Abstract
Numerous age-dependent alterations at the molecular, cellular, tissue and organ systems levels underlie the pathophysiology of aging. Herein, the focus is upon the secreted protein thrombospondin-1 (TSP1) as a promoter of aging and age-related diseases. TSP1 has several physiological functions in youth, including promoting neural synapse formation, mediating responses to ischemic and genotoxic stress, minimizing hemorrhage, limiting angiogenesis, and supporting wound healing. These acute functions of TSP1 generally require only transient expression of the protein. However, accumulating basic and clinical data reinforce the view that chronic diseases of aging are associated with accumulation of TSP1 in the extracellular matrix, which is a significant maladaptive contributor to the aging process. Identification of the relevant cell types that chronically produce and respond to TSP1 and the molecular mechanisms that mediate the resulting maladaptive responses could direct the development of therapeutic agents to delay or revert age-associated maladies.
Keywords: aging, cardiovascular and metabolic disease, CD47, self-renewal, senescence, thrombospondin-1
INTRODUCTION
US President Theodore Roosevelt is popularly credited with saying “Old age is like everything else. To make a success of it, you’ve got to start young.” Advanced age is the main risk factor for cardiovascular, neurodegenerative, metabolic, and malignant disease, and the elderly are the fastest growing segment of the population worldwide. Aging entails loss of the ability to repair and recuperate after stress and injury. It is associated with multiple molecular changes in cells, including decreased telomere length, dysregulated energy balance and mitochondrial function, genetic instability, loss of stemness, and increased cell senescence. These age-related changes occur at varying rates in different cell types and organs and between individuals, indicating that biological and chronological aging can diverge. Genetic diversity and environment each contribute to this variation.
All organ systems are negatively impacted by aging, and the physiological consequences are well characterized. The cardiovascular system of aged individuals is less elastic, more fibrotic, impaired in maintaining blood flow, hypertensive, and less responsive to vasoactive agents (224). The lungs of the elderly are also more fibrotic and less elastic, have reduced functional capacity, and present an increasing barrier to gas exchange (144). Other vital organs deteriorate with aging, including loss of filtering capacity and fibrosis of the glomeruli in the kidneys (190), impaired hematopoiesis and immune responses to infectious agents and malignancies (9), impaired memory and cognition, and decreased bone and muscle mass and strength in the musculoskeletal system (33, 127). Furthermore, it is quite rare for people to age without developing one or more age-related disease. This suggests a limit to human age that may not be extendable. Indeed, molecular studies have identified a programmed global inactivation of anti-aging mechanisms by the sixth decade of life (210). Nonetheless, understanding the mechanisms that accelerate and worsen age-related disease should allow for interventions that lengthen the healthy, active portion of life.
THROMBOSPONDIN-1
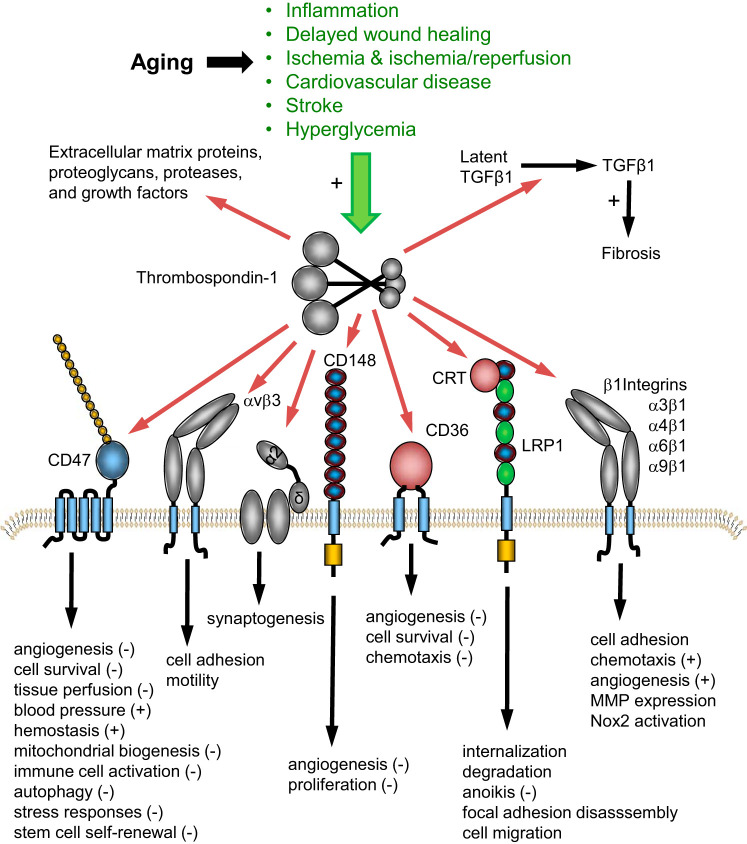
Thrombospondin-1 (TSP1) is a 480-kDa trimeric secreted protein that can engage extracellular matrix and cell surface molecules (180). TSP1 exists in a matrix-bound form as well as a soluble protein that circulates in body fluids. Proteolytic fragments of TSP1 have been identified in the extracellular space in the context of aging (26), although their physiological significance remains unknown. Apart from megakaryocytes, which package TSP1 into platelet α-granules, most cells produce low or undetectable basal levels of TSP1 in vivo but rapidly upregulate expression and/or secretion in response to cell injury and stress. TSP1 modulates cell function indirectly by binding to specific matrix proteins and growth factors that alter the properties of the extracellular matrix and directly by engaging signaling receptors on the cell surface (180, 183). As such, it is classified as a matricellular protein (18). Soluble and matrix-bound TSP1 binds to multiple cell receptors, including several integrins, cluster of differentiation 47 (CD47), CD36, CD148, the gabapentin receptor α2δ1, low-density lipoprotein receptor-related protein 1 (LRP1), stromal interaction molecule 1 (STIM1), and possibly signal regulatory protein-α (SIRPα) (Fig. 1). These receptors regulate signaling pathways involved in cell survival, redox signaling, pluripotency and self-renewal, mitochondrial function and metabolism, responses to stress and tissue injury, and sensitivity to genotoxic agents such as radiation and cytotoxic chemotherapy. Because several of these systems are degraded with aging, a role for TSP1 in promoting the aging process is now emerging.
Fig. 1.
Aging increases thrombospondin-1 (TSP1) expression and dysregulates its physiological functions. TSP1 is transiently secreted into the extracellular matrix during embryonic development and in response to wounding in adults, but it is efficiently cleared by cell uptake via calreticulin (CRT) and the LDL receptor-related protein receptor (LRP1). Various physiological functions of TSP1 are mediated by its interactions with specific cell surface receptors and by modulating the function of other extracellular matrix proteins and growth factors. Several chronic and acute conditions associated with aging result in increased TSP1 biosynthesis and accumulation in the extracellular matrix, which perturb TSP1 signaling functions mediated by cell surface receptors and increase the activation of latent TGFβ1.
MATRICELLULAR PROTEINS AND CELLULAR SENESCENCE
Senescence is defined as permanent, stress-mediated cell cycle arrest (76). This process has been noted in multiple cell types spanning people and in the animal and plant kingdoms. Senescent cells accumulate with age and are characterized by a lack of proliferation, changes in cell morphology, increased expression of proliferation-inhibiting proteins (e.g., p16, ARF, interleukins, and chemokines), accumulation of senescence-associated β-galactosidase and NF-κB, and DNA damage (25, 44, 147).
The matricellular family of proteins includes thrombospondins, fibulins, osteopontin, the CCN family, periostin, tenascin, and SPARC (184). In addition to the role of TSP1 reviewed here, age-dependent alterations in the expression of other matricellular proteins have been reported, and some functional roles in aging have been established. Dermal fibroblasts produced increased thrombospondin-2 (TSP2) in proportion to the duration of culture and the age of the donating animals from which cells were harvested (4). Fibroblasts from young 4- to 6-mo-old, but not aged 22- to 27-mo-old, Sparc-null mice had better replicative capacity than cells from wild-type animals. SPARC expression was increased in endothelial cells and fibroblasts from older (mean age: >65 yr) compared with younger individuals (mean age: <30 yr) and mice (179) (see Table 1 for age ranges in animal and human studies). Aged 18- to 29-mo-old Sparc-null mice were resistant to cardiac fibrosis (211). Unlike aged Cd47-null animals, old Sparc-null mice resembled aged controls in angiogenic capability (179). Also, SPARC decreased TSP1 mRNA (125), suggesting possible cross-talk. Exogenous cellular communication network factor 1 (CCN1) promoted senescence of young human fibroblasts along with enhanced expression of NADPH oxidase 1 (Nox1) and p16 (103). Similarly, myocyte precursors treated with exogenous CCN1 showed decreased replication and increased levels of p16, p53, and β-galactosidase (46). Skin samples, from individuals with sun-mediated aging, displayed increased CCN1 along with increased IL-1β (176). Of note is that TSP1 also stimulates IL-1β production (199), which can induce cell cycle arrest via p16 (174). In contrast to the former matricellular proteins that increase with cell age and promote senescence, periostin protein levels were decreased in adipose precursor cells from aged 14-mo-old compared with young 1.4-mo-old mice (68). Together, these studies suggest a complex role for several matricellular proteins in cellular aging and senescence.
Table 1.
Age ranges in human subject and animal studies
| Young | Intermediate | Old/Aged | |
|---|---|---|---|
| Mice, months | 0.5–8 | 9–11 | 12–29 |
| Rats, months | 2 | NA | 22 |
| Humans, years | 9–30 | 45–50 | 64–83 |
NA, not applicable.
TSP1 AND CELLULAR SENESCENCE
TSP1 was identified as an inhibitor of endothelial cell proliferation (10, 65, 208), and this activity localized to the NH2-terminal domain and central type 1 repeats (87, 212). This is of interest in light of data indicating that TSP1 limits pluripotency (reviewed below; Fig. 2). Likewise, absence of the transcription factor Dmp1, which activates senescence-associated ARF, was found to be concurrent with decreased pulmonary TSP1 (139). Senescent human fibroblasts showed increased TSP1 mRNA and soluble protein in conditioned medium, whereas conditioned medium from senescent cells spiked with a TSP1 antibody (clone C6.7), when applied to proliferating fibroblasts, was incapable of promoting senescence (151). The TSP1 receptor CD36 was upregulated in late-passage senescent cells and associated with increased expression of p16 and senescence-associated β-galactosidase protein and senescence-associated secretory phenotype transcripts (30), although changes in TSP1 were not determined. Interestingly, treatment of nonsenescent cells with amyloid β-peptide (Aβ1–42) activated senescence-promoting NF‐κB. Cerebral expression of TSP1 was associated with senile plaques in individuals with Alzheimer’s disease (AD) (23). More recently, increased expression of TSP1, CD36, CD47, and SIRPα was demonstrated in a subset of cerebral vessels in tissue samples from AD patients carrying the ApoE3/4 allele (128). Likewise, Aβ, via CD36-CD47 cross-talk, is known to limit nitric oxide (NO) signaling (152) and phagocytosis (101). The status of ligand TSP1, while not determined in these experiments, may be inferred since TSP1 is increased in senescent cells, and treatment with exogenous TSP1 increased senescence of human pulmonary (146) and murine brain microvascular endothelial cells (59) along with upregulating p16, p21, and p53. Furthermore, Aβ stimulated capillary constriction in individuals with AD and decreased cognition through redox dysregulation and enhanced endothelin-1 (ET-1) signaling (161). TSP1 also promotes increased ET-1 signaling in other vascular beds (187). Conversely, TSP1 protected certain neural cells from Aβ-mediated injury in an α2δ1-dependent manner (108). It should be emphasized that TSP1 effects on cell senescence are cell type and concentration dependent. Thus, in AD, TSP1 is likely both harmful and protective.
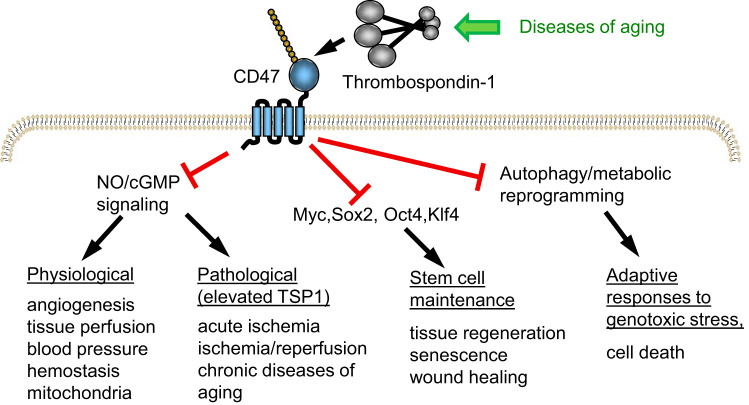
Fig. 2.
CD47 signaling pathways mediating effects of elevated thrombospondin-1 (TSP1) in aging. Physiological levels of TSP1 control nitric oxide (NO) biosynthesis and downstream cGMP signaling in vascular cells to regulate vascular homeostasis and mitochondrial biogenesis. Chronically, elevated TSP1 levels in aging impair these functions and contribute to age-related impairment of recovery from ischemic injuries and cardiovascular diseases of aging. Suppression of the stem cell transcription factors octamer-binding transcription factor 3/4 (Oct3/4), sex-determining region Y-box 2 (Sox2), Kruppel like factor 4 (Klf4), and V-myc myelocytomatosis viral oncogene homolog (cMyc), by increased TSP1/cluster of differentiation 47 (CD47) signaling, results in loss of stem cells, which are required for wound repair and tissue regeneration. Inhibition of protective autophagy responses and metabolic adaptation to stress impair recovery from the genotoxic stress caused by radiotherapy and chemotherapy.
Strikingly, replicative capacity was maintained in pulmonary endothelial cells from young 2- to 3-mo-old Thbs1- and Cd47-null mice for ≥6 mo (112). When exposed to cell passage stress, lung endothelial cells from young TSP1- and CD47-replete wild-type mice quickly assumed a senescent phenotype with cell flattening, β-galactosidase activation, and vacuole formation, while null cells maintained a normal cobblestone appearance. Enhanced replicative capacity was also reported in renal tubular epithelial cells (RTEC) from young Cd47-null mice compared with RTEC from wild-type mice. Furthermore, treating RTEC from young wild-type mice with an antibody that blocks TSP1-CD47 interaction, or with a CD47 siRNA, increased cell replication (188). Protein levels of Ki-67, a marker of cell proliferation, were increased in the tracheal epithelium from young 3- to 3.5-mo-old Cd47-null mice compared with wild types (122). Thus in vitro, and in null cells ex vivo, TSP1 acts through several receptors and intermediates to inhibit cell replication and promote senescence.
TSP1 AND STEM CELL DIFFERENTIATION
Signaling cues modulate the differentiation of embryonic stem cells along specific pathways. Following embryogenesis, differentiation is associated with loss of replicative potential in primary cells. The finding that lung endothelial cells from 2- to 3-mo old mice that lacked TSP1 or CD47, but not CD36, maintained cell replication with weekly passage for over 6 mo (112) suggests an important role for these genes in this process. However, the finding implies a possible role for these genes in cell differentiation. In a novel study of umbilical cord-derived endothelial progenitor cells from preterm neonates, increased TSP1 was associated with decreased cell replication, whereas silencing of TSP1 restored replication (132). Interestingly, TSP1 levels were noted lower in dedifferentiated breast cancer stem cells (bCSCs) compared with differentiated MDA-MB-231 cells (109). Exogenous TSP1 also promoted the differentiation of bronchial-alveolar stem cells (passage 2–6) from 0.5- to 1-mo-old mice (129) and mouse embryonic stem cell (ESC)-derived auditory neurons via the α2δ1 receptor (134). Parenthetically, CD47 expression was increased during the controlled differentiation of mesenchymal stem cell (MSCs) to chondrocytes (64). In contrast, exogenous TSP1 inhibited MSC differentiation into osteoblasts (11). In this same line, adipose-derived stem cells isolated from white fat from obese individuals (mean age, 41.56 ± 3.07 yr; BMI > 40 kg/m2) expressed increased TSP1 protein and mRNA and showed decreased differentiation into adipocytes compared with stem cells from similar fat from nonobese individuals (mean age, 22.26 ± 0.88 yr) (170). Furthermore, human MSCs treated with exogenous TSP1 had increased replication (16). These data suggest cell- and perhaps cell age-specific effects of TSP1 on stem cell replication and differentiation.
TSP1 AND PLURIPOTENCY
Pluripotency is the ability to differentiate into the three primary embryonic cell lineages. Stem cells can undergo replication many times longer than non-stem cells but, unless embryonic, are not pluripotent, and cells from young donors replicate more than those from old donors. The first data suggesting a role for TSP1-CD47 in necessarily blocking dedifferentiation came from cultured lung endothelial cell from young 3- to 3.5-mo-old wild-type and Cd47-null mice. Under extended passaging, a proportion of these cells continued to express the endothelial precursor stem cell markers Sca-1, CD14, and CD11 (112). Surprisingly, Cd47-null but not wild-type endothelial cells grown in serum-free medium formed floating cell aggregates resembling embryoid bodies (EBs), and this was associated with upregulation of stem cell-associated genes, including nestin, SSEA-1, and c-Kit. Consistent with this, Cd47-null EBs showed asymmetric cell division. When cultured in specific differentiation mediums, null EBs selectively expressed genes unique to endo-, ecto-, and mesodermal cells, confirming pluripotency. It was not clear whether similar results would be obtained growing CD47-null cells in TSP1-free serum. Forced expression of a panel of stem cell transcription factors (Oct3/4, Sox2, Kl4, and cMyc, abbreviated OSKM) in human somatic cells dedifferentiates these into induced pluripotent stem cells (iPSCs) (205). Cells and organs from young Cd47-null mice have markedly increased OSKM levels compared with the same samples from wild-type mice. Because Cd47-null mice do express TSP1, albeit at a lower level than wild-type mice, these results indicated a CD47-dependent role for TSP1 in inhibiting pluripotency. Subsequent studies support this view. Primary RTEC from young Cd47-null mice were found to overexpress OSKM. Likewise, TSP1 treatment of RTEC from wild-type but not Cd47-null mice suppressed OSKM expression (188). Interestingly, in CD47-positive cells, TSP1 destabilized cMyc to lower protein levels. These data suggest a method for the production of human iPSCs by lowering/blocking TSP1-CD47 signaling.
TSP1 AND AGE-RELATED DISEASES
The major age-related diseases include cardiovascular disease (myocardial ischemia/infarct, heart failure, peripheral vascular disease, stroke, and hypertension), chronic obstructive pulmonary disease, adult cancer, arthritis and osteoporosis, diabetes and metabolic syndrome, dementia and Alzheimer’s disease, and glaucoma and cataracts. TSP1 is recognized to mediate important pathways in the acute setting. Yet the role of TSP1 in age-related processes, especially in regard to human disease, is just now being appreciated (Table 2).
Table 2.
Site-specific TSP1 expression in human aging and age-associated diseases
| Fluid/Cell/Tissue/Organ | Age-Related Disease |
|---|---|
| Upregulation | |
| Interstitial skeletal muscle fluid | PAD |
| Circulating extracellular vesicles | PAD, hypercholesteremia, aging |
| Platelets | Aging |
| Stromal cells, adipose tissue | Obesity |
| Monocytes | Rheumatoid arthritis, glaucoma |
| Fibroblasts | Rheumatoid arthritis |
| Vascular smooth muscle cells | PAD |
| Plasma | PAD, CVD ± diabetes, HFpEF, hypertension, prediabetes, rheumatoid arthritis, glaucoma |
| Artery, systemic | PAH, PVD, aortic dissection and aneurysm, aging |
| Artery, cerebral | Alzheimer’s disease |
| Synovial artery | Rheumatoid arthritis |
| Subcutaneous adipose tissue | Obesity |
| Skeletal muscle | PAD, aging (expression not exercise responsive) |
| Visceral adipose tissue | Metabolic syndrome |
| Cartilage | Osteoarthritis, early |
| Kidney | Diabetic nephropathy |
| Heart | Organ transplantation, heart failure |
| Downregulation | |
| Vitreous fluid | Proliferative diabetic retinopathy |
| Eye | Macular degeneration |
| Cartilage | Osteoarthritis, late |
CVD, cardiovascular disease; HFpEF, heart failure with preserved ejection fraction; PAD, peripheral arterial disease; PAH, pulmonary arterial hypertension; TSP1, thrombospondin-1.
Vascular Disease
Plasma TSP1 levels were higher in individuals with peripheral arterial disease compared with those without (80, 195) and in patients following acute ischemic stroke (58). Plasma TSP1 was elevated in individuals with cardiovascular disease (CVD) receiving dialysis for end-stage renal disease compared with individuals undergoing dialysis alone (84). Indeed, in this study, plasma TSP1 levels within the top quartile strongly correlated with all-cause and CVD-related mortality and predicted adverse outcomes. These data raise the possibility that hemodialysis may concentrate plasma TSP1 to promote adverse cardiovascular events. As pointed out, TSP1 is found preformed in platelets, requiring that platelet activation be minimized when assessing plasma TSP1.
A role for vascular parenchymal TSP1 has also been noted. TSP1 expression in human pulmonary arteries correlated positively with age (146). TSP1 mRNA and protein were significantly increased in lower extremity tissue samples from a cohort of elderly individuals with advanced, noncorrectable peripheral arterial disease (PAD) (51). In situ hybridization showed marked TSP1 mRNA expression in the blood vessels of ischemic muscle samples, whereas immunoreactive TSP1 protein, characterized by immunofluorescent tissue imaging, was found in the media (vascular smooth muscle cells) of the vessels. This is of likely clinical import, as TSP1 efficiently limits nitric oxide (NO)-mediated signaling in both endothelial (96) and vascular smooth muscle cells (99). Similarly, TSP1 mRNA was significantly increased in femoral arteries from individuals (mean age, 76.6 ± 3.79 yr) with advanced PAD (57). TSP1 protein was increased in lower limb calf muscle biopsies from individuals (mean age, 64 ± 7 yr) with PAD and decreased ankle brachial index compared with age-matched controls (189). Human carotid arteries, with confirmed alterations in laminar blood flow, showed increased TSP1 expression (114). In atherosclerotic vessels, plaque size correlated positively with TSP1 expression (42). A TSP1 single-nucleotide polymorphism (SNP) was strongly associated with coronary vascular disease in people with familial premature myocardial infarction (213), raising the idea that altered TSP1 activity may accelerate vascular aging. In a cohort of individuals with aortic dissection (mean age, 50.5 yr), TSP1 was found to be increased, particularly at regions of vessel wall disruption (231). In this setting, platelet activation may have contributed to increased TSP1 expression in areas of aortic wall damage. Related to this, vascular TSP1 protein levels were elevated in older individuals (mean age, 71.3 yr) with thoracic aortic aneurism (113).
Age alone and age-related vascular disease also modify TSP1 expression in circulating and noncirculating cells. In platelets from healthy adults, TSP1 was increased compared with platelets from healthy children (32). Individuals with familial hypercholesterolemia, a disease associated with accelerated vascular aging, displayed increased levels of TSP1-positive platelet microparticles (202). Also, individuals with increased arterial vasculopathy, as determined by MRI, had increased levels of circulating platelet-derived microparticles positive for TSP1. It is not clear whether microparticle TSP1 would signal through canonical TSP1 receptors. Conditioned medium from adipocyte-derived MSCs (AMSCs), obtained from individuals with overt coronary artery disease (CAD), maintained TSP1 expression concurrent with a decrease in vascular endothelial growth factor (VEGF) expression compared with AMSC-conditioned medium from individuals without CAD (49). Circulating TSP1 levels were elevated in individuals with both coronary artery disease and type 2 diabetes (mean age, 65.8 ± 9.7 yr) (29). Thrombospondin was increased in plasma from type 1 diabetics, but it is not clear whether this was exclusively TSP1 (14). Hutchinson-Gilford Progeria Syndrome (HGPS) drives accelerated aging, leading to diffuse vasculopathy and a shorted lifespan. Analysis of circulating cells from individuals with HGPS (9–14 yr of age) found increased expression of TSP2 (35).
Hypertension
The role of TSP1 in age-associated hypertension has not been directly addressed. In individuals with essential hypertension, thigh skeletal muscle capillary area and density were decreased compared with normotensive controls, although differences in skeletal muscle TSP1 expression among the cohorts were not detected (62). Related to this, in old (mean age, 65 ± 1 yr) inactive individuals, TSP1 levels in skeletal muscle interstitial fluid remained unchanged after 8 wk of exercise (63). In contrast, skeletal muscle intestinal fluid TSP1 levels were increased in individuals with peripheral arterial disease (mean age, 68 ± 2 yr) compared with similarly aged individuals without overt vascular disease (80). The vascular networks of the skeletal muscle groups of the trunk and extremities provide the primary vascular resistance for maintenance of systemic blood pressure. Although these preliminary studies are indeterminant, the question of whether and how muscle or perimuscle fluid TSP1 modulates vascularity and vascular resistance to modify blood pressure deserves further study. Hypertensive individuals (mean age, 61.24 ± 12.3 yr), treated with the ACE inhibitor Perindopril, had significant elevation in plasma TSP1 compared with untreated hypertensives (22). Conversely, 12 wk of oral vitamin D in normotensive vitamin D-deficient mid-aged adults (mean age, 45 ± 11 yr) resulted in lower blood pressure and markedly (∼2.5-fold) lower plasma TSP1 levels (7). In children with sickle cell disease (SCD), TSP1 levels were increased compared with non-SCD controls. Within the SCD group, those with vaso-occlusive crisis had the highest TSP1 levels, and this varied inversely with vitamin D (3). This is relevant given recent findings of a high incidence (80%) of undiagnosed hypertension and prehypertension in children with SCD (mean age, 11.9 ± 4.5 yr) (154). The finding that vitamin D administration lowered plasma TSP1 levels is intriguing, as adult (163) and pediatric sickle cell patients (66) have increased plasma TSP1 levels and dysregulated blood pressure. It is not clear whether TSP1 contributes to altered blood pressure in these situations.
Myocardial Infarction and Left and Right Heart Failure
Individual homozygotes for the TSP1 SNP N700S were found to be at increased risk for coronary artery disease and premature (≤45 yr of age) myocardial infarction (MI) (213). In a larger cohort, a single allele of the N700S SNP increased the risk of early MI (234). However, in a Chinese Han population, the associated risk in early MI (<60 yr of age) with TSP1 N700S was not found (233). Also, N700S did not increase the overall MI risk (118). Interestingly, the recombinant N700S variant of TSP1 promoted faster, more robust platelet aggregation compared with native TSP1 (159) and bound Ca2+ less (73) but did not alter the von Willebrand Factor (VWF) multimer size (234). In contrast, individuals with SCD were found to have decreased to no ADAMTS13 activity associated with increased circulating TSP1 and vaso-occlusive crisis (162). ADAMTS13 targets VWF multimers for proteolysis, whereas SCD may be associated with early MI (167). Thus, TSP1 and a SNP variant of TSP1 function in diverse ways to promote vascular occlusion. The TSP1 N700S SNP results in decreased Ca2+ binding by the protein’s signature domain and likely alters the shape of the molecule (73), leading to altered receptor interactions and increased protein half-life (2). High circulating TSP1 was associated with increased cardiovascular-related death in individuals (mean age, 64.0 ± 10.4 yr) with end-stage renal disease on dialysis (84).
Cardiac transplantation is an option for individuals with end-stage left heart failure. Cardiac transplants were found to have increased TSP1 mRNA levels, and the persistence of this was associated with worse coronary vasculopathy (232).
Lung Disease
Pulmonary parenchymal TSP1 expression correlated positively with age (146). Pulmonary arterial hypertension (PAH) is characterized by a loss of pulmonary microvasculature (ischemia), increased vascular resistance, and progressive right heart failure. The disease is found predominantly in mid-aged and older individuals and is fatal. Distal pulmonary arteries from PAH subjects (mean age, 58.4 ± 12.6 yr) showed marked induction of TSP1 and CD47 and were less sensitive to vasorelaxants compared with arteries from non-PAH controls (mean age, 36.3 ± 14.8 yr) (187), whereas treating diseased human pulmonary arteries with a CD47-blocking antibody partially restored NO-mediated vasorelaxation (187). Plasma TSP1 levels were also increased in PAH subjects (104). A role for TSP1 in chronic obstructive pulmonary disease (COPD) or emphysema has not been defined. However, both conditions have been linked to TGFβ signaling (28), and TSP1 promotes TGFβ signaling through activating the latent protein (158). Furthermore, COPD is characterized by vascular dysfunction, and loss of NO signaling and can be complicated by PAH. It is possible that TSP1 is contributing to the vasculopathy of COPD and, through its effects on inflammatory cells, contributing to the pulmonary parenchymal disease as well.
Eye Disease
TSP1 was increased in the ocular trabecular meshwork (54) and in blood mononuclear cells and plasma of individuals with glaucoma (100). Conversely, immunoreactive TSP1 expression was decreased in eyes from individuals with age-related macular degeneration (ARMD) compared with control eyes (mean age, 83.9 yr) (17, 215). The investigators proposed that loss of antiangiogenic TSP1 accounted for the abnormal choroidal neovascularization in these cases. The reason for downregulation of TSP1 in ARDM remains unknown, although VEGF and NO signaling are increased in the retinal pigment epithelial cells of ARMD eyes (74), and exogenous NO, in an ERK-dependent manner, suppressed TSP1 mRNA and secreted TSP1 in cultured endothelial cells (182). A proangiogenic proteolytic fragment of TSP1 was also implicated in the pathogenesis of ARMD (26), which might account for the reported loss of immunoreactive TSP1.
As with other proliferative retinal vasculopathies, TSP1 was not detected in the vitreous fluid of eyes from individuals with diabetic retinopathy (DR) (1). Also, COX-2 was increased in retinal cells from individuals (mean age, 66.3 yr) with DR, and inhibiting COX-2 resulted in upregulation of TSP1 (191). Thus, TSP1 appears to limit and promote age-related ocular complications.
Muscle, Joint, and Bone Disease
Sarcopenia, the loss of skeletal muscle mass and function, is common with aging and contributes to loss of bone mass and increased fractures in the elderly (126). Age-related sarcopenia arises in part from a loss of muscle neural innervation. Other factors, including altered endocrine function, metabolism, and nutrition, contribute to age-related sarcopenia. A specific role for TSP1 in motor neuron innervation with aging has not been determined. Skeletal muscle mass and function determines, in part, exercise capacity. Conversely, exercise is known to maintain muscle mass and function in the elderly (20, 172). In young men, exercise training was associated with decreased thigh muscle TSP1 mRNA (79). In contrast, in elderly individuals (mean age, 68 ± 1 yr) with peripheral vascular disease, skeletal muscle TSP1 mRNA did not decrease after exercise (80), suggesting that age-associated skeletal muscle TSP1 expression may be unresolvable by intervention and could contribute to vascular diminution.
TSP1 expression was increased in cartilage from knee joints showing early osteoarthritis but decreased in cases of severe disease (range 59–79 yr) compared with samples from nonarthritic joints (173). TSP1 was also increased in the synovial vessels of arthritic rheumatoid (RA) joints (67) as well as in in the plasma (mean age, 51 ± 8.16 yr) (181) and circulating monocytes from individuals with RA (201). However, others noted that immunoreactive TSP1 was decreased in RA synovial samples (mean age, 56 yr; range 29–82 yr) (145). For comparison control, “normal” synovial samples were obtained from individuals undergoing surgery for ligamentous disruption in the knee, which may account for the disparity in findings in this study. RA-derived fibroblast overexpression of hypoxia inducible factor-1α upregulated TSP1 (83) and a CD47-binding peptide from the COOH terminus of TSP1 decreased human T cell adhesion to RA-derived fibroblasts (216). This is relevant, as RA-derived fibroblasts promote T cell activation and inflammation in RA.
Osteoporosis, whether senile or postmenopausal, is defined as a decrease in bone density and mass and, as with age-related sarcopenia, is associated with increased long bone and lumbar fractures and delayed fracture healing. Several SNPs of the TSP1 type 1 domain were associated with a greater decrease in bone density in Japanese women with osteoporosis (mean age, 72.7 ± 7.3 yr) (155). The type 1 domain of TSP1 can engage CD36, TGFβ, and β1 integrin, all of which have been implicated in the regulation of bone density. As noted, increased vitamin D intake, which itself increases bone density, was associated with decreased plasma TSP1 levels (7). These results are worth replicating and expanding to aged individuals. Diabetes is also associated with osteoporosis, and TSP1 is upregulated in individuals with diabetes (reviewed below).
Obesity, Metabolic Syndrome, and Diabetes
Metabolic syndrome comprises elevated cholesterol/triglycerides, blood glucose, and blood pressure. TSP1 was found in complex with the anti-obesity hormone adiponectin in serum from healthy and diabetic individuals, and this interaction was confirmed via coimmunoprecipitation (225); however, the possible clinical implications of this are unknown. White adipose-derived stromal cells (ADSCs) from obese individuals (mean age, 41.56 ± 3.07 yr; BMI > 40 kg/m2) had increased TSP1 mRNA and decreased angiogenic capacity compared with ADSCs from nonobese individuals (mean age, 38.28 ± 2.48 yr; BMI < 25 kg/m2) (170). Subcutaneous adipose TSP1 mRNA levels were positively associated with obesity and insulin resistance in individuals throughout the aging spectrum (218), whereas in elderly subjects (mean age, 50.4 ± 13.6 yr; mean BMI 29.8 ± 6.7 kg/m2) visceral adipose TSP1 mRNA levels were positively associated with hyperglycemia, hypertension, and abdominal obesity (142). Hypoxia-inducible factor is increased in fat and, paradoxically, drives fibrosis (226) and upregulates TSP1 (123), all of which may promote obesity-related heart failure with preserved ejection fraction (HFpEF). Notably, in individuals with HFpEF elevated plasma TSP2 levels were associated with increased cardiac complications (115). In this same study, circulating TSP1 protein levels were associated with several metabolic syndrome-related abnormalities. However, TSP1 protein levels were assessed in serum (following blood coagulation) thus complicating data interpretation. Regardless, these findings are pertinent since TSP2 can engage CD47, albeit with less affinity than TSP1, to limit NO signaling (89). Mass spectrometry analysis of plasma samples from 439 individuals (mean age, 63 ± 8.5 yr) found that TSP1 positively correlated with prediabetes as defined by elevated fasting (≥ 6.1 mmol/L) and 2-h oral glucose tolerance test blood glucose (220). Diabetes can arise from loss of pancreatic islet β-cells and decreased insulin production and/or decreased peripheral insulin sensitivity. Interestingly, human islets obtained from donors without diabetes (mean age, 50 ± 9 yr; mean BMI 23.6 ± 1.8 kg/m2), when cultured in high glucose (16.7 mmol/L), displayed increased TSP1 and decreased VEGF mRNA and decreased CD31+ cells (an endothelium-specific marker) compared with islets cultured in low glucose (47). Transient suppression of TSP1 in human islets increased palmitate- (39), cytokine-, and endoplasmic reticulum-mediated apoptosis (40). Diabetes is associated with end-organ injury and functional loss. Kidney biopsies from individuals with diabetic nephropathy (mean age, 53.3 yr; range 33–66 yr) (222) confirmed progressive upregulation of TSP1 mRNA in the glomeruli and renal cortex (78). Individuals that are heterozygous for a mutation in β-globin [sickle cell trait (SCT)] and that have type 2 diabetes (T2D) (mean age, 42.4 ± 9.3 yr) experienced greater impairment of cardiovascular dynamics compared with individuals with either condition alone (43). Also, the number of individuals with both SCT and T2D is increasing. As reported, TSP1 is upregulated in the blood and tissues in both conditions.
Cancer
In contrast to most other diseases of aging where TSP1 levels are elevated, malignant progression is often associated with a loss of TSP1 expression (92). One mechanism proposed to explain this inverse correlation is based on the antiangiogenic activity of TSP1. Cancers require angiogenesis to supply nutrients and oxygen for tumor growth, and loss of TSP1 combined with induction of proangiogenic factors can drive angiogenesis. TSP1 expression was lower in triple-native breast cancer patients who relapsed following neoadjuvant chemotherapy (71). In this case, TSP1 signaling via CD47 was implicated in limiting the escape of tumor cells from chemotherapy-induced senescence.
The loss of TSP1 expression is usually the result of altered epigenetic regulation of THBS1 caused by mutated oncogenes or tumor suppressor genes. Several noncoding polymorphisms in the gene have been associated with risk for developing gastric cancer (81). A noncoding polymorphism was similarly associated with risk for developing bladder cancer (70), and another THBS1 polymorphism was associated with risk for prostate cancer in the context of a polymorphism in the angiogenic gene VEGFA (192).
Mutational-Driven Accelerated Aging
Progeriod syndromes, such as Hutchinson-Gilford Progeria and Werner and Cockayne syndrome, manifest in accelerated aging in certain tissues and organs in children and young adults and result in decreased longevity. These diseases are linked to single mutations that lead to increased DNA oxidative modifications that accelerate telomere shortening and cellular senescence. TSP1 has not been interrogated in progeriod syndromes. Yet the role of TSP1 in suppressing cMyc and in promoting permanent cell cycle arrest and senescence, which itself increases telomerase reverse transcriptase activity to lengthen telomeres (223), and in stimulating pathologic reactive oxygen species (ROS) production suggests that it may contribute to accelerated aging in progeriod diseases.
TSP1 IN CHRONOLOGICALLY AGED ANIMALS AND AGE-RELATED DISEASE MODELS
TSP1 in Aged Animals
In 3-mo-old versus 24-mo-old animals (169), age-related expression of TSP1 was associated with decreased renal function and vascularity, fewer proliferating endothelial cells, and less VEGF expression (107). These findings are of some relevance given that in endothelial cells, TSP1, via CD47, limits VEGF and VEGF-R2 signaling (110). The skin of aged 18-mo-old Thbs1- and Cd47-null mice is more vascular and shows enhanced vasoactivity in response to temperature flux and exogenous NO compared with skin from wild-type mice of similar age (186). Age-related loss of vasoactivity and blood flow in skin from wild-type mice was associated with increased TSP1 and CD47 protein and mRNA expression. Consistent with its known role to mediate angiogenesis through inhibition of NO and VEGF signaling, 12-mo-old wild-type mice were found to have less pancreatic islet vascularity compared with islets from similarly aged Thbs1-null mice (45). Interestingly, 12- to 18-mo-old Thbs1-null mice had maintenance of soft tissue blood flow and improved tissue survival under ischemic stress compared with wild-type controls (91). Aged null animals were found to have inherently more of the critical NO second-messenger cGMP, and this was preserved in the face of tissue ischemia. Young 3- to 4.5-mo-old wild-type mice experienced increased endothelial cell TSP1 expression within several days of disruption of femoral artery blood flow (19). Aged 12- to 18-mo-old Cd47-null mice also enjoyed increased soft tissue blood flow under ischemic conditions and, along with Thbs1-nulls, displayed more hindlimb perfusion after acute femoral artery ligation versus aged wild-type mice. Interestingly, aged wild-type and ApoE-null mice, which are subject to accelerated vascular aging, when treated with a translation-blocking CD47-targeting morpholino oligonucleotide, experienced improved tissue survival in response to ischemia (91). Related to this, ApoE-null mice displayed increased TSP1 protein in major arteries (21). TSP1 mRNA levels were found to increase to a greater degree in the fat from LPS-treated mice aged 24-mo-old versus young 4-mo-old mice (198). Mesenchymal stem cells from older 18- to 24-mo-old wild-type mice showed increased TSP1 expression compared with cells from young 1- to 3-mo-old animals (50). At the same time, 12-mo-old Cd47-null mice were found to be lighter and have less body fat than similarly aged wild-type animals (56). In rats, ADSCs from aged 24-mo-old animals had decreased angiogenic activity and increased TSP1 expression compared with cells from young animals (6). Treatment of ADSCs from aged animals with a TSP1-blocking antibody enhanced angiogenic capacity. In aged neonatal cardiac myocytes (4 days versus 21 days), TSP1 mRNA and protein levels were increased, whereas in 26-mo-old C57Bl6 × 129Sv mice, cardiac TSP1 protein levels were increased compared with younger controls (217). This finding was confirmed in 30-mo-old Kunming mice (24), a strain favored in aging studies (36). Aged mice also demonstrated increased levels of the oxidative stress marker malondialdehyde. This finding is important in that TSP1 is an activator of NADPH oxidase to increase pathological ROS production (34, 230). Although TSP1 and CD47 mRNA and protein levels did not change in coronary or cardiac tissue samples in young 4-mo-old versus old 24-mo-old female rats, TSP1 inhibited NO-mediated vasodilation more effectively in coronaries from aged animals. This finding may have been secondary to increased ROS production noted in older vessels exposed to exogenous TSP1 (160). Interestingly, ex vivo treatment of vessels from aged rats with a CD47 blocking antibody attenuated TSP1-driven superoxide production and in aged animals improved coronary blood flow reserve.
TSP1 in Age-Related Disease Models
Blood pressure, pulmonary and systemic hypertension, and heart failure.
A possible role for TSP1 in the regulation of blood pressure under stress was revealed in studies that assessed blood pressure in Thbs1- and Cd47-null mice. When young animals were administered an exogenous NO donor molecule, Thbs1- and Cd47-null mice displayed a greater decrease in mean arterial blood pressure (MABP) compared with wild-type mice (95). Loss of autonomic neural support through pharmacological blockade in the null mice resulted in an exaggerated decrease in systemic blood pressure compared with wild-type controls. Conversely, awake-cycle mean arterial blood pressure was increased in null mice, likely as a compensatory defense against their inherent blood pressure lability. Furthermore, on administration of the endothelial vasodilator acetylcholine, Thbs1-null mice experienced a greater decrease in MABP compared with wild types, whereas an exogenous bolus of TSP1 (22 pmol/g body wt) increased MABP in both null and wild types (12). In 6-mo-old OLETF rats that develop obesity and hypertension, TSP1 was increased in visceral fat (77). In rats, aortic constriction-mediated hypertension was associated with increased angiotensin levels and left ventricular TSP1 expression (15), although these data may have been confounded by streptozotocin (STZ)-mediated hyperglycemia. As both TSP1 and angiotensin II limit NO-mediated activation of soluble guanylyl cyclase (178), an inhibitory effect upon NO signaling is expected. Independent of glycemic dysregulation, it would be valuable to expose aged Thbs1- and Cd47-null mice to angiotensin II and determine cardiac and renal function. Spontaneously hypertensive heart failure rats treated from 2 to 22 mo of age with eplerenone and a standard heart failure program (quinapril-torasemide-carvedilol) developed less heart failure along with suppression of injury-induced TSP1 (156).
Left ventricle heart failure can lead to pulmonary congestion. Chronic hypoxia increases pulmonary vascular resistance and leads to pulmonary hypertension (PH). Thbs1-null mice were resistant to the effects of chronic hypoxia, including pulmonary remodeling and increased right ventricle pressure, and were resistant to acute hypoxia-induced vasoconstriction (165). Treating wild-type mice with a CD47-blocking antibody mitigated hypoxic PH (13). Animals carrying the human sickle gene (BERK mice) develop PH. Interestingly, transplanting bone marrow from BERK mice into Cd47-null mice blocked the development of SCD-associated PH (164). Parenthetically, 13- to 14-mo-old but not young 4-mo-old and intermediate aged 9- to-11-mo-old female BERK mice showed increased pulmonary TSP1 and CD47 expression compared with age-matched hemi-BERK and wild-type animals. Here, too, TSP1 expression increased with age. Aortic constriction (AC) leads to left ventricular (LV) heart failure. Cd47-null mice were protected from AC-mediated LV failure, as were wild-type mice given a CD47-blocking antibody (193).
Vascular disease models.
Age-related models of vasculopathy impart a range of injury through acute and chronic as well as partial and complete alterations in blood flow. Skin grafts are an example of complete disruption in blood flow and, although avascular at first, undergo gradual reperfusion secondary to angiogenesis. Acute ligation of the femoral artery is followed by recruitment of secondary blood vessels and restoration of hindlimb blood flow within 10–14 days in rodents. Transient arterial occlusion of the renal, hepatic, and coronary arteries induces an ischemia-reperfusion (IR) injury. Arterial endothelial disruption, commonly via an intraluminal wire, induces injury that results in arterial remodeling and luminal narrowing. Partial arterial occlusion via an externally applied clip increases vascular resistance and may induce general vasculopathy. Animal models of dysregulated glucose and lipid metabolism and blood pressure are also associated with varying degrees of vasculopathy.
In dogs and 2- to 3-mo-old mice, cardiac IR was associated with increased TSP1 expression at the infarct border zones (55). Interestingly, mortality and infarct size, but not LV remodeling, were the same between wild-type and Thbs1-null mice following cardiac IR. In Cnr2-null mice, IR-mediated cardiac induction of TSP1 protein and mRNA at the infarct boarder zone was diminished, and this was associated with adverse remodeling and decreased cardiac function (48). Likewise, in renal (60, 209, 228) and liver (93) IR, TSP1 expression was increased and associated with greater organ injury. Knockdown of miR-21 resulted in increased TSP1 expression and worse renal function after IR (228). In mice, cerebral IR resulted in TSP1 overexpression concurrent with decreased VEGF levels (75). In 2- to 2.5-mo-old rats exposed to cerebral IR, TSP1 expression peaked early in endothelial cells and later in neuronal cells (133). Lack of TSP1 (90) or CD47 (93) or interruption of TSP1-CD47 signaling (93) mitigated soft tissue and visceral organ IR injury and was associated with decreased levels of pathological ROS, accelerated restoration of blood flow, and increased OSKM self-renewal signaling (188). Furthermore, TSP1 was increased in the hindlimb skeletal muscle of 2- to 3-mo-old wild-type mice following femoral artery ligation (31). Lack of TSP1-CD47 signaling, but not TSP1-CD36 signaling, in mice resulted in improved soft tissue and hindlimb tissue survival following fixed ischemia. Treatment of wild-type mice with a TSP1 or CD47 blocking antibody or a CD47-targeting morpholino oligonucleotide maintained tissue viability after permanent vascular disruption (97). Nitrite, in combination with a TSP1-blocking antibody or a CD47-suppressing morpholino oligonucleotide, further enhanced ischemic soft tissue survival versus solo therapy (98). Skin grafts displayed more complete healing when grafted onto wound beds in mice lacking TSP1 or CD47 and when grafted on wound beds in wild-type mice that were treated with a CD47 blocking antibody or CD47 morpholino oligonucleotide (94). TSP1 engaged cell receptor SIRPα to promote renal IR (230), and 2.5- to 3-mo-old mice lacking functional SIRPα were protected from renal IR (61). These results are intriguing, particularly as mice lacking functional SIRPα still produce TSP1 and CD47, suggesting that CD47-SIRPα cross-talk may mediate certain TSP1 signals. Lack of endothelial Dicer, a gene involved in miRNA regulation, led to decreased hindlimb blood flow and greater tissue injury following femoral artery resection, whereas in endothelial cells, knockdown of Dicer increased TSP1 expression and decreased VEGF sensitivity (203). Conversely, following femoral artery ligation, ischemia-mediated induction of TSP1 protein in calf muscle was suppressed in 2.5- to 3-mo-old endothelial Foxo1-null mice, and this was associated with improved blood flow compared with controls (189). Collagen matrix seeded with adipose stromal cells (ASC) from aged rats treated with a TSP1-blocking antibody displayed more angiogenesis when transplanted into subcutaneous wounds compared with ASC-seeded implants treated with a control antibody. At the same time, stromal cells from aged animals showed increased TSP1 expression versus similar cells from young animals (6). Related to this, carotid arteries from wild-type mice subjected to partial ligation developed more stiffening compared with arteries from Thbs1-null mice. Partially ligated wild-type vessels had increased expression of TSP1 compared with controls. Although interesting, results in rodent vessels are confounded by the fact that vessel ligation is likely to induce hypoxia, a known activator of the TSP1 promoter, in the adjacent arterial wall.
Obesity, metabolic syndrome, and diabetes disease models.
In wild-type mice on a high-fat diet from 1.25 to 5 mo of age, and in 3.75-mo-old ob/ob mice that spontaneously develop obesity, TSP1 expression increased in subcutaneous adipose tissue (221). Fed a high-fat diet (HFD) for 14 wk, 2-mo-old male wild-type and Thbs1-null mice gained weight at the same rate and degree, but null mice were more glucose tolerant and insulin sensitive (131). Conversely, male and female Thbs1-null mice showed less weight gain after 5 mo on an HFD or high-carbohydrate/low-fat diet compared with wild-type mice (119), indicating that the effects of TSP1 on fat-induced weight gain may be time dependent. Obesity-related renal impairment was less in 6-mo-old Thbs1- (37) and Cd36-null mice (38). In 10-mo-old animals with targeted deletion of Thbs1 in immune cells, but not adipocytes, TSP1 provided protection from HFD-induced inflammation and insulin resistance (148). Finally, 6-mo-old Cd47-null mice had less weight gain when fed an HFD compared with wild types (137). In contrast to the above, 2.3- to 3-mo-old Thbs1-null mice were found to be glucose intolerant and had lower basal and stimulated insulin levels despite greater islet mass (168). Within the pancreas of 1.75- to 3-mo old Zucker diabetic fatty rats, the number of islet endothelial cells decreased with time as TSP1 mRNA increased (130). In 2.5- to 3.75-mo-old mice, streptozotocin (STZ)-induced diabetes was associated with increased calf muscle TSP1 mRNA that did not diminish after exercise (116). Two-month-old diabetic NOS3-null mice displayed increased renal TSP1 that correlated positively with tubular damage (120), not unexpected findings given that NO decreases TSP1 expression, whereas high glucose increases it. Additionally, 8-mo-old Thbs1-null mice were resistant to STZ-mediated renal damage (41). Also, treatment of 3.25- to 4.25-mo-old diabetic Akita mice with a peptide derived from TSP1, that limits TFG-β activation, was associated with decreased proteinuria (135). Interestingly, 3-mo-old Akita/Thbs1-null mice were found, by measuring renal nicotinamide adenine dinucleotide and flavin adenine dinucleotide, to have dysregulated metabolism (138). In this study, changes in creatinine, blood urea nitrogen, and renal histology would have been helpful to confirm organ damage. Diabetic 4.75-mo-old db/db mice treated with dRK6, which binds VEGFA, were found to have increased immunoreactive cardiac TSP1 compared to controls (171). This is of interest since heart failure is increased in diabetics, and loss of VEGF signaling and VEGF-driven NO production promotes TSP1 upregulation. Biobreeding rats, which develop autoimmune diabetes, had loss of skeletal muscle capillaries concurrent with increased muscle TSP1 protein (5). Also, there was a positive correlation in these animals between the degree of hyperglycemia and the amount of muscle TSP1 protein. Vascular smooth muscle cell TSP1 protein and mRNA were increased in thoracic aortas from 7.25-mo-old diabetic rats, and this was associated with increased transmural fibrosis and decreased vessel compliance (204). The vaso vasorum traverses the arterial wall to supply oxygen and nutrients to major conduit arteries. Transmural vasa vasorum were decreased in aortas from 2.25- to 3-mo-old diabetic rats, whereas TSP1 was increased (200). These data suggest that TSP1 may promote metabolic syndrome, diabetes, and diabetic-associated vascular complications.
Muscle, joint, and bone disease models.
Mitochondria size and number were increased in calf muscle samples from 3-mo-old male Thbs1- and Cd47-null mice compared with control samples, and these changes translated into greater running endurance in Cd47-null animals (56). However, by 12 mo of age, muscle mitochondrial changes in null mice, compared with wild types, were no longer evident. Also, mitochondria from Cd47-null vascular smooth muscle cells produced less ROS. Muscle wasting has been studied in rodents employing activity (loading) and inactivity (unloading). When unloaded, hindlimb skeletal muscle TSP1 (105) and receptor CD36 (106) increased, whereas VEGF decreased compared with expression levels in loaded muscle groups (105), suggesting that activity limits TSP1 signaling in skeletal muscle. These data are of further relevance as decreased activity is a feature of aged 18- to 24-mo-old mice (85) and people. In rats with adjuvant-driven arthritis, ankle swelling was increased by direct administration of exogenous TSP1 (117). In line with TSP1 promoting inflammation, rats given a TSP1-derived peptide daily experienced less peptidoglycan/polysaccharide-mediated joint swelling and inflammation, which was associated with decreased plasma TSP1 levels (140). In contrast, 2.5-mo-old rats given collagen/Freund’s incomplete adjuvant and a TSP1 overexpressing viral vector had less arthritis compared with those given a control vector (102). This same group reported that knee osteoarthritis following surgical injury to the anterior cruciate ligament was decreased in 2-mo-old rats treated locally with a TSP1 viral vector (82). These contradictory results may be the result of the different arthritis models. Study duration may also account for these variations, as the proinflammatory and antiangiogenic actions of TSP1 evolve differently.
Bone mass loss and delayed fracture healing are endemic changes found in older individuals. Although a role for TSP1 in this regard has not been widely studied, preliminary results are available. Mice null for TSP2 had faster fracture healing, increased wound vascularity and cell proliferation, and more final bone mass compared with controls (149). Likewise, 2- to 8-mo-old Thbs1- and 2-mo-old Cd47-null mice had denser long bones compared with wild types (8, 214), and Thbs1- and Cd47-null osteoclast function was deemed less. This latter finding was ascribed to variations in NO signaling and loss of CD47-SIRPα signaling (136).
Eye disease models.
TSP1 was found in the aqueous humor outflow tract of murine eyes primarily in the extracellular areas of the juxtacanalicular (cribriform) part of the trabecular meshwork (54), whereas 1.75- to 2.75-mo-old Thbs1- and Thbs2-null mice had lower intraocular pressures (72). Induction of experimental glaucoma in 6.5-mo-old mice, secondary to anterior chamber injection, increased ocular TSP1 expression (166).
TSP1 IN ACCELERATED AGING ASSOCIATED WITH GENOTOXIC STRESS
Increased exposure to solar and cosmic radiation during space flight and life in planetary orbit induces genomic mutations and redox stress, resulting in physiological changes similar to accelerated aging (219). Wild-type mice in orbit for 13 days had increased pulmonary TSP1 expression and lung remodeling compared with controls maintained in an earth-based vivarium (69). It would be of interest to see whether the space flight-associated upregulation of TSP1 subsided with the return to Earth. The role of TSP1 in adaptation to this mutagenic environment merits further investigation. Similarly, premature aging of cancer patients is associated with the genotoxic stress caused by radiation and chemotherapy. In addition to the known protection mediated by enhancing autophagy and metabolic reprogramming (52, 153, 196, 197), therapeutic targeting of TSP1-CD47 may protect noncancer cells and organs by enhancing the DNA damage response (111).
TSP1 AND CIRCADIAN RHYTHMS
Diurnal cycles (circadian rhythms) in physiological processes are controlled by genes encoding circadian locomotor output cycle kaput (Clock) and aryl hydrocarbon receptor nuclear translocase-like protein (Bmal1) (185). Furthermore, circadian rhythms are dysregulated with aging (143), and this may promote age-related diseases. In a cohort of young, healthy individuals (mean age 26.5 ± 8 yr; BMI 23.2 ± 2.5 kg/m2), plasma TSP1 was measured every 2 h over 24 h (206). Consistent with circadian regulation, plasma TSP1 levels were lower in the morning and higher at night, peaking between 8 and 10 PM, before subsiding again to an early morning nadir. Coincidently, individuals with obstructive sleep apnea experience a peak in MI onset at night (121). Human arterial endothelial cells exposed to intermittent hypoxic perfusion, as a mimic of sleep apnea, showed increased TSP1 expression (175). These data indicate that circulating TSP1 levels follow a circadian rhythm and encourage additional study. Many cardiovascular parameters are also variant along circadian periods. For example, sensitivity to an endothelial nitric oxide synthase (eNOS)-stimulated increase in blood flow is less in evening hours (194). Furthermore, in healthy individuals, plasma TSP1 levels, although still maintaining a circadian rhythm, were found to increase significantly (2.5-fold) following administration of insulin (206). These findings deserve follow-up, as TSP1 promotes insulin resistance in animals (86). After 3 wk on an HFD, 2.75-mo-old Thbs1-null mice had a more balanced circadian rhythm compared with wild types (86). In cell-based systems, self-renewal transcription factors such as OSKM have been found to control and be controlled by clock genes. Expression of OSKM in differentiated cells resulted in loss of circadian clock genes (229), whereas expression of the clock gene Period 2 suppressed cMyc (227). Conversely, loss of Sox2 in certain brain neurons was associated with decreased clock gene expression (27). Given the link between TSP1, CD47, and the OSKM factors, it is possible that TSP1 is both an effector and target of circadian cycle dysregulation.
PARADOXICAL FINDINGS AND FURTHER AREAS OF INQUIRY
A growing body of clinical, translational, and basic science research supports a role of TSP1 in promoting aging and age-related diseases. One aspect of all of the reported work is the absence of analysis of sex difference in this process. Women outlive men and, for a substantial period of their adult lives, have a lower rate of cardiovascular disease. Hormonal differences are thought to be a key factor in the delayed rate of cardiovascular disease in women compared with men. TSP1 expression is suppressed by exogenous estrogen (207) but increased in endometrial samples from women 29–74 yr of age stimulated by progesterone (88), providing a rationale for testing in animal and clinical studies the hypothesis that sex hormone status impacts age-related TSP1 effects.
Nonetheless, it is unclear what factors drive the inappropriate induction of TSP1 signaling with aging. The THBS1 promoter is sensitive to several factors known to change with aging. TSP1 is increased by factors that increase with age, including ROS, glucose, and hypoxia. At the same time, TSP1 stimulates ROS, limits NO to decrease blood flow, promotes hypoxia and vascular pruning, and encourages metabolic syndrome and glucose intolerance. Thus, with aging, alterations in any or several of these moieties may provide a stimulus for increased TSP1 that, in a feed-forward manner, could amplify the process and provoke further TSP1 expression. Still, this begs the question as to why compensatory pathways cannot modulate and curtail age-related increases in TSP1. Secreted TSP1 is internalized and removed from the extracellular space through the interaction of the NH2-terminal domain of the protein with cell surface low-density lipoprotein receptor-related protein (LRP1) (150). Data indicate that LRP1 expression is decreased with aging (53, 177). This may prevent normal healthy cell clearance of secreted TSP1 and contribute to the progressive age-related increase in TSP1. Alternatively, age-related fixed epigenetic changes, such as decreased DNA methylation, may undermine homeostatic mechanisms and increase THBS1 promoter activity (124). These questions are worth further study.
While lack of TSP1 and CD47 confers protection from obesity and hyperglycemia in animals, these findings should be studied independently of each other and in people. In addition to the known regulation of TSP1 expression by circulating glucose, it would be interesting to examine whether excess calories or fat alone can increase TSP1 expression. Complementary studies should consider the role of healthy dietary and activity modifications upon existent and potential age-related TSP1 signaling.
CONCLUSIONS
Aging involves multiple organ systems and is mediated by dysregulation of multiple molecular pathways. Interpretation of existing data from animal models of age-related diseases is often limited by a lack of precise data for animal ages. The increased emphasis on transparency in scientific data presentation will hopefully correct this deficiency going forward. Although no single gene or environmental factor can be ascribed primacy, consistent changes in TSP1 expression have been identified in multiple age-related pathologies. Age-related increases in tissue TSP1 levels contribute to the dysregulation of multiple physiological processes, including angiogenesis, stem cell self-renewal, metabolism, immunity, and blood flow. Elevated TSP1 also contributes to ischemia, inflammation, and overproduction of ROS (Fig. 3). Thus, TSP1 merits further consideration as an important mediator of aging and age-related diseases. Conversely, the ability of agents that limit the TSP1/CD47 signaling axis to mitigate effects of aging in animal models and human cells supports the further evaluation of therapeutics targeting TSP1 and CD47 for treating age-related diseases. Therapeutics targeting CD47 are currently in multiple clinical trials for enhancing antitumor immunity (141, 157). Such agents could potentially be repurposed to treat nonmalignant diseases of aging.
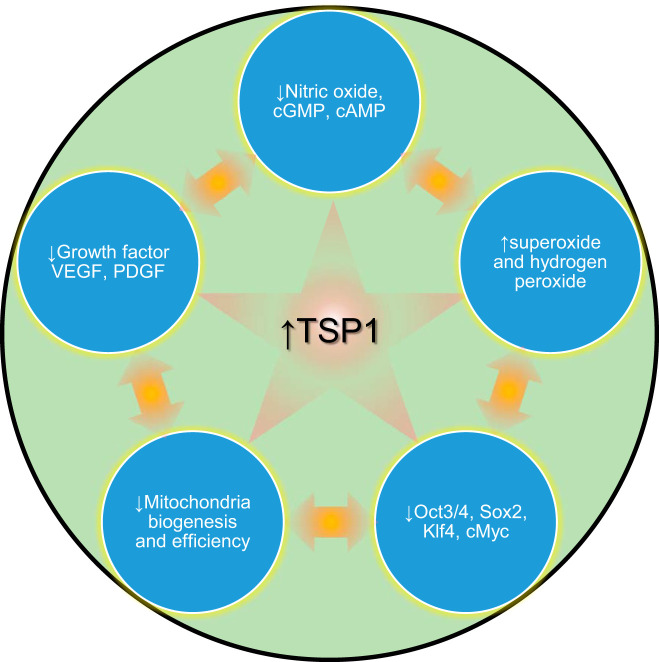
Fig. 3.
Age-related induction of thrombospondin-1 (TSP1) limits key survival and renewal pathways to propel the aging wheel. Age increases TSP1 expression widely, directly limiting 1) biogas nitric oxide (NO) and downstream intermediates cGMP and cAMP, 2) growth factors VEGF/PDGF, and 3) self-renewal transcription factors octamer-binding transcription factor 3/4 (Oct3/4), sex-determining region Y-box 2 (Sox2), Kruppel like factor 4 (Klf4), and V-myc myelocytomatosis viral oncogene homolog (cMyc; OSKM). Age-related overactive TSP1 directly stimulates increased NADPH-oxidase production of reactive oxygen species (ROS), which then act to impede these same survival/renewal moieties. Further, TSP1, in part via negative effects on mitochondria, dysregulates glucose homeostasis and metabolism.
GRANTS
This work was supported by the Intramural Research Program of the NIH/National Cancer Institute (ZIA SC009172).
DISCLOSURES
J.S.I. serves as Chief Science Officer of Radiation Control Technologies, Inc. J.S.I. and D.D.R. are co-inventors of CD47 technology patents assigned to the United States of America.
AUTHOR CONTRIBUTIONS
J.S.I. and D.D.R. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Abu El-Asrar AM, Nawaz MI, Kangave D, Siddiquei MM, Ola MS, Opdenakker G. Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol 50: 545–551, 2013. doi: 10.1007/s00592-011-0330-9. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC, Bentley AA, Kvansakul M, Hatherley D, Hohenester E. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J Cell Sci 121: 784–795, 2008. doi: 10.1242/jcs.021006. [DOI] [PubMed] [Google Scholar]
- 3.Adegoke SA, Smith OS, Adeniyi AT, Adekile AD. Thrombospondin-1 and vitamin D in children with sickle cell anemia. J Pediatr Hematol Oncol 41: e525–e529, 2019. doi: 10.1097/MPH.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 4.Agah A, Kyriakides TR, Letrondo N, Björkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol 22: 539–547, 2004. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Aiken J, Mandel ER, Riddell MC, Birot O. Hyperglycaemia correlates with skeletal muscle capillary regression and is associated with alterations in the murine double minute-2/forkhead box O1/thrombospondin-1 pathway in type 1 diabetic BioBreeding rats. Diab Vasc Dis Res 16: 28–37, 2019. doi: 10.1177/1479164118805928. [DOI] [PubMed] [Google Scholar]
- 6.Aird AL, Nevitt CD, Christian K, Williams SK, Hoying JB, LeBlanc AJ. Adipose-derived stromal vascular fraction cells isolated from old animals exhibit reduced capacity to support the formation of microvascular networks. Exp Gerontol 63: 18–26, 2015. doi: 10.1016/j.exger.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amarasekera AT, Assadi-Khansari B, Liu S, Black M, Dymmott G, Rogers NM, Sverdlov AL, Horowitz JD, Ngo DTM. Vitamin D supplementation lowers thrombospondin-1 levels and blood pressure in healthy adults. PLoS One 12: e0174435, 2017. doi: 10.1371/journal.pone.0174435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amend SR, Uluckan O, Hurchla M, Leib D, Novack DV, Silva M, Frazier W, Weilbaecher KN. Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts. J Bone Miner Res 30: 106–115, 2015. doi: 10.1002/jbmr.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol 152: 448–455, 2008. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagavandoss P, Wilks JW. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun 170: 867–872, 1990. doi: 10.1016/0006-291X(90)92171-U. [DOI] [PubMed] [Google Scholar]
- 11.Bailey DuBose K, Zayzafoon M, Murphy-Ullrich JE. Thrombospondin-1 inhibits osteogenic differentiation of human mesenchymal stem cells through latent TGF-β activation. Biochem Biophys Res Commun 422: 488–493, 2012. doi: 10.1016/j.bbrc.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88: 471–481, 2010. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 93: 682–693, 2012. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayraktar M, Dündar S, Kirazli S, Teletar F. Platelet factor 4, beta-thromboglobulin and thrombospondin levels in type I diabetes mellitus patients. J Int Med Res 22: 90–94, 1994. doi: 10.1177/030006059402200204. [DOI] [PubMed] [Google Scholar]
- 15.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol 171: 777–789, 2007. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belotti D, Capelli C, Resovi A, Introna M, Taraboletti G. Thrombospondin-1 promotes mesenchymal stromal cell functions via TGFβ and in cooperation with PDGF. Matrix Biol 55: 106–116, 2016. doi: 10.1016/j.matbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Bhutto IA, Uno K, Merges C, Zhang L, McLeod DS, Lutty GA. Reduction of endogenous angiogenesis inhibitors in Bruch’s membrane of the submacular region in eyes with age-related macular degeneration. Arch Ophthalmol 126: 670–678, 2008. doi: 10.1001/archopht.126.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130: 503–506, 1995. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bréchot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Bréchot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One 3: e3950, 2008. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol (1985) 69: 1725–1733, 1990. doi: 10.1152/jappl.1990.69.5.1725. [DOI] [PubMed] [Google Scholar]
- 21.Bu DX, Rai V, Shen X, Rosario R, Lu Y, D’Agati V, Yan SF, Friedman RA, Nuglozeh E, Schmidt AM. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res 106: 1040–1051, 2010. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buda V, Andor M, Petrescu L, Cristescu C, Baibata DE, Voicu M, Munteanu M, Citu I, Muntean C, Cretu O, Tomescu MC. Perindopril induces TSP-1 expression in hypertensive patients with endothelial dysfunction in chronic treatment. Int J Mol Sci 18: 348, 2017. doi: 10.3390/ijms18020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buée L, Hof PR, Roberts DD, Delacourte A, Morrison JH, Fillit HM. Immunohistochemical identification of thrombospondin in normal human brain and in Alzheimer’s disease. Am J Pathol 141: 783–788, 1992. [PMC free article] [PubMed] [Google Scholar]
- 24.Cai H, Yuan Z, Fei Q, Zhao J. Investigation of thrombospondin-1 and transforming growth factor-β expression in the heart of aging mice. Exp Ther Med 3: 433–436, 2012. doi: 10.3892/etm.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 75: 685–705, 2013. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CY, Melo E, Jakob P, Friedlein A, Elsässer B, Goettig P, Kueppers V, Delobel F, Stucki C, Dunkley T, Fauser S, Schilling O, Iacone R. N-Terminomics identifies HtrA1 cleavage of thrombospondin-1 with generation of a proangiogenic fragment in the polarized retinal pigment epithelial cell model of age-related macular degeneration. Matrix Biol 70: 84–101, 2018. doi: 10.1016/j.matbio.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Cheng AH, Bouchard-Cannon P, Hegazi S, Lowden C, Fung SW, Chiang CK, Ness RW, Cheng HM. SOX2-dependent transcription in clock neurons promotes the robustness of the central circadian pacemaker. Cell Rep 26: 3191–3202.e8, 2019. doi: 10.1016/j.celrep.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CH, Chuang CH, Liu SL. Transforming growth factor-β1 and tumor necrosis factor-α are associated with clinical severity and airflow limitation of COPD in an additive manner. Hai 192: 95–102, 2014. doi: 10.1007/s00408-013-9520-2. [DOI] [PubMed] [Google Scholar]
- 29.Choi KY, Kim DB, Kim MJ, Kwon BJ, Chang SY, Jang SW, Cho EJ, Rho TH, Kim JH. Higher plasma thrombospondin-1 levels in patients with coronary artery disease and diabetes mellitus. Korean Circ J 42: 100–106, 2012. doi: 10.4070/kcj.2012.42.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong M, Yin T, Chen R, Xiang H, Yuan L, Ding Y, Pan CC, Tang Z, Alexander PB, Li QJ, Wang XF. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep 19: e45274, 2018. doi: 10.15252/embr.201745274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu LH, Vijay CG, Annex BH, Bader JS, Popel AS. PADPIN: protein-protein interaction networks of angiogenesis, arteriogenesis, and inflammation in peripheral arterial disease. Physiol Genomics 47: 331–343, 2015. doi: 10.1152/physiolgenomics.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cini C, Yip C, Attard C, Karlaftis V, Monagle P, Linden M, Ignjatovic V. Differences in the resting platelet proteome and platelet releasate between healthy children and adults. J Proteomics 123: 78–88, 2015. doi: 10.1016/j.jprot.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43: 748–759, 2014. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csányi G, Yao M, Rodríguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 32: 2966–2973, 2012. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csoka AB, English SB, Simkevich CP, Ginzinger DG, Butte AJ, Schatten GP, Rothman FG, Sedivy JM. Genome-scale expression profiling of Hutchinson-Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell 3: 235–243, 2004. doi: 10.1111/j.1474-9728.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 36.Cui LB, Zhou XY, Zhao ZJ, Li Q, Huang XY, Sun FZ. The Kunming mouse: as a model for age-related decline in female fertility in human. Zygote 21: 367–376, 2013. doi: 10.1017/S0967199412000123. [DOI] [PubMed] [Google Scholar]
- 37.Cui W, Maimaitiyiming H, Qi X, Norman H, Wang S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am J Physiol Renal Physiol 305: F871–F880, 2013. doi: 10.1152/ajprenal.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui W, Maimaitiyiming H, Zhou Q, Norman H, Zhou C, Wang S. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim Biophys Acta 1852: 1323–1333, 2015. doi: 10.1016/j.bbadis.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunha DA, Cito M, Carlsson PO, Vanderwinden JM, Molkentin JD, Bugliani M, Marchetti P, Eizirik DL, Cnop M. Thrombospondin 1 protects pancreatic β-cells from lipotoxicity via the PERK-NRF2 pathway. Cell Death Differ 23: 1995–2006, 2016. doi: 10.1038/cdd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunha DA, Cito M, Grieco FA, Cosentino C, Danilova T, Ladrière L, Lindahl M, Domanskyi A, Bugliani M, Marchetti P, Eizirik DL, Cnop M. Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J Biol Chem 292: 14977–14988, 2017. doi: 10.1074/jbc.M116.769877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes 56: 2982–2989, 2007. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 42.Della-Morte D, Beecham A, Dong C, Wang L, McClendon MS, Gardener H, Blanton SH, Sacco RL, Rundek T. Association between variations in coagulation system genes and carotid plaque. J Neurol Sci 323: 93–98, 2012. doi: 10.1016/j.jns.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaw M, Pialoux V, Martin C, Samb A, Diop S, Faes C, Mury P, Sall Diop N, Diop SN, Ranque B, Mbaye MN, Key NS, Connes P. Sickle cell trait worsens oxidative stress, abnormal blood rheology, and vascular dysfunction in type 2 diabetes. Diabetes Care 38: 2120–2127, 2015. doi: 10.2337/dc15-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drott CJ, Olerud J, Emanuelsson H, Christoffersson G, Carlsson PO. Sustained beta-cell dysfunction but normalized islet mass in aged thrombospondin-1 deficient mice. PLoS One 7: e47451, 2012. doi: 10.1371/journal.pone.0047451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du J, Klein JD, Hassounah F, Zhang J, Zhang C, Wang XH. Aging increases CCN1 expression leading to muscle senescence. Am J Physiol Cell Physiol 306: C28–C36, 2014. doi: 10.1152/ajpcell.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubois S, Madec AM, Mesnier A, Armanet M, Chikh K, Berney T, Thivolet C. Glucose inhibits angiogenesis of isolated human pancreatic islets. J Mol Endocrinol 45: 99–105, 2010. doi: 10.1677/JME-10-0020. [DOI] [PubMed] [Google Scholar]
- 48.Duerr GD, Heinemann JC, Gestrich C, Heuft T, Klaas T, Keppel K, Roell W, Klein A, Zimmer A, Velten M, Kilic A, Bindila L, Lutz B, Dewald O. Impaired border zone formation and adverse remodeling after reperfused myocardial infarction in cannabinoid CB2 receptor deficient mice. Life Sci 138: 8–17, 2015. doi: 10.1016/j.lfs.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Efimenko A, Dzhoyashvili N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V, Parfyonova Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med 3: 32–41, 2014. doi: 10.5966/sctm.2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med 9: 10, 2011. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol 207: 358–366, 2005. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 52.Feliz-Mosquea YR, Christensen AA, Wilson AS, Westwood B, Varagic J, Meléndez GC, Schwartz AL, Chen QR, Mathews Griner L, Guha R, Thomas CJ, Ferrer M, Merino MJ, Cook KL, Roberts DD, Soto-Pantoja DR. Combination of anthracyclines and anti-CD47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat 172: 69–82, 2018. doi: 10.1007/s10549-018-4884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Field PA, Gibbons GF. Decreased hepatic expression of the low-density lipoprotein (LDL) receptor and LDL receptor-related protein in aging rats is associated with delayed clearance of chylomicrons from the circulation. Metabolism 49: 492–498, 2000. doi: 10.1016/S0026-0495(00)80014-1. [DOI] [PubMed] [Google Scholar]
- 54.Flügel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lüssen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res 79: 649–663, 2004. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 111: 2935–2942, 2005. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 56.Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol 30: 154–161, 2011. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu S, Zhao H, Shi J, Abzhanov A, Crawford K, Ohno-Machado L, Zhou J, Du Y, Kuo WP, Zhang J, Jiang M, Jin JG. Peripheral arterial occlusive disease: global gene expression analyses suggest a major role for immune and inflammatory responses. BMC Genomics 9: 369, 2008. doi: 10.1186/1471-2164-9-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao JB, Tang WD, Wang HX, Xu Y. Predictive value of thrombospondin-1 for outcomes in patients with acute ischemic stroke. Clin Chim Acta 450: 176–180, 2015. doi: 10.1016/j.cca.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Gao Q, Chen K, Gao L, Zheng Y, Yang YG. Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis 7: e2368, 2016. doi: 10.1038/cddis.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geng X, Xu X, Fang Y, Zhao S, Hu J, Xu J, Jia P, Ding X, Teng J. Effect of long non-coding RNA growth arrest-specific 5 on apoptosis in renal ischaemia/reperfusion injury. Nephrology (Carlton) 24: 405–413, 2019. doi: 10.1111/nep.13476. [DOI] [PubMed] [Google Scholar]
- 61.Ghimire K, Chiba T, Minhas N, Meijles DN, Lu B, O’Connell P, Rogers NM. Deficiency in SIRP-α cytoplasmic recruitment confers protection from acute kidney injury. FASEB J 33: 11528–11540, 2019. doi: 10.1096/fj.201900583R. [DOI] [PubMed] [Google Scholar]
- 62.Gliemann L, Buess R, Nyberg M, Hoppeler H, Odriozola A, Thaning P, Hellsten Y, Baum O, Mortensen SP. Capillary growth, ultrastructure remodelling and exercise training in skeletal muscle of essential hypertensive patients. Acta Physiol (Oxf) 214: 210–220, 2015. doi: 10.1111/apha.12501. [DOI] [PubMed] [Google Scholar]
- 63.Gliemann L, Olesen J, Biensø RS, Schmidt JF, Akerstrom T, Nyberg M, Lindqvist A, Bangsbo J, Hellsten Y. Resveratrol modulates the angiogenic response to exercise training in skeletal muscles of aged men. Am J Physiol Heart Circ Physiol 307: H1111–H1119, 2014. doi: 10.1152/ajpheart.00168.2014. [DOI] [PubMed] [Google Scholar]
- 64.Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Hörmann K, Riedel F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med 21: 271–279, 2008. doi: 10.3892/ijmm.21.3.271. [DOI] [PubMed] [Google Scholar]
- 65.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 87: 6624–6628, 1990. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol 83: 15–18, 2008. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gotis-Graham I, Hogg PJ, McNeil HP. Significant correlation between thrombospondin 1 and serine proteinase expression in rheumatoid synovium. Arthritis Rheum 40: 1780–1787, 1997. doi: 10.1002/art.1780401009. [DOI] [PubMed] [Google Scholar]
- 68.Graja A, Garcia-Carrizo F, Jank AM, Gohlke S, Ambrosi TH, Jonas W, Ussar S, Kern M, Schürmann A, Aleksandrova K, Blüher M, Schulz TJ. Loss of periostin occurs in aging adipose tissue of mice and its genetic ablation impairs adipose tissue lipid metabolism. Aging Cell 17: e12810, 2018. doi: 10.1111/acel.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gridley DS, Mao XW, Tian J, Cao JD, Perez C, Stodieck LS, Ferguson VL, Bateman TA, Pecaut MJ. Genetic and apoptotic changes in lungs of mice flown on the STS-135 mission in space. In Vivo 29: 423–433, 2015. [PubMed] [Google Scholar]
- 70.Gu J, Tao J, Yang X, Li P, Yang X, Qin C, Cao Q, Cai H, Zhang Z, Wang M, Gu M, Lu Q, Yin C. Effects of TSP-1-696 C/T polymorphism on bladder cancer susceptibility and clinicopathologic features. Cancer Genet 207: 247–252, 2014. doi: 10.1016/j.cancergen.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 71.Guillon J, Petit C, Moreau M, Toutain B, Henry C, Roché H, Bonichon-Lamichhane N, Salmon JP, Lemonnier J, Campone M, Verrièle V, Lelièvre E, Guette C, Coqueret O. Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment. Cell Death Dis 10: 199, 2019. doi: 10.1038/s41419-019-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haddadin RI, Oh DJ, Kang MH, Villarreal G Jr, Kang JH, Jin R, Gong H, Rhee DJ. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci 53: 6708–6717, 2012. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hannah BL, Misenheimer TM, Pranghofer MM, Mosher DF. A polymorphism in thrombospondin-1 associated with familial premature coronary artery disease alters Ca2+ binding. J Biol Chem 279: 51915–51922, 2004. doi: 10.1074/jbc.M409632200. [DOI] [PubMed] [Google Scholar]
- 74.Hattenbach LO, Falk B, Nürnberger F, Koch FH, Ohrloff C. Detection of inducible nitric oxide synthase and vascular endothelial growth factor in choroidal neovascular membranes. Ophthalmologica 216: 209–214, 2002. doi: 10.1159/000059634. [DOI] [PubMed] [Google Scholar]
- 75.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23: 166–180, 2003. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 76.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621, 1961. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 77.Hida K, Wada J, Zhang H, Hiragushi K, Tsuchiyama Y, Shikata K, Makino H. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res 41: 1615–1622, 2000. [PubMed] [Google Scholar]
- 78.Hohenstein B, Daniel C, Hausknecht B, Boehmer K, Riess R, Amann KU, Hugo CP. Correlation of enhanced thrombospondin-1 expression, TGF-beta signalling and proteinuria in human type-2 diabetic nephropathy. Nephrol Dial Transplant 23: 3880–3887, 2008. doi: 10.1093/ndt/gfn399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoier B, Passos M, Bangsbo J, Hellsten Y. Intense intermittent exercise provides weak stimulus for vascular endothelial growth factor secretion and capillary growth in skeletal muscle. Exp Physiol 98: 585–597, 2013. doi: 10.1113/expphysiol.2012.067967. [DOI] [PubMed] [Google Scholar]
- 80.Hoier B, Walker M, Passos M, Walker PJ, Green A, Bangsbo J, Askew CD, Hellsten Y. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol (1985) 115: 1777–1787, 2013. doi: 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong BB, Chen SQ, Qi YL, Zhu JW, Lin JY. Association of THBS1 rs1478605 T>C in 5′-untranslated regions with the development and progression of gastric cancer. Biomed Rep 3: 207–214, 2015. doi: 10.3892/br.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsieh JL, Shen PC, Shiau AL, Jou IM, Lee CH, Wang CR, Teo ML, Wu CL. Intraarticular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis. J Orthop Res 28: 1300–1306, 2010. doi: 10.1002/jor.21134. [DOI] [PubMed] [Google Scholar]
- 83.Hu F, Liu H, Xu L, Li Y, Liu X, Shi L, Su Y, Qiu X, Zhang X, Yang Y, Zhang J, Li Z. Hypoxia-inducible factor-1α perpetuates synovial fibroblast interactions with T cells and B cells in rheumatoid arthritis. Eur J Immunol 46: 742–751, 2016. doi: 10.1002/eji.201545784. [DOI] [PubMed] [Google Scholar]
- 84.Huang CL, Jong YS, Wu YW, Wang WJ, Hsieh AR, Chao CL, Chen WJ, Yang WS. Association of plasma thrombospondin-1 level with cardiovascular disease and mortality in hemodialysis patients. Acta Cardiol Sin 31: 113–119, 2015. doi: 10.6515/acs20140630d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging 2: 221–227, 1981. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- 86.Inoue M, Jiang Y, Barnes RH II, Tokunaga M, Martinez-Santibañez G, Geletka L, Lumeng CN, Buchner DA, Chun TH. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology 154: 4548–4559, 2013. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 100: 1423–1431, 1999. doi: 10.1161/01.CIR.100.13.1423. [DOI] [PubMed] [Google Scholar]
- 88.Iruela-Arispe ML, Porter P, Bornstein P, Sage EH. Thrombospondin-1, an inhibitor of angiogenesis, is regulated by progesterone in the human endometrium. J Clin Invest 97: 403–412, 1996. doi: 10.1172/JCI118429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 284: 1116–1125, 2009. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 109: 1945–1952, 2007. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol 27: 2582–2588, 2007. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- 92.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer 9: 182–194, 2009. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 144: 752–761, 2008. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg 247: 180–190, 2008. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 28: 110–119, 2009. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA 102: 13141–13146, 2005. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res 100: 712–720, 2007. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 98.Isenberg JS, Shiva S, Gladwin M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide 21: 52–62, 2009. doi: 10.1016/j.niox.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res 71: 785–793, 2006. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 100.Jeoung JW, Ko JH, Kim YJ, Kim YW, Park KH, Oh JY. Microarray-based analysis of gene expression profiles in peripheral blood of patients with acute primary angle closure. Ophthalmic Genet 38: 520–526, 2017. doi: 10.1080/13816810.2017.1300922. [DOI] [PubMed] [Google Scholar]
- 101.Jones RS, Minogue AM, Connor TJ, Lynch MA. Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol 8: 301–311, 2013. doi: 10.1007/s11481-012-9427-3. [DOI] [PubMed] [Google Scholar]
- 102.Jou IM, Shiau AL, Chen SY, Wang CR, Shieh DB, Tsai CS, Wu CL. Thrombospondin 1 as an effective gene therapeutic strategy in collagen-induced arthritis. Arthritis Rheum 52: 339–344, 2005. doi: 10.1002/art.20746. [DOI] [PubMed] [Google Scholar]
- 103.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010. [Erratum in Nat Cell Biol 12: 1249, 2010]. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaiser R, Frantz C, Bals R, Wilkens H. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir Res 17: 96, 2016. doi: 10.1186/s12931-016-0412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanazashi M, Okumura Y, Al-Nassan S, Murakami S, Kondo H, Nagatomo F, Fujita N, Ishihara A, Roy RR, Fujino H. Protective effects of astaxanthin on capillary regression in atrophied soleus muscle of rats. Acta Physiol (Oxf) 207: 405–415, 2013. doi: 10.1111/apha.12018. [DOI] [PubMed] [Google Scholar]
- 106.Kaneguchi A, Ozawa J, Kawamata S, Kurose T, Yamaoka K. Intermittent whole-body vibration attenuates a reduction in the number of the capillaries in unloaded rat skeletal muscle. BMC Musculoskelet Disord 15: 315, 2014. doi: 10.1186/1471-2474-15-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 37: 601–611, 2001. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 108.Kang S, Byun J, Son SM, Mook-Jung I. Thrombospondin-1 protects against Aβ-induced mitochondrial fragmentation and dysfunction in hippocampal cells. Cell Death Discov 4: 31, 2018. doi: 10.1038/s41420-017-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, Manu N, Wu W, Mannan P, Garfield SH, Roberts DD. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 7: 10133–10152, 2016. doi: 10.18632/oncotarget.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J Biol Chem 285: 38923–38932, 2010. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaur S, Schwartz AL, Jordan DG, Soto-Pantoja DR, Kuo B, Elkahloun AG, Mathews Griner L, Thomas CJ, Ferrer M, Thomas A, Tang SW, Rajapakse VN, Pommier Y, Roberts DD. Identification of schlafen-11 as a target of CD47 signaling that regulates sensitivity to ionizing radiation and topoisomerase inhibitors. Front Oncol 9: 994, 2019. doi: 10.3389/fonc.2019.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep 3: 1673, 2013. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kessler K, Borges LF, Ho-Tin-Noé B, Jondeau G, Michel JB, Vranckx R. Angiogenesis and remodelling in human thoracic aortic aneurysms. Cardiovasc Res 104: 147–159, 2014. doi: 10.1093/cvr/cvu196. [DOI] [PubMed] [Google Scholar]
- 114.Kim CW, Pokutta-Paskaleva A, Kumar S, Timmins LH, Morris AD, Kang DW, Dalal S, Chadid T, Kuo KM, Raykin J, Li H, Yanagisawa H, Gleason RL Jr, Jo H, Brewster LP. Disturbed flow promotes arterial stiffening through thrombospondin-1. Circulation 136: 1217–1232, 2017. doi: 10.1161/CIRCULATIONAHA.116.026361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kimura Y, Izumiya Y, Hanatani S, Yamamoto E, Kusaka H, Tokitsu T, Takashio S, Sakamoto K, Tsujita K, Tanaka T, Yamamuro M, Kojima S, Tayama S, Kaikita K, Hokimoto S, Ogawa H. High serum levels of thrombospondin-2 correlate with poor prognosis of patients with heart failure with preserved ejection fraction. Heart Vessels 31: 52–59, 2016. doi: 10.1007/s00380-014-0571-y. [DOI] [PubMed] [Google Scholar]
- 116.Kivelä R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J 20: 1570–1572, 2006. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- 117.Koch AE, Szekanecz Z, Friedman J, Haines GK, Langman CB, Bouck NP. Effects of thrombospondin-1 on disease course and angiogenesis in rat adjuvant-induced arthritis. Clin Immunol Immunopathol 86: 199–208, 1998. doi: 10.1006/clin.1997.4480. [DOI] [PubMed] [Google Scholar]
- 118.Koch W, Hoppmann P, de Waha A, Schömig A, Kastrati A. Polymorphisms in thrombospondin genes and myocardial infarction: a case-control study and a meta-analysis of available evidence. Hum Mol Genet 17: 1120–1126, 2008. doi: 10.1093/hmg/ddn001. [DOI] [PubMed] [Google Scholar]
- 119.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab 305: E439–E450, 2013. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kosugi T, Heinig M, Nakayama T, Connor T, Yuzawa Y, Li Q, Hauswirth WW, Grant MB, Croker BP, Campbell-Thompson M, Zhang L, Atkinson MA, Segal MS, Nakagawa T. Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol 174: 1221–1229, 2009. doi: 10.2353/ajpath.2009.080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 52: 343–346, 2008. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kutten J. Role of TSP1-CD47 Signaling in Tracheal Repair and Regeneration (Dissertation) Pittsburgh, PA: University of Pittsburgh, 2016. [Google Scholar]
- 123.Labrousse-Arias D, Castillo-González R, Rogers NM, Torres-Capelli M, Barreira B, Aragonés J, Cogolludo Á, Isenberg JS, Calzada MJ. HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc Res 109: 115–130, 2016. doi: 10.1093/cvr/cvv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lan CC, Huang SM, Wu CS, Wu CH, Chen GS. High-glucose environment increased thrombospondin-1 expression in keratinocytes via DNA hypomethylation. Transl Res 169: 91–101.e3, 2016. doi: 10.1016/j.trsl.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 125.Lane TF, Iruela-Arispe ML, Sage EH. Regulation of gene expression by SPARC during angiogenesis in vitro. Changes in fibronectin, thrombospondin-1, and plasminogen activator inhibitor-1. J Biol Chem 267: 16736–16745, 1992. [PubMed] [Google Scholar]
- 126.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99: 427–511, 2019. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol 46: 451–456, 1979. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 128.Lee G. The Anatomic Distribution and Expression of Matricellular Proteins in the Cerebral Vasculature of Alzheimer’s Disease Subjects (Undergraduate thesis) Pittsburgh, PA: University of Pittsburgh, 2018. [Google Scholar]
- 129.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156: 440–455, 2014. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes 55: 2965–2973, 2006. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One 6: e26656, 2011. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, Lamy E, Sarlon G, Quemener C, Bikfalvi A, Marcelli M, Pascal A, Dizier B, Simeoni U, Dignat-George F, Anfosso F. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood 118: 1699–1709, 2011. doi: 10.1182/blood-2010-12-325142. [DOI] [PubMed] [Google Scholar]
- 133.Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 34: 177–186, 2003. doi: 10.1161/01.STR.0000047100.84604.BA. [DOI] [PubMed] [Google Scholar]
- 134.Liu Z, Jiang Y, Li X, Hu Z. Embryonic stem cell-derived peripheral auditory neurons form neural connections with mouse central auditory neurons in vitro via the alpha2delta1 receptor. Stem Cell Reports 11: 157–170, 2018. doi: 10.1016/j.stemcr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol 178: 2573–2586, 2011. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maile LA, DeMambro VE, Wai C, Lotinun S, Aday AW, Capps BE, Beamer WG, Rosen CJ, Clemmons DR. An essential role for the association of CD47 to SHPS-1 in skeletal remodeling. J Bone Miner Res 26: 2068–2081, 2011. doi: 10.1002/jbmr.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maimaitiyiming H, Norman H, Zhou Q, Wang S. CD47 deficiency protects mice from diet-induced obesity and improves whole body glucose tolerance and insulin sensitivity. Sci Rep 5: 8846, 2015. doi: 10.1038/srep08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maleki S, Sepehr R, Staniszewski K, Sheibani N, Sorenson CM, Ranji M. Mitochondrial redox studies of oxidative stress in kidneys from diabetic mice. Biomed Opt Express 3: 273–281, 2012. doi: 10.1364/BOE.3.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mallakin A, Sugiyama T, Kai F, Taneja P, Kendig RD, Frazier DP, Maglic D, Matise LA, Willingham MC, Inoue K. The Arf-inducing transcription factor Dmp1 encodes a transcriptional activator of amphiregulin, thrombospondin-1, JunB and Egr1. Int J Cancer 126: 1403–1416, 2010. doi: 10.1002/ijc.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Manns JM, Uknis AB, Rico MC, Agelan A, Castaneda J, Arango I, Barbe MF, Safadi FF, Popoff SN, DeLa Cadena RA. A peptide from thrombospondin 1 modulates experimental erosive arthritis by regulating connective tissue growth factor. Arthritis Rheum 54: 2415–2422, 2006. doi: 10.1002/art.22021. [DOI] [PubMed] [Google Scholar]
- 141.Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev 276: 145–164, 2017. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 142.Matsuo Y, Tanaka M, Yamakage H, Sasaki Y, Muranaka K, Hata H, Ikai I, Shimatsu A, Inoue M, Chun TH, Satoh-Asahara N. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metabolism 64: 1490–1499, 2015. doi: 10.1016/j.metabol.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mattis J, Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab 27: 192–203, 2016. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol (1985) 78: 1957–1968, 1995. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- 145.McMorrow JP, Crean D, Gogarty M, Smyth A, Connolly M, Cummins E, Veale D, Fearon U, Tak PP, Fitzgerald O, Murphy EP. Tumor necrosis factor inhibition modulates thrombospondin-1 expression in human inflammatory joint disease through altered NR4A2 activity. Am J Pathol 183: 1243–1257, 2013. doi: 10.1016/j.ajpath.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 146.Meijles DN, Sahoo S, Al Ghouleh I, Amaral JH, Bienes-Martinez R, Knupp HE, Attaran S, Sembrat JC, Nouraie SM, Rojas MM, Novelli EM, Gladwin MT, Isenberg JS, Cifuentes-Pagano E, Pagano PJ. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci Signal 10: eaaj1784, 2017. doi: 10.1126/scisignal.aaj1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65: 510–520, 2004. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 148.Memetimin H, Li D, Tan K, Zhou C, Liang Y, Wu Y, Wang S. Myeloid-specific deletion of thrombospondin 1 protects against inflammation and insulin resistance in long-term diet-induced obese male mice. Am J Physiol Endocrinol Metab 315: E1194–E1203, 2018. doi: 10.1152/ajpendo.00273.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miedel E, Dishowitz MI, Myers MH, Dopkin D, Yu YY, Miclau TS, Marcucio R, Ahn J, Hankenson KD. Disruption of thrombospondin-2 accelerates ischemic fracture healing. J Orthop Res 31: 935–943, 2013. doi: 10.1002/jor.22302. [DOI] [PubMed] [Google Scholar]
- 150.Mikhailenko I, Krylov D, Argraves KM, Roberts DD, Liau G, Strickland DK. Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J Biol Chem 272: 6784–6791, 1997. doi: 10.1074/jbc.272.10.6784. [DOI] [PubMed] [Google Scholar]
- 151.Mikuła-Pietrasik J, Sosińska P, Janus J, Rubiś B, Brewińska-Olchowik M, Piwocka K, Książek K. Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-β1. Int J Biochem Cell Biol 45: 2087–2096, 2013. doi: 10.1016/j.biocel.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 152.Miller TW, Isenberg JS, Shih HB, Wang Y, Roberts DD. Amyloid-β inhibits No-cGMP signaling in a CD36- and CD47-dependent manner. PLoS One 5: e15686, 2010. doi: 10.1371/journal.pone.0015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, Wink DA, Roberts DD. CD47 globally regulates metabolic pathways that control resistance to ionizing radiation. J Biol Chem 290: 24858–24874, 2015. doi: 10.1074/jbc.M115.665752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moodalbail DG, Falkner B, Keith SW, Mathias RS, Araya CE, Zaritsky JJ, Stuart MJ. Ambulatory hypertension in a pediatric cohort of sickle cell disease. J Am Soc Hypertens 12: 542–550, 2018. doi: 10.1016/j.jash.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mori S, Kou I, Sato H, Emi M, Ito H, Hosoi T, Ikegawa S. Association of genetic variations of genes encoding thrombospondin, type 1, domain-containing 4 and 7A with low bone mineral density in Japanese women with osteoporosis. J Hum Genet 53: 694–697, 2008. doi: 10.1007/s10038-008-0300-4. [DOI] [PubMed] [Google Scholar]
- 156.Muñoz-Pacheco P, Ortega-Hernández A, Caro-Vadillo A, Casanueva-Eliceiry S, Aragoncillo P, Egido J, Fernández-Cruz A, Gómez-Garre D. Eplerenone enhances cardioprotective effects of standard heart failure therapy through matricellular proteins in hypertensive heart failure. J Hypertens 31: 2309–2319, 2013. doi: 10.1097/HJH.0b013e328364abd6. [DOI] [PubMed] [Google Scholar]
- 157.Murata Y, Saito Y, Kotani T, Matozaki T. CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci 109: 2349–2357, 2018. doi: 10.1111/cas.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-β activation: A therapeutic target for fibrotic disease. Matrix Biol 68-69: 28–43, 2018. doi: 10.1016/j.matbio.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Narizhneva NV, Byers-Ward VJ, Quinn MJ, Zidar FJ, Plow EF, Topol EJ, Byzova TV. Molecular and functional differences induced in thrombospondin-1 by the single nucleotide polymorphism associated with the risk of premature, familial myocardial infarction. J Biol Chem 279: 21651–21657, 2004. doi: 10.1074/jbc.M311090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nevitt C, McKenzie G, Christian K, Austin J, Hencke S, Hoying J, LeBlanc A. Physiological levels of thrombospondin-1 decrease NO-dependent vasodilation in coronary microvessels from aged rats. Am J Physiol Heart Circ Physiol 310: H1842–H1850, 2016. doi: 10.1152/ajpheart.00086.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, Attwell D. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365: eaav9518, 2019. doi: 10.1126/science.aav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Novelli EM, Kato GJ, Hildesheim ME, Barge S, Meyer MP, Lozier J, Hassett AC, Ragni MV, Isenberg JS, Gladwin MT. Thrombospondin-1 inhibits ADAMTS13 activity in sickle cell disease. Haematologica 98: e132–e134, 2013. doi: 10.3324/haematol.2013.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Novelli EM, Kato GJ, Ragni MV, Zhang Y, Hildesheim ME, Nouraie M, Barge S, Meyer MP, Hassett AC, Gordeuk VR, Gladwin MT, Isenberg JS. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol 87: 326–330, 2012. doi: 10.1002/ajh.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Novelli EM, Little-Ihrig L, Knupp HE, Rogers NM, Yao M, Baust JJ, Meijles D, St. Croix CM, Ross MA, Pagano PJ, DeVallance ER, Miles G, Potoka KP, Isenberg JS, Gladwin MT. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 316: L1150–L1164, 2019. doi: 10.1152/ajplung.00302.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg 5: 32, 2010. doi: 10.1186/1749-8090-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oglesby EN, Tezel G, Cone-Kimball E, Steinhart MR, Jefferys J, Pease ME, Quigley HA. Scleral fibroblast response to experimental glaucoma in mice. Mol Vis 22: 82–99, 2016. [PMC free article] [PubMed] [Google Scholar]
- 167.Ogunbayo GO, Misumida N, Olorunfemi O, Elbadawi A, Saheed D, Messerli A, Elayi CS, Smyth SS. Comparison of outcomes in patients having acute myocardial infarction with versus without sickle-cell anemia. Am J Cardiol 120: 1768–1771, 2017. doi: 10.1016/j.amjcard.2017.07.108. [DOI] [PubMed] [Google Scholar]
- 168.Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for β-cell function. Diabetes 60: 1946–1954, 2011. doi: 10.2337/db10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olson BA, Day JR, Laping NJ. Age-related expression of renal thrombospondin 1 mRNA in F344 rats: resemblance to diabetes-induced expression in obese Zucker rats. Pharmacology 58: 200–208, 1999. doi: 10.1159/000028282. [DOI] [PubMed] [Google Scholar]
- 170.Oñate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Díez-Caballero A, Ballesta-López C, Moscatiello F, Herrero J, Badimon L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J 26: 4327–4336, 2012. doi: 10.1096/fj.12-207217. [DOI] [PubMed] [Google Scholar]
- 171.Park CW, Kim HW, Lim JH, Yoo KD, Chung S, Shin SJ, Chung HW, Lee SJ, Chae CB, Kim YS, Chang YS. Vascular endothelial growth factor inhibition by dRK6 causes endothelial apoptosis, fibrosis, and inflammation in the heart via the Akt/eNOS axis in db/db mice. Diabetes 58: 2666–2676, 2009. doi: 10.2337/db09-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 43: 249–258, 2011. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pfander D, Cramer T, Deuerling D, Weseloh G, Swoboda B. Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Ann Rheum Dis 59: 448–454, 2000. doi: 10.1136/ard.59.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Philipot D, Guérit D, Platano D, Chuchana P, Olivotto E, Espinoza F, Dorandeu A, Pers YM, Piette J, Borzi RM, Jorgensen C, Noel D, Brondello JM. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res Ther 16: R58, 2014. doi: 10.1186/ar4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Polotsky VY, Savransky V, Bevans-Fonti S, Reinke C, Li J, Grigoryev DN, Shimoda LA. Intermittent and sustained hypoxia induce a similar gene expression profile in human aortic endothelial cells. Physiol Genomics 41: 306–314, 2010. doi: 10.1152/physiolgenomics.00091.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qin Z, Okubo T, Voorhees JJ, Fisher GJ, Quan T. Elevated cysteine-rich protein 61 (CCN1) promotes skin aging via upregulation of IL-1β in chronically sun-exposed human skin. Age (Dordr) 36: 353–364, 2014. doi: 10.1007/s11357-013-9565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci 7: 136, 2015. doi: 10.3389/fnagi.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramanathan S, Mazzalupo S, Boitano S, Montfort WR. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry 50: 7787–7799, 2011. doi: 10.1021/bi201060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reed MJ, Bradshaw AD, Shaw M, Sadoun E, Han N, Ferara N, Funk S, Puolakkainen P, Sage EH. Enhanced angiogenesis characteristic of SPARC-null mice disappears with age. J Cell Physiol 204: 800–807, 2005. doi: 10.1002/jcp.20348. [DOI] [PubMed] [Google Scholar]
- 180.Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol 37: 83–91, 2014. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 181.Rico MC, Manns JM, Driban JB, Uknis AB, Kunapuli SP, Dela Cadena RA. Thrombospondin-1 and transforming growth factor beta are pro-inflammatory molecules in rheumatoid arthritis. Transl Res 152: 95–98, 2008. doi: 10.1016/j.trsl.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 102: 13147–13152, 2005. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Roberts DD, Kaur S, Soto-Pantoja DR. Thrombospondin-1. In: Encyclopedia of Signaling Molecules, edited by Choi S. New York: Springer, 2018, p. 5400–5409. [Google Scholar]
- 184.Roberts DD, Lau LF. Matricellular proteins. In: The Extracellular Matrix: An Overview, edited by Mecham RP. Berlin: Springer-Verlag, 2011, p. 369–413. [Google Scholar]
- 185.Rodrigo GC, Herbert KE. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic Biol Med 119: 115–120, 2018. doi: 10.1016/j.freeradbiomed.2017.10.381. [DOI] [PubMed] [Google Scholar]
- 186.Rogers NM, Roberts DD, Isenberg JS. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann Surg 258: 184–191, 2013. doi: 10.1097/SLA.0b013e31827e52e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J, Novelli EM, Ross M, St. Croix C, Kutten JC, Czajka CA, Sembrat JC, Rojas M, Labrousse-Arias D, Bachman TN, Vanderpool RR, Zuckerbraun BS, Champion HC, Mora AL, Straub AC, Bilonick RA, Calzada MJ, Isenberg JS. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res 113: 15–29, 2017. doi: 10.1093/cvr/cvw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int 90: 334–347, 2016. doi: 10.1016/j.kint.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 189.Roudier E, Milkiewicz M, Birot O, Slopack D, Montelius A, Gustafsson T, Paik JH, DePinho RA, Casale GP, Pipinos II, Haas TL. Endothelial FoxO1 is an intrinsic regulator of thrombospondin 1 expression that restrains angiogenesis in ischemic muscle. Angiogenesis 16: 759–772, 2013. doi: 10.1007/s10456-013-9353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010. doi: 10.7326/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sennlaub F, Valamanesh F, Vazquez-Tello A, El-Asrar AM, Checchin D, Brault S, Gobeil F, Beauchamp MH, Mwaikambo B, Courtois Y, Geboes K, Varma DR, Lachapelle P, Ong H, Behar-Cohen F, Chemtob S. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 108: 198–204, 2003. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- 192.Sfar S, Saad H, Mosbah F, Chouchane L. Combined effects of the angiogenic genes polymorphisms on prostate cancer susceptibility and aggressiveness. Mol Biol Rep 36: 37–45, 2009. doi: 10.1007/s11033-007-9149-4. [DOI] [PubMed] [Google Scholar]
- 193.Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, Champion HC, Isenberg JS. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc 3: e000670, 2014. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shaw JA, Chin-Dusting JP, Kingwell BA, Dart AM. Diurnal variation in endothelium-dependent vasodilatation is not apparent in coronary artery disease. Circulation 103: 806–812, 2001. doi: 10.1161/01.CIR.103.6.806. [DOI] [PubMed] [Google Scholar]
- 195.Smadja DM, d’Audigier C, Bièche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol 31: 551–559, 2011. doi: 10.1161/ATVBAHA.110.220624. [DOI] [PubMed] [Google Scholar]
- 196.Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, Abu-Asab M, Wink DA, Tsokos M, Roberts DD. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy 8: 1628–1642, 2012. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep 3: 1038, 2013. doi: 10.1038/srep01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Starr ME, Hu Y, Stromberg AJ, Carmical JR, Wood TG, Evers BM, Saito H. Gene expression profile of mouse white adipose tissue during inflammatory stress: age-dependent upregulation of major procoagulant factors. Aging Cell 12: 194–206, 2013. doi: 10.1111/acel.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stein EV, Miller TW, Ivins-O’Keefe K, Kaur S, Roberts DD. Secreted thrombospondin-1 regulates macrophage interleukin-1β production and activation through CD47. Sci Rep 6: 19684, 2016. doi: 10.1038/srep19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 107: 3209–3215, 2003. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 201.Stuhlmüller B, Ungethüm U, Scholze S, Martinez L, Backhaus M, Kraetsch HG, Kinne RW, Burmester GR. Identification of known and novel genes in activated monocytes from patients with rheumatoid arthritis. Arthritis Rheum 43: 775–790, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 202.Suades R, Padró T, Alonso R, Mata P, Badimon L. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb Haemost 114: 1310–1321, 2015. doi: 10.1160/TH15-04-0325. [DOI] [PubMed] [Google Scholar]
- 203.Suárez Y, Fernández-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci USA 105: 14082–14087, 2008. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun H, Zhao Y, Bi X, Li S, Su G, Miao Y, Ma X, Zhang Y, Zhang W, Zhong M. Valsartan blocks thrombospondin/transforming growth factor/Smads to inhibit aortic remodeling in diabetic rats. Diagn Pathol 10: 18, 2015. doi: 10.1186/s13000-015-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 206.Tan BK, Syed F, Lewandowski KC, O’Hare JP, Randeva HS. Circadian oscillation of circulating prothrombotic thrombospondin-1: ex vivo and in vivo regulation by insulin. J Thromb Haemost 6: 1827–1830, 2008. doi: 10.1111/j.1538-7836.2008.03123.x. [DOI] [PubMed] [Google Scholar]
- 207.Tan XJ, Lang JH, Zheng WM, Leng JH, Zhu L. Ovarian steroid hormones differentially regulate thrombospondin-1 expression in cultured endometrial stromal cells: implications for endometriosis. Fertil Steril 93: 328–331, 2010. doi: 10.1016/j.fertnstert.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 208.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol 111: 765–772, 1990. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Timmons JA, Volmar CH, Crossland H, Phillips BE, Sood S, Janczura KJ, Törmäkangas T, Kujala UM, Kraus WE, Atherton PJ, Wahlestedt C. Longevity-related molecular pathways are subject to midlife “switch” in humans. Aging Cell 18: e12970, 2019. doi: 10.1111/acel.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Toba H, de Castro Brás LE, Baicu CF, Zile MR, Lindsey ML, Bradshaw AD. Increased ADAMTS1 mediates SPARC-dependent collagen deposition in the aging myocardium. Am J Physiol Endocrinol Metab 310: E1027–E1035, 2016. doi: 10.1152/ajpendo.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol 122: 497–511, 1993. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, Freedman M, Metivier J, Cannata R, O’Donnell CJ, Kottke-Marchant K, Murugesan G, Plow EF, Stenina O, Daley GQ. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 104: 2641–2644, 2001. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- 214.Uluçkan O, Becker SN, Deng H, Zou W, Prior JL, Piwnica-Worms D, Frazier WA, Weilbaecher KN. CD47 regulates bone mass and tumor metastasis to bone. Cancer Res 69: 3196–3204, 2009. doi: 10.1158/0008-5472.CAN-08-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Uno K, Bhutto IA, McLeod DS, Merges C, Lutty GA. Impaired expression of thrombospondin-1 in eyes with age related macular degeneration. Br J Ophthalmol 90: 48–54, 2006. doi: 10.1136/bjo.2005.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vallejo AN, Yang H, Klimiuk PA, Weyand CM, Goronzy JJ. Synoviocyte-mediated expansion of inflammatory T cells in rheumatoid synovitis is dependent on CD47-thrombospondin 1 interaction. J Immunol 171: 1732–1740, 2003. doi: 10.4049/jimmunol.171.4.1732. [DOI] [PubMed] [Google Scholar]
- 217.van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, Schroen B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 10: 769–779, 2011. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ III, Lee MJ, Fried SK, McGehee RE Jr, Peterson CA, Kern PA. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57: 432–439, 2008. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vernikos J, Schneider VS. Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontology 56: 157–166, 2010. doi: 10.1159/000252852. [DOI] [PubMed] [Google Scholar]
- 220.von Toerne C, Huth C, de Las Heras Gala T, Kronenberg F, Herder C, Koenig W, Meisinger C, Rathmann W, Waldenberger M, Roden M, Peters A, Thorand B, Hauck SM. MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: the KORA F4 study. Diabetologia 59: 1882–1892, 2016. doi: 10.1007/s00125-016-4024-2. [DOI] [PubMed] [Google Scholar]
- 221.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 222.Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia 48: 2650–2660, 2005. doi: 10.1007/s00125-005-0006-5. [DOI] [PubMed] [Google Scholar]
- 223.Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev 12: 1769–1774, 1998. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 111: 245–259, 2012. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 225.Wang Y, Xu LY, Lam KS, Lu G, Cooper GJ, Xu A. Proteomic characterization of human serum proteins associated with the fat-derived hormone adiponectin. Proteomics 6: 3862–3870, 2006. doi: 10.1002/pmic.200500840. [DOI] [PubMed] [Google Scholar]
- 226.Warbrick I, Rabkin SW. Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes Rev 20: 701–712, 2019. doi: 10.1111/obr.12828. [DOI] [PubMed] [Google Scholar]
- 227.Xiang R, Cui Y, Wang Y, Xie T, Yang X, Wang Z, Li J, Li Q. Circadian clock gene Per2 downregulation in non-small cell lung cancer is associated with tumour progression and metastasis. Oncol Rep 40: 3040–3048, 2018. doi: 10.3892/or.2018.6704. [DOI] [PubMed] [Google Scholar]
- 228.Xu X, Song N, Zhang X, Jiao X, Hu J, Liang M, Teng J, Ding X. Renal protection mediated by hypoxia inducible factor-1α depends on proangiogenesis function of miR-21 by targeting thrombospondin 1. Transplantation 101: 1811–1819, 2017. doi: 10.1097/TP.0000000000001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yagita K, Horie K, Koinuma S, Nakamura W, Yamanaka I, Urasaki A, Shigeyoshi Y, Kawakami K, Shimada S, Takeda J, Uchiyama Y. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc Natl Acad Sci USA 107: 3846–3851, 2010. doi: 10.1073/pnas.0913256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yao M, Rogers NM, Csányi G, Rodriguez AI, Ross MA, St. Croix C, Knupp H, Novelli EM, Thomson AW, Pagano PJ, Isenberg JS. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 25: 1171–1186, 2014. doi: 10.1681/ASN.2013040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zeng T, Yuan J, Gan J, Liu Y, Shi L, Lu Z, Xue Y, Xiong R, Huang M, Yang Z, Lin Y, Liu L. Thrombospondin 1 Is Increased in the Aorta and Plasma of Patients With Acute Aortic Dissection. Can J Cardiol 35: 42–50, 2019. doi: 10.1016/j.cjca.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 232.Zhao XM, Hu Y, Miller GG, Mitchell RN, Libby P. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation 103: 525–531, 2001. doi: 10.1161/01.CIR.103.4.525. [DOI] [PubMed] [Google Scholar]
- 233.Zhou X, Huang J, Chen J, Zhao J, Ge D, Yang W, Gu D. Genetic association analysis of myocardial infarction with thrombospondin-1 N700S variant in a Chinese population. Thromb Res 113: 181–186, 2004. doi: 10.1016/j.thromres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 234.Zwicker JI, Peyvandi F, Palla R, Lombardi R, Canciani MT, Cairo A, Ardissino D, Bernardinelli L, Bauer KA, Lawler J, Mannucci P. The thrombospondin-1 N700S polymorphism is associated with early myocardial infarction without altering von Willebrand factor multimer size. Blood 108: 1280–1283, 2006. doi: 10.1182/blood-2006-04-015701. [DOI] [PMC free article] [PubMed] [Google Scholar]