Abstract
It is widely agreed that breathing is subject to circadian regulation. Circadian differences in respiratory physiology significantly impact a number of diseases including sleep apnea, asthma, and seizure-induced death. The effect of time of day on breathing has been previously characterized; however, an endogenous free-running respiratory rhythm in mammals has not previously been described. Furthermore, it is assumed that circadian rhythms in breathing are dependent on the hypothalamic suprachiasmatic nucleus (SCN), the home of the mammalian central circadian oscillator, but this has not been shown experimentally. The breathing of mice was monitored during wakefulness using whole body plethysmography at six times of day while housed under light-dark conditions and at six circadian phases while housed under constant darkness. Respiratory frequency and minute ventilation, but not tidal volume, were significantly higher during the active phase in both entrained and free-running conditions. To determine whether circadian regulation of breathing requires the SCN, in separate sets of animals this structure was electrolytically lesioned bilaterally or a sham surgery was performed, and breathing was measured at six different time points. Time-dependent oscillations in breathing were lost in SCN-lesioned animals, but not those subjected to sham surgery. These results suggest that breathing is subject to circadian regulation via the SCN. Mechanistic insights into the circadian regulation of breathing may lead to targeted interventions to reduce the morbidity and mortality associated with diseases with respiratory pathophysiology.
NEW & NOTEWORTHY It has long been appreciated that breathing is altered by time of day. This study demonstrates that rhythmicity in breathing persists in constant darkness but is dependent on the suprachiasmatic nucleus in the hypothalamus. Understanding circadian rhythms in breathing may be important for the treatment and prevention of diseases such as sleep apnea and sudden unexpected death in epilepsy.
Keywords: breathing, circadian rhythms, suprachiasmatic nucleus
INTRODUCTION
Circadian rhythms are near-24 h oscillations that influence practically every aspect of biological function and can be found in most organisms from prokaryotes to primates (5, 51, 68). Circadian rhythmicity is evolutionarily advantageous as it allows an organism to predictively adapt to changes in its environment and isolate processes or activities that cannot be conducted simultaneously (53). Many physiologic processes such as sleep, thermoregulation, stress hormone release, and cardiac function have been shown to exhibit circadian rhythmicity in the absence of environmental cues (20, 21, 58, 70). In mammals, circadian rhythmicity is coordinated by the central circadian oscillator in the suprachiasmatic nucleus (SCN) in the ventral-anterior hypothalamus (39, 65). SCN ablation eliminates, or at least attenuates, circadian rhythmicity in processes such as cardiac function, sleep, thermoregulation, and gastrointestinal function (19, 33, 37).
Breathing, under normal healthy conditions and in response to challenges, is modulated in a time of day-dependent fashion (7, 40). Sleep also alters breathing (44, 73). Sleep usually occurs during the night in diurnal organisms such as humans, causing sleep and night to often be considered together; however, sleep and time of day independently affect breathing (66). In nocturnal rodents entrained to a typical 12:12-h light-dark (12:12 LD) cycle, respiratory frequency (fR) and minute ventilation (V̇e) are greater during the dark phase when the animals are more active and lower during the light phase when rodents tend to be less active (60, 66). This is inverted in humans and other diurnal animals such that ventilation is greater during the light phase when these species are more active (64, 66).
A defining diagnostic criterion of circadian rhythms is that they are endogenously generated and continue to oscillate in constant environmental conditions without indicators of the passage of time (71). A substantial body of work has characterized daily differences in breathing in organisms that were kept under normal conditions with exposure to temporal cues; however, investigations of breathing in free-running conditions are lacking (7, 40, 60, 66). To date, the only vertebrate organism for which free-running breathing rhythms have been examined is the garter snake (Thamnophis sirtalis). In these snakes, free-running rhythms of fR and tidal volume (VT) were seen. Unfortunately, this difference was not shown to be independent of differences in the quantity of time spent in the animals’ “sleeping” state in which breathing is altered (30). It is assumed that previously described day-night changes in mammalian breathing are endogenously circadian; however, whether these rhythms in breathing persist in the absence of external time cues has not been examined.
In the current study, we hypothesized that day-night differences in breathing are endogenously circadian and dependent on the SCN. To test this hypothesis, breathing was monitored using whole body plethysmography with concomitant EEG and electromyogram (EMG) recording at different time points in mice entrained to normal light-dark conditions, maintained in free-running conditions of constant darkness (DD), and maintained in constant darkness after bilateral electrolytic lesion of the SCN.
METHODS
Ethical approval.
All procedures and protocols performed in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa in accordance with Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) international guidelines. At the end of the experimental paradigm all mice were euthanized with an overdose of ketamine (100 mg/kg ip; Ketaset; Zoetis Inc., Kalamazoo, MI) and xylazine (10 mg/kg; Anased; Akorn Animal Health, Lake Forest, IL). All steps were taken to minimize the animals’ pain and suffering and to use the minimum number of animals possible.
Experimental animals.
Adult (7–8-wk) male C57BL/6J mice were obtained from the Jackson Laboratory (000664; Bar Harbor, ME). Mice were singly housed in cages (Maximiser no. 9; Thoren Caging Systems Inc., Hazelton, PA) equipped with running wheels (CC01A02EXERNC; Thoren Caging Systems Inc.). Food (NIH-31 Mouse Diet) and water were available ad libitum. Single housing was necessary to prevent animals from damaging their cohabitants’ EEG-EMG head mounts following the surgery and to allow wheel-running behavior to be monitored for each animal individually. The wheel-running home cages were placed in custom-made, fan-ventilated, light-impermeable boxes equipped with independently programmable lighting (1.5 m × 48 cm × 45 cm). These lights were set to either 12:12 LD (lights on at 0600, lights off at 1800) or DD conditions.
EEG-EMG surgery.
In animals in which breathing was recorded in LD and DD conditions without an SCN lesion or sham surgery, EEG and EMG electrodes were implanted without additional instrumentation. In animals undergoing SCN lesion or sham surgery, the SCN lesion or sham insertion was performed immediately before EEG and EMG electrode implantation during the same surgical session. Electrode implantation was conducted in accordance with previously described methods in the laboratory (9). Briefly, four pilot holes were bored into the surface of the skull (1 mm anterior to bregma/lambda; ±2 mm from midline) using a 35-gauge hypodermic needle (ref. 305145; Becton, Dickinson and Company, Franklin Lakes, NJ). An EEG-EMG head mount (no. 8201; Pinnacle Technology Inc., Lawrence, KS) was secured into these four holes and adhered to the skull with dental acrylic (ref. 1320; Lang Dental Manufacturing Co., Wheeling, IL). Screws secure the head mount to the skull and serve as the EEG electrodes (no. 8209/no. 8212; Pinnacle Technology Inc.). Electrical conductivity between the screws and the metal contacts of the head mount was enhanced with silver epoxy (8331-14G; Digi-Key Electronics, Thief River Falls, MN). EMG wire electrodes were inserted bilaterally into the nuchal muscles of the neck and secured with absorbable sutures (029292; Henry Schein Inc., Dublin, OH). A layer of dental acrylic was used to coat the EEG-EMG head mount to electrically insolate it from the skin. The incision was closed using nonabsorbable sutures (669H 3-0; Ethicon Inc., Cincinnati, OH). Ophthalmic ointment was applied to the eyes before surgery, to avoid corneal drying (R1112; Dechra Veterinary Products, Overland Park, KS). Mice were allowed to recover for at least 1 wk before experimentation.
EEG and EMG recording.
EEG and EMG signals were acquired and processed using previously described methods in our laboratory (9). Briefly, the preamplifier (8202-SL; Pinnacle Technology Inc.) was attached to the exposed plug on the implanted EEG-EMG head mount. The preamplifier cord was passed through an airtight gasket in the custom plethysmography chamber and then attached to a six-channel commutator (no. 8204; Pinnacle Technology Inc.). The signal was passed to a conditioning amplifier (model 440 Instrumentation Amplifier; Brownlee Precision, San Jose, CA). The EEG signals were amplified (100 times), band-pass filtered (0.3–200 Hz for EEG; 10–300 Hz for EMG), and digitized (1,000 samples/s; NI USB-6008; National Instruments, Austin, TX). The digitized signal was transferred to a desktop computer and recorded using software custom written in MATLAB (R2018b; MathWorks, Natick, MA). The sleep-wake state of the animal was determined post hoc using the frequency and amplitude characteristics of the EEG and EMG traces as has been previously described (9, 25). Briefly, periods of time in which EMG tone was high with low-amplitude, high-frequency EEG activity were categorized as wakefulness, and the corresponding respiratory data were included in the analysis. Conversely, periods of time characterized by either low EMG tone and high-amplitude, low-frequency EEG activity [non-rapid eye movement (NREM) sleep] or low EMG tone with moderate-frequency, moderate-amplitude EEG activity [rapid eye movement (REM) sleep] were categorized as sleep, and the corresponding respiratory data were excluded from the analysis (9).
SCN lesion.
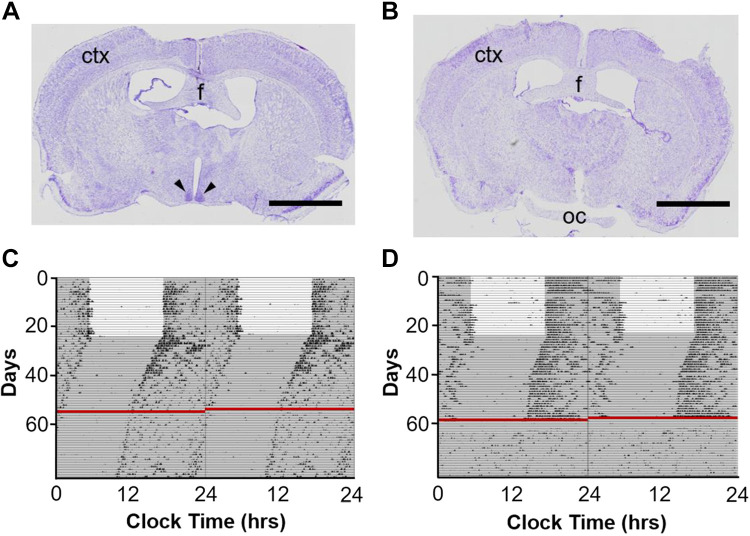
The SCN was electrolytically lesioned using methods described previously (61). Briefly, using aseptic technique under isoflurane anesthesia, a midline skin incision was made in the scalp. Small bur holes (1-mm diameter) were drilled in the skull above the SCN bilaterally (in mm from bregma: AP −0.2, ML ±0.2) with care taken not to puncture the dura mater. An insulated wire (010SW/2, 0.254 mm diameter; Plastics One Inc., Roanoke, VA), with the distalmost 0.5 mm of insulation removed, was lowered through the bur holes and inserted into the SCN (in mm from bregma: AP −0.2, ML ± 0.2, DV −5.3). For sham surgeries, the electrode was lowered into the SCN, but current was not delivered (Fig. 1A). SCN lesions (Fig. 1B) were performed bilaterally using anodal constant current (1 mA, 15 s) delivered by an isolated pulse stimulator (2100; A-M Systems, Sequim, WA). The negative lead from the isolated pulse stimulator was attached to the fur caudal to the incision using an alligator clip wrapped with saline-moistened gauze. After the electrode was retracted, the bur holes were sealed with dental acrylic. The incision was closed using nonabsorbable sutures. Ophthalmic ointment was applied to eyes before surgery, to avoid corneal drying. Animals were kept in DD following the SCN lesion or sham surgery for at least 14 days before respiratory monitoring to confirm the arrhythmic, or rhythmic, behavioral phenotype.
Fig. 1.
Rhythms in circadian wheel-running behavior are abolished following suprachiasmatic nucleus (SCN) lesion. Cresyl violet-stained 25-µm coronal brain section from a mouse subjected to sham (A) or SCN lesion (B) surgery. Scale bars, 2 mm; ctx, cortex; f, fornix; oc, optic chiasm. Black arrowheads point to SCN. Representative double-plotted wheel-running data from a mouse subjected to sham (C) or SCN lesion (D) surgery, 80 days. Red lines indicate the day of surgery. White background depicts lights on; gray background depicts lights off.
Whole body plethysmography.
During each monitoring session, animals were removed from their home cage and placed in a cast acrylic recording chamber (350 cm3). The plethysmography chamber was contained in a custom-made, light-impermeable chamber (40 cm × 30 cm × 30 cm). The transfer from the home cage to the recording chamber was performed under dim red light (635–700 nm, <4 lux). Compressed air (21% O2-79% N2; Praxair Inc., Cedar Rapids, IA) flowed through the chamber at 0.40–0.45 L/min. The balance of air flowing in and out of the chamber was maintained with two flowmeters (32907-67; Cole-Parmer; Vernon Hills, IL) and a vacuum regulator (V-800-30; Airtrol Components Inc., New Berlin, WI). The recording chamber was outfitted with an ultralow-volume pressure transducer (DC002NDR5; Honeywell International Inc., Morris Plains, NJ) to detect small pressure waves associated with breathing. The signal was amplified (100 times), band-pass filtered (0.3–30 Hz), and digitized as above before being recorded with the same custom MATLAB software mentioned above and stored on a desktop computer. These methods have been described previously (8, 9).
The animals were placed in the plethysmography chamber followed by 200 s of calibration and 600 s of respiratory monitoring. No fewer than 60 s of breathing were quantified from each respiratory monitoring session. The fR, VT, and V̇e were determined from artifact-free epochs of respiratory data during quiet wakefulness in which the animal was not sniffing or moving around the chamber. It took a period of several minutes for the animal to be removed from its home cage and set up in the plethysmography chamber. As a result, if the animal had been asleep before the trial, the animal would have been aroused for several minutes before the start of the recording thereby eliminating any potential latent effects from the preceding sleep period. VT was calculated using a barometric flow-through method (18) of incorporating breath amplitude, barometric pressure (https://www.wunderground.com/), flow rate, mouse temperature, air temperature, and the amplitude of metered calibration air pulses (300 μL, 180/min) that were delivered to the recording chamber using a rodent mechanical ventilator (MiniVent 845; Hugo Sachs Elektronik, Grünstraße, Germany). The air temperature of the room was monitored during the experiment with a digital thermometer (401014; ExTech Instruments, Nashua, NH) and ranged from 20 to 22°C regardless of season or time of day. Animal temperature was sampled during the experiment with the aid of a subcutaneous temperature telemeter (IPTT-300; Bio Medic Data Systems Inc., Seaford, DE). Animals were habituated to the recoding chamber and the stress of handling in two 30-min sessions. Monitoring sessions were conducted in order (with each coming 4 h after the last) with the starting time point randomized to avoid any sequencing effects. Between monitoring sessions, the animals were returned to their home cage.
Wheel-running behavior.
Animals were singly housed in cages equipped with a running wheel (11.5-cm diameter) suspended from the cage top. A rare earth magnet (5-mm diameter, 0.75 mm thick, neodymium; K & J Magnetics, Plumsteadville, PA) was attached to the wheel and closed the circuit of a hermetically sealed reed switch (MITI-3v1–6-12.5; Littelfuse Inc., Schiphol, The Netherlands) with each wheel revolution. Switch closures, signifying wheel revolutions, were relayed to a multiplexer (ACTI-556B; Actimetrics, Wilmette, IL) and ultimately to a desktop computer running ClockLab Data Collection software (version 3; Actimetrics). Each 24-h day was plotted serially with the running wheel revolutions being summed over each minute and plotted as a histogram (Fig. 1, C and D). Wheel-running activity was double plotted to facilitate visual identification of the rhythm (each 24-h segment of data is plotted twice, once in each vertical column; Fig. 1, C and D). The onset of activity was used to determine the free-running period of each animal. Circadian time 12 (CT 12) was defined as the time of the onset of activity by convention. Running wheels were locked into place on the first day to prevent novelty-induced phase shifts (48). Animals were allowed to acclimate to 12:12 LD conditions for at least 14 days before respiratory monitoring to confirm adequate entrainment to environmental conditions. Once released into DD, animals were allowed to free-run for at least 14 days before respiratory monitoring to confirm a free-running phenotype with stable periodicity.
Tissue fixation and anatomical confirmation.
Tissue processing and anatomical confirmation of lesion size and shape were conducted in accordance with previously described methods in our laboratory (62). Briefly, mice were anesthetized using ketamine (50–75 mg/kg ip)-xylazine (5–7.5 mg/kg ip) and intracardially perfused with phosphate-buffered saline followed by 4% paraformaldehyde. Brains were extracted, cryoprotected with 30% sucrose, frozen in medium (4583; Sakura Finetek Inc., Torrance, CA), and stored at −80°C until sectioned (20–40 µm) with a cryostat (CM1520; Leica Biosystems Inc., Buffalo Grove, IL). Sections were stained with cresyl violet, and the electrode track and lesion sizes were observed in relation to known anatomical landmarks such as the optic chiasm (Fig. 1, A and B).
Statistical analysis.
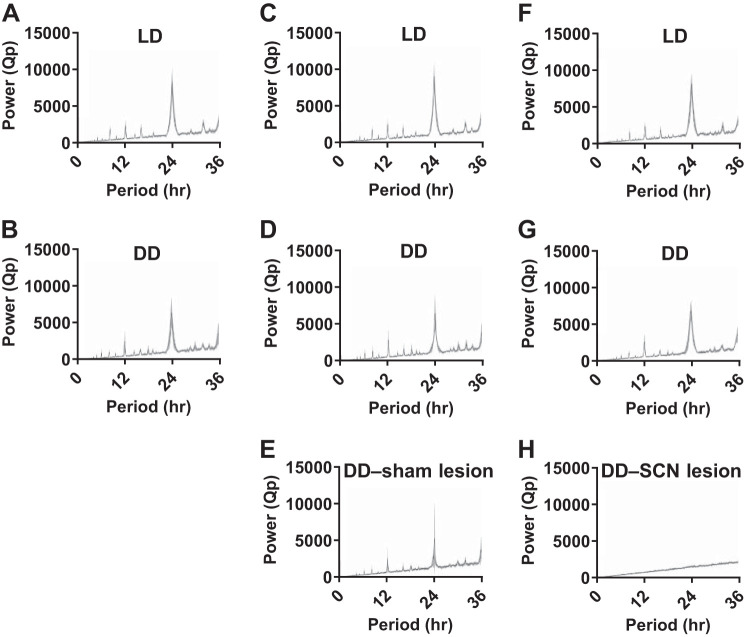
Data scoring and analysis were conducted with the investigator blind to experimental condition. All statistical analyses were completed using GraphPad Prism (version 7; GraphPad Software Inc., La Jolla, CA). Circadian rhythms in respiratory parameters were assessed using the cosinor method as previously described (13, 50). Briefly, a cosine wave with 95% confidence interval that best fits the 24-h time series data is generated using the least squares method. The range of the 95% confidence interval is compared with the midline estimating statistic of rhythm (MESOR) line for that data set. If the 95% confidence interval of the fitted curve does not overlap the MESOR line, then a circadian rhythm is inferred with a P < 0.05 (50). ClockLab analysis software (version 6.0.52; Actimetrics) was used to generate periodograms from the wheel-running behavior data using the chi-square method (Fig. 2, A–H).
Fig. 2.
Circadian wheel-running periodicity is lost following suprachiasmatic nucleus (SCN) lesion. Chi-square wheel-running periodograms for 14 days of data depicted as mean power (Qp, black curve) with 95% confidence interval (gray area) from animals in the normal light-dark (LD) and constant darkness (DD) respiratory recording group in LD (A) and DD (B); from animals in the sham lesion group in LD (C), in DD before the surgery (D), and in DD after the surgery (E); and from animals in the SCN lesion group in LD (F), in DD before the surgery (G), and in DD after the surgery (H).
RESULTS
Breathing in mice entrained to a light-dark cycle.
Wheel-running activity was used to verify that mice were appropriately entrained to the light-dark cycle. Wheel-running activity of mice entrained normally under standard 12:12 LD conditions with a period (τ) of almost exactly 24 h (LD and DD respiratory recording group, n = 12 mice, τ = 24.00 ± <0.01 h, Fig. 2A).
To examine the effect of time of day on breathing, whole body plethysmography was performed during wakefulness on a group of surgically instrumented mice (which did not undergo SCN lesion or sham surgery) concomitant with EEG and EMG recording at six times of day [n = 12 mice; Zeitgeber time (ZT) 2, 6, 10, 14, 18, and 22]. Respiratory monitoring sessions during the dark phase (ZT 14, 18, and 22) were performed in darkness. The mouse was transferred from its home cage to the recording chamber under dim red light. Respiratory monitoring sessions in normally entrained mice were conducted between December and March.
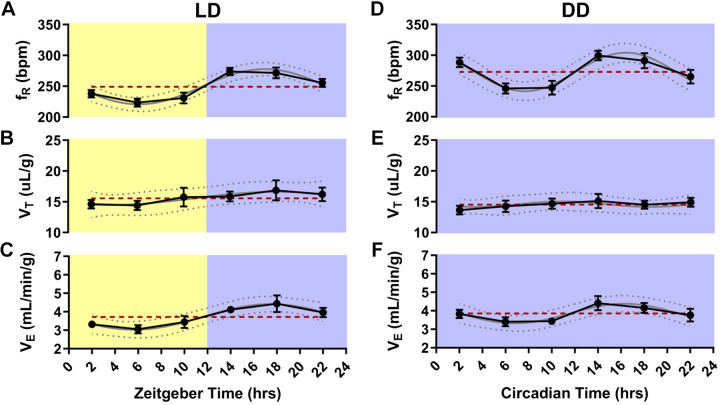
Cosinor analysis revealed a statistically significant daily oscillation in fR (P < 0.001, MESOR = 249.2, R2 = 0.39; Fig. 3A) and V̇e (P = 0.002, MESOR = 3.72, R2 = 0.21; Fig. 3C). There was not a significant daily oscillation in VT (P = 0.38, MESOR = 15.56, R2 = 0.05; Fig. 3B).
Fig. 3.
Circadian breathing rhythms free-run in constant darkness. Respiratory frequency (fR) in breaths per minute (bpm), tidal volume (VT), and ventilation (V̇e) at different times of day in animals entrained to normal light-dark conditions (LD; A–C) and at different circadian phases in animals kept in constant darkness (DD; D–F). n = 12 mice. Values are depicted as means (●) ± SE (black error bars). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted gray curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line.
Breathing in mice free-run in constant conditions.
After completion of the respiratory monitoring sessions conducted under LD conditions (described above), the instrumented mice used in the LD experiments were placed into DD. Wheel-running activity was used to determine the animals’ free-running circadian behavior rhythm in DD. Mice free-ran with a period slightly less than 24 h (LD and DD respiratory recording group, n = 12 mice, τ = 23.92 ± 0.03 h, Fig. 2B). To examine the effect of circadian phase on breathing, whole body plethysmography was performed at six circadian phases (n = 12 mice; CT 2, 6, 10, 14, 18, and 22). All respiratory monitoring sessions were performed in darkness. The mouse was transferred from its home cage to the recording chamber under dim red light. Respiratory monitoring sessions in free-running mice were conducted between January and March.
Cosinor analysis revealed a statistically significant oscillation in fR (P < 0.001, MESOR = 272.8, R2 = 0.29; Fig. 3D) and V̇e (P = 0.041, MESOR = 3.85, R2 = 0.12; Fig. 3F), but not VT (P = 0.871, MESOR = 14.55; Fig. 3E) with fR and V̇e being highest during the animals’ active phase.
Circadian breathing rhythms were eliminated by SCN lesion.
To determine a role of the SCN in circadian regulation of breathing, the SCN was lesioned bilaterally or a sham surgery was performed in separate groups of animals, and breathing was measured at different time points. Prior to surgery, animals were first entrained to LD and then allowed to free-run in DD (Fig. 1, C and D). Both groups entrained normally to LD (sham surgery group, n = 6 mice, τ 24.01 ± <0.01 h, Fig. 2C; and SCN lesion group, n = 7 mice, τ = 24.00 ± <0.01 h, Fig. 2F) and displayed comparable free-running periods in DD (sham surgery group, n = 6 mice, τ = 23.91 ± 0.05 h, Fig. 2D; and SCN lesion group, n = 7 mice, τ = 23.87 ± 0.03 h, Fig. 2G). After surgery, the wheel-running activity of the mice was monitored to assess circadian periodicity or lack thereof (Fig. 1, C and D). The free-running period of animals following the sham surgery was comparable to presurgical periodicity (τ = 23.93 ± 0.08 h; Fig. 2, D and E). Circadian rhythmicity in wheel-running behavior was lost following SCN lesion as assessed by analysis of wheel-running activity via chi-square periodogram (Figs. 1D and 2H). The size and location of lesion were histologically verified post hoc (Fig. 1, A and B). The mean body temperature of SCN-lesioned animals was not statistically significantly different from sham-surgerized animals [unpaired t test; t(11) = 1.14, P = 0.28, data not shown].
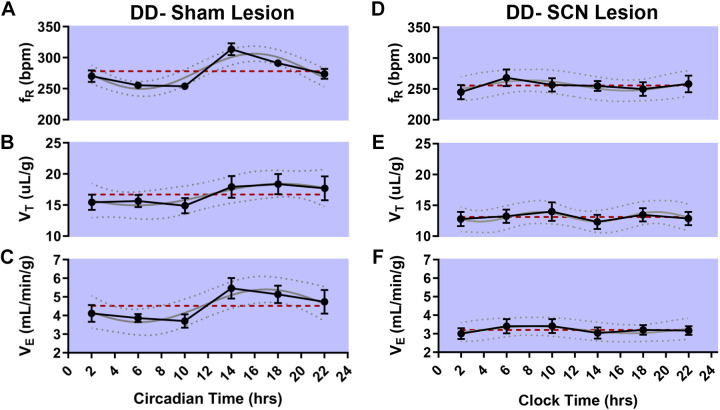
Cosinor analysis of the breathing of animals in which a sham surgery was performed revealed a statistically significant oscillation in fR (n = 6 mice, P < 0.001, MESOR = 277.1, R2 = 0.53; Fig. 4A) and V̇e (P = 0.032, MESOR = 4.52, R2 = 0.25; Fig. 4C), but not VT (P = 0.231, MESOR = 16.68, R2 = 0.13; Fig. 4B). Cosinor analysis of the breathing of animals in which the SCN was lesioned revealed no statistically significant oscillation in fR (n = 7 mice, P = 0.692, MESOR = 255.6, R2 = 0.04; Fig. 4D), VT (P = 0.779, MESOR = 13.11, R2 = 0.03; Fig. 4E), or V̇e (P = 0.777, MESOR = 3.21, R2 = 0.03; Fig. 4F). Respiratory monitoring sessions in SCN-lesioned and sham-surgerized animals were conducted between August and December.
Fig. 4.
Circadian breathing rhythms are eliminated by suprachiasmatic nucleus (SCN) lesion. Respiratory frequency (fR) in breaths per minute (bpm), tidal volume (VT), and ventilation (V̇e) at different circadian time points in sham animals in constant darkness (DD; A–C; n = 6 mice) and at different clock times in SCN-lesioned animals in DD (D–F, n = 7 mice). Values are depicted as means (●) ± SE (black error bars). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line.
DISCUSSION
It has long been appreciated that breathing changes over the 24-h day in diurnal (e.g., humans and bees; 64, 69) and nocturnal (e.g., mice and rats; 16, 66) organisms. It is assumed that these day-night alterations in breathing are endogenously circadian in mammals (7, 40), but this has not been demonstrated empirically.
Here we demonstrate that daily oscillations in breathing in mice persist in the absence of time cues, suggesting that breathing is subject to endogenous circadian regulation. We further observed that circadian rhythms in ventilation in mice are primarily driven by changes in fR. Interestingly, prior investigations into breathing at different times of day in rats found that rhythms in V̇e were driven both by fR and VT; this may be due to an interspecies difference (59). In general, smaller animals are able to increase their fR with less energetic expenditure than larger animals (14). Perhaps the larger size of rats necessitates circadian modulation of breathing by both volume and frequency. Only epochs of plethysmography data that occurred during wakefulness, as assessed by concomitant EEG-EMG recording, were analyzed in this study. Therefore, the time of day and circadian differences in breathing observed in this study are not attributable to the altered distribution of vigilance states seen at different times of day. These data corroborate previous rodent studies indicating that time of day affects breathing independent of sleep state (66). The free-running circadian oscillations in fR and V̇e are comparable in amplitude and shape to the oscillation in fR and V̇e observed in LD conditions indicating that the presence or absence of light does not dramatically alter circadian rhythms in breathing. The ablating or at least attenuating effect of SCN lesion on circadian rhythms in body temperature has been the subject of a considerable body of research (19, 26, 36, 49, 57); however, less attention has been given to whether lesioning of the SCN alters mean body temperature. We did not observe any effect of SCN lesion on mean body temperature; this finding is consistent with prior reports in rats and golden hamsters (49, 57).
Recently, the O2 consumption, CO2 production, and activity of mice kept in conditions of constant darkness were examined (2). It was observed that rhythmicity in these metabolic parameters persisted in constant conditions indicating that these processes are subject to endogenous circadian regulation. Furthermore, it was demonstrated that timed feeding was able to restore rhythmicity in O2 consumption and CO2 production in arrhythmic Period gene mutant animals (Per1/2−/−) (2).
Circadian rhythmicity in breathing is dependent on the SCN.
The SCN houses the central mammalian circadian pacemaker and coordinates the temporal organization of a wide range of physiologic processes (10, 19, 39, 65); however, this has not yet been shown to be true of breathing. In this study we demonstrate that circadian rhythms in breathing are abolished by SCN lesion. Identifying the SCN as necessary for circadian rhythmicity in breathing is an important step in mapping the mechanistic pathway by which circadian regulation in breathing is implemented. There are several ways in which circadian rhythms might alter breathing: Circadian regulation of breathing might occur at the level of the neuronal breathing network via direct projections from the SCN or local changes in protein, transmitter, or metabolite levels within individual respiratory nuclei. Conversely, circadian differences in breathing might be mediated by circadian differences in overall metabolism. These potential mechanisms for circadian modulation of breathing will be discussed below.
Potential circadian modulation of the neural respiratory network and respiratory tissues.
There are a number of brain stem nuclei that shape the pattern and rhythm of breathing. These are subdivided into the dorsal respiratory group, which contains the nucleus tractus solitarius, and the ventral respiratory group, which contains nucleus retroambiguus, the Bötzinger complex, and the pre-Bötzinger complex (22, 24). The pre-Bötzinger complex is essential for the generation of the respiratory rhythm (15, 63). The rhythm of the pre-Bötzinger complex is modulated by endogenous serotonin (45). There are circadian variations in serotonergic tone and in the metabolic activity of the serotonergic raphe nuclei (54, 56). It may be that circadian oscillations in serotonergic neurotransmission contribute to circadian oscillations in breathing. The circadian regulation of breathing observed in this investigation may be happening at the level of respiratory central pattern generation in the brain; however, it should be noted that circadian rhythms alter the properties of peripheral respiratory tissues, such as the lungs and larynx (43, 67). Respiratory tissues such as the larynx, trachea, and lung have peripheral circadian oscillators characterized by robust rhythms in circadian clock genes Per1, Per2, Bmal1, and Clock (4). Circadian rhythms in these tissues are likely important for daily changes in mucin secretion and airway patency (4, 35). Genetic deletion of the clock genes Cry1 and Cry2 disables the peripheral oscillators in these tissues as does bilateral SCN lesion indicating that these oscillators operate under the purview of the central circadian clock in the SCN (4). Future investigations into clock gene expression profiles in respiratory nuclei and the ability of respiratory nuclei to maintain Per1 oscillations in vitro may provide insights into the possible neural contributions to circadian oscillations in breathing (1).
Metabolism as a possible mediator for circadian rhythms in breathing.
In addition to circadian modulation of breathing at the level of the neural circuit as described above, circadian differences in breathing may be at least in part mediated by circadian changes in metabolism. In both humans and rodents, ventilation is elevated during the active phase raising the possibility that circadian differences in breathing are entirely explicable by differences in the animal’s activity. However, it is unlikely that the SCN-dependent circadian modulation of breathing observed in this study is due to differences in activity for the following reasons. First, all breathing analyzed in this study was taken from periods of quiet wakefulness in which the animal was not actively moving around. Second, analysis of breathing during the day or night with equivalent activity in rats (e.g., comparing periods of high activity during the day with periods of high activity during the night) has revealed that time-of-day differences in breathing are independent of daily changes in activity (40, 60).
Certainly, breathing is altered by changes in metabolic activity and body temperature. Cellular respiration produces the ATP needed to fuel cellular processes. Cellular respiration expends O2 and produces CO2. The levels of these gasses in the blood are closely regulated by breathing. Changes in metabolic rate necessitate a change in breathing if blood gas levels are to be maintained. Body temperature, activity, and oxygen consumption display time-of-day differences with a pattern very similar to that seen in breathing (60). It is possible that the circadian differences in breathing seen in this investigation are partially or entirely the result of an underlying circadian oscillation in metabolism. Circadian differences in metabolism, oxygen consumption, and body temperature are not present in the absence of a circadian clock (2, 12, 42, 49). It is possible that the absence of circadian oscillations in these metabolic parameters is responsible for the lack of circadian rhythms in breathing in the SCN-lesioned animals examined in this study. Parsing the effects that the SCN might be having on breathing via modulation of the respiratory nuclei or peripheral respiratory tissues from the potential mediating effects of circadian rhythms in metabolic parameters is beyond the scope of this initial investigation. Future studies might be able to resolve this question with in vitro experiments. Many nuclei in the brain continue to exhibit rhythmic oscillations in clock gene expression even in incubated slices (1). If respiratory nuclei in incubated slices continue to exhibit circadian oscillations in clock gene expression and oscillations in fictive breathing that match the pattern of clock gene expression, it would suggest that circadian oscillations in breathing are not entirely the result of alterations in metabolism.
Nocturnal exacerbation of disorders associated with respiratory dysfunction.
Time of day modulates the pathophysiology of several diseases associated with respiratory problems and high mortality. Obstructive sleep apnea (OSA) is characterized by occlusion of the upper airway during sleep; duration of the resulting apneas is altered by circadian rhythmicity (11, 72). Conversely, asthma is characterized by excessive airway responsiveness, bronchoconstriction, and inflammation of the airway that worsens during the night (17, 27). Patients with asthma are more likely to suffer ventilatory arrest and die during the night (17). The leading cause of death in patients with refractory epilepsy is sudden unexpected death in epilepsy (SUDEP; 29). Mortality in SUDEP occurs consequent to respiratory arrest in the time following a seizure and most often occurs at night (3, 34, 47, 55). There is evidence in rodent models to suggest that adverse respiratory outcomes following a seizure may be more common when seizures happen or are induced at certain times of day (28, 38, 46). Future experimentation is needed to elucidate how circadian changes in breathing might contribute to the nocturnality of SUDEP. Sudden infant death syndrome (SIDS) shares many common features with SUDEP, most notably, a form of respiratory failure implicated in its etiology and primarily nocturnal occurrence (6, 31, 52). Improving our understanding of how and why breathing changes with time of day may be important to preventing the morbidity and mortality associated with diseases characterized by respiratory dysfunction.
Limitations.
This study is not without limitations. Respiratory data were not examined during sleep in this study. Sleep alters breathing independent of circadian rhythmicity in ways that are potentially significant to human health and disease (66). Because of the acute monitoring paradigm employed in this study there were not enough data recorded from NREM and REM sleep for a comparison to be made between breathing during sleep and wake epochs at different time points. A fixed calibration frequency and volume were used across all plethysmograph monitoring sessions. Calibration air pulses result in greater changes in pressure when administered at a higher frequency and vice versa. In an optimal calibration paradigm, the frequency of calibration would be equivalent to the animal’s fR for the trial in question (23, 32, 41). Because fR was observed to be different across time points, it is possible that the fixed calibration frequency influenced the measurement of VT. Future investigations might circumvent this issue by calibrating at a frequency close to expected fR on the basis of pilot data or by calibrating at a range of possible frequencies in separate stages and using the calibration data closest to the animal’s own fR during data analysis. Furthermore, electrolytic lesions destroy axons passing through the target area in addition to local cell bodies. The disruption of these fibers of passage may result in off-target effects. Future investigations would do well to use an alternative lesioning method, such as ibotenic acid, to avoid this issue. Only males were used in this investigation. There may be sex differences in the circadian regulation of breathing, which would make the inclusion of female animals instructive in future studies.
Summary.
The effect of time of day and vigilance state on breathing in normally entrained animals has been the subject of thorough prior investigation (40, 59, 60, 66). In the current study, differences in breathing during wakefulness across a variety of circadian phases in free-running animals were examined. It was found that the rhythmicity in respiratory parameters persisted in DD, indicating that breathing is subject to endogenous circadian regulation. This investigation also demonstrates that these circadian differences in breathing are dependent on the SCN. Broadening our comprehension of the mechanisms by which breathing is different at different times of day may provide insights into the pathomechanisms and treatment of respiratory disorders that display nocturnality.
Future directions.
Future investigations using a constant recording paradigm such as those used by previous studies (66) may allow conclusions to be drawn regarding the interplay between sleep and circadian rhythms in respiratory regulation in DD and in the absence of the SCN. Food access was not restricted in this investigation; however, it may be that temporally restricting food access in our SCN-lesioned mice would have restored aspects of respiratory rhythmicity (2). Additional experimentation will be required to determine whether circadian rhythms in breathing are due to underlying circadian differences in metabolism. Most respiratory phenotypes are temporally inverted between nocturnal and diurnal mammals, which is to say that humans and rodents have reversed daily patterns of breathing. In the future, investigation into the circadian rhythmicity in breathing of a diurnal animal, such as the fat sand rat (Psammomys obesus) or the Nile grass rat (Arvicanthis niloticus), could provide results that may yield more easily translatable insights into the circadian rhythms of human breathing. Additional experimentation is needed to determine whether and, mechanistically, how circadian rhythmicity in breathing contributes to the nocturnal morbidity and mortality seen in diseases such as SUDEP and SIDS.
GRANTS
This work was supported by a University of Iowa Graduate College Post-Comprehensive Exam Research Fellowship and NIH/National Institute of Neurological Disorders and Stroke (NINDS) Grants T32-NS-007421 and F31-NS-106819 to B.S.P. and by NIH/NINDS Grant R01-NS-095842 and the Beth L. Tross Epilepsy Professorship to G.F.B.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S.P. and G.F.B. conceived and designed research; B.S.P. performed experiments; B.S.P. analyzed data; B.S.P. and G.F.B. interpreted results of experiments; B.S.P. prepared figures; B.S.P. drafted manuscript; B.S.P. and G.F.B. edited and revised manuscript; B.S.P. and G.F.B. approved final version of manuscript.
REFERENCES
- 1.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 22: 350–356, 2002. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamovich Y, Ladeuix B, Sobel J, Manella G, Neufeld-Cohen A, Assadi MH, Golik M, Kuperman Y, Tarasiuk A, Koeners MP, Asher G. Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab 29: 1092–1103.e3, 2019. doi: 10.1016/j.cmet.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Ali A, Wu S, Issa NP, Rose S, Towle VL, Warnke P, Tao JX. Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav 76: 1–6, 2017. doi: 10.1016/j.yebeh.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci 27: 4359–4365, 2007. doi: 10.1523/JNEUROSCI.4131-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544–556, 2005. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan GF. Impaired CO2-induced arousal in SIDS and SUDEP. Trends Neurosci 42: 242–250, 2019. doi: 10.1016/j.tins.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan GF. Timing, sleep, and respiration in health and disease. Prog Mol Biol Transl Sci 119: 191–219, 2013. doi: 10.1016/B978-0-12-396971-2.00008-7. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 592: 4395–4410, 2014. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA 107: 16354–16359, 2010. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 464: 36–48, 2003. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 11.Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep (Basel) 38: 1793–1801, 2015. doi: 10.5665/sleep.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, Willems van Dijk K, Romijn JA, Meijer JH. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 62: 1102–1108, 2013. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model 11: 16, 2014. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol 158: 1–14, 1961. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining preBötzinger complex rhythm- and pattern-generating neural microcircuits in vivo. Neuron 91: 602–614, 2016. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis EM, Locke LW, McDowell AL, Strollo PJ, O’Donnell CP. Obesity accentuates circadian variability in breathing during sleep in mice but does not predispose to apnea. J Appl Physiol (1985) 115: 474–482, 2013. doi: 10.1152/japplphysiol.00330.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas NJ. Nocturnal asthma. Thorax 48: 100–102, 1993. doi: 10.1136/thx.48.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 19.Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav 32: 357–368, 1984. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- 20.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev 93: 107–135, 2013. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav 50: 373–378, 1991. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- 22.Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I: Nucleus ambiguus and ventral respiratory group. J Comp Neurol 294: 202–211, 1990. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- 23.Epstein RA, Epstein MA, Haddad GG, Mellins RB. Practical implementation of the barometric method for measurement of tidal volume. J Appl Physiol 49: 1107–1115, 1980. doi: 10.1152/jappl.1980.49.6.1107. [DOI] [PubMed] [Google Scholar]
- 24.Feldman JL, Speck DF. Interactions among inspiratory neurons in dorsal and ventral respiratory groups in cat medulla. J Neurophysiol 49: 472–490, 1983. doi: 10.1152/jn.1983.49.2.472. [DOI] [PubMed] [Google Scholar]
- 25.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol Regul Integr Comp Physiol 275: R1127–R1137, 1998. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 26.Fuller CA, Lydic R, Sulzman FM, Albers HE, Tepper B, Moore-Ede MC. Circadian rhythm of body temperature persists after suprachiasmatic lesions in the squirrel monkey. Am J Physiol Regul Integr Comp Physiol 241: R385–R391, 1981. doi: 10.1152/ajpregu.1981.241.5.R385. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg H, Cohen RI. Nocturnal asthma. Curr Opin Pulm Med 18: 57–62, 2012. doi: 10.1097/MCP.0b013e32834d098e. [DOI] [PubMed] [Google Scholar]
- 28.Hajek MA, Buchanan GF. Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol 115: 2286–2293, 2016. doi: 10.1152/jn.00011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, Walczak TS, Beghi E, Brodie MJ, Hauser A; ILAE Commission on Epidemiology; Subcommission on Mortality . Combined analysis of risk factors for SUDEP. Epilepsia 52: 1150–1159, 2011. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- 30.Hicks JW, Riedesel ML. Diurnal ventilatory patterns in the garter snake, Thamnophis elegans. J Comp Physiol 149: 503–510, 1983. doi: 10.1007/BF00690009. [DOI] [Google Scholar]
- 31.Holt RL, Arehart E, Hunanyan A, Fainberg NA, Mikati MA. Pediatric sudden unexpected death in epilepsy: what have we learned from animal and human studies, and can we prevent it? Semin Pediatr Neurol 23: 127–133, 2016. doi: 10.1016/j.spen.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Jacky JP. Barometric measurement of tidal volume: effects of pattern and nasal temperature. J Appl Physiol 49: 319–325, 1980. doi: 10.1152/jappl.1980.49.2.319. [DOI] [PubMed] [Google Scholar]
- 33.Janssen BJ, Tyssen CM, Duindam H, Rietveld WJ. Suprachiasmatic lesions eliminate 24-h blood pressure variability in rats. Physiol Behav 55: 307–311, 1994. doi: 10.1016/0031-9384(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 34.Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 53: 253–257, 2012. doi: 10.1111/j.1528-1167.2011.03360.x. [DOI] [PubMed] [Google Scholar]
- 35.Larsen KR, Moore JG, Dayton MT. Circadian rhythms of gastric mucus efflux and residual mucus gel in the fasting rat stomach. Dig Dis Sci 36: 1550–1555, 1991. doi: 10.1007/BF01296396. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Chen XM, Yoda T, Nagashima K, Fukuda Y, Kanosue K. Involvement of the suprachiasmatic nucleus in body temperature modulation by food deprivation in rats. Brain Res 929: 26–36, 2002. doi: 10.1016/S0006-8993(01)03374-1. [DOI] [PubMed] [Google Scholar]
- 37.Malloy JN, Paulose JK, Li Y, Cassone VM. Circadian rhythms of gastrointestinal function are regulated by both central and peripheral oscillators. Am J Physiol Gastrointest Liver Physiol 303: G461–G473, 2012. doi: 10.1152/ajpgi.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore BM, Jerry Jou C, Tatalovic M, Kaufman ES, Kline DD, Kunze DL. The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP). Epilepsia 55: 1808–1816, 2014. doi: 10.1111/epi.12793. [DOI] [PubMed] [Google Scholar]
- 39.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–206, 1972. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 40.Mortola JP. Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol 91: 119–129, 2004. doi: 10.1007/s00421-003-0978-0. [DOI] [PubMed] [Google Scholar]
- 41.Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol 76: 937–944, 1998. doi: 10.1139/y99-001. [DOI] [PubMed] [Google Scholar]
- 42.Nagai K, Nagai N, Sugahara K, Niijima A, Nakagawa H. Circadian rhythms and energy metabolism with special reference to the suprachiasmatic nucleus. Neurosci Biobehav Rev 18: 579–584, 1994. doi: 10.1016/0149-7634(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 43.Nishio T, Bando H, Bamba H, Hisa Y, Okamura H. Circadian gene expression in the murine larynx. Auris Nasus Larynx 35: 539–544, 2008. doi: 10.1016/j.anl.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis 118: 909–939, 1978. doi: 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- 45.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purnell BS, Hajek MA, Buchanan GF. Time-of-day influences on respiratory sequelae following maximal electroshock-induced seizures in mice. J Neurophysiol 118: 2592–2600, 2017. doi: 10.1152/jn.00039.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purnell BS, Thijs RD, Buchanan GF. Dead in the night: sleep-wake and time-of-day influences on sudden unexpected death in epilepsy. Front Neurol 9: 1079, 2018. doi: 10.3389/fneur.2018.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reebs SG, Mrosovsky N. Large phase-shifts of circadian rhythms caused by induced running in a re-entrainment paradigm: the role of pulse duration and light. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 165: 819–825, 1989. doi: 10.1007/BF00610880. [DOI] [PubMed] [Google Scholar]
- 49.Refinetti R, Kaufman CM, Menaker M. Complete suprachiasmatic lesions eliminate circadian rhythmicity of body temperature and locomotor activity in golden hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 175: 223–232, 1994. doi: 10.1007/BF00215118. [DOI] [PubMed] [Google Scholar]
- 50.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38: 275–325, 2007. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 52.Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia 52, Suppl 1: 28–38, 2011. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson I, Reddy AB. Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett 588: 2477–2483, 2014. doi: 10.1016/j.febslet.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Rosenwasser AM, Trubowitsch G, Adler NT. Circadian rhythm in metabolic activity of suprachiasmatic, supraoptic and raphe nuclei. Neurosci Lett 58: 183–187, 1985. doi: 10.1016/0304-3940(85)90161-2. [DOI] [PubMed] [Google Scholar]
- 55.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Høgenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 12: 966–977, 2013. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 56.Sánchez S, Sánchez C, Paredes SD, Cubero J, Rodríguez AB, Barriga C. Circadian variations of serotonin in plasma and different brain regions of rats. Mol Cell Biochem 317: 105–111, 2008. doi: 10.1007/s11010-008-9836-z. [DOI] [PubMed] [Google Scholar]
- 57.Satinoff E, Prosser RA. Suprachiasmatic nuclear lesions eliminate circadian rhythms of drinking and activity, but not of body temperature, in male rats. J Biol Rhythms 3: 1–22, 1988. doi: 10.1177/074873048800300101. [DOI] [PubMed] [Google Scholar]
- 58.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol 280: H1391–H1399, 2001. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 59.Seifert EL, Knowles J, Mortola JP. Continuous circadian measurements of ventilation in behaving adult rats. Respir Physiol 120: 179–183, 2000. doi: 10.1016/S0034-5687(00)00108-0. [DOI] [PubMed] [Google Scholar]
- 60.Seifert EL, Mortola JP. The circadian pattern of breathing in conscious adult rats. Respir Physiol 129: 297–305, 2002. doi: 10.1016/S0034-5687(01)00316-4. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu K, Fukada Y. Stereotaxic surgery for suprachiasmatic nucleus lesions in mice. Bio Protoc 7: e2346, 2017. doi: 10.21769/BioProtoc.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith HR, Leibold NK, Rappoport DA, Ginapp CM, Purnell BS, Bode NM, Alberico SL, Kim YC, Audero E, Gross CT, Buchanan GF. Dorsal raphe serotonin neurons mediate CO2-induced arousal from sleep. J Neurosci 38: 1915–1925, 2018. doi: 10.1523/JNEUROSCI.2182-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol 526: 683–694, 2000. doi: 10.1111/j.1469-7793.2000.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. J Physiol 536: 225–235, 2001. doi: 10.1111/j.1469-7793.2001.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sundar IK, Yao H, Sellix MT, Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol 309: L1056–L1075, 2015. doi: 10.1152/ajplung.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179, 2017. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teixeira LV, Waterhouse JM, Marques MD. Respiratory rhythms in stingless bee workers: circadian and ultradian components throughout adult development. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197: 361–372, 2011. doi: 10.1007/s00359-010-0620-7. [DOI] [PubMed] [Google Scholar]
- 70.Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. Neuromethods 50: 217–235, 2011. doi: 10.1007/978-1-60761-883-6_9. [DOI] [Google Scholar]
- 71.Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health 25: 85–93, 2001. [PMC free article] [PubMed] [Google Scholar]
- 72.Waite PD. Obstructive sleep apnea: a review of the pathophysiology and surgical management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85: 352–361, 1998. doi: 10.1016/S1079-2104(98)90056-7. [DOI] [PubMed] [Google Scholar]
- 73.White DP, Weil JV, Zwillich CW. Metabolic rate and breathing during sleep. J Appl Physiol (1985) 59: 384–391, 1985. doi: 10.1152/jappl.1985.59.2.384. [DOI] [PubMed] [Google Scholar]