Abstract
We evaluated the effects of differential muscle architectural adaptations on neuromuscular fatigue resistance. Seven young males and six females participated in this study. Using a longitudinal within-subject design, legs were randomly assigned to perform isometric training of the tibialis anterior (TA) three times per week for 8 wk at a short (S-group) or long muscle-tendon unit length (L-group). Before and following training, fascicle length (FL) and pennation angle (PA) of the TA were assessed. As well, fatigue-related time course changes in isometric maximal voluntary contraction (MVC) torque and isotonic peak power (20% MVC resistance) were determined before, immediately after, and 1, 2, 5, and 10 min following task failure. The fatiguing task consisted of repeated maximal effort isotonic (20% MVC resistance) contractions over a 40° range of motion until the participant reached a 40% reduction in peak power. Although there was no clear improvement in neuromuscular fatigue resistance following training in either group (P = 0.081; S-group: ∼20%; L-group: ∼51%), the change in neuromuscular fatigue resistance was related positively to the training-induced increase in PA (∼6%, P < 0.001) in the S-group (r = 0.739, P = 0.004) and negatively to the training-induced increase in FL (∼4%, P = 0.001) in the L-group (r = −0.568, P = 0.043). Both groups recovered similarly for MVC torque and peak power after the fatiguing task as compared with before training. We suggest that the relationships between the changes in muscle architecture and neuromuscular fatigue resistance depend on the muscle-tendon unit lengths at which the training is performed.
NEW & NOTEWORTHY Eight weeks of isometric training at a long or short muscle-tendon unit length increased and did not change fascicle length, respectively. The “width” of the torque-angle relationship plateau became broader following isometric training at the long length. Despite marked differences in muscle architecture and functional adaptations between the groups, there was only a small-magnitude improvement in neuromuscular fatigue resistance, which was surprisingly negatively related to increased fascicle length in the long length-training group.
Keywords: dorsiflexion, fascicle length, isometric and isotonic contractions, number of repetitions to failure, pennation angle
INTRODUCTION
Neuromuscular fatigue is defined as any exercise-induced reduction in the ability to generate force or power, regardless of whether or not the task can be sustained (34). Fatigue is highly task dependent (28), and recently, the use of isotonic contractions to quantify neuromuscular fatigue has gained popularity, owing to their closer approximation of “normal everyday movements” as compared with isometric and isokinetic contractions (2, 17, 21, 45, 46, 66–68, 76). However, despite the numerous studies using this paradigm, something that has not been considered with this approach is that the imposed isotonic resistance will represent different relative levels of maximal strength throughout a given range of motion. This would be particularly true at the starting and end points of the range of motion, owing to the distinct torque-angle relationship of the muscle group tested (11, 24). This novel assumption may be one potential explanation for divergent neuromuscular fatigability across various populations such as young versus old (21, 68, 71, 76), male versus female (18, 50, 66, 67), or trained versus untrained (37). If this is true, given that a change in muscle fascicle length (FL) presumably affects the torque-angle relationship of a muscle group (13), the muscle’s architecture could have profound influences on neuromuscular fatigue resistance when assessed via a series of maximal isotonic contractions. Therefore, it would be reasonable to hypothesize that individuals with longer fascicles would have reduced neuromuscular fatigability during an isotonic task as compared with shorter fascicles, owing to a more optimal (i.e., larger plateau region) torque-angle relationship (11, 24). This larger plateau would provide less “variability” in the imposed relative resistance throughout a set joint excursion (i.e., 20% would stay closer to that value throughout most of the range of motion). To test this hypothesis, we exploited the specific muscle architectural adaptations to isometric resistance training at short and long muscle-tendon unit (MTU) lengths.
Few studies have investigated length-dependent muscle architectural adaptations following isometric training (5, 55), and no clear consensus has yet been reached regarding consistent adaptations (57). Alegre et al. (5) did not observe a change in FL of the vastus lateralis muscle when isometric training of the knee extensors was performed at both a short and long MTU length, although an increase in pennation angle (PA) of the vastus lateralis muscle was observed when the training was performed at a long MTU length. In the study by Noorkõiv et al. (55), FL of the vastus lateralis muscle was measured at proximal, middle, and distal sites, and FL of the rectus femoris muscle was determined at only one site before and after isometric training of the knee extensors. They found an increase in FL at the middle site when the training was performed at a short MTU length and at the distal site when the training was performed at a long MTU length, with no other FL adaptations. It is important to consider that the vastus lateralis and rectus femoris muscles act in synergy with other knee extensors, and thus adaptations may have been distributed across other knee extensors, as shown in other studies (13, 26, 60). To combat this limitation, we propose the tibialis anterior (TA) as a more suitable muscle to assess functional muscle architectural adaptations. The TA occupies more than half of the total dorsiflexor muscle volume (54), and most dorsiflexion strength comes from the TA (32, 40). In the present study, we assessed, in a longitudinal design, neuromuscular fatigue resistance of the ankle dorsiflexors and muscle architecture of the TA before and following isometric ankle dorsiflexion training at a short and long MTU length. For a schematic representation of the hypothesis, please refer to Supplemental Figure S1 (Supplemental Material for this article is available online at https://doi.org/10.6084/m9.figshare.12117072.v1).
MATERIALS AND METHODS
Data accessibility.
Individual values of all supporting data are available upon request.
Participants.
Seven males (age: 27 ± 7 yr; height: 178.5 ± 6.3 cm; body mass: 78.8 ± 11.0 kg; means ± SD) and six females (age: 22 ± 3 yr; height: 169.7 ± 8.3 cm; body mass: 63.2 ± 4.2 kg; mean ± SD) were recruited to participate in this study, and both legs of each participant were assigned to a group training at a short MTU length (S-group; n = 13) or a long MTU length (L-group; n = 13). Dominant legs for four males and nondominant legs for the other 3 males were assigned to S-group, and the opposite legs were assigned to the L-group randomly. Furthermore, dominant legs for three females and nondominant legs for the other three females were assigned to the S-group, and the opposite legs were assigned to L-group randomly. Here, we defined the preferred kicking leg as the dominant leg. The participants were free of cardiovascular and neuromuscular diseases, were recreationally active, and did not perform any specific training regarding ankle dorsiflexion. Participants provided informed written consent. This study was approved by the University of Guelph Research Ethics Board (REB no. 19-06-002).
Experimental design.
Supplemental Figure S1 (https://doi.org/10.6084/m9.figshare.12117072.v1) shows a schematic of the experimental design.
Both before and after the 8-wk training period (outlined below: 0 wk and 8 wk), measurements for both legs were performed on 2 separate days in a random order. The measurements at 8 wk were conducted 2–6 days after the last training day.
On each testing day, muscle size, PA, and FL of the TA were determined using a B-mode ultrasound diagnostic system (MicrUs EXT-1H; Telemed), followed by determining peak-to-peak amplitudes of the compound muscle action potential (Mmax) of the TA and the soleus muscle (SOL) to normalize surface electromyography (EMG) data across legs and participants and before and after training. Baseline neuromuscular measurements included electrically evoked peak torque, isometric maximal voluntary contraction (MVC) peak torque, voluntary activation (VA), a torque-angle relationship across nine ankle joint angles, and isotonic peak power. Isotonic peak power measurements were performed using a 20% MVC resistance. These measures were followed by a fatiguing task consisting of repeated maximal effort isotonic contractions (20% MVC resistance) to task failure (defined below). Immediately after the fatiguing task (post) and throughout recovery [1 (R1), 2 (R2), 5 (R5) and 10 min (R10) following task failure], evoked torque, isometric MVC torque with VA, and isotonic parameters were reassessed. During isometric and isotonic contractions, EMG signals were recorded from the TA and SOL.
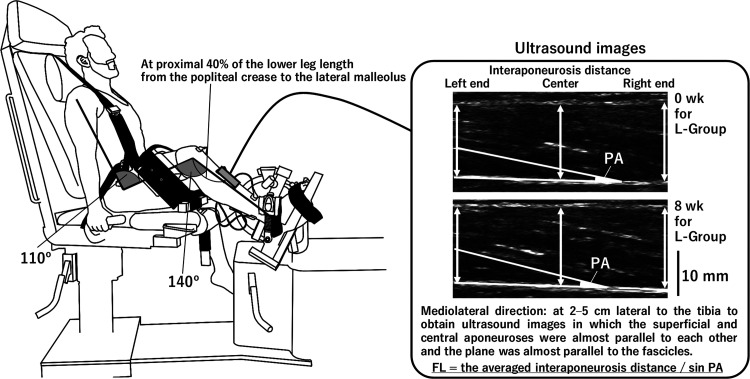
A Humac NORM dynamometer (CSMi Medical Solutions) was used to determine joint torque (Nm), power (W), and angular velocity (rad/s). The participants sat on the dynamometer with their hip flexed at 110°, knee flexed to 140°, and medial malleolus aligned with the dynamometer’s axis of rotation (180° being full extension) (Fig. 1). Care was taken to adjust the center of rotation of the ankle joint with that of the dynamometer. The pelvis and ankle were secured on the seat and a foot pedal adapter, respectively, with a seat belt and/or nonelastic straps. The relative position of the participant in relation to the dynamometer was carefully matched using scales attached to the dynamometer between the measurements at 0 wk and 8 wk and throughout the training period.
Fig. 1.
Experimental setup. Participants performed all testing and training while sitting on the dynamometer with a hip and knee angle of 110° and 140°, respectively. Ultrasound images were collected while the ankle was positioned at 15°and 40° of plantar flexion. 0 wk and 8 wk, before and after an 8-wk training; FL, fascicle length, L-group, a training group at long muscle-tendon unit length; PA, pennation angle.
The experimental data other than ultrasound data were stored at a sampling frequency of 2,000 Hz on a personal computer using LabChart software (version 8.1.11; ADInstruments) after the A/D conversion (PowerLab16/35; ADInstruments). The torque, angular velocity, and power data were smoothed using a 10-point moving average, and the EMG data were band-pass filtered between 20 and 450 Hz. Only when analyzing evoked torque and VA offline, the torque data were low-pass filtered at 500 Hz instead of the smoothing. During the voluntary isometric and isotonic measurements, strong verbal encouragement and visual feedback were provided to the participants at all times.
Muscle size and architecture of the TA.
To determine the measurement site of muscle size and architecture of the TA, the proximal 30% (1, 75) and 40% (56) of the leg length from the popliteal crease to the lateral malleolus for each participant were measured to the nearest 0.5 cm with a steel tape when standing upright. First, cross-sectional ultrasound images of the TA were obtained at the proximal 30% of the leg length twice to determine muscle thickness, which is defined as the distance from the subcutaneous adipose tissue-muscle interface to the deep aponeurosis of the TA (1, 75) and is an index of muscle size. During thickness measurements, the participants stood quietly. Afterwards, the participants sat on the Humac NORM dynamometer (CSMi Medical Solutions), and longitudinal ultrasound images of the TA were collected to measure PA and FL. These measures were performed at rest with the ankle joint positioned to 15° and 40° (anatomic position = 0°, positive value = plantar flexion). We obtained three images at each angle in a random order. Imaging was obtained using a 65-mm-wide linear array probe (LV8-4L65S-3; frequency: 4–8 MHz; Telemed), which was placed at the proximal 40% of the leg length and at 2–5 cm lateral to the tibia to obtain ultrasound images in which the superficial and central aponeuroses were almost parallel to each other and the plane was almost parallel to the fascicles, with water-soluble transmission gel (Fig. 1). When obtaining the ultrasound images, the examiner avoided compressing the muscle. The same position was used both before and after training.
The obtained images were stored on a personal computer as a DICOM file and analyzed using an image analysis software package (sliceOmatic version 5.0 Rev-2d; TomoVision). The average value of two measurements was used for the muscle thickness. PA and FL were determined in accordance with previous studies (16, 48, 56). The angle between the fascicle and central aponeurosis was defined as PA, and FL was calculated as the interaponeurosis distance divided by the sine component of PA (Fig. 1). Here, the averaged interaponeurosis distance around the right and left ends and center was used to calculate FL. On each image, analyses of muscle thickness, PA, and FL were performed once. At each angle of 15° and 40°, the three values of PA and FL were averaged. Given that we used repeated isotonic dorsiflexions as the fatiguing task and that the angle at instantaneous peak power at each time point was 26.5 ± 3.2° (14.6–35.1°), the average values of PA and FL at 15° and 40° were used as the representative values. The coefficient of variation (CV) and intraclass correlation coefficient (ICC) for the measured values of each parameter related to ultrasound images were calculated to evaluate the accuracy of the examiner. The CV and ICC (type 1, type 2) for the two values of muscle thickness (13 participants × 2 legs) were 0.6 ± 0.5% and 0.993 (P < 0.001). The CVs and ICCs (type 1, type 3) for the three values of PA and FL (13 participants × 2 legs × 2 angles) were 2.6 ± 1.3% and 0.988 (P < 0.001) for PA and 3.0 ± 1.5% and 0.982 (P < 0.001) for FL, respectively.
Mmax of the TA and SOL.
After ultrasound images were obtained, Mmax of the TA and SOL were determined using an EMG system (Octal Bioamp FE238; ADInstruments) and a constant-current variable voltage stimulator (DS7AH, Digitimer Ltd.), which were connected to the A/D converter (PowerLab16/35; ADInstruments). The participants sat on the dynamometer with the ankle at 40°. The sites of placement of Ag/AgCl surface EMG electrodes (20 mm diameter, Cleartrace, 1700-030; CONMED) were in accordance with a previous study of Chen and Power (16). One electrode was placed ∼7 cm inferior and 2 cm lateral to the tibial tuberosity, and a second electrode was placed over the tendon for the TA, and one electrode was placed ∼2 cm inferior to the border of the gastrocnemius and a second electrode over the Achilles tendon for the SOL. A ground electrode was positioned over the patella. Before the electrode placements, all recording areas were shaved and thoroughly cleaned with rubbing alcohol. Subsequently, the site to stimulate the deep fibular nerve (innervating the TA) was identified by palpating the region posterolateral to the fibular head and moving distally. The site to stimulate the tibial nerve (innervating the SOL) was identified by palpating deep in the popliteal fossa, lateral to the semitendinosus tendon. Once located, a standard clinical bar electrode (interelectrode distance of 30 mm; Empi) was coated in conductive gel and positioned over the nerve to elicit an involuntary twitch response via a 400-V, 200-μs-width rectangular pulse. Stimulus intensity was increased until plateaus in Mmax and twitch torque amplitude were reached, and this Mmax obtained from a single trial was used to normalize agonist (TA) or antagonist (SOL) EMG signals.
Measurements before the fatiguing task and torque-angle relationship.
Dorsiflexion peak doublet twitches were evoked via deep fibular nerve stimulation at 100 Hz at a joint angle of 40° using supramaximal stimulus intensity (the current at the time of obtaining the Mmax multiplied by 1.4; 28–140 mA). After doublet torque was determined from a single trial, the participants performed two 3-s isometric dorsiflexion MVCs at 40° with a 3-min rest interval, and the highest torque value was used as the before the fatiguing task (pre) value. Here, all of the differences between two values of isometric MVC torque were <10% of the higher one for each participant at both 0 wk and 8 wk. On the second MVC attempt, VA was assessed using the interpolated twitch technique (ITT). Supramaximal doublet stimulations were interpolated ∼2 s after the beginning and end of contraction, respectively, for VA assessment using the following formula: VA (%) = [1 – (interpolated twitch torque/control twitch torque)] × 100. A value of 95% VA or higher was deemed “near maximal” (16), and all participants met this condition at pre at both 0 wk and 8 wk. The reliability and validity of the ITT were ensured elsewhere (12). The root mean square values of EMG signals (RMS-EMGs) for the TA and SOL during the highest MVC were evaluated over a 0.5-s period around the peak torque, excluding the time point of stimulation. After two isometric MVCs were conducted, the participants performed 3-s isometric dorsiflexion MVCs at nine ankle joint angles (−10°, 0°, 5°, 10°, 15°, 20°, 25°, 30°, and 40°) in a random order, with a 2-min rest interval between maximal efforts. Following a 3-min rest, MVC torque and VA were reassessed at 40° to ensure that no fatigue was present. For all participants, isometric MVC torque was >90% of the pre value, and VA was near maximal (i.e., >95%) at both 0 wk and 8 wk. Thus, after it was confirmed that there was no fatigue, the participants were instructed to perform maximal effort isotonic dorsiflexions with a resistance set to 20% MVC three times every 3–4 s from an ankle joint angle of 40° to 0°. Here, the first decimal place of 20% MVC was rounded off to set the resistance value due to the specification of the Humac NORM dynamometer. The foot pedal adapter was automatically (passively) returned to its initial position (i.e., 40° ankle joint angle) 1 s after a single dorsiflexion. Peak power was calculated using the product of angular velocity and torque. The isotonic resistance at 8 wk was programmed into the dynamometer to match 0 wk (i.e., 20% MVC at 0 wk). The average of the three values of peak power was used as pre, and the corresponding average of angular velocity and dynamic torque at instantaneous peak power were reported. In addition, RMS-EMGs for the TA and SOL during each isotonic contraction were assessed over a 0.5-s period around the peak power, and the three values were averaged for further analysis.
Fatiguing task.
The participants repeated maximal-effort 20% MVC isotonic dorsiflexions over the 40° range of motion (40°–0° of ankle joint angle) until peak power fell below 60% of pre for two consecutive contractions (2). As described above, the foot pedal adapter was passively returned to its initial position (i.e., 40° ankle joint angle) 1 s after a single dorsiflexion. Then, the participants performed dorsiflexion again as soon as the foot pedal adapter was returned. The value of 60% of pre was calculated using peak power at pre for 0 wk and 8 wk, respectively. Neuromuscular fatigue resistance was assessed by the number of repetitions to failure during the fatiguing task.
Measurements at post and R1–R10.
Immediately following task failure (post) and throughout recovery [i.e., 1 (R1), 2 (R2), 5 (R5) and 10 min (R10)], doublet peak torque was obtained once, and then a 3-s MVC with ITT and two maximal effort isotonic contractions were performed. There was a ∼3–5 s delay between the end of the fatiguing task and the doublet stimulation at post. The highest peak power, and the corresponding angular velocity and dynamic torque were evaluated at each time point, except for post. The peak power, and the angular velocity and dynamic torque at instantaneous peak power of the last contraction of the fatiguing task were used as the immediate post values. At each time point, RMS-EMGs for the TA and SOL during the isometric MVC or the selected isotonic contraction were evaluated over a 0.5-s period around the peak torque or power, respectively.
Training.
Participants completed three sets of 8 (for the 1st to 2nd wk), 9 (for the 3rd to 4th wk), or 10 (for the 5th to 8th wk) isometric dorsiflexion MVCs three times/wk, with 1–2 days between sessions for 8 wk. They performed MVCs at 0° (S-group) or 40° (L-group) ankle joint angles as fast and forcefully as possible and held the contraction for 5 s, with a 10-s rest between contractions and a 1-min rest period between sets. Both legs were trained on the same day, and it was randomly decided which leg to train first in each session. A 3-min rest period was provided when changing legs. Encouragement and visual feedback was provided to the participants throughout the training sessions.
In each training session, total areas under the time-torque curve of 24 (for the 1st to 2nd wk), 27 (for the 3rd to 4th wk), or 30 (for the 5th to 8th wk) contractions were calculated. To highlight the progressive overload and any differences between the groups, the sum of these total areas for 8 wk (24 sessions) were normalized to the sum of the total areas in the 1st wk, which was defined as the training volume. All participants were able to complete all of the training sessions.
Statistical analyses.
Whereas others (e.g., see Refs. 18, 66, and 67) found a sex difference in neuromuscular fatigability, in the present study there was no effect of sex on the number of repetitions to failure at either 0 wk or 8 wk when using a two-way repeated-measures analysis of variances (ANOVA) with a within-group factor [time (0 wk and 8 wk)] and a between-group factor [sex (male and female)]. Therefore, all data were collapsed across sex when the following analyses were performed.
The main outcomes of this study were neuromuscular fatigue resistance (the no. of repetitions to task failure) and muscle architectural parameters (muscle thickness, PA, and FL) before and after the isometric training. Hence, an a priori sample size estimation was performed for a two-way repeated measures ANOVA using the G*Power software package (version 3.1.9.4; Kiel University) before other statistical analyses. The input parameters were as follows: Statistical test = ANOVA: Repeated measures, within factors; Effect size f = 0.25; α err prob = 0.05; Power (1 − β err prob) = 0.80; Number of groups = 2; Number of measurements = 2; Corr among rep measures = 0.70; Nonsphericity correction e = 1. As a result, the total sample size (n = 26) was satisfied with this condition (n = 22).
The Shapiro-Wilk test was used to check normality of the data. The data of some parameters were not normally distributed. Considering the present experimental design, two-way or three-way repeated-measures ANOVAs (i.e., parametric tests) were required. Therefore, the corresponding data were log-transformed before analyses as needed. For ease of interpretation, data in the text and figures are presented as means ± SD of raw data, unless noted otherwise. Statistical significance was set to P < 0.05. To avoid misrepresentation of null-hypothesis significance testing, indices of effect size were also calculated. For the main effect and interaction of two-way or three-way ANOVAs, η2 was calculated as an index of the effect size. In addition, when a paired t-test with Bonferroni or Holm-Bonferroni corrections revealed a significant difference between the two values, Cohen’s d was calculated as another index of the effect size. The values of η2 or d were interpreted as η2 < 0.01 or d < 0.20 for trivial, 0.01 ≤ η2 < 0.06 or 0.20 ≤ d < 0.50 for small, 0.06 ≤ η2 < 0.14 or 0.50 ≤ d < 0.80 for medium, and 0.14 ≤ η2 or 0.80 ≤ d for large effects (19). In accordance with a previous study (38), we considered the main effects, interactions, or differences to be substantial if both effect size was ≥ small (i.e., η2 ≥ 0.01 or d ≥ 0.20) and P < 0.05. Statistical analyses were performed using statistical analysis software (SPSS 25.0; IBM) and an Excel calculator for Holm-Bonferroni correction (doi:10.13140/RG.2.2.28346.49604).
Torque-angle relationships for each group were evaluated by the absolute and relative values. When using the absolute value, we found the optimum angle where the highest average value of torque for all participants was observed in each group at each of 0 wk and 8 wk. When using the relative value, for each participant we found the highest value of torque, and each value of torque was normalized to the highest value in each group at each of 0 wk and 8 wk. Subsequently, we found the optimum angle where the highest average value of normalized torque for all participants was observed in each group and time (0 wk and 8 wk). In both cases, differences between the optimum angle and the other angles were examined using a paired t test with Holm-Bonferroni correction and Cohen’s d. We defined the region, including no substantial differences from the optimum angle as the plateau region of torque-angle relationship.
Training effects on muscle size and architecture of the TA, the number of repetitions during the fatiguing task, and each parameter (isometric MVC torque, doublet torque, VA, isotonic peak power, angular velocity, dynamic torque, and RMS-EMGs for the TA and SOL) at pre were investigated using a two-way repeated-measures ANOVA with a within-group factor [time (0 wk and 8 wk)] and a between-group factor [group (S-Group and L-Group)]. In this case, Cohen’s d was also calculated using the data at 0 wk and 8 wk for each group as needed.
To investigate effects of changes in muscle architecture on a change in neuromuscular fatigue resistance, the ratios of PA and FL and the number of repetitions at 8 wk to at 0 wk were expressed as a percentage and then log-transformed, and their relationships were examined using Pearson’s product-moment correlation coefficients in the S-group and L-group. These figures are presented using the log-transformed value.
A three-way repeated-measures ANOVA with two within-group factors [time (0 wk and 8 wk) and time point (pre, post, R1, R2, R5, and R10)] and a between-group factor [group (short and long)] was used to evaluate time course changes in each parameter (isometric MVC torque, doublet torque, VA, isotonic peak power, angular velocity, dynamic torque, and RMS-EMGs for the TA and SOL) and training effects on them. Here, the post–R10 values relative to the pre value at 0 wk and 8 wk for each group were used. When a main effect of time point was significant without any significant interactions, Bonferroni multiple-comparison test was used to determine the differences in each parameter between pre and the other time points. In addition, Cohen’s d was also calculated to determine the corresponding differences at 0 wk and 8 wk for each group.
If there was a difference in the training volume between the groups, it might affect the interpretation of the results of this study. Therefore, an unpaired t test was used to investigate the presence of the difference.
RESULTS
Training volume.
The training volumes (i.e., the sum of the total area under the time-torque curve of each contraction from the 1st to the 8th wk) were 9.5 ± 1.4 times and 9.6 ± 0.7 times higher than the sum of the total area in the 1st wk for S-group and L-group, respectively. There was no difference in training volume between groups.
Training-induced changes in parameters related to evoked, isometric, and isotonic contractions at pre.
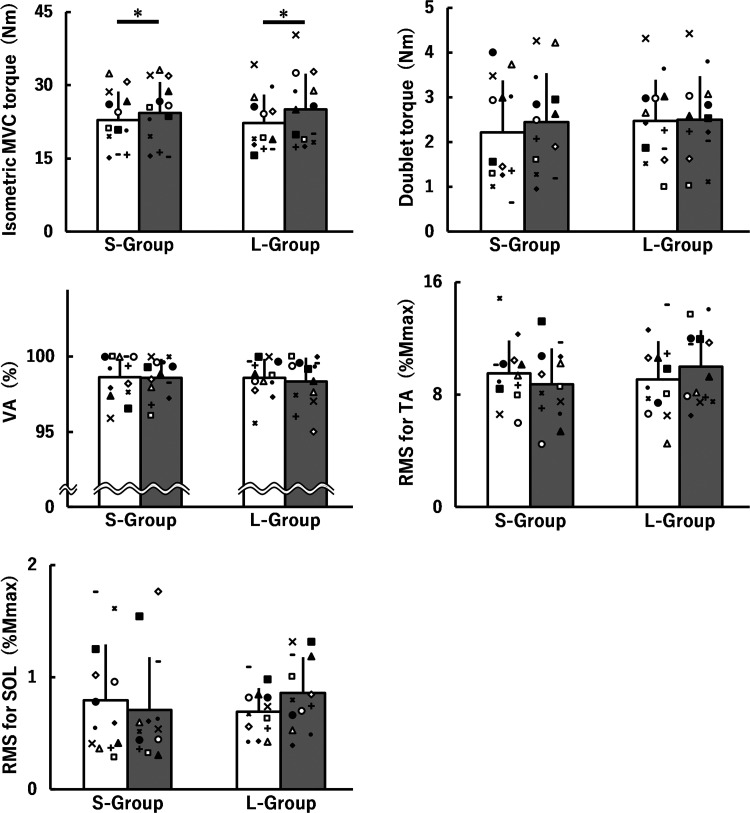
Isometric MVC torque, doublet torque, VA, and RMS-EMGs normalized to Mmax during isometric MVC at pre at 0 wk and 8 wk in the S-group and L-group are shown in Fig. 2. There was no time × group interaction or main effect of group for isometric MVC torque, doublet torque, VA, or RMS-EMG for the TA or SOL. A main effect of time was found for isometric MVC torque [F(1, 24) = 16.215, P < 0.001, η2 = 0.03], indicating a training-induced increase in isometric MVC torque at pre for the S-group (d = 0.25) and L-group (d = 0.42). There were no main effects of time for doublet torque, VA, or RMS-EMGs for the TA or SOL.
Fig. 2.
Isometric maximal voluntary contraction (MVC) torque, doublet torque, voluntary activation (VA), and root mean square values of electromyography signals (RMS-EMGs) for the tibialis anterior (TA) and soleus (SOL) muscles during isometric MVCs before (white) and after (gray) 8 weeks of isometric training at short (S-group) and long (L-group) muscle-tendon unit lengths. The data of VA and RMS for SOL were nonnormally distributed and, therefore, log-transformed when analyzing. All data are presented as means ± SD of raw data. *Significant main effect of time with ≥ small effect size. Mmax, muscle action potential. The symbols indicate the same individual's data points before and after 8 weeks of isometric training.
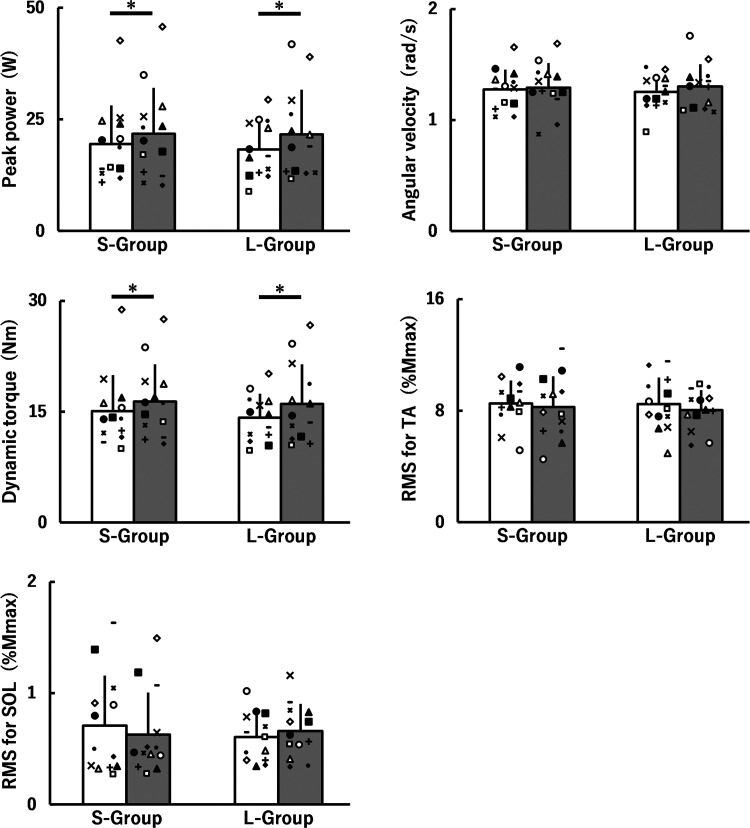
Figure 3 shows peak power, angular velocity, dynamic torque, and RMS-EMGs during isotonic contraction at pre at 0 wk and 8 wk in the S-group and L-group. A time × group interaction and a main effect of group were not found for all parameters. Main effects of time for peak power [F(1, 24) = 9.694, P = 0.005, η2 = 0.02] and dynamic torque [F(1, 24) = 11.494, P = 0.002, η2 = 0.03] demonstrated training-induced increases at pre for the S-group (peak power: d = 0.20; dynamic torque: d = 0.30) and L-group (peak power: d = 0.35; dynamic torque: d = 0.37). There were no corresponding main effects for angular velocity or RMS-EMGs for the TA and SOL.
Fig. 3.
Peak power, angular velocity, dynamic torque, and root mean square values of electromyography signals (RMS-EMGs) for the tibialis anterior (TA) and soleus (SOL) muscles during isometric MVCs before (white) and after (gray) 8 weeks of isometric training at short (S-group) and long (L-group) muscle-tendon unit lengths. The data of peak power, dynamic torque, and RMS for SOL were nonnormally distributed and, therefore, log-transformed when analyzing. All data are presented as means ± SD of raw data. *Significant main effect of time with ≥ small effect size. Mmax, peak-to-peak amplitudes of the compound muscle action potential. The symbols indicate the same individual's data points before and after 8 weeks of isometric training.
Torque-angle relationships.
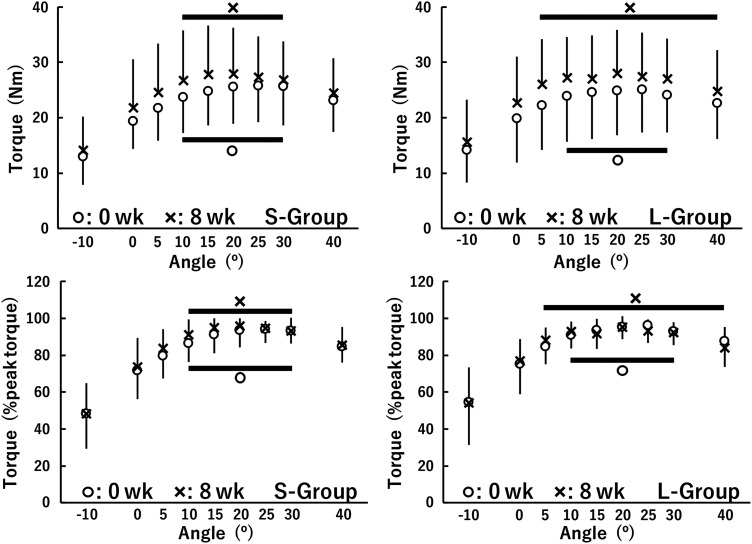
Figure 4 indicates torque-angle relationships at 0 wk and 8 wk in the S-group and L-group. Here, the region including no substantial differences from the optimum angle was defined as the plateau region of torque-angle relationship. In both groups, the optimum angles determined by the absolute or normalized torque were 25° at 0 wk and 20° at 8 wk. In the S-group, the values of absolute and normalized torques at −10°, 0°, 5°, and 40° were lower than that at the optimum angle (P ≤ 0.044, d = 0.44–3.01), and there were no differences in the corresponding torque values between 10°–30° at either 0 wk (compared with 25°) or 8 wk (compared with 20°), indicating no change in the plateau region (10°–30°) between 0 wk and 8 wk. Similarly to the S-group, the lower values of absolute and normalized torques at −10°, 0°, 5°, and 40° compared with at the optimum angle (25°) were found (P ≤ 0.015, d = 0.34–2.39), without differences between 10°–30°, at 0 wk in the L-group. Meanwhile, in the L-group, the corresponding torque value at the optimum angle (20°) was higher than those at −10° and 0° (P = 0.017–0.019, d = 0.71–1.51) but not at the other angles (5°–40°) at 8 wk. Thus, the plateau region became broader at 8 wk (5°–40°) than at 0 wk (10°–30°) in the L-Group. In other words, whereas there were minimal shifts in optimum angle of torque production (from 25° to 20°), there were robust effects of training on the “width” of the plateau such that the L-group had a larger plateau region than the S-group following the 8-wk training.
Fig. 4.
Torque-angle relationships for the dorsiflexors before and after 8 weeks of isometric training (0 wk and 8 wk) at short (S-group) and long (L-group) muscle-tendon unit lengths. A thick solid line indicates the plateau region of the torque-angle relationship. The data were nonnormally distributed and, therefore, log-transformed when analyzed and are presented as means ± SD of raw data.
Muscle size and architecture and neuromuscular fatigue resistance.
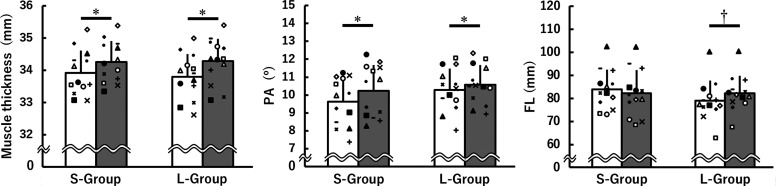
Figure 5 shows muscle thickness, PA, and FL of the TA at 0 wk and 8 wk in the S-group and L-group. There was no time × group interaction for muscle thickness or PA without a main effect of group. A main effect of time was found for muscle thickness [F(1, 24) = 64.573, P < 0.001, η2 = 0.08] and PA [F(1, 24) = 29.472, P < 0.001, η2 = 0.03], demonstrating training-induced increases in muscle thickness and PA regardless of MTU length [muscle thickness: d = 0.48 (S-group) and 0.69 (L-group); PA: d = 0.43 (S-group) and 0.26 (L-group)]. For FL, a time × group interaction was observed [F(1, 24) = 14.458, P = 0.001, η2 = 0.02]. There was no difference in FL at 0 wk between the groups (P = 0.166). The greater FL at 8 wk than at 0 wk was found in the L-group (P = 0.001, d = 0.39), without the corresponding change in the S-group.
Fig. 5.
Muscle thickness, pennation angle (PA), and fascicle length (FL) of the tibialis anterior muscle before (white) and after (gray) 8 weeks of isometric training at short (S-group) and long (L-group) muscle-tendon unit lengths. Data are presented as means ± SD. *Significant main effect of time with ≥ small effect size; †significant difference with ≥ small effect size between before and after the 8 weeks of isometric training. The symbols indicate the same individual's data points before and after 8 weeks of isometric training.
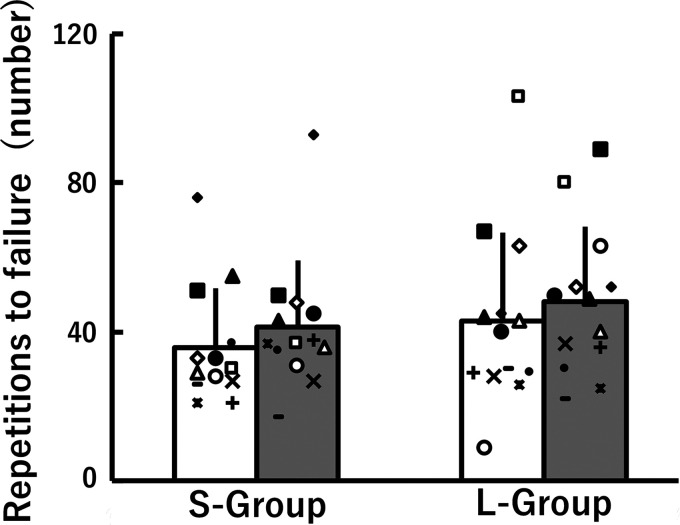
The number of repetitions to task failure at 0 wk and 8 wk in the S-group and L-group is shown in Fig. 6. There was no clear time × group interaction nor main effects of time or group; however, the main effect of time almost reached statistical significance [F(1, 24) = 3.325, P = 0.081, η2 = 0.03] with small effects of η2 (0.03) and d [0.37 (S-group) and 0.36 (L-Ggroup)], indicating a small magnitude improvement in fatigue resistance following training for both groups.
Fig. 6.
No. of repetitions to failure during repeated isotonic dorsiflexion before (white) and after (gray) 8 weeks of isometric training at short (S-group) and long (L-group) muscle-tendon unit lengths. The data were nonnormally distributed and, therefore, log-transformed when analyzed and are presented as means ± SD of raw data. The symbols indicate the same individual's data points before and after 8 weeks of isometric training.
Relationships between training-induced changes in muscle architecture and neuromuscular fatigue resistance.
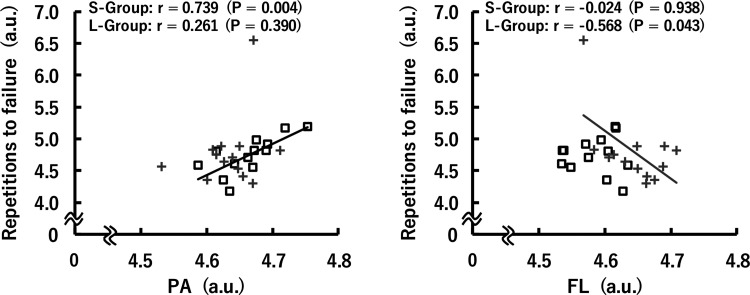
Figure 7 indicates the relationships between PA or FL and the number of repetitions to task failure at 8 wk normalized to 0 wk, which are presented using log-transformed values. In the S-group, there was a significant positive correlation between PA and the number of repetitions (r = 0.739, P = 0.004). On the other hand, FL significantly and negatively correlated with the number of repetitions in the L-group (r = −0.568, P = 0.043).
Fig. 7.
Relationships of the ratios of pennation angle (PA) and fascicle length (FL) of the tibialis anterior muscle with the ratio of the no. of repetitions during repeated isotonic dorsiflexion following 8 weeks of isometric training (after/before the training) at short (S-group; □) and long (L-group; +) muscle-tendon unit lengths. The data were nonnormally distributed and, therefore, log-transformed when analyzed and are presented using log-transformed values. a.u., arbitrary units.
Recovery from fatigue.
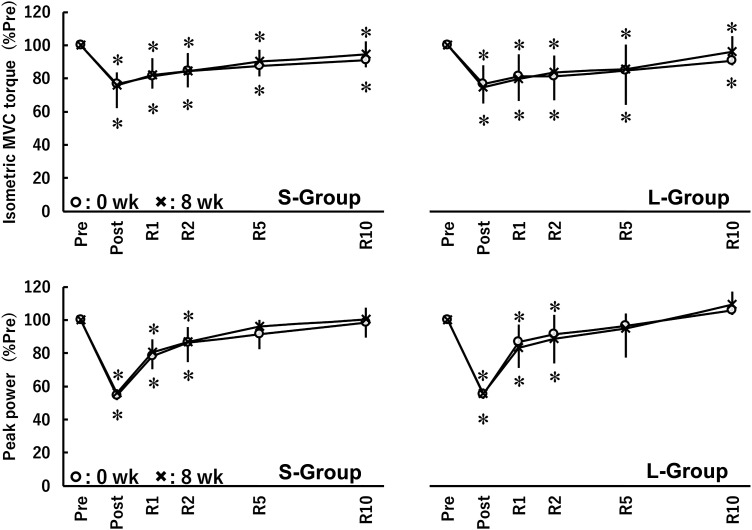
Figure 8 displays the time course changes in isometric MVC torque during isometric MVC and peak power during the isotonic contractions at 0 wk and 8 wk in the S-group and L-group. Considering the priority and readability of the results, the corresponding data of all other parameters are shown in Supplemental Fig. S2 (doublet torque, VA, and RMS-EMGs during isometric MVC) or Supplemental Fig. S3 (angular velocity, dynamic torque. and RMS-EMGs during isotonic contractions). The related values of F and P and effect size are summarized in Supplemental Table S1 or Supplemental Table S2. There were no interactions or main effect of group for all parameters. A main effect of time point was found for all parameters other than VA. Isometric MVC torque did not recover by R10, and peak power recovered by R5.
Fig. 8.
Time course changes in isometric maximal voluntary contraction (MVC) torque during isometric MVC and peak power during maximal isotonic contraction before and after 8 weeks of isometric training (0 wk and 8 wk) at short (S-group) and long (L-group) muscle-tendon unit lengths. There were 6 time points: before (pre) and immediately after (post) and 1 min (R1), 2 min (R2), 5 min (R5), and 10 min (R10) after a fatiguing task. The data were nonnormally distributed and, therefore, log-transformed when analyzed and are presented as means ± SD of raw data. *Significant difference from pre with ≥ small effect size at the same time.
DISCUSSION
Main findings.
The main findings of this study were the following. 1) There was a training-induced increase in PA in both groups, whereas FL only increased in the group training at long MTU lengths, and although the training-induced change in optimum angle of torque production was similar between both groups, this increase in FL corresponded with a wider plateau region of the torque-angle relationship; 2) there was a small magnitude improvement in fatigue resistance, which was positively related to the increase in PA in the group training at short MTU lengths and negatively related to the increased FL in the group training at long MTU lengths; and 3) following training, both groups recovered similarly following the fatigue task for isometric MVC torque and peak power following the fatigue task. These results do not support our hypothesis. Despite increasing FL and the width of the plateau region of the torque-angle relationship in the group training at long MTU lengths, these changes appear to have a negative effect on individual differences in training-induced changes in fatigue resistance.
Isometric training-induced changes in muscle thickness, PA, and FL of the TA.
In the present study, muscle thickness and PA increased in both groups following the 8 wk of training (Fig. 5). An increase in PA is considered to be a space-saving strategy for packing muscle fibers with increased diameters into a limited area of aponeurosis (35), and the PA increase is often accompanied with muscle hypertrophy following resistance training (27, 29), and these assumptions are consistent with the present results. On the other hand, training-induced increases in FL were observed only for the L-group (Fig. 5). Training-induced muscle architectural adaptions are disparate across studies and training modalities (25). Some studies report increases in both PA and FL (7, 49, 77), whereas others report no change (51, 64, 69, 72) or only increases in FL (73) or PA (27, 30, 60). Based on previous studies (49, 74), a greater FL increase induced by training is suggested to occur when training is performed at long MTU lengths due to a longer muscle stretch. The present study is in line with this suggestion but is inconsistent with other previous studies performing isometric training at short and long MTU lengths (5, 55). In both previous studies, participants were laid supine with the knees fully extended (i.e., at a knee joint angle of 0°) during the FL measurements, whereas the isometric training was performed at ∼40–50° (short MTU length) or ∼90° (long MTU length) of knee joint angles. That is, there was a great difference between the angles where the measurement and the training were performed. If this is one of the reasons for not finding the isometric training-induced increase in FL in the previous studies (5, 55), it is not surprising that the corresponding FL increase (the average value at 15° and 40°) was found in the L-group (40°) but not in the S-group (0°) in the present study. Of course, as described in the introduction, the difference in the examined muscles is likely to be another reason for the discrepancy between the training-induced adaptation in FL in the present (the dorsiflexors) and the previous studies (the knee extensors) (5, 55).
Effects of changes in FL of the TA on changes in torque-angle relationships for the dorsiflexors.
The torque-angle relationship for the dorsiflexors at 0 wk was similar to those reported in previous studies (43, 47). Following training, FL of the TA increased (Fig. 5), and the torque-angle relationship for the dorsiflexors showed adaptations for the L-group but not for the S-group (Fig. 4). The training-induced increase in FL of the TA resulted in a broader plateau region of torque-angle relationship of the dorsiflexors. Ultimately, longer FLs increased the torque-producing potential throughout a range of motion (11), which led to the expectation of less fatigability during the isotonic task as compared with shorter FLs, as described in the introduction. Nevertheless, this hypothesis was rejected.
Effects of training-induced changes in PA on neuromuscular fatigue resistance.
Many studies have examined the effects of resistance training on fatigability (e.g., see Refs. 23, 33, 39, 59, and 65), but results are equivocal. In the present study, although as a group the training-induced improvement of neuromuscular fatigue resistance (i.e., the training-induced increase in the number of repetitions) did not reach statistical significance (a main effect of time: P = 0.081), there were small effects [η2 (0.03) and d (0.37) (S-group) and 0.36 (L-Group); Fig. 6)] due to large variability between participants. Additionally, the training-induced increase in PA was found in both groups (Fig. 5), and there was a positive relationship between the training-induced increase in PA and the small-magnitude improvement of neuromuscular fatigue resistance in the S-group (Fig. 7). Thus, PA, rather than FL, seems to have a greater effect on neuromuscular fatigability. PA has important physiological effects on the force-velocity relationship and thus peak power (20). Considering that an increase in curvature of the force-velocity relationship is induced by fatigue (41, 44), PA is also expected to influence neuromuscular fatigue resistance. PA relates to muscle strength (14, 70), owing to the muscle size-strength association (3, 4, 31). Furthermore, the increased PA allows for muscle fibers to shorten less for a given tendon displacement due to the rotation of pennate muscle fibers during contraction (52), increasing the likelihood that a fascicle with a greater PA operates closer to its optimum length and, based on the force-length relationship, is able to generate more force (13, 52). Additionally, muscle gearing that is influenced by PA (8, 9) allows for an uncoupling of external angular velocity and internal muscle fiber shortening velocity. This is a potential unexplored “dynamic” architectural adaptation promoting neuromuscular fatigue resistance with increasing PA.
Blazevich et al. (13) examined the changes in PA and FL of the vastus lateralis muscle induced by 10 wk of concentric contraction- or eccentric contraction-only training. In this previous study, PA increased 5 wk after the start of the training and continued to increase further after 5 wk for both training groups. On the other hand, FL increased 5 wk after the start of training but did not change thereafter, regardless of training modality. If these results apply to other muscles even when isometric training is performed, further increases in PA can be expected by carrying out the isometric training longer than 8 wk, possibly resulting in a clearer improvement of neuromuscular fatigue resistance.
Isometric training-induced changes in the parameters related to isometric and isotonic contractions at pre.
Isometric MVC torque increased after the 8 wk of isometric training for both groups (Fig. 2). The present results showing the improvement of isometric MVC torque with no changes in doublet torque and RMS-EMGs are in line with those previously reported (36) following 4 wk of explosive strength training for the TA. Moreover, complete VA is often observed with the dorsiflexors (10, 16, 17) during isometric MVCs, and therefore, no further neural adaptations may have been observed, as shown in the results of VA and RMS-EMGs (Fig. 2). Both groups increased peak power and dynamic torque, with no changes in angular velocity (Fig. 3). Muscle hypertrophy was likely to increase dynamic torque as well as isometric MVC torque, and correspondingly, peak power also seemed to be increased in both groups.
Effects of isometric training on time course changes in the parameters following the fatiguing task.
Recovery from neuromuscular fatigue can be evaluated as time course changes in isometric MVC torque (or force) and/or power during and following a fatiguing task (2, 45, 46, 66, 76). The participants repeated maximal-effort 20% MVC isotonic dorsiflexions until peak power fell below 60% of Pre twice in a row, and the values of peak power at Pre were used for 0 wk and 8 wk. Correspondingly, for each group, the present study indicates that the time course changes in isometric MVC torque and peak power following the fatiguing task (at post, R1, R2, R5, and R10) did not change before or after the 8-wk training period (Fig. 8). Moreover, the corresponding changes in other indices of peripheral fatigue (doublet torque; see Refs. 15 and 61) or central fatigue (VA; see Refs. 15 and 61; and RMS-EMGs normalized to Mmax of an agonist muscle; see Refs. 58 and 62) were also not found for both groups. That is, the effects of the 8 wk of isometric training at short or long MTU lengths on the recovery aspect following the fatiguing task were not observed in the present study.
Limitations.
In the present study, FL was estimated as the interaponeurosis distance divided by the sine component of PA due to the limited width of the ultrasound probe, which could involve a large error (6). However, the values of PA and FL obtained here (Fig. 5) were consistent with those of previous studies (22, 42, 48, 53, 56). Furthermore, the changes in FL (Fig. 5) corresponded to those in torque-angle relationships (Fig. 4), and the correlations between PA or FL and the number of repetitions were found using the log-transformed values (Fig. 7). Based on these results, the effect of the aforementioned possible error on the interpretation of the present results seems to be small. Another limitation is that we did not determine PA and FL during contractions. Previous studies (48, 53, 63) found contraction-induced increases in PA and decreases in FL. For instance, Maganaris and Baltzopoulos (48) reported that PA increased by more than 60% and FL decreased by nearly 40% during MVC compared with at rest. As described previously, evaluation of the system’s gear ratio during contraction against a range of loads for the TA, and length-dependent alterations to muscle energetics should be essential to investigate the effect of muscle architectural adaptation induced by training on the neuromuscular fatigue resistance more precisely. Therefore, if there are individual differences in the modifiability of PA and FL during contraction, we have to clarify this point to better understand the findings of the present study.
Conclusions.
In the present study, following 8 wk of isometric training at a short or long MTU length, we observed an increase in PA for both groups and an increase in FL for the L-group. Although there were minimal shifts in optimum angle of torque production, there were robust effects of training on the “width” of the plateau of the torque-angle relationship such that the L-group had a larger plateau region than the S-group following the 8-wk training. Despite the differences in the training-induced architectural and functional changes between the groups, there was only a small magnitude improvement in neuromuscular fatigue resistance in either group. However, this change in neuromuscular fatigue resistance was positively related to increases in PA for the S-group and negatively related to increases in FL for the L-group. Thus, it is suggested that the relationships between the changes in muscle architecture and neuromuscular fatigue resistance depend on the MTU lengths where the training is performed.
GRANTS
This study was supported by JSPS KAKENHI Grant No. JP17KK0174 (Fund for the Promotion of Joint International Research, Fostering Joint International Research) and the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A., A.H., and G.A.P. conceived and designed research; R.A., A.H., and G.A.P. performed experiments; R.A. analyzed data; R.A., A.H., and G.A.P. interpreted results of experiments; R.A. and G.A.P. prepared figures; R.A. drafted manuscript; R.A., A.H., and G.A.P. edited and revised manuscript; R.A., A.H., and G.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brooke Davidson for assistance with the experiment and Dr. Brian Dalton for prereview comments on the manuscript.
REFERENCES
- 1.Abe T, Kondo M, Kawakami Y, Fukunaga T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol 6: 161–170, 1994. doi: 10.1002/ajhb.1310060204. [DOI] [PubMed] [Google Scholar]
- 2.Akagi R, Hinks A, Davidson B, Power GA. Differential contributions of fatigue-induced strength loss and slowing of angular velocity to power loss following repeated maximal shortening contractions. Physiol Rep 8: e14362, 2020. doi: 10.14814/phy2.14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akagi R, Takai Y, Ohta M, Kanehisa H, Kawakami Y, Fukunaga T. Muscle volume compared to cross-sectional area is more appropriate for evaluating muscle strength in young and elderly individuals. Age Ageing 38: 564–569, 2009. doi: 10.1093/ageing/afp122. [DOI] [PubMed] [Google Scholar]
- 4.Akagi R, Tohdoh Y, Takahashi H. Muscle strength and size balances between reciprocal muscle groups in the thigh and lower leg for young men. Int J Sports Med 33: 386–389, 2012. doi: 10.1055/s-0031-1299700. [DOI] [PubMed] [Google Scholar]
- 5.Alegre LM, Ferri-Morales A, Rodriguez-Casares R, Aguado X. Effects of isometric training on the knee extensor moment-angle relationship and vastus lateralis muscle architecture. Eur J Appl Physiol 114: 2437–2446, 2014. doi: 10.1007/s00421-014-2967-x. [DOI] [PubMed] [Google Scholar]
- 6.Ando R, Taniguchi K, Saito A, Fujimiya M, Katayose M, Akima H. Validity of fascicle length estimation in the vastus lateralis and vastus intermedius using ultrasonography. J Electromyogr Kinesiol 24: 214–220, 2014. doi: 10.1016/j.jelekin.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Angleri V, Ugrinowitsch C, Libardi CA. Crescent pyramid and drop-set systems do not promote greater strength gains, muscle hypertrophy, and changes on muscle architecture compared with traditional resistance training in well-trained men. Eur J Appl Physiol 117: 359–369, 2017. doi: 10.1007/s00421-016-3529-1. [DOI] [PubMed] [Google Scholar]
- 8.Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci USA 105: 1745–1750, 2008. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azizi E, Roberts TJ. Variable gearing in a biologically inspired pneumatic actuator array. Bioinspir Biomim 8: 026002, 2013. doi: 10.1088/1748-3182/8/2/026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol 100: 515–525, 2007. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 11.Baxter JR, Hullfish TJ, Chao W. Functional deficits may be explained by plantarflexor remodeling following Achilles tendon rupture repair: Preliminary findings. J Biomech 79: 238–242, 2018. doi: 10.1016/j.jbiomech.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Behm DG, St-Pierre DM, Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol (1985) 81: 2267–2273, 1996. doi: 10.1152/jappl.1996.81.5.2267. [DOI] [PubMed] [Google Scholar]
- 13.Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol (1985) 103: 1565–1575, 2007. doi: 10.1152/japplphysiol.00578.2007. [DOI] [PubMed] [Google Scholar]
- 14.Bloomquist K, Langberg H, Karlsen S, Madsgaard S, Boesen M, Raastad T. Effect of range of motion in heavy load squatting on muscle and tendon adaptations. Eur J Appl Physiol 113: 2133–2142, 2013. doi: 10.1007/s00421-013-2642-7. [DOI] [PubMed] [Google Scholar]
- 15.Burnley M, Vanhatalo A, Jones AM. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol (1985) 113: 215–223, 2012. doi: 10.1152/japplphysiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Power GA. Modifiability of the history dependence of force through chronic eccentric and concentric biased resistance training. J Appl Physiol (1985) 126: 647–657, 2019. doi: 10.1152/japplphysiol.00928.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng AJ, Rice CL. Isometric torque and shortening velocity following fatigue and recovery of different voluntary tasks in the dorsiflexors. Appl Physiol Nutr Metab 34: 866–874, 2009. doi: 10.1139/H09-085. [DOI] [PubMed] [Google Scholar]
- 18.Clark BC, Manini TM, Thé DJ, Doldo NA, Ploutz-Snyder LL. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol (1985) 94: 2263–2272, 2003. doi: 10.1152/japplphysiol.00926.2002. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, MI: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 20.Cormie P, McGuigan MR, Newton RU. Developing maximal neuromuscular power: Part 1–biological basis of maximal power production. Sports Med 41: 17–38, 2011. doi: 10.2165/11537690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Dalton BH, Power GA, Vandervoort AA, Rice CL. Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol (1985) 109: 1441–1447, 2010. doi: 10.1152/japplphysiol.00335.2010. [DOI] [PubMed] [Google Scholar]
- 22.de Boer MD, Seynnes OR, di Prampero PE, Pisot R, Mekjavić IB, Biolo G, Narici MV. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol 104: 401–407, 2008. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- 23.de Menezes Bassan N, Denadai BS, de Lima LCR, Caritá RAC, Abdalla LHP, Greco CC. Effects of resistance training on impulse above end-test torque and muscle fatigue. Exp Physiol 104: 1115–1125, 2019. doi: 10.1113/EP087204. [DOI] [PubMed] [Google Scholar]
- 24.Drazan JF, Hullfish TJ, Baxter JR. Muscle structure governs joint function: linking natural variation in medial gastrocnemius structure with isokinetic plantar flexor function. Biol Open 8: bio048520, 2019. doi: 10.1242/bio.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ema R, Akagi R, Wakahara T, Kawakami Y. Training-induced changes in architecture of human skeletal muscles: current evidence and unresolved issues. J Phys Fit Sports Med 5: 37–46, 2016. doi: 10.7600/jpfsm.5.37. [DOI] [Google Scholar]
- 26.Ema R, Saito I, Akagi R. Neuromuscular adaptations induced by adjacent joint training. Scand J Med Sci Sports 28: 947–960, 2018. doi: 10.1111/sms.13008. [DOI] [PubMed] [Google Scholar]
- 27.Ema R, Wakahara T, Miyamoto N, Kanehisa H, Kawakami Y. Inhomogeneous architectural changes of the quadriceps femoris induced by resistance training. Eur J Appl Physiol 113: 2691–2703, 2013. doi: 10.1007/s00421-013-2700-1. [DOI] [PubMed] [Google Scholar]
- 28.Enoka RM. Mechanisms of muscle fatigue: Central factors and task dependency. J Electromyogr Kinesiol 5: 141–149, 1995. doi: 10.1016/1050-6411(95)00010-W. [DOI] [PubMed] [Google Scholar]
- 29.Erskine RM, Fletcher G, Folland JP. The contribution of muscle hypertrophy to strength changes following resistance training. Eur J Appl Physiol 114: 1239–1249, 2014. doi: 10.1007/s00421-014-2855-4. [DOI] [PubMed] [Google Scholar]
- 30.Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol 110: 1117–1125, 2010. doi: 10.1007/s00421-010-1601-9. [DOI] [PubMed] [Google Scholar]
- 31.Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand 172: 249–255, 2001. doi: 10.1046/j.1365-201x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 32.Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Edgerton VR. Specific tension of human plantar flexors and dorsiflexors. J Appl Physiol (1985) 80: 158–165, 1996. doi: 10.1152/jappl.1996.80.1.158. [DOI] [PubMed] [Google Scholar]
- 33.Gacesa JZ, Jakovljevic DG, Kozic DB, Dragnic NR, Brodie DA, Grujic NG. Morpho-functional response of the elbow extensor muscles to twelve-week self-perceived maximal resistance training. Clin Physiol Funct Imaging 30: 413–419, 2010. doi: 10.1111/j.1475-097X.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- 34.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 35.Gans C, Gaunt AS. Muscle architecture in relation to function. J Biomech 24, Suppl 1: 53–65, 1991. doi: 10.1016/0021-9290(91)90377-Y. [DOI] [PubMed] [Google Scholar]
- 36.Geertsen SS, Lundbye-Jensen J, Nielsen JB. Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol (1985) 105: 915–922, 2008. doi: 10.1152/japplphysiol.01155.2007. [DOI] [PubMed] [Google Scholar]
- 37.Hartman MJ, Ryan ED, Cramer JT, Bemben MG. The effects of fatigue of the plantar flexors on peak torque and voluntary activation in untrained and resistance-trained men. J Strength Cond Res 25: 527–532, 2011. doi: 10.1519/JSC.0b013e3181bf3bc7. [DOI] [PubMed] [Google Scholar]
- 38.Häuser W, Wolfe F, Tölle T, Uçeyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 26: 297–307, 2012. doi: 10.2165/11598970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Izquierdo M, Ibañez J, Calbet JA, González-Izal M, Navarro-Amézqueta I, Granados C, Malanda A, Idoate F, González-Badillo JJ, Häkkinen K, Kraemer WJ, Tirapu I, Gorostiaga EM. Neuromuscular fatigue after resistance training. Int J Sports Med 30: 614–623, 2009. doi: 10.1055/s-0029-1214379. [DOI] [PubMed] [Google Scholar]
- 40.Jeng CL, Thawait GK, Kwon JY, Machado A, Boyle JW, Campbell J, Carrino JA. Relative strengths of the calf muscles based on MRI volume measurements. Foot Ankle Int 33: 394–399, 2012. doi: 10.3113/FAI.2012.0394. [DOI] [PubMed] [Google Scholar]
- 41.Jones DA. Changes in the force-velocity relationship of fatigued muscle: implications for power production and possible causes. J Physiol 588: 2977–2986, 2010. doi: 10.1113/jphysiol.2010.190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klimstra M, Dowling J, Durkin JL, MacDonald M. The effect of ultrasound probe orientation on muscle architecture measurement. J Electromyogr Kinesiol 17: 504–514, 2007. doi: 10.1016/j.jelekin.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Koh TJ, Herzog W. Evaluation of voluntary and elicited dorsiflexor torque-angle relationships. J Appl Physiol (1985) 79: 2007–2013, 1995. doi: 10.1152/jappl.1995.79.6.2007. [DOI] [PubMed] [Google Scholar]
- 44.Kristensen AM, Nielsen OB, Pedersen TH, Overgaard K. Fatiguing stimulation increases curvature of the force-velocity relationship in isolated fast-twitch and slow-twitch rat muscles. J Exp Biol 222: jeb204545, 2019. doi: 10.1242/jeb.204545. [DOI] [PubMed] [Google Scholar]
- 45.Lanning AC, Power GA, Christie AD, Dalton BH. Influence of sex on performance fatigability of the plantar flexors following repeated maximal dynamic shortening contractions. Appl Physiol Nutr Metab 42: 1118–1121, 2017. doi: 10.1139/apnm-2017-0013. [DOI] [PubMed] [Google Scholar]
- 46.Lee A, Baxter J, Eischer C, Gage M, Hunter S, Yoon T. Sex differences in neuromuscular function after repeated eccentric contractions of the knee extensor muscles. Eur J Appl Physiol 117: 1119–1130, 2017. doi: 10.1007/s00421-017-3599-8. [DOI] [PubMed] [Google Scholar]
- 47.Maganaris CN. Force-length characteristics of in vivo human skeletal muscle. Acta Physiol Scand 172: 279–285, 2001. doi: 10.1046/j.1365-201x.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 48.Maganaris CN, Baltzopoulos V. Predictability of in vivo changes in pennation angle of human tibialis anterior muscle from rest to maximum isometric dorsiflexion. Eur J Appl Physiol Occup Physiol 79: 294–297, 1999. doi: 10.1007/s004210050510. [DOI] [PubMed] [Google Scholar]
- 49.McMahon G, Morse CI, Burden A, Winwood K, Onambélé GL. Muscular adaptations and insulin-like growth factor-1 responses to resistance training are stretch-mediated. Muscle Nerve 49: 108–119, 2014. doi: 10.1002/mus.23884. [DOI] [PubMed] [Google Scholar]
- 50.Metcalf E, Hagstrom AD, Marshall PW. Trained females exhibit less fatigability than trained males after a heavy knee extensor resistance exercise session. Eur J Appl Physiol 119: 181–190, 2019. doi: 10.1007/s00421-018-4013-x. [DOI] [PubMed] [Google Scholar]
- 51.Morales-Artacho AJ, Padial P, García-Ramos A, Pérez-Castilla A, Argüelles-Cienfuegos J, De la Fuente B, Feriche B. Intermittent resistance training at moderate altitude: effects on the force-velocity relationship, isometric strength and muscle architecture. Front Physiol 9: 594, 2018. doi: 10.3389/fphys.2018.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhl ZF. Active length-tension relation and the effect of muscle pinnation on fiber lengthening. J Morphol 173: 285–292, 1982. doi: 10.1002/jmor.1051730305. [DOI] [PubMed] [Google Scholar]
- 53.Muraoka T, Muramatsu T, Kanehisa H, Fukunaga T. Transverse strain of aponeurosis in human tibialis anterior muscle at rest and during contraction at different joint angles. J Appl Biomech 19: 39–48, 2003. doi: 10.1123/jab.19.1.39. [DOI] [Google Scholar]
- 54.Nakagawa Y, Ratkevicius A, Mizuno M, Quistorff B. ATP economy of force maintenance in human tibialis anterior muscle. Med Sci Sports Exerc 37: 937–943, 2005. [PubMed] [Google Scholar]
- 55.Noorkõiv M, Nosaka K, Blazevich AJ. Neuromuscular adaptations associated with knee joint angle-specific force change. Med Sci Sports Exerc 46: 1525–1537, 2014. doi: 10.1249/MSS.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 56.Oda T, Himeno R, C Hay D, Chino K, Kurihara T, Nagayoshi T, Kanehisa H, Fukunaga T, Kawakami Y. In vivo behavior of muscle fascicles and tendinous tissues in human tibialis anterior muscle during twitch contraction. J Biomech 40: 3114–3120, 2007. doi: 10.1016/j.jbiomech.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Oranchuk DJ, Storey AG, Nelson AR, Cronin JB. Isometric training and long-term adaptations: Effects of muscle length, intensity, and intent: A systematic review. Scand J Med Sci Sports 29: 484–503, 2019. doi: 10.1111/sms.13375. [DOI] [PubMed] [Google Scholar]
- 58.Papaiordanidou M, Guiraud D, Varray A. Does central fatigue exist under low-frequency stimulation of a low fatigue-resistant muscle? Eur J Appl Physiol 110: 815–823, 2010. doi: 10.1007/s00421-010-1565-9. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen KK, Madsen MK, Hvid LG, Overgaard K. Concentric strength training at optimal or short muscle length improves strength equally but does not reduce fatigability of hamstring muscles. Physiol Rep 7: e14196, 2019. doi: 10.14814/phy2.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelzer T, Ullrich B, Pfeiffer M. Periodization effects during short-term resistance training with equated exercise variables in females. Eur J Appl Physiol 117: 441–454, 2017. doi: 10.1007/s00421-017-3544-x. [DOI] [PubMed] [Google Scholar]
- 61.Pethick J, Winter SL, Burnley M. Effects of ipsilateral and contralateral fatigue and muscle blood flow occlusion on the complexity of knee-extensor torque output in humans. Exp Physiol 103: 956–967, 2018. doi: 10.1113/EP086960. [DOI] [PubMed] [Google Scholar]
- 62.Place N, Yamada T, Bruton JD, Westerblad H. Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol 110: 1–15, 2010. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- 63.Raiteri BJ, Cresswell AG, Lichtwark GA. Three-dimensional geometrical changes of the human tibialis anterior muscle and its central aponeurosis measured with three-dimensional ultrasound during isometric contractions. PeerJ 4: e2260, 2016. doi: 10.7717/peerj.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raj IS, Bird SR, Westfold BA, Shield AJ. Effects of eccentrically biased versus conventional weight training in older adults. Med Sci Sports Exerc 44: 1167–1176, 2012. doi: 10.1249/MSS.0b013e3182442ecd. [DOI] [PubMed] [Google Scholar]
- 65.Roman WJ, Fleckenstein J, Stray-Gundersen J, Alway SE, Peshock R, Gonyea WJ. Adaptations in the elbow flexors of elderly males after heavy-resistance training. J Appl Physiol (1985) 74: 750–754, 1993. doi: 10.1152/jappl.1993.74.2.750. [DOI] [PubMed] [Google Scholar]
- 66.Senefeld J, Pereira HM, Elliott N, Yoon T, Hunter SK. Sex Differences in Mechanisms of Recovery after Isometric and Dynamic Fatiguing Tasks. Med Sci Sports Exerc 50: 1070–1083, 2018. doi: 10.1249/MSS.0000000000001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senefeld J, Yoon T, Bement MH, Hunter SK. Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle Nerve 48: 436–439, 2013. doi: 10.1002/mus.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Senefeld J, Yoon T, Hunter SK. Age differences in dynamic fatigability and variability of arm and leg muscles: Associations with physical function. Exp Gerontol 87: 74–83, 2017. doi: 10.1016/j.exger.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seymore KD, Domire ZJ, DeVita P, Rider PM, Kulas AS. The effect of Nordic hamstring strength training on muscle architecture, stiffness, and strength. Eur J Appl Physiol 117: 943–953, 2017. doi: 10.1007/s00421-017-3583-3. [DOI] [PubMed] [Google Scholar]
- 70.Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 35: 2377–2388, 2013. doi: 10.1007/s11357-013-9517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sundberg CW, Kuplic A, Hassanlouei H, Hunter SK. Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults. J Appl Physiol (1985) 125: 146–158, 2018. doi: 10.1152/japplphysiol.01141.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka H, Ikezoe T, Nakamura M, Yanase K, Fujita K, Motomura Y, Kusano K, Araki K, Umehara J, Saeki J, Morishita K, Ichihashi N. Improvement in muscle strength with low-load isotonic training depends on fascicle length but not joint angle. Muscle Nerve 57: 83–89, 2018. doi: 10.1002/mus.25601. [DOI] [PubMed] [Google Scholar]
- 73.Ullrich B, Holzinger S, Soleimani M, Pelzer T, Stening J, Pfeiffer M. Neuromuscular Responses to 14 Weeks of Traditional and Daily Undulating Resistance Training. Int J Sports Med 36: 554–562, 2015. doi: 10.1055/s-0034-1398529. [DOI] [PubMed] [Google Scholar]
- 74.Valamatos MJ, Tavares F, Santos RM, Veloso AP, Mil-Homens P. Influence of full range of motion vs. equalized partial range of motion training on muscle architecture and mechanical properties. Eur J Appl Physiol 118: 1969–1983, 2018. doi: 10.1007/s00421-018-3932-x. [DOI] [PubMed] [Google Scholar]
- 75.Wakahara T, Takeshita K, Kato E, Miyatani M, Tanaka NI, Kanehisa H, Kawakami Y, Fukunaga T. Variability of limb muscle size in young men. Am J Hum Biol 22: 55–59, 2010. doi: 10.1002/ajhb.20951. [DOI] [PubMed] [Google Scholar]
- 76.Wallace JW, Power GA, Rice CL, Dalton BH. Time-dependent neuromuscular parameters in the plantar flexors support greater fatigability of old compared with younger males. Exp Gerontol 74: 13–20, 2016. doi: 10.1016/j.exger.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Wells AJ, Fukuda DH, Hoffman JR, Gonzalez AM, Jajtner AR, Townsend JR, Mangine GT, Fragala MS, Stout JR. Vastus lateralis exhibits non-homogenous adaptation to resistance training. Muscle Nerve 50: 785–793, 2014. doi: 10.1002/mus.24222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual values of all supporting data are available upon request.