Abstract
During the last decades, the impact of hyperthermophiles and their enzymes has been intensively investigated for implementation in various high-temperature biotechnological processes. Biocatalysts of hyperthermophiles have proven to show extremely high thermo-activities and thermo-stabilities and are identified as suitable candidates for numerous industrial processes with harsh conditions, including the process of an efficient plant biomass pretreatment and conversion. Already-characterized archaea-originated glycoside hydrolases (GHs) have shown highly impressive features and numerous enzyme characterizations indicated that these biocatalysts show maximum activities at a higher temperature range compared to bacterial ones. However, compared to bacterial biomass-degrading enzymes, the number of characterized archaeal ones remains low. To discover new promising archaeal GH candidates, it is necessary to study in detail the microbiology and enzymology of extremely high-temperature habitats, ranging from terrestrial to marine hydrothermal systems. State-of-the art technologies such as sequencing of genomes and metagenomes and automated binning of genomes out of metagenomes, combined with classical microbiological culture-dependent approaches, have been successfully performed to detect novel promising biomass-degrading hyperthermozymes. In this review, we will focus on the detection, characterization and similarities of archaeal GHs and their unique characteristics. The potential of hyperthermozymes and their impact on high-temperature industrial applications have not yet been exhausted.
Keywords: Archaea, Glycoside hydrolases, Hyperthermozymes, Hydrothermal systems, Bioeconomy
Background
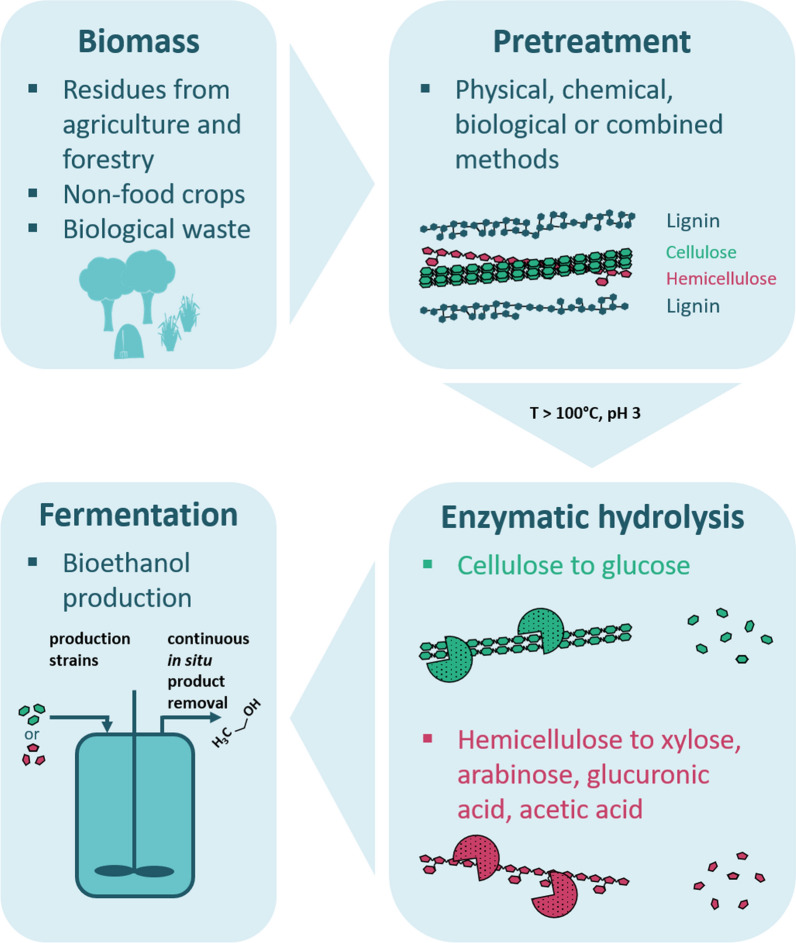
Fossil resources are still the main source of energy as well as for the production of many chemicals. To develop a sustainable economy without the use of these limited resources, governments worldwide initiated research and development strategies for the transition from an oil-based to a circular bio-based economy [1]. A central element of this bioeconomy is the development of sustainable biorefineries, which use renewable resources as feedstock, such as plant biomass, instead of oil [2] (Fig. 1).
Fig. 1.
Application of plant-degrading hyperthermozymes in second-generation biorefinery
The first generation of biofuels uses plant biomass from sugarcane, sugar beet, wheat and crops. Hence, first-generation biofuels, including bioethanol and biodiesel, are mainly produced from starch and vegetable oils [3, 4]. Nevertheless, since biomass for first-generation biofuels consists of potentially edible plant material and, further, requires large areas of agriculture fields, other sources of biomass had to be considered. This led to the development of the second generation of biofuels, which is based on lignocellulosic biomass. Lignocellulose consists of valuable polysaccharides, is abundant in agricultural residues and wood materials, and can be obtained from non-food feedstocks [5]. Despite these advantages, a major challenge is formed by the recalcitrant character of lignocellulose, which necessitates a pretreatment of this substrate for fractionation, for example, by combining physical and chemical pretreatment methods [6–8].
Efficient pretreatment results in the cleavage of lignocellulose enabling the enzymatic accessibility of its components: cellulose, hemicellulose and lignin. The first two components can be enzymatically hydrolyzed to yield hexose and pentose monomers, which can subsequently be fermented to ethanol or other alcohols and chemicals by anaerobic bacteria and fungi [9–14]. To save energy and avoid expensive cooling steps, combinatorial approaches for physicochemical biomass pretreatment with simultaneous enzymatic hydrolysis were developed [15, 16]. For this purpose, extremely heat-active and heat-stable GHs are needed. Since archaea have been significantly less studied than bacteria and eukaryotes, they present a so-far underexploited source of novel hyperthermozymes particularly useful for biorefineries [17].
Biorefinery concepts depend on the applied renewable resources including plant polymers such as cellulose, starch, xylan and mannan. Since these differ in the glycosidic linkages of their backbones, many different kinds of GHs are needed to hydrolyze these polysaccharides [17]. Therefore, integrated biorefinery processes need a variety of GHs, including cellulases, amylases, mannosidases and pullulanases, which are stable towards the respective process conditions. Many biotechnological processes are performed at elevated temperatures to improve the solubility and bioavailability of organic compounds and biomass [18]. Further benefits of high process temperatures include increased diffusion rates and a significantly reduced contamination risk [19, 20]. As a consequence, enzymes derived from hyperthermophilic microorganisms have become highly popular for such high-temperature industrial processes since hyperthermozymes exhibit maximum activity at temperatures around 100 °C and are extremely thermostable [21]. Furthermore, when simultaneously applying hyperthermozymes with different substrate specificities, but similar temperature and pH preferences, synergistic effects can be especially beneficial for the efficient utilization of plant biomass [22] (Fig. 1). In this review, we are highlighting the impressive characteristics of already characterized hyperthermozymes obtained from archaea, and summarize some interesting strategies to discover novel ones.
Discovery of the potential of archaea and their glycoside hydrolases
The full potential of the extreme lifestyle at elevated temperatures was recognized in 1981, when Karl Stetter and Wolfram Zillig discovered life above a temperature of 80 °C with the isolation of the first hyperthermophilic species Methanothermus fervidus, which was unimaginable before [23, 24]. Later it was impressively shown that some hyperthermophiles are even growing at temperatures around (or above) 100 °C, like heterotrophic members of the genera Pyrococcus [25, 26] and Thermococcus [27], or the chemolithoautotroph Pyrolobus fumarii [28]. Beside the impact of these impressive findings for ecology [29] and evolution [30–33] and specially for our understanding of microbial physiology and metabolisms at extreme habitats [34–36], the discovery of hyperthermophiles also paved the path for the finding and characterization of extremely heat-stable biocatalysts, which are naturally produced by these microorganisms. While some hyperthermophiles, such as Methanopyrus kandleri are strictly chemolithoautotrophic [37], also heterotrophic hyperthermophilic representatives were isolated and characterized. Since a heterotrophic metabolism requires enzyme machineries which are able to degrade and utilize organic biomass, the use of such heterotrophic archaea or their recombinantly produced extremely heat-stable GHs was investigated for high-temperature industrial processes such as production of food, beverage, detergent and chemical products, as well as biomass pretreatment for biofuel generation [17, 38, 39]. Therefore, genes encoding GHs, such as amylases and xylanases, were cloned from isolated and known hyperthermophiles, produced (mostly in E. coli) and characterized. Interestingly, since some polysaccharide-degrading enzymes are secreted by their native organisms, biochemical characterizations of these extracellular enzymes revealed that their optimal activity can be found close to the temperature of the optimal growth rate of the respective microorganism (Table 1). Furthermore, besides the impressive heat activity, features of some archaeal plant-biomass-degrading enzymes revealed a concomitant high activity under acidic conditions and high pressure [40–42].
Table 1.
Archaeal heterotrophic hyperthermophiles and characterized GHs
| Species* | Growth range [°C] | Topt [°C] | Characterized GH derived from organism** | Topt [°C] | Tstab | Genbank accession no.*** |
|---|---|---|---|---|---|---|
| Pyrococcus furiosus [25] | 70–103 | 100 |
Endoglucanase (GH12) extracellular enzyme [46] No Genbank |
100 | T1/2 40 h at 95 °C | WP_011011985.1 (from CAZy website) |
| Endoglucanase (GH16) extracellular enzyme [47] | 105 | 100% of residual activity after incubation at 80 °C for 110 h | AF013169 | |||
| Amylase extracellular enzyme [44] | 100 | > 60% relative activity at 120 °C after incubation for 1 h | n.l. | |||
| Amylase intra- and extracellular enzymes [43] | 106 | 30% residual activity after incubation for 8 h at 98 °C | n.l. | |||
| Amylase extracellular enzyme [48] | 100 | 80% residual activity after incubation for 6 h at 100 °C | U96622 | |||
| Amylase intracellular enzyme [49] | 100 | > 80% residual activity after incubation for 2 h at 100 °C | n.l. | |||
| Glucosidase intracellular enzyme [50] | 102–105 | T1/2 85 h at 100 °C | n.l. | |||
| Glucosidase secretion n.l. [51] | 100 | 80% residual activity after incubation for 1 h at 100 °C | AF013169 | |||
| Pyrococcus horikoshii [26] | < 80–102 | 98 | Endoglucanase (GH5) extracellular enzyme [52] | 97 | Relative activity 80% after incubation at 97 °C for 3 h | AAQ31833.1 (from CAZy website) |
| Pyrococcus woesei [53] | n.l. | 100–103 | Amylase extracellular enzyme [54] | 100 | No loss of activity after incubation at 90 °C for 6 h | n.l. |
| Palaeococcus pacificus [55] | 50–90 | 80 | Cyclodextrinase PpCD (GH13) secretion n.l. [56] | 95 | > 90% relative activity after 8 h at 85 °C | WP_048164969 |
| Thermofilum pendens [57] | 85–90 | Up to 95 | Glucosidase (GH 3) intracellular enzyme [51] | 90 | 50% residual activity after incubation for 60 min at 95 °C | YP_920894 |
| Staphylothermus marinus [58] | n.l. | 98 | Amylase SMMA (GH13) secretion n.l. [59] | 100 | Melting point Tm 109 °C | WP_011838911.1 |
| Thermococcus chitonophagus [60] | 60–93 | 85 | Chitinase chi70 associated with outer cell membrane [61] | 70 | > 50% activity after incubation at 120 °C for 1 h | n.l. |
| Thermococcus kodakarensis [62] | 60–100 | 85 | Pullulanase TK-PUL (GH13) secretion n.l. [63] | 95–100 | T1/2 45 min at 100 °C | BAD85166.1 |
| Saccharolobus shibatae [64] | Up to 86 | 81 | Endoglucanase (GH 12) secretion n.l. [65] | 100 | 100% activity after incubation at 85 °C for 1 h | LT221867 |
| Sulfolobus acidocaldarius [66] | 55–80 | 70–77 | Amylase (GH 57) membrane-bound enzyme [67] | 105 | T1/2 2.5 h at 100 °C | AAY80509.1 (from CAZy website) |
| Saccharolobus solfataricus [68] | n.l. | 70 | Xylanase membrane-bound enzyme [69] | 90 | T1/2 47 min at 100 °C | n.l. |
| Endoglucanase SSO1949 (GH 12) extracellular enzyme [41] | 80 | T1/2 8 h at 80 °C (pH 1.8) | AAK42142.1; NP_343352.1 (from CAZy website) | |||
| Galactosidase intracellular enzyme [70] | 95 | > 3 h at 75 °C | n.l. | |||
| Galactosidase secretion n.l. [71] | 90 | > 2 h at 75 °C | n.I. | |||
| Galactosidase (GH36) intracellular enzyme [72] | 90 | T1/2 30 min at 90 °C | n.l. | |||
| Xylosidase (GH 31) secretion n.l. [73] | 90 | T1/2 38 h at 90 °C | AJ251975 | |||
| Mannosidase (GH 38) intracellular enzyme [74] | 85 | > 20% activity after 10 min at 80 °C | AAK43108.1; AIX48014.1; CAC24028.1; NP_344318.1 (from CAZy website) | |||
| Caldivirga maquilingensis [75] | 60–92 | 85 | Galactosidase (GH1) secretion n.l. [76] | 110 | 100% relative activity after 120 min at 90 °C | ABW01253.1 |
| Pyrobaculum aerophilum [77] | 75–104 | 100 | Glucosidase (GH31) intracellular [78] | 90 | 46% relative activity after 1 h at 110 °C | gi:18160499 |
| Picrophilus torridus [79] | 45–65 | 60 | Glucosidase (GH31) intracellular enzyme [80] | 87 | 81% relative activity after 120 min at 80 °C | AAT42677.1 (from CAZy website) |
| Mannosidase (GH38) intracellular enzyme [80] | 70 | 50% relative activity after 1 h at 80 °C (with Cd2+) | AAT42676.1 |
| Functional screening of metagenomes derived from hydrothermal systems | ||||||
|---|---|---|---|---|---|---|
| Metagenome | Characterized GH derived from metagenome (archaeal origin) | Topt [°C] | Tstab | Genbank accession no.*** | ||
| Deep sea hydrothermal vent | Endoglucanase (GH 12) extracellular enzyme [81] | 92 | > 80% activity after incubation at 80 °C for 4,5 h | LN850140 (fosmid insert) | ||
| Hot spring | Glucosidase (GH 1) secretion n.l. [40] | 90 | > 67% activity after incubation at 90 °C for 1.5 h | HG326254 | ||
| Combinatorial approach of enrichment culture and screening | ||||||
|---|---|---|---|---|---|---|
| Enrichment culture* | Archaeon | Characterized GH derived from enrichment culture | Topt [°C] | Tstab | Genbank accession no.*** | |
| Shallow marine vent sample from Vulcano Island, incubation 90 °C, cellulosic substrate, anoxic [82] | Unknown, not cultivated in pure culture, probably related to Thermococcus | Endoglucanase Vul_Cel5A (GH 5) extracellular enzyme [42] | 115 | T1/2 43 min at 100 °C | MH910342 | |
| Glucosidase (GH 1) intracellular enzyme [22] | 105 | T1/2 99 min at 75 °C | MN329095 | |||
| Sample from 94 °C geothermal pool of northern Nevada, incubated at 90 °C, cellulosic substrates, anoxic [83] | Unknown, not cultivated in pure culture, probably related to Ignisphaera | Endoglucanase EBI-244 (multidomain) extracellular enzyme [83] | 109 | T1/2 4.5 h at 100 °C | JF509452 | |
| In situ enrichment culture in hot vent (76–99 °C, Kuril archipelago) using xylan as carbon source [84] | Thermococcus sp. Strain 2319X1 |
Multidomain glycosidase MDG (Multidomain) extracellular enzyme Full-length protein Truncated GH5 version [84] |
60 90 |
n.l. n.l. |
CP012200 (genome) | |
Selection criteria of the reported GHs shown in the table are a high thermo-activity and thermo-stability. GH families (in brackets) and Genbank accession numbers are provided if the respective articles contain this information
n.l. not listed in the original paper
* Literature source reports the first description of the organism
** Literature source reports the enzyme characterization
*** As provided by the literature source or CAZy
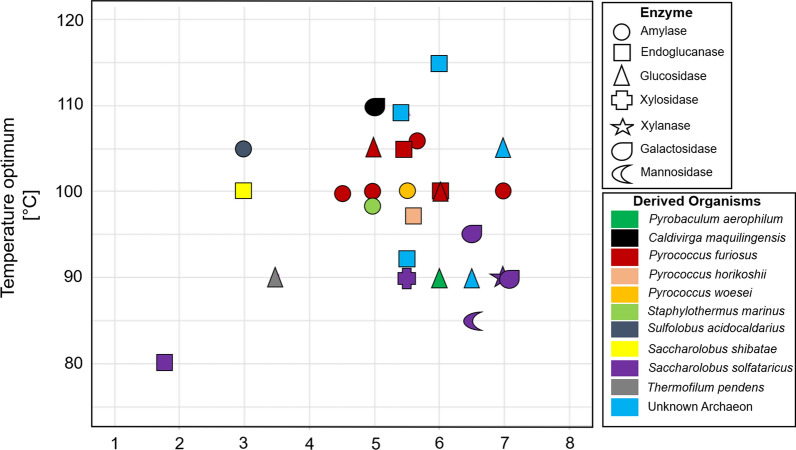
Within the last 30 years, numerous GHs of already isolated hyperthermophiles were produced using sequence- or function-based screening methods (Table 1). Among the first-characterized GHs were amylases from the hyperthermophile Pyrococcus furiosus, which were obtained by performing cultivations on carbohydrates with subsequent GH detection and activity tests of the crude cell extract as well as the supernatant of the culture. The detected and characterized amylases exhibited maximum activity at temperatures higher than 99 °C [43, 44] (Table 1). Within the last decades, more GHs of Pyrococcus furiosus were discovered and characterized, including endoglucanases, amylases and glucosidases, which are all working optimally at temperatures around 100 °C (Table 1). Additionally, numerous characterized archaeal GHs, including endoglucanases, amylases, mannosidases and glucosidases, were characterized from pure strains of Saccharolobus solfataricus (previously Sulfolobus solfataricus [45]) Saccharolobus shibatae (previously Sulfolobus shibatae [45]), Pyrococcus horikoshii, Pyrococcus woesei, Sulfolobus acidocaldarius, Staphylothermus marinus and Thermofilum pendens (Table 1, Fig. 2). However, due to the fact that cultivation of new hyperthermophiles in pure cultures is most often a challenging task, many thermozymes and some hyperthermozymes were detected using culture-independent metagenomic approaches.
Fig. 2.
Biochemical characteristics of known biomass-degrading GHs of archaeal origin. The following GHs were included in the figure: endoglucanases of Pyrococcus furiosus [46, 47], Pyrococcus horikoshii [52], Saccharolobus shibatae [65], Saccharolobus solfataricus [41], and three unknown archaea [42, 81, 83]; amylases of Pyrococcus furiosus [43, 44, 48, 49], Sulfolobus acidocaldarius [67], Pyrococcus woesei [54], and Staphylothermus marinus [59]; sylanase of Saccharolobus solfataricus [69]; glucosidases of Pyrococcus furiosus [50, 51], Pyrobaculum aerophilum [78] and two unknown archaea [22, 40]; galactosidases of Saccharolobus solfataricus [70, 71] and Caldivirga maquilingensis [76]; xylosidase of Saccharolobus solfataricus [73]; mannosidase of Saccharolobus solfataricus [74]
Classification of GHs demonstrates a small number of characterized archaeal enzymes
GHs are classified based on primary sequences (Carbohydrate-Active enZyme database CAZy, www.cazy.org) or function (Enzyme Commission EC, established in 1992) [85]. The amino acid structure-based classification of CAZy is an adequate way to predict and identify mechanisms and specificities of glycoside hydrolases (GHs) [86, 87]. So far, CAZy divides GHs into 167 GH families, and the GH 5, 13, 16, 30 and 43 families are again divided into several subfamilies [88–92] (www.cazy.org). GH family 5 represents one of the largest GH families and consists of 16,520 enzymes as noted in June 2020. The deposited primary protein sequences of GH family 5 demonstrate the lack of knowledge about archaeal GH since 99 archaeal protein sequences are vastly outnumbered by 13,137 bacterial ones. From these deposited sequences, 5 archaeal GHs are listed as characterized (two cellulases and three endoglucanases), compared to 559 bacterial ones. Regarding the activity and function of the structurally related proteins of GH5, cellulolytic and hemicellulolytic enzymes are present, including endo-β-1,4-glucanases (EC 3.2.1.4), β-glucosidases (EC 3.2.1.21), licheninases (EC 3.2.1.73), and endo-β-1,4-mannosidases (EC 3.2.1.78), and are, therefore, of industrial interest. GH5 subfamily 1 contains the extremely stable endoglucanase of Pyrococcus horikoshii [52], whose structure was analyzed via X-ray crystallography in 2008 [93, 94] confirming the typical GH5 (β/α)8 fold. In a recent study of Strazzulli and colleagues, a novel archaeal mannanase was discovered, belonging to the subfamily GH5_19 [95].
One explanation for the imbalance of archaeal:bacterial GHs is probably the cultivation challenge that comes along with many archaeal species, which are mainly found in extreme habitats in terms of pH, salinity and temperature and pressure. However, within the last years, the distribution of archaeal species was focused in the research field of microbial ecology [96], and numerous studies detected, with cultivation-independent methods, a distribution of archaea in non-extreme environments, such as sulfur-rich lakes [97], marine sediments and water columns [98, 99], estuarine ecosystems [100] or the grass-root zone [101]. Biotechnology will benefit from this increasing knowledge of archaeal ecology since more and more binned genomes of uncultivated archaea and novel Candidatus species will be published that can be used for enzyme screening. To date, 367 genomes (CAZomes) of archaea are published in CAZy, compared to 17,054 bacterial ones. The listed genomes of CAZy of heterotrophic archaea are exhibiting diverse GH families with interest of various biotechnological disciplines. For example, the archaeon Staphylothermus marinus (Taxonomy ID 399550) contains a total number of 11 GHs of family 1, 4, 13, 38, 57, 84, and 122 and, therefore, offers potentially heat-stable glucosidases, galactosidases, amylases, mannosidases and glucosaminidases. One additional challenge of understanding and making use of archaeal GH machineries lies in the whole metabolism of archaea, which is considered to be a complex “mixture” of bacterial- and eukaryotic-like pathways resulting in modified pathways [102–104]. Studies focusing on transcriptomics of cultured archaea or reassembling of uncultured archaeal genomes will provide highly useful insights into new archaeal metabolisms, and novel catabolism reactions could be investigated for degradation of complex substrates [105].
Culture-dependent approaches coupled with metagenomics for the identification of promising extremozymes
The revolution of enzyme discovery and microbial diversity analysis came along with the next-generation sequencing (NGS) technology, which is based on the fragmentation of (meta)genomic DNA, followed by sequencing of the resulting fragments, thus allowing millions of (high-throughput) sequencing reactions in parallel [106]. With sinking costs for NGS, this technology became the gold standard in all areas of life sciences, reaching from the analysis of human microbiome consortia [107] to microbial communities in extreme hydrothermal ecosystems [108]. Furthermore, this method allowed a deeper insight, not just into genera abundance of the composition of microbial communities, but also into the metabolic pathways of these consortia [109]. Bioinformatic tools including MG-RAST [110] and MEGAN [111, 112] were developed as valuable means for calculating the taxonomy of metagenomes based on known sequences of a database (reference-based classification). Since metagenomic sequences cannot only be used for diversity analysis, but also for identification of putative carbohydrate-encoding genes, the metagenomic era supported the detection of a huge number of novel enzyme candidates [113].
In addition, it was shown that interdisciplinary approaches, consisting of microbial diversity analyses and screening for novel GH-encoding genes, are a promising combination which leads to successful identification of novel enzymes, in particular when analyzing extreme habitats such as hydrothermal systems [83, 95, 114]. Environmental samples of extremely hot ecosystems contain a huge variety of microorganisms with different metabolisms, ranging from chemolithoautotrophy to heterotrophy, and the pool of coding sequences in such a sample is huge in relation to the extreme conditions of such an environment. Thus, to focus on selected target enzymes, enrichment cultures with conditions that support only the microbes with metabolisms of interest can be applied, and, therefore, select a defined microbial pool [115]. In the following, we will focus on three specific archaeal plant-biomass degrading enzymes that were detected by performing culture-dependent approaches:
The research team of Frank Robb aimed to gain novel insights into archaeal degradation of crystalline cellulose, which still is a very unexploited field of research [83]. The team performed strictly anaerobic enrichment cultures at a temperature of 90 °C using sediment samples from a terrestrial geothermal source of Nevada. Enrichment cultures were transferred several times into fresh medium and microcrystalline cellulose and Whatman filter paper were used as carbon and energy source. Using this approach, a three-species consortium was enriched, whose 16S rRNA genes showed highest similarities to the archaeal genera Ignisphaera, Pyrobaculum and Thermofilum. Using a metagenomic approach, cellulase-encoding genes of GH family 5 were screened and one predicted GH, labeled as EBI-244, represented a potential multidomain cellulase. This cellulase seemed to consist of four structural domains, and while one of these domains showed similarities to GH5, the three remaining domains did not show any similarity to known GH families. Characterization of the heterologously produced protein in E. coli revealed highly impressive characteristics: a temperature optimum of 109 °C, a temperature melting point of 113 °C, a half-life time of 5 h at 100 °C, as well as resistance against ionic liquids, detergents and salts, and finally, a high activity towards crystalline cellulose (Avicel). The unique composition of the different domains of this enzyme proved to be interesting, and furthermore, the catalytic domain and the whole sequence showed high similarities to non-thermophilic eukaryotic mannanases.
Another combinatorial approach linking cultivation and genomics for the identification of novel archaeal plant-degrading enzymes was used by the team of Bettina Siebers [84]. An in situ enrichment culture was performed in a hot vent of the Kuril archipelago using birchwood xylan as substrate. This sophisticated experimental setup resulted in the successful isolation of Thermococcus sp. strain 2319X1, which is able to grow on xylan as sole carbon and energy source. By performing genome sequencing and genome reassembly of this specific strain, a multi-domain glycosidase (MDG) was detected. The protein contains three GH domains, one of GH family 5 and two of GH family 12, which could explain the impressive multifunctionality towards a broad substrate spectrum, including Avicel, carboxymethyl cellulose, β-1,4 linked and β-1,3 linked glucose polysaccharides, as well as xylose-based and mannose-based carbohydrates. The enzyme showed, in contrast to most endoglucanases of archaeal origin, optimal activity in alkaline milieu (optimum pH 8.5).
A third example for a combinatorial and multidisciplinary approach in regard to cultivation and omics technologies for archaeal enzyme discovery was performed using samples of the extremely shallow marine vents of Vulcano Island, which were taken at a temperature of 100 °C [22, 42, 82]. Enrichment cultures of samples were performed under anoxic conditions at a temperature of 90 °C, using carboxymethyl cellulose as carbon source, and the diversity of the enrichment culture was analyzed using a metagenomic approach. The diversity analysis performed with MEGAN6 revealed that the community consisted of more than 96% of archaeal microorganisms with the hyperthermophilic genera Thermococcus and Palaeococcus showing the highest abundance in these cultures [82]. Afterwards, the metagenome was used for a sequence-based screening for GH5 endoglucanases, and the putative endoglucanase Vul_Cel5A was detected, which showed highest similarity to a putative endoglucanase of Thermococcus sp. and 56% identity to the characterized endoglucanase of Pyrococcus furiosus [42]. Production and characterization of the recombinantly produced enzyme Vul_Cel5A revealed impressive characteristics in regard to thermo-activity (Topt of 115 °C), thermo-stability (T1/2 of 43 min at 100 °C) and resistance towards a broad range of detergents, as well as an extremely high relative activity and stability under acidic conditions. In addition, the metagenome was binned using Maxbin [116], which resulted in a reassembly of four genomes. Further genes encoding putative biomass-degrading enzymes were identified in the partially reassembled genome in which Vul_Cel5A was located. Cloning of the genes and production of the respective enzymes resulted in the identification and characterization of the archaeal β-glucosidase Vul_Bgl1A, which exhibited highest activity at 105 °C towards 4-nitrophenyl β-d-glucopyranoside and cellobiose [22]. Interestingly, by simultaneously applying Vul_Cel5A and Vul_Bgl1A, a significant increase of glucose formation was monitored indicating synergistic effects of the two enzymes.
All three combinatorial approaches highlight the importance of combining cultivation with state-of-the-art (meta-)genomic analyses to identify novel archaeal enzyme candidates. The detected and produced enzymes are highly promising candidates for industrial processes since all three enzymes exhibited, besides their high thermo-activity, a very broad biomass substrate spectrum and a relatively high activity in acidic or alkaline milieu. Regarding the sequence composition of the mentioned archaeal GH5 enzymes, xylanase MDG [84] showed a sequence identity of 46% with endoglucanase Vul_Cel5A [42], while the similarity of EBI-244 [83] to these enzymes was very low (23% to MDG and 19% to Vul_Cel5A). Improved combinatorial approaches, such as metatranscriptomic- and proteomic-based screening coupled with prior high-temperature cultivation on plant biomass, will probably have a high impact on prospective identification of novel hyperthermozymes for application in various biotechnological processes including biorefineries. The successful application of such a combinatorial approach was recently described by Zayulina and colleagues, who coupled cultivation of a novel archaeon Thermofilum adornatum with proteomic analyses to identify four novel cellulolytic enzymes [117].
Protein engineering to tailor plant-degrading enzymes for industrial processes
While the implementation of plant-degrading hyperthermozymes in integrated biorefineries is a straightforward application of these robust biocatalysts, even these naturally already thermo-active and thermo-stable enzymes need further improvement before being subjected to the biorefinery process (Fig. 1) [1, 118]. In general, wild-type enzymes are not directly suitable for industrial application but have to be modified prior to usage in biotechnological processes due to oxidation sensitivity and generally low activities of the native enzymes. The replacement of oxidation-sensitive methionine residues is performed by site-directed mutagenesis, whereas improvements of substrate specificity and activity are gained by various rounds of protein-engineering coupled with screening for desired activities and/or stabilities [119, 120]. Applied protein engineering approaches need different levels of previous knowledge of the target enzyme, ranging from directed evolution applying random mutagenesis, which requires only the DNA sequence, to rational protein design, such as site-directed mutagenesis, which relies on the X-ray structure of the target enzyme. Nowadays, combinations of directed evolution and rational protein design are frequently applied with the aim to gain maximum benefit from each of the protein engineering techniques [121, 122].
In particular, rational protein-engineering methods have been successfully applied for improving plant-degrading enzymes since X-ray structures of hyperthermozymes are often being resolved and analyzed with the aim to understand the mechanisms that lead to their superior stability properties [123, 124]. One example of a promising plant-degrading hyperthermozyme with potential for further optimization is a β-glycosidase from the extreme thermoacidophilic archaeon Saccharolobus solfataricus. This hyperthermozyme was reported to exhibit maximal activity above 95 °C and remarkable stability towards detergents [125]. However, alkaline pH values seemed to have a strong destabilizing effect on this archaeal GH, which belongs to GH family 1 [126]. It was, therefore, concluded that the enzyme’s stability resulted from ionic interactions on the surface, which would be perturbated at alkaline pH [125].
However, the β-glycosidase from Saccharolobus solfataricus proved to be an excellent example for the successful heterologous production of an archaeal plant-degrading enzyme applying a yeast expression system [127]. The application of mesophilic Saccharomyces cerevisiae as host for industrial-scale fermentation enabled a fast and efficient purification strategy of the target enzyme by taking advantage of its exceptional heat stability when applying a heat precipitation of the host proteins [127]. The reasons for the enzyme’s heat stability were analyzed by creating mutants of the hyperthermozyme, which, for example, hampered the formation of an ion pair network at the tetrameric interface of the enzyme and led to heat-sensitive mutants [128]. These results further supported the current hypothesis that ionic interactions are of major importance for protein stability of hyperthermophiles. Furthermore, when comparing the enzyme to β-glycosidases from Thermosphaera and Pyrococcus furiosus, it was deduced that oligomerization could be another general factor for protecting hyperthermozymes from degradation [128–130]. General mechanisms of protein unfolding were analyzed by creating mutants of the N and C terminii of the β-glycosidase from Saccharolobus solfataricus [128, 131]. The respective studies showed that the quaternary structure was crucial for stability of this hyperthermozyme [128] and that fraying of the polypeptide chain termini played an important role in protein unfolding [131].
In addition to rational approaches, also random mutagenesis studies involving suitable in vivo selection mechanisms were conducted with hyperthermozymes. One study focusing on the β-glycosidase from Saccharolobus solfataricus showed that mutations far from the active site may have crucial impact on the enzyme’s activity and stability profiles [132]. While a mutant with three random mutations showed a twofold enhanced specific activity towards galactosides at 85 °C, the higher flexibility of the enzyme variant that enabled this increase in substrate turnover also led to an almost 300-fold reduced thermal stability. Directed evolution was also applied in a study focusing on broadening the temperature profile of a β-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Here, the low-temperature activity of the hyperthermozyme was significantly increased with twofold enhanced activity towards cellobiose at 20 °C [133].
Successful engineering of another hyperthermozyme was previously demonstrated by Kang et al. when further improving the thermo-active and thermo-stable cellulase from Pyrococcus horikoshii [52, 134]. In this study, rational protein design was performed by eliminating cysteine residues and adding a carbohydrate-binding domain to increase the cellulase’s activity. The successful approach led to the remarkable observation that the thermo-stability of the enzyme was not significantly impaired by removing disulfide bonds. Furthermore, increased affinity towards crystalline cellulose was obtained by the addition of a chitin-binding domain from another hyperthermozyme, leading to the conclusion that the generation of sophisticated fusion proteins might be a suitable means to tailor hyperthermozymes for industrial application [134].
Despite the fact that hyperthermozymes offer a huge potential for industrial application, there are only few examples of actual utilization of these enzymes. This is mainly due to the fact that in contrast to their mesophilic counterparts, a significantly lower number of hyperthermozymes is known to date. Furthermore, they are often more difficult to produce at high amounts as there are no industrially approved extremophilic production strains available yet. However, it was shown that expression problems with mesophilic hosts, such as E. coli, might be overcome by designing synthetic genes, which was successfully applied for a phosphopantetheine adenylyltransferase from the hyperthermophilic archaeon Pyrococcus abyssi [135]. In a different approach, careful adjustment of expression conditions was sufficient to produce an archaeal chitinases with E. coli expression strains [136].
Another example highlighting the potential of hyperthermozymes for industrial application is the development of a continuous process for lactulose production by implementation of immobilized thermostable β-glycosidase from Pyrococcus furiosus [137]. With the advance of more thorough analyses of archaea, including the Archaeal Proteome Project (ArcPP), further insights into the mechanisms and beneficial properties of archaeal enzymes are expected in the near future [102, 138].
Conclusion
Characterizations of already known archaeal thermo-active and thermo-stable biomass-degrading GHs have highlighted their potential for high-temperature industrial processes. Archaeal GH properties provide interesting features for an efficient biomass conversion and biofuel generation. To discover new promising candidates, it is necessary to study in detail the microbiology, physiology and enzymology of microorganisms of extremely hot habitats, and to combine and implement this generated knowledge for an efficient screening for novel promising GH candidates. Combinatorial approaches of cultivation and omics technologies lead to the discovery of highly interesting archaeal GHs with outstanding characteristics. Current and future global challenges require sustainable biobased solutions, and bioeconomy is becoming an important field to meet these challenges. Still, one of the major challenges is the efficient transformation of recalcitrant plant biomass to polysaccharides that can be used as a resource for countless fermentation processes. Archaeal hyperthermozymes represent an ideal platform to support this crucial step in an eco-friendly way.
Acknowledgements
The authors would like to thank their colleagues for constructive feedback.
Abbreviations
- GH
glycoside hydrolases
- CBM
carbohydrate-binding modules
Authors’ contributions
MS was the major contributor in writing the manuscript. AK wrote two sections for this manuscript. MS, AK and GA drafted the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Projekt DEAL.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krüger A, Schäfers C, Schröder C, Antranikian G. Towards a sustainable biobased industry—highlighting the impact of extremophiles. Nat Biotechnol. 2018;40:144–153. doi: 10.1016/J.NBT.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Schieb P-A, Lescieux-Katir H, Thénot M, Clément-Larosière B. An original business model: the integrated biorefinery. Biorefinery 2030 Futur. Prospect. Bioeconomy, Berlin, Heidelberg: Springer Berlin Heidelberg; 2015, p. 25–66. 10.1007/978-3-662-47374-0_2.
- 3.Cherubini F. The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Convers Manage. 2010;51:1412–1421. doi: 10.1016/J.ENCONMAN.2010.01.015. [DOI] [Google Scholar]
- 4.Naik SN, Goud VV, Rout PK, Dalai AK. Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev. 2010;14:578–597. doi: 10.1016/J.RSER.2009.10.003. [DOI] [Google Scholar]
- 5.Carriquiry MA, Du X, Timilsina GR. Second generation biofuels: economics and policies. Energy Policy. 2011;39:4222–4234. doi: 10.1016/J.ENPOL.2011.04.036. [DOI] [Google Scholar]
- 6.Mussatto SI, Carneiro LM, Silva JPA, Roberto IC, Teixeira JA. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr Polym. 2011;83:368–374. doi: 10.1016/j.carbpol.2010.07.063. [DOI] [Google Scholar]
- 7.Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 8.Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB. Biomass pretreatment: fundamentals toward application. Biotechnol Adv. 2011;29:675–685. doi: 10.1016/J.BIOTECHADV.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M, Shi D, Lu X, Zong H, Zhuge B. Co-production of 1,2,4-butanetriol and ethanol from lignocellulose hydrolysates. Bioresour Technol. 2019;282:433–438. doi: 10.1016/j.biortech.2019.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Liu T, Peng B, Geng A. Enhanced ethanol production from industrial lignocellulose hydrolysates by a hydrolysate-cofermenting Saccharomyces cerevisiae strain. Bioprocess Biosyst Eng. 2019;42:883–896. doi: 10.1007/s00449-019-02090-0. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y-G, Jin Y-S, Cha Y-L, Seo J-H. Bioethanol production from cellulosic hydrolysates by engineered industrial Saccharomyces cerevisiae. Bioresour Technol. 2017;228:355–361. doi: 10.1016/j.biortech.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Rahayu F, Kawai Y, Iwasaki Y, Yoshida K, Kita A, Tajima T, et al. Thermophilic ethanol fermentation from lignocellulose hydrolysate by genetically engineered Moorella thermoacetica. Bioresour Technol. 2017;245:1393–1399. doi: 10.1016/j.biortech.2017.05.146. [DOI] [PubMed] [Google Scholar]
- 13.Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN. pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? In: Olsson L, editor. Biofuels. Berlin Heidelberg: Springer; 2007. pp. 67–93. [DOI] [PubMed] [Google Scholar]
- 14.Olsson L, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol. 1996;18:312–331. doi: 10.1016/0141-0229(95)00157-3. [DOI] [Google Scholar]
- 15.Wang P, Liu C, Chang J, Yin Q, Huang W, Liu Y, et al. Effect of physicochemical pretreatments plus enzymatic hydrolysis on the composition and morphologic structure of corn straw. Renew Energy. 2019;138:502–508. doi: 10.1016/j.renene.2019.01.118. [DOI] [Google Scholar]
- 16.Kirsch C, Zetzl C, Smirnova I. Development of an integrated thermal and enzymatic hydrolysis for lignocellulosic biomass in fixed-bed reactors. Holzforschung. 2011;65:483–489. doi: 10.1515/HF.2011.061. [DOI] [Google Scholar]
- 17.Elleuche S, Schäfers C, Blank S, Schröder C, Antranikian G. Exploration of extremophiles for high temperature biotechnological processes. Curr Opin Microbiol. 2015;25:113–119. doi: 10.1016/J.MIB.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Niehaus F, Bertoldo C, Kähler M, Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 19.Krahe M, Antranikian G, Märkl H. Fermentation of extremophilic microorganisms. FEMS Microbiol Rev. 1996;18:271–285. doi: 10.1111/j.1574-6976.1996.tb00243.x. [DOI] [Google Scholar]
- 20.Becker P, Abu-Reesh I, Markossian S, Antranikian G, Märkl H. Determination of the kinetic parameters during continuous cultivation of the lipase-producing thermophile Bacillus sp. IHI-91 on olive oil. Appl Microbiol Biotechnol. 1997;48:184–190. doi: 10.1007/s002530051036. [DOI] [PubMed] [Google Scholar]
- 21.Adams MW, Kelly RM. Finding and using hyperthermophilic enzymes. Trends Biotechnol. 1998;16:329–332. doi: 10.1016/S0167-7799(98)01193-7. [DOI] [PubMed] [Google Scholar]
- 22.Schröder C, Eixenberger D, Suleiman M, Schäfers C, Antranikian G. Characterization of an extremely thermo-active archaeal β-glucosidase and its activity towards glucan and mannan in concert with an endoglucanase. Appl Microbiol Biotechnol. 2019;103:9505–9514. doi: 10.1007/s00253-019-10218-1. [DOI] [PubMed] [Google Scholar]
- 23.Stetter KO. Hyperthermophiles in the history of life. Philos Trans R Soc B Biol Sci. 2006;361:1837–1842. doi: 10.1098/rstb.2006.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stetter KO, Thomm M, Winter J, Wildgruber G, Huber H, Zillig W, et al. Methanothermus fervidus, sp. nov., a novel extremely thermophilic methanogen isolated from an icelandic hot spring. Zentralblatt Für Bakteriol Mikrobiol Und Hyg I Abt Orig C Allg Angew Und Ökologische Mikrobiol. 1981;2:166–178. doi: 10.1016/s0721-9571(81)80038-5. [DOI] [Google Scholar]
- 25.Fiala G, Stetter KO. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100 °C. Arch Microbiol. 1986;145:56–61. doi: 10.1007/BF00413027. [DOI] [Google Scholar]
- 26.González JM, Masuchi Y, Robb FT, Ammerman JW, Maeder DL, Yanagibayashi M, et al. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles. 1998;2:123–130. doi: 10.1007/s007920050051. [DOI] [PubMed] [Google Scholar]
- 27.Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea. 2004;1:263–267. doi: 10.1155/2004/204953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blöchl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch HW, Stetter KO. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 °C. Extremophiles. 1997;1:14–21. doi: 10.1007/s007920050010. [DOI] [PubMed] [Google Scholar]
- 29.Huber H, Stetter KO, Huber R. Towards the ecology of hyperthermophiles: biotopes, new isolation strategies and novel metabolic properties. FEMS Microbiol Rev. 2000;24:615–623. doi: 10.1111/j.1574-6976.2000.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 30.Lecompte O, Ripp R, Puzos-Barbe V, Duprat S, Heilig R, Dietrich J, et al. Genome evolution at the genus level: comparison of three complete genomes of hyperthermophilic Archaea. Genome Res. 2001;11:981–993. doi: 10.1101/gr.GR1653R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forterre P. A hot topic: the origin of hyperthermophiles. Cell. 1996;85:789–792. doi: 10.1016/S0092-8674(00)81262-3. [DOI] [PubMed] [Google Scholar]
- 32.Schwartzman DW, Lineweaver CH. The hyperthermophilic origin of life revisited. Biochem Soc Trans. 2004;32:168–171. doi: 10.1042/bst0320168. [DOI] [PubMed] [Google Scholar]
- 33.Schönheit P, Buckel W, Martin WF. On the origin of heterotrophy. Trends Microbiol. 2016;24:12–25. doi: 10.1016/j.tim.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Schönheit P, Schäfer T. Metabolism of hyperthermophiles. World J Microbiol Biotechnol. 1995;11:26–57. doi: 10.1007/BF00339135. [DOI] [PubMed] [Google Scholar]
- 35.Amend JP, Shock EL. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev. 2001;25:175–243. doi: 10.1111/j.1574-6976.2001.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 36.Schut GJ, Boyd ES, Peters JW, Adams MWW. The modular respiratory complexes involved in hydrogen and sulfur metabolism by heterotrophic hyperthermophilic archaea and their evolutionary implications. FEMS Microbiol Rev. 2013;37:182–203. doi: 10.1111/j.1574-6976.2012.00346.x. [DOI] [PubMed] [Google Scholar]
- 37.Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, et al. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruins ME, Janssen AEM, Boom RM. Thermozymes and their applications. Appl Biochem Biotechnol. 2001;90:155–186. doi: 10.1385/ABAB:90:2:155. [DOI] [PubMed] [Google Scholar]
- 39.Bai Y, Wang J, Zhang Z. Biotechnologically relevant enzymes and proteins A novel family 9 β-1,3 (4)-glucanase from thermoacidophilic Alicyclobacillus sp. A4 with potential applications in the brewing industry. Appl Microbiol. 2010;3:251–259. doi: 10.1007/s00253-010-2452-3. [DOI] [PubMed] [Google Scholar]
- 40.Schröder C, Elleuche S, Blank S, Antranikian G. Characterization of a heat-active archaeal β-glucosidase from a hydrothermal spring metagenome. Enzyme Microb Technol. 2014;57:48–54. doi: 10.1016/j.enzmictec.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Krauss G, Cottaz S, Driguez H, Lipps G. A highly acid-stable and thermostable endo-beta-glucanase from the thermoacidophilic archaeon Sulfolobus solfataricus. Biochem J. 2005;385:581–588. doi: 10.1042/BJ20041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suleiman M, Schröder C, Klippel B, Schäfers C, Krüger A, Antranikian G. Extremely thermoactive archaeal endoglucanase from a shallow marine hydrothermal vent from Vulcano Island. Appl Microbiol Biotechnol. 2018 doi: 10.1007/s00253-018-9542-z. [DOI] [PubMed] [Google Scholar]
- 43.Brown SH, Costantino HR, Kelly RM. Characterization of amylolytic enzyme activities associated with the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990;56:1985 LP–1991 LP. doi: 10.1128/AEM.56.7.1985-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch R, Zablowski P, Spreinat A, Antranikian G. Extremely thermostable amylolytic enzyme from the archaebacterium Pyrococcus furiosus. FEMS Microbiol Lett. 1990;71:21–26. doi: 10.1111/j.1574-6968.1990.tb03792.x. [DOI] [Google Scholar]
- 45.Sakai HD, Kurosawa N. Saccharolobus caldissimus gen. nov., sp. nov., a facultatively anaerobic iron-reducing hyperthermophilic archaeon isolated from an acidic terrestrial hot spring, and reclassification of sulfolobus solfataricus as saccharolobus solfataricus comb. nov. and. Int J Syst Evol Microbiol. 2018;68:1271–1278. doi: 10.1099/ijsem.0.002665. [DOI] [PubMed] [Google Scholar]
- 46.Bauer MW, Driskill LE, Kelly RM. An endoglucanase, EglA, from the hyperthermophilic archaeon pyrococcus furiosus hydrolyzes beta-1,4 bonds in mixed-linkage (133), (134)-beta-d-glucans and cellulose. J Bacteriol. 1999;181:284. doi: 10.1128/JB.181.1.284-290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gueguen Y, Voorhorst WGB, Van der Oost J, De Vos WM. Molecular and biochemical characterization of an endo-β-1,3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1997;272:31258–31264. doi: 10.1074/jbc.272.50.31258. [DOI] [PubMed] [Google Scholar]
- 48.Jørgensen S, Vorgias CE, Antranikian G. Cloning, sequencing, characterization, and expression of an extracellular amylase from the hyperthermophilic archaeon Pyrococcus furiosus in Escherichia coli and Bacillus subtilis * 1997;272:16335–42. [DOI] [PubMed]
- 49.Laderman KA, Davis BR, Krutzsch HC, Lewis MS, Griko YV, Privalov PL, et al. The purification and characterization of an extremely thermostable α- amylase from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Biol Chem. 1993;268:24394–24401. [PubMed] [Google Scholar]
- 50.Kengen SWM, Luesink EJ, Stams AJM, Zehnder AJB. Purification and characterization of an extremely thermostable P-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem. 1993;312:305–312. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- 51.Li D, Li X, Dang W, Tran PL, Park S-H, Oh B-C, et al. Characterization and application of an acidophilic and thermostable β-glucosidase from Thermofilum pendens. J Biosci Bioeng. 2013;115:490–496. doi: 10.1016/j.jbiosc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Ando S, Ishida H, Kosugi Y, Ishikawa K. Hyperthermostable endoglucanase from Pyrococcus horikoshii. Appl Environ Microbiol. 2002;68:430–433. doi: 10.1128/AEM.68.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zillig W, Holz I, Klenk H-P, Trent J, Wunderl S, Janekovic D, et al. Pyrococcus woesei, sp. nov., an ultra-thermophilic marine archaebacterium, representing a novel order, Thermococcales. Syst Appl Microbiol. 1987;9:62–70. doi: 10.1016/S0723-2020(87)80057-7. [DOI] [Google Scholar]
- 54.Koch R, Spreinat A, Lemke K, Antranikian G. Purification and properties of a hyperthermoactive α-amylase from the archaeobacterium Pyrococcus woesei. Arch Microbiol. 1991;155:572–578. doi: 10.1007/BF00245352. [DOI] [Google Scholar]
- 55.Zhang X, Zhang X, Jiang L, Alain K, Jebbar M, Shao Z. Palaeococcus pacificus sp. nov., an archaeon from deep-sea hydrothermal sediment xiang zeng. Int J Syst Evol Microbiol. 2013;63:2155–2159. doi: 10.1099/ijs.0.044487-0. [DOI] [PubMed] [Google Scholar]
- 56.Ji H, Bai Y, Li X, Wang J, Xu X, Jin Z. Preparation of malto-oligosaccharides with specific degree of polymerization by a novel cyclodextrinase from Palaeococcus pacificus. Carbohydr Polym. 2019;210:64–72. doi: 10.1016/J.CARBPOL.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 57.Zillig W, Gierl A, Schreiber G, Wunderl S, Janekovic D, Stetter KO, et al. The archaebacterium Thermofilum pendens represents, a novel genus of the thermophilic, anaerobic sulfur respiring thermoproteales. Syst Appl Microbiol. 1983;4:79–87. doi: 10.1016/S0723-2020(83)80035-6. [DOI] [PubMed] [Google Scholar]
- 58.Fiala G, Stetter KO, Jannasch HW, Langworthy TA, Madon J. Staphylothermus marinus sp. nov. represents a novel genus of extremely thermophilic submarine heterotrophic archaebacteria growing up to 98 °C. Syst Appl Microbiol. 1986;8:106–113. doi: 10.1016/S0723-2020(86)80157-6. [DOI] [Google Scholar]
- 59.Li D, Park J-T, Li X, Kim S, Lee S, Shim J-H, et al. Overexpression and characterization of an extremely thermostable maltogenic amylase, with an optimal temperature of 100 °C, from the hyperthermophilic archaeon Staphylothermus marinus. N Biotechnol. 2010;27:300–307. doi: 10.1016/J.NBT.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Huber R, Stöhr J, Hohenhaus S, Rachel R, Burggraf S, Jannasch HW, et al. Thermococcus chitonophagus sp. nov., a novel, chitin-degrading, hyperthermophilic archaeum from a deep-sea hydrothermal vent environment. Arch Microbiol. 1995;164:255–264. doi: 10.1007/BF02529959. [DOI] [Google Scholar]
- 61.Andronopoulou E, Vorgias ÆCE. Purification and characterization of a new hyperthermostable, allosamidin-insensitive and denaturation-resistant chitinase from the hyperthermophilic archaeon Thermococcus chitonophagus 2003: 43–53. 10.1007/s00792-002-0294-3. [DOI] [PubMed]
- 62.Takemasa R, Yokooji Y, Yamatsu A, Atomi H, Imanaka T. Thermococcus kodakaraensis as a host for gene expression and protein secretion. Appl Environ Microbiol. 2011;77:2392 LP–2398 LP. doi: 10.1128/AEM.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad N, Rashid N, Haider MS, Akram M, Akhtar M. Novel maltotriose-hydrolyzing thermoacidophilic type III pullulan hydrolase from Thermococcus kodakaraensis. Appl Environ Microbiol. 2014;80:1108 LP–1115 LP. doi: 10.1128/AEM.03139-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grogan D, Palm P, Zillig W. Isolate B12, which harbours a virus-like element, represents a new species of the archaebacterial genus Sulfolobus, Sulfolobus shibatae, sp. nov. Arch Microbiol. 1990;154:594–599. doi: 10.1007/BF00248842. [DOI] [PubMed] [Google Scholar]
- 65.Boyce A, Walsh G. Expression and characterisation of a thermophilic endo-1,4-β-glucanase from Sulfolobus shibatae of potential industrial application. Mol Biol Rep. 2018;45:2201–2211. doi: 10.1007/s11033-018-4381-7. [DOI] [PubMed] [Google Scholar]
- 66.Brock TD, Brock KM, Belly RT, Weiss RL. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 67.Choi KH, Cha J. Membrane-bound amylopullulanase is essential for starch metabolism of Sulfolobus acidocaldarius DSM639. Extremophiles. 2015;19:909–920. doi: 10.1007/s00792-015-0766-x. [DOI] [PubMed] [Google Scholar]
- 68.Zillig W, Stetter KO, Wunderl S, Schulz W, Priess H, Scholz I. The Sulfolobus-``Caldariella’’ group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch Microbiol. 1980;125:259–269. doi: 10.1007/BF00446886. [DOI] [Google Scholar]
- 69.Cannio R, Di Prizito N, Rossi M, Morana A. A xylan-degrading strain of Sulfolobus solfataricus: isolation and characterization of the xylanase activity. Extremophiles. 2004;8:117–124. doi: 10.1007/s00792-003-0370-3. [DOI] [PubMed] [Google Scholar]
- 70.Pisani FM, Rella R, Rata CA, Rozzo C, Nucci R, Gambacorta A, et al. Thermostable β-galactosidase from the archaebacterium Sulfolobus solfataricus Purification and properties. Eur J Biochem. 1990;187:321–328. doi: 10.1111/j.1432-1033.1990.tb15308.x. [DOI] [PubMed] [Google Scholar]
- 71.Moracci M, La Volpe A, Pulitzer JF, Rossi M, Ciaramella M. Expression of the thermostable beta-galactosidase gene from the archaebacterium Sulfolobus solfataricus in Saccharomyces cerevisiae and characterization of a new inducible promoter for heterologous expression. J Bacteriol. 1992;174:873–882. doi: 10.1128/jb.174.3.873-882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brouns SJJ, Smits N, Wu H, Snijders APL, Wright PC, de Vos WM, et al. Identification of a Novel α-galactosidase from the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 2006;188:2392–2399. doi: 10.1128/JB.188.7.2392-2399.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moracci M, Ponzano BC, Trincone A, Fusco S, De Rosa M, Van Der Oost J, et al. Identification and molecular characterization of the first α-xylosidase from an Archaeon. J Biol Chem. 2000;275:22082–22089. doi: 10.1074/jbc.M910392199. [DOI] [PubMed] [Google Scholar]
- 74.Cobucci-Ponzano B, Conte F, Strazzulli A, Capasso C, Fiume I, Pocsfalvi G, et al. The molecular characterization of a novel GH38 α-mannosidase from the crenarchaeon Sulfolobus solfataricus revealed its ability in de-mannosylating glycoproteins. Biochimie. 2010;92:1895–1907. doi: 10.1016/j.biochi.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 75.Itoh T, Suzuki K, Sanchez PC, Nakase T. Caldivirga maquilingensis gen. nov., sp. nov., a new genus of rod-shaped crenarchaeote isolated from a hot spring in the Philippines. Int J Syst Evol Microbiol. 1999;49:1157–1163. doi: 10.1099/00207713-49-3-1157. [DOI] [PubMed] [Google Scholar]
- 76.Letsididi R, Hassanin HAM, Koko MYF, Ndayishimiye JB, Zhang T, Jiang B, et al. Characterization of a thermostable glycoside hydrolase (CMbg0408) from the hyperthermophilic archaeon Caldivirga maquilingensis IC-167. J Sci Food Agric. 2017;97:2132–2140. doi: 10.1002/jsfa.8019. [DOI] [PubMed] [Google Scholar]
- 77.Volkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, et al. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol. 1993;59:2918–2926. doi: 10.1128/aem.59.9.2918-2926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeon H, Lee H, Byun D, Choi H, Shim JH. Molecular cloning, characterization, and application of a novel thermostable α-glucosidase from the hyperthermophilic archaeon Pyrobaculum aerophilum strain IM2. Food Sci Biotechnol. 2015;24:175–182. doi: 10.1007/s10068-015-0024-0. [DOI] [Google Scholar]
- 79.Schleper C, Puehler G, Holz I, Gambacorta A, Janekovic D, Santarius U, et al. Picrophilus gen. nov., fam. nov.: a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J Bacteriol. 1995;177:7050–7059. doi: 10.1128/jb.177.24.7050-7059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angelov A, Putyrski M, Liebl W. Molecular and biochemical characterization of α-glucosidase and α-mannosidase and their clustered genes from the thermoacidophilic archaeon Picrophilus torridus. J Bacteriol. 2006;188:7123–7131. doi: 10.1128/JB.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leis B, Heinze S, Angelov A, Pham VTT, Thürmer A, Jebbar M, et al. Functional Screening of Hydrolytic Activities Reveals an Extremely Thermostable Cellulase from a Deep-Sea Archaeon. Front Bioeng Biotechnol. 2015;3:95. doi: 10.3389/fbioe.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antranikian G, Suleiman M, Schäfers C, Adams MWW, Bartolucci S, Blamey JM, et al. Diversity of bacteria and archaea from two shallow marine hydrothermal vents from Vulcano Island. Extremophiles. 2017;21:733–742. doi: 10.1007/s00792-017-0938-y. [DOI] [PubMed] [Google Scholar]
- 83.Graham JE, Clark ME, Nadler DC, Huffer S, Chokhawala HA, Rowland SE, et al. Identification and characterization of a multidomain hyperthermophilic cellulase from an archaeal enrichment. Nat Commun. 2011;2:375–379. doi: 10.1038/ncomms1373. [DOI] [PubMed] [Google Scholar]
- 84.Gavrilov SN, Stracke C, Jensen K, Menzel P, Kallnik V, Slesarev A, et al. Isolation and characterization of the first xylanolytic hyperthermophilic euryarchaeon Thermococcus sp. strain 2319x1 and its unusual multidomain glycosidase. Front Microbiol. 2016;7:1–17. doi: 10.3389/fmicb.2016.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Webb EC. Enzyme nomenclature 1992. Recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the Nomenclature and Classification of Enzymes. Acad Press 1992; No. Ed. 6.
- 86.Henrissat B. A classification of glycosyl hydrolases based sequence similarities amino acid. Biochem J. 1991;280:309–316. doi: 10.1007/s007920050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aspeborg H, Coutinho PM, Wang Y, Brumer H, Henrissat B. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5) BMC Evol Biol. 2012 doi: 10.1186/1471-2148-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viborg AH, Terrapon N, Lombard V, Michel G, Czjzek M, Henrissat B, et al. A subfamily roadmap of the evolutionarily diverse glycoside hydrolase family 16 (GH16) J Biol Chem. 2019;294:15973–15986. doi: 10.1074/jbc.RA119.010619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mewis K, Lenfant N, Lombard V, Henrissat B. Dividing the large glycoside hydrolase family 43 into subfamilies: a motivation for detailed enzyme characterization. Appl Environ Microbiol. 2016;82:1686–1692. doi: 10.1128/AEM.03453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stam MR, Danchin EGJ, Rancurel C, Coutinho PM, Henrissat B. Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of α-amylase-related proteins. Protein Eng Des Sel. 2006;19:555–562. doi: 10.1093/protein/gzl044. [DOI] [PubMed] [Google Scholar]
- 92.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2008;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim H, Mino K, Ishikawa K. crystallization communications Crystallization and preliminary X-ray analysis of endoglucanase from Pyrococcus horikoshii crystallization communications. Struct Biol Cryst Commun. 2008;64:1169–1171. doi: 10.1107/S1744309108036919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim H-W, Ishikawa K. Structure of hyperthermophilic endocellulase from Pyrococcus horikoshii. Proteins Struct Funct Bioinforma. 2010;78:496–500. doi: 10.1002/prot.22602. [DOI] [PubMed] [Google Scholar]
- 95.Strazzulli A, Cobucci-Ponzano B, Iacono R, Giglio R, Maurelli L, Curci N, et al. Discovery of hyperstable carbohydrate-active enzymes through metagenomics of extreme environments. FEBS J. 2020;287:1116–1137. doi: 10.1111/febs.15080. [DOI] [PubMed] [Google Scholar]
- 96.Adam PS, Borrel G, Brochier-Armanet C, Gribaldo S. The growing tree of Archaea: new perspectives on their diversity, evolution and ecology. ISME J. 2017;11:2407–2425. doi: 10.1038/ismej.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Llirós M, Casamayor EO, Borrego C. High archaeal richness in the water column of a freshwater sulfurous karstic lake along an interannual study. FEMS Microbiol Ecol. 2008;66:331–342. doi: 10.1111/j.1574-6941.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- 98.Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci. 2005;102:14683–14688. doi: 10.1073/pnas.0506625102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoshino T, Inagaki F. Abundance and distribution of Archaea in the subseafloor sedimentary biosphere. ISME J. 2019;13:227–231. doi: 10.1038/s41396-018-0253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X, Pan J, Liu Y, Li M, Gu J-D. Diversity and distribution of Archaea in global estuarine ecosystems. Sci Total Environ. 2018;637–638:349–358. doi: 10.1016/j.scitotenv.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Butterfield CN, Li Z, Andeer PF, Spaulding S, Thomas BC, Singh A, et al. Proteogenomic analyses indicate bacterial methylotrophy and archaeal heterotrophy are prevalent below the grass root zone. PeerJ. 2016;2016:1–28. doi: 10.7717/peerj.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bräsen C, Esser D, Rauch B, Siebers B. Carbohydrate metabolism in archaea: current insights into unusual enzymes and pathways and their regulation. JAMA Ophthalmol. 2014;132:326–331. doi: 10.1128/MMBR.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spang A, Saw JH, Jørgensen SL, Zaremba-Niedzwiedzka K, Martijn J, Lind AE, et al. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature. 2015;521:173. doi: 10.1038/nature14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spang A, Stairs CW, Dombrowski N, Eme L, Lombard J, Caceres EF, et al. Proposal of the reverse flow model for the origin of the eukaryotic cell based on comparative analyses of Asgard archaeal metabolism. Nat Microbiol. 2019;4:1138–1148. doi: 10.1038/s41564-019-0406-9. [DOI] [PubMed] [Google Scholar]
- 105.Reinhardt A, Johnsen U, Schönheit P. l-Rhamnose catabolism in archaea. Mol Microbiol. 2019;111:1093–1108. doi: 10.1111/mmi.14213. [DOI] [PubMed] [Google Scholar]
- 106.Jünemann S, Kleinbölting N, Jaenicke S, Henke C, Hassa J, Nelkner J, et al. Bioinformatics for NGS-based metagenomics and the application to biogas research. J Biotechnol. 2017;261:10–23. doi: 10.1016/j.jbiotec.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 107.Jervis-Bardy J, Leong LEX, Marri S, Smith RJ, Choo JM, Smith-Vaughan HC, et al. Deriving accurate microbiota profiles from human samples with low bacterial content through post-sequencing processing of Illumina MiSeq data. Microbiome. 2015;3:19. doi: 10.1186/s40168-015-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie W, Wang F, Guo L, Chen Z, Sievert SM, Meng J, et al. Comparative metagenomics of microbial communities inhabiting deep-sea hydrothermal vent chimneys with contrasting chemistries. ISME J. 2011;5:414–426. doi: 10.1038/ismej.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vanwonterghem I, Jensen PD, Ho DP, Batstone DJ, Tyson GW. Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr Opin Biotechnol. 2014;27:55–64. doi: 10.1016/J.COPBIO.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Meyer F, Paarmann D, D’Souza M, Olson R, Glass EM, Kubal M, et al. The metagenomics RAST server-a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huson DH, Beier S, Flade I, Górska A, El-Hadidi M, Mitra S, et al. MEGAN Community edition—interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput Biol. 2016;12:1–12. doi: 10.1371/journal.pcbi.1004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmeisser C, Steele H, Streit WR. Metagenomics, biotechnology with non-culturable microbes. Appl Microbiol Biotechnol. 2007;75:955–962. doi: 10.1007/s00253-007-0945-5. [DOI] [PubMed] [Google Scholar]
- 114.Knapik K, Becerra M, González-Siso MI. Microbial diversity analysis and screening for novel xylanase enzymes from the sediment of the Lobios Hot Spring in Spain. Sci Rep. 2019;9:11195. doi: 10.1038/s41598-019-47637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Knietsch A, Waschkowitz T, Bowien S, Henne A, Daniel R. Construction and screening of metagenomic libraries derived from enrichment cultures: generation of a gene bank for genes conferring alcohol oxidoreductase activity on Escherichia coli. Appl Environ Microbiol. 2003;69:1408 LP–1416 LP. doi: 10.1128/AEM.69.3.1408-1416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Y, Tang Y, Tringe SG, Simmons BA, Singer SW. MaxBin: an automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm 2014:1–18. [DOI] [PMC free article] [PubMed]
- 117.Zayulina KS, Kochetkova TV, Piunova UE, Ziganshin RH, Podosokorskaya OA, Kublanov IV. Novel hyperthermophilic crenarchaeon Thermofilum adornatum sp. nov. Uses GH1, GH3, and two novel glycosidases for cellulose hydrolysis. Front Microbiol. 2020;10:2972. doi: 10.3389/fmicb.2019.02972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dumorné K, Córdova DC, Astorga-Eló M, Renganathan P. Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol. 2017;27:649–659. doi: 10.4014/jmb.1611.11006. [DOI] [PubMed] [Google Scholar]
- 119.Contesini FJ, de Melo RR, Sato HH. An overview of Bacillus proteases: from production to application. Crit Rev Biotechnol. 2018;38:321–334. doi: 10.1080/07388551.2017.1354354. [DOI] [PubMed] [Google Scholar]
- 120.Packer MS, Liu DR. Methods for the directed evolution of proteins. Nat Rev Genet. 2015;16:379–394. doi: 10.1038/nrg3927. [DOI] [PubMed] [Google Scholar]
- 121.Bornscheuer UT, Pohl M. Improved biocatalysts by directed evolution and rational protein design. Curr Opin Chem Biol. 2001;5:137–143. doi: 10.1016/S1367-5931(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 122.Böttcher D, Bornscheuer UT. Protein engineering of microbial enzymes. Curr Opin Microbiol. 2010;13:274–282. doi: 10.1016/j.mib.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 123.Jaenicke R, Böhm G. The stability of proteins in extreme environments. Curr Opin Struct Biol. 1998;8:738–748. doi: 10.1016/S0959-440X(98)80094-8. [DOI] [PubMed] [Google Scholar]
- 124.Sandgren M, Ståhlberg J, Mitchinson C. Structural and biochemical studies of GH family 12 cellulases: improved thermal stability, and ligand complexes. Prog Biophys Mol Biol. 2005;89:246–291. doi: 10.1016/j.pbiomolbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 125.D’Auria S, Moracci M, Febbraio F, Tanfani F, Nucci R, Rossi M. Structure-function studies on β-glycosidase from Sulfolobus solfataricus. Molecular bases of thermostability. Biochimie. 1998;80:949–957. doi: 10.1016/S0300-9084(00)88892-6. [DOI] [PubMed] [Google Scholar]
- 126.Cobucci-Ponzano B, Perugino G, Rossi M, Moracci M. Engineering the stability and the activity of a glycoside hydrolase. Protein Eng Des Sel. 2010;24:21–26. doi: 10.1093/protein/gzq085. [DOI] [PubMed] [Google Scholar]
- 127.Morana A, Moracci M, Ottombrino A, Ciaramella M, Rossi M, De Rosa M. Industrial-scale production and rapid purification of an archaeal beta-glycosidase expressed in Saccharomyces cerevisiae. Biotechnol Appl Biochem. 1995;22:261–268. [PubMed] [Google Scholar]
- 128.Cobucci-Ponzano B, Moracci M, Di Lauro B, Ciaramella M, D’Avino R, Rossi M. Ionic network at the C-terminus of the β-glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus: functional role in the quaternary structure thermal stabilization. Proteins Struct Funct Bioinforma. 2002;48:98–106. doi: 10.1002/prot.10128. [DOI] [PubMed] [Google Scholar]
- 129.Pouwels J, Moracci M, Cobucci-Ponzano B, Perugino G, van der Oost J, Kaper T, et al. Activity and stability of hyperthermophilic enzymes: a comparative study on two archaeal β-glycosidases. Extremophiles. 2000;4:157–164. doi: 10.1007/s007920070030. [DOI] [PubMed] [Google Scholar]
- 130.Chi YI, Martinez-Cruz LA, Jancarik J, Swanson RV, Robertson DE, Kim SH. Crystal structure of the β-glycosidase from the hyperthermophile Thermosphaera aggregans: insights into its activity and thermostability. FEBS Lett. 1999;445:375–383. doi: 10.1016/S0014-5793(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 131.Ausili A, Cobucci-Ponzano B, Di Lauro B, D’Avino R, Scirè A, Rossi M, et al. Structural basis of the destabilization produced by an amino-terminal tag in the β-glycosidase from the hyperthermophilic archeon Sulfolobus solfataricus. Biochimie. 2006;88:807–817. doi: 10.1016/j.biochi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 132.Perugino G, Strazzulli A, Mazzone M, Rossi M, Moracci M. Effects of random mutagenesis and in vivo selection on the specificity and stability of a thermozyme. Catalysts. 2019;9:1–15. doi: 10.3390/catal9050440. [DOI] [Google Scholar]
- 133.Lebbink JHG, Kaper T, Bron P, van der Oost J, de Vos WM. Improving Low-Temperature Catalysis in the Hyperthermostable Pyrococcus furiosus β-Glucosidase CelB by Directed Evolution. Biochemistry. 2000;39:3656–3665. doi: 10.1021/bi991483q. [DOI] [PubMed] [Google Scholar]
- 134.Kang H-J, Uegaki K, Fukada H, Ishikawa K. Improvement of the enzymatic activity of the hyperthermophilic cellulase from Pyrococcus horikoshii. Extremophiles. 2007;11:251–256. doi: 10.1007/s00792-006-0033-2. [DOI] [PubMed] [Google Scholar]
- 135.Nálezková M, de Groot A, Graf M, Gans P, Blanchard L. Overexpression and purification of Pyrococcus abyssi phosphopantetheine adenylyltransferase from an optimized synthetic gene for NMR studies. Protein Expr Purif. 2005;39:296–306. doi: 10.1016/j.pep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 136.García-Fraga B, da Silva AF, López-Seijas J, Sieiro C. Optimized expression conditions for enhancing production of two recombinant chitinolytic enzymes from different prokaryote domains. Bioprocess Biosyst Eng. 2015;38:2477–2486. doi: 10.1007/s00449-015-1485-5. [DOI] [PubMed] [Google Scholar]
- 137.Mayer J, Kranz B, Fischer L. Continuous production of lactulose by immobilized thermostable β-glycosidase from Pyrococcus furiosus. J Biotechnol. 2010;145:387–393. doi: 10.1016/j.jbiotec.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 138.Schulze S, Adams Z, Cerletti M, De Castro R, Ferreira-Cerca S, Fufezan C, et al. The Archaeal Proteome Project advances knowledge about archaeal cell biology through comprehensive proteomics. Nat Commun. 2020;11:3145. doi: 10.1038/s41467-020-16784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.