Abstract
To explore the vitamin D levels of periodontitis patients in comparison with periodontally healthy ones, and to assess the influence of vitamin D supplementation as an adjunctive during nonsurgical periodontal treatment (NSPT). Five databases (Pubmed, Embase, Scholar, Web of Sciences, and Cochrane Library) were searched until May 2020. Mean difference (MD) meta-analysis with corresponding 95% confidence interval (95% CI) and sensitivity tests via meta-regression were used. We followed Strength of Recommendation Taxonomy (SORT) to appraise the strength and quality of the evidence. Sixteen articles were included, fourteen case-control and two intervention studies, all reporting 25-hydroxyvitamin D (25(OH)D) levels. Compared with the healthy controls, the circulating 25(OH)D levels were significantly lower in chronic periodontitis patients (pooled MD = −6.80, 95% CI: −10.59 to −3.02). Subgroup analysis revealed differences among 25(OH)D measurements, with liquid chromatography-mass spectrometry being the most homogeneous method (pooled MD = −2.05, 95% CI: −3.40 to −0.71). Salivary levels of 25(OH)D showed no differences between groups. Due to the low number of studies, conclusions on aggressive periodontitis and in the effect of vitamin D supplementation after NSPT were not possible to ascribe. Compared with healthy controls, 25(OH)D serum levels are significantly lower in chronic periodontitis patients, with an overall SORT A recommendation. Future studies are needed to clarify the effect of vitamin D supplementation and the biological mechanisms linking vitamin D to the periodontium.
Keywords: vitamin D, vitamin D deficiency, 25(OH)D, periodontal disease, periodontitis, systematic review, meta-analysis
1. Introduction
Vitamin D is a fat-soluble hormone primarily obtained from exposure to sunlight, and additionally from the diet and nutritional supplements [1,2,3]. Vitamin D is a universal term employed to describe the compound that exhibits the biological activity of cholecalciferol in animals (vitamin D3). This vitamin is a key factor to the calcium-phosphate homeostasis regulation and mineral bone metabolism [4,5]. In this sense, vitamin D increases the intestinal absorption of calcium and decreases the secretion of parathyroid hormone, which consequently decreases systemic bone resorption [6,7]. In addition, vitamin D stimulates osteoblastic bone production and alkaline phosphatase activity, optimizes bone remodeling and covers bone mass by increasing bone matrix proteins [2,3,8,9].
Public awareness about vitamin D has increased over the last years due to the prevalence of its deficiency [3,10,11,12,13,14]. Accordingly, vitamin D deficiency may play an important biological and metabolic role in reducing bone mineral density, total mineral content, and, consequently, may represent a risk factor against bone healing [3,14].
Periodontitis (PD) is a complex polymicrobial disease induced by an unbalanced interaction between the oral microbial and the individual inflammatory response [15,16]. The onset of this pandemic non-communicable disease [17,18] is characterized by gum inflammation (gingivitis), and the progression results in loss of the supporting tissues of the teeth, and, if untreated, ultimately leads to tooth loss [19,20]. Furthermore, to prevent disease progression, to minimize symptoms and possibly to restore lost tissues, it requires a combination of periodontal therapeutic modalities according to patient periodontal status. The treatment can include oral hygiene instruction, subgingival instrumentation to remove plaque and calculus, local and/or systemic pharmacotherapy and periodontal surgery [21].
The nutritional consequences of vitamin D levels on periodontal health represent a matter of interest [22,23,24,25,26,27]. Over decades, lower vitamin D levels have been associated with higher periodontal destruction and severe periodontitis stages [28,29,30,31,32,33,34,35,36]. Others supported the idea that patients with higher levels of vitamin D were related to less bleeding on probing (BoP) comparing to patients with lower levels [34]. In addition, in vitro studies demonstrated that vitamin D may decrease the number of Porphyromonas gingivalis through active autophagy [37] and might decrease the inflammatory burden of periodontitis in rodent models [38,39,40,41]. Furthermore, the association between vitamin D levels and periodontitis has been systematically investigated [42,43,44,45]; however, none of these studies were able to produce quantitative synthesis. Additionally, the impact of vitamin D supplementation during nonsurgical periodontal therapy (NSPT) has never been appraised in a systematic way.
Therefore, the aim of this systematic review was two-fold. The primary objective was to render robust synthesis on the association between vitamin D levels and periodontitis. The secondary objective was to assess the influence of vitamin D supplementation as an adjunctive during NSPT.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review protocol was defined a priori and no deviations from the protocol were made. The work followed the Cochrane Handbook of Systematic Reviews of Interventions [46] and the report was made according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [47] (Supplementary Material Table S1).
2.2. Focused Questions and Eligibility Criteria
The following focused questions were addressed:
“Are 25-hydroxyvitamin D (25[OH]D) levels associated with periodontitis?”
Chronic and aggressive periodontitis patients (Patients—P); periodontitis (Exposure—E); periodontal healthy patients (Comparison—C); serum and salivary 25-hydroxyvitamin D (25(OH)D) levels (Outcome—O).
-
2.
“Does vitamin D supplementation have an adjunctive effect on NSPT?”
Patients with periodontitis supplemented with vitamin D (Patients—P); NSPT (Exposure—E); patients with periodontitis supplemented with placebo (Comparison—C); periodontal probing depth (PPD), clinical attachment loss (CAL), BoP levels (Outcome—O).
Inclusion criteria were determined as follows:
Design: Intervention trials (randomized clinical trials (RCTs) and non-randomized studies of interventions (NRSI)) and observational studies (case-control, cohort studies);
Studies in humans reporting 25(OH)D levels in patients with and without periodontitis;
Studies in humans reporting the effect of vitamin D supplementation as an adjunctive of NSPT;
Studies describing vitamin D units of measurement and measurement methodology.
The search was conducted without any restrictions regarding year of publication or language.
2.3. Search Strategy
We searched Pubmed, MEDLINE (Medical Literature Analysis and Retrieval System Online), CENTRAL (The Cochrane Central Register of Controlled Trials), EMBASE (The Excerpta Medica Database), Web of Science from the earliest data available until May 2020. We merged keywords and the Medical Subject Headings (MeSHs) regarding the periodontal disease (periodontitis OR gingivitis OR periodontal health OR periodontal diseases [MeSH]) and Vitamin D (vitamin D OR (vitamin D [MeSH]) OR 25-hydroxy-vitamin OR calcitriol OR vitamin D supplementation OR Vitamin D deficiency OR vitamin D receptor) in accordance with the thesaurus of each database. Grey literature was searched through the OpenGrey portal [48]. Additional appropriate literature was included after a manual search of the reference lists of the final included articles. Periodontology-and nutrition-specific journals were hand-searched to identify additional articles.
2.4. Study Selection and Data Extraction
Two researchers (S.L. and V.M.) independently selected the relevant articles through titles and abstracts and excluded unrelated studies. A third author (J.B.) checked the eligible studies and any disagreement was resolved through discussion. If there were multiple publications for the same study, data from the largest sample were used.
Two researchers (S.L. and V.M.) independently extracted the relevant data from the studies. Any disagreement was resolved through discussion with a third researcher (J.B). A predefined table was used to extract necessary data from each eligible study, including the first author’s name, publication year, the country where the study was conducted, exclusion criteria, number of participants, gender, mean age, percentage of smokers, periodontal case definition, sample type (saliva or serum), measurement of 25(OH)D levels and laboratory analysis. Clinical periodontal measures included PPD, CAL and BoP. All data were independently extracted by two reviewers with a consensus in all aspects. The authors were contacted for additional data clarification when necessary.
2.5. Risk of Bias (RoB) in Individual Studies
Methodological quality assessment was independently performed by two calibrated authors (V.M. and J.B.) using the Cochrane risk-of-bias tool 2 (RoB2) for RCTs [46] or ROBINS-I tool for NRSI [49]. For case-control and cohort studies, we used the Newcastle-Ottawa Scale (NOS). Regarding this last tool, we scored across three categories: studies with 7–9 stars were deemed of low RoB, studies with 5–6 stars of moderate RoB, whilst studies with less than 5 stars were deemed of high RoB. Any doubt was resolved by discussion with a third author.
2.6. Statistical Analysis
Statistical analysis was performed using R version 4.0.0 (R Studio Team 2018). For continuous data, mean values and standard deviations (SD) were used and analyzed with mean differences (MD) and correspondent estimates by 95% confidence interval (95% CI). The unit of measurement used in the MD meta-analysis was ng/mL. In the case of median and interquartile range report, we converted to mean and SD following Hozo et al. [50]. DerSimonian-Laird random-effects meta-analysis [51] and forest plots were performed using the ‘meta’ package [52]. Statistical heterogeneity was inspected through the I2 index and Cochrane’s Q statistic (p < 0.1). The overall homogeneity was calculated through the χ2 test [53]. All tests were two-tailed, with alpha set at 0.05. Further, the weight percentage given to each study in each analysis was provided in the forest plots. Meta-regression was performed towards the influence of smoking in serum 25(OH)D levels. Publication bias was planned if at least 10 or more studies were included [53]. In the case of lacking data amenable to meta-analysis, we followed the Synthesis Without Meta-analysis (SWiM) guidelines to synthesize quantitative data [54].
2.7. Strength of Recommendations
We employed the Strength of Recommendation Taxonomy (SORT) to appraise the strength and quality of the evidence [55]. The outcomes of the present systematic review, clinical recommendations, and future necessary research were discussed.
3. Results
3.1. Study Selection
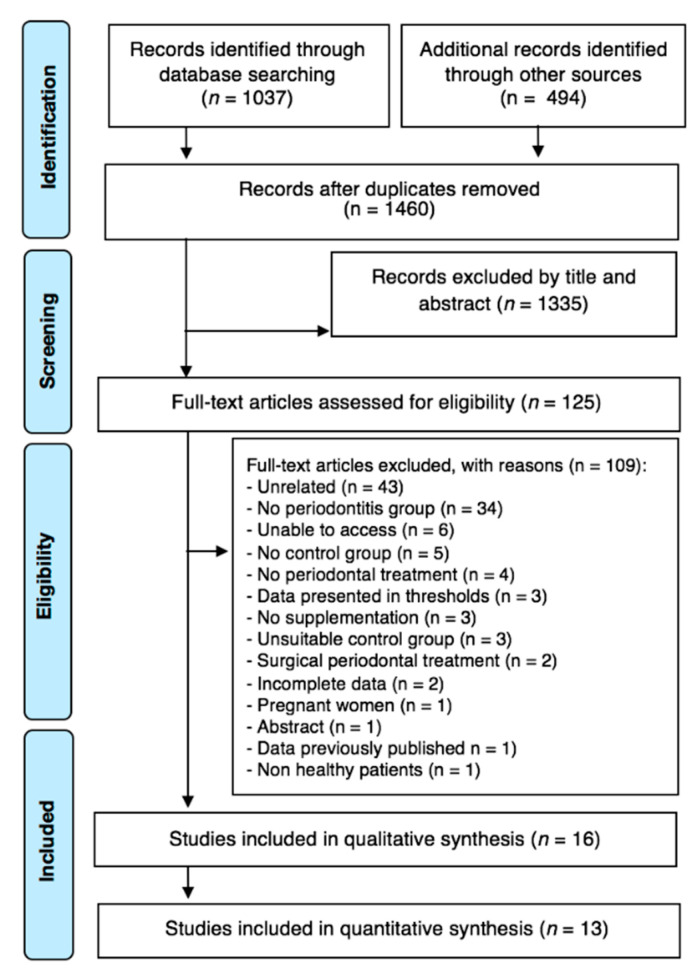
Overall, the search yielded a total of 1531 studies (Figure 1). After duplicate removal, 1460 were screened for titles and abstracts, and 125 articles fulfilled the inclusion criteria (1335 were excluded). These 125 articles were subjected to full paper review eligibility and 109 were excluded as they did not approach the research questions (Supplementary Material Table S2). Then, 16 articles were included for the qualitative analysis, of which two concerned Vitamin D supplementation. A total of 13 studies were included in the quantitative synthesis regarding 25(OH)D levels in patients with and without periodontitis (Figure 1).
Figure 1.
Article selection flow chart for the systematic review.
3.2. Studies Characteristics
Overall, the included case-control studies were from ten different countries, across Asia, Europe and America (Table 1), with a total of 10,597 participants included in this review. Two articles [28,56] reported 91 aggressive periodontitis cases—one assessed the circulating 25(OH)D levels [28], and the other assessed salivary levels [56]. Quantitative analysis included a total of 10,506 subjects (subcategorised as 9718 periodontal healthy patients and 788 patients with chronic periodontitis) from 13 studies, of which three [33,57,58] assessed 25(OH)D levels through salivary samples, nine through serum levels [28,29,30,31,32,36,59,60,61], and one assessed both methods [62].
Table 1.
Characteristics of the included studies regarding 25-hydroxyvitamin D (25(OH)D) levels.
| Authors, Year, Country | Funding | N. of Subjects | N. of Healthy/ CP/AgP |
Male/Female | Smokers n (%) | Mean Age ± SD | PD Diagnostic Criteria | Method | Samples |
|---|---|---|---|---|---|---|---|---|---|
| Constantini et al., 2020, Italy | None | 42 | 21/21/- | 14/28 | 5 (11.9) | 54.3 ± 5.0/56.9 ± 5.4/- | EFP/AAP 2018 | ELISA | Saliva |
| Isola et al., 2020, Italy | University of Catania | 89 | 43/46/- | 33/56 | 24 (27.9) | 53.7 ± 4.5/53.1 ± 4.2/- | EFP/AAP 2018 | ELISA | Serum |
| Agrawal et al., 2019, India | NR | 40 | 20/20/- | NR | 0 (0) | 44.7/39.3/- | GI ≥ 1, PI ≥ 1, PPD ≥ 5 mm and CAL ≥ 5 mm | ELISA | Serum |
| Ketharanathan et al., 2019 (Tamil) | Institute of Clinical Odontology, Faculty of Dentistry, University of Oslo, Norway | 48 | 21/27/- | 48/0 | 2 (4.6) | 41.1 ± 5.7/42.6 ± 6.7/- | CAL ≥ 6 mm in 2 or more teeth and 1 or more sites with PPD ≥ 5 mm | LC-MS | Serum |
| Ketharanathan et al., 2019 (Norwegian), Norway | 44 | 23/21/- | 44/0 | 4 (8.3) | 50.3 ± 13.1/52.1 ± 9.0/- | ||||
| Ebersole et al., 2018, USA | U.S.P.H.S. grant GM103538, GM103440, and Center for Oral Health Research (University of Kentucky College of Dentistry) | 9696 | 9308/388/- | NR | NR | NR | NHANES 1999–2004: CAL ≥ 3 mm and PPD ≥ 4 mm; 1999–2000 and 2003–2004: PPD ≥ 3 mm and CAL ≥ 4 mm; NHANES 2001–2004: Page and Eke 2007 | LC-MS | Serum |
| Anbarcioglu et al., 2018, Turkey | Ondokuz Mayıs University Scientific Research Projects Foundation | 156 | 27/55/74 | 74/82 | 0 (0) | 30.9 ± 3.8/39.4 ± 4.7/29.9 ± 5.2 | AAP 1999 | LC-MS | Serum |
| Yuce et al., 2017, Turkey | Gaziosmanpasa University Unit of Scientific Research Projects | 36 | 18/18/- | 18/18 | 4 (22.2) | 48.8 ± 9.6/49.5 ± 9.4 /- | AAP 1999 | ELISA | Saliva and Serum |
| Laky et al., 2017, Austria | Austrian National Bank (n°. 12986) | 58 | 29/29/- | 20/38 | 19 (32.8) | 35.5 ± 7.4/35.4 ± 7.7/- | PPD ≥ 5 mm | ELISA | Serum |
| Abreu et al., 2016, Puerto Rico | National Institute on Minority Health and Health Disparities of the National Institutes of Health | 38 | 19/19/- | 10/28 | 0 (0) | 46.7 ± 8.2/47.6 ± 8.7/- | CDC—AAP 2003 | CLIA | Serum |
| Gümüş et al., 2016, Turkey | Research foundation of Ege University, Izmir, Turkey (n°. 2013DIS029) | 42 | 27/15/- | 0/42 | 0 (0) | 25.0 ± 4.0/-/40.0 ±10. 0 | Armitage 1999 | ELISA | Saliva |
| Joseph et al., 2015, India | SBMR, Directorate of Medical Education, Government of Kerala | 98 | 48/50/- | 46/52 | 9 (9,2) | 40.77 ± 5.1/40.76 ± 7.8 /- | Armitage 1999 | CLIA | Serum |
| Antonoglou et al., 2015, Finland | CIMO, Finnish Ministry of Education and Culture, Finnish Dental Society Apollonia | 84 | 30/54/- | 32/52 | 40 (47,6) | 41.9 ± 12.7/46.3 ± 13.7 /- | Page and Eke 2007 | CLIA | Serum |
| Miricescu et al., 2014, Romania | European Social Fund and Romanian Government (POSDRU/6/1.5/S/S17) | 50 | 25/25/- | 16/34 | 0 (0) | 18.66 ± 2/-/51.26 ± 7.4 | At least 6 sites with PPD ≥ 4 mm; bone loss > 30% and gingival inflammation | ELISA | Saliva |
| Zhang et al., 2012, China | Natural Science Foundations of China; National Key Project of Scientific and Technical Supporting Programs of China; Clinical Research Fund, Ministry of Health | 76 | 32/-/44 | 29/47 | 7 (10,1) | 24.3 ± 0.8 /-/ 26.8 ± 1.7 | Classification of Periodontal Diseases and Conditions 1999 | ELISA | Saliva |
AAP—American Academy of Periodontology; AgP—aggressive periodontitis; CAL—clinical attachment loss; CDC—Centers for Disease Control; CIMO—Centre for International Mobility; CLIA—chemiluminescence immunoassay; CP—chronic periodontitis; EFP/AAP—European Federation of Periodontology/American Academy of Periodontology; ELISA—enzyme-linked immunosorbent assay; GI—gingival index; LC-MS—liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry; NHANES—National Health and Nutrition Examination Survey; NR—not reported; PI—periodontal index; PD—periodontitis; PPD—probing periodontal depth; SBMR—State Board of Medical Research; SD—standard deviation.
In Vitamin D supplementation on NSPT, two studies were included [63,64]. Overall, 557 patients suffering from chronic periodontitis were submitted to NSPT and 276 participants were medicated with Vitamin D3 supplements. Gao et al. [63] searched the effect of two different concentrations of Vitamin D3 supplements in a controlled design (Table 2).
Table 2.
Characteristics of the included studies regarding the 25(OH)D supplementation after nonsurgical periodontal treatment (NSPT).
| Authors, Year, Country | Funding | N. of Subjects | N. of Control/CP | Male/Female | Smokers n (%) | Mean Age ± SD | PD Diagnostic Criteria | PD Treatment | Method | Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Gao et al., 2020, China | Beijing Science and Technology Program and the National Natural Science Foundation of China | 240 | 120/120 | 119/121 | 5 (11.9) | 53.0 ± 5.2/51.0 ± 6.3 | At least 6 sites with PPD > 6 mm, CAL > 4 mm, X-ray showing at least 6 sites with alveolar bone loss more than one third of the root length | Control—NSPT and placebo; Test group—NSPT and 1000 IU/day vitamin D3 | ELISA | Serum (Baseline and 3 months after NSPT) |
| 240 | 120/120 | 116/125 | 5 (11.9) | 53.0 ± 5.2/49.0 ± 5.4 | Control—NSPT and placebo; Test group—NSPT and 2000 IU/day vitamin D3 | |||||
| Perayil et al., 2015, India | None | 77 | 41/36 | NR | NR | NR | One or more teeth with chronic moderate periodontitis, CAL of 3–4 m | Control – NSPT and placebo; Test group—NSPT and 60,000–120,000 IU/day vitamin D3 | ELISA | Serum (Baseline and 3 months after NSPT) |
CAL—clinical attachment loss; ELISA—enzyme-linked immunosorbent assay; IU—international unit; NR—not reported; NSPT—nonsurgical periodontal treatment; PPD—probing periodontal depth; SD—standard deviation.
3.3. Risk of Bias within Studies
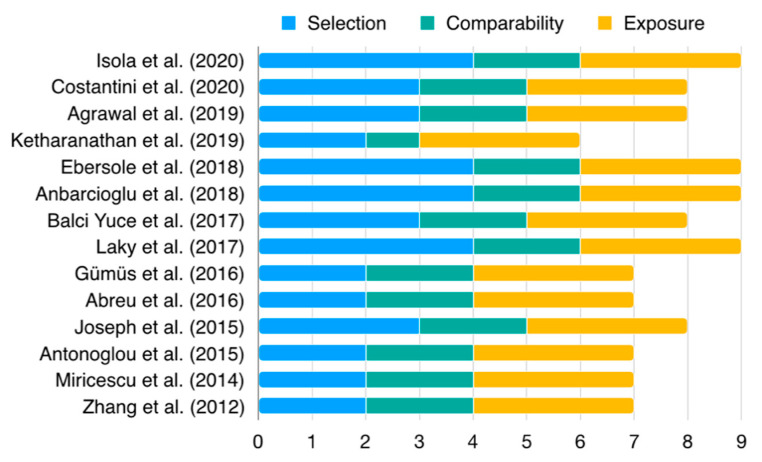
Thirteen articles presented with low RoB (four with 9/9, four with 8/9 and five with 7/9 scores) (Figure 2, Supplementary Material Table S3). Only one article presented moderate risk of bias, with an overall score of 6/9 [32]. The main reason for bias arose from the representativeness of the cases. Overall, articles adopted an adequate periodontitis case definition (100%, n = 14) and definition of control. A considerable number of studies failed to include representative samples (57.1%, n = 8) and the selection of controls (42.9%, n = 6). In the ascertainment of exposure, usability of the same method of ascertainment for cases and controls, and non-response rate, all articles presented low RoB (100.0%, n = 14). Two intervention trials presented low risk of bias, one RCT (Supplementary Material Table S4) and one NRSI (Supplementary Material Table S5).
Figure 2.
Newcastle Ottawa-Scale (NOS) for case-control studies. Detailed information is presented in the Supplementary Information (Table S3).
3.4. Synthesis of Results
3.4.1. Vitamin D Levels and Periodontitis
Serum Levels
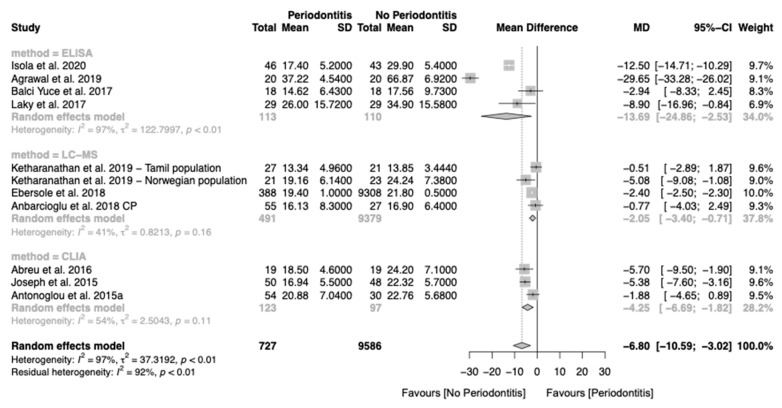
In our analysis, chronic periodontitis was associated with average lower serum 25(OH)D levels (MD of −6.80, 95% CI: −10.59; −3.02), but with high heterogeneity (I2 = 97%) (Figure 2). To mitigate the level of heterogeneity, we performed a subgroup analysis according to the method of 25(OH)D measurement (Figure 3). The liquid chromatography–mass spectrometry (LC-MS) method presented the lower differences and a moderate heterogeneity (MD of −2.05, 95% CI: −3.40; −0.71, I2 = 41%). The enzyme-linked immunosorbent Assay (ELISA) (MD of −13.69, 95% CI: −24.86; −2.53, I2 = 97%) and chemiluminescence immunoassay (CLIA) (MD of −4.25, 95% CI: −6.69; −1.82, I2 = 54%) presented higher differences but also higher heterogeneity. Univariate meta-regression found that the decrease in serum levels of 25(OH)D was not associated with smokers (Supplementary Material Table S6).
Figure 3.
Forest plot of studies evaluating serum 25(OH)D levels in patients with and without chronic periodontitis (p-value < 0.001). Mean effect size estimates have been calculated with the correspondent 95% confidence intervals (95% CI). Area of squares represents sample size, continuous horizontal lines and diamonds width represents 95% CI. The diamond and the vertical dotted line represent the overall pooled estimate.
Due to the existence of only two articles [28,56] regarding the comparison of serum levels of 25(OH)D of patients diagnosed with aggressive periodontitis compared to healthy periodontal patients, the meta-analysis was not deemed possible (Table 1). While one study [28] reported lower levels of 25(OH)D serum levels, the other, based on salivary measurements, showed opposite results [56].
Salivary Levels
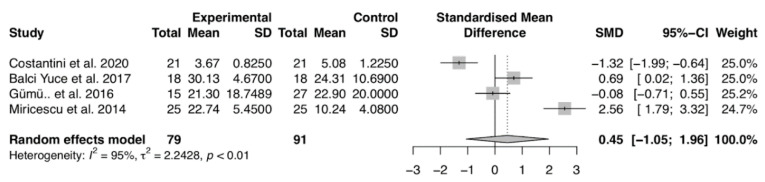
Regarding salivary 25(OH)D levels, our analysis did not report differences comparing chronic periodontitis to healthy periodontal subjects (MD of 0.45, 95% CI: −1.05; 1.96) (Figure 4).
Figure 4.
Forest plot of studies evaluating salivary 25(OH)D levels in patients with and without periodontitis (p-value = 0.5545). Mean effect size estimates have been calculated with 95% confidence intervals and are shown in the figure. Area of squares represents sample size, continuous horizontal lines and diamonds width represents 95% confidence interval. The diamond and the vertical dotted line represent the overall pooled estimate.
3.4.2. Vitamin D Supplementation as an Adjunctive in NSPT
Due to the lack of data amenable to perform a meta-analysis about vitamin D supplementation on NSPT, we therefore decided to synthesize evidence without analysis. Two studies fulfilled the inclusion criteria, one RCT (Gao et al., 2020) and one NRSI (Perayil et al., 2015), both of low RoB (Supplementary Material Tables S4 and S5).
Gao et al. [63] applied vitamin D supplementation on 360 patients with moderate or severe periodontitis following NSPT. Patients were randomly assigned to 2000 international units (IU)/d vitamin D3, 1000 IU/d vitamin D3, or placebo. The effect of vitamin D supplementation tended to be modest with limited periodontal clinical relevance and long-term efficacy towards PPD and CAL (Gao et al., 2020).
Furthermore, Perayil et al. [64] investigated, in 82 moderate chronic periodontitis patients, if vitamin D supplementation plus calcium (Shelcal-D 500 mg calcium + 250 IU vitamin D once daily) compared to placebo following NSPT. Despite PPD and CAL having no differences between groups, the authors reported significantly better results for the vitamin D group in relation to gingival inflammation and bone density (measured using panoramic x-ray).
3.5. Additional Analyses
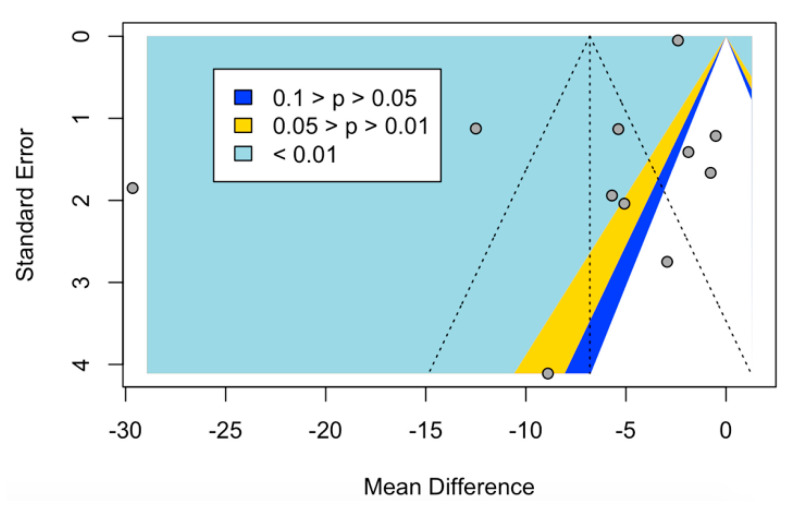
We confirmed that no publication bias was assessed in meta-analysis regarding the serum 25(OH)D levels (p = 0.1174) (Figure 5).
Figure 5.
Funnel plot of studies evaluating serum vitamin D levels in patients with and without chronic periodontitis. Overall, this analysis showed no signs of publication bias.
3.6. Reporting on Strength of Recommendation
Using the SORT recommendation, we concluded that chronic periodontitis is strongly associated with lower serum levels of 25(OH)D (SORT A) [55].
4. Discussion
4.1. Summary of Main Findings and Quality of the Evidence
This systematic review supported an association between serum vitamin D levels (measured in ng/mL of 25(OH)D) and chronic periodontitis, with an overall SORT A recommendation. Within the lack of the available studies, salivary levels of 25(OH)D did not present an association with chronic periodontitis. In addition, there was a scarcity of studies regarding the association of aggressive periodontitis and 25(OH)D levels, and the influence of vitamin D supplementation precluded any definitive conclusion. Analyzing the impact of smoking in serum 25(OH)D level changes in chronic periodontitis, meta-regression analysis revealed that smoking had no meaningful impact.
Overall, the results of this systematic review support a link between 25(OH)D serum levels and chronic periodontitis. That is, patients diagnosed with chronic periodontitis presented lower serum levels of 25(OH)D than periodontal healthy patients. These results are clinically relevant considering the crucial role of vitamin D in bone maintenance and in the immune system [2,3,8,65,66]. In addition, several studies have unveiled the potential harmful impact of vitamin D deficiency on the periodontium, especially after periodontal surgery where this baseline decrease might result in undesirable outcomes [67,68].
A possible mechanism for this association whereby vitamin D reduces the risk of periodontitis is through the induction of cathelicidin [69,70,71,72,73]. The vitamin D pathway has been shown to exist in human gingival fibroblasts and periodontal ligaments cells, playing an important role in immune defense in periodontal soft tissues via the activation of the human cationic antimicrobial protein cathelicidin [69,70,71,72]. Recently, serum 25(OH)D deficiency was associated with decreased hBD−2 and cathelicidin levels in periodontal tissues in gingivitis and chronic periodontitis [73].
Several limitations, however, should be reported. Firstly, the level of heterogeneity observed was high and can limit the validity and robustness of these quantitative analyses. The lack of consistency in the periodontitis case definition precluded more robust analyses of the extent and severity of periodontitis with 25(OH)D serum levels. Thus, future studies should accommodate the up-to-date consensus [74], because of its upgraded characteristics [75,76], as well providing more in-depth data on the extent and severity of periodontitis and its relevant periodontal clinical measures (such as PPD, CAL and BoP). Secondly, the main analysis in this systematic review was derived from observational studies, that only inform the association between periodontitis and 25(OH)D serum changes. Therefore, studies with longer follow-ups are mandatory to clarify this matter. Furthermore, the included studies showed multiple quantification method of 25(OH)D levels, and this may have contributed to the heterogeneity. In the future, studies should harmonize the measurement method of 25(OH)D levels. On the other hand, there are strengths in this evidence-based study. This review was designed a priori and followed a strict protocol, updated international reporting guidelines, and had an extensive literature search.
Considering the existing evidence, this is the first review to render a magnitude effect on the association between serum 25(OH)D levels and chronic periodontitis. Overall, four systematic reviews have analyzed such association [42,43,44,77], but without success in pursuing meta-analysis. Further, these reviews have reported contradictory conclusions, wherein Van der Putten et al. (2009) found no evidence of an association of vitamin D with periodontal disease in non-institutionalized elderly people, Pinto et al. [44] and Perić et al. [43] found insufficient data to provide a conclusion, while Varela-López et al. [77] reported a potential association.
With regard to periodontal treatment, our narrative synthesis provides a small view on the potential characteristics of vitamin D supplementation on NSPT. One the one hand, the shortage of literature was already highlighted, which precludes any conclusion on the effect of serum vitamin D levels on periodontal treatment [43,78]. On the other hand, a previous review found that baseline vitamin D deficiency at the time of the periodontal treatment, especially in surgical procedures, negatively affected treatment outcomes [45]. However, more randomized clinical trials are warranted to provide a robust conclusion.
4.2. Clinical and Research Implications
Public awareness of vitamin D levels is high due to the worrisome prevalence of vitamin D deficiency worldwide [3,10,11]. Nevertheless, it is important to highlight that to confirm the decreased total levels of 25(OH)D in a more complete way, studies may combine total 25(OH)D with parathyroid hormone (PTH), calcium and phosphate levels [79]. Thereafter, the results of this systematic review are clinically relevant because they link these low vitamin D levels to chronic periodontitis, an inflammatory condition that figures as one of the most prevalent disease in the world [18,80]. In other words, periodontitis patients are very likely to have lower serum levels of 25(OH)D, though the clinical consequences are still unclear in their entirety, and for this reason larger and well-designed RCTs are warranted.
Vitamin D levels have been consistently associated with several systemic diseases, such as rheumatic [81,82,83], cardiovascular [84,85,86,87], diabetes mellitus [87,88,89], inflammatory bowel disease [90,91], or female-related conditions [92,93,94,95]. Further, other factors such as seasonal variation [96], race and vitamin D binding protein [97] can also affect 25(OH)D levels. Likewise, periodontitis is also a relevant risk factor in these pathologies [98,99,100,101,102,103,104,105], and a potential mediation effect of periodontitis-vitamin D may be considered and further developed in clinical studies.
Our results can also serve as clinical and research guidelines for expected differences in vitamin D levels in periodontitis patients, as well as clarifying the importance of knowing the method to measure vitamin D, as this may impact vitamin D differences. At this stage, the LC-MS measurement method showed moderate heterogeneity (I2 = 41%), regardless of the multiple periodontal diagnostic criteria observed in this subgroup; therefore, we might expect more homogeneous results when studies employ the same case definition [28,30,32]. In view of these results, LC-MS might be seen as the most consistent method to measure 25(OH)D serum levels, both in clinical practice as well as in research studies, and is in agreement with a previous reliability study on vitamin D quantification [106].
While 25(OH)D serum levels of periodontitis patients were decreased compared to healthy controls, the 25(OH)D levels in whole saliva had no significant differences, and the results of the included studies are quite heterogeneous in saliva. A reasonable explanation for such differences can be the expression of vitamin-D binding protein (DBP) locally versus systemically. In this sense, a previous study on healthy periodontal patients showed that the levels of DBP in gingival crevicular fluid were higher than serum levels [107], and therefore periodontal tissue might be another source of DBP [108]. Furthermore, the levels of DBP were found to be lower in periodontitis cases, presumably due to the lack of effective production or an increase in local consumption [107]. The results on salivary levels show some heterogeneity among the studies, in particular [33,58], as well a low number of participants, which limits the validity of this finding. A possible explanation for the discrepancy between Miricescu et al., 2014 [58] and Costantini et al., 2020 [33] is the difference between mean age, gender distribution and periodontal diagnosis between these two studies.
Notwithstanding these issues, more studies are warranted to explore the role of DBP expression on the salivary and serum levels of 25(OH)D on periodontal patients.
Mindful of the inflammation surrounding teeth, peri-implantitis is a pathological condition characterized by the progressive loss of supporting peri-implant bone [109], and strong evidence has suggested that PD is a risk factor for implant loss [110]. A single study found that 25(OH)D levels are significantly decreased in peri-implantitis patients comparing with peri-implant healthy patients and, consequently, might be important indicators of peri-implant diseases [111]. Nevertheless, further studies are needed to confirm if such an association with vitamin D follows the same fashion as PD.
Importantly, future studies on the impact of vitamin D supplementation should bear in mind the baseline status of the patients. Currently, vitamin D supplementation studies support the arbitrary application of vitamin D supplements as an adjunct in NSPT, though studies have seemed to disregard the baseline vitamin D levels, and this might lead to inevitable bias of analysis. In this sense, future studies should define a priori which patients have vitamin D deficiency (<20 ng/mL) or insufficiency (<30 ng/mL) to clarify whether the restoration of DV levels will result in superior periodontal clinical results. Moreover, studies must consider the initial periodontal status and the interplay of 25(OH)D with key periodontal clinical measures. Therefore, intervention studies using vitamin D supplementation should define with a clear threshold alike patients according to the baseline 25(OH)D levels and the periodontal status.
5. Conclusions
Periodontitis is associated with lower 25(OH)D serum levels. The effect of vitamin D supplementation as an adjunct of nonsurgical periodontal treatment remains unclear due to the shortage of available studies. Future studies are needed regarding the effect of vitamin D supplementation and the biological mechanisms linking vitamin D to the periodontium.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/8/2177/s1, Table S1. PRISMA 2009 Checklist; Table S2. List of potentially relevant studies not included in the systematic review, along with the reasons for exclusion; Table S3. Newcastle—Ottawa Scale; Table S4. RoB2 Tool; Table S5. ROBINS-I Tool; Table S6. Summary of estimates of meta-regression to assess the influence of smoking on 25(OH)D serum level.
Author Contributions
Conceptualization, V.M. and J.B.; methodology, V.M., S.L. and J.B.; software, V.M., L.P. and J.B.; validation, J.J.M. and J.B.; formal analysis, V.M., L.P. and J.B.; investigation, V.M. and S.L.; resources, none; data curation, V.M., S.L. and J.B.; writing—original draft preparation, V.M. and S.L.; writing—review and editing, J.B.; visualization, J.J.M.; supervision, J.B.; project administration, V.M.; funding acquisition, None. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Borel P., Caillaud D., Cano N.J. Vitamin D Bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015;55:1193–1205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 2.Holick F.M. Vitamin D Deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Holick M.F., Chen T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87:1080–1086. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari H.A., Kiel D.P., Dawson-Hughes B., Orav J.E., Li R., Spiegelman D., Dietrich T., Willett W.C. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J. Bone Miner. Res. 2009;24:935–942. doi: 10.1359/jbmr.081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid I.R., Bolland M.J., Grey A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-Analysis. Lancet. 2014;383:146–155. doi: 10.1016/S0140-6736(13)61647-5. [DOI] [PubMed] [Google Scholar]
- 6.Kogawa M., Findlay D.M., Anderson P.H., Ormsby R., Vincent C., Morris H.A., Atkins G.J. Osteoclastic metabolism of 25(OH)-vitamin D3: A potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–4625. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- 7.Haussler M.R., Whitfield G.K., Kaneko I., Haussler C.A., Hsieh D., Hsieh J.C., Jurutka P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 8.Girgis C.M., Clifton-Bligh R.J., Hamrick M.W., Holick M.F., Gunton J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013;34:33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari H.A., Willett W.C., Wong J.B., Giovannucci E., Dietrich T., Dawson-Hughes B. Fracture Prevention With Vitamin D Supplementation. JAMA. 2005;293:2257. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 10.EFSA Panel on Dietetic Products. Nutrition and Allergies (EFSA NDA Panel) Turck D., Bresson J.L., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., et al. Update of the tolerable upper intake level for vitamin D for infants. EFSA J. 2018;16:e05365. doi: 10.2903/j.efsa.2018.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanham-New S.A., Wilson L.R. Vitamin D-has the new dawn for dietary recommendations arrived? Nutr. Bull. 2016;41:2–5. doi: 10.1111/nbu.12185. [DOI] [PubMed] [Google Scholar]
- 12.McKenna M.J., Murray B. Vitamin D Deficiency. Springer; New York, NY, USA: 2014. [Google Scholar]
- 13.Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A., Pierroz D.D., Weber P., Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 14.Mogire R.M., Mutua A., Kimita W., Kamau A., Bejon P., Pettifor J.M., Adeyemo A., Williams T.N., Atkinson S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health. 2020;8:e134–e142. doi: 10.1016/S2214-109X(19)30457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darveau R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 16.Caton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., Mealey B.L., Papapanou P.N., Sanz M., Tonetti M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018;45:1–8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 17.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-analysis. JDR Clínical Res. Suppl. 2014;93:20s–28s. doi: 10.1177/0022034514537828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 19.Ramseier C.A., Anerud A., Dulac M., Lulic M., Cullinan M.P., Seymour G.J., Faddy M.J., Bürgin W., Schätzle M., Lang N.P. Natural history of periodontitis: Disease progression and tooth loss over 40 years. J. Clin. Periodontol. 2017;44:1182–1191. doi: 10.1111/jcpe.12782. [DOI] [PubMed] [Google Scholar]
- 20.Helal O., Göstemeyer G., Krois J., Fawzy El Sayed K., Graetz C., Schwendicke F. Predictors for tooth loss in periodontitis patients: Systematic review and meta-analysis. J. Clin. Periodontol. 2019;46:699–712. doi: 10.1111/jcpe.13118. [DOI] [PubMed] [Google Scholar]
- 21.Graziani F., Karapetsa D., Alonso B., Herrera D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontology 2000. 2017;75:152–188. doi: 10.1111/prd.12201. [DOI] [PubMed] [Google Scholar]
- 22.Najeeb S., Zafar M.S., Khurshid Z., Zohaib S., Almas K. The role of nutrition in periodontal health: An update. Nutrients. 2016;8:530. doi: 10.3390/nu8090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagelavičienė E., Vaitkevičienė I., Šilingaitė D., Šinkūnaitė E., Daugėlaitė G. The relationship between vitamin D and periodontal pathology. Medicina. 2018;54:45. doi: 10.3390/medicina54030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia M.N., Hildebolt C.F., Miley D.D., Dixon D.A., Couture R.A., Anderson Spearie C.L., Langenwalter E.M., Shannon W.D., Deych E., Mueller C., et al. One-Year Effects of Vitamin D and Calcium Supplementation on Chronic Periodontitis. J. Periodontol. 2011;82:25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant W.B., Boucher B.J. Are hill’s criteria for causality satisfied for vitamin D and periodontal disease? Dermatoendocrinology. 2010;2:30–36. doi: 10.4161/derm.2.1.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein S.H., Livada R., Tipton D.A. Re-evaluating the role of vitamin D in the periodontium. J. Periodontal Res. 2014;49:545–553. doi: 10.1111/jre.12149. [DOI] [PubMed] [Google Scholar]
- 27.Botelho J., Machado V., Proença L., Delgado A.S., Mendes J.J. Vitamin D deficiency and oral health: A comprehensive review. Nutrients. 2020;12:1471. doi: 10.3390/nu12051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anbarcioglu E., Kirtiloglu T., Öztürk A., Kolbakir F., Acıkgöz G., Colak R. Vitamin D deficiency in patients with aggressive periodontitis. Oral Dis. 2018;25:242–249. doi: 10.1111/odi.12968. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A.A., Kolte A.P., Kolte R.A., Chari S., Gupta M., Pakhmode R. Evaluation and comparison of serum vitamin D and calcium levels in periodontally healthy, chronic gingivitis and chronic periodontitis in patients with and without diabetes mellitus–a cross-sectional study. Acta Odontol. Scand. 2019;77:592–599. doi: 10.1080/00016357.2019.1623910. [DOI] [PubMed] [Google Scholar]
- 30.Ebersole J.L., Lambert J., Bush H., Huja P.E., Basu A. Serum nutrient levels and aging effects on periodontitis. Nutrients. 2018;10:1986. doi: 10.3390/nu10121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isola G., Alibrandi A., Rapisarda E., Matarese G., Williams R.C., Leonardi R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020:1–11. doi: 10.1111/jre.12746. [DOI] [PubMed] [Google Scholar]
- 32.Ketharanathan V., Torgersen G.R., Petrovski B.É., Preus H.R. Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D 3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health. 2019;19:1–7. doi: 10.1186/s12903-019-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini E., Sinjari B., Piscopo F., Porreca A., Reale M., Caputi S., Murmura G. Evaluation of salivary cytokines and vitamin D levels in periodontopathic patients. Int. J. Mol. Sci. 2020;21:2669. doi: 10.3390/ijms21082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietrich T., Joshipura K.J., Dawson-Hughes B., Bischoff-Ferrari H.A. Association between serum concentrations of 25-hydroxyvitamin D 3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 35.Millen A.E., Hovey K.M., LaMonte M.J., Swanson M., Andrews C.A., Kluczynski M.A., Genco R.J., Wactawski-Wende J. Plasma 25-Hydroxyvitamin D Concentrations and Periodontal Disease in Postmenopausal Women. J. Periodontol. 2013;84:1243–1256. doi: 10.1902/jop.2012.120445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonoglou G.N., Knuuttila M., Niemelä O., Raunio T., Karttunen R., Vainio O., Hedberg P., Ylöstalo P., Tervonen T. Low serum level of 1,25(OH)2D is associated with chronic periodontitis. J. Periodontal Res. 2015;50:274–280. doi: 10.1111/jre.12207. [DOI] [PubMed] [Google Scholar]
- 37.Hu X., Niu L., Ma C., Huang Y., Yang X., Shi Y., Pan C., Liu J., Wang H., Li Q., et al. Calcitriol decreases live Porphyromonas gingivalis internalized into epithelial cells and monocytes by promoting autophagy. J. Periodontol. 2019;91:956–966. doi: 10.1002/JPER.19-0510. [DOI] [PubMed] [Google Scholar]
- 38.Han J., Cheng C., Zhu Z., Lin M., Zhang D.X., Wang Z.M., Wang S. Vitamin D reduces the serum levels of inflammatory cytokines in rat models of periodontitis and chronic obstructive pulmonary disease. J. Oral Sci. 2019;61:53–60. doi: 10.2334/josnusd.17-0357. [DOI] [PubMed] [Google Scholar]
- 39.Oh C., Kim H.J., Kim H.M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α. J. Periodontal Implant Sci. 2019;49:270–286. doi: 10.5051/jpis.2019.49.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Zhou X., Zhang P., Zhao P., Nie L., Ji N., Ding Y., Wang Q. 25-Hydroxyvitamin D3 positively regulates periodontal inflammaging via SOCS3/STAT signaling in diabetic mice. Steroids. 2020;156:108570. doi: 10.1016/j.steroids.2019.108570. [DOI] [PubMed] [Google Scholar]
- 41.Li H., Zhong X., Li W., Wang Q. Effects of 1,25-dihydroxyvitamin on experimental periodontitis and ahr/nf-κb/nlrp3 inflammasome pathway in a mouse model. J. Appl. Oral Sci. 2019;27:1–10. doi: 10.1590/1678-7757-2018-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Putten G.J., Vanobbergen J., De Visschere L., Schols J., de Baat C. Association of some specific nutrient deficiencies with periodontal disease in elderly people: A systematic literature review. Nutrition. 2009;25:717–722. doi: 10.1016/j.nut.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Perić M., Cavalier E., Toma S., Lasserre J.F. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population—a systematic review. J. Periodontal Res. 2018;53:645–656. doi: 10.1111/jre.12560. [DOI] [PubMed] [Google Scholar]
- 44.Pinto J.P.N.S., Goergen J., Muniz F.W.M.G., Haas A.N. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018;53:298–305. doi: 10.1111/jre.12531. [DOI] [PubMed] [Google Scholar]
- 45.Fakheran O., Khodadadi-Bohlouli Z., Khademi A. Effect of Vitamin D level on periodontal treatment outcomes: A systematic review. Gen. Dent. 2019;67:64–67. [PubMed] [Google Scholar]
- 46.Higgins P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. 4th ed. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 47.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.OpenGrey. [(accessed on 20 April 2020)]; Available online: http://www.opengrey.eu.
- 49.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:4–10. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarzer G., Carpenter J.R., Rücker G. Meta-Analysis with R. Springer; Berlin/Heidelberg, Germany: 2015. [Google Scholar]
- 52.Schwarzer G. meta: An R Package for Meta-Analysis. R News. 2007;7:40–45. [Google Scholar]
- 53.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. The Cochrane Collaboration ’s tool for assessing risk of bias in randomised trials. BMJ Br. Med. J. 2011;343:1–9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S., Hartmann-Boyce J., Ryan R., Shepperd S., Thomas J., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ. 2020;368:16890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman M.G., Weyant R., Hujoel P. JEBDP Improves Grading System and Adopts Strength of Recommendation Taxonomy Grading (SORT) for Guidelines and Systematic Reviews. J. Evid. Based. Dent. Pract. 2007;7:147–150. doi: 10.1016/j.jebdp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X., Meng H., Sun X., Xu L., Zhang L., Shi D., Feng X., Lu R., Chen Z. Elevation of vitamin D-binding protein levels in the plasma of patients with generalized aggressive periodontitis. J. Periodontal Res. 2012;48:74–79. doi: 10.1111/j.1600-0765.2012.01505.x. [DOI] [PubMed] [Google Scholar]
- 57.Gumus P., Huseyinalemdaroglu B., Buduneli N. The role of oxidative stress in the interaction of periodontal disease with systemic diseases or conditions. Oxid. Antioxid. Med. Sci. 2016;5:33. doi: 10.5455/oams.310516.rv.024. [DOI] [Google Scholar]
- 58.Miricescu D., Totan A., Calenic B., Mocanu B., Didilescu A., Mohora M., Spinu T., Greabu M. Salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol. Scand. 2014;72:42–47. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 59.Laky M., Bertl K., Haririan H., Andrukhov O., Seemann R., Volf I., Assinger A., Gruber R., Moritz A., Rausch-Fan X. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin. Oral Investig. 2017;21:1553–1558. doi: 10.1007/s00784-016-1965-2. [DOI] [PubMed] [Google Scholar]
- 60.Abreu O.J., Tatakis D.N., Elias-Boneta A.R., López Del Valle L., Hernandez R., Pousa M.S., Palacios C. Low vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health. 2016;16:1–5. doi: 10.1186/s12903-016-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joseph R., Nagrale A., Joseraj M., Pradeep Kumar K., Kaziyarakath J., Chandini R. Low levels of serum Vitamin D in chronic periodontitis patients with type 2 diabetes mellitus: A hospital-based cross-sectional clinical study. J. Indian Soc. Periodontol. 2015;19:501–506. doi: 10.4103/0972-124X.167162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuce H.B., Gokturk O., Turkal H.A., Inanir A., Benli I., Demir O. Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. J. Oral Sci. 2017;59:397–404. doi: 10.2334/josnusd.16-0677. [DOI] [PubMed] [Google Scholar]
- 63.Gao W., Tang H., Wang D., Zhou X., Song Y., Wang Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal Res. 2020;55:354–362. doi: 10.1111/jre.12719. [DOI] [PubMed] [Google Scholar]
- 64.Perayil J., Menon K.S., Kurup S., Thomas A.E., Fenol A., Vyloppillil R., Bhaskar A., Megha S. Influence of Vitamin D & calcium supplementation in the management of periodontitis. J. Clin. Diagn. Res. 2015;9:ZC35–ZC38. doi: 10.7860/JCDR/2015/12292.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonnet C., Rabbani R., Moffatt M.E.K., Kelekis-Cholakis A., Schroth R.J. The Relation Between Periodontal Disease and Vitamin D. J. Can. Dent. Assoc. 2019;84:j4. [PubMed] [Google Scholar]
- 66.Khammissa R.A.G., Ballyram R., Jadwat Y., Fourie J., Lemmer J., Feller L. Vitamin D Deficiency as It Relates to Oral Immunity and Chronic Periodontitis. Int. J. Dent. 2018;2018 doi: 10.1155/2018/7315797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bashutski J.D., Eber R.M., Kinney J.S., Benavides E., Maitra S., Braun T.M., Giannobile W.V., McCauley L.K. Teriparatide and Osseous Regeneration in the Oral Cavity. N. Engl. J. Med. J Med. 2010;363:2396–2405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bashutski J.D., Eber R.M., Kinney J.S., Benavides E., Maitra S., Braun T.M., Giannobile W.V., McCauley L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011;90:1007–1012. doi: 10.1177/0022034511407771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tada H., Shimizu T., Nagaoka I., Takada H. Vitamin D3 analog maxacalcitol (OCT) induces hCAP-18/LL-37 production in human oral epithelial cells. Biomed. Res. 2016;37:199–205. doi: 10.2220/biomedres.37.199. [DOI] [PubMed] [Google Scholar]
- 70.Tada H., Shimizu T., Matsushita K., Takada H. Porphyromonas gingivalis-induced IL-33 down-regulates hCAP-18/LL-37 production in human gingival epithelial cells. Biomed. Res. 2017;38:167–173. doi: 10.2220/biomedres.38.167. [DOI] [PubMed] [Google Scholar]
- 71.Gao Z., Liu K., Meng H. Preliminary investigation of the vitamin D pathway in periodontal connective tissue cells. J. Periodontol. 2018;89:294–302. doi: 10.1002/JPER.17-0530. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X., Zhang P., Wang Q., Xia S., Ji N., Ding Y., Wang Q. 25-hydroxyvitamin D3 alleviates experimental periodontitis via promoting expression of cathelicidin in mice with type 2 diabetic mellitus. J. Nutr. Sci. Vitaminol. (Tokyo) 2018;64:307–315. doi: 10.3177/jnsv.64.307. [DOI] [PubMed] [Google Scholar]
- 73.Bayirli B.A., Öztürk A., Avci B. Serum vitamin D concentration is associated with antimicrobial peptide level in periodontal diseases. Arch. Oral Biol. 2020;117:104827. doi: 10.1016/j.archoralbio.2020.104827. [DOI] [PubMed] [Google Scholar]
- 74.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018;45:149–161. doi: 10.1111/jcpe.12945. [DOI] [PubMed] [Google Scholar]
- 75.Graetz C., Mann L., Krois J., Sälzer S., Kahl M., Springer C., Schwendicke F. Comparison of periodontitis patients’ classification in the 2018 versus 1999 classification. J. Clin. Periodontol. 2019;46:908–917. doi: 10.1111/jcpe.13157. [DOI] [PubMed] [Google Scholar]
- 76.Botelho J., Machado V., Proença L., Mendes J.J. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci. Rep. 2020;10:7093. doi: 10.1038/s41598-020-63700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varela-López A., Navarro-Hortal M.D., Giampieri F., Bullón P., Battino M., Quiles J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules. 2018;23:1226. doi: 10.3390/molecules23051226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia M.N., Hildebolt C. Limited Evidence Suggests That Vitamin D May Help Prevent and Treat Periodontal Disease in Adults. J. Evid. Based Dent. Pract. 2020;20:101342. doi: 10.1016/j.jebdp.2019.101342. [DOI] [PubMed] [Google Scholar]
- 79.Jassil N.K., Sharma A., Bikle D., Wang X. Vitamin D binding protein and 25-hydroxyvitamin D levels: Emerging clinical applications. Endocr. Pract. 2017;23:605–613. doi: 10.4158/EP161604.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global Burden of Severe Periodontitis in 1990-2010. J. Dent. Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bae S.-C., Lee Y.H. Association between Vitamin D level and/or deficiency, and systemic lupus erythematosus: A meta-analysis. Cell. Mol. Biol. 2018;51:7–13. doi: 10.14715/cmb/2018.64.1.2. [DOI] [PubMed] [Google Scholar]
- 82.Lee H.J., Je D.I., Won S.J., Paik D.I., Bae K.H. Association between Vitamin D deficiency and periodontal status in current smokers. Community Dent. Oral Epidemiol. 2015;43:471–478. doi: 10.1111/cdoe.12173. [DOI] [PubMed] [Google Scholar]
- 83.Lin J., Liu J., Davies M.L., Chen W. Serum Vitamin D level and rheumatoid arthritis disease activity: Review and meta-analysis. PLoS ONE. 2016;11:e0146351. doi: 10.1371/journal.pone.0146351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L., Song Y., Manson J.A.E., Pilz S., März W., Michaëlsson K., Lundqvist A., Jassal S.K., Barrett-Connor E., Zhang C., et al. Circulating 25-Hydroxy-Vitamin D and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ. Cardiovasc. Qual. Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou R., Wang M., Huang H., Li W., Hu Y., Wu T. Lower vitamin D status is associated with an increased risk of ischemic stroke: A systematic review and meta-analysis. Nutrients. 2018;10:277. doi: 10.3390/nu10030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brøndum-Jacobsen P., Nordestgaard B.G., Schnohr P., Benn M. 25-Hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann. Neurol. 2013;73:38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 87.Autier P., Boniol M., Pizot C., Mullie P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 88.Lips P., Eekhoff M., van Schoor N., Oosterwerff M., de Jongh R., Krul-Poel Y., Simsek S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017;173:280–285. doi: 10.1016/j.jsbmb.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 89.Santos R.K.F., Brandão-Lima P.N., Tete R.M.D.D., Freire A.R.S., Pires L.V. Vitamin D ratio and glycaemic control in individuals with type 2 diabetes mellitus: A systematic review. Diabetes. Metab. Res. Rev. 2018;34:1–11. doi: 10.1002/dmrr.2969. [DOI] [PubMed] [Google Scholar]
- 90.Fabisiak N., Fabisiak A., Watala C., Fichna J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017;51:878–889. doi: 10.1097/MCG.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 91.Del Pinto R., Pietropaoli D., Chandar A.K., Ferri C., Cominelli F. Association between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2015;21:2708–2717. doi: 10.1097/MIB.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lerchbaum E., Rabe T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014;26:145–150. doi: 10.1097/GCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 93.Trummer C., Pilz S., Schwetz V., Obermayer-Pietsch B., Lerchbaum E. Vitamin D, PCOS and androgens in men: A systematic review. Endocr. Connect. 2018;7:R95–R113. doi: 10.1530/EC-18-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He C., Lin Z., Robb S.W., Ezeamama A.E. Serum vitamin d levels and polycystic ovary syndrome: A systematic review and meta-analysis. Nutrients. 2015;7:4555–4577. doi: 10.3390/nu7064555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Irani M., Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014;102:460–468. doi: 10.1016/j.fertnstert.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 96.Klingberg E., Oleröd G., Konar J., Petzold M., Hammarsten O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine. 2015;49:800–808. doi: 10.1007/s12020-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yousefzadeh P., Shapses S.A., Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int. J. Endocrinol. 2014;2014:1–6. doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muñoz Aguilera E., Suvan J., Buti J., Czesnikiewicz-Guzik M., Barbosa Ribeiro A., Orlandi M., Guzik T.J., Hingorani A.D., Nart J., D’Aiuto F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 99.Machado V., Aguilera E.M., Hussain S.B. Association between Periodontitis and High Blood Pressure: Results from the Study of Periodontal Health in Almada-Seixal (SoPHiAS) J. Clin. Med. 2020;9:1585. doi: 10.3390/jcm9051585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Machado V., Botelho J., Lopes J., Patrão M., Alves R., Chambrone L., Alcoforado G., Mendes J.J. Periodontitis impact in interleukin-6 serum levels in solid organ transplanted patients: A systematic review and meta-analysis. Diagnostics. 2020;10:184. doi: 10.3390/diagnostics10040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Machado V., Escalda C., Proença L., Mendes J.J., Botelho J. Is There a Bidirectional Association between Polycystic Ovarian Syndrome and Periodontitis? A Systematic Review and Meta-analysis. J. Clin. Med. 2020;9:1961. doi: 10.3390/jcm9061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hussain S.B., Botelho J., Machado V., Zehra S.A., Mendes J.J., Ciurtin C., Orlandi M., Aiuto F.D. Seminars in Arthritis and Rheumatism. WB Saunders; Philadelphia, PA, USA: 2020. Is there a bidirectional association between rheumatoid arthritis and periodontitis? A systematic review and meta-analysis. [DOI] [PubMed] [Google Scholar]
- 103.Machado V., Lopes J., Patrão M., Botelho J., Proença L., Mendes J.J. Validity of the association between periodontitis and female infertility conditions: A concise review. Reproduction. 2020 doi: 10.1530/REP-20-0176. [DOI] [PubMed] [Google Scholar]
- 104.Sanz M., Marco del Castillo A., Jepsen S., Gonzalez-Juanatey J.R., D’Aiuto F., Bouchard P., Chapple I., Dietrich T., Gotsman I., Graziani F., et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020;47:268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Preshaw P.M., Bissett S.M. Periodontitis and diabetes. Br. Dent. J. 2019;227:577–584. doi: 10.1038/s41415-019-0794-5. [DOI] [PubMed] [Google Scholar]
- 106.Snellman G., Melhus H., Gedeborg R., Byberg L., Berglund L., Wernroth L., Michaëlsson K. Determining vitamin D status: A comparison between commercially available assays. PLoS ONE. 2010;5:3–9. doi: 10.1371/annotation/23307aa4-726e-4f11-86c0-8a292be33517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X., Meng H., Xu L., Zhang L., Shi D., Feng X., Lu R., Chen Z. Vitamin D-binding protein levels in plasma and gingival crevicular fluid of patients with generalized aggressive periodontitis. Int. J. Endocrinol. 2014;2014 doi: 10.1155/2014/783575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rafique S., Hingorjo M.R., Mumtaz M., Qureshi M.A. The relationship of 1,25-dihydroxyvitamin D and vitamin D binding protein in periodontitis. Pakistan J. Med. Sci. 2019;35:847–851. doi: 10.12669/pjms.35.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwarz F., Derks J., Monje A., Wang H.L., Wolfgang J. Peri-implantitis. J. Clin. Periodontol. 2018;45:S246–S266. doi: 10.1111/jcpe.12954. [DOI] [PubMed] [Google Scholar]
- 110.Sgolastra F., Petrucci A., Severino M., Gatto R., Monaco A. Periodontitis, implant loss and peri-implantitis: A meta-analysis. Clin. Oral Implants Res. 2013;26:e8–e16. doi: 10.1111/clr.12319. [DOI] [PubMed] [Google Scholar]
- 111.Acipinar S., Karsiyaka Hendek M., Olgun E., Kisa U. Evaluation of FGF-23 and 25(OH)D3 levels in peri-implant sulcus fluid in peri-implant health and diseases. Clin. Implant Dent. Relat. Res. 2019;21:1106–1112. doi: 10.1111/cid.12832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.