Abstract
The intestinal tract contains over half of all immune cells and peripheral nerves and manages the beneficial interactions between food compounds and the host. Paramylon is a β-1,3-glucan storage polysaccharide from Euglena gracilis (Euglena) that exerts immunostimulatory activities by affecting cytokine production. This study investigated the signaling mechanisms that regulate the beneficial interactions between food compounds and the intestinal tract using cell type-specific calcium (Ca2+) imaging in vivo and in vitro. We successfully visualized Euglena- and paramylon-mediated Ca2+ signaling in vivo in intestinal epithelial cells from mice ubiquitously expressing the Yellow Cameleon 3.60 (YC3.60) Ca2+ biosensor. Moreover, in vivo Ca2+ imaging demonstrated that the intraperitoneal injection of both Euglena and paramylon stimulated dendritic cells (DCs) in Peyer’s patches, indicating that paramylon is an active component of Euglena that affects the immune system. In addition, in vitro Ca2+ imaging in dorsal root ganglia indicated that Euglena, but not paramylon, triggers Ca2+ signaling in the sensory nervous system innervating the intestine. Thus, this study is the first to successfully visualize the direct effect of β-1,3-glucan on DCs in vivo and will help elucidate the mechanisms via which Euglena and paramylon exert various effects in the intestinal tract.
Keywords: β-1,3-glucan; Euglena gracilis; Ca2+ signaling; intestinal epithelial cell; intravital imaging; small intestine; immune system
1. Introduction
The intestinal tract is the first line of defense against pathogenic microorganisms and manages beneficial interactions between food compounds and the host [1]. These interactions are mediated by the neural, endocrine, and immune systems to maintain intestinal homeostasis [2]; however, homeostatic dysfunction can significantly affect host immunity and the course of chronic inflammation [3]. Some probiotics and polysaccharides have been found to regulate intestinal and immune homeostasis; for instance, probiotic Bifidobacterium bifidum alleviates dysbiosis and constipation in mice induced by a low-fiber diet [4], while grains fermented with Aspergillus oryzae can protect against chronic constipation and gastrointestinal damage [5,6]. In addition, the soluble dietary fiber β-1,3-glucan from seaweed has been shown to suppress intestinal inflammation in mouse models of human inflammatory bowel disease by increasing the Lactobacillus population and the number of regulatory T cells in the colon [7]. However, different polysaccharides can exert varying effects on immune homeostasis [8,9,10].
Euglena gracilis (Euglena) is a microalga that contains a wide range of nutrients, including vitamins, minerals, amino acids, and fatty acids. Since it combines the properties of both plants and animals, it is often used as a food or dietary supplement [11]. The storage polysaccharide paramylon is an insoluble dietary fiber unique to Euglena that has a triple-helical polymer structure composed of straight-chain β-1,3-glucans. Like other β-1,3-glucans, paramylon has various beneficial effects on health, such as modulating immune function [12,13,14,15] and suppressing visceral fat accumulation, likely by improving the intestinal environment [16,17,18,19]. In addition, paramylon can bind to and stimulate Dectin-1, the primary receptor on epithelial cells, macrophages, and dendritic cells (DCs) to exert immunomodulatory effects [20,21,22]. Following their ingestion, polysaccharides and probiotics are thought to be taken up into M cells, a type of intestinal epithelial cell (IEC) that lies over Peyer’s patches (PPs), allowing them to access gut immune cells such as macrophages and DCs [23,24]; however, it is difficult to monitor these biological events in real time.
Calcium imaging using genetically encoded calcium biosensors has enabled nutrition-sensing mechanisms in the intestinal tract to be visualized. For example, Calcium ion (Ca2+) signaling in specific cell populations has been visualized in conditional transgenic mice expressing Yellow Cameleon 3.60 (YC3.60) [25,26]. Ca2+ is a universal secondary messenger that performs multiple functions in most cells, including lymphocytes, epithelial cells, and neurons [27,28,29,30]. Intravital Ca2+ imaging in transgenic mice expressing YC3.60 under the control of the CD11c gene promoter has demonstrated that oral propolis administration stimulates DCs in lymphoid tissues [31], while in vivo Ca2+ imaging has revealed that probiotics induce Ca2+ signaling in IECs under physiological conditions [32]. Enteroendocrine cells are a form of IEC that constitute the largest endocrine organ in the human body, while the autonomic nervous system that innervates the intestine exerts strong modulatory effects on the motor, secretory, and immunologic functions of the intestinal tract [33,34]. To elucidate the complex sensing mechanisms that recognize Euglena and paramylon in the intestinal tract, we performed cell type-specific Ca2+ imaging in three conditional transgenic mouse lines with specific or ubiquitous YC3.60 expression [26,31] to visualize Euglena- and paramylon-induced Ca2+ signaling in IECs, DCs in intestinal PPs, and nerve cells innervating the intestine.
2. Materials and Methods
2.1. Animals
Conditional YC3.60 expressing transgenic mice were as described previously [26]. The floxed YC3.60 reporter (YC3.60flox) mouse line was crossed with cell type-specific Cre mouse lines (CD11c-Cre, Nestin-Cre, and CAG-Cre) to produce YC3.60flox/CD11c-Cre, YC3.60flox/Nestin-Cre, and YC3.60flox/CAG-Cre mice, respectively [26,31]. These mice were maintained in our animal facility under specific pathogen-free conditions in accordance with the animal care guidelines of the Tokyo Medical and Dental University, while animal procedures were approved by its Animal Care Committee (approval number A2019-207C4, date of approval 3 December 2019).
2.2. Dorsal Root Ganglia (DRG) Cells
Dorsal root ganglia (DRG) cells were prepared from YC3.60flox/Nestin-Cre mice as described previously [35]. Briefly, mice were euthanized by cervical dislocation and their DRG excised before being treated with collagenase (1 mg/mL) and trypsin (0.25 mg/mL), as described previously [36]. The cells were then washed twice with Dulbecco’s modified Eagle’s medium (DMEM) and cultured at 37 °C on a gelatin-coated plate with DMEM containing 10% fetal calf serum and 100 ng/mL of nerve growth factor.
2.3. Test Substances
Euglena powder and paramylon were obtained from Euglena Co., Ltd. (Tokyo, Japan). The nutritional composition of the Euglena powder was as follows: carbohydrates 55.0%, protein 29.9%, and lipid 9.0%. Approximately 70–80% of the carbohydrate content was paramylon. Paramylon was prepared according to the standard method, as follows: cultured Euglena gracilis Z cells were collected by continuous centrifugation and washed with water. After being suspending in water, the cells were disintegrated using ultrasonic waves and the cell contents (containing paramylon) were collected. The crude paramylon preparation was treated with 1% sodium dodecyl sulfate (SDS) solution for 1 h at 95 °C followed by 0.1% SDS for 30 min at 50 °C to remove lipids and proteins. After centrifugation, paramylon was refined by repeated washing with water, acetone, and ether.
2.4. Intravital and In Vitro Imaging
IECs and PPs from anesthetized mice were imaged as described previously [32]. Small intestinal tracts were surgically opened lengthwise, placed on a glass cover slip, and immobilized on a microscope stage. To observe IECs, 0.1 mL of Euglena or paramylon in phosphate buffered saline (PBS; 1 mg/mL) was added to the intestinal tract, with PBS as a control. Images were acquired using a Nikon A1 laser-scanning confocal microscope with a 20× objective lens, dichronic mirrors (DM457/514), and two bandpass emission filters (482/35 for cyan fluorescent protein, CFP, 540/30 for yellow fluorescent protein, YFP), as described previously [26]. The YFP/CFP ratio was obtained by excitation at 458 nm. Images of purified spleen cells in PBS were obtained using the same method. Acquired images were analyzed using NIS-Elements software (Nikon, Tokyo, Japan).
2.5. In Vivo Stimulation Assay
To observe PPs, the peritoneal cavity of each mouse was injected with 200 μg of Euglena or paramylon in PBS (1 mg/mL), with PBS as a control. After 2 hours, the mice were subjected to intravital imaging analysis, as described previously [31].
2.6. Statistical Analysis
Statistical analysis was performed with Pearson’s chi-square test to compare the proportions of cells. R version 3.4.1 were used to conduct the statistical analyses. p < 0.05 was considered significant.
3. Results
3.1. Euglena and Paramylon Induce Ca2+ Signaling in the IECs of Mice With Ubiquitous Yc3.60 Expression
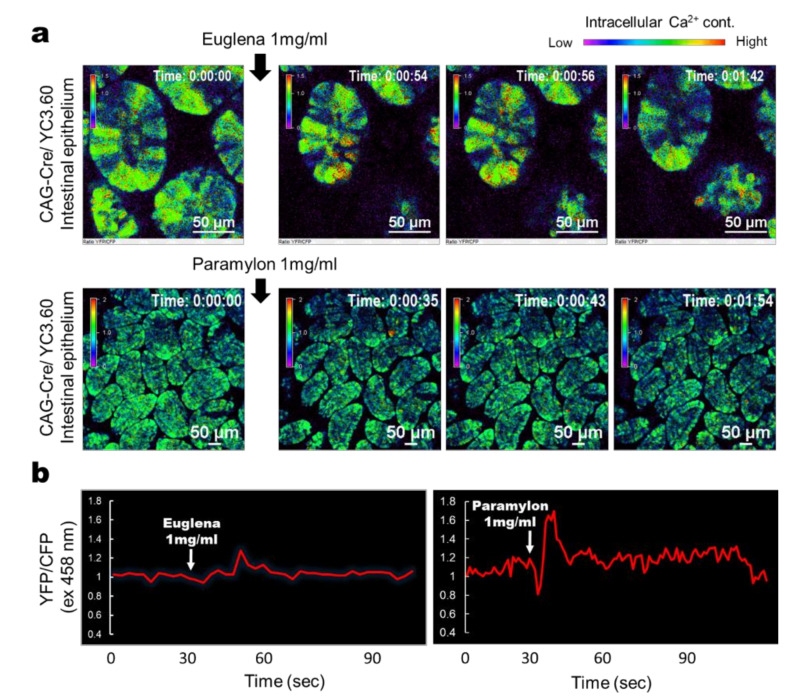
To examine whether Euglena and paramylon directly stimulate IECs, we carried out intravital Ca2+ imaging. Euglena induced transient Ca2+ signaling in most IECs (Figure 1a) and intracellular Ca2+ levels increased following stimulation (Figure 1b). Conversely, paramylon induced robust but sparse Ca2+ signaling limited to minor IEC subpopulations (Figure 1). Together, these findings suggest that Euglena and paramylon directly stimulate IECs.
Figure 1.
Intravital calcium (Ca2+) signaling mediated by Euglena gracilis (Euglena) or paramylon in the intestinal tract. (a) Representative ratiometric Ca2+ signaling images from the intestinal tract of a YC3.60flox/CAG-Cre mouse with ubiquitous YC3.60 expression showing yellow fluorescent protein/cyan fluorescent protein (YFP/CFP) intensity at 458 nm excitation. Euglena or paramylon (0.1 mL) in phosphate buffered saline (PBS) (1 mg/mL) was added at the indicated time point. The color scale indicates relative Ca2+ concentration. (b) Time course of YFP/CFP fluorescence intensity at 458 nm excitation. Euglena or paramylon (0.1 mL) in PBS (1 mg/mL) was added at the indicated time point. Results are representative of at least three independent experiments (n = 3 mice; scale bars, 50 μm).
3.2. Euglena and Paramylon Induce Ca2+ Signaling in DCs
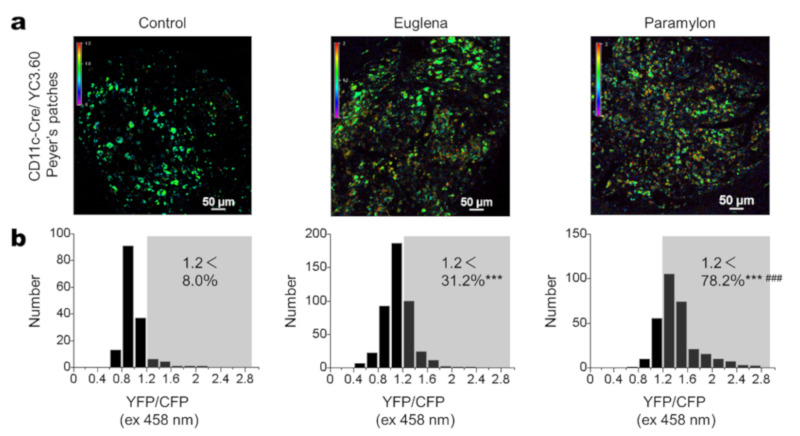
To assess the potential immune-stimulatory effects of Euglena and paramylon on immune cells in vivo, we performed intravital Ca2+ imaging on PPs in YC3.60flox/CD11c-Cre mice [31] injected intraperitoneally (IP) with Euglena or paramylon (1 mg/mL). After 2 hours of intraperitoneal administration, intravital imaging analysis showed that Euglena increased intracellular Ca2+ levels in DCs, with 31.2% of DCs exhibiting higher intracellular Ca2+ concentrations (Figure 2). However, paramylon induced robust Ca2+ signaling in 78.2% of DCs, thus exerted stronger effects than Euglena (Figure 2b). These results indicate that Euglena and paramylon possess immune-stimulatory functions.
Figure 2.
Intravital Ca2+ signaling in Peyer’s patches (PPs). (a) Representative Ca2+ signaling images of PPs in YC3.60flox/CD11c-Cre mice intraperitoneally injected with PBS (control, left) Euglena/PBS (center), or paramylon/PBS (right). Intravital ratiometric imaging was carried out 2 hours after injection and shows YFP/CFP excitation at 458 nm. The results are representative of at least three independent experiments (n = 3 mice; scale bars, 50 μm). (b) Distribution of intracellular Ca2+ levels in randomly selected cells (control, n = 152; Euglena, n = 455; paramylon, n = 303). YFP/CFP > 1.2 was defined as cells of relatively high Ca2+ concentration. Pearson’s chi-square test, *** p < 0.001 vs. Control, ### p < 0.001 Euglena vs. Paramylon.
3.3. Euglena Elicits In Vitro Ca2+ Signaling in DRG-Derived Neurons From YC3.60flox/Nestin-Cre Mice
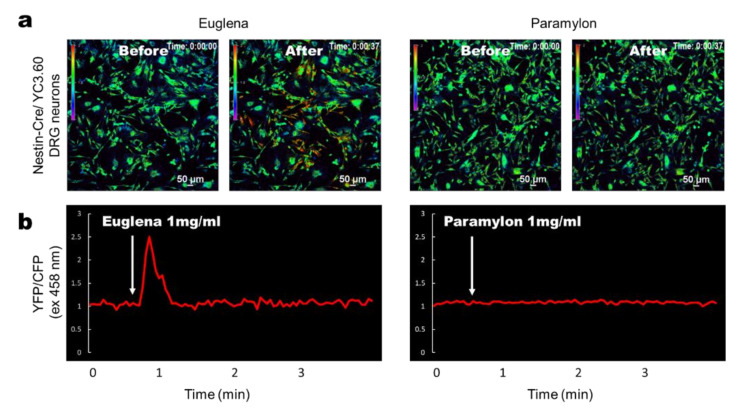
To test whether Euglena or paramylon have the potential to stimulate sensory neurons in the intestine, we performed Ca2+ imaging on primary neurons dissected from the DRG of YC3.60flox /Nestin-Cre mice [32] and cultured on a dish for several days [35]. Euglena induced robust Ca2+ signaling in the DRG neurons (Figure 3a), whereas no Ca2+ signaling was induced by paramylon (Figure 3b), indicating that Euglena stimulates sensory neurons but its component paramylon does not.
Figure 3.
Ca2+ signaling images using Euglena and Paramylon in dorsal root ganglia (DRG) neurons in vitro. (a) Representative ratiometric Ca2+ signaling images in DRG cells from YC3.60flox/Nestin-Cre mice showing YFP/CFP excitation at 458 nm. Euglena or paramylon (0.1 mL) in PBS (1 mg/mL) was added to the cell culture at the indicated time point. The color scale indicates relative Ca2+ concentration. (b) Time course of ratiometric YFP/CFP fluorescence intensity at 458 nm excitation. Results are representative of at least three independent experiments (n = 9 mice; scale bars, 50 μm).
4. Discussion
This study successfully visualized Euglena- and paramylon-mediated Ca2+ signaling in IECs, DCs, and DRG-derived neurons using Ca2+ imaging in YC3.60 mice. Euglena and paramylon both exhibited stimulatory activities in IECs in vivo and possessed immune-stimulating properties against DCs in PPs in vivo. Furthermore, Euglena directly induced Ca2+ signaling in DRG-derived neurons.
Understanding the mechanisms underlying the interaction between food compounds and IECs is important for evaluating the physiological benefits of food compounds, since stimulated IECs can produce cytokines and/or peptide hormones [30]. Probiotic microbes bind with a series of pattern recognition receptors, including toll-like receptors, nucleotide-binding sites, leucine-rich repeat-containing receptors, and retinoic acid-inducible gene-I-like receptors [37]. On IECs, the β-glucan receptor Dectin-1 triggers the secretion of pro-inflammatory cytokines, such as Interleukin-8 (IL-8) and monocyte chemoattractant protein 1 (CCL2) [22]. Although enteroendocrine cells make up less than 1% of the IEC population, they form the largest endocrine organ in the body and secrete multiple peptide hormones such as ghrelin, serotonin (5-hydroxytryptamine), Cholecystokinin (CCK), peptide tyrosine-tyrosine (PYY), glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) [38]. In response to food intake, enteroendocrine cells produce GLP-1, a 30-amino acid peptide hormone that exerts various metabolic actions, such as reducing appetite and food intake [39]. In addition, GLP-1 promotes insulin secretion and may contribute toward improved insulin sensitivity [40]. Thus, food components and dietary supplements that modulate nutrient-sensing pathways may have therapeutic potential for treating obesity and metabolic diseases [38,41]. A previous study showed that Bacillus subtilis var. natto, which has been shown to modulate immune responses, triggers gradual and sustained Ca2+ signaling in IECs [32]. Conversely, the probiotic Lactococcus lactis, which is similar to commensal bacteria, does not induce Ca2+ signaling in IECs, likely due to hyporesponsiveness following chronic exposure to bacteria [32]. In this study, we found that both Euglena and paramylon evoked Ca2+ signaling in IECs, similar to other prebiotics, suggesting that they can directly access IECs. It should be noted that Euglena and paramylon showed different bioactivity for IECs. Euglena stimulation evoked transient Ca2+ signaling throughout IECs with distinct Ca2+ signaling kinetics to those evoked by paramylon. Previous studies have shown that oral paramylon and Euglena intake exert preventive effects against obesity, likely due to the presence of paramylon [17,18]. Here, paramylon induced sparse transient Ca2+ signaling in minor IEC subpopulations, with a similar spatial pattern of Ca2+ signaling to the cellular distribution of enteroendocrine cells [42]. Thus, nutrient sensing in the intestinal tract may transmit neuronal signals to the brain by secreting multiple gastrointestinal hormones to modulate the physiological response to food components. However, further studies of specific cell types are required to clarify the biological properties of paramylon.
Biologically-active polysaccharides may be a potential method of preventing dysfunctional immune homeostasis [9,43]. For instance, β-glucan extracted from the maitake mushroom (Grifola frondosa) has been shown to control the cytokine balance between T lymphocyte Th-1 and Th-2 subsets, resulting in enhanced cellular immunity [8]. In addition, the subcutaneous application of β-1,3-glucan extracted from Saccharomyces cerevisiae in 20 children with asthma increased serum levels of the anti-inflammatory cytokine IL-10 and simultaneously reduced symptom scores [10]. The oral intake of paramylon has been found to reduce cytokine secretion and relieve arthritis symptoms in mice by modulating Th17 immunity [44], while studies have suggested that Euglena and paramylon could reduce upper respiratory tract infection symptoms and protect against influenza virus infection [13,45]. Here, we found that IP Euglena and paramylon injection stimulated DCs in PPs in vivo, with stronger Ca2+ signaling observed with paramylon treatment; thus, paramylon may be the active component of Euglena that affects the immunological system. This finding is consistent with previous studies indicating that β-1,3-glucans stimulate macrophages and DCs via a Dectin-1-dependent pathway [20,22]. Orally administered β-glucans are thought to access gut immune cells, such as macrophages and DCs, by being taken up into M cells, a type of IEC that lies over PPs [23,24]. On macrophages, the β-glucan receptor Dectin-1 specifically binds to paramylon and exerts immune stimulatory effects [20,46]. Alongside phagocytosis in macrophages, Dectin-1 also induces proinflammatory cytokine secretion [47]. Due to its crystalline structure, paramylon typically exists in the form of insoluble granules that are 2–3 μm in size, suggesting that paramylon may be transported across IECs via the same mechanism as pathogenic bacteria. Our findings, together with those of previous studies, suggest that oral paramylon intake could stimulate intestinal DCs in PPs to enhance host immune function. Consistently, some studies in human subjects suggest that Euglena and paramylon may support a healthy immune system and protect overall health [45,48]. Intravital imaging using several transgenic mice with biosensors expressed in the intestinal epithelium, nervous system, and immune cells will be a powerful tool to dissect the mechanism of a beneficial effect of the active ingredient in food products on human immunity [32].
In this study, we demonstrated that Euglena, but not paramylon, directly triggers Ca2+ signaling in DRG neurons, suggesting that Euglena can excite visceral afferents. Although paramylon has various bioactive functionalities [21], other bioactive components of water-soluble fraction seem to activate the neurons. Indeed, the water extract partially purified from Euglena also contains bioactive materials, as this is crucial for preventing lung carcinoma growth and intracellular lipid accumulation [19,49]. The sensory innervation of the small intestine is due to spinal and vagus nerves, which have cell bodies in the DRG and nodose ganglion, respectively [50]. DRG afferents are largely peptidergic and express the calcitonin gene-related peptide Substance P and/or transient receptor potential vanilloid 1 [51,52]. The calcitonin gene-related peptide (CGRP) neuropeptide modifies macrophages, DCs, and other immune cells, suggesting that it plays a key role in neuro-immune cross-talk and allows sensory fibers to mediate immune function [52]. The release of neuropeptides from sensory afferents has been associated to nociceptive transmission, energy homeostasis, and longevity [53,54], whereas the vagal afferent is required for the activity of sympathetic nerves innervating brown adipose tissue triggered by a capsaicin analog, indicating that sensory afferents play important roles in inducing autonomous nerve activity [55]. Some prebiotic bacteria have been shown to alter emotional behavior by regulating the vagal nerve [56], whereas capsaicin in hot peppers can trigger Ca2+ signaling in the intestinal tract, which is transmitted to the nervous system and results in a transient shift toward higher arousal levels in the brain [35]. Further studies will be required to better understand the physiological role of food compound-mediated visceral afferents in homeostasis, behavior, emotion or cognitive function. Although the mechanisms underlying our observations remain unclear, the ability of Euglena to excite neurons may underlie its beneficial effects on health-related Quality of Life observed in human subjects [45]. In the near future, after the components involved in Euglena responsible for these signaling responses have been identified and a method for extracting the active ingredients has been established, Euglena can be utilized to produce useful ingredients on an industrial scale [57].
5. Conclusions
Interactions between food and the intestinal tract transmit signals via the gut–immune–brain axis by secreting multiple cytokines and hormones that modulate physiological homeostasis [38,58]. Thus, our findings help to elucidate the mechanisms via which Euglena and paramylon exert various effects from the intestinal tract.
Author Contributions
Conceptualization, K.Y., A.N., A.M., K.S. and T.A.; methodology, T.A.; formal analysis, T.A.; resources, K.Y., A.N., A.M. and K.S.; original draft preparation and writing, K.Y.; review and editing, K.Y., A.N., A.M., K.S. and T.A.; supervision, K.S. and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
K.Y., A.N., A.M. and K.S. are salaried employees of Euglena Co., Ltd. which produced some of the Euglena used in this study. All research funding for this study was provided by Euglena Co., Ltd.
References
- 1.Mitsuoka T. Development of Functional Foods. Biosci. Microbiota Food Health. 2014;33:117–128. doi: 10.12938/bmfh.33.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furness J.B., Rivera L.R., Cho H.J., Bravo D.M., Callaghan B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013;10:729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 3.Ott S.J., Musfeldt M., Wenderoth D.F., Hampe J., Brant O., Fölsch U.R., Timmis K.N., Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makizaki Y., Maeda A., Oikawa Y., Tamura S., Tanaka Y., Nakajima S., Yamamura H. Alleviation of low-fiber diet-induced constipation by probiotic Bifidobacterium bifidum G9-1 is based on correction of gut microbiota dysbiosis. Biosci. Microbiota Food Health. 2019;38:49–53. doi: 10.12938/bmfh.18-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochiai M., Shiomi S., Ushikubo S., Inai R., Matsuo T. Effect of a fermented brown rice extract on the gastrointestinal function in methotrexate-treated rats. Biosci. Biotechnol. Biochem. 2013;77:243–248. doi: 10.1271/bbb.120638. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka K., Ogasa S., Kuwahara T., Bando Y., Hagiwara M., Arimochi H., Nakanishi S., Iwasaki T., Ohnishi Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig. Dis. Sci. 2008;53:1601–1608. doi: 10.1007/s10620-007-0063-3. [DOI] [PubMed] [Google Scholar]
- 7.Tang C., Kamiya T., Liu Y., Kadoki M., Kakuta S., Oshima K., Hattori M., Takeshita K., Kanai T., Saijo S., et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18:183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A., Kodama N., Nanba H. Effect of maitake (Grifola frondosa) D-Fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol. Pharm. Bull. 2002;25:536–540. doi: 10.1248/bpb.25.536. [DOI] [PubMed] [Google Scholar]
- 9.Jesenak M., Banovcin P., Rennerova Z., Majtan J. β-Glucans in the treatment and prevention of allergic diseases. Allergol. Immunopathol. (Madr.) 2014;42:149–156. doi: 10.1016/j.aller.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Sarinho E., Medeiros D., Schor D., Rego Silva A., Sales V., Motta M.E., Costa A., Azoubel A., Rizzo J.A. Production of interleukin-10 in asthmatic children after Beta-1-3-glucan. Allergol. Immunopathol. 2009;37:188–192. doi: 10.1016/j.aller.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzbach S.D., Shigeoka S. Euglena: Biochemistry, Cell and Molecular Biology. Springer International Publishing; Cham, Switzerland: 2017. [Google Scholar]
- 12.Kondo Y., Kato A., Hojo H., Nozoe S., Takeuchi M., Ochi K. Cytokine-Related Immunopotentiating Activities of Paramylon, a (3-1-≫3)-d-Glucan from Euglena gracilis. J. Pharmacobiodyn. 1992;15:617–621. doi: 10.1248/bpb1978.15.617. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima A., Suzuki K., Asayama Y., Konno M., Saito K., Yamazaki N., Takimoto H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017;494:379–383. doi: 10.1016/j.bbrc.2017.09.167. [DOI] [PubMed] [Google Scholar]
- 14.Russo R., Barsanti L., Evangelista V., Frassanito A.M., Longo V., Pucci L., Penno G., Gualtieri P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2017;5:205–214. doi: 10.1002/fsn3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasuda K., Ogushi M., Nakashima A., Nakano Y., Suzuki K. Accelerated wound healing on the skin using a film dressing with β-glucan paramylon. In Vivo. 2018;32:799–805. doi: 10.21873/invivo.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoe S., Yamanaka C., Nishioka M., Onaka N., Nishida N., Takahashi M. Effects of paramylon extracted from Euglena gracilis EOD-1 on parameters related to metabolic syndrome in diet-induced obese mice. Nutrients. 2019;11:1674. doi: 10.3390/nu11071674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakanoi Y., Shuang E., Yamamoto K., Ota T., Seki K., Imai M., Ota R., Asayama Y., Nakashima A., Suzuki K., et al. Simultaneous intake of Euglena gracilis and vegetables synergistically exerts an anti-inflammatory effect and attenuates visceral fat accumulation by affecting gut microbiota in mice. Nutrients. 2018;10:1417. doi: 10.3390/nu10101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okouchi R., Shuang E., Yamamoto K., Ota T., Seki K., Imai M., Ota R., Asayama Y., Nakashima A., Suzuki K., et al. Simultaneous intake of Euglena gracilis and vegetables exerts synergistic anti-obesity and anti-inflammatory effects by modulating the gut microbiota in diet-induced obese mice. Nutrients. 2019;11:204. doi: 10.3390/nu11010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto R., Ishibashi-Ohgo N., Atsuji K., Miwa Y., Iwata O., Nakashima A., Suzuki K. Euglena extract suppresses adipocyte-differentiation in human adipose-derived stem cells. PLoS ONE. 2018;13:1–14. doi: 10.1371/journal.pone.0192404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown G.D., Gordon S. Immune recognition: A new receptor for β-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima A., Yamada K., Iwata O., Sugimoto R., Atsuji K., Ogawa T., Ishibashi-Ohgo N., Suzuki K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. (Tokyo) 2018;64:8–17. doi: 10.3177/jnsv.64.8. [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Kedar S., Baram L., Elad H., Brazowski E., Guzner-Gur H., Dotan I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur. J. Immunol. 2014;44:3729–3740. doi: 10.1002/eji.201444876. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson A.J., Harris N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 24.Yanagihara S., Kanaya T., Fukuda S., Nakato G., Hanazato M., Wu X.R., Yamamoto N., Ohno H. Uromodulin-SlpA binding dictates Lactobacillus acidophilus uptake by intestinal epithelial M cells. Int. Immunol. 2017;29:357–363. doi: 10.1093/intimm/dxx043. [DOI] [PubMed] [Google Scholar]
- 25.Nagai T., Yamada S., Tominaga T., Ichikawa M., Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa S., Usami T., Kikuta J., Ishii M., Sasano T., Sugiyama K., Furukawa T., Nakasho E., Takayanagi H., Tedder T.F., et al. Intravital imaging of Ca2+ signals in lymphocytes of Ca2+ biosensor transgenic mice: Indication of autoimmune diseases before the pathological onset. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurosaki T., Shinohara H., Baba Y.B. Cell Signaling and Fate Decision. Annu. Rev. Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 28.Nathanson M.H. Cellular and subcellular calcium signaling in gastrointestinal epithelium. Gastroenterology. 1994;106:1349–1364. doi: 10.1016/0016-5085(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 29.Silva A.J., Paylor R., Wehner J.M., Tonegawa S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 30.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 31.Adachi T., Yoshikawa S., Tezuka H., Tsuji N.M., Ohteki T., Karasuyama H., Kumazawa T. Propolis induces Ca2+ signaling in immune cells. Biosci. Microbiota Food Health. 2019;38:141–149. doi: 10.12938/bmfh.19-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adachi T., Kakuta S., Aihara Y., Kamiya T., Watanabe Y., Osakabe N., Hazato N., Miyawaki A., Yoshikawa S., Usami T., et al. Visualization of probiotic-mediated Ca2+ signaling in intestinal epithelial cells in vivo. Front. Immunol. 2016;7:601. doi: 10.3389/fimmu.2016.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 34.Horii Y., Nakakita Y., Misonou Y., Nakamura T., Nagai K. The serotonin receptor mediates changes in autonomic neurotransmission and gastrointestinal transit induced by heat-killed Lactobacillus brevis SBC8803. Benef. Microbes. 2015;6:817–822. doi: 10.3920/BM2015.0031. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura Y., Fukuda Y., Okonogi T., Yoshikawa S., Karasuyama H., Osakabe N., Ikegaya Y., Sasaki T., Adachi T. Dual real-time in vivo monitoring system of the brain-gut axis. Biochem. Biophys. Res. Commun. 2020;524:340–345. doi: 10.1016/j.bbrc.2020.01.090. [DOI] [PubMed] [Google Scholar]
- 36.Okazawa M., Takao K., Hori A., Shiraki T., Matsumura K., Kobayashi S. Ionic Basis of Cold Receptors Acting as Thermostats. J. Neurosci. 2002;22:3994–4001. doi: 10.1523/JNEUROSCI.22-10-03994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavelle E.C., Murphy C., O’Neill L.A.J., Creagh E.M. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010;3:17–28. doi: 10.1038/mi.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raka F., Farr S., Kelly J., Stoianov A., Adeli K. Metabolic control via nutrient-sensing mechanisms: Role of taste receptors and the gut-brain neuroendocrine axis. Am. J. Physiol. Endocrinol. Metab. 2019;317:E559–E572. doi: 10.1152/ajpendo.00036.2019. [DOI] [PubMed] [Google Scholar]
- 39.Holst J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 40.Cani P., Delzenne N. The Role of the Gut Microbiota in Energy Metabolism and Metabolic Disease. Curr. Pharm. Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 41.Shibakami M., Shibata K., Akashi A., Onaka N., Takezaki J., Tsubouchi G., Yoshikawa H. Correction to: Creation of Straight-Chain Cationic Polysaccharide-Based Bile Salt Sequestrants Made from Euglenoid β-1,3-Glucan as Potential Antidiabetic Agents. Pharm. Res. 2019;36:31. doi: 10.1007/s11095-018-2559-2. [DOI] [PubMed] [Google Scholar]
- 42.Gerbe F., Legraverend C., Jay P. The intestinal epithelium tuft cells: Specification and function. Cell. Mol. Life Sci. 2012;69:2907–2917. doi: 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vetvicka V., Vannucci L., Sima P., Richter J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules. 2019;24:1251. doi: 10.3390/molecules24071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki K., Nakashima A., Igarashi M., Saito K., Konno M., Yamazaki N., Takimoto H. Euglena gracilis Z and its carbohydrate storage substance relieve arthritis symptoms by modulating Th17 immunity. PLoS ONE. 2018;13:e0191462. doi: 10.1371/journal.pone.0191462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishibashi K., Nishioka M., Onaka N., Takahashi M., Yamanaka D., Adachi Y., Ohno N. Effects of Euglena gracilis EOD-1 Ingestion on Salivary IgA Reactivity and Health-Related Quality of Life in Humans. Nutrients. 2019;11:1144. doi: 10.3390/nu11051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ujita M., Nagayama H., Kanie S., Koike S., Ikeyama Y., Ozaki T., Okumura H. Carbohydrate binding specificity of recombinant human macrophage β-glucan receptor dectin-1. Biosci. Biotechnol. Biochem. 2009;73:237–240. doi: 10.1271/bbb.80503. [DOI] [PubMed] [Google Scholar]
- 47.Kankkunen P., Teirilä L., Rintahaka J., Alenius H., Wolff H., Matikainen S. (1,3)-β-Glucans Activate Both Dectin-1 and NLRP3 Inflammasome in Human Macrophages. J. Immunol. 2010;184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 48.Evans M., Falcone P.H., Crowley D.C., Sulley A.M., Campbell M., Zakaria N., Lasrado J.A., Fritz E.P., Herrlinger K.A. Effect of a Euglena gracilis Fermentate on Immune Function in Healthy, Active Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019;11:2926. doi: 10.3390/nu11122926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishiguro S., Upreti D., Robben N., Burghart R., Loyd M., Ogun D., Le T., Delzeit J., Nakashima A., Thakkar R., et al. Water extract from Euglena gracilis prevents lung carcinoma growth in mice by attenuation of the myeloid-derived cell population. Biomed. Pharmacother. 2020;127:110166. doi: 10.1016/j.biopha.2020.110166. [DOI] [PubMed] [Google Scholar]
- 50.Riera C.E., Dillin A. Emerging Role of Sensory Perception in Aging and Metabolism. Trends Endocrinol. Metab. 2016;27:294–303. doi: 10.1016/j.tem.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Christianson J.A., Davis B.M. Translational Pain Research: From Mouse to Man. CRC Press; Bosca Raton, FL, USA: 2009. The role of visceral afferents in disease; pp. 51–76. [Google Scholar]
- 52.Assas B.M., Pennock J.I., Miyan J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014;8:23. doi: 10.3389/fnins.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riera C.E., Huising M.O., Follett P., Leblanc M., Halloran J., Van Andel R., De Magalhaes Filho C.D., Merkwirth C., Dillin A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 54.Benemei S., Nicoletti P., Capone J.G., Geppetti P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009;9:9–14. doi: 10.1016/j.coph.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Ono K., Tsukamoto-Yasui M., Hara-Kimura Y., Inoue N., Nogusa Y., Okabe Y., Nagashima K., Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J. Appl. Physiol. 2011;110:789–798. doi: 10.1152/japplphysiol.00128.2010. [DOI] [PubMed] [Google Scholar]
- 56.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harada R., Nomura T., Yamada K., Mochida K. Genetic engineering strategies for Euglena gracilis and its industrial contribution to sustainable development goals: A review. Front. Bioeng. Biotechnol. 2020;8:1–10. doi: 10.3389/fbioe.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Sadelhoff J.H.J., Pardo P.P., Wu J., Garssen J., Van Bergenhenegouwen J., Hogenkamp A., Hartog A., Kraneveld A.D. The gut-immune-brain axis in autism spectrum disorders; a focus on amino acids. Front. Endocrinol. (Lausanne) 2019;10:247. doi: 10.3389/fendo.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]