1:1,000 dilution of DAPI (Invitrogen) was added and incubated for 5 min. Samples were washed twice, mounted with PBS, and covered with a glass slip to prevent sample dehydration during imaging. The protected device was fitted directly into the mounting frame for fluorescence microscopy.
RNA isolation, RT-PCR, and quantitative PCR analysis for ECs.
RNA was collected and purified using the RNAeasy Mini Kit (Qiagen), and residual DNA was removed by on-column DNase digestion (Qiagen). RNA quantity and quality were measured with a NanoDrop ND 1000 Spectrophotometer. RT-PCR was performed using a Biometra T-Personal Thermal Cycler with iScript Reverse Transcription Supermix (Bio-Rad). Quantitative PCR was performed using the Real-time PCR System (Applied Biosystems) with Fast SYBR Green Master Mix (Applied Biosystems) and primers (RealTimePrimers). Gene expression was quantified according to MIQE guidelines, and the expression of each gene was determined relative to the reference gene GAPDH (10).
Microscopy and image processing.
ECs were imaged using a Nikon High Resolution Widefield microscope with a high-resolution charge-couple device camera. Brightness/contrast adjustments were made uniformly across all images in Fiji (ImageJ). To calculate the cell size for HKMEC monoculture without VEGF conditions, a random cell region on a ×10 image was chosen, cells were counted, and the average cell size was determined by the region area/DAPI count. For other culture conditions where HKMECs were confluent, the average cell size was determined by the field of view/total DAPI count.
Statistical analysis.
Quantitative data were graphed and analyzed using GraphPad Prism 7 software. In Fig. 2, each plotted point represents the average of ~3.5 × 104–8.0 × 104 cells analyzed within a device. HKMEC count and size were compared using a one-way ANOVA test followed by Tukey's multiple comparisons. In Figs. 3 and 4, statistical tests were run to compare the expression in coculture versus monoculture (all VEGF-free) as follows: due to the distribution of data from multiple human donors, the coculture expression ratios for the three donors were log transformed to better fulfill the assumptions of the t test. The log-transformed coculture data were then compared with 0 using a two-tailed one-sample t test using “QuickCalcs one-sample t test” on the GraphPad website. (Note that the coculture data had previously been normalized to monoculture data, which was set to 1 as described in the figures; log (1) = 0, and hence we compared with 0 using the one-sample t test.)
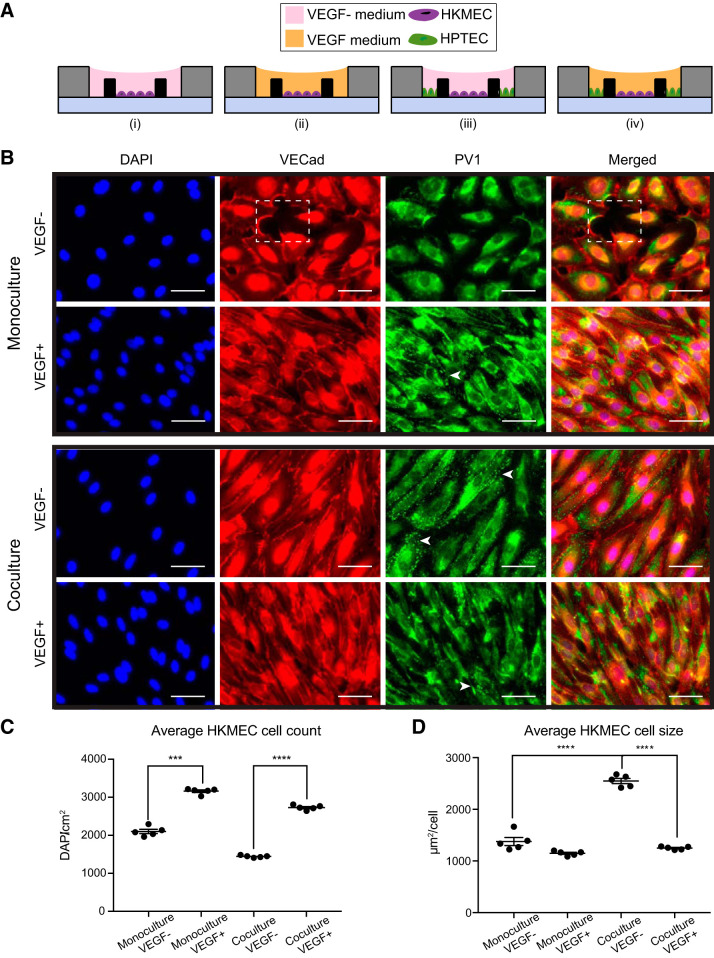
Fig. 2.
Coculture with human kidney proximal tubule epithelial cells (HPTECs) preserves human kidney peritubular microvascular endothelial cell (HKMEC) morphology in coculture. A: device cross-sectional views showing the four culture conditions of HKMECs and HPTECs: monoculture of HKMECs in media without (i) or with (ii) exogeneous VEGF and segregated coculture of HKMECs and HPTECs in media without (iii) or with (iv) exogeneous VEGF. B: immunofluorescence images of HKMECs under differential culture conditions. Blue, nuclear stain (DAPI); red, vascular-endothelial cadherin (VECad). The dotted box indicates a region without cells. Green, plasmalemma vesicle-associated protein (PV1). Arrows indicate example regions with clear PV1 structures (punctate staining). Scale bars = 50 μm. The images shown are from donor C and are representative of experiments with cells from three different human donors each with five device replicates per experiment. C: quantitative comparison of HKMEC count between “monoculture, VEGF−” and “monoculture VEGF+” conditions and “coculture, VEGF−” and “coculture, VEGF+” conditions. D: quantitative comparison of HKMEC size between “coculture, VEGF−” and “monoculture, VEGF−” conditions and “coculture, VEGF−” and “coculture, VEGF+” conditions. Error bars represent the SEs of five device replicates for a single human donor (donor C) in a single experiment. Data sets were analyzed using a one-way ANOVA test; P values are indicated for Tukey’s multiple-comparisons tests. ***P ≤ 0.001; ****P ≤ 0.0001.
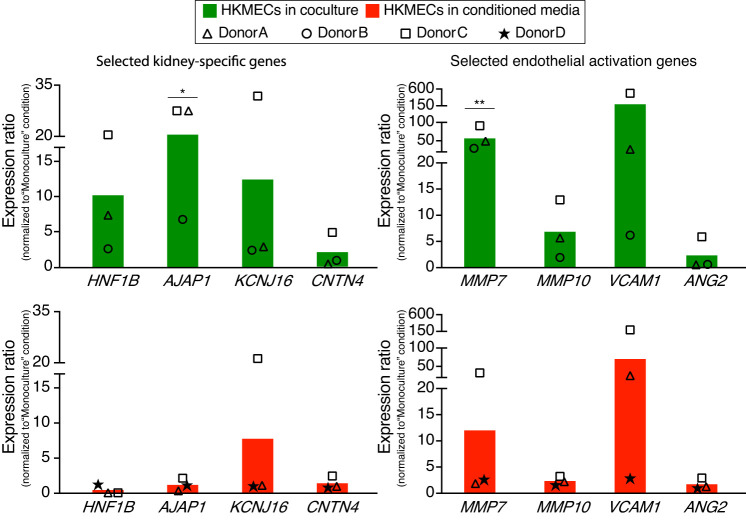
Fig. 3.
Transcriptional changes of human kidney peritubular microvascular ECs (HKMECs) by coculturing with human kidney proximal tubule epithelial cells (HPTECs). Quantitative RT-PCR of selected HKMEC-specific genes (top) and activation genes (bottom) showed upregulation in the “coculture, VEGF−” condition (green) compared with the “monoculture, VEGF−” condition (red). Each plotted point represents data from an independent human donor (pooled from 5 replicate microculture devices per donor, average of three quantitative RT-PCR technical replicates), with each normalized to the “monoculture, VEGF−” condition (which was set to 1). Each colored bar represents the average ratio of the three donors. Statistical comparisons (as described in materials and methods) are shown for genes showing significant differences between “coculture, VEGF−” and “monoculture, VEGF−” as described in materials and methods. *P ≤ 0.05; **P ≤ 0.01. HNFB1, hepatocyte nuclear factor 1 homeobox B; AJAP1, adherens junctions-associated protein 1; KCNJ16, potassium voltage-gated channel subfamily J member 16; CNTN4, contactin 4; MMP7, matrix metalloproteinase-7; MMP10, matrix metalloproteinase-10; VCAM1, vascular cell adhesion molecule-1; ANG2, angiopoietin 2.
Fig. 4.
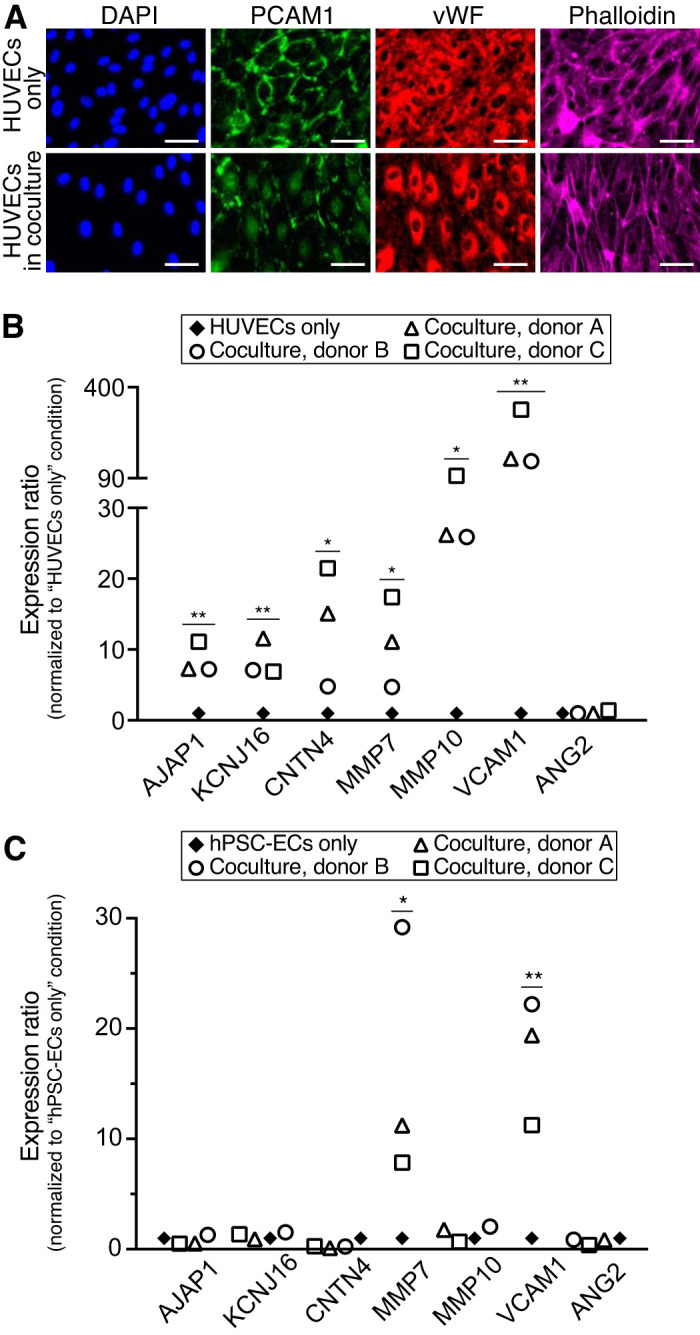
Human kidney proximal tubule epithelial cells (HPTECs) activate other types of endothelial cells (ECs) in coculture. A: immunofluorescence images of human umbilical vein ECs (HUVECs) in monoculture and coculture condition with HPTECs. Scale bars = 50 μm. B: selected kidney-specific markers and endothelial activation markers in HUVECs cocultured with HPTECs from three different donors. C: selected kidney-specific markers and endothelial activation markers in human pluripotent stem cell-derived ECs (hPSC-ECs) cocultured with HPTECs from three different donors. Quantitative RT-PCR samples were pooled from five replicate microculture devices per donor, with an average of three quantitative RT-PCR technical replicates. Gene expression in both B and C was normalized to monoculture, which was set to 1. Statistical comparisons (as described in materials and methods) are shown for genes showing significant differences between coculture and monoculture. *P ≤ 0.05; **P ≤ 0.01. PCAM-1, platelet-EC adhesion molecule; vWF, von Willebrand factor; AJAP1, adherens junctions-associated protein 1; KCNJ16, potassium voltage-gated channel subfamily J member 16; CNTN4, contactin 4; MMP7, matrix metalloproteinase-7; MMP10, matrix metalloproteinase-10; VCAM1, vascular cell adhesion molecule-1; ANG2, angiopoietin 2.
RESULTS AND DISCUSSION
Open microfluidic device design and workflow.
Open microfluidic systems are characterized by the introduction of an air-liquid interface, where liquid flows in microscale channels devoid of at least one side wall (2, 5, 6, 13). Unlike typical microfluidic devices with enclosed culture chambers, open devices offer increased pipette accessibility at any point along the culture chamber and simpler fabrication processes, such as straightforward micromilling and injection molding, allowing open microfluidic devices to become efficient tools with versatility and transferability across different research disciplines (5a, 11, 18, 30, 31, 40, 58).
In the present study, we develop a user-friendly platform to maintain and evaluate kidney EC organ specificity under various microenvironments. Our device consists of two cell culture regions, a center chamber and a side chamber, segregated by a half wall (Fig. 1A and Supplemental Fig. S1A). The width and height of the side chamber are designed to allow filling by a single pipette-dispensing step based on the concept of spontaneous capillary flow in open microchannels (see the Supplemental Material for the equation describing the conditions for capillary flow and illustrations in Supplemental Fig. S1 and Supplemental Video S1). Two cell types are selectively pipetted into the center (ECs) and side chamber (HPTECs), respectively; after adherence, the cells are placed into paracrine signaling contact by filling cell culture medium over the half wall (Fig. 1B). The notched features for each chamber are designed to accommodate a pipette tip for ease of use when pipetting media. Figure 1C shows a device with a 5 × 4 array of the wells. The replicating wells allow for testing multiple culture conditions in a single experiment. Additionally, the dimensions of the device plate are designed to fit into a standard petri dish (88 mm in diameter, shown in Fig. 1C) to maintain sterility and control evaporation during incubation. The device plate dimensions (60 × 50 mm) are also compatible with universal microscope mounting frames for convenient end point imaging.
Importantly, the coculture device is fabricated with PS, which is the standard material for cell culture due to its biocompatibility and good optical properties for medium-resolution imaging, which is used for standard kidney EC immunocytochemistry and morphology studies (17). In contrast to microfluidic platforms made of polydimethylsiloxane, the device used in this work mitigates problems with oligomer leaching and adsorption of small molecules (such as potential drugs or toxicants that this device could be used to screen in future studies) (7, 25, 45, 54). The ECs (HKMECs, HUVECs, and hPSC-ECs) and HPTEC coculture model presented in this study demonstrates that the coculture device as well as our culture protocols to avoid evaporation and ensure nutrient availability in microscale culture are compatible with cells from different sources. Additionally, to demonstrate the versatility of the device for culturing different cells in the side chamber, we cultured freshly isolated human pericytes in the side chamber and HKMECs in the middle chamber for 72 h; this is a biologically relevant coculture model because kidney pericytes play a profound role in inflammation and the renal pericyte-endothelial cross-talk contributes to fibrogenesis during kidney disease progression (29). The results showed that incorporation of pericytes increased HKMEC density in the device (Supplemental Fig. S2).
Although Transwells have been widely used in coculture studies to study soluble factor signaling, our open microfluidic coculture system offers additional features that address several limitations of the Transwell system. Specifically, our system provides a tissue culture-treated PS substrate to culture both cell types, avoiding the potential complication of culturing sensitive cell types on Transwell insert membranes; the coculture device enables imaging both cell types in the same plane of focus, enabling efficient imaging during culture; the open microfluidic design allows for a simple workflow for media changes, minimizing the operation time and the risk of drying the cells in culture; and, finally, the size and ratio of the two cell culture regions can be tuned easily in the computer-aided design (CAD) file, offering the flexibility for researchers to customize the system suitable for their own research purpose. However, a potential limitation for this present open microfluidic system is that it does offer the mechanical capacity (which requires syringe pumps and valves) to address the effect of shear stress caused by the tubular flow and blood flow in the in vivo kidney proximal tubules and peritubular microvessels, respectively. A pending design challenge we seek to solve in future studies is to combine shear flow and real time paracrine signaling events in microfluidic coculture models.
HKMEC and HPTEC coculture in an open microscale device preserves endothelial morphology.
A central goal of our study was to understand to what extent EC specificity can be modified by parenchymal cells in vitro and potentially to develop a method to maintain kidney specificity of HKMECs in vitro. The loss of the microvascular endothelium in renal disease progression is directly related to altered local secretion of VEGF in vivo, which is mainly produced by the proximal tubule epithelium (21, 46, 57). We used the open microscale coculture devices to examine the morphology of HKMECs under four differential cultural conditions (Fig. 2A). Briefly, HKMECs were cultured in the center chambers of the device alone or with HPTECs cultured in the side chambers, both in media with and without exogeneous VEGF. We used immunocytochemistry to qualitatively compare the expression of vascular-endothelial cadherin (VECad), an endothelial integrity marker that is an essential component of endothelial intercellular adherens junctions and critical to endothelium integrity and barrier function (12), and the expression of plasmalemma vesicle-associated protein (PV1), a marker critical to the formation of stomatal (in the perinuclear cytosol) and fenestral (in the periphery of EC membranes) diaphragms on EC membranes (50).
As shown in Fig. 2B, without exogenous VEGF, HKMECs cultured alone (i.e., “monoculture, VEGF−”) lost consistent expression of VECad between adjacent cells, and gaps between the cells were observed (Fig. 2B, box), indicating a disrupted EC barrier. Additionally, in the “monoculture, VEGF−” condition, the majority of PV1 expression was perinuclear, suggesting limited fenestrae formation. The addition of exogenous VEGF in monoculture enabled a contiguous monolayer without gaps (as visualized in the VECad staining) and partially rescued PV1 expression. With the presence of HPTECs in VEGF-free media (i.e., “coculture, VEGF−”), HKMECs maintained integrated cell-cell contact (VECad staining) and showed clusters of clear PV1 expression (Fig. 2B, arrows, punctate staining), indicating that soluble factors from the neighboring but physically separated HPTECs helped maintain important EC function without exogenous VEGF. In addition to qualitatively comparing immunostaining of HKMECs, we also quantitatively compared the cell count and cell size for HKMECs in differential culture conditions. We found that HKMECs cultured with “VEGF+” media yielded higher cell counts than HKMECs in “VEGF−” media, regardless of the presence of HPTECs in the coculture device (P < 0.001 and P < 0.0001; Fig. 2C). The increase in cell number corresponding to the addition of exogeneous VEGF in the media suggested that VEGF had essential role in HKMEC proliferation. Moreover, we quantified average HKMEC cell size and found that in the “coculture, VEGF−” condition, HKMECs had larger cell size compared with the “monoculture, VEGF−” and “coculture, VEGF+” conditions (P < 0.0001; Fig. 2D). This suggests that HPTEC coculture promoted HKMEC quiescence and maturation (35). The addition of VEGF alone did not have the same influence on cell size as the coculture condition, suggesting that HPTECs affect HKMECs via different or additional small molecule signaling than VEGF alone. A similar trend was observed across donors, and additional data from donor A are provided in Supplemental Fig S3. Taken together, our results show the utility of the coculture device as a simplified in vitro platform able to recapitulate key kidney endothelial protein expression and morphology sustained by soluble factor cross-talk with kidney epithelial cells.
HPTECs support expression of key HKMEC organ-specific and endothelium-remodeling genes in coculture.
Previous studies have observed that during organ development, ECs from different organs are heterogeneous in both morphology and gene expression patterns and participate in cross-talk with surrounding microenvironments to form the organ-specific vasculature (14, 39). Multiple kidney-specific endothelial genes are highly expressed in freshly isolated primary kidney endothelial tissues yet become significantly downregulated in expanded culture (37). We wanted to determine if coculture with HPTECs could help rescue this loss in parenchymal gene expression by soluble factor signaling. With the same setup shown in Fig. 2A, we cultured HKMECs under the four differential conditions (Fig. 2A) and collected RNA for quantitative gene expression analysis of selected genes.
We chose to focus on a panel of genes that were previously identified as representative molecular markers for HKMECs (33, 37). The quantitative PCR data shown in Fig. 3, top, demonstrated that compared with HKMECs in monoculture VEGF-free media (red bars), HKMECs in coculture VEGF-free media (green bars) exhibited upregulation of selective kidney-specific genes, including a 3- to 21-fold increase in hepatocyte nuclear factor 1 homeobox B (HNF1B), a transcription factor important to nephron development, a 7- to 28-fold increase in adherens junctions-associated protein 1 (AJAP1), an adhesion junctional protein precursor crucial to endothelium barrier function, and a 2- to 32-fold increase in potassium voltage-gated channel subfamily J member 16 (KCNJ16), a gene encoding K+ channel protein essential to fluid regulation and pH balance. Key endothelial activation genes (Fig. 3, bottom) followed a similar trend of upregulation in the coculture (green bars) versus monoculture (red bars) condition, including a 33- to 95-fold increase in matrix metalloproteinase-7 (MMP7) and a 2- to 13-fold increase in matrix metalloproteinase-10 (MMP10) as well as a 6- to 526-fold increase in vascular cell adhesion molecule-1 (VCAM1), which regulates leucocyte adhesion to the vascular wall (15, 19, 26, 42, 51). Additionally, HKMECs from donor C (squares) showed a fivefold upregulation of the kidney-specific gene contactin 4 (CNTN4), which is linked to normal kidney development, and a sixfold upregulation in the endothelial activation gene angiopoietin 2 (ANG2), which regulates angiogenesis and vascular inflammation, in coculture versus monoculture (24). Interestingly, HKMECs cocultured in VEGF-supplemented media (blue bars) showed less upregulation on average of the aforementioned genes, except for ANG2, compared with HKMECs cocultured in VEGF-free media (green bars). These data suggest that in coculture, HPTECs secrete not only VEGF but also other soluble factors supporting HKMECs to maintain key kidney endothelial features via paracrine signaling (22, 43, 56).
In contrast to the genes shown in Fig. 3, a subset of genes including PV1, VECad, nitric oxide synthase 3 (NOS3; an endothelial proliferation gene), and VEGF receptor 2 (VEGFR2) were upregulated when cultured in “VEGF+” media compared with “VEGF−” media, for both monoculture (orange vs. red bars) and coculture (blue vs. green bars) conditions (Supplemental Fig. S4). The result suggests the critical role of VEGF in HKMEC proliferation and certain aspects of kidney function development.
Figure 3 and Supplemental Fig. S4 also demonstrate the variance across different human donors, which highlights the importance of studying human cell signaling pathways with patient-specific cells to understand patient-dependent disease mechanisms. Due to the variation across human donors, only AJAP1 and MMP7 showed statistical significance between the “monoculture, VEGF−” condition (red bars) and “coculture, VEGF−” condition (green bars); however, from a biological perspective, it is meaningful that all three human donors showed at least twofold higher expression in the latter condition than the former condition for HNF1B, AJAP1, KCNJ16, VCAM1, MMP7, and MMP10. The response among donors for CNTN4 and ANG2 was mixed, with only donor C (squares) showing upregulated expression. Moreover, donor C showed the most pronounced upregulation in the “coculture, VEGF−” condition than the other two donors across all genes of interest in Fig. 3.
HPTECs induce key kidney-specific markers in other types of ECs.
As shown in Figs. 1−3, HKMECs displayed plasticity in the coculture device and demonstrated that paracrine signaling with HPTECs led to enhanced parenchymal properties. Furthermore, we wanted to investigate if parenchymal cells (i.e., HPTECs) have inherent potential to tune human nonkidney ECs through paracrine signaling.
HUVECs have been widely used as a microvascular EC surrogate in traditional kidney research (19, 26, 28, 52, 53). We determined whether coculture with HPTECs could induce parenchymal changes (i.e., kidney-specific gene expression) in HUVECs through paracrine signaling. We placed HUVECs in the center chamber of the coculture device with or without HPTECs seeded in the side chamber. After 3 days of incubation, we used immunocytochemistry to evaluate expression of platelet-EC adhesion molecule (PECAM-1, also known as CD31), blood glycoprotein vWF, and actin cytoskeleton molecule (phalloidin staining) in differential culture conditions (16, 47). Figure 4A shows that HUVECs in monoculture had robust PECAM-1 localization at intercellular junctions, whereas in coculture, very low levels of PECAM-1 resided at the intercellular junctions, suggesting junctional instability and cell activation. In addition, HUVECs in coculture showed less vWF in the cell cytosol than in monoculture and more aligned phalloidin structure, suggesting that HPTECs perturbed endothelial quiescence and led to endothelial activation (4). Together, the data suggest that HPTEC perturbed HUVEC quiescence in coculture and that HPTECs secreted soluble factors that may stimulate endothelial activation in HUVECs.
We also compared the expression of the same panel of kidney-specific and activation genes in HUVECs cultured alone or with HPTECs. The results shown in Figure 4B suggest that similar to HKMECs in coculture with HPTECs, HUVECs expressed higher levels of kidney organ-specific genes, including AJAP1 (7- to 11-fold), KCNJ16 (7- to 12-fold), and CNTN4 (5- to 22-fold), and endothelial activation genes, including MMP7 (5- to 17-fold), MMP10 (26- to 99-fold), and VACM1 (148- to 323-fold), in coculture. However, none of the donor cells led to upregulation of ANG2. Expression of HNF1B, a kidney-specific gene, was also detected in HUVECs after coculture, whereas no detectable expression in the HUVEC-alone condition (data not shown). Consequently, HPTECs showed potential to induce parenchymal characteristics in HUVECs through paracrine signaling.
Generating ECs de novo using hPSCs has emerged as a tool in the past decade to study vascular pathogenesis and construct tissues for drug screening and other therapeutic applications (32, 41). Here, we explored whether hPSC-ECs can develop kidney-specific features when cocultured with HPTECs. Briefly, we differentiated hPSCs to ECs following the protocol adapted from Palpant et al. (41) and cultured hPSC-ECs in our microscale device in the center well alone (monoculture) or cocultured with HPTECs. We showed that compared with in monoculture, hPSC-ECs in coculture exhibited upregulation of MMP7 and VCAM1, whereas no obvious changes of AJAP1, KCNJ16, CNTN4, MMP10, and ANG2 across the three human donors were observed (Fig. 4C). Similar to HUVECs, expression of HNF1B was not detected in the hPSC-EC monoculture sample but at a detectable level in the coculture samples (data not shown). Hence, through the coculture of nonkidney ECs (i.e., HUVECs and hPSC-ECs) with HPTECs, we demonstrated a proof of concept that HPTECs can induce changes in EC phenotype and that ECs can acquire kidney specificity by the support of HPTECs in the microenvironment. Moreover, parenchymal tissue coculture (i.e., HPTEC and nonkidney EC coculture) has the potential to tune human generic cells through paracrine signaling to develop organ-specific physiological features. The present open microfluidic coculture system also provides opportunities for future investigation of the effects of paracrine signaling on developing tissue-specific features in other cell types.
It should be noted that cross-contamination of the cell types could be a potential issue during the media changing step, as unattached cells or debris could be flushed into the other cell culture chamber when fresh media is pipetted directly from the top of the device; if this occurred, the purity of the cell lysate sample used for quantitative PCR analysis would be affected. In this study, since the immunofluorescence images of the ECs (Fig. 2) did not show any presence of HPTECs, we are confident about the purity of the EC lysate collected from the center chamber. Furthermore, we have explored this coculture effect via conditioned media cultures as below, to further support the changes of ECs by HPTECs.
HPTEC coculture and conditioned media culture lead to different effects.
Historically, transferring conditioned media from one cell type to another has been a common approach to recapitulating cell-cell interactions via soluble signaling mediators. Although conditioned media experiments have facilitated the understanding of cellular signaling mechanisms and the discovery of biomarkers and therapeutic targets in diseases, they are inadequate to capture the effects of fast decaying factors or dynamic bidirectional signaling due to the temporal segregation of the cell populations (8, 59). Using the segregated coculture device, we were able to compare the difference between HKMECs in shared media coculture with HPTECs and HKMECs in HPTEC conditioned media culture. To model conditioned media culture, HPTECs were plated in the side chamber of the device overnight, and conditioned media were collected to feed HKMECs seeded in the center chamber of a separate device. HPTEC conditioned media were collected every 24 h, and the freshly collected media were used to replace the HKMEC culture media. HKMECs were collected for quantiative RT-PCR analysis after 3 days of culture with HPTEC conditioned media.
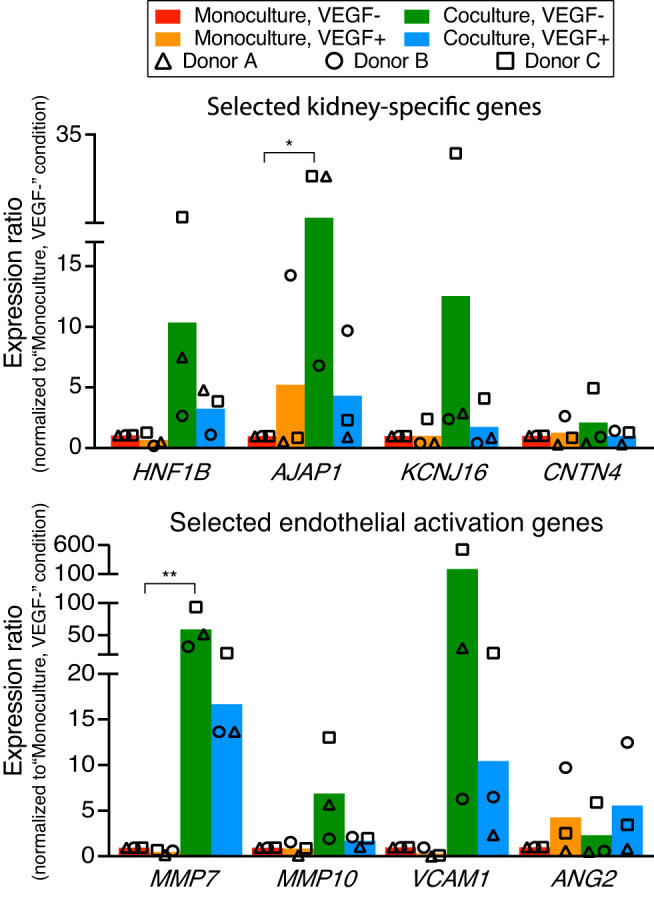
As shown in Fig. 5, HKMECs in conditioned media culture (red) showed a similar trend with noticeable upregulation of kidney-specific genes, particularly KCNJ16, MMP7, and VCAM1, whereas the changes of HNF1B, AJAP1, and MMP10 were evidently less pronounced in conditioned media culture (red) than in coculture (green). These data suggest that the signaling between HKMECs and HPTECs involves both unidirectional signaling (in conditioned media) and bidirectional signaling (in shared media) pathways. It is also possible that some of the key signaling factors are short lived and are therefore lost in the conditioned media experiments while still present in the coculture experiments. Although potential improvements in our system can be made to include the features of tissue-specific architecture, three-dimensional structure, and proximity of two cell populations to better resemble the in vivo condition, our system provides an opportunity to investigate the bidirectional signaling molecule interactions in real time, which is a step forward compared with the traditional one-way signaling in conditioned media studies.
Fig. 5.
Selected gene expression profiles for human kidney peritubular microvascular endothelial cells (HKMECs) in coculture and conditioned media culture. HKMECs showed higher upregulation of kidney-specific (left) and endothelial activation (right) genes in coculture with human kidney proximal tubule epithelial cells (HPTECs) (green) compared with in HPTEC conditioned media (red). Each plotted point represents data from an independent human donor (pooled from five replicate microculture devices per donor, with an average of three quantitative RT-PCR technical replicates). Each colored bar represents the average of three human donors. Gene expression was normalized to monoculture, which was set to 1. Statistical comparisons (as described in materials and methods) are shown for genes showing significant differences between coculture and monoculture. *P ≤ 0.05; **P ≤ 0.01. HNFB1, hepatocyte nuclear factor 1 homeobox B; AJAP1, adherens junctions-associated protein 1; KCNJ16, potassium voltage-gated channel subfamily J member 16; CNTN4, contactin 4; MMP7, matrix metalloproteinase-7; MMP10, matrix metalloproteinase-10; VCAM1, vascular cell adhesion molecule-1; ANG2, angiopoietin 2.
Conclusions.
Here, we present an open microscale coculture device that provides spatial segregation for different cell types and supports paracrine signaling in the shared microenvironment. With relatively small cell samples and regular pipetting actions (no sophisticated external pumps or valves), a coculture configuration with operation efficiency can be achieved. Using the microscale device, we cocultured HPTECs with ECs from different sources. We observed that signaling molecules secreted by HPTECs enhanced some level of kidney-specific characteristics in generic human ECs (HUVECs and hPSC-ECs) and led to endothelial remodeling, indicating a supporting role of HPTECs to the functional development of ECs. Since adult human kidney ECs are similar to fetal kidney ECs in morphology and protein expression profile (33), we expect to see that HPTEC coculture would lead to similar changes in adult human kidney ECs. Future studies would be interesting to examine the different level of genetic modification for different types of ECs in the coculture model.
Moreover, we observed higher upregulation in certain kidney-specific genes and endothelial activation genes when HKMECs were cultured with HPTECs in shared media (coculture) than in conditioned media, suggesting a bidirectional paracrine signaling mechanism in the system. In future work, we envision performing proteomic analyses of the conditioned media collected from HPTEC monoculture and HPTEC-HKMEC coculture, which will provide more specific insights into the mediators of paracrine signaling in the system.
Due to the functional differences among ECs from different organs, it is important to use organ-specific ECs to understand specific vascular injury and regeneration mechanisms. For the study of the interaction between the human kidney proximal tubule epithelium and peritubular microvascular endothelium, this is the first time that primary human kidney cell types have been cocultured in shared media, more precisely resembling the paracrine signaling effect in the in vivo microenvironment. Our findings suggest that parenchymal cells have the capability to tune generic human cells in vitro through paracrine signaling.
Our culture system is simple to use, and we have translated it to biological collaborators in other application areas who are able to operate the device in their own laboratories without assistance. In future work, we envision that our microscale device will be batch fabricated by injection molding (18, 30, 31) and easily adapted to tune parenchymal characteristics in in vitro cell culture studies and construct patient-specific tissue paracrine signaling models for pathological research and therapeutic applications.
GRANTS
This work was supported by National Institutes of Health Grants UG3-TR-002158 (to J. Himmelfarb), UH2-DK-107343 (to Y. Zheng), and R35-GM-128648 (to A. B. Theberge, specifically for the development of the fundamental coculture platform), grants from the Kidney Research Institute and University of Washington Department of Chemistry, and an unrestricted gift from the Northwest Kidney Centers to the UW Kidney Research Institute.
DISCLOSURES
The authors acknowledge the following potential conflicts of interest in companies pursuing open microfluidic technologies: E. Bertheir, Tasso Inc., Salus Discovery LLC, and Stacks to the Future LLC; and A. B. Theberge, Stacks to the Future LLC.
AUTHOR CONTRIBUTIONS
T.Z., D.L., E.B., J.H., Y.Z., and A.B.T. conceived and designed research; T.Z., D.L., and J.X. performed experiments; T.Z. analyzed data; T.Z., R.J.N., Y.Z., and A.B.T. interpreted results of experiments; T.Z. prepared figures; T.Z., D.L., Y.Z., and A.B.T. drafted manuscript; T.Z., D.L., R.J.N., J.H., Y.Z., and A.B.T. edited and revised manuscript; T.Z., D.L., R.J.N., J.X., E.B., J.H., Y.Z., and A.B.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Day and J. Lee for device milling support, and S. Rayner for imaging support.
REFERENCES
- 1.Al-Awqati Q. Goldman's Cecil Medicine. Philadelphia, PA: Elsevier, 2012. [Google Scholar]
- 2.Álvarez-García YR, Ramos-Cruz KP, Agostini-Infanzón RJ, Stallcop LE, Beebe DJ, Warrick JW, Domenech M. Open multi-culture platform for simple and flexible study of multi-cell type interactions. Lab Chip 18: 3184–3195, 2018. doi: 10.1039/C8LC00560E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bayless KJ, Johnson GA. Role of the cytoskeleton in formation and maintenance of angiogenic sprouts. J Vasc Res 48: 369–385, 2011. doi: 10.1159/000324751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry SB, Zhang T, Day JH, Su X, Wilson IZ, Berthier E, Theberge AB. Upgrading well plates using open microfluidic patterning. Lab Chip 17: 4253–4264, 2017. doi: 10.1039/C7LC00878C. [DOI] [PubMed] [Google Scholar]
- 5a.Berthier J, Brakke KA, Berthier E. Open Microfluidics. Hoboken, NJ: Wiley, 2012. doi: 10.1002/9781118720936. [DOI] [Google Scholar]
- 6.Berthier E, Dostie AM, Lee UN, Berthier J, Theberge AB. Open microfluidic capillary systems. Anal Chem 91: 8739–8750, 2019. doi: 10.1021/acs.analchem.9b01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthier E, Young EW, Beebe D. Engineers are from PDMS-land, biologists are from polystyrenia. Lab Chip 12: 1224–1237, 2012. doi: 10.1039/c2lc20982a. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 32: 760–772, 2014. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Falchuk KH, Keimowitz RI, Berliner RW. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest 48: 1519–1531, 1969. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622, 2009. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 11.Byrne MB, Leslie MT, Patel HS, Gaskins HR, Kenis PJA. Design considerations for open-well microfluidic platforms for hypoxic cell studies. Biomicrofluidics 11: 054116, 2017. doi: 10.1063/1.4998579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J, Ehling M, März S, Seebach J, Tarbashevich K, Sixta T, Pitulescu ME, Werner AC, Flach B, Montanez E, Raz E, Adams RH, Schnittler H. Polarized actin and VE-cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis. Nat Commun 8: 2210, 2017. doi: 10.1038/s41467-017-02373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casavant BP, Berthier E, Theberge AB, Berthier J, Montanez-Sauri SI, Bischel LL, Brakke K, Hedman CJ, Bushman W, Keller NP, Beebe DJ. Suspended microfluidics. Proc Natl Acad Sci USA 110: 10111–10116, 2013. doi: 10.1073/pnas.1302566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 100: 10623–10628, 2003. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol 11: 102–112, 2015. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 16.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol 77: 487–495, 2005. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis AS, Forrester JV, McInnes C, Lawrie F. Adhesion of cells to polystyrene surfaces. J Cell Biol 97: 1500–1506, 1983. doi: 10.1083/jcb.97.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day JH, Nicholson TM, Su X, van Neel TL, Clinton I, Kothandapani A, Lee J, Greenberg MH, Amory JK, Walsh TJ, Muller CH, Franco OE, Jefcoate CR, Crawford SE, Jorgensen JS, Theberge AB. Injection molded open microfluidic well plate inserts for user-friendly coculture and microscopy. Lab Chip 20: 107–119, 2020. doi: 10.1039/C9LC00706G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood 104: 2385–2393, 2004. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun 8: 14361, 2017. doi: 10.1038/ncomms14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 140: 947–959, 1998. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 77: 527–543, 1999. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56: 794–814, 1999. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 24.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, Christiaans SC, Bir ND, Pittet JF. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg 247: 320–326, 2008. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 25.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 63: 218–231, 2015. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Ito TK, Ishii G, Saito S, Yano K, Hoshino A, Suzuki T, Ochiai A. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 113: 2363–2369, 2009. doi: 10.1182/blood-2008-08-172742. [DOI] [PubMed] [Google Scholar]
- 27.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kim BS, Chen J, Weinstein T, Noiri E, Goligorsky MS. VEGF expression in hypoxia and hyperglycemia: reciprocal effect on branching angiogenesis in epithelial-endothelial co-cultures. J Am Soc Nephrol 13: 2027–2036, 2002. doi: 10.1097/01.ASN.0000024436.00520.D8. [DOI] [PubMed] [Google Scholar]
- 29.Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 34: 374–383, 2014. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee UN, Su X, Guckenberger DJ, Dostie AM, Zhang T, Berthier E, Theberge AB. Fundamentals of rapid injection molding for microfluidic cell-based assays. Lab Chip 18: 496–504, 2018. doi: 10.1039/C7LC01052D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y, Choi JW, Yu J, Park D, Ha J, Son K, Lee S, Chung M, Kim HY, Jeon NL. Microfluidics within a well: an injection-molded plastic array 3D culture platform. Lab Chip 18: 2433–2440, 2018. doi: 10.1039/C8LC00336J. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Wingert RA. Regenerative medicine for the kidney: stem cell prospects & challenges. Clin Transl Med 2: 11, 2013. doi: 10.1186/2001-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ligresti G, Nagao RJ, Xue J, Choi YJ, Xu J, Ren S, Aburatani T, Anderson SK, MacDonald JW, Bammler TK, Schwartz SM, Muczynski KA, Duffield JS, Himmelfarb J, Zheng Y. A novel three-dimensional human peritubular microvascular system. J Am Soc Nephrol 27: 2370–2381, 2016. doi: 10.1681/ASN.2015070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd AC. The regulation of cell size. Cell 154: 1194–1205, 2013. doi: 10.1016/j.cell.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 36.Maeshima Y, Makino H. Angiogenesis and chronic kidney disease. Fibrogenesis Tissue Repair 3: 13, 2010. doi: 10.1186/1755-1536-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcu R, Choi YJ, Xue J, Fortin CL, Wang Y, Nagao RJ, Xu J, MacDonald JW, Bammler TK, Murry CE, Muczynski K, Stevens KR, Himmelfarb J, Schwartz SM, Zheng Y. Human organ-specific endothelial cell heterogeneity. iScience 4: 20–35, 2018. doi: 10.1016/j.isci.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 39.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219, 2013. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh S, Ryu H, Tahk D, Ko J, Chung Y, Lee HK, Lee TR, Jeon NL. “Open-top” microfluidic device for in vitro three-dimensional capillary beds. Lab Chip 17: 3405–3414, 2017. doi: 10.1039/C7LC00646B. [DOI] [PubMed] [Google Scholar]
- 41.Palpant NJ, Pabon L, Friedman CE, Roberts M, Hadland B, Zaunbrecher RJ, Bernstein I, Zheng Y, Murry CE. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat Protoc 12: 15–31, 2017. doi: 10.1038/nprot.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: e92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SA, Jeong MS, Ha KT, Jang SB. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep 51: 73–78, 2018. doi: 10.5483/BMBRep.2018.51.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redd MA, Zeinstra N, Qin W, Wei W, Martinson A, Wang Y, Wang RK, Murry CE, Zheng Y. Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat Commun 10: 584, 2019. doi: 10.1038/s41467-019-08388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip 9: 2132–2139, 2009. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudnicki M, Perco P, Enrich J, Eder S, Heininger D, Bernthaler A, Wiesinger M, Sarközi R, Noppert SJ, Schramek H, Mayer B, Oberbauer R, Mayer G. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest 89: 337–346, 2009. doi: 10.1038/labinvest.2008.158. [DOI] [PubMed] [Google Scholar]
- 47.Rusu L, Minshall R. Endothelial cell von Willebrand factor secretion in health and cardiovascular disease. Endothelial Dysfunction: Old Concepts and New Challenges. London, UK: IntechOpen, 2018. doi: 10.5772/intechopen.74029. [DOI] [Google Scholar]
- 48.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature 507: 181–189, 2014. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 50.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, Kobayashi T, Shipman SL, Moodie KL, Daghlian CP, Ernst PA, Lee HK, Suriawinata AA, Schned AR, Longnecker DS, Fiering SN, Noelle RJ, Gimi B, Shworak NW, Carrière C. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell 23: 1203–1218, 2012. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szmitko PE, Wang C-H, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation. Circulation 108: 1917–1923, 2003. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 52.Tasnim F, Zink D. Cross talk between primary human renal tubular cells and endothelial cells in cocultures. Am J Physiol Renal Physiol 302: F1055-F1062, 2012. doi: 10.1152/ajprenal.00621.2011. [DOI] [PubMed] [Google Scholar]
- 53.Tourovskaia A, Fauver M, Kramer G, Simonson S, Neumann T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp Biol Med (Maywood) 239: 1264–1271, 2014. doi: 10.1177/1535370214539228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Midwoud PM, Janse A, Merema MT, Groothuis GM, Verpoorte E. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal Chem 84: 3938–3944, 2012. doi: 10.1021/ac300771z. [DOI] [PubMed] [Google Scholar]
- 55.Vedula EM, Alonso JL, Arnaout MA, Charest JL. A microfluidic renal proximal tubule with active reabsorptive function. PLoS One 12: e0184330, 2017. doi: 10.1371/journal.pone.0184330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev 25: 1–19, 2014. doi: 10.1016/j.cytogfr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int 67: 449–457, 2005. doi: 10.1111/j.1523-1755.2005.67101.x. [DOI] [PubMed] [Google Scholar]
- 58.Walsh EJ, Feuerborn A, Wheeler JHR, Tan AN, Durham WM, Foster KR, Cook PR. Microfluidics with fluid walls. Nat Commun 8: 816, 2017. doi: 10.1038/s41467-017-00846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 60.Young EW, Berthier E, Beebe DJ. Assessment of enhanced autofluorescence and impact on cell microscopy for microfabricated thermoplastic devices. Anal Chem 85: 44–49, 2013. doi: 10.1021/ac3034773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young EWK, Beebe DJ. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem Soc Rev 39: 1036–1048, 2010. doi: 10.1039/b909900j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, Zhao H, Zhang Y, Tsatralis T, Cao Q, Wang Y, Wang Y, Wang YM, Alexander SI, Harris DC, Zheng G. Isolation and epithelial co-culture of mouse renal peritubular endothelial cells. BMC Cell Biol 15: 40, 2014. doi: 10.1186/s12860-014-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]