Abstract
Vasopressin (AVP) and oxytocin (OXT) regulate social behavior by binding to their canonical receptors, the vasopressin V1a receptor (V1aR) and oxytocin receptor (OTR), respectively. Recent studies suggest that these neuropeptides may also signal via each other’s receptors. The extent to which such cross-system signaling occurs likely depends on anatomical overlap between AVP/OXT fibers and V1aR/OTR expression. By comparing AVP/OXT fiber densities with V1aR/OTR binding densities throughout the rat social behavior neural network (SBNN), we propose the potential for cross-system signaling in four regions: the medial amygdala (MeA), bed nucleus of the stria terminalis (BNSTp), medial preoptic area, and periaqueductal grey. We also discuss possible implications of corresponding sex (higher in males versus females) and age (higher in adults versus juveniles) differences in AVP fiber and OTR binding densities in the MeA and BNSTp. Overall, this review reveals the need to unravel the consequences of potential cross-system signaling between AVP and OXT systems in the SBNN for the regulation of social behavior.
Keywords: Oxytocin, Vasopressin, V1a receptor, oxytocin receptor, social behavior, cross-talk, medial amygdala, bed nucleus of the stria terminalis, medial preoptic area, periaqueductal grey
Graphical abstract
1. Introduction
Vasopressin (AVP) and oxytocin (OXT) regulate social behavior across a wide array of vertebrate species by acting at their centrally expressed receptors, namely the vasopressin V1a receptor (V1aR) and the oxytocin OT receptor (OTR), respectively (Albers, 2015; Numan & Young, 2016; Veenema & Neumann, 2008; Goodson, 2005). However, recent studies have provided evidence that AVP can act via the OTR and OXT can act via the V1aR to modulate social behavior (reviewed in Song & Albers, 2018). For example, in male Syrian hamsters, central AVP administration extends the time interval over which a social odor can be recognized, an effect that is blocked by co-administration of a specific OTR, but not V1aR, antagonist (Song et al., 2016a). AVP in the ventral tegmental area (VTA) of male Syrian hamsters modulates social reward in an OTR, but not V1aR, dependent manner (Song et al., 2016b). Deficits in social interaction and social recognition can be restored by central OXT administration in male C57BL/6 OTR knock-out mice, an effect that is eliminated by pretreatment with a V1aR antagonist (Sala et al., 2011). In male Syrian hamsters, central administration of OXT enhances flank marking behavior, an effect that is inhibited by central administration of a V1aR, but not an OTR, antagonist (Song et al., 2014). In female meadow voles, same-sex peer affiliation is reduced by OXT acting at the V1aR rather than the OTR in the LS (Anacker et al., 2016). In macaque monkeys, AVP or OXT injected into the anterior cingulate gyrus increased behavioral synchrony within a pair of males, an effect that is likely mediated via binding to the abundantly expressed V1aR rather than the OTR, which is absent in this region (Jiang & Platt, 2018).
The above-mentioned pharmacological studies do not provide evidence for endogenous cross-system signaling. However, the possibility that endogenous AVP and OXT may regulate social behavior by promiscuously signaling at the OTR or the V1aR, respectively, is plausible given the high structural similarity between AVP and OXT (differ in only 2 out of 9 amino acids) and the high sequence homology (80%) between the OTR and V1aR (Koehbach et al., 2013). Furthermore, in vitro receptor binding assays have been performed to compare the affinity of AVP and OXT for the V1aR and the OTR in human, rat and mouse tissue. These assays have demonstrated that only OXT is selective for the human OTR while the selectivity criterion (affinity values differing by two orders of magnitude for OTR and V1aR in the same species) was not reached for AVP and OXT binding to human V1aR, rat V1aR, rat OTR, mouse V1aR, and mouse OTR (Manning et al., 2008; 2012). Together, these data suggest the possibility that both V1aR and OTR should be considered target receptors for endogenously released AVP and OXT.
We propose that endogenous cross-system signaling may be particularly likely to occur under conditions in which AVP/OXT is axonally released in brain regions that primarily contain either the V1aR or the OTR. Thus, mapping the anatomical overlap between AVP fibers and OTR binding as well as OXT fibers and V1aR binding will provide valuable information about brain regions where potential cross-system signaling may occur. This knowledge will also have important consequences for better understanding the role of these neuropeptides in the regulation of social behavior. Therefore, in section 2 of this review, we compare AVP and OXT fiber densities with V1aR and OTR binding densities in the adult male rat brain to determine the extent of overlap, or lack thereof, between the AVP and OXT systems. We chose to focus on the adult male rat because of available data on AVP and OXT fiber densities as well as V1aR and OTR binding densities (DiBenedictis et al., 2017; Smith et al., 2017) and the well-known roles of AVP/OXT and their receptors in regulating diverse social behaviors in adult male rats (Caldwell & Albers, 2016; Veenema & Neumann, 2008; Bielsky & Young, 2004).
Social behavior repertoires differ between the sexes and across developmental time points. AVP and OXT systems also differ between the sexes and change over the course of development (Smith et al., 2017; DiBenedictis et al., 2017; Dumais et al., 2013; Dumais & Veenema, 2016a,b; Hammock, 2015; Vaidyanathan & Hammock, 2017). This suggests that AVP and OXT systems may be involved in the sex- and age-specific regulation and/or expression of social behaviors (For review see: Dumais & Veenema, 2016a, b; Hammock, 2015; Vaidyanathan & Hammock, 2017; Grinevich et al., 2015; Bredewold & Veenema, 2018). Thus, it is also critical to understand how potential overlap, or lack thereof, between AVP/OXT fiber densities and V1aR/OTR binding densities vary by sex and age. Therefore, in section 3 we compare the density patterns of AVP and OXT fibers with V1aR and OTR binding across sex and age (juvenile and adult) in rats.
The focus of this review is on brain regions comprising the social behavior neural network (SBNN). The SBNN was first proposed by Sara Newman (Newman, 1999) as a reciprocally interconnected set of brain regions involved in the regulation of diverse social behaviors such as aggression, sexual behavior, and parental behavior. The SBNN consists of the posterior aspect of the medial amygdala (MeP), posterior bed nucleus of the stria terminalis (BNSTp), lateral septum (LS), medial preoptic area (MPOA), ventromedial hypothalamus (VMH), anterior hypothalamus (AH), and periaqueductal grey (PAG). Importantly, AVP and/or OXT-immunoreactive (-ir) fibers and V1aR and/or OTR binding are found in all seven nodes of the rat SBNN (Gerstberger & Fahrenholz, 1989; Schmidt et al., 1991; Insel et al., 1994; Freund-Mercier et al., 1987; Tribollet et al., 1988; Smith et al., 2017; DiBenedictis et al., 2017). It has been proposed that activation across the interconnected nodes of the SBNN leads to the expression of social behavior and that differences in the relative activation of these nodes lead to the display of distinct forms of social behavior (e.g., sexual behavior or aggressive behavior; Newman, 1999; Goodson et al., 2005; Crews et al., 2006; O’Connell & Hofmann, 2012).
Recent evidence suggests that the SBNN shows remarkable homology across vertebrate species (O’Connell & Hofmann, 2011; 2012; Goodson & Kingsbury, 2013). Likewise, AVP and OXT regulate social behaviors by acting in nodes of the SBNN in species ranging from teleost fish to mammals (For review see: Goodson & Bass, 2001; Godwin & Thompson, 2012; Albers, 2015). Although the genetic sequences of AVP and OXT genes are highly conserved, AVP and OXT systems in the brain have changed significantly over the course of evolution. One particular evolutionary change highly relevant to this review has been the expansion of neuropeptide axonal projections to forebrain regions in mammals (Knobloch & Grinevich, 2014). Given the potential similarities in behavioral functions of AVP and OXT systems across species, we also discuss the extent to which distribution patterns, as well as sex and age differences, in AVP and OXT system parameters found in rats hold across species.
2. Comparing density patterns of AVP and OXT fibers with V1aR and OTR binding in the adult male rat SBNN
In this section, we discuss the extent of the anatomical overlap between AVP/OXT fibers and V1aR/OTR receptors in the adult male Wistar rat SBNN, by comparing data from two previous studies from our lab that quantified AVP and OXT fiber density using immunohistochemistry (DiBenedictis et al., 2017) and V1aR and OTR binding density using receptor autoradiography (Smith et al., 2017). It should be noted that this comparison has several limitations. First, it is unclear whether fibers visualized with immunohistochemistry represent axon terminals, axon fibers-of-passage, or dendrites. Therefore, we can only speculate about potential axonal or dendritic neuropeptide release in the region where fibers are observed. Second, due to the lack of reliable antibodies for V1aR and OTR, receptor autoradiography is currently the best proxy method to determine protein levels of V1aR and OTR in an anatomical manner. However, this method requires brain preservation procedures that are different than those required for immunohistochemistry. Therefore, immunohistochemical fiber density and autoradiographic receptor binding density measures were obtained from separate sets of adult male rats. Third, comparisons between fiber densities and receptor binding densities in a given brain region will be qualitative at most. Both immunohistochemistry and receptor autoradiography involve signal amplification, and not necessarily to the same degree. Thus, when we talk about high or low density of fibers/receptor binding in a given node of the SBNN, this is defined as relative to the density of fibers/receptor binding in the other nodes of the SBNN. Fourth, adult male rats were housed in same sex pairs and isolated for five days before brain collection at 70 days of age for immunohistochemistry (DiBenedictis et al., 2017) or were maintained in same sex pairs until brain collection at 84 days of age for receptor autoradiography (Smith et al., 2017). The age of the rats and the specific housing conditions may have influenced the fiber and receptor binding density patterns reported in these two studies. Additionally, V1aR and OTR binding densities can vary depending on the social experiences an animal has been exposed to (Curley et al., 2009; Lukas et al., 2010). Therefore, the fiber and receptor binding patterns reported here may not be the same in rats of other ages and/or that have been exposed to different housing and social experiences. Last, this review will not include comparisons with the two other AVP receptor subtypes, namely the V1b receptor and the V2 receptor. Due to the lack of specific antibodies and ligands, little is known about V1b receptor protein levels or V1b receptor binding density in the SBNN. The recent development of potent rat fluorescent ligands suggests the expression of the V1b receptor in the lateral septum, while other nodes of the SBNN haven’t been explored (Corbani et al., 2018). Even so, V1b receptor activation in two other SBNN nodes (MPOA and BNST) has been shown to modulate social behaviors such as maternal behavior (Bayerl et al., 2016). Furthermore, pharmacological studies suggest the presence of the V2 receptor in the lateral septum and PAG (Landgraf et al., 1991; Yang et al., 2006). It would therefore be of interest for future studies to explore the distribution and function of V1b and V2 receptors in the SBNN.
Below, we first compare density patterns between AVP fibers and V1aR binding (subsections 2.1-2.3), we then compare density patterns between OXT fibers and OTR binding (subsections 2.4-2.6), and lastly, we report high-density patterns of fibers and receptor binding across the AVP and OXT systems (subsections 2.7 and 2.8). Where possible, we compare our findings in the adult male Wistar rat SBNN with other studies to determine consistency in fiber and receptor binding density patterns in the SBNN.
2.1. High AVP fiber density and high V1aR binding density in the LS and PAG
The LS and PAG of adult male rats show a relatively high density of AVP-ir fibers and V1aR binding compared to the other nodes of the SBNN (Fig. 1; Table 1). These findings are in line with previous studies in adult male rats (DeVries et al., 1981, 1983; Caffe et al., 1983; Tribollet et al., 1988; Snijdewint et al., 1989; Veenema et al., 2012; Dumais & Veenema et al., 2016; DeVries & Al-Shamma et al., 1990), except that we believe that we are the first to describe V1aR binding in the PAG. These density patterns suggest that AVP signals at the V1aR within the LS and PAG to mediate its effects on behavior and other processes. To the best of our knowledge, no studies to date have determined the role of AVP acting at the V1aR in the PAG of adult male rats. However, AVP acting at V2 receptors rather than V1 receptors in the PAG modulates pain in adult male rats (Yang et al., 2006). In hamsters, microinjection of AVP into the PAG increases flank-marking, a form of social communication (Hennessey et al., 1992). Yet, it is unclear how this finding in hamsters translates to rats because no studies have described or quantified AVP fibers in the PAG of hamsters and it is unclear whether this effect is mediated via the V1aR. In contrast to the PAG, AVP and V1aR in the LS of adult male rats are well known to be involved in the regulation of social behaviors, most notably social recognition and aggression. For example, AVP administered into the LS facilitates (Dantzer et al., 1988; Veenema et al., 2012) while a V1aR antagonist administered into the LS impairs (Veenema et al., 2012) social recognition in adult male rats. Moreover, impairments in social recognition induced by exposure to early life stress are accompanied by a decrease in extracellular LS-AVP release in adult male rats, and social recognition can be restored by AVP administration into the LS (Lukas et al., 2011). AVP administered into the LS rescues progesterone-induced deficits in social memory in adult male rats (Bychowski et al., 2013). Furthermore, AVP administered into the LS increases inter-male aggression (Koolhaas et al., 1998) and extracellular AVP release in the LS correlates positively with levels of inter-male aggression (Veenema et al., 2010) in adult male rats. Finally, administration of a V1aR antagonist into the LS reduces inter-male aggression in highly aggressive adult male rats (Veenema et al., 2010). Although opposite behavioral effects are found between administration of AVP into the LS and administration of a V1aR antagonist into the LS, none of these studies provide direct evidence that AVP is mediating these behavioral effects via the V1aR. This is relevant because OXT-ir fibers and OTR binding are present in the LS (see 2.5) and OXT/OTR play a similar role in social recognition as AVP/V1aR in the LS of adult male rats (Lukas et al., 2013). Albeit in adult female meadow voles, some behavioral effects mediated by OXT administered into the LS can be blocked by co-administration of a V1aR, but not OTR, antagonist (Anacker et al., 2016). Future studies are required to determine whether endogenously released AVP in the LS and in the PAG regulates social behavior by acting on the V1aR in the LS in rats.
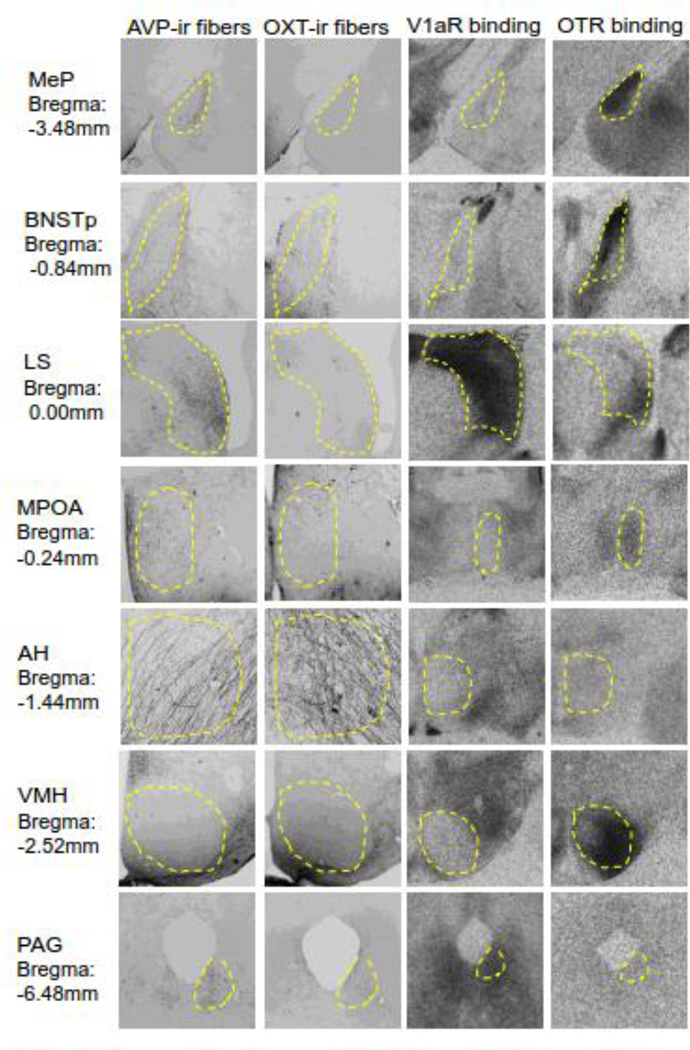
Figure 1.
Representative images of AVP-ir fibers, OXT-ir fibers, V1aR binding, and OTR binding in the regions of the adult male rat SBNN. Dashed outlines indicate region boundaries. Bregma distances are based on the Paxinos and Watson Rat Brain Atlas (2007).
Table 1.
Comparisons of relative densities of AVP-ir and OXT-ir fibers and V1aR and OTR binding in the SBNN of adult male rats based on DiBenedictis et al. (2017) and Smith et al. (2017).
| Brain Region | AVP-ir fibers | V1aR binding | OXT-ir fibers | OTR binding |
|---|---|---|---|---|
| MeP | Dense | Sparse | Sparse | Dense |
| BNSTp | Dense | Sparse | Dense | Dense |
| LS | Dense | Dense | Sparse | Dense |
| MPOA | Dense | Sparse | Sparse | Dense |
| AH | Dense | Sparse | Dense | Sparse |
| VMH | Sparse | Sparse | Sparse | Dense |
| PAG | Dense | Dense | Dense | Sparse |
Blue color indicates increased potential for cross-talk between AVP and OTR; yellow color indicates increased potential for cross-talk between OXT and V1aR; see text for discussion.
2.2. High AVP fiber density and low V1aR binding density in the MeP, BNSTp, MPOA, and AH
There is a mismatch in density patterns between AVP-ir fibers and V1aR binding in the MeP, BNSTp, MPOA, and AH. In detail, AVP-ir fiber density is high while V1aR binding density is low compared to other nodes of the SBNN (Fig. 1; Table 1). The high AVP-ir fiber density in these four regions confirms previous neuroanatomical studies using adult male rats as well as adult male mice (De Vries et al., 1983; Rood et al., 2011; Otero-Garcia et al., 2014). The observation that V1aR binding has been detected and/or quantified in many forebrain regions except the MeP, BNSTp, MPOA, and AH in previous studies using adult male rats (Dumais & Veenema, 2016a; Lukas et al., 2010; Veinante & Freund-Mercier, 1997; Tribollet et al., 1988; Tribollet et al., 1999; Kremarik et al., 1993) could provide support for our detection of low V1aR binding densities in these four regions.
A possible explanation for the mismatch between relatively high AVP-ir fiber density and relatively low V1aR binding density is that not all AVP fibers in these regions release AVP. This could be the case if these AVP-ir fibers represent dendrites and/or axonal fibers-of-passage. Indeed, the majority of AVP-ir fibers in the MeP and BNSTp could represent dendrites from AVP producing cell bodies within these regions while only a few may represent axonal projections from hypothalamic AVP producing cell bodies (Hernandez et al., 2015). AVP-ir fibers in the MPOA and AH could represent long-range fibers of passage, traveling from the PVN and supraoptic nucleus of the hypothalamus (SON) to the median eminence, pituitary gland, spinal cord and dorsomedial medulla (Sawchenko & Swanson, 1982; Swanson & Sawchenko, 1980; DeVries & Buijs, 1983; Palkovitz, 1984). Such fibers-of-passage may have few synaptic boutons, resulting in lower AVP release within the MPOA and AH. Electron microscopy experiments could be conducted in order to determine which of these brain regions contain releasable AVP (in vesicles). These and other studies would be critical to advancing our understanding of where in the brain AVP is coming from when binding to V1aRs.
Another possibility is that AVP fibers in the BNSTp, MeP, MPOA and AH release AVP, which then diffuses via volume transmission (Ludwig & Leng, 2006; Landgraf & Neumann, 2004) to nearby areas expressing the V1aR. V1aR binding is indeed relatively dense in areas adjacent to the MPOA and AH, such as the lateral hypothalamus and arcuate nucleus (Smith et al., 2017). A third possibility is that AVP acts on V1b receptors in addition to V1aR in these brain regions. V1b receptor mRNA expression has been detected in the medial amygdala, BNST, and MPOA of adult male rats (Arakawa et al., 2010).
Whether AVP signals at the V1aR in the MeP, BNSTp, MPOA, and AH to modulate social behavior in adult male rats is largely unknown. Administration of a V1aR-specific antagonist into the anterior medial amygdala reduces natural avoidance of illness-related social odor in adult male rats (Arakawa et al., 2010). There might be spill-over of the antagonist to the MeP, but that is speculative at best. Administration of a V1aR antagonist into the MPOA reduces maternal behavior in rats (Pedersen et al., 1994). However, lactating female rats have denser MPOA-V1aR binding compared to virgin female rats (Bosch et al., 2010), and, thus, these findings may be less informative as to the functional role of V1aR in the MPOA in adult male rats. Further research is required to determine whether AVP is released from AVP fibers in the MeP, BNSTp, MPOA, and AH, and whether AVP acting on V1aR and/or V1b receptors in these regions modulates social behavior in adult male rats.
2.3. Low AVP fiber density and low V1aR binding density in VMH
Sparse AVP-ir fibers and low V1aR binding density are found in the VMH compared to other SBNN nodes (Fig. 1; Table 1). The finding of sparse AVP-ir fibers in the VMH is in line with a previous study in adult male mice in which only very sparse AVP-ir fibers were observed in this brain region (Rood and DeVries, 2011). Previous forebrain-wide studies of V1aR binding in adult male rats did not include quantification of V1aR binding in the VMH (Lukas et al., 2010; Dumais & Veenema, 2016), which seems to support the relatively low V1aR binding density in the VMH that we observed. Given its anatomical location, AVP-ir fibers in the VMH could originate from median eminence-projecting AVP cell bodies located in the PVN and/or SON (Sawchencko and Swanson, 1982) and thus, may represent fibers-of-passage. As mentioned above, the V1aR is highly expressed in the lateral hypothalamus and arcuate nucleus (Smith et al., 2017), brain regions adjacent to the VMH. This raises the possibility that if AVP is released in the VMH, it may activate V1aRs in brain regions nearby via diffusion following local axonal release in the VMH.
To the best of our knowledge no studies have directly tested the functional role of AVP acting on V1aR in the VMH in rats. However, AVP injected into the VMH facilitates aggression in adult male hamsters (Ferris & Delville, 1994). Moreover, using slice preparations from adult male and female 129/Sv × Black Swiss mice it was shown that excitation of VMH neurons induced by application of an AVP receptor agonist could be blocked by an AVP receptor antagonist when applied prior to the agonist (Ragnauth et al., 2004). It is currently not known whether these findings in hamsters and mice occur via activation of the V1aR.
2.4. High OXT fiber density and high OTR binding density in the BNSTp
OXT-ir fiber density and OTR binding density are both high in the BNSTp compared to other nodes of the SBNN (Fig. 1; Table 1). OXT-ir fibers have been reported in the anterolateral BNST of adult male rats (Dabrowska et al., 2011) but we found no other rodent studies reporting OXT-ir fibers in the BNSTp. In contrast, dense OTR binding in the BNSTp has previously been described in adult male rats (Uhl-Bronner et al., 2005; Dumais et al., 2013). Microdialysis studies demonstrate an increase in extracellular OXT release in the BNSTp during exposure to a social stimulus in adult male rats (Dumais et al., 2016c). Furthermore, administration of OXT into the BNSTp improves, while administration of an OTR antagonist into the BNSTp impairs social recognition in adult male rats (Dumais et al., 2016c). Together, these findings provide credit to the notion that OXT is released from local OXT fibers and binds OTR in the BNSTp to modulate social behavior in adult male rats.
2.5. High OXT fiber density and low OTR binding density in the AH and PAG
OXT-ir fiber density is high while OTR binding density is low in the AH and PAG compared to other nodes of the SBNN (Fig. 1; Table 1). To the best of our knowledge, no previous work has characterized OXT-ir fiber density in the AH or PAG of adult male rats. However, OXT-ir fibers have been observed in the PAG of adult male mice (Nasanbyan et al., 2018). OTR binding in the AH and PAG has not been reported in adult male rats, despite qualification or quantification of OTR binding in other hypothalamic and brainstem regions (Lukas et al., 2010; Dumais et al., 2013; Shapiro & Insel, 1989; Tribollet et al., 1992; Smith et al., 2017), suggesting low OTR binding in the AH and PAG in these studies as well. The AH is located directly ventral to the PVN and dorsal to the median eminence. Thus, it is likely that the high density of OXT-ir fibers observed in this region represent median-eminence projecting fibers originating from OXT cell bodies in the PVN (Laqueur, 1954; Vandesande et al., 1977). If so, OXT may be released en passant from neurohypophyseal axons in the AH to regulate social behavior. To the best of our knowledge, no studies to date have investigated the role of AH-OXT or PAGOXT in social behavior regulation in adult male rats. However, administration of an OTR antagonist into the PAG decreases, while administration of OXT increases, pain thresholds in adult male rats (Yang et al., 2011). This suggests functional signaling via the OTR even in a brain region with low OTR binding density.
2.6. Low OXT fiber density and high OTR binding density in the MeP, LS, MPOA, and VMH
Relatively sparse OXT-ir fibers, but relatively dense OTR binding are observed in the MeP, LS, MPOA, and VMH compared to other SBNN nodes (Fig. 1; Table 1). These findings are in line with studies in adult female rats and adult male mice. In detail, lactating rats showing sparse OXT-ir fibers in the MPOA and VMH (Knobloch et al., 2012) and adult male mice showing a lack of OXT-ir fibers in the MeP (Castel & Morris, 1988) and only very sparse OXT-ir fibers in the VMH (Otero-Garcia et al., 2016, Nasanbuyan et al., 2018). High OTR binding densities in the MeP, MPOA, and VMH have been consistently observed in adult male and female rats as well as adult male mice (Dumais et al., 2013; Lukas et al., 2010; Campbell et al., 2009; Uhl-Bronner et al., 2005; Veinante & Freund-Mercier, 1997; Elands et al., 1988; Tribollet et al., 1992; Shapiro & Insel, 1989).
Increases in OTR binding density enabled OXT to induce physiological effects at lower concentrations in human myometrium (Maggi et al., 1990). Perhaps this could be true in the brain as well, such that OXT released from only a few OXT fibers might be sufficient to mediate a response due to dense OTR binding. Additionally, OXT could reach these brain regions via volume transmission following somatodendritic release from the magnocellular neurons in the PVN and SON (Pow & Morris 1989; Ludwig et al., 2002; Ludwig et al., 1997; Engelmann et al., 2000; Landgraf & Neumann, 2004; Ludwig & Leng, 2006; Herkenham, 1987; van den Pol et al., 2014). However, calculations based on axonal release from the ventral hippocampus suggest that extra-synaptically released OXT has a very limited radius at which OXT is effective (Chini et al., 2017). Further research would be needed to determine whether OXT may or may not reach these brain regions through volume transmission.
Despite low OXT-ir fiber density in the LS, increases in extracellular LS-OXT release have been observed in response to a social encounter in adult male rats (Lukas et al., 2013). Furthermore, blocking OTR function in the LS or medial amygdala, with either an OTR antagonist or OTR antisense, impairs social recognition in adult male rats and mice (Ferguson et al., 2001; Choleris et al., 2007; Takayanagi et al., 2017; Lukas et al., 2013). Moreover, administration of OXT into the MeP is sufficient to restore social recognition in OXT knockout mice (Ferguson et al., 2001). In the MPOA, OXT administration facilitates both sexual behavior (Gil et al., 2011) and social recognition (Popik & van Ree, 1991) in adult male rats. These behaviors are dependent on signaling at the OTR, as they are reduced by administration of an OTR antagonist into the MPOA (Gill et al., 2011; Popik & van Ree, 1991). Surprisingly, no studies to date have determined the involvement of OXT and OTR in the VMH in the regulation of social behavior in adult male rats. However, the OXT system in the VMH regulates sexual receptivity in adult female rats (Schulze & Gorzalka, 1991; McCarthy et al., 1994). Overall, despite scarce OXT fibers in the MeP, LS, MPOA, and VMH of rats and mice, these studies demonstrate an important role for OXT and the OTR in these SBNN nodes in regulating diverse social behaviors.
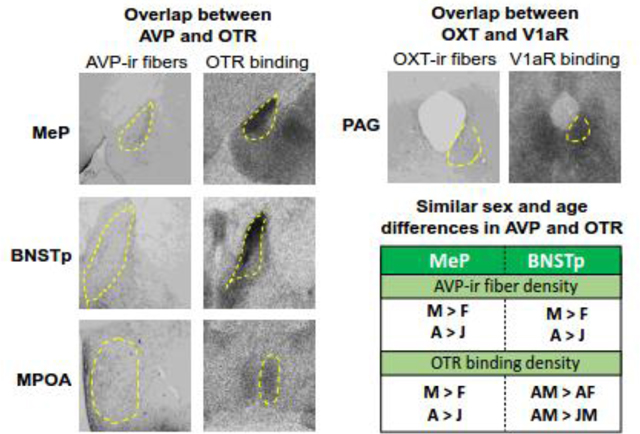
2.7. High AVP fiber density and high OTR binding density in the MeP, BNSTp, and MPOA
Intriguingly, dense AVP-ir fibers and OTR binding, but sparse V1aR binding and OXT fibers were observed in the MeP, BNSTp and MPOA relative to other SBNN nodes (Fig. 1; Table 1). If the dense AVP fibers correspond with high AVP release, then this pattern suggests that these brain regions are potential sites of AVP signaling at the OTR. As discussed above, action across AVP and OXT systems is mechanistically plausible (Manning et al., 2008, 2012) and studies in rodents have shown that AVP signaling can occur via the OTR to modulate social behavior, albeit in brain regions outside of the SBNN (Song et al., 2016a, b; Sala et al., 2011; Manzano-Garcia et al., 2018).
At present, it is unclear whether the AVP-ir fibers represent dendrites, axon terminals or long-range fibers. Because AVP cell bodies are present in the MeP and BNSTp, it is possible that a portion of the AVP-ir fibers are dendrites. In that case, AVP could be released somatodendritically to bind to nearby OTRs. This has been shown to occur in the supraoptic nucleus, where AVP released from magnocellular dendrites modulates the activity of local neurons through the OTR (Hirasawa et al., 2003). AVP-ir fibers in the MPOA may be long-range fibers of passage traveling from the PVN to the median eminence (Sawchenko & Swanson, 1982; Swanson & Sawchenko, 1980; DeVries & Buijs, 1983; Palkovitz, 1984), but may be released locally via en passant boutons, as was proposed for OXT released in the nucleus accumbens (Ross & Young, 2009). AVP and OTR in these brain regions play a role in the regulation of social behavior in adult male rats (Arawaka et al., 2010; Popik & van Ree, 1991; see 2.2 and 2.5 for more details) as well as in female rats and other rodent species (Ferris et al., 1984; Albers et al., 1986; Gill et al., 2011; Schulze & Gorzalka, 1991; Whitman & Albers, 1998). However, the effects of AVP administration and V1aR/OTR blockade on social behavior are seldom studied in a single design. Given the dense AVP-ir fibers and OTR binding in the MeP, BNSTp, and MPOA, combined with evidence that AVP can act via the OTR in other brain regions, it would be highly interesting to determine whether, how, and under what conditions AVP signaling occurs at the OTR within these nodes of the SBNN.
2.8. High OXT fiber density and high V1aR binding density in the PAG
Dense OXT-ir fibers and V1aR binding, but sparse OTR binding were observed in the PAG relative to other SBNN nodes (Fig. 1; Table 1). Dense OXT-ir fibers were also found in the PAG of adult male C57Bl/6J mice relative to other SBNN nodes analyzed (Nasanbyan et al., 2018). Dense V1aR binding was found in the PAG of adult male Sprague-Dawley rats and adult male ICR mice, while OTR binding seems absent from the PAG in these rodent species (Tribollet et al., 1988; 2002). If the dense OXT fibers correspond with high OXT release, then it could be that OXT signals via the V1aR rather than, or in addition to, the OTR in the PAG. As mentioned in section 2.5, OXT administered into the PAG increases pain thresholds in adult male rats (Yang et al., 2011), but it is unclear whether this is mediated by binding to the OTR or the V1aR, or both. In fact, to the best of our knowledge, no studies to date have determined the functional role of the V1aR in the PAG of adult male rats in any type of behavior. However, administration of an OTR antagonist into the PAG decreases pain thresholds in adult male rats (Yang et al., 2011). The authors used desGly-NH2-d(CH2)5[D-Tyr2, Thr4]OVT, which is 95 times more potent as an OTR antagonist than as V1aR antagonist; Manning et al., 2012). This suggests functional signaling via the OTR even in a brain region with low OTR binding density. Given the presence of dense OXT fibers and V1aR binding in rats and mice, it would be of interest to determine potential cross-system signaling in this SBNN node.
2.9. How do density patterns of AVP and OXT fibers/receptor binding compare with other species?
One outstanding question is how the correspondence between AVP/OXT fibers and V1aR/OTR binding, or lack thereof, in rats compares to other species. This is particularly relevant given that much of the pharmacological evidence for cross-talk in these systems comes from other rodent species, i.e., hamsters, mice and voles (Sala et al., 2011; Song et al., 2014; 2016a,b; Anacker et al., 2016). Furthermore, understanding the extent to which mismatches in the AVP/OXT systems are conserved across species may provide additional insights into where one might expect to consistently find cross-talk. Unfortunately, and to the best of our knowledge, parallel studies in which both AVP and OXT or V1aR and OTR are compared in nodes of the SBNN have not been conducted in species other than rats. For example, AVP fiber densities have been analyzed in the MeP, BNSTp, and MPOA in numerous species and across diverse taxa (reviewed by De Vries & Panzica, 2006; Albers, 2015). However, there are no corresponding studies (except those in the rat) that have assessed the binding densities of V1aR and OTR relative to each other in these areas. This makes it difficult to determine whether cross-talk is more or less likely in these species. Evidence does, however, suggest that there are likely to be some species differences. For example, in Syrian hamsters, there are virtually no AVP cell bodies or fibers in the MeP, BNSTp, or LS (Dubois-Dauphin et al., 1990; Albers et al., 1991; Ferris et al., 1995). Interestingly, despite this lack of AVP fiber density, V1aR binding density is present in the LS of Syrian hamsters (Johnson et al., 1995), as it is in rats (Smith et al., 2017). Taken together, studies are required in which AVP and OXT fibers as well as V1aR and OTR binding (or mRNA expression) are analyzed in nodes of the SBNN and across various species. This will lend valuable insights into potential sites of crosstalk between AVP and OXT systems across the evolutionary continuum.
Comparing sex and age differences in density patterns of AVP and OXT fibers with V1aR and OTR binding in the rat SBNN
Sex differences have been found in AVP-ir cell number and fiber density in nodes of the SBNN across many adult species, including rats (reviewed in DeVries & Panzica, 2006). In contrast, no sex differences have been found in OXT-ir cell number and fiber density in nodes of the rat SBNN (DiBenedictis et al., 2017) and very little is known about potential sex differences in OXT-ir cell number and fiber densities in nodes of the SBNN of other species (Table 2 for overview and citations). Yet, there are sex differences in V1aR and OTR binding densities in nodes of the SBNN in rats (Uhl-Bronner et al., 2005; Dumais et al., 2013; Dumais & Veenema, 2016a; Smith et al., 2017) and other species (Table 2 for overview and citations). Several studies have compared the developmental trajectories of AVP and OXT parameters in the rat brain, but these comparisons were mostly qualitative (Tribollet et al., 1989; Snijdewint et al., 1989; Shapiro & Insel, 1989; Tribollet et al., 1991; Tribollet et al., 1992). We recently quantified AVP-ir and OXT-ir cell number and fiber density (DiBenedictis et al., 2017) and V1aR and OTR binding density (Smith et al., 2017) in the SBNN of juvenile and adult male and female rats. Analysis of findings from both studies reveals three important points. First, sex and/or age differences in AVP-ir fiber and OTR binding densities are found in five of the seven nodes of the SBNN (i.e., MeP, BNSTp, LS, MPOA, and VMH; Fig. 2), while no sex or age differences are observed for OXT-ir fiber density and V1aR binding density in the same regions (with the notable exceptions of V1aR in the MeP and LS; Fig. 2, Fig. 3B). Second, a similar pattern of sex and age differences is found for AVP-ir cell number/fiber density and OTR binding density (higher in males and adults) in the MeP and BNSTp. Last, an opposite pattern of sex and age differences is found for AVP-ir fibers density (higher in males and adults) and OTR binding density (higher in juveniles and females) in the LS. These three points and their relevance for potential functional interaction between AVP and OXT systems are discussed below.
Table 2.
Overview of sex and age (adults versus juveniles) differences in AVP and OXT systems in nodes of the SBNN across vertebrate species.
| Brain Region | Species | AVP cells/fibers | V1aR binding | OXT cells/ fibers | OTR binding | Citations | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Sex | Age | Sex | Age | Sex | Age | |||
| MeP | Rats | M>F | A>J | n.d. | n.d. | n.d. | n.d. | M>F | A>J | 1–12 |
| C57/Bl6 Mice | M>F | 13,14 | ||||||||
| CD1 mice | M>F | 17 | ||||||||
| Singing Mice | n.d. | M>F | 15 | |||||||
| Garden Dormice | M>F | 16 | ||||||||
| European Hamsters | M>F | 18 | ||||||||
| Long-tailed Hamsters | n.d. | 19 | ||||||||
| Golden Hamsters | n.d. | 25 | ||||||||
| Prairie Voles | M>F | n.d. | 20, 21 | |||||||
| Meadow Voles | M>F | 20 | ||||||||
| Brandt’s Voles | n.d. | 19 | ||||||||
| Mandarin Voles | M>F | 22 | ||||||||
| Naked mole-rats | n.d. | M>F | 23,24 | |||||||
| Macaques | n.d. | n.d. | 26,38 | |||||||
| Marmosets | n.d. | 27 | ||||||||
| Tree Lizards | M>F | 28 | ||||||||
| Newts | M>F | n.d. | 29, 30 | |||||||
| Bullfrogs | M>F | 31,32 | ||||||||
| BNSTp | Rats | M>F | A>J | n.d. | n.d. | n.d. | n.d. | M>F | A>J | 1,2,4,5,7–12, 33–36,40 |
| C57/Bl6 Mice | M>F | 13,14 | ||||||||
| CD1 Mice | F>M | 37 | ||||||||
| Singing Mice | n.d. | 15 | ||||||||
| Prairie Voles | M>F | n.d. | n.d. | n.d. | 20,21,39 | |||||
| Meadow Voles | M>F | n.d. | 20,39 | |||||||
| Montane Voles | n.d. | 39 | ||||||||
| Pine Voles | n.d. | 39 | ||||||||
| Naked mole-rats | n.d. | 23 | ||||||||
| Macaques | n.d. | n.d. | 26, 38 | |||||||
| Marmosets | M>F | n.d. | 27 | |||||||
| Humans | n.d. | 41 | ||||||||
| Chickens | M>F | 42,43 | ||||||||
| Japanese Quail | M>F | 44,45,46 | ||||||||
| Zebra Finches | M>F | n.d. | 47,48, 49 | |||||||
| Spice Finches | n.d. | 49 | ||||||||
| Melba Finches | n.d. | 49 | ||||||||
| Violet-eared Waxbill | n.d. | 49 | ||||||||
| Angolan blue Waxbill | n.d. | 49 | ||||||||
| Sparrows | M>F | 50 | ||||||||
| Penduline Tits | M>F | 51 | ||||||||
| Blue Tits | n.d. | 51 | ||||||||
| Tree Lizards | M>F | 28 | ||||||||
| Newts | M>F | 30 | ||||||||
| Geckos | M>F | n.d. | 52 | |||||||
| LS | Rats | M>F | A>J | F>M | A>J | n.d. | n.d. | F>M | J>A | 1,7–12,35, 36,40,53–55 |
| C57/Bl6 Mice | M>F | n.d. | J>A | 13,14,56,57 | ||||||
| CD1 Mice | M>F | F>M | 17,37 | |||||||
| MF1 Mice | M>F | 58 | ||||||||
| ICR Mice | n.d. | 59 | ||||||||
| California Mice | n.d. | 60 | ||||||||
| Garden Dormice | M>F | 16 | ||||||||
| Gerbils | M>F | 61 | ||||||||
| European Hamster | M>F | 18 | ||||||||
| Golden Hamsters | n.d. | 25 | ||||||||
| Prairie Voles | M>F | n.d. | n.d. | 20,21,39,62 | ||||||
| Meadow Voles | M>F | 20,39 | ||||||||
| Montane Voles | M>F | 39 | ||||||||
| Pine Voles | M>F | 39 | ||||||||
| Naked mole-rats | n.d. | 23 | ||||||||
| Tuco-tucos | n.d. | 63 | ||||||||
| Macaques | n.d. | 38 | ||||||||
| Humans | n.d. | n.d. | 41 | |||||||
| Japanese Quail | M>F | 44,45 | ||||||||
| Zebra Finches | n.d. | n.d. | 49,64 | |||||||
| Spice Finches | n.d. | 49 | ||||||||
| Melba Finches | n.d. | 49 | ||||||||
| Violet-eared Waxbill | n.d. | 49 | ||||||||
| Angolan blue Waxbill | n.d. | 49 | ||||||||
| Sparrows | M>F | 50 | ||||||||
| Penduline Tits | M>F | 51 | ||||||||
| Blue Tits | n.d. | 51 | ||||||||
| Canaries | M>F | 65 | ||||||||
| Tree Lizards | M>F | 28 | ||||||||
| Newts | n.d. | 30,66 | ||||||||
| Geckos | M>F | 67 | ||||||||
| Bullfrogs | n.d. | 31,32,66 | ||||||||
| Turtles | M>F | 68 | ||||||||
| Snakes | M>F | 68 | ||||||||
| MPOA | Rats | M>F | A>J | n.d. | n.d. | n.d. | n.d. | M>F | A>J | 1,7,12,11, |
| C57/Bl6 Mice | M>F | F>M | n.d. | 13,14, 57, 69 | ||||||
| Mongolian Gerbils | n.d. | n.d. | 70 | |||||||
| Chinese Hamsters | n.d. | n.d. | 70 | |||||||
| Long-tailed Hamsters | n.d. | 19 | ||||||||
| Prairie Voles | n.d. | 39 | ||||||||
| Meadow Voles | n.d. | 39 | ||||||||
| Brandt’s Voles | n.d. | 19 | ||||||||
| Pine Voles | n.d. | 39 | ||||||||
| Montane Voles | n.d. | 39 | ||||||||
| Naked mole-rats | n.d. | 23 | ||||||||
| Macaques | n.d. | 38 | ||||||||
| Japanese Quail | M>F | 45,46 | ||||||||
| Zebra Finches | n.d. | 49 | ||||||||
| Spice Finches | n.d. | 49 | ||||||||
| Melba Finches | n.d. | 49 | ||||||||
| Violet-eared Waxbill | n.d. | 49 | ||||||||
| Angolan blue Waxbill | n.d. | 49 | ||||||||
| Penduline Tits | M>F | 51 | ||||||||
| Blue Tits | n.d. | 51 | ||||||||
| Tree Lizards | M>F | 28 | ||||||||
| Newts | M>F | 30 | ||||||||
| Blue Wrasse | F<M | 71 | ||||||||
| Rock Hind | n.d. | 72 | ||||||||
| Medaka | M>F | 73 | ||||||||
| Goldfish | n.d. | 74 | ||||||||
| VMH | Rats | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | AM>AF | A>J | 1,7–12,35,36,40,76,77 |
| C57/Bl6 Mice | EF>JF | 57 | ||||||||
| ICR Mice | F>M | 59 | ||||||||
| Siberian Hamsters | M>F | 75 | ||||||||
| Macaques | n.d. | 38 | ||||||||
| AH | Rats | n.d. | JM>A M | n.d. | n.d. | n.d. | n.d. |
n.d. |
n.d. | 1,12, |
| C57/Bl6 Mice | n.d. | 13 | ||||||||
| Singing Mice | n.d. | 15 | ||||||||
| Mongolian Gerbils | n.d. | n.d. | 70 | |||||||
| Chinese Hamsters | n.d. | n.d. | 70 | |||||||
| Long-tailed Hamsters | n.d. | 19 | ||||||||
| Mandarin Voles | n.d. | 79 | ||||||||
| Brandt’s Voles | n.d. | 19 | ||||||||
| Naked mole-rats | n.d. | 23 | ||||||||
| Chickens | M>F | 78 | ||||||||
| Rock Hind | n.d. | 72 | ||||||||
| PAG | Rats | M>F | n.d. | n.d. | n.d. | n.d. | 12,81 | |||
| C57/BL6 Mice | n.d. | n.d. | J>A | 13,57 | ||||||
| Singing Mice | n.d. | 15 | ||||||||
| Naked-mole rats | n.d. | 23 | ||||||||
| Macaques | n.d. | 26 | ||||||||
| Geckos | M>F | 67 | ||||||||
| Newts | n.d. | 66 | ||||||||
| Frogs | n.d. | 66 | ||||||||
| Turtles | M>F | 68 | ||||||||
| Snakes | M>F | 68 | ||||||||
| Fish | M>F | 80 | ||||||||
Note the lack of studies addressing age differences in AVP and OXT systems. A: adult, F: female, J: juvenile, M: male, n.d. none detected (comparisons have been made, but no differences were observed).
References for Table 2
Caffe et al., 1989
Haussler et al., 1990
Dumais & Veenema, 2016
Olazabal & Alsina-Llanes, 2013
Voorhuis et al., 1988
Gonzalez & Smeets, 1992
Godwin et al., 71
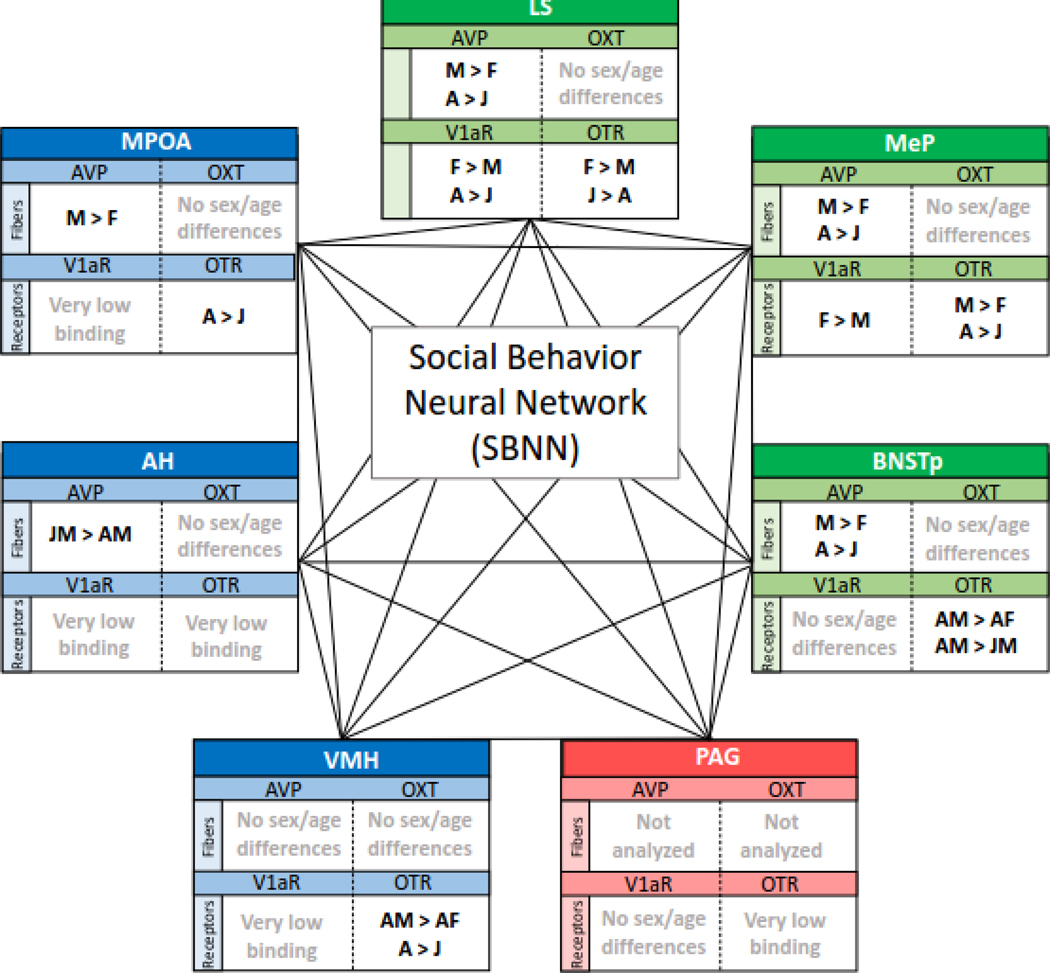
Figure 2.
Overview of sex and age differences in AVP-ir and OXT-ir cells/fiber density (measured as cell number/pixels: DiBenedictis et al., 2017) and in OTR and V1aR binding density (measured as disintegrations per minute/milligram tissue: Smith et al., 2017) in nodes of the rat SBNN. Significant main effects of sex (M = males, F = females) and of age (J = juveniles, A = adults) are indicate with the symbol >. Significant sex*age interaction effects are denoted by the inclusion of the appropriate sex + age. Green boxes represent cortico-striatal regions; blue boxes represent hypothalamic regions; red box represents midbrain region.
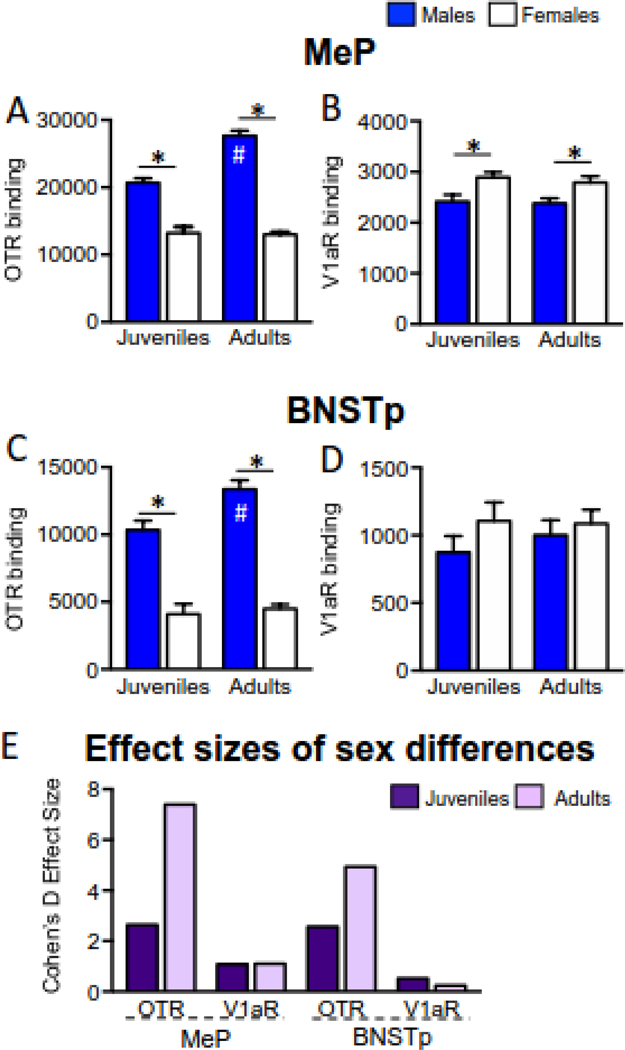
Figure 3.
Robust male-biased sex differences in OTR and more limited female-biased sex differences in V1aR. (A) OTR binding density in the MeP is higher in males than in females and higher in adult males than in juvenile males (Smith et al., 2017). (B) V1aR binding density in the MeP is higher in females than in males (Sex effect: F(1,43) = 13.4, p<0.001), in both juveniles and adults (Age effect: F(1,43) = 0.31, p=0.58; Interaction effect: F(1,43) = 0.06, p=0.80). (C) OTR binding density in the BNSTp is higher in males than in females and higher in adults than in juveniles (Smith et al., 2017). (D) V1aR binding density in the BNSTp does not differ with sex or age (Sex effect: F(1,41) = 1.70, p=0.20; Age effect: F(1,41) = 0.20, p=0.66; Interaction effect: F(1,41) = 0.37, p=0.55). (E) Cohen’s D effect sizes for significant sex differences. Data presented in B and D was conducted according to Smith et al. (2017). *p<0.05, #p<0.05 versus respective juvenile group, 2-way ANOVA, values indicate means (disintegrations per minute /milligram tissue) + SEM.
3.1. Sex and/or age differences in AVP fiber and OTR binding density in five of the seven nodes of the rat SBNN
Our analysis revealed sex and/or age differences in AVP-ir fibers and OTR binding density in five of the seven nodes of the SBNN, i.e., the MeP, BNSTp, LS, MPOA, and VMH (Fig. 2; DiBenedictis et al., 2017; Smith et al., 2017). This is in contrast to OXT-ir fiber and V1aR binding densities that are similar between the sexes and between juveniles and adults in these regions, with the exception of denser V1aR binding in females versus males in the MeP (Fig. 3B) and the LS, and in adults versus juveniles in the LS (Smith et al., 2017). This suggests that AVP and OTR in these brain regions play roles in sex- and age-specific regulation and/or expression of social behaviors. In support, AVP and OTR have both been implicated in behaviors that differ in their regulation and/or expression with sex and age, such as olfactory social communication, social play, aggression, and sexual behavior (Arawaka et al., 2010; Ferris et al., 1984; Albers et al., 1986; Gill et al., 2011; Popik & van Ree, 1991; Schulze & Gorzalka, 1991; Whitman & Albers, 1998; Veenema et al., 2013). Current knowledge about the potential underlying mechanisms and behavioral consequences of these sex and age differences in AVP-ir fiber and OTR binding densities is discussed in more detail in the following sections.
3.2. Similar patterns of sex and age differences in AVP cell number/fiber density and OTR binding density in the rat MeP and BNSTp
AVP-ir cell number (in the MeP and BNSTp) and fiber density (in the MeP only) is higher in adult males compared to adult females and compared to juvenile males (Fig. 2; DiBenedictis et al., 2017). Likewise, in both the MeP and BNSTp, OTR binding density is higher in adult males compared to adult females and compared to juvenile males (Fig. 2, Fig. 3A, C; Smith et al., 2017). In contrast, no sex and age differences were found for OXT-ir cell number and fiber density (DiBenedictis et al., 2017) nor for V1aR binding density in the BNSTp (Fig. 3D) while V1aR binding is denser in females than in males at both juvenile and adult ages in the MeP (Fig. 3B).
AVP and OTR are both regulated by gonadal steroid hormones. Specifically, neonatal and adult gonadectomy substantially reduce AVP-ir cell number and fiber density in the MeP and BNSTp of adult male rats (Bingham & Viau, 2008; De Vries et al., 1984, 1994; Rood et al., 2013; Wang & De Vries, 1995). Neonatal testosterone treatment increases AVP mRNA expression in the BNSTp and MeP of neonatally gonadectomized adult male and female rats (Han & De Vries, 2003). Similarly, gonadectomy of adult male rats reduced OTR, but not V1aR, binding density in several brain regions, although the MeP and BNSTp were not included in this analysis (Tribollet et al., 1990). Neonatal testosterone treatment increases OTR binding density in the BNSTp and MeP in postpubertal female rats (Uhl-Bronner et al., 2005). Thus, regulation by gonadal steroid hormones during both early development and adulthood may be a shared potential mechanism that underlies the higher AVP-ir fiber density and OTR binding density in the MeP and BNSTp in males compared to females and adults compared to juveniles.
Given that AVP and OTR in the MeP and BNSTp are both involved in the modulation of similar social behaviors (Arawaka et al., 2010; Popik & van Ree, 1991; Ferris et al., 1984; Albers et al., 1986; Gill et al., 2011; Schulze & Gorzalka, 1991; Whitman & Albers, 1998) and the similar sex and age differences in AVP-ir cell number/fiber density and OTR binding density in the MeP and BNSTp, we speculate that AVP and OTR in these regions serve similar functions and may do so by interacting with each other. It could be that AVP predominantly signals at the OTR in the MeP and BNSTp, particularly in adult males that show the highest AVP-ir fiber and OTR binding densities. It may also be possible that OTR activation modulates the activity of AVP-producing neurons in the MeP and BNSTp. Both possibilities, which may not be mutually exclusive, and are only speculations at this point, would allow for sex- and age-specific modulation of neuronal activity of the MeP and BNSTp, namely via AVPinduced OTR signaling and/or via OTR-induced AVP signaling. The BNSTp and MeP are reciprocally interconnected structures (Weller & Smith, 1982; Canteras et al., 1992; Dong et al., 2001; Coolen & Wood, 1998). Therefore, a third possibility could be that OTR-expressing neurons in the MeP synapse onto AVP neurons in the BNSTp and/or that AVP neurons in the BNSTp synapse onto OTR-expressing neurons in the MeP. This would allow yet another way in which AVP and OTR might interact. Further research is needed to provide evidence for these diverse speculations.
Even if AVP and OTR do not directly interact with one another in the MeP and BNSTp, we propose that the observed similar sex and age differences in AVP cell number/fiber density and OTR binding density suggest that AVP and OTR may be involved in the regulation of the same types of sex- and age-specific behaviors. No studies to date have determined the impact of sex or age differences in AVP-ir cell number or AVP-ir fiber density in the MeP and BNSTp for the regulation of behavior. However, activity of BNSTp-AVP cells in adult male C57Bl/6 mice, which have more AVP-ir cells than females (Rood et al., 2013), is increased in response to sexual interaction and non-aggressive social interaction (Ho et al., 2010). OTR in the MeP and BNSTp has been shown to be involved in the regulation of social recognition in adult male and female rats (Lukas et al., 2013; Dumais et al., 2016c). Moreover, OXT administered into the BNSTp prolongs the duration of social recognition in adult male, but not female, rats (Dumais et al., 2016c). This suggests that males are more sensitive to the behavioral effects of exogenous OXT, which could be due to their denser OTR binding compared to females. Unfortunately, and to the best of our knowledge, no studies have investigated the role of AVP and OTR in the MeP and BNSTp in the regulation of the same type of behavior. Clearly, more research is needed at the cellular, pharmacological, and behavioral level to test the intriguing hypothesis that AVP and OTR may interact in the MeP and BNSTp to regulate the same type(s) of social behavior and possibly do so in sex- and age-specific ways.
3.2.1. How do sex and age differences in AVP cell number/fibers and OTR binding in the rat MeP and BNSTp compare with other species?
The sex difference in AVP-ir cell number/fiber density in the MeP and BNSTp is one of the best characterized and conserved sex differences in the brain (van Leeuwen et al., 1985; Miller et al., 1989; Szot & Dorsa, 1993; Wang & De Vries, 1995; Taylor et al., 2012) with males having higher AVP-ir (or its homologous vasotocin) cell number and/or fiber density in the MeP and/or BNSTp than females in 15 out of 18 vertebrate species analyzed across the evolutionary continuum (for overview and references, see Table 2). The sex difference in OTR binding density in the MeP and BNSTp in rats (Dumais et al., 2013; Smith et al., 2017) is also found in naked-mole rats (Mooney et al., 2015), singing mice (Campbell et al., 2009) and mandarin voles (Smeltzer et al., 2006) (Table 2). However, no sex differences in OTR binding density in the medial amygdala and BNSTp were found in prairie voles (Bales et al., 2007) and Syrian hamsters (Dubois-Dauphin et al., 1992).
The age difference in AVP-ir cell number/fiber density and OTR binding density in the MeP and BNSTp (DiBenedictis et al., 2017; Smith et al., 2017) confirms previous studies in male rats showing higher AVP mRNA expression in the MeP and BNSTp in adults than in juveniles (Pak et al., 2009; Szot & Dorsa, 1993) and higher OTR binding density and OTR mRNA expression in the BNSTp in adults than in 10-day or 3-week-old males (qualitative measures only; Yoshimura et al., 1991; Tribollet et al., 1992). To the best of our knowledge, no other species were studied to investigate potential age differences in AVP cell number/fiber density and OTR binding density in the MeP and BNSTp (Table 2), making it unclear whether the observed age differences in rats are conserved across species.
Overall, these findings demonstrate that the sex differences in AVP-ir cell number/fiber density and OTR binding density in the MeP and BNSTp are found across multiple rodent species. This makes it plausible that a possible cross-talk between AVP and OTR in the MeP and BNSTp could occur in several species and that potential sex-specific functional consequences could be similar across these species.
3.3. Opposite patterns of sex and age differences in AVP fiber density and OTR binding density in the rat LS
The pattern of sex and age differences for AVP-ir fiber density in the LS is the same as for AVP-ir cell number/fiber density in the BNST and MeP, i.e., higher in males than in females and higher in adults than in juveniles (DiBenedictis et al., 2017). Given this pattern and the notion that AVP neurons in the BNST and MeP project to the LS (De Vries & Buijs, 1983; Caffé et al., 1987), it is likely that this pathway plays an important role in the sex- and age-specific regulation and/or expression of social behaviors.
OTR binding density shows a pattern of sex and age differences in the LS that is opposite to OTR binding density in the BNSTp and MeP and opposite to LS-AVP fiber density, i.e., higher in females than in males and higher in juveniles than in adults (Smith et al., 2017). These findings demonstrate a highly brain region-specific regulation of OTR by sex and age. This may indicate that a sex- and age-specific functional cross-talk between AVP and OTR, as was suggested for the MeP and BNSTp, is less likely to occur in the LS.
Given the similar age patterns between LS-AVP fiber density and LS-V1aR binding density (i.e., denser in adults than in juveniles; Fig. 2; Smith et al., 2017), it could be that adults show stronger AVP-V1aR signaling in the LS than juveniles. This, in turn, may allow for some behaviors to be regulated in an age-specific way. In support, administration of a V1aR antagonist into the LS impairs social recognition in adult male rats (Dantzer et al., 1998; Everts & Koolhaas, 1997; Veenema et al., 2012; Lukas et al., 2013), but not in juvenile male rats (Veenema et al., 2012). Administration of AVP into the LS improves social recognition in adult, but not in juvenile, male rats (Veenema et al., 2012). Moreover, the AVP-V1aR system in the LS facilitates intermale aggression in adult male rats (Koolhaas et al., 1991; Veenema et al., 2010) and reduces social play behavior in juvenile male rats (Veenema et al., 2013; Bredewold et al., 2014). If denser LS-AVP-ir fibers and LS-V1aR binding in adults versus juveniles reflect higher AVP-V1aR signaling in the LS, then such higher signaling may serve to facilitate the developmental transition from juvenile social play behavior into adult aggression in males.
Although female rats show an age pattern in LS-AVP fiber density and LS-V1aR binding that is similar to male rats (Smith et al., 2017), it is less clear what the functional implications of this age pattern are. For example, administration of AVP into the LS improves social recognition similarly in adult and juvenile female rats (Veenema et al., 2012). Yet, administration of a V1aR antagonist into the LS impaires social recognition in adult female rats, but not in juvenile female rats (Veenema et al., 2012). Administration of a V1aR antagonist into the LS decreases social play behavior in juvenile female rats (Veenema et al., 2013; Bredewold et al., 2014, 2018). V1aR binding in the LS increases at parturition and correlates positively with maternal aggression in Sprague-Dawley rats (Caughey et al., 2011). This suggests that LS-V1aR activation facilitates juvenile-specific as well as adult-specific social behaviors in female rats.
The denser OTR binding observed in the LS of juveniles compared to adults (Smith et al., 2017) coincides with relatively sparse OXT-ir and AVP-ir fibers in the LS of juveniles compared to adults (DiBenedictis et al., 2017). Denser OTR binding could perhaps compensate for sparse fibers to facilitate OTR-mediated neuronal signaling, which, in turn, may play a role in juvenile-specific behaviors. However, administration of an OTR antagonist into the LS does not change social play behavior, a juvenile-specific behavior, nor does it change social preference or social novelty preference in juvenile male and female rats (Bredewold et al., 2014; Smith et al., 2015). OXT administered into the LS has no effect on social play behavior in juvenile male rats, but it reduces social play behavior in juvenile female rats (Bredewold et al., 2014). Thus, more research is needed to determine whether denser OTR binding in the LS of juveniles versus adults translates into age differences in OTR signaling and if so, what the age specific behavioral consequences may be.
3.3.1. How do sex and age differences in fibers and receptors for AVP and OXT systems in the rat LS compare with other species?
The LS is a well-characterized sexually dimorphic projection target of AVP synthesizing neurons in the MeP/BNSTp of rats (DeVries et al., 1981; Caffe et al., 1987) as well as of other rodent species with higher AVP-ir fiber density in adult males versus adult females in mice (Rood et al., 2013), gerbils (Crenshaw et al., 1992), European hamsters (Buijs et al., 1986) and prairie, pine, meadow and montane voles (Table 2; Wang et al., 1996). Males of most non-mammalian species analyzed also show denser vasotocin (homologue of AVP)-ir fibers in the LS than females (Table 2) including Japanese quail (Aste et al., 1998; Panzica et al., 2001), sparrows (Maney et al., 2005), canaries (Voorhuis et al., 1988), geckos (Stoll & Voorn, 1985), turtles (Smeets et al., 1990) and snakes (Smeets et al., 1990).
The sex differences in LS-OTR and LS-V1aR binding densities in rats (both higher in females than males) are largely driven by juveniles (Smith et al., 2017). This may explain why a similar sex difference in LS-V1aR binding density was found in another rat study analyzing juveniles and adults (Veenema et al., 2012: sex difference in V1aR binding density), but not in two other rat studies using adults only (Dumais et al., 2013: no sex difference in OTR binding density; Dumais & Veenema, 2016a: no sex difference in V1aR binding density), nor in studies using adults of other species, such as mice (Tribollet et al., 2002), golden hamsters (Dubois-Dauphin et al., 1992), prairie voles (Bales et al., 2007), tuco-tucos (Beery et al., 2008), finches (Goodson et al., 2006), waxbills (Goodson et al., 2006), macaques (Young et al., 1999), or humans (Table 2; Loup et al., 1991).
The age difference in LS-AVP-ir fiber density is in line with a previous qualitative study in rats (De Vries et al., 1981) and a study on vasotocin-ir fiber density in chickens (Aste et al., 2016). The age difference in OTR binding density in the LS (Smith et al., 2017) confirms previous findings in rats (Lukas et al., 2010; Yoshimura et al., 1991) and mice (Hammock & Levitt, 2012; Olazabal & Alsina-llanes, 2016). To the best of our knowledge, there are no data on age differences in V1aR binding density in the LS of species other than the rat.
Overall, in those instances where data is available in multiple species, there is considerable consistency across species in patterns of sex and age differences in the LS-AVP and LS-OXT systems. If these sex and age differences have functional relevance for the sex- and age-specific regulation of social behavior, then we would expect to see a similar functional relevance across these species. Given the complexity of the sex and age differences in AVP and OXT systems, combined with the variety of behaviors the LS can modulate, further studies addressing the presence of both canonical and cross-talk AVP/OXT signaling will be critical to understanding the roles of these neuropeptides in the LS for behavioral regulation.
4. Conclusions and future directions
We have discussed several intriguing patterns of AVP/OXT-ir fiber and V1aR/OTR binding densities in the rat SBNN. First, few SBNN nodes showed a match between relative densities of fibers and receptors within a system (i.e., the LS and PAG for the AVP system and the BNSTp for the OXT system). Second, several SBNN nodes showed a mismatch between relative densities of fibers and receptors (i.e., the MeP, BNSTp, MPOA, and AH for the AVP system and the MeP, LS, MPOA, and VMH for the OXT system). Third, similarly high densities were found for fibers and receptors across systems in three SBNN nodes that showed a mismatch (i.e., high AVP-ir fiber density and high OTR binding density in the MeP, BNSTp, and MPOA). Fourth, in two of these three SBNN nodes (i.e., the MeP and BNSTp), AVP-ir fiber and OTR binding densities changed in similar ways with sex and age (higher in males and higher in adults). These findings in rats strongly suggest the potential of AVP and OXT systems to interact with each other and that AVP may signal at the OTR rather than, or in addition to, the V1aR in these SBNN nodes.
This review sheds light on important outstanding questions, especially regarding the pharmacological aspects of ligand-receptor interactions and the extent of conservation of cross-talk between AVP and OXT systems. For example, it is unclear how AVP/OXT fiber density relates to extracellular and synaptic AVP/OXT release. Studies comparing differences in AVP/OXT fiber density with extracellular AVP/OXT release using intracerebral microdialysis may provide some insight into this. Yet another issue is that the source of either AVP or OXT released within a given region remains difficult to identify. Electron microscopy studies, as well as studies in which tools are used to induce endogenous ligand release will be critical to answering this question. Once released, it also remains unclear how far these neuropeptides can travel to act at their target receptors. Our review of the existing literature across species makes it clear that characterization of the relative densities of AVP/OXT fibers and V1aR/OTR binding in one design will be necessary in order to determine which mismatches between fibers and receptors in the SBNN are consistent across species, and which are species-specific.
We further discussed the implications of matches and mismatches between AVP/OXT fibers and V1aR/OTR in the rat SBNN for the regulation of social behavior. This highlighted a need to elucidate whether the behavioral effects of local AVP or OXT administration are mediated a) via activation of the V1aR, OTR, or both, and b) are mediated by endogenous AVP, OXT, or both. Interestingly, most pharmacological based evidence for cross system signaling comes from species other than rats, namely hamsters, mice, and voles (Sala et al., 2011; Song et al., 2014; 2016a,b; Anacker et al., 2016). Therefore, studies are needed to test to extent to which cross-talk may actually occur within the rat SBNN.
The similar sex and age differences in AVP and OTR in specific nodes of the rat SBNN suggest the potential of AVP and OXT systems to interact with each other in sex- and age-specific ways. We speculated that such interactions may facilitate sex- and age-specific regulation of social behavior. We also discussed that, with the exception of sex differences in AVP-ir cell number/fiber density in some SBNN nodes, very little is known about sex differences in OXT-ir fiber density and V1aR and OTR binding densities (or mRNA expression) in species other than the rat. Likewise, we highlighted that virtually nothing is known about developmental trajectories of AVP and OXT systems in species other than the rat.
If we gain more evidence of the existence of cross system signaling in rats and other species and better insights into their functional consequences, then this would allow us to address questions related to the evolutionary relevance of the development of the cross-talk between the AVP and OXT systems and addressing the implications for basic and clinical studies. Overall, we hope that the analyses herein will serve as a springboard for further studies in rats and other species aimed at unraveling the roles of AVP and OXT systems in the regulation of social behavior, via potential cross-talk and in possibly sex- and age-specific ways.
Highlights:
A better understanding of cross-system signaling of AVP and OXT systems is needed
There is a mismatch between AVP/OXT fibers and V1aR/OTR binding in rat SBNN nodes
Cross-system signaling of AVP with OTR may occur in the MeA, BNST, and MPOA
Cross-system signaling of OXT with V1aR may occur in the PAG
AVP fiber and OTR binding densities change similarly with sex and age in MeA and BNST
Acknowledgements:
We would like to thank the members of the Veenema Lab for their critical reading of the manuscript.
Funding: This work was supported by the National Institutes of Health [grant number R01MH102456 to AHV]; and the National Science Foundation [grant number IOS1253385 to AHV].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Albers HE, Pollock J, Simmons WH, Ferris CF (1986) A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus, J Neurosci. 6(7): 2085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Rowland CM, Ferris CF (1991) Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus). Brain Research. 539(1): 137–42. [DOI] [PubMed] [Google Scholar]
- Albers HE (2015) Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network, Front Neuroendocrinol. 36:49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Christensen JD, LaFlamme EM, Grunberg DM, Beery AK (2016) Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles, Psychoneuroendocrinology. 68:156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Deak T (2010) Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor, Neuroscience. 171(4):1141–51. [DOI] [PubMed] [Google Scholar]
- Aste N, Balthazart J, Absil P, Grossmann R, Mülhbauer E, Viglietti-Panzica C, Panzica GC (1998) Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica), J Comp Neurol. 396(2):141–57. [PubMed] [Google Scholar]
- Aste N, Yoshioka N, Sakamoto E, Saito N (2016) Female-biased sex difference in vasotocin-immunoreactive neural structures in the developing quail brain, J Chem Neuroanat. 77:41–54. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C, (2006) Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens, Nature Neuroscience. 9(2):220–6. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS (2007) Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors, Neuroscience. 144(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM (1995) Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites, Endocrinology. 136(11):5135–8. [DOI] [PubMed] [Google Scholar]
- Bayerl DS, Kaczmarek V, Jurek B, van den Burg EH, Neumann ID, Gaßner BM, Klampfl SM, Bosch OJ (2016) Antagonism of V1b receptors promotes maternal motivation to retrieve pups in the MPOA and impairs pup-directed behavior during maternal defense in the mpBNST of lactating rats, Horm Behav.79:18–27. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD (2008). Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J. Comp. Neurol. 507:1847–1859. [DOI] [PubMed] [Google Scholar]
- Bingham B, Viau V. Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2008. July;149(7):3581–91. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pförtsch J, Beiderbeck DI, Landgraf R, Neumann ID (2010) Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat, J. Neuroendocrinol. 22(5):420–9. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Moore FL (1991) Gonadectomy reduces the concentrations of putative receptors for arginine vasotocin in the brain of an amphibian, Brain Res. 541(2):193–7. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Moore FL (1992) Sexually dimorphic concentrations of arginine vasotocin in sensory regions of the amphibian brain, Brain Res. 588(2):304–6. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Tyler CJ, De Vries GJ (1992) Sexual dimorphism in the vasotocin system of the bullfrog (Rana catesbeiana), J Comp Neurol. 325(2):313–25. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH, (2014) Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context, Frontiers in Behavioral Neuroscience. 8(216):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM (1980) Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron microscopy, The Journal of Histochemistry and Cytochemistry. 28(4):357–60. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Pevet P, Masson-Pevet M, Pool CW, de Vries GJ, Canguilhem B, Vivien-Roels B (1986) Seasonal variation in vasopressin innervation in the brain of the European hamster (Cricetus cricetus), Brain Research. 371:193–6. [DOI] [PubMed] [Google Scholar]
- Bychowski ME, Mena JD, Auger CJ (2013) Vasopressin infusion into the lateral septum of adult male rats rescues progesterone-induced impairment in social recognition, Neuroscience. 246:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffé AR, van Leeuwen FW, Luiten PG (1987) Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus, J Comp Neurol. 261(2): 237–52. [DOI] [PubMed] [Google Scholar]
- Caffé AR, van Ryen PC, van der Woude TP, van Leeuwen FW (1989) Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis, J Comp Neurol. 287(3): 302–25. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE (2004) Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters, Horm Behav. 46(4): 444–9. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Barakat AS, Smith DD, Hruby VJ, Pedersen CA (1990) A uterotonic antagonist blocks the oxytocin-induced facilitation of female sexual receptivity, Brain Res. 512(2):291–6. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA (1989) Medial preoptic area oxytocin and female sexual receptivity, Behav Neurosci. 103(3):655–62. [DOI] [PubMed] [Google Scholar]
- Campbell P, Ophir AG, Phelps SM (2009) Central vasopressin and oxytocin receptor distributions in two species of singing mice, J Comp Neurol. 516(4):321–33. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW (1992) Connections of the posterior nucleus of the amygdala, J Comp Neurol. 324(2):143–79. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF (1988) The neurophysin-containing innervation of the forebrain of the mouse, Neuroscience. 24(3):937–66. [DOI] [PubMed] [Google Scholar]
- Chini B, Verhage M, Grinevich V (2017) The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Behavior, Trends Pharmacol Sci. 38(11):982–991. [DOI] [PubMed] [Google Scholar]
- Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW (2007) Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice, Proc Natl Acad Sci U S A. 104(11):4670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaan JC, Buijs RM, Pool CW, De Ruiter AJ, Koolhaas JM (1993) Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice, Brain Res Bull. 30(1–2):1–6. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI (1998) Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing, J Comp Neurol. 399(2):189–209. [DOI] [PubMed] [Google Scholar]
- Corbani M, Marir R, Trueba M, Chafai M, Vincent A, Borie AM, Desarménien MG, Ueta Y, Tomboly C, Olma A, Manning M, Guillon G (2018) Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands, Gen Comp Endocrinol. 258:15–32. [DOI] [PubMed] [Google Scholar]
- Crenshaw BJ, De Vries GJ, Yahr P (1992) Vasopressin innervation of sexually dimorphic structures of the gerbil forebrain under various hormonal conditions, J Comp Neurol. 332: 589–98. [DOI] [PubMed] [Google Scholar]
- Crews D, Lou W, Fleming A, Ogawa S (2006) From gene networks underlying sex determination and gonadal differentiation to the development of neural networks regulating sociosexual behavior, Brain Res. 1126(1):109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA (2009) Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice, Front Behav Neurosci, 15:3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R (1998) Vasopressin, gonadal steroids and social recognition, Prog Brain Res. 119: 409–14. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthé RM, Le Moal M (1988) Septal vasopressin modulates social memory in male rats, Brain Res. 457(1):143–7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Al-Shamma HA (1990) Sex differences in hormonal responses of vasopressin pathways in the rat brain, J Neurobiol. 21(5):686–93. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF (1981) Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain- presence of a sex difference in the lateral septum, Brain Research. 218: 67–78. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM (1983) The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum, Brain Research. 273:307–317. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Van Leeuwen FW (1984) Sex differences in vasopressin and other neurotransmitter systems in the brain, Prog Brain Res. 61:185–203. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC (2006) Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints, Neuroscience. 138(3):947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang L, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits, J Neuroscience. 22(20):9005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH (2017) Quantitative analysis of age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network, J Comp Neurol, 525(11):2549–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis, Brain Res Brain Res Rev. 38(1–2):192–246. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Barberis C, de Bilbao F (1996) Vasopressin receptors in the mouse (Mus musculus) brain: sex-related expression in the medial preoptic area and hypothalamus, Brain Res. 743(12): 32–9. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pévet P, Barberis C, Tribollet E, Dreifuss JJ (1992) Localization of binding sites for oxytocin in the brain of the golden hamster, Neuroreport. 3(9):797–800. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Tribollet E, Dreifuss JJ (1990) Vasopressin in the brain of the golden hamster: the distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide, J Comp Neurol. 300(4):535–48. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Theler JM, Zaganidis N, Dominik W, Tribollet E, Pévet P, Charpak G, Dreifuss JJ (1991) Expression of vasopressin receptors in hamster hypothalamus is sexually dimorphic and dependent upon photoperiod. Proc Natl Acad Sci U S A. 1991 (24):11163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013) Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways, Hormones and Behavior. 64(4): 693–701. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH (2016a) Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 40:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. (2016b) Presence and absence of sex differences in structure and function of the brain oxytocin system: Implications for understanding social behavior In: Sex Differences in the Central Nervous System, Elsevier, Shansky R & Johnson J (Eds), p. 247–284. [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH (2016c) Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition, Psychoneuroendocrinology.64:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elands J, Beetsma A, Barberis C, de Kloet ER (1988) Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist, J Chem Neuroanat. 1(6):293–302. [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Ebner K, Landgraf R (2000) Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol. Spec No:125S–130S. [DOI] [PubMed] [Google Scholar]
- Everett NA, McGregor IS, Baracz SJ, Cornish JL (2018) The role of the vasopressin V1A receptor in oxytocin modulation of methamphetamine primed reinstatement, Neuropharmacology. 133:1–11. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM (1999) Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats, Behav Brain Res. 99(1):7–16. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ (2001) Oxytocin in the medial amygdala is essential for social recognition in the mouse, Journal of Neuroscience. 21(20):8278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE (1984) Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters, Science, 224(4648):521–3. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y (1994) Vasopressin and serotonin interactions in the control of agonistic behavior, Psychoneuroendocrinology. 19(5–7):593–601. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Miller MA, Dorsa DM, De Vries GJ (1995) Distribution of small vasopressinergic neurons in golden hamsters, J Comp Neurol. 360(4):589–98. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y (1997) Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters, 17(11):4331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Potegal M (1988) Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters, Physiol Behav, 44(2):235–9. [DOI] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal N, Swaab DF (1986) Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis, Brain Res, 375(2):363–7. [DOI] [PubMed] [Google Scholar]
- Floody OR, Cooper TT, Albers HE (1998) Injection of oxytocin into the medial preoptic-anterior hypothalamus increases ultrasound production by female hamsters, Peptides. 19(5):833–9. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, Richard P (1987) Pharmacological characteristics and anatomical distribution of [3H] oxytocin-binding sites in the Wistar rat brain studied by autoradiography, Neuroscience. 20(2):599–614. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF (2006) Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice, J Neuroscience. 26(8): 2335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger R, Fahrenholz F (1989) Autoradiographic localization of V1 vasopressin binding sites in rat brain and kidney, Eur J Pharmacol. 167(1): 105–16. [DOI] [PubMed] [Google Scholar]
- Gil M, Bhatt R, Picotte KB, Hull EM (2011) Oxytocin in the medial preoptic area facilitates male sexual behavior in the rat, Horm Behav. 59(4):435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z (2009) Anterior hypothalamic vasopressin regulates pairbonding and drug-induced aggression in a monogamous rodent, Proc Natl Acad Sci U S A. 106(45):19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J, Sawby R, Warner RR, Crews D, Grober MS (2000) Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish, Brain Behav Evol. 55(2):77–84. [DOI] [PubMed] [Google Scholar]
- Godwin J, Thompson R (2012) Nonapeptides and social behavior in fishes, Horm Behav. 61(3):230–8. [DOI] [PubMed] [Google Scholar]
- González A, Smeets WJ (1992) Comparative analysis of the vasotocinergic and mesotocinergic cells and fibers in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii, J Comp Neurol. 315(1):53–73. [DOI] [PubMed] [Google Scholar]
- Goodson JL (2005) The vertebrate social behavior network: evolutionary themes and variations, Horm Behav. 48(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH (2001) Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates, Brain Res Brain Res Rev. 35(3): 246–65. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y (2006) Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes, Horm Behav. 50(2):223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA (2013) What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks, Horm Behav. 64(1):103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Desarménien MG, Chini B, Tauber M, Muscatelli F (2015) Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders, Front Neuroanat. 20;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA (2015) Developmental perspectives on oxytocin and vasopressin, Neuropsychopharmacology. 40(1):24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Levitt P (2012) Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR), Neuroscience. 222: 20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RT, Kim HB, Kim YB, Choi K, Park GY, Lee PR, Lee J, Kim HY, Park CK, Kang Y, Oh SB Na HS (2018) Oxytocin produces thermal analgesia via vasopressin-1a receptor by modulating TRPV1 and potassium conductance in the dorsal root ganglion neurons, Korean J Physiol Pharmacol. 22(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ohno N, Aoki Y, Kageyama J, Takahara J, Ofuji T (1982) Distribution and characterization of corticotrophin-releasing factor and arginine vasopressin in the rat hypothalamic nuclei, Neuroendocrinology. 34:32–7. [DOI] [PubMed] [Google Scholar]
- Hӓussler HU, Jirikowski GF, Caldwell JD (1990) Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus, Journal of Chemical Neuroanatomy. 3: 271–6. [PubMed] [Google Scholar]
- Hennessey AC, Huhman KL, Albers HE (1994) Vasopressin and sex differences in hamster flank marking, Physiol Behav. 55(5):905–11. [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Whitman DC, Albers HE (1992) Microinjection of arginine-vasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus), Brain Res. 569(1):136–40. [DOI] [PubMed] [Google Scholar]
- Herkenham M (1987) Mismatches between neurotransmitter and receptor localizations in brain: observations and implications, Neuroscience. 23(1):1–38. [DOI] [PubMed] [Google Scholar]
- Hermes MLHJ, Buijs RM, Masson-Pevet M, Pevet P (1990) Seasonal changes in vasopressin in the brain of the garden dormouse (Eliomys Quercinus L.), J Comp Neurol. 293: 340–6. [DOI] [PubMed] [Google Scholar]
- Hernández VS, Vázquez-Juárez E, Márquez MM, Jáuregui-Huerta F, Barrio RA, Zhang L (2015) Extra-neurohypophyseal axonal projections from individual vasopressin-containing magnocellular neurons in rat hypothalamus. Front Neuroanat. 6: 9–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE (1991) The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice, Neuroscience. 43(2–3), 623–30. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Mouginot D, Kozoriz MG, Kombian SB, Pittman QJ (2003) Vasopressin differentially modulates non-NMDA receptors in vasopressin and oxytocin neurons in the supraoptic nucleus, J Neurosci. 23(10):4270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JM, Murray JH, Demas GE, Goodson JL (2010) Vasopressin cell groups exhibit strongly divergent responses to copulation and male-male interactions in mice, Horm Behav. 58(3):368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF (1994) Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents, J Neurosci. 14(9):5381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Platt ML (2018) Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex, Sci Rep. 8(1):820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkevich A, Barth SW, Grossmann R (1997) Sexual dimorphism of arg-vasotocin gene expressing neurons in the telencephalon and dorsal diencephalon of the domestic fowl. An immunocytochemical and in situ hybridization study, Cell Tissue Res. 287(1):69–77. [DOI] [PubMed] [Google Scholar]
- Jurkevich A, Barth SW, Kuenzel WJ, Köhler A, Grossmann R (1999) Development of sexually dimorphic vasotocinergic system in the bed nucleus of stria terminalis in chickens. J Comp Neurol. 408(1):46–60. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Weiss SL, Moore MC (2008) Arginine vasotocin (AVT) immunoreactivity relates to testosterone but not territorial aggression in the tree lizard, Urosaurus ornatus. Brain Behav Evol. 72(4):283–94. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Morrison JA, Goodson JL (2010) Cryptic regulation of vasotocin neuronal activity but not anatomy by sex steroids and social stimuli in opportunistic desert finches, Brain Behav Evol. 75(1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Okanoya K, Wada M (1999) Effect of testosterone on the distribution of vasotocin immunoreactivity in the brain of the zebra finch, Taeniopygia guttata castanotis, Life Sci. 65(16):1663–70. [DOI] [PubMed] [Google Scholar]
- Kline RJ, O’Connell LA, Hofmann HA, Holt GJ, Khan IA (2011) The distribution of an AVT V1a receptor in the brain of a sex changing fish, Epinephelus adscensionis, J Chem Neuroanat. 42(1):72–88. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response, Neuron. 73(3): 553–66. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V (2014) Evolution of oxytocin pathways in the brain of vertebrates, Front Behav Neurosci. 14: 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW (2013) Insights into the molecular evolution of oxytocin receptor ligand binding, Biochem Soc Trans. 41(1):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Moor E, Hiemstra Y, Bohus B, 1991. The testosterone-dependent vasopressinergic neurons in the medial amygdala and lateral septum: involvement in social behaviour of male rats In: Jard S, Jamison R (Eds.), vasopressin. INSERM/ John Libbey, Paris/London, pp. 213–219. [Google Scholar]
- Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B (1998) Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex, Prog Brain Res. 119:437–48. [DOI] [PubMed] [Google Scholar]
- Krémarik P, Freund-Mercier MJ, Stoeckel ME (1993) Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat, J Comp Neurol. 333(3):343–59. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Ramirez AD, Ramirez VD (1991) The positive feedback action of vasopressin on its own release from rat septal tissue in vitro is receptor-mediated, Brain Res. 545:137–141. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID (2004) Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication, Front Neuroendocrinol. 25(3–4):150–76. [DOI] [PubMed] [Google Scholar]
- Laqueur GL (1954) Neurosecretory pathways between the hypothalamic paraventricular nucleus and the neurohypophysis, J Comp Neurol. 101:543–554. [DOI] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL (2009) Neural distribution of nonapeptide binding sites in two species of songbird, J Comp Neurol. 513(2):197–208. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ (1999) Sex differences in the parental behavior of adult virgin prairie voles: Independence from gonadal hormones and vasopressin, Journal of Neuroendocrinology. 11: 441–9. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ (1991) Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study, Brain Research. 555(2):220–32. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Brown CH, Russell JA, Leng G (1997) Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J Physiol. 505:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours, Nat Rev Neurosci. 7(2):126–36. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Dayanithi G, Russell JA, Leng G (2002) The active role of dendrites in the regulation of magnocellular neurosecretory cell behavior, Prog Brain Res. 139:247–56. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH (2010) Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats, Neuropharmacology, 58(1):78–87. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH (2011a) Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats, Psychoneuroendocrinology. 36(6): 843–53. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011b) The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice, Neuropsychopharmacology. 36(11):2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID (2013) Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics, Psychoneuroendocrinology. 38(6), 916–26. [DOI] [PubMed] [Google Scholar]
- Maggi M, Del Carlo P, Fantoni G, Giannini S, Torrisi C, Casparis D, Massi G, Serio M (1990) Human myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptors. J Clin Endocrinol Metab. 70(4):1142–54. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT (2005) Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows, Horm Behav. 48(2):196–206. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G (2012) Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics, J Neuroendocrinology. 24(4):609–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G (2008) Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents, Prog Brain Res. 170:473–512. [DOI] [PubMed] [Google Scholar]
- Manzano-García A, González-Hernández A, Tello-García IA, Martínez-Lorenzana G, Condés-Lara M (2018) The role of peripheral vasopressin 1A and oxytocin receptors on the subcutaneous vasopressin antinociceptive effects, Eur J Pain. 22(3):511–526. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Kleopoulos SP, Mobbs CV, Pfaff DW (1994) Infusion of antisense oligodeoxynucleotides to the oxytocin receptor in the ventromedial hypothalamus reduces estrogen-induced sexual receptivity and oxytocin receptor binding in the female rat, Neuroendocrinology. 59(5):432–40. [DOI] [PubMed] [Google Scholar]
- Miller MA, DeVries GJ, al-Shamma HA, Dorsa DM (1992) Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration, J Neurosci. 12(8):2881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Urban JH, Dorsa DM (1989) Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization, Peptides. 10:615–619. [DOI] [PubMed] [Google Scholar]
- Montagnese CM, Székely T, Gray D, Balázsa T, Zachar G (2014) Immunoreactivity distribution of vasotocin and vasoactive intestinal peptide in brain nuclei of two songbird species with different breeding systems, Brain Behav Evol. 83(2):140–9. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Coen CW, Holmes MM, Beery AK (2015) Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents, Neuroscience. 303:261–9. [DOI] [PubMed] [Google Scholar]
- Moore FL, Richardson C, Lowry CA (2000) Sexual dimorphism in numbers of vasotocin-immunoreactive neurons in brain areas associated with reproductive behaviors in the roughskin newt. Gen Comp Endocrinol. 117(2):281–98. [DOI] [PubMed] [Google Scholar]
- Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, Onaka T. (2018) Oxytocin-Oxytocin Receptor Systems Facilitate Social Defeat Posture in Male Mice, Endocrinology. 159:763–775. [DOI] [PubMed] [Google Scholar]
- Newman SW (1999) The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network, Ann N Y Acad Sci. 29;877:242–57. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ (2016) Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications, Horm Behav. 77:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis, J Comp Neurol. 519(18):3599–639. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA (2012) Evolution of a vertebrate social decision-making network, Science. 336(6085):1154–7. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Kow LM, Pfaff DW (1992) Effects of lordosis-relevant neuropeptides on midbrain periaqueductal gray neuronal activity in vitro, Peptides. 13(5):965–75. [DOI] [PubMed] [Google Scholar]
- Ohya T, Hayashi S (2006) Vasotocin/isotocin-immunoreactive neurons in the medaka fish brain are sexually dimorphic and their numbers decrease after spawning in the female, Zoolog Sci. 23(1):23–9. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Alsina-Llanes M (2016) Are age and sex differences in brain oxytocin receptors related to maternal and infanticidal behavior in naïve mice? Horm Behav. 77:132–40. [DOI] [PubMed] [Google Scholar]
- Otero-García M, Agustín-Pavón C, Lanuza E, Martínez-García F (2016) Distribution of oxytocin and co-localization with arginine vasopressin in the brain of mice. Brain Struct Funct. 221(7):3445–73. [DOI] [PubMed] [Google Scholar]
- Otero-Garcia M, Martin-Sanchez A, Fortes-Marco L, Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F (2014) Extending the socio-sexual brain: arginine-vasopressin immunoreactive circuits in the telencephalon of mice, Brain Struct Funct. 219(3):1055–81. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Hinds LR, Handa RJ (2009) Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3beta-diol, Am J Physiol Endocrinol Metab. 296(6): E1409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovitz M (1984) Neuropeptides in the hypothalamo-hypophyseal system: lateral retrochiasmatic area as a common gate for neuronal fibers towards the median eminence, Peptides. 5 Suppl 1:35–9. [DOI] [PubMed] [Google Scholar]
- Panzica G, Viglietti-Panzica C, Balthazart J (2001) Sexual dimorphism in the neuronal circuits of the quail preoptic and limbic regions, Microsc Res Tech. 54(6):364–74. [DOI] [PubMed] [Google Scholar]
- Parhar IS, Tosaki H, Sakuma Y, Kobayashi M (2001) Sex differences in the brain of goldfish: gonadotropin-releasing hormone and vasotocinergic neurons, Neuroscience. 104(4):1099–110. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA (1994) Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas, Behav Neurosci. 108(6):1163–71. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM (1991) Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain, Eur Neuropsychopharmacol. 1(4):555–60. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF (1989) Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis, Neuroscience. 32(2):435–9. [DOI] [PubMed] [Google Scholar]
- Qiao X, Yan Y, Wu R, Tai F, Hao P, Cao Y, Wang J (2014) Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles, Journal of Comparative Physiology, 200:149–59. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Goodwillie A, Brewer C, Muglia LJ, Pfaff DW, Kow LM (2004) Vasopressin stimulates ventromedial hypothalamic neurons via oxytocin receptors in oxytocin gene knockout male and female mice. Neuroendocrinology. 80(2):92–9. [DOI] [PubMed] [Google Scholar]
- Robinzon B, Sayag N, Koike TI, Kinzler SL, Marks PA (1992) Embryonic differentiation of sexual dimorphism in vasotocin and mesotocin levels in chickens, Pharmacol Biochem Behav. 42(4):823–9. [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ (2011) Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord, J Comp Neurol, 519(12):2434–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ (2013) Site of origin of sex diffrences in the vasopressin innervation of the mouse (Mus musculus) brain, J Comp Neurol. 521(10): 2321–58. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG (2008) Distribution of oxytocin in the brain of a eusocial rodent, Neuroscience. 155, 809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ (2009) Characterization of the oxytocin system regulating affiliative behavior in female prairie voles, Neuroscience. 162(4):892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B (2011) Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism, Biol Psychiatry. 69(9):875–82. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW (1982) Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat, J Comp Neurol. 205(3):260–72. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Audigier S, Barberis C, Jard S, Manning M, Kolodziejczyk AS, Sawyer WH (1991) A radioiodinated linear vasopressin antagonist: a ligand with high affinity and specificity for V1 receptors, FEBS Lett. 282(1):77–81. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupré A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS (2010) Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse, J Neurosci. 30(24):8274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze HG, & Gorzalka BB (1991) Oxytocin effects on lordosis frequency and lordosis duration following infusion into the medial pre-optic area and ventromedial hypothalamus of female rats. Neuropeptides, 18(2): 99–106. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR (1989) Ontogeny of oxytocin receptors in rat forebrain: a quantitative study, Synapse. 4(3):259–66. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Sevensma JJ, Jonker AJ (1990) Comparative analysis of vasotocin-like immunoreactivity in the brain of the turtle Pseudemys scripta elegans and the snake Python regius, Brain Behav Evol. 35(2):65–84. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z, (2006) Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles, Neuroscience Letters. 394:146–51. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Wilkins KB, Mogavero JN, Veenema AH (2015) Social Novelty Investigation in the Juvenile Rat: Modulation by the μ-Opioid System, J Neuroendocrinol. 27(10):752–64. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH (2017. ) Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network, Brain Struct Funct. 222(2):981–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint FG, Van Leeuwen FW, Boer GJ (1989) Ontogeny of vasopressin and oxytocin binding sites in the brain of Wistar and Brattleboro rats as demonstrated by lightmicroscopical autoradiography, J Chem Neuroanat. 2(1):3–17. [PubMed] [Google Scholar]
- Song Z, Albers HE (2018) Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery, Front Neuroendocrinol. 51:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE (2016a) Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions, Psychoneuroendocrinology. 74:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Larkin TE, Malley MO, Albers HE (2016b) Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus), Horm Behav. 81:20–7. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JK 4th, Larkin TE 2nd, Huhman KL, Albers HE (2014) Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors, Psychoneuroendocrinology. 50:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll CJ, Voorn P (1985) The distribution of hypothalamic and extrahypothalamic vasotocinergic cells and fibers in the brain of a lizard, Gekko gecko: presence of a sex difference, J Comp Neurol. 239(2):193204. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms, Neuroendocrinology. 31(6):410–7. [DOI] [PubMed] [Google Scholar]
- Szot P, Dorsa DM (1993) Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain, Brain Res Dev Brain Res. 73(2):177–83. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Takashima A, Takanami K, Yoshida S, Nishimori K, Nishijima I, Sakamoto H, Yamagata T, Onaka T (2016) Activation of Supraoptic Oxytocin Neurons by Secretin Facilitates Social Recognition, Biol Psychiatry. 81(3):243–251. [DOI] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ (2012) Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats, Biol Sex Differ. 3(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepen T, Voorn P, Stoll CJ, Sluiter AA, Pool CW, Lohman AH (1987) Mesotocin and vasotocin in the brain of the lizard Gekko gecko. An immunocytochemical study, Cell Tissue Res. 250(3):649–56. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ (1988) Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography, Brain Res. 442(1):105–18. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ (1989) Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology, J Neurosci. 9(5):1764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S (1992) Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann N Y Acad Sci. 652:29–38. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dreifuss JJ (1991) Appearance and transient expression of vasopressin and oxytocin receptors in the rat brain, J Recept Res. 11(1–4): 333–46. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Raufaste D, Maffrand J, Serradeil-Le Gal C. (1999) Binding of the non-peptide vasopressin V1a receptor antagonist SR-49059 in the rat brain: an in vitro and in vivo autoradiographic study, Neuroendocrinology. 69(2):113–20. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Ueta Y, Heitz F, Marguerat A, Koizumi K, Yamashita H (2002) Up-regulation of vasopressin and angiotensin II receptors in the thalamus and brainstem of inbred polydipsic mice, Neuroendocrinology. 75:113–123. [DOI] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martínez-Lorenzana G, Condes Lara M, Freund-Mercier MJ (2005) Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat, Neuroscience. 135(1):147–54. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, Hammock EA (2017) Oxytocin receptor dynamics in the brain across development and species, Dev Neurobiol. 77(2):143–157. [DOI] [PubMed] [Google Scholar]
- van den Pol AN (2012) Neuropeptide transmission in brain circuits, Neuron. 76(1):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K, De Mey J (1977) The origin of the vasopressinergic and oxytocinergic fibres of the external region of the median eminence of the rat hypophysis, Cell Tissue Res. 180(4):443–52. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffe AR, De Vries GJ (1985) Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res, 325(1–2): 391–4. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID (2010) Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermal aggression, Horm Behav. 58(2):273–81. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ (2012) Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways, Horm Behav. 61(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ (2013) Sex-specific modulation of juvenile social play by vasopressin, Psychoneuroendocrinology, 38(11):2554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID (2008) Central vasopressin and oxytocin release: regulation of complex social behaviours, Prog Brain Res. 170:261–76. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ (1997) Distribution of oxytocin- and vasopressin- binding sites in the rat extended amygdala: A histoautoradiographic study, J Comp Neurol. 383(3):305–25. [PubMed] [Google Scholar]
- Voorhuis TA, de Kloet ER, de Wied D (1991) The distribution and plasticity of [3H] vasopressin-labelled specific binding sites in the canary brain, Brain Res. 457(1):148–53. [DOI] [PubMed] [Google Scholar]
- Wang Z (1995) Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus), Behav Neurosci. 109(2): 305–11. [DOI] [PubMed] [Google Scholar]
- Wang Z, De Vries GJ (1995) Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats, Journal of Neuroendocrinology. 7:827–831. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR (1997) Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus), Synapse. 27(1):14–25. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pan Y, Wang Z, Zhang Z (2013) Species differences in the immunoreactive expression of oxytocin, vasopressin, tyrosine hydroxylase and estrogen receptor alpha in the brain of Mongolian gerbils (Meriones unguiculatus) and chinese striped hamsters (Cricetulus barabensis), Plos One. 8(6):65807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR (1996) Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study, The Journal of Comparative Neurology. 366:726–37. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA (1982) Afferent connections to the bed nucleus of the stria terminalis, Brain Res. 232(2):255–70. [DOI] [PubMed] [Google Scholar]
- Whitman DC, Albers HE (1998) Oxytocin immunoreactivity in the hypothalamus of female hamsters, Cell Tissue Res. 291(2):231–7. [DOI] [PubMed] [Google Scholar]
- Whitman DC, Albers HE (1995) Role of oxytocin in the hypothalamic regulation of sexual receptivity in hamsters, Brain Res. 680(1–2):73–9. [DOI] [PubMed] [Google Scholar]
- Xu L, Pan Y, Young KA, Wang Z, Zhang Z (2010) Oxytocin and vasopressin immunoreactive staining in the brains of Brandt’s voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton), Neuroscience. 169(3):1235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen JM, Liu WY, Song CY, Lin BC (2006) Through V2, not V1 receptor relating to endogenous opiate peptides, arginine vasopressin in periaqueductal gray regulates antinociception in the rat, Regul Pept. 137(3):156–61. [DOI] [PubMed] [Google Scholar]
- Yang J, Li P, Liang JY, Pan YJ, Yan XQ, Yan FL, Hao F, Zhang XY, Zhang J, Qiu PY, Wang DX (2011) Oxytocin in the periaqueductal grey regulates nociception in the rat, Regul Pept. 169(13):39–42. [DOI] [PubMed] [Google Scholar]
- Yao Y, Fu LY, Zhang X, van den Pol AN (2012) Vasopressin and oxytocin excite MCH neurons, but not other lateral hypothalamic GABA neurons, Am J Physiol Regul Integr Comp Physiol. 302(7):815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Kimura T, Watanabe D, Kiyama H (1996) Differential expression of oxytocin receptor mRNA in the developing rat brain, Neurosci Res. 24(3):291–304. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR (1999) Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain, J Neuroendocrinol. 11(4), 291–7. [DOI] [PubMed] [Google Scholar]