Abstract
Dietary fibres are an integral part of a balanced diet. Consumption of a high-fibre diet confers many physiological and metabolic benefits. However, fibre is generally avoided by individuals with gastrointestinal motility disorders like gastroparesis due to increased likelihood of exacerbated symptoms. Low-viscosity soluble fibres have been identified as a possible source of fibre tolerable for these individuals. The aim of this study is to determine the rheological properties of 10 common commercially available soluble fibres in chemically simulated digestive conditions and evaluate their suitability for individuals with mild to moderate gastroparesis, a gastric motility disorder. Rheological testing under neutral condition (distilled water pH 7) and chemically simulated gastric digestion were evaluated to determine the yield point and relative viscosity of each fibre. Our results reveal two rheological categories of soluble fibres; pseudoplastic and dilatant. Simulated digestion was shown to significantly alter the yield-points of psyllium husk, iota-carrageenan, beta-glucan, apple-fibre pectin, and inulin. Gum Arabic and partially hydrolysed guar gum showed the lowest viscosities and were not affected under simulated digestion, characteristics that make them potential candidate fibres for patients with gastroparesis. Altogether, our results demonstrate that digestion can have a significant impact on fibre viscosity and should be taken into consideration when evaluating the suitability of fibres for patients with gastric motility disorders.
Keywords: dietary fibres, soluble fibre, gastroparesis, motility disorder, rheology
1. Introduction
Dietary fibres are an integral part of a balanced diet. An adequate daily intake of fibre is essential to maintain good gut health, reduce the risk of diabetes, heart disease and colorectal cancer [1,2,3,4]. Dietary fibres are defined by Food Standards Australia New Zealand (FSANZ) as “The fraction of edible parts of plants or their extracts, or synthetic analogues, that are resistant to digestion and absorption in the small intestine, usually with complete or partial fermentation in the large intestine. This includes polysaccharides, oligosaccharides (degree of polymerisation >2) and lignans” [5]. Such a broad definition of dietary fibres indicates that there are many different types with attendant structural chemistry, rheological properties and physiological effects. Physicians recommend that healthy adults should consume 25–30 g of fibre daily as part of their balanced diet, but most adults and children fail to meet their daily intake requirement [6,7]. Shortfalls in dietary fibre intake can lead to poor gut health, increased risk of obesity and chronic gastrointestinal (GI) disorders such as constipation, diarrhoea and IBS [8].
There are primarily two types of fibre in food—soluble and insoluble. Both these types of dietary fibre are indigestible in the GI tract but play an important role in gut health and motility. Soluble fibres become ‘sticky’ and absorb water in the digestive system to form gel-like substances that help modulate blood glucose, reduce cholesterol and reduce gastroesophageal reflux disease symptoms [9,10,11]. Soluble fibres play a role in improving lower GI health through fermentation by gut bacteria and the release of beneficial by-products, including short chain fatty acid (SCFA) acetates, propionates and butyrates [12,13]. Soluble fibres such as psyllium, β-glucan, pectin and guar gum form colloidal gels and hydrocolloid pastes that are ideally suited to provide cholesterol reduction, glycaemic control and early satiety in patients with type 2 diabetes mellitus, laxation for patients with constipation and irritable bowel syndrome and colonic relief in patients with diarrhoea [14,15]. The addition of dietary fibres has been shown to affect the viscosity of small intestinal digesta and the bioavailability of antioxidants [16,17].
Gastroparesis is a GI motility disorder where there is reduced gastric functionality and varying degrees of stomach paralysis. Sufferers experience a range of post-prandial symptoms associated with delayed gastric emptying [18]. Despite the benefits of soluble fibres, they are not recommended to patients with gastroparesis due to the risk of exacerbated symptoms such as bloating, nausea, abdominal pains and vomiting [18,19,20,21,22]. Certain soluble fibres are shown to cause delayed gastric emptying, and this has been verified using 99mTc-sulphur colloid radio-labelled meals and gastric-emptying scintigraphy [23]. Gastroparesis patients also suffer from 30–40% co-morbidity with either type 1 or 2 diabetes mellitus where soluble dietary fibres can play an important part in glucose modulation [24]. A long-term lack of fibre can lead to dysbiosis and poor gut health; therefore, viable soluble dietary fibre options are needed for gastroparesis sufferers [21,24].
Properties such as the molecular weight (MW), chain length, chemical structure and branching, the preparation solvent, pH and the thermodynamic configuration of constituent polysaccharides in solution play a significant role in determining the rheological properties of a dietary fibre preparation [25,26,27]. A category of soluble fibre called “low-viscosity fibres” have recently gained significant interest due to the ease in which they can be emptied through the GI tract, while displaying significant metabolic and physiological benefits. Dilution and hydrolysis of dietary fibres are often employed commercially in order to decrease the viscosity of food preparations, a prominent example of this being partially hydrolysed guar gum (PHGG) soluble fibre, which is manufactured by the hydrolysis of guar gum soluble fibre. The beneficial effects of low-viscosity type soluble dietary fibres have been documented in the literature [28,29,30]. Low-viscosity fibres have been used to alter the texture, rheology, taste and colour of manufactured food products to assist in glucose modulation, body weight management and as prebiotic supplements.
The sum of existing literature indicates a need to characterise the rheological behaviour of certain commonly available soluble dietary fibres in depth. Such characterisations would greatly aid healthcare providers in determining whether certain soluble dietary fibres can be incorporated into foods designed for gastroparesis patients. It has been noted in the literature that early satiety, a symptom of gastroparesis, is better correlated with the viscosity of gastric-phase digestion rather than the viscosity of intestinal-phase digestion [31,32]. Hence, the rheological properties of fibres under simulated upper GI digestion would provide a more accurate assessment of fibre suitability for gastroparesis patients [33].
The primary aim of this study is to determine the rheological properties of 10 soluble fibres during chemically simulated upper GI digestion. Chemical digestion is performed in order to identify candidate fibres suitable for patients with mild-to-moderate gastroparesis symptoms who are capable of oral feeding. By examining the rheological behaviour of the selected fibres at varying concentrations and conditions, the authors hypothesise that “low-viscosity” soluble fibres would retain low yield-point shear stress values at crossovers during simulated digestion.
2. Materials and Methods
2.1. Instrumentation and Equipment
The primary instrument was the Dynamic Stress Rheometer (DSR)™ and CPU from Rheometric Scientific, Texas Instruments (Piscataway, NJ, USA) which included a Neslab RTE11 water bath and fibre Dry™ air-dryer from Pisco (Elmhurst, IL, USA). A 40.0-mm-diameter, smooth, chrome-finished aluminium bottom plate; 40.0-mm-diameter, smooth, hard-coated aluminium upper plate; and the hard-coated aluminium adapter for the upper plate used in the analysis were all acquired from Rheometric Scientific, Texas Instruments (Piscataway, NJ, USA).
2.2. Chemicals and Reagents
The dietary fibre samples were purchased from various commercial suppliers in Australia and the labelled dietary information on the products is shown in Table 1. The following chemical standards with reported analytical purity were procured from multiple suppliers. Sodium chloride (99.7%), potassium chloride (99.0%), sodium bicarbonate (99.7%) and hydrochloric acid (32.0%) were purchased from Chem-Supply (Gillman, SA, Australia). The pepsin (99.0%) reference standard was procured from European Pharmacopoeia (Strasbourg, France) and α-amylase (99.0%) was purchased from Sigma-Aldrich (St Louis, MO, USA). The purified de-ionized water used in the analyses (>18 MΩ.cm) was obtained from a MilliQ™ Advantage A10 system with a Q-POD from Merck (Darmstadt, Germany).
Table 1.
Labelled nutritional content of tested dietary fibre supplements.
| Product (Name) Qty (per 100 g) |
Commercial Supplier (Name) | Total Dietary Fibre (%) | Energy (kJ) |
Carbohydrates, Sugars (g) | Protein (g) |
Total Fat, Saturated Fat (g) | Essential Nutrients (mg) * |
|---|---|---|---|---|---|---|---|
| Guar gum | Ceres Organics | 77.3 | 743.0 | 0.5, 0.5 | 4.7 | 0.3, 0.0 | 13.0 |
| Iota-carrageenan | The Melbourne Food Ingredient Depot | 76.0 | 1298.0 | 76.0, 0.0 | 0.0 | 0.0, 0.0 | 640.0 |
| Xanthan gum | Myprotein | 62.0 | 832.0 | 78.0, 0.0 | 3.3 | 0.0, 0.0 | 0.0 |
| Psyllium husk | SF Health Foods | 80.0 | 802.0 | 0.0, 0.0 | 3.0 | 3.0, 0.0 | 79.0 |
| Citrus pectin | Lotus Pantry | 55.0 | 1005.0 | 30.0, 30.0 | 0.0 | 0.0, 0.0 | 1000.0 |
| Beta-glucan | Blooms Health Products | 44.0 | 1251.0 | 22.0, 0.0 | 20.0 | 5.0, 1.0 | 1088.0 |
| Apple-fibre pectin | Myprotein | 40.0 | 1850.0 | 90.0, 40.0 | 0.0 | 0.0, 0.0 | 0.0 |
| Inulin | Myprotein | 89.0 | 848.0 | 8.0, 8.0 | 0.0 | 0.0, 0.0 | 0.0 |
| Gum Arabic | New Directions Australia | 80.0 | 1339.0 | 80.0, 0.0 | 0.0 | 0.0, 0.0 | 0.0 |
| Partially hydrolysed guar gum (PHGG) | Healthy Origins | 80.0 | 1691.0 | 93.0, 13.0 | 0.0 | 0.0, 0.0 | 0.0 |
* Essential nutrients are the cumulative Na, Mg, Ca, K, Fe and Zn present in the commercial fibre supplement in mg.
2.3. Experimental Procedure
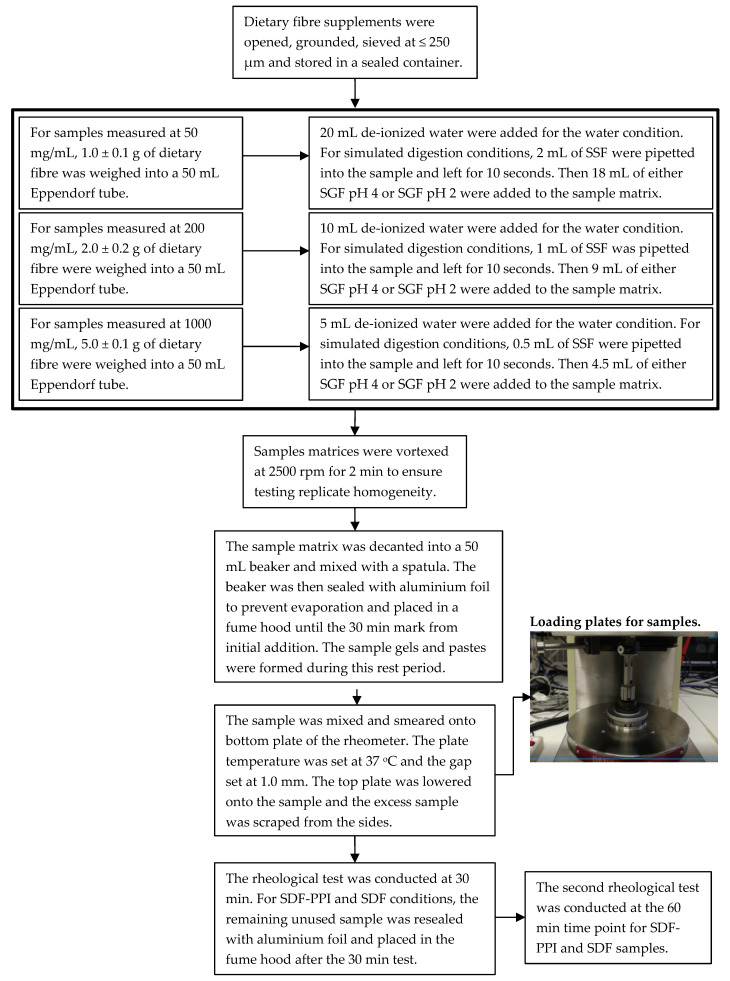
The simulated salivary fluid (SSF) and simulated gastric fluids (SGF) were prepared in accordance with the Davis and Minekus methods reported in the literature [34,35]. Distilled water (pH 7) was collected in a 1-L Schott bottle from de-ionized water. The SSF was prepared by accurately weighing out potassium chloride (0.4 mM), sodium chloride (0.4 mM), sodium bicarbonate (5.0 mM) and α-amylase (2.0% w/v) into a 250-mL Schott bottle and made up to volume with 200 mL of de-ionized water. The SGF solutions were prepared by accurately weighing out sodium chloride (34.2 mM) and pepsin (0.0525% w/v) into a 1-L Schott bottle. The SGF solution was adjusted to physiological gastric pH 2 and made to volume with 1 L of de-ionized water. An additional SGF solution was made to pH 4 to simulate gastric condition of individuals on proton-pump inhibitors (PPI). It has been reported that approximately 60% of gastroparesis patients also suffer gastro-oesophageal reflux disorder [24] and are therefore prescribed PPI, which reduces gastric acid production and elevates gastric pH from 2 to 4 [36]. The SGF at pH 4 was used to accurately represent the physiological environment within these patients. The SGF solutions (pH 4 and pH 2) were created by the dropwise addition of dilute hydrochloric acid (6.4% v/v) and pH adjustment using a calibrated pH meter (Mettler Toledo, Port Melbourne, VIC, Australia). All the prepared solutions were then sonicated at 60 °C for 30 min and then cooled for 20 min. The sonication and cooling procedures were performed each time the solutions were used for analysis. The solutions were re-sealed and stored in a storage cabinet after each use with a 1-week expiry. Before analysis under the SDF-PPI (simulated digestion fluid proton pump inhibitor at pH 4) and SDF (simulated digestion fluid pH 2) conditions, the SSF and SGF solutions were sealed and placed in a water bath at 37 °C. The experimental rubric for the preparation of the dietary fibre samples is shown in Figure 1. Under each condition, rheological measurements were taken at 30 min after sample preparation. The sample beakers were sealed with aluminium foil and stored in a fume hood and then re-opened for rheological measurements at 60 min.
Figure 1.
Procedural flowchart for sample preparation and rheological analysis. Keywords as follows: SSF (simulated salivary fluid), SGF (simulated gastric fluid), SDF-PPI (simulated digestion fluid proton pump inhibitor at pH 4), SDF (simulated digestion fluid at pH 2).
2.4. Rheological Method
The data acquisition and analysis were performed using the RSI Orchestrator v6.5.8 software from Rheometric Scientific (Piscataway, NJ, USA). The sample geometry was initialized using the stored geometry “[Para Plate] 40 mm dia PP Geometry P0019”. The smooth parallel-plate geometry was selected for the rheological analysis since a large gap of 0.3–1.0 mm can be used to reduce shear during loading and axial stresses during oscillation, which is proven effective for larger particle-size colloidal gels and hydrocolloid pastes that are formed by dietary fibre preparations [37,38,39]. The plate diameter was set at 40.0 mm and the gap between the smooth parallel plates was set at 1.0 mm and auto calibrated. The minimum sample volume was 1.257 cm3 and the tool serial number was 0019. The pre-defined “[DStresSwp] Dynamic Stress Sweep Test” test setup was used in the method.
2.5. Data Acquisition and Analysis
The dynamic stress sweep test was stress-controlled with a frequency of oscillation (ω) of 45.0 rad/s (7.28 Hz) and the sweep mode was linear. The rotation of the smooth parallel plates created the flow behaviour needed to measure the viscoelastic properties of the sample and the oscillation enables destruction free, highly precise movements that are used to measure within the sample’s viscoelastic range. The lower plate temperature was set at 37 °C to match standard human biological temperature. The samples were analysed rapidly (1–2 min) to ensure that they did not dry out on the plate, leading to consistent measurement across all sample matrices. The measurement concentration, sample state at measured concentration, rheological behaviour type, shear stress increment, initial applied shear stress (min: 0.078 Pa), final applied shear stress (max: 3901.942 Pa) and measurement time period for each dietary fibre are shown in Table 2. The target sample concentration for each dietary fibre preparation was gradually increased from 50 to 1000 mg/mL until a yield-point crossover was observed. For the sake of analysis, measurements were taken at 50, 200 and 1000 mg/mL concentration thresholds to compare yield points among the selected soluble dietary fibres.
Table 2.
Measured concentration, colloidal state, rheological behaviour and method analysis parameters under parallel plate configuration.
| Dietary Fibre (Name) | Measurement Concentration (mg/mL) | Sample State at Measured Concentration |
Rheological Behaviour at Concentration | Shear Stress Increment (Pa/s) | Initial Applied Shear Stress (Pa) |
Final Applied Shear Stress (Pa) |
Measurement Time Period (s) |
|---|---|---|---|---|---|---|---|
| Guar gum | 50.0 | Colloidal gel | Pseudoplastic | 30.0 | 400.0 | 1200.0 | 26.6 |
| Iota-carrageenan | 50.0 | Colloidal gel | Pseudoplastic | 25.0 | 400.0 | 1300.0 | 36.0 |
| Xanthan gum | 50.0 | Hydrocolloid paste | Pseudoplastic | 20.0 | 50.0 | 400.0 | 17.5 |
| Psyllium husk | 50.0 | Hydrocolloid paste | Pseudoplastic | 4.0 | 1.0 | 180.0 | 44.8 |
| Citrus pectin | 200.0 | Hydrocolloid paste | Pseudoplastic | 100.0 | 1500.0 | 3900.0 | 24.0 |
| Beta-glucan | 200.0 | Hydrocolloid paste | Pseudoplastic | 20.0 | 100.0 | 900.0 | 40.0 |
| Apple-fibre pectin | 200.0 | Hydrocolloid paste | Pseudoplastic | 1.0 | 1.0 | 100.0 | 99.0 |
| Inulin | 1000.0 | Hydrocolloid paste | Pseudoplastic | 8.0 | 20.0 | 240.0 | 27.5 |
| Gum Arabic | 1000.0 | Hydrocolloid paste | Dilatant | 0.4 | 10.0 | 50.0 | 100.0 |
| Partially hydrolysed guar gum (PHGG) | 1000.0 | Hydrocolloid paste | Dilatant | 0.4 | 10.0 | 40.0 | 75.0 |
The collected rheological measurements were statistically fitted by the application of Hook’s law for viscoelastic materials [40]. The thixotropy of the soluble dietary fibres was determined by the application of the Cox-Merz rule, where the functional dependence of complex viscosity (η*) magnitude is expressed as a function of frequency (ω) which is identical to functional dependence of the steady shear viscosity (η) which is expressed as a function of shear rate (γ) [41]. The equation for the Cox-Merz rule [42] reads as:
| |η*(ω)| = η(γ) | γ = ω |
After data acquisition, G’ (the elastic modulus responsible for energy storage in the sample), G” (the viscous modulus responsible for energy loss in the sample) and Tan (δ) (the loss or damping factor at phase angle δ) were logarithmically plotted using the in-built RSI orchestrator software. At the initial applied shear stress, the behaviour of G’ and G” was linear and these moduli deformed to produce the Gc (the crossover modulus) as oscillatory shear stress was gradually increased over time. The yield-point shear stress (τy) and Gc at the phase transition (or sol-gel transition) point were interpolated in each plot for each sample where:
| G’ = G” | Tan (δ) = 1 |
The complex viscosity (η*) was also plotted against increasing oscillatory shear stress to determine the type of rheological behaviour. The yield points and Gc values were tabulated and analysed using Microsoft Excel (Office 2016). The p-values reported in the results and discussion section were generated using a two-tailed, homoscedastic (two sample equal variance) Student’s t-test.
3. Results
3.1. Rheological Plots
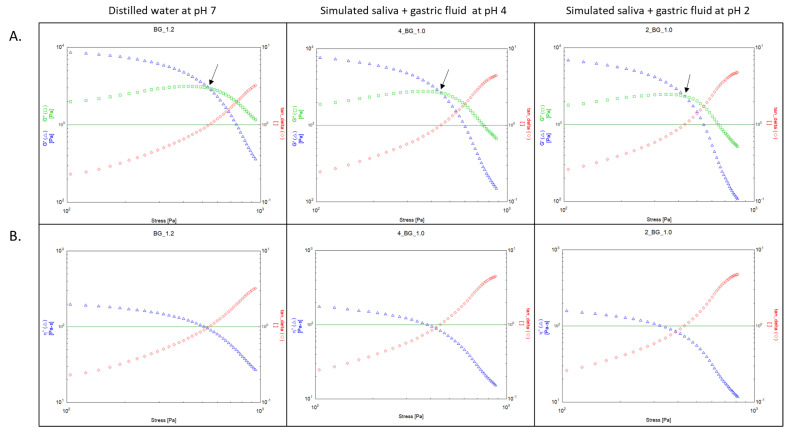
Rheological analysis of the soluble dietary fibres produced two distinct types of thixotropic phase transitions, pseudoplastic (shear-thinning) and dilatant (shear-thickening), which occur in non-Newtonian type viscoelastic gels and pastes. Figure 2 and Figure 3 show examples of each type of phase transition with arrows indicating the yield point. In both these figures, the elastic G’ modulus, the viscous G” modulus, the complex viscosity (η*) and Tan (δ) are tracked as the shear stress is increased. The majority of soluble fibres took the form of a pseudoplastic (shear-thinning) phase transition shown in Figure 2 at the sample concentration when the yield point was achieved, where initially G’ is greater than G”. As greater oscillatory shear stress (τ) is applied to the sample, G’ gradually decreases, and after the yield-point crossover, G” is greater. Once the yield point is crossed, the complex viscosity (η*) decreases rapidly as shown in Figure 2, indicating a complete breakdown in the molecular associations of the constituent dietary polysaccharides and therefore the sample begins to the “flow” from this point onward with G” dominating the rheological behaviour.
Figure 2.
An example of pseudoplastic rheological behaviour observed in beta-glucan. Graphs show (A) rheological yield points (top half, y-axis left: G’ and G” in Pa, y-axis right: Tan (δ), x-axis bottom: shear stress in Pa) and (B) complex viscosity (bottom half, y-axis left: η* in Pa.s, y-axis right: Tan (δ), x-axis bottom: shear stress in Pa) plots as shear stress is gradually increased in distilled water (pH 7), simulated digestions with SDF-PPI (simulated digestion fluid proton pump inhibitor at pH 4) and SDF (simulated digestion fluid at pH 2). The black arrow in each plot indicates the yield point where Tan (δ) = 1. Note that the rheological behaviour does not change under chemically simulated digestion.
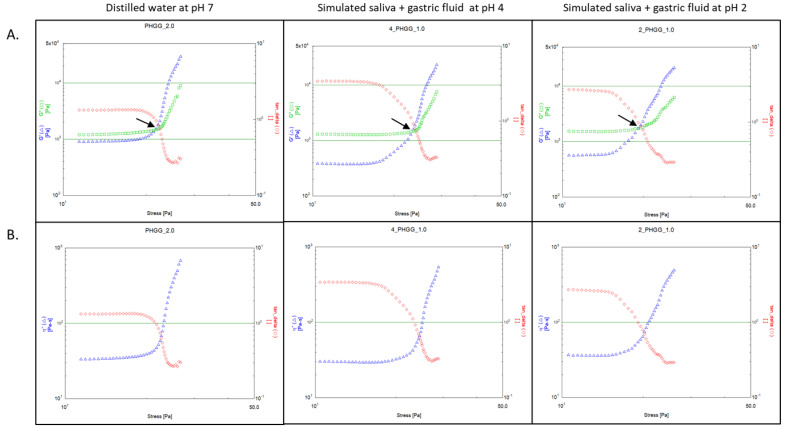
Figure 3.
An example of dilatant rheological behaviour observed in partially hydrolysed guar gum (PHGG). Graphs show (A) rheological yield points (top half, y-axis left: G’ and G” in Pa, y-axis right: Tan (δ), x-axis bottom: shear stress in Pa) and (B) complex viscosity (bottom half, y-axis left: η* in Pa.s, y-axis right: tan (δ), x-axis bottom: shear stress in Pa) plots as shear stress is gradually increased in distilled water (pH 7), simulated digestions with SDF-PPI (simulated digestion fluid proton pump inhibitor at pH 4) and SDF (simulated digestion fluid at pH 2). The black arrow in each plot indicates the yield point where Tan (δ) = 1. Note that the rheological behaviour does not change under chemically simulated digestion.
For PHGG and gum Arabic at 50 mg/mL, G” is greater than G’ with no yield point observed in the acquisition range for shear stress (0.078–3901.942 Pa). This type of viscoelastic behaviour at low concentration is almost entirely viscous and Newtonian, since the fibre is completely dissolved in solution. This viscous Newtonian behaviour at 50 mg/mL was also found in the dietary fibres which produced pseudoplastic yield-point crossovers at 200 and 1000 mg/mL. As concentrations of PHGG and gum Arabic preparations were increased to 1000 mg/mL phase transitions and yield points were observed as shown in Figure 3. As the shear stress (τ) was increased, the complex viscosity (η*) of the sample increased until a steady-state was reached, indicating complex shear behaviour that is initially dilatant (or shear thickening). The type of behaviour where the G’ contribution is higher than the G” contribution is a non-Newtonian, thixotropic characteristic of gum Arabic and PHGG in the distilled water (pH 7), which is consistent with previous rheological studies of these fibres [43]. The dilatant rheological behaviour of gum Arabic and PHGG remained relatively consistent during simulated digestion.
3.2. Rheological Yield Points
The tabulated summary of the yield-point shear stress (τy) and crossover modulus (Gc) values under distilled water and simulated digestion are shown in Table 3. The sample measurements were acquired from sequential samples prepared in triplicate at both 30 and 60 min. The percentage relative standard deviation (%RSD) values across all measurements for yield shear stress measurements does not exceed ±12.81% (Citrus pectin in SDF-PPI). The %RSD values across all sample measurements for crossover modulus (Gc) does not exceed ±11.80% (Apple-fibre pectin in distilled water). The low %RSD values for both yield-point shear stress and Gc in the measurements across all dietary fibre preparations indicate good method precision, repeatability, and reliability [37].
Table 3.
Tabulated summary of yield-point shear stresses (τy), crossover moduli (Gc), and percentage relative standard deviation (%RSD) for the dietary fibre preparations in distilled water, and simulated digestion with gastric fluid at pH 4 and gastric fluid at pH 2.
| Dietary Fibre (Name) |
Sample Concentration (mg/mL) a |
Rheological Condition (pH; time point) b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled Water (pH 7; 30 min) |
Simulated Digestion (pH 4; 30 min) | Simulated Digestion (pH 2; 30 min) | Simulated Digestion (pH 4; 60 min) |
Simulated Digestion (pH 2; 60 min) |
|||||||
| τy (Pa) ± (%RSD) |
Gc (Pa) ± (%RSD) |
τy (Pa) ± (%RSD) |
Gc (Pa) ± (%RSD) |
τy (Pa) ± (%RSD) |
Gc (Pa) ± (%RSD) |
τy (Pa) ± (%RSD) |
Gc (Pa) ± (%RSD) |
τy (Pa) ± (%RSD) |
Gc (Pa) ± (%RSD) |
||
| Guar gum | 50.0 | 994.51 (4.56) | 1573.43 (5.46) | 1011.12 (6.01) | 796.77 (4.31) | 946.93 (2.14) | 810.08 (9.31) | 1057.50 (3.02) | 872.16 (7.47) | 975.59 (5.91) | 807.69 (7.28) |
| Iota-carrageenan | 50.0 | 700.08 (4.30) | 819.82 (2.18) | 758.00 (0.95) |
2050.50 (7.79) |
1232.03 (4.16) | 782.68 (8.07) | 921.82 (1.85) |
1480.77 (9.95) |
1068.17 (7.18) | 528.68 (5.28) |
| Xanthan gum | 50.0 | 156.89 (7.16) |
114.16 (7.48) |
160.99 (6.63) |
125.16 (9.63) |
180.37 (3.94) |
129.33 (4.41) |
174.83 (1.47) |
139.27 (11.63) |
183.16 (9.40) |
128.65 (11.64) |
| Psyllium husk | 50.0 | 56.31 (6.78) |
134.31 (3.12) |
51.38 (9.49) |
117.06 (4.04) |
80.85 (4.19) |
119.94 (0.88) |
62.70 (9.90) |
116.84 (3.11) |
104.49 (10.24) |
123.44 (1.66) |
| Citrus pectin | 200.0 | 3049.43 (5.36) |
2043.93 (6.72) |
2848.50 (8.41) |
2234.63 (1.99) |
2996.10 (9.38) |
2036.47 (6.30) |
2725.07 (12.81) |
1578.80 (1.97) |
2911.83 (3.50) |
1743.17 (8.00) |
| Beta-glucan | 200.0 | 544.58 (6.08) |
2938.67 (1.22) |
454.57 (3.41) |
2653.37 (2.88) |
419.02 (7.57) |
2427.70 (9.47) |
614.19 (3.88) |
2952.63 (1.11) |
545.38 (5.28) |
2784.37 (6.67) |
| Apple-fibre pectin | 200.0 | 23.27 (8.49) |
343.67 (11.80) |
19.29 (2.53) |
226.21 (11.16) |
22.99 (4.88) |
295.24 (5.07) |
39.27 (6.56) |
451.10 (9.76) |
36.51 (2.13) |
385.13 (10.23) |
| Inulin | 1000.0 | 47.36 (4.64) |
98.01 (9.52) |
103.87 (7.40) |
151.04 (9.14) |
105.15 (6.37) |
143.09 (8.68) |
123.48 (0.94) |
222.70 (1.07) |
126.41 (4.22) |
197.24 (1.03) |
| Gum Arabic | 1000.0 | 31.76 (7.94) |
794.02 (6.41) |
36.09 (5.39) |
805.88 (2.53) |
33.83 (4.36) |
815.66 (4.55) |
35.66 (8.82) |
784.39 (8.11) |
31.15 (8.08) |
893.63 (5.75) |
| Partially hydrolysed guar gum (PHGG) | 1000.0 | 20.01 (7.52) |
1460.33 (3.79) |
21.99 (9.00) |
1504.47 (4.17) |
20.55 (8.78) |
1699.07 (2.86) |
23.51 (4.28) |
1522.40 (3.29) |
21.22 (2.30) |
1904.53 (10.41) |
a Fibre concentration in solution was increased when no crossover modulus (Gc) was observed in the 0.08–3901.01 Pa shear stress (τ) acquisition range; b Measurements taken at each condition with n = 3 replicates for yield shear stress (τy) and crossover modulus (Gc) in Pa with ± %RSD at the yield point (sol-gel transition point).
Among the 10 selected fibres in this study, preparations with yield points at 50 mg/mL are labelled as ‘high-viscosity’, preparations with yield points at 200 mg/mL are labelled as ‘medium-viscosity’ and preparations with yield points at 1000 mg/mL are labelled as ‘low-viscosity’. Such descriptions are relative terms with respect to the possible clinical use and does not describe the range of distinct rheological properties and behaviours, which is largely dependent on the preparation concentration and chemical composition of a dietary fibre supplement.
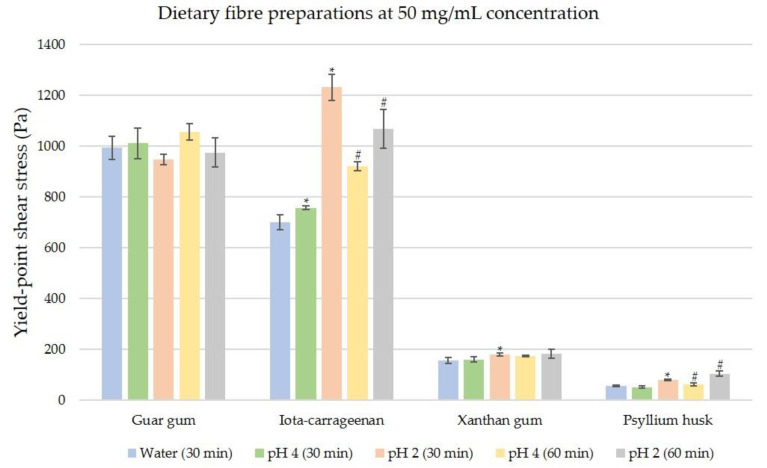
For the soluble dietary fibre preparations measured at 50 mg/mL, as shown in Figure 4, under distilled water conditions, guar gum exhibits the highest yield-point shear stress (994.51 Pa) and psyllium husk exhibits the lowest (56.31 Pa). At 30 min, no significant differences in shear stress yield points were observed between simulated digestions (pH 4) and in distilled water (p > 0.05), apart from iota-carrageenan (p = 0.03). Under simulated digestion (pH 2), psyllium husk (80.85 Pa) and iota-carrageenan (1232.03 Pa) display significantly increased yield stress (p < 0.01) along with xanthan gum (p < 0.05), while no changes were observed in guar gum. At 60 min for both simulated digestive conditions, changes in yield stress were observed for psyllium husk and iota-carrageenan (p < 0.05) while no major changes were observed for xanthan gum and guar gum (p > 0.05).
Figure 4.
Comparisons of yield-point shear stresses of four ‘high-viscosity’ fibres; guar gum, iota-carrageenan, xanthan gum, and psyllium husk at concentration of 50 mg/mL. Test conditions are in distilled water (pH 7), and simulated digestion at pH 4 and pH 2. The data tip (*) indicates significant difference between the distilled water condition and the simulated digestion condition (pH 4 or pH 2) at 30 min. The data tip (#) indicates significant difference between the simulated digestion condition at 30 min (pH 4 or pH 2) and its corresponding simulated digestion condition at 60 min. The significance level is p ≤ 0.05 for both (*) and (#).
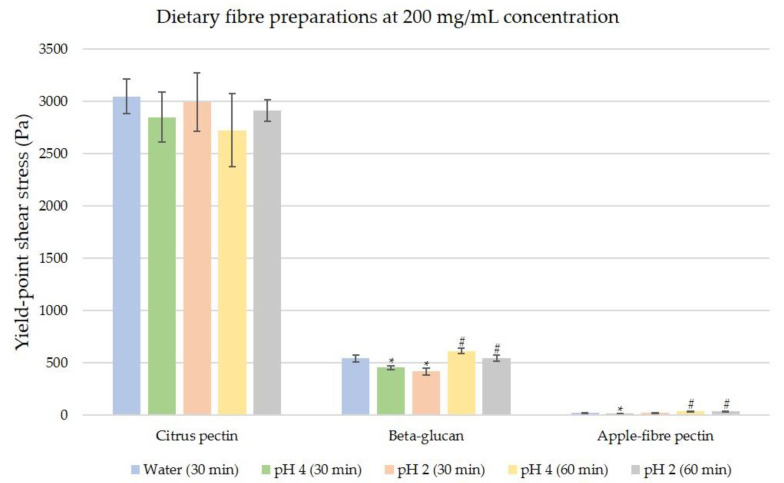
When the sample concentration was increased to 200 mg/mL as shown in Figure 5, yield points were observed for citrus pectin, apple-fibre pectin and beta-glucan. Under distilled water (pH 7), citrus pectin exhibited the highest yield-point shear stress (3049.43 Pa) and apple-fibre pectin exhibited the lowest yield shear stress (23.27 Pa). At 30 min under simulated digestive conditions, citrus pectin did not show any major changes in yield-point stress (p > 0.05), while apple-fibre pectin showed a minor decrease in yield-point shear stress in simulated digestion at pH 4 (p = 0.03). Beta-glucan showed a stepwise decrease in yield-point stress from distilled water (pH 7) to simulated digestion at pH 4 (p < 0.05) and then pH 2 (p = 0.01). At 60 min, citrus pectin exhibited no significant changes in yield-point stress (p > 0.05) while beta-glucan and apple-fibre pectin exhibited a significant increase in yield-point stress compared to the previous time point (p < 0.01).
Figure 5.
Comparisons of yield-point shear stresses of three ‘medium-viscosity’ fibres; citrus pectin, beta-glucan, and apple-fibre pectin at 200 mg/mL. Test conditions are in distilled water (pH 7), and simulated digestion at pH 4 and pH 2. The data tip (*) indicates significant difference between the distilled water condition and the simulated digestion condition (pH 4 or pH 2) at 30 min. The data tip (#) indicates significant difference between the simulated digestion condition at 30 min (pH 4 or pH 2) and its corresponding simulated digestion condition at 60 min. The significance level is p ≤ 0.05 for both (*) and (#).
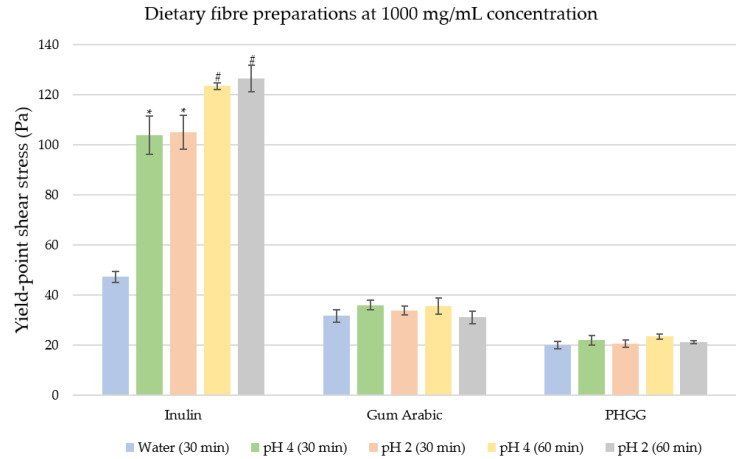
At 1000 mg/mL as shown in Figure 6, yields were then observed for inulin, gum Arabic and PHGG, with pseudoplastic rheological behaviour in inulin and dilatant behaviour in gum Arabic and PHGG. In distilled water (pH 7), inulin displayed the highest yield-point shear stress (47.36 Pa) and PHGG displayed the lowest (20.01 Pa). At 30 min in simulated digestion, no significant changes in yield-point stress were observed for either gum Arabic or PHGG compared to water (p > 0.05). On the other hand, inulin exhibits a significant increase in yield-point stress in simulated digestions at pH 4 and pH 2 (p < 0.01), relative to water. At 60 min, the yield-point stress changes were not significant for PHGG and gum Arabic (p > 0.05), while there was a significant increase in yield-point stress for inulin in both simulated digestion solutions compared to its 30-min timepoint (p = 0.01).
Figure 6.
Comparisons of yield-point shear stresses of three ‘low-viscosity’ fibres; inulin, gum Arabic, and partially hydrolysed guar gum (PHGG) at 1000 mg/mL. Test conditions are in distilled water (pH 7), and simulated digestion at pH 4 and pH 2. The data tip (*) indicates significant difference between the distilled water condition and the simulated digestion condition (pH 4 or pH 2) at 30 min. The data tip (#) indicates significant difference between the simulated digestion condition at 30 min (pH 4 or pH 2) and its corresponding simulated digestion condition at 60 min. The significance level is p ≤ 0.05 for both (*) and (#).
4. Discussion
The rheology of 10 commercially available soluble dietary fibres were comprehensively studied under neutral (distilled water, pH 7) and simulated digestion conditions. The study identified gum Arabic and PHGG as two promising candidates for gastroparesis patients based on their low-viscosity behaviour in both distilled water and simulated digestion conditions.
In general, the rheological parameters of yield-point shear stress and complex viscosity (η*) are affected by the chemical structure, preparation concentration, pH and the presence of cations at steady biological temperature (37 °C). Due to these factors, significant variability in rheological properties were observed between our 10 soluble fibres. Fibres such as guar gum and psyllium husk required relatively high shear stresses to achieve their yield points at 50 mg/mL, suggesting that a large amount of mechanical force is required to breakdown their molecular associations. The physiological digestion of food bolus in the stomach requires the breakdown of particles to an average size of 2.0 mm, which is required for transit through the pylorus [44,45,46]. The shear stress requirement for a yield point in dietary fibre preparations can be analogous to the mechanical force needed by the stomach to churn and breakdown the molecular associations of the dietary fibre. When gastric motility is impaired or absent (as is the case in gastroparesis), fibres with high yield points may not be sufficiently broken down, which leads to delayed gastric emptying and associated symptoms [47].
It can be ascertained from Figure 4, Figure 5 and Figure 6 that the chemical structure and composition of dietary fibres play an important role in the shear stress requirements for a yield point in distilled water (pH 7). Guar gum and PHGG are good examples to demonstrate this, as PHGG is a short chain hydrolysed version of the guar gum polysaccharide, which consists of a mannose backbone and a galactose side chain (2:1). PHGG shows a far lower yield-point stress and lower viscosity than guar gum, demonstrating that shortening the length of major polysaccharides in a dietary fibre directly affects the rheological properties [48]. It must be noted that hydrolysing guar gum into PHGG also changed the sample state from a colloidal gel into a hydrocolloid paste. Such de-gelling helps lower the viscosity of PHGG and allows it to be incorporated into yogurts in order to reduce viscosity and improve texture quality [49]. Inulin is a linear short chain β-2,1 fructan polysaccharide with variable degrees of polymerisation. The alpha-inulin variety used in this study is water soluble at room temperature unlike delta-inulin, which is insoluble at temperatures below 40 °C. Inulin polysaccharides are of low molecular weight, with a range of 0.6 kDa to 7.2 kDa, leading to low yield-point stress values in distilled water (pH 7) [50].
The Gum Arabic dietary polysaccharide has a large molecular weight of 240–580 kDa but has been shown to demonstrate low-viscosity rheological behaviour in distilled water (pH 7) and in various dietary preparations [51]. This rheological behaviour is due to the extensively branched arabinogalactan polysaccharide which consists of a backbone of (1,3)-linked β-d-galactopyranosyl units, a side chain of between two to five (1,3)-linked β-d-galactopyranosyl units which is joined to the backbone with (1,6)-linkages and monomer residues of rhamnose, arabinose, glucuronic acid. The proportion of the amphiphilic micellar structured arabinogalactan protein (AGP) complex in gum Arabic has been shown to be correlated with increased shear-thinning thixotropy and a reduction in viscosity [52]. This effect is due to AGP micelles in solution being disrupted by the application of steady shear stress. When the steady shear stress is ceased, the AGP polysaccharide returns to its original micelle configuration. Interestingly, significantly different yield points were observed in pectin fibres from apple and citrus (Figure 5). The discrepancy may be due to varying proportions of the constituent pectin polysaccharides homogalacturonan, xylogalacturonan, apiogalacturonan rhamnogalacturonan I and rhamnogalacturonan II [53], as well as differences in the total dietary fibre content (apple fibre 40% vs citrus fibre 55%) in their respective supplements, as reported in Table 1.
It has been documented in the literature that the rheological properties of some water-soluble polysaccharides (such as, pectins, gums, mucilages) are affected by the adjustment of pH, the presence of cations and temperature [54]. In this study, the presence of H+, Na+ and K+ cations within the simulated gastric fluids are the primary factors that affect molecular associations of the fibre polysaccharides, but this effect is greatly dependent on whether the cations present in solution can disrupt and rearrange the default thermodynamic configuration of a polysaccharide [55]. In the polysaccharides of gum fibres (gum Arabic, PHGG, guar gum, xanthan gum), which lowered pH and increased cations, seems to have little to no effect on the yield-point shear stress requirements or the sample viscosity due to the hydrogen and covalent bonding in micellar configurations being stable and undisrupted [56,57]. The pectin-type dietary fibres (apple-fibre and citrus pectin) were similarly unaffected by the simulated gastric conditions, but these pectin dietary fibres did not form the colloidal gels reported in the “egg-box” model [58,59] and instead formed hydrocolloidal pastes, which may have affected their rheology [58]. The rheological properties of high-methoxy pectins (like apple-fibre and citrus pectin) are largely unaffected by the decrease in pH and increased cation presence and are shown in the literature to be more affected by substances like the food additive gelatine [53]. It must also be noted that the solutions used in our experiments contained little to no free Ca2+ ions, which are required for pectin to form the colloidal gels found in the “egg-box” model where the Ca2+ ions interact with COO− ions in-between sliding sheets of polysaccharide chains, which then stack over each other and result in increased viscosity.
Fibres such as iota-carrageenan, inulin, beta-glucan and psyllium husk display significant changes in yield-point shear stress in simulated digestion conditions compared with distilled water. For the iota-carrageenan polysaccharide, the mechanism of gel formation and solubility is primarily affected by the presence of the OSO3− ester sulphate group on its O-3-substituted β-d-galactopyranosyl and O-4-substituted 3,6-anhydro-α-d-galactopyranosyl dimer backbone with a double helical configuration [60]. The iota-carrageenan variety is more sulphated than kappa-carrageenan, forming a soft gel rather than the rigid gel found in kappa-carrageenan [61]. Abundance of free cations in solution results in cationic interaction where the sulphate ester groups on the crosslinked double helix configuration are aggregated, especially by K+. The sol-gel transition points after such interactions have been studied in the literature using the photon transmission technique [62] and are shown to increase gel formation, decrease solubility and significantly increase yield-point shear stress. Similarly, inulin also exhibited increased yield-point shear stress due to its six-turn helical configuration of the alpha-inulin polysaccharide in aqueous solution, which is affected by the aggregation of cations to the helix in a manner very similar to iota-carrageenan [63,64,65]. Although inulin has relatively low viscosity, the significant increase in viscosity under simulated digestion is not ideal for gastroparesis patients. Given the delayed gastric emptying characteristics of gastroparesis, inulin may be exposed to gastric fluids for over 4 h in these patients, resulting in increased viscosity and likelihood of associated symptoms. In addition, inulin can cause severe reactions in people with fructan allergies and is, therefore, excluded from any potential clinical study involving gastroparesis patients [66,67].
Psyllium husk is an anionic mucilage polysaccharide with many COO− groups and a great deal of electrostatic repulsion, which causes the polysaccharide strands to expand and become interpenetrated. The microstructure of psyllium husk is extensively porous in distilled water and in alkaline conditions (pH > 7). When the pH is sufficiently decreased, the net intermolecular electrostatic repulsion in the polysaccharide is reduced causing the colloidal material to become more rigid, increasing the yield-point shear stress and increasing viscosity [68]. Such intermolecular associations explain its increased yield-point stress at low pH solutions. Beta-glucan is a water-soluble mucilage polysaccharide with repeating β-D-glucose monomer units with (1,3) and (1,4) glycosidic linkages that can be branched [69]. Literature investigations of beta-glucan report that the rheological viscosity is increased under acidic conditions and decreased under alkaline conditions [70]. This runs counter to the result obtained in this study, where the yield-point shear stress values and viscosity after yield are reduced more when pH is lowered in simulated digestion conditions. Such an effect may be due to the presence of cations in solution since their effect on beta-glucan has not been investigated extensively. The reduced viscosity may also be due to the low percentage of total dietary fibre present in beta-glucan (44%) and other constituents within the supplement. The other polysaccharides present in the beta-glucan supplement (22%) and their intermolecular interactions with the beta-glucan (22%) polysaccharide in aqueous solution may also have affected the rheology when the pH is reduced.
Simulated digestions were carried out over the course of 60 min, iota-carrageenan, psyllium husk, beta-glucan, apple-fibre pectin showed significant increases in yield-point shear stress compared to their 30 min timepoint results. The primary factors playing a role in yield points which increase at 60 min are the water-binding ability of constituent polysaccharides and the syneresis effect. The binding of water by dietary fibres can be done through anionic interactions, ionic interactions involving carboxyl groups with cations, strong and weak types of hydrogen bonding, hydrophobic interactions involving the formation of water clathrates on gel surfaces and the enclosure of water through capillary action [71]. Some soluble dietary fibres experience rapid syneresis during the stabilization or rest period where the sample matrix expels water bound to the constituent polysaccharides. This causes colloidal gels and hydrocolloid pastes to become brittle and dried out, increasing the yield-point shear stress and the viscosity, though this process is reversible [72]. Therefore, the water-binding capacity of soluble dietary fibres and any observed syneresis during rheological measurements are largely dependent on the chemical structure and thermodynamic configuration of constituent polysaccharides in aqueous solution.
There are some limitations in this study. While the rheological properties of the dietary fibres under neutral and simulated digestive conditions were studied, the direct physiological effects of these fibres in the upper gastrointestinal tract have not been evaluated. Though the yield-point shear stress values provide valuable rheological information for pre-clinical evaluation, they may not be an accurate representation of the peristaltic forces in the stomach. Evaluation of the physiological effects may require either a simulated stomach like a SIMulator Gastro-Intestinal (SIMGI) compartment [73] or clinical study involving human participants. Despite these limitations, the rheological data presented in this research can prove useful for clinicians and dietitians in assessing the suitability of certain dietary fibre supplements for patients with upper gastrointestinal disorders such as gastroparesis where peristaltic activity is greatly reduced or impaired.
5. Conclusions
Altogether, our results demonstrate the rheological heterogeneity within a range of soluble fibres. We propose that digestion may have a significant impact on fibre viscosity and should be taken into consideration when evaluating the suitability of fibres for patients with gastric motility disorders. Although the physiological benefits of soluble fibres in gastroparesis will need to be evaluated, our results provide evidence for gum Arabic and PHGG to be tolerable sources of soluble fibre for patients with gastroparesis. Future clinical studies would involve introducing our candidate fibres (gum Arabic and PHGG) into the diets of gastroparesis patients in order to evaluate their effects on blood glucose, gastric motility and associated gastric symptoms.
Acknowledgments
We would like to acknowledge the research colleagues and administrative staff of the GI Motility Disorders Unit at the School of Medicine, Western Sydney University (WSU). This research work was supported by Western Sydney University (WSU), Australian Rotary Health (ARH), and Devonport Rotary Club as part of a joint PhD scholarship.
Author Contributions
Conceptualization, H.S., V.H., J.Z.; methodology, H.S.; formal analysis, H.S., J.Z.; investigation, H.S., J.Z.; resources, V.H., J.Z.; data curation, H.S.; writing—original draft preparation H.S.; writing, H.S., V.H., J.Z.; visualization, H.S., J.Z.; supervision, V.H., J.Z.; project administration, V.H., J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
All authors declare no financial interest or benefit that has arisen from the direct applications of this research.
References
- 1.Wieckert M.O., Pfeiffer A.F.H. Metabolic Effects of Dietary Fiber Consumption and Prevention of Diabetes. J. Nutr. 2008;138:439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 2.Jensen M.K., Koh-Banerjee P., Hu F.B., Franz M., Sampson L., Gronbaeck M., Rimm E.B. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004;80:1492–1499. doi: 10.1093/ajcn/80.6.1492. [DOI] [PubMed] [Google Scholar]
- 3.Dahm C.C., Keogh R.H., Spencer E.A., Greenwood D.C., Key T.J., Fentiman I.S., Shipley M.J., Brunner E.J., Cade J.E., Burley V.J., et al. Dietary Fiber and colorectal cancer risk: A Nested case–control Study Using Food Diaries. J. Natl. Cancer Inst. 2010;102:614–626. doi: 10.1093/jnci/djq092. [DOI] [PubMed] [Google Scholar]
- 4.Murphy N., Norat T., Ferrari P., Jenab M., Bueno-de-Mesquita B., Skeie G., Dahm C.C., Overvad K., Olsen A., Tonneland A., et al. Dietary Fibre Intake and Risks of Cancers of the Colon and Rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC) PLoS ONE. 2012;7:e39361. doi: 10.1371/journal.pone.0039361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health and Medical Research Council . Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Australian Government Department of Health; Canberra, Australia: 2017. [(accessed on 1 March 2020)]. Available online: https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes/ [Google Scholar]
- 6.Howarth N.C., Saltzman E., Roberts S.B. Dietary Fiber and Weight Regulation. Nutr. Rev. 2001;59:129–139. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 7.Fayet-Moore F., Cassettari T., Tuck K., McConnell A., Petocz P. Dietary Fibre Intake in Australia. Paper I: Associations with Demographic, Socio-Economic, and Anthropometric Factors. Nutrients. 2018;10:599. doi: 10.3390/nu10050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrera-Bastos P., Fontes-Villalba M., O’Keefe J.H., Lindeberg S., Cordain L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011;2:15–35. doi: 10.2147/RRCC.S16919. [DOI] [Google Scholar]
- 9.Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017;57:3543–3564. doi: 10.1080/10408398.2016.1180501. [DOI] [PubMed] [Google Scholar]
- 10.Chater P.I., Wilcox M.D., Pearson P.J., Brownlee I.A. The impact of dietary fibres on the physiological processes governing small intestinal digestive processes. Bioact. Carbohydr. Diet. Fibre. 2015;6:117–132. doi: 10.1016/j.bcdf.2015.09.002. [DOI] [Google Scholar]
- 11.Morozov S., Isakov V., Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J. Gastroenterol. 2018;24:2291–2299. doi: 10.3748/wjg.v24.i21.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamargo A., Cueva C., Alvarez M.D., Herranz B., Moreno-Arribas M.V., Laguna L. Physical effects of dietary fibre on simulated luminal flow, studied by in vitro dynamic gastrointestinal digestion and fermentation. Food Funct. 2019;10:3452–3465. doi: 10.1039/C9FO00485H. [DOI] [PubMed] [Google Scholar]
- 13.Chambers E.S., Byrne C.S., Morrison D.J., Murphy K.G., Preston T., Tedford C., Garcia-Perez I., Fountana S., Serrano-Contreras J.I., Holmes E., et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut. 2019;68:1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McRorie J.W., McKeown N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017;117:251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 15.El-Salhy M., Ystad S.O., Mazzawi T., Gundersen D. Dietary fiber in irritable bowel syndrome (Review) Int. J. Mol. Med. 2017;40:607–613. doi: 10.3892/ijmm.2017.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikeman C.L., Murphy M.R., Fahey G.C. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J. Nutr. 2006;136:913–919. doi: 10.1093/jn/136.4.913. [DOI] [PubMed] [Google Scholar]
- 17.Palafox-Carlos H., Ayala-Zavala J.F., González-Aguilar G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011;76:R6–R15. doi: 10.1111/j.1750-3841.2010.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parrish C.R., McCray S. Gastroparesis & nutrition: The art. Pract. Gastroenterol. 2011;35:26–41. [Google Scholar]
- 19.Müller M., Canfora E.E., Blaak E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients. 2018;10:275. doi: 10.3390/nu10030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu K., Ke M.Y., Li W.H., Zhang S.Q., Fang X.C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia. Pac. J. Clin. Nutr. 2014;23:210–218. doi: 10.6133/apjcn.2014.23.2.01. [DOI] [PubMed] [Google Scholar]
- 21.Benini L., Castellani G., Brighenti F., Heaton K.W., Brentegani M.T., Casiraghi M.C., Sembenini C., Pellegrini N., Fioretta A., Minniti G., et al. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut. 1995;36:825–830. doi: 10.1136/gut.36.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waseem S., Moshiree B., Draganov P.V. Gastroparesis: Current diagnostic challenges and management considerations. World J. Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ménard O., Famelart M.H., Deglaire A., Le Gouar Y., Guerin S., Malbert C.H., Dupont D. Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition. Nutrients. 2018;10:1308. doi: 10.3390/nu10091308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fass R., McCallum R.W., Parkman H.P. Treatment Challenges in the Management of Gastroparesis-Related GERD. Gastroenterol. Hepatol. 2009;10:4–16. [PMC free article] [PubMed] [Google Scholar]
- 25.Poutanen K.S., Fiszman S., Marsaux C.F.M., Pentikainen S.P., Steinert R.E., Mela D.J. Recommendations for characterization and reporting of dietary fibers in nutrition research. Am. J. Clin. Nutr. 2018;108:437–444. doi: 10.1093/ajcn/nqy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augusto P.E.D., Cristianini M., Ibarz A. Effect of temperature on dynamic and steady-state shear rheological properties of siriguela (Spondias purpurea L.) pulp. J. Food Eng. 2012;108:283–289. doi: 10.1016/j.jfoodeng.2011.08.015. [DOI] [Google Scholar]
- 27.Repin N., Cui S.W., Goff H.W. Rheological behavior of dietary fibre in simulated small intestinal conditions. Food Hyd. 2018;76:216–225. doi: 10.1016/j.foodhyd.2016.10.033. [DOI] [Google Scholar]
- 28.Yoon S.J., Chu D.C., Raj Juneja L. Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. J. Clin. Biochem. Nutr. 2008;42:1–7. doi: 10.3164/jcbn.2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babiker R., Merghani T.H., Elmusharaf K., Badi R.M., Lang F., Saeed A.M. Effects of Gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: Two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 2012;11:111. doi: 10.1186/1475-2891-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan K., Wright A.J., Goff H.D. Correlating the structure and in vitro digestion viscosities of different pectin fibers to in vivo human satiety. Food Funct. 2015;6:63–71. doi: 10.1039/C4FO00543K. [DOI] [PubMed] [Google Scholar]
- 32.Fabek H. Master’s Thesis. The University of Guelph; Guelph, ON, Canada: 2011. [(accessed on 1 April 2020)]. Effect of In Vitro Human Digestion on the Viscosity of Hydrocolloids in Solution: A Dietary Fibre Study. Available online: https://atrium.lib.uoguelph.ca/xmlui/handle/10214/3094/ [Google Scholar]
- 33.Poutanen K.S., Dussort P., Erkner A., Fiszman S., Karnik K., Kristensen M., Marsaux C.F.M., Miquel-Kergoat S., Pentikainen S.P., Putz P., et al. A review of the characteristics of dietary fibers relevant to appetite and energy intake outcomes in human intervention trials. Am. J. Clin. Nutr. 2017;106:747–754. doi: 10.3945/ajcn.117.157172. [DOI] [PubMed] [Google Scholar]
- 34.Davis R.E., Hartman C.W., Fincher J.H. Dialysis of Ephedrine and Pentobarbital from Whole Human Saliva and Simulated Saliva. J. Pharm. Sci. 1971;60:429–432. doi: 10.1002/jps.2600600318. [DOI] [PubMed] [Google Scholar]
- 35.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carriere F., Boutrou R., Corredig M., Dupont D., et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M., Parkman H.P., Shafi M.A., Abell T.L., Gerson L. Clinical guideline: Management of gastroparesis. Am. J. Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbosa-Canovas G.V., Kokini J.L., Ma L., Ibarz A. The Rheology of Semiliquid Foods. Adv. Food Nutr. Res. 1996;39:1–69. doi: 10.1016/S1043-452660073-X. [DOI] [PubMed] [Google Scholar]
- 38.Kravchuk O., Stokes J.R. Review of algorithms for estimating the gap error correction in narrow gap parallel plate rheology. J. Rheol. 2013;57:365. doi: 10.1122/1.4774323. [DOI] [Google Scholar]
- 39.De Souza Mendes P.R., Alicke A.A., Thompson R.L. Parallel-plate Geometry Correction for Transient Rheometric Experiments. Appl. Rheol. 2014;24:52721. doi: 10.3933/ApplRheol-24-52721. [DOI] [Google Scholar]
- 40.Doraiswamy D., Mujumdar A.N., Tsao I., Beris A.N., Danforth S.C., Metzner A.B. The Cox–Merz rule extended: A rheological model for concentrated suspensions and other materials with a yield stress. J. Rheol. 1991;35:647–685. doi: 10.1122/1.550184. [DOI] [Google Scholar]
- 41.Li S.-P., Zhao G., Chen H.-Y. The Relationship between Steady Shear Viscosity and Complex Viscosity. J. Disp. Sci. Technol. 2005;26:415–419. doi: 10.1081/DIS-200054555. [DOI] [Google Scholar]
- 42.Cox W.P., Merz E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958;28:619–622. doi: 10.1002/pol.1958.1202811812. [DOI] [Google Scholar]
- 43.Li X., Fang Y., Zhang H., Nishinari K., Al-Assaf S., Phillips G.O. Rheological properties of gum arabic solution: From Newtonianism to thixotropy. Food Hydrocoll. 2011;25:293–298. doi: 10.1016/j.foodhyd.2010.06.006. [DOI] [Google Scholar]
- 44.Kong F., Singh R.P. Disintegration of solid foods in human stomach. J. Food Sci. 2008;73:R67–R80. doi: 10.1111/j.1750-3841.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 45.Olausson E.A., Alpsten M., Larsson A., Mattson H., Andersson H., Attvall S. Small particle size of a solid meal increases gastric emptying and late postprandial glycaemic response in diabetic subjects with gastroparesis. Diabetes Res. Clin. Pract. 2008;80:231–237. doi: 10.1016/j.diabres.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Olausson E.A., Störsrud S., Grundin H., Isaksson M., Attvall S., Simren M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: A randomized controlled trial. Am. J. Gastroenterol. 2014;109:375–385. doi: 10.1038/ajg.2013.453. [DOI] [PubMed] [Google Scholar]
- 47.Abrahamsson H. Treatment options for patients with severe gastroparesis. Gut. 2007;56:877–883. doi: 10.1136/gut.2005.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mudgil D., Barak S., Khatkar B.S. Guar gum: Processing, properties and food applications-A Review. J. Food Sci. Technol. 2014;51:409–418. doi: 10.1007/s13197-011-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mudgil D., Barak S., Khatkar B.S. Texture profile analysis of yogurt as influenced by partially hydrolyzed guar gum and process variables. J. Food Sci. Technol. 2017;54:3810–3817. doi: 10.1007/s13197-017-2779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mensink M.A., Frijlink H.W., van der Voort Maarschalk K., Hinrichs W.L.J. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carb. Polym. 2015;130:405–419. doi: 10.1016/j.carbpol.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed J., Ramaswamy H.S., Ngadi M.O. Rheological Characteristics of Arabic Gum in Combination with Guar and Xanthan Gum Using Response Surface Methodology: Effect of Temperature and Concentration. Int. J. Food Prop. 2005;8:179–192. doi: 10.1081/JFP-200060234. [DOI] [Google Scholar]
- 52.Li X., Zhang H., Fang Y., Al-Assaf S., Phillips G.O., Nishinari K. Gum Arabic. 1st ed. Royal Society of Chemistry Publishing; Cambridge, UK: 2011. pp. 229–238. [Google Scholar]
- 53.Gawkowska D., Cybulska J., Zdunek A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers. 2018;10:762. doi: 10.3390/polym10070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhingra D., Michael M., Rajput H., Patil R.T. Dietary fibre in foods: A review. J. Food Sci. Tech. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindman B., Medronho B., Alves L., Costa C., Edlund H., Norgren M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. PCCP. 2017;19:23704–23718. doi: 10.1039/C7CP02409F. [DOI] [PubMed] [Google Scholar]
- 56.Grein A., da Silva B.C., Wendel C.F., Tischer C.A., Sierakowski M.R., Moura A.B.D., Iacomini M., Gorin P.A.J., Simas-Tosin F.F., Riegel-Vidotti I.C. Structural characterization and emulsifying properties of polysaccharides of Acacia mearnsii de Wild gum. Carb. Polym. 2013;92:312–320. doi: 10.1016/j.carbpol.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 57.Rowe R.C. Handbook of Pharmaceutical Excipients. 6th ed. Pharmaceutical Press; London, UK: 2009. [Google Scholar]
- 58.Warwicker J.O., Wright A.C. Function of sheets of cellulose chains in swelling reactions on cellulose. J. Appl. Polym. Sci. 1967;11:659–671. doi: 10.1002/app.1967.070110504. [DOI] [Google Scholar]
- 59.Cardoso S.M., Coimbra M.A., Lopes da Silva J.A. Temperature dependence of the formation and melting of pectin–Ca2+ networks: A rheological study. Food Hydrocoll. 2003;17:801–807. doi: 10.1016/S0268-005X(03)00101-2. [DOI] [Google Scholar]
- 60.Dea I.M.C. Industrial Gums. 3rd ed. Academic Press; Cambridge, MA, USA: 1993. pp. 21–52. [Google Scholar]
- 61.Guo M.Q., Hu X., Wang C., Ai L. Solubility of Polysaccharides. 1st ed. Intech Open; London, UK: 2017. pp. 7–21. [Google Scholar]
- 62.Kara S., Arda E., Kavzak B., Pekcan O. Phase transitions of κ-carrageenan gels in various types of salts. J. Appl. Polym. Sci. 2006;102:3008–3016. doi: 10.1002/app.24662. [DOI] [Google Scholar]
- 63.Cooper P.D., Barclay T.G., Ginic-Markovic M., Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23:1164–1174. doi: 10.1093/glycob/cwt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper P.D., Barclay T.G., Ginic-Markovic M., Gerson A.R., Petrovsky N. Inulin isoforms differ by repeated additions of one crystal unit cell. Carb. Polym. 2014;103:392–397. doi: 10.1016/j.carbpol.2013.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petroski N. Immunopotentiators in Modern Vaccines. 2nd ed. Academic Press; Cambridge, MA, USA: 2017. pp. 199–210. [Google Scholar]
- 66.Fedewa A., Rao S.S. Dietary fructose intolerance, fructan intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014;16:370. doi: 10.1007/s11894-013-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coudray C., Tressol J.C., Gueux E., Rayssiguier Y. Effects of inulin-type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur. J. Nutr. 2003;42:91–98. doi: 10.1007/s00394-003-0390-x. [DOI] [PubMed] [Google Scholar]
- 68.Fischer M.H., Yu N., Gray G.R., Ralph J., Anderson L., Marlett J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk) Carb. Res. 2004;339:2009–2017. doi: 10.1016/j.carres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 69.Anttila H., Sontag-Strohm T., Salovaara H. Viscosity of beta-glucan in oat products. Agric. Food Sci. 2004;13:80–87. doi: 10.2137/1239099041838012. [DOI] [Google Scholar]
- 70.Zarzycki P., Sobota A. Effect of pH on Apparent Viscosity of Wholemeal Oat Flour Water Dispersions. Int. J. Food Prop. 2014;18:303–315. doi: 10.1080/10942912.2013.809538. [DOI] [Google Scholar]
- 71.Chaplin M.F. Fibre and water binding. Proc. Nutr. Soc. 2003;62:223–227. doi: 10.1079/PNS2002203. [DOI] [PubMed] [Google Scholar]
- 72.Mizrahi S. Chemical Deterioration and Physical Instability of Food and Beverages. 1st ed. Woodhead Publishing; Cambridge, UK: 2010. pp. 324–348. [Google Scholar]
- 73.Faintuch J., Faintuch S. Microbiome and Metabolome in Diagnosis, Therapy, and other Strategic Applications. 1st ed. Academic Press; Cambridge, MA, USA: 2019. [Google Scholar]