Abstract
The vasa vasorum (VV), the microvascular network around large vessels, has been recognized as an important contributor to the pathological vascular remodeling in cardiovascular diseases. In bovine and rat models of hypoxic pulmonary hypertension (PH), we have previously shown that chronic hypoxia profoundly increased pulmonary artery (PA) VV permeability, associated with infiltration of inflammatory and progenitor cells in the arterial wall, perivascular inflammation, and structural vascular remodeling. Extracellular adenosine was shown to exhibit a barrier-protective effect on VV endothelial cells (VVEC) via cAMP-independent mechanisms, which involved adenosine A1 receptor-mediated activation of Gi-phosphoinositide 3-kinase-Akt pathway and actin cytoskeleton remodeling. Using VVEC isolated from the adventitia of calf PA, in this study we investigated in more detail the mechanisms linking Gi activation to downstream barrier protection pathways. Using a small-interference RNA (siRNA) technique and transendothelial electrical resistance assay, we found that the adaptor protein, engulfment and cell motility 1 (ELMO1), the tyrosine phosphatase Src homology region 2 domain-containing phosphatase-2, and atypical Gi- and Rac1-mediated protein kinase A activation are implicated in VVEC barrier enhancement. In contrast, the actin-interacting GTP-binding protein, girdin, and the p21-activated kinase 1 downstream target, LIM kinase, are not involved in this response. In addition, adenosine-dependent cytoskeletal rearrangement involves activation of cofilin and inactivation of ezrin-radixin-moesin regulatory cytoskeletal proteins, consistent with a barrier-protective mechanism. Collectively, our data indicate that targeting adenosine receptors and downstream barrier-protective pathways in VVEC may have a potential translational significance in developing pharmacological approach for the VV barrier protection in PH.
Keywords: adenosine, endothelial barrier, Gi, Rac1, vasa vasorum
INTRODUCTION
The vasa vasorum (VV) is a microvascular network, which provides oxygen and nutrients to the adventitial and medial layers of large blood vessels. Although it was originally recognized as the guardian of vascular integrity, the VV has recently emerged as an important contributor to the initiation and progression of both the systemic and pulmonary circulation diseases, including atherosclerosis, aortic aneurysm, and pulmonary hypertension (PH), in which vascular remodeling and inflammation play a prominent role (7, 14, 62, 78, 83). Our previous studies demonstrated that the VV expansion observed in the pulmonary artery (PA) of chronically hypoxic hypertensive calves was associated with the impaired endothelial barrier function. The pathophysiological consequence of this hypoxia-induced VV barrier dysfunction was infiltration and homing of inflammatory cells in the PA wall, sustained perivascular inflammation, structural arterial remodeling, and hemodynamic changes in pulmonary circulation, which ultimately lead to increased pulmonary arterial pressure and right ventricular dysfunction (15, 22, 52, 65, 83).
There has been much research into mechanism of the vascular leak; however, the understanding is far from complete, necessitating an urgent need for developing better drugs to combat vascular dysfunction in cardiovascular diseases, including PH. Although the vascular leak mechanisms have been intensively investigated in various endothelial cell (EC) types, including lung microvascular EC, the data are lacking on VVEC (47, 59, 93). In addition, the mechanisms of endothelial barrier protection are less well understood than the mechanisms of endothelial barrier disruption and require further investigation. Barrier-protective mechanisms presented in the majority of endothelial cells include the activation of cAMP/protein kinase A (PKA), cAMP/exchange protein activated by cAMP) (EPAC)/Rab, glycogen synthase kinase 3-β (GSK3β)/catenin, and/or Rac-Arp2/3-WASP, leading to actin rearrangement, attenuation of RhoA/ROCK-dependent stress fiber formation, and VE-cadherin junctional integrity. G protein-coupled receptor (GPCR) ligands, including adrenomedullin, somatostatin, glucagon-like peptide-1 receptor, melanocortin, 12-hydroxyheptadecatrenoic acid (12-HHT), and sphingosine-1 phosphate (S1P) (to name a few), enhance endothelial barrier function by counteracting the RhoA/ROCK pathway, causing a redistribution of tight junctional proteins or by activating cAMP/PKA signaling (8, 59, 82, 93, 98). The effects of somatostatin, 12-HHT, and S1P1 are mediated via Gαi proteins (33, 82, 88). Recent studies demonstrated that engulfment and cell motility 1 (ELMO1) and dedicator of cytokinesis 1 (DOCK180) proteins form an evolutionarily conserved complex controlling Rac1 GTPase signaling during cell adhesion, migration, phagocytosis, and myoblast fusion. Complex formation between G protein-coupled receptor GPR124/ADGRA2 and ELMO/Dock controls cytoskeletal dynamics during during endothelial cell adhesion in angiogenesis (38). In cancer cells, the association between ELMO/Dock180 protein and Gαi subunits is involved in chemokine signaling and cytoskeletal rearrangements, leading to metastasis (51, 81). Whether ELMO/Dock 180 complex participates in Gi- and Rac1-mediated endothelial barrier enhancement is not known.
Extracellular purines (ATP, ADP, and adenosine) have emerged as critical signaling mediators in the vasculature. Adenosine, a product of ATP hydrolysis by ecto-ATP/ADPases, exhibits anti-inflammatory and barrier-protective effects in different organs, including the lung (12, 26, 36, 70, 89, 90). Alterations in extracellular adenosine levels have been observed in hypoxia-associated vascular and blood diseases (25, 55). The effects of adenosine are mediated through the P1 family of G protein-coupled adenosine receptors (ARs), which are subdivided into A1, A2A, A2B, and A3 subtypes (28, 54). Activation of A1 and A3 receptors leads to a decrease in cAMP concentration via inhibition of adenylate cyclase and to a rise in intracellular Ca2+ levels by a pathway involving phospholipase C activation (28, 60). In contrast, stimulation of A2A and A2B receptors leads to the activation of adenylate cyclase and generation of cAMP (37). In endothelial cells, elevations of cAMP levels positively correlate with the assembly of the adherence and tight junction and the enhancement of endothelial barrier function (2, 9, 11, 76, 90). These mechanisms of cAMP-dependent barrier enhancement involve the activation of myosin light chain (MLC) phosphatase (MLCP) and EPAC (a cAMP-activated factor for Rap GTPase) that lead to inhibition of endothelial contractility and stabilization of endothelial junctions (9–11, 20).
In contrast to the abovementioned observations, our previous studies on VVEC suggested cAMP-independent barrier regulation (100). Furthermore, it was found that adenosine-induced barrier enhancement in VVEC occurs via an atypical mechanism that involves adenosine A1 receptor-mediated activation of Gi/phosphoinositide 3-kinase (PI3K)/Akt pathway (92). In the present study, we extended our previous observations and investigated this mechanism in more detail using endothelial cells cultured from normal bovine pulmonary artery vaso vasorum, assuming that the mechanisms of barrier protection we identify in these endothelial cells also provide insight into mechanisms in the vaso vasorum of animals with pulmonary hypertension. Using the siRNA-mediated gene silencing approach and transendothelial electrical resistance (TER) assay, we identified that ELMO1-Rac1-Akt-GSK3 and ELMO1-Rac1-PKA represent novel signaling modules involved in adenosine-mediated VVEC barrier enhancement. With keeping in mind the importance of the barrier-protective effect of adenosine against hypoxia-induced VV hyperpermeability, the results from our study may suggest novel VV normalization therapies for PH and potentially other cardiovascular diseases.
MATERIALS AND METHODS
Materials.
Fetal bovine serum, antibiotic-antimycotic solution, sodium pyruvate, and l-glutamine were purchased from Gibco (Gaithersburg, MD). Medium 199 (M199) and siPORT Amine Transfection Agent were from Thermo Fisher Scientific (Waltham, MA). Trypsin-EDTA solution, horseradish-peroxidase (HRP)-conjugated anti-mouse and anti-rabbit IgG, and adenosine were obtained from Sigma-Aldrich (St. Louis, MO). Anti-β-tubulin was obtained from EMD Millipore (Darmstadt, Germany). 4–20% Mini-PROTEAN® TGX Gel, 0.2-µM pore size PVDF membrane, 2× Laemmli sample buffer, and Trans-Blot® Turbo Transfer System, were purchased from Bio-Rad (Hercules, CA). Enhanced chemiluminescence (ECL) reagent was from Pierce (Rockford, IL). Anti-ppMLCT18/S19, anti-Rac1, LIM kinase 1 (LIMK1), green fluorescent protein (GFP), growth factor receptor binding 2 (Grb2)-associated protein 1 (Gab1), Src homology region 2 domain-containing phosphatase-2 (SHP2), β-actin, Hsp90, p-GSK3β, p-Akt, p-cofilin, p-vasodilator-stimulated phosphoprotein (VASP), and p-ezrin-radixin-moesin (pERM) were from Cell Signaling (Beverly, MA). Catalog numbers and working dilutions for all antibodies are provided in Table 1. Nonspecific (scrambled) siRNA, LIMK1, and Gab-1 siRNAs were purchased from Dharmacon Research (Lafayette, CO). HyBlot® ES autoradiography film was from Denville Scientific, Inc. (Holliston, MA).
Table 1.
Sources of validated antibodies
| Antibody | Source | Catalog No. | Dilution |
|---|---|---|---|
| p-AktSer473 | CST | 4060S | 1:1,000 |
| p-GSK-3β | CST | 5558S | 1:1,000 |
| p-Cofilin | CST | 3313S | 1:1,000 |
| p-VASP | CST | 84519S | 1:1,000 |
| p-ERM | CST | 3726S | 1:1,000 |
| pp-MLC | CST | 3674S | 1:1,000 |
| β-Tubulin | Sigma | 05-661 | 1:2,000 |
| GAB1 | CST | 3232S | 1:1,000 |
| ELMO1 | CST | 14457S | 1:1,000 |
| LIMK1 | CST | 3842S | 1:1,000 |
| β-Actin | CST | 4970S | 1:2,000 |
| Rac1 | CST | 2465S | 1:1,000 |
| HSP90 | CST | 4877S | 1:1,000 |
| Girdin | CST | 14200S | 1:1,000 |
| Anti-rabbit IgG | CST | 7074S | 1:4,000 |
| Anti-mouse IgG | CST | 7076S | 1:4,000 |
Isolation and culture of bovine bovine pulmonary artery vasa vasorum endothelial cells.
VVEC were isolated from the pulmonary artery adventitia of Holstein calves (males, 1–15 days old), obtained from a Laluna Dairy (Fort Collins, CO), as described previously (31, 92). Institutional guidelines were followed, and the procedure was approved by the Institutional Animal Care and Use Committee (Department of Physiology, School of Veterinary Medicine, Colorado State University, Ft. Collins, CO). All studies were performed on cells from passages 3 to 7. Under these conditions, cells sustained consistent functional, morphological, and phenotypic characteristics.
Transendothelial electrical resistance measurement.
TER of VVEC monolayers was measured as previously described (5, 92). Briefly, VVEC (3 × 104 cells/well) were seeded on 8W10E electric cell substrate impedance-sensing (ECIS) arrays. The subsequent day the media were changed to fresh complete culture media and the cells were incubated in serum-free medium for 1 h and challenged with vehicle or adenosine (100 µM) for indicated time, and TER was measured using Model 1600R station controller (Applied BioPhysics, Troy, NY). After treatment, the collected data were normalized to the initial resistance values and plotted as normalized resistance. Pharmacologic inhibitors were added prior or simultaneously to adenosine stimulation as defined in the specific experiments.
In experiments with adenoviral transduction, cells were incubated in complete media for 1 day, then were transduced with GFP-containing control adenoviral vector or with adenoviral vector containing Rac1 dominant-negative (DN) mutant (GFP-Rac1-N17) (multiplicity of infection with titer 3 × 1011). Twenty-four hours after transduction the cells were incubated in serum-free medium for 1 h and challenged with vehicle or adenosine (100 µM), followed by TER measurements as described above.
In experiments with depletion of proteins of interest, VVEC seeding on ECIS plates were transfected with scrambled (nsRNA) or siRNA of a specific protein (both 20–50 nM) for 72 h as described below, treated with vehicle or adenosine, and TER was monitored for indicated time periods. The efficiency of silencing was determined by immunoblotting with antibodies specific for proteins of interest with β-actin or tubulin as loading controls.
Immunoblotting.
VVEC on 12-well or ECIS plates were washed with ice-cold PBS on ice, then lysed immediately and transferred to 0.2 μM pore size PVDF membrane by TransBlot® Turbo Transfer System at 25 V, 1 mA for 30 min. Subsequently, the membranes were blocked with 5% nonfat dry milk (m/v) in TBS-Tween 20 (TBST) and incubated with specific antibodies overnight at 4°C. After incubation with the primary antibodies, the membranes were washed three times with TBST and incubated with the corresponding HRP-conjugated secondary antibody. Immunoreactive proteins were visualized by ECL based on autoradiography. ImageJ software (Research Services Branch, National Institutes of Health, Bethesda, MD) was used for quantitative densitometric analysis. The primary antibodies are listed in Table 1. All antibodies were validated by vendors.
Immunoprecipitation.
VVEC transduced with adenoviral DN Rac1 construct or empty-vector control were lysed in 0.1% (vol/vol) Triton X-100, 150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 20 mM EDTA, and 0.5% (vol/vol) and 0.5% (vol/vol) protease inhibitor cocktail containing buffer. Immunoprecipitation of ectopically expressed GFP-Rac1-N17 was carried out using anti-GFP antibody, conjugated to protein-G Sepharose magnetic beads. After 3 h of incubation at 4°C, the beads were washed three times with 0.1% (vol/vol) Triton X-100, 0.5 mM NaCl, 50 mM Tris·HCl (pH 7.4), 20 mM EDTA, and 0.5% (vol/vol) protease inhibitor cocktail containing buffer and boiled in 100 μl 2× Laemmli buffer for 5 min at 100°C. Immunoprecipitates were subsequently subjected to immunoblotting with specific antibodies as described in the figure legends.
Gene silencing.
VVEC seeded on ECIS plates were transfected with siRNA (50 nM final concentration) using siPORT Amine transfection reagent according to the manufacturer’s instructions. Nonspecific (scrambled) siRNA (same final concentration) was used as a negative control. Briefly, siPORT Amine transfection reagent and siRNAs were diluted in Opti-MEM in separate tubes. After 5 min of incubation at room temperature, diluted transfection reagent was added to the diluted siRNA, followed by incubation at room temperature for 20 min. When the transfection complex was formed, the transfection mixture was added to VVEC in serum-free medium. After 6 h of incubation, the serum free-medium was changed to complete EBM-2 supplemented with the components of EGM-MV BulletKit, and the cells were incubated for 72 h at 37°C. The transfected cells were used for further TER measurements as described above.
PKA activity measurement.
VVEC seeded on six-well plates were incubated in the absence or presence of 50 μM adenosine or for 30 min; then cells were washed three times with 1 ml ice-cold PBS on ice and lysed with buffer containing 20 mM MOPS, 50 mM β-glycerol phosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 5 mM EGTA, 2 mM EDTA, 1% NP-40, 1 mM AEBSF, and 1% (vol/vol) protease inhibitor cocktail. PKA activity was measured using a commercial kit (Enzo Life Sciences) according to the manufacturer's instructions. Briefly, 96-well plates precoated with PKA-specific synthetic peptide substrate were incubated with the extracted protein (1 μg/well) in the presence of adenosine, and PKA activity was determined with a phospho-specific substrate antibody.
Statistical analysis.
Data are presented as means ± SE of at least three independent experiments. Statistical analyses of differences were performed using GraphPad Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA) by analysis of variance (ANOVA) followed by Newman-Keuls post hoc test. Results with P < 0.05 were considered statistically significant.
RESULTS
Rac1 and ELMO1 are involved in adenosine-induced Gαi-mediated VVEC barrier enhancement.
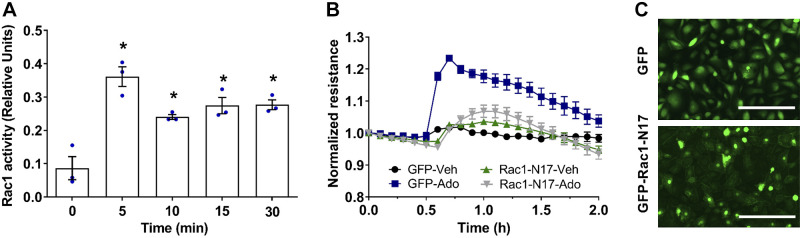
Considering that Gi proteins regulate barrier-protective effect in VVEC via PI3K/Akt pathway (92), we hypothesize that the mechanism may involve Gβγ subunits, known to activate PI3Kβ isoform. To investigate this possibility, we tested the specific Gβγ inhibitor, gallein (84). Our data showed that gallein had no effect on adenosine-induced TER increase (Fig. 1), indicating that Gβγ subunits are not involved in adenosine-induced VVEC barrier regulation. As Gαi subunits mediate Rac1 GTPase-dependent barrier enhancement in the PA and lung microvascular EC (30, 46), we focused on the role of Gαi. As shown in Fig. 2, adenosine activates Rac1 in a time-dependent manner with the maximal effect preceding a maximal TER increase. Inhibition of Rac1 by transducing dominant-negative (DN) adenoviral Rac1 construct (Rac N17) almost completely abolished adenosine-induced TER increase (Fig. 2B). In addition, pharmacologic inhibition of p21-activated kinase 1 (PAK1) with IPA, a well-known downstream target of Rac1 (67, 80), decreased basal TER and abolished adenosine-induced TER increase (Fig. 3A). However, depletion of PAK1 downstream substrate, LIM kinase (LIMK) (71), had no effect on adenosine-induced TER increase, indicating that Rac1/PAK1-induced barrier enhancement is not mediated by LIMK activity (Fig. 3B).
Fig. 1.
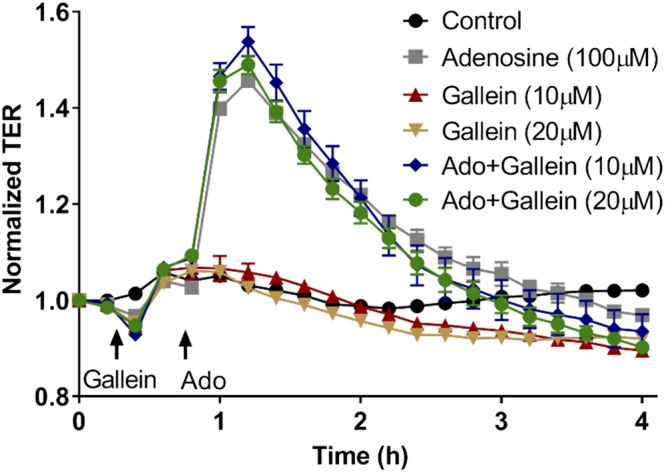
Inhibition of Gβ/γ subunits has no effect on adenosine-induced increase in transendothelial electrical resistance (TER). Vasa vasorum endothelial cell (VVEC) monolayers were incubated in serum-free medium for 1 h. Cells were pretreated with gallein (10 and 20 µM) for 30 min before the addition of adenosine (Ado; 100 µM). Values are means ± SE (n = 3 experiments).
Fig. 2.
Rac1 is involved in adenosine-mediated vasa vasorum endothelial cell (VVEC) barrier enhancement. A: adenosine increases Rac1 activity in VVEC. Cells were incubated with adenosine (100 μM) in serum-free medium for 5, 10, 15, and 30 min. Rac1 activation was measured using G-LISA Rac1 activity assay (cytoskeleton) according to the manufacturer’s instructions. Absorbance was read at 490 nm, and the background was subtracted. The data are expressed as means ± SE (n = 3 experiments), one-way ANOVA. *P < 0.05 compared with 0 min. B: overexpression of dominant-negative mutant Rac1 attenuates adenosine-induced barrier enhancement in VVEC. VVEC were seeded in electric cell substrate impedance-sensing arrays and transduced with adenovirus-containing green fluorescent protein (GFP) or dominant-negative mutant Rac1 (GFP-Rac1-N17). 24 h after transduction, VVEC were incubated in serum-free medium for 1 h and then stimulated with vehicle or adenosine (100 µM). Transendothelial electrical resistance was monitored for ~2.0 h. C: immunofluorescence images show the transduction efficiency (scale bar = 200 μm).
Fig. 3.
p21-activated kinase 1 (PAK1), but not LIM kinase (LIMK), is involved in adenosine-induced vasa vasorum endothelial cell (VVEC) barrier enhancement. A: PAK1 is involved in adenosine-induced VVEC barrier enhancement. VVEC monolayers on electric cell substrate impedance-sensing (ECIS) chambers were incubated in a serum-free medium for 1 h. Adenosine (100 μM) was added after 30 min pretreatment with PAK inhibitor, IPA3 (10 μM); n = 4 experiments; Values are means ± SE. B: depletion of LIMK1 has no effect on adenosine-induced transendothelial electrical resistance (TER) increase. VVEC seeding on ECIS plates was transfected with scrambled (nsRNA) or LIMK1 siRNA (siLIMK1) (both 20 nM) for 72 h, then treated with vehicle (Veh) or adenosine (Ado, 100 µM), and TER was monitored for 1.5 h. The efficiency of silencing was determined by immunoblotting with LIMK1-specific antibodies, and β-actin was used as a loading control (inset); (n = 3 experiments; means ± SE).
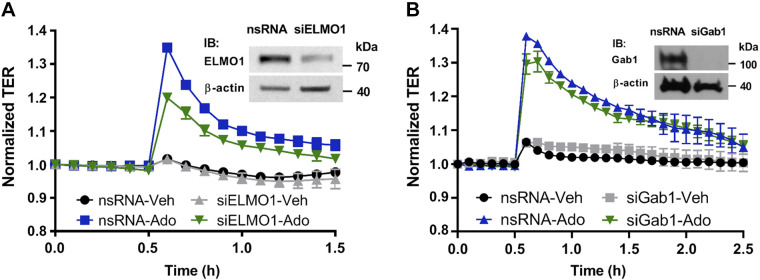
While the molecular mechanisms of adenosine-induced Gαi-mediated Rac1 activation are unknown, it was recently suggested that ELMO1/Dock180 complex acts as the specific bipartite guanidine exchange factor (GEF) for Rac1, thus providing its activation in cancer cells (51). Depletion of ELMO1 by siRNA significantly attenuated adenosine-induced increase in TER in VVEC, supporting the involvement of this adapter protein in the adenosine-induced VVEC barrier enhancement (Fig. 4A).
Fig. 4.
Effect of engulfment and cell motility protein 1 (ELMO1) and growth factor receptor binding 2-associated protein 1 (GAB1) depletion on adenosine-induced vasa vasorum endothelial cell (VVEC) permeability. Confluent cells in electric cell substrate impedance-sensing arrays were treated with siRNA specific to ELMO1 (siELMO1) (A) or GAB1 (siGAB1) (B) or scrambled control RNA (nsRNA). 72 h later VVEC were stimulated with vehicle or adenosine (Ado, 100 µM). Changes in transendothelial electrical resistance (TER) were monitored 30 min before and 1–2 h after adenosine addition. The efficiency of silencing was determined by immunoblotting with GAB1-specific antibodies and β-actin was used as a loading control (insets); (n = 3 experiments, means ± SE).
GAB1 and LIMK are not involved in Gαi-mediated adenosine-induced VVEC barrier enhancement.
Studies on human umbilical vein ECs (HUVEC) and macrophages have demonstrated that, alternatively, Gαi can be involved in Rac1 activation through complex formation with the adaptor protein GAB1 [growth factor receptor binding 2 (Grb2)-associated protein] following by PI3K/Akt and Rac1 activation (13, 21, 53). Depletion of GAB1 had no effect on the adenosine-induced TER increase, indicating that GAB1 does not mediate a barrier-protective effect of adenosine in VVEC (Fig. 4B).
Akt, GSK3, and VASP are downstream targets of Rac1-mediated pathways of VVEC barrier enhancement.
To further evaluate barrier-protective adenosine signaling downstream to Rac1, we introduced adenoviral DN GFP-Rac N17 and performed immunoprecipitation under nondenaturing conditions using GFP-specific antibody. Western blot analysis of Rac1-associated proteins showed that Rac1 inhibition decreased phosphorylation of Akt and GSK3 but increased phosphorylation of cofilin, indicating that Akt/GSK3 pathway is downstream of Rac1 (Fig. 5). In addition, inhibition of Rac1 attenuated phosphorylation of the PKA target, vasodilator-stimulated phosphoprotein (VASP) (35), suggesting the existence of Rac1-PKA axis in VVEC (Fig. 5). Finally, inhibition of Rac1 increased phosphorylation of ezrin-radixin-moesin (ERM) myosin light chain (MLC) proteins, known cytoskeletal targets of MLC phosphatase (MLCP) (43), which mediates endothelial barrier protection in vitro and in vivo (49).
Fig. 5.
Effect of Rac1 inhibition on vasa vasorum endothelial cell (VVEC) signaling. Representative immunoblots show the expression level of endogenous Rac1 and green fluorescent protein (GFP)-Rac1-N17 in control and Rac-N17-transduced cells, as well as changes in phosphorylation level of p-glycogen synthase kinase 3 (GSK)-3β-Ser9, p-Akt-Ser473, p-cofilin-Ser3, p-vasodilator-stimulated phosphoprotein (VASP)-Ser157, p-ezrin-radixin-moesin (ERM), and pp-myosin light chain (MLC)-Thr18/Ser19 resulting from Rac1 inhibition. Hsp90 was used as a loading control.
Adenosine inactivates ERM proteins.
ERM proteins have distinct roles in endothelial barrier regulation. Phosphorylation of moesin, a predominantly expressed ERM protein in human pulmonary endothelium, is involved in LPS-induced endothelial barrier dysfunction. Conversely, dephosphorylation of moesin by MLCP has a barrier-protective role in LPS-induced acute lung injury (ALI) in vitro and in vivo (1, 49). Adenosine transiently decreased phosphorylation of moesin (reflecting its inactivation) (Fig. 6), which suggested that Rac1 is upstream of MLCP in adenosine-induced VVEC barrier enhancement.
Fig. 6.

Adenosine decreases ezrin-radixin-moesin (ERM) phosphorylation in a time-dependent manner. Vasa vasorum endothelial cells were treated with adenosine (100 μM) in serum-free media, and cell lysates were subjected to immunoblotting with phospho-ERM or β-tubulin (loading control) antibodies. Phospho-ERM expression was normalized to actin. The data are expressed as means ± SE, n = 3 experiments; *P < 0.05 compared with 0 min (one-way ANOVA).
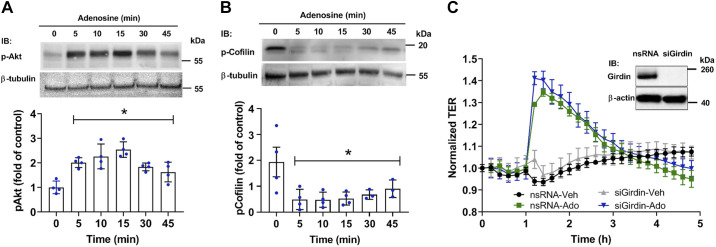
Akt and cofilin, but not the Akt target, girdin, are involved in adenosine-induced VVEC barrier enhancement.
We then demonstrated that adenosine stimulated, in a time-dependent manner, the activation of Akt (Fig. 7A), and this response paralleled adenosine-induced changes in TER. In addition, adenosine-induced TER increase and Akt phosphorylation were accompanied by dephosphorylation (activation) of actin-severing protein, cofilin (61, 86), which may be involved in cytoskeletal rearrangement downstream of the Akt/glycogen synthase kinase-3 βa (GSK3β) pathway (73, 74) (Fig. 7B). Notably, inhibition of Rac1 led to the dephosphorylation of Akt and GSK3β but increased cofilin phosphorylation (Fig. 5). These data support the involvement of the Rac1/Akt/GSK3/cofilin pathway in Ado-induced VVEC cytoskeletal reorganization and barrier enhancement.
Fig. 7.
Akt, cofilin, but not girdin, are involved in adenosine (Ado)-induced vasa vasorum endothelial cell (VVEC) barrier regulation. A: VVEC were treated with Ado (100 μM) for various time points. Total cell lysates were subjected to immunoblotting with phospho-Akt (Ser473) and β-tubulin antibodies. Phospho-Akt was normalized to β-tubulin and expressed as a fold change of the control (0 min) (one-way ANOVA, means ± SE) (n = 4 experiments), *P < 0.05. B: experiments were performed and quantified as described in A. Shown are the results of immunoblotting with phospho-cofilin (Ser3) and β-tubulin antibodies. The data are expressed as means ± SE (n = 4 experiments), one-way ANOVA, *P < 0.05 compared with 0 min. C: girdin is not involved in adenosine-induced barrier enhancement in VVEC. Cell monolayers seeding on electric cell substrate impedance-sensing arrays were transfected with scrambled (nsRNA) or girdin siRNA (both 20 nM for 48 h), treated with vehicle or adenosine (100 μM), and transendothelial electrical resistance (TER) was monitored for an additional 5 h. The data are expressed as means ± SE (n = 4 experiments), one-way ANOVA. Inset: representative immunoblots (in duplicates) of the girdin siRNA-mediated protein depletion in VVEC. β-actin was used as a loading control.
Some reports indicate that the mechanisms of PI3K/Akt-mediated cytoskeleton rearrangement include phosphorylation of the actin-binding protein, girdin, a recently discovered Akt cytoskeletal target (39). However, girdin depletion did not attenuate the adenosine-induced increase in TER in VVEC that excludes its role in adenosine-induced VVEC barrier enhancement (Fig. 7C).
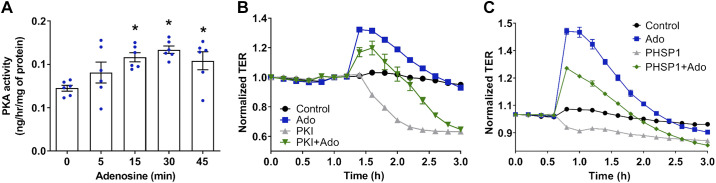
PKA activation is involved in adenosine-induced VVEC barrier enhancement.
cAMP/PKA pathway promotes barrier integrity in pulmonary endothelium (29, 68, 69); however, whether PKA may mediate cAMP-independent regulation of VVEC barrier remains unknown. Our data demonstrated that adenosine significantly increases PKA activity in a time-dependent manner (Fig. 8A). Importantly, maximal PKA activation (30 min) corresponds to maximal adenosine-induced TER increase, and inhibition of PKA significantly attenuated the basal and adenosine-induced increase in TER (Fig. 8B), indicating a role of PKA in adenosine-induced barrier enhancement. It was shown that the catalytic subunit of PKA (PKAc) forms a complex with GAB1 and Shp2 (Src homology-2-containing protein Tyr phosphatase 2), leading to PKA activation (23, 57). Though our data indicated that GAB is not involved in adenosine-induced VVEC barrier protection (Fig. 4B), specific inhibition of Shp2 with PHSP1 significantly attenuated the barrier-protective effect of adenosine on TER (Fig. 8C).
Fig. 8.
PKA is involved in adenosine-induced vasa vasorum endothelial cell (VVEC) barrier enhancement. A: effect of adenosine on PKA activity in VVEC lysates. EC were treated with 100 µM adenosine for indicated time periods in a serum-free media. Cell lysates (1 µg) were subjected to ELISA-based PKA activity assay (Enzo Life Sciences). PKA activity is represented as ng/h/μg of cell lysate; one-way ANOVA, means ± SE, n = 6 experiments, *P < 0.05. B: effect of PKA inhibition on transendothelial electrical resistance (TER) in adenosine-treated and control VVEC. Cell monolayers were incubated in serum-free media for 1 h. Adenosine (Ado) (100 µM) was added after 30 min pretreatment with specific PKA inhibitor, myrPKI14-22 (200 µM). The data are expressed as means ± SE, n = 4 experiments. C: effect of Src homology region 2 domain-containing phosphatase-2 (Shp2) inhibition on adenosine-induced VVEC barrier enhancement. EC monolayers were incubated in serum-free medium for 1 h and then treated with Shp2 inhibitor, PHSP1 (20 μM) ± adenosine (100 μM). The data are expressed as means ± SE (n = 4 experiments).
DISCUSSION
Our previous studies suggested that hypoxia-induced PA VV permeability may be one of the critical pathogenic factors in the development of perivascular inflammation and pathological structural remodeling in PH (15, 52). Considering that VV support the integrity of the vascular wall by providing oxygen and nutrients to the adventitial and medial layers, it would be necessary to better understand the mechanisms of VVEC barrier regulation. It is important to mention that, despite the mechanisms of endothelial permeability having been intensively investigated (41, 59, 94), the mechanisms involved in endothelial barrier strengthening remain largely unknown. In this study, we used PA VVEC, as distinctive endothelial cell model, enabling investigation of previously unrecognized mechanisms of VV barrier protection.
Based on our prior findings (92), this study was undertaken to further investigate the signaling mechanisms of a barrier-protective effect of adenosine, known to be a critical signaling mediator in the vasculature (26, 32, 56, 89, 91, 92). Adenosine promotes a barrier-protective, anti-inflammatory, and anti-remodeling effect in pulmonary vasculature under various pathological conditions (26, 56, 90), including pulmonary arterial hypertension (79, 99), ischemia/reperfusion (66, 101), sepsis (95), and endotoxin (LPS)-induced lung injury (63, 64). While in the PA and microvascular EC adenosine enhances endothelial barrier via the Gs/cAMP-dependent mechanism (5, 44, 50, 56, 90), here we provided new evidence on the barrier-protective mechanism that involves Gi-dependent activation of small GTPase Rac1 upstream of Akt/GSK3β. The involvement of Gi/Rac1 pathway has been described just for two agonists like sphingosine-1 phosphate (S1P) and stromal cell-derived factor (SDF) (30, 45, 75). However, the actual mechanism of Gi-mediated Rac1 activation, including Gi-mediated GEF for Rac1, remains not investigated (72). Recent data showed that ELMO1/Dock180 bipartite complex is involved in Rac1 activation in the protection of EC against apoptosis, as well as in netrin-induced signaling cascade, leading to blood vessel formation during embryogenesis in zebrafish (77). In addition, ELMO/Dock-mediated Rac1 activation was reported as a part of the GPCR mechanism involved in the maintenance of the blood-brain barrier (38). Our data extended the observations of others by demonstrating the role of ELMO1 in physiological signaling of adenosine-mediated VVEC barrier protection and providing a mechanistic link between Gi activation and the established downstream signaling components.
We also showed that adenosine/Gi-induced VVEC barrier enhancement involved activation of protein kinase A (PKA). These data are somewhat surprising because Gi activation may lead to a decrease in cAMP production. The mechanisms of cAMP-independent PKA activation are largely unknown. One published study has demonstrated that PKA activation may also occur independently of cAMP elevation by the coupling of Gi to the adapter protein GAB1 (53). GAB1 forms a complex with Shp2 and PKAc (PKA catalytic subunit), leading to PKA activation (23, 57). However, here we showed that the depletion of GAB1 had no effect on adenosine-induced VVEC barrier enhancement. In contrast, we demonstrated that pharmacological inhibition of Shp2 decreased basal TER and attenuated the adenosine-induced TER increase, thus supporting the involvement of Shp2 in VVEC barrier enhancement. Consistent with this observation, some studies indicate a role of Shp2 in the stabilization of EC adherens junctions (17). Additionally, it has been demonstrated that constitutively active Shp2 blocks LPS-induced EC barrier compromise in vitro and in vivo (18), whereas inhibition of Shp2 led to the disruption of the EC barrier via catenin and cadherin Tyr phosphorylation (18). Therefore, Shp2 activity is critical to EC barrier maintenance. Meanwhile, the role of Shp2 alone and in Gi-mediated adenosine-induced PKA activation remains to be determined.
Our recently published data in human lung microvascular EC strongly suggest the specific involvement of PKA anchoring protein 2 (AKAP2) in Gi-mediated PKA activation induced by nonhydrolyzable ATP analog, ATPγS (5). Scaffolding PKA-binding AKAP proteins exerted their activities mainly via directing PKA to the specific subcellular location (targets) and serving as a platform for biochemical interactions (19). AKAP2 seems to act as a scaffold in the organization of functional complex between Gαi, PKA, and myosin phosphatase regulatory subunit (MYPT1), thus facilitating Gi-mediated PKA and MLCP activation. Whether or not specific AKAPs are involved in PKA scaffolding in VVEC barrier enhancement mechanisms requires further investigation.
Current data indicating the links between Rac1/PAK1 signaling, PKA activity, and PI3K/Akt pathway are controversial. For example, in glioma cells, PKA stimulates Rac1 activity through phosphorylation of Rac1, GEF, Dock180 (27). Other studies demonstrate that Rac1 acts as a dual-kinase scaffold for PAK1 and PKA. Activation of PKA leads to dissociation of Rac1/PKA complex, and PKA can phosphorylate and activate PAK1 (3, 4). In contrast, recent data demonstrated that Rac1 is an upstream regulator of PKA, and PKA is upstream of Akt in the regulation of NO signaling in EC (48). Here we showed that downregulation of Rac1 attenuates phosphorylation of VASP, a well-known PKA substrate (16, 34), suggesting the existence of Rac1-PKA axis in VVEC. Maximal Rac1 activation precedes maximal PKA activation, supports this hypothesis.
We have previously shown that adenosine-induced VVEC barrier enhancement is critically dependent upon actin but not microtubule cytoskeleton and was determined as a significant increase of the polymerized cortical actin formation in the area of the cell-cell junctions (92). Rac1 activation leading to cortical actin formation was reported to be important for EC barrier strengthening in response to various stimuli, including purinergic receptor agonists (40, 58, 91, 96). However, the intermediate signaling events leading to cytoskeletal rearrangement and key cytoskeletal targets may be endothelial type specific and remained not investigated in VVEC. For instance, the mechanisms of PI3K/Akt-mediated cytoskeleton rearrangements are not well defined but shown to be critical to VVEC barrier enhancement. It was shown that Akt phosphorylates and inhibits GSK3 (85), which may lead to activation (deinhibition) of slingshot protein phosphatase slingshot protein phosphatase (SSH1), followed by dephosphorylation and activation of the actin-severing protein cofilin (61, 86). Another study implicated GSK3 inhibition and cofilin phosphorylation (inhibition) in endothelial barrier enhancement by stress fiber dissolution (73). Our data demonstrated that VVEC stimulation with adenosine decreased cofilin phosphorylation, whereas inhibition of Rac1 by transduction of dominant-negative Rac1 mutant (GFP-Rac-N17) leads to dephosphorylation of both Akt and GSK3 but increased cofilin phosphorylation. Together, these data support the involvement of Rac1/Akt/GSK3/cofilin pathway in adenosine-induced VVEC cytoskeletal reorganization and barrier enhancement. Akt-mediated cytoskeleton rearrangement may also include phosphorylation of the actin-binding protein, girdin, a recently discovered Akt target (39). However, girdin depletion had no effect on TER increase, indicating that girdin is not involved in adenosine-induced barrier enhancement in VVEC.
In human pulmonary EC, Rac1 and MLCP are involved in Gs-mediated adenosine-induced EC barrier enhancement (5, 43, 46). Our results demonstrated that Rac1 inhibition is accompanied by an increase in MLC phosphorylation and almost completely abolishes the adenosine-induced increase in TER, supporting the role of MLCP in the adenosine-induced Rac1-mediated barrier enhancement. The involvement of MLCP activation in VVEC barrier enhancement is further supported by our data, demonstrating that adenosine-induced TER increase is accompanied by dephosphorylation of unconventional MLCP substrates, cytoskeletal regulatory ERM (ezrin-radixin-moesin) proteins. However, detailed mechanisms of MLC-dependent VVEC barrier protection, including PKA (97) or phosphatase 2A (PP2A) (24, 42, 87), require further investigation.
In conclusion, this study defined specific signaling mechanisms of adenosine-induced VVEC barrier enhancement that involve adaptor protein ELMO1, Rac1/Akt pathway, atypical cAMP-independent PKA activation, and Rac1/PKA-mediated cytoskeleton rearrangements (Fig. 9). It can be expected that these studies will extend the knowledge on VV endothelial barrier regulation by adenosine and may suggest novel therapeutic options for preserving VV barrier integrity in PH and possibly other vascular lung diseases.
Fig. 9.
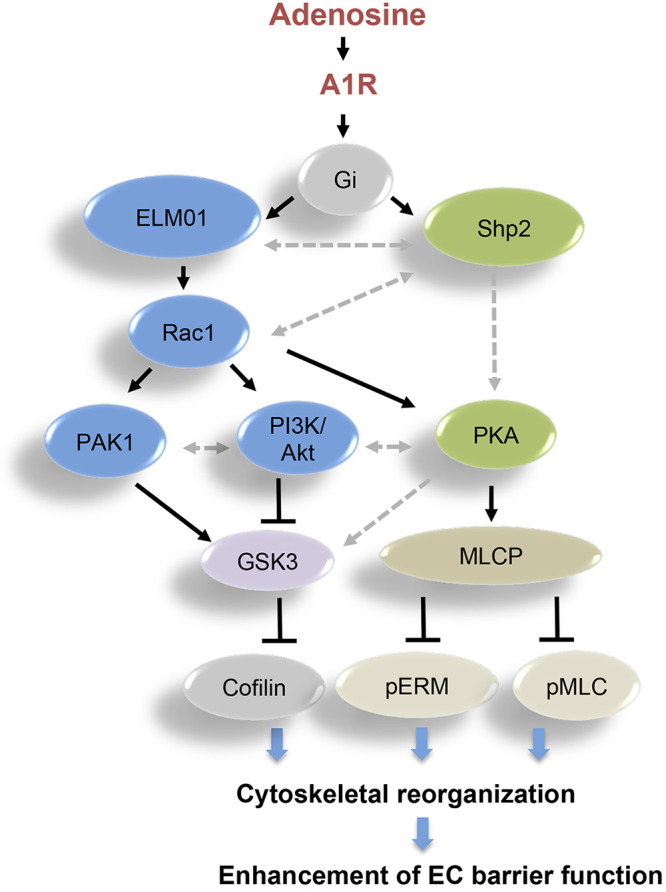
Schematic illustration of adenosine-mediated signaling pathways leading to vasa vasorum endothelial cell (EC) barrier enhancement.
GRANTS
This study was supported by National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL086783 (E.G.), American Heart Association (AHA) Postdoctoral Fellowship 20POST35210753 (R.B.), NHLBI Program Project Grant HL101902 (A.V.), and AHA Grant AHA00161 (A.K.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.D.V. and E.G. conceived and designed research; R.B., A.K.-K., M.C.-S., S.K., I.C., V.K., and D.S. performed experiments; A.D.V., R.B., A.K.-K., M.C.-S., S.K., I.C., V.K., D.S., and E.G. analyzed data; A.D.V., A.K.-K., K.R.S., and E.G. interpreted results of experiments; A.D.V., R.B., A.K.-K., M.C.-S., S.K., I.C., V.K., and D.S. prepared figures; A.D.V., R.B., and E.G. drafted manuscript; A.D.V., R.B., D.S., K.R.S., and E.G. edited and revised manuscript; A.D.V., R.B., A.K.-K., M.C.-S., S.K., I.C., V.K., D.S., K.R.S., and E.G. approved final version of manuscript.
REFERENCES
- 1.Adyshev DM, Dudek SM, Moldobaeva N, Kim KM, Ma SF, Kasa A, Garcia JG, Verin AD. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am J Physiol Lung Cell Mol Physiol 305: L240–L255, 2013. doi: 10.1152/ajplung.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslam M, Tanislav C, Troidl C, Schulz R, Hamm C, Gündüz D. cAMP controls the restoration of endothelial barrier function after thrombin-induced hyperpermeability via Rac1 activation. Physiol Rep 2: e12175, 2014. doi: 10.14814/phy2.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann VA, Bister K, Stefan E. Interplay of PKA and Rac: fine-tuning of Rac localization and signaling. Small GTPases 4: 247–251, 2013. doi: 10.4161/sgtp.27281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann VA, Riml A, Huber RG, Baillie GS, Liedl KR, Valovka T, Stefan E. Reciprocal regulation of PKA and Rac signaling. Proc Natl Acad Sci USA 110: 8531–8536, 2013. doi: 10.1073/pnas.1215902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bátori R, Kumar S, Bordán Z, Cherian-Shaw M, Kovács-Kása A, MacDonald JA, Fulton DJR, Erdődi F, Verin AD. Differential mechanisms of adenosine- and ATPγS-induced microvascular endothelial barrier strengthening. J Cell Physiol 234: 5863–5879, 2019. doi: 10.1002/jcp.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billaud M, Hill JC, Richards TD, Gleason TG, Phillippi JA. Medial hypoxia and adventitial vasa vasorum remodeling in human ascending aortic aneurysm. Front Cardiovasc Med 5: 124, 2018. doi: 10.3389/fcvm.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J 21: 2776–2786, 2007. doi: 10.1096/fj.06-7660com. [DOI] [PubMed] [Google Scholar]
- 9.Birukova AA, Burdette D, Moldobaeva N, Xing J, Fu P, Birukov KG. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res 79: 128–138, 2010. doi: 10.1016/j.mvr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215: 715–724, 2008. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol 221: 750–759, 2009. doi: 10.1002/jcp.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112: 358–404, 2006. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet E, Calvayrac O, Mazières J, Lajoie-Mazenc I, Boubekeur N, Favre G, Pradines A. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A inhibition. Oncogene 35: 1760–1769, 2016. doi: 10.1038/onc.2015.240. [DOI] [PubMed] [Google Scholar]
- 14.Boyle EC, Sedding DG, Haverich A. Targeting vasa vasorum dysfunction to prevent atherosclerosis. Vascul Pharmacol 96-98: 5–10, 2017. doi: 10.1016/j.vph.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol 297: L238–L250, 2009. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269: 14509–14517, 1994. [PubMed] [Google Scholar]
- 17.Chattopadhyay R, Raghavan S, Rao GN. Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox Biol 12: 438–455, 2017. doi: 10.1016/j.redox.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chichger H, Braza J, Duong H, Harrington EO. SH2 domain-containing protein tyrosine phosphatase 2 and focal adhesion kinase protein interactions regulate pulmonary endothelium barrier function. Am J Respir Cell Mol Biol 52: 695–707, 2015. doi: 10.1165/rcmb.2013-0489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol 9: 216–221, 1999. doi: 10.1016/S0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 20.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 21.Dance M, Montagner A, Yart A, Masri B, Audigier Y, Perret B, Salles JP, Raynal P. The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor receptor-2 to the activation of phosphoinositide 3-kinase. J Biol Chem 281: 23285–23295, 2006. doi: 10.1074/jbc.M600987200. [DOI] [PubMed] [Google Scholar]
- 22.Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006. doi: 10.2353/ajpath.2006.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, Hassid A, Busse R, Fleming I. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res 97: 1236–1244, 2005. doi: 10.1161/01.RES.0000195611.59811.ab. [DOI] [PubMed] [Google Scholar]
- 24.Doherty DF, Nath S, Poon J, Foronjy RF, Ohlmeyer M, Dabo AJ, Salathe M, Birrell M, Belvisi M, Baumlin N, Kim MD, Weldon S, Taggart C, Geraghty P. Protein phosphatase 2A reduces cigarette smoke-induced cathepsin s and loss of lung function. Am J Respir Crit Care Med 200: 51–62, 2019. doi: 10.1164/rccm.201808-1518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubey R, Mi Z, Gillespie DG, Jackson EK. Dysregulation of extracellular adenosine levels by vascular smooth muscle cells from spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 21: 249–254, 2001. doi: 10.1161/01.ATV.21.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng H, Hu B, Vuori K, Sarkaria JN, Furnari FB, Cavenee WK, Cheng SY. EGFRvIII stimulates glioma growth and invasion through PKA-dependent serine phosphorylation of Dock180. Oncogene 33: 2504–2512, 2014. doi: 10.1038/onc.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001. [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 30.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 11: 169–182, 2008. doi: 10.1007/s10456-007-9087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gessi S, Merighi S, Fazzi D, Stefanelli A, Varani K, Borea PA. Adenosine receptor targeting in health and disease. Expert Opin Investig Drugs 20: 1591–1609, 2011. doi: 10.1517/13543784.2011.627853. [DOI] [PubMed] [Google Scholar]
- 33.González-Mariscal L, Raya-Sandino A, González-González L, Hernández-Guzmán C. Relationship between G proteins coupled receptors and tight junctions. Tissue Barriers 6: e1414015, 2018. doi: 10.1080/21688370.2017.1414015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res 99: 845–852, 2006. doi: 10.1161/01.RES.0000245189.21703.c0. [DOI] [PubMed] [Google Scholar]
- 35.Harbeck B, Hüttelmaier S, Schluter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J Biol Chem 275: 30817–30825, 2000. doi: 10.1074/jbc.M005066200. [DOI] [PubMed] [Google Scholar]
- 36.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25: 33–39, 2004. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Headrick JP, Ashton KJ, Rose’meyer RB, Peart JN. Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Ther 140: 92–111, 2013. doi: 10.1016/j.pharmthera.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Hernández-Vásquez MN, Adame-García SR, Hamoud N, Chidiac R, Reyes-Cruz G, Gratton JP, Côté JF, Vázquez-Prado J. Cell adhesion controlled by adhesion G protein-coupled receptor GPR124/ADGRA2 is mediated by a protein complex comprising intersectins and Elmo-Dock. J Biol Chem 292: 12178–12191, 2017. doi: 10.1074/jbc.M117.780304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichimiya H, Maeda K, Enomoto A, Weng L, Takahashi M, Murohara T. Girdin/GIV regulates transendothelial permeability by controlling VE-cadherin trafficking through the small GTPase, R-Ras. Biochem Biophys Res Commun 461: 260–267, 2015. doi: 10.1016/j.bbrc.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol 291: L289–L295, 2006. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kása A, Csortos C, Verin AD. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers 3: e974448, 2015. doi: 10.4161/21688370.2014.974448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kása A, Czikora I, Verin AD, Gergely P, Csortos C. Protein phosphatase 2A activity is required for functional adherent junctions in endothelial cells. Microvasc Res 89: 86–94, 2013. doi: 10.1016/j.mvr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KM, Csortos C, Czikora I, Fulton D, Umapathy NS, Olah G, Verin AD. Molecular characterization of myosin phosphatase in endothelium. J Cell Physiol 227: 1701–1708, 2012. doi: 10.1002/jcp.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal 14: 99–108, 2002. doi: 10.1016/S0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi K, Sato K, Kida T, Omori K, Hori M, Ozaki H, Murata T. Stromal cell-derived factor-1α/C-X-C chemokine receptor type 4 axis promotes endothelial cell barrier integrity via phosphoinositide 3-kinase and Rac1 activation. Arterioscler Thromb Vasc Biol 34: 1716–1722, 2014. doi: 10.1161/ATVBAHA.114.303890. [DOI] [PubMed] [Google Scholar]
- 46.Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, Natarajan V, Pearse DB, Garcia JG, Verin AD. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res 97: 115–124, 2005. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- 47.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72: 463–493, 2010. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 48.Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and beta3-adrenergic receptors in endothelial NO signaling. J Biol Chem 282: 32719–32729, 2007. doi: 10.1074/jbc.M706815200. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs-Kasa A, Gorshkov BA, Kim KM, Kumar S, Black SM, Fulton DJ, Dimitropoulou C, Catravas JD, Verin AD. The protective role of MLCP-mediated ERM dephosphorylation in endotoxin-induced lung injury in vitro and in vivo. Sci Rep 6: 39018, 2016. doi: 10.1038/srep39018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacs-Kasa A, Kim KM, Cherian-Shaw M, Black SM, Fulton DJ, Verin AD. Extracellular adenosine-induced Rac1 activation in pulmonary endothelium: Molecular mechanisms and barrier-protective role. J Cell Physiol 233: 5736–5746, 2018. doi: 10.1002/jcp.26281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Yang L, Fu H, Yan J, Wang Y, Guo H, Hao X, Xu X, Jin T, Zhang N. Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat Commun 4: 1706, 2013. doi: 10.1038/ncomms2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol 187: 2711–2722, 2011. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Wang D, Chen Z, Lu E, Wang Z, Duan J, Tian W, Wang Y, You L, Zou Y, Cheng Y, Zhu Q, Wan X, Xi T, Jiang M, Han Y, Cao C, Birnbaumer L, Chu WM, Yang Y. Gαi1 and Gαi3 regulate macrophage polarization by forming a complex containing CD14 and Gab1. Proc Natl Acad Sci USA 112: 4731–4736, 2015. [Erratum in Proc Natl Acad Sci USA 115: E5252, 2018]. doi: 10.1073/pnas.1503779112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41: 775–787, 2001. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 55.Liu H, Zhang Y, Wu H, D’Alessandro A, Yegutkin GG, Song A, Sun K, Li J, Cheng NY, Huang A, Edward Wen Y, Weng TT, Luo F, Nemkov T, Sun H, Kellems RE, Karmouty-Quintana H, Hansen KC, Zhao B, Subudhi AW, Jameson-Van Houten S, Julian CG, Lovering AT, Eltzschig HK, Blackburn MR, Roach RC, Xia Y. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 134: 405–421, 2016. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Q, Harrington EO, Newton J, Casserly B, Radin G, Warburton R, Zhou Y, Blackburn MR, Rounds S. Adenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancement. Am J Physiol Lung Cell Mol Physiol 298: L755–L767, 2010. doi: 10.1152/ajplung.00330.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y, Xiong Y, Huo Y, Han J, Yang X, Zhang R, Zhu DS, Klein-Hessling S, Li J, Zhang X, Han X, Li Y, Shen B, He Y, Shibuya M, Feng GS, Luo J. Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway. Proc Natl Acad Sci USA 108: 2957–2962, 2011. doi: 10.1073/pnas.1009395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal 17: 131–139, 2005. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 60.Merighi S, Mirandola P, Varani K, Gessi S, Leung E, Baraldi PG, Tabrizi MA, Borea PA. A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol Ther 100: 31–48, 2003. doi: 10.1016/S0163-7258(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 61.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal 25: 457–469, 2013. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Mulligan-Kehoe MJ, Simons M. Vasa vasorum in normal and diseased arteries. Circulation 129: 2557–2566, 2014. doi: 10.1161/CIRCULATIONAHA.113.007189. [DOI] [PubMed] [Google Scholar]
- 63.Neely CF, Jin J, Keith IM. A1-adenosine receptor antagonists block endotoxin-induced lung injury. Am J Physiol 272: L353–L361, 1997. doi: 10.1152/ajplung.1997.272.2.L353. [DOI] [PubMed] [Google Scholar]
- 64.Ngamsri KC, Wagner R, Vollmer I, Stark S, Reutershan J. Adenosine receptor A1 regulates polymorphonuclear cell trafficking and microvascular permeability in lipopolysaccharide-induced lung injury. J Immunol 185: 4374–4384, 2010. doi: 10.4049/jimmunol.1000433. [DOI] [PubMed] [Google Scholar]
- 65.Nijmeh H, Balasubramaniam V, Burns N, Ahmad A, Stenmark KR, Gerasimovskaya EV. High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am J Physiol Lung Cell Mol Physiol 306: L661–L671, 2014. doi: 10.1152/ajplung.00244.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park SW, Chen SW, Kim M, Brown KM, D’Agati VD, Lee HT. Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther 333: 736–747, 2010. doi: 10.1124/jpet.110.166884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parrini MC, Matsuda M, de Gunzburg J. Spatiotemporal regulation of the Pak1 kinase. Biochem Soc Trans 33: 646–648, 2005. doi: 10.1042/BST0330646. [DOI] [PubMed] [Google Scholar]
- 68.Patterson CE, Garcia JG. Regulation of thrombin-induced endothelial cell activation by bacterial toxins. Blood Coagul Fibrinolysis 5: 63–72, 1994. doi: 10.1097/00001721-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 7: 287–308, 2000. doi: 10.3109/10623320009072215. [DOI] [PubMed] [Google Scholar]
- 70.Paty PS, Sherman PF, Shepard JM, Malik AB, Kaplan JE. Role of adenosine in platelet-mediated reduction in pulmonary vascular permeability. Am J Physiol Heart Circ Physiol 262: H771–H777, 1992. doi: 10.1152/ajpheart.1992.262.3.H771. [DOI] [PubMed] [Google Scholar]
- 71.Petrilli A, Copik A, Posadas M, Chang LS, Welling DB, Giovannini M, Fernández-Valle C. LIM domain kinases as potential therapeutic targets for neurofibromatosis type 2. Oncogene 33: 3571–3582, 2014. doi: 10.1038/onc.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reinhard NR, Mastop M, Yin T, Wu Y, Bosma EK, Gadella TWJ Jr, Goedhart J, Hordijk PL. The balance between Gαi-Cdc42/Rac and Gα12/13-RhoA pathways determines endothelial barrier regulation by sphingosine-1-phosphate. Mol Biol Cell 28: 3371–3382, 2017. doi: 10.1091/mbc.e17-03-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rom S, Fan S, Reichenbach N, Dykstra H, Ramirez SH, Persidsky Y. Glycogen synthase kinase 3β inhibition prevents monocyte migration across brain endothelial cells via Rac1-GTPase suppression and down-regulation of active integrin conformation. Am J Pathol 181: 1414–1425, 2012. doi: 10.1016/j.ajpath.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 75.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 278: 47281–47290, 2003. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 76.Sayner SL. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol 300: L667–L678, 2011. doi: 10.1152/ajplung.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schäker K, Bartsch S, Patry C, Stoll SJ, Hillebrands JL, Wieland T, Kroll J. The bipartite rac1 Guanine nucleotide exchange factor engulfment and cell motility 1/dedicator of cytokinesis 180 (elmo1/dock180) protects endothelial cells from apoptosis in blood vessel development. J Biol Chem 290: 6408–6418, 2015. doi: 10.1074/jbc.M114.633701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J. Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol 9: 706, 2018. doi: 10.3389/fimmu.2018.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shang P, He ZY, Chen JF, Huang SY, Liu BH, Liu HX, Wang XT. Absence of the adenosine A2A receptor confers pulmonary arterial hypertension through RhoA/ROCK signaling pathway in mice. J Cardiovasc Pharmacol 66: 569–575, 2015. doi: 10.1097/FJC.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 80.Sheehan KA, Ke Y, Solaro RJ. p21-Activated kinase-1 and its role in integrated regulation of cardiac contractility. Am J Physiol Regul Integr Comp Physiol 293: R963–R973, 2007. doi: 10.1152/ajpregu.00253.2007. [DOI] [PubMed] [Google Scholar]
- 81.Shi L, Zhang B, Sun X, Zhang X, Lv S, Li H, Wang X, Zhao C, Zhang H, Xie X, Wang Y, Zhang P. CC chemokine ligand 18(CCL18) promotes migration and invasion of lung cancer cells by binding to Nir1 through Nir1-ELMO1/DOC180 signaling pathway. Mol Carcinog 55: 2051–2062, 2016. doi: 10.1002/mc.22450. [DOI] [PubMed] [Google Scholar]
- 82.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 104: 978–986, 2009. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75: 23–47, 2013. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Surve CR, Lehmann D, Smrcka AV. A chemical biology approach demonstrates G protein βγ subunits are sufficient to mediate directional neutrophil chemotaxis. J Biol Chem 289: 17791–17801, 2014. doi: 10.1074/jbc.M114.576827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan J, Geng L, Yazlovitskaya EM, Hallahan DE. Protein kinase B/Akt-dependent phosphorylation of glycogen synthase kinase-3beta in irradiated vascular endothelium. Cancer Res 66: 2320–2327, 2006. doi: 10.1158/0008-5472.CAN-05-2700. [DOI] [PubMed] [Google Scholar]
- 86.Tang W, Zhang Y, Xu W, Harden TK, Sondek J, Sun L, Li L, Wu D. A PLCβ/PI3Kγ-GSK3 signaling pathway regulates cofilin phosphatase slingshot2 and neutrophil polarization and chemotaxis. Dev Cell 21: 1038–1050, 2011. doi: 10.1016/j.devcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tar K, Csortos C, Czikora I, Olah G, Ma SF, Wadgaonkar R, Gergely P, Garcia JG, Verin AD. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem 98: 931–953, 2006. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 88.Thennes T, Mehta D. Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc Res 83: 31–44, 2012. doi: 10.1016/j.mvr.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200: 1395–1405, 2004. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vascul Pharmacol 52: 199–206, 2010. doi: 10.1016/j.vph.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Umapathy NS, Zemskov EA, Gonzales J, Gorshkov BA, Sridhar S, Chakraborty T, Lucas R, Verin AD. Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J Cell Physiol 223: 215–223, 2010. doi: 10.1002/jcp.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Umapathy NS, Kaczmarek E, Fatteh N, Burns N, Lucas R, Stenmark KR, Verin AD, Gerasimovskaya EV. Adenosine A1 receptors promote vasa vasorum endothelial cell barrier integrity via Gi and Akt-dependent actin cytoskeleton remodeling. PLoS One 8: e59733, 2013. [Erratum in PLoS One 8: e63, 2013.] doi: 10.1371/journal.pone.0059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 94.Vogel SM, Malik AB. Cytoskeletal dynamics and lung fluid balance. Compr Physiol 2: 449–478, 2012. doi: 10.1002/cphy.c100006. [DOI] [PubMed] [Google Scholar]
- 95.Wilson CN, Vance CO, Lechner MG, Matuschak GM, Lechner AJ. Adenosine A1 receptor antagonist, L-97-1, improves survival and protects the kidney in a rat model of cecal ligation and puncture induced sepsis. Eur J Pharmacol 740: 346–352, 2014. doi: 10.1016/j.ejphar.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 39: 187–199, 2002. doi: 10.1016/S1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 97.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 98.Xing J, Birukova AA. ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc Res 79: 56–62, 2010. doi: 10.1016/j.mvr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu MH, Gong YS, Su MS, Dai ZY, Dai SS, Bao SZ, Li N, Zheng RY, He JC, Chen JF, Wang XT. Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J Vasc Res 48: 171–183, 2011. doi: 10.1159/000316935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yegutkin GG, Helenius M, Kaczmarek E, Burns N, Jalkanen S, Stenmark K, Gerasimovskaya EV. Chronic hypoxia impairs extracellular nucleotide metabolism and barrier function in pulmonary artery vasa vasorum endothelial cells. Angiogenesis 14: 503–513, 2011. doi: 10.1007/s10456-011-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yildiz G, Demiryürek AT, Gümüşel B, Lippton H. Ischemic preconditioning modulates ischemia-reperfusion injury in the rat lung: role of adenosine receptors. Eur J Pharmacol 556: 144–150, 2007. doi: 10.1016/j.ejphar.2006.11.008. [DOI] [PubMed] [Google Scholar]