Abstract
Postexercise protein ingestion can elevate rates of myofibrillar protein synthesis (MyoPS), mTORC1 activity, and mTOR translocation/protein-protein interactions. However, it is unclear if leucine-enriched essential amino acids (LEAA) can similarly facilitate intracellular mTOR trafficking in humans after exercise. The purpose of this study was to determine the effect of postexercise LEAA (4 g total EAAs, 1.6 g leucine) on acute MyoPS and mTORC1 translocation and signaling. Recreationally active men performed lower-body resistance exercise (5 × 8–10 leg press and leg extension) to volitional failure. Following exercise participants consumed LEAA (n = 8) or an isocaloric carbohydrate drink (PLA; n = 10). MyoPS was measured over 1.5–4 h of recovery by oral pulse of l-[ring-2H5]-phenylalanine. Phosphorylation of proteins in the mTORC1 pathway were analyzed via immunoblotting and mTORC1-LAMP2/WGA/Rheb colocalization via immunofluorescence microscopy. There was no difference in MyoPS between groups (LEAA = 0.098 ± 0.01%/h; PL = 0.090 ± 0.01%/h; P > 0.05). Exercise increased (P < 0.05) rpS6Ser240/244(LEAA = 35.3-fold; PLA = 20.6-fold), mTORSer2448(LEAA = 1.8-fold; PLA = 1.2-fold) and 4EBP1Thr37/46(LEAA = 1.5-fold; PLA = 1.4-fold) phosphorylation irrespective of nutrition (P > 0.05). LAT1 and SNAT2 protein expression were not affected by exercise or nutrient ingestion. mTOR-LAMP2 colocalization was greater in LEAA preexercise and decreased following exercise and supplement ingestion (P < 0.05), yet was unchanged in PLA. mTOR-WGA (cell periphery marker) and mTOR-Rheb colocalization was greater in LEAA compared with PLA irrespective of time-point (P < 0.05). In conclusion, the postexercise consumption of 4 g of LEAA maintains mTOR in peripheral regions of muscle fibers, in closer proximity to its direct activator Rheb, during prolonged recovery independent of differences in MyoPS or mTORC1 signaling compared with PLA ingestion. This intracellular localization of mTOR may serve to “prime” the kinase for future anabolic stimuli.
NEW & NOTEWORTHY This is the first study to investigate whether postexercise leucine-enriched amino acid (LEAA) ingestion elevates mTORC1 translocation and protein-protein interactions in human skeletal muscle. Here, we observed that although LEAA ingestion did not further elevate postexercise MyoPS or mTORC1 signaling compared with placebo, mTORC1 peripheral location and interaction with Rheb were maintained. This may serve to “prime” mTORC1 for subsequent anabolic stimuli.
Keywords: anabolic signaling, essential amino acids, leucine, mTORC1, muscle protein synthesis
INTRODUCTION
Muscle protein synthesis is the main cellular process influencing net protein balance in healthy humans (10, 38) and is predominantly altered by two “anabolic” stimuli, mechanical loading/resistance exercise and amino acid ingestion (11). Both amino acid ingestion and resistance exercise can acutely stimulate muscle protein synthesis (especially of the contractile myofibrillar fraction; MyoPS) independently with their combination acting synergistically to further enhance MyoPS (10, 37). The essential amino acid (EAA) leucine is purported to be the most potent activator of protein synthesis (13, 14) as its ingestion alone can elevate MyoPS at rest (58) whereas its removal from a protein supplement significantly attenuates MyoPS (13, 14). Based on previous dose-response studies (15, 59), as little as ~1 g of leucine can enhance postexercise mixed muscle protein synthesis and MyoPS above placebo with an equivalent of ~2–3 g maximizing the synthetic response. Interestingly, however, the duration of MyoPS is sustained after exercise to a greater degree when other EAAs are ingested alongside leucine (14), likely due to the needs for these EAAs as substrates for protein translation. For example, while postexercise rates of MyoPS generally peak 1–3 h after exercise with amino acid ingestion, these rates may be sustained for up to 5 h with the ingestion of a full complement of EAAs (13, 14). Consuming an adequate quantity of leucine and the associated EAA substrates is required to maximize postexercise MyoPS (13, 52) and potentially sustain rates during the latter acute recovery period. Thus it is plausible that enriching smaller doses of EAAs with high amounts of leucine can be as effective as whole protein sources.
At the molecular level, muscle protein synthesis is believed to be primarily regulated by the mechanistic target of rapamycin complex 1 (mTORC1) (17, 18), an evolutionarily conserved serine/threonine kinase complex whose downstream targets are implicated in the control of translation initiation and elongation (26). Both mechanical loading and amino acids (AAs) activate this kinase complex through differing mechanisms. For example, AAs increase mTORC1’s recruitment to the lysosome (44, 45) and initiate mTORC1 translocation toward the cell periphery (25, 30) whereas mechanical loading activates Ras homolog enriched in brain (Rheb) (28), a direct mTORC1 activator while also initiating mTORC1 translocation toward the cell periphery (48). Importantly, in vitro investigations in HeLa cells have identified a leucine-specific mechanism of mTORC1 activation whereby its binding with Sestrin proteins leads to the activation of Rag proteins at the lysosomal membrane and subsequent recruitment of mTORC1 to these areas (12, 60). These findings have since been replicated in skeletal muscle both in vitro (5) and in vivo (4, 33) observing mTORC1 activation following leucine administration/ingestion alone. Furthermore, the removal of leucine from an EAA beverage attenuates S6K1Thr389 phosphorylation (common readout of mTORC1 activity) whereas the coingestion of complementary EAAs with leucine further elevates mTORC1 activation compared with leucine alone (4, 33, 34). Collectively, these data suggest that leucine is a major activator of mTORC1 and subsequently MyoPS but other EAAs are also required to maximize these responses. However, it is yet to be established if leucine-enriched amino acid (LEAA) supplements affect the intracellular mechanisms by which EAAs, and in particular leucine, are believed to have their effects, namely elevated mTORC1-lysosome colocalization and translocation toward peripheral regions where Rheb resides (44, 48).
The influx of EAAs, and in particular leucine, into skeletal muscle cells is primarily coordinated by L-type amino acid transporter 1 (LAT1) and sodium-coupled neutral amino acid transporter 2 (SNAT2) (21, 31, 39), the latter of which coordinates the transport of glutamine into the cell via sodium-coupled transport to be used as an antiport substrate for the uptake of leucine by the former (6). The effects of anabolic stimuli on the content of these two amino acid transporters (AATRs) have been investigated but with equivocal findings as some report elevations (3, 7, 16, 19, 20) while others suggest no change or decreases (3, 19, 40, 41, 53) in the protein content of LAT1 and/or SNAT2 after EAA ingestion and/or exercise. As these AATRs are suggested to also act as nutrient sensors (51), it is important to establish if enriching a small AA supplement with high amounts of leucine can affect the content of either transporter.
The primary objective of this study was to investigate the effects of a small LEAA dose (1.6 g leucine, 4 g total EAAs) or isocaloric carbohydrate placebo beverage on acute (≤4 h) MyoPS following an unaccustomed bout of resistance exercise in young men. Our secondary objectives were to then determine if the MyoPS response to exercise and supplementation were underpinned by alterations in intracellular signaling, AA transporter content and/or mTOR localization with LAMP2, Rheb, and the cell periphery. We measured MyoPS from 1.5 to 4 h of recovery as it has been reported that consuming a complete protein and/or a complement of EAA sustains postexercise MyoPS up to 5 h when fasted exercised rates may begin to wane (13, 14). Furthermore, we utilized a novel oral pulse tracer method to circumvent the need for intravenous administration (49, 62) and were cognizant of the need to maintain detectable isotopic enrichment over the duration of the measurement period, which otherwise subsides over the traditional 60–90 min bolus tracer administration (46, 47). We hypothesized that LEAA ingestion would enhance MyoPS and mTORC1 activity and elevate mTORC1’s association with Rheb and the lysosome in peripheral regions of muscle fibers.
MATERIALS AND METHODS
Participants and ethical approval.
The procedures of this study were in conformity with the Declaration of Helsinki and were reviewed and approved by the Research Ethics Board of the University of Toronto (REB# 00034365) and the Institutional Review Board of Ajinomoto Co., Inc (No. 2016–036), and registered at https://clinicaltrials.gov (NCT03319147). Prior to participation, all subjects were given verbal and written explanation of the study objectives, familiarized with experimental procedures to be used, any potential risks, and provided written informed consent.
Eighteen healthy recreationally active young men [LEAA: age 24.5 ± 4.21 yr, body mass – 81.6 ± 9.8 kg, body fat% - 19.8 ± 7.7%, leg press 1 repetition maximum (1RM) 262.35 ± 41.3 kg, leg extension 1RM 115.95 ± 20.18 kg; placebo (PLA): age 24 ± 1 yr, body mass 80.8 ± 2.2 kg, body fat% 20 ± 1.6%, leg press 1RM 257.55 ± 48.32 kg, leg extension 1RM 106.41 ± 26.38 kg; means ± SEM] who had no history of participating in past stable isotope amino acid tracer experiments participated in this study. Participants were a subset of those included in a recent investigation from our laboratory (54). All subjects were considered healthy and physically active based on responses to the Physical Activity Readiness Questionnaire for Everyone (PARQ+) and International Physical Activity Questionnaire (iPAQ). Participants also had not completed any lower-body resistance training for the previous 6 mo, were nonsmokers, and had no previous usage of performance enhancing drugs/steroids.
Preliminary assessments and exercise familiarization.
Prior to the pretrial assessment sessions, participants completed a 3-day diet and physical activity log to assess habitual energy intake and physical activity levels. Participants attended the laboratory in the fasted state (~10 h) and underwent body composition analysis via air displacement plethysmography (BodPod, Cosmed) to determine body mass, fat mass (FM), and fat-free mass (FFM). Following this, resting energy expenditure (REE) was determined via continuous, open-circuit indirect calorimetry (GA-300, iWorx), with participants in a seated position, using the abbreviated Weir equation (55). The average of two 10-min measurements was taken to enhance accuracy of our measurements. Participants then completed 1 repetition maximum (1RM) strength testing for 45° leg press (Hammer Strength, Life Fitness, Rosemont, IL) and leg extension (PRECOR, Woodinville, WA) exercises. The 1RM testing protocol consisted of a warm-up set at a comfortable load before load was progressively increased until a single repetition could no longer be performed. Each 1RM attempt was separated by 3 min. Approximately 1 wk later, participants completed an exercise familiarization session consisting of 5 bilateral sets of leg press and leg extension at 70%1RM, to volitional failure, with 90 s rest-interval between sets.
Experimental protocol.
In a double-blind manner, subjects were randomized into one of two groups: to ingest an amino acid drink consisting of 4 g of essential amino acids (EAAs) (1.6 g of which was leucine) immediately following exercise (LEAA, n = 8, 24 ± 1 yr, 81.6 ± 3.5 kg, 19.8 ± 2.7%BF) or an isocaloric carbohydrate placebo (PLA, n = 10, 24 ± 2 yr, 80.2 ± 2.9 kg, 20.2 ± 1.9%BF). The present study represents a subset of participants from a larger study that investigated free-living MyoPS at rest and up to 96 h after exercise with muscle strength and performance measures (see Table 1 for drink compositions).
Table 1.
LEAA and PLA drink composition
| Ingredients | LEAA, mg | Placebo, mg |
|---|---|---|
| Histidine/H2O/HCl | 68.2 | |
| Isoleucine | 425 | |
| Leucine | 1,600 | |
| Lysine HCl | 668 | |
| Methionine | 131 | |
| Phenylalanine | 269 | |
| Threonine | 371 | |
| Tryptophan | 28.1 | |
| Valine | 440 | |
| Tang | 20,000 | 20,000 |
| Splenda | 5,000 | 5,000 |
| PFD | 2,000 | 2,000 |
| Polycal | 4,000 |
Tang: artificially flavored orange carbohydrate mix (Kraft Foods, Chicago, IL). Splenda: sucralose-based artificial sweetener (Heartland Food Products Group. Carmel, IN). PFD: protein- and amino acid free powder (Mead Johnson Nutrition). Polycal: high-energy, unflavored carbohydrate powder (SHS International, Liverpool, UK).
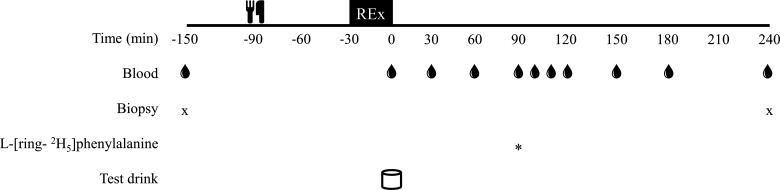
Four weeks following the exercise familiarization session, participants arrived at the laboratory at 0800, following an overnight fast (~10 h). The day before the experimental trial, participants consumed a control diet consisting of 1.2 g·kg−1·day−1 protein, 4 g·kg−1·day−1 carbohydrate with the remaining calories to achieve an energy intake of 1.6 × REE coming from fat. Upon arrival, participants were placed in the supine position and a 21G venous catheter (BD, Mississauga, ON, Canada) was inserted into the antecubital vein for repeated blood sampling. Following a baseline blood sample, a skeletal muscle biopsy sample was obtained from the vastus lateralis of the participant’s nondominant leg, under local anesthesia, using a Bergstrom needle (50) modified for suction. Participants then ingested a standardized breakfast consisting of ~0.3 g/kg protein and 25% daily caloric intake 90 min before initiating the acute exercise bout, which was identical to that completed during exercise familiarization. This breakfast was provided to increase the ecological validity of a larger exercise recovery study and is in line with the recent approach of other laboratories in the field (27, 59). Participants ingested their assigned supplement immediately following completion of the exercise session. To avoid excessive tracer dilution from the LEAA beverage and to capture the presumed more prolonged exercise and nutrient interaction on MyoPS (13, 37), participants consumed 200 mg of l-[ring-2H5]phenylalanine 90 min after the test beverage ingestion to simulate a pulse-dose isotope infusion protocol (49, 62) to measure MyoPS. A second skeletal muscle biopsy sample was obtained from a separate incision of the vastus lateralis 240 min after the test beverage (150 min after tracer ingestion). As the present study represented a subset of a larger study we were limited by the number and timing of biopsies we were able to obtain in the present study. Therefore, a 4-h biopsy was selected to represent a balance between maintaining adequate plasma enrichment (47, 62) while also capturing the 1.5–4 h recovery period that would presumably differentiate postexercise MyoPS between adequate and inadequate EAA ingestion (13, 49). Venous blood samples were obtained at 0, 30, 60, 90, 100, 110, 120, 150, 180, and 240 min after test drink ingestion. All blood samples were collected into an EDTA-coated vacutainer (BD, Mississauga, ON, Canada) and placed on ice before processing. Plasma was isolated via centrifugation at 1,258 g for 10 min at 4°C. Plasma was then placed in 3 separate aliquots and frozen at −80°C until analysis. Skeletal muscle biopsy samples were blotted free of blood and excess fat/connective tissue removed before immediate freezing in liquid nitrogen and stored at −80°C until further analysis. A separate piece of the skeletal muscle samples was placed in optimal cutting temperature (OCT) compound (VWR International, Mississauga, ON, Canada) and frozen in liquid nitrogen-cooled isopentane before storage at −80°C for immunofluorescence analysis. A detailed schematic of the experimental protocol is displayed in Fig. 1.
Fig. 1.
Experimental protocol schematic. Rex, resistance exercise.
Plasma analyses.
For plasma l-[ring-2H5]phenylalanine enrichments, free amino acids were isolated from 50 µl plasma using 500 µl acetonitrile and centrifuged for 10 min at 3,220 g. The supernatant was transferred and dried under nitrogen and the amino acids were converted to their heptoflurobutryic derivative before GC-MS analysis. Plasma phenylalanine enrichments were determined by selective ion monitoring at m/z 148 and m/z 153 for unlabeled and labeled (m+5) phenylalanine, respectively. For plasma amino acid concentrations, 150 µl plasma was added to 300 µl of 10% trichloroacetic acid (TCA). The solution was briefly vortexed, then centrifuged for 20 min at 4°C and 9,100 g. Three-hundred microliters of the supernatant was filtrated with Amicon Ultra Centrifugal Filters 0.5 mL, 10K (14,000 G, for 30 min at 4°C). Amino acid concentrations were then determined using an automated amino acid analyzer (L8900; Hitachi High-Tech Science Corporation, Tokyo, Japan) as previously described (29).
Muscle analyses.
For myofibrillar protein extraction, ~30 mg muscle tissue was homogenized in RadioImmunoprecipitation assay (65 mM Tris-base, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) buffer supplemented with protease and phosphatase inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany) using a teflon pestle. Samples were then centrifuged at 700 g for 5 min at 4°C to separate soluble and insoluble proteins. The supernatant (soluble, cytosolic fraction) was placed in a separate centrifuge and stored at −80°C until immunoblotting analysis. The pellet (insoluble fraction) was then rinsed in ddH2O (recentrifuged and supernatant combined with previous for immunoblotting analysis) before 0.3 M sodium hydroxide (NaOH) was added. This solution was then vortexed and incubated at 50°C for 30 min to solubilize the myofibrillar fraction (vortexed at 10 min intervals). Samples were then centrifuged and the supernatant (myofibrillar fraction) transferred to glass test-tubes. Perchloric acid (1 M) (Sigma-Aldrich, Poole, UK) was then added and samples centrifuged at 2,500 g for 15 min to precipitate myofibrillar proteins. The supernatant was then removed and the pelleted proteins were rinsed in 70% ethanol. Following a final centrifugation, the supernatant was removed and 1 M HCl/DOWEX slurry (Sigma-Aldrich) added for hydrolysis. Myofibrillar proteins were subsequently hydrolyzed at 110°C for 48 h (vortexed at 24 h).
Following hydrolysis, constituent myofibrillar amino acids were purified on cation-exchange columns (Dowex50W-X8–200; Sigma-Aldrich) constructed in 5 mL syringes. Purified amino acids were dried under nitrogen, reconstituted in 600 µL 0.1 M HCl and stored in four equal aliquots of 150 µL at −80°C until mass spectrometry analysis.
Samples were diluted 10x in mobile phase B (10 mM ammonium formate pH 3.2 in 10/90 water/acetonitrile) and analyzed by LC-MS/MS using an Agilent 1290 series HPLC stack coupled to a SCIEX 5500QTRAP mass spectrometer. From each sample 10 µL was injected onto a Phenomenex Kinetex HILIC (50 × 4.6 mm, 2.65 µm) column with mobile phases A: 5 mM ammonium formate pH 3.2 in 90/10 water/acetonitrile and B: 10 mM ammonium formate pH 3.2 in 10/90 water/acetonitrile. Data were analyzed using Analyst software from SCIEX, version 1.6.2.
Calculations.
The oral tracer L-[ring-2H5]phenylalanine was used to determine the FSR of myofibrillar proteins 4 h post exercise using the following equation:
where EB is the bound enrichment (EBt2 = 4 h post muscle bound enrichment; EBt1 = preexercise muscle bound enrichment) and Ep is the precursor enrichment obtained from plasma l-[ring-2H5]phenylalanine enrichments [Smith et al. (46)]. The change in bound l-[ring-2H5]phenylalanine enrichment and the AUC (i.e., sum of linear AUC divided by sum of delta t) for average precursor enrichments was determined over the period of incorporation (t = 150 min), starting from the consumption of the oral tracer (t = 90 min) until the biopsy (t = 240 min).
Immunofluorescence.
Serial cross-sections (7 µm) were collected from muscle samples that were embedded in OCT compound onto room temperature uncoated glass slides (ThermoFisher SuperFrost+, Fisher Scientific, Rockford, IL). Sections were air-dried at room temperature for ~15 min to remove excess water within sections. Sections were then fixed in a solution of acetone-ethanol (3:1) and stained for mTOR colocalization with LAMP2, WGA, and Rheb as previously described (1, 48).
Image capture and analysis.
Image capture was conducted as described previously (1, 2). Using an EVOS FL Auto Cell imaging microscope (Fisher Scientific) at 40x 0.75NA magnification, slides were observed and at least 6 images were captured per section with each image including ~8 fibers on average. Samples from both time points within each participant were stained on the same slide with one participant from each supplementation cohort placed on each slide to ensure reliable comparisons. The average number of fibers analyzed per subject, per time point was ~122 (range: 46–206) for colocalization. Image capturing parameters such as exposure time, gain, image frame, and light intensity were consistent between images.
All image processing and quantitation was completed on ImagePro Premier v 9.2 (Media Cybernetics, Inc., Rockville, MD). Prior to image analysis, each image underwent deconvolution via Hi-Gaussian correction and despeckling. To quantify colocalization, pixel intensities, of all pixels in each image, from two channels were plotted against one another and the corresponding Pearson’s correlation coefficient used as a readout of colocalization. Theoretically, increases in colocalization between two timepoints would result in r values moving closer to 1. This was completed for the following stains: mTOR-LAMP2, mTOR-Rheb, and mTOR-WGA.
Immunoblotting.
Immunoblotting procedures were conducted as previously described (1). Briefly, the cytosolic fraction of muscle homogenates isolated during myofibrillar protein extraction was used for analysis. Protein concentrations of these fractions were determined using a commercially available bicinchoninic acid assay (BCA, Fisher Scientific) and working samples at equal concentrations were prepared for all samples in 1x Laemmli Sample Buffer and denatured at 95°C for 5 min. Equal amounts of protein were then separated by SDS-PAGE and transferred to nitrocellulose membranes (100 V for 60 min). Membranes were then blocked in 5% bovine serum albumin (BSA) in Tris-buffered saline supplemented with 0.1% Tween (TBST) and incubated in primary antibodies overnight at 4°C. Antibodies utilized were as follows: mTORSer2448 (#2971), eEF2Thr56 (#2331), rpS6Ser240/244 (#5364), ERK1/2Thr202/Tyr204 (#4377), 4EBP1Thr37/46 (#9459, diluted in 5% BSA in TBST), LAT1 [ab85226, Abcam (Toronto, Canada) diluted in 5% BSA], and SNAT2 (ab90677, Abcam, diluted in 5%BSA) and were all purchased from Cell Signaling Technology (Danvers, MA) and diluted at 1:1,000 in TSBT unless otherwise stated. Membranes were then washed and incubated in appropriate horseradish peroxidase-conjugated secondary antibody (1:1000 in TBST) for 60 min at room temperature. Bands were then visualized using chemiluminescent substrate (Millipore; cat. WBKLS0500) and imaged using a Fluorochem E Imaging system (Protein Simple; Alpha Innotech, Santa Clara, CA). Bands were quantified using Protein Simple AlphaView SA software and normalized to Ponceau S and a gel control (identical generic sample run on every gel) (43).
Statistical analysis.
Statistical analyses were analyzed in SPSS (Version 24, IBM, Armonk, NY), with significance set at P < 0.05. All data sets were tested for normality via the Shapiro-Wilks test, with subsequent statistical analysis conducted on logarithmically transformed data if this test was violated. An independent t-test was used to examine the effect of supplementation on MyoPS and the relative change in colocalization measures. Other variables were examined by two-way repeated-measures ANOVA, with treatment and time as within-subject factors. When a significant main or interaction effect was observed, post hoc tests with Bonferroni correction for multiple comparisons were conducted. Data are reported as means ± SEM, unless otherwise stated.
RESULTS
Plasma amino acid concentrations.
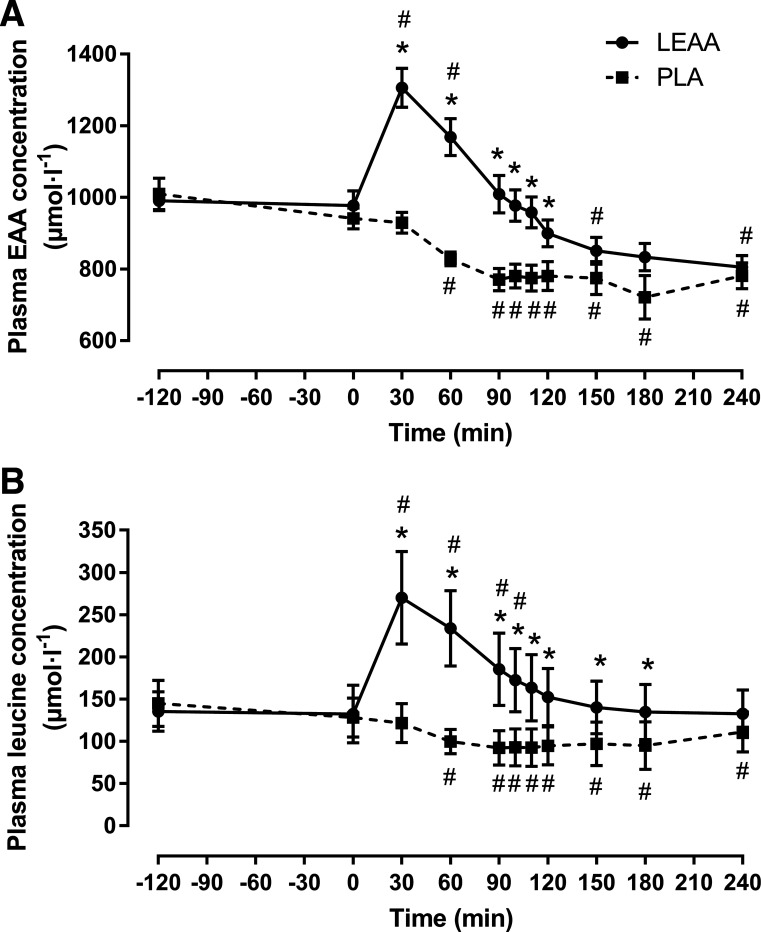
There were group × time interaction effects (P < 0.001) present for plasma EAA concentrations and leucine concentrations (Fig. 2, A and B). Fasted (t = −120) and pre-beverage (t = 0) plasma amino acid concentrations were not different between groups (P > 0.05). Plasma EAA and leucine concentrations increased in LEAA and peaked at ~1.3-fold and ~2-fold, respectively, above basal concentrations at ~30 min postingestion before reaching a nadir at 240 min after ingestion. In contrast, plasma EAA and leucine concentrations decreased over time in PLA. Plasma EAA concentrations were greater in LEAA compared with PLA (P < 0.01) up to 120 min postingestion. Plasma leucine concentrations were greater in LEAA compared with PLA (P < 0.001) up to 180 min postingestion.
Fig. 2.
Plasma essential amino acids (EAA) concentrations (A) and plasma leucine concentrations (B) before and after resistance exercise in response to leucine-enriched essential amino acid (LEAA) or placebo (PLA) consumption. Data were analyzed using a 2-way repeated-measures ANOVA (group × time) with post hoc tests completed with Bonferroni correction for multiple comparisons. *Significantly different from PLA at corresponding time point (P < 0.001). Values are means (± SEM). #Significantly different from baseline. N = 8 for LEAA; N = 10 for PLA.
Plasma l-[ring-2H5]phenylalanine enrichment and myofibrillar fractional synthetic rate.
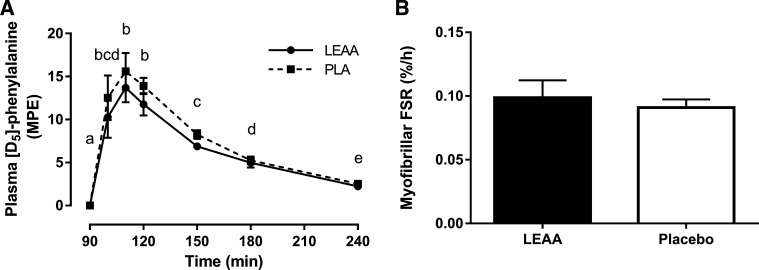
There was a main effect of time (P < 0.001) for l-[ring-2H5]phenylalanine enrichment (Fig. 3A). Irrespective of supplementation, plasma l-[ring-2H5]phenylalanine enrichment rapidly increased and peaked ~20 min after tracer ingestion (110 min after supplement ingestion) and decayed over time before returning close to basal values 150 min after tracer ingestion (240 min after supplement ingestion). Plasma l-[ring-2H5]phenylalanine enrichment was not different between supplementation cohorts at any time point (P > 0.05). MyoPS was only ~8% greater in LEAA than PLA and did not approach significance (P = 0.59, Fig. 3B).
Fig. 3.
Plasma l-[ring-2H5]phenylalanine enrichment (means ± SEM) (A) and myofibrillar fractional synthetic rate (FSR) (B) using oral l -[ring-2H5]phenylalanine tracer 1.5–4 h after resistance exercise in response to leucine-enriched essential amino acid (LEAA) or placebo (PLA) consumption. Data were analyzed using a 2-way repeated measures ANOVA (group × time) (A) and independent samples t-tests (B). MPE, mole percent excess. Means with different letters are significantly different from each other (main effect of time, P < 0.001). N = 8 for LEAA; N = 10 for PLA.
Muscle anabolic signaling and amino acid transporter expression.
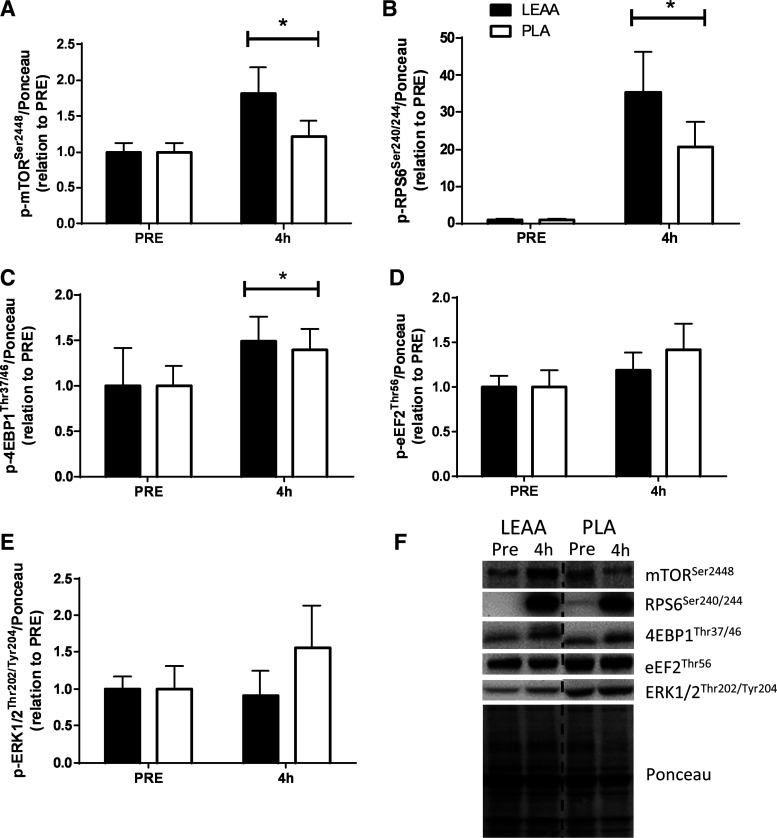
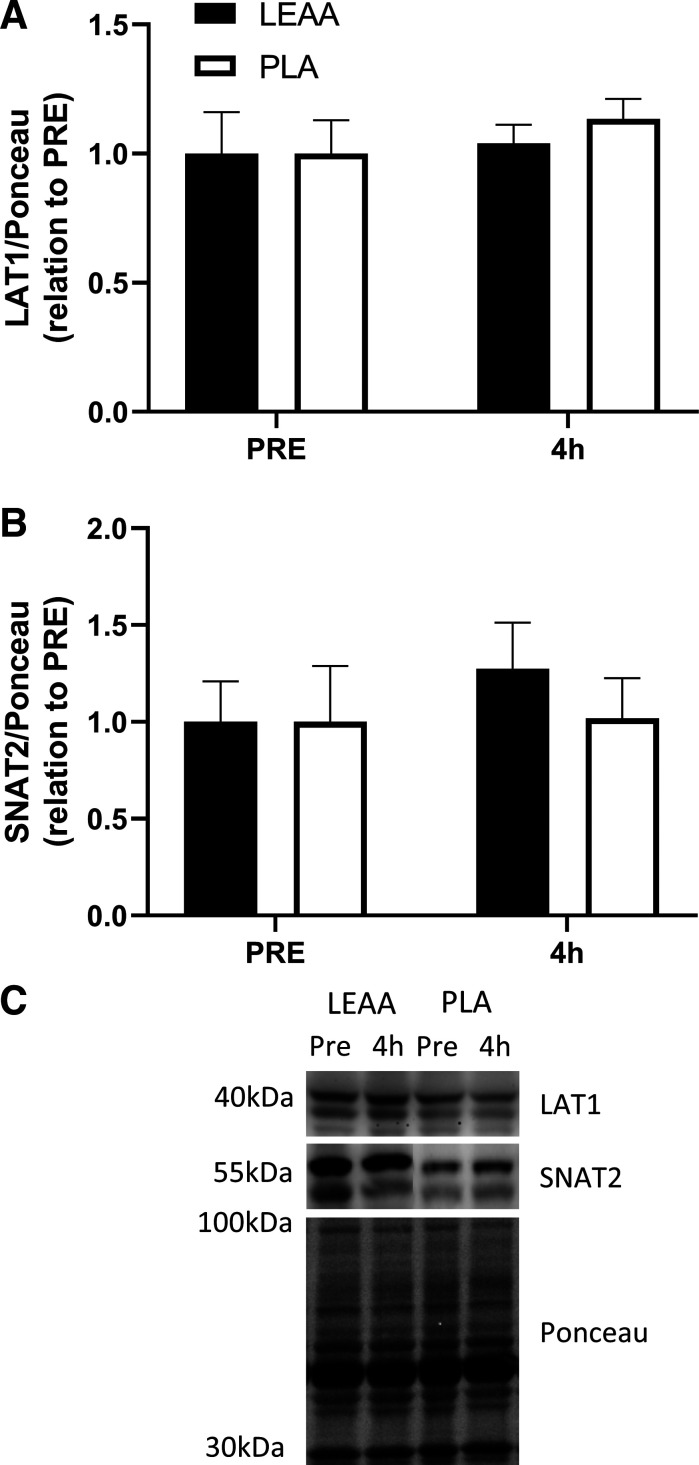
mTORSer2448, rpS6Ser240/244 and 4EBP1Thr37/46 phosphorylation increased 4 h after resistance exercise/feeding in relation to PRE irrespective of supplementation (P = 0.039, P = 0.001, and P = 0.041, respectively, Fig. 4, A, B, and C). However no differences between time points or supplements were observed for other targets measured (eEF2Thr56 and ERK1/2Thr202/Tyr204, both P > 0.05, Fig. 4, D and E). Furthermore, the protein expression of LAT1 and SNAT2 did not differ from pre- to postexercise (P = 0.252 and P = 0.612, respectively) or between supplementation groups (P = 0.572 and P = 0.511, respectively, Fig. 5, A and B).
Fig. 4.
Phosphorylation status of p-mTORSer2448/Ponceau (A), p-RPS6Ser240/244/Ponceau (B), p-4EBP1Thr37/46/Ponceau (C), p-eEF2Thr56/Ponceau (D), and p-ERK 1/2Thr202/Tyr204/Ponceau (E) before and 4 h post resistance exercise and consumption of leucine-enriched essential amino acids (LEAA) or placebo (PLA) in young men. All values are expressed in relation to Ponceau (loading control) and corresponding PRE values. *Main effect of time (P < 0.05). Values are means ± SE. Data were analyzed using 2-way repeated-measures ANOVA (group × time). F: representative blots for each anabolic marker of mTOR with corresponding time points. N = 8 for LEAA; N = 9 for PLA.
Fig. 5.
Protein content of LAT1/Ponceau (A) and SNAT2/Ponceau (B) before and 4 h post resistance exercise and consumption of leucine-enriched essential amino acids (LEAA) or placebo (PLA) in young men. All values are expressed in relation to Ponceau (loading control) and corresponding PRE values. Values are means ± SE. Data were analyzed using 2-way repeated-measures ANOVA (group × time). C: representative blots for each target. N = 8 for LEAA; N = 9 for PLA.
Immunofluorescence.
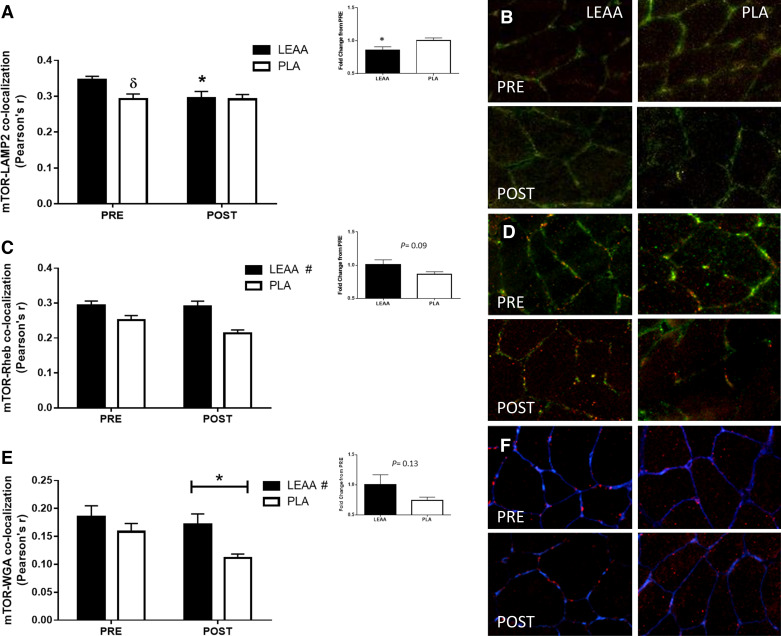
An interaction effect was observed for mTOR-LAMP2 colocalization (P = 0.026). Post hoc analysis revealed that at PRE, mTOR-LAMP2 colocalization was greater in LEAA compared with PLA (P = 0.008, Fig. 6A). Furthermore, following resistance exercise, mTOR-LAMP2 colocalization decreased in LEAA (P = 0.004), but remained unchanged in PLA (P = 0.982). When expressed in relation to basal levels, the reduction in mTOR-LAMP2 colocalization in LEAA was greater than PLA (P = 0.03, Fig. 6A, inset). mTOR colocalization with Rheb was unchanged from pre- to postexercise (P = 0.09) (Fig. 6C). However, a main effect of group (P = 0.001) was apparent where, with time points combined, mTOR-Rheb colocalization was greater in the LEAA cohort compared with PLA (Fig. 6C). When expressed relative to PRE, a trend toward a greater reduction in mTOR-Rheb in PLA, compared with LEAA, was observed (P = 0.09, Fig. 6C, inset). A group difference (P = 0.046) was also observed for mTOR colocalization with WGA (a marker of the cell periphery) where again colocalization in the LEAA cohort was greater. A time effect was also found here (P = 0.026) suggesting that, with groups combined, mTOR-WGA colocalization decreased postexercise (Fig. 6E). When expressed relative to PRE, changes in mTOR-WGA colocalization were not different between supplements (P = 0.13, Fig. 6E, inset).
Fig. 6.
The effect of leucine-enriched essential amino acids (LEAA) and placebo (PLA) on mTOR colocalization with LAMP2, Rheb, and WGA following a novel bout of resistance exercise. Quantification of mTOR-LAMP2 (A), mTOR-Rheb (C), and mTOR-WGA (E) colocalization is presented as Pearson’s correlation coefficient. Data are presented as means ± SE. Representative images displayed as a composite (merged) of mTOR (red) and LAMP2 (green) interaction (B), mTOR (red) and Rheb (green) interaction (D), and mTOR (red) and WGA (blue) stains (F) at rest (PRE) and 4 h following resistance exercise (POST). Yellow/orange regions represent mTOR-LAMP2 or mTOR-Rheb colocalization, in their respective images. Purple regions represent mTOR-WGA colocalization. Group data are quantified and reported; solid bars represent LEAA, and open bars represent PLA. δSignificantly different from LEAA at this time point, P = 0.008. #Main effect of group (P ≤ 0.028). *Significantly different from PRE or between groups for fold change (inset) (P < 0.05). N = 8 for LEAA; N = 9 for PLA.
DISCUSSION
LEAA resulted in a rapid (i.e., within ~30 min) but transient (i.e., ~120–180 min duration) ~2-fold increase in plasma leucine and EAA concentration that would be characteristic of the ingestion of crystalline AA and/or a rapidly digested protein isolate (e.g., whey) (14, 37, 57), which is a plasma AA profile that has been suggested to maximize postexercise MyoPS (56). While we observed a ~8% greater MyoPS with LEAA supplementation compared with placebo, this difference was not significant and was of lower magnitude than that previously reported with ~1.1 g (~15%) and up to ~2.6 g of leucine (~22–49%) (27, 36, 59). It is important to note that our stable isotope protocol only allowed us to measure MyoPS for a 2.5 h period as l-[ring-2H5]phenylalanine was ingested 90 min after supplement ingestion. This time-frame was chosen to match that previously utilized using an intravenous pulse tracer injection (120 min post-exercise/supplement consumption) that was successful in capturing greater mixed muscle protein synthesis rates following the ingestion of only 10 g of whey and carbohydrate ingestion compared with a placebo (62). In the current study, the tracer was administered slightly earlier (90 min post-exercise/supplementation) as a balance between increasing the incorporation time for a more compete and robust MyoPS while ensuring we did not lose tracer enrichment in our plasma precursor pool at the biopsy, which we were successful in achieving (Fig. 3A). In addition, postexercise MyoPS are generally greater over a more prolonged postexercise recovery time period (i.e., 3–5 h) with adequate protein/amino acid ingestion (13, 37). Our oral pulse tracer approach, which induced a rapid peak (i.e., within 10 min) in plasma enrichment similar to intravenous pulse (62) or flooding dose (46) approaches, allowed us to capture physiological MyoPS (i.e., ~0.06–0.12%/h) as traditional primed-constant intravenous infusions using a similar time course (27, 37, 40). However, it is possible that we were unable to capture the true postexercise MyoPS response (i.e., 0–4 h) with LEAA given that our measurement period began after the peak plasma EAA/leucine concentrations. We also cannot discount the possibility that the protein-containing breakfast, which was included for ecological validity, resulted in an expansion of the intracellular free amino acid pool that allowed a greater postexercise MyoPS in the placebo group and, thus, minimized differences between conditions.
To evaluate whether more subtle effects of LEAA ingestion were observed at the molecular level, we probed commonly reported targets of mTORC1 activity by immunoblot. We observed that two readouts of mTORC1 activation, rpS6Ser240/244 (~30 fold) and 4EBP1Thr37/46 (~1.5 fold), only displayed a main effect of time, suggesting the resistance exercise bout itself was the main driver of these responses despite being numerically higher following LEAA ingestion. This is not necessarily a unique finding as others have shown resistance exercise to supersede the effects of nutritional stimuli (8, 22, 33, 42, 61), in particular when lower protein doses are ingested (59, 61). However, these signaling responses are often observed to peak within 1–2 h of resistance exercise/feeding (14, 26, 33) and we may therefore have missed any supplementation effects. For example, we have previously shown S6K1 kinase activity, a highly sensitive readout of mTORC1 activity (32), to be equal 3 h postexercise in cohorts who either completed a resistance exercise bout and remained fasted or ingested a protein-carbohydrate beverage following the bout (48) despite its kinase activity being greater at 1 h in the nutritional group. As such, it would be remiss if we did not acknowledge the possibility of missing the window to accurately measure these variables. In addition, we also investigated whether LEAA ingestion postexercise altered the content of two main AATRs in skeletal muscle. Although alterations in LAT1 and SNAT2 content have been previously reported following anabolic stimuli (7, 16, 20), we did not observe any change in the total protein expression of these AATRs similar to other reports (16, 19, 40, 41, 53). Importantly, however, AATRs are only “active” when associated with the plasma membrane (51), an outcome we were unable to measure in the current study due to tissue constraints. However, we have previous observed that LAT1 resides in close proximity to capillaries (23), which may suggest that a greater mTOR peripheral localization could position it in close proximity to substrates (e.g., leucine) of this transporter. Therefore, the lack of change in the expression of LAT1 and SNAT2 in the present study suggests the previously observed ability of skeletal muscle to rapidly increase the influx of amino acids of these transporters (e.g., leucine, alanine) early (i.e., 3 h) (9, 10) after resistance exercise with and without EAA is unrelated to acute changes in total AATR abundance. It is possible that, in contrast to some previous observations (16, 20) but consistent with others (19, 40), the window within which we measured changes in AATR expression was too short to detect meaningful changes in protein content in response to our anabolic stimuli of exercise and LEAA. Several studies, however, have reported consistent elevations in the mRNA expression of AATRs following acute resistance exercise (16, 19, 20, 41). As such, it could be hypothesized that acute AATR adaptations to anabolic stimuli occur at the level of gene expression which only manifest into alterations in protein content following repeated exposures to anabolic stimuli (i.e., chronic resistance exercise training).
The kinase activity of mTORC1 and its downstream targets are dynamically regulated by protein-protein interactions and intracellular location (24, 25, 48). Accordingly, we next explored whether acute postexercise LEAA ingestion altered mTORC1 cellular location and interaction with the lysosome (LAMP2) and Rheb. The canonical in vitro mechanism of mTORC1 activation by EAAs is via enhanced lysosomal targeting of mTORC1 (44, 45). Herein, however, mTOR-LAMP2 colocalization decreased following LEAA ingestion and remained unchanged following PLA ingestion, contrary to our hypothesis. This would suggest reduced mTORC1 activity following LEAA ingestion, a finding which was not apparent in our immunoblot data (given several readouts of mTORC1 activity were elevated). Furthermore, the MyoPS rates observed here are at least ~2-fold greater than previously reported nonexercised, fasted rates (35) thereby suggestive of an enhancement in this measure postexercise. We have previously observed no effect of anabolic stimuli on mTOR-LAMP2 in human skeletal muscle (24, 48) and believe this to be due to a lack of dissociation of mTOR from the lysosome during the mild nutrient deprivation of the postabsorptive state in vivo as compared with complete nutrient removal in vitro (30).
In the current study, we observed no change in mTOR-Rheb colocalization from pre- to postexercise, in contrast to previous literature (1, 2, 48). We have previously observed mTOR-Rheb colocalization to peak 60–120 min postexercise (1, 48), which could suggest our 4 h postexercise time point may not have been sufficient to capture changes in this measure. We did however, observe a supplement effect on mTOR-Rheb colocalization suggesting that, with time points combined, LEAA supplementation elicited greater colocalization of these two proteins. This effect is likely to be primarily driven by the difference in mTOR-Rheb colocalization at 4 h post-exercise/feeding [LEAA – 0.29 ± 0.01 versus PLA 0.21 ± 0.01, Cohen’s d effect size (95% CI): 2.14 (1.21–3.08)], as analysis of the relative change in this measure from pre- to postexercise suggested a trend toward a greater decrease in PLA (P = 0.09). A similar effect was observed for mTOR-WGA colocalization where a supplement effect was apparent (main effect) but potentially driven primarily by the difference between supplementation cohorts at the 4 h postexercise time point [LEAA 0.17 ± 0.02 versus PLA 0.11 ± 0.01, ES (95% CI) 1.506 (0.649–2.363), relative change P = 0.13]. We have previously reported a similar notion, whereby mTOR-WGA colocalization 3 h following a combination of resistance exercise and protein-carbohydrate feeding was greater when compared with feeding alone (24), suggesting that amino acids (e.g., LEAA) may maintain mTOR in peripheral regions of muscle fibers for a greater duration after exercise. Collectively, the greater mTOR-Rheb colocalization and peripheral localization (i.e., mTOR-WGA) at 4 h where AATR reside (23) could suggest that LEAA ingestion (either in isolation or within a mixed macronutrient meal) could act synergistically with exercise to “prime” mTORC1 for future activation by maintaining the kinase complex in cellular locations where efficient activation can occur. However, consistent with previous reports of a peak mTOR-WGA colocalization at ~1 h of recovery with a return to (1, 24, 48) or further reduction below (1, 24) basal levels by 3–5 h, the main effect of time in the present study would suggest the peripheral localization of mTOR is a transient event that may require greater time resolution to elucidate its complete profile.
Conclusion.
In conclusion, we demonstrate that the consumption of 4 g of LEAA containing 1.6 g of leucine following an unaccustomed bout of resistance exercise did not induce a differential MyoPS, mTORC1 signaling, or AATR expression during the late postprandial period compared with an isocaloric placebo. LEAA supplementation did seem to maintain mTORC1 in peripheral regions of skeletal muscle fibers, and in closer proximity to Rheb, suggesting mTORC1 may be primed for further anabolic stimuli. Future research should aim to determine the full time course of these intracellular mechanisms in response to varying AA/protein supplements.
GRANTS
This study was financially supported by Ajinomoto Co., Inc., and a Natural Sciences and Engineering Research Council of Canada Discovery Grant awarded to D. R. Moore. N. Hodson is a postdoctoral research fellow funded through the Mitacs Accelerate Program (No. IT15730).
DISCLOSURES
H. Kato and K. Matsunaga are employees of Ajinomoto Co. Inc. Study results support in part Japan patent JP 6431670. No conflicts of interest, financial or otherwise, are declared by the other authors.
AUTHOR CONTRIBUTIONS
N.H., H.K., and D.R.M. conceived and designed research; S.J.H., S.A.S., M.M., M.W.-F., J.D., D.A.K., and D.R.M. performed experiments; S.J.H., N.H., S.A.S., M.M., K.M., and M.W.-F. analyzed data; S.J.H., N.H., and D.R.M. interpreted results of experiments; S.J.H. and N.H. prepared figures; S.J.H. and N.H. drafted manuscript; S.J.H., N.H., H.K., and D.R.M. edited and revised manuscript; S.J.H., N.H., S.A.S., M.M., H.K., K.M., M.W.-F., J.D., D.A.K., and D.R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the time and effort of the participants.
REFERENCES
- 1.Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, Philp A, Li Z, Paluska SA, Burd NA, Moore DR. Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep 6: e13628, 2018. doi: 10.14814/phy2.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou Sawan S, van Vliet S, West DWD, Beals JW, Paluska SA, Burd NA, Moore DR. Whole egg, but not egg white, ingestion induces mTOR colocalization with the lysosome after resistance exercise. Am J Physiol Cell Physiol 315: C537–C543, 2018. doi: 10.1152/ajpcell.00225.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agergaard J, Bülow J, Jensen JK, Reitelseder S, Bornø A, Drummond MJ, Schjerling P, Holm L. Effect of light-load resistance exercise on postprandial amino acid transporter expression in elderly men. Physiol Rep 5: e13444, 2017. doi: 10.14814/phy2.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apró W, Moberg M, Hamilton DL, Ekblom B, Rooyackers O, Holmberg HC, Blomstrand E. Leucine does not affect mechanistic target of rapamycin complex 1 assembly but is required for maximal ribosomal protein S6 kinase 1 activity in human skeletal muscle following resistance exercise. FASEB J 29: 4358–4373, 2015. doi: 10.1096/fj.15-273474. [DOI] [PubMed] [Google Scholar]
- 5.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38: 1533–1539, 2010. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 6.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab 297: E822–E829, 2009. doi: 10.1152/ajpendo.00330.2009. [DOI] [PubMed] [Google Scholar]
- 7.Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet S, Young JR, Ulanov AV, Li Z, Paluska SA, De Lisio M, Burd NA. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am J Clin Nutr 104: 1014–1022, 2016. doi: 10.3945/ajcn.116.130385. [DOI] [PubMed] [Google Scholar]
- 8.Bell KE, Brook MS, Snijders T, Kumbhare D, Parise G, Smith K, Atherton PJ, Phillips SM. Integrated myofibrillar protein synthesis in recovery from unaccustomed and accustomed resistance exercise with and without multi-ingredient supplementation in overweight older men. Front Nutr 6: 40, 2019. doi: 10.3389/fnut.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 10.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 11.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol (1985) 106: 1692–1701, 2009. doi: 10.1152/japplphysiol.91351.2008. [DOI] [PubMed] [Google Scholar]
- 12.Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9: 1–8, 2014. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99: 276–286, 2014. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- 14.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 590: 2751–2765, 2012. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson JM, Drummond MJ, Coben JR, Volpi E, Rasmussen BB. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr 32: 273–280, 2013. doi: 10.1016/j.clnu.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141: 856–862, 2011. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985) 111: 135–142, 2011. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudani AK, Prasad R. Amino acid transport: its role in cell division and growth of Saccharomyces cerevisiae cells. Biochem Int 7: 15–22, 1983. [PubMed] [Google Scholar]
- 22.Edman S, Söderlund K, Moberg M, Apró W, Blomstrand E. mTORC1 signaling in individual human muscle fibers following resistance exercise in combination with intake of essential amino acids. Front Nutr 6: 96, 2019. doi: 10.3389/fnut.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodson N, Brown T, Joanisse S, Aguirre N, West DWD, Moore DR, Baar K, Breen L, Philp A. Characterisation of L-type amino acid transporter 1 (LAT1) expression in human skeletal muscle by immunofluorescent microscopy. Nutrients 10: 23, 2017. doi: 10.3390/nu10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Rüegg MA, Hamilton DL, Phillips SM, Philp A. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 313: C604–C611, 2017. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodson N, Philp A. The Importance of mTOR Trafficking for Human Skeletal Muscle Translational Control. Exerc Sport Sci Rev 47: 46–53, 2019. doi: 10.1249/JES.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodson N, West DWD, Philp A, Burd NA, Moore DR. Molecular regulation of human skeletal muscle protein synthesis in response to exercise and nutrients: a compass for overcoming age-related anabolic resistance. Am J Physiol Cell Physiol 317: C1061–C1078, 2019. doi: 10.1152/ajpcell.00209.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackman SR, Witard OC, Philp A, Wallis GA, Baar K, Tipton KD. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front Physiol 8: 390, 2017. doi: 10.3389/fphys.2017.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs BL, You JS, Frey JW, Goodman CA, Gundermann DM, Hornberger TA. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol 591: 4611–4620, 2013. doi: 10.1113/jphysiol.2013.256339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato H, Suzuki H, Inoue Y, Suzuki K, Kobayashi H. Leucine-enriched essential amino acids augment mixed protein synthesis, but not collagen protein synthesis, in rat skeletal muscle after downhill running. Nutrients 8: 399, 2016. doi: 10.3390/nu8070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O’Kane CJ, Deretic V, Rubinsztein DC. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol 13: 453–460, 2011. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch 447: 784–795, 2004. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 32.McGlory C, White A, Treins C, Drust B, Close GL, Maclaren DP, Campbell IT, Philp A, Schenk S, Morton JP, Hamilton DL. Application of the [γ-32P] ATP kinase assay to study anabolic signaling in human skeletal muscle. J Appl Physiol (1985) 116: 504–513, 2014. doi: 10.1152/japplphysiol.01072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moberg M, Apró W, Ekblom B, van Hall G, Holmberg HC, Blomstrand E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am J Physiol Cell Physiol 310: C874–C884, 2016. doi: 10.1152/ajpcell.00374.2015. [DOI] [PubMed] [Google Scholar]
- 34.Moberg M, Apró W, Ohlsson I, Pontén M, Villanueva A, Ekblom B, Blomstrand E. Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Appl Physiol Nutr Metab 39: 183–194, 2014. doi: 10.1139/apnm-2013-0244. [DOI] [PubMed] [Google Scholar]
- 35.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 36.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 37.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587: 897–904, 2009. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips SM, McGlory C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 592: 5341–5343, 2014. doi: 10.1113/jphysiol.2014.273615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poncet N, Mitchell FE, Ibrahim AF, McGuire VA, English G, Arthur JS, Shi YB, Taylor PM. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS One 9: e89547, 2014. doi: 10.1371/journal.pone.0089547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, Rasmussen BB. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol (1985) 116: 1353–1364, 2014. doi: 10.1152/japplphysiol.01093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberson PA, Haun CT, Mobley CB, Romero MA, Mumford PW, Martin JS, Roberts MD. Skeletal muscle amino acid transporter and BCAT2 expression prior to and following interval running or resistance exercise in mode-specific trained males. Amino Acids 50: 961–965, 2018. doi: 10.1007/s00726-018-2570-2. [DOI] [PubMed] [Google Scholar]
- 42.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 38: 120–125, 2013. doi: 10.1139/apnm-2012-0092. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 44.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol Endocrinol Metab 262: E372–E376, 1992. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 47.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab 275: E73–E78, 1998. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 48.Song Z, Moore DR, Hodson N, Ward C, Dent JR, O’Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff Y-G, Hornberger TA, Spriet LL, Heigenhauser GJ, Philp A. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep 7: 5028, 2017. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab 32: 1132–1138, 2007. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]
- 50.Tarnopolsky MA, Pearce E, Smith K, Lach B. Suction-modified Bergström muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve 43: 716–725, 2011. doi: 10.1002/mus.21945. [DOI] [PubMed] [Google Scholar]
- 51.Taylor PM. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr 99: 223S–230S, 2014. doi: 10.3945/ajcn.113.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem 10: 89–95, 1999. doi: 10.1016/S0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 53.van Vliet S, Skinner SK, Beals JW, Pagni BA, Fang H-Y, Ulanov AV, Li Z, Paluska SA, Mazzulla M, West DWD, Moore DR, Wilund KR, Burd NA. Dysregulated handling of dietary protein and muscle protein synthesis after mixed-meal ingestion in maintenance hemodialysis patients. Kidney Int Rep 3: 1403–1415, 2018. doi: 10.1016/j.ekir.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waskiw-Ford M, Hannaian S, Duncan J, Kato H, Abou Sawan S, Locke M, Kumbhare D, Moore D. Leucine-enriched essential amino acids improve recovery from post-exercise muscle damage independent of increases in integrated myofibrillar protein synthesis in young men. Nutrients 12: 1061, 2020. doi: 10.3390/nu12041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94: 795–803, 2011. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson DJ, Bukhari SSI, Phillips BE, Limb MC, Cegielski J, Brook MS, Rankin D, Mitchell WK, Kobayashi H, Williams JP, Lund J, Greenhaff PL, Smith K, Atherton PJ. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin Nutr 37, 6 Pt A: 2011–2021, 2018. doi: 10.1016/j.clnu.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 591: 2911–2923, 2013. doi: 10.1113/jphysiol.2013.253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 60.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48, 2016. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108: 1780–1788, 2012. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X-J, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab 283: E753–E764, 2002. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]