Abstract
Structural changes to airway morphology, such as increased bronchial wall thickness (BWT) and airway wall area, are cardinal features of chronic obstructive pulmonary disease (COPD). Ferrets are a recently established animal model uniquely exhibiting similar clinical and pathological characteristics of COPD as humans, including chronic bronchitis. Our objective was to develop a microcomputed tomography (µCT) method for evaluating structural changes to the airways in ferrets and assess whether the effects of smoking induce changes consistent with chronic bronchitis in humans. Ferrets were exposed to mainstream cigarette smoke or air control twice daily for 6 mo. µCT was conducted in vivo at 6 mo; a longitudinal cohort was imaged monthly. Manual measurements of BWT, luminal diameter (LD), and BWT-to-LD ratio (BWT/LD) were conducted and confirmed by a semiautomated algorithm. The square root of bronchial wall area (√WA) versus luminal perimeter was determined on an individual ferret basis. Smoke-exposed ferrets reproducibly demonstrated 34% increased BWT (P < 0.001) along with increased LD and BWT/LD versus air controls. Regression indicated that the effect of smoking on BWT persisted despite controlling for covariates. Semiautomated measurements replicated findings. √WA for the theoretical median airway luminal perimeter of 4 mm (Pi4) was elevated 4.4% in smoke-exposed ferrets (P = 0.015). Increased BWT and Pi4 developed steadily over time. µCT-based airway measurements in ferrets are feasible and reproducible. Smoke-exposed ferrets develop increased BWT and Pi4, changes similar to humans with chronic bronchitis. µCT can be used as a significant translational platform to measure dynamic airway morphological changes.
Keywords: bronchial wall thickness, chronic bronchitis, COPD
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction and is currently the third leading cause of death in the United States (25). COPD, which is most frequently caused by cigarette smoking, results in emphysema that principally features destruction of lung parenchyma; chronic bronchitis, characterized by chronic mucus hypersecretion and expectoration (9, 25); or often a mix of these phenotypes. The protean effects of emphysema and chronic bronchitis that vary in severity on an individual basis make it challenging to subphenotype and monitor COPD, thus limiting the ability to characterize relevant mechanisms, the severity of disease expression and progression, and response to targeted treatment (8). This is particularly important for the diagnosis of chronic bronchitis, which afflicts ~60% of individuals with COPD and in which specific therapies are only beginning to emerge.
Computed tomography (CT) is increasingly being used to visualize structural changes associated with emphysema (parenchymal destruction) and airway remodeling (bronchial wall thickening, airway narrowing, or even loss of small airways in its extreme form) (1). Recent advances in CT image-processing techniques have led to accurate segmentation of airways and lung structures in patients, thus enabling the characterization of COPD population into emphysema and chronic bronchitis predominant subgroups. Chronic bronchitis-associated airway remodeling in COPD has been successfully quantified by estimating bronchial diameters and airway wall thickness on CT images acquired at full inspiration (10, 15). Pi10 measurements, where multiple measurements of the relationship between airway perimeter and bronchial wall area are determined on an individual basis and then calculated for a theoretical airway with a luminal circumference of 10 mm (approximating a segmental airway), were devised to avoid bias due to between-subject differences in airway sizes. Using this calculation, Pi10 increases with worsening chronic bronchitis and airway disease and can be used as an effective outcome measure (7, 12, 14, 19). These techniques have improved our understanding of COPD progression in patients but have not yet been coupled to experimental models with prominent airway disease to reveal novel pathophysiological targets.
Animal models that recapitulate the morphological changes to the airways and exhibit spontaneous lung disease due to cigarette smoking are needed to accelerate our mechanistic understanding and help evaluate novel therapeutic targets of COPD-related airway disease (4). While mice have been a powerful model, particularly for lung matrix destruction that accompanies emphysema and the genetic contribution to these pathways, they do not exhibit many of the mucosal abnormalities present in ferrets, including clinical and mechanistic features of chronic bronchitis (5). Previously, we have demonstrated that ferrets chronically exposed to cigarette smoke exhibit similar clinical and pathological features associated with COPD in humans (20). With smoke exposure, ferrets develop chronic bronchitis, including the presence of chronic cough, histologic evidence of chronic mucus hypersecretion, and glandular hyperplasia, in addition to emphysematous lung parenchyma. To fully exploit the model, novel biomarkers of pathophysiology and clinical response are needed.
We hypothesized that microcomputed tomography (µCT) imaging of the ferret model of COPD could enable noninvasive demonstration of key features of airway remodeling, including increased bronchial wall thickness (BWT) that progresses longitudinally, providing a crucial, noninvasive, and potentially responsive serial biomarker with direct clinical relevance. In this article, we establish increased bronchial wall thickness and elevated bronchial wall area in smoke-exposed ferrets that is dynamic, providing a longitudinal outcome measure well suited for the evaluation of novel therapies.
METHODS
Ferret model of COPD.
All animal protocols were reviewed and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC). Age- and sex-matched wild-type ferrets (Mustela putorius furo) were exposed to diluted mainstream cigarette smoke following age of maturity (17–20 wk of age) from 3R4F research cigarettes (Univ. of Kentucky, Lexington, KY) using an automated cigarette smoking apparatus (TSE Systems, Chesterfield, MO). Ferrets were exposed to 200 μg/L of total particulate matter (35-mL puffs of 2-s duration at a rate of 3 L/s), as previously described (20). All ferrets were imaged by µCT at 6 mo of cigarette smoke exposure. A subcohort underwent µCT at baseline and monthly thereafter through 6 mo. Ferret weights by sex were recorded after 6 mo of smoke exposure; smokers showed no statistical difference when compared with controls (23 smoke, 14 control) (Supplemental Table S1; all supplemental material is available at https://doi.org/10.5281/zenodo.3751865).
µCT imaging.
We conducted noncontrast CT imaging of ferrets under inhaled isoflurane anesthesia and prospectively gated for a single inspiratory phase of respiration using a µCT scanner (MiLabs, Utrecht, The Netherlands) (Supplemental Fig. S1). All images were acquired at ultra-focused magnification with respiratory gating and the following parameters: tube voltage (55 kV), tube current (0.19 mA), scan angle (360°), and 20 ms of exposure. All images were reconstructed using vendor software at 40 μm/voxel resolution for manual measurements and 80 μm/voxel for semiautomated measurements at a single respiratory phase; reconstruction at lower voxel counts did not impact resolution due to limitations in respiratory gating of spontaneously breathing animals. After reconstruction, images were filtered using 0.5 mm Gaussian smoothing kernel on PMOD software (PMOD Technologies LLC, Zurich, Switzerland).
Manual measurements of airway morphology.
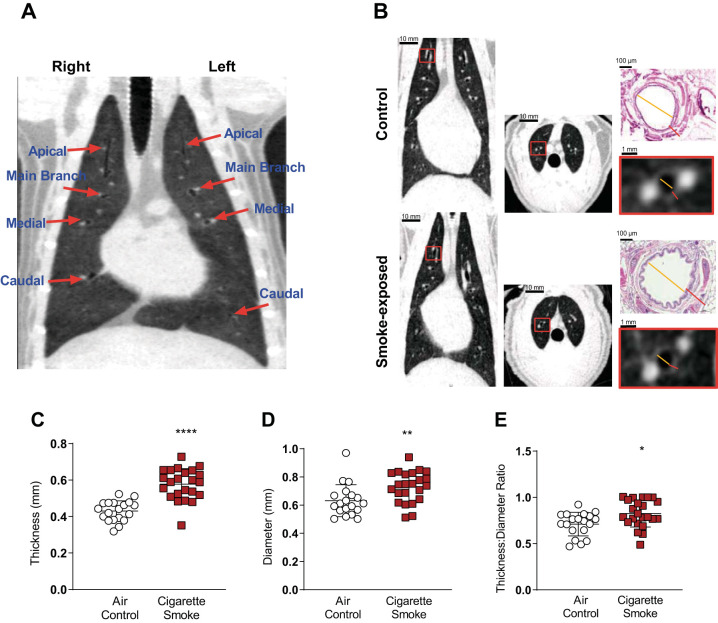
The segmental and subsegmental bronchial wall thickness is commonly measured to assess degree of airway remodeling in COPD patients (1). To conduct this in ferrets, airways were selected based on the ability to routinely visualize them by µCT and produce the measurements at specific anatomic locations in a reproducible fashion between ferrets. Lungs were divided into apical, medial, and caudal sections and a single 5th (or 6th when the 5th was not well visualized) generation airway from each region was measured for both the left and right lung. Typical 5th to 6th generation airways (using the sum of airway wall plus lumen) are estimated between 1.4 and 1.6 mm in males, which is ~50% of the size of the corresponding airway in humans but otherwise similar in histological structure. Noting that length is 25–50% smaller in females and airway lumen is ~50% of the total airway diameter, this corresponds to luminal airway measurements between 0.4 and 0.8 mm in females (17). The analyst was blinded to exposure type. For each selected airway, the axial image view was used for bronchial wall thickness (BWT) and luminal diameter (LD) measurements at the midpoint of each segment, confirmed by segmental image view so as to avoid the impact of airway branching, and BWT-to-LD ratio (BWT/LD) calculated (Fig. 1A). This resulted in six airway measurements for each animal (n = 23 smoke, 19 control for left and right apical, medial, and caudal lobes, respectively). We compared the mean values of these six airway measurements between smoke-exposed ferrets and controls, both with each airway treated as an individual variable and averaged as a single point estimate of these parameters for each ferret. A second analyst, also blinded to treatment assignment, recapitulated measurements from a subset of ten ferrets (n = 5 smoke, 5 control). The airway perimeter was calculated by LD × π and the square root of the bronchial wall area (√WA) by √[π × (BWT2 + LD × BWT)]. A regression line was then generated between airway perimeter and √WA for each ferret. Because the average mean internal luminal perimeter in control ferrets was ~2 mm for the manual method, we estimated the √WA of airways with internal perimeters of 2 mm, termed Pi2.
Fig. 1.
Structural analysis of smoke-exposed ferret airways using microcomputed tomography (µCT). A: representative image of ferret lung µCT scan. Red arrows show the locations where airway measurements were taken. B: representative coronal and axial projections of air control (top) and 6 mo smoke-exposed (bottom) ferrets. Lower insets are magnified views of a representative airway selected for measurements. Yellow line indicates airway luminal diameter (LD) and red line represents bronchial wall thickness (BWT). Upper insets demonstrate the same measurements by histopathological analysis for comparison. C: manual BWT of smoke-exposed and air control ferrets. Each point represents mean BWT of a single ferret derived from 6 airway measurements per ferret; n = 42 ferrets (23 smoke, 19 control). ****P < 0.0001. D: manual mean luminal diameter. **P < 0.01. E: manual mean ratio of BWT to LD. *P < 0.05. Inferential comparisons by Student’s t test.
Semiautomated measurement of bronchial wall thickness.
We authored a customized semiautomated version to recapitulate the measurement of ferret bronchial wall thickness but limited this to the apical lobe due to reproducibility of the procedure. First, the entire region of the airway lumen was segmented using a region-growing method (22) when the seed pixel was selected at the center of the cranial trachea [threshold: ≤ −900 Hounsfield units (HU)] to initiate airway mapping (Supplemental Fig. S2A). Then, the 4th to 6th generations of apical lobe airway in both the left and right lungs were selected for analysis (Supplemental Fig. S2B), noting that this is more proximal than could be reliably achieved with manual measurements enabled by the region-growing method. The three-dimensional (3-D) CT image was vertically reoriented for each airway generation so that it was vertical to the image slices. Vertical reorientation was a 3-D rotation of the image so the selected airway was vertical to the axial image slices, facilitating sequential axial cuts to determine bronchial wall area. Vertical reorientation was implemented in the following steps. First, the centroid of the airway lumen was determined in each image slice. Second, a line fitting the centroids of the image slices was determined, and the angle between the fitted line and the vertical axis was calculated (Supplemental Fig. S2C). Third, the 3-D image was rotated to align with the vertical axis (Supplemental Fig. S2D).
In each image slice, the binary luminal region was obtained using a region-growing method after the seed pixel was selected at the center of trachea (threshold: ≤ −900 HU) and dilated 12 times sequentially (40-μm increase at each dilation) (3), creating an iso-distance shell region (40-μm thickness) at each iteration by SRn = DLRn – DLRn−1, where SRn and DLRn are the shell region and the dilated luminal region, respectively, at the nth iteration (Supplemental Fig. S3, A and 3B). The pixel values of the bronchial wall on each shell region were averaged, and the bronchial wall thickness (BWT) was determined as twice the distance from the original luminal boundary to the shell region having the maximum mean pixel value (Supplemental Fig. S3C). The luminal diameter (LD) and perimeter were calculated by 2√(LA/π) and 2√(πLA), respectively, where LA is the luminal area, so that comparisons could be made to manual measurements. The square root of the wall area, √WA, was calculated by √[π × (BWT2 + LD × BWT)]. Approximately 200 image slices were analyzed for each animal at one airway per apical lobe between the 4th and 6th generations. The √WA of all image slices was plotted over the luminal perimeter for each animal, and a linear regression line was retrieved. The √WA at various luminal perimeters (e.g., Pi2 and Pi4) was estimated on the regression line. Since the mean luminal perimeter with the automated method approximated 4 mm, Pi4 was used as the primary analysis and Pi2 was used as a sensitivity analysis. The segmentation of the airway lumen was conducted using ImageJ (National Institutes of Health, Bethesda MD), and the other image processing and analysis were fully automated using a laboratory-made computer software package based on LabVIEW, version 17.0 (National Instrument, Austin, TX).
Histopathologic analysis.
Whole left lungs were inflated to a pressure of 25 cm of water and instilled by 70% alcoholic formalin for a minimum of 48 h before sectioning and paraffin embedding. Tissues were hematoxylin-eosin (HE) stained and airway wall thickness was determined from red-green-blue (RGB) color images of HE-stained sections using Image Pro Plus 7 image analysis software (Media Cybernatics). Images were made with ×4, ×10, ×20, or ×40 objectives and were obtained from each airway sectioned at approximately perpendicularly in the sections provided. For each airway, epithelial type (cuboidal or ciliated respiratory), and presence or absence of cartilage, mucus glands, and goblet cells were recorded (Supplemental Fig. S4A). Using image editing software, images were prepared for analysis by erasing the area external to the wall so as to leave airway adventitia, cartilage, mucus glands, and smooth muscle. Material in the lumen was erased and the lumen filled with solid red (Supplemental Fig. S4B). To measure the total area of the airway, the image was gray scaled and thresholded so as to form a representative black object (Supplemental Fig. S4C), and the total area (in µm2) was exported to an Excel spreadsheet data template. The software was appropriately calibrated for each objective magnification. The software was configured not to fill holes or spaces, as the majority of apparently empty space is due to shrinkage during fixation and processing and is thus not present in life. The area of the lumen was determined by selecting the red object representing the lumen, then gray scaling, thresholding, and exporting the area in µm2 for analysis. The perimeter of the luminal object was also collected and exported (Supplemental Fig. S4D). From these raw data, we calculated estimated wall thickness as (total area – lumen area)/lumen perimeter; estimated airway luminal diameter as lumen perimeter/π and the ratio of wall thickness/luminal diameter. Use of the perimeter of the luminal object estimates the luminal diameter, and thus wall thickness, of the airway at full diameter, without mucosal folding, which provides better estimates of size and wall thickness in life than circumference of a circle of the area of the luminal object. The data from the airways for each animal were collected individually. Ten to 25 airways (average 16.75) were analyzed per ferret.
Statistical analysis.
Descriptive analysis was conducted with Graphpad Prism V7 (La Jolla, CA), and inferential statistics were conducted by Student’s t test or ANOVA with Tukey’s post hoc evaluation, as appropriate. Results are presented as means ± SD, unless indicated otherwise. P < 0.05 was considered statistically significant. Regression analysis was conducted using SPSS V19 (IBM). Univariate regression was used to determine factors related to determinants of airway morphology, including smoking status, sex, and anatomic location (apical, medial, or caudal, as categorical variables) and laterality. Subsequently, multivariate backward regression was used to assess the independent contributors to airway morphology.
RESULTS
Bronchial wall thickness and luminal diameter.
Bronchial wall thickness is an important marker of airway wall injury and is associated with chronic bronchitis in patients (7, 12, 14, 23). As depicted in representative images, after 6 mo of cigarette smoke exposure, smoke-exposed ferrets demonstrated greater bronchial wall thickness as compared with air controls (Fig. 1B). Manual evaluation of BWT revealed cigarette smoke-exposed ferrets had elevated mean BWT by 34% (0.58 ± 0.09 mm, n = 23 vs. 0.43 ± 0.06 mm, n = 19 control; P < 0.001) (Fig. 1C). Since BWT was partially dependent on airway size, we also examined luminal diameter (LD) and BWT/LD. LD was 15% larger in smoke-exposed ferrets measured at the same anatomic locations (0.73 ± 0.11 mm smoke vs. 0.63 ± 0.11 mm air controls; P = 0.003; Fig. 1D), which may be an early indicator of altered airway tone or, alternatively, airway dilatation induced by chronic bronchitis. Despite these changes, mean BWT/LD was 16% higher in smoke-exposed ferrets (0.82 ± 0.15 mm smoke exposed vs. 0.71 ± 0.13 mm controls; P = 0.013) (Fig. 1E), indicating BWT is induced by smoking even when controlled for changes in airway size.
To compare with µCT, we conducted histopathological analysis of airway wall thickness by morphometry, capturing the distance from the surface of the epithelial cells to the surrounding smooth muscle but not overlying mucus since histopathological fixation disrupts mucus continuity with the epithelium (see Fig. 1B, inset), by a veterinary pathologist familiar with the ferret lung and blinded to exposure assignment. Results in airways from 0.53–0.93 mm luminal diameter (chosen to reflect approximate size of airways measured by µCT) indicated smoke-exposed ferrets had no increase in airway wall thickness compared with air control ferrets (0.35 ± 0.03 mm smoke exposed vs. 0.52 ± 0.16 mm controls; P = 0.067), indicating that the principle reason for increased BWT by µCT was likely due to mucosal thickening.
Regional differences in airway wall thickness.
Having established that smoke-exposed airways exhibited significantly greater bronchial wall thickening as compared with air control-exposed ferrets, we next sought to determine whether particular lobes were more affected, as seen in human COPD patients (18, 24). Likely related to differences in airflow in ferrets that alter deposition of cigarette smoke constituents, increased BWT performed by manual measurement disproportionately affected the caudal (40% increase, P < 0.001) and apical lobes (37% increase, P = 0.001) of ferrets exposed to cigarette smoke as compared with controls, whereas the medial lobe (25% increase, P = 0.005) was less severely affected by smoking (Fig. 2A). The effect of smoking on airway caliber varied substantially by anatomic location; the apical lobe of smoke-exposed ferrets exhibited increased diameter (by 23%), whereas the medial (8%) and caudal (19%) lobes were less dilated (Fig. 2B). Consequently, ratiometric BWT/LD measurements were also increased by smoking when all airways were considered (Fig. 2C) and were relatively consistent by anatomic location (13%, 17%, and 19% increased by smoke exposure over air controls at the apical, medial, and caudal lobes, respectively); considered individually, the changes in BWT/LD at each individual anatomic location were not statistically significant, but they were as a group (Supplemental Table S2).
Fig. 2.
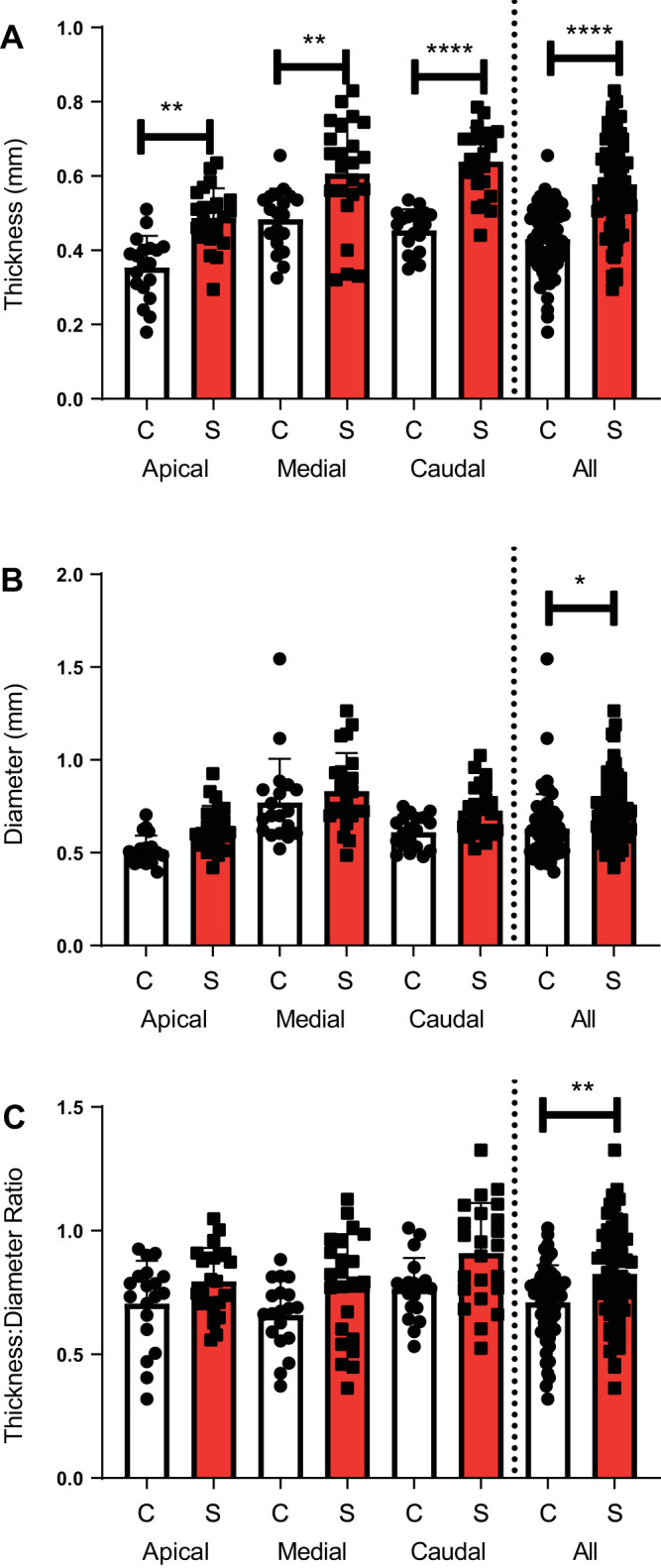
Anatomic location specific structural analysis of smoke-exposed ferret airways using microcomputed tomography. A: manual bronchial wall thickness (BWT) of air control and smoke-exposed ferrets by anatomic location. B: manual luminal diameter. C: manual ratio of BWT to luminal diameter. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Each region had 2 measurements per ferret (i.e., left, right) that were averaged to a single point estimate per ferret [n = 23 smoke (S), 19 control (C)]. A single point estimate for each of 3 regions was included for each ferret in the all-region analysis (n = 69 S, 57 C). Inferential comparisons by ANOVA with Tukey’s post hoc test.
Regression analysis.
As multiple different variables could be influencing airway dimensions, we next performed regression analysis to account for potential factors independently, using every airway measurement in the data set. Univariate analysis for an effect on BWT demonstrated smoke exposure (β = 0.148, indicating smoking had increased BWT by 0.148 mm on average; P < 0.001) and apical anatomic location (β = 0.064, P < 0.001) each had significant contributions to bronchial thickness in ferrets, whereas laterality and sex had no effect (Table 1). Similar to BWT, BWT/LD ratio was also significantly affected by smoke exposure (β = 0.080; P = 0.003) and anatomic location (β = 0.044; P = 0.007). Likewise, smoking (β = 0.099, P < 0.001) and male sex were associated with increased airway dilation (β = 0.119, P < 0.001).
Table 1.
Manual regression analysis of determinants of bronchial anatomy by CT
| Predictor | β | Standard Error | R2 | P Value |
|---|---|---|---|---|
| Univariate regression | ||||
| Smoking status | 0.148 | 0.016 | 0.241 | <0.001 |
| Anatomical location | 0.064 | 0.011 | 0.124 | <0.001 |
| Male sex | 0.025 | 0.019 | 0.007 | 0.184 |
| Laterality | 0.511 | 0.009 | 0 | 0.830 |
| Multivariate backward regression | ||||
| Smoking status | 0.015 | 0.002 | 0.368 | <0.001 |
| Anatomical location | 0.006 | 0.001 | 0.368 | <0.001 |
In the univariate regression analysis of determinants of bronchial wall thickness (BWT), all variables, including smoke status, sex, anatomic location (apical vs. medial vs. caudal lung lobes), and laterality (left vs. right), were modeled for contribution in BWT. In the multivariate backward regression model for significant determinants of BWT in chronic obstructive pulmonary disease (COPD) ferrets, all variables, including smoke status, sex, and anatomic location and laterality, were considered in the original model. n = 252 ferrets, P < 0.0001, R2 = 0.368.
To account for each of these variables independently, we then conducted multivariate backward regression to determine dominant contributors to airway morphology, starting with all variables noted in Table 1. In the most parsimonious model for determinants of BWT (R2 = 0.368, P < 0.001, n = 252 ferrets), smoke exposure and lung region were each independent determinants of increased airway thickness (Table 1). For BWT/LD (R2 = 0.141, P < 0.001, n = 252 airways), smoke exposure (β = 1.192; P < 0.001), female sex (β = 0.855; P < 0.001), and anatomic location (β = 0.444; P < 0.005) each remained in the final model. Similarly, for LD (R2 = 0.166, P < 0.001), smoke exposure (β = 0.093; P < 0.001), anatomic location (β = 0.049; P = 0.001), and male sex (β = 0.115; P < 0.001) were significant. In aggregate, these findings indicate smoke exposure has a strong effect on BWT and BWT/LD, even when controlled for covariates that influence these parameters.
Airway wall thickness as measured by Pi2.
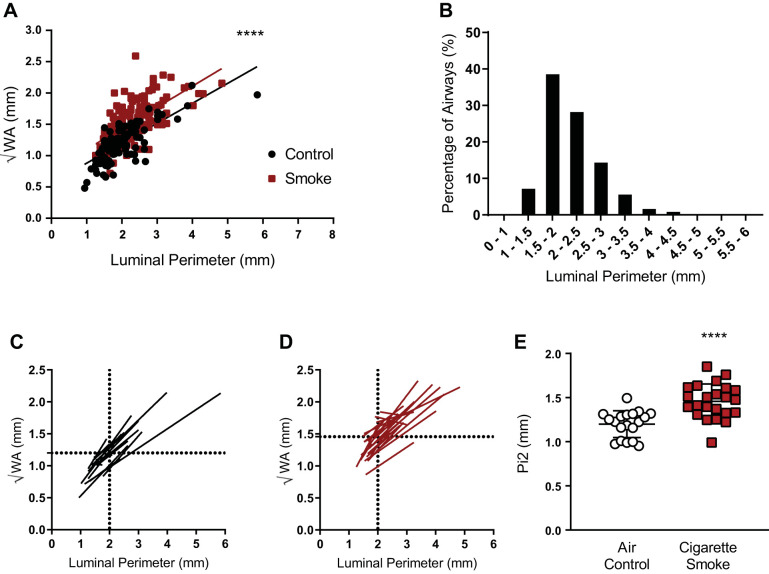
Next, we standardized measurements of airway abnormality in ferrets that closely modeled human airway disease detection using calculated airway wall thickness expressed as the square root of the wall area of a theoretical airway, as described in detail in methods; in the case of humans, this is a 10-mm internal perimeter airway (Pi10), as previously described (7, 12, 14, 19). For the ferret equivalent, we first observed pronounced differences between smoke-exposure groups indicating a consistent increase in wall area across various luminal perimeters (Fig. 3A). The probability density function of the inner airway perimeter of control ferrets indicated 65% of the airways measured were between 1.5 and 2.5 mm, with a mean perimeter of 1.94 mm (Fig. 3B); thus, we used calculation of √WA of a theoretical 2-mm perimeter airway for each ferret (i.e., Pi2) to assess difference between groups. Regression analysis on an individual ferret basis was used to calculate the √WA for the theoretical 2-mm perimeter airway (Fig. 3, C and D). Pi2 was 21.5% higher in smoke-exposed ferrets as compared with air controls (1.458 ± 0.196 mm smoke exposed vs. 1.199 ± 0.151 mm controls; P < 0.001, Fig. 3E). These findings indicated Pi2 provided a strong indicator of the effects of smoking, with excellent discrimination between groups using a method that is translatable to human studies and incorporates those advantages.
Fig. 3.
Differences in calculated bronchial wall area (√WA) for theoretical airways in ferrets. A: manual √WA vs. luminal perimeter plotted for each individual ferret airway (6 airways/ferret, n = 23 smoke, 19 control) by smoke exposure status. y-intercept (√WA) of the regression line was significantly greater in smoke-exposed ferrets as compared with air control. ****P < 0.0001 by linear regression slope comparison. B: manual probability density function of airway luminal perimeter sizes of air control ferrets. C and D: manual regression lines for each air control (C) and smoke-exposed ferret (D). Dotted lines show mean √WA for 2-mm perimeter airways (Pi2). E: manual calculated √WA of the theoretical Pi2 from ferrets exposed to cigarette smoke vs. airway control. ****P < 0.0001 by Student’s t test.
Interanalyst reproducibility.
To assess the influence of analyzer on manual measurements, we trained a second analyzer on a representative data set of 10 ferrets and assessed reproducibility of mean airway wall measurements per ferret between operators after blinding to exposure assignment. Although there were systematic differences in the measurement of BWT, LD, and BWT/LD between analyzers, the relative difference in BWT (30.9% increase in smoke-exposed ferrets vs. air controls by analyst no. 1 vs. 38% by analyst no. 2) and BWT/LD (19.4% vs. 41.7%) between smoke-exposed ferrets as compared with air controls remained relatively consistent and detectable (Supplemental Fig. S5, A and B). Similar conclusions were true for interanalyst differences in Pi2 measurements (Supplemental Fig. S5C). A Bland–Altman plot showed that differences between analysts were not related to airway thickness and were within 95% confidence of each other (Supplemental Fig. S5D). This suggested that airway morphometry measurements were robust in terms of ability to distinguish disease phenotype, but also suggested that semiautomated measurement could be potentially advantageous to avoid analyst-dependent differences in absolute quantitation.
Semiautomated airway analysis.
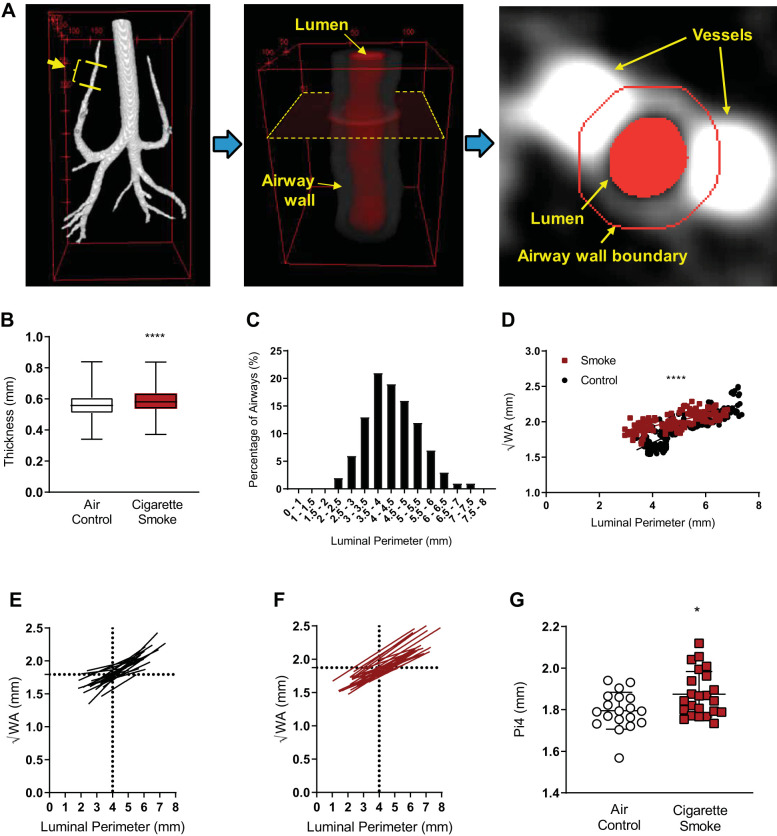
To address the between-operator differences in airway measurements, increase the number of measurements possible in a given data set, and transition to actual measurement versus a calculated assessment of airway wall area (important in the case of asymmetry), we developed a semiautomated airway wall measurement algorithm and applied it to the upper airways. Figure 4A illustrates the process for semiautomatic BWT measurement. The individual BWT measurements (n = 200 axial slices per ferret × 23 smoke, 19 control) were elevated in smoke-exposed ferrets (0.590 ± 0.077 mm) as compared to that of the air control group (0.561 ± 0.074 mm; P < 0.0001; Fig. 4B). As compared with manual measurements in the same ferrets, semiautomated measurements of bronchial wall thickness were larger, reflecting that the algorithm calculated the thickness of airways more proximal than could reliably be obtained by evaluating a single axial slice but continued to distinguish BWT in smoke-exposed ferrets versus air controls (Supplemental Fig. S6).
Fig. 4.
Semiautomated analysis of airway wall anatomy in smoke-exposed and air control ferrets. A: semiautomated image processing for measurement of bronchial wall thickness (BWT) is illustrated in 3 steps: first, the entire airway luminal region is segmented, and a target airway generation is specified as indicated with yellow lines. Second, the 3-dimensional computed tomography image is reoriented for the target airway region (lumen and wall) to be vertical to the image slices. A representative image slice is indicated with a dotted rectangle. Third, the bronchial wall boundary is automatically determined in each image slice. Typically, ~200 image slices were analyzed for each animal at one airway per apical lobe between the 4th and 6th generations. B: box plots of the semiautomated BWT in the control and smoke groups; n = 6,648 airways; ****P < 0.0001 by Student’s t test. Boxes denote the 25th–75th percentile and error bars represent the range. C: semiautomated probability density function of the luminal perimeter when the 4th–6th generations of the apical airway were analyzed in the control group. D: semiautomated scatter plots of calculated bronchial wall area (√WA) vs. airway luminal perimeter of a representative smoke-exposed and air control ferret, each plotted with a linear regression line. ****P < 0.0001. E and F: semiautomated linear regression lines of √WA vs. airway luminal perimeters of each ferret from the air control (E) and smoke-exposed (F) groups. Mean √WA at 4-mm luminal perimeter is indicated with dotted lines in each panel. G: semiautomated calculated √WA of the theoretical 4-mm perimeter airway (Pi4) for each ferret in the air control and smoke-exposed groups. *P = 0.012 by Student’s t test.
To conduct Pi measurements with the semiautomated methods, we conducted a similar analysis procedure as performed with manual measurements but used a larger Pi to reflect the fact that more proximal airways were captured. The probability density function (PDF) of luminal perimeter in the control group indicated 40% of the airways measured were between 3.5 and 4.5 mm, with a mean perimeter of 4.293 mm; thus, we used calculation of √WA of a theoretical 4-mm perimeter airway for each ferret (i.e., Pi4) to assess differences between groups (Fig. 4C). Figure 4D shows a scatter plot of measured √WA versus luminal perimeter of a representative animal in each group together with a linear regression line, suggesting the relationship is linear in each case (P < 0.001 for each). Figure 4, E and F, shows the √WA versus luminal perimeter regression lines of each animal in the control (n = 19) and smoke groups (n = 23), respectively, each constructed from ~200 data points. The mean √WA for a 4-mm luminal perimeter airway is indicated with a horizontal dotted line. The Pi4 of animals in the smoke-exposed group was 1.874 ± 0.110 mm, significantly larger than that in the control group (1.795 ± 0.090 mm; P = 0.015; Fig. 4G). As a sensitivity analysis and to compare with Pi estimates conducted with the manual measurements, the Pi2 in the smoke group was also higher than that in the control group, approaching statistical significance (1.622 ± 0.111 mm vs. 1.533 ± 0.184 mm; P = 0.061). Overall, these results confirmed findings with manual airway measurements in a fashion that was more robust and that could be applied to a greater number of representative airways.
Longitudinal changes in airway dimensions with smoke exposure.
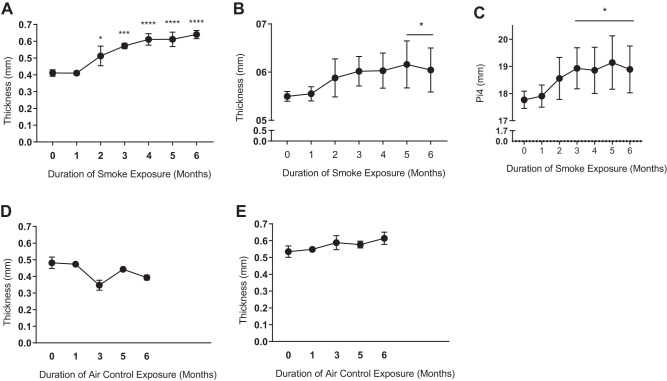
To assess whether airway thickness evolves over time in smoke-exposed ferrets with chronic bronchitis, we next performed quantitative µCT analysis at baseline and monthly thereafter through 6 mo in a subcohort of smoke-exposed ferrets (n = 3). As seen in Fig. 5A, mean BWT by manual measurement method steadily increased over time, with initial changes detectable as early as 2 mo of cigarette smoke exposure (24% increase, P < 0.05) that plateaued by 4 mo (48.8% increase). Semiautomated measurements showed BWT steadily increased with cigarette smoke exposure and became statistically significant at 5 mo (Fig. 5B, 10% increase, P = 0.016). Similarly, automated Pi4 measurements also increased over time (Fig. 5C), becoming statistically significant (P < 0.05) at 3 mo and peaking with a 7.7% increase (5 mo, P = 0.006). In contrast to smoke-exposed ferrets, air control ferrets did not have an increase in BWT over time, either by manual (Fig. 5D) or semiautomated measurements (Fig. 5E). These results showed cigarette smoke exposure induces airway disease as detected by several complimentary measures of airway wall abnormalities readily detected by µCT and is first evident by 2–3 mo of exposure.
Fig. 5.
Longitudinal cigarette smoke exposure increases bronchial wall thickness (BWT) and 4-mm perimeter airways (Pi4). A: 6-mo longitudinal study of mean BWT derived from all anatomic locations manually measured in a subset of ferrets (n = 3) followed over the course of smoking-induced COPD. *P < 0.05, ***P < 0.001, ****P < 0.0001. B: 6-mo longitudinal study plotting mean BWT of all anatomic locations by semiautomated analysis. *P < 0.05. C: calculated bronchial wall area (√WA) of theoretical Pi4 in the same longitudinal cohort by semiautomated analysis. *P < 0.05. Inferential comparisons by one-way ANOVA and Tukey’s post hoc test. D: mean BWT derived from all anatomic locations as a single value per ferret manually measured in an age-matched air control cohort of 9 ferrets (n = 1–5 measures at each time point). E: semiautomated BWT of same as in D.
DISCUSSION
Here we have demonstrated a technique to study structural airway changes using high-resolution µCT in a ferret model of smoking-induced chronic bronchitis, a technique highly sensitive to airway disease in humans with COPD (7, 12, 14, 19). This was successfully implemented by the manual measurement of 6 segmental airways, similar to a human study that established the correlation of bronchial wall thickness with airway reactivity (19), and also by semiautomated data extraction that improved the number of airways assessed, the range of airways measured, and the throughput and potential bias of the assessments. Both between-analyst reproducibility and the use of a semiautomated version of the method demonstrated complementary findings that were also reproducible, providing definitive conclusions. We further showed that cigarette smoke exposure in ferrets caused an increase in bronchial wall thickness that progressed over the course of a longitudinal 6-mo study as compared with air control ferrets, expanding our prior understanding of airway disease in the model (20) and firmly establishing causality. As compared with histopathological analysis that did not show that smoke exposure increased airway wall thickness but did not capture the overlying mucus layer, our results suggest that mucus accumulation may be the dominant factor underlying the difference in µCT parameters, although other aspects, including differences related to in vivo measurements for CT imaging versus ex vivo measurements for histology or technical issues related to tissue processing and variable washout of mucus, could also have contributed to this discrepancy. Of note, this is consistent with our prior report where global changes in epithelial thickness were not observed, even though epithelial cell height was increased (20).
The segmental and subsegmental airway wall thickness is commonly measured using Pi10 in human subjects and has proved to be a more superior biomarker of airway disease than other CT-based metrics (7, 12, 14, 19). Several studies demonstrated the significance of Pi10 measurements toward estimating airway narrowing associated with COPD (7, 12, 14, 19), and Pi10 has major advantages for sensitively detecting disease while avoiding bias in the estimate of between-subject differences in airway sizes. As an additional method developed in our study, in an effort to recapitulate the measurement of bronchial wall area in humans to estimate airway size for a standardized airway caliber on an individual basis (7, 12, 14, 19), we successfully implemented theoretical airway caliber calculations for the square root of bronchial wall area for the six medium-sized airways we calculated manually, as well as the ~200 semiautomated measurements of bronchial wall area (BWA) we measured directly in each ferret. Both the manual measure of bronchial wall area for theoretical airways on an individualized ferret basis (Pi2) and the semiautomated version (Pi4) demonstrated similar findings, in that they each indicated a 5–20% increase in smoke-exposed ferrets as compared with air controls and did so as soon as 2–4 mo following initiation of smoke exposure. These results provided a nonbiased and potentially powerful approach to quantify airway changes over time and clearly demonstrated the deleterious effects of smoking on airway morphometry that occur soon after first exposure, even though the intensity of smoking was not massive. Noting that the semiautomated measure was based on ~200 points to determine the regression line necessary to calculate theoretical airway perimeter, as compared with many fewer points with manual measurements, we suspect semiautomated Pi4 will be particularly valuable in future studies of pathophysiological change or therapeutic response, noting that Pi4 did include airways with a larger airway circumference, and thus may also report on distinct pathophysiological processes occurring in medium-sized airways.
The protean effects of cigarette smoking on airway pathophysiology continue to emerge and have been delayed in part by the challenges of modeling the disorder in humans (2). Development of the ferret model that exhibits chronic bronchitis in addition to emphysema, a unique pathophysiology compared with rodents but one that closely resembles human disease, provides an opportunity to better understand the human condition if appropriate end points can be developed and implemented, overcoming modest limitations posed by limited availability of ferret-specific protein reagents (20). In ferrets, the effects of the cigarette smoke were most pronounced in the caudal and apical regions of the lungs, which are areas most prone to particle deposition in the former (6) and consistent with central airway disease in humans in the case of the latter (21). More detailed analysis of how pathophysiology might differ by anatomic region provide an avenue for future research. Interestingly, the effects of sex were not prominent (except for differences in BWT/LD ratio), suggesting that despite sexually dimorphic characteristics with respect to overall animal size, the effects of smoking were prominent in each. This sex-based difference in airway changes is also consistent with that seen in human airways (11, 13). While we focused on airway disease, there may be other radiographic findings warranting attention in future work, including radiographic estimates of emphysema or the characterization of patchy alveolar opacities that are transient in nature.
A potential limitation of our study is that the resolution of the µCT scans do not yet fulfill the full capability of the instrumentation, principally due to limitations imposed by motion artifacts (i.e., beaming) associated with imaging a breathing animal, despite use of respiratory gating. This was noted as smaller voxel reconstitution did not improve end resolution. The limit for reliability in airway wall measurements will be primarily determined by the spatial resolution of image. With current methods, the spatial resolution is ~0.12 mm; further improvements would necessitate longer imaging times and subsequently computational power for image processing. Improved gating procedures, or institution of breath-hold maneuvers to pause breathing during image acquisition, could also be developed to improve limits of resolution, allowing sufficient resolution to ultimately address new questions, such as the severity of emphysema regional differences in disease expression or the occurrence of airway drop out detectable in human specimen by frozen tissue analysis and ultra-high resolution procedures (9, 10, 15, 16). While manual measurements were limited by throughput and the ability to measure the exact same location over time, using semiautomated measurements, we were able to increase the number of measures and allow for improved estimates of Pi4 that proved sensitive to disease as it emerged. However, our semiautomated method for bronchial wall thickness measurements was developed under the assumption that the airway is relatively straight at that location. Thus, this method may be suboptimal for curved airways.
In summary, we successfully developed µCT-based metrics of airway disease in a COPD ferret model, allowing within- and between-subject comparisons in vivo and enabling longitudinal studies as pathology evolves. Results in comparison to pathological interpretation demonstrated that airway disease is principally due to changes in the airway mucosa, as opposed to structural changes in the smooth muscle, and is clearly induced by cigarette smoking in the model. We further show that these metrics are dynamic, and that quantification of airway thickening can readily be performed on an individual ferret basis, providing a viable in vivo biomarker that can be exploited in future studies. This sets the stage for use of airway wall parameters as a sensitive metric for understanding biology that also has the potential for measuring the effects of novel interventions affecting airway mucus or epithelial function.
GRANTS
This work was supported in part by NIH National Heart, Lung, and Blood Institute (NHLBI) Grant R35HL135816 (to S.M.R.), National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK072482 (to S.M.R.), NHLBI Grant K23HL133438 (to S.P.B.), and National Institute on Alcohol Abuse and Alcoholism Grant 1R01AA027528 (to S.V.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., H.K., S.V.R., and S.M.R. conceived and designed research; D.S., J.L., and S.A.B. performed experiments; D.S., H.K., E.S.H., and S.M.R. analyzed data; S.V.R. and S.M.R. interpreted results of experiments; T.R.S. prepared figures; D.S., H.K., S.B., S.P.B., and S.M.R. drafted manuscript; D.S., H.K., S.B., J.L., S.A.B., E.S.H., H.P.N., S.P.B., S.V.R., and S.M.R. edited and revised manuscript; D.S., H.K., S.B., J.L., S.A.B., E.S.H., H.P.N., S.P.B., S.V.R., and S.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge institutional support through the University of Alabama Health Services Foundation Institutional Endowment to purchase the µCT instrument.
REFERENCES
- 1.Bodduluri S, Reinhardt JM, Hoffman EA, Newell JD Jr, Bhatt SP. Recent advances in computed tomography imaging in chronic obstructive pulmonary disease. Ann Am Thorac Soc 15: 281–289, 2018. doi: 10.1513/AnnalsATS.201705-377FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Churg A, Wright JL. Animal models of cigarette smoke-induced chronic obstructive lung disease. Contrib Microbiol 14: 113–125, 2007. doi: 10.1159/000107058. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty ER. An Introduction to Morphological Image Processing. Bellingham, WA: SPIE Optical Engineering Press, 1992. [Google Scholar]
- 4.Fisher JT, Zhang Y, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol 742: 311–334, 2011. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fricker M, Deane A, Hansbro PM. Animal models of chronic obstructive pulmonary disease. Expert Opin Drug Discov 9: 629–645, 2014. doi: 10.1517/17460441.2014.909805. [DOI] [PubMed] [Google Scholar]
- 6.Ganguly K, Carlander U, Garessus ED, Fridén M, Eriksson UG, Tehler U, Johanson G. Computational modeling of lung deposition of inhaled particles in chronic obstructive pulmonary disease (COPD) patients: identification of gaps in knowledge and data. Crit Rev Toxicol 49: 160–173, 2019. doi: 10.1080/10408444.2019.1584153. [DOI] [PubMed] [Google Scholar]
- 7.Grydeland TB, Thorsen E, Dirksen A, Jensen R, Coxson HO, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative CT measures of emphysema and airway wall thickness are related to DLCO. Respir Med 105: 343–351, 2011. doi: 10.1016/j.rmed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, Make BJ, Rabe KF, Rennard SI, Sciurba FC, Silverman EK, Vestbo J, Washko GR, Wouters EF, Martinez FJ. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 182: 598–604, 2010. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 10.Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest 143: 1436–1443, 2013. doi: 10.1378/chest.12-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim V, Davey A, Comellas AP, Han MK, Washko G, Martinez CH, Lynch D, Lee JH, Silverman EK, Crapo JD, Make BJ, Criner GJ; COPDGene Investigators . Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res 15: 52, 2014. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim V, Desai P, Newell JD, Make BJ, Washko GR, Silverman EK, Crapo JD, Bhatt SP, Criner GJ; COPDGene Investigators . Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res 15: 84, 2014. doi: 10.1186/s12931-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YI, Schroeder J, Lynch D, Newell J, Make B, Friedlander A, Estépar RS, Hanania NA, Washko G, Murphy JR, Wilson C, Hokanson JE, Zach J, Butterfield K, Bowler RP; COPDGene Investigators . Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD 8: 285–292, 2011. doi: 10.3109/15412555.2011.586658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, MacNee W. Airway dimensions in COPD: relationships with clinical variables. Respir Med 104: 1683–1690, 2010. doi: 10.1016/j.rmed.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 15.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Paré PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 365: 1567–1575, 2011. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 94: 782–787, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Ou C, Li Y, Wei J, Yen HL, Deng Q. Numerical modeling of particle deposition in ferret airways: a comparison with humans. Aerosol Sci Technol 51: 477–487, 2017. doi: 10.1080/02786826.2016.1265913. [DOI] [Google Scholar]
- 18.Owsijewitsch M, Ley-Zaporozhan J, Kuhnigk JM, Kopp-Schneider A, Eberhardt R, Eichinger M, Heussel CP, Kauczor HU, Ley S. Quantitative emphysema distribution in anatomic and non-anatomic lung regions. COPD 12: 260–266, 2015. doi: 10.3109/15412555.2014.933950. [DOI] [PubMed] [Google Scholar]
- 19.Patel BD, Coxson HO, Pillai SG, Agustí AG, Calverley PM, Donner CF, Make BJ, Müller NL, Rennard SI, Vestbo J, Wouters EF, Hiorns MP, Nakano Y, Camp PG, Nasute Fauerbach PV, Screaton NJ, Campbell EJ, Anderson WH, Paré PD, Levy RD, Lake SL, Silverman EK, Lomas DA; International COPD Genetics Network . Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178: 500–505, 2008. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 20.Raju SV, Kim H, Byzek SA, Tang LP, Trombley JE, Jackson P, Rasmussen L, Wells JM, Libby EF, Dohm E, Winter L, Samuel SL, Zinn KR, Blalock JE, Schoeb TR, Dransfield MT, Rowe SM. A ferret model of COPD-related chronic bronchitis. JCI Insight 1: e87536, 2016. doi: 10.1172/jci.insight.87536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith BM, Traboulsi H, Austin JHM, Manichaikul A, Hoffman EA, Bleecker ER, Cardoso WV, Cooper C, Couper DJ, Dashnaw SM, Guo J, Han MK, Hansel NN, Hughes EW, Jacobs DR Jr, Kanner RE, Kaufman JD, Kleerup E, Lin CL, Liu K, Lo Cascio CM, Martinez FJ, Nguyen JN, Prince MR, Rennard S, Rich SS, Simon L, Sun Y, Watson KE, Woodruff PG, Baglole CJ, Barr RG; MESA Lung and SPIROMICS investigators . Human airway branch variation and chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 115: E974–E981, 2018. doi: 10.1073/pnas.1715564115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan O, Duan HL, Lu WX. [Review: segmentation and classification methods of 3D medical images]. Zhongguo Yi Liao Qi Xie Za Zhi 26: 197–206, 2002. [PubMed] [Google Scholar]
- 23.Teerapuncharoen K, Wells JM, Raju SV, Raraigh KS, Atalar M, Cutting GR, Rasmussen L, Nath PH, Bhatt SP, Solomon GM, Dransfield MT, Rowe SM. Acquired CFTR dysfunction and radiographic bronchiectasis in current and former smokers: a cross-sectional study. Ann Am Thorac Soc 16: 150–153, 2019. doi: 10.1513/AnnalsATS.201805-325RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tho NV, Trang TH, Murakami Y, Ogawa E, Ryujin Y, Kanda R, Nakagawa H, Goto K, Fukunaga K, Higami Y, Seto R, Nagao T, Oguma T, Yamaguchi M, Lan TT, Nakano Y. Airway wall area derived from 3-dimensional computed tomography analysis differs among lung lobes in male smokers. PLoS One 9: e98335, 2014. doi: 10.1371/journal.pone.0098335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med 195: 557–582, 2017. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]