Abstract
Furfural chemistry is one of the most promising platforms directly derived from lignocellulose biomass. In this study, a niobium-based catalyst (mNb-bc) was synthesized by a new fast and simple method. This new method uses microemulsion to obtain a catalyst with a high specific surface area (340 m2 g–1), defined mesoporosity, and high acidity (65 μmol g–1). Scanning electron microscopy revealed that mNb-bc has a rough surface. The mNb-bc was used to catalyze the conversion reaction of xylose into 2-furfuraldehyde in a monophasic system using water as a green solvent. This reaction was investigated using a 23 experimental design by varying the temperature, time, and catalyst-to-xylose ratio (CXR). The responses evaluated were xylose conversion (Xc), reaction yield (Y), and selectivity to 2-furfuraldehyde (S). The optimized reaction conditions were used to evaluate the reaction kinetics. At milder reaction conditions of 140 °C, 2 h, and a CXR of 10%, mNb-bc led to an Xc value of 41.2%, an S value of 77.1%, and a Y value of 31.8%.
1. Introduction
In the group of aldehydes, 2-furfuraldehyde stands out as one of the top added-value chemicals obtained from lignocellulose biomass according to the U.S. Department of Energy.1 It has various applications in several fields, such as pharmaceutics, agrochemistry, plastics, and polymers.2,3 In addition, 2-furfuraldehyde is a renewable chemical product used in the synthesis of important industrial C4 and C5 chemicals, such as tetrahydrofuran and furoic acid. In this way, 2-furfuraldehyde and its derivatives can be considered as promising substitutes to petroleum-based products.4,5
In the early 1920s, the first industrial production of 2-furfuraldehyde was established based on the Quaker Oats method.6 Nowadays, China, Dominican Republic, and South Africa are the main producers of 2-furfuraldehyde and its derivatives. These countries still have industrial processes based on the original Quaker Oats method, with some modifications.7 The process consists of digesters loaded with lignocellulose biomass and a solution containing acid catalyst at specific pressures and temperatures, where stream stripping and distillation steps are used to recover 2-furfuraldehyde.8
The current production of 2-furfuraldehyde still has some drawbacks, such as low yield and poor selectivity. In addition, the use of homogeneous catalysts based on mineral acids and steam as a stripping agent causes equipment corrosion and environmental problems due to emission of toxic effluents with low pH.9 Therefore, more technological development is needed to increase commercial viability of the process and improve competition with petrochemical products. To this end, recent studies have been focused on the use of heterogeneous catalysts,10 different separation processes,8,11 or other types of reactions12 to improve the selectivity and yield of xylose dehydration reactions without relying upon acid solutions.
Concerning the catalysts, their pivotal advantages include surface acidity that can replace acid solutions, possibility of their recovery and reuse in the process, and the potential for obtaining better performance compared to homogeneous catalysts.13 In addition, heterogeneously catalyzed reactions are more environmentally friendly, allowing the development of green chemistry-based processes.14 Some heterogeneous niobium-based catalysts have been used for xylose dehydration reactions. Niobium-based catalysts stood out in heterogeneous catalysis, demonstrating high catalytic activity, selectivity, and chemical stability,15 as well as high acidity including both Lewis and Brønsted sites. This outstanding performance has also been reported for xylose dehydration reactions, as shown in Table 1.
Table 1. Matrix of Experiments for the 23 Experimental Design and Responses (Conversion, Yield, and Selectivity) Obtained for the Dehydration Reaction of Xylose to 2-Furfuraldehyde as well as Comparison with Other Heterogeneous Catalysts Reported in the Literature.
| catalyst type | reaction medium | T (°C) | t (min) | CXR (%) | Xc (%) | S (%) | Y (%) | refs |
|---|---|---|---|---|---|---|---|---|
| mNb-bc | water | 140 | 15 | 10 | 7.33 | 65.17 | 4.78 | this study |
| 140 | 120 | 90 | 63.98 | 2.08 | 1.33 | |||
| 140 | 15 | 90 | 34.83 | 16.67 | 5.81 | |||
| 140 | 120 | 10 | 26.83 | 75.27 | 20.20 | |||
| 180a | 67.5a | 50a | 91.90 | 28.53 | 26.20 | |||
| 180a | 67.5a | 50a | 94.37 | 26.83 | 25.32 | |||
| 180a | 67.5a | 50a | 91.72 | 21.90 | 20.09 | |||
| 220 | 120 | 90 | 92.07 | 22.49 | 20.71 | |||
| 220 | 120 | 10 | 99.17 | 33.55 | 33.27 | |||
| 220 | 15 | 90 | 91.72 | 19.78 | 18.14 | |||
| 220 | 15 | 10 | 91.47 | 35.84 | 32.78 | |||
| NbP | water | 160 | 60 | 7 | 17.4 | 39.5 | 6.8 | (21) |
| MCM12Nb | water | 160 | 60 | 30 | 8.7 | 58.0 | 5.1 | (22) |
| SBA-12Nb | water | 160 | 60 | 30 | 4.8 | 95.0 | 5.0 | (22) |
| Si-12Nb | water | 160 | 60 | 30 | 9.6 | 80.0 | 7.7 | (22) |
| Al-12Nb | water | 160 | 60 | 30 | 7.8 | 60.0 | 4.7 | (22) |
| NbOc | water | 130 | 360 | 67 | 43.0 | 53.1 | 22.8 | (23) |
| Nb/SZsg | water | 130 | 360 | 67 | 24.0 | 61.5 | 14.8 | (23) |
| Nb/SZi | water | 130 | 360 | 67 | 21.0 | 60.0 | 12.6 | (23) |
| MIL-101(Cr)-SO3H | cyclopentyl methyl ether/H2O–NaCl solvent system | 170 | 180 | 67.5 | 97.8 | 72.4 | 70.8 | (24) |
Center point of the 23 experimental design.
The aim of this study is to synthesize a mesoporous niobium-based catalyst (mNb-bc) and investigate its application for the dehydration reaction of xylose to 2-furfuradehyde. Generally, the methods reported in the literature for preparation of the niobium-based catalysts include a calcination step that requires a high amount of electrical energy. On the other hand, a new synthesis method employing low temperatures and cetyltrimethylammonium bromide (CTAB) was developed in the present study, yielding a catalyst with high surface area and acidity. The effect of the reaction parameters, such as temperature, time, and catalyst-to-xylose ratio (CXR), on the dehydration of xylose to 2-furfuraldehyde was investigated using a 23 experimental design. The responses evaluated were the conversion of xylose (Xc), selectivity (S) of the mNb-bc for 2-furfuraldehyde production, and yield of the reaction (Y).
2. Results and Discussion
2.1. Characterization of the Catalyst
The mNb-bc synthesized in this study was characterized to determine its textural and acidic properties. The N2 adsorption and desorption isotherms on mNb-bc and the infrared spectrum of pyridine adsorbed on mNb-bc are presented in Figure 1a,b, respectively.
Figure 1.
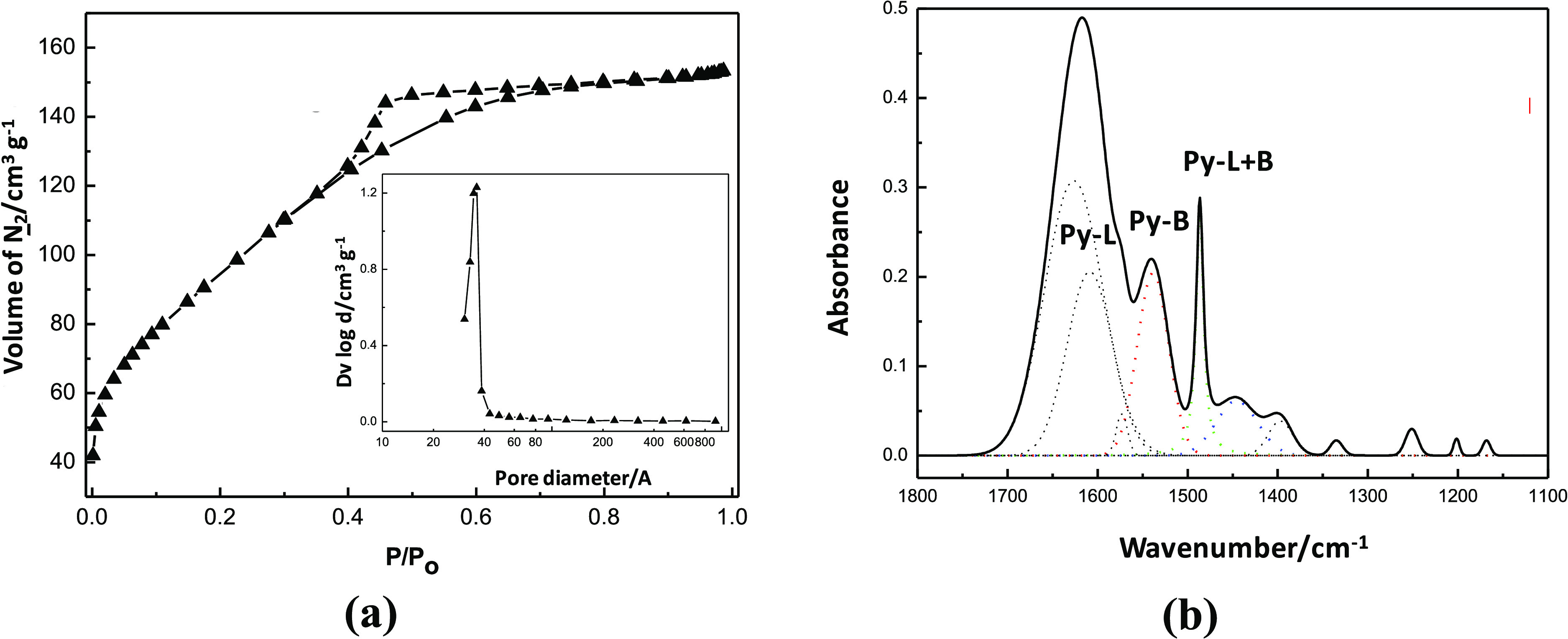
(a) Adsorption and desorption isotherms of N2 on mNb-bc and (b) infrared spectrum of pyridine (Py) adsorbed on mNb-bc (Py-L = Lewis site, Py-B = Brønsted site).
The mNb-bc had a specific surface area of 340 m2 g–1 and an average pores size of 40 Å. This catalyst can be classified as a mesoporous material according to the International Union of Pure and Applied Chemistry (IUPAC). According to the original IUPAC classification,16 Brunauer–Emmett–Teller (BET) isotherms were similar to type IV that are related to multilayer adsorption on mesoporous solids. High specific surface area and large pores size are the desired properties for catalysts to obtain high conversion.
Pyridine adsorption on mNb-bc was used for the identification and quantification of Lewis and Brønsted acid catalytic sites. The infrared spectrum (Figure 1b) shows a band at 1540 cm–1, which is related to the vibrational mode of the pyridinium ion. This band is associated with the Brønsted sites (Py-B) on the catalyst surface. The bands at 1445 and 1610 cm–1 are related to the vibrational modes of pyridine coordinated to Lewis acid sites (Py-L), while the band at 1488 cm–1 is related to vibrational modes of both protonated and coordinated pyridine (Py-L + B).17 The bands at 1446 and 1539 cm–1 have been used for the quantification of acid sites, which showed that mNb-bc had acidity, with a total amount of acid sites of 65 μmol g–1. In fact, this high acidity value can promote a conversion of substrates without extensive formation of byproducts, as it was reported for catalysts with high acidity.18
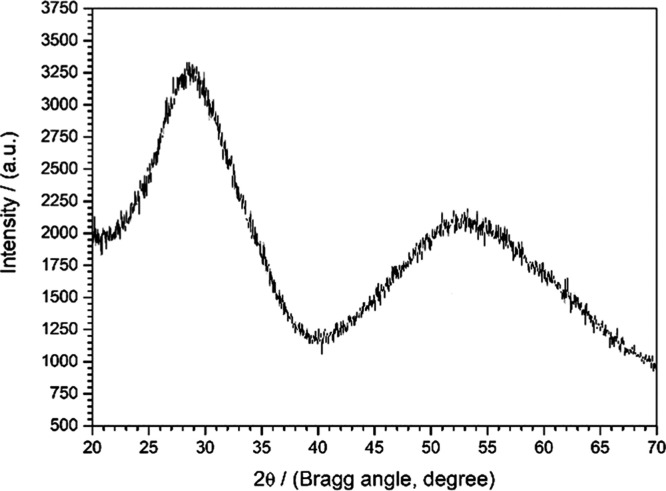
The diffractogram of mNb-bc is shown in Figure 2. The synthesis of mNb-bc was not carried out at high temperatures, and the mNb-bc did not undergo a hydrothermal or a thermal treatment process before the catalytic tests. Therefore, it is an amorphous catalyst, different from those catalysts reported in the literature. This structural characteristic is interesting, as it may be one of the characteristics responsible for the activity of the mNb-bc, as well as for its high specific area.
Figure 2.
Diffractogram of the mNb-bc.
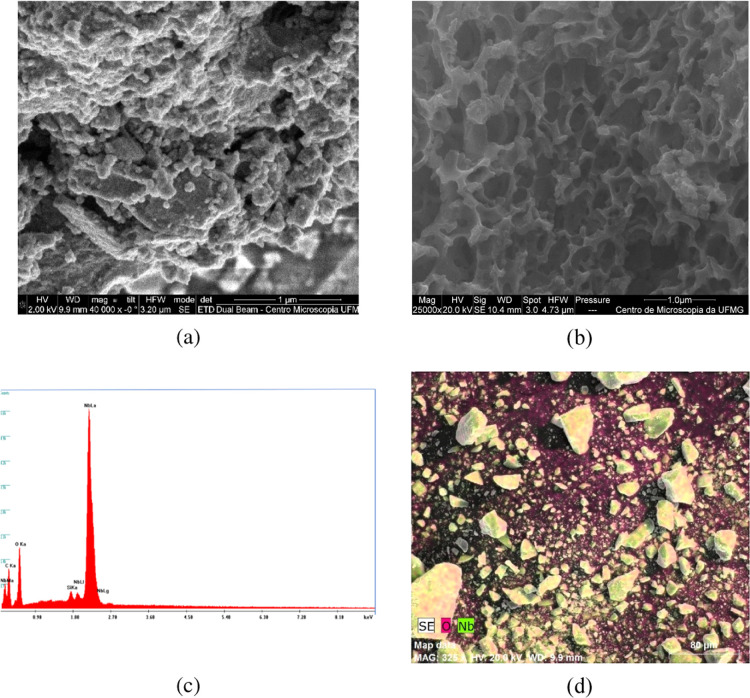
The surface of the mNb-bc was examined by scanning electron microscopy (SEM). In addition, the Nb loaded on the mNb-bc was analyzed by energy-dispersive X-ray (EDX). SEM and SEM-EDX micrographs with surface mapping are shown in Figure 3.
Figure 3.
Micrographs of the mNb-bc at magnifications of 40 000× (a) and 25 000× (b) obtained by scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) spectrum of the mNb-bc (c), and SEM-EDX image of the mNb-bc (Nb, light green).
The SEM micrographs of the mNb-bc (Figure 3a,b) revealed that it has a rough surface. The spongy surface of the mNb-bc was produced by the synthesis method used. The EDX spectrum and surface mapping analysis of the mNb-bc (Figure 3c,d) showed the presence of niobium and oxygen, with a high dispersion in the material.
2.2. Effect of Temperature, Time, and Catalyst-to-Xylose Ratio on Xylose Conversion, Yield, and Selectivity
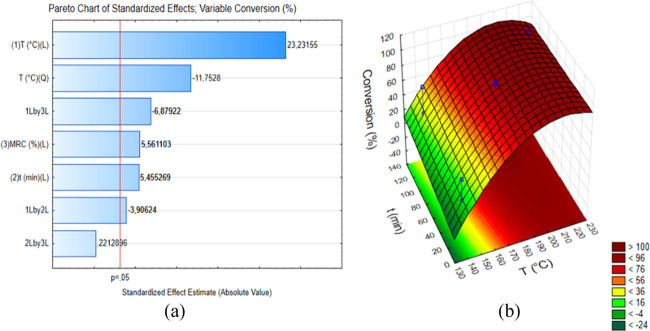
The effects of temperature, time, and CXR on Xc, Y, and S were evaluated using a 23 experimental design. The Pareto chart of standardized effects for response Xc is presented in Figure 4a. The variable T was of the variable T at the higher level increased the Xc value. Figure 4b shows that as the reaction temperature was increased, the Xc value increased for a CXR value fixed at 10%. As shown in Table 1, from a reaction temperature of 180 °C, Xc values higher than 91% were obtained for reaction times from 15 to 120 min and CXR values from 10 to 90%.
Figure 4.
(a) Pareto chart of standardized effects for xylose conversion and (b) surface response for xylose conversion with a catalyst-to-xylose ratio fixed at 10%.
The variable CXR had a positive significant effect (5.56) on Xc, which indicates that the use of the variable CXR at the higher level increased the Xc value. However, the effect of the variable CXR on Xc was lower than the effect of the variable T on Xc. As shown in Table 1, the use of low temperatures (140 °C) did not lead to high Xc values (<64%) for CXR values from 10 to 90%. Although one of the major advantages of the use of a heterogeneous catalyst to convert xylose into 2-furfuraldehyde is the possibility of recovery and reuse of the solid catalyst at the end of the reaction, using low amounts of the catalyst increases the feasibility of the process.
As expected, the variable t also had a positive significant effect (5.45) on Xc, which indicates that the use of the variable t at the higher level increased the Xc value. Figure 4b shows that as the reaction time was increased the Xc value increased for a CXR value fixed at 10%. However, as the effect of T (23.23) was greater than the effect of t (5.45) on Xc, the Xc value was less affected by an increase of t than an increase of T. This is an important finding, as longer reaction times lead to an elevated cost to the process due to the high energy consumption.
For the effect of interactions between the variables, the interactions of T with CXR (−6.88) and T with t (−3.91) had negative significant effects on the Xc value. This indicates that use of both variables T and CXR or T and t at the higher levels did not lead to an increase in the Xc value. Dias et al.19 reported similar behavior using Lewis and Brønsted acid catalysts. These authors concluded that the use of acid catalysts can contribute to decrease the activation energy for conversion of xylose into xylulose, which is the first step of the conversion reaction of xylose into 2-furfuraldehyde. However, with the decreased activation energy, the use of the variables T and CXR or T and t at the higher levels may lead to the formation of undesired byproducts.
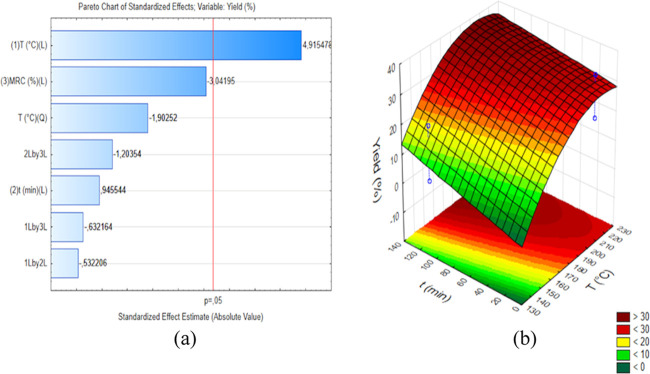
The Pareto chart of standardized effects for response Y is shown in Figure 5a, which shows that the independent variable that had the most significant effect on Y was variable T (4.91). This indicates that the use of variable T at the higher level for the dehydration reaction of xylose to 2-furfuraldehyde led to an increase in Y value. The higher effect of variable T on the Y value can be observed by comparing the experiment performed at 140 °C, 15 min, and a CXR of 10% (Y = 4.78%) with the experiment performed at 220 °C, 15 min, and a CXR of 10% (Y = 32.78%). As the dehydration of xylose to 2-furfuraldehyde is an endothermic reaction, with activation energies varying from 68.520 to 123.3 kJ mol–121 depending on the presence of an acid catalyst (homogeneous or heterogeneous) and extractant agents, the increase in temperature displaces the chemical reaction equilibrium to the product, which contributes to obtaining higher yields.
Figure 5.
(a) Pareto chart of standardized effects for yield and (b) surface response for the yield with a catalyst-to-xylose ratio fixed at 10%.
As shown in Figure 5a, the independent variable that presented the second most significant effect on Y was variable CXR. However, differently from what was observed for variable T, the effect of variable CRX was negative (−3.04), which means that using variable CXR at the higher level decreased the Y value. The negative effect of variable CXR can be explained by the catalytic effect of acid sites on the surface of mNb-bc since a higher acidity favors some undesired parallel and consecutive reactions, such as the formation of furan resins and condensation products, among others, contributing to lower yields of 2-furfuraldehyde.9 This can be observed by comparing the experiments performed at 140 °C and 120 min with CXR values of 90 and 10%, respectively. The experiment performed using a lower CXR value had a Y value of 20.20%, while the experiment performed using a higher CXR value had a Y value of 1.33% (Table 1). The same behavior was observed for experiments carried out using more severe reaction conditions, such as 220 °C and 120 min with CXR values of 90 and 10%, respectively. The experiment carried out using a lower CXR value had a Y value of 33.27%, while the experiment carried out using a higher CXR value had a Y value of 20.71% (Table 1).
For the effect of interactions between variables (Figure 5a), the interactions of t with CXR (−1.20), T with CXR (−0.63), and T with t (−0.53) had negative insignificant effects on the Y value. This indicates that using both variables t and CXR, T and CXR, or T and t at the higher levels did not lead to an increase in Y value. The response surface for Y is shown in Figure 5b. It is observed that high Y values (>30%) can only be obtained from reaction conditions using temperatures higher than 180 °C and times longer than 15 min for a CXR value of 10%.
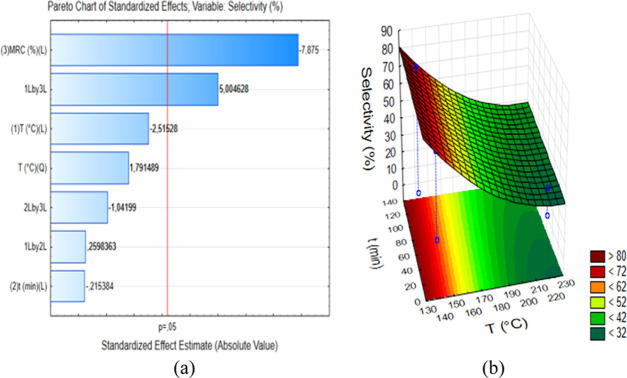
The Pareto chart of standardized effects for response S is shown in Figure 6a, which shows that the variable CXR was the only independent variable that had a significant effect. This variable had a negative effect (−7.88) on S, which shows that the use of variable CXR at the higher level resulted in a lower S value. The use of a higher CXR value led to a dehydration reaction of lower selectivity because it seemed to favor parallel and consecutive reactions, thus decreasing the selectivity of conversion of xylose into 2-furfuraldehyde. This is an important finding as it suggests that to obtain higher values of Y and S, lower CXR values are required. As already discussed, lower amounts of the catalyst lead to lower process costs. Catalysts with more effective reaction sites are used in lower amounts in the process, as the case of mNb-bc used in the present study.
Figure 6.
(a) Pareto chart of standardized effects for selectivity and (b) surface response of selectivity for 2-furfuraldehyde production with a xylose-to-catalyst ratio fixed at 10%.
As shown in Figure 6b, for a CXR value of 10%, higher S values (>65%) were obtained at temperatures lower than 150 °C and reaction times longer than 15 min. As shown in Table 1, the experiment performed at 140 °C and 120 min had the highest S value (75.27%), with a Y value of 20.20%. High selectivity is a desirable characteristic for catalysts to be applied in industrial processes since an industrial process employing catalysts with high selectivity has reduced costs. If a catalyst has a poor selectivity, an additional step for separation and purification of the product is required after the reaction is complete. In this way, the reaction condition performed at 140 °C and 120 min with a CXR value of 10% was chosen as the optimum condition for the dehydration reaction of xylose to 2-furfuraldehyde using mNb-bc.
The p-values for lack of fit of the mathematical models generated for all responses evaluated (Y, Xc, and S) were higher than 0.05, as shown in Supporting Information Tables 2–4. The p-values suggested that the mathematical models exhibited a satisfactory fit for experimental data of all responses evaluated. The R2 values for the responses Y, Xc, and S were 0.9303, 0.9963, and 0.9702, respectively.
In Table 1, it is observed there are other reaction conditions with higher values of xylose conversion. However, reaction conditions with higher values of conversion and lower values of selectivity can lead to higher costs for the process. In large-scale processes, problems related to lower values of conversion can be minimized by recirculating the residual output running from the separation unit, which contains a high amount of unconverted substrate that can be used for a new cycle of conversion in the reactor.
After the statistical analysis of the experimental data, the desirability tool of Statistica software was used to predict, through the interaction of all mathematical models generated, the best reaction condition to obtain the highest values of Y and S and a moderate value of Xc. The higher values of Xc require higher reaction temperatures. However, as discussed earlier, an increase in temperature promoted a decrease in the values of S and Y. The desirability tool (Supporting Information Figure 1) indicated that the best condition was 152 °C, 120 min, and a CXR of 10%. The predicted values for the responses Xc, Y, and S were 53, 33, and 75%, respectively. The predicted condition had higher values of Xc and Y compared to the experimental condition of 140 °C, 120 min, and a CXR of 10% (Table 1). For response S, no significant difference was observed. The parameter S is an important for the process since a lower value of S is associated with more complex and expensive separation units for 2-furfuraldehyde. Therefore, the experimental condition of 140 °C, 120 min, and a CXR of 10% was chosen as a better condition since the temperature is 12 °C lower and, consequently, the energy viability is higher. In addition, the experimental condition of 140 °C, 120 min, and a CXR of 10% was chosen to study the effect of the reaction time on Xc, S, and Y.
As shown in Table 1, there is a significant improvement in the yield when a biphasic system is used instead of a monophasic system. This is explained by the occurrence of secondary reactions in water, which produce furan resins and condensation products from polymerization and degradation of 2-furfuraldehyde in the acidic reaction medium.24 Furthermore, when toluene is used as a cosolvent in the reaction medium due to its lower polarity and higher affinity for 2-furfuraldehyde compared to water, it is able to extract 2-furfuraldehyde from the aqueous medium, thus avoiding such undesired reactions.5 This leads to an improvement in the selective dehydration of xylose to 2-furfuraldehyde.2,3,6 However, it should be emphasized that toluene is an organic solvent with high toxicity, showing serious harmful effects on the environment and humans, which limits its industrial applications.24
Although using water as the only solvent in the reaction medium resulted in lower Y values than biphasic systems shown in Table 1, it is noticed that there is a significant improvement in the values of Y and S when dehydration was performed at higher temperatures and for longer times. This is an opposite behavior to that reported in the literature for reaction conditions employing high temperatures. For example, a niobium oxide-based catalyst (NbOc) had yield values of 43 and 53.1% of selectivity for 2-furfuraldehyde production in monophasic systems.
Different studies available in the literature reported on the use of heterogeneous catalysts for the dehydration reaction of xylose to 2-furfuraldehyde.9 These studies pointed out that Brønsted acid sites are responsible for protonation of xylose hydroxyl groups, promoting dehydration of xylose via a unimolecular elimination reaction24 On the other hand, Lewis acid sites are responsible for faster dehydration of xylose than Brønsted acid sites, mainly via isomerization of xylose to xylulose. However, xylulose is faster dehydrated by Brønsted acid sites via protonation than xylose, leading to the formation of 2-furfuraldehyde. Due to this synergism between Lewis and Brønsted sites, mesoporous catalysts containing both acid sites are of great interest for conversion of xylose into 2-furfuraldehyde.9 In this way, this is the key to the excellent performance exhibited by the NbP catalyst, added to the advantage of its use in a lower CXR value compared to other catalysts shown in Table 1.
Compared to other catalysts shown in Table 1, higher Xc values observed in the present study were obtained using reaction conditions employing higher temperatures (180 and 220 °C), which led to Xc values higher than 90%. However, the use of higher temperatures also led to lower values of Y and S compared to some catalysts shown in Table 1.
2.3. Effect of the Reaction Time on Conversion, Selectivity, and Yield of 2-Furfuraldehyde
After optimization of the reaction conditions for the dehydration of xylose to 2-furfuraldehyde using the 23 experimental design, a kinetic study was carried out using optimized reaction parameters (T of 140 °C and a CXR of 10%). The study of conversion versus time of xylose dehydration to 2-furfuraldehyde is shown in Figure 7.
Figure 7.
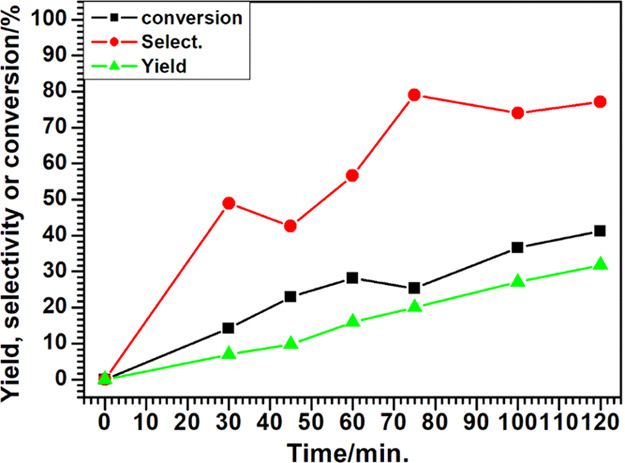
Conversion, selectivity, and yield versus time of xylose dehydration to 2-furfuraldehyde at 140 °C with a xylose-to-catalyst ratio of 10%.
In Figure 7, it is observed that xylose conversion increased with increasing reaction time until 120 min. At 120 min of reaction, 41.2% of xylose conversion was achieved. The tendency of xylose conversion selectivity is in agreement with the results obtained from the 23 experimental design (Table 1). The highest selectivity (79.1%) for the dehydration reaction of xylose to 2-furfuraldehyde was attained at 75 min of reaction. After this time, selectivity decreased from 79.1% (75 min) to 77.1% (120 min), despite the increase in xylose conversion. This indicates that some xylose molecules may have been converted into 2-furfuraldehyde and posteriorly the 2-furfuraldehyde molecules formed may have been converted into furan resins and/or other condensation products.9 The highest yield (31.8%) was observed at 120 min.
For comparison purposes, a commercial niobic acid was tested as a heterogeneous catalyst for dehydration of xylose to 2-furfuraldehyde. The commercial niobic acid achieved a Xc value of 15.5% with an S value to 2-furfuraldehyde of 10.8% at 140 °C and 120 min of reaction.
The turnover number (TON) calculated for the best reaction conditions (140 °C, 120 min, a CXR of 10%) considering a xylose conversion of 41.2% was 460, showing that mNb-bc prepared in the present study exhibited a great performance for dehydration of xylose to 2-furfuraldehyde.
3. Conclusions
In this study, a niobium-based catalyst (mNb-bc) was prepared by a simple method. The characterization of mNb-bc showed that it has high specific surface area and acidity. X-ray diffraction analysis showed that mNb-bc is an amorphous material. SEM and SEM-EDX analyses showed that mNb-bc has a rough surface, with high dispersion of niobium and oxygen in the material. In addition, mNb-bc was able to dehydrate xylose to 2-furfuraldehyde, achieving a xylose conversion of 41.2% and a selectivity of 77.1% in the kinetic study carried out under optimized reaction conditions obtained from the 23 experimental design (140 °C, 120 min, a CXR of 10%). At these reaction conditions, mNb-bc had a very high TON value (460). Since selective dehydration of xylose to 2-furfuraldehyde is very important for the synthesis of intermediates and precursors to various industrially added-value products, the results obtained here may contribute to the development of most stable, selective, and active catalysts under milder reaction conditions.
4. Material and Methods
4.1. Material
NbCl5 (99%) and chromatography-grade standards β-d-xylose (≥99%) and 2-furfuraldehyde (≥98.5%) were purchased from Sigma-Aldrich. Butanol (99%), hexanol (99%), and cetyltrimethylammonium bromide (CTAB, 99%) were purchased from Vetec (Brazil). NH4OH (28 wt % NH3 in water) was purchased from Qhemis (Brazil). Nitrocellulose membranes (0.22 μm) were purchased from Unifil (Brazil). Ultrapure water was used in all experiments (Gehaka, model MS2000). Commercial niobic acid was kindly provided by the Companhia Brasileira de Metalurgia e Mineração (Araxá, Minas Gerais, Brazil).
4.2. Synthesis of the Catalyst
mNb-bc was synthesized in a water–oil surfactant system. First, a mixture (%, v/v) consisting of ultrapure water (17%), butanol (22%), and hexanol (61%) was prepared in a 1 L beaker. This mixture was heated to 65 °C under constant magnetic stirring (200 rpm). Then, 41.2 mmol CTAB was added to the mixture. After CTAB was completely solubilized, 51.8 mmol NbCl5 was added to the mixture. Then, 100 mL of ultrapure water was added, leading to the formation of two phases. Next, the mixture was neutralized with NH4OH until a pH of 7.5 was reached. Ultrapure water was added to the mixture to complete the volume to 1 L. Finally, the mixture was kept at 65 °C under constant magnetic stirring (200 rpm) for 48 h. Then, solid and liquid phases were separated by a vacuum filtration in a Büchner funnel and the solid (catalyst) was washed with excess of ultrapure water and then dried in an oven at 60 °C for 12 h.
4.3. Characterization of the Catalyst
Adsorption and desorption of N2 over mNb-bc were measured at 77 K using a pore and surface area analyzer (Quantachrome, model Autosorb iQ2). The catalyst surface area was determined by the Brunauer–Emmett–Teller25 (BET) method, and the pore size distribution was determined by the Barrett–Joyner–Halenda26 (BJH) method.
The identification of the acidic sites of mNb-bc was performed by adsorption of pyridine followed by spectroscopy in the infrared region. Approximately 10 mg of sample was treated at 100 °C for 2 h under N2 flow (g) at 80–100 mL min–1. Then, the adsorption of pyridine was performed at a temperature of 55 °C for 1 h. Then, the removal of the physissor-living pyridine was carried out by heating the catalysts at 100 °C for 1 h. The samples were prepared in the form of KBr tablets and analyzed using infrared spectroscopy in the 1800–1400 cm–1 region, with a resolution of 4 cm–1 and 16 scans. This procedure was carried out with the catalyst sample and with a commercial Nb2O5 standard provided by CBMM. With the acidity provided by CBMM, the sum of the areas obtained by IR was used to determine the amount of acidic sites by comparing the commercial standard of Nb2O5 and the catalysts used in this work.
The surface of the mNb-bc was examined using a field-effect gun (FEG) scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer (EDX) (ThermoFisher Scientific Quanta, model 200 FEG-FEI). The microscope was operated at a voltage of 30 keV, with a resolution of 1.6 nm. The structure of the mNb-bc was analyzed by X-ray diffraction (Siemens, model D5000). The diffractogram was recorded at 2θ (Bragg’s angle) from 20 to 70°, with a scan rate of 2.0° min–1, using Kα Cu radiation (λ = 1.542 Å).
4.4. Catalytic Tests
The dehydration reaction of xylose to 2-furfuraldehyde catalyzed by mNb-bc was investigated using a 316L stainless steel tubular reactor of 15 mL. Figure 8 shows the reaction setup used.
Figure 8.
Diagram of the reaction setup used for catalytic tests.
In a typical run, the reactor was loaded with an amount of xylose, 10 mg of mNb-bc, and 12 mL of ultrapure water. The amount of xylose loaded into the reactor depended on the CXR evaluated. Then, the reactor was placed into a thermostatic glycerol bath (Marconi, model MA159) and heated to a defined reaction temperature and for a defined time. At the end of the reaction time, the reaction was immediately stopped by quenching the reactor in an ice-water bath. Then, mNb-bc was recovered by centrifugation using a centrifuge (FANEM, model excels II) at 250 rpm for 15 min and the supernatant was collected, filtered through nitrocellulose membranes (0.22 μm), and stored at −20 °C for characterization by high-performance liquid chromatography (HPLC). The turnover number (TON) was calculated for the highest value of xylose conversion. For calculation of TON (eq 1), it was considered as the number of moles of acid sites (L + B) determined by infrared spectroscopy using a pyridine probe molecule.
| 1 |
where nxylose converted and nacid sites (L+B) are the number of moles of xylose converted and the number of moles of acid sites (Lewis (L) + Brønsted (B)).
4.5. Design of Experiments
The dehydration reaction of xylose to 2-furfuraldehyde was evaluated using a 23 experimental design. The independent variables (reaction parameters) evaluated were temperature (T, °C), time (t, min), and CXR (%, w/w). The levels of the independent variables studied were based on the previous studies reported in the literature, as shown in Table 1. The matrix of experiments is presented in Table 1. The dependent variables (responses) evaluated were xylose conversion (Xc, %), yield (Y, %), and selectivity (S, %).
The results were analyzed with Statistica software (StatSoft Inc., version 10) with a 95% confidence level using pure error. The second-degree polynomial equation used to model the responses is shown eq 2.
| 2 |
where DV is the dependent variable evaluated (Xc, Y, or S) and b0, b1, b2,..., b9 are the regression coefficients of the model.
4.6. Analytical Methods
The quantification of xylose and 2-furfuraldehyde in liquid phase after the reaction was performed by HPLC (Agilent, model 1260 infinity II). The chromatography system was equipped with an Aminex HPX-87H column (300 × 7.8 mm2, Bio-Rad) maintained at 55 °C by an oven with forced air circulation, a refractive index detector for detection of xylose, and a ultraviolet–visible (UV–vis) detector set at 274 nm for detection of 2-furfuraldehyde. An aqueous sulfuric acid (5 mmol L–1) solution was used as the eluent at a constant flow rate of 0.6 mL min–1.
Xylose conversion (Xc) was calculated using eq 3
| 3 |
where nxylose,0 and nxylose,t are the number of moles of xylose initially and at time t.
The selectivity (S) of mNb-bc for 2-furfuraldehyde production was calculated using eq 4
| 4 |
where nfufural,0 is the number of moles of 2-furfuraldehyde at time t.
The reaction yield (Y) for 2-furfuraldehyde production was calculated using eq 5
| 5 |
Acknowledgments
The authors are grateful to the Federal University of Ouro Preto (UFOP grant number 23109.003209/2016-98), the Brazilian National Council for Scientific and Technological Development (CNPq grant number 443426/2014-7), the Minas Gerais Research Foundation (FAPEMIG grant number APQ-00847-14; APQ-00369-14), and the National Coordination for Higher Education Staff Improvement (CAPES, Finance Code 001) for funding this research.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01547.
Regression coefficients for the responses evaluated in the 23 experimental design, ANOVA results for the response yield (%) evaluated in the 23 experimental design, ANOVA results for response conversion (%) evaluated in the experimental design, ANOVA results for response selectivity (%) evaluated in the 23 experimental design, and predicted values by the desirability tool for all responses studied (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bozell J. J.; Petersen G. R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. 10.1039/b922014c. [DOI] [Google Scholar]
- Eseyin A. E.; Philip H. S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. 10.14419/ijac.v3i2.5048. [DOI] [Google Scholar]
- Li X.; Jia P.; Wang T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. 10.1021/acscatal.6b01838. [DOI] [Google Scholar]
- Luo Y.; Li Z.; Li X.; Liu X.; Fan J.; Clark J. H.; Hu C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. 10.1016/j.cattod.2018.06.042. [DOI] [Google Scholar]
- Machado G.; Leon S.; Santos F.; Lourega R.; Dullius J.; Mollmann M. E.; Eichler P. Literature review on furfural production from lignocellulosic biomass. Nat. Resour. 2016, 7, 115–129. 10.4236/nr.2016.73012. [DOI] [Google Scholar]
- Dashtban M.; Gilbert A.; Fatehi P. Production of furfural: Overview and challenges. J. Sci. Technol. Forest Prod. Process. 2012, 2, 44–53. [Google Scholar]
- Crõnert H.; Loeper D.; Wyss E. Production en continu de furfural. Inf. Chim. 1972, 106, 171–179. [Google Scholar]
- Hu S.; Guan Y.; Cai D.; Li S.; Qin P.; Karim M. N.; Tan T. A novel method for furfural recovery via gas stripping assisted vapor permeation by a polydimethylsiloxane membrane. Sci. Rep. 2015, 5, 9428 10.1038/srep09428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariscal R.; Maireles-Torres P.; Ojeda M.; Sádaba I.; López Granados M. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. 10.1039/C5EE02666K. [DOI] [Google Scholar]
- Agirrezabal-Telleria I.; Gandarias I.; Arias P. L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. 10.1016/j.cattod.2013.11.027. [DOI] [Google Scholar]
- Bhanja P.; Bhaumik A. Porous nanomaterials as green catalyst for the conversion of biomass to bioenergy. Fuel 2016, 185, 432–441. 10.1016/j.fuel.2016.08.004. [DOI] [Google Scholar]
- Demesa A. G.; Laari A.; Tirronen E.; Turunen I. Comparison of solvents for the recovery of low-molecular carboxylic acids and furfural from aqueous solutions. Chem. Eng. Res. Des. 2015, 93, 531–540. 10.1016/j.cherd.2014.04.033. [DOI] [Google Scholar]
- Yemiş O.; Mazza G. Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction. Bioresour. Technol. 2011, 102, 7371–7378. 10.1016/j.biortech.2011.04.050. [DOI] [PubMed] [Google Scholar]
- Karinen R.; Vilonen K.; Niemelä M. Biorefining: Heterogeneously Catalyzed Reactions of Carbohydrates for the Production of Furfural and Hydroxymethylfurfural. ChemSusChem 2011, 4, 1002–1016. 10.1002/cssc.201000375. [DOI] [PubMed] [Google Scholar]
- Lopes O. F.; Mendonça V. R.; Silva F. B. F.; Paris E. C.; Ribeiro C. Niobium oxides: an overview of the synthesis of nb2o5 and its application in heterogeneous photocatalysis. Quím. Nova 2015, 38, 106–117. 10.5935/0100-4042.20140280. [DOI] [Google Scholar]
- Sing K. S. W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. 10.1351/pac198557040603. [DOI] [Google Scholar]
- Anunziata O. A.; Eimer G. A.; Pierella L. B. Catalytic conversion of natural gas with added ethane and LPG over Zn-ZSM-11. Appl. Catal., A 2000, 190, 169–176. 10.1016/S0926-860X(99)00297-5. [DOI] [Google Scholar]
- Lima A. L. D.; Batalha D. C.; Fajardo H. V.; Rodrigues J. L.; Pereira M. C.; Silva A. C. Room temperature selective conversion of aniline to azoxybenzene over an amorphous niobium oxyhydroxide supported on δ-FeOOH. Catal. Today 2020, 344, 118–123. 10.1016/j.cattod.2018.10.035. [DOI] [Google Scholar]
- Dias A. S.; Lima S.; Pillinger M.; Valente A. A.. Furfural and furfural-based industrial chemicals. In Ideas in Chemistry and Molecular Sciences: Advances in Synthetic Chemistry; Pignataro B., Ed.; Wiley-VCH: Weinheim, 2010; pp 165–186. [Google Scholar]
- Hua D.-R.; Wu Y.-L.; Liu Y.-F.; Chen Y.; Yang M.-D.; Lu X.-N.; Li J. Preparation of furfural and reaction kinetics of xylose dehydration to furfural in high-temperature water. Pet. Sci. 2016, 13, 167–172. 10.1007/s12182-015-0069-y. [DOI] [Google Scholar]
- Jing Q.; LÜ X. Kinetics of non-catalyzed decomposition of D-xylose in high temperature liquid water. Chin. J. Chem. Eng. 2007, 15, 666–669. 10.1016/S1004-9541(07)60143-8. [DOI] [Google Scholar]
- a Li J.; Lu S.; Liu G.; Zhou Y.; Lv Y.; She J.; Fan R. Co-exposure to polycyclic aromatic hydrocarbons, benzene and toluene and their dose–effects on oxidative stress damage in kindergarten-aged children in Guangzhou, China. Sci. Total Environ. 2015, 524–525, 74–80. 10.1016/j.scitotenv.2015.04.020. [DOI] [PubMed] [Google Scholar]; b Win-Shwe T.-T.; Fujimaki H. Neurotoxicity of toluene. Toxicol. Lett. 2010, 198, 93–99. 10.1016/j.toxlet.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Dias A. S.; Pillinger M.; Valente A. A. Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. J. Catal. 2005, 229, 414–423. 10.1016/j.jcat.2004.11.016. [DOI] [Google Scholar]
- Liu Y.; Ma C.; Huang C.; Fu Y.; Chang J. Efficient Conversion of Xylose into Furfural Using Sulfonic Acid-Functionalized Metal–Organic Frameworks in a Biphasic System. Ind. Eng. Chem. Res. 2018, 57, 16628–16634. 10.1021/acs.iecr.8b04070. [DOI] [Google Scholar]
- Brunauer S.; Emmett P. H.; Teller E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. 10.1021/ja01269a023. [DOI] [Google Scholar]
- Barrett E. P.; Joyner L. G.; Halenda P. P. The determination of pore volume and area distributions in porous substances. I. Computations from Nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. 10.1021/ja01145a126. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.