Abstract
Neutrophil extracellular traps (NETs) provide host defense but can contribute to the pathobiology of diverse human diseases. We sought to determine the extent and mechanism by which NETs contribute to human airway cell inflammation. Primary normal human bronchial epithelial cells (HBEs) grown at air-liquid interface and wild-type (wt)CFBE41o- cells (expressing wtCFTR) were exposed to cell-free NETs from unrelated healthy volunteers for 18 h in vitro. Cytokines were measured in the apical supernatant by Luminex, and the effect on the HBE transcriptome was assessed by RNA sequencing. NETs consistently stimulated IL-8, TNF-α, and IL-1α secretion by HBEs from multiple donors, with variable effects on other cytokines (IL-6, G-CSF, and GM-CSF). Expression of HBE RNAs encoding IL-1 family cytokines, particularly IL-36 subfamily members, was increased in response to NETs. NET exposure in the presence of anakinra [recombinant human IL-1 receptor antagonist (rhIL-1RA)] dampened NET-induced changes in IL-8 and TNF-α proteins as well as IL-36α RNA. rhIL-36RA limited the increase in expression of proinflammatory cytokine RNAs in HBEs exposed to NETs. NETs selectively upregulate an IL-1 family cytokine response in HBEs, which enhances IL-8 production and is limited by rhIL-1RA. The present findings describe a unique mechanism by which NETs may contribute to inflammation in human lung disease in vivo. NET-driven IL-1 signaling may represent a novel target for modulating inflammation in diseases characterized by a substantial NET burden.
Keywords: airway inflammation, IL-1, IL-8, IL-36, neutrophil extracellular trap
INTRODUCTION
Neutrophils are the first cells recruited and activated in response to diverse microbial pathogens, playing a critical role in innate host defense. Polymorphonuclear cells (PMNs) use several mechanisms to limit infection including release of reactive oxygen species, phagocytosis of microorganisms, and secretion of antimicrobial proteins (12). Neutrophils have recently been described to produce extracellular traps to kill microbes. Neutrophil extracellular traps (NETs) contain a mixture of nuclear, granular, and cytoplasmic cellular material that is released during infection (10). They are indispensable for control of fungal infections in patients with chronic granulomatous disease and are required to limit bacterial dissemination in murine models of Escherichia coli sepsis (5, 38). The deployment of NETs exposes the extracellular environment to a network of DNA strands studded with enzymes, e.g., myeloperoxidase (MPO) and neutrophil elastase (NE), and cytokines, e.g., IL-8. Many proteins identified in NETs have been demonstrated to retain biologic activity (46). NETs in blood and joints of patients with rheumatoid arthritis serve as a source of autoantigens, which can contribute to disease pathology (32), and NET-bound antibodies can contribute to venous thrombosis in murine models of heparin-induced thrombocytopenia (22). Together, these findings support the notion that excessive NET activity may contribute to the pathogenesis of diseases characterized by increased inflammation.
Defining pathways that perpetuate neutrophilic lung inflammation has the potential to identify therapeutic targets that could limit tissue damage. Although neutrophilic inflammation contributes to lung injury in acute respiratory distress syndrome (ARDS) and cystic fibrosis (CF), the role of NETs and the mechanisms by which they interact with pulmonary cells remain unclear (20, 33, 52). NETs are detected in mouse and human lungs, but their impact on primary human airway epithelial cell function requires further study (30, 35, 36). Increased NET complexes (DNA-MPO) have been described in the blood of patients with transfusion-associated lung injury (TRALI), and NET complexes (DNA-NE) were increased in bronchoalveolar lavage (BAL) of patients who developed primary graft dysfunction after lung transplantation (11, 47). On the basis of these previous observations, we hypothesized that NETs are sufficient to produce airway epithelial inflammatory responses in the absence of infection or other stimuli. We characterized the impact of NETs alone and NET-epithelial interactions using human airway epithelial cell monolayers in vitro. Our studies identified IL-1 signaling as a key airway epithelial response to NET exposure that was sensitive to IL-1 inhibition with recombinant human IL-1 receptor antagonist (rhIL-1RA). Collectively, our results demonstrate that NETs are sufficient to stimulate a specific airway cell inflammatory response.
MATERIALS AND METHODS
Generation of human NETs.
The study protocol, no. 2016-3837, was approved by the University of Cincinnati institutional review board (IRB). Up to 60 mL of peripheral blood were collected from healthy adult human donors in sodium citrate vacuum tubes (Fisher Scientific). Neutrophils were isolated using MACSxpress Neutrophil Isolation Kit, Human (Miltenyi), by negative magnetic bead selection according to the manufacturer’s instructions. Trypan blue, 0.4% (Fisher Scientific), was used to dilute neutrophils for counting with a hemocytometer and to assess viability. PMNs were suspended in RPMI-1640 media (GIBCO) with 3% fetal bovine serum (Fisher Scientific) at a concentration of 5 × 106 cells/mL in 100-mm tissue culture-treated petri dishes (Fisher Scientific). Phorbol 12-myristate 13-acetate (PMA; Sigma) was added to the neutrophils at a final concentration of 500 nM and incubated at 37°C and 5% CO2 for 4 h to induce NET generation (41). Following incubation, media were aspirated, the viscous surface layer of NETs was gently washed twice with PBS, and the wash was discarded. The NET layer was scraped from the dish, collected in a centrifuge tube, and mixed vigorously. The sample was then centrifuged at 450 g for 10 min at 22°C, after which the supernatant was collected. This centrifugation step was repeated to eliminate cell debris. NET dosage was defined by DNA concentration, as described by Saffarzadeh et al. (46). DNA content was quantified by averaging the result of the QuantiFluor ONE dsDNA System (Promega) and SYTOX green nucleic acid stain (Fisher Scientific), both to manufacturer’s specifications. For most experiments, epithelial cells were incubated with NET doses of ~5 μg/mL for 18 h at 37°C. Anakinra (rhIL-1RA), purchased from the Cincinnati Children’s Hospital Medical Center (CCHMC) research pharmacy, was diluted in PBS. Human bronchial epithelial cells (HBEs) were exposed to 5 μg/mL NETs + 100 ng/mL rhIL-1RA or 100 ng/mL rhIL-1RA alone for 18 h at 37°C (43). To determine the effect of inhibiting the IL-36 axis, rhIL-36RA (R&D Systems) was activated per the manufacturer’s directions, and then HBEs were exposed to 5 μg/mL NETs + 50 μM rhIL-36RA or 50 μM rhIL-36RA alone for 18 h at 37°C. To inhibit neutrophil elastase, HBEs were exposed to 5 μg/mL NETs + 100 μg/mL sivelestat (Sigma) or 100 μg/mL sivelestat alone for 18 h at 37°C.
Primary HBE and wild-type CFTR CFBE41o- monolayers.
CFBE41o- cells stably transduced with wild-type CFTR (wtCFBE41o-) were provided by the CCHMC CF Research Development Program Translational and Model Systems Cores. Cells were cultured as previously described (8). Primary HBEs (from non-CF donors) were obtained from University of North Carolina Airway Cell Core and were utilized at passages 2–3. HBEs were differentiated and grown at an air-liquid interface (ALI) on Transwell inserts by the CCHMC Pulmonary Core as described (8). Primary HBE use was approved by the CCHMC, Pulmonary Biorepository Core, and University of Cincinnati IRBs. The apical surfaces of HBEs were exposed to human NETs +/− dornase alfa (Pulmozyme) purchased from the CCHMC pharmacy or control conditions including PBS, 5 μg/mL human genomic DNA (gDNA; Promega) +/− 500 nM PMA, or Ultroser G-based media for 18 h at 37°C, unless otherwise specified. Every experiment was done with a different HBE donor, but we were not able to test all analytes in every experiment.
Collection and characterization of cell supernatants.
After exposure to NETs or controls, supernatants were collected from the apical surface of inserts and centrifuged to remove cellular debris. Supernatants were divided into aliquots and stored at −80°C. Human neutrophil elastase (NE) activity was measured using NE activity fluorescence assay (Cayman Chemical). Human neutrophil myeloperoxidase (MPO) activity was measured by MPO activity kinetic absorbance assay (Cayman Chemical). Luminex assay was performed for all cytokines by Cincinnati Children’s Hospital Research Flow Cytometry Core per kit instructions (Millipore). Confirmation of IL-8 and IL-1RA concentrations was performed, where indicated, by ELISA performed per the manufacturer’s instructions (R&D Systems).
RNA isolation and sequence analysis.
RNA sequencing (RNA-seq) was performed on primary HBEs from three healthy control subjects exposed to PBS or 5 μg/mL human NETs isolated from three unrelated healthy donors for 18 h. RNA sequencing was performed by CCHMC’s Gene Expression Core utilizing the Illumina HiSeq2500. RNA-seq FASTQ files were aligned using Bowtie to human reference genome GRCh37. Normalized gene expressions were generated using Cufflinks (51). Gene counts were obtained using Bioconductor’s GenomicAlignments (21). DESeq was used to analyze the raw gene counts and calculate differentially expressed genes (1). Genes were deemed differentially expressed that had a fold change >2.5, an nbinomTest P value <0.01, and reads per kilobase million (RPKMs) >2 for 50% of the samples in at least one condition. Revised metadata and processed data files were submitted to Gene Expression Omnibus (GEO), accession no. GSE124378. A heat map of differentially expressed genes was z-score normalized and generated using Partek Genomics Suite (https://www.partek.com/pgs). ToppGene’s ToppFun was used to identify functional enrichment hits of significantly altered RNAs (13). P values of functional enrichment hits were –log10 transformed for graphical visualization. Predicted upstream regulators and networks were generated using Ingenuity Pathway Analysis (IPA) suites. To verify RNA-seq results, RT-PCR was performed with TaqMan master mix and primers IL-36α (HS00205367_m1), IL-36γ (HS00219742_m1), IL-36RN (encoding IL-36RA; HS01104220_g1), IL-1α (HS00174092_m1), IL-1β (HS01555410_m1), IL-1RN (HS00893626_m1), CXCL-8 (HS00174103_m1), and TNF-α (HS00174128_m1), normalized to EUK 18S rRNA AB (1207030; Fisher Scientific).
Immunofluorescence microscopy.
NET images were captured on a Zeiss Axioplan 2 microscope using antibodies to Hoechst (Invitrogen) and NE (Abcam) with Alexa Fluor 488-conjugated secondary antibody (Invitrogen).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software v7.04. Groups were compared using one-way ANOVA with Bonferroni correction for multiple comparisons or a Student’s t test where indicated. Data are expressed as means ± SE. A P value of <0.05 was considered significant. Graphs include data from at least three separate experiments unless indicated. Each experiment included conditions run on triplicate or more wells (as noted in figure legends).
RESULTS
Isolated human NETs exhibit characteristic staining and contain enzymatically active MPO and NE.
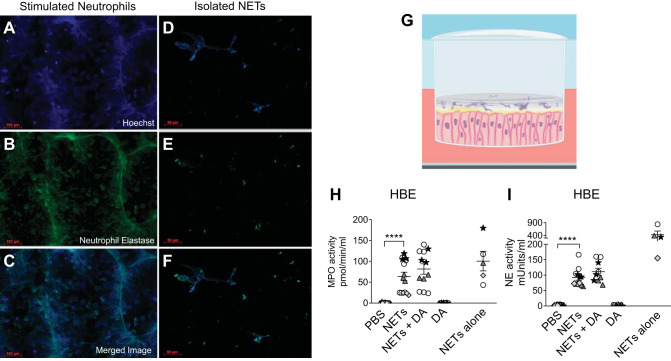
We generated human NETs from freshly harvested PMNs isolated from eight different healthy adult subjects, with each donor represented in the figures by different symbols. NETs were then separated from remaining neutrophils to produce a cell-free NET preparation as previously described, allowing isolation of NET-specific effects and minimizing the effects of necrotic or intact PMNs (41). As shown in Fig. 1, A–F, both PMA-stimulated neutrophils and isolated cell-free NETs demonstrated characteristic NET morphology, including an extracellular DNA backbone studded with neutrophil elastase (NE; 46). Figure 1, A–C, demonstrates that stimulated neutrophils contain significant cellular debris as well as neutrophils that did not form NETs, which is not seen in the isolated NET preparation used in our experiments (Fig. 1, D–F).
Fig. 1.
Characterization of cell-free human neutrophil extracellular traps (NETs) and experimental design. A–F: fluorescence microscopy of neutrophils stimulated to form NETs or isolated NETs. Representative images depicting colocalization of DNA (Hoechst) and NET-related protein neutrophil elastase (Alexa Fluor 488). Freshly harvested neutrophils were stimulated with 500 nM PMA for 4 h to induce NET formation directly on a slide (A–C) or in a dish, and then cell-free NETs were isolated (D–F). G: schema illustrating the experimental design. NETs in PBS were added to the apical surface of human bronchial epithelial cells (HBEs) grown at air-liquid interface (ALI). H and I: HBEs were grown at ALI and exposed to PBS, 5 µg/mL NETs, 5 µg/mL NETs + 0.5 µg/mL dornase alfa (DA), or 0.5 µg/mL DA for 18 h. NETs alone were simultaneously incubated for 18 h without epithelial cells. Enzymatic activity of myeloperoxidase (MPO) and neutrophil elastase (NE) was measured in the supernatant and analyzed by one-way ANOVA. Each symbol represents NETs isolated from a different donor that were exposed to unrelated HBE donor cells in triplicate wells. (HBE donors = 4–5, NET donors = 3–4.) ****P < 0.0001.
Polarized HBEs from 14 normal donors were exposed to human NETs from unrelated (normal) donors or control conditions on their apical surface for 18 h (Fig. 1G; 26). We included primary HBEs from many donors to capture the spectrum of biologic variability. The conditions tested included NETs, NETs coincubated with dornase alfa (DA; recombinant human DNase), DA alone, and PBS controls. DA is a common therapy in CF and was added to determine whether treatment with DNase impacted HBE responses to NET exposure. To assess enzymatic activity in the NETs, we assayed NE and MPO activity in NETs alone and in the apical supernatant of HBEs exposed to NETs +/− DA. NET-derived MPO activity was largely retained, whereas NE activity decreased when NETs were coincubated with HBEs for 18 h (Fig. 1, H and I). Neither NE nor MPO activity was detected in the basal compartment of HBEs (data not shown). The extent of enzymatically active MPO and NE varied between NET donors both at baseline and after NETs were incubated with HBEs (Fig. 1, H and I).
Human NETs stimulate secretion of proinflammatory cytokines by airway epithelial cells.
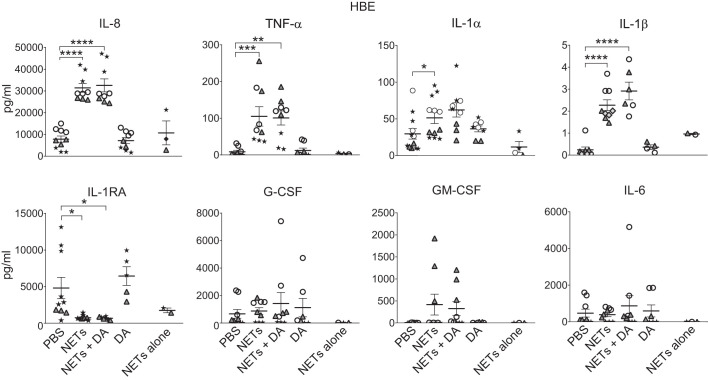
NET treatment consistently led to IL-8 release into the apical compartment across multiple donors compared with PBS controls (Fig. 2). TNF-α level was also significantly increased following exposure to NETs, but with more variability among donors (Fig. 2). Levels of IL-1α and, to a lesser magnitude, IL-1β were increased in HBE supernatant in response to NETs (Fig. 2). The level of anti-inflammatory cytokine IL-1 receptor antagonist (IL-1RA) was significantly decreased in HBEs exposed to NETs (Fig. 2), but there were no consistent differences in secretion of G-CSF, GM-CSF, or IL-6 by HBEs following NET exposure (Fig. 2). To determine whether NETs were a significant source of these cytokines, NETs alone were incubated on inserts without HBEs for 18 h. NETs alone did not contribute significant levels of TNF-α, G-CSF, GM-CSF, or IL-6 proteins, but NETs did contribute measurable levels of IL-8, IL-1RA, and IL-1α (Fig. 2).
Fig. 2.
Human neutrophil extracellular traps (NETs) alter cytokine secretion by human bronchial epithelial cells (HBEs). HBEs were grown at air-liquid interface and exposed to PBS, 5 μg/mL NETs, 5 μg/mL NETs + 0.5 μg/mL dornase alfa (DA), or 0.5 μg/mL DA for 18 h. NETs alone were simultaneously incubated for 18 h without epithelial cells. Cytokine concentrations were measured in the apical supernatant by Luminex, and IL-8 and IL-1 receptor antagonist (IL-1RA) concentrations were confirmed by ELISA. Results were analyzed by one-way ANOVA. (HBE donors = 6, NET donors = 5.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
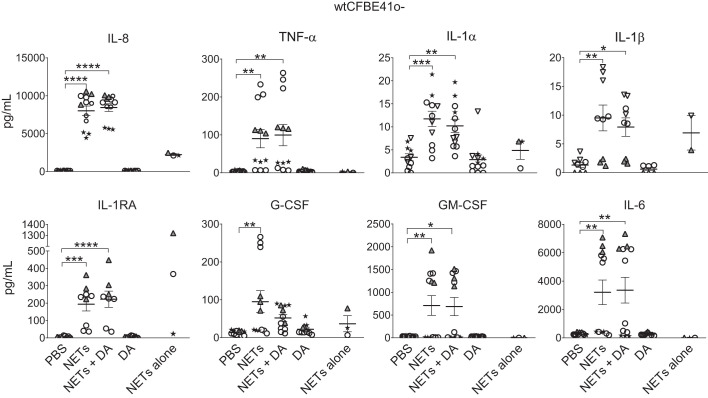
Our studies in primary human samples identified significant biologic variability in cytokine production by different NET and airway epithelial donors (Fig. 2). To understand the relative contribution of NET versus epithelial donors to this variability, we measured cytokine levels in the apical supernatants of a common airway epithelial cell line (wtCFBE41o- cells) exposed to NETs from several donors. This cell line was chosen as it readily forms monolayers and maintains important features of primary human bronchial epithelia including apical and basal compartments and tight junctions (4, 23). Similar to findings with HBEs, wtCFBE41o- cells exposed to NETs had increased secretion of IL-8, TNF-α, IL-1α, and IL-1β compared with PBS controls (Fig. 3). In contrast to primary HBEs, NETs stimulated IL-1RA, G-CSF, GM-CSF, and IL-6 in wtCBE41o- cells. The increases in IL-1RA, G-CSF, GM-CSF, and IL-6 primarily represent the results from two NET donors, whereas NETs from the other donors elicited small or no amounts of these cytokines from wtCFBE41o- cells (Fig. 3). These results demonstrate that the variability in cytokine responses evoked by NET-airway epithelial interactions depends on both the NET and airway epithelial cell donors.
Fig. 3.
Human neutrophil extracellular traps (NETs) alter cytokine secretion by wild-type (wt)CFBE41o- cells. wtCFBE41o- cells were exposed to PBS, 5 μg/mL NETs, 5 μg/mL NETs + 0.5 μg/mL dornase alfa (DA), or 0.5 μg/mL DA for 18 h in triplicate wells. NETs alone were simultaneously incubated for 18 h without epithelial cells. Cytokine concentrations were measured in triplicate wells of apical supernatant by Luminex, and IL-8 concentration was confirmed by ELISA. Data were analyzed by one-way ANOVA. Each symbol represents NETs isolated from a different donor. IL-1RA, IL-1 receptor antagonist. (NET donors = 4.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Simultaneous treatment of NETs with DA did not significantly impact apical cytokine concentrations in HBEs or wtCFBE41o- cells. Epithelial exposure to DA alone yielded similar cytokine results to those seen with PBS controls (Figs. 2 and 3).
NETs stimulate proinflammatory transcriptional changes in primary HBEs.
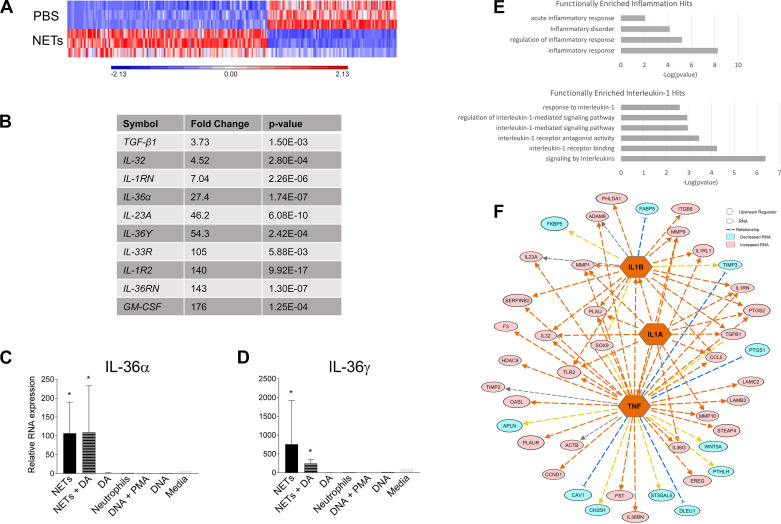
Since NETs stimulated secretion of a select repertoire of inflammatory cytokines from airway cells, we tested whether NETs regulated the expression of RNAs encoding specific inflammatory mediators. We measured the effect of NETs on the transcriptome of primary HBEs (from 3 unrelated donors) via RNA-seq. Figure 4A demonstrates that 247 genes were differentially expressed in HBEs exposed to NETs compared with HBEs exposed to PBS (>2.5-fold cutoff for significant expression change). One hundred fifty genes were upregulated, most of which were related to IL-1 family members and GM-CSF. The RNAs with greatest increase after NET exposure compared with PBS controls are summarized in Fig. 4B. Expression of IL-1RN RNA (encoding IL-1RA), IL-1R2 decoy receptor, IL-36α, IL-36γ, and IL-36RN was markedly increased by NETs. Increases in IL-36α and IL-36γ expression were confirmed by RT-PCR (Fig. 4, C and D). The effects of NETs on the IL-1-associated genes were not observed with DA alone, unstimulated human neutrophils, gDNA + PMA, human gDNA alone, or media (Fig. 4, C and D). NETs did not contain detectable RNA, confirming that the effects of NETs on the HBE transcriptome represented changes in epithelial cell gene expression.
Fig. 4.
Neutrophil extracellular traps (NETs) influence gene expression in human bronchial epithelial cells (HBEs). HBEs grown at air-liquid interface were exposed to PBS, 5 μg/mL NETs, 5 μg/mL NETs + 0.5 μg/mL dornase alfa (DA), or 0.5 μg/mL DA for 18 h in triplicate wells. Differential gene expression was assessed by RNA sequencing. A: expression of 150 RNAs was increased, whereas expression of 97 RNAs was decreased, after exposure to 5 μg/mL NETs compared with PBS (P < 0.01 and a fold change >2.5). B: the most highly induced RNAs in response to NETs, particularly IL-1 family members (IL-36α, IL-36γ, etc.) and GM-CSF. C and D: HBEs were exposed to the above conditions as well as unstimulated human neutrophils, 0.5 µg/mL genomic DNA (gDNA) + 500 nM PMA, 0.5 µg/mL gDNA, or media control for 18 h. IL-36α and IL-36γ gene expression was assessed by RT-PCR, normalized to 18S, and analyzed by one-way ANOVA comparing each condition relative to RNA expression in HBEs exposed to PBS. Measured in biologic triplicate wells and run in experimental triplicates. E: functional enrichment analysis using ToppGene indicated that IL-1 cytokines and their corresponding antagonists were increased from all three HBE donors. F: schematic representation of the upstream regulators (orange) predicted by Ingenuity Pathway Analysis to influence the transcriptional changes seen in HBEs exposed to NETs. Pink indicates increased expression of RNAs, and aqua indicates decreased expression of RNAs. (HBE donors = 3, NET donors = 3.) *P < 0.05.
Transcriptome analysis indicated that the pathways most functionally enriched in HBEs by NETs were related to inflammatory responses, particularly IL-1 signaling (Fig. 4E). Figure 4F indicates that TNF-α, IL-1α, and IL-1β were the upstream regulators predicted to control the transcriptional changes produced by NETs in HBEs (Fig. 4, A and B). Consistent with these transcriptional findings, increases in TNF-α, IL-1α, and IL-1β protein cytokine levels were detected in supernatants of HBEs exposed to NETs (Fig. 2).
rhIL-1RA inhibits NET-induced cytokine changes.
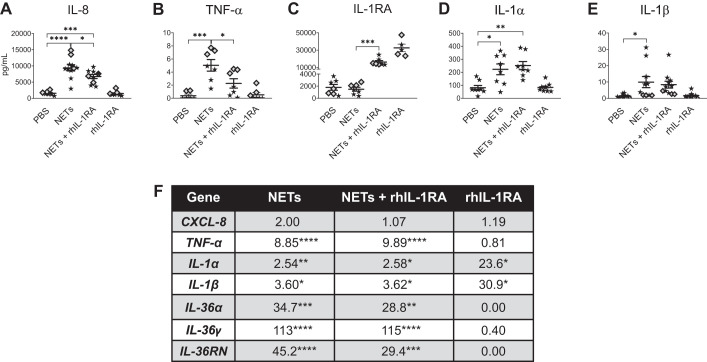
On the basis of the distinct signature of secreted cytokines and transcriptional changes produced by isolated NETs, we decided to test whether inhibition of IL-1 signaling with rhIL-1RA would impact NET effects on airway epithelia. Treatment with rhIL-1RA decreased the secreted IL-8 and TNF-α concentrations in HBEs exposed to NETs (Fig. 5, A and B), whereas IL-1α and IL-1β protein concentrations were unchanged (Fig. 5, D and E). In control experiments, exogenous rhIL-1RA protein remained detectable after 18 h of incubation (Fig. 5C), and HBE treatment with rhIL-1RA alone (without NETs) did not alter protein cytokine concentrations of IL-8 or TNF-α (Fig. 5, A and B) or IL-1 agonists (Fig. 5, D and E). HBEs treated with rhIL-1RA and exposed to NETs trended toward lower concentrations of IL-1RA protein in apical supernatants compared with those observed in HBEs treated with rhIL-1RA alone (Fig. 5C).
Fig. 5.
Recombinant human IL-1 receptor antagonist (rhIL-1RA) blunts neutrophil extracellular trap (NET)-induced cytokine changes in human bronchial epithelial cells (HBEs). HBEs grown at air-liquid interface were pretreated for 1 h with 100 ng/mL rhIL-1RA or PBS and then exposed to 5 μg/mL NETs +/− 100 ng/mL rhIL-1RA for 18 h in triplicate or more wells. A–E: supernatant IL-8 and IL-1RA concentrations were measured by ELISA, and the remaining cytokine concentrations were measured by Luminex and analyzed by one-way ANOVA. F: RNA expression of cytokine genes was measured by RT-PCR, and fold changes were calculated relative to HBEs exposed to PBS and analyzed by one-way ANOVA. (HBE donors = 3, NET donors = 3.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We then tested the effects of exogenous rhIL-1RA on NET-induced gene expression in HBEs exposed to NETs. rhIL-1RA did not alter NET-induced increases in expression of IL-8 or TNF-α RNAs at 18 h. rhIL-1RA reduced NET-induced increases in expression of IL-36α and IL-36RN RNAs (Fig. 5F). rhIL-1RA alone did not influence epithelial expression of CXCL-8, TNF-α, or IL-36 cytokine RNAs (Fig. 5F). Exposure to rhIL-1RA alone significantly increased IL-1α and IL-1β transcripts (Fig. 5F), potentially through previously described feedback inhibition (19).
NET-induced IL-36 signaling increases expression of proinflammatory cytokine RNAs.
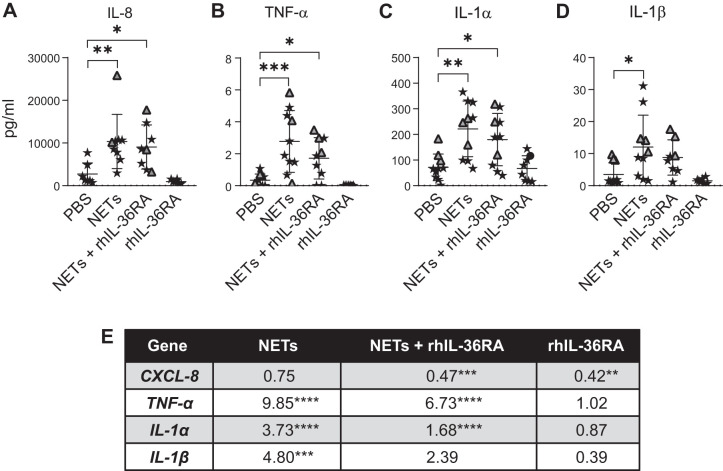
A key finding of our work was that NETs increased expression of RNAs of several IL-1 subfamily members, including agonists IL-36α and IL-36γ and the competitive antagonist IL-36RN (Fig. 4, B–D). To determine the impact of this, we limited IL-36 agonist binding to the IL-36 receptor (IL-36R) by exposing HBEs to NETs in the presence of rhIL-36RA. No significant changes were seen in IL-8 protein, but there was a trend toward decreased TNF-α, IL-1α, and IL-1β protein levels at 18 h (Fig. 6, A–D). rhIL-36RA added to NETs significantly decreased expression of HBE RNAs for CXCL-8, TNF-α, and IL-1α by 30–50% with a trend toward decreasing IL-1β RNA expression (Fig. 6E). HBEs exposed to rhIL-36RA alone reduced CXCL-8 RNA expression, but expression of other cytokine RNAs was unchanged (Fig. 6E).
Fig. 6.
Recombinant human IL-36 receptor antagonist (rhIL-36RA) decreases expression of proinflammatory cytokine RNAs in human bronchial epithelial cells (HBEs) exposed to neutrophil extracellular traps (NETs). HBEs grown at air-liquid interface were exposed to PBS, NETs, NETs + 1 μg/mL activated rhIL-36RA, or rhIL-36RA alone for 18 h in triplicate wells. A–D: cytokine concentrations in apical supernatant of HBEs were measured by Luminex, and IL-8 concentration was confirmed by ELISA and analyzed by one-way ANOVA. E: RNA expression of cytokine genes was measured by RT-PCR, and fold changes were calculated relative to HBEs exposed to PBS and analyzed by one-way ANOVA. (HBE donors = 3, NET donors = 3.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
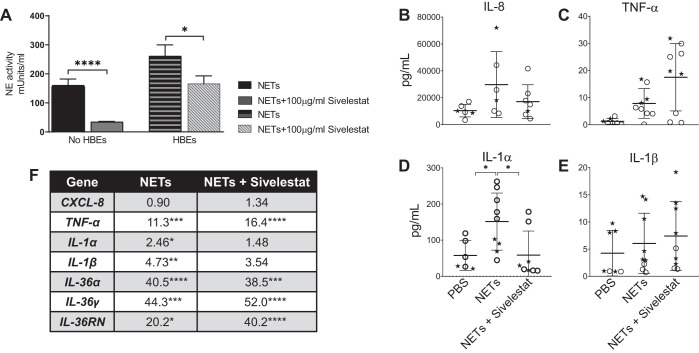
Inhibition of neutrophil elastase in NETs reduces NET-induced IL-1α levels.
We sought to determine whether NET-bound neutrophil elastase contributed to NET-induced proinflammatory cytokine changes in HBEs. Figure 7A demonstrates that the NE inhibitor sivelestat effectively reduced the NE activity of NETs in both the presence and absence of epithelial cells. Inhibition of NE activity in NETs significantly reduced IL-1α protein levels (Fig. 7D) with a corresponding trend toward decreasing expression of IL-1α, IL-1β, and IL-36α RNAs (Fig. 7F). Sivelestat did not significantly decrease levels of other cytokine proteins or RNAs (Fig. 7, B, C, E, and F) at 18 h.
Fig. 7.
Neutrophil elastase (NE) in neutrophil extracellular traps (NETs) contributes to cytokine changes in human bronchial epithelial cells (HBEs). A: neutrophil elastase activity was measured in NETs in the absence and presence of HBE, both with and without the NE inhibitor sivelestat 100 μg/mL for 18 h. B–E: HBEs grown at air-liquid interface were exposed to PBS, NETs, or NETs + 100 μg/mL sivelestat for 18 h. Cytokine concentrations in apical supernatant of HBEs were measured by Luminex, and IL-8 concentration was confirmed by ELISA and analyzed by Student’s t test. F: RNA expression of cytokine genes was measured by RT-PCR, and fold changes were calculated relative to HBEs exposed to PBS and analyzed by one-way ANOVA. (HBE donors = 2, NET donors = 2.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
DISCUSSION
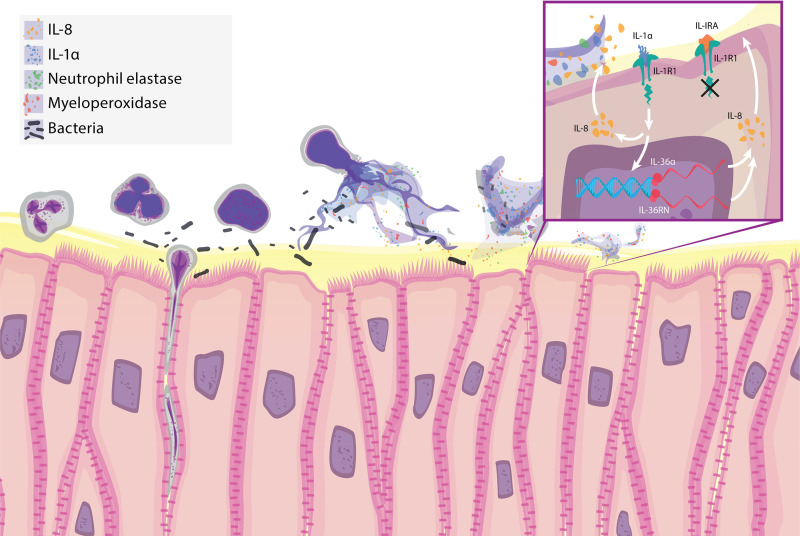
NETs are a recently described mechanism by which neutrophils trap and kill microorganisms in various tissue compartments (29, 53). NETs may contribute to inflammatory injury in autoimmune disorders and have been shown to enhance lung injury under certain circumstances (33, 50). We hypothesized that isolated NETs could contribute to inflammatory signaling and cytokine responses in human airway epithelial cells. Our results indicate that NETs caused airway epithelia to secrete the proinflammatory cytokines IL-8, TNF-α, IL-1α, and IL-1β. Isolated human NETs also contained measurable amounts of IL-8, IL-1α, and IL-1RA, but these levels were small relative to those produced by NETs combined with HBEs or wtCFBE41o- cells (Figs. 2 and 3). In complementary transcriptional studies, NETs increased airway epithelial expression of IL-1 family members, particularly from the IL-36 subfamily (Fig. 4). Inhibition of IL-1 signaling decreased NET-induced epithelial cytokine changes (Fig. 5), highlighting the specificity of the NET-HBE interaction and importance of this pathway in pulmonary epithelial responses to NETs. Competitive inhibition of the NET-induced IL-36 axis decreased expression of IL-1 agonist RNAs in HBEs (Fig. 6). The neutrophil elastase in NETs contributed to increases in IL-1α levels in airway epithelia (Fig. 7). Collectively, these findings support the hypothesis that NETs are sufficient to drive airway inflammation, with airway IL-1 family signaling serving as an important driver of cytokine release (Fig. 8).
Fig. 8.
Neutrophil extracellular traps (NETs) increase inflammatory cytokine levels in human airway epithelium. Schematic illustration summarizing present findings. Neutrophils release NETs that contain cytokines [IL-8, IL-1α, and IL-1 receptor antagonist (IL-1RA)] and enzymatically active neutrophil elastase (NE) and myeloperoxidase (MPO). Inset: NETs induce airway epithelia to release increased levels of IL-1α, which binds to IL-1R1 and activates IL-1 signaling to increase airway IL-8 levels. Enhanced IL-1 signaling also increases expression of IL-36α RNA, which further drives IL-8 expression. The addition of the competitive inhibitor rhIL-1RA (anakinra) dampens NET-induced IL-1 signaling, which leads to decreases in levels of IL-8 protein and IL-36α and IL-36RN RNAs.
NETs increased the levels of both IL-1 agonists, IL-1α and IL-1β, which bind to IL-1 receptor 1 (IL-1R1) and activate intracellular signaling to increase expression of proinflammatory cytokines (19). IL-8 and TNF-α release was stimulated by NETs (Fig. 2), and both have previously been correlated with severity of inflammatory lung diseases such as ARDS and CF (6, 14, 18). NET-induced IL-8 has been reported by other groups in pulmonary cell lines, but our work builds on these findings by providing mechanistic insights into this process (42, 45). NETs have been reported to stimulate IL-1β from diseased macrophages, but, to our knowledge, herein is the initial demonstration that NETs drive IL-1 cytokine family content in primary human airway epithelia (2). IL-1RA is the naturally occurring competitive inhibitor that binds IL-1R1 without producing signal transduction (24). Our results indicate that NET-induced IL-8 release and TNF-α release were both sensitive to inhibition by rhIL-1RA treatment (Fig. 5, A and B), highlighting the downstream nature of these cytokines to IL-1. The results reported herein build on our prior findings in murine models of acute lung injury in which intratracheal rhIL-1RA decreased BAL CXCL-1 (IL-8 homolog) and neutrophil counts and previous reports that greater IL-1RA expression correlates with a lower risk of ARDS (27, 39). Together, the results of the present and prior studies help to characterize a novel mechanism to potentially limit chemoattractant signaling in a selective manner.
Another novel finding was that NETs increased IL-36 subfamily RNA expression, likely mediated by IL-1 signaling. Our pathway analysis predicted that IL-1α and IL-1β regulate many of the NET-induced changes in HBE RNAs, including both IL-36α and IL-36γ (Fig. 4). This is consistent with previous reports that IL-1β increased expression of IL-36α and IL-36γ RNAs and separately that rhIL-36α and rhIL-36γ increased IL-8 protein levels in human bronchial epithelia (15, 54). Inhibition of the IL-1 pathway (with rhIL-1RA) decreased expression of both IL-36α and IL-36RN (Fig. 5F), confirming the central role of IL-1 regulation of IL-36 subfamily members by NETs. Increased IL-36 signaling has recently been described in the pathology of the autoinflammatory skin disease generalized pustular psoriasis (GPP; 28). This disorder can be caused by mutations in the IL-36RN gene, which reduce expression of the IL-36 receptor antagonist. Keratinocytes from patients with GPP exposed to IL-36 or IL-1β demonstrate heightened IL-8 production. Subcutaneous rhIL-1RA treatment reduced inflammation, skin lesions, and blood leukocytosis in patients with GPP (28, 37). Our results suggest a possible mechanism by which rhIL-1RA impacted GPP disease pathology and why increased NETs correlated with disease severity in psoriasis vulgaris (25, 28). On the basis of our results in Figs. 5 and 6, we speculate that NET-induced IL-1 signaling could also stimulate IL-36α and IL-36γ production, leading to enhanced CXCL-8 transcription and increased downstream neutrophil chemotaxis in human airways.
We found that NE in NETs is partially responsible for NET-induced cytokine changes in HBEs. Free and NET-bound neutrophil proteases have also been described to modulate the activity of IL-1 and IL-36 family members, and NE could further enhance IL-1 signaling via this mechanism (16, 17). The lack of effect of DA on NET-induced cytokines is consistent with previous reports in pulmonary cell lines exposed to NETs in the presence of nucleases. Together, these results suggest that NET DNA does not stimulate epithelial cytokines (45). To our knowledge, the data presented here are the first to demonstrate that NETs are sufficient to stimulate the expression of IL-1 agonists, TNF-α, and IL-36 agonists in primary human airway epithelia.
A strength of this work was the use of primary neutrophils and primary HBEs from many different donors demonstrating the spectrum of biologic variability, readily interpretable in our graphs. The cytokine-stimulatory effects of NETs on human airway epithelia were variable by donor (Fig. 2). By testing primary HBEs and an established epithelial cell line, we demonstrated that both NETs and airway cells independently contributed to response variability. Neutrophil responses are known to vary between donors and within the same donor from day to day (49). Variability in NET production between individuals has also been previously reported, and primary HBEs from different donors have variable biologic responses (9, 24). We attempted to limit some of the variability contributed by PMNs by harvesting cells at the same time each day from healthy volunteers without recent exposure to NSAIDs, alcohol, or illness, but clearly our data indicate that additional factors can influence neutrophil and epithelial cell responses (49). It is theoretically possible that epithelial cells could detect that unrelated NETs are “nonself” and stimulate an immune response from the HBEs. We are, however, unaware of a recognition mechanism by which this could occur, and there are no antigen-presenting cells to detect a “mismatch.”
In our study, dosing of NETs was defined by DNA content. Although this is considered the standard in the field, this may underestimate the effects of the complex NET structure and NET composition, which vary among individuals (3, 7). Using primary human cells from many donors not only strengthens the validity of the conclusion that NETs induce specific and consistent changes in IL-1 family members and IL-8 across many subjects but also highlights that NETs and epithelia may produce additional cytokine responses that are unique. Future work could determine whether differing NET-epithelial responses represent phenotypes that occur in vivo that could be exploited using precision medicine.
Our in vitro studies were not designed to determine or predict whether NETs produce negative or positive host effects in vivo. We speculate that NET effects are dependent on the nature and duration of NET-epithelial interactions and their role in modulating inflammation is likely context dependent. For example, NETs have been demonstrated to have an anti-inflammatory effect in some models of gout (48). In our studies we have examined fundamental questions regarding isolated NETs and their interactions with polarized human airway epithelia, demonstrating that they can drive inflammatory responses. As NETs are an important but incompletely understood component of the host response to pathogens, additional studies will be necessary to better understand the relative protective and injurious aspects of NET biology in more complex systems.
In conclusion, isolated human NETs were sufficient to activate inflammatory signaling in primary polarized human bronchial epithelia. NETs contributed to inflammation by providing inflammatory cytokines within their structure and, to a greater extent, by driving an HBE inflammatory response characterized by increased IL-8 and TNF-α expression secondary to IL-1 signaling. NETs also increased expression of RNAs encoding IL-36 subfamily members, a group of cytokines known to stimulate IL-8 production in bronchial epithelia (54). Our results may be of particular relevance to lung disorders including ARDS and cystic fibrosis, which are characterized by airway neutrophilia, abundant NET stimuli, and elevated IL-8 and IL-1 cytokine concentrations (31, 40, 44).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 1K08HL124191, Cystic Fibrosis Foundation Grant HUDOCK15I0, a Parker B. Francis fellowship, University of Cincinnati Clinical and Translational Science Award CT2 Scholar Award KL2TR001426, University of Cincinnati, and Cincinnati Children’s Hospital Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.H., G.S.W., B.C.T., and J.P.C. conceived and designed research; K.M.H., M.S.C., M.I., K.G., C.M., A.J.O., E.J.K., C.R.D., A.S., and P.A. performed experiments; K.M.H., M.S.C., M.I., J.S., E.L.K., J.J.B., E.J.K., A.S., and Y.X. analyzed data; K.M.H., M.S.C., M.I., J.S., E.L.K., J.J.B., K.G., E.J.K., S.S., and J.P.C. interpreted results of experiments; K.M.H., M.S.C., M.I., and J.S. prepared figures; K.M.H. drafted manuscript; K.M.H., E.L.K., J.J.B., P.A., S.S., G.S.W., and J.P.C. edited and revised manuscript; K.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Jessica Moncivaiz and Lauren Strecker of the CCHMC Pulmonary Core for providing cells for this work. We thank Laura Collins for creating our illustrations.
REFERENCES
- 1.Anders S, Huber W. Differential Expression of RNA-Seq Data at the Gene Level: the DESeq Package. Heidelberg, Germany: European Molecular Biology Laboratory, 2012. [Google Scholar]
- 2.Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, Chrysanthopoulou A, Nakos K, Tsironidou V, Koffa M, Boumpas DT, Ritis K. Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever. Ann Rheum Dis 75: 269–277, 2016. doi: 10.1136/annrheumdis-2014-205958. [DOI] [PubMed] [Google Scholar]
- 3.Barrientos L, Marin-Esteban V, de Chaisemartin L, Le-Moal VL, Sandré C, Bianchini E, Nicolas V, Pallardy M, Chollet-Martin S. An improved strategy to recover large fragments of functional human neutrophil extracellular traps. Front Immunol 4: 166, 2013. doi: 10.3389/fimmu.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, Sorscher EJ, Clancy JP. Failure of cAMP agonists to activate rescued ΔF508 CFTR in CFBE41o− airway epithelial monolayers. J Physiol 569: 601–615, 2005. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114: 2619–2622, 2009. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 152: 2111–2118, 1995. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 7.Bowers EC, McCullough SD, Morgan DS, Dailey LA, Diaz-Sanchez D. ERK1/2 and p38 regulate inter-individual variability in ozone-mediated IL-8 gene expression in primary human bronchial epithelial cells. Sci Rep 8: 9398, 2018. doi: 10.1038/s41598-018-27662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewington JJ, Backstrom J, Feldman A, Kramer EL, Moncivaiz JD, Ostmann AJ, Zhu X, Lu LJ, Clancy JP. Chronic β2AR stimulation limits CFTR activation in human airway epithelia. JCI Insight 3: e93029, 2018. doi: 10.1172/jci.insight.93029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkmann V, Goosmann C, Kühn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol 3: 413, 2013. doi: 10.3389/fimmu.2012.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 11.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 122: 2661–2671, 2012. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatfield SM, Thieblemont N, Witko-Sarsat V. Expanding neutrophil horizons: new concepts in inflammation. J Innate Immun 10: 422–431, 2018. doi: 10.1159/000493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37: W305–W311, 2009. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun 61: 4553–4559, 1993. doi: 10.1128/IAI.61.11.4553-4559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chustz RT, Nagarkar DR, Poposki JA, Favoreto S Jr, Avila PC, Schleimer RP, Kato A. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol 45: 145–153, 2011. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy DM, Henry CM, Sullivan GP, Martin SJ. Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J 284: 1712–1725, 2017. doi: 10.1111/febs.14075. [DOI] [PubMed] [Google Scholar]
- 17.Clancy DM, Sullivan GP, Moran HB, Henry CM, Reeves EP, McElvaney NG, Lavelle EC, Martin SJ. Extracellular neutrophil proteases are efficient regulators of IL-1, IL-33, and IL-36 cytokine activity but poor effectors of microbial killing. Cell Reports 22: 2937–2950, 2018. doi: 10.1016/j.celrep.2018.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Dean TP, Dai Y, Shute JK, Church MK, Warner JO. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res 34: 159–161, 1993. doi: 10.1203/00006450-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281: 8–27, 2018. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dwyer M, Shan Q, D’Ortona S, Maurer R, Mitchell R, Olesen H, Thiel S, Huebner J, Gadjeva M. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J Innate Immun 6: 765–779, 2014. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gollomp K, Kim M, Johnston I, Hayes V, Welsh J, Arepally GM, Kahn M, Lambert MP, Cuker A, Cines DB, Rauova L, Kowalska MA, Poncz M. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 3: 99445, 2018. doi: 10.1172/jci.insight.99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haws C, Krouse ME, Xia Y, Gruenert DC, Wine JJ. CFTR channels in immortalized human airway cells. Am J Physiol Lung Cell Mol Physiol 263: L692–L707, 1992. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann JH, Schaekel K, Gaiser MR, Enk AH, Hadaschik EN. Interindividual variation of NETosis in healthy donors: introduction and application of a refined method for extracellular trap quantification. Exp Dermatol 25: 895–900, 2016. doi: 10.1111/exd.13125. [DOI] [PubMed] [Google Scholar]
- 25.Hu Z, Murakami T, Tamura H, Reich J, Kuwahara-Arai K, Iba T, Tabe Y, Nagaoka I. Neutrophil extracellular traps induce IL-1β production by macrophages in combination with lipopolysaccharide. Int J Mol Med 39: 549–558, 2017. doi: 10.3892/ijmm.2017.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudock KM, Margaret S. Collins MS, Imbrogno M, Kopras EJ, Kramer EL, Brewington JJ, Ostmann AJ, McCarthy C, Moncivaiz J, Srdiharan A, Davidson C, Clancy JP. Neutrophil extracellular traps increase proinflammatory cytokine expression by human airway epithelial cells in vitro (Abstract). Am J Respir Crit Care Med 197: A3689, 2018. [Google Scholar]
- 27.Hudock KM, Liu Y, Mei J, Marino RC, Hale JE, Dai N, Worthen GS. Delayed resolution of lung inflammation in Il-1rn−/− mice reflects elevated IL-17A/granulocyte colony-stimulating factor expression. Am J Respir Cell Mol Biol 47: 436–444, 2012. doi: 10.1165/rcmb.2012-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hüffmeier U, Wätzold M, Mohr J, Schön MP, Mössner R. Successful therapy with anakinra in a patient with generalized pustular psoriasis carrying IL36RN mutations. Br J Dermatol 170: 202–204, 2014. doi: 10.1111/bjd.12548. [DOI] [PubMed] [Google Scholar]
- 29.Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth HV, Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 6: e24437, 2017. doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan MA, Ali ZS, Sweezey N, Grasemann H, Palaniyar N. Progression of cystic fibrosis lung disease from childhood to adulthood: neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes (Basel) 10: 183, 2019. doi: 10.3390/genes10030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151: 1075–1082, 1995. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 32.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5: 178ra40, 2013. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 3: 98178, 2018. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, Wu D, Liu B, Li H, Li H, Li Y, Dai M, Li Y, Hu C, Tsung A. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep 6: 37252, 2016. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, Huml M, Stoiber W, Hector A, Griese M, Hannig M, Studnicka M, Vitkov L, Hartl D. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros 11: 84–92, 2012. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, Towne JE, Douangpanya J, Pons C, Mansour S, Serre V, Makni H, Mahfoudh N, Fakhfakh F, Bodemer C, Feingold J, Hadj-Rabia S, Favre M, Genin E, Sahbatou M, Munnich A, Casanova JL, Sims JE, Turki H, Bachelez H, Smahi A. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 365: 620–628, 2011. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 38.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12: 324–333, 2012. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Meyer NJ, Feng R, Li M, Zhao Y, Sheu CC, Tejera P, Gallop R, Bellamy S, Rushefski M, Lanken PN, Aplenc R, O’Keefe GE, Wurfel MM, Christiani DC, Christie JD. IL1RN coding variant is associated with lower risk of acute respiratory distress syndrome and increased plasma IL-1 receptor antagonist. Am J Respir Crit Care Med 187: 950–959, 2013. doi: 10.1164/rccm.201208-1501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery ST, Dittrich AS, Garratt LW, Turkovic L, Frey DL, Stick SM, Mall MA, Kicic A; Australian Respiratory Early Surveillance Team for Cystic Fibrosis . Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J Cyst Fibros 17: 715–722, 2018. doi: 10.1016/j.jcf.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Najmeh S, Cools-Lartigue J, Giannias B, Spicer J Ferri LE. Simplified human neutrophil extracellular traps (NETs) isolation and handling. J Vis Exp (98): e52687, 2015. doi: 10.3791/52687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham DL, Ban GY, Kim SH, Shin YS, Ye YM, Chwae YJ, Park HS. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin Exp Allergy 47: 57–70, 2017. doi: 10.1111/cea.12859. [DOI] [PubMed] [Google Scholar]
- 43.Piper SC, Ferguson J, Kay L, Parker LC, Sabroe I, Sleeman MA, Briend E, Finch DK. The role of interleukin-1 and interleukin-18 in pro-inflammatory and anti-viral responses to rhinovirus in primary bronchial epithelial cells. PLoS One 8: e63365, 2013. doi: 10.1371/journal.pone.0063365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reilly JP, Anderson BJ, Hudock KM, Dunn TG, Kazi A, Tommasini A, Charles D, Shashaty MG, Mikkelsen ME, Christie JD, Meyer NJ. Neutropenic sepsis is associated with distinct clinical and biological characteristics: a cohort study of severe sepsis. Crit Care 20: 222, 2016. doi: 10.1186/s13054-016-1398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabbione F, Keitelman IA, Iula L, Ferrero M, Giordano MN, Baldi P, Rumbo M, Jancic C, Trevani AS. Neutrophil extracellular traps stimulate proinflammatory responses in human airway epithelial cells. J Innate Immun 9: 387–402, 2017. doi: 10.1159/000460293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7: e32366, 2012. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, Ross DJ, Lynch JP III, Saggar R, Ardehali A, Ware LB, Christie JD, Belperio JA, Looney MR; Lung Transplant Outcomes Group Investigators . Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 191: 455–463, 2015. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20: 511–517, 2014. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 49.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 127: 2173–2181, 2016. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 50.Skopelja S, Hamilton BJ, Jones JD, Yang ML, Mamula M, Ashare A, Gifford AH, Rigby WF. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 1: e88912, 2016. doi: 10.1172/jci.insight.88912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515, 2010. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 167: 1567–1574, 2003. doi: 10.1164/rccm.200207-664OC. [DOI] [PubMed] [Google Scholar]
- 53.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One 6: e23637, 2011. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Yin Y, Lin X, Yan X, Xia Y, Zhang L, Cao J. IL-36 induces cytokine IL-6 and chemokine CXCL8 expression in human lung tissue cells: implications for pulmonary inflammatory responses. Cytokine 99: 114–123, 2017. doi: 10.1016/j.cyto.2017.08.022. [DOI] [PubMed] [Google Scholar]